Abstract
The mechanisms behind cancer initiation and progression are not clear. Therefore, development of clinically relevant models to study cancer biology and drug response in tumors is essential. In vivo models are very valuable tools for studying cancer biology and for testing drugs; however, they often suffer from not accurately representing the clinical scenario because they lack either human cells or a functional immune system. On the other hand, two dimensional (2D) in vitro models lack the three dimensional (3D) network of cells and extracellular matrix (ECM) and thus do not represent the tumor microenvironment (TME). As an alternative approach, 3D models have started to gain more attention, as such models offer a platform with the ability to study cell-cell and cell-material interactions parametrically, and possibly include all the components present in the TME. Here, we first give an overview of the breast cancer TME, and then discuss the current state of the pre-clinical breast cancer models, with a focus on the engineered 3D tissue models. We also highlight two engineering approaches that we think are promising in constructing models representative of human tumors: 3D printing and microfluidics. In addition to giving basic information about the TME in the breast tissue, this review article presents the state-of-the-art tissue engineered breast cancer models.
Keywords: Breast cancer, tumor microenvironment, tissue engineering, 3D tumor models, bioprinting, microfluidics
Graphical abstract
1. Introduction
Breast cancer is one of the most common cancer types. In 2018, the estimated number of new breast cancer cases was 270,000 in the United States alone [1], and 2.1 million across the globe [2]. In addition, breast cancer has the potential to metastasize to secondary tissues such as bone, lung, and liver [3], which is the main cause of cancer-related deaths [4].
Finding an efficient treatment method for cancer is not easy, since it is a complex set of diseases, with patient-to-patient variance and heterogeneity between cells within the tumor [5–7]. Pre-clinical studies on cancer drug development have traditionally been based on the drug’s in vitro cytotoxicity in two dimensional (2D) models [8], which do not recapitulate the three dimensional (3D) tumor microenvironment (TME) and thus fail to reflect the actual response of tumors in the body to these drugs. This discrepancy highly contributes to the inefficient translation of pre-clinical findings, where 95% of the drugs that are effective in preclinical trials have proven ineffective in clinic [9], and only 7.5% of drugs tested in Phase 1 trials eventually get approval for clinical use [5,10].
Although 2D models are less complex and more useful in dissecting the individual effect of each parameter tested, cell-cell and cell-material interactions in 2D are different than those actually taking place in vivo, because cells adapt to the 2D monolayer environment and thus poorly retain their original phenotype [11]. In vivo models are extremely useful in understanding the mechanisms of tumor initiation and behavior, and drug metabolism. However, these models are more costly, laborious, and time-consuming for the researchers to produce and maintain. Most importantly, they fail to reflect the human response to drug treatment, because either they lack human cells or their immune system is compromised, which also contributes to the failure in translating the pre-clinical findings. In addition, the high number of animals killed raises ethical concerns for their use.
Thus, more reliable drug testing platforms are required. Three dimensional in vitro models that mimic the TME are crucial for the development of effective treatment strategies and for studying the molecular mechanisms behind tumor formation, progression, and metastasis. In fact, results generated from the 3D in vitro models have been reported to show good correlation with the in vivo studies and clinical outcomes [12,13]. Engineering a human representative 3D model, at least for specific applications or pathophysiological conditions, is now possible, thanks to the advancements in biomaterials and tissue engineering (TE). In this review article, we first describe the components of TME and their effects on tumor progression, and then explain the strategies to engineer 3D models that recapitulate the TME.
2. The tumor microenvironment
Normal epithelium is composed of epithelial (luminal) and myoepithelial (basal) cells tightly attached to each other via cell junctions with the help of cell adhesion molecules (CAMs), such as cadherins, to form a hollow tubular structure [14] (Figure 1a). These cells are connected to the basement membrane, a thin layer of extracellular matrix (ECM) separating the epithelium from the surrounding stromal (i.e. adipose and fibrous) tissues. This organization is important for a functional mammary gland.
Figure 1.
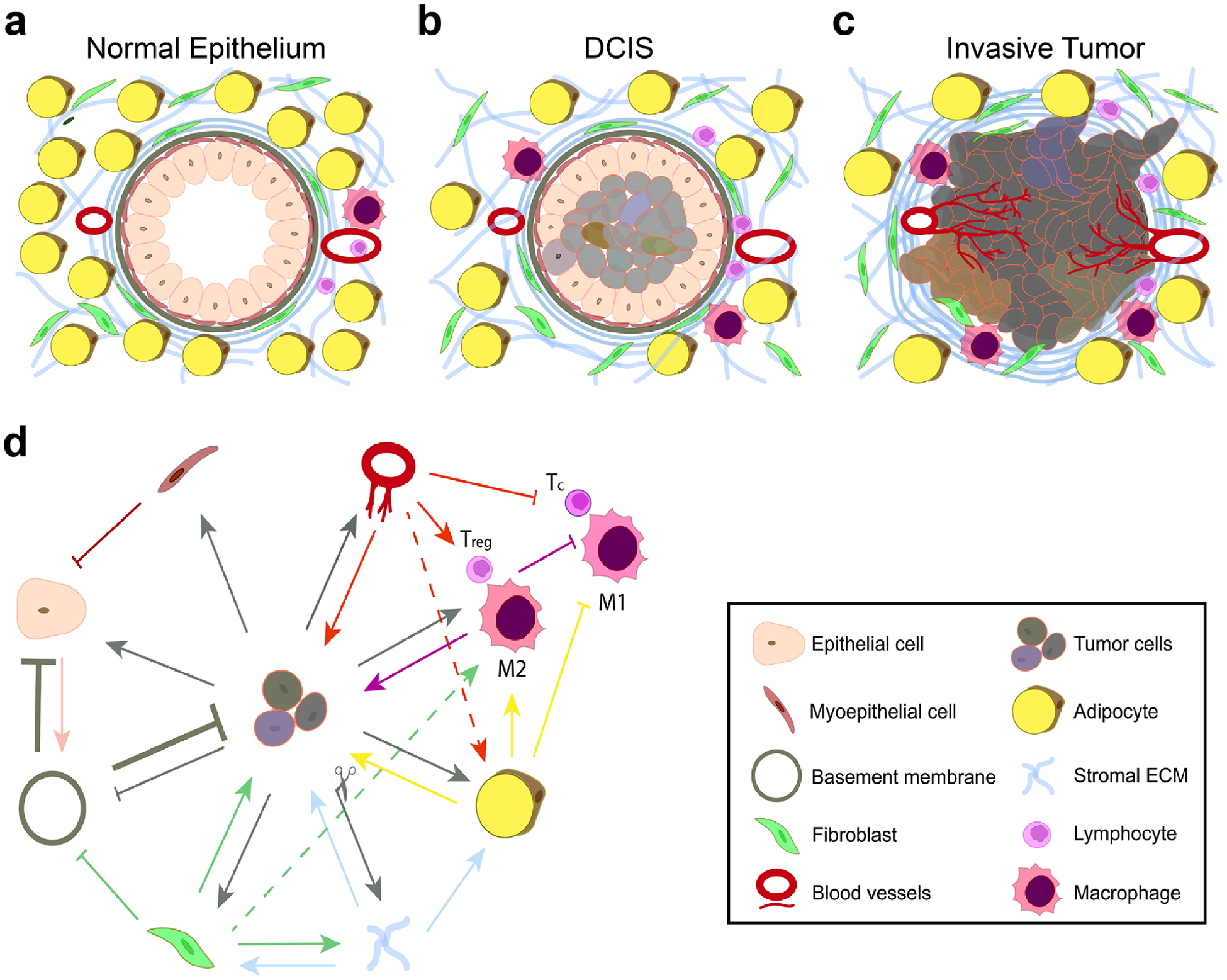
Interactions between cells and ECM lead to alteration of normal epithelium towards the tumor. (a) Normal epithelium, (b) ductal carcinoma in situ (DCIS), and (c) invasive tumor. (d) Simplified illustration of the network of interactions between cells and the ECM. Cross-talk between the tumor cells, stromal cells (fibroblasts and adipocytes), immune cells (regulatory T cells (Treg), and type 1 (M1) and type 2 (M2) macrophages), and the endothelial cells alters the microenvironment.
Cell adhesion, especially cell-cell adhesion, is reduced in tumor cells, leading to their dissociation from the epithelium and from each other, rapid proliferation, and formation of ductal carcinoma in situ (DCIS) –solid tumor masses in the lumens of epithelial tissues [15,16] (Figure 1b). Due to uncontrolled cell division, these cells are inherently heterogenic, and this heterogeneity is further fortified by the differential oxygen and nutrient supply to cells at different sites in the tumor. Cells in the core of the solid tumors receive less oxygen and thus are necrotic, cells in the middle layer are senescent, and those in the outer layer are proliferating [17]. The hypoxic environment in the tumor also alters protein expression in the tumor cells, further deviating them from the normal cell phenotype [5,18,19].
Tumor cells and stromal cells secrete soluble factors (growth factors and cytokines) affecting both the other cells and the ECM, which eventually leads to the disruption of normal epithelial organization. The newly created environment after tumor formation, namely the TME, plays a crucial role in tumor progression and metastasis. TME is a complex environment, with dynamic cell-cell and cell-ECM interactions contributing to cancer initiation. Cross-talk between stromal and immune cells leads to a cascade of events that favors the tumor [20,21]. These cells, as well as tumor cells, produce soluble factors that immunosuppress the immune cells or direct the other cells to proliferate, migrate, differentiate, and produce or degrade the ECM [22]. This complex interaction between the cells and the ECM eventually leads to more invasive tumor cells that can break the connective tissue and metastasize [14] (Figure 1c). In this section, we briefly explain how tumors manipulate the TME to survive, proliferate, migrate and invade through the stroma, and at the same time evade the surveillance mechanisms in the body.
2.1. Cellular composition of the breast tissue
Cancer originates from an altered phenotype and/or genotype due to cellular mutations that results in uncontrolled cell division [3,4,23]. This cellular growth then leads to further mutations and tumor development within the diseased tissue [3,4]. The degree of mutation is closely correlated with breast cancer progression [3,4]. As mutations accumulate, tumors become increasingly malignant and more difficult to treat [24]. For example, breast tumors that still express hormone and growth receptors (i.e. ER+, PR+, and HER2+) are much less aggressive and have more treatment options than tumors not expressing these receptors (triple negative), which are highly metastatic and therapy-resistant [25–29]. This variance between different tumors, as well as tumor cell heterogeneity within the tumor, makes treatment extremely difficult. Therefore, identifying the changes in cell properties for each type and stage of breast tumors is extremely important.
2.1.1. Breast cancer cells
The epithelial-mesenchymal transition (EMT) has an effect not only on the transforming cell, but also on the neighboring cells [30,31]. As the EMT progresses, cells lose their polarity and endogenous cuboidal shape, and adopt a more disorganized grape-like (non-invasive) to stellate (invasive) morphology depending on aggressiveness [32] (Table 1). In breast tissue, one or a few layers of epithelial cells line the basement membrane to form the lumen [33,34]. After EMT, however, cells fill this lumen and form the DCIS (Figure 1b), through which blood does not flow easily. This results in a hypoxic microenvironment in the core of the tumor, which enhances cellular heterogeneity and leads to a more aggressive tumor phenotype [19]. In addition to phenotype, cell stiffness is also altered upon EMT. Transformed cells are softer and more deformable than the endogenous epithelial cells, potentially increasing their motility [16,35]. Overall, the changes in phenotype and stiffness result in aggressive cancer cells that are able to squeeze through the ECM, enter the circulatory system and invade blood and lymph vessels, and migrate through the vessels and metastasize to secondary organs [28,29,36,37].
Table 1.
Cell and tissue differences between the normal and tumor microenvironments.
| Epithelium | Stroma | Reference | ||||
|---|---|---|---|---|---|---|
| Normal | Non-invasive Tumor | Invasive Tumor | Normal | Tumor-Associated | ||
| Cell Morphology | Cuboidal Polar | Grape-like Non-polar | Stellate Non-polar | - | - | [32] |
| Cell Organization | 1–2 layers Lumen Organized nuclei | Dense Necrotic core Disorganized nuclei Poor cell adhesion | Very dense Necrotic core Disorganized nuclei | Not dense | Dense | [32–34] |
| Cell Stiffness | Normal (1.97 ± 0.70 kPa) | Soft | Very soft (0.53 ± 0.10 kPa) | - | - | [16,102] |
| ECM Composition | High collagen IV & laminin-1 Low Laminin 5 | Intermediate | Low collagen IV & laminin-1 High Laminin 5 | Low collagen I, low MMPs | High collagen I, high MMPs | [83–85,89,90,94,96] |
| ECM Organization | Permissive Thin | Dense Thick | Dense Thick | Permissive | Dense | [32,34] |
| ECM Stiffness | Soft (0.15–0.20 kPa) | Intermediate | Stiff (1.0–4.0 kPa) | Soft (~0.2 kPa) | Stiff (0.4–1.0 kPa) | [103,104] |
| Vasculature and Oxygen supply | Organized, Normoxia | Disorganized, Hypoxic core Interstitial pressure | Disorganized, Hypoxic core Interstitial pressure | Organized | Directed to tumor | [123,125] |
The success of breast cancer metastasis relies on the ability of the tumor cells to modulate their interaction with endogenous cells (Figure 1d). As the tumor progresses, these cells activate stromal cells, transforming them into tumor (or cancer)-associated fibroblasts (TAFs) and adipocytes (TAA), leading to an increased expression of proteins such as alpha-smooth muscle actin (α-SMA) and matrix metalloproteinases (MMPs), and increasing tumor invasiveness (Figure 2a) [38]. Additionally, the hypoxic environment in the tumor core leads to endothelial induction of angiogenesis, allowing easy access to blood and lymph vessels for metastasis [36,37]. The metastasizing tumor cells can interact with the immune cells and induce the release of immunosuppressive cytokines, which help them evade the immune system [39].
Figure 2.
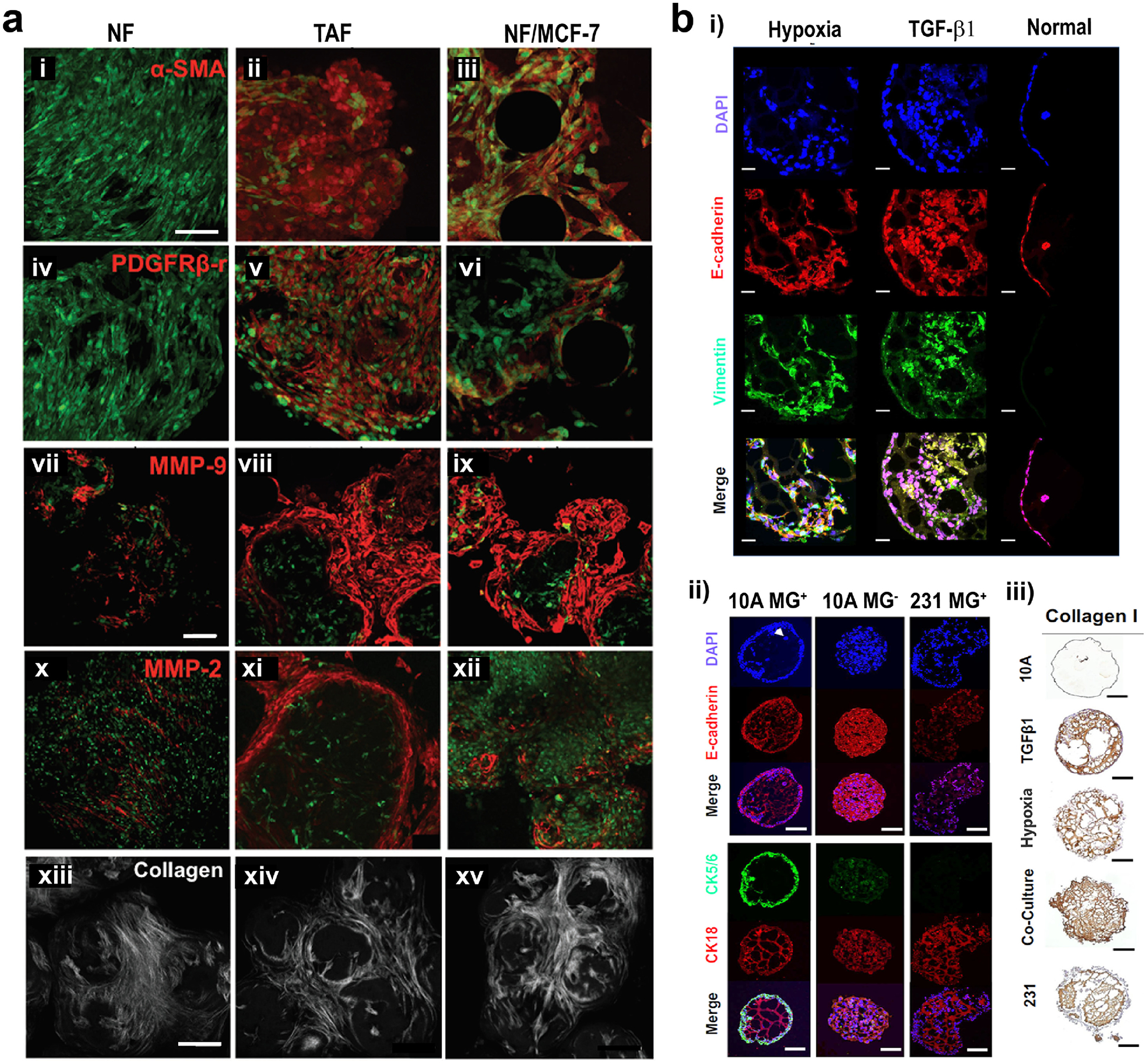
Protein expression changes in the tumor microenvironment. (a) In TME, normal fibroblasts (NF) turn into tumor-associated fibroblasts (TAFs), with increased expression of (i-iii) α-SMA, (iv-vi) PDGF receptor, (vii-xii) MMPs and (xiii-xv) collagen. When NFs are co-cultured with MCF-7 tumor cells, expression of these factors also increase. Green: actin, red: proteins. Scale bars: 100 μm. Reproduced with permission from [38]. Copyright 2016 John Wiley and Sons. (b) Differential expression of some markers in non-tumorigenic (normal, MCF-10A) and malignant breast cancer (tumor, MDA-MB-231) cells. Expression of some markers (i) when MCF-10A cells were cultured under hypoxic conditions or stimulated with TGF-β1, and (ii, iii) when MCF-10A and MDA-MB-231 cells were incubated under various conditions. MG+: in Matrigel (normal environment), MG−: Matrigel-free (DCIS environment). Scale bars: 100 μm for MG− in image (ii), and 200 μm for other images. Reproduced with permission from [86]. Copyright 2018 Springer Nature.
2.1.2. Stromal cells
Along with the epithelial cells, stromal cells including fibroblasts, adipocytes, endothelial, and immune cells within the TME play a significant role in tumor progression and metastasis.
2.1.2.1. Fibroblasts
Fibroblasts are a significant component of the connective tissue. Upon tissue insult, they are activated and converted to myofibroblasts in order to promote the recovery of an injured site through ECM production [40,41]. Myofibroblasts, along with other cell types including bone marrow-derived mesenchymal stem cells, hematopoietic stem cells, epithelial cells and endothelial cells within the TME could transform, or help in transformation of other cells, to TAFs [42,43]. The presence of TAFs, in conjunction with inflammation results in tissue fibrosis, which in turn increases the risk of tumorigenesis [44]. TAFs secrete growth factors promoting angiogenesis, EMT, immunosuppression, as well as tumor cell proliferation, survival, invasion, and metastasis [45–47]. In addition, TAF signaling pathways such as oxidative stress, autophagy, and glycolysis promote the generation of a microenvironment suitable for tumor cell growth and expansion [48].
2.1.2.2. Adipocytes
Adipocytes, an abundant cell type found within the mammary gland, have recently been shown to secrete hormones and growth factors that facilitate breast tumor growth [49–51]. Adipocytes are typically enriched within the TME and subsequently transform to TAAs in response to cytokines received from other cells occupying the TME [50,52]. Upon transformation, TAAs secrete various cytokines and adipokines that promote tumor progression [53,54]. This abnormal cytokine and adipokine secretion, in combination with the release of free fatty acids (FAAs) and MMPs, results in the recruitment of immune cells to the TME, creating an environment akin to chronic inflammation, promoting EMT and an invasive tumor phenotype [52,55,56].
2.1.2.3. Vascular cells
Vascular endothelial cells line the luminal side of blood vessels and are a crucial component of angiogenesis [57]. Cells within the TME stimulate angiogenesis by secreting vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF-2) [58]. The newly formed blood vessels carry oxygen and nutrients to the growing tumor, and since they are extremely leaky due to their rapid growth and a lack of some cytokines, they help the invasive tumor cells intravasate through the vessels [14,58,59]. This, along with additional endothelial-tumor cell interactions further accelerate tumor growth and metastasis [60,61].
Pericytes are located on the basal side of blood vessels and serve as a stabilizing unit to the vessel structure [5]. In tumor vasculature, the pericyte population is significantly decreased [62], increasing vessel permeability, which in turn enhances tumor cell growth and intravasation [61,63].
2.1.2.4. Blood cells
In the early stages of tumor formation, the altered expression of cytokines and growth factors within the TME produces an environment similar to what is observed in sites of chronic inflammation [56]. This leads to the recruitment of T lymphocytes, natural killer cells, and macrophages that target tumor-associated antigens [64]. However, tumor cells within the microenvironment promote the polarization of macrophages toward the immunosuppressive M2 subtype. The mixed population of M1/M2 macrophages, making up the tumor-associated macrophages (TAMs), leads to the expression of immunosuppressive cytokines, assisting tumor cell evasion from immune surveillance [39,65,66]. TAMs and other immune cells, including regulatory T lymphocytes and neutrophils, promote angiogenesis and cell proliferation, contributing to tumor metastasis [67–69] (Figure 1d).
In addition to immune cells, recent work has also demonstrated the contribution of platelets to tumor progression and metastasis [28,29]. Platelet activation is significantly increased and has been associated with poor clinical outcome in many cancers including breast cancer [70,71]. The biological role of platelets is to halt bleeding in response to an insult to blood vessels. In a tumor, the leaky vasculature mimics a vascular insult, promoting platelet activation and subsequent coagulation [72]. Within the TME, these coagulation factors interact with tumor cells, enhancing tumor progression and malignancy [73].
2.2. Extracellular Matrix
The ECM is a 3D network of proteins like collagen, laminin, and fibronectin, and glycosaminoglycans (GAGs) like hyaluronic acid (HA), chondroitin sulfate, and heparan sulfate, providing physical support and presenting biomechanical and biochemical cues for the cells to attach, proliferate, and migrate [74]. ECM also acts as a reservoir for the growth factors, which are necessary for the cells to survive and function properly. These growth factors are released as a result of ECM degradation by MMPs [74,75]. ECM properties such as composition, stiffness, topography, as well as the microarchitecture of the ECM, affect the cell behavior and may contribute to tumor progression [76–78].
2.2.1. Biochemical Composition
The composition of the ECM gives the tissue its specific characteristic and it varies depending on the location in the tissue. For example, the basement membrane is rich in type IV collagen (collagen IV), laminin, and entactin, which play a role in the polarization of the cells and formation and maintenance of acini – the lobules in the mammary gland [79] (Figure 1a). The stromal tissue, on the other hand, is rich in type I collagen (collagen I, fibrous tissue), lipids (adipose tissue) and some proteoglycans including perlecan and tenascin [80–82].
In the TME, the biochemical composition of the ECMs is altered, which affects the behavior of cells. For instance, reduced expression of E-cadherin and laminin 1 in the basal cells leads to reduced cell adhesion and disruption of cell polarity in the acini, a hallmark of EMT [83–86] (Figure 2b). In a laminin 1-rich environment, breast cancer cells revert to normal phenotype [87], showing the significant role of this protein in maintaining the normal phenotype. Moreover, the loss of laminin 1 in the basement membrane enables direct contact of cells with the stromal ECM [88], which leads to EMT. Conversely, increased expression of laminin 5, a type of laminin that mediates the interaction between epithelial and mesenchymal cells, correlates with increased tumor invasiveness and reduced prognosis [89,90].
Integrin-mediated attachment of cells to ECM components such as collagen, fibronectin, HA, and proteoglycans confers resistance against apoptosis and plays an important role in tumor cell survival [91–93]. Although collagen I in the stroma serves as a physical barrier against tumor cell invasion [5], epithelial cell binding to collagen I induces EMT [94,95], which leads to secretion of ECM-degrading MMPs and eventually to stromal invasion by tumor cells [38,86,96,97] (Figure 2). Enzymatic degradation of the ECM opens a path for the tumor cells to travel through, and thus increases invasiveness. The degradation products, usually oligopeptides, might help in this process. Cleavage of perlecan, for example, promotes the invasive phenotype of tumor cells [98]. The peptide motifs glycine-phenylalanine-hydroxyproline-glycine-glutamate-arginine (GFOGER) (specific for collagen I) and isoleucine-lysine-valine-alanine-valine (IKVAV) (specific for laminin), but not the arginine-glycine-aspartic acid (RGD) (found mainly in fibronectin but also in collagen), were shown to enhance the invasiveness of the aggressive cancer cells [99].
ECM components also affect the penetration of immune cells, antibodies, and drugs to the tumor site by acting as a physical barrier [18]. Collagen (e.g. collagen I) [5], which forms a fibrous network, and proteoglycans (e.g. tenascin) [80] and/or GAGs (e.g. HA) [100], which imbibe a large amount of water, are some of the ECM components that serve as physical barriers. However, the molecular size of these components may change their effects. For example, the ultra-high molecular weight HA present in the stroma of naked mole rats confers resistance against cancer in these animals [101]. This resistance is mainly due to the inability of tumor cells to degrade these HA molecules because of low hyaluronidase expression in these animals, eliminating the risk of stromal invasion.
It is extremely important, thus, to take into account the biochemical composition of the tumor-associated stroma when designing in vitro models closely mimicking the TME. Reproducing the biochemical composition of the TME would enable creation of more realistic and clinically relevant cancer drug responses.
2.2.2. Stiffness
Individual cancer cells are softer than the benign cells [16,102]. Conversely, the breast tumor tissue is much stiffer (elastic modulus of 1000–4000 Pa) than the normal mammary gland (elastic modulus of 150–200 Pa) [103,104] (Table 1). The stiffness of the tumor-associated stroma (elastic modulus of 400–1000 Pa) is also higher than the normal stroma (elastic modulus of 200 Pa) [103,104]. The increased stiffness in the TME could be caused by the increased expression of collagen in the ECM, and crosslinking of this collagen as a result of lysyl oxidase (LOX) and transglutaminase activity, and non-enzymatic glycation [104–107]. It could also be due to the buildup of interstitial pressure within the tumor as a result of rapid growth of the tumor and the ingrowth of blood vessels [108]. The stiffer matrix increases the migration speed of the tumor cells [109], further contributing to their invasiveness [110], which also promotes angiogenesis within the tumor [111]. Similar to ECM composition, stiffness is also effective in preventing immune cell surveillance along with drug and antibody penetration to the tumor site [112] and should be taken into consideration when designing engineered tumor models.
2.2.3. Organization
Microarchitecture (i.e. fiber structure, porosity and pore size) is an important mediator of cancer cell invasion and migration. For example, the presence of dense collagen structures in the stroma promotes tumor invasiveness in vivo [107], since the confinement of tumor cells in small pores triggers ECM degradation by MMP activity [113,114]. Tumor cells and tumor-associated stromal cells remodel the ECM such that radially aligned collagen fibers are formed [107]. These aligned fibers act as highways through which tumor cells can travel [114,115]. ECM alignment in vitro was shown to result in an elongated cell morphology and a higher migration speed, with the most aligned cells migrating faster [116]. In a study, alignment of collagen was reported to enhance migration of the invasive breast cancer cells (MDA-MB-231) more than the non-invasive cells (MCF-7) [117], suggesting that a selective environment for the migration of the invasive cancer cells is created. In fact, these results are supported by a recent study, in which collagen alignment in breast stromal tissues of 227 women patients with DCIS was evaluated [97]. The study showed that patients with poor prognosis had higher amounts of collagen fibers that were perpendicular to the ducts.
In addition to ECM alignment, pore size is also important in regulating the tumor cell behavior. For example, stiffness-driven migration of the tumor cells is pore-size dependent; stiff collagen gels promote tumor cell invasion when their pores are large, while reducing cell migration when their pores are small [118]. Nevertheless, small pores that prevent the migration of tumor cells trigger secretion of MMPs, which degrade the ECM and eventually promote invasion [113]. Surface topography, the nano- or micro-scale orientation of supramolecular structures, also affects cell migration; branched and hydrophilic structures hamper cell motility due to steric effect [18].
2.2.4. Vasculature
Solid tumors need access to blood vessels to grow and metastasize [58]. At the early stages, a solid tumor is a multicellular spheroid without a vascular system [119]. However, without developing a vascular network, tumors cannot grow beyond 2–3 mm in diameter [120]. Cells in the tumors, as well as other tissues, are not merely in need of oxygen and nutrients to keep growing, but also need a way of disposing of carbon dioxide and metabolic waste [121]. Therefore, angiogenesis is crucial for tumor growth [59]. Breast cancer is considered among the cancer types that are more angiogenesis-dependent [122].
Tumor vasculature shows abnormal morphological characteristics compared to that in the healthy tissue [123] (Table 1). In normal tissue, vasculature is organized in an order of arteries, arterioles, capillaries, venules and veins, and the intercapillary distance controls its growth. In contrast, there is no control on the growth of tumor vasculature, resulting in a disorganized development and heterogeneity. As a consequence, necrotic and low microvessel density regions may exist [124]. This non-uniformity in the vasculature of tumors results in spatially and temporally heterogeneous blood flow, leading to acute or perfusion-limited hypoxia [125].
Hypoxia induces the formation of hypoxia inducible factor (HIF), a key factor regulating tumor angiogenesis [126]. The increase in levels of HIF proteins results in the transcription of genes associated with cellular adaptation such as angiogenesis, survival, and cell proliferation [127]. HIF-1, which is highly expressed in breast cancer [128], induces the expression of VEGF [129]. Hypoxia also leads to dedifferentiation of cells. Tumor cells isolated from the hypoxic regions of xenografts contain high number of cells with stem cell-like properties [130]. These cancer stem cells remain stable in vitro for several passages.
The disorganized vascular network results in an increased influx of fluids into the tumor, and reduced efficiency in the outflow of these fluids [5,131]. This imbalanced flow leads to interstitial fluid pressure (IFP), which in turn may alter cell proliferation and protease expression that help in metastasis [132]. Another effect of this abnormal vascular organization is shear stress-induced differential gene expression, which further contributes to the invasion of the tumor cells [133].
2.3. Signaling molecules
In addition to the ECM, signaling molecules also have been shown to play a significant role in the induction and progression of tumors and the immune response against them. Considering the increasing complexity of tumor models, it is crucial to have a thorough biological understanding of the roles of these molecules within the TME.
Although the exact mechanisms responsible for tumorigenesis and metastasis in breast tissue are not fully understood, inflammation has been hypothesized to play a predominant role [56,134]. The release of growth factors or cytokines like transforming growth factor-beta (TGF-β), tumor necrotic factor-alpha (TNF-α), bone morphogenetic proteins (BMPs), epidermal growth factor (EGF), hepatocyte growth factor (HGF), FGF, leukemia inhibitory factor (LIF), and interleukins (IL), which in turn leads to the introduction of the anti-inflammatory cells, has been shown to facilitate tumor formation [135–138].
In addition to their contribution to tumorigenesis, these signaling molecules, the most prominent and most studied of which being TGF-β, play a significant role in EMT [14,86,134] (Figure 2b). Most human tumors (including breast cancer) either secrete or induce secretion of TGF-β, which accumulates in the TME [139,140]. Interaction of TGF-β and its receptors induces the EMT by promoting various cytokines and activating various transcription factors [30,31,141]. One of the key characteristics of the EMT is the increased mobility of tumor cells as a result of reduced cadherin expression, which supports the metastatic phenotype of these cells [142].
Once a tumor has undergone EMT, it can begin to metastasize to other parts of the body, leading to the formation of secondary tumors, and eventually to death of the patient. The process of metastasis is extremely complex and requires cancer cells to leave their niche, enter the vasculature, home onto a target, invade and then proliferate at that target [143]. Many signaling molecules are required for this process to properly take place, including a myriad of cytokines, chemokines, MMPs, and related receptors [4,144]. TGF-β and some BMPs (such as BMP4) are crucial for intravasation, metastasis target preference, and tumor aggressiveness [4,14,30,33,144–146]. Additionally, other factors such as EGF, HGF, and FGF have been shown to significantly increase cancer cell invasiveness [5,147,148]. Many of these cytokines, along with others have been shown to be secreted by endothelial or endothelial-like cells, suggesting that the vasculature plays a significant role in the aggressiveness of breast cancer [5,147–149].
The immune system plays a significant role in nearly every aspect of breast cancer progression, from tumorigenesis to metastasis. Besides being actively involved in the inflammation process, immune cells are also affected by and respond to inflammation, and thus are believed to be key contributors of tumor formation [14,65,135–137,150,151]. In the TME, some of the cytokines (TNF-α, IL-1, IL-6, IL-10, LIF, etc.) serve an immunosuppressive role, preventing a proper response against the tumor by the body [65,66,137,138,152–154] (Figure 1c). Finally, there is evidence that paracrine factors secreted by immune cells actually promote invasion of new niches by the metastatic cancer cells [4,66,147,152,153,155].
Cell signaling is a very complex and intricate process, which has been shown to play a significant role in all portions of cancer biology. As tumor models, both in vitro and in vivo, become increasingly complex, researchers will need to be more cognizant of how the various portions of their model are communicating, and if there is any significant communication that is being missed due to a lack of a cytokine.
3. Breast cancer models
Although the TME is extremely complex, it is possible to recapitulate at least the basic components that play a role in tumor progression. There are currently three approaches to model the TME: in vivo models, ex vivo models, and in vitro models. Each of these models has their own specific strengths and weakness when modeling the TME and investigators should be careful in choosing the proper model that best correlates with the phenomena being observed [156]. Together, these models enable the study of cancer biology and tumorigenesis, as well as screening and discovery of new drugs and therapies [157].
3.1. In vivo models
In vivo breast cancer models are the gold standard and the final test that must be passed before any treatment can move on to clinical trials. Currently, both xenograft and syngeneic in vivo models are used to study breast cancer, with each providing its own set of advantages and disadvantages.
Xenografts, through the use of immunocompromised mice, allow researchers to study the response and behavior of human cells (preferentially patient-derived cells) in vivo, which cannot be done otherwise [158]. Patient-derived xenografts (PDX) allow for the study of a specific line of cancer that is currently unavailable as an animal model [159]. In recent years, these models have been crucial in better elucidating the role of many important signaling pathways in cancer development, including the AKT/mTOR and GSK3β/β-catenin/cyclin D1 pathways [154,159–166]. Unfortunately, xenograft models come with their own challenges. Several studies have shown distinct disadvantages of working with xenografts, the most significant being the lack of immune cells which influence breast cancer cell behavior and play important roles in tumor development and progression [161,167–169].
The other commonly used in vivo model of breast cancer over the past few decades has been the syngeneic mouse model. This model aims to recapitulate breast tumors in mice through the use of reductionist cell lines, genetic engineering, and environmental induction [170]. In contrast to xenografts, syngeneic mouse models have been used mostly to study the basic biology of breast cancer [161,170,171], instead of drug response. These models are uniquely suited for this aim as they allow for the recapitulation of metastasis in its entirety in a single organism, without any cross species interactions [157,172]. Unfortunately, the greatest advantage of the model also turns out to be its greatest weakness; the lack of human cells in the model prevents any results from being directly translatable to the clinic.
In addition to the advantages and disadvantages previously mentioned, both of these in vivo models share significant disadvantages that have promoted the development of in vitro methods for studying breast cancer. Although looking at a whole organism is beneficial, it is extremely difficult to look at any individual mechanism during tumor progression using in vivo models. In the same vein, this also makes it difficult to control specific variables in an entire organism. Besides, animal models have the significant issue of species-to-species variability, which is commonly seen in poor translation of cancer drugs and treatments from animal models to clinical trials [173,174].
3.2. Ex vivo models
In ex vivo culture, thin slices of the animal- or human-origin tumors, sometimes embedded in a gel, are used to study cancer biology and/or test drug efficacy [175–177]. Ex vivo models are thought to preserve the native ECM composition and structure in the TME, and thus support the native cell phenotype and heterogeneity, presenting a realistic gene expression [178]. Although the use of ex vivo models is restricted to the availability of explants from animal and human subjects, banks of explants have recently been established [179], making it easier to use this model for drug screening. Additionally, recent advances enable longer culture of explants without loss of phenotype [180]. Nonetheless, variance between the patients from which the tumor has been obtained makes it difficult to compare the experimental results [157].
3.3. In vitro models
In vivo models are valuable tools to study cancer biology; however, they are not efficient in predicting drug efficacy in humans, due to differences in physiology, metabolism, immune response, and cell types and behavior between animals and humans [176,181]. Moreover, the high number of subjects used in animal experimentation raises ethical concerns.
In vitro models enable the use of primary human cells and/or cell lines, co-cultures of cells, growth factors and materials in a controlled environment, allowing for dissection of the molecular mechanisms by reducing the complexity of the system [182]. In vitro models of breast cancer were also shown to successfully predict the clinical efficacy of drugs [174], and mimic tumor behavior [183–185], although pharmacokinetics and pharmacodynamics could be different in vitro than in the body.
3.3.1. Two dimensional (2D) models
For many decades, 2D cell culture models have been the main workhorse for biological research. In breast cancer, this is no different, with nearly all studies starting off with simple single cell line monolayer and trans-well studies, before moving on to more complicated 3D and in vivo models. The advantages of these 2D models include ease of use, easier measurement of specific changes in cell behavior and parameters, easier manipulation of specific mechanisms for a better understanding, reduced cost, and faster experiment time [186,187]. In addition, 2D culture allows for easier manipulation of the cell culture substrate to chemically, mechanically, or electrically manipulate cells in a non-physiological way to better understand the role of a specific substrate in cellular behavior [186,188–190]. However, specifically in the realm of breast cancer biology, these cell culture methods come with a host of problems, with many 2D results being directly contradicted by results from in vivo and clinical models [158]. The 2D models allow for random cell morphology, lacking cell shape and orientation-related signaling [191,192]. Besides, several studies have shown that when grown in 2D, cancer cells do not exhibit their native signaling response in several biological aspects, including growth, morphology, metabolism, and differentiation [192–194]. With these significant disadvantages, and the growing acceptance of 3D cell culture techniques, 2D cell culture seems to be useful only as an early investigative or purely mechanistic model.
3.3.2. Three dimensional (3D) models
Two dimensional models provide cells with a substrate to attach only in 2D (e.g. surface of the flask or well plate). This one-face binding changes the cell morphology, phenotype and gene expression profile of cells. Besides, the cells are half-exposed in the culture media, which makes them more sensitive to drugs [158,195]. In the native tissue, however, cells bind to each other and to ECM, forming a dynamic 3D network. The 3D in vitro models enable the recapitulation of the TME by providing these cell-cell and cell-material interactions, perfusion, and hypoxic conditions. For instance, in spheroids, tumor cells assume a rounded shape and cluster to form solid tumor-like structures [176]. When compared to those in 2D models, gene expression, cell proliferation, cell migration or invasion, cell morphology and heterogeneity in 3D in vitro models are closer to in vivo [195,196]. All these make 3D in vitro models a very valuable tool for cancer research.
Since 3D models closely recapitulate the tumor in vivo, these models can be used in drug screening and selection. Tumor cells in 3D models are usually more resistant against drugs and more invasive compared to those in 2D models [110,158,197,198], but in some cases they may be less resistant. In a study, tumor cells in 3D culture were reported to be less resistant to tirapazamine, a cytotoxic drug in hypoxic conditions, than those in 2D culture [199]. In another study, gene expression profiles of patient samples were analyzed in a 3D in vitro model, and different genes were identified for a better prognosis in ER- and ER+ cancers [12]. The genes identified for each type of cancer were targeted using specific drugs, and the responses of tumors to these drugs were predictive of the clinical outcomes.
The 3D models can be useful in dissecting the interactions of the tumor cells with immune and stromal cells and ECM, and in solving the signal transduction pathways involved in tumor initiation and progression [167]. The 3D in vitro models can also be designed to study angiogenesis and drug efficacy. These models can be categorized as tumor-mimicking spheroids and TME-mimicking engineered tissue models, each having its own advantages and disadvantages in different applications [200]. These benefits and drawbacks should be considered when choosing the right approach for a particular application.
3.3.2.1. Spheroids
Spheroids, also known as multicellular tumor spheroids, are cancer cells that are packed closely together to form 100–600 micron aggregates. Unlike other models, spheroids favor cell-cell interactions rather than cell-material interactions, and thus are similar in stiffness, structure, oxygen and cell proliferation gradients, and cell heterogeneity to the tumors in vivo [201]. Their gene expression profiles are also closer to tumors in animal and human subjects [202], although some differences have been reported [156]. As a result, spheroids are widely used as 3D in vitro models, despite their expensive and time consuming production.
Methods to produce spheroids include the hanging drop method, cell suspension culture using spinner flasks, basement membrane extracts, non-adherent surfaces, magnetic levitation, 3D printing, and the aqueous two phase systems [17,157,158,196] (Figure 3).
Figure 3.
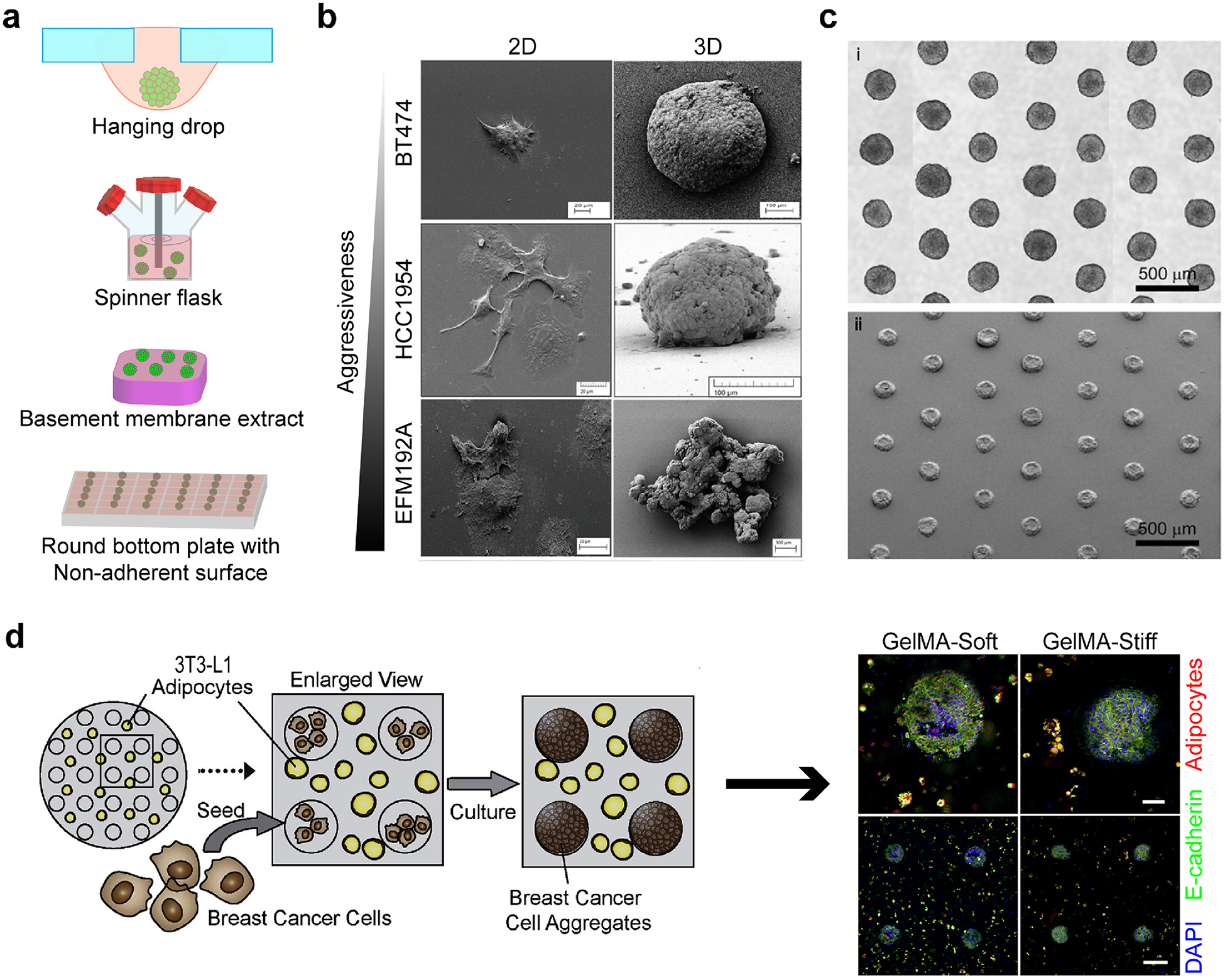
Spheroids as 3D in vitro models. (a) Spheroid production methods. (b) Morphology of cells changes with respect to aggressiveness in 2D and 3D cultures. Reproduced with permission from [196]. Copyright 2016 Breslin and O’Driscoll. Creative Commons Attribution 3.0 License. (c) Several spheroids with uniform size can be produced using high throughput fabrication methods. Reproduced with permission from [17]. Copyright 2010 Elsevier Science. (d) Tumor spheroids were produced in microwells of 3T3-L1 preadipocyte-containing soft or stiff GelMA hydrogels. Adipose differentiation was hampered with the increasing stiffness of hydrogels. Red: adipocytes, green: E-cadherin, and blue: DAPI. Reproduced with permission from [226]. Copyright 2018 Elsevier Science.
In the hanging drop method, cells are seeded on a plate and cultured vertically in an upside-down position [203]. With the help of gravity, the cells aggregate and form spheroids. In suspension culture, cells are continuously agitated in a spinner flask and not allowed to attach on its surface [204]. Thus, they adhere to each other and form spheroids. Non-adherent surfaces are created by coating round bottom plates with hydrophilic polymers, such as agarose or polyethylene glycol (PEG), to reduce cell attachment to the surface of the plate [205,206]. In magnetic levitation, cells are brought together by the help of magnetic particles and cultured that way to form spheroids [207,208]. In 3D printing, cells or cell-hydrophilic polymer suspensions are printed intermittently as small droplets [209]. In the aqueous two phase system, two aqueous solutions are used to entrap cells in the more hydrophilic phase [184,185].
While small spheroids (100–200 μm) are used to study cell-cell and cell-material interactions and test anticancer drugs, larger ones (400–600 μm) have an oxygen gradient with a necrotic core and a 100–300 μm thick proliferating outer shell, and thus are used to study the effects of hypoxia as well [187,201,210]. The size of the tumor is also associated with its aggressiveness. Spheroids were shown to demonstrate a more aggressive phenotype (i.e. higher collective migration and expression of mesenchymal markers) in hormone receptor-positive cells when their size was larger [211]. Similar to what is observed in the clinic, the behavior of the triple-negative breast cancer cells was not dependent on the speroid size.
Spheroids can be used to test the effect of each component on tumor progression. For example, the benign breast epithelial cell line, MCF-10A, was used to form normal acinus structures under various conditions [86]. When MCF-10A cells were co-cultured with mesenchymal stem cells (MSCs) or when they were supplemented with TGF-β or cobalt chloride, a hypoxia-inducing agent, they showed a neoplastic phenotype. Spheroids can also be used in drug efficacy and screening studies. For example, in one study, the uptake and efficacy of antisense oligonucleotides (ODNs) in three formulations: free ODNs, those encapsulated in lipid, and those encapsulated in polyethyleneimine-based carriers, were tested in vitro on tumor spheroids [212]. The size of the carrier was shown to be very important for the penetration and efficacy of the ODNs, and thus should be taken into consideration for in vivo and human applications.
The type of cells used is also important when creating 3D breast cancer models. In a study, the efficacy of paclitaxel and doxorubicin (DOX) was tested on spheroids of six breast cancer cell lines [195]. Spheroids of BT-549, BT-474 and T-47D cells exhibited lower sensitivity to the drugs compared to 2D culture, while spheroids of the more aggressive MCF-7, HCC1954, and MDA-MB‑ 231 cells showed high sensitivity to drugs due to loose packing. This study shows that sensitivity to drugs is not necessarily correlated with the aggressiveness of breast cancer cells. An interesting platform for drug screening was described by Eckhardt et al., who first created xenografts of inflammatory breast cancer (IBC) patients, and then produced a 3D spheroid model by dissociating the cells of xenografts and re-aggregating them using magnetic levitation [208]. They tested several drugs on the spheroid model and showed that their model was representative of the original tumor. Moreover, they claimed that some drugs such as bortezomib, romidepsin, and flovopiridol would be more effective than the standard drugs such as DOX and paclitaxel.
Although difficult to prepare, spheroids can be produced reproducibly with uniform sizes, enabling high throughput analysis [17] (Figure 3c).
3.3.2.2. Engineered 3D models
Tissue engineering combines materials sciences and biology with the aim of creating an entire tissue or a part of it [213]. To fabricate tissues in vitro, cells are seeded on or embedded in natural or synthetic polymer scaffolds, which provide physical, mechanical and biochemical cues that guide the cells. Signaling molecules may also be added to direct the cells toward a specific functional direction. Advances in TE and cell biology enable fabrication of mimetic TME, on which one can study the interaction of the tumor with the surrounding ECM, and test the effects of drugs on the breast cancer cells. TE-based strategies concentrate more on interactions of the tumor with the stroma and immune system and can be predictive of the in vivo response. Thus, the dynamics between the cells, ECM, vasculature and signaling molecules in the TME are taken into consideration when designing a TE-based model.
3.3.2.2.1. Biomaterials used to engineer the TME
When engineering a 3D model, the choice of biomaterial used is of utmost importance to provide the cells with an environment resembling the native TME. The biomaterials used to construct a 3D model could be naturally-derived or synthetic polymers. Natural polymers include collagen, gelatin, hyaluronic acid, agarose, alginate, and chitosan, while the most common synthetic polymers used for TME engineering are polyethylene glycol (PEG), polylactic acid (PLA), polycaprolactone (PCL), and polyurethane (PU).
Naturally-derived polymers intrinsically recapitulate the tissue microenvironment, and thus support cancer cell adhesion, proliferation, and migration or invasion [5,76,214]. The main drawbacks of the natural polymers are their low mechanical properties which lead to altered cell behavior, and batch-to-batch differences which reduce consistency and reproducibility [5,110].
On the other hand, synthetic materials can be produced with controlled properties such as stiffness, degradation rate, and structure [214]. The main drawback of the synthetic polymers is that they lack bioactive groups that are otherwise present in the normal or tumorous ECM [110]. Nonetheless, they can be functionalized or decorated with bioactive groups, which in turn allows for testing the effect of that specific group on tumor progression [215].
3.3.2.2.1.1. Hydrogels
Hydrogels, which have the potential to bind a large amount of water upon crosslinking, are suitable for engineering the TME, since they are soft and they provide a highly aqueous environment just like the ECM [216]. Hydrogels allow for embedding of one or more types of cells (e.g. adipocytes, fibroblasts, and macrophages) or tumor spheroids, biochemical and mechanical cues, and thus closely mimic the TME. Since hydrogels could be produced in various forms, shapes, and components, they allow for the recapitulation of tumor complexity and heterogeneity, which is one of the main reasons for the ineffectiveness of drugs.
Collagen I is the most abundant structural protein in the human body and has bioactive sites that support cell attachment and proliferation as well as ECM production [217]. It allows for construction of biologically relevant tumor models, and thus is one of the most widely used polymers to engineer the TME. Collagen can also be produced to have different stiffness and pore sizes in order to study the effects of these properties on tumor progression, migration and invasion [104,112,218]. Moreover, collagen hydrogels can be used to study the effect of fiber alignment on cancer cell migration. Alignment of the collagen fibers was shown to block cell protrusions and induce cell alignment along the fibers by forcing directional movement [219]. In a study, it was shown that when myoepithelial and luminal cells isolated from mammoplasty specimens were embedded in collagen gels, they self-assembled into bilayer acini structures [220], suggesting that collagen supports native cell organization. After inducing HER2 expression by IL-4 treatment, the researchers observed formation of DCIS, which could be cured with anti-HER2 treatment. This study also showed the role of cytokines in tumor progression.
Incorporation of immune cells into 3D models has recently gained attention in cancer research. Incorporation of M2 macrophages into tumor cell-loaded collagen gels induced matrix degradation due to increased MMP activity and tumor cell invasion [221]. Similarly, in a recent study, Neal and colleagues embedded tumor fragments from mice or humans into collagen gels to form organoids [222]. The organoids contained tumor-infiltrating lymphocytes similar to those in the original tumors in terms of gene expression. Upon activation of the immune cells by blocking programmed cell death-1 (PD-1) receptor and its ligand on tumor cells, PD-L1, the researchers observed a stronger immune reaction against the tumor. This study showed that the 3D in vitro models can successfully recapitulate the TME, and can be used to test drugs or study cancer biology.
Gelatin, the denatured form of collagen, is also widely used to engineer the TME. Brancato et al., studied the efficacy of DOX on a spheroid model and a gelatin-based engineered model [223]. The engineered 3D model demonstrated a higher sensitivity to DOX than the spheroid model, and a lower expression of epithelial biomarkers, similar to what they observed in the xenograft control. The methacrylated form of gelatin, GelMA, has also gained attention in the biomedical field due to its photoactivity [224]. GelMA can be crosslinked upon light exposure, which makes it valuable especially when spatial control over the biochemical factors or on the stiffness is desired. For example, Casey et al. used a GelMA-based microwell system with low or high stiffness to encapsulate the mouse mammary organoids or HCC1806 cells to recreate the normal and tumor breast microenvironments, respectively [225]. They formed hollow tumor spheroids of 300 μm diameter with high cell viability especially at the outer surface of the spheroids. A cell viability gradient was created, similar to what is observed in tumors. In another study by the same group, tumor spheroids (using human cell lines, HCC1806 and MDA-MB-231) or mouse mammary organoids were seeded in microwells of soft (mimicking the normal stroma) or stiff (mimicking the tumor-associated stroma) GelMA gels, in which pre-adipocytes were encapsulated [226] (Figure 3d). Tumor spheroids were shown to block adipogenesis when seeded in the stiff GelMA while this effect diminished when the GelMA gel was soft. This study showed that the effect of the tumor on stromal cells is stiffness dependent.
HA is another polymer used to construct 3D tumor models. In a study, MCF-7 cells showed greater migration/invasion and higher expression of VEGF, IL-8, and FGF-2 in the HA-based model than they did in 2D culture, suggesting that the 3D environment in HA gels enhanced invasiveness [227].The study also showed that cancer cells tended to proliferate and form clusters in the HA gels. In another study, an EGF gradient was created toward the center of the HA hydrogels that have MMP-cleavable sites, and varying responses were observed by the different cancer cells to the EGF gradient [228]. The MDA-MB-231 cells, which normally express EGFR to a moderate level, showed increased invasion toward the EGF gradient, while MDA-MB-468 cells, which highly express EGFR, exhibited reduced invasion. Interestingly, MDA-MB-231 cell invasion was reduced in response to cetuximab drug, while MDA-MB-468 cell invasion was increased. This study showed the importance of drug testing in 3D in vitro platforms and with various cell types before in vivo and clinical settings. In another study, GelMA and the methacrylated HA were used to encapsulate isogenic primary (21PT) and metastatic (21MT-2) cell lines [229]. Under hypoxic conditions, the metastatic breast cancer cells exhibited enhanced cell migration and LOX expression, indicative of EMT. By applying LOX inhibitor, they showed reduced breast cancer cell viability, migration, and EMT.
Another polymer used to produce 3D tumor models is alginate. Alginate crosslinks in response to bivalent cations, such as calcium or barium, which allows spatial crosslinking of the polymer. A new approach involved the use of electrostatic encapsulation to embed tumor cells in alginate solution, which was then sprayed in a calcium bath to crosslink the alginate [230]. Culture of the alginate beads resulted in tumor spheroid formation, and the size of spheroids determined the response of tumor cells to radiotherapy. Cells in large spheroids survived better against radiation, and the resistance of the cells was explained by the hypoxic core in the large spheroids, although it could also be due to reduced penetration of radiation to the core of the large spheroids. Regardless of the underlying mechanism, this study could help in calculation of the dose of radiotherapy needed to clinically treat a certain size tumor. Alginate could also be blended with other polymers such as collagen and chitosan to control its stiffness and microarchitecture. In a study, MDA-MB-231 cells and human mammary fibroblasts (HMFs) were embedded in alginate-collagen gels with stiffness mimicking that of the tumor and tumor-associated stroma [231]. The fibroblasts were shown to lead the way to invasion, while the tumor cells were the followers. In a similar study, chitosan-alginate blends were used to co-culture TAFs, breast cancer cells, and T-lymphocytes [232]. The study showed that TAFs can hamper the production of TNF-α by T cells. These studies showed the role of fibroblasts in tumor progression.
In addition to the natural polymers, synthetic polymers are also used to engineer the TME. PEG is the most commonly used synthetic polymer, with many advantages including reproducibility, tailorable mechanical properties, pore size, pore shapes, and degradation rates, and the option for surface modification, which allows for attaching the desired functional groups or molecules [5,99,186,233]. PEG can be synthesized to be degradable or to carry bioactive groups or functional groups that confer photoactive or adhesive properties. For instance, in a study, star PEG, a branched form of the molecule, was modified with growth factor-encapsulated heparin that enables sustained release of these growth factors [234]. This system was shown to support blood vessel formation. Similarly, other researchers also functionalized the heparin-modified and MMP-degradable PEGs with RGD, GFOGER, and IKVAV to test the effects of these peptides on the invasiveness of cancer cells [99]. The presence of GFOGER and IKVAV resulted in increased invasiveness of the aggressive cancer cells as characterized with higher MMP activity. In another study, MMP-sensitive PEG/heparin hydrogels enriched with various growth factors including VEGF, FGF-2 and stromal cell-derived factor 1 (SDF-1) were used to engineer a 3D in vitro tumor model [233]. Tri-culture of breast tumor cells (MCF-7 or MDA-MB-231) with the human umbilical vein endothelial cells (HUVECs) and MSCs resulted in formation of vascularized tumor spheroids. More resistance to epirubicin drug treatment was reported when the cells were tri-cultured in 3D microenvironment compared to 2D.
In brief, hydrogels are very useful in engineering the TME, because they are not complex and enable precise control over the biochemical composition, allowing easy dissection of the effect of each component on tumor progression.
3.3.2.2.1.2. Basement membrane extracts and decellularized ECM
Engineered hydrogels explained in the previous section usually comprise of one or two types of polymers in contrast to the native ECM, which contains several proteins, GAGs, and growth factors. Therefore, researchers have also explored using native ECM parts such as basement membrane extract (BME) and decellularized whole ECM, which contain most of the proteins that are normally found in the native tissues. The BME is obtained from murine Engelbreth-Holm-Swarm (EHS) tumor cell cultures and contains laminin (60%), collagen type IV (30%), entactin (8%), and growth factors [235,236]. The BME is commercially available as MatrigelTM, Cultrex®, and Geltrex®, and has widely been used to support the growth of epithelial cells, stem cells, and cancer cells in culture [225,226,237–239].
In one study, pre-neoplastic (MCF10AT1-EIII8) breast epithelial cells were embedded in Matrigel in the presence of HUVECs, and normal or tumor-associated fibroblasts [240]. The presence of TAFs increased the responsiveness of the EIII8 cells to estrogen, and introduction of HUVECs enhanced their invasiveness and induced MMP production by these pre-neoplastic cells. Similarly, co-culture of breast cancer cells with pro-monocytes in Matrigel resulted in increased aggressiveness of the cancer cells due to higher MMP and cyclooxygenase (an inflammatory factor) expression [241]. The researchers also showed that transferring the media of the breast cancer/pro-monocyte culture to the non-invasive MCF-10A cells resulted in disruption of the MCF-10A acini.
In a recent study, metastasis of mouse (4T1) and human (MDA-MB-231) breast cancer cells in Matrigel was examined [242]. Asparagine was shown to increase the invasive/metastatic potential of cells. Interestingly, blocking of asparagine synthase resulted in reduced invasion without affecting the growth of tumor, suggesting that asparagine is involved only in the metastasis process. The results were verified in vivo in a mouse model, highlighting the predictive potential of this 3D in vitro model.
Matrigel has also been blended with other hydrogels to increase its stiffness. For example, MCF-10A cells were embedded in Matrigel/alginate to form normal acini [243]. The gel system contained calcium-entrapped light-sensitive liposomes, which were triggered to release calcium and further crosslink the gel system. Increasing the stiffness to the tumor level induced an invasive phenotype, characterized by enhanced collective migration of the cells. Similarly, alginate was blended with Matrigel in various ratios to engineer the TME [244]. When the MDA-MB-231 cells were embedded in a one-to-one mixture of these materials, they exhibited nuclear fragmentation, an altered morphology, invadopodia expression, and increased invasion through the gel system toward blood vessel-mimetic membranes.
Another biomaterial used to engineer the TME is the decellularized ECM and its hydrogel made after solubilizing it. In one study, decellularized adipose tissues from human abdomen were used to engineer a 3D breast cancer model [245]. MCF-7, BT474, and SKBR3 cells seeded on the decellularized tissues exhibited a closer proliferation and gene expression profile, cell morphology and spheroid forming potential to the in vivo xenograft model than the Matrigel and 2D substrates. In another study, MCF-7 cells were seeded on decellularized porcine lungs and formed large spheroids upon culture [246]. Breast cancer markers, BRCA1 and HER2, were reported to increase after culturing these cells on the decellularized tissues compared to 2D substrates. The deeper regions of the tissues exhibited reduced oxygen levels, and the cells in those regions expressed HIF-1α, similar to the TME. Treatment of cells with the drug, 5-fluorouracil, resulted in higher survival on these matrices compared to that in the 2D substrates.
Every tissue has a unique ECM composition, and thus cells respond to ECMs derived from various tissues in a tissue-specific manner [247]. Cells derived from a specific tissue would maintain their native phenotypes and functions when seeded on the decellularized ECM of that tissue. Hence, the use of decellularized ECM from the mammary gland would enable engineering of a 3D model closely resembling the breast TME. However, the amount of native tissue that could be obtained especially from human sources could be a limiting factor for clinically relevant applications of this approach.
3.3.2.2.1.3. Solid scaffolds
Solid scaffolds are generally used to model migration, invasion, and metastasis of breast cancer cells. For example, a gelatin construct was engineered via electrospinning, coated with collagen I and seeded with MCF-7 to produce a breast cancer model [248]. Estrogen treatment was shown to induce the expression of the metastasis related gene, MMP-2, and reduce E-cadherin expression, while progesterone treatment had the opposite effect.
In another study, the aggressive MDA-MB-231 cell line was co-cultured with osteoblast-like MG63 cells in a silk fibroin-based 3D scaffold to evaluate the interaction of these cells during metastasis and to test the efficacy of targeted delivery of DOX via folate-conjugated nanoparticles [249]. They showed reduced VEGF production in the presence of nanoparticles. Similarly, polyurethane [250] and nanoclay-based PCL [251] scaffolds were used to model the breast cancer-to-bone metastasis microenvironment and study the cell-cell and cell-material interactions. Both studies showed significant differences in cell proliferation and protein expression profiles of the 2D and 3D models.
In the above sections, we explain the materials used to engineer the TME. In the next section, we point out two very promising approaches used to engineer the TME; three dimensional (3D) printing and microfluidics.
3.3.2.2.2. Three dimensional printing for breast TME engineering
Three dimensional printing is an additive manufacturing technique, in which computer-aided 3D geometries are produced through layer-by-layer deposition of the materials often referred to as bioinks. Using 3D printing, complex 3D structures can be created with living cells, an approach called bioprinting. Recently, bioprinting has gained popularity in medical and scientific societies because of its advantages over other traditional biofabrication methods, including the ability to create complex structures with viable cells, accurate reproducibility, low cost, high throughput and efficiency [252,253]. In addition, bioprinting systems allow for using multiple cartridges that can be filled with different bioinks, and thus it is possible to spatially and temporally control the location of the cells, proteins, growth factors, and other bioactive elements to fabricate physiologically relevant tissue constructs [254–257].
3D printing has been used to investigate breast cancer from different perspectives. For example, Reid et al. were able to create reproducible and reliable arrays of human mammary organoids inside 3D collagen matrices via the bioprinting method [258]. They compared the printed models with manual matrix-embedded ones and demonstrated the superiority of the former in terms of efficiency and consistency in organoid morphology. In another study, sacrificial gelatin arrays were used to fabricate concave wells, into which MCF-7 cells were seeded in situ and tumor spheroids were created [259]. This high throughput system was shown to allow for uniform cell seeding and have the potential for tumor-on-chip fabrication.
In another study, pre-formed spheroids of MCF-10A and MDA-MB-231 cells were printed in gelatin/alginate or collagen/alginate bioinks without affecting the cell viability and morphology [209]. The printed spheroids exhibited higher resistance to paclitaxel treatment than the individually printed cells. In the presence of HUVECs, this difference was not observed. They suggested that their system could be applied to engineer physiologically relevant TME for use in drug screening. Wang et al. also utilized 3D bioprinting to fabricate breast cancer models representing in vivo conditions, which can be used in drug screening [255]. They printed a stromal compartment using adipose-derived mesenchymal stem cells (ADSC) and a tumor compartment in the center using 21PT breast cancer cells, and tested the response of cells to DOX (Figure 4a). They showed that, at low DOX concentrations, ADSCs prevented the tumor cells from going through apoptosis. Moreover, they showed that both cells expressed LOX, regardless of treatment with DOX. Interestingly, while ADSCs responded to LOX inhibitors as characterized by lowered matrix stiffness in the vicinity of these cells, the breast cancer cells did not respond to LOX inhibitors [255].
Figure 4.
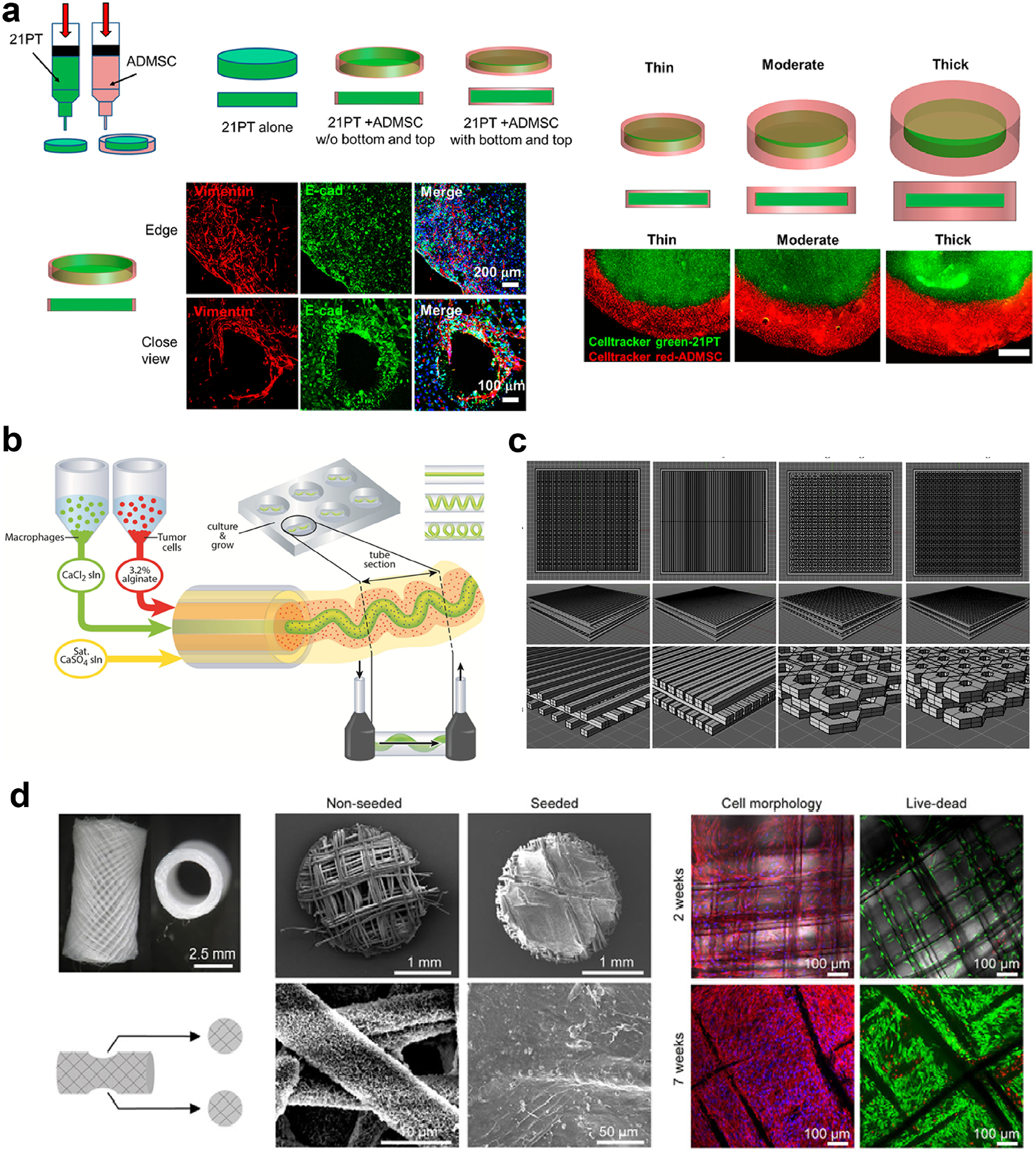
Three dimensional printing is an efficient method in modeling the TME, as it enables spatial and morphological control on the printed materials. Multiple print heads enable (a) bioprinting of the tumor cells in the center and the mesenchymal stem cells (MSCs) in the outer region to mimic the TME (reproduced with permission from [255], Copyright 2018 American Chemical Society) or (b) co-bioprinting of the immune cells and the tumor cells, the immune cells being printed as blood vessel-like channels passing through the tumor (reproduced with permission from [260], Copyright 2015 John Wiley and Sons). (c) Scaffolds can be printed in various shapes thanks to computer aided design (CAD). Reproduced with permission from [261]. Copyright 2016 Elsevier Science. (d) 3D printing can also be used to recreate the metastasis process. Here, breast-to-bone metastasis of tumor cells is simulated using the 3D printed humanized bone. Reproduced with permission from [263]. Copyright 2014 The Company of Biologists Ltd. Creative Commons Attribution 3.0 License.
In another study, a co-extrusion based bioprinting approach was used to fabricate different types of geometries using human breast cancer cells (MDA-MB-231) and mouse macrophages for anti-cancer drug screening (Figure 4b) [260]. This study showed that the shape of the bioprinted microchannel, which contained macrophages in the center, affected their migration profile and interaction with breast cancer cells in the circumferential shell layer. Moreover, the researchers demonstrated that a paracrine loop was formed between the cancer cells and macrophages, which in turn improved cell motility.
3D printed tumor models can also be used to study metastasis of breast cancer cells to other tissues, mainly bone. For example, to study the invasion of breast cancer to bone in vivo, Zhu et al. fabricated PEG/hydroxyapatite (HAp)-based bone mimics with a highly controlled structure (Figure 4c) [261]. They showed that cells cultured in 3D matrices exhibited higher migration compared to 2D culture. Co-culturing of human fetal osteoblasts (hFOB) with breast cancer cells (MDA-MB-231) resulted in reduced proliferation of the osteoblasts, but increased proliferation of the breast cancer cells. The same group also used a GelMA-HAp system to print osteoblasts or MSCs as the bone stromal compartment and seeded the MDA-MB-231 cells on the matrices [262]. They showed that co-culturing of breast cancer cells with osteoblasts or MSCs in 3D printed matrices resulted in higher VEGF expression compared to mono-cultured breast cells. In another study, Thibaudeau et al. used melt electrospun PCL fibers seeded with human primary osteoblasts to create a humanized bone and implanted it into NOD/SCID mice (Figure 4d) [263]. Four weeks after injection of the invasive breast cancer cells (MCF-10A, MDA-MB-231, and the bone-seeking sub-strain MDA-MB-231BO) into the hearts of the mice, the MDA-MB-231BO cells were shown to have metastasized to the humanized tissue engineered bone in all of the mice. The same group also used printed PCL/tricalcium phosphate (TCP) scaffolds to create a bone mimic and seeded them with human osteoblasts (hOB) to create a humanized bone model, which was implanted into mice [264]. Breast cancer cells, MDA-MB-231, SUM1315, and MDA-MB-231BO, were encapsulated in PEG and implanted adjacent to the printed bone mimic in the mice. The number of MDA-MB-231BO infiltrating the humanized bone was higher than that of the other cell lines used. Introducing the breast cancer cells into bone microenvironment resulted in increased osteoclastic activity, which was similar to what occurs in the body.
3D printing is an extremely useful approach to engineer breast cancer models, since it allows for spatial control on the cell types, biochemical composition and stiffness of printed models. Large tumor models and the interation of these tumors with the surrounding tissues is also possible.
3.3.2.2.3. Microfluidic systems for breast TME engineering
Organ-on-chip microfluidic devices simulate the TME in a small chip with microchannels allowing perfusion. The channels are often filled with a photoactive hydrogel precursor-cell suspension, which is then gelled after exposure to light. Since the channels can be perfused at tailorable flow rates, they are widely used for studying angiogenesis, intravasation, extravasation, mechanotransduction pathways, cancer cell behavior, and drug response under shear stress [265–268]. The effects of material type, cell type and flow on tumor behavior could easily be studied in microfluidic devices. For example, Peela et. al. microfabricated a microfluidic device filled with GelMA-based gels with tunable stiffness, and studied the behavior of malignant (MDA-MB-231, MCF-7) and non-malignant (MCF-10A) breast epithelial cell lines in the device [269]. They showed that while MCF-7 and MDA-10A formed clusters within the circular tumor compartment, MDA-MB-231 did not cluster and they migrated in the stroma-like compartment at a significantly higher rate than the other two cell lines. Lanz et. al. examined the effects of material type (Matrigel, Cultrex, and collagen I) and flow conditions on the viability of various breast cancer cell lines (MDA-MB-453, MDA-MB-231, and HCC1937) in a microfluidic device [270]. They showed that breast cancer cell survival was the highest in the presence of Matrigel, and perfusion increased the viability of cells in the Matrigel and Geltrex, but reduced that of the cells in collagen I gels. The study showed that the response of tumor cells depended on the material used and the flow conditions. The researchers also used these results to treat breast cancer in a PDX model and showed significant improvements in the outcome.
Microfluidic systems also allow for spatial control on the cell distribution, matrix composition and matrix stiffness, and enable the study of site-specific cell and material interactions [176]. In a study, MDA-MB-231 cells were shown to migrate from a confined environment to one that has more free space, while their benign counterparts did not exhibit the same behavior [271]. This study showed how the aggressive cancer cells can easily relocate to a more favorable environment to survive. In another study, a microfluidic device with 3 channels was used to create a DCIS model (Figure 5a) [272]. The central channel was coated first with a layer of Collagen I and then with a Matrigel layer to mimic the basement membrane. The Matrigel layer was seeded with MCF-10A and then with MCF10ADCIS.com cells to mimic the lumen in DCIS. The two side channels were coated with Collagen I and seeded with HMFs to mimic the stroma. The devices containing DCIS cells showed substantial invasion toward the stroma-mimicking channels, while the controls missing these cells did not show any sign of invasion. Invasive lesions facing the HMFs exhibited reduced E-cadherin expression, highlighting the effect of stroma on the tumor phenotype. In another study, a microfluidic platform with three microchannels connected with micropillars was produced in an attempt to simulate metastasis of breast cancer cells (Figure 5b) [273]. The central microchannel was filled with aggregates of the metastatic breast cancer cell line, MX1, surrounded by a hydrogel of the cationic methylated Collagen I and anionic terpolymer of hydroxylethylmethacrylate–methylmethacrylate–methylacrylic acid (HEMA-MMA-MAA) as a barrier to keep the cells in the central microchannel. The side microchannels were used to perfuse the system. When cells were cultured in the presence of a chemoattractant, the researchers showed that cells remodeled the collagen and invaded the gel. This system could be used to monitor cell migration/invasion in real-time, enabling researchers to analyze the mechanism of cell invasion and the cell behavior in response to drugs.
Figure 5.
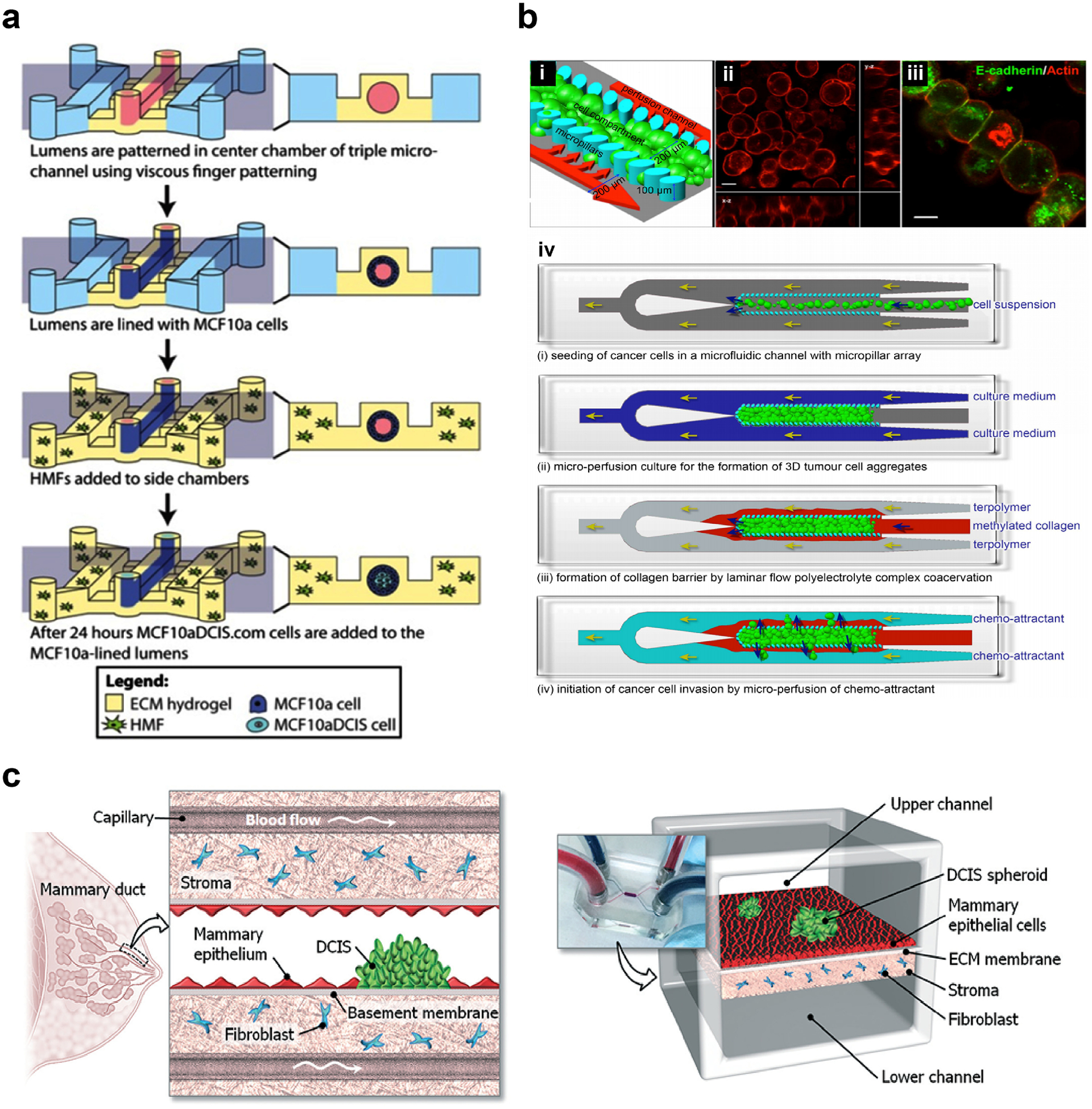
Microfluidic devices introduce flow to breast cancer models. (a) A microfluidic device with three channels used to study the interaction of the tumor cells, non-tumor cells and fibroblasts. Tumor cells in the vicinity of the stromal fibroblasts were more aggressive and migrated a greater distance. Reproduced with permission from [272]. Copyright 2015 Springer Nature. Creative Commons Attribution 4.0 International License. (b) Another design in which tumor cell aggregates in the center channel (mimicking the tumor) were surrounded by collagen-based gel in the side channels (mimicking the stroma) that enables the study of tumor cell invasion through the stroma in response to a chemoattractant. (i) The model. (ii, iii) Actin (red) and E-cadherin (green) expression in tumor cells. (iv) Preparation of the model. Reproduced with permission from [273]. Copyright 2018 Toh, Raja, Yu, Van Noort. Creative Commons Attribution 3.0 License. (c) A DCIS model showing the effect of flow on 3D interactions on drug sensitivity of the tumor cells. Tumor cells were more sensitive to drugs after interacting with the epithelial cells and then with stromal fibroblasts. Reproduced with permission from [274]. Copyright 2015 Royal Society of Chemistry.
The effects of stromal cells on the tumor cell behavior and drug response could be studied in the microfluidic devices. For example, a device with two separate compartments was fabricated and seeded with either normal fibroblasts or TAFs in the stromal compartment, and with MCF-7 in the tumor compartment [38]. Normal fibroblasts expressed α-SMA and PDGF, markers of fibroblast activation, while TAFs expressed MMP-2 and MMP-9, markers of invasiveness. Choi and colleagues fabricated a microfluidic device with two compartments, and co-cultured the MDA10ADCIS.com cells with human mammary ductal epithelial cells in one channel and HMFs in the other channel (Figure 5c) [274]. They tested the efficacy of paclitaxel on the DCIS cells and showed that DCIS cells were more sensitive to the drug in the presence of epithelial cells.
Similar to 3D printing, microfluidic devices can also be useful in studying metastasis to other tissues or organs. Breast cancer cells metastasize mostly to bone; therefore, these models mainly focus on recapitulating breast-to-bone metastasis. Bersini et al. fabricated a Collagen I-based microfluidic device, in which they evaluated the metastasis of breast cancer cells in the presence or absence of oste-differentiated MSCs [275]. MDA-MB-231 cells exhibited significantly higher extravasation through the vasculature-like endothelial cell barrier in the presence of osteo-differentiated MSCs than in their absence, and formed microtumors after extravasating to the collagen gels. The same group also compared these bone-mimicking devices with the muscle-mimicking environment and showed significantly higher extravasation to bone than to muscle [276]. They also showed that adenosine increased the permeability of vasculature but decreased the extravasation rate of breast cancer cells. The introduction of flow to the model reduced the vascular permeability and thus extravasation of the cancer cells, but increased the distance they traveled.
Although in their infancy period, microfluidics studies have been a very important tool in breast cancer research, having contributed to the discovery of cell migration-related genes and receptors [157]. Yet, the very small number of cells and volume of samples that can be filled into the channels makes it difficult to derive statistically meaningful conclusions. Thus, integration of sensors that allow for sensitive detection, and software enabling easy analysis of these data can enhance the utility of such microfluidic-based TME models in years to come.
4. Conclusions and future prospects
In vivo models provide a dynamic environment involving the immune system, vasculature, and other naturally occurring events in the TME; however, they are time-consuming, laborious and costly to prepare. Syngeneic mouse models do not reflect the human response against tumors, while xenograft models lack the immune system, which is an essential component of tumor progression. Besides, in vivo models are so complex with many variables in effect that it is very difficult to dissect the contribution of one factor, and there are growing ethical concerns in response to using more and more animal subjects. In vitro models are simpler, and enable easier analysis of the outcomes and the possible reasons for these outcomes. Engineered 3D models involve the use of biomaterials (mainly hydrogels) and multiple cell types at once in the form of co-cultures, thus enabling these cells to assume their native morphology and achieve cell-cell and cell-ECM interactions. Among the fabrication methods for 3D in vitro models, bioprinting and microfabrication techniques such as microfluidics are promising, since they enable spatial control on the biochemical composition (tumor vs stroma), cells (tumor cells, fibroblasts, adipocytes, and immune cells), and flow (vasculature). These techniques bring in the advantage of engineering 3D models closely resembling the TME, although there is some space for improvement.
One problem related to 3D in vitro models is the lack of media suitable for all the cell types when co-culture of cells is aimed [167]. This issue can be addressed by spatially incorporating the necessary growth factors, cytokines and MMPs into the stromal or tumor compartments, which will support the growth of cells at each compartment. These factors can either be immobilized to the hydrogels or encapsulated in carriers that release them sustainably and in a controlled manner.
Another point to be improved is the involvement of M2 macrophages and regulatory T cells, which regulate the reaction of immune cells such as M1 macrophages and other lymphocytes, and of endothelial cells and pericytes, which regulate angiogenesis. These cells can be incorporated into the design of the engineered 3D models in order to better recapitulate the complexity of TME. Incorporating TAAs and TAFs in the engineered models is as crucial as including immune cells in studying how these cells impact tumor cell invasiveness and resistance to drugs [277].
Immunoeditting, an approach that involves the blocking of receptors (PD-1) on M2 macrophages and T cells that are otherwise immunosuppressed by ligands (PD-L1, and PD-L2) on tumor cells, or blocking these ligands, is gaining attention in tumor biology [278,279]. This concept can also be applied to stromal cells such as TAAs and TAFs to block the tumor from controlling their functions. Susceptibility of similar receptors on the stromal cells can be investigated and, if present, can be targeted using the engineered 3D models.
Complex microfluidic systems incorporating liver cell and renal cell compartments, together with the breast TME compartment, can be engineered to study the pharmacokinetics and pharmacodynamics of the drugs. Finally, incorporating sensors into the microfluidic systems may also enable monitoring of the response of cells to specific treatments. Sensors can be useful in microfluidic devices, to analyze the large amount of data produced in these small devices.
Finally, applying decreasing doses of drugs during chemotherapy to mice was shown to be more effective than administering the same dose repeatedly, which leads tumors to develop resistance against the drugs [280]. This evolution-guided treatment strategy could be incorporated to the engineered 3D models to explore the applicability of sustained drug release approach with decreasing doses of the drugs.
Statement of Significance.
Involvement of biomaterials and tissue engineering fields in cancer research enables realistic mimicry of the cell-cell and cell-extracellular matrix (ECM) interactions in the tumor microenvironment (TME), and thus creation of better models that reflect the tumor response against drugs. Engineering the 3D in vitro models also requires a good understanding of the TME. Here, an overview of the breast cancer TME is given, and the current state of the pre-clinical breast cancer models, with a focus on the engineered 3D tissue models is discussed. This review article is useful not only for biomaterials scientists aiming to engineer 3D in vitro TME models, but also for cancer researchers willing to use these models for studying cancer biology and drug testing.
Acknowledgements
This work is supported by NIH (Award No: 1R01EB027660-01), NSF (Award No: 1805157), Mike and Josie Harper Cancer Research Institute and Walther Cancer Foundation Cancer Cure Venture Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest None
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer Statistics, 2018, CA. Cancer J. Clin 68 (2018) 7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- [2].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA. Cancer J. Clin 68 (2018) 394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- [3].Weigelt B, Peterse JL, Van’t Veer LJ, Breast cancer metastasis: Markers and models, Nat. Rev. Cancer (2005). doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- [4].Chaffer CL, Weinberg RA, A Perspective on Cancer Cell Metastasis, Science (80-. ). 331 (2011) 1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- [5].Fong ELS, Harrington DA, Farach-Carson MC, Yu H, Heralding a new paradigm in 3D tumor modeling, Biomaterials. 108 (2016) 197–213. doi: 10.1016/j.biomaterials.2016.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kamb A, What’s wrong with our cancer models?, Nat. Rev. Drug Discov 4 (2005) 161–165. doi: 10.1038/nrd1635. [DOI] [PubMed] [Google Scholar]
- [7].Rubin EH, Gilliland DG, Drug development and clinical trials—the path to an approved cancer drug, Nat. Rev. Clin. Oncol 9 (2012) 215–222. doi: 10.1038/nrclinonc.2012.22. [DOI] [PubMed] [Google Scholar]
- [8].Debeir OD, Decaestecker C, Debeir O, Adanja I, Kiss R, Models of cancer cell migration and cellular imaging and analysis, in: Lambrechts A, Ampe C (Eds.), Motile Actin Syst. Heal. Dis, Transworld Research Network, Kerala, 2008: pp. 123–156. https://www.researchgate.net/publication/262858925. [Google Scholar]
- [9].Unger C, Kramer N, Walzl A, Scherzer M, Hengstschläger M, Dolznig H, Modeling human carcinomas: Physiologically relevant 3D models to improve anti-cancer drug development, Adv. Drug Deliv. Rev 79–80 (2014) 50–67. doi: 10.1016/J.ADDR.2014.10.015. [DOI] [PubMed] [Google Scholar]
- [10].Toniatti C, Jones P, Graham H, Pagliara B, Draetta G, Oncology Drug Discovery: Planning a Turnaround, Cancer Discov 4 (2014) 397–404. doi: 10.1158/2159-8290.CD-13-0452. [DOI] [PubMed] [Google Scholar]
- [11].Sia D, Moeini A, Labgaa I, Villanueva A, The future of patient-derived tumor xenografts in cancer treatment, Pharmacogenomics. 16 (2015) 1671–1683. doi: 10.2217/pgs.15.102. [DOI] [PubMed] [Google Scholar]
- [12].Martin KJ, Patrick DR, Bissell MJ, Fournier MV, Prognostic breast cancer signature identified from 3D culture model accurately predicts clinical outcome across independent datasets, PLoS One. (2008). doi: 10.1371/journal.pone.0002994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Han J, Chang H, Giricz O, Lee GY, Baehner FL, Gray JW, Bissell MJ, Kenny PA, Parvin B, Molecular Predictors of 3D Morphogenesis by Breast Cancer Cell Lines in 3D Culture, PLoS Comput. Biol 6 (2010) e1000684. doi: 10.1371/journal.pcbi.1000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alkasalias T, TUMOR MICROENVIRONMENT: THE PARADOXICAL ACTION OF FIBROBLASTS, Karolinska Institute, 2018. [Google Scholar]
- [15].Okegawa T, Pong R-C, Li Y, Hsieh J-T, The role of cell adhesion molecule in cancer progression and its application in cancer therapy., Acta Biochim. Pol 51 (2004) 445–57. doi:035001445. [PubMed] [Google Scholar]
- [16].Cross SE, Jin Y-S, Tondre J, Wong R, Rao J, Gimzewski JK, AFM-based analysis of human metastatic cancer cells, Nanotechnology. 19 (2008) 384003. doi: 10.1088/0957-4484/19/38/384003. [DOI] [PubMed] [Google Scholar]
- [17].Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA, Multicellular tumor spheroids: An underestimated tool is catching up again, J. Biotechnol (2010). doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- [18].Holle AW, Young JL, Spatz JP, In vitro cancer cell-ECM interactions inform in vivo cancer treatment, Adv. Drug Deliv. Rev 97 (2016) 270–279. doi: 10.1016/j.addr.2015.10.007. [DOI] [PubMed] [Google Scholar]
- [19].Semenza GL, The hypoxic tumor microenvironment: A driving force for breast cancer progression, Biochim. Biophys. Acta - Mol. Cell Res 1863 (2016) 382–391. doi: 10.1016/j.bbamcr.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hanahan D, Weinberg RA, Hallmarks of Cancer: The Next Generation, Cell. 144 (2011) 646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [21].Junttila MR, de Sauvage FJ, Influence of tumour micro-environment heterogeneity on therapeutic response, Nature. 501 (2013) 346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- [22].Albritton JL, Miller JS, 3D bioprinting: improving in vitro models of metastasis with heterogeneous tumor microenvironments., Dis. Model. Mech 10 (2017) 3–14. doi: 10.1242/dmm.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shriver M, Stroka KM, Vitolo MI, Martin S, Huso DL, Konstantopoulos K, Kontrogianni-Konstantopoulos A, Loss of giant obscurins from breast epithelium promotes epithelial-to-mesenchymal transition, tumorigenicity and metastasis, Oncogene. 34 (2015) 4248–4259. doi: 10.1038/onc.2014.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L, Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease, Nat. Rev. Clin. Oncol 13 (2016) 674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mallini P, Lennard T, Kirby J, Meeson A, Epithelial-to-mesenchymal transition: What is the impact on breast cancer stem cells and drug resistance, Cancer Treat. Rev 40 (2014) 341–348. doi: 10.1016/j.ctrv.2013.09.008. [DOI] [PubMed] [Google Scholar]
- [26].Onitilo AA, Engel JM, Greenlee RT, Mukesh BN, Breast Cancer Subtypes Based on ER/PR and Her2 Expression: Comparison of Clinicopathologic Features and Survival, Clin. Med. Res 7 (2009) 4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Youk JH, Son EJ, Chung J, Kim J-A, Kim E, Triple-negative invasive breast cancer on dynamic contrast-enhanced and diffusion-weighted MR imaging: comparison with other breast cancer subtypes, Eur. Radiol 22 (2012) 1724–1734. doi: 10.1007/s00330-012-2425-2. [DOI] [PubMed] [Google Scholar]
- [28].Konstantopoulos K, Thomas SN, Cancer Cells in Transit: The Vascular Interactions of Tumor Cells, Annu. Rev. Biomed. Eng 11 (2009) 177–202. doi: 10.1146/annurev-bioeng-061008-124949. [DOI] [PubMed] [Google Scholar]
- [29].Gay LJ, Felding-Habermann B, Contribution of platelets to tumour metastasis, Nat. Rev. Cancer. 11 (2011) 123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pang M-F, Georgoudaki A-M, Lambut L, Johansson J, Tabor V, Hagikura K, Jin Y, Jansson M, Alexander JS, Nelson CM, Jakobsson L, Betsholtz C, Sund M, Karlsson MCI, Fuxe J, TGF-β1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis, Oncogene. 35 (2016) 748–760. doi: 10.1038/onc.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Das V, Bhattacharya S, Chikkaputtaiah C, Hazra S, Pal M, The basics of epithelial–mesenchymal transition (EMT): A study from a structure, dynamics, and functional perspective, J. Cell. Physiol (2019) In press. doi: 10.1002/jcp.28160. [DOI] [PubMed] [Google Scholar]
- [32].Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, Lorenz K, Lee EH, Barcellos-Hoff MH, Petersen OW, Gray JW, Bissell MJ, The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression, Mol. Oncol (2007). doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, Gong Z, Zhang S, Zhou J, Cao K, Li X, Xiong W, Li G, Zeng Z, Guo C, Role of tumor microenvironment in tumorigenesis, J. Cancer. (2017). doi: 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lazzari G, Couvreur P, Mura S, Multicellular tumor spheroids: A relevant 3D model for the: In vitro preclinical investigation of polymer nanomedicines, Polym. Chem (2017). doi: 10.1039/c7py00559h. [DOI] [Google Scholar]
- [35].Ren X, Ghassemi P, Kanaan YM, Naab T, Copeland RL, Dewitty RL, Kim I, Strobl JS, Agah M, Kernel-Based Microfluidic Constriction Assay for Tumor Sample Identification, ACS Sensors. 3 (2018) 1510–1521. doi: 10.1021/acssensors.8b00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].van de Ven S, Smit VTHBM, Dekker TJA, Nortier JWR, Kroep JR, Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer, Cancer Treat. Rev 37 (2010) 422–430. doi: 10.1016/j.ctrv.2010.11.006. [DOI] [PubMed] [Google Scholar]
- [37].Kakarala M, Wicha MS, Implications of the Cancer Stem-Cell Hypothesis for Breast Cancer Prevention and Therapy, J. Clin. Oncol 26 (2008) 2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gioiella F, Urciuolo F, Imparato G, Brancato V, Netti PA, An Engineered Breast Cancer Model on a Chip to Replicate ECM-Activation In Vitro during Tumor Progression, Adv. Healthc. Mater 5 (2016) 3074–3084. doi: 10.1002/adhm.201600772. [DOI] [PubMed] [Google Scholar]
- [39].Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, Pasqualini F, Nebuloni M, Chiabrando C, Mantovani A, Allavena P, Tumor-Conditioned Macrophages Secrete Migration-Stimulating Factor: A New Marker for M2-Polarization, Influencing Tumor Cell Motility, J. Immunol 185 (2010) 642–652. doi: 10.4049/jimmunol.1000413. [DOI] [PubMed] [Google Scholar]
- [40].Li B, Wang JH-C, Fibroblasts and myofibroblasts in wound healing: Force generation and measurement, J. Tissue Viability. 20 (2011) 108–120. doi: 10.1016/j.jtv.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mehner C, Radisky DC, Triggering the landslide: The tumor-promotional effects of myofibroblasts, Exp. Cell Res 319 (2013) 1657–1662. doi: 10.1016/j.yexcr.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sugimoto H, Mundel TM, Kieran MW, Kalluri R, Identification of fibroblast heterogeneity in the tumor microenvironment., Cancer Biol. Ther 5 (2006) 1640–1646. http://www.ncbi.nlm.nih.gov/pubmed/17106243 (accessed March 2, 2019). [DOI] [PubMed] [Google Scholar]
- [43].Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H, Cancer-associated fibroblasts: Their characteristics and their roles in tumor growth, Cancers (Basel). 7 (2015) 2443–2458. doi: 10.3390/cancers7040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Radisky DC, Kenny PA, Bissell MJ, Fibrosis and cancer: Do myofibroblasts come also from epithelial cells via EMT?, J. Cell. Biochem 101 (2007) 830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ishii G, Ochiai A, Neri S, Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment, Adv. Drug Deliv. Rev 99 (2016) 186–196. doi: 10.1016/J.ADDR.2015.07.007. [DOI] [PubMed] [Google Scholar]
- [46].Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, Marini F, Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression., PLoS One. 4 (2009) e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Erez N, Truitt M, Olson P, Hanahan D, Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner, Cancer Cell. 17 (2010) 135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- [48].Martinez-Outschoorn UE, Lisanti MP, Sotgia F, Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth, Semin. Cancer Biol 25 (2014) 47–60. doi: 10.1016/j.semcancer.2014.01.005. [DOI] [PubMed] [Google Scholar]
- [49].Rajala MW, Scherer PE, Minireview: The Adipocyte—At the Crossroads of Energy Homeostasis, Inflammation, and Atherosclerosis, Endocrinology. 144 (2003) 3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- [50].Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C, Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion, Cancer Res 71 (2011) 2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- [51].Hao J, Yan F, Zhang Y, Triplett A, Zhang Y, Schultz DA, Sun Y, Zeng J, Silverstein KAT, Zheng Q, Bernlohr DA, Cleary MP, Egilmez NK, Sauter E, Liu S, Suttles J, Li B, Expression of Adipocyte/Macrophage Fatty Acid–Binding Protein in Tumor-Associated Macrophages Promotes Breast Cancer Progression, Cancer Res 78 (2018) 2343–2355. doi: 10.1158/0008-5472.CAN-17-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Corrêa LH, Corrêa R, Farinasso CM, de Sant’Ana Dourado LP, Magalhães KG, Adipocytes and Macrophages Interplay in the Orchestration of Tumor Microenvironment: New Implications in Cancer Progression, Front. Immunol 8 (2017) 1129. doi: 10.3389/fimmu.2017.01129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Arendt LM, McCready J, Keller PJ, Baker DD, Naber SP, Seewaldt V, Kuperwasser C, Obesity Promotes Breast Cancer by CCL2-Mediated Macrophage Recruitment and Angiogenesis, Cancer Res 73 (2013) 6080–6093. doi: 10.1158/0008-5472.CAN-13-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Obesity is associated with macrophage accumulation in adipose tissue, J. Clin. Invest 112 (2003) 1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang Y-Y, Lehuédé C, Laurent V, Dirat B, Dauvillier S, Bochet L, Le Gonidec S, Escourrou G, Valet P, Muller C, Adipose tissue and breast epithelial cells: A dangerous dynamic duo in breast cancer, Cancer Lett 324 (2012) 142–151. doi: 10.1016/j.canlet.2012.05.019. [DOI] [PubMed] [Google Scholar]
- [56].Nieman KM, Romero IL, Van Houten B, Lengyel E, Adipose tissue and adipocytes support tumorigenesis and metastasis, Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1831 (2013) 1533–1541. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hoffman BD, Grashoff C, Schwartz MA, Dynamic molecular processes mediate cellular mechanotransduction., Nature. 475 (2011) 316–323. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jain RK, Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy, Science (80-. ). 307 (2005) 58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- [59].Carmeliet P, Jain RK, Angiogenesis in cancer and other diseases, Nature. 407 (2000) 249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- [60].Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DYR, Chen EI, Lyden D, Bissell MJ, The perivascular niche regulates breast tumour dormancy., Nat. Cell Biol 15 (2013) 807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Maishi N, Hida K, Tumor endothelial cells accelerate tumor metastasis, Cancer Sci 108 (2017) 1921–1926. doi: 10.1111/cas.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG, Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies., Cancer Res 60 (2000) 1388–1393. http://www.ncbi.nlm.nih.gov/pubmed/10728704 (accessed March 5, 2019). [PubMed] [Google Scholar]
- [63].Meng M-B, Zaorsky NG, Deng L, Wang H-H, Chao J, Zhao L-J, Yuan Z-Y, Ping W, Pericytes: a double-edged sword in cancer therapy, Futur. Oncol 11 (2015) 169–179. doi: 10.2217/fon.14.123. [DOI] [PubMed] [Google Scholar]
- [64].DeNardo DG, Coussens LM, Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression, Breast Cancer Res 9 (2007) 212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bruttel VS, Wischhusen J, Cancer Stem Cell Immunology: Key to Understanding Tumorigenesis and Tumor Immune Escape?, Front. Immunol 5 (2014) 360. doi: 10.3389/fimmu.2014.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Janssen LME, Ramsay EE, Logsdon CD, Overwijk WW, The immune system in cancer metastasis: friend or foe?, J. Immunother. Cancer. 5 (2017) 79. doi: 10.1186/s40425-017-0283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Coussens LM, Werb Z, Inflammation and cancer., Nature. 420 (2002) 860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pollard JW, Tumour-educated macrophages promote tumour progression and metastasis, Nat. Rev. Cancer. 4 (2004) 71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- [69].Shitara K, Nishikawa H, Regulatory T cells: a potential target in cancer immunotherapy, Ann. N. Y. Acad. Sci 1417 (2018) 104–115. doi: 10.1111/nyas.13625. [DOI] [PubMed] [Google Scholar]
- [70].Sierko E, Wojtukiewicz MZ, Platelets and angiogenesis in malignancy, Semin. Thromb. Hemost 30 (2004) 95–108. doi: 10.1055/s-2004-822974. [DOI] [PubMed] [Google Scholar]
- [71].Taucher S, Salat A, Gnant M, Kwasny W, Mlineritsch B, Menzel RC, Schmid M, Smola MG, Stierer M, Tausch C, Galid A, Steger G, Jakesz R, Impact of pretreatment thrombocytosis on survival in primary breast cancer, Thromb. Haemost 89 (2003) 1098–1106. doi: 10.1055/s-0037-1613413. [DOI] [PubMed] [Google Scholar]
- [72].Ruggeri ZM, Mendolicchio GL, Adhesion mechanisms in platelet function, Circ. Res 100 (2007) 1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- [73].Hu L, Lee M, Campbell W, Perez-Soler R, Karpatkin S, Role of endogenous thrombin in tumor implantation, seeding, and spontaneous metastasis, Blood. 104 (2004) 2746–2751. doi: 10.1182/blood-2004-03-1047. [DOI] [PubMed] [Google Scholar]
- [74].Farach-Carson MC, Warren CR, Harrington DA, Carson DD, Border patrol: Insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders, Matrix Biol 34 (2014) 64–79. doi: 10.1016/J.MATBIO.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bonnans C, Chou J, Werb Z, Remodelling the extracellular matrix in development and disease, Nat. Rev. Mol. Cell Biol 15 (2014) 786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bahcecioglu G, Hasirci N, Bilgen B, Hasirci V, Hydrogels of agarose, and methacrylated gelatin and hyaluronic acid are more supportive for in vitro meniscus regeneration than three dimensional printed polycaprolactone scaffolds, Int. J. Biol. Macromol 122 (2019) 1152–1162. doi: 10.1016/j.ijbiomac.2018.09.065. [DOI] [PubMed] [Google Scholar]
- [77].Bahcecioglu G, Hasirci N, Hasirci V, Effects of microarchitecture and mechanical properties of 3D microporous PLLA-PLGA scaffolds on fibrochondrocyte and L929 fibroblast behavior, Biomed. Mater 13 (2018) 035005. doi: 10.1088/1748-605X/aaa77f. [DOI] [PubMed] [Google Scholar]
- [78].Bahcecioglu G, Hasirci N, Hasirci V, Cell behavior on the alginate-coated PLLA/PLGA scaffolds, Int. J. Biol. Macromol 124 (2019) 444–450. doi: 10.1016/j.ijbiomac.2018.11.169. [DOI] [PubMed] [Google Scholar]
- [79].Mak KM, Mei R, Basement Membrane Type IV Collagen and Laminin: An Overview of Their Biology and Value as Fibrosis Biomarkers of Liver Disease, Anat. Rec 300 (2017) 1371–1390. doi: 10.1002/ar.23567. [DOI] [PubMed] [Google Scholar]
- [80].Conklin MW, Keely PJ, Why the stroma matters in breast cancer, Cell Adh. Migr 6 (2012) 249–260. doi: 10.4161/cam.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Polyak K, Kalluri R, The Role of the Microenvironment in Mammary Gland Development and Cancer, Cold Spring Harb. Perspect. Biol 2 (2010) a003244–a003244. doi: 10.1101/cshperspect.a003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Arendt LM, Rudnick JA, Keller PJ, Kuperwasser C, Stroma in breast development and disease., Semin. Cell Dev. Biol 21 (2010) 11–8. doi: 10.1016/j.semcdb.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wong SHM, Fang CM, Chuah L-H, Leong CO, Ngai SC, E-cadherin: Its dysregulation in carcinogenesis and clinical implications, Crit. Rev. Oncol. Hematol 121 (2018) 11–22. doi: 10.1016/j.critrevonc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- [84].Horne HN, Sherman ME, Garcia-Closas M, Pharoah PD, Blows FM, Yang XR, Hewitt SM, Conway CM, Lissowska J, Brinton LA, Prokunina-Olsson L, Dawson S-J, Caldas C, Easton DF, Chanock SJ, Figueroa JD, Breast cancer susceptibility risk associations and heterogeneity by E-cadherin tumor tissue expression, Breast Cancer Res. Treat 143 (2014) 181–187. doi: 10.1007/s10549-013-2771-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Akhavan A, Griffith OL, Soroceanu L, Leonoudakis D, Luciani-Torres MG, Daemen A, Gray JW, Muschler JL, Loss of Cell-Surface Laminin Anchoring Promotes Tumor Growth and Is Associated with Poor Clinical Outcomes, Cancer Res 72 (2012) 2578–2588. doi: 10.1158/0008-5472.CAN-11-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Djomehri SI, Burman B, Gonzalez ME, Takayama S, Kleer CG, A reproducible scaffold-free 3D organoid model to study neoplastic progression in breast cancer, J. Cell Commun. Signal 13 (2019) 129–143. doi: 10.1007/s12079-018-0498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Howlett AR, Petersen OW, Steeg PS, Bissell MJ, A novel function for the nm23-H1 gene: overexpression in human breast carcinoma cells leads to the formation of basement membrane and growth arrest., J. Natl. Cancer Inst 86 (1994) 1838–44. http://www.ncbi.nlm.nih.gov/pubmed/7990158 (accessed January 31, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Gudjonsson T, Rønnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW, Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition., J. Cell Sci 115 (2002) 39–50. http://www.ncbi.nlm.nih.gov/pubmed/11801722 (accessed January 31, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kwon S-Y, Chae SW, Wilczynski SP, Arain A, Carpenter PM, Philip M, Laminin 332 expression in breast carcinoma., Appl. Immunohistochem. Mol. Morphol. AIMM. 20 (2012) 159–164. doi: 10.1097/PAI.0b013e3182329e8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Carpenter PM, Ziogas A, Markham EM, Cantillep AS, Yan R, Anton-Culver H, Laminin 332 expression and prognosis in breast cancer, Hum. Pathol 82 (2018) 289–296. doi: 10.1016/j.humpath.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Damiano JS, Integrins as novel drug targets for overcoming innate drug resistance., Curr. Cancer Drug Targets. 2 (2002) 37–43. http://www.ncbi.nlm.nih.gov/pubmed/12188919 (accessed January 31, 2019). [DOI] [PubMed] [Google Scholar]
- [92].Said G, Guilbert M, Morjani H, Garnotel R, Jeannesson P, El Btaouri H, Extracellular Matrix Proteins Modulate Antimigratory and Apoptotic Effects of Doxorubicin, Chemother. Res. Pract 2012 (2012) 1–10. doi: 10.1155/2012/268681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Misra S, Ghatak S, Toole BP, Regulation of MDR1 expression and drug resistance by a positive feedback loop involving hyaluronan, phosphoinositide 3-kinase, and ErbB2., J. Biol. Chem 280 (2005) 20310–20315. doi: 10.1074/jbc.M500737200. [DOI] [PubMed] [Google Scholar]
- [94].Carey SP, Martin KE, Reinhart-King CA, Three-dimensional collagen matrix induces a mechanosensitive invasive epithelial phenotype, Sci. Rep (2017). doi: 10.1038/srep42088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Medici D, Nawshad A, Type I collagen promotes epithelial-mesenchymal transition through ILK-dependent activation of NF-κB and LEF-1, Matrix Biol 29 (2010) 161–165. doi: 10.1016/j.matbio.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Clark AG, Vignjevic DM, Modes of cancer cell invasion and the role of the microenvironment, Curr. Opin. Cell Biol 36 (2015) 13–22. doi: 10.1016/j.ceb.2015.06.004. [DOI] [PubMed] [Google Scholar]
- [97].Conklin MW, Gangnon RE, Sprague BL, Van Gemert L, Hampton JM, Eliceiri KW, Bredfeldt JS, Liu Y, Surachaicharn N, Newcomb PA, Friedl A, Keely PJ, Trentham-Dietz A, Collagen Alignment as a Predictor of Recurrence after Ductal Carcinoma In Situ, Cancer Epidemiol. Biomarkers Prev 27 (2018) 138–145. doi: 10.1158/1055-9965.EPI-17-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Grindel BJ, Martinez JR, Pennington CL, Muldoon M, Stave J, Chung LW, Farach-Carson MC, Matrilysin/matrix metalloproteinase-7(MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior, Matrix Biol 36 (2014) 64–76. doi: 10.1016/J.MATBIO.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Taubenberger AV, Bray LJ, Haller B, Shaposhnykov A, Binner M, Freudenberg U, Guck J, Werner C, 3D extracellular matrix interactions modulate tumour cell growth, invasion and angiogenesis in engineered tumour microenvironments, Acta Biomater (2016). doi: 10.1016/j.actbio.2016.03.017. [DOI] [PubMed] [Google Scholar]
- [100].Singha NC, Nekoroski T, Zhao C, Symons R, Jiang P, Frost GI, Huang Z, Shepard HM, Tumor-Associated Hyaluronan Limits Efficacy of Monoclonal Antibody Therapy, Mol. Cancer Ther 14 (2015) 523–532. doi: 10.1158/1535-7163.MCT-14-0580. [DOI] [PubMed] [Google Scholar]
- [101].Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V, Seluanov A, High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat, Nature. 499 (2013) 346–349. doi: 10.1038/nature12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Cross SE, Jin Y-S, Rao J, Gimzewski JK, Nanomechanical analysis of cells from cancer patients, Nat. Nanotechnol 2 (2007) 780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- [103].Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM, Tensional homeostasis and the malignant phenotype, Cancer Cell. 8 (2005) 241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- [104].Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM, Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling, Cell. 139 (2009) 891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zhang DY, Friedman SL, Fibrosis-dependent mechanisms of hepatocarcinogenesis, Hepatology. 56 (2012) 769–775. doi: 10.1002/hep.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Cornelissen LAM, Van Vliet SJ, A bitter sweet symphony: Immune responses to altered o-glycan epitopes in cancer, Biomolecules. (2016). doi: 10.3390/biom6020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ, Collagen density promotes mammary tumor initiation and progression, BMC Med 6 (2008) 11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Milosevic M, Fyles A, Hedley D, Hill R, The human tumor microenvironment: invasive (needle) measurement of oxygen and interstitial fluid pressure, Semin. Radiat. Oncol 14 (2004) 249–258. doi: 10.1016/J.SEMRADONC.2004.04.006. [DOI] [PubMed] [Google Scholar]
- [109].Stroka KM, Wong BS, Shriver M, Phillip JM, Wirtz D, Kontrogianni-Konstantopoulos A, Konstantopoulos K, Loss of giant obscurins alters breast epithelial cell mechanosensing of matrix stiffness, Oncotarget. 8 (2017) 54004–54020. doi: 10.18632/oncotarget.10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ma X, Liu J, Zhu W, Tang M, Lawrence N, Yu C, Gou M, Chen S, 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling, Adv. Drug Deliv. Rev 132 (2018) 235–251. doi: 10.1016/j.addr.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bordeleau F, Mason BN, Lollis EM, Mazzola M, Zanotelli MR, Somasegar S, Califano JP, Montague C, LaValley DJ, Huynh J, Mencia-Trinchant N, Negrón Abril YL, Hassane DC, Bonassar LJ, Butcher JT, Weiss RS, Reinhart-King CA, Matrix stiffening promotes a tumor vasculature phenotype, Proc. Natl. Acad. Sci (2017). doi: 10.1073/pnas.1613855114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Lam CRI, Wong HK, Nai S, Chua CK, Tan NS, Tan LP, A 3D Biomimetic Model of Tissue Stiffness Interface for Cancer Drug Testing, Mol. Pharm 11 (2014) 2016–2021. doi: 10.1021/mp500059q. [DOI] [PubMed] [Google Scholar]
- [113].Sabeh F, Shimizu-Hirota R, Weiss SJ, Protease-dependent versus - independent cancer cell invasion programs: three-dimensional amoeboid movement revisited, J. Cell Biol 185 (2009) 11–19. doi: 10.1083/JCB.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Paul CD, Mistriotis P, Konstantopoulos K, Cancer cell motility: lessons from migration in confined spaces., Nat. Rev. Cancer. 17 (2017) 131–140. doi: 10.1038/nrc.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Sidani M, Wyckoff J, Xue C, Segall JE, Condeelis J, Probing the Microenvironment of Mammary Tumors Using Multiphoton Microscopy, J. Mammary Gland Biol. Neoplasia. 11 (2006) 151–163. doi: 10.1007/s10911-006-9021-5. [DOI] [PubMed] [Google Scholar]
- [116].Wang WY, Pearson AT, Kutys ML, Choi CK, Wozniak MA, Baker BM, Chen CS, Extracellular matrix alignment dictates the organization of focal adhesions and directs uniaxial cell migration, APL Bioeng (2018). doi: 10.1063/1.5052239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Campbell JJ, Husmann A, Hume RD, Watson CJ, Cameron RE, Development of three-dimensional collagen scaffolds with controlled architecture for cell migration studies using breast cancer cell lines, Biomaterials. 114 (2017) 34–43. doi: 10.1016/j.biomaterials.2016.10.048. [DOI] [PubMed] [Google Scholar]
- [118].Tan PHS, Aung KZ, Toh SL, Goh JCH, Nathan SS, Three-dimensional porous silk tumor constructs in the approximation of in vivo osteosarcoma physiology, Biomaterials. 32 (2011) 6131–6137. doi: 10.1016/J.BIOMATERIALS.2011.04.084. [DOI] [PubMed] [Google Scholar]
- [119].Welter M, Rieger H, Computer Simulations of the Tumor Vasculature: Applications to Interstitial Fluid Flow, Drug Delivery, and Oxygen Supply, in: Adv. Exp. Med. Biol, 2016: pp. 31–72. doi: 10.1007/978-3-319-42023-3_3. [DOI] [PubMed] [Google Scholar]
- [120].Wang M, Liu Y, Cheng Y, Wei Y, Wei X, Immune checkpoint blockade and its combination therapy with small-molecule inhibitors for cancer treatment, Biochim. Biophys. Acta - Rev. Cancer. 1871 (2019) 199–224. doi: 10.1016/j.bbcan.2018.12.002. [DOI] [PubMed] [Google Scholar]
- [121].Fakhrejahani E, Asao Y, Toi M, Tumor Microvasculature Characteristics Studied by Image Analysis: Histologically-Driven Angiogenic Profile, Int. J. Biol. Markers. 29 (2014) 204–207. doi: 10.5301/jbm.5000098. [DOI] [PubMed] [Google Scholar]
- [122].Fakhrejahani E, Toi M, Antiangiogenesis Therapy for Breast Cancer: An Update and Perspectives from Clinical Trials, Jpn. J. Clin. Oncol 44 (2014) 197–207. doi: 10.1093/jjco/hyt201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Viallard C, Larrivée B, Tumor angiogenesis and vascular normalization: alternative therapeutic targets, Angiogenesis. 20 (2017) 409–426. doi: 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- [124].Nagy JA, Chang S-H, Dvorak AM, Dvorak HF, Why are tumour blood vessels abnormal and why is it important to know?, Br. J. Cancer. 100 (2009) 865–869. doi: 10.1038/sj.bjc.6604929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Welter M, Fredrich T, Rinneberg H, Rieger H, Computational Model for Tumor Oxygenation Applied to Clinical Data on Breast Tumor Hemoglobin Concentrations Suggests Vascular Dilatation and Compression, PLoS One. 11 (2016) e0161267. doi: 10.1371/journal.pone.0161267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian Y-M, Lanahan AA, Pollard P, Ruiz de Almodovar C, De Smet F, Vinckier S, Aragonés J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P, Carmeliet P, Heterozygous Deficiency of PHD2 Restores Tumor Oxygenation and Inhibits Metastasis via Endothelial Normalization, Cell. 136 (2009) 839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Marín-Hernández A, Gallardo-Pérez JC, Ralph SJ, Rodríguez-Enríquez S, Moreno-Sánchez R, HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms., Mini Rev. Med. Chem 9 (2009) 1084–101. http://www.ncbi.nlm.nih.gov/pubmed/19689405 (accessed February 15, 2019). [DOI] [PubMed] [Google Scholar]
- [128].Gasparini G, Fanelli M, Boracchi P, Morabito A, Locopo N, Biganzoli E, Behaviour of metastasis in relation to vascular index in patients with node-positive breast cancer treated with adjuvant tamoxifen., Clin. Exp. Metastasis. 18 (2000) 15–20. http://www.ncbi.nlm.nih.gov/pubmed/11206833 (accessed February 15, 2019). [DOI] [PubMed] [Google Scholar]
- [129].Darby IA, Hewitson TD, Hypoxia in tissue repair and fibrosis, Cell Tissue Res 365 (2016) 553–562. doi: 10.1007/s00441-016-2461-3. [DOI] [PubMed] [Google Scholar]
- [130].Kim H, Lin Q, Glazer PM, Yun Z, The hypoxic tumor microenvironment in vivo selects the cancer stem cell fate of breast cancer cells, Breast Cancer Res (2018). doi: 10.1186/s13058-018-0944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Munson J, Shieh A, Interstitial fluid flow in cancer: implications for disease progression and treatment, Cancer Manag. Res 6 (2014) 317–328. doi: 10.2147/CMAR.S65444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Rutkowski JM, Swartz MA, A driving force for change: interstitial flow as a morphoregulator, Trends Cell Biol 17 (2007) 44–50. doi: 10.1016/j.tcb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- [133].Rizvi I, Gurkan UA, Tasoglu S, Alagic N, Celli JP, Mensah LB, Mai Z, Demirci U, Hasan T, Flow induces epithelial-mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules, Proc. Natl. Acad. Sci 110 (2013) E1974–E1983. doi: 10.1073/pnas.1216989110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Grivennikov SI, Greten FR, Karin M, Immunity, Inflammation, and Cancer, Cell. 140 (2010) 883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Zarzynska JM, Two Faces of TGF-Beta1 in Breast Cancer, Mediators Inflamm 2014 (2014) 1–16. doi: 10.1155/2014/141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Esquivel-Velázquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J, The Role of Cytokines in Breast Cancer Development and Progression, J. Interf. Cytokine Res 35 (2015) 1–16. doi: 10.1089/jir.2014.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Gyamfi J, Eom M, Koo J-S, Choi J, Multifaceted Roles of Interleukin-6 in Adipocyte–Breast Cancer Cell Interaction, Transl. Oncol 11 (2018) 275–285. doi: 10.1016/j.tranon.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Sheikhpour E, Noorbakhsh P, Foroughi E, Farahnak S, Nasiri R, Neamatzadeh H, A Survey on the Role of Interleukin-10 in Breast Cancer: A Narrative., Reports Biochem. Mol. Biol 7 (2018) 30–37. http://www.ncbi.nlm.nih.gov/pubmed/30324115 (accessed February 25, 2019). [PMC free article] [PubMed] [Google Scholar]
- [139].Gallina G, Dolcetti L, Serafini P, Santo CD, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V, Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells, J. Clin. Invest. 116 (2006) 2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Serafini P, Borello I, Bronte V, Myeloid suppressor cells in cancer: Recruitment, phenotype, properties, and mechanisms of immune suppression, Semin. Cancer Biol 16 (2006) 53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- [141].Wang Y, Zhou BP, Epithelial-mesenchymal transition in breast cancer progression and metastasis, Chin. J. Cancer 30 (2011) 603–611. doi: 10.5732/cjc.011.10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Lamouille S, Xu J, Derynck R, Molecular mechanisms of epithelial-mesenchymal transition., Nat. Rev. Mol. Cell Biol 15 (2014) 178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Yankaskas CL, Thompson KN, Paul CD, Vitolo MI, Mistriotis P, Mahendra A, Bajpai VK, Shea DJ, Manto KM, Chai AC, Varadarajan N, Kontrogianni-Konstantopoulos A, Martin SS, Konstantopoulos K, A microfluidic assay for the quantification of the metastatic propensity of breast cancer specimens, Nat. Biomed. Eng 3 (2019) 452–465. doi: 10.1038/s41551-019-0400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R, Badve S, Nakshatri H, CD44+/CD24-Breast cancer cells exhibit enhanced invase properties: An early step necessary for metastasis, Breast Cancer Res 8 (2006) R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Tian M, Schiemann WP, TGF-β stimulation of EMT programs elicits non-genomic ER-α activity and anti-estrogen resistance in breast cancer cells, J. Cancer Metastasis Treat 3 (2017) 150–160. doi: 10.20517/2394-4722.2017.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Ampuja M, Alarmo EL, Owens P, Havunen R, Gorska AE, Moses HL, Kallioniemi A, The impact of bone morphogenetic protein 4 (BMP4) on breast cancer metastasis in a mouse xenograft model, Cancer Lett 375 (2016) 238–244. doi: 10.1016/j.canlet.2016.03.008. [DOI] [PubMed] [Google Scholar]
- [147].Truong D, Puleo J, Llave A, Mouneimne G, Kamm RD, Nikkhah M, Breast cancer cell invasion into a three dimensional tumor-stroma microenvironment, Sci. Rep (2016). doi: 10.1038/srep34094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Ali R, Wendt MK, The paradoxical functions of EGFR during breast cancer progression., Signal Transduct. Target. Ther 2 (2017) 16042. doi: 10.1038/sigtrans.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Wagenblast E, Soto M, Gutiérrez-Ángel S, Hartl CA, Gable AL, Maceli AR, Erard N, Williams AM, Kim SY, Dickopf S, Harrell JC, Smith AD, Perou CM, Wilkinson JE, Hannon GJ, Knott SRV, A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis, Nature. 520 (2015) 358–362. doi: 10.1038/nature14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Petkau K, Ferguson M, Guntermann S, Foley E, Constitutive Immune Activity Promotes Tumorigenesis in Drosophila Intestinal Progenitor Cells, Cell Rep 20 (2017) 1784–1793. doi: 10.1016/j.celrep.2017.07.078. [DOI] [PubMed] [Google Scholar]
- [151].Upadhyay S, Sharma N, Gupta KB, Dhiman M, Role of immune system in tumor progression and carcinogenesis, J. Cell. Biochem 119 (2018) 5028–5042. doi: 10.1002/jcb.26663. [DOI] [PubMed] [Google Scholar]
- [152].Gonzalez H, Hagerling C, Werb Z, Roles of the immune system in cancer: from tumor initiation to metastatic progression, Genes Dev 32 (2018) 1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Li R, Hebert JD, Lee TA, Xing H, Boussommier-Calleja A, Hynes RO, Lauffenburger DA, Kamm RD, Macrophage-Secreted TNFα and TGFβ1 Influence Migration Speed and Persistence of Cancer Cells in 3D Tissue Culture via Independent Pathways, Cancer Res 77 (2017) 279–290. doi: 10.1158/0008-5472.CAN-16-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Li X, Yang Q, Yu H, Wu L, Zhao Y, Zhang C, Yue X, Liu Z, Wu H, Haffty BG, Feng Z, Hu W, LIF promotes tumorigenesis and metastasis of breast cancer through the AKT-mTOR pathway, Oncotarget. 5 (2014) 788–801. doi: 10.18632/oncotarget.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Bent R, Moll L, Grabbe S, Bros M, Interleukin-1 Beta—A Friend or Foe in Malignancies?, Int. J. Mol. Sci 19 (2018) 2155. doi: 10.3390/ijms19082155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Boghaert ER, Lu X, Hessler PE, McGonigal TP, Oleksijew A, Mitten MJ, Foster-Duke K, Hickson JA, Santo VE, Brito C, Uziel T, Vaidya KS, The Volume of Three-Dimensional Cultures of Cancer Cells In Vitro Influences Transcriptional Profile Differences and Similarities with Monolayer Cultures and Xenografted Tumors, Neoplasia (United States). 19 (2017) 695–706. doi: 10.1016/j.neo.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Katt ME, Placone AL, Wong AD, Xu ZS, Searson PC, In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform, Front. Bioeng. Biotechnol (2016). doi: 10.3389/fbioe.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Asghar W, El Assal R, Shafiee H, Pitteri S, Paulmurugan R, Demirci U, Engineering cancer microenvironments for in vitro 3-D tumor models, Mater. Today. 18 (2015) 539–553. doi: 10.1016/j.mattod.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Dobrolecki LE, Airhart SD, Alferez DG, Aparicio S, Behbod F, Bentires-Alj M, Brisken C, Bult CJ, Cai S, Clarke RB, Dowst H, Ellis MJ, Gonzalez-Suarez E, Iggo RD, Kabos P, Li S, Lindeman GJ, Marangoni E, McCoy A, Meric-Bernstam F, Piwnica-Worms H, Poupon M-F, Reis-Filho J, Sartorius CA, Scabia V, Sflomos G, Tu Y, Vaillant F, Visvader JE, Welm A, Wicha MS, Lewis MT, Patient-derived xenograft (PDX) models in basic and translational breast cancer research, Cancer Metastasis Rev 35 (2016) 547–573. doi: 10.1007/s10555-016-9653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Davis NM, Sokolosky M, Stadelman K, Abrams SL, Libra M, Candido S, Nicoletti F, Polesel J, Maestro R, D’Assoro A, Drobot L, Rakus D, Gizak A, Laidler P, Dulińska-Litewka J, Basecke J, Mijatovic S, Maksimovic-Ivanic D, Montalto G, Cervello M, Fitzgerald TL, Demidenko ZN, Martelli AM, Cocco L, Steelman LS, McCubrey JA, Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: possibilities for therapeutic intervention, Oncotarget. 5 (2014) 4603–4650. doi: 10.18632/oncotarget.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Holen I, Speirs V, Morrissey B, Blyth K, In vivo models in breast cancer research: progress, challenges and future directions, Dis. Model. Mech 10 (2017) 359–371. doi: 10.1242/dmm.028274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Peng F, Li T-T, Wang K-L, Xiao G-Q, Wang J-H, Zhao H-D, Kang Z-J, Fan W-J, Zhu L-L, Li M, Cui B, Zheng F-M, Wang H-J, Lam EW-F, Wang B, Xu J, Liu Q, H19/let-7/LIN28 reciprocal negative regulatory circuit promotes breast cancer stem cell maintenance, Cell Death Dis 8 (2017) e2569–e2569. doi: 10.1038/cddis.2016.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Xu L-Z, Li S-S, Zhou W, Kang Z-J, Zhang Q-X, Kamran M, Xu J, Liang D-P, Wang C-L, Hou Z-J, Wan X-B, Wang H-J, Lam EW-F, Zhao Z-W, Liu Q, p62/SQSTM1 enhances breast cancer stem-like properties by stabilizing MYC mRNA, Oncogene. 36 (2017) 304–317. doi: 10.1038/onc.2016.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Gao S, Ge A, Xu S, You Z, Ning S, Zhao Y, Pang D, PSAT1 is regulated by ATF4 and enhances cell proliferation via the GSK3β/β-catenin/cyclin D1 signaling pathway in ER-negative breast cancer, J. Exp. Clin. Cancer Res 36 (2017) 179. doi: 10.1186/s13046-017-0648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Moore KM, Thomas GJ, Duffy SW, Warwick J, Gabe R, Chou P, Ellis IO, Green AR, Haider S, Brouilette K, Saha A, Vallath S, Bowen R, Chelala C, Eccles D, Tapper WJ, Thompson AM, Quinlan P, Jordan L, Gillett C, Brentnall A, Violette S, Weinreb PH, Kendrew J, Barry ST, Hart IR, Jones JL, Marshall JF, Therapeutic Targeting of Integrin αvβ6 in Breast Cancer, JNCI J. Natl. Cancer Inst 106 (2014) dju169. doi: 10.1093/jnci/dju169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].De Francesco E, Maggiolini M, Musti A, Crosstalk between Notch, HIF-1α and GPER in Breast Cancer EMT, Int. J. Mol. Sci 19 (2018) 2011. doi: 10.3390/ijms19072011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Holliday DL, Speirs V, Choosing the right cell line for breast cancer research, Breast Cancer Res 13 (2011) 215. doi: 10.1186/bcr2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Kuperwasser C, Dessain S, Bierbaum BE, Garnet D, Sperandio K, Gauvin GP, Naber SP, Weinberg RA, Rosenblatt M, A Mouse Model of Human Breast Cancer Metastasis to Human Bone, Cancer Res 65 (2005) 6130–6138. doi: 10.1158/0008-5472.CAN-04-1408. [DOI] [PubMed] [Google Scholar]
- [169].Jacobsen BM, Harrell JC, Jedlicka P, Borges VF, Varella-Garcia M, Horwitz KB, Spontaneous Fusion with, and Transformation of Mouse Stroma by, Malignant Human Breast Cancer Epithelium, Cancer Res 66 (2006) 8274–8279. doi: 10.1158/0008-5472.CAN-06-1456. [DOI] [PubMed] [Google Scholar]
- [170].Gengenbacher N, Singhal M, Augustin HG, Preclinical mouse solid tumour models: status quo, challenges and perspectives, Nat. Rev. Cancer. 17 (2017) 751–765. doi: 10.1038/nrc.2017.92. [DOI] [PubMed] [Google Scholar]
- [171].Manning HC, Buck JR, Cook RS, Mouse Models of Breast Cancer: Platforms for Discovering Precision Imaging Diagnostics and Future Cancer Medicine, J. Nucl. Med 57 (2016) 60S–68S. doi: 10.2967/jnumed.115.157917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Arrigoni C, Bersini S, Gilardi M, Moretti M, In vitro co-culture models of breast cancer metastatic progression towards bone, Int. J. Mol. Sci 17 (2016) 1405. doi: 10.3390/ijms17091405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Day C-P, Carter J, Bonomi C, Hollingshead M, Merlino G, Preclinical therapeutic response of residual metastatic disease is distinct from its primary tumor of origin, Int. J. Cancer. 130 (2012) 190–199. doi: 10.1002/ijc.25978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Voskoglou-Nomikos T, Pater JL, Seymour L, Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models, in: Clin. Cancer Res, 2003. doi: 10.1111/j.1532-5415.2011.03779.x. [DOI] [PubMed] [Google Scholar]
- [175].Li Z, Wang Y, Dong S, Ge C, Xiao Y, Li R, Ma X, Xue Y, Zhang Q, Lv J, Tan Q, Zhu Z, Song X, Tan J, Association of CXCR1 and 2 expressions with gastric cancer metastasis in ex vivo and tumor cell invasion in vitro, Cytokine. 69 (2014) 6–13. doi: 10.1016/j.cyto.2014.05.004. [DOI] [PubMed] [Google Scholar]
- [176].Carvalho MR, Lima D, Reis RL, Correlo VM, Oliveira JM, Evaluating Biomaterial- and Microfluidic-Based 3D Tumor Models, Trends Biotechnol 33 (2015) 667–678. doi: 10.1016/j.tibtech.2015.09.009. [DOI] [PubMed] [Google Scholar]
- [177].Centenera MM, Hickey TE, Jindal S, Ryan NK, Ravindranathan P, Mohammed H, Robinson JL, Schiewer MJ, Ma S, Kapur P, Sutherland PD, Hoffmann CE, Roehrborn CG, Gomella LG, Carroll JS, Birrell SN, Knudsen KE, Raj GV, Butler LM, Tilley WD, A patient-derived explant (PDE) model of hormone-dependent cancer, Mol. Oncol 12 (2018) 1608–1622. doi: 10.1002/1878-0261.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].DeRose YS, Wang G, Lin Y-C, Bernard PS, Buys SS, Ebbert MTW, Factor R, Matsen C, Milash BA, Nelson E, Neumayer L, Randall RL, Stijleman IJ, Welm BE, Welm AL, Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes, Nat. Med 17 (2011) 1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Bruna A, Rueda OM, Greenwood W, Batra AS, Callari M, Batra RN, Pogrebniak K, Sandoval J, Cassidy JW, Tufegdzic-Vidakovic A, Sammut S-J, Jones L, Provenzano E, Baird R, Eirew P, Hadfield J, Eldridge M, McLaren-Douglas A, Barthorpe A, Lightfoot H, O’Connor MJ, Gray J, Cortes J, Baselga J, Marangoni E, Welm AL, Aparicio S, Serra V, Garnett MJ, Caldas C, A Biobank of Breast Cancer Explants with Preserved Intra-tumor Heterogeneity to Screen Anticancer Compounds, Cell. 167 (2016) 260–274. doi: 10.1016/j.cell.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Jardé T, Lloyd-Lewis B, Thomas M, Kendrick H, Melchor L, Bougaret L, Watson PD, Ewan K, Smalley MJ, Dale TC, Wnt and Neuregulin1/ErbB signalling extends 3D culture of hormone responsive mammary organoids, Nat. Commun 7 (2016) 13207. doi: 10.1038/ncomms13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Yamada KM, Cukierman E, Modeling Tissue Morphogenesis and Cancer in 3D, Cell. 130 (2007) 601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- [182].Shologu N, Szegezdi E, Lowery A, Kerin M, Pandit A, Zeugolis DI, Recreating complex pathophysiologies in vitro with extracellular matrix surrogates for anticancer therapeutics screening, Drug Discov. Today. 21 (2016) 1521–1531. doi: 10.1016/j.drudis.2016.06.001. [DOI] [PubMed] [Google Scholar]
- [183].Estrada MF, Rebelo SP, Davies EJ, Pinto MT, Pereira H, Santo VE, Smalley MJ, Barry ST, Gualda EJ, Alves PM, Anderson E, Brito C, Modelling the tumour microenvironment in long-term microencapsulated 3D co-cultures recapitulates phenotypic features of disease progression, Biomaterials. 78 (2016) 50–61. doi: 10.1016/j.biomaterials.2015.11.030. [DOI] [PubMed] [Google Scholar]
- [184].Ham SL, Thakuri PS, Plaster M, Li J, Luker KE, Luker GD, Tavana H, Three-dimensional tumor model mimics stromal - breast cancer cells signaling, Oncotarget. 9 (2018) 249–267. doi: 10.18632/oncotarget.22922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Ham SL, Joshi R, Luker GD, Tavana H, Engineered Breast Cancer Cell Spheroids Reproduce Biologic Properties of Solid Tumors, Adv. Healthc. Mater 5 (2016) 2788–2798. doi: 10.1002/adhm.201600644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Duval K, Grover H, Han L-H, Mou Y, Pegoraro AF, Fredberg J, Chen Z, Modeling Physiological Events in 2D vs. 3D Cell Culture, Physiology. 32 (2017) 266–277. doi: 10.1152/physiol.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- [187].Edmondson R, Broglie JJ, Adcock AF, Yang L, Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors, Assay Drug Dev. Technol 12 (2014) 207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Acun A, Zorlutuna P, Engineered myocardium model to study the roles of HIF-1α and HIF1A-AS1 in paracrine-only signaling under pathological level oxidative stress, Acta Biomater 58 (2017) 323–336. doi: 10.1016/j.actbio.2017.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Mabry KM, Payne SZ, Anseth KS, Microarray analyses to quantify advantages of 2D and 3D hydrogel culture systems in maintaining the native valvular interstitial cell phenotype, Biomaterials. 74 (2016) 31–41. doi: 10.1016/j.biomaterials.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Kolind K, Leong KW, Besenbacher F, Foss M, Guidance of stem cell fate on 2D patterned surfaces, Biomaterials. 33 (2012) 6626–6633. doi: 10.1016/j.biomaterials.2012.05.070. [DOI] [PubMed] [Google Scholar]
- [191].Ellem SJ, De-Juan-Pardo EM, Risbridger GP, In vitro modeling of the prostate cancer microenvironment, Adv. Drug Deliv. Rev 79–80 (2014) 214–221. doi: 10.1016/j.addr.2014.04.008. [DOI] [PubMed] [Google Scholar]
- [192].Luca AC, Mersch S, Deenen R, Schmidt S, Messner I, Schäfer K-L, Baldus SE, Huckenbeck W, Piekorz RP, Knoefel WT, Krieg A, Stoecklein NH, Impact of the 3D Microenvironment on Phenotype, Gene Expression, and EGFR Inhibition of Colorectal Cancer Cell Lines, PLoS One. 8 (2013) e59689. doi: 10.1371/journal.pone.0059689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Bellis SL, Variant glycosylation: an underappreciated regulatory mechanism for β1 integrins, Biochim. Biophys. Acta - Biomembr 1663 (2004) 52–60. doi: 10.1016/j.bbamem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- [194].Benton G, George J, Kleinman HK, Arnaoutova IP, Advancing science and technology via 3D culture on basement membrane matrix, J. Cell. Physiol 221 (2009) 18–25. doi: 10.1002/jcp.21832. [DOI] [PubMed] [Google Scholar]
- [195].Imamura Y, Mukohara T, Shimono Y, Funakoshi Y, Chayahara N, Toyoda M, Kiyota N, Takao S, Kono S, Nakatsura T, Minami H, Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer, Oncol. Rep 33 (2015) 1837–1843. doi: 10.3892/or.2015.3767. [DOI] [PubMed] [Google Scholar]
- [196].Breslin S, O’Driscoll L, The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance, Oncotarget. (2016). doi: 10.18632/oncotarget.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Lovitt CJ, Shelper TB, Avery VM, Evaluation of chemotherapeutics in a three-dimensional breast cancer model, J. Cancer Res. Clin. Oncol (2015). doi: 10.1007/s00432-015-1950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Falkenberg N, Höfig I, Rosemann M, Szumielewski J, Richter S, Schorpp K, Hadian K, Aubele M, Atkinson MJ, Anastasov N, Three-dimensional microtissues essentially contribute to preclinical validations of therapeutic targets in breast cancer, Cancer Med (2016). doi: 10.1002/cam4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Tung Y-C, Hsiao AY, Allen SG, Torisawa Y, Ho M, Takayama S, High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array, Analyst. 136 (2011) 473–478. doi: 10.1039/c0an00609b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Stock K, Estrada MF, Vidic S, Gjerde K, Rudisch A, Santo VE, Barbier M, Blom S, Arundkar SC, Selvam I, Osswald A, Stein Y, Gruenewald S, Brito C, Van Weerden W, Rotter V, Boghaert E, Oren M, Sommergruber W, Chong Y, De Hoogt R, Graeser R, Capturing tumor complexity in vitro: Comparative analysis of 2D and 3D tumor models for drug discovery, Sci. Rep 6 (2016) 28951. doi: 10.1038/srep28951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA, Spheroid-based drug screen: considerations and practical approach, Nat. Protoc 4 (2009) 309–324. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- [202].V LaBarbera D, Reid BG, Yoo BH, The multicellular tumor spheroid model for high-throughput cancer drug discovery, Expert Opin. Drug Discov 7 (2012) 819–830. doi: 10.1517/17460441.2012.708334. [DOI] [PubMed] [Google Scholar]
- [203].Hsiao AY, Tung Y-C, Kuo C-H, Mosadegh B, Bedenis R, Pienta KJ, Takayama S, Micro-ring structures stabilize microdroplets to enable long term spheroid culture in 384 hanging drop array plates, Biomed. Microdevices. 14 (2012) 313–323. doi: 10.1007/s10544-011-9608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Lin RZ, Chang HY, Recent advances in three-dimensional multicellular spheroid culture for biomedical research, Biotechnol. J (2008). doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- [205].Metzger W, Sossong D, Bächle A, Pütz N, Wennemuth G, Pohlemann T, Oberringer M, The liquid overlay technique is the key to formation of co-culture spheroids consisting of primary osteoblasts, fibroblasts and endothelial cells, Cytotherapy. 13 (2011) 1000–1012. doi: 10.3109/14653249.2011.583233. [DOI] [PubMed] [Google Scholar]
- [206].Hribar KC, Finlay D, Ma X, Qu X, Ondeck MG, Chung PH, Zanella F, Engler AJ, Sheikh F, Vuori K, Chen SC, Nonlinear 3D projection printing of concave hydrogel microstructures for long-term multicellular spheroid and embryoid body culture., Lab Chip. 15 (2015) 2412–8. doi: 10.1039/c5lc00159e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Tasoglu S, Yu CH, Gungordu HI, Guven S, Vural T, Demirci U, Guided and magnetic self-assembly of tunable magnetoceptive gels, Nat. Commun 5 (2014) 4702. doi: 10.1038/ncomms5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Eckhardt BL, Gagliardi M, Iles LK, Evans K, Ivan C, Liu X, Liu CG, Souza G, Rao A, Meric-Bernstam F, Ueno NT, Bartholomeusz GA, Clinically relevant inflammatory breast cancer patient-derived xenograft–derived ex vivo model for evaluation of tumor-specific therapies, PLoS One. 13 (2018) e0195932. doi: 10.1371/journal.pone.0195932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Swaminathan S, Hamid Q, Sun W, Clyne AM, Bioprinting of 3D breast epithelial spheroids for human cancer models, Biofabrication. 11 (2019) 025003. doi: 10.1088/1758-5090/aafc49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Gottfried E, Kunz-Schughart LA, Andreesen R, Kreutz M, Brave Little World: Spheroids as an in vitro Model to Study Tumor-Immune-Cell Interactions, Cell Cycle. 5 (2006) 691–695. doi: 10.4161/cc.5.7.2624. [DOI] [PubMed] [Google Scholar]
- [211].Singh M, Mukundan S, Jaramillo M, Oesterreich S, Sant S, Three-dimensional breast cancer models mimic hallmarks of size-induced tumor progression, Cancer Res (2016). doi: 10.1158/0008-5472.CAN-15-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Carver K, Ming X, Juliano RL, Multicellular tumor spheroids as a model for assessing delivery of oligonucleotides in three dimensions, Mol. Ther. - Nucleic Acids. (2014). doi: 10.1038/mtna.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Langer R, Vacanti JP, Tissue engineering., Science. 260 (1993) 920–926. http://www.ncbi.nlm.nih.gov/pubmed/8493529 (accessed March 10, 2019). [DOI] [PubMed] [Google Scholar]
- [214].Keane TJ, Badylak SF, Biomaterials for tissue engineering applications, Semin. Pediatr. Surg 23 (2014) 112–118. doi: 10.1053/j.sempedsurg.2014.06.010. [DOI] [PubMed] [Google Scholar]
- [215].Zhu J, Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering, Biomaterials. 31 (2010) 4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Hasirci N, Kilic C, Kömez A, Bahcecioglu G, Hasirci V, Hydrogels in Regenerative Medicine, in: Demirci U, Khademhossini A (Eds.), Gels Handb. Fundam. Prop. Appl, World Scientific, Singapour, 2016: pp. 1–52. doi: 10.1142/9789813140394_0001. [DOI] [Google Scholar]
- [217].Liu Y, Chan-Park MB, A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture, Biomaterials. 31 (2010) 1158–1170. doi: 10.1016/j.biomaterials.2009.10.040. [DOI] [PubMed] [Google Scholar]
- [218].Lang NR, Skodzek K, Hurst S, Mainka A, Steinwachs J, Schneider J, Aifantis KE, Fabry B, Biphasic response of cell invasion to matrix stiffness in three-dimensional biopolymer networks, Acta Biomater 13 (2015) 61–67. doi: 10.1016/j.actbio.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Riching KM, Cox BL, Salick MR, Pehlke C, Riching AS, Ponik SM, Bass BR, Crone WC, Jiang Y, Weaver AM, Eliceiri KW, Keely PJ, 3D Collagen Alignment Limits Protrusions to Enhance Breast Cancer Cell Persistence, Biophys. J 107 (2014) 2546–2558. doi: 10.1016/j.bpj.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Carter EP, Gopsill JA, Gomm JJ, Jones JL, Grose RP, A 3D in vitro model of the human breast duct: A method to unravel myoepithelial-luminal interactions in the progression of breast cancer, Breast Cancer Res (2017). doi: 10.1186/s13058-017-0843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Linde N, Gutschalk CM, Hoffmann C, Yilmaz D, Mueller MM, Integrating Macrophages into Organotypic Co-Cultures: A 3D In Vitro Model to Study Tumor-Associated Macrophages, PLoS One. 7 (2012) e40058. doi: 10.1371/journal.pone.0040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, Liu IH, Chiou S-H, Salahudeen AA, Smith AR, Deutsch BC, Liao L, Zemek AJ, Zhao F, Karlsson K, Schultz LM, Metzner TJ, Nadauld LD, Tseng Y-Y, Alkhairy S, Oh C, Keskula P, Mendoza-Villanueva D, De La Vega FM, Kunz PL, Liao JC, Leppert JT, Sunwoo JB, Sabatti C, Boehm JS, Hahn WC, Zheng GXY, Davis MM, Kuo CJ, Organoid Modeling of the Tumor Immune Microenvironment, Cell. 175 (2018) 1972–1988.e16. doi: 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Brancato V, Gioiella F, Imparato G, Guarnieri D, Urciuolo F, Netti PA, 3D breast cancer microtissue reveals the role of tumor microenvironment on the transport and efficacy of free-doxorubicin in vitro, Acta Biomater. (2018). doi: 10.1016/j.actbio.2018.05.055. [DOI] [PubMed] [Google Scholar]
- [224].Yang Y, Yang X, Zou J, Jia C, Hu Y, Du H, Wang H, Evaluation of photodynamic therapy efficiency using an in vitro three-dimensional microfluidic breast cancer tissue model, Lab Chip. (2015). doi: 10.1039/c4lc01065e. [DOI] [PubMed] [Google Scholar]
- [225].Casey J, Yue X, Nguyen TD, Acun A, Zellmer VR, Zhang S, Zorlutuna P, 3D hydrogel-based microwell arrays as a tumor microenvironment model to study breast cancer growth, Biomed. Mater 12 (2017) 025009. doi: 10.1088/1748-605X/aa5d5c. [DOI] [PubMed] [Google Scholar]
- [226].Yue X, Nguyen TD, Zellmer V, Zhang S, Zorlutuna P, Stromal cell-laden 3D hydrogel microwell arrays as tumor microenvironment model for studying stiffness dependent stromal cell-cancer interactions, Biomaterials. (2018). doi: 10.1016/j.biomaterials.2018.04.001. [DOI] [PubMed] [Google Scholar]
- [227].Suo A, Xu W, Wang Y, Sun T, Ji L, Qian J, Dual-degradable and injectable hyaluronic acid hydrogel mimicking extracellular matrix for 3D culture of breast cancer MCF-7 cells, Carbohydr. Polym (2019). doi: 10.1016/J.CARBPOL.2019.01.115. [DOI] [PubMed] [Google Scholar]
- [228].Fisher SA, Tam RY, Fokina A, Mahmoodi MM, Distefano MD, Shoichet MS, Photo-immobilized EGF chemical gradients differentially impact breast cancer cell invasion and drug response in defined 3D hydrogels, Biomaterials. 178 (2018) 751–766. doi: 10.1016/j.biomaterials.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Wang Y, Mirza S, Wu S, Zeng J, Shi W, Band H, Band V, Duan B, 3D hydrogel breast cancer models for studying the effects of hypoxia on epithelial to mesenchymal transition, 2018. www.oncotarget.comwww.oncotarget.com. [DOI] [PMC free article] [PubMed]
- [230].Read GH, Miura N, Carter JL, Kines KT, Yamamoto K, Devasahayam N, Cheng JY, Camphausen KA, Krishna MC, Kesarwala AH, Three-dimensional alginate hydrogels for radiobiological and metabolic studies of cancer cells, Colloids Surfaces B Biointerfaces. 171 (2018) 197–204. doi: 10.1016/j.colsurfb.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Liu C, Lewin Mejia D, Chiang B, Luker KE, Luker GD, Hybrid collagen alginate hydrogel as a platform for 3D tumor spheroid invasion, Acta Biomater 75 (2018) 213–225. doi: 10.1016/j.actbio.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Phan-Lai V, Florczyk SJ, Kievit FM, Wang K, Gad E, Disis ML, Zhang M, Three-Dimensional Scaffolds to Evaluate Tumor Associated Fibroblast-Mediated Suppression of Breast Tumor Specific T Cells, Biomacromolecules. 14 (2013) 1330–1337. doi: 10.1021/bm301928u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Bray LJ, Binner M, Holzheu A, Friedrichs J, Freudenberg U, Hutmacher DW, Werner C, Multi-parametric hydrogels support 3D invitro bioengineered microenvironment models of tumour angiogenesis, Biomaterials. (2015). doi: 10.1016/j.biomaterials.2015.02.124. [DOI] [PubMed] [Google Scholar]
- [234].Chwalek K, Tsurkan MV, Freudenberg U, Werner C, Glycosaminoglycan-based hydrogels to modulate heterocellular communication in in vitro angiogenesis models, Sci. Rep 4 (2015) 4414. doi: 10.1038/srep04414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR, Basement membrane complexes with biological activity., Biochemistry. 25 (1986) 312–318. http://www.ncbi.nlm.nih.gov/pubmed/2937447 (accessed February 14, 2019). [DOI] [PubMed] [Google Scholar]
- [236].Benton G, Arnaoutova I, George J, Kleinman HK, Koblinski J, Matrigel: From discovery and ECM mimicry to assays and models for cancer research, Adv. Drug Deliv. Rev (2014). doi: 10.1016/j.addr.2014.06.005. [DOI] [PubMed] [Google Scholar]
- [237].Gaiko-Shcherbak A, Fabris G, Dreissen G, Merkel R, Hoffmann B, Noetzel E, The Acinar Cage: Basement Membranes Determine Molecule Exchange and Mechanical Stability of Human Breast Cell Acini, PLoS One. 10 (2015) e0145174. doi: 10.1371/journal.pone.0145174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Ellis BW, Acun A, Can UI, Zorlutuna P, Human iPSC-derived myocardium-on-chip with capillary-like flow for personalized medicine, Biomicrofluidics. 11 (2017) 024105. doi: 10.1063/1.4978468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Acun A, Zorlutuna P, CRISPR/Cas9 edited hiPSC-based vascular tissues to model aging and disease-dependent impairment, Tissue Eng. Part A. (2019) In press. doi: 10.1089/ten.TEA.2018.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L, Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression., Cancer Res 61 (2001) 1320–1326. http://www.ncbi.nlm.nih.gov/pubmed/11245428 (accessed February 14, 2019). [PubMed] [Google Scholar]
- [241].Chimal-Ramírez GK, Espinoza-Sánchez NA, Utrera-Barillas D, Benítez-Bribiesca L, Velázquez JR, Arriaga-Pizano LA, Monroy-García A, Reyes-Maldonado E, Domínguez-López ML, Piña-Sánchez P, Fuentes-Pananá EM, MMP1, MMP9, and COX2 Expressions in Promonocytes Are Induced by Breast Cancer Cells and Correlate with Collagen Degradation, Transformation-Like Morphological Changes in MCF-10A Acini, and Tumor Aggressiveness, Biomed Res. Int 2013 (2013) 279505. doi: 10.1155/2013/279505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Knott SRV, Wagenblast E, Khan S, Kim SY, Soto M, Wagner M, Turgeon MO, Fish L, Erard N, Gable AL, MacEli AR, DIckopf S, Papachristou EK, D’Santos CS, Carey LA, Wilkinson JE, Harrell JC, Perou CM, Goodarzi H, Poulogiannis G, Hannon GJ, Asparagine bioavailability governs metastasis in a model of breast cancer, Nature. (2018). doi: 10.1038/nature25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Stowers RS, Allen SC, Sanchez K, Davis CL, Ebelt ND, Van Den Berg C, Suggs LJ, Extracellular Matrix Stiffening Induces a Malignant Phenotypic Transition in Breast Epithelial Cells, Cell. Mol. Bioeng 10 (2017) 114–123. doi: 10.1007/s12195-016-0468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Cavo M, Caria M, Pulsoni I, Beltrame F, Fato M, Scaglione S, A new cell-laden 3D Alginate-Matrigel hydrogel resembles human breast cancer cell malignant morphology, spread and invasion capability observed “in vivo,” Sci. Rep (2018). doi: 10.1038/s41598-018-23250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Dunne LW, Huang Z, Meng W, Fan X, Zhang N, Zhang Q, An Z, Human decellularized adipose tissue scaffold as a model for breast cancer cell growth and drug treatments, Biomaterials. 35 (2014) 4940–4949. doi: 10.1016/j.biomaterials.2014.03.003. [DOI] [PubMed] [Google Scholar]
- [246].Li W, Hu X, Yang S, Wang S, Zhang C, Wang H, Cheng YY, Wang Y, Liu T, Song K, A novel tissue-engineered 3D tumor model for anti-cancer drug discovery, Biofabrication. 11 (2018) 015004. doi: 10.1088/1758-5090/aae270. [DOI] [PubMed] [Google Scholar]
- [247].Crapo PM, Gilbert TW, Badylak SF, An overview of tissue and whole organ decellularization processes, Biomaterials. 32 (2011) 3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Janani G, Pillai MM, Selvakumar R, Bhattacharyya A, Sabarinath C, An in vitro 3D model using collagen coated gelatin nanofibers for studying breast cancer metastasis, Biofabrication. 9 (2017) 015016. doi: 10.1088/1758-5090/aa5510. [DOI] [PubMed] [Google Scholar]
- [249].Subia B, Dey T, Sharma S, Kundu SC, Target specific delivery of anticancer drug in silk fibroin based 3D distribution model of bone-breast cancer cells, ACS Appl. Mater. Interfaces. (2015). doi: 10.1021/am506094c. [DOI] [PubMed] [Google Scholar]
- [250].Angeloni V, Contessi N, De Marco C, Bertoldi S, Tanzi MC, Daidone MG, Farè S, Polyurethane foam scaffold as in vitro model for breast cancer bone metastasis, Acta Biomater 63 (2017) 306–316. doi: 10.1016/j.actbio.2017.09.017. [DOI] [PubMed] [Google Scholar]
- [251].Kar S, Molla MDS, Katti DR, Katti KS, Tissue-engineered nanoclay based 3D in vitro breast cancer model for studying breast cancer metastasis to bone, J. Tissue Eng. Regen. Med 13 (2019) 119–130. doi: 10.1002/term.2773. [DOI] [PubMed] [Google Scholar]
- [252].Knowlton S, Onal S, Yu CH, Zhao JJ, Tasoglu S, Bioprinting for cancer research, Trends Biotechnol 33 (2015) 504–513. doi: 10.1016/j.tibtech.2015.06.007. [DOI] [PubMed] [Google Scholar]
- [253].Belgodere JA, King CT, Bursavich JB, Burow ME, Martin EC, Jung JP, Engineering Breast Cancer Microenvironments and 3D Bioprinting, Front. Bioeng. Biotechnol 6 (2018) 66. doi: 10.3389/fbioe.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Peng W, Unutmaz D, Ozbolat IT, Bioprinting towards Physiologically Relevant Tissue Models for Pharmaceutics, Trends Biotechnol 34 (2016) 722–732. doi: 10.1016/j.tibtech.2016.05.013. [DOI] [PubMed] [Google Scholar]
- [255].Wang Y, Shi W, Kuss M, Mirza S, Qi D, Krasnoslobodtsev A, Zeng J, Band H, Band V, Duan B, 3D Bioprinting of Breast Cancer Models for Drug Resistance Study, ACS Biomater. Sci. Eng 4 (2018) 4401–4411. doi: 10.1021/acsbiomaterials.8b01277. [DOI] [PubMed] [Google Scholar]
- [256].Bahcecioglu G, Hasirci N, Bilgen B, Hasirci V, A 3D printed PCL/hydrogel construct with zone-specific biochemical composition mimicking that of the meniscus, Biofabrication. 11 (2019) 025002. doi: 10.1088/1758-5090/aaf707. [DOI] [PubMed] [Google Scholar]
- [257].Bahcecioglu G, Bilgen B, Hasirci N, Hasirci V, Anatomical meniscus construct with zone specific biochemical composition and structural organization, Biomaterials. 218 (2019) 119361. doi: 10.1016/j.biomaterials.2019.119361. [DOI] [PubMed] [Google Scholar]
- [258].Reid JA, Mollica PA, Bruno RD, Sachs PC, Consistent and reproducible cultures of large-scale 3D mammary epithelial structures using an accessible bioprinting platform, Breast Cancer Res 20 (2018) 122. doi: 10.1186/s13058-018-1045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Ling K, Huang G, Liu J, Zhang X, Ma Y, Lu T, Xu F, Bioprinting-Based High-Throughput Fabrication of Three-Dimensional MCF-7 Human Breast Cancer Cellular Spheroids, Engineering. 1 (2015) 269–274. doi: 10.15302/J-ENG-2015062. [DOI] [Google Scholar]
- [260].Grolman JM, Zhang D, Smith AM, Moore JS, Kilian KA, Rapid 3D Extrusion of Synthetic Tumor Microenvironments, Adv. Mater 27 (2015) 5512–5517. doi: 10.1002/adma.201501729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Zhu W, Holmes B, Glazer RI, Zhang LG, 3D printed nanocomposite matrix for the study of breast cancer bone metastasis, Nanomedicine Nanotechnology, Biol. Med 12 (2016) 69–79. doi: 10.1016/j.nano.2015.09.010. [DOI] [PubMed] [Google Scholar]
- [262].Zhou X, Zhu W, Nowicki M, Miao S, Cui H, Holmes B, Glazer RI, Zhang LG, 3D Bioprinting a Cell-Laden Bone Matrix for Breast Cancer Metastasis Study, ACS Appl. Mater. Interfaces 8 (2016) 30017–30026. doi: 10.1021/acsami.6b10673. [DOI] [PubMed] [Google Scholar]
- [263].Thibaudeau L, Taubenberger AV, Holzapfel BM, Quent VM, Fuehrmann T, Hesami P, Brown TD, Dalton PD, Power CA, Hollier BG, Hutmacher DW, A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone, Dis. Model. Mech 7 (2014) 299–309. doi: 10.1242/dmm.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Quent VMC, Taubenberger AV, Reichert JC, Martine LC, Clements JA, Hutmacher DW, Loessner D, A humanised tissue-engineered bone model allows species-specific breast cancer-related bone metastasis in vivo, J. Tissue Eng. Regen. Med 12 (2018) 494–504. doi: 10.1002/term.2517. [DOI] [PubMed] [Google Scholar]
- [265].Paul CD, Hung W-C, Wirtz D, Konstantopoulos K, Engineered Models of Confined Cell Migration, Annu. Rev. Biomed. Eng 18 (2016) 159–180. doi: 10.1146/annurev-bioeng-071114-040654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Kim S, Kim W, Lim S, Jeon JS, Vasculature-on-a-chip for in vitro disease models, Bioengineering. 4 (2017) 8. doi: 10.3390/bioengineering4010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Damiati S, Kompella UB, Damiati SA, Kodzius R, Microfluidic devices for drug delivery systems and drug screening, Genes (Basel). 9 (2018) E103. doi: 10.3390/genes9020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Ma Y-HV, Middleton K, You L, Sun Y, A review of microfluidic approaches for investigating cancer extravasation during metastasis, Microsystems Nanoeng 4 (2018) 17104. doi: 10.1038/micronano.2017.104. [DOI] [Google Scholar]
- [269].Peela N, Sam FS, Christenson W, Truong D, Watson AW, Mouneimne G, Ros R, Nikkhah M, A three dimensional micropatterned tumor model for breast cancer cell migration studies, Biomaterials. 81 (2016) 72–83. doi: 10.1016/j.biomaterials.2015.11.039. [DOI] [PubMed] [Google Scholar]
- [270].Lanz HL, Saleh A, Kramer B, Cairns J, Ng CP, Yu J, Trietsch SJ, Hankemeier T, Joore J, Vulto P, Weinshilboum R, Wang L, Therapy response testing of breast cancer in a 3D high-throughput perfused microfluidic platform, BMC Cancer. 17 (2017) 709. doi: 10.1186/s12885-017-3709-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Mak M, Reinhart-King CA, Erickson D, Microfabricated Physical Spatial Gradients for Investigating Cell Migration and Invasion Dynamics, PLoS One. 6 (2011) e20825. doi: 10.1371/journal.pone.0020825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Bischel LL, Beebe DJ, Sung KE, Microfluidic model of ductal carcinoma in situ with 3D, organotypic structure, BMC Cancer. 15 (2015) 12. doi: 10.1186/s12885-015-1007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Toh Y-C, Raja A, Yu H, van Noort D, A 3D Microfluidic Model to Recapitulate Cancer Cell Migration and Invasion, Bioengineering. 5 (2018) 29. doi: 10.3390/bioengineering5020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Choi Y, Hyun E, Seo J, Blundell C, Kim HC, Lee E, Lee SH, Moon A, Moon WK, Huh D, A microengineered pathophysiological model of early-stage breast cancer, Lab Chip (2015). doi: 10.1039/c5lc00514k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, Charest JL, Moretti M, Kamm RD, A microfluidic 3D invitro model for specificity of breast cancer metastasis to bone, Biomaterials. (2014). doi: 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD, Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation, Proc. Natl. Acad. Sci (2015). doi: 10.1073/pnas.1417115112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Kalluri R, The biology and function of fibroblasts in cancer, Nat. Rev. Cancer. (2016). doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- [278].Iwai Y, Hamanishi J, Chamoto K, Honjo T, Cancer immunotherapies targeting the PD-1 signaling pathway, J. Biomed. Sci 24 (2017) 26. doi: 10.1186/s12929-017-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, Cormier JN, Lewis C, Hwu WJ, Hanna E, Diab A, Wong MK, Royal R, Gross N, Weber R, Lai SY, Ehlers R, Blando J, Milton DR, Woodman S, Kageyama R, Wells DK, Hwu P, Patel SP, Lucci A, Hessel A, Lee JE, Gershenwald J, Simpson L, Burton EM, Posada L, Haydu L, Wang L, Zhang S, Lazar AJ, Hudgens CW, Gopalakrishnan V, Reuben A, Andrews MC, Spencer CN, Prieto V, Sharma P, Allison J, Tetzlaff MT, Wargo JA, Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma, Nat. Med 24 (2018) 1649–1654. doi: 10.1038/s41591-018-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Enriquez-Navas PM, Kam Y, Das T, Hassan S, Silva A, Foroutan P, Ruiz E, Martinez G, Minton S, Gillies RJ, Gatenby RA, Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer, Sci. Transl. Med 8 (2016) 327ra24 www.ScienceTranslationalMedicine.org. [DOI] [PMC free article] [PubMed] [Google Scholar]


