Abstract
Objective
To review the laboratory and clinical evidence of the medicinal value of zinc for the treatment of the common cold.
Data Sources
Published articles identified through Medline (1980–2003) using the search terms zinc, rhinovirus, and other pertinent subject headings. Additional sources were identified from the bibliographies of the retrieved articles.
Study Selection
By the author.
Data Extraction
By the author.
Data Synthesis
Human rhinoviruses, by attaching to the nasal epithelium via the intracellular adhesion molecule-1 (ICAM-1) receptor, cause most colds. Ionic zinc, based on its electrical charge, also has an affinity for ICAM-1 receptor sites and may exert an antiviral effect by attaching to the ICAM-1 receptors in the rhinovirus structure and nasal epithelial cells. Clinical tests of zinc for treatment of common colds have been inconsistent, primarily because of study design, blinding, and lozenge contents. Early formulations of lozenges also were unpalatable. In three trials with similar study designs, methodologies, and efficacy assessments, zinc effectively and significantly shortened the duration of the common cold when it was administered within 24 hours of the onset of symptoms. Recent reports of trials with zinc gluconate administered as a nasal gel have supported these findings; in addition, they have shown that treatment with zinc nasal gel is effective in reducing the duration and severity of common cold symptoms in patients with established illness.
Conclusion
Clinical trial data support the value of zinc in reducing the duration and severity of symptoms of the common cold when administered within 24 hours of the onset of common cold symptoms. Additional clinical and laboratory evaluations are warranted to further define the role of ionic zinc for the prevention and treatment of the common cold and to elucidate the biochemical mechanisms through which zinc exerts its symptom-relieving effects.
Keywords: Zinc, common cold, infectious diseases, dietary supplements, vitamins, and minerals
The common cold is one of the most frequent illnesses among humans, with an estimated 1 billion colds occurring annually in the United States.1 , 2 According to a 1996 survey, approximately 62 million cases of the common cold require medical attention each year, of which 27 million occur in individuals under the age of 17 years.2 Preschool-age children average about five to seven colds each year, compared with two or four colds annually in adults.2 , 3 The common cold has a significant socioeconomic impact, accounting annually for 20 million days of missed work, 22 million days of missed school, 148 million days of restricted activity, and approximately $17 billion in expenditures for physician visits.2
At a Glance
Synopsis: Laboratory and clinical evidence supports the medicinal value of zinc for the treatment of the common cold. Early studies reported inconsistent findings, often because of design or lozenge content inconsistencies. Three recent clinical trials with similar study designs, methodologies, and efficacy assessments provided evidence that zinc can effectively and significantly shorten the duration of the common cold if it is administered within 24 hours of the onset of symptoms. In addition, reports of trials with zinc gluconate administered as a nasal gel have supported these findings.
Analysis: Zinc preparations are widely available and promoted for the treatment of the common cold. Pharmacists should advise patients to begin zinc treatment at the very first sign of cold symptoms, ideally within 24 hours of onset. Zinc products should be continued until symptoms resolve or until otherwise advised by a physician. Pharmacists should reassure patients that the use of zinc at dosages consistent with product labeling is safe. Adverse effects are mild and are generally confined to the gastrointestinal tract. Zinc formulations for the common cold have not been systematically studied in pregnant and lactating females.
Etiology of the Common Cold
Among the viral etiologic agents implicated in the common cold are more than 100 different serotypes of rhinoviruses.2 , 3 Rhinoviruses account for about 30% to 50% of all respiratory illnesses, but in autumn they can cause up to 80% of all upper respiratory infections.3 Corona viruses cause 10% to 15% of upper respiratory infections, and respiratory syncytial virus, parainfluenza viruses, and adenoviruses each cause about 5% of these infections.3
Rhinovirus infection begins with entry of the virus into the anterior nasal mucosa or mucous membrane of the eye, transportation by mucociliary action to the posterior nasopharynx, and invasion of the nasal mucosal epithelium, where the virus replicates rapidly—producing symptoms within 10 to 12 hours.3
Astringent Properties of Zinc
The in vitro effects of zinc (Zn2+) ions and their role in the treatment of the common cold have been researched extensively.4 Zn2+ ions are known for their astringent properties. Loosely bound complexes with hydrated Zn2+ ions, such as zinc acetate and zinc chloride, are astringent, whereas most tightly bound or lipophilic zinc complexes are not.5
The beneficial effects of Zn2+ appear to take place exclusively at the cell membrane.4., 5., 6., 7., 8., 9., 10. Zn2+ reduces the permeability of the cell membrane without penetration into or damage to the cell. Like other astringents, Zn2+ alters the capillary epithelium, thus inhibiting transcapillary movement of plasma protein and reducing local edema, inflammation, exudation, and mucus secretion.5 , 8
Mechanism of Zinc Action
Rhinovirus is transmitted to a susceptible host by either direct contact or large particle aerosols.11 Entry of rhinovirus into the nasal epithelium is mediated by binding to a cellular receptor, intercellular adhesion molecule-1 (ICAM-1).5 , 12 The rhinovirus protein capsid contains four viral proteins arranged in a symmetrical pattern with deep “canyons” descending from the surface.5 , 12 The outer end of the ICAM-1 has a narrow, wedge-shaped segment that can reach and bind with side chains on the canyon floor of the rhinovirus, allowing the virus to penetrate the cell and replicate.12 ICAM-1 also plays a role in inflammatory processes and in the T-cell-mediated host defense system.13 ICAM-1 is used as a receptor for leukocyte function-associated antigen (LFA-1) to bind with leukocytes and initiate and sustain inflammation.12 , 14
The exact biochemical, immunologic, or virologic basis for the action of zinc in the common cold has not been elucidated. However, a leading hypothesis is that Zn2+ is a competitive inhibitor of ICAM-1 in both rhinovirus particles and the nasal epithelium.5 , 12 Zinc ions are small, positively charged spheres with an affinity for ICAM-1 receptor sites that can easily reach the rhinovirus canyon floor.5 By attaching to the ICAM-1 receptor sites, zinc ions prevent the rhinovirus from binding with ICAM-1 and also from effectively entering the cell and replicating.5 , 15 By competing with LFA-1 for ICAM-1 receptor sites, zinc ions are also thought to disrupt the binding of LFA-1 to ICAM-1 and suppress inflammation; this may explain the reduction of inflammation reported by patients treated with zinc for the common cold.12 At least one clinical trial of zinc in the common cold correlated plasma levels of zinc and proinflammatory cytokines.16
Development of Zinc Formulations
The effectiveness of products containing ionic zinc for reducing symptom severity and duration in the common cold may depend on the formulation of zinc and its route of administration.5 Research shows that efficacy is contingent on the use of an ionic form of zinc and that the Zn2+ ions must be delivered to the nasal mucosa to reach and maintain contact with ICAM-1 receptor sites.5 Zinc ions are readily adsorbed into the mucous membranes of the oropharyngeal cavity when applied directly to those tissues.12
The potential medicinal value of zinc has been explored in the laboratory and in clinical trials, and several formulations of zinc have been developed as a treatment for the common cold.5 , 15 , 17 These trials are summarized in Table 1 . Maintaining the ionic availability of zinc has been a challenge to the formulation of effective zinc products.5 Zinc has an offensive metallic taste, and to mask these properties, flavorings and chelating agents were added to some products.5 However, masking the unpleasant taste of zinc reduced the concentration of ionic zinc in some products, thereby reducing efficacy.5 More recently developed oral zinc products have been formulated to provide high levels of ionic zinc and tissue affinity in a pleasant-tasting base.
Table 1.
Overview of Randomized, Placebo-Controlled, Double-Blind, Clinical Trials of Zinc for Treatment of Common Colds
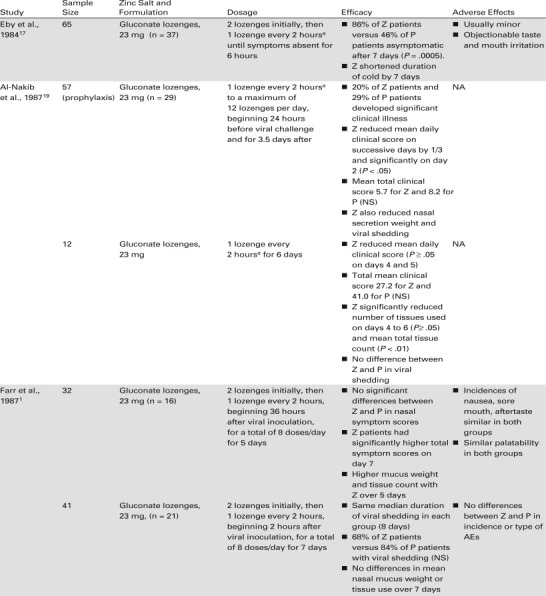
Zinc acetate or gluconate lozenges have been evaluated clinically in patients with the common cold since 1984.1 , 16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26. More recently, researchers theorized that if rhinovirus replication takes place in the nasal mucosa, and zinc's efficacy against the common cold resides in its ability to inhibit viral replication, intranasal administration of zinc may optimize the effectiveness of zinc on cold symptoms.15 , 27 Intranasal preparations of Zn2+ ions—nasal spray and gel formulations—have been evaluated to determine whether direct delivery of ionic zinc to the site of viral infection may be more effective than oral preparations.15 , 27 , 28 The results of the first clinical trial of intranasal zinc for the common cold were reported in 2000.28
Clinical Trials of Zinc
The effects of lozenge or intranasal zinc formulations on the duration or severity of symptoms of the common cold have been evaluated in numerous clinical trials, 15 of which were randomized, double-blind, placebo-controlled studies.1 , 18., 19., 20., 21., 22., 23., 24., 25., 26., 27., 28., 29.
Efficacy of Zinc Lozenge Formulations
Eby and associates were the first to study zinc tablets formulated to dissolve in the mouth.17 Their findings showed that ionic zinc significantly shortened the duration of a cold and reduced the severity of its symptoms; however, the unflavored zinc gluconate was unpalatable and led to a high patient dropout rate.17 Several years later, Al-Nakib et al.19 found that zinc gluconate lozenges in a flavored sugar base that released 23 mg of elemental zinc were effective for the prophylaxis and treatment of induced rhinovirus colds in a small group of volunteers.
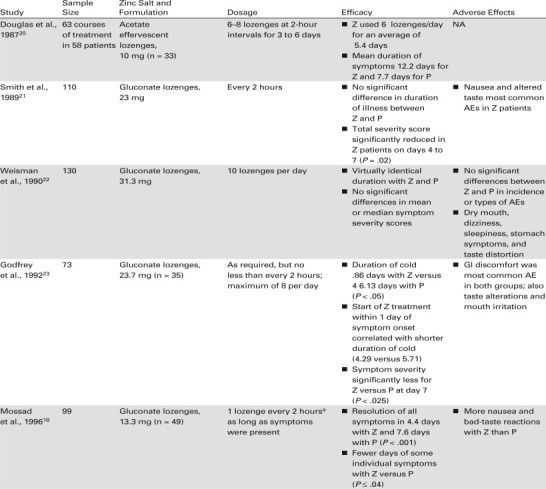
Subsequently, a number of groups conducted trials in patients with experimentally induced and natural colds using other flavoring agents with strong complexing components, such as citric acid, tartaric acid, or mannitol/sorbitol. However, these formulations resulted in highly bound zinc complexes that released very little ionic zinc in the mouth, and the trials were unable to demonstrate treatment effects different from placebo lozenges.1 , 20 , 21 In addition, several trials were considered flawed by the use of a relatively low dose (4.5 mg) of zinc22 , 30 or faulty methodologies.30
Speculating that the lack of effectiveness of zinc in these early studies was the result of inactivation by chelating agents, researchers developed formulations that readily released most zinc ions (90%–93%) from the complex and evaluated them in clinical trials.23 In the first of these Godfrey et al.23 found that zinc gluconate in a hard candy base (elemental zinc 23.7 mg) used every 2 hours while awake significantly reduced the duration of the cold and the severity of symptoms. Another key finding of the study was that zinc reduced the duration of the cold by an additional 1.42 days (P = .035) if treatment was initiated within 24 hours of the onset of cold symptoms. 23
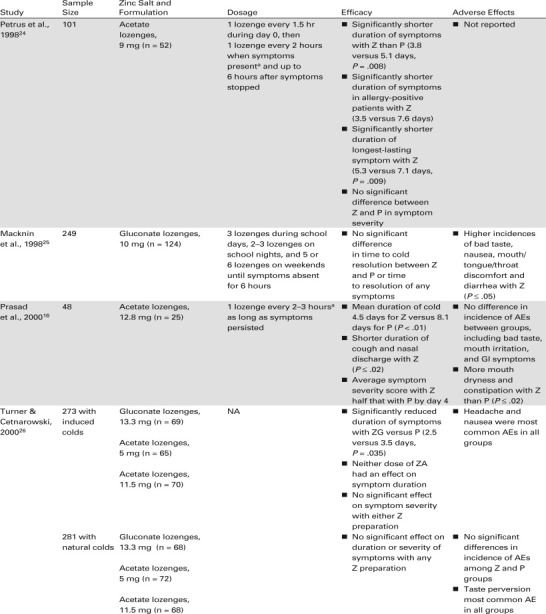
The results of the study by Godfrey et al. were corroborated by three of five studies published over the next 8 years, all of which were comparable in study design to the earlier trial.16 , 18 , 23 , 24 The three studies used zinc gluconate or zinc acetate lozenges (9 mg to 13.3 mg elemental zinc) in a calcium lactate or dextrose base.16 , 18 , 24 They enrolled 50 or more patients, used the same 4-point scale to rate symptom severity (0, none; 1, mild; 2, moderate; 3, severe), began treatment within 24 hours of symptom onset, and had virtually identical dosage regimens (1 lozenge every 2 hours as long as symptoms persisted). Zinc consistently and significantly reduced the duration of the cold and the duration of cold symptoms in all three studies. The duration of the cold was reduced by 1.3 to 3.6 days (P < .008 to P < .001).
Two other randomized, placebo-controlled studies failed to find consistently significant differences between zinc and placebo in the duration of the cold and the duration of cold symptoms.25 , 26 Both studies differed considerably in methodology from the three studies that showed significant differences between zinc and placebo treatment. For example, a study by Turner and Cetnarowski26 evaluated zinc gluconate (elemental zinc 13.3 mg) and zinc acetate (elemental zinc 5 and 11.5 mg) lozenges in adults with induced or natural colds. Zinc gluconate significantly reduced the duration of induced colds compared with placebo (P = .035) but not the severity of cold symptoms, and neither zinc formulation affected the duration or severity of natural colds. These zinc acetate lozenges may have been formulated in a base that contained plant-derived oils.31 The high temperatures used in manufacturing the lozenge may have caused the oils to react with positively charged zinc ions, yielding fat complexes that were incapable of releasing Zn2+ ions.31
Efficacy of Intranasal Formulations
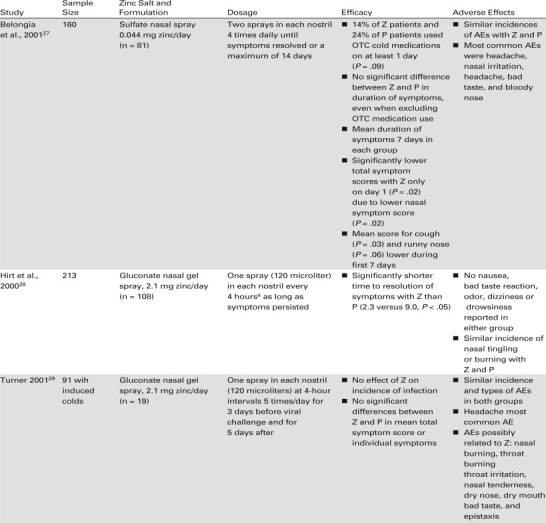
The inconsistent results with zinc lozenges, coupled with the uncertain mechanism of action of ionic zinc, led researchers to explore delivery of Zn2+ ions directly to the nasal mucosa, the site of rhinovirus infection.15 , 27., 28., 29.
An early intranasal spray formulation contained 0.l2% zinc as zinc sulfate (elemental zinc 0.011 mg per dose), given as two inhalations in each nostril four times daily until symptoms resolved.27 The only clinical trial of this formulation was a randomized, double-blind, placebo-controlled study in which patients were enrolled within 24 hours of symptom onset, and symptoms were evaluated on a 4-point scale. There were no differences between the zinc nasal spray and placebo spray in the median duration of all symptoms nor in the median duration of nasal symptoms. A post hoc analysis found that after adjustment for differences in baseline symptom severity, the zinc spray significantly reduced the total symptom and total nasal symptom scores on day 1 only. The total maximum daily dose of zinc delivered in this preparation (0.044 mg) may have been too low to be effective.15 , 27
Further evaluations of intranasal zinc gel in randomized, double-blind trials have been published.15 , 28 , 29 The intranasal gel formulation consisted of 33 mM ionic zinc in an emulsion with a pH of 7.2 and delivering 120 microliters per spray.15 , 28 , 29 The nasal gel spray has been investigated in three randomized, double-blind, placebo-controlled studies: two for the treatment of natural colds and one as prophylaxis and treatment of induced colds.15 , 28 , 29
The design of the first study, by Hirt et al.,28 was similar to that of the evaluation of zinc gluconate lozenges by Mossad et al. in 1996.18 More than 100 patients in each group were treated with either the nasal gel spray or placebo, beginning less than 24 hours after the onset of cold symptoms, and rated their symptoms on a 4-point scale twice daily.28 They were instructed to use the zinc nasal gel every 4 hours while awake until they no longer experienced symptoms. Using an efficacy endpoint of complete resolution of symptoms, the investigators found that the duration of symptoms in patients treated with the zinc nasal gel was 2.3 days, compared with 9.0 days in the placebo group (difference: 6.7 days (P < .05). This 75% reduction in the duration of symptoms was consistent with that reported in the preliminary study of the nasal gel formulation for patients treated within 24 hours of the onset of symptoms.28
A study by Turner29 evaluated the prophylactic effectiveness of intranasal zinc gluconate in healthy volunteers, and treatment consisted of a single spray per nostril 5 times a day for 3 days prior to rhinovirus inoculation and for 5 days afterward. Of those patients who developed rhinovirus infection following inoculation, administration of the nasal gel had no effect on the total and nasal symptom scores.29
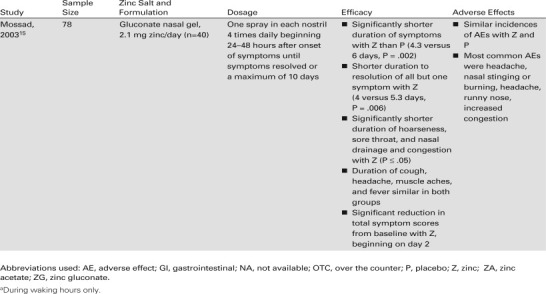
The results of the most recent clinical trial of the zinc nasal gel formulation were reported by Mossad. in 2003.15 This trial demonstrated that the same nasal gel formulation, delivering a total daily dose of elemental zinc 2.1 mg, was effective when administered after cold symptoms had been present for 24 to 48 hours. As in the study by Hirt et al.,28 the patients used the nasal gel four times a day until their symptoms resolved and rated their symptoms on a 4-point scale twice daily.15 The zinc nasal gel significantly reduced the median duration of symptoms from 6 days with placebo to 4.3 days, a difference of 1.7 days (P = .002). Treatment with the spray gel also significantly reduced the time to resolution of all but one cold symptom by 1.3 days (P = .006). In addition to its effect on symptom duration, zinc nasal gel also significantly reduced the time to resolution of nasal drainage and congestion, hoarseness, and sore throat by 1.5 to 2.0 days (P ≤ .05). Scores for nasal symptoms in patients treated with the zinc nasal gel were significantly lower than those in patients treated with the placebo nasal gel on days 3 through 8; similarly, scores for throat symptoms were significantly lower on days 2 through 4.
Safety of Zinc Formulations
No serious adverse events have been reported in clinical trials using either the zinc lozenge or the zinc nasal gel for treatment of the common cold. The more common adverse effects in clinical trials of the lozenge and nasal spray formulations included headache, dry mouth and/or throat, taste alteration, nasal irritation or burning, and mild gastrointestinal complaints.1 , 16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26., 27.
In studies of the zinc nasal gel formulation, the type and incidence of adverse effects were similar in the zinc and placebo groups. Nasal tingling, stinging, or burning sensation was the most frequently reported adverse event, but patients reported no cases of dry mouth or throat or altered sense of taste.15 , 28 , 29
Although isolated, anecdotal reports in the lay media have alleged an association between the use of zinc nasal gel and the onset of anosmia, in no clinical trial of intranasal zinc gluconate gel has there been a single report of lost or diminished smell. In at least once instance, this association was based on findings from 1930s-era studies evaluating the nasal administration of an entirely different compound—zinc sulfate—for polio prevention.32 Zinc sulfate is a mineral salt that reacts with water to produce sulfuric acid and zinc oxide. By comparison, zinc gluconate is a weak organic salt that dissolves to form positively charged zinc ions and negatively charged gluconate—a naturally occurring, nontoxic compound found in all human tissues.
Conclusion
Rhinoviruses are believed to be the primary etiologic agent of the common cold. ICAM-1 is a receptor present on both the nasal epithelium and in the rhinovirus structure. Attachment of the rhinovirus to endothelial cells by ICAM-1 produces inflammation and other immunologic responses characteristic of the common cold. Proposed mechanisms of action for ionic zinc are competition with ICAM-1 receptors to prevent rhinovirus attachment and replication in nasal epithelial cells and reduction of inflammation in nasal tissues.
Double-blind, randomized, placebo-controlled clinical trials have been inconsistent in their findings with regard to the effectiveness of zinc gluconate or acetate lozenges. However, two well-designed trials provide evidence that zinc gluconate can shorten the duration of the common cold when administered within 24 hours of the onset of symptoms.15 , 28 Inconsistent results are likely attributable to reductions in the efficacy of zinc secondary to changes in formulation in efforts to mask the unpleasant properties of zinc.
Recent reports have demonstrated that treatment with zinc nasal gel is effective in reducing the duration and severity of common cold symptoms within 24 hours of symptom onset.15 , 28
Zinc preparations are widely available in retail outlets and are promoted to the general public. Pharmacists are likely to receive inquiries from patients regarding the safety and efficacy of zinc for the common cold. As discussed in this report, evidence exists to support the therapeutic effect of zinc when started early in the course of a cold, ideally in the prodromal period. Pharmacists should reassure patients of zinc's safety when used at dosages consistent with product labeling. Adverse effects are mild and are generally confined to the gastrointestinal tract. Patients should be counseled to begin zinc at the very first sign of cold symptoms, ideally within 24 hours of onset of cold symptoms. Zinc products should be continued until symptoms resolve or until otherwise advised by a physician. Zinc formulations for the common cold have not been systematically studied in pregnant and lactating females.
Footnotes
Disclosure: Dr. Hulisz has received either research support, consulting, and/or speaking honoraria from the following pharmaceutical companies in the past 48 months: AstraZeneca, Aventis, Improvita, Matrixx, Merck, Novartis, Pfizer, and Wyeth.
References
- 1.Farr B.M., Conner E.M., Betts R.F. Two randomized controlled trials of zinc gluconate lozenge therapy of experimentally induced rhinovirus colds. Antimicrob Agents Chemother. 1987;31:1183–1187. doi: 10.1128/aac.31.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fendrick A.M. Viral respiratory infections due to rhinoviruses: current knowledge, new developments. Am J Ther. 2003;10:193–202. doi: 10.1097/00045391-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Heikkinen T., Järvinen A. The common cold. Lancet. 2003;361:51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merluzzi V.J., Cipriano D., McNeil D. Evaluation of zinc complexes on the replication of rhinovirus 2 in vitro. Res Comm Chem Pathol Pharmacol. 1989;66:425–440. [PubMed] [Google Scholar]
- 5.Novick S.G., Godfrey J.C., Godfrey N.J. How does zinc modify the common cold? Clinical observations and implications regarding mechanisms of action. Med Hypotheses. 1996;46:295–302. doi: 10.1016/s0306-9877(96)90259-5. [DOI] [PubMed] [Google Scholar]
- 6.Sugarman B. Zinc and infection. Rev Infect Dis. 1983;5:137–147. doi: 10.1093/clinids/5.1.137. [DOI] [PubMed] [Google Scholar]
- 7.Bashford C.L., Alder G.M., Graham J.M. Ion modulation of membrane permeability: effect of cations on intact cells and on cells and phospholipid bilayers treated with pore-forming agents. J Membrane Biol. 1988;103:79–94. doi: 10.1007/BF01871934. [DOI] [PubMed] [Google Scholar]
- 8.Bashford C.L., Alder G.M., Menestrina G. Membrane damage by hemolytic viruses, toxins, complement, and other cytotoxic agents. J Biol Chem. 1986;261:9300–9308. [PubMed] [Google Scholar]
- 9.Bashford C.L., Menestrina G., Henkart P.A., Pasternak C.A. Cell damage by cytolysin. Spontaneous recovery and reversible inhibition by divalent cations. J Immunol. 1988;141:3965–3974. [PubMed] [Google Scholar]
- 10.Pasternak C.A. Virus, toxin, complement: common actions and their prevention by Ca2 + or Zn2 + Bioassays. 1987;6:14–19. doi: 10.1002/bies.950060105. [DOI] [PubMed] [Google Scholar]
- 11.Turner R.B. The treatment of rhinovirus infections: progress and potential. Antiviral Res. 2001;49:1–14. doi: 10.1016/S0166-3542(00)00135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novick S.G., Godfrey J.C., Pollack R.L. Zinc-induced suppression of inflammation in the respiratory tract, caused by infection with human rhinovirus and other irritants. Med Hypotheses. 1997;49:347–357. doi: 10.1016/s0306-9877(97)90201-2. [DOI] [PubMed] [Google Scholar]
- 13.van de Stolpe A., van der Saag P.T. Intercellular adhesion molecule-1. J Mol Med. 1996;74:13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- 14.Bella J., Kolatkar P.R., Marlor C.W. The structure of the two aminoterminal domains of human ICAM-1 suggests how it functions as a rhinovirus receptor and as an LFA-1 integrin ligand. Proc Natl Acad Sci USA. 1998;95:4140–4145. doi: 10.1073/pnas.95.8.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mossad S.B. Effect of zincum gluconicum nasal gel on the duration and symptom severity of the common cold in otherwise healthy adults. QJM. 2003;96:35–43. doi: 10.1093/qjmed/hcg004. [DOI] [PubMed] [Google Scholar]
- 16.Prasad A.S., Fitzgerald J.T., Bao B. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. Ann Intern Med. 2000;133:245–252. doi: 10.7326/0003-4819-133-4-200008150-00006. [DOI] [PubMed] [Google Scholar]
- 17.Eby G.A., Davis D.R., Halcomb W.W. Reduction in duration of common colds by zinc gluconate lozenges in a double-blind study. Antimicrob Agents Chemother. 1984;25:20–24. doi: 10.1128/aac.25.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mossad S.B., Macknin M.L., Medendorp S.V. Zinc gluconate lozenges for treating the common cold. A randomized, double-blind, placebocontrolled study. Ann Intern Med. 1996;125:81–88. doi: 10.7326/0003-4819-125-2-199607150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Al-Nakib W., Higgins P.G., Barrow I. Prophylaxis and treatment of rhinovirus colds with zinc gluconate lozenges. J Antimicrob Chemother. 1987;20:893–901. doi: 10.1093/jac/20.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douglas R.M., Miles H.B., Moore B.W. Failure of effervescent zinc acetate lozenges to alter the course of upper respiratory tract infections in Australian adults. Antimicrob Agents Chemother. 1987;31:1263–1265. doi: 10.1128/aac.31.8.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith D.S., Helzner E.C., Nuttall C.E., Jr. Failure of zinc gluconate in treatment of acute upper respiratory tract infections. Antimicrob Agents Chemother. 1989;33:646–648. doi: 10.1128/aac.33.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weismann K., Jakobsen J.P., Weismann J.E. Zinc gluconate lozenges for common cold. A double-blind clinical trial. Dan Med Bull. 1990;37:279–281. [PubMed] [Google Scholar]
- 23.Godfrey J.C., Sloane B.C., Smith D.S. Zinc gluconate and the common cold: a controlled clinical study. J Intern Med Res. 1992;20:234–246. doi: 10.1177/030006059202000305. [DOI] [PubMed] [Google Scholar]
- 24.Petrus E.J., Lawson K.A., Bucci L.R. Randomized, double-masked, placebo-controlled clinical study of the effectiveness of zinc acetate lozenges on common cold symptoms in allergy-tested subjects. Curr Ther Res. 1998;59:595–607. doi: 10.1016/S0011-393X(98)85058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macknin M.L., Piedmonte M., Calendine C. Zinc gluconate lozenges for treating the common cold in children. A randomized controlled trial. JAMA. 1998;279:1962–1967. doi: 10.1001/jama.279.24.1962. [DOI] [PubMed] [Google Scholar]
- 26.Turner R.B., Cetnarowski W.E. Effect of treatment with zinc gluconate or zinc acetate on experimental and natural colds. Clin Infect Dis. 2000;31:1202–1208. doi: 10.1086/317437. [DOI] [PubMed] [Google Scholar]
- 27.Belongia E.A., Berg R., Liu K. A randomized trial of zinc nasal spray for the treatment of upper respiratory illness in adults. Am J Med. 2001;111:103–108. doi: 10.1016/s0002-9343(01)00765-3. [DOI] [PubMed] [Google Scholar]
- 28.Hirt M., Nobel S., Barron E. Zinc nasal gel for the treatment of common cold symptoms: a double-blind, placebo-controlled trial. Ear Nose Throat J. 2000;79:778–780. 782. [PubMed] [Google Scholar]
- 29.Turner R.B. Ineffectiveness of intranasal zinc gluconate for prevention of experimental rhinovirus colds. Clin Infect Dis. 2001;33:1865–1870. doi: 10.1086/324347. [DOI] [PubMed] [Google Scholar]
- 30.Jackson J.L., Peterson C., Lesho E. A meta-analysis of zinc salts lozenges and the common cold. Arch Intern Med. 1997;157:2373–2376. [PubMed] [Google Scholar]
- 31.Eby G.A. Elimination of efficacy by additives in zinc acetate lozenges for common colds [letter] Clin Infect Dis. 2001;32:1520. doi: 10.1086/320177. [DOI] [PubMed] [Google Scholar]
- 32.Rutty C.J. The middle-class plague: epidemic polio and the Canadian State, 1936–1937. Canadian Bull Med Hist. 1996;13:277–314. doi: 10.3138/cbmh.13.2.277. [DOI] [PubMed] [Google Scholar]


