Abstract
Despite optimal use of currently available therapy for stable COPD, acute exacerbations are common events that constitute a major health burden. The development of highly sensitive diagnostic tools highlighted the role of viral infections in inducing COPD exacerbations, with rhinoviruses being the most frequently identified virus type. So far, little is known about the mechanisms of virus-induced exacerbations. The recent development of the first human rhinovirus-induced COPD exacerbation model represents an invaluable tool towards increasing our knowledge of immunological and inflammatory mechanisms of COPD exacerbation. The model will give us the opportunity to highlight key inflammatory mediators representing possible therapeutic targets for the development of novel drugs able to treat and prevent acute episodes in COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterised by fixed airway obstruction associated with abnormal inflammation of the airways that occurs in susceptible subjects exposed to noxious particles or gases, most frequently cigarette smoke. The clinical history of COPD is punctuated by recurrent acute exacerbations — episodes of acute onset of worsening of the patient's clinical status, characterised by dyspnea, wheeze, cough or sputum production [1]. Besides acute clinical presentation, assessment of the severity of the exacerbation is also based on a patient's medical history before the exacerbation (including severity of airway obstruction), pre-existing co-morbidities, duration and magnitude of symptoms, physical examination and arterial blood gas measurement. Acute exacerbations are a common occurrence in COPD patients and the frequency of exacerbations increases with the severity of the disease [2, 3, 4]. In addition to being the major cause of COPD-associated morbidity and mortality, exacerbations contribute to impaired health status, loss of lung function [3, 5, 6], and thus to disease progression. It has been estimated that acute exacerbations are also the cause of ∼70% of health-care costs associated with COPD [7].
Thanks to the development of highly sensitive diagnostic tools, there is now evidence that not only bacterial but also viral infections are major causes of COPD exacerbations (Table 1 ). Although several mechanisms have been proposed to explain how bacterial infection can trigger COPD exacerbations (including induction of mucus hypersecretion [8], reduction of ciliary beat frequency [9] and enhancement of neutrophilic inflammation [10]), very few data are available on the pathogenesis of virus-induced exacerbations.
Table 1.
Pathogens identified at COPD exacerbations and methods used for their detection.
| Pathogen | Method |
|---|---|
| Extracellular bacteria | |
| H. influenzae | Sputum and bronchial secretion cultures |
| S. pneumoniae | |
| M. catarrhalis | |
| Staphylococcus aureus | |
|
P. aeruginosa | |
| Viruses | |
| Rhinovirus | |
| Influenza | |
| Parainfluenza | PCR |
| Coronavirus | Cell cultures |
| Adenovirus | Serology |
| Respiratory syncytial virus | |
| Picornavirus | |
| Metapneumovirus |
|
| Intracellular bacteria | |
| Mycoplasma pneumoniae | Serology |
| Chlamydia pneumoniae | PCR |
Modified from [50•].
Bronchodilators, corticosteroids and, when indicated, antibiotics represent the currently recommended pharmacological treatment for COPD exacerbations. To date, no treatment has been evaluated/designed specifically to prevent or treat virus-induced COPD exacerbations [1]. Thus, the development of models of virus-induced COPD exacerbations is a fundamental step towards increasing our knowledge of the relevant pathogenetic mechanisms, as well as providing an invaluable tool with which to identify and test novel pharmacological targets.
In our current review, we briefly review the data currently available on the role of respiratory viruses in COPD exacerbations. We focus particularly on in vitro and in vivo models of virus-induced COPD exacerbations.
Virus infection in COPD
COPD exacerbations are associated with an increased number of activated inflammatory cells in the airways [11]. Chronic inflammation in COPD is characterized by increased numbers of CD8+ T lymphocytes infiltrating both central [12] and peripheral airways [13], which are then further increased upon exacerbation [14].
Among the possible causes of CD8+ recruitment to the airways, chronic viral infection has been suggested as being particularly important, and attention has been given to the possible role of latent adenoviral infection in the pathogenesis of COPD [15, 16]. Recently, it has been documented that COPD patients with repeated presence of respiratory syncytial virus in sputum over two years had faster lung function decline over time [17••]. Taken together, these data indicate that, in response to repeated/latent viral infections, an excessive recruitment of CD8+ T lymphocytes might occur in the tracheo-bronchial tree. This CD8+-driven inflammation can damage the lung in susceptible smokers, leading to COPD progression [18]. The use of highly sensitivity diagnostic methods such as PCR for viral detection in biological samples has shown that the proportion of virus-induced COPD exacerbations is likely to be ≥ 40% in an outpatient setting [19•] and ≥ 50% in hospitalised patients [20, 21••], with rhinoviruses being the most frequently identified virus type.
A recent study has addressed the relative importance of viral versus bacterial infections in the aetiology of severe (hospitalised) COPD exacerbations [21••]. Viral and/or bacterial infection was detected in approximately 78% of COPD exacerbations (29.7% bacterial; 23.4% viral; 25% viral/bacterial co-infection). Infectious exacerbations led to longer hospitalisations and greater impairments of several measures of lung function than did non-infectious exacerbations. Importantly, exacerbations with co-infection resulted in more marked lung function impairment and longer hospitalisations. Similarly, in an outpatient setting of COPD exacerbations, a greater decline in lung function was documented in those patients in which both bacterial infection and cold symptoms were present simultaneously [19•].
Mechanisms of virus-induced COPD exacerbations
Our understanding of the mechanisms of virus-associated asthma exacerbations is relatively poor [22•], and even less is known about the pathogenesis of virus-associated COPD exacerbations.
Inflammatory mechanisms
During COPD exacerbations, several inflammatory mediators are increased in the airways. However, little is known about the inflammatory mediators specific to virus-induced COPD exacerbations. One study found increased sputum interleukin (IL)-6 in virus-associated acute episodes when compared to non-viral exacerbations [23]. Recently, the presence of both rhinovirus and Haemophilus influenzae at exacerbation has been associated with increased levels of serum IL-6, suggesting that viruses and bacteria can synergistically interact to increase the severity of inflammation that occurs during exacerbations [19•].
The major group of rhinoviruses (accounting for 90% of total rhinovirus types) attaches to airway epithelium through intercellular adhesion molecule 1 (ICAM-1) [24]. Interestingly, rhinovirus infection induces expression of its own receptor (ICAM-1) [25, 26], which might promote inflammatory cell recruitment and activation. Indeed, there is evidence for upregulation of ICAM-1 in the bronchial mucosa of patients with chronic bronchitis [27], and thus one of the possible mechanisms for increased airway inflammation is rhinovirus-induced lower airway inflammation, which could occur via upregulation of ICAM-1.
Cellular mechanisms
The nature of the inflammatory cells recruited to the lung during COPD exacerbations has not been fully clarified. Few studies have analysed bronchial biopsies at exacerbations because of the difficulties associated with performing an invasive procedure in acutely ill patients. Two studies using bronchial biopsies during exacerbations of chronic bronchitis from a single cohort of patients reported prominent airway eosinophilia, together with increased numbers of neutrophils and T lymphocytes, in the exacerbated group when compared with stable patients [28, 29]. Another recent study showed increased number of neutrophils in sputum during exacerbations; this increase was independent of the pathogen detected. The same study documented that virus-induced COPD exacerbations, with or without concomitant bacterial infection, are associated with increased numbers of eosinophils in sputum, suggesting that sputum eosinophilia could be a marker of viral infection during COPD exacerbations [21••].
Interestingly, increased numbers of sputum CD8+ T lymphocytes have been reported during COPD exacerbations, with a relative reduction in the ratio of interferon (IFN)-γ/IL-4-expressing CD8+ T lymphocytes [14]. Thus, a switch towards a Th2 phenotype immune response during exacerbation could be one of the potential mechanisms involved in the recruitment of eosinophils, a classical Th2 effector cell, during virus-induced COPD exacerbations.
Susceptibility to virus infections in COPD patients
Whether COPD patients are more susceptible to virus infection compared with normal subjects is strongly debated. A recent study documented that patients with frequent COPD exacerbations have more frequent episodes of naturally occurring colds when compared with patients with infrequent exacerbations [30•]. These results suggest that COPD subjects with frequent exacerbations represent a subgroup particularly susceptible to viral infections. Thus, although there is solid evidence of impaired innate [31••, 32••] and (possibly) acquired [33, 34] immune responses to viral infection in asthmatic patients, it is not yet clear whether COPD patients have increased susceptibility to viral infections. Intriguingly, patients experiencing frequent colds had a significantly higher exposure to cigarette smoke [30•]. Using a mouse model of cigarette smoke exposure, it has been recently demonstrated that cigarette smoke increases susceptibility to viral infections, possibly via alteration and/or inhibition of immune responses [35]. Therefore, we could speculate that cigarette smoke exposure, which is the most important risk factor for the development of COPD, might cause impaired immune response to viral infections in COPD patients.
Another mechanism that might lead to increased susceptibility to viral infections is the upregulation of ICAM-1, the receptor for the major group of human rhinoviruses. Latent expression of adenoviral E1A protein in alveolar epithelial cells of patients with emphysema increases ICAM-1 expression, which could explain the greater susceptibility to rhinovirus infection in COPD [16]. Solid evidence shows that patients with COPD are chronically colonized with airway bacteria, and the bacterial load is related to airway inflammation and disease progression [36]. It has been postulated that bacterial colonisation contributes to increased susceptibility to viral infection in COPD patients, for example, by increasing ICAM-1 expression in bronchial epithelial cells, either directly or through induced inflammation [37••].
Further studies are required to investigate the interaction between chronic bacterial colonisation and respiratory viral infection and, in particular, whether chronic bacterial colonisation can increase susceptibility to viral infection or vice versa.
In vivo model of virus-induced COPD exacerbation
In vitro respiratory virus infection of both bronchial epithelial cells [26, 38, 39] and macrophages [40] induces the production of several pro-inflammatory molecules relevant to the pathogenesis of COPD exacerbation. However, although in vitro models can provide important insights into the molecular mechanisms of inflammatory and antiviral responses to viral and bacterial infections, any such insights require validation in in vivo models. At present, there is no published animal model of COPD exacerbation for either virus-associated or bacteria-induced COPD exacerbations, and thus we rely exclusively on human studies for what little data we have available to date.
Performing studies of naturally occurring COPD exacerbations has proved difficult for several reasons, including non-reporting of exacerbations by patients, lack of baseline data before exacerbations, wide variation in etiology, variation in timing of sampling relative to onset of exacerbation and, finally, the difficulty of carrying out invasive airway investigations in acutely unwell patients. One way to overcome these obstacles is the development of a human experimental model that would allow studies to take place under controlled conditions. The first step towards development of such a model has been realised recently [41••] with the reporting of the first study evaluating the effects of an experimental viral infection in COPD patients.
In this pilot study, mild COPD patients were selected for experimental infection with the purpose of, firstly, evaluating whether the procedure is safe in COPD patients and, secondly, providing preliminary data on whether experimental rhinovirus infection in COPD patients is sufficient to trigger exacerbations per se. As safety was the prime concern, a carefully designed rhinovirus dose-escalation study was performed in small groups of subjects (n=5) to determine the minimum dose of virus able to induce clinical colds in 80% of inoculated subjects (i.e. 4 out of 5). Surprisingly, all of the first four patients exposed to the initial lowest dose of rhinovirus inoculum experienced not only cold symptoms but also lower respiratory tract symptoms, including shortness of breath, wheeze, cough and increased sputum production (e.g. symptoms of COPD exacerbation) [41••]. Moreover, experimental rhinovirus infection induced a significant fall in lung function, typical of naturally occurring exacerbations [42]. The severity of the exacerbations induced was mild-to-moderate, thus achieving the primary aim of the study — to show that experimental rhinovirus infection could be safely carried out in mild COPD patients.
This model also showed that experimental rhinovirus infection can cause exacerbations in COPD patients and that, when performed using a larger number of patients and including assessment of lower airway inflammatory responses, has the potential to provide a valid model of naturally occurring COPD exacerbation. For COPD, this is, as yet, the only scenario in which a specific aetiology has been experimentally proven to induce exacerbation. This model offers the possibility to investigate the specific immunologic and inflammatory events that drive the transition from stable state to exacerbation after rhinovirus infection.
Two potentially important preliminary observations arose from this study: COPD patients developed colds and exacerbations with 100- to 1000-fold lower doses of virus than used in previous studies in asthmatic and normal volunteers; and there was a 3–4 day gap between the peak of cold symptoms and the peak of lower respiratory symptoms (Figure 1 ) [41••]. These data suggest that COPD patients may be highly susceptible to virus infection and that, if an effective antiviral or anti-inflammatory treatment were given at the onset of cold symptoms, this might possibly change the clinical outcome of the infection in COPD. These preliminary findings clearly require confirmation with a larger number of patients.
Figure 1.
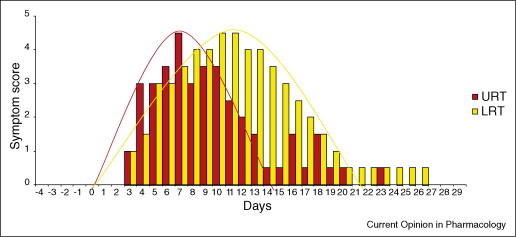
Upper (URT) and lower respiratory tract (LRT) symptoms of COPD patients in vivo experimentally infected with rhinovirus. A 3- to 4-day gap between the peak of cold symptoms and the peak of lower respiratory symptoms was documented (modified from [41••]). These data suggest that, if an effective antiviral or anti-inflammatory treatment could be given at the onset of cold symptoms, this could possibly change the clinical outcome of the viral infection in COPD.
With a similar experimental model for asthmatic patients, we recently documented that the severity of the virus-induced exacerbations, both in terms of symptoms, lung function reduction and inflammation in the airways, was inversely related to the production of a novel class of interferon called IFN-λ [32••]. An impaired innate immune response might therefore be one of the mechanisms by which asthmatic patients shown increased susceptibility to respiratory viral infections. The new human model for virus-induced COPD exacerbation will give us the opportunity to test whether similar or different immune deficiencies are present in COPD and, if so, whether modulation and/or restoration of the immune response could represent an effective and novel pharmacological approach to treat or prevent COPD exacerbations.
The development of an experimental model in which causation is clearly defined, and in which detailed clinical studies on mechanisms of disease can be carried out, is a major step forward in our ability to perform studies aimed at increasing our understanding of this clinically relevant condition.
Treatment of COPD exacerbations: impact on virus-induced COPD exacerbations
Bronchodilators are the gold standard for COPD treatment. When the disease is more severe, International Guidelines recommend combination therapy with bronchodilator and inhaled steroids [1]. These therapeutic regimens have provided beneficial effects on symptom control in COPD patients, and there is also evidence that they can reduce the frequency of exacerbations [43, 44]. Nevertheless despite optimized therapy, exacerbations still occur.
The aims of COPD exacerbation management are to relieve symptoms and airway obstruction, to correct hypoxia and hypercapnia (when present), and to treat any precipitating factors and/or comorbidities. This involves a number of modalities of treatment including pharmacological therapies, oxygen therapy and, when indicated, mechanical ventilation. Inhaled bronchodilators and systemic corticosteroids have been shown to be beneficial during acute exacerbation of COPD [45, 46]. Conversely, the role and effectiveness of antibiotics in acute exacerbations of COPD are still debated. A recent meta-analysis supported the use of antibiotics in COPD exacerbation for those patients moderate-to-severely ill with increased cough and sputum purulence. Analysis restricted to community-based studies did not find any differences between antibiotic and placebo [47]. However, surveys of antibiotic prescribing in COPD exacerbations in clinical practice indicate that they are used in the majority of exacerbations, both in the community and in hospital practice. Thus, a large number of patients are probably receiving inappropriate antibiotic therapy, with the associated increase in cost and risk of antibiotic resistance. Therefore, there is an urgent need to develop simple clinical or biological markers to identify those patients at highest risk of bacterial infection, as well as those who will benefit most from antibiotic therapy.
None of the clinical trials conduced so far have investigated whether currently available therapy for COPD can specifically prevent and/or treat virus-induced exacerbations. Thus, further studies are needed to elucidate the mechanisms that lead viral infections to trigger COPD exacerbations and thus to identify key inflammatory mediators and/or immunological pathways for the development of novel drugs able to treat and prevent acute episodes of COPD. Indeed, so far, anti-viral therapy has focused mainly on vaccination as the optimal prevention strategy, and subsequently the development of antiviral agents has lagged behind that of anti-bacterial chemotherapy. Vaccination might not be a realistic option for the treatment of respiratory viral infections in view of the variety of different viruses that cause similar clinical syndromes. Development of a vaccine for rhinoviruses is especially problematic because of the large number (>100) of different serotypes. The recognition that rhinoviruses are associated with more severe clinical syndromes than just upper respiratory tract infections has stimulated research into anti-rhinoviral agents. A number of anti-rhinoviral drugs are in development but, to date, no agent has been approved for the treatment of rhinovirus infections [48]. A specific strategy able to interfere with the pathogenesis of virus-induced exacerbations is the real future challenge.
Conclusions
By 2020, it is expected that COPD will be the third greatest cause of death worldwide [49], with the major morbidity, mortality and health-care costs of COPD being caused by exacerbations [1]. Thanks to the use of novel and highly sensitive diagnostic tools, the role of respiratory viruses in COPD exacerbations has emerged during the past few years. Nevertheless, the mechanisms that lead to COPD exacerbations after respiratory virus infections are still largely unknown. The recent development of the first human model of virus-induced COPD exacerbation [41••], which has been demonstrated to be feasible and (so far) safe, should facilitate identification of novel pharmacological targets that will provide opportunities to develop new treatments for exacerbations of COPD.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
References
- 1.National Institute of Health: National Heart Lung, and Blood Institute: Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Workshop report. NIH Publication No 2701A, March 2001. Update 2006.
- 2.Donaldson G.C., Seemungal T.A., Patel I.S., Lloyd-Owen S.J., Wilkinson T.M., Wedzicha J.A. Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir J. 2003;22:931–936. doi: 10.1183/09031936.03.00038303. [DOI] [PubMed] [Google Scholar]
- 3.Seemungal T.A., Donaldson G.C., Paul E.A., Bestall J.C., Jeffries D.J., Wedzicha J.A. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 4.Anthonisen N.R., Manfreda J., Warren C.P., Hershfield E.S., Harding G.K., Nelson N.A. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson G.C., Seemungal T.A., Bhowmik A., Wedzicha J.A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanner R.E., Anthonisen N.R., Connett J.E. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164:358–364. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan S.D., Ramsey S.D., Lee T.A. The economic burden of COPD. Chest. 2000;117(2 Suppl):5S–9S. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- 8.Adler K.B., Hendley D.D., Davis G.S. Bacteria associated with obstructive pulmonary disease elaborate extracellular products that stimulate mucin secretion by explants of guinea pig airways. Am J Pathol. 1986;125:501–514. [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson R., Roberts D., Cole P. Effect of bacterial products on human ciliary function in vitro. Thorax. 1985;40:125–131. doi: 10.1136/thx.40.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Read R.C., Wilson R., Rutman A., Lund V., Todd H.C., Brain A.P., Jeffery P.K., Cole P.J. Interaction of nontypable Haemophilus influenzae with human respiratory mucosa in vitro. J Infect Dis. 1991;163:549–558. doi: 10.1093/infdis/163.3.549. [DOI] [PubMed] [Google Scholar]
- 11.Wedzicha J.A., Donaldson G.C. Exacerbations of chronic obstructive pulmonary disease. Respir Care. 2003;48:1204–1213. [PubMed] [Google Scholar]
- 12.O'Shaughnessy T.C., Ansari T.W., Barnes N.C., Jeffery P.K. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155:852–857. doi: 10.1164/ajrccm.155.3.9117016. [DOI] [PubMed] [Google Scholar]
- 13.Saetta M., Di Stefano A., Turato G., Facchini F.M., Corbino L., Mapp C.E., Maestrelli P., Ciaccia A., Fabbri L.M. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:822–826. doi: 10.1164/ajrccm.157.3.9709027. [DOI] [PubMed] [Google Scholar]
- 14.Tsoumakidou M., Tzanakis N., Chrysofakis G., Kyriakou D., Siafakas N.M. Changes in sputum T-lymphocyte subpopulations at the onset of severe exacerbations of chronic obstructive pulmonary disease. Respir Med. 2005;99:572–579. doi: 10.1016/j.rmed.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Matsuse T., Hayashi S., Kuwano K., Keunecke H., Jefferies W.A., Hogg J.C. Latent adenoviral infection in the pathogenesis of chronic airways obstruction. Am Rev Respir Dis. 1992;146:177–184. doi: 10.1164/ajrccm/146.1.177. [DOI] [PubMed] [Google Scholar]
- 16.Retamales I., Elliott W.M., Meshi B., Coxson H.O., Pare P.D., Sciurba F.C., Rogers R.M., Hayashi S., Hogg J.C. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med. 2001;164:469–473. doi: 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- 17••.Wilkinson T.M., Donaldson G.C., Johnston S.L., Openshaw P.J., Wedzicha J.A. Respiratory syncytial virus, airway inflammation, and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:871–876. doi: 10.1164/rccm.200509-1489OC. [DOI] [PubMed] [Google Scholar]; This paper demonstrated that persistent respiratory syncytial virus infection could contribute to the progression of COPD severity.
- 18.Majo J., Ghezzo H., Cosio M.G. Lymphocyte population and apoptosis in the lungs of smokers and their relation to emphysema. Eur Respir J. 2001;17:946–953. doi: 10.1183/09031936.01.17509460. [DOI] [PubMed] [Google Scholar]
- 19•.Wilkinson T.M., Hurst J.R., Perera W.R., Wilks M., Donaldson G.C., Wedzicha J.A. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest. 2006;129:317–324. doi: 10.1378/chest.129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article showed that rhinovirus and bacterial infections interact to increase the severity of COPD exacerbation.
- 20.Rohde G., Wiethege A., Borg I., Kauth M., Bauer T.T., Gillissen A., Bufe A., Schultze-Werninghaus G. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58:37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Papi A., Bellettato C.M., Braccioni F., Romagnoli M., Casolari P., Caramori G., Fabbri L.M., Johnston S.L. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]; This study investigated the inflammatory response and clinical outcomes of COPD exacerbations caused by viral and/or bacterial infection. Results showed that dual infection is common, that sputum eosinophilia can be a marker of viral infection, and that when both pathogens are present the exacerbation is more severe.
- 22•.Contoli M., Caramori G., Mallia P., Johnston S., Papi A. Mechanisms of respiratory virus-induced asthma exacerbations. Clin Exp Allergy. 2005;35:137–145. doi: 10.1111/j.1365-2222.2005.02163.x. [DOI] [PubMed] [Google Scholar]; In this review, inflammatory and immunological mechanisms of virus-induced asthma exacerbations are presented.
- 23.Seemungal T.A., Harper-Owen R., Bhowmik A., Jeffries D.J., Wedzicha J.A. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J. 2000;16:677–683. doi: 10.1034/j.1399-3003.2000.16d19.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greve J.M., Davis G., Meyer A.M., Forte C.P., Yost S.C., Marlor C.W., Kamarck M.E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 25.Grunberg K., Sharon R.F., Hiltermann T.J., Brahim J.J., Dick E.C., Sterk P.J., Van Krieken J.H. Experimental rhinovirus 16 infection increases intercellular adhesion molecule-1 expression in bronchial epithelium of asthmatics regardless of inhaled steroid treatment. Clin Exp Allergy. 2000;30:1015–1023. doi: 10.1046/j.1365-2222.2000.00854.x. [DOI] [PubMed] [Google Scholar]
- 26.Papi A., Johnston S.L. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J Biol Chem. 1999;274:9707–9720. doi: 10.1074/jbc.274.14.9707. [DOI] [PubMed] [Google Scholar]
- 27.Vignola A.M., Campbell A.M., Chanez P., Bousquet J., Paul-Lacoste P., Michel F.B., Godard P. HLA-DR and ICAM-1 expression on bronchial epithelial cells in asthma and chronic bronchitis. Am Rev Respir Dis. 1993;148:689–694. doi: 10.1164/ajrccm/148.3.689. [DOI] [PubMed] [Google Scholar]
- 28.Saetta M., Di Stefano A., Maestrelli P., Turato G., Ruggieri M.P., Roggeri A., Calcagni P., Mapp C.E., Ciaccia A., Fabbri L.M. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med. 1994;150:1646–1652. doi: 10.1164/ajrccm.150.6.7952628. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J., Qiu Y.S., Majumdar S., Gamble E., Matin D., Turato G., Fabbri L.M., Barnes N., Saetta M., Jeffery P.K. Exacerbations of bronchitis: bronchial eosinophilia and gene expression for interleukin-4, interleukin-5, and eosinophil chemoattractants. Am J Respir Crit Care Med. 2001;164:109–116. doi: 10.1164/ajrccm.164.1.2007050. [DOI] [PubMed] [Google Scholar]
- 30•.Hurst J.R., Donaldson G.C., Wilkinson T.M., Perera W.R., Wedzicha J.A. Epidemiological relationships between the common cold and exacerbation frequency in COPD. Eur Respir J. 2005;26:846–852. doi: 10.1183/09031936.05.00043405. [DOI] [PubMed] [Google Scholar]; This article suggests that, in COPD subjects, exacerbation risk is associated with the frequency of acquiring the common cold. Patients experiencing frequent colds had a significantly higher exposure to cigarette smoke, suggesting that cigarette smoke can contribute to the increased susceptibility of COPD patients to respiratory viral infections.
- 31••.Wark P., Johnston S.L., Bucchieri F., Powell R., Puddicombe S., Laza-Stanca V., Holgate S.T., Davies D.E. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first paper showing that bronchial epithelial cells from asthmatic patients have a deficient innate immune response to in vitro rhinovirus infection.
- 32••.Contoli M., Message S.D., Laza-Stanca V., Edwards M.R., Wark P.A., Bartlett N.W., Kebadze T., Mallia P., Stanciu L.A., Parker H.L. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]; This paper documented that asthmatic patients have a deficient production of IFN-λ following rhinovirus infection. The production of IFN-λ was inversely related to the severity of the exacerbation in experimental rhinovirus-infected asthmatic patients, indicating that impaired innate immune responses may explain the increased susceptibility of asthmatic patients to respiratory viral infections.
- 33.Papadopoulos N.G., Stanciu L.A., Papi A., Holgate S.T., Johnston S.L. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002;57:328–332. doi: 10.1136/thorax.57.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gern J.E., Vrtis R., Grindle K.A., Swenson C., Busse W.W. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 35.Robbins C.S., Dawe D.E., Goncharova S.I., Pouladi M.A., Drannik A.G., Swirski F.K., Cox G., Stampfli M.R. Cigarette smoke decreases pulmonary dendritic cells and impacts antiviral immune responsiveness. Am J Respir Cell Mol Biol. 2004;30:202–211. doi: 10.1165/rcmb.2003-0259OC. [DOI] [PubMed] [Google Scholar]
- 36.Patel I.S., Seemungal T.A., Wilks M., Lloyd-Owen S.J., Donaldson G.C., Wedzicha J.A. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;57:759–764. doi: 10.1136/thorax.57.9.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Sajjan U.S., Jia Y., Newcomb D.C., Bentley J.K., Lukacs N.W., LiPuma J.J., Hershenson M.B. H. influenzae potentiates airway epithelial cell responses to rhinovirus by increasing ICAM-1 and TLR3 expression. FASEB J. 2006;20:2121–2123. doi: 10.1096/fj.06-5806fje. [DOI] [PubMed] [Google Scholar]; This paper provides a cellular mechanism by which bacterial infection might increase the susceptibility of COPD patients to rhinovirus-induced exacerbations.
- 38.Donninger H., Glashoff R., Haitchi H.M., Syce J.A., Ghildyal R., van Rensburg E., Bardin P.G. Rhinovirus induction of the CXC chemokine epithelial-neutrophil activating peptide-78 in bronchial epithelium. J Infect Dis. 2003;187:1809–1817. doi: 10.1086/375246. [DOI] [PubMed] [Google Scholar]
- 39.Johnston S.L., Papi A., Bates P.J., Mastronarde J.G., Monick M.M., Hunninghake G.W. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J Immunol. 1998;160:6172–6181. [PubMed] [Google Scholar]
- 40.Laza-Stanca V., Stanciu L.A., Message S.D., Edwards M.R., Gern J.E., Johnston S.L. Rhinovirus replication in human macrophages induces NF-kappaB-dependent tumor necrosis factor alpha production. J Virol. 2006;80:8248–8258. doi: 10.1128/JVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Mallia P., Message S.D., Kebadze T., Parker H.L., Kon O.M., Johnston S.L. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir Res. 2006;7:116. doi: 10.1186/1465-9921-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first in vivo model of virus-induced COPD exacerbation. The study documented that low-dose experimental rhinovirus infection in patients with COPD induces symptoms and lung function changes typical of an acute exacerbation of COPD, appears safe, and provides evidence of causation.
- 42.Seemungal T.A., Donaldson G.C., Bhowmik A., Jeffries D.J., Wedzicha J.A. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 43.Sin D.D., McAlister F.A., Man S.F., Anthonisen N.R. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA. 2003;290:2301–2312. doi: 10.1001/jama.290.17.2301. [DOI] [PubMed] [Google Scholar]
- 44.Calverley P., Pauwels R., Vestbo J., Jones P., Pride N., Gulsvik A., Anderson J., Maden C. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 45.McCrory D.C., Brown C., Gelfand S.E., Bach P.B. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest. 2001;119:1190–1209. doi: 10.1378/chest.119.4.1190. [DOI] [PubMed] [Google Scholar]
- 46.Davies L., Angus R.M., Calverley P.M. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet. 1999;354:456–460. doi: 10.1016/s0140-6736(98)11326-0. [DOI] [PubMed] [Google Scholar]
- 47.Ram FS, Rodriguez-Roisin R, Granados-Navarrete A, Garcia-Aymerich J, Barnes NC: Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006 19:CD004403. [DOI] [PubMed]
- 48.Mallia P., Contoli M., Caramori G., Pandit A., Johnston S.L., Papi A. Exacerbations of asthma and chronic obstructive pulmonary disease (COPD): focus on virus induced exacerbations. Curr Pharm Des. 2007;13:73–97. doi: 10.2174/138161207779313777. [DOI] [PubMed] [Google Scholar]
- 49.Murray C.J., Lopez A.D. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 50•.Sapey E., Stokley R.A. COPD exacerbations 2: aetiology. Thorax. 2006;61:250–258. doi: 10.1136/thx.2005.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]; An updated and exhaustive review of COPD exacerbation aetiology.


