Abstract
Air cleaning techniques have been applied worldwide with the goal of improving indoor air quality. The effectiveness of applying these techniques varies widely, and pollutant removal efficiency is usually determined in controlled laboratory environments which may not be realized in practice. Some air cleaners are largely ineffective, and some produce harmful by-products. To summarize what is known regarding the effectiveness of fan-driven air cleaning technologies, a state-of-the-art review of the scientific literature was undertaken by a multidisciplinary panel of experts from Europe, North America, and Asia with expertise in air cleaning, aerosol science, medicine, chemistry and ventilation. The effects on health were not examined. Over 26,000 articles were identified in major literature databases; 400 were selected as being relevant based on their titles and abstracts by the first two authors, who further reduced the number of articles to 160 based on the full texts. These articles were reviewed by the panel using predefined inclusion criteria during their first meeting. Additions were also made by the panel. Of these, 133 articles were finally selected for detailed review. Each article was assessed independently by two members of the panel and then judged by the entire panel during a consensus meeting. During this process 59 articles were deemed conclusive and their results were used for final reporting at their second meeting. The conclusions are that: (1) None of the reviewed technologies was able to effectively remove all indoor pollutants and many were found to generate undesirable by-products during operation. (2) Particle filtration and sorption of gaseous pollutants were among the most effective air cleaning technologies, but there is insufficient information regarding long-term performance and proper maintenance. (3) The existing data make it difficult to extract information such as Clean Air Delivery Rate (CADR), which represents a common benchmark for comparing the performance of different air cleaning technologies. (4) To compare and select suitable indoor air cleaning devices, a labeling system accounting for characteristics such as CADR, energy consumption, volume, harmful by-products, and life span is necessary. For that purpose, a standard test room and condition should be built and studied. (5) Although there is evidence that some air cleaning technologies improve indoor air quality, further research is needed before any of them can be confidently recommended for use in indoor environments.
Keywords: Indoor air quality (IAQ), Air cleaner, By-product, High efficiency particulate air (HEPA), Sorption, Ultraviolet germicidal irradiation (UVGI), Photocatalytic oxidation (PCO), Thermal catalytic oxidation (TCO), Plasma, Ozone, Ion generator, Electrostatic precipitator, Clean air delivery rate (CADR)
Abbreviations: AC, activated carbon; BTEX, benzene, toluene, ethyl benzene, and xylene; CADR, clean air delivery rate; CFM, cubic feet per minute; DBD, dielectric barrier discharge; EPA, Environmental Protection Agency; ESP, electrostatic precipitator; IAQ, indoor air quality; HEPA, high efficiency particulate air; PCO, photocatalytic oxidation; TCO, thermal catalytic oxidation; VOC, volatile organic compound; SOA, secondary organic aerosol; SP, submicron particles; SVOC, semi-volatile organic compound; TVOC, total volatile organic compound; UV-C, ultraviolet C, wavelength range: 280–100 nm; UVGI, ultraviolet germicidal irradiation; WHO, World Health Organization
Highlights
► Active air cleaning technologies are reviewed by an inter multidisciplinary panel. ► The performances including CADR, efficiency, by-products are summarized and compared. ► The problems such as producing harmful by-products etc. are pointed out and analyzed. ► Benchmarks, standard procedure for labeling air cleaner performance are necessary.
1. Introduction
Because indoor air quality is an important determinant of human health, comfort and productivity, high quality indoor air is desirable. Air cleaning technologies are of increasing importance, especially when building ventilation rates are being reduced to conserve energy. Numerous air cleaning technologies have been developed and used, but there have been no systematic assessments of these technologies. This is particularly true with regard to (1) application at realistic indoor conditions, (2) long-term performance, and (3) production of unwanted by-products during operation. The lack of widespread acceptance of reliable protocols for estimating the effectiveness of air cleaning systems has made it difficult to develop a standardized labeling system for indoor air cleaners, including standard methods for estimating Clean Air Delivery Rate (CADR). Consequently, a literature review was undertaken to collect state-of-the-art information on air cleaning technologies focusing on both their effects at removing indoor air pollutants and the problems that may occur during their application.
2. Methods
The scientific peer-reviewed literature on the effects of commonly-used gas-phase and particle phase air cleaners on indoor air pollutants in non-industrial indoor environments was reviewed by a multidisciplinary group of scientists with expertise in medicine, epidemiology, toxicology and engineering. The focus was only on air cleaning techniques for which indoor air flows through a device and is returned to the indoor environment (“fan-driven” air cleaners). Technologies like “catalyst in paint” or other passive air purification materials, masks and other personal protective devices were not included. Botanic air cleaners that did not involve flow-through systems were also excluded. Air cleaning devices that are intended only for outdoor air intakes (e.g., filters in mechanical ventilation system) were not considered. Consequently the air cleaners reviewed included only: high efficiency particulate air (HEPA), adsorption, ultraviolet germicidal irradiation (UVGI), photocatalytic oxidation (PCO), thermal catalytic oxidation (TCO), plasma, botanic air cleaners, ion generators, and electrostatic precipitators.
The selected air cleaning technologies were reviewed regarding their efficiency to reduce/remove indoor air pollutants including particles, microorganisms, inorganic and organic gases; radon was not included. The effects on health and/or occupant performance were not considered. For example, we did not consider articles which only reported the effects of an air cleaning device on health unless the effects on air pollutants were also reported. The selected articles were limited to those which reported the tests involving pollutant concentrations within an order of magnitude of concentrations reported by the US EPA, WHO, and others to be typical in non-industrial indoor environments. This approach may have excluded some information related to particle filtration because standard test protocols are completed at elevated particle concentrations and there is general consensus that removal efficiency is not affected by standard test concentrations.
Only demonstrated changes of concentration of one or more pollutants due to the use of an air cleaning device were considered, where “demonstrated” means that the methodology was validated and other effects such as air leakage and natural decay were considered. Demonstrated changes in odor intensity or perceived indoor air quality were also considered as evidence in this review.
3. Literature review
The scientific literature was gathered by searching through the following databases: ISI Web of Science (1910–present), ScienceDirect (1823–present), MEDLINE (1965–present) and Engineering village 2 (1884–present). Google Scholar was used as a supplementary search. As a source of search records, the following keywords were used:
-
•
Keywords related to air pollutants: formaldehyde, benzene, toluene, volatile organic compound, semi-volatile organic compound, total volatile organic compound, VOC, SVOC, TVOC, ammonia, carbon monoxide, gaseous pollutant, particulate matter, particulates, gas-phase pollutant, particle, dust, PM10, PM2.5, odor, bacteria, virus, fungi, fungus, microorganism, mold, pollen, droplet, droplet nuclei, aerosol, bio-aerosol, air pollutant, air contaminant, airborne pollutant, airborne contaminant, nitrogen oxides, CO, NO2, nitrogen dioxide, nitrogen monoxide, NOx, sulfur dioxide, tobacco smoke, amoebae, algae, mite, protozoa, insect feces, arthropods, asbestos, respirable suspended particulate, RSP, ozone.
-
•
Keywords related to air cleaning: air filter, filtration, high efficiency particulate air, HEPA, adsorption, ultraviolet, UV, ultraviolet germicidal irradiation, UVGI, photocatalytic oxidation, photocatalytic oxidation, PCO, UVPCO, thermal catalytic oxidation, TCO, catalysis, catalyst, plasma, ozone, botanic, air cleaning, air purification, air purifier, air ionizer, ionic air purifier, Electrostatic precipitator, activated carbon, zeolite, molecular sieve.
-
•
Keywords related to indoor environments: hospital, home, house, dwelling, residence, apartment, office, residence, school, building, aircraft, cabin, car, ship, subway, church, jail, indoor air, indoor, indoor environment, enclosure, room, vehicle, train, railway, clinic, classroom, university, laboratory, barrack, castle, temple, airport and stadium.
The bibliography of retrieved articles was also reviewed to identify references that were otherwise missed.
Articles and/or publications were considered for inclusion based on the following criteria:
-
•
Original research articles in English;
-
•
Articles relevant to the key research questions identified;
-
•
Publications up to June 2009;
-
•
Textbooks, design guidelines, standards, and review articles were excluded;
-
•
Articles without information on indoor air pollutants were excluded;
-
•
Abstracts and purely descriptive articles without a detailed analytic component were excluded;
-
•
Conference papers were excluded.
During the literature search, over 26,000 articles were identified. 400 articles were selected as relevant based on their titles and abstracts by the first two authors, who also further reduced the number of articles to 160 based on the full text. These articles were reviewed by the panel using predefined inclusion criteria during their first meeting and some articles were found to be outside the scope of this review. Additions were also made by the panel. In this process 133 articles were selected for thorough review. Each article was reviewed by two scientists, one assigned to be a prime reviewer and the other one assigned to be secondary reviewer. Each scientist reviewed 17 to 18 articles. The articles were assigned to the reviewers completely at random and not depending only on their expertise; no article was assigned to a scientist if he was one of the authors. When reviewing the article, information on different aspects of the study was collected including design, methods, data analysis, measurements of airflow rates and air pollutants, possible bias, single-pass removal efficiency, CADR, creation of by-products, results and main conclusions. Reviewed articles were then classified as: relevant and conclusive – providing sufficient information on air cleaning effect, data processing and reporting; relevant but non-informative – lacking essential information; relevant but inconclusive – with incomplete data processing or reporting. Classification of each article was first made independently by each reviewer. Then, during the plenary meetings, the whole group agreed on a final classification. The articles judged during plenary discussions of the whole group as conclusive were used to formulate the final consensus statement and conclusions.
During the review process the following definitions and terms were used:
-
•
Indoor air pollutants refer to contamination of the indoor environment by any chemical, physical or biological agent that is harmful to human health or uncomfortable to humans, and new airborne pollutants with unknown health effects. (ISIAQ, 2010, WHO, 2010)
-
•
Secondary indoor air pollutants refer to the intermediates or by-products produced by air cleaning devices, and released to indoor air.
-
•
Single-pass removal efficiency is defined as the percentage (or fraction) of the target pollutant that enters an air cleaner and is removed by the cleaner.
-
•
Effectiveness is defined as the fractional reduction in indoor pollutant concentration that results from application of a control device relative to the identical conditions without the control device in place (Nazaroff, 2000).
-
•
Clean air delivery rate (CADR) is the equivalent clean airflow rate delivered by an air cleaner in which “clean” only refers to the absence of the target pollutants removed by the air cleaner. It is equal to the single-pass efficiency multiplied by the airflow rate through the device.
-
•
Single-pass or flow-through test method is a method in which an air cleaner is placed between two tightly sealed chambers in such a way that one chamber is connected to the air cleaner intake and the other to the air cleaner outlet. The pressure drop between the chambers is adjusted to zero, so that the air cleaner operates as it would in a normal room. There is a constant airflow. Target pollutants (particles or gases) are constantly generated upstream and the concentration is measured in the upstream and downstream chambers.
-
•
Decay test method involves an air cleaner that is positioned in a sealed chamber. The airborne pollutant(s) is (are) injected as a short-term release and mixed with the chamber air before activating the air cleaner. The concentration of the pollutant in the chamber air is measured over a specific time period. The CADR is calculated from the decay curve accounting for losses by deposition to chamber surfaces and air exchange rate, if any (Niu et al., 1998).
-
•
Non-industrial indoor environments include any indoor environment not related to industrial exposures.
4. Results and discussions
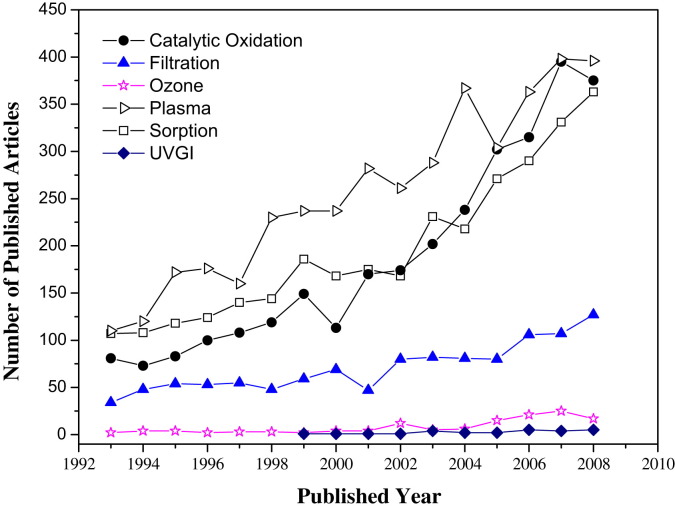
The number of published articles found using the above-mentioned keywords in ISI Web of Science (1910–present) vs. publication year is shown in Fig. 1 . Although studies and articles related to indoor air cleaning have increased rapidly since 1993, we have not been able to read and analyze carefully all these articles as this was beyond our collective ability.
Fig. 1.
. Number of published journal articles (in English) according to ISI Web of Science (1993–2008).
Of the 133 articles thoroughly reviewed and discussed by the panel, 59 articles were judged relevant and conclusive. We found that the number of articles with real environment data (field test) depends on the specific air cleaning technologies. For example, catalytic oxidation, ozone, and plasma are mostly tested in laboratory settings. For the traditional technologies such as filtration, sorption and UVGI, many studies were in completed in real environments. Table 1 summarizes the distribution of reviewed articles in either laboratory or real environments.
Table 1.
Number of included articles in laboratory or real environmental setting.
| Technology | Laboratory |
Field test | Total | |
|---|---|---|---|---|
| Single-pass test | Chamber test | |||
| Catalytic oxidation (including PCO) | 18 | 3 | 0 | 21 |
| Filtration | 5 | 2 | 8 | 15 |
| Ozone-related | 2 | 0 | 0 | 2 |
| Plasma | 3 | 0 | 0 | 3 |
| Sorption | 5 | 2 | 1 | 8 |
| UVGI | 3 | 4 | 3 | 10 |
| Total | 36 | 11 | 12 | 59 |
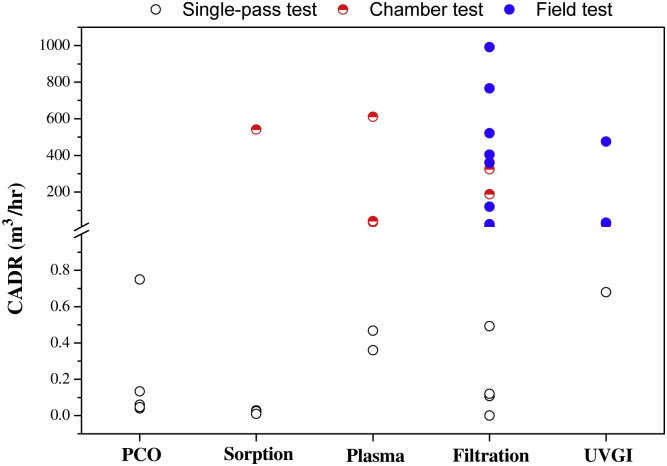
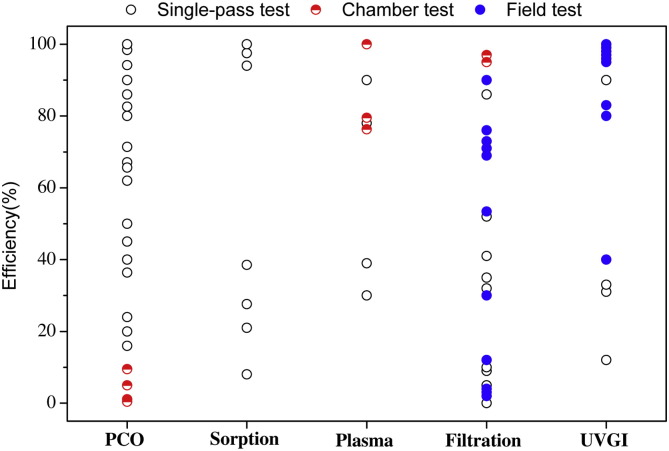
The performance of air cleaners is best measured and compared (between cleaning devices) by a Clean Air Delivery Rate (CADR) which is defined as the product of the single-pass removal efficiency and volumetric airflow rate through the cleaning device. However, many of the articles that were reviewed in this study did not include an explicit determination of CADR, nor were single-pass removal efficiency or volumetric flow rates provided to allow for an implicit calculation of CADR. Fig. 2, Fig. 3 summarize the reported CADR and single-pass removal efficiency in the 59 reviewed articles (Table 2, Table 3, Table 4, Table 5, Table 6 ).
Fig. 2.
Summary of reported CADR values in the reviewed articles.
Fig. 3.
Summary of reported efficiency values in the reviewed articles.
Table 2.
Catalytic oxidation air cleaning technology. Catalytic oxidation refers to a set of chemical treatment procedures designed to remove organic and inorganic materials in gas by catalysts. The common types for indoor air cleaning are photocatalytic oxidation (PCO), thermal catalytic oxidation (TCO) and ozone-catalytic oxidation.
| Papers | Results by the authors | Research type/test procedure | Target pollutants/concentration | Airflow rate, Air velocity, or residence time | CADR (m3 h−1)/efficiency (%) | By-product tested or not and results |
|---|---|---|---|---|---|---|
| Photocatalytic oxidation | ||||||
| Ao and Lee (2003) | UV-TiO2/AC was more effective in BTEX removal and less affected by the increasing humidity than AC alone. AC acted as a local pollutant concentrator by adsorbing pollutants from the air stream. | Laboratory; single-pass test. Primary UV wavelength: 365 nm; UV intensity: 0.75 mW cm−2; temperature: 25 ± 1 °C. | NO: 200 ppbv; BTEX: 20 ppbv; humidity: 2100–22000 ppmv. | Airflow rate: 5–30 L min. Residence time: 0.6–3.7 min. |
50% to over 90%. Varies with residence time and humidity levels. When the residence time is 1.2 min, the efficiencies of TiO2/AC are: BETX: over 60%, NO: over 70%. |
Yes. NO2 is an intermediate from photodegradation of NO. If combining UV-TiO2 and activated carbon for BTEX and NO removal, there was no deactivation. But deactivation occurred if only using UV-TiO2. |
| Ao et al. (2003) | Humidity and residence time had significant influence on the conversion rate by UVPCO, especially for BTEX compounds. The presence of NO reduced the photodegradation of BTEX at moderate humidity levels. | Laboratory; Single-pass test. PCO reactor: plate type; Primary UV wavelength: 365 nm; UV intensity: 0.6 mW cm−2. | NO: 200 ppbv; BTEX: <100 ppbv; Humidity: 2100–22000 ppmv. | Residence time: 2.85–11.4 min. | 24–86% for BTEX and over 90% for NO at low humidity level. | Yes. The reaction between NO and BTEX will generate NO2. |
| Ao et al. (2004) | The presence of SO2 inhibited the conversion of BTEX and NO compounds by UVPCO, but increased the generation of NO2 as a by-product from NO. | Laboratory; Single-pass test. PCO reactor: plate type. Germicidal UV lamp. Temperature: 25 ± 1 °C. | NO: 200 ppbv; SO2: 200 ppbv; BTEX: 20 ppbv; Humidity; 2100–22000 ppmv. | Residence time:1.24 min. | 40% to >80% for TiO2. After irradiation by UV for 120 min, the efficiencies are: Benzene: 30–70%; Toluene: 50–80%. Ethyl benzene: 60–80%; o-xylene: 65–80% (Residence time: 1.2 min; Humidity: 2100 ppmv). |
Yes. NO2 is the by-product from the degradation of NO. |
| Chen and Zhang (2008) | The interference effect among VOCs was small in 2-VOC and 3-VOC mixture tests. However, such effect became significant in 16-VOC mixture. There is a competitive adsorption among different VOCs. |
Laboratory; Chamber test. PCO reactor: Honeycomb monoliths; Primary UV wavelength: 365 nm; UV intensity: 0.6 mW cm−2; Temperature: 23 ± 0.5 °C; Humidity: 50 ± 5%. | Single compound: 1 mg m−3 for each compound except formaldehyde and acetaldehyde. 16 VOCs mixture test: 2 mg m−3 for each compound. |
Airflow rate: 1360 m3 h−1. Face velocity: 1.05 m s−1. |
1.1–9.5% for all VOCs. | No. |
| Jeong et al. (2005) | The photodegradation of toluene and benzene by UV254+185 nmwas much higher than by UV254 nm or UV365 nm. Ozone generated by the UV-185 nm lamp enhanced this photodegradation effect. | Laboratory; Single-pass test. PCO reactor: Pyrex glass cylinder; Primary UV wavelength: 185–254–365 nm; Temperature: 24.9 ± 1 °C. Humidity: <1–90%. | Toluene, benzene: 0.6–20 ppmv. | Airflow rate: 1.0–4.0 L min−1. Residence time: 33.0–8.3 s. |
Varies with airflow rate. At 1 L min−1, inlet concentration = 0.6 ppmv, RH = 40%, Toluene: 82.6–99.9%; Benzene: 67.1–94.2%. |
Yes. CO2 and CO are the main degradation products, with some water-soluble organic intermediates. |
| Hodgson et al. (2007) | UVPCO can effectively reduce many VOCs, with conversion efficiency greatest for alcohols and glycol ethers and lowest for halogenated aliphatic hydrocarbons. | Laboratory; Single-pass test in dust. PCO reactor: Honeycomb monoliths; Primary UV wavelength: 254 nm; UV intensity: 6.0–6.5 mW cm−2. Temperature: 19.5–25 °C; Humidity: 42–65%. |
Mixture contained 27 VOCs from office buildings; Mixture including 10 VOCs emitted by cleaning products. | Airflow rate: 165–580 m3 h−1 for 27 VOCs; 165–580 m3 h−1 for 10 VOCs. Face velocity: 0.51–1.79 m s−1. |
Given for all VOCs in study. Toluene: 16%–45% | Yes. Formaldehyde, acetaldehyde, formic acid and acetic acid are the main by-products from the degradation of 27 VOCs. Formaldehyde, acetaldehyde and acetone are from 10 VOCs. |
| Mo et al. (2009) | The by-products of toluene by UVPCO were identified, such as benzaldehyde, methanol, acetaldehyde and acetone. | Laboratory; Single-pass test. PCO reactor: Plate type; Primary UV wavelength: 254 nm; UV intensity; 0.43–0.95 mW cm−2; Temperature: 24–26 °C; Humidity: 1.1–84%. | Toluene: 450–8000 ppbv. | Airflow rate: 0.55 L min−1. Residence time: 0.2 s. | – | Yes. Benzaldehyde, methanol, acetaldehyde etc. are the main gas-phase by-product. |
| Muggli et al. (1998) | A possible reaction mechanism of ethanol decomposed by UVPCO was proposed. The intermediates of ethanol such as acetaldehyde, acetic acid (acetate), formaldehyde, and formic acid (formate) were identified. | Laboratory; Single-pass test. PCO reactor: annular Pyrex reactor. Primary UV wavelength: 356 nm; UV intensity; 0.3 mW cm−2; Room temperature. |
Ethanol. | – | – | Yes. Part of the ethanol reacts on the surface through the pathway: acetaldehyde → acetic acid → CO2 +formaldehyde → formic acid → CO2. |
| Obee and Brown (1995) | Competitive adsorption between water and trace (sub-ppmv) contaminants has a significant effect on the oxidation rate. | Laboratory; Single-pass test. PCO reactor: Plate type; Primary UV wavelength: 250–350 nm; UV intensity; <0.01–0.125 mW cm−2. Humidity: 0–20000 ppmv; Temperature: 12.8–60 °C. |
Formaldehyde; toluene and 1,3-butadiene: 0–<20 ppmv. | Face velocity; 2.6–12 cm s−1. | Varies with initial pollutant concentrations, humidity levels, and temperature. | No. |
| Tsai et al. (2008) | The application of UV/TiO2/quartz or UV/TiO2/MCM-41 in the process to control toluene, ethyl benzene, xylene was viable and effective. UV/TiO2/quartz had a better reaction rate than that of UV/TiO2/MCM-41. | Laboratory; Cycling or decay. PCO reactor: batch packed-bed reactor; Primary UV wavelength: 365 nm; UV intensity; 1.67 mW cm−2; Humidity: 0–20000 ppmv; Temperature: 15–35 °C. | Toluene, ethyl benzene, xylene: 2–10 ppmv. | Residence time: 8.5–20 s. | Varies with residence time. Toluene: 71.4–98.4%; Ethyl benzene: 54.4–94.8%; Xylene: 56.4–95.1%. |
No. |
| Tsoukleris et al. (2007) | P25 TiO2 nanoparticle paste is an effective photocatalyst for removal of VOCs. | Laboratory; Single-pass test. PCO reactor: packed-bed reactor; Primary UV wavelength: 350 nm; UV intensity; maximum 0.0715 mW cm−2; Humidity: 60%; Temperature: 25 ± 2 °C. | Toluene: 1170.4–1321.7 μg m−3; Benzene: 701–775.2 μg m−3; Xylene: 0–45.4 μg m−3. | – | Toluene: 86%; Benzene 100%; Xylene: 100% in 3 min. | No. |
| Wisthaler et al. (2007) | The concentration of most organic pollutants present in aircraft cabin was efficiently reduced by PCO and adsorption air cleaning. PCO had intermediate products of acetaldehyde and formaldehyde by degrading ethanol. | Laboratory; Chamber test. PCO reactor and adsorptive prefilter. Humidity: 21 ± 2%; Temperature: 23.2 ± 0.1 °C. |
Ethanol, monoterpenes, acetaldehyde, acetone, formaldehyde, methanol and isoprene: less than 200 ppbv. | Face velocity: 50.3–64.3 cm s−1. | – | Yes. PCO produces un acceptably high levels of acetaldehyde and formaldehyde. |
| Yang et al. (2007) | Vacuum ultraviolet (VUV) improved the conversion of formaldehyde markedly on the basis of TiO2/UV. The hybrid process was more economical than the TiO2/UV process. | Laboratory; Single-pass test. PCO reactor: annular type; Primary UV wavelength: 254 nm; UV intensity; 0.25–2.8 mW cm−2; Humidity: 30–80%. |
Formaldehyde: 150–500 ppbv. | Face velocity: 0.3–0.94 m s−1. | Varies with airflow rate, UV intensity etc. 20% (0.94 m s−1)–62% (0.3 m s−1). | Yes. Ozone was produced by VUV. |
| Yu et al. (2006a) | The rate constants of PCO for toluene, xylene and mesitylene ranged from 1.22 to 4.00 μmol m−1 s−1 and were proportional to kOH (VOC-OH· rate constants). | Laboratory; Single-pass test. PCO reactor: plate type; Primary UV wavelength: 254 nm; UV intensity; 0.25–2.8 mW cm−2; Humidity: dry-humid; Temperature: 25 ± 0.5 °C. | n-Hexane, Iso-butanol, Toluene, p-Xylene, m-Xylene, Mesitylene: 0.1–9.0 ppmv. | Airflow rate: 200–1200 mL min−1. | – | Yes. The photodegradation of VOC results in CO2 and residual intermediates at different rates. |
| Yu et al. (2006b) | Both toluene and formaldehyde can be removed using photocatalytic filters in a simulated HVAC system. The VOC removal efficiency increases with RH. | Laboratory; Single-pass test. A mechanical filter coated with P25 and 2 commercial photocatalytic filters; Primary UV wavelength: 254 nm; UV intensity: average 0.0489 mW cm−2; Total air changer rate: 0.5–1.5 h−1; Temperature: average 25.65; RH: 30–70%. | Toluene, formaldehyde: 2 ppmv. | Face velocity: 177–532 m h−1 (0.05–0.15 m s−1). | CADRs varies with face velocity. At a face velocity of 177 m h−1, Toluene: 0.0466–0.0840 m3 h−1; Formaldehyde: 0.0732–0.0947 m3 h−1. | Yes. The ozone concentration is less than 25 ppbv in the test chamber. |
| Zhang et al. (2003a) | Introduction of O3 to TiO2/UV systems can enhance the degradation of toluene. | Laboratory; Single-pass test. Primary UV wavelength: 254 nm and 365 nm; Humidity: 20–60%; Temperature: 20–22 °C. | Toluene: 1.0–20 ppmv. | Airflow rate: 1.0–5.0 L min−1. Residence time: 17.3–86.4 s. | Varied from less than 5% for ozone alone to > 80% with ozone and UV-TiO2. | No. |
| Zhang et al. (2003b) | A simple PCO reactor model was developed to analyze the VOC removal performance in PCO reactors with experimental validation. | Laboratory; Chamber test. PCO reactor: annular type. Primary UV wavelength: 254 nm; Humidity: 37–50%; Temperature: 24–26 °C. | Toluene: 7.66 ppmv; Formaldehyde 1.77–1.85 ppmv. | 25 m3 h−1. | Toluene: 0.4%; Formaldehyde: 5%. | No. |
| Zhang et al. (2007) | An analysis of the UVPCO behavior for two compounds (toluene and benzene) was provided. The component impact factor between binary compounds was defined to describe the influence of one compound on the reaction coefficient of another compound. | Laboratory; Single-pass test. PCO reactor: plate type; Primary UV wavelength: 254 nm; UV intensity: 0.56 mW cm−2; Humidity: ∼40%; Temperature: 25–27 °C. | Toluene: 4.48 mg m−3; Benzene 1.82–4.08 mg m−3. | Airflow rate: 3 L min−1. Face velocity: 1 m s−1. |
Varies with reaction conditions. | No. |
| Other catalytic oxidation | ||||||
| Ellis and Tometz (1972) | The room temperature catalytic efficiency in decomposing ozone of 35 materials was investigated. Activated carbon/charcoal removes most O3 under room temperature, while zeolites, glass wool, and several others remove less. | Laboratory; Single-pass test. Humidity: 15–20%; Temperature: 23 ± 2 °C. |
O3: 45–1000 ppbv. | Airflow rate: 0.14–1.17 ft3 min−1 (3.96–33.1 L min−1). | 0–100% depending on the materials. The efficiency of all materials tested degraded with time. | No. |
| Kwong et al. (2008a) | Catalytic oxidation rate of toluene was enhanced over 3 types of adsorbent: NaX, NaY and MCM-41 when ozone was injected (6 ppmv). | Laboratory; Single-pass test. Humidity: 0% (dry condition), 50%; Temperature: 25 °C. |
O3: 6 ± 0.1 ppmv in regular tests and 24 ± 0.5 ppmv was also used in some cases; Toluene: 1.5 ± 0.03 ppmv. | Airflow rate: 0.21 m3 h−1; Residence time: 0.13 s. Face velocity: 1.54 m s−1. |
For toluene under dry conditions without ozone, at a 200 mm bed length: 73% (MCM-41), 53% (NaY) and 45% (NaX). Dry condition with ozone, at a 200 mm bed length: 90% (MCM-41), 78% (NaY) and 71.6% (NaX); Over 98% inlet ozone was consumed. |
Yes. Some aldehyde species were generated, such as formaldehyde, acetaldehyde and benzaldehyde. |
| Kwong et al. (2008b) | Use of zeolite and MCM-41 catalytic sorbents, during ozonation, can reduce by-product formation while removing toluene. | Laboratory test/single-pass. Humidity: 0%, 50%; Temperature: 23–25 °C. |
Toluene: 0.3–4.5 ppmv; O3: 0–80 ppmv. | Airflow rate: 0.39–0.12 m3 h−1. Residence time: 0.07–0.23 s. | 50% toluene via adsorption and another 20–40% was decomposed by ozonation. | Yes. Acetaldehyde, formaldehyde, benzaldehyde and formic acid are the main by-products. |
By-products (e.g., formaldehyde, acetaldehyde, formic and acetic acid, etc.) were generated during the PCO decomposition of various pollutants. Combining TiO2 with adsorption material (activated carbon etc.) may lower the generation of the by-products. The effect of multiple indoor pollutants on UVPCO performance needs further investigation and should not be neglected. Most of the researches on UVPCO are laboratory studies.
Table 3.
Filtration air cleaning technology. Filtration is a mechanical or physical operation which is used for the removal of particles by physical separation from air by interposing a medium through which only the air can pass.
| Papers | Results by the authors | Research type/test procedure | Target pollutants/concentration | Airflow rate, air velocity, or residence time | CADR (m3 h−1)/efficiency (%) | By-product tested or not and results |
|---|---|---|---|---|---|---|
| Batterman et al. (2005) | Filter decreased PM concentrations in field tests (ETS) by 30–70%, depending on size fraction and occupant activities, and significantly reduced the half-life of PM 0.3–1. No evidence of VOC removal. | Field test, homes. Decay and modeling. Stand alone HEPA, with activated carbon prefilter, max airflow 745 m3 h−1. |
−1: 110000–340000 counts L−1; PM1–5: 450–2400 counts L−1; Toluene: 26–33 μg m−3; 2–5 dimethyl furan (2–5 DMF): 0.60–1.09 μg m−3. | – | CADR is 374 m3 h−1 at airflow of about 700 m3 h−1 for PM 0.3–1. | No. |
| Bekö et al. (2008) | Bag filters in combination with activated carbon downstream of the particle filter can remove particles odor, and part of ozone. | Laboratory/field test, Sensory assessment. EU5, EU7 and combination of EU7 and activated carbon. | Odor. | 0.2 m s−1 for sensory assessment. 2.0 m s−1 during soiling period (5 months, outdoor air). | – | Yes. By sensory assessment. |
| Bekö et al. (2009) | Filters containing activated carbon downstream can remove particles odors and part of ozone (more with more AC). | Laboratory test, Sensory assessment. F7 filter and F7 with activated carbon. | O3: 15–25 ppbv, odor. | 0.2 m s−1 for sensory assessment, 2.0 m s−1 for soiling (3 and 6 months, outdoor air). | – | Yes. By sensory assessment. |
| Bekö et al. (2006) | Oxidation by O3 of organic compounds adsorbed on filters resulted in increased odor. | Laboratory; Single-pass test. EU7. | O3: 75 ppbv. | 0.125 m s−1. | 5–10% (ozone). | No. |
| Cheng et al. (1998) | HEPA filters reduced particle (fungal spores and pollens) concentration by 80% of which settling accounts for 50% at an air change rate of 1–1.2 ach. At a low air change rate no difference, as the particles settle rapidly. | Field test, home, Portable HEPA filter (404 m3 h−1). | Pollens and fungal spores. | – | – | No. |
| Davis et al. (1994) | The experimental data significantly deviated from model predictions. No significant difference in filtration efficiency between different types of filters (standard, electret, electrostatically enhanced). | Laboratory test, of 11 ducted commercial residential filters, including standard mechanical, electret, and electrostatically enhanced filters. Single-pass, validation modeling. | PM 0.5–4. | 2.3–3.8 m s−1. | PM 0.5: 0–32%; PM 4: 35–86%. | No. |
| Kujundzic et al. (2005) | HEPA-UV air filter can reduce the concentration of culturable and total bacteria, but not of airborne endotoxin. | Field and laboratory test. | Mycobacterium parafortuitum cells. | – | 12–76%. | No. |
| Lee et al. (2004) | Unipolar ion emission produced by corona discharge can be efficient in controlling indoor particles. | Laboratory; Chamber test. | Fine and ultrafine particles. | – | PM 0.1: 97%; PM 1: 95% (in 30 min). Strong ion source! Particles settle on surfaces including humans. | No. |
| Lorimier et al. (2008) | Fiber arrangement has an impact on filtration efficiency: the more homogeneous the better; many layers of media can give high efficiency. | Laboratory; Single-pass test; activated carbon. | PM 0.1–2.5: 2500 particles cm−3. | 0.37–0.50 m s−1. | 52–86%. | No. |
| Miller-Leiden et al. (1996) | Filtration was effective in reducing airborne particle (droplet nuclei) concentration. The degree of protection provided by in room air filtration may not be sufficient for tuberculosis infection control. | Field test, 4 portable, and three mounted air cleaners, 5 with HEPA filters. Max airflow 250–1175 m3 h−1. | Chemical and Bacterial particles with an aerodynamic diameter of 0.7 μm and 1.3 μm. | – | 30–90%. | No. |
| Offermann et al. (1985) | Panel filters were largely ineffective at removing ETS. Extended surface filters and electrostatic precipitators had a high efficiency. | Field test, commercial product/decay; 4 panel filters, 2 extended surface filters (one with HEPA), 2 electrostatic precipitators, 2 ion generators. | Cigarette smoke particles: 1–2×105 particles cm−3. | – | Panel filters 0–12 m3 h−1; extended surface filters 97–306 m3 h−1 (HEPA); electrostatic precipitators 197–207 m3 h−1; ion generators, residential type 2 m3 h−1, commercial type 51 m3 h−1. | No. |
| Offermann et al. (1992) | Extended surface filters, (one HEPA) and electrostatic precipitators were more effective than single panel filters. For HEPA filters, the measured efficiency (73%) was significantly lower than according to the manufacturers’ data (99.97%, of DOP particles). | Field test of 6 ducted air cleaners, 2 panel filters, 2 extended surface filters, and 2 electrostatic precipitators. | Cigarette smoke particles: 1–2×105 particles cm−3. | – | Panel filters 2–3%; Extended surface filters 71–73%, electrostatic precipitators, 4% (two stage foam filters), 69% (two stage flat plate collector). | No. |
| Schleibinger and Rüden (1999) | A test of VOC emissions from used and unused (cellulose fiber) filters. Formaldehyde and acetone were the main products from dust loaded filters. The emission of acetaldehyde was higher from new filters. Chemical reactions or microbial activity may be the cause. | Field test; Filter class EU6, EU7. | Carbonyl compounds (14 aldehydes and two ketones). | Normal velocity in HVAC ducts. | – | Yes. Formaldehyde, acetaldehyde, acetone. |
| Waring et al. (2008) | The pollutant removal benefits of ozone-generating air cleaners may be outweighed by the generation of indoor pollution. | Laboratory; Chamber test. Commercial products. | Particles with sizes 12.6–514 nm diameter. | – | CADRs: HEPA:188–324 m3 h−1; Electrostatic precipitator 284 m3 h−1; Ion generators 35–41 m3 h−1. | Yes. Ozone. |
| Zhao et al. (2007) | Ozone can to some extent be removed by common HVAC filters. Loaded filters are more effective than new. | Laboratory test/single-pass; 21 MERV 4 and one MERV 8 filters. | O3:80 ppbv. | Face velocity: 0.004 m s. | Ozone removal, 0–9% for new filters, 10–41% for loaded particle filters. | No. |
Formaldehyde and acetone were the main by-products from dust loaded filters. Emission of VOC from the filter material should be very low. However, there were unknown oxidation products due to reactions between O3 and adsorbed organic compounds.
Table 4.
Ozone-oxidation and plasma air cleaning technology. Ozone has a very high oxidation potential and can react with some indoor pollutants. The common types of ozone generator for indoor uses are the corona discharge method and ultraviolet light method. Plasma is a gas produced by electrical charge, in which a certain proportion of its composition (atoms, molecules, or ions) is ionized. The atoms, molecules, or ions with unpaired electrons cause them to be highly chemically reactive.
| Papers | Results by the authors | Research type/test procedure | Target pollutants/concentration | Airflow rate, air velocity, or residence time | CADR (m3 h−1)/efficiency (%) | By-product tested or not and results |
|---|---|---|---|---|---|---|
| Ozone oxidation | ||||||
| Biey and Verstraete (1999) | O3 generated from UV lamp can reduce odor from organic waste (e.g., grass, fruit, kitchen waste). | Laboratory; Chamber test. | Odors from ethyl acetate, E. coli. | – | E. coli: almost 100% after 14 days. | No. |
| Boelter and Davidson (1997) | Negative polarity can generate more O3 than positive polarity; Current level, wire diameter, operating temperature and relative humidity were studied. | Laboratory; Single-pass test. | O3. | 0.20–6.20 m s−1. | – | Yes. Ozone. |
| Plasma | ||||||
| Park et al. (2008) | Plasma (dielectric barrier discharge, DBD) combined with electrostatic precipitator (ESP), remove particles, and in combination with UV-photocatalyst (UVP), also gases. | Laboratory; Chamber test. Dielectric barrier discharge. |
PM2.5: 500 μg m−3; Submicron particles (SP); Formaldehyde and BTX (benzene, toluene and xylene): 1 ppmv. | 0.38 m s−1 | PM2.5: 79.5% SP: 76.3% in 5 h. With UV-photocatalyst HCHO, and BTX are removed 100% in respectively 20, and 40 min. | Yes. With DBD + ESP 0.4–0.7 ppmv ozone, with also UVP, 0.01 ppmv ozone. |
| Van Durme et al. (2007) | Plasma catalytic hybrid system can increase toluene removal efficiency and decrease NOx and ozone production in dry air. Humidity has a positive influence on removal of NOx, and decreases production of ozone, and a strong negative effect on removal of toluene. | Laboratory test/single-pass; corona discharge. | Toluene: 0.5 ppmv. | 0.12 m s−1. | Post plasma catalyst 10 g CuOMnO2/TiO2 and with an energy density of 2.5 J L−1) reduces toluene 78% in dry air and 30% at RH 27%. | Yes. Ozone, NOx. |
| Van Durme et al. (2009) | Humidity has a significantly negative effect on toluene decomposition. Humidity does not influence the removal of ozone. | Laboratory test/single-pass; Corona discharge. | O3: 25 ppmv; toluene: 0.5 ppmv. | 0.12 m s−1. | Post plasma catalyst Pd/Al2O3 at an energy density of 10 J L−1, reduces toluene 90% in dry air, and 39% at an RH of 74%. | Yes. Ozone. |
The production of harmful pollutants from ozone is a major problem. Ozone generated by-products include a wide range of carbonyls, dicarbonyls, carboxylic acids, secondary organic aerosols, peroxides and more. Negative ion generators produced excessive concentration of O3 and NOx. For VOC removal, other harmful by-products were generated in addition to CO, CO2, NOx and O3.
Table 5.
Sorption air cleaning technology. Sorption is the collection of a substance onto the surface of adsorbent solids. It is a removal process where certain compounds or particles are bound to an adsorbent surface by either chemical or physical attraction.
| Papers | Results by the authors | Research type/test procedure | Target pollutants/concentration | Airflow rate, air velocity, or residence time | CADR (m3 h−1)/efficiency (%) | By-product tested or not and results |
|---|---|---|---|---|---|---|
| Chen et al. (2005) | Very volatile gases, such as dichloromethane, formaldehyde, and acetaldehyde, could not be efficiently removed by activated carbon alone. However, they could be removed if specific sorption media (e.g., activated alumina impregnated with potassium permanganate) were added. Botanic air cleaner significantly removed n-hexanal, formaldehyde, and acetaldehyde. | Laboratory test, commercial products/single-pass or decay. | 16 VOCs and ozone/2 mg m−3 for formaldehyde and acetaldehyde; 1 mg m−3 for the others. | Face velocity: 1.01 m s−1; flow rate: 14-335 CFM. | 0–611 m3 h−1 for each individual compound. | Yes. Air ionizer produces ozone. |
| Fang et al. (2008) | Some VOCs and sensory pollutants (odor) were both removed effectively by the desiccant wheel. | Laboratory test/single-pass; sensory assessment. | Pollutants from human subjects (bioeffluents) and flooring materials; formaldehyde; ethanol; toluene and 1,2-dichloroethane/toluene: 0.1–5 ppmv. | 190 L s−1. | Mean single-pass efficiency > 94%. | Yes. But no by-products were detected. |
| Gesser and Fu (1990) | Plasticized polyethylenimine is effective in chemical adsorption of formaldehyde as well as for acidic gases such as H2S, SO2 and NO2. | Laboratory or Field test/decay or single-pass. | Formaldehyde: 085–10.0 ppmv; H2S: 75 ppmv; SO2: 125 ppmv; NO2: 250 ppmv. | – | CADRs: Formaldehyde: 0.029 m3 h−1; H2S: 0.024 m3 h−1; SO2: 0.0096 m3 h−1. | No |
| Metts and Batterman (2006) | Activated carbon is effective at removing ozone, even at high concentrations. The presence of VOCs has an adverse effect for ozone removal. | Laboratory test/single-pass. | O3: 1.1–8.9 ppmv. | 0.15 m s−1. | – | No. |
| Metts (2007) | O3–limonene reactions occur heterogeneously on activated carbon but to a much lesser extent. Further studies are needed to investigate whether O3 enhances desorption of VOCs from activated carbon. | Laboratory test. | O3. | 0.15 m s−1. | – | Yes. VOC-loaded AC air filters exposed to O3 are not likely to emit significant amounts of secondary pollutants. |
| Parmar and Grosjean (1991) | Sorbents can generally remove indoor air pollutants in museum air. Activated carbon has good performance for all tested pollutants. | Laboratory test/decay or single-pass. | O3: 60–300 ppbv; NO2: 75–200 ppbv; SO2 120–180 ppbv; formaldehyde 160–1200 ppbv; H2S 60–260 ppbv. | 0.65–2.0 L min−1 for active mode. | 0.039–0.12 m3 h−1/97.5% (for formaldehyde) and 100% (for the others). | No. |
| Yao et al. (2009) | Activated carbon fiber cloth has stable adsorption performance for tested pollutants in 300 heating (150 °C) and cooling cycles. | Laboratory test/single-pass. | Initial inlet concentration: toluene 100 ppbv; limonene 100 ppbv; methyl ethyl ketone (MEK) 100 ppbv. | 92 L min−1, 184 L min−1. | 27.6–38.5% for airflow rate 92 L min−1; 8-21 for airflow rate 184 L min−1. | No. |
| Zhang et al. (2008) | A model was developed to evaluate the VOCs removal performance of a desiccant wheel. | Validated modeling. | Inlet concentrations: Toluene-467 μg m−3; 1,1,1-trichloroethane-289 μg m−3. | 160 L s−1 (5.78 m3 h−1). | ≥94% | No. |
Sorption is effective for VOC removal. The sorbed VOCs and O3 may generate some low level reaction by-products, including particles. However, some adsorbents, e.g., activated carbon, can also be effective at removing reactant species such as ozone by surface reactions. Humidity generally has a negative effect on VOC removal due to the sorption competition.
Table 6.
UVGI air cleaning technology. Ultraviolet germicidal irradiation (UVGI) is a sterilization method that uses ultraviolet (UV) light at sufficiently short wavelength to break down microorganisms.
| Papers | Results by the authors | Research type/test procedure | Target pollutants/concentration | Airflow rate, air velocity, or residence time | CADR (m3 h−1)/efficiency (%) | By-product tested or not and results |
|---|---|---|---|---|---|---|
| Harstad et al. (1954) | Ultraviolet air conditioner will reduce the number of airborne microorganisms in room air. | Field test, commercial product/cycling. | Serratia indica. | 280 CFM circulating air and 30 CFM fresh air. | 99.95–99.98%. | No. |
| Kujundzic et al. (2006) | UVGI had high efficiency in reducing the concentration of bacteria and fungi. Ionizer had low efficiency. | Laboratory test, commercial product/cycling. | Mycobacterium parafortuitum; Micrococcus luteus; Aspergillus versicolor | Each air cleaner: 856 m3 h−1. | 1480–2370 m3 h−1 when using air cleaners in combination with upper-room air UVGI. | No. |
| Lai et al. (2003) | UV can effectively inactivate Staphylococcus aureus bio-aerosols both in laboratory test and in occupied bedroom or bathroom. | Laboratory and Field test/cycling. | Staphylococcus aureus: 5000–15000 CFU m−3. | 19 CFM with the effectiveness of 71%. | – | No. |
| McDevitt et al. (2007) | UVC was effective in reducing the survival ratio of vaccinia virus aerosols. | Laboratory test/single-pass. | Vaccinia virus. | 28.3 L min−1. | Various with different UV intensities. | No. |
| Menzies et al. (2003) | UVGI reduces surface contamination of microbial and endotoxin concentrations in ventilation systems | Field test/cycling, sensory assessment. | Microbial and endotoxin. | – | Operation of UVGI resulted in 99% reduction of microbial and endotoxin concentrations on irradiated surfaces within the ventilation systems. | No. |
| Paschoalino and Jardim (2008) | The polyester supported TiO2 is efficient for bacteria but less efficient for fungi. Doping silver in the catalyst has no increase in biocide activity. | Field test/decay. | Bacteria and fungi. | 200 L min−1. | Bacteria inactivation is over 99% after 1 h operation. | No. |
| Tseng and Li (2005) | The relationship between survival fraction and UV intensity, UV exposure time and microorganism susceptibility is presented | Laboratory; Single-pass test. | Four virus (bacterial phages): ssRNA, ssDNA, dsRNA and dsDNA. | – | Survival fraction is less than 10%. | No. |
| Walker and Ko (2007) | Air disinfection using 254 nm UVC may be effective for inactivating viral aerosols. Of the three viruses examined, adenovirus was the most resistant to 254 nm UVC and should be exposed to high UV doses for complete inactivation. Unlike bacterial aerosols, there was no significant protective effect of high RH on UV susceptibility of the tested viral aerosols. | Laboratory; Single-pass test. | Bacteriophage MS2, adenovirus, and coronavirus. | 12.5 L min−1. | Survival fractions of MS2, adenovirus and coronavirus are about 31%, 33% (2608 μW s cm−2 UVC exposure) and 12% (599 μW s cm−2 UV-C). | No. |
| Xu et al. (2005) | Performance of the UVGI system degraded significantly when RH was increased from 50% to 75–90%. The inactivation rate increased linearly with effective UV fluence rate up to 5 μW cm−2; an increase in the fluence rate above this level did not yield a proportional increase in inactivation rate. | Field test, commercial product/decay. | Mycobacterium parafortuitum cells. | – | 40–95% (in 20 min). | No. |
| Xu et al. (2003) | UVGI removes or inactivate airborne bacteria spores and mycobacteria cells. | Field test, commercial product/decay. | Bacillus subtilis (spores), Mycobacterium parafortuitum, and Mycobacterium bovis BCG cells. | – | Bacillus subtilis (spores): 46–80%, Mycobacterium parafortuitum: 83–98%, and Mycobacterium bovis BCG cells: 96–97%. | No. |
O3 and dioxin are possible pollutants induced by UV. However, few measurement or tests were done.
4.1. Catalytic oxidation
Most catalytic oxidation air cleaning studies focus on photocatalytic oxidation (Table 1). TiO2 is the most commonly-used material in PCO research. In some studies ozone was applied to enhance the performance of the catalysts (Ellis and Tometz, 1972, Kwong et al., 2008b). PCO is a general air cleaning technology, which can degrade almost all contaminants (such as aldehyde, aromatics, alkanes, olefins, halogenated hydrocarbons, odor compounds etc.). The competitive adsorption effect for contaminants and water vapor has a significant effect on the oxidation rate (Obee and Brown, 1995). Hybrid catalysts (combined TiO2 with adsorption materials such as activated carbon and zeolite) are used to enhance the PCO degradation of VOCs (Ao and Lee, 2003). In most studies only a single compound was tested, although often with good results. However, indoor air contains numerous contaminants, so tests of only one or a few compounds may be misleading. Furthermore, the generation of by-products is a serious problem for catalytic oxidation processes. Indeed, PCO can generate by-products (formaldehyde, acetaldehyde etc.) that are more harmful than the target pollutant (Hodgson et al., 2007, Muggli et al., 1998, Mo et al., 2009). Table 1shows that there is lack of field studies for PCO air cleaning in the 59 reviewed papers. Most of the studies were performed in small-scale laboratory settings, which resulted in their low CADR values (Fig. 2). PCO has high efficiency in the single-pass tests, but its efficiency is significantly reduced in the chamber tests (Fig. 3). This indicates that PCO technology is not ready for practical application.
4.2. Filtration
Fifteen articles on air filters (some with activated carbon) underwent a detailed review process; recall that filters used on outdoor air intakes were not included in the review. Some of the articles focused on particle removal efficiency, but the particles studied were quite different, ranging from large microbes to very small particles. They all report a positive effect with regard to particle removal (higher removal efficiencies for larger particles), but sometimes not as high as the manufacturers’ data indicate. VOC removal was investigated in four studies, with results ranging from zero removal (no activated carbon) (Batterman et al., 2005) to some removal (with activated carbon) (e.g., Bekö et al., 2008). Removal of ozone by reactions with filters has also been observed by Bekö et al. (2006) and Zhao et al. (2007). However, ozone reaction products released from filters have been reported (Bekö et al., 2006, Bekö et al., 2008, Bekö et al., 2009, Hyttinen et al., 2007, Schleibinger and Rüden, 1999). In summary, mechanical filters can efficiently remove particles, but are not as effective for organic and inorganic chemical pollutants. The main problem with mechanical filters is that they act as a pollution source if they are not properly used. A solution seems to be a combination of particulate filter and activated carbon, as shown by Bekö et al. (2009), and Metts and Batterman (2006).
4.3. Ozone-oxidation
Ozone is an oxidant that can react with some indoor pollutants. The combined use of ozone and various micro- or meso-porous adsorbents can take advantage of the oxidizing capability of ozone and reduce the residual ozone due to enhanced catalytic reaction in the porous structure (Kwong et al., 2008a). Considering that ozone itself is quite harmful and that reactions with compounds such as terpenes can produce potentially harmful secondary organic aerosol (SOA) in the ultra fine and fine size ranges (Waring et al., 2008), as well as reactive organic compounds, caution should be taken when using ozone-emitting air cleaning techniques (e.g., UVGI, plasma, electrostatic precipitator, and ion generators) in indoor environments, no matter whether they intentionally or unintentionally produce ozone.
4.4. Plasma
There are several ways to generate plasma for air purification: corona discharge with alternating current, direct current and dielectric barrier discharge (DBD). Plasma air cleaners have been reported to remove particles at high efficiency, e.g., within the range of 76–99% (Park et al., 2008, Van Durme et al., 2007, Van Durme et al., 2009). The technology is not efficient at removing gas-phase pollutants (Park et al., 2008). When combined with catalytic technology, plasma air cleaners have been observed to more effectively remove VOCs, such as toluene (Van Durme et al., 2007). If plasma is combined with UV-catalytic technology, the improved removal efficiencies for formaldehyde, benzene, toluene and xylene is promising (Park et al., 2008). The performance of plasma-catalyst technology for VOC removal can be inhibited by humidity (Van Durme et al., 2009). In general, plasma technologies can enhance the performance of filters for particle removal and catalysts for gas-phase pollutants. However, the production of secondary pollutants such as NOx and O3 is a major drawback of plasma technology (Van Durme et al., 2007).
4.5. Sorption
Eight articles involved investigations of sorption air cleaning techniques. Sorption is good for gas pollutant removal. For adsorption and chemisorption, the following factors are involved: sorption mechanism (e.g., strong chemisorption vs. weaker hydrogen bonding), specific sorbent surface area, porosity, specific equilibrium adsorption quantity, diffusion coefficient of target pollution in adsorbent and half-life (Parmar and Grosjean, 1991). Desiccant wheels may be promising in controlling indoor air humidity and removing indoor VOCs simultaneously (Fang et al., 2008). There are some problems when applying such techniques in practice: (1) The sorbed VOCs and O3 may generate reaction by-products, such as particles. Adsorbents such as activated carbon can also be effective at removing reactant species (such as O3) by surface reactions; (2) Humidity and/or other indoor pollutants generally have a negative effect on target indoor pollutant removal due to competitive sorption; (3) The removal effect for multiple indoor compounds remains unknown; (4) For a target pollutant, the criteria for selecting the best sorption material to optimize removal over a given time period are generally not available; and (5) The lifetime for sub-ppbv level indoor air pollutants removal is unknown.
4.6. UVGI
The use of ultraviolet (UV) wavelengths of light in the germicidal range (200–365 nm) for air or surface disinfection is referred to as UVGI. Though the germicidal ability of UV (200–320 nm) has been known for more than 100 years (Kowalski, 2009), conclusive field data are still lacking to demonstrate the effectiveness of UVGI. None of the 10 studies included in this review studied the formation of possible secondary pollutants by UV, e.g., as initiated by ozone chemistry.
5. Overall comments and suggestions
The characteristics of commonly-used indoor air cleaning technologies were compared in Table 7 . Some “new” air cleaning technologies, such as PCO, plasma, and ozone-related, can handle more than one type of indoor air pollutant. For example, PCO can decompose almost all indoor organic compounds, and can also sterilize indoor microbes. This more “general” potential has made them a hot research topic (Fig. 1). However, they all produce harmful by-products and there is a lack of data on practical applications. Fig. 2 shows PCO and plasma have low CADR values, even if they achieve high single-pass efficiencies in laboratory studies (Fig. 3). For the “typical” air cleaning technologies, such as sorption, filtration and UVGI, they generally can remove one type of indoor air pollutant. Many studies show that filtration and UVGI have high single-pass efficiencies in real environment applications (Fig. 3).
Table 7.
Comparison of the commonly-used indoor air cleaning technologies reviewed.
| Technology name | Indoor air pollutants removed | Advantages | Disadvantages | Potential development |
|---|---|---|---|---|
| Catalytic oxidation (including PCO) | Gas pollutants: organic, inorganic Airborne microbe. |
Active at room temperature. Can degrade various contaminants (such as aldehyde, aromatics, alkanes, olefins, halogenated hydrocarbons, odor compounds, airborne microbes). Does not need regeneration. |
Can generate harmful by-products such as formaldehyde, acetaldehyde, and acetone. Catalyst may be poisoned, resulting in decreased performance. |
Combined with other air cleaning technologies, such as adsorption and thermal catalytic oxidation, to reduce by-products and enhance performance. |
| Plasma | Gas pollutants: organic, inorganic Airborne microbe. |
Can simultaneously remove gas pollutants, airborne microbe and even particles. High single-pass removal efficiency. |
May produce O3, NOx and other harmful by-products. High voltage and high energy consumption. |
Combined with particle filter to increase filter performance and reduce pressure drop. Combined with catalysts to reduce or remove ozone. |
| Ozone-related | Gas pollutants: organic, inorganic Airborne microbe. |
Can reduce some targeted odors; Can enhance some catalytic oxidation reactions for VOC removal. |
Ozone itself is a harmful pollutant and may react with other indoor pollutants to produce harmful pollutants such as carbonyls, dicarbonyls, carboxylic acids, and secondary organic aerosols. | Combined with catalysts to reduce or remove ozone. |
| Sorption | Gas pollutants: organic, inorganic. | No harmful by-products. Good performance for gas-phase pollutants. |
Must be regenerated after long-term operation. May produce some airborne pollutants. Reactions with ozone may generate gaseous secondary pollutants. |
Dynamic continual or intermittent generation systems need to be developed. |
| Filtration | Particles | Good at removing particles in the range from 0.1 μm to 4 μm. | Used particle filters are sources of sensory pollutants. No evidence of VOC removal by filter alone, except when filter is combined with other materials, such as activated carbon. |
Combined with electrostatic field. |
| UVGI | Airborne microbe | Good for inactivation of some airborne microorganisms (bacteria, fungal, virus). | May generate O3 and dioxin. | – |
In addition, many of the articles reviewed in this study did not include an explicit determination of CADR, nor were single-pass removal efficiency or volumetric flow rates provided to allow for an implicit calculation of CADR. Though the potentially harmful by-products created by the air cleaners are important, only a few articles refer to secondary products associated with the air cleaning process. Energy consumption by air cleaners is often overlooked and will be increasingly important as building energy consumption is reduced.
We believe that researchers who study air cleaning devices should determine and report CADR, by-products, and energy requirements (perhaps CADR/energy consumption), and that peer reviewers of related submissions should require this information.
6. Conclusions
The following consensus statements were made based on the 59 articles identified as conclusive and relevant: (1) Filtration is an efficient technology for removing particles, although used particle filters can be a source of sensory pollution. (2) Sorption is an efficient technology for removing some gaseous pollutants, including VOCs, formaldehyde, O3, NO2, SO2, and H2S, provided that the sorption system is properly designed and operated. It may produce pollutants, especially if ozone reacts with contaminants that deposit on or are sorbed by the media. More information is needed on the long-term performance of air cleaners using sorption principles. (3) UVGI is a proven technology for inactivation of some airborne microorganisms such as bacteria, fungi and viruses, but ozone may be produced during operation. (4) PCOs can reduce concentrations of some gaseous pollutants (e.g., BTEX, ethanol, formaldehyde), but may generate harmful by-products. By-products need to be identified and controlled and catalyst poisoning must be understood when this technique is applied for prolonged periods. (5) Laboratory tests show that plasma air cleaning can reduce concentrations of some gaseous pollutants such as BTEX, ethanol, and formaldehyde. It can also produce harmful by-products such as ozone and oxidation intermediates, and its energy consumption tends to be high. (6) Ozone is not recommended for indoor air cleaning because of the harmful by-products. (7) The performance criteria of commonly-used fan-driven air cleaners, such as efficiency and CADR, strongly depend on the type of indoor air pollutants and the test conditions. Benchmarks and standard condition and procedures for evaluating air cleaner performance are necessary, and labeling of air cleaners will be valuable in the future.
This review has shown that the question “Can commonly-used, fan-driven air cleaning technologies improve indoor air quality?” does not yet have an answer. Once researchers in the indoor air cleaning field have solved the problems identified in our review, the answer will be clear.
Acknowledgments
This literature review was financially supported by a research project of the National Natural Science Foundation of China (Project Number: 50725620, 51006057). Thanks to Prof. J. Zhang, Syracuse University, for providing the space and on-site support for the 2nd expert meeting, Dr. Jeffery Siegel for the additional paper-selection, and thanks to Ph. D candidates J. Pei of Syracuse University and Z. Liu of Virginia Tech for their assistance.
Contributor Information
Yinping Zhang, Email: zhangyp@tsinghua.edu.cn.
Yuguo Li, Email: liyg@hku.hk.
Jan Sundell, Email: ja.sundell@gmail.com.
Primary references (reviewed articles by the panel)
- Ao C.H., Lee S.C. Enhancement effect of TiO2 immobilized on activated carbon filter for the photodegradation of pollutants at typical indoor air level. Applied Catalysis B – Environmental. 2003;44:191–205. [Google Scholar]
- Ao C.H., Lee S.C., Mak C.L., Chan L.Y. Photodegradation of volatile organic compounds (VOCs) and NO for indoor air purification using TiO2: promotion versus inhibition effect of NO. Applied Catalysis B – Environmental. 2003;42:119–129. [Google Scholar]
- Ao C.H., Lee S.C., Zou S.C., Mak C.L. Inhibition effect of SO2 on NOx and VOCs during the photodegradation of synchronous indoor air pollutants at parts per billion (ppb) level by TiO2. Applied Catalysis B – Environmental. 2004;49:187–193. [Google Scholar]
- Batterman S., Godwin C., Jia C.R. Long duration tests of room air filters in cigarette smokers’ homes. Environmental Science & Technology. 2005;39:7260–7268. doi: 10.1021/es048951q. [DOI] [PubMed] [Google Scholar]
- Bekö G., Clausen G., Weschler C.J. Sensory pollution from bag filters, carbon filters and combinations. Indoor Air. 2008;18:27–36. doi: 10.1111/j.1600-0668.2007.00501.x. [DOI] [PubMed] [Google Scholar]
- Bekö G., Fadeyi M.O., Clausen G., Weschler C.J. Sensory pollution from bag-type fiberglass ventilation filters: conventional filter compared with filters containing various amounts of activated carbon. Building and Environment. 2009;44:2114–2120. [Google Scholar]
- Bekö G., Halás O., Clausen G., Weschler C.J. Initial studies of oxidation processes on filter surfaces and their impact on perceived air quality. Indoor Air. 2006;16:56–64. doi: 10.1111/j.1600-0668.2005.00401.x. [DOI] [PubMed] [Google Scholar]
- Biey E.M., Verstraete W. The use of a UV lamp for control of odour decomposition of kitchen and vegetable waste. Environmental Technology. 1999;20:331–335. [Google Scholar]
- Boelter K.J., Davidson J.H. Ozone generation by indoor, electrostatic air cleaners. Aerosol Science and Technology. 1997;27:689–708. [Google Scholar]
- Chen W., Zhang J.S. UV-PCO device for indoor VOCs removal: investigation on multiple compounds effect. Building and Environment. 2008;43:246–252. [Google Scholar]
- Chen W., Zhang J.S., Zhang Z. Performance of air cleaners for removing multiple volatile organic compounds in indoor air. ASHRAE Transactions. 2005;111:1101–1114. [Google Scholar]
- Cheng Y.S., Lu J.C., Chen T.R. Efficiency of a portable indoor air cleaner in removing pollens and fungal spores. Aerosol Science and Technology. 1998;29:92–101. [Google Scholar]
- Davis W.T., Cornell C., Dever M. Comparison of Experimental and Theoretical efficiencies of residential air filters. TAPPI Journal. 1994;77:180–186. [Google Scholar]
- Ellis W.D., Tometz P.V. Room-temperature catalytic decomposition of ozone. Atmospheric Environment. 1972;6:707. [Google Scholar]
- Fang L., Zhang G., Wisthaler A. Desiccant wheels as gas-phase absorption (GPA) air cleaners: evaluation by PTR-MS and sensory assessment. Indoor Air. 2008;18:375–385. doi: 10.1111/j.1600-0668.2008.00538.x. [DOI] [PubMed] [Google Scholar]
- Gesser H.D., Fu S. Removal of Aldehydes and acidic pollutants from indoor air. Environmental Science and Technology. 1990;24:495–497. [Google Scholar]
- Harstad J.B., Decker H.M., Wedum A.G. Use of ultraviolet irradiation in a room air Conditioner for removal of bacteria. Applied Microbiology. 1954;2:148–151. doi: 10.1128/am.2.3.148-151.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson A.T., Destaillats H., Sullivan D.P., Fisk W.J. Performance of ultraviolet photocatalytic oxidation for indoor air cleaning applications. Indoor Air. 2007;17:305–316. doi: 10.1111/j.1600-0668.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- Jeong J., Sekiguchi K., Lee W., Sakamoto K. Photodegradation of gaseous volatile organic compounds (VOCs) using TiO2 photoirradiated by an ozone-producing UV lamp: decomposition characteristics, identification of by-products and water-soluble organic intermediates. Journal of Photochemistry and Photobiology A – Chemistry. 2005;169:279–287. [Google Scholar]
- Kujundzic E., Zander D.A., Hernandez M., Angenent L.T., Henderson D.E., Miller S.L. Effects of ceiling-mounted HEPA-UV air filters on airborne bacteria concentrations in an indoor therapy pool building. Journal of the Air & Waste Management Association. 2005;55:210–218. doi: 10.1080/10473289.2005.10464612. [DOI] [PubMed] [Google Scholar]
- Kujundzic E., Matalkah F., Howard C.J., Hernandez M., Miller S.L. UV air cleaners and upper-room air ultraviolet germicidal irradiation for controlling airborne bacteria and fungal spores. Journal of Occupational and Environmental Hygiene. 2006;3:536–546. doi: 10.1080/15459620600909799. [DOI] [PubMed] [Google Scholar]
- Kwong C., Chao C.Y.H., Hui K.S., Wan M.P. Removal of VOCs from indoor environment by ozonation over different porous materials. Atmospheric Environment. 2008;42:2300–2311. [Google Scholar]
- Kwong C.W., Chao C.Y.H., Hui K.S., Wan M.P. Catalytic ozonation of toluene using zeolite and MCM-41 materials. Environmental Science & Technology. 2008;42:8504–8509. doi: 10.1021/es801087f. [DOI] [PubMed] [Google Scholar]
- Lai M.H., Moschandreas D.J., Pagilla K.R. Airborne bacteria control under chamber and test-home conditions. Journal of Environmental Engineering-Asce. 2003;129:202–208. [Google Scholar]
- Lee B.U., Yermakov M., Grinshpun S.A. Removal of fine and ultra fine particles from indoor air environments by the unipolar ion emission. Atmospheric Environment. 2004;38:4815–4823. doi: 10.1016/j.atmosenv.2004.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimier C., Le Coq L., Subrenat A., Le Cloirec P. Indoor air particulate filtration onto activated carbon fiber media. Journal of Environmental Engineering – ASCE. 2008;134:126–137. [Google Scholar]
- McDevitt J.J., Lai K.M., Rudnick S.N., Houseman E.A., First M.W., Milton D.K. Characterization of UVC light sensitivity of vaccinia virus. Applied and Environmental Microbiology. 2007;73:5760–5766. doi: 10.1128/AEM.00110-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies D., Popa J., Hanley J.A., Rand T., Milton D.K. Effect of ultraviolet germicidal lights installed in office ventilation systems on workers’ health and wellbeing: double-blind multiple crossover trial. Lancet. 2003;362:1785–1791. doi: 10.1016/S0140-6736(03)14897-0. [DOI] [PubMed] [Google Scholar]
- Metts T.A. Heterogeneous reactions of ozone and d-limonene on activated carbon. Indoor Air. 2007;17:362–371. doi: 10.1111/j.1600-0668.2007.00484.x. [DOI] [PubMed] [Google Scholar]
- Metts T.A., Batterman S.A. Effect of VOC loading on the ozone removal efficiency of activated carbon filters. Chemosphere. 2006;62:34–44. doi: 10.1016/j.chemosphere.2005.04.049. [DOI] [PubMed] [Google Scholar]
- Miller-Leiden S., Lobascio C., Nazaroff W.W., Macher J.M. Effectiveness of in-room air filtration and dilution ventilation for tuberculosis infection control. J Air Waste Manag Assoc. 1996;46:869–882. doi: 10.1080/10473289.1996.10467523. [DOI] [PubMed] [Google Scholar]
- Mo J.H., Zhang Y.P., Xu Q.J., Zhu Y.F., Lamson J.J., Zhao R.Y. Determination and risk assessment of by-products resulting from photocatalytic oxidation of toluene. Applied Catalysis B – Environmental. 2009;89:570–576. [Google Scholar]
- Muggli D.S., McCue J.T., Falconer J.L. Mechanism of the photocatalytic oxidation of ethanol on TiO2. Journal of Catalysis. 1998;173:470–483. [Google Scholar]
- Obee T.N., Brown R.T. TiO2 photocatalysis for indoor air applications – effects of humidity and trace contaminant levels on the oxidation rates of formaldehyde, toluene, and 1,3-butadiene. Environmental Science & Technology. 1995;29:1223–1231. doi: 10.1021/es00005a013. [DOI] [PubMed] [Google Scholar]
- Offermann F.J., Sextro R.G., Fisk W.J., Grimsrud D.T., Nazaroff W.W., Nero A.V., Revzan K.L., Yater J. Control of respirable particles in indoor air with portable air cleaners. Atmospheric Environment. 1985;19:1761–1771. [Google Scholar]
- Offermann F.J., Loiselle S.A., Sextro R.G. Performance of air cleaners in a residential forced air system. ASHRAE Journal. 1992;34:51–57. [Google Scholar]
- Park J.H., Byeon J.H., Yoon K.Y., Hwang J. Lab-scale test of a ventilation system including a dielectric barrier discharger and UV-photocatalyst filters for simultaneous removal of gaseous and particulate contaminants. Indoor Air. 2008;18:44–50. doi: 10.1111/j.1600-0668.2007.00503.x. [DOI] [PubMed] [Google Scholar]
- Parmar S.S., Grosjean D. Sorbent removal of air pollutants from museum display cases. Environment International. 1991;17:39–50. [Google Scholar]
- Paschoalino M.P., Jardim W.F. Indoor air disinfection using a polyester supported TiO2 photo-reactor. Indoor Air. 2008;18:473–479. doi: 10.1111/j.1600-0668.2008.00548.x. [DOI] [PubMed] [Google Scholar]
- Schleibinger H., Rüden H. Air filters from HVAC systems as possible source of volatile organic compounds (VOC) – laboratory and field assays. Atmospheric Environment. 1999;33:4571–4577. [Google Scholar]
- Tsai C.W., Chang C.T., Chiou C.S., Shie J.L., Chang Y.M. Study on the indoor volatile organic compound treatment and performance assessment with TiO2/MCM-41 and TiO2/quartz photoreactor under ultraviolet irradiation. Journal of the Air & Waste Management Association. 2008;58:1266–1273. doi: 10.3155/1047.3289.58.10.1266. [DOI] [PubMed] [Google Scholar]
- Tseng C.C., Li C.S. Inactivation of virus-containing aerosols by ultraviolet germicidal irradiation. Aerosol Science and Technology. 2005;39:1136–1142. [Google Scholar]
- Tsoukleris D.S., Maggos T., Vassilakos C., Falaras P. Photocatalytic degradation of volatile organics on TiO2 embedded glass spherules. Catalysis Today. 2007;129:96–101. [Google Scholar]
- Van Durme J., Dewulf J., Sysmans W., Leys C., Van Langenhove H. Efficient toluene abatement in indoor air by a plasma catalytic hybrid system. Applied Catalysis B – Environmental. 2007;74:161–169. [Google Scholar]
- Van Durme J., Dewulf J., Demeestere K., Leys C., Van Langenhove H. Post-plasma catalytic technology for the removal of toluene from indoor air: effect of humidity. Applied Catalysis B: Environmental. 2009;87:78–83. [Google Scholar]
- Walker C.M., Ko G. Effect of ultraviolet germicidal irradiation on viral aerosols. Environmental Science & Technology. 2007;41:5460–5465. doi: 10.1021/es070056u. [DOI] [PubMed] [Google Scholar]
- Waring M.S., Siegel J.A., Corsi R.L. Ultrafine particle removal and generation by portable air cleaners. Atmospheric Environment. 2008;42:5003–5014. [Google Scholar]
- Wisthaler A., Strøm-Tejsen P., Fang L., Arnaud T.J., Hansel A., Märk T.D., Wyon D.P. PTR-MS assessment of photocatalytic and sorption-based purification of recirculated cabin air during simulated 7-h flights with high passenger density. Environmental Science & Technology. 2007;41:229–234. doi: 10.1021/es060424e. [DOI] [PubMed] [Google Scholar]
- Xu P., Peccia J., Fabian P., Martyny J.W., Fennelly K.P., Hernandez M., Miller S.L. Efficacy of ultraviolet germicidal irradiation of upper-room air in inactivating airborne bacterial spores and mycobacteria in full-scale studies. Atmospheric Environment. 2003;37:405–419. [Google Scholar]
- Xu P., Kujundzic E., Peccia J., Schafer M.P., Moss G., Hernandez M., Miller S.L. Impact of environmental factors on efficacy of upper-room air ultraviolet germicidal irradiation for inactivating airborne mycobacteria. Environmental Science & Technology. 2005;39:9656–9664. doi: 10.1021/es0504892. [DOI] [PubMed] [Google Scholar]
- Yang L.P., Liu Z.Y., Shi J.W., Zhang Y.Q., Hu H., Shangguan W.F. Degradation of indoor gaseous formaldehyde by hybrid VUV and TiO2/UV processes. Separation and Purification Technology. 2007;54:204–211. [Google Scholar]
- Yao M., Zhang Q., Hand D.W., Perrarn D., Taylor R. Adsorption and regeneration on activated carbon fiber cloth for volatile organic compounds at indoor concentration levels. Journal of the Air & Waste Management Association. 2009;59:31–36. doi: 10.3155/1047-3289.59.1.31. [DOI] [PubMed] [Google Scholar]
- Yu K.P., Lee G.W.M., Huang W.M., Wu C.C., Lou C.L., Yang S.H. Effectiveness of photocatalytic filter for removing volatile organic compounds in the heating, ventilation, and air conditioning system. Journal of the Air & Waste Management Association. 2006;56:666–674. doi: 10.1080/10473289.2006.10464482. [DOI] [PubMed] [Google Scholar]
- Yu K.P., Lee G.W.M., Huang W.M., Wu C.C., Yang S.H. The correlation between photocatalytic oxidation performance and chemical/physical properties of indoor volatile organic compounds. Atmospheric Environment. 2006;40:375–385. [Google Scholar]
- Zhang P.Y., Liang F.Y., Yu G., Chen Q., Zhu W.P. A comparative study on decomposition of gaseous toluene by O3/UV, TiO2/UV and O3/TiO2/UV. Journal of Photochemistry and Photobiology A – Chemistry. 2003;156:189–194. [Google Scholar]
- Zhang Y.P., Yang R., Zhao R.Y. A model for analyzing the performance of photocatalytic air cleaner in removing volatile organic compounds. Atmospheric Environment. 2003;37:3395–3399. doi: 10.1080/10473289.2004.10471016. [DOI] [PubMed] [Google Scholar]
- Zhang Y.P., Yang R., Xu Q.J., Mo J.H. Characteristics of photocatalytic oxidation of toluene, benzene, and their mixture. Journal of the Air & Waste Management Association. 2007;57:94–101. doi: 10.1080/10473289.2007.10465302. [DOI] [PubMed] [Google Scholar]
- Zhang G., Zhang Y.F., Fang L. Theoretical study of simultaneous water and VOCs adsorption and desorption in a silica gel rotor. Indoor Air. 2008;18:37–43. doi: 10.1111/j.1600-0668.2007.00502.x. [DOI] [PubMed] [Google Scholar]
- Zhao P., Siegel J.A., Corsi R.L. Ozone removal by HVAC filters. Atmospheric Environment. 2007;41:3151–3160. [Google Scholar]
Secondary references (articles used in this paper)
- Hyttinen M., Pasanen P., Björkroth M., Kalliokoski P. Odors and volatile organic compounds released from ventilation filters. Atmospheric Environment. 2007;41:4029–4039. [Google Scholar]
- ISIAQ . 2010. Vocabulary of the Indoor Air Sciences by International Society of Indoor Air Quality and Climate (ISIAQ)http://www.isiaq.org/publications/VocaboftheIndoorAirSciences.pdf [Google Scholar]
- Kowalski W. Springer; 2009. Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection. [Google Scholar]
- Nazaroff W.W. Effectiveness of air cleaning technologies. Proceedings of 6th International Conference of Healthy Buildings, Helsinki. 2000;vol. 2:49–54. [Google Scholar]
- Niu J., Tung T.C.W., Chui V.W.Y. Using large environmental chamber technique for gaseous contaminant removal equipment test. ASHRAE Transactions. 1998;104:1289–1296. [Google Scholar]
- WHO . 2010. World Health Organization: The Definition of Air Pollution.http://www.who.int/topics/air_pollution/en [Google Scholar]