Abstract
With rabbit patients, as in other species, analyzing blood and urine samples can be useful and informative, although interpretation of the results is sometimes challenging. This article summarizes the interpretation of laboratory results from rabbits. Hematological parameters can yield information about the red blood cell population and leukocyte response to stress and pathogens. Biochemistry evaluation can be used to investigate liver, kidney, and other organ function, and urinalysis results may yield additional information about kidney function and electrolyte imbalances. Serological tests are available for several pathogens of rabbits, including Encephalitozoon cuniculi, although the significance of positive results and antibody titers is not clear. Serum protein electrophoresis aids the understanding of protein disorders and the immune response to acute and chronic inflammation.
Key words: biochemistry, blood sampling, hematology, rabbit, serum protein electrophoresis, urinalysis
Rabbits can mask signs of illness or show few or confusing clinical signs. Additional information may be gained from laboratory tests, and in-house analyzers can provide a complete profile with a small volume of the patient’s blood. Unfortunately, most of the published data on rabbit hematology and biochemistry values are descriptions of the effects of toxins on hematological and biochemical parameters of laboratory rabbits. There is little information available that describes the effect of clinical disease on the blood parameters of companion rabbits, or on the use of blood tests as diagnostic and prognostic indicators. The lack of biochemical data for pet rabbits is changing as practitioners collect information and researchers are more cognizant of diagnostic and prognostic hematologic indicators.
Blood Sampling: Sites and Techniques
The blood volume of a healthy rabbit is approximately 55 to 65 mL/kg, and 6% to 10% of the blood volume may be safely collected. Many sites are described for blood collection in rabbits. Cardiac puncture is used in laboratory rabbits but is not recommended for pet animals. Both the marginal ear vein and central ear artery are easily accessible, but they may be difficult to access in some patients. Collecting a sufficient volume of blood from these sites to perform all of the desired clinical tests may also be difficult, especially from dwarf breeds with small ears. Sampling from ear vessels can occasionally result in thrombosis and subsequent avascular necrosis of parts of affected pinna tissue. Blood may be collected from the cephalic vein, which is straight and easily accessible, but, because of the short antebrachium, occlusion of the vessel by encircling the limb at the elbow is difficult. The cephalic vein is also small and easily collapses. The jugular veins are large and allow for ample-sized blood volumes to be collected, but jugular phlebotomy can be stressful for rabbits and may require chemical restraint. The dewlap may interfere with jugular vein visualization, especially in obese does. An accessible and efficient site for blood sampling in rabbits is the lateral saphenous vein (Fig 1).
Figure 1.
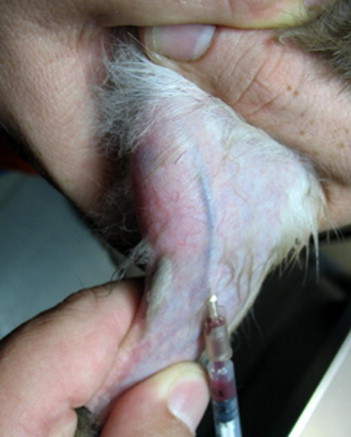
Blood collection from the saphenous vein.
Most biochemical parameters of rabbits can be measured from serum or plasma. Rabbit blood clots easily at room temperature and will coagulate quickly if not mixed with anticoagulant during collection. Heparin is a suitable anticoagulant because it does not alter biochemical parameters, even though the anticoagulant/blood ratio is not always optimal. Hemolysis can be prevented by letting the blood drop from the needle into the tube, but often, only a few drops can be collected before the blood clots. The technique can be improved by heparinizing syringes and needles through aspiration of a few drops of heparin into the syringe, then removing the excess with injection pressure. The small amount of heparin remaining in the needle prevents clotting without significant alterations in biochemical parameters.
It is important to make several air-dried blood smears at the time of venipuncture before the anticoagulant in the tube and transport can modify red and white cell morphology. Most of the standard blood stains work well. Automated flow cytometry is reliable for analyzing most hematological parameters for rabbit patients.
Artifactual Changes
Age, sex, breed, and circadian rhythms all affect hematological and biochemical parameters in rabbits. Rabbits under 12 weeks of age have lower red blood cell (RBC) and white blood cell (WBC) counts. The total WBC and lymphocyte counts are lowest in the late afternoon and evening, when the heterophils and eosinophil counts rise. Urea and cholesterol levels tend to increase at the end of the day.
Stress can alter many different hematological parameters (e.g., blood glucose). Prolonged stress, such as transportation, unfamiliar noises, smell, chronic pain, and poor environment, can induce heterophilia, lymphopenia, and leukocytosis. Simple handling does not induce this response, but several muscle enzymes including lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and creatine kinase elevate after physical restraint of the rabbits, especially if they are fractious or unfamiliar with handling. Sedation or general anesthesia can be helpful. Isoflurane anesthesia does not appear to affect blood parameters in rabbits.
Hemolysis can induce several artifacts, such as decreased RBC and amylase values and increased LDH, AST, creatine kinase, total protein, and potassium levels. In-house analyzers can be very sensitive to hemolysis, thereby altering the true blood parameters of the patient.
Hematology
Hematology results of rabbits can be difficult to interpret. Most reference ranges are from experimental laboratory studies that are run on homogeneous groups of rabbits belonging to the same breed, strain, age, and environmental conditions. This can be very different from the clinician’s situation of dealing with a heterogeneous population of pet rabbits. Many texts amalgamate reference ranges from different sources to create ranges so wide that they include almost any result. Another problem is that healthy pet rabbits are very hard to find, so samples from rabbits with an apparently acute condition may also show changes due to an underlying chronic problem, such as malnutrition, improper husbandry, or subclinical disease. For example, Harcourt-Brown and Baker1 showed that rabbits that were caged, fed on commercial mixes, and suffered from dental disease had consistently lower packed cell volumes (PCV), RBC counts, hemoglobin values, and lymphocyte counts in comparison with rabbits kept outside with a more natural diet and exercise.
Morphology of Red Blood Cells
Rabbit erythrocytes are typical mammalian anucleate biconcave discs with an average diameter of 6.8 μm, which is midway between cats and dogs.2 Erythrocyte size varies between 5.0 and 7.8 μm, which is often reported as a marked anisocytosis (Fig 2). The short life span (57 days) and high turnover of erythrocytes is reflected as polychromasia, which is not clinically significant. The presence of a few nucleated RBCs (1-2 × 100 leukocytes) and the occasional Howell-Jolly bodies (Fig 2) should be considered within the normal reference range for rabbits and not an indicator of cellular regeneration.
Figure 2.
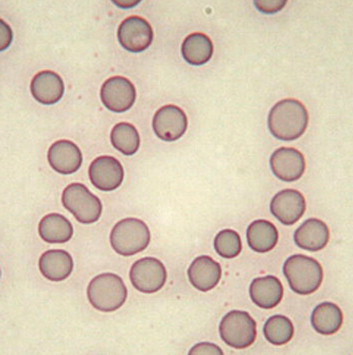
Anisocytosis and a Howell-Jolly body. In domestic carnivores, anisocytosis usually reflects the presence of reticulocytes and indicates regenerative anemia. This is not true in rabbits in which 1% to 4% of the circulating erythrocytes may be reticulocytes. The occasional Howell-Jolly body is not clinically significant either.
Anemia
The published reference range for PCV in the rabbit is 30% to 50%, but pet rabbits often have lower values of 30% to 40%.2 Values higher than 45% may indicate dehydration, which is usually linked to gastrointestinal (GI) stasis. Combined PCV and total protein (TP) is useful to differentiate acute conditions from subclinical chronic diseases that have suddenly deteriorated. A PCV of less than 30% indicates anemia, especially if the RBC and hemoglobin levels are low as well. Nonregenerative anemia associated with chronic disease is common in pet rabbits. Otitis media, dental disease with or without abscesses, pneumonia, pododermatitis, mastitis, endometritis and pyometra, renal disease, and osteomyelitis are all examples of chronic infections that can be associated with nonregenerative anemia in pet rabbits.
Regenerative anemia is manifested by significant and rapid reticulocyte production and usually indicates blood loss. Causes of external hemorrhage in rabbits include trauma and severe flea infestation. Common internal causes include hematuria due to kidney or bladder stones or bleeding uterine adenocarcinomas or endometrial aneurysms in does. Intravascular hemolysis is an unusual cause of regenerative anemia after ingestion of leaves and stems of potato plants and possibly other Solanaceae. Alliums (onion, garlic, and chives) may also cause Heinz body anemia.3 Autoimmune hemolytic anemia has been reported in laboratory rabbits in association with lymphosarcoma, and isolated cases have been treated in private practice (Harcourt-Brown, personal communication, November, 2006).4 Lead toxicosis is a cause of regenerative anemia, characterized by many nucleated erythrocytes, hypochromasia, poikilocytosis, and basophilic cytoplasmatic stippling.2 Nucleated red cells of more than 1% to 2% of the RBC can be linked with the acute, septicemic phase of an infectious disease, although this is unusual because of the presence of a nonregenerative anemia from underlying chronic disease.
White Blood Cell Count
A well-prepared air-dried smear is required for an accurate differential white cell count and evaluation of the cytological appearance of each cell type (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6). Differential white cell counts and cell morphology can be used to develop a differential diagnoses list and to determine the general condition of the patient.
Figure 3.
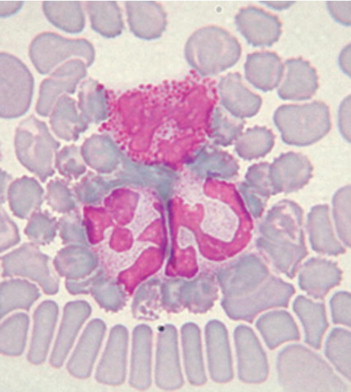
Neutrophils. Rabbit neutrophils are rounded cells with a diameter of 10 to 15 μm. The nucleus of rabbit neutrophils is distinctly segmented and stained a deep purple-blue; the segments are connected by thin strands of chromatin. In the clear cytoplasm, variable numbers of granules can be seen, ranging in size and color (small and pink or large and reddish). The relative number of each granule population may alter the staining characteristics of the cell. In some cases, laboratories report rabbit neutrophils as heterophils or acidophils because of the deep pink to red stained granules in the cells. Regardless of how the cells are labeled, there is no functional difference between the cells.
Figure 4.
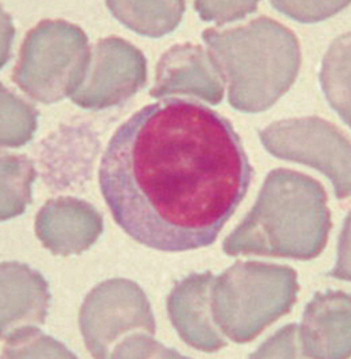
Large lymphocyte. Rabbits have 2 distinct populations of lymphocytes: small (7-10 μm) and large (10-15 μm). Lymphocytes are round cells with a round or slightly oval deep purple nucleus. Cytoplasm is scarce or absent in the small lymphocytes and more abundant and bluish in the large ones. Some large lymphocytes may present a clear halo around the nucleus and even a few azurophilic granules.
Figure 5.
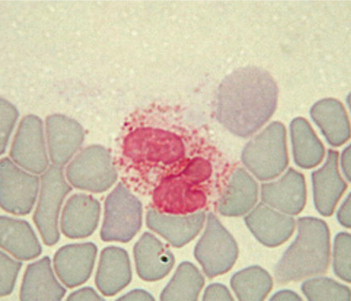
Eosinophil. Rabbit eosinophils measure 10 to 16 μm in diameter and have a purple bilobed or horseshoe-shaped nucleus. The cytoplasm is obscured by so many granules that the cell looks orange-pink and foamy. The abundance of granules is the main difference between the eosinophils and the neutrophils. Removal of histamine and histamine-like toxins is the most important function of the eosinophils, suggesting that they therefore play an important role in controlling allergic reactions.
Figure 6.
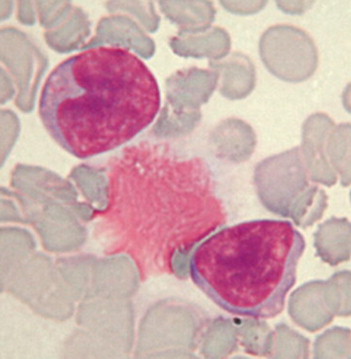
Monocytes. Monocytes are the largest of the leukocytes, with an average diameter of 15 to 18 μm. Their purple nucleus is usually lobulated, but may be horseshoe or bean shaped. Monocytes have more cytoplasm than lymphocytes, and their cytoplasm stains a blue-gray mottled color.
Interpretation of WBC data from rabbits is different from other domestic species including dogs, cats, and birds, in which a leukocytosis is the response to inflammation. With rabbits, although leukocytosis can be identified in cases that have been diagnosed with lymphosarcoma, it is not the usual response to inflammation. Laboratory investigations have shown no increase in the total number of circulating leukocytes in rabbits injected with bacteria or yeast, although fever, increased plasma cortisol concentrations, neutrophilia, and lymphopenia were observed.5, 6 In clinical practice, rabbits with sepsis can show a variety of responses including a neutropenia, normal neutrophilic count, or a mature neutrophilia, accounting for more than 90% of WBCs. Band neutrophils appear to be a rare finding in clinical infection, and the absence of a left shift does not rule out an infectious problem. An alteration of the neutrophil/lymphocyte ratio showing a relative neutrophilia coupled with lymphopenia may indicate a response to infection. This ratio is approximately 1:1 in an adult healthy rabbit, but stress can alter the neutrophil/lymphocyte ratio. Transport, waiting in a room full of unfamiliar sounds and smells, or even restraint for clinical examination can change the ratio. Gentle clinical examination and blood collection do not seem to affect the differential white cell count, whereas more prolonged stress like travel or exposure to barking dogs in the waiting room can induce a lymphopenia and relative neutrophilia, that may persist for up to 24 to 48 hours.7 The total WBC can be used to further characterize acute stress from chronic stress (e.g., malnutrition, improper husbandry, prolonged social stress, dental disease), as both a leukopenia and lymphopenia are more common with chronic stress. Chronic stress is often reported as general leukopenia and lymphopenia in a rabbit’s differential WBC evaluation.
The primary role of the lymphocytes is to respond to those activities that stimulate the immune system. In rabbits, lymphocytes are primarily found in the blood, spleen, bone marrow, lymph nodes and the lymphatic tissues in the GI tract. The number of circulating lymphocytes is a balance between the cells entering and leaving the bloodstream and does not necessarily reflect a change in lymphopoiesis. In rabbits, it has been shown that an increase in adrenaline levels (acute stress) induces lymphocytosis, whereas raised cortisol levels (chronic stress) leads to lymphopenia. Viral diseases may result in a normal or higher lymphocyte count. Other causes of lymphocytosis are lymphoma and lead poisoning.8
Eosinophilia in rabbits can occur when tissues rich in mast cells, such as the skin, lungs, GI tract, or uterus, are involved in disease. Eosinophilia can indicate the presence of an abscess and may be found during wound healing. In other species, eosinophilia is linked to parasitic diseases, especially when larvae are moving through tissues, but this is rare in domestic rabbits. Encephalitozoon cuniculi does not stimulate an eosinophilic response. In clinically healthy rabbits, a very low eosinophilic count, even 0, is a common finding. High levels of cortisol (chronic stress) can induce eosinopenia.
Monocytosis is linked with chronic inflammation (e.g., abscesses, mastitis, tympanic bulla empyema). However, the absence of a monocytosis does not rule out inflammation. Monocyte counts within the normal range are a common finding in rabbits with osteomyelitis due to dental disease.
The diameter of rabbit basophil measures 8 to 12 μm. Its nucleus is less segmented than the eosinophil or heterophil and difficult to see because of the many deep purple granules that obscure the light gray cytoplasm. As in other species, the function of the rabbit basophil is not fully understood, but these cells are often present in large numbers of rabbit blood smears. Basophilia with concurrent eosinophilia has been described in rabbits with chronic skin problems (e.g., atopy, pyoderma).3
Biochemistry Reference Ranges
Enzymes
In rabbits, AST is widely distributed in many tissues. It is present in cardiac tissue and muscle, as well as the liver, and has a short half-life (5 hours). Although higher AST levels may be found in patients diagnosed with liver damage, struggling during collection or hemolysis of the sample also raises AST levels. Creatine phosphokinase levels also increase after restraint and are purely muscular in origin. LDH is produced by muscle and liver cells in rabbits and therefore is not beneficial as a diagnostic tool for many disease evaluations. Reference levels for AST, CK, and LDH can be found in Table 1.
Table 1.
Biochemical Reference Ranges
| AST (IU/L) | 35-130 |
| ALT (IU/L) | 45-80 |
| ALP (IU/L) | 12-96 |
| CPK (IU/L) | 140-372 |
| GGT (IU/L) | 0-7.0 |
| T. BIL (mg/dL) | 0-0.7 |
| Bile acids (mMol/L) | <40 |
| Amylase (U/L) | 200-400 |
| Cholesterol (mg/dL) | 10-80 |
| Urea (mg/dL) | 20-45 |
| CREA (mg/dL) | 0.5-2.5 |
| Ca (mg/dL) | 11-14 |
| Phos (mg/dL) | 4.0-6.5 |
| Na (mEq/L) | 138-150 |
| K (mEq/L) | 3.5-6.9 |
| Glucose (mg/dL) | 75-155 |
| Total protein (g/dL) | 5.4-7.5 |
| Albumin (g/dL) | 2.7-5.0 |
| Globulin (g/dL) | 1.5-2.7 |
AST = aspartate aminotransferase, ALT = alanine aminotransferase, ALP = alkaline phosphatase, CPK = creatine phosphokinase, GGT = glutamyltransferase, T. BIL = total bilirubin, CREA = creatinine, CA = calcium, Phos = phosphorous, Na = sodium, K = potassium.
In many mammalian species, alanine aminotransferase (ALT) is a useful indicator of hepatocyte damage because of its specificity for liver tissue and its long half-life (45-60 hours in dogs). In rabbits, ALT is not as useful as an indicator of liver damage as with other species because, like other herbivores (e.g., horses, cattle, guinea pigs), ALT is not liver specific and has a shorter half-life (around 5 hours). However, ALT concentrations are not affected by restraint and therefore can be used as a diagnostic tool. Slightly increased ALT levels are a common finding in apparently healthy rabbits. Mildly increased ALT levels in healthy rabbits have been attributed to exposure to low concentration of toxic substances, such as resins in wood-based litter or aflatoxins in food.9 Raised ALT levels (with alkaline phosphatase (ALP), bilirubin and glutamyltransferase [GGT]) can be associated with hepatic lipidosis or may be found in patients with hepatic coccidiosis (Eimeria steidae) or torsion of a liver lobe. ALP is a widely distributed enzyme; liver and bone contain the highest concentrations, but it is also found in bowel epithelium, kidney tubules, and placenta. A physiological cause of high-serum ALP concentrations is osteoblastic activity in growing animals. Animals with bone lesions will show raised ALP levels. As a liver enzyme, ALP does not increase because of hepatocellular damage but is indicative of bile stasis (e.g., hepatic coccidiosis, liver abscesses, neoplasia, lipidosis). Extrahepatic causes, such as abscesses or neoplasia, can cause bile stasis by occluding the bile ducts. Rabbits produce 2 ALP isoenzymes in the liver. An intestinal isoenzyme is quite abundant, so serum ALP concentrations are actually the sum of these 3 isoenzymes, which may explain why many reference ranges are vague and wide and why raised ALP levels in clinically healthy animals are a common finding. ALP does have a diagnostic value because it is not altered by restraint and thus is considered a good indicator of real tissue damage. Reference levels for ALT and ALP can be found in Table 1.
GGT is a useful indicator of chronic liver disease with bile stasis in horses, cattle, and domestic carnivores; however, in rabbits, the activity of GGT is low. Activity of this enzyme is high in the kidney, yet renal GGT does not reach the circulation because it is eliminated with the urine. Therefore, elevated GGT levels in the rabbit are most often linked to obstructive lesions of the bile ducts, but with a lower sensitivity than that found in other species. Reference levels for GGT can be found in Table 1.
Bile Pigments
Bile pigments are produced during the breakdown of the heme molecule by hepatocytes and are excreted into the bowel. Bilirubin levels reflect either hepatocyte or bile tract function. Rabbits produce a large amount of bile for their weight, and the main compound is biliverdin, for which there is no commercial laboratory test. About 30% of biliverdin is converted to bilirubin, which is found in the blood in measurable amounts. The main cause of hyperbilirubinemia is bile flow obstruction. In young rabbits, hepatic coccidiosis is the most common cause of biliary obstruction, while in adult rabbits it is biliary neoplasia. Cellular causes of hyperbilirubinemia, and rarely icterus, are aflatoxicosis (e.g., eating moldy food), which induces hepatic fibrosis (ALT is usually raised too), and viral hemorrhagic disease, which causes acute hepatic necrosis with concurrent high levels of all hepatocellular enzymes. If the rabbit survives long enough, icterus may be seen. Bilirubin may also be increased in diseases that cause hemolysis (e.g., immune-mediated hemolytic anemia).
Bile Acids
In other species, a comparison of preprandial and postprandial serum bile acid concentrations is used as an indicator of liver function. In rabbits, cecotrophy makes it almost impossible to fast a rabbit for the preprandial sample, so bile acid measurement is not a routine procedure in clinical practice, although persistently raised bile acids have been reported in association with hepatic disease.10
Cholesterol and Triglycerides
Cholesterol is synthesized in the liver or obtained from the diet and is a precursor of steroids. It is metabolized by the liver and excreted in bile. In carnivores, hypercholesterolemia is linked with several metabolic diseases, such as hypothyroidism, hyperadrenocorticism, diabetes, and hepatopathies. Hypocholesterolemia indicates liver failure. Cholesterol and triglycerides levels peak after a meal and fasting is needed for accurate measurement, which limits their diagnostic value in rabbits because of cecotrophy. Abnormal levels of cholesterol and triglycerides can be related to a diet rich in fats, obese patients, or hepatic disease. In anorexic patients, hypercholesterolemia carries a poor prognosis because it indicates end-stage hepatic lipidosis. Hypercholesterolemia has also been linked with pancreatitis, diabetes mellitus, nephrotic syndrome, and chronic renal failure.3 Decreased cholesterol levels in rabbits might be found in cases of liver failure, chronic malnutrition, and even pregnancy (up to 30% below the range).
Amylase and Lipase
Unlike other species, amylase is an almost pure pancreatic enzyme in rabbits, with little or no content in salivary glands, intestinal tissue, or liver. Therefore, raised amylase levels in rabbits reflects pancreatic damage from pancreatitis, pancreatic duct obstruction, peritonitis, or abdominal trauma. Renal failure can also cause hyperamylasemia because this enzyme is cleared by renal filtration. Corticosteroids (exogenous or endogenous) can raise amylase values in serum, whereas hemolysis lowers it.
There is little information on the function and diagnostic value of lipase in rabbits. Increased lipase values may indicate cellular damage to the pancreas as it does in other species. As with amylase, lipase is artifactually elevated by corticosteroids.
Urea and Creatinine
Urea is a by-product of protein catabolism and is excreted by the kidneys into the urine. Urea levels in rabbits depend on the circadian rhythm (peak in late afternoon and early evening), quantity and quality of proteins in the diet, nutritional status, liver function, intestinal absorption, urease activity of the caecal flora, and hydration status. Often, small changes in urea levels are difficult to interpret. Reference ranges have been determined from laboratory rabbits fed on a standardized diet and bled at the same time of the day, whereas clinicians see pet rabbits fed on a variety of foods and samples are taken at random. Slight elevations in blood urea are a common finding. Reference levels for urea can be found in Table 1.
Creatinine is a protein catabolite that is produced from the muscle creatine and excreted by glomerular filtration at a constant rate. Creatinine is a more reliable test of renal function than blood urea. In rabbits, prerenal azotemia can be caused by dehydration because rabbits have a limited ability to concentrate urine. Only a few hours without drinking or losing fluids, as in cases of ileus or diarrhea, may cause an increase of urea and creatinine to levels compatible with renal failure. Urea and creatinine levels rapidly return to normal once the dehydration deficit is corrected. Stress can also induce shock and cardiac disease, classified as prerenal disease, causing a decrease in renal perfusion. Another potential cause of prerenal azotemia is GI hemorrhage, which results in increased protein digestion. Azotemia is also indicative of renal disease, usually affecting the rabbit patient in association with hyperkalemia or hypokalemia, hypercalcemia and coexisting hyperphosphatemia, nonregenerative anemia, and isostenuric urine. The most common cause of renal failure in rabbit patients is E. cuniculi, which causes granulomatous and then fibrotic lesions in the renal parenchyma. Other possible causes of renal failure are chronic interstitial nephritis, glomerulonephritis, pyelonephritis, nephrolithiasis, renal cysts, and lymphosarcoma. Postrenal azotemia can occur because of obstruction to urine flow as a complication of bladder sludge or urolithiasis. An abdominal radiograph is mandatory in any azotemic rabbit. Blood urea levels below the reference range indicate hepatic insufficiency or muscle mass loss (e.g., dental disease). Reference levels for creatinine can be found in Table 1.
Glucose
Glucose metabolism in rabbits is different from dogs or cats. Not only do rabbits eat continuously during the day, but they also use volatile fatty acids produced by cecal flora as a primary energy source. A fasting blood sample is impossible to obtain because rabbits ingest fecal pellets. A rabbit that is not given food can continue to ingest cecotrophs. It has been shown that 4 days of starvation does not reduce blood glucose levels in rabbits.11
Diabetes mellitus is rare in rabbits, although hyperglycemia is a common finding and may be associated with glucosuria. Reports of confirmed diabetes mellitus are from laboratory strains bred as a model for human diabetes. Clinical signs commonly observed in pet rabbits with diabetes mellitus are polyphagia, polyuria, polydipsia, very high blood glucose levels (>500 mg/dL), and glycosuria with significantly elevated glycosylated hemoglobin and raised triglycerides; however, obesity and ketoacidosis are not observed.12 In clinical practice, most cases of hyperglycemia are due to stress (e.g., transport, handling, venipuncture, underlying disease). A marked hyperglycemia (around 350 mg/dL) is reported in cases of acute intestinal blockage by a foreign body.10 Early mucoid enteropathy may be associated with hyperglycemia. In rabbits with GI stasis, hyperglycemia carries a bad prognosis because it may indicate hepatic lipidosis. Other causes of raised serum glucose levels are traumatic or hypovolemic shock and hyperthermia. Acute pancreatitis could cause blood glucose abnormalities, even though the role of the pancreas in glucose metabolism in rabbits is less important than in other species. Glucocorticoids and other drugs can raise blood glucose. Hyperadrenocorticism has not been described in rabbits.
Hypoglycemia is an important finding. In anorexic patients, it indicates that the rabbit is using adipose tissue and is at risk of developing hepatic lipidosis. Hypoglycemia may occur in terminal mucoid enteropathy, liver failure, or other chronic diseases. Rabbits with acute sepsis may be hypoglycemic too.3 Insulinoma has not been described in rabbits. Reference levels for glucose can be found in Table 1.
Electrolyte Abnormalities
The complex GI physiology and the limited ability of the kidneys to correct acid-base alterations make rabbits susceptible to electrolyte imbalances. Any disturbance of serum electrolytes should alarm the clinician to possible pathology of the digestive or excretory system. Anorexia can rapidly lead to metabolic acidosis.
Sodium
The diagnostic value of sodium for rabbit patients is low. Hypernatremia can be due to dehydration or loss of fluids (e.g., diarrhea, peritonitis, burns, myiasis). Hyponatremia is usually associated with polyuric renal failure (acute or more commonly chronic), when urine flow in the renal tubules is too fast to impede the sodium-potassium exchange. Lipemia or hyperproteinemia can artifactually decrease sodium levels in serum. Reference levels for sodium can be found in Table 1.
Potassium
Potassium is homeostatically important because it is essential for maintenance of membrane potential. Changes in membrane potential can be lethal (e.g., impaired contractility of myocardial cells due to hyperkalemia can cause arrhythmias and cardiac arrest). Intracellular and extracellular potassium levels are maintained by a complex mechanism of exchange between cells and microenvironment, and regulated by several hormones such as aldosterone, insulin, and catecholamines. Hypoadrenocorticism has not been described in rabbits. Instead, raised potassium levels are more often due to acute renal failure or urine flow obstruction. Severe tissue damage can also cause hyperkalemia by dispersing potassium into the extracellular space. For the same reason, hemolysis (e.g., intravascular, bad sampling technique, letting the sample wait too long before separating the serum) can artifactually raise potassium levels. Another indirect cause of higher potassium levels in serum is metabolic acidosis, which increases the exchange of potassium ions across the cell membrane. Reference levels for potassium can be found in Table 1.
Causes of hypokalemia in rabbits include dietary insufficiency and loss of fluids from the GI system (e.g., saliva, mucoid diarrhea) or the kidneys (e.g., renal failure, diuretic drugs). Stress-induced increases in catecolamine levels can also cause hypokalemia. Alkalosis, although rare in rabbits, decreases the blood potassium concentrations by stimulating the cellular uptake of potassium ions. Hyperproteinemia and lipemia can artifactually decrease blood potassium levels that may present clinically as sensory depression and muscle weakness. A correlation between low potassium levels in blood and “floppy rabbit syndrome” has been reported.10 In other herbivorous species (e.g., horses), blood potassium levels fluctuate widely, depending on physical activity or even on the quantity of saliva produced during the meals.13 These examples of serum potassium level fluctuation are purely physiological and may occur in rabbits.
Calcium
Calcium is found in blood either bound to serum proteins or ionized free in the serum. Most laboratories report a total serum calcium value, which is the sum of bound and ionized calcium. Ionized calcium is a more precise measurement but is a more difficult and expensive parameter to test. Total serum calcium is influenced by dietary intake, serum protein levels, and other metabolic conditions. Calcium metabolism in rabbits is different from that in other animals. Blood calcium levels are influenced more by the calcium content of the diet than in the dog or the cat. Rabbits absorb calcium in proportion to the concentration of the ion in the gut, and the kidney eliminates the excess. Vitamin D is not important in calcium absorption if dietary levels are high, yet it does play an important role if dietary levels are low. Vitamin D is also important in calcium distribution within the body. As with other species, parathyroid hormone regulates blood calcium levels, but the level at which calcium is moved from the blood to the bones is high in rabbits. Consequently, blood calcium levels are higher, and the normal range is broader than that in other species. Growing youngsters and pregnant does use more calcium, resulting in lower blood calcium concentrations. In these rabbits blood calcium concentrations rarely rise above 14 mg/dL, even when fed calcium-rich diets, whereas adult rabbits on a varied diet can show calcium levels up to 16 to 17 mg/dL (Table 1). The urinary excretion rate of calcium is around 45% to 60% for rabbits, whereas most mammals excrete no more than 2% of their calcium through renal filtration. This predisposes rabbits to the formation of sludge and stones in the rabbit urinary system. Although the constant excretion of calcium could be a cause of renal failure in rabbits fed unbalanced diets, hypercalcemia is also a consequence of renal disease in rabbits because of the inability of the kidney to eliminate excess calcium. Measurement of blood calcium is essential to diagnose and treat renal disease in rabbits.
Hypocalcemia is rare but is reported in rabbits. The most frequent cause of low blood calcium levels is hypoalbuminemia due to poor nutrition. Hypocalcemic seizures have been described in late-pregnant and lactating does.13
Phosphorus
Phosphorus is involved in many enzymatic systems in rabbits, but its main function is contributing to the proper formation of bones and teeth. Because phosphorus is present inside cells, blood phosphate concentrations are easily increased by hemolysis (e.g., spontaneous or sampling problems). Blood phosphate values should always be evaluated with blood calcium levels to determine the mineral balance in patients diagnosed with urinary tract stones, dental disease, or other signs of nutritional secondary hyperparathyroidism. Because the kidney is the main organ involved in phosphorus balance by regulation of glomerular filtration and tubular reabsorption, blood phosphate levels can be an indirect measurement of kidney function and, in general, parallel azotemia. Serum phosphorus levels can be elevated as a result of prerenal, renal, and postrenal effects. Hyperphosphatemia usually indicates chronic kidney failure (a loss of more than 80% of nephrons) given that serum phosphorus levels are normalized by compensatory mechanisms in early-onset renal disease. Hyperphosphatemia may also be an indicator of soft tissue trauma. Reference levels for phosphorus can be found in Table 1.
Hypophosphatemia is not rare, but its clinical significance is unknown at this time, with dietary deficiencies or reduced intestinal absorption possibly involved.
Protein Disorders
Total protein, consisting of the sum of albumin and globulin, is an important parameter in any species of animal. Many factors (e.g., age of the animal, reproductive status, pregnancy) can affect TP levels. Total serum protein levels can be artifactually raised by hemostasis at the sample site through fluid loss because of digital pressure on the vessel from which the blood is collected. Reference levels for TP, albumin and globulins can be found in Table 1.
The main cause of hyperproteinemia in rabbits is dehydration. Raised TP levels may also indicate a chronic infectious or metabolic process. Measuring albumin and globulin fractions helps to differentiate the causes of hyperproteinemia.
Hypoproteinemia is usually due to chronic malnutrition or protein loss. If both albumin and globulin are low, hemorrhage or protein loss through exudative skin lesions such as burns or flystrike should be considered. Other possible causes must be examined in cases of hypoalbuminemia with normal or raised globulins. Because the liver is the only site of albumin synthesis, a lowered albumin level may indicate an advanced hepatic disease, such as hepatic coccidiosis (E stiedae) or scarring and necrosis due to the migrations of Cysticercus (Taenia) pisiformis larvae. Protein-losing nephropathies (glomerulonephropathy) and enteropathies are rarely diagnosed in rabbits. A common cause of hypoalbuminemia in pet rabbits is chronic malnutrition either from poor diet or advanced dental disease. All causes of reduced cecotrophy (e.g., dental disease, obesity, back pain) can be reflected as low protein, especially albumin levels.
A method of evaluating serum proteins is by electrophoresis (EPH), which divides the globulins into distinct fractions. In acute disease, alpha-globulins are elevated; consequently, a rabbit with an alpha-globulin peak may have a bacterial infection or a developing abscess, and/or present febrile. The beta portion of globulins consists of several proteins classified as “acute-phase” proteins including fibrinogen. Fibrinogen correlates with inflammation in rabbits, although the correlation is not as evident as in other species. Plasma is preferable to serum for EPH because it does include fibrinogen. Gamma-globulins are mainly antibodies, and a peak of this fraction indicates a subacute to chronic inflammation, especially when associated with a bacterial infection. Coronavirus infections lead to an impressive increase of rabbit globulins as feline coronavirus does in cats, but this disease is probably limited to laboratory settings. The correlation between EPH curves and different rabbit pathologies is unknown and currently under investigation.
Serology
There are serological tests for antibodies against E cuniculi, Toxoplasma gondii, Treponema cuniculi, myxomatosis, viral hemorragic disease, and Pasteurella multocida, although their availability varies in different parts of the world. The most common serological assay used in private practice is for the antibodies to E cuniculi. Laboratory studies have shown that infected rabbits start to develop a measurable serological immune response 4 weeks after infection, 2 weeks before E cuniculi is found in the kidney or in the urine, and at least 8 weeks before any brain lesion.14, 15 If a serologic test for E cuniculi is negative for a neurological rabbit patient, one could assume, with confidence, that this patient does not have the disease. If the test is positive, neurological signs may be due to E cuniculi or a different disease process. The seropositive result may be due to past exposure to the pathogen, which is common within most rabbit populations. Further laboratory tests may be helpful to determine a definitive diagnosis. A correlation between antibody titers and neurological signs is hard to prove. A rising titer after 2 weeks is often considered diagnostic in suspect cases. The kidney is a target organ for E cuniculi, so evaluation of renal function and structure by biochemistry, urinalysis, and ultrasound may aid one’s diagnostic overview. Inflammatory changes in the cerebrospinal fluid are suggestive of E cuniculi but are nonspecific. At the present time, the definitive diagnosis of rabbit encephalitozoonosis requires histopathology or isolation of the spores from the urine by microscopy or polymerase chain reaction assay.
Although serology for T cuniculi can be used to screen a breeding facility, it has little value in clinical practice. At least 3 months are needed for antibody levels to become measurable in the blood even with clinically evident dermatological lesions. This substantial lag in the development of antibody titers can lead to false-negative test results. Positive titers without skin lesions can indicate subclinical disease or previous infection in which the skin lesions are missed. Antibody titers fall rapidly once the rabbit is treated for T cunicoli.2
Serological testing for P multocida is available in some countries, but its use in clinical practice is limited. A single positive response has no clinical significance because many rabbits harbor the bacteria in the nasal cavities without illness. A positive titer can also indicate previous exposure to the bacteria rather than clinical infection. Repeat testing after 2 weeks, while looking for a rising titer, can be helpful in obtaining a correct diagnostic evaluation. Results from rabbits younger than 2 months can be difficult to interpret because maternal antibodies are still present. A high antibody titer is not protective and may indicate subclinical disease.
Urine Analysis
Urine samples can be collected from spontaneous micturition, bladder expression, catheterization (quite difficult because sedation/anesthesia is required), or cystocentesis, with the latter being the preferred method for obtaining a sample for bacterial culture. Care should be taken when performing a cystocentsis, because microtrauma to the bladder wall can stimulate local mineralization and it is possible to puncture the cecum, an enlarged uterus, or even an abscess. If bacteriology is not required, free catch from a clean litter tray is the easiest way to obtain a urine sample. Manual expression of the bladder should be done carefully because the wall is thin and ruptures easily, especially if an obstruction is present or the rabbit struggles. In cases of chronic cystitis, the risk for bladder rupture is lower because the bladder is thickened. It is preferable to collect the first urine of the morning and to run the analysis as soon as possible. Normal rabbit urine is dense and rich in minerals, so it should be centrifuged or filtered for biochemical analysis or examinations. Clear urine indicates low calcium excretion, which may be pathological because of renal failure or physiological in the case of growing or lactating rabbits. The color may range from light yellow to reddish brown. In most cases, dark urine is caused by dietary pigments; however, the urine should be checked for hematuria, which may be caused by uroliths, urinary tract inflammation/infection, uterine problems, or anticoagulants.
Test dipsticks work well to evaluate blood, glucose, ketone, and pH levels in rabbit urine but are not accurate for other parameters. Glucose may be found as a consequence of stress hyperglycemia; checking urine collected at home can help to rule out stress-related problems. True glucosuria indicates altered energy metabolism, such as hepatic lipidosis, or, very rarely, diabetes mellitus. Ketones are always abnormal and indicate anorexia (even short duration), hepatic lipidosis, pregnancy toxemia, or diabetes.
The pH of rabbit urine tends to be high (7.5 to 9) in rabbits fed a correct diet. Acidic urine indicates acidosis due to anorexia, fever, pregnancy toxemia, or hepatic lipidosis. It is possible that, because a normal kidney would eliminate acidic urine in cases of acidosis, the finding of alkaline urine in an anorexic rabbit could indicate compromised renal function. Specific gravity (SG) indicates the ability to concentrate urine. A refractometer is a more reliable tool to measure SG than dipsticks. Most normal rabbit urine is quite dilute, with an average SG of 1.015 (range, 1.003-1.036). Prerenal azotemia is associated with raised SG (>1.030), whereas true azotemia linked to renal failure is associated with dilute urine (SG <1.013).
Urine-specific gravity is useful if it is examined alongside urine protein concentrations. Protein traces in clinically normal rabbits, especially the young, are not significant, whereas a dilute urine (<1.020) with proteins is very significant. Proteinuria appears earlier than biochemical changes in renal disease, making this test useful in clinical practice. Measuring urine proteins/urine creatinine ratio (<0.6 is suggested as normal) may further improve the sensitivity of urine-specific gravity testing.
Sediment examination can differentiate normal urine that is rich in crystals from sludge. After centrifugation, normal crystals should resuspend when shaken, while sludge remains as a solid mass. Cytology is not very different from that of other mammals; a small number of leukocytes are considered normal in rabbits. Gram or trichrome stains can reveal E cuniculi spores.3
References
- 1.Harcourt-Brown F.M., Baker S.J. Parathyroid hormone, hematological and biochemical parameters in relation to dental disease and husbandry in pet rabbits. J Small Anim Pract. 2001;42:130–136. doi: 10.1111/j.1748-5827.2001.tb02009.x. [DOI] [PubMed] [Google Scholar]
- 2.Fudge A.M. Rabbit hematology. In: Fudge A.M., editor. Laboratory Medicine: Avian and Exotic Pets. WB Saunders Company; Philadelphia, PA: 2000. pp. 273–275. [Google Scholar]
- 3.Saunders R.A., Davies R.R. Blackwell Publishing; Oxford, UK: 2005. Notes on Rabbit Internal Medicine. [Google Scholar]
- 4.Weisbroth S.H. Neoplastic diseases. In: Manning P.J., Ringler D.H., Newcomer C.E., editors. The Biology of Laboratory Rabbit. (ed 2) Academic Press; New York, NY: 1994. pp. 259–292. [Google Scholar]
- 5.Toth L.A., Krueger J.M. Alteration of sleep in rabbits by Staphylococcus aureus infection. Infect Immun. 1988;56:1785–1791. doi: 10.1128/iai.56.7.1785-1791.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toth L.A., Krueger J.M. Hematological effects of exposure to three infective agents in rabbits. J Am Vet Med Assoc. 1988;195:981–985. [PubMed] [Google Scholar]
- 7.Toth L.A., January B. Physiological stabilization of rabbits after shipping. Lab Anim Sci. 1990;40:384–387. [PubMed] [Google Scholar]
- 8.Reavill D.R., Schmidt R.E. Rabbit surgical pathology. In: Fudge A.M., editor. Laboratory Medicine: Avian and Exotic Pets. WB Saunders; Philadelphia, PA: 2000. pp. 353–366. [Google Scholar]
- 9.Fekete S., Huszenicza G. Effects of T-2 toxin on ovarian activity and some metabolic variables of rabbits. Lab Anim Sci. 1993;43:646–649. [PubMed] [Google Scholar]
- 10.Harcourt-Brown F. Butterworth–Heinemann; Oxford, UK: 2002. Textbook of Rabbit Medicine. [Google Scholar]
- 11.Kozma C., Macklin W., Cummins L.M. The anatomy, physiology and the biochemistry of the rabbit. In: Weisbroth S.H., Flatt R.E., Kraus A.L., editors. The Biology of the Laboratory Rabbit. ed 1. Academic Press; San Diego, CA: 1974. pp. 59–64. [Google Scholar]
- 12.Roth S., Conaway H.H. Spontaneous diabetes mellitus in the New Zealand White rabbit. Am J Pathol. 1982;109:359–363. [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr M. Blackwell Scientific Publications; 1989. Veterinary Laboratory Medicine: Clinical Biochemistry and Haematology. [Google Scholar]
- 14.Cox J.C., Gallicchio H.A. Serological and histological studies on adult rabbits with recent naturally acquired encephalitozoonosis. Res Vet Sci. 1978;24:260–261. [PubMed] [Google Scholar]
- 15.Quesenberry K.E., Carpenter J.W. (ed 2) Saunders, Elsevier; St Louis: 2003. Ferrets, Rabbits and Rodents Clinical Medicine and Surgery. [Google Scholar]


