Highlights
-
•
Transmission of viruses in water has a significant impact on public health.
-
•
Contaminated groundwater and recreational water cause majority of virus-related WBDOs.
-
•
Noroviruses are the dominant cause of WBDOs in high-income countries.
-
•
Application of a true viral indicator would allow for better protection of public health.
Abstract
The public health impact of the transmission of viruses in water is significant worldwide. Waterborne viruses can be introduced into our recreational and finished drinking water sources through a variety of pathways ultimately resulting in the onset of illness in a portion of the exposed population. Although there have been advances in both drinking water treatment technologies and source water protection strategies, waterborne disease outbreaks (WBDOs) due to viral pathogens still occur each year worldwide. By highlighting the prevalence of viral pathogens in water as well as (1) the dominant viruses of concern, (2) WBDOs due to viruses, and (3) available water treatment technologies, the goal of this review is to provide insight into the public health impact of viruses in water.
Current Opinion in Virology 2013, 4:50–57
This review comes from a themed issue on Environmental virology
Edited by Lee-Ann Jaykus and John Scott Meschke
For a complete overview see the Issue and the Editorial
Available online 14th January 2014
1879-6257/$ – see front matter, © 2014 Elsevier B.V. All rights reserved.
Introduction
Waterborne viruses are frequently implicated as the cause of water-related gastrointestinal illness. Waterborne disease outbreaks (WBDOs) are reported each year and are associated with recreational water (RW), treated drinking water (DW), and ground water (treated and untreated). Depending on the water source, the actual source of contamination can vary; however, the two common threads are (1) the introduction of fecal material into the water source and (2) inadequate or interrupted treatment of water intended for drinking [1, 2, 3, 4]. In 2003, the World Health Organization estimated that worldwide 3.4 million deaths each year can be attributed to the water-related (water, sanitation, hygiene) transmission of pathogens (all pathogens, not just enteric viruses) [5]. For the European Union (EU), the European Environment and Health Information System estimated the annual burden of disease due to water-related pathogens at 13,548 deaths for children 0–14 years old. For the United States, Reynolds (2008) estimated 7 million illnesses and more than 1000 deaths each year were due to waterborne pathogens though these are based on model simulations and not actual values. Unfortunately, the number of illnesses and deaths due specifically to waterborne viruses is difficult to determine and thus basically unknown.
The current review (Figure 1 ) focuses on (1) the occurrence of viral pathogens of primary concern in various water sources; (2) virus-related WBDOS by water type reported worldwide over the past decade (from approximately 2000 to 2012); and (3) DW treatment options for the inactivation or removal of viruses. Finally, this review briefly discusses how we may better understand the public health impact of waterborne viruses as well as potential measures that can be taken to reduce the impact of viral pathogens in water.
Figure 1.
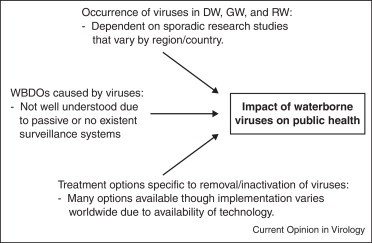
Summary of key factors effecting the impact of waterborne viruses on public health. DW: drinking water; RW: recreational water; GW: groundwater; WBDO: waterborne disease outbreak.
Waterborne viruses of primary concern
Viruses most often implicated in WBDOs include (but are not restricted to) noroviruses (NoV), Hepatitis A virus (HAV), Hepatitis E virus (HEV), adenovirus (AdV), astrovirus, enteroviruses (EV), and rotavirus (RV) (Table 1 ). Although viruses implicated in WBDOs are capable of causing a variety of acute illnesses (Table 1), acute gastrointestinal illness (AGI) is most commonly reported. Enteric viruses are host-specific (i.e. in this instance, specific to humans) and are not capable of replicating in the environment outside of its host. In addition, enteric viruses have a presumed low infectious dose (i.e. <10–103 virus particles) [6, 7, 8, 9]; prolonged (3–4 weeks), asymptomatic periods of shedding; and enhanced environmental stability due to their non-enveloped capsid structure [10]. These characteristics allow enteric viruses to play a significant role in water-related outbreaks. Noroviruses have been the largest cause of virus-related WBDOs in the U.S. since 2003, and data indicate a similar trend in selected countries (France, Japan, Sweden, Switzerland, The Netherlands, UK) [11•, 12, 13]. Aside from NoVs, HAV, HEV, and RV are still of significant concern in low-income countries without adequate water and sanitation. Additional viruses of lesser epidemiologic importance though still capable of waterborne transmission include human reovirus, parvovirus, parechovirus, polyomavirus, coronavirus, and torovirus [14, 15].
Table 1.
Viruses of primary concern for waterborne disease outbreaks.
| Family | Virus group | Properties | Associated illnesses | Public health impact |
|---|---|---|---|---|
| Adenoviridae | Adenoviruses | 90–100 nm, dsDNA | Conjunctivitis, gastroenteritis, respiratory disease | Outbreaks not common in the U.S. though sporadic illnesses do occur; respiratory disease most common |
| Astroviridae | Astroviruses | 28–30 nm, ssRNA | Gastroenteritis | Predominantly impacts children ≤2 years of age; possibly higher prevalence in settings outside of U.S. (i.e. China, India, Egypt) |
| Picornaviridae | Enterovirusesa | 24–30 nm, ssRNA | Gastroenteritis, HFMD, encephalitis, meningitis, conjunctivitis | 10–15 million infections in the U.S. each year (all routes of transmission, not just water)b |
| Picornaviridae | HAV | 25-30 nm, ssRNA | Hepatitis | 17,000 new cases in the U.S. in 2010 (person-to-person, food and water)c; in developing countries nearly all children are infect with HAV by 9 years of aged |
| Picornaviridae | HEV | 25–30 nm, ssRNA | Acute viral hepatitis | Rare in the U.S. though it is very common in many parts of the world due to inadequate sanitation; 20 million cases globally each yeare |
| Caliciviridae | Noroviruses | 27–38 nm, ssRNA | Gastroenteritis | Leading cause of reported outbreaks of gastroenteritis in the U.S. and primary cause of viral gastroenteritis and foodborne outbreaks worldwide |
| Reoviridae | Rotaviruses | 70–75 nm, dsRNA | Gastroenteritis | Before introduction of vaccine in 2006, resulted in hospitalization 55,000 children each year in the U.S. and caused 527,000 deaths in children each year worldwidef |
ds: double stranded; ss: single stranded; HFMD: hand, foot, and mouth disease.
Non-polio enteroviruses: Coxsackievirus A and B, Echoviruses.
Occurrence of viral pathogens in water
Human enteric viruses may be introduced into the water environment through various routes. One obvious route of transmission is through the discharge of sewage-contaminated water into RW and/or DW sources. Viruses may also be introduced by land application of municipal biosolids [16, 17]; groundwater impacted by surface water or in proximity to faulty septic systems and leaking sewers [18, 19, 20, 21]; and discharge of untreated wastewater [22] or inadequately treated wastewater effluent [23, 24•, 25]. The occurrence of human enteric viruses in water remains largely unknown unless an outbreak is reported and samples are collected since water sources are not routinely tested for viruses. Moreover, there are challenges related to sampling studies to determine virus presence due to both differences and limitations in recovery and concentration methods for the detection of viruses in water [26•]. Regardless of these challenges, a snapshot of the occurrence of enteric viruses in water sources over the past decade is provided below.
Treated drinking water
In this section, there is a specific focus on DW derived from treated surface water as opposed to treated ground water that is used as DW. Keswick et al. (1984) — one of the seminal publications on the prevalence of viruses in DW in the U.S. — reported 83% of the samples to be positive for either RV or EVs [27]. Shortly thereafter, Bitton et al. (1986) followed up with a review on viruses in DW both in the U.S. and internationally [28]. Aside from these earlier studies, few studies on virus occurrence in DW in the U.S. have been reported since, and of those, such as Gibson and Schwab (2011), no viruses were detected [29]. This paucity of available data for viruses in DW can most likely be attributed to the need for very large volumes (>100 to 6000 L) of water to be concentrated followed by subsequent recovery and detection of virus targets — a process that is challenging often resulting in low recovery efficiency and detection sensitivity [26•].
In international settings, there is more research related to determining the occurrence of viral pathogens in DW. Again, Bitton et al. (1986) highlighted several studies out of the E.U. In particular, research from 1965 in Paris, France was the driving force for the investigation of the occurrence of viruses in DW worldwide for the next 20 years and beyond. As this review is primarily focused on the past decade, data published no later than 2002 have been included. Lee and Kim (2002) reported 4 and 7% of tested DW samples positive for NoV GI and GII, respectively, in South Korea [30]. Studies on viruses in DWTPs in Egypt [31] reported no viruses positive samples. Conversely, in South Africa [32], 11–16% of DWTP samples were positive for enteric viruses (predominantly coxsackievirus B). In 2009, Albinana-Gimenez et al. reported 11% of DW samples collected in Spain positive for AdV [33]. Additionally, a study by Dong et al. (2010) on viruses in New Zealand DW found 18–36% of samples positive for AdV depending on the method of analysis [34]. Last, Ye et al. (2012) reported the presence of human enteric viruses in DW from Wuhan, China with 100% of the 48 samples positive for RVs and AdVs and 21% of samples positive for EVs [35]. Overall, depending on the type of DW treatment process, source water quality, and sampling and detection methods, there is a wide range of viral pathogen occurrence in DW from around the world.
Ground water-treated and untreated
One of the greatest fallacies regarding groundwater (GW) as drinking water is that GW is more likely to be free of pathogenic microorganisms due in part to the presumed natural filtering abilities of subsurface environments [19]. Pathogen contaminated GW can often be attributed to failing or poorly sited septic systems in karst settings that do not allow for proper attenuation of pathogens, especially viruses, due to their rapid movement from surface to aquifer [36•, 37]. Several studies over the past decade have demonstrated widespread occurrence of human enteric viruses in both individual and municipal wells in the U.S. In 2003, Abbaszadegan et al. reported 4.8% and 32% of 448 GW samples to be virus positive by infectivity assay and PCR, respectively [20]. Additional U.S. centric studies have reported 8–49% virus positive GW sites [38, 39, 40, 41, 42]. A more recent study by Borchardt et al. (2012) reported 24% of the samples (n = 1200) positive for human enteric viruses (AdV, EV, and NoV GI) and also estimated 6–22% of the AGI in the study communities (n = 14) to be attributable to exposure to viruses in nondisinfected tap water [43••]. One reason for this vulnerability — aside from the complexity of pathogen–subsurface interactions — is that before the Ground Water Rule (GWR) of 2006 [44], public utilities with a GW source were not required to disinfect their water supply; moreover, many individual wells are still used untreated. As reported in Fout et al. (2003), untreated GW was responsible for approximately 50% of WBDOs in the U.S. and this trend continues today [38].
For an international perspective, a study on NoV occurrence in GW sources in South Korea reported 17–22% NoV positive out of 300 samples collected and analyzed [45•]. Another study out of South Korea [46] reported similar levels of GW contamination with 18%, 5.1%, and 7.7% of samples positive for NoV, EV, and AdV, respectively.
Recreational water
Most data available on the occurrence of enteric viruses in RW sources is related to untreated venues such as lakes, rivers, marine beaches, among others as opposed to treated venues (i.e. waterparks, pools); however, virus-related WBDOs can occur in both venues as outlined in the next section. Moreover, the quantity of data available on human enteric virus occurrence in RW sources is quite significant; therefore, a portion of these data has been compiled in Table 2 . Overall, RW have a high occurrence of human enteric viruses depending on the water type and virus; however, when considering these studies in Table 2, one should take into account the differences in sampling and detection methodologies which are beyond the scope of this review.
Table 2.
Occurrence of human enteric viruses in recreational water sources — U.S. and international combined.
| Water type | Sampling year | Description of results | Reference |
|---|---|---|---|
| Urban Rivers | 2000 | 52, 62, and 76% of samples (total of 21) positive for AdV, EVs, and HAV, respectively | [66] |
| River | 2002 | 57 and 37% of samples (total of 30) positive for HEV and AdV, respectively | [67] |
| Lake/River | 2002–2003 | 0 to 76% positive samples depending on sampling site and virus (AdV, astroviruses, EVs, HAV, NoVs, RVs) | [68] |
| Fresh | 2004 | 24% samples (14 of 58) virus positive for AdVs | [69] |
| River | 2007–2008 | 90 and 40% positive for AdV and human polyomaviruses, respectively | [33] |
| Marine | 2007 | No virus positive samples (total of 12) for NoV and HAV | [70] |
| Lake | 2007 | 50% of samples (25 of 58) virus positive (AdV, EV, RV) | [71] |
| Coastal/Stream | 2002–2007 | 53% of samples (8 of 15) positive for AdV by integrated cell culture — nested PCR | [34] |
| River | 2008–2009 | 18 to 96% positive depending on the virus with AdV being most prevalent followed by polyomaviruses, RV, NoV, and EVs | [72] |
| River | 2008–2009 | 20 and 36% of samples (total of 85) positive for AdV and EVs, respectively | [73] |
| Urban Rivers | 2006–2009 | 83% of samples (70 of 84) were positive for at least one enteric virus (AdV, astrovirus, EV, NoV, HAV) | [74] |
| Fresh/Marine | 2006 | Nearly 40% of samples virus positive; AdVs were more prevalent than NoVs in both marine and fresh waters | [75•] |
| River | 2009–2010 | 54, 63, and 44% of samples (total of 52) positive for NoV GI, NoV GII, and AdV, respectively | [76] |
| River | 2007–2008 | Nearly 31% of samples (20 of 65) positive for NoV | [25] |
| River | 2010–2011 | 100% of samples (total of 12) were positive for AdV and RV while 50% were positive for EVs | [35] |
AdV: adenovirus; EV: enteroviruses; HAV: Hepatitis A virus; NoV: norovirus; RV: rotaviruses.
Waterborne disease outbreaks due to viruses — United States
Table 3 details the reported WBDOs due to viral pathogens from 2003 to 2010 — the most recent reporting period for WBDOs related to DW available from the Centers for Disease Control and Prevention (CDC). For 2011 to 2013, there are no official numbers available aside from sporadic peer-reviewed publications and news media reports as detailed below. The relatively low number of WBDOs may be misleading as a result of voluntary reporting of WBDOs to the CDC [47]. Therefore, in general, outbreak data — such as what is presented here — may not be as comprehensive [11•]. Additionally, total morbidity and mortality are uncertain as virus-related illnesses and outbreaks are often unrecognized, unreported, or not even monitored.
Table 3.
Waterborne disease outbreaks due to viral pathogens in the United States — 2003–2010.a
| Location | Year | Water type | Virus | No. of cases | Reference |
|---|---|---|---|---|---|
| Connecticut | 2003 | RW-T | Echovirus 9 | 36 | [77] |
| Florida | 2004 | RW-T | NoV | 42 | [77] |
| Idaho | 2004 | RW-T | NoV | 140 | [77] |
| Minnesota | 2004 | RW-U | NoV | 9 | [77] |
| Oregon | 2004 | RW-U | NoV | 39 | [77] |
| Pennsylvania | 2004 | DW, pond | NoV | 70 | [78] |
| Vermont | 2004 | RW-T | NoV | 70 | [78] |
| Minnesota | 2005 | RW-U | NoV | 8 | [80] |
| Florida | 2006 | RW-U | NoV GII | 50 | [80] |
| Maryland | 2006 | DW, well | NoV GI | 148 | [79] |
| Minnesota | 2006 | RW-U | NoV GI | 10 | [80] |
| North Carolina | 2006 | DW, spring | HAV | 16 | [79] |
| Oregon | 2006 | DW, well | NoV GI | 48 | [79] |
| Wisconsin | 2006 | RW-T | NoV | 18 | [80] |
| California | 2007 | RW-T | NoV GII | 6 | [81] |
| Colorado | 2007 | DW, well | NoV GII | 77 | [82] |
| Idaho | 2007 | RW-T | NoV GII | 50 | [81] |
| Maryland | 2007 | DW, well | NoV GII | 94 | [82] |
| Washington | 2007 | DW, well | NoV | 32 | [82] |
| Wisconsin | 2007 | DW, well | NoV GI | 229 | [82] |
| Connecticut | 2008 | RW-U | NoV GI | 16 | [81] |
| Minnesota | 2008 | RW-U | NoV | 26 | [81] |
| Ohio | 2008 | RW-U | NoV GI | 54 | [81] |
| Oklahoma | 2008 | DW, well | NoV GI.4 | 62 | [82] |
| Tennessee | 2008 | DW, well | HAV | 9 | [82] |
| Wisconsin | 2008 | RW-U | NoV GI | 23 | [81] |
| Maine | 2009 | DW, well | HAV | 2 | [83] |
| California | 2010 | DW, well | NoV | 47 | [83] |
RW-T: treated recreational water; RW-U: untreated recreational water; DW: drinking water; NoV: human norovirus; HAV: Hepatitis A virus.
Includes only outbreaks reported by the CDC in official surveillance summaries.
Drinking water sources
From 2003 to 2010, 12 virus-related WBDOs were caused by contaminated DW — all from GW sources (Table 3). Reported outbreaks were caused predominantly by NoVs with two outbreaks caused by HAV. The dominance of NoV in DW related WBDOs may be strictly due to its status as the primary cause of AGI in the population or there may be other intrinsic factors involved such as the environmental stability of NoV and related viral ecology.
Recreational water sources
Of the 26 virus-related WBDOs reported from 2003 to 2008, more than half (16 of 26; 62%) were due to contaminated RW sources — both treated and untreated (Table 3). The dominant viral etiologic agents were human NoVs followed by one outbreak due to an EV, Echovirus 9. As highlighted by Sinclair et al. (2009), the apparent increase in virus etiologies accounting for recreational WBDOs is most likely the result of improved detection methods, but may also represent a true increase in incidence related to shifts in viral ecology or changes in population behavior patterns [48]. Other outbreaks that have not yet been reported by the U.S. WBDOSS include a NoV GI outbreak at Lake Wazee in Jackson County Wisconsin that sickened at least 200 people [49]. Interestingly, Matthews et al. (2012) determined that NoV GI outbreaks were significantly more likely to be associated with waterborne transmission (worldwide) compared to GII NoVs — the dominant circulating NoV genogroup (i.e. NoV GII.4) worldwide [11•].
Waterborne disease outbreaks due to viruses — international (not U.S.)
Determining the occurrence of virus-related WBDOs outside of the U.S. is somewhat challenging with more data available for high-income as opposed to low-income countries — most likely related to the capacity of surveillance systems within each country. Data available through the European Environment and Health Information System [50] states that from 2000 to 2007 there were 148 WBDOs caused by enteric viruses — 136 and 12 due to contaminated DW and RW sources, respectively — accounting for 42% of all reported WBDOs. However, these data only represent 14 countries, and within these countries, the efficiency of surveillance systems, as well as the reporting period, is variable. More recent, official data on WBDOs could not be ascertained though some peer-reviewed publications on outbreaks are highlighted here.
High-income countries
In 2005, Fretz et al. reported on NoV outbreaks in Switzerland caused by NoV from 2001 to 2003 [13]. The authors identified 2 outbreaks caused by the waterborne transmission of NoV out of 73 total outbreaks. Hoebe et al. (2004) described another NoV WBDO in The Netherlands in 2002 in which a recreational fountain was determined to be the common source of exposure for approximately 100 children with AGI [51]. In 2008, a massive WBDO due to contaminated municipal DW was investigated in Podgorica, Montenegro where nearly 1700 people fell ill with NoV [52]. Last, Koh et al. (2011) describe a WBDO at a waterpark where at least 67 cases of AGI were caused by GW contaminated with NoV [53].
Low-income countries
As one might expect, estimates on virus-related WBDOs in low-income, developing countries are difficult to determine due to the lack of surveillance systems and complexity of pathogen transmission when both adequate drinking water and sanitation are lacking. Ashbolt et al. (2004) provides a comprehensive review of the microbial contamination of DW in developing countries and highlights RV as one of the primary etiologic agents in children while HAV, HEV, and EVs are more common in adults [54].
Treatment options for virus removal and inactivation
The options available to treat water for the removal and/or inactivation of enteric viruses range from low-tech (i.e. household water treatment) to high-tech (i.e. DWTPs utilizing advanced membrane filtration processes). Treatment options tend to focus solely on removal of bacteria therefore, some of the technologies described below were never initially designed for the removal of viruses.
Point-of-use
Point-of-use (POU), or household water, treatment options are primarily implemented in low-income countries that (1) do not have a centralized DW treatment and distribution system or (2) do have a centralized DW distribution system, but one that may inadequately treat water and is unreliable. One of the most recent, comprehensive reviews on POU water treatment options is by Sobsey et al. (2008). Sobsey and others systematically evaluated five separate POU options including chlorination with safe storage, coagulant — chlorine disinfection systems (i.e. Watermaker and PuR packets), SODIS (transparent PET bottles filled with water and exposed to sunlight), ceramic filters, and biosand filters [55]. Point-of-use technologies involving the use of chlorine were determined to be the most effective for virus reduction (2 to ≥6 logs) while the available filtration methods were the least effective (0.5–4 log removal). Additional research on the efficacy of these various POU water treatment technologies to remove human enteric viruses has been published in the past 5 years [56, 57, 58, 59] which further support the conclusions of Sobsey et al. (2008).
Community-based
Community-based water treatment systems have also been implemented in rural and peri-urban areas of low-income countries over the past decade and are designed to target rural communities with limited opportunity to hook up to a centralized DWTP. Opryszko et al. (2012) investigated the impact of water-vending kiosks in rural Ghana. The water-vending kiosks were designed as ‘mini’ DWTPs including surface water treatment using multi-stage filtration and ultraviolet light disinfection [60]. A companion study by Gibson et al. (2011) evaluated the efficacy of the water-vending kiosks in Ghana to remove human enteric viruses and reported the presence of NoV GII and human polyomavirus in 1 of 6 of DW samples analyzed [61•]. Additional studies on the efficacy of community-based water treatment systems for the reduction of human enteric viruses are non-existent — most likely due to the methods needed for viral recovery and detection in water.
Full-scale DWTPs
Conventional DWTPs are designed for reliable physicochemical removal (5–7-log10) of microorganisms — bacteria, protozoa, viruses — from public DW supplies during optimal operation [62]. However, in the past decade, more advanced processes for DW treatment have been implemented including alternative disinfectants (combined chlorine, ozone, UV radiation) and membrane filtration (low-pressure microfiltration and ultrafiltration) technologies. However, enteric viruses are more resistant to inactivation by both UV and combined chlorine (monochloramine) when compared to chlorine [62]. On the other hand, advanced membrane filtration technologies, especially ultrafiltration, allow for the simultaneous removal of all classes of microorganisms from DW sources [63]. Numerous studies highlighted in recent review articles [62, 64] have reported on the efficacy of advanced membrane technologies and select alternative disinfectants for the removal and inactivation of enteric viruses in DW sources.
Concluding remarks
Viral pathogens in the water environment will continue to adversely impact public health. Even though viruses are not the only pathogens present in water that can cause disease, the risk of illness is 10–10,000 times greater for viruses than bacteria at a similar level of exposure [65]. Because of this increased risk and in consideration of the data presented herein, there are a few points of discussion that can be made. First, human enteric viruses are clearly a concern for both DW and RW microbial water quality; however, we still rely on a bacterial indicator system to alert us if there is a potential contamination issue. The use of bacterial indicators has been debated for decades, and we are still at a stalemate when it comes to actually predicting the presence of human viral pathogens in water. For the protection of public health, a true viral indicator should continue to be pursued. Second, in order to move toward an indicator for viruses, we need to harmonize the concentration, recovery, and detection methods employed for the analysis of waterborne viruses. Harmonizing steps have been taken by the U.S. Environmental Protection Agency with the introduction of Method 1615 though this method is specific to EVs and NoVs. Last, countries should start investing in both aging DW distribution systems and wastewater infrastructure, especially in large cities, as this would likely decrease a portion of the WBDOs that are reported each year, at least in high income countries.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This work was supported by the Arkansas Biosciences Institute and 104b funding from the Arkansas Water Resources Center.
References
- 1.Craun G.F., Brunkard J.M., Yoder J.S., Roberts V.A., Carpenter J., Wade T., Calderon R.L., Roberts J.M., Beach M.J., Roy S.L. Causes of outbreaks associated with drinking water in the United States from 1971 to 2006. Clin Microbiol Rev. 2010;23:507–528. doi: 10.1128/CMR.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambertini E., Spencer S.K., Kieke B.A., Loge F.J., Borchardt M.A. Virus contamination from operation and maintenance events in small drinking water distribution systems. J Water Health. 2011;9:799–812. doi: 10.2166/wh.2011.018. [DOI] [PubMed] [Google Scholar]
- 3.Okoh A.I., Sibanda T., Gusha S.S. Inadequately treated wastewater as a source of human enteric viruses in the environment. Int J Environ Res Public Health. 2010;7:2620–2637. doi: 10.3390/ijerph7062620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds K.A., Mena K.D., Gerba C.P. Risk of waterborne illness via drinking water in the United States. Rev Environ Contam Toxicol. 2008;192:117–158. doi: 10.1007/978-0-387-71724-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO/OECD . IWA Publishing; London, UK: 2003. Assessing Microbial Safety of Drinking Water: Improving Approaches and Methods. [Google Scholar]
- 6.Teunis P.F.M., Moe C.L., Liu P., Miller S.E., Lindesmith L., Baric R.S., Le Pendu J., Calderon R.L. Norwalk virus: how infectious is it? J Med Virol. 2008;80:1468–1476. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- 7.Ward R.L., Bernstein D.I., Young E.C., Sherwood J.R., Knowlton D.R., Schiff G.M. Human rotavirus studies in volunteers: determination of infectious dose and serological response to infection. J Infect Dis. 1986;154:871–880. doi: 10.1093/infdis/154.5.871. [DOI] [PubMed] [Google Scholar]
- 8.Schiff G.M., Stefanovic’ G.M., Young E.C., Sander D.S., Pennekamp J.K., Ward R.L. Studies of echovirus-12 in volunteers: determination of minimal infectious dose and the effect of previous infection on infectious dose. J Infect Dis. 1984;150:858–866. doi: 10.1093/infdis/150.6.858. [DOI] [PubMed] [Google Scholar]
- 9.Acheson D., Fiore A.E. Hepatitis A transmitted by food. Clin Infect Dis. 2004;38:705–715. doi: 10.1086/381671. [DOI] [PubMed] [Google Scholar]
- 10.Rzezutka A., Cook N. Survival of human enteric viruses in the environment and food. FEMS Microbiol Rev. 2004;28:441–453. doi: 10.1016/j.femsre.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 11•.Matthews J.E., Dickey B.W., Miller R.D., Felzer J.R., Dawson B.P., Lee A.S., Rocks J.J., Kiel J., Montes J.S., Moe C.L. The epidemiology of published norovirus outbreaks: a review of risk factors associated with attack rate and genogroup. Epidemiol Infect. 2012;140:1161–1172. doi: 10.1017/S0950268812000234. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reports a greater incidence of NoV genogroup I in water-related outbreaks even though NoV genogroup II is the dominant circulating group.
- 12.Bon F., Ambert-Balay K., Giraudon H., Kaplon J., Le Guyader S., Pommepuy M., Gallay A., Vaillant V., de Valk H., Chikhi-Brachet R. Molecular epidemiology of caliciviruses detected in sporadic and outbreak cases of gastroenteritis in France from December 1998 to February 2004. J Clin Microbiol. 2005;43:4659–4664. doi: 10.1128/JCM.43.9.4659-4664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fretz R., Svoboda P., L¸thi T.M., Tanner M., Baumgartner A. Outbreaks of gastroenteritis due to infections with Norovirus in Switzerland, 2001–2003. Epidemiol Infect. 2005;133:429–437. doi: 10.1017/s0950268804003619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosch A. Human enteric viruses in the water environment: a minireview. Int Microbiol. 1998;1:191–196. [PubMed] [Google Scholar]
- 15.La Rosa G., Fratini M., della Libera S., Iaconelli M., Muscillo M. Emerging and potentially emerging viruses in water environments. Annali dell’Istituto Superiore di Sanità. 2012;48:397–406. doi: 10.4415/ANN_12_04_07. [DOI] [PubMed] [Google Scholar]
- 16.Wong K., Onan B.M., Xagoraraki I. Quantification of enteric viruses, pathogen indicators, and salmonella bacteria in class B anaerobically digested biosolids by culture and molecular methods. Appl Environ Microbiol. 2010;76:6441–6448. doi: 10.1128/AEM.02685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chetochine A.S., Brusseau M.L., Gerba C.P., Pepper I.L. Leaching of phage from Class B biosolids and potential transport through soil. Appl Environ Microbiol. 2006;72:665–671. doi: 10.1128/AEM.72.1.665-671.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong T., Mansfield L.S., Wilson D.L., Schwab D.J., Molloy S.L., Rose J.B. Massive microbiological groundwater contamination associated with a waterborne outbreak in Lake Erie, South Bass Island, Ohio. Environ Health Perspect. 2007;115:856–864. doi: 10.1289/ehp.9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds K. Groundwater vulnerability to microbial contamination. Water Condition Purif. 2004:28–30. [Google Scholar]
- 20.Abbaszadegan M., LeChevallier M., Gerba C. Occurrence of viruses in US groundwaters. J AWWA. 2003;95:107–120. [Google Scholar]
- 21.Rutsch M., Rieckermann J., Cullmann J., Ellis J.B., Vollertsen J., Krebs P. Towards a better understanding of sewer exfiltration. Water Res. 2008;42:2385–2394. doi: 10.1016/j.watres.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Kessler R. Stormwater strategies: cities prepare aging infrastructure for climate change. Environ Health Perspect. 2011;119:A514–A519. doi: 10.1289/ehp.119-a514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crockett C.S. The role of wastewater treatment in protecting water supplies against emerging pathogens. Water Environ Res. 2007;79:221–232. doi: 10.2175/106143006x111952. [DOI] [PubMed] [Google Scholar]
- 24•.Cheng H.A., Lucy F.E., Broaders M.A., Mastitsky S.E., Chen C., Murray A. Municipal wastewater treatment plants as pathogen removal systems and as a contamination source of noroviruses and Enterococcus faecalis. J Water Health. 2012;10:380–389. doi: 10.2166/wh.2012.138. [DOI] [PubMed] [Google Scholar]; Demonstrates municipal wastewater treatment plants as a source of human enteric viruses in both recreational and drinking water sources.
- 25.Maunula L., Söderberg K., Vahtera H., Vuorilehto V., von Bonsdorff C., Valtari M., Laakso T., Lahti K. Presence of human noro- and adenoviruses in river and treated wastewater, a longitudinal study and method comparison. J Water Health. 2012;10:87–99. doi: 10.2166/wh.2011.095. [DOI] [PubMed] [Google Scholar]
- 26•.Julian T.R., Schwab K.J. Challenges in environmental detection of human viral pathogens. Curr Opin Virol. 2012;2:78–83. doi: 10.1016/j.coviro.2011.10.027. [DOI] [PubMed] [Google Scholar]; Highlights the challenges and limitations surrounding the recovery and detection of human enteric viruses in environmental water sources.
- 27.Keswick B.H., Gerba C.P., Dupont H.L., Rose J.B. Detection of enteric viruses in treated drinking water. Appl Environ Microbiol. 1984;47:1290–1294. doi: 10.1128/aem.47.6.1290-1294.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bitton G., Farrah S.R., Montague C.L., Akin E.W. Viruses in drinking water. Environ Sci Technol. 1986;20:216–222. doi: 10.1021/es00145a605. [DOI] [PubMed] [Google Scholar]
- 29.Gibson K.E., Schwab K.J. Tangential-flow ultrafiltration with integrated inhibition detection for recovery of surrogates and human pathogens from large-volume source water and finished drinking water. Appl Environ Microbiol. 2011;77:385–391. doi: 10.1128/AEM.01164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S., Kim S. Detection of infectious enteroviruses and adenoviruses in tap water in urban areas in Korea. Water Res. 2002;36:248–256. doi: 10.1016/s0043-1354(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 31.Ali M.A., Al-Herrawy A., El-Hawaary S. Detection of enteric viruses, Giardia and Cryptosporidium in two different types of drinking water treatment facilities. Water Res. 2004;38:3931–3939. doi: 10.1016/j.watres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Vivier J.C., Ehlers M.M., Grabow W.O.K. Detection of enteroviruses in treated drinking water. Water Res. 2004;38:2699–2705. doi: 10.1016/S0043-1354(01)00433-X. [DOI] [PubMed] [Google Scholar]
- 33.Albinana-Gimenez N., Miagostovich M.P., Calgua B., Huguet J.M., Matia L., Girones R. Analysis of adenoviruses and polyomaviruses quantified by qPCR as indicators of water quality in source and drinking-water treatment plants. Water Res. 2009;43:2011–2019. doi: 10.1016/j.watres.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 34.Dong Y., Kim J., Lewis G.D. Evaluation of methodology for detection of human adenoviruses in wastewater, drinking water, stream water and recreational waters. J Appl Microbiol. 2010;108:800–809. doi: 10.1111/j.1365-2672.2009.04477.x. [DOI] [PubMed] [Google Scholar]
- 35.Ye X.Y., Ming X., Zhang Y.L., Xiao W.Q., Huang X.N., Cao Y.G., Gu K.D. Real-time PCR detection of enteric viruses in source water and treated drinking water in Wuhan, China. Curr Microbiol. 2012;65:244–253. doi: 10.1007/s00284-012-0152-1. [DOI] [PubMed] [Google Scholar]
- 36•.Borchardt M.A., Bradbury K.R., Alexander E., Jr., Kolberg R.J., Alexander S.C., Archer J.R., Braatz L.A., Forest B.M., Green J.A., Spencer S.K. Norovirus outbreak caused by a new septic system in a dolomite aquifer. Ground Water. 2011;49:85–97. doi: 10.1111/j.1745-6584.2010.00686.x. [DOI] [PubMed] [Google Scholar]; Utilizes epidemiological data as well as tracer dye test to determine contamination of a fractured rock aquifer with human norovirus.
- 37.Gupta V., Johnson W.P., Shafieian P., Ryu H., Alum A., Abbaszadegan M., Hubbs S.A., Rauch-Williams T. Riverbank filtration: comparison of pilot scale transport with theory. Environ Sci Technol. 2009;43:669–676. doi: 10.1021/es8016396. [DOI] [PubMed] [Google Scholar]
- 38.Fout G., Martinson B., Moyer M., Dahling D. A multiplex reverse transcription-PCR method for detection of human enteric viruses in groundwater. Appl Environ Microbiol. 2003;69:3158–3164. doi: 10.1128/AEM.69.6.3158-3164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borchardt M., Bertz P., Spencer S., Battigelli D. Incidence of enteric viruses in groundwater from household wells in Wisconsin. Appl Environ Microbiol. 2003;69:1172–1180. doi: 10.1128/AEM.69.2.1172-1180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunt R.J., Borchardt M.A., Richards K.D., Spencer S.K. Assessment of sewer source contamination of drinking water wells using tracers and human enteric viruses. Environ Sci Technol. 2010;44:7956–7963. doi: 10.1021/es100698m. [DOI] [PubMed] [Google Scholar]
- 41.Futch J.C., Griffin D.W., Lipp E.K. Human enteric viruses in groundwater indicate offshore transport of human sewage to coral reefs of the Upper Florida Keys. Environ Microbiol. 2010;12:964–974. doi: 10.1111/j.1462-2920.2010.02141.x. [DOI] [PubMed] [Google Scholar]
- 42.Gibson K.E., Schwab K.J. Detection of bacterial indicators and human and bovine enteric viruses in surface water and groundwater sources potentially impacted by animal and human wastes in Lower Yakima Valley, Washington. Appl Environ Microbiol. 2011;77:355–362. doi: 10.1128/AEM.01407-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Borchardt M.A., Spencer S.K., Kieke B.J.A., Lambertini E., Loge F.J. Viruses in non-disinfected drinking water from municipal wells and community incidence of acute gastrointestinal illness. Environ Health Perspect. 2012;120:1272–1279. doi: 10.1289/ehp.1104499. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides evidence that populations served by groundwater-source public water systems producing water without disinfection are exposed to waterborne viruses along with an increased incidence of AGI.
- 44.USEPA National primary drinking water regulations — ground water rule, final rule. Fed Reg. 2006;71(216):65574–65660. [Google Scholar]
- 45•.Lee S.G., Jheong W.H., Suh C.I., Kim S.H., Lee J.B., Jeong Y.S., Ko G., Jang K.L., Lee G.C., Paik S.Y. Nationwide groundwater surveillance of noroviruses in South Korea, 2008. Appl Environ Microbiol. 2011;77:1466–1474. doi: 10.1128/AEM.01996-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive review of groundwater contamination with viruses in a high-income country other than the United States.
- 46.Jung J.H., Yoo C.H., Koo E.S., Kim H.M., Na Y., Jheong W.H., Jeong Y.S. Occurrence of norovirus and other enteric viruses in untreated groundwaters of Korea. J Water Health. 2011;9:544–555. doi: 10.2166/wh.2011.142. [DOI] [PubMed] [Google Scholar]
- 47.Ford T., Harrington W., Olson E., Reichard E. US drinking water challenges in the twenty-first century. Environ Health Perspect. 2002;110(Suppl 1):43–52. doi: 10.1289/ehp.02110s143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinclair R.G., Jones E.L., Gerba C.P. Viruses in recreational water-borne disease outbreaks: a review. J Appl Microbiol. 2009;107:1769–1780. doi: 10.1111/j.1365-2672.2009.04367.x. [DOI] [PubMed] [Google Scholar]
- 49.Food Safety News: 200 Ill With Norovirus Infections After Swimming in WI Lake. 2012, Available at: http://www.foodsafetynews.com/2012/07/200-ill-with-norovirus-infections-after-swimming-in-wi-lake/#.Ui01XxaFbdl Accessed on: September 8, 2013.
- 50.WHO: Outbreaks of Waterborne Diseases, Fact Sheet 1.1. 2009, RPG1_WatSan_E1
- 51.Hoebe C.J.P.A., Vennema H., de R.H., van Duynhoven Y.T.H.P. Norovirus outbreak among primary schoolchildren who had played in a recreational water fountain. J Infect Dis. 2004;189:699–705. doi: 10.1086/381534. [DOI] [PubMed] [Google Scholar]
- 52.Werber D., Lausevic D., Mugosa B., Vratnica Z., Ivanovic-Nikolic L., Zizic L., Alexandre-Bird A., Fiore L., Ruggeri F.M., Di Bartolo I. Massive outbreak of viral gastroenteritis associated with consumption of municipal drinking water in a European capital city. Epidemiol Infect. 2009;137:1713–1720. doi: 10.1017/S095026880999015X. [DOI] [PubMed] [Google Scholar]
- 53.Koh S., Cho H.G., Kim B.H., Choi B.Y. An outbreak of gastroenteritis caused by norovirus-contaminated groundwater at a waterpark in Korea. J Korean Med Sci. 2011;26:28–32. doi: 10.3346/jkms.2011.26.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ashbolt N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicol. 2004;198:229–238. doi: 10.1016/j.tox.2004.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sobsey M.D., Stauber C.E., Casanova L.M., Brown J.M., Elliott M.A. Point of use household drinking water filtration: a practical, effective solution for providing sustained access to safe drinking water in the developing world. Environ Sci Technol. 2008;42:4261–4267. doi: 10.1021/es702746n. [DOI] [PubMed] [Google Scholar]
- 56.Harding A.S., Schwab K.J. Using limes and synthetic psoralens to enhance solar disinfection of water (SODIS): a laboratory evaluation with Norovirus, Escherichia coli, and MS2. Am J Trop Med Hyg. 2012;86:566–572. doi: 10.4269/ajtmh.2012.11-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown J., Sobsey M.D. Microbiological effectiveness of locally produced ceramic filters for drinking water treatment in Cambodia. J Water Health. 2010;8:1–10. doi: 10.2166/wh.2009.007. [DOI] [PubMed] [Google Scholar]
- 58.Heaselgrave W., Kilvington S. The efficacy of simulated solar disinfection (SODIS) against coxsackievirus, poliovirus and hepatitis A virus. J Water Health. 2012;10:531–538. doi: 10.2166/wh.2012.128. [DOI] [PubMed] [Google Scholar]
- 59.Jenkins M.W., Tiwari S.K., Darby J. Bacterial, viral and turbidity removal by intermittent slow sand filtration for household use in developing countries: experimental investigation and modelling. Water Res. 2011;45:6227–6239. doi: 10.1016/j.watres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 60.Opryszko M.C., Guo Y., MacDonald L., MacDonald L., Kiihl S., Schwab K.J. Impact of water-vending kiosks and hygiene education on household drinking water quality in rural Ghana. Am J Trop Med Hyg. 2013;88:651–660. doi: 10.4269/ajtmh.12-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Gibson K.E., Opryszko M.C., Schissler J.T., Guo Y., Schwab K.J. Evaluation of human enteric viruses in surface water and drinking water resources in southern Ghana. Am J Trop Med Hyg. 2011;84:20–29. doi: 10.4269/ajtmh.2011.10-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the only published studies investigating the presence of enteric viruses in treated drinking water in a low-income country.
- 62.Shannon M.A., Bohn P.W., Elimelech M., Georgiadis J.G., Mariñas B.J., Mayes A.M. Science and technology for water purification in the coming decades. Nature. 2008;452:301–310. doi: 10.1038/nature06599. [DOI] [PubMed] [Google Scholar]
- 63.Jacangelo J.G., Brown L.P., Madec A. International Water Association; London, UK: 2007. Micro and Ultrafiltration Performance Specifications Based on Microbial Removal. [Google Scholar]
- 64.Leiknes T. The effect of coupling coagulation and flocculation with membrane filtration in water treatment: a review. J Environ Sci (China) 2009;21:8–12. doi: 10.1016/s1001-0742(09)60003-6. [DOI] [PubMed] [Google Scholar]
- 65.Haas C.N., Rose J.B., Gerba C., Regli S. Risk assessment of virus in drinking water. Risk Anal. 1993;13:545–552. doi: 10.1111/j.1539-6924.1993.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 66.Jiang S.C., Chu W. PCR detection of pathogenic viruses in southern California urban rivers. J Appl Microbiol. 2004;97:17–28. doi: 10.1111/j.1365-2672.2004.02269.x. [DOI] [PubMed] [Google Scholar]
- 67.Fong T., Lipp E.K. Enteric viruses of humans and animals in aquatic environments: health risks, detection, and potential water quality assessment tools. Microbiol Mol Biol Rev. 2005;69:357–371. doi: 10.1128/MMBR.69.2.357-371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pusch D., Oh D., Wolf S., Dumke R., Schröter-Bobsin U., Höhne M., Röske I., Schreier E. Detection of enteric viruses and bacterial indicators in German environmental waters. Arch Virol. 2005;150:929–947. doi: 10.1007/s00705-004-0467-8. [DOI] [PubMed] [Google Scholar]
- 69.Xagoraraki I., Kuo DH-, Wong K., Wong M., Rose J.B. Occurrence of human adenoviruses at two recreational beaches of the great lakes. Appl Environ Microbiol. 2007;73:7874–7881. doi: 10.1128/AEM.01239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abdelzaher A., Wright M., Ortega C., Solo-Gabriele H., Miller G., Elmir S., Newman X., Shih P., Bonilla J.A., Bonilla T.D. Presence of pathogens and indicator microbes at a non-point source subtropical recreational marine beach. Appl Environ Microbiol. 2010;76:724–732. doi: 10.1128/AEM.02127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong M., Kumar L., Jenkins T.M., Xagoraraki I., Phanikumar M.S., Rose J.B. Evaluation of public health risks at recreational beaches in Lake Michigan via detection of enteric viruses and a human-specific bacteriological marker. Water Res. 2009;43:1137–1149. doi: 10.1016/j.watres.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 72.Jurzik L., Hamza I.A., Puchert W., Uberla K., Wilhelm M. Chemical and microbiological parameters as possible indicators for human enteric viruses in surface water. Int J Hyg Environ Health. 2010;213:210–216. doi: 10.1016/j.ijheh.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 73.Dorevitch S., Panthi S., Huang Y., Li H., Michalek A.M., Pratap P., Wroblewski M., Liu L., Scheff P.A., Li A. 2011: Water ingestion during water recreation. Water Res. 2011;45:2020–2028. doi: 10.1016/j.watres.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 74.Aw T.G., Gin K.Y.-H. Prevalence and genetic diversity of waterborne pathogenic viruses in surface waters of tropical urban catchments. J Appl Microbiol. 2011;110:903–914. doi: 10.1111/j.1365-2672.2011.04947.x. [DOI] [PubMed] [Google Scholar]
- 75•.Wyn-Jones A., Carducci A., Cook N., D’Agostino M., Divizia M., Fleischer J., Gantzer C., Gawler A., Girones R., Höller C. Surveillance of adenoviruses and noroviruses in European recreational waters. Water Res. 2011;45:1025–1038. doi: 10.1016/j.watres.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive, multi-national study on the occurrence of human enteric viruses (adenoviruses and noroviruses) in recreational waters.
- 76.Kishida N., Morita H., Haramoto E., Asami M., Akiba M. One-year weekly survey of noroviruses and enteric adenoviruses in the Tone River water in Tokyo metropolitan area, Japan. Water Res. 2012;46:2905–2910. doi: 10.1016/j.watres.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 77.Dziuban E.J., Liang J.L., Craun G.F., Hill V., Yu P.A., Painter J., Moore M.R., Calderon R.L., Roy S.L., Beach M.J., Centers for Disease Control and Prevention, C.D.C. Surveillance for waterborne disease and outbreaks associated with recreational water — United States, 2003–2004. MMWR Surveill Summ. 2006;55:1–30. [PubMed] [Google Scholar]
- 78.Liang J.L., Dziuban E.J., Craun G.F., Hill V., Moore M.R., Gelting R.J., Calderon R.L., Beach M.J., Roy S.L., Centers for Disease Control and Prevention, C.D.C. Surveillance for waterborne disease and outbreaks associated with drinking water and water not intended for drinking — United States, 2003–2004. MMWR Surveill Summ. 2006;55:31–65. [PubMed] [Google Scholar]
- 79.Yoder J., Roberts V., Craun G.F., Hill V., Hicks L.A., Alexander N.T., Radke V., Calderon R.L., Hlavsa M.C., Beach M.J. Surveillance for waterborne disease and outbreaks associated with drinking water and water not intended for drinking — United States, 2005–2006. MMWR Surveill Summ. 2008;57:39–62. [PubMed] [Google Scholar]
- 80.Yoder J.S., Hlavsa M.C., Craun G.F., Hill V., Roberts V., Yu P.A., Hicks L.A., Alexander N.T., Calderon R.L., Roy S.L. Surveillance for waterborne disease and outbreaks associated with recreational water use and other aquatic facility-associated health events — United States, 2005–2006. MMWR Surveill Summ. 2008;57:1–29. [PubMed] [Google Scholar]
- 81.Hlavsa M.C., Roberts V.A., Anderson A.R., Hill V.R., Kahler A.M., Orr M., Garrison L.E., Hicks L.A., Newton A., Hilborn E.D. Surveillance for waterborne disease oubreaks and other health events associated with recreational water — United States, 2007–2008. MMWR Surveill Summ. 2011;60:1–32. [PubMed] [Google Scholar]
- 82.Brunkard J.M., Ailes E., Roberts V.A., Hill V., Hilborn E.D., Craun G.F., Rajasingham A., Kahler A., Garrison L., Hicks L. Surveillance for waterborne disease outbreaks associated with drinking water — United States, 2007–2008. MMWR Surveill Summ. 2011;60:38–68. [PubMed] [Google Scholar]
- 83.Hilborn E.D., Wade T.J., Hicks L., Garrison L., Carpenter J., Adam E., Mull B., Yoder J., Roberts V., Gargano J.W. Surveillance for waterborne disease outbreaks associated with drinking water and other nonrecreational water — United States, 2009–2010. MMWR Surveill Summ. 2013;62:714–720. [Google Scholar]


