Graphical abstract
Keywords: Elderberry, Chemical composition, Nutritional value, Bioactive compounds, Antibacterial and antiviral properties, Medicinal use
Highlights
-
•
Elderberry as a rich source of antioxidant compounds and natural dyes used in food industry.
-
•
The influence of processing on the content of antioxidants in elderberry fruit and flowers.
-
•
The anti-microbiological activity and healing potential of elderberry.
-
•
The use of elderberry raw material in fruit processing and pharmaceutical industry.
-
•
Toxic glycosides and lectins as compounds making all parts of elder potentially life threatening.
Abstract
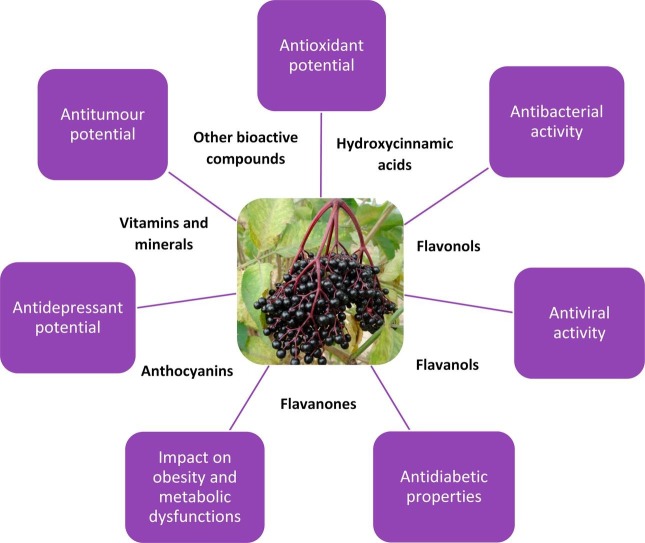
European black elderberry naturally occurs in most of Europe and has been introduced into various parts of the world for fruit and flower production. Elderberry is rich in nutrients, such as carbohydrates, proteins, fats, fatty acids, organic acids, minerals, vitamins and essential oils. Elderberry also contains cyanogenic glycosides which are potentially toxic. Polyphenols, known for their free radical scavenging (antioxidant) activity, are the most important group of bioactive compounds present in elderberry in relatively high concentration. The high antioxidant activity of elderberry fruit and flowers is associated with their therapeutic properties. Elderberry has for a long time been used in folk medicine as a diaphoretic, antipyretic and diuretic agent. In recent years it was also found to have antibacterial, antiviral antidepressant and antitumour and hypoglycemic properties, and to reduce body fat and lipid concentration. Due to its health-promoting and sensory properties, elderberry is used primarily in food and pharmaceutical industry.
1. Taxonomy, geographical distribution, habitat
The genus Sambucus L. (elderberry) belongs to the Adoxaceae family, which consists of 7 different genera that have berry fruit (NRCS Natural Resources Conservation Service, 2016a). Sambucus L. consist of 5–30 species and 6–11 subspecies, depending on the taxonomy system (NPGS, 2016, NRCS, 2016b). The most commonly occurring species is Sambucus nigra L. Its common names include elder, elderberry, black elder, European elder, European elderberry and European black elderberry (ITIS, 2016). Sambucus nigra L. has three subspecies: S. nigra L. ssp. nigra, S. nigra L. ssp. canadensis, S. nigra L. ssp. cerulea. The two latter ones, which were in the past considered separate species, are native to North America. European black elderberry naturally occurs in most of Europe (except for certain parts of Scandinavia and Russia) and in North Africa (the Atlas Mountains of Algeria, Morocco and Tunisia). In the east of Europe, the plant’s distribution ends on approximately 55°E. The altitude limit depends on the latitude (e.g. 900 m a.s.l. in the Tatra Mountains and 2200 a.s.l. in the Atlas Mountains). Sambucus nigra L. ssp. nigra has been introduced into various parts of the world including E. Asia, N. America, New Zealand and the southern part of Australia (Hultén & Fries, 1986).
Seed maturation in October is determined by mean temperatures. Below 7.2 °C seeds are unable to mature (Lennon & Turner, 1995), and the maximum mean temperature in which seed maturation is possible is 15 °C, as is the case in South Europe (Atkinson & Atkinson, 2002). Sambucus nigra L. usually grows in open fields or at woodland edges. It sometimes occurs under deep shade in woodland but such bushes are often survivors of former more open conditions. In Central Europe elder is commonly found in woodlands of various mixtures of willow, poplar, elm and oak (Ellenberg, 1988). Black elderberry favours disturbed, base-rich and nitrogen-rich soils, and phosphate-rich soils. High levels of available phosphate and potassium and of mineralizable nitrogen were observed in a series of soils from S. nigra sites (Atkinson & Atkinson, 2002). Nevertheless, despite its preferred natural environment, elderberry can be grown on a wide variety of soils (Charlebois, Byers, Finn, & Thomas, 2010).
2. Production and harvest
The parts of plant material that are relevant for production are fruit and flower extract (Christensen, Kaack, & Fretté, 2008). In many countries, large quantities of raw material are collected from wild plants. However, since the beginning of 1980s, black elderberry has been planted and grown on experimental and commercial plots in some European countries and in the USA, Canada, New Zealand and Chile (Finn, Thomas, Byers, & Kemal, 2008). In Central Europe, the most popular cultivars are 'Alleso', 'Korsor' ‘Samyl’, ’Sampo’, all of them bred in Denmark. Total fruit production as compared to other berry crops is rather small and, therefore, the relevant statistical data are scarce or incomplete. The biggest producers are Germany (Höhne, 2014), Denmark (Kaack, Fretté, Christensen, Landbo, & Meyer, 2008), Poland (Waźbińska & Puczel, 2002), Italy (Mohebalian, Cernusca, & Aguilar, 2012), Austria and the Czech Republic (Vítová, Divišová, Sůkalová, & Matějíček, 2013). According to the German Federal Statistical Office, the production of elderberry fruit amounted to 1576 tonnes (580 ha) in 2013 and to 1.759 tonnes (583 ha) in 2015 (DStatis, 2016). In Austria, the total crop of elderberry fruit varied between 7.3 and 10.1 thousand tonnes between 2013 and 2015 (Statistics Austria, 2016).
In the USA, elderberries are grown commercially on a small scale in the states of New York, Ohio, Oregon (Way, 1981), Missouri and Kentucky. However, in the USA, the most commonly grown are hybrids between S. nigra ssp. nigra and ssp. canadensis. Breeders at the Experiment Station in Geneva (Cornell University) discovered that such hybrids are resistant to viruses spread in elderberry plantations by nematodes (Way, 1981). Elderberries are planted in single rows. The distance between bushes in the row is between 1.5 and 2.5 m (Kaack, 1984). Well developed bushes require a spacing of 2–2.5 meters increased by the width of machines (Roper & McManus, 1998). The recommended maximum density of elderberry bushes is approximately 1200 bushes/ha (Charlebois et al., 2010).
Plants come into full production after 3–4 years. The elderberry fruit is not well suited for mechanical harvesting because it does not separate readily from the pedicels (Charlebois et al., 2010), however in some countries it is collected using machinery constructed for other berry crops and adapted for elderberry (McKay, 2001). The yield depends on the growing site, growing condition, planting distance and cultivar, and can vary from 1.3 kg/bush for wild-harvested genotypes to 23.0 kg/bush for some cultivars (Kaack, 1984, Kollanyi et al., 2005, Waźbińska et al., 2004). Elderberry flowering cymes harvested for fresh use or drying are clipped when all flowers are open. Individual flowers are easily removed from the cyme by rubbing over screens. The flowers may then be dried or frozen for future use (Byers, Thomas, & Gold, 2014).
3. Chemical composition and nutritional value
The chemical composition of Sambucus nigra is rich and depends on different factors, such as cultivar, location, ripening stage and climatic conditions. In respect of carbohydrates, elderberry berries contain 7.86–11.50% of total sugar and 2.8–8.55% of reducing sugar. According to Veberic et al., the content of total sugar in elderberries ranged from 68.53 to 104.16 g/kg FW, depending on the cultivar or selection. The main sugars identified were glucose (33.33–50.23 g/kg FW) and fructose (33.99–52.25 g/kg FW), whereas only small amounts of sucrose were present in the fruit (0.47–1.68 g/kg FW, which made up 0.33% according to Vulić et al.). Carbohydrates found in Sambucus nigra fruit also include dietary fibre, in particular, pectin, pectic acid, protopectin, Ca-pectate and cellulose. Elderberry is a source of whole protein – its content is 2.7–2.9% in berries, 2.5% in flowers and 3.3% in leaves. This protein includes sixteen amino acids, nine of which are essential; the total content of the essential amino acids is approx. 9% in flowers and 11.5% in leaves. Glutamic acid, aspargic acid and alanine were reported as the dominant amino acids. Fats are accumulated mostly in elderberry seeds (fat content: 22.4%) and seed flour (fat content: 15.9%). The major fatty acids are polyunsaturated fatty acids, which constitute 75.15% and 21.54% of total fatty acids in seeds and seed flour, respectively, whereas monounsaturated fatty acids (14.21% and 4.21%) and saturated fatty acids (10.64% and 4.81%) make up a significantly smaller share. Polyunsaturated fatty acids that are present in highest concentrations in seeds are α-linolenic, linoleic and oleic acid. Organic acids constitute 1.0–1.3% of the berry content. Veberic et al. detected four organic acids in elderberry fruit. The most abundant of them was citric acid (3.08–4.81 g/kg FW), whose contents was quite high in comparison to other fruits. The next one was malic acid (0.97–1.31 g/kg FW) and the remaining two acids were shikimic acid (0.14–0.93 g/kg FW) and fumaric acid (0.10–0.29 g/kg FW). Minerals are located both in berries and flowers. The mineral matter content represents 0.90–1.55% of the fruit mass, and includes K (2953–5494 mg/kg FW), P (735–1337), Ca (574–1528), Na (13–146), Mg (396–739), Fe (12.4–84.7), Zn (1.9–11.3), Mn (3.6–9.5), Cu (1.7–2.9). Additionally, berries contain also heavy metals such as lead (0.016–0.098 mg/kg FW) and cadmium (0.039–0.053), as well as nitrates (V) (2.63–3.21 mg/kg FW) and nitrates (III) (0.69–0.86) (Akbulut et al., 2009, Borowska et al., 2000, Diviš et al., 2015, Dulf et al., 2013, Fazio et al., 2013, Kislichenko and Vel’ma, 2006, Kołodziej et al., 2012, Mratinić and Fotirić, 2007, Ognik et al., 2006, Veberic et al., 2009, Vulić et al., 2008). Elderberry fruit and flowers also include essential oils (around 0.01% in fruit), consisting of approx. 53 compounds in berries and 58 compounds in flowers (Kaack, 2008, Kaack and Christensen, 2008).
With regard to vitamins present in S. nigra, several studies mention ascorbic acid, but the quantitative findings are pretty inconsistent. According to Kaack and Austed, the content of ascorbic acid ranges between 6 and 25 mg/100 g of elderberry fruit and depends on the cultivar. The highest content of this vitamin was found in ‘Mammut’ and the lowest–in ‘Sambu’ and ‘Haschberg’ (Kaack & Austed, 1998). According to other studies, vitamin C content in wild elderberry is 13–35 mg/100 g and 28–34 mg/100 g, depending on the location (Akbulut et al., 2009, Mratinić and Fotirić, 2007). Still others report a vitamin C concentration between 32.75–44.42 mg/100 g, with the highest content being found in the ‘Samyl’ cultivar and the lowest amounts in wild elderberries (Borowska et al., 2000, Vulić et al., 2008). Furthermore, elderberry seed flour is a source of α-Tocopherol (0.49 µg/g of oil), which has the highest vitamin E bioactivity, as well as γ-Tocopherol (2.63 µg/g), which shows better antioxidant potential (Fazio et al., 2013).
4. Harmful compounds
All parts of the elderberry contain cyanogenic glycosides, the most abundant of which are sambunigrin and prunasin. Furthermore, elderberry contains m-hydroxysubstituted glycosides, such as zierin and holocalin (DellaGreca et al., 2000, Jensen and Nielsen, 1973). These compounds are potentially toxic and life-threatening, because they can be hydrolysed resulting in the release of cyanide (Bromley, Hughes, Leong, & Buckley, 2005). However, they occur primarily in unripe berries and are degraded during heat treatment (Williamson, Driver, & Baxter, 2009). The highest amounts of sambunigrin are present in elder leaves (27.68–209.61 µg/g FW), lower amounts were found in flowers (1.23–18.88 µg/g FW), whereas berries contain the lowest amounts of this compound (0.08–0.77 µg/g FW). It was also found that the content of sambunigrin in elderberry changes depending on the growing altitude. The highest content of sambunigrin was recorded on a hilltop (1048 and 1077 m a.s.l.), which had lower temperatures and higher solar radiation as compared to other altitudes studied (209–858 m a.s.l.) (Senica, Stampar, Veberic, & Mikulic-Petkovsek, 2016). Other toxic compounds present in elderberry are lectins sharing a high amino acids sequence homology and some of them having the N-glycosidase activity, which is typical for type II ribosome-inactivating proteins (RIPs). Type II RIPs occur in elderberry bark (e.g. nigrin b-SNA V, SNA I), fruit (nigrin f) and seeds (nigrin s) (Stripe, 2004, Tejero et al., 2015). Moreover, elderberry contains the allergen Sam n1, which causes type 1 allergy and some tryptic peptides of Sam n1 demonstrate a high amino acid sequence with lectins and type 2 RIPs located in Sambucus (Förster-Waldl et al., 2003, Jiménez et al., 2013, Jimenez et al., 2013). However, it was discovered that incubation in a boiling water bath for 5–10 min, made lectins completely sensitive to hydrolytic enzymes in vitro and thus reduced the risk of allergenicity (Jiménez et al., 2017).
5. Content of bioactive compounds and their stability during processing
The bioactive compounds found in elderberry are primarly polyphenols and anthocyanins (their content is presented in Table 1 ). The fruit of Sambucus nigra is an important source of phenolic compounds – their content in the elderberries is relatively high in comparison to other fruits (Leja, Mareczek, & Nanaszko, 2007). The main polyphenols in elderberry fruit are chlorogenic acid, neochlorogenic acid, cryptochlorogenic acid, quercetin, quercetin-3-rutinoside (rutin), quercetin-3-glucoside (isoquercitrin), kaempferol-3-rutinoside, kaempferol-3-glucoside (astragaline), isorhamnetin-3-rutinoside and isorhamnetin-3-glucoside. The primary flavonol in this plant is rutin, while the other flavonols, isoquercitrin and astragaline occur in elderberries in smaller amounts (Dawidowicz et al., 2006, Kaack and Austed, 1998, Kaack et al., 2008, Lee and Finn, 2007, Pietta and Bruno, 1992, Veberic et al., 2009). The concentration of quercetin measured in the fruit of thirteen elderberry cultivars ranged from 29 (‘Gentofte’) to 60 mg/100 g (‘Haschberg’) of fruit (Kaack & Austed, 1998). Furthermore, Sambucus nigra berries contain small amounts of tannins with a low condensation degree. These are procyanidins, such as epicatechin (88.4% of total tannins) and catechin (11.6% of total tannins) and their thiol derivatives (Oszmiański & Moutounet, 1995).
Table 1.
The content of phenolic compounds in elderberry fruit and flowers.
| Compound | Content in fruit | Content in flowers | Source |
|---|---|---|---|
| Total polyphenolics | 364–582 mg GAE/100 g FW, 4917–8974 mg GAE/100 g DW extr, 2684–4480 mg CAE/100 g FW, 622–672 mg CE/100 g FW | 1021.7 mg GAE/100 g FW, 3702–5333 mg CAE/100 g FW, 194 mg GAE/g dry extr | 68,25,64,74,89,16 |
| 3-caffeoylquinic acid (neochlorogenic acid) | 0.7–4.4 mg ChAE/100 g FW, 0.05–0.40 mg ChAE/g DW | 510.6 mg/kg FW, 0.8–2.4 mg/g DW | 68,59,74,17 |
| 4-caffeoylquinic acid (cryptochlorogenic acid) | 1.2–2.5 mg ChAE/100 g FW | 31.4 mg/kg FW, 0.6–1.5 mg/g DW | 68,74,17 |
| 5-caffeoylquinic acid (chlorogenic acid) | 26.4–35.9 mg ChAE/100 g FW, 0.53–1.22 mg ChAE/g DW | 10.1–20.7 mg/g DW | 68,59,17 |
| 5-caffeoylquinic acid 1 | 2779.3 mg/kg FW | 74 | |
| 5-caffeoylquinic acid 2 | 187.2 mg/kg FW | 74 | |
| p-coumaric acid hexoside | 28.9 mg/kg FW | 74 | |
| 3-p-coumaroylquinic acid | 40.5 mg/kg FW, 0.4–1.9 mg/g DW | 74,17 | |
| 5-p-coumaroylquinic acid 1 | 80.9 mg/kg FW, 0.5–1.2 mg/g DW | 74,17 | |
| 5-p-coumaroylquinic acid 2 | 14.6 mg/kg FW | 74 | |
| 3-feruloylquinic acid | 717.3 mg/kg FW | 74 | |
| 5-feruloylquinic acid | 370.6 mg/kg FW | 74 | |
| 1,5-Di-caffeoylquinic acid | 8.0–13.9 mg/g DW | 17 | |
| 3,4-Di-caffeoylquinic acid | 0.4–1.2 mg/g DW | 17 | |
| 3,5-Di-caffeoylquinic acid | 0.5–3.2 mg/g DW | 17 | |
| 4,5-Di-caffeoylquinic acid | 0.2–1.5 mg/g DW | 17 | |
| Dicaffeoylquinic acid 1 | 217.4 mg/kg FW | 74 | |
| Dicaffeoylquinic acid 2 | 64.6 mg/kg FW | 74 | |
| Dicaffeoylquinic acid 3 | 43.1 mg/kg FW | 74 | |
| p-Coumaroyl-caffeoylquinic acid 1 | 11.7 mg/kg FW | 74 | |
| p-Coumaroyl-caffeoylquinic acid 2 | 10.6 mg/kg FW | 74 | |
| Kaempferol dihexoside 1 | 40.2 mg/kg FW | 74 | |
| Kaempferol dihexoside 2 | 33.5 mg/kg FW | 74 | |
| Kaempferol triglycoside | 44.3 mg/kg FW | 74 | |
| Kaempferol-3-rutinoside | 0.7–1.2 mg rutin/100 g FW | 646.9 mg/kg FW, 0.2–3.0 mg/g DW | 68,74,17 |
| Kaempferol-3-glucoside | 1.05–1.79 g/100 g extr | 3.5 mg/kg FW, 1.28–2.50 g/100 g extr | 74,20 |
| Kaempferol acetylhexoside | 13.5 mg/kg FW | 74 | |
| Quercetin | 2.7–4.5 mg CGE/100 g FW, 29–60 mg/100 g FW | 107,55 | |
| Quercetin hexoside pentoside 1 | 15.1 mg/kg FW | 74 | |
| Quercetin hexoside pentoside 2 | 2.0 mg/kg FW | 74 | |
| Quercetin-3-rutinoside (rutin) | 42.6–95.6 mg rutin/100 g FW, 6–14 mg rutin/g DW, 10.86–15.39 g/100 g extr, 35.59–52.02 mg CGE/100 g FW, 1.94–6.31 mg rutin/g DW | 3265.1 mg/kg FW, 11.6–42.3 mg/g DW, 132.69–202.08 g/100 g extr | 68,107,97,74,59,17,20 |
| Quercetin-3-galactoside | 10.3 mg/kg FW | 74 | |
| Quercetin-3-glucoside (isoquercitrin) | 3.9–14.9 mg rutin/100 g FW, 1.79–3.01 g/100 g extr, 6.4–26.5 mg CGE/100 g FW, 0.11–1.08 mg rutin/g DW | 20.2 mg/kg FW, 0.4–1.9 mg/g DW, 5.37–9.67 g/100 g extr | 68,107,74,59,17,20 |
| Quercetin dihexoside | 12.2 mg/kg FW | 74 | |
| Quercetin acetylglucoside | 94.8 mg/kg FW | 74 | |
| Quercetin-3-6-acetylglucoside | 0.9–2.8 mg/g DW | 17 | |
| Isorhamnetin dihexoside | 24.5 mg/kg FW | 74 | |
| Isorhamnetin-3-rutinoside | 0.3–2.2 mg rutin/100 g FW | 888.0 mg/kg FW, 2.0–7.5 mg/g DW | 68,74,17 |
| Isorhamnetin-3-glucoside | 0.1–0.3 mg rutin/100 g FW | 61.6 mg/kg FW, 0.2–1.0 mg/g DW | 68,74,17 |
| Isorhamnetin pentoside | 3.4 mg/kg FW | 74 | |
| Isorhamnetin acetylhexoside | 37.8 mg/kg FW | 74 | |
| Catechin | 10.7 mM/kg FW | 6.8 mg/kg FW | 74,86 |
| Epicatechin | 81.3 mM/kg FW | 254.3 mg/kg FW | 74,86 |
| Procyanidin trimer | 155.6 mg/kg FW | 74 | |
| Naringenin | 734.2 mg/kg FW | 74 | |
| Total anthocyanins | 170–343 mg CGE/100 g FW, 518–1028 mg CGE/100 g FW, 408.6–1066.6 mg CGE/100 g DW, 285–1326 mg CGE/100 g extr, 39–153 mg CGE/g DW, 664–1816 mg CGE/100 g FW, 8.33–101.40 mg CSE/g DW, 1.9–20.2 g CSE/kg FW, 48.46–52.89 g/100 g extra, 602.9–1265.3 mg CGE/100 g FW, 254–841 mg/100 g FW | 68,107,97,25,53,59,20,55 | |
| Cyanidin-3-sambubioside-5-glucoside | 16.0–59.2 mg CGE/100 g FW, 14–47 mg CGE/100 g FW, 0.86–11.50 mg CSE/g DW, 19.52–53.49 mg CGE/100 g FW | 68,107,59,55 | |
| Cyanidin-3,5-diglucoside | 8.2–19.5 mg CGE/100 g FW, 0.12–5.22 mg CSE/g DW, 5–36 mg CGE/100 g FW, 7.41–23.29 mg CGE/100 g FW | 68,107,59,55 | |
| Cyanidin-3-sambubioside | 122.2–269.1 mg CGE/100 g FW, 4.62–40.30 mg CSE/g DW, 269–656 mg CGE/100 g FW, 27.05–27.70 g/100 g extr, 270.8–630.8 mg CGE/100 g FW, 15–59 mg CGE/g DW | 68,107,97,59,20,55 | |
| Cyanidin-3-glucoside | 204.6–481.4 mg CGE/100 g FW, 2.74–49.50 mg CSE/g DW, 361–1266 mg CGE/100 g FW, 21.41–25.18 g/100 g extr, 221.4–586.4 mg CGE/100 g FW, 14–78 mg CGE/g DW, 42.4–254.3 mg/100 g extr | 68,107,97,25,59,20,55 | |
| Cyanidin-3-rutinoside | 1.49–9.63 mg CGE/100 g FW, Tr | 68,107 | |
| Pelargonidin-3-glucoside | Tr | 68 | |
| Delphinidin-3-rutinoside | Tr | 68 |
* Abbreviation: GAE – gallic acid equivalents, CAE – caffeic acid equivalents, ChAE – chlorogenic acid equivalents, CGE – cyanidin-3-glucoside equivalents, CSE – cyanidin-3-sambubioside equivalents, DW – dry weight FW – fresh weight.
Phenolic compounds include anthocyanins, which are water-soluble glycosides or acylglycosides of anthocyanidins being oxygenated derivatives of flavylium salts (Prior, 2003). They are well-known functional compounds used as food colorants, and they reduce the oxidative stress by scavenging free radicals, which makes them potential chemopreventive agents. Therefore, as excellent food colorants with antioxidative properties, anthocyanins are a good alternative to synthetic dyes due to their safety as well as potential nutritional and therapeutic effect (Bridle and Timberlake, 1997, Espín et al., 2000, Stintzing et al., 2002).
Elderberry is a very rich source of anthocyanins in comparison to other fruits. The fruit of Sambucus nigra contains anthocyanins, especially cyanidin-3-glucoside and cyanidin-3-sambubioside. Two other (minor) anthocyanins are cyanidin-3,5-diglucoside, and cyanidin-3-sambubioside-5-glucoside. In addition, trace quantities of cyanidin-3-rutinoside, pelargonidin-3-glucoside and delphinidin-3-rutinoside were identified in the fruit of certain elderberry cultivars. Their content depends on cultivar/variety, ripening stage, growing season or kind and method of extraction (Borowska et al., 2000, Duymuş et al., 2014, Fiorini, 1995, Kaack, 1997, Kaack and Austed, 1998, Kaack et al., 2008, Lee and Finn, 2007, Leja et al., 2007, Pliszka et al., 2005, Seabra et al., 2010, Veberic et al., 2009, Youdim et al., 2000). The cultivars most abundant in anthocyanins are ‘Mammut’, ‘Samdal’, ‘Sampo’ and ‘Samyl’, while ‘Finn Sam’, ‘Haschberg’, ‘Allesoe’, but also wild elderberry have the lowest quantities of these bioactive compounds. Total anthocyanin content varies almost threefold depending on the cultivar, e.g. ‘Haschberg’ was found to contain 664 mg of total anthocyanins/100 g, while the concentration of these compounds in ‘Sampo’ was 1816 mg/100 g. (Kaack, 1997, Kaack and Austed, 1998, Pliszka et al., 2005). The polyphenol content changes at different stages of fruit ripening, and each compound shows its own individual change pattern during this process. The content of anthocyanins, especially cyanidin-3-sambubioside-5-glucoside, cyanidin-3-sambubioside and cyanidin-3-glucoside, usually increases as berries ripen (Kaack et al., 2008). The content of polyphenols and anthocyanins is also dependent on the growing season. Lee and Finn (2007) found that the total anthocyanin content in the fruit of ‘Korsør’ in 2005 was almost twice higher (343 mg of cyanidin-3-glucoside/100 g) than in 2004 (176 mg of cyanidin-3-glucoside/100 g). Total phenolic content was also much higher in 2005 (510 and 582 mg of gallic acid/100 g) than in 2004 (364 and 387 mg of gallic acid/100 g), respectively, for ‘Haschberg’ and ‘Korsør’. The same applied to individual anthocyanins and almost all investigated polyphenols in the berries of both cultivars. The predominant anthocyanin found in elderberry juice concentrate or must is cyanidin-3-sambubioside, while cyanidin-3-glucoside was found to be the most abundant anthocyanin in elderberry wine (Bermúdez-Soto and Thomás-Barberán, 2004, Schmitzer et al., 2010) and in the fruit of thirteen elderberry cultivars except ‘Allesøe’ (Kaack & Austed 1998).
Alcoholic fermentation of berries causes changes in the content of phenolic compounds and anthocyanins, which also leads to colour changes. The concentration of polyphenols, such as neochlorogenic acid, chlorogenic acid, quercetin-3-rutinoside, quercetin-3-glucoside, kaempferol-3-rutinoside, cyanidin-3-sambubioside-5-glucoside, cyanidin-3,5-diglucoside, cyanidin-3-glucoside or cyanidin-3-rutinoside, was observed to be higher in wine than in must, except cyanidin-3-sambubioside, which significantly decreased during alcoholic fermentation. The storage and aging of elderberry wine resulted in the decrease in the content of each analysed compound, and after three years the total phenolic content fell by 21%, whereas the total anthocyanins content was lower even by 94% compared to the young wine. Due to the degradation of anthocyanins, the colour of elderberry wine also changed from bright-red to brown–red hues. Antioxidant activity was also higher in young wine than in must and dropped after aging (Schmitzer et al., 2010).
The method of juice production affects the content of bioactive compounds. For instance, elderberry juices processed using enzymatic treatment (pectinolysis) demonstrated a lower average content of most investigated phenolic compounds compared to the juices produced without enzymatic treatment (Kaack et al., 2008).
The content of anthocyanins in elderberry products is affected by pH, storage time and storage temperature. It was observed that the extension of storage time and increase in pH and storage temperature resulted in the decrease in the content of anthocyanins in elderberry juice concentrate (Walkowiak-Tomczak, Czapski, & Młynarczyk, 2016).
Elderberry pomace, although it makes up 25–40% of total fruit weight, is very rich in anthocyanins: it contains 75–98% of total anthocyanins found in fresh elderberries (Brønnum-Hansen, Jacobsen, & Flink, 1985). The major anthocyanin in elderberry pomace is usually cyanidin-3-glucoside (14–78 mg/g DW) and the sum of this compound and cyanidin-3-sambubioside (15–61 mg/g DW) represents approximately 90% of the total anthocyanin content, which ranges from 39 to 153 mg/g of dry matter, depending on the extraction method. On the other hand, the content of rutin in elderberry pomace is 6–14 mg/g DW (Seabra et al., 2010).
Sambucus nigra flowers contain even higher amounts of phenolic compounds in comparison to the fruit and leaves of this species (Dawidowicz et al., 2006, Kołodziej and Drożdżal, 2011). The main group of phenolic compounds found in elderflowers are hydroxycinnamic acids, the most important of which is chlorogenic acid. Other hydroxycinnamic acids present in elderflowers include, among others, neochlorogenic acid, cryptochlorogenic acid, 3- and 5-feruloylquinic acid, and dicaffeoylquinic acids, including notably 1,5-Di-caffeoylquinic acid. The second important group of polyphenols comprise flavonols, from which several glycosides of quercetin, kaempferol and isorhamnetin were detected in elderflower extracts. The major polyphenol from this group is quercetin-3-rutinoside (rutin). This flavonol together with kaempferol-3-rutinoside and isorhamnetin-3-rutinoside constitute more than 90% of total flavonoids in most analysed genotypes. Other groups of phenolic compounds occurring in elderflowers are flavanols, which include catechin, epicatechin and procyanidin trimer, and flavanones, e.g. naringenin. Elderflowers contain more of certain phenolic compounds (rutin, isoquercitrin and astragaline) than berries or leaves (Christensen et al., 2008, Dawidowicz et al., 2006, Mikulic-Petkovsek et al., 2015). The content of phenolic compounds in elderflowers, similarly to elderberries, depends on the genotype as well as on liquid composition and extraction conditions. For instance, the highest content of chlorogenic acid was found in the Virum genotype (20.7 mg/g DW), while the lowest in the Sambu genotype (10.1 mg/g DW). On the other hand, the Samocco genotype was shown to have the highest (42.3 mg/g DW) whereas the Jaegerspris genotype the lowest (11.6 mg/g DW) concentration of rutin (Christensen et al., 2008). Moreover, the highest content of flavonols in elderflowers was a result of higher temperature of extraction – 214.25 and 139.32 g/100 g extr. for 100 °C and 20 °C, respectively (Dawidowicz et al. 2006).
6. Antioxidant potential
Elderberry fruit is characterized by high antioxidant activity, which ranges from 82.08 to 89.25% of inhibition in relation to the DPPH radical. The antioxidant properties of elderberries are primarily attributable to the presence of phenolic compounds and depend largely on the chemical structure of molecules and composition of individual berries (Pliszka et al., 2005, Rice-Evans et al., 1996, Zheng and Wang, 2003). Anthocyanins significantly influence the antioxidant activity of elderberries. With the increase of anthocyanin concentration the antioxidant activity also increases, but only to a certain level above which this parameter starts to fall (Pliszka et al., 2005).
According to Duymuş et al., elderberry extracts show antiradical activity (towards DPPH), but not as strong as ascorbic acid used as standard. The IC50 value (concentration needed to scavenge 50% of free radicals) was 117 µg/ml for 70% acetone extract and 123 µg/ml for water extract of elderberry, while it was 8 µg/ml for ascorbic acid. As for TEAC values (ABTS radical scavenging activity), these ranged from 0.89 (acidified methanol extract) to 1.96 mmol Trolox equiv./l (70% acetone extract) and the highest value was almost the same as for ascorbic acid (1.97 mM). Additionally, the TEAC value for cyanidin-3-glucoside standard was nearly the same as for water extract (1.87 and 1.85 mM, respectively). Elderberries are also able to inhibit the linoleic acid oxidation. The highest inhibition rate (58%) was observed for 70% ethanol extract; however, none of the analysed elderberry extracts was as active as BHT control (Duymuş et al., 2014). A commercial concentrate of elderberry fruit demonstrated lower radical scavenger capacity towards DPPH compared to other studied sources of anthocyanins (extracts of fresh chokeberries, blackthorn fruit and strawberries) and natural and synthetic antioxidants (α-tocopherol, BHT and BHA) (Espin et al., 2000).
The antioxidant potential of elderberry was also investigated in in vitro cultured human colonic mucosa cells. An elderberry extract at a concentration of 1 mg of freeze-dried elderberry powder/ml reduced the excessive intracellular reactive oxygen species production by 22% and oxidative DNA damage by 46% in the colonic cells. Additionally, the inhibition of oxidant-induced mutagenicity by 26% in the Salmonella typhimurium strain was observed. These results indicate that elderberry fruit is able to protect colon cells against the harmful effects of oxidative stress (Olejnik et al., 2016).
Flowers of Sambucus nigra usually have higher antioxidant activity than berries and leaves. According to Kołodziej and Drożdżal, TEC50 (time required to reduce the initial DPPH concentration by 50%) was 23–75 s for flowers, whereas TEC50 for fruit was 91–133 s. In another study, elderflowers exhibited stronger DPPH radical inhibition activity (91.95–94.15%) in comparison to berries (50.25–67.69%) and leaves (16.76–48.52%). These values also depended on the temperature of alcoholic extraction–in each case, extraction at a temperature of 100 °C resulted in a higher percentage rate of free radical inhibition than extraction at a lower temperature (20 °C) (Dawidowicz et al., 2006, Kołodziej and Drożdżal, 2011). The results obtained by Stoilova et al. demonstrate that the extract of elderflowers possess greater radical scavenging properties in comparison to rutin, BHT and BHA used as standards. Elderflower extract showed the highest inhibition of DPPH (97.70%) at the lowest concentration (10 µg/ml) in comparison with the standards (77.47; 82.40; 89.98% and 40; 20; 20 µg/ml, respectively) and the IC50 concentration of S. nigra extract for the inhibition was the lowest (0.152 µg/ml) compared to IC50 of rutin, BHT and BHA (14.65; 4.407; 1.120 µg/ml, respectively). In addition, this extract eliminated the highly reactive hydroxyl radicals, thus preventing the deoxyribose degradation, more effectively as compared to rutin and quercetin (IC50 = 0.0122; 0.505; 4.61 µg/ml, for elderflower, rutin and quercetin extracts, respectively). Furthermore, extract of elderflowers showed metal-chelative properties, preventing the initiation of hydroxyl radicals, that were also stronger than those of rutin and quercetin standards (Stoilova, Wilker, Stoyanova, Krastanov, & Stanchev, 2007).
7. Bioavailability of elderberry antioxidants
In a study which investigated the metabolism of various anthocyanins after oral administration of elderberry juice, small amounts of Sambucus nigra anthocyanins were identified in human urine. Seven volunteers took 4 g of spray-dried elderberry juice, corresponding to 50 ml of fresh juice and containing 500 mg of anthocyanins (12.5%). Several peaks appeared in the chromatogram and two of them had the same retention time as the major cyanidin-3-glucoside and cyanidin-3-sambubioside from juice. Only 0.04% of the consumed cyanidin-3-sambubioside and 0.01% of cyanidin-3-glucoside were excreted into urine and the highest excretion of anthocyanins was noted 3–4 h after the intake of elderberry juice. Even lower amounts of unmetabolized cyanidin-3,5-diglucoside and cyanidin-3-sambubioside-5-gluside were identified in a few samples; however, other substances shown in the chromatogram that were possible metabolites of Sambucus nigra anthocyanins were not identified (Murkovic, Mülleder, Adam, & Pfannhauser, 2001). According to another study concerning the absorption of anthocyanins, these antioxidants are ingested by humans in their original, unchanged glycosylated forms. The content of anthocyanins in blood plasma and urine was analysed in four women treated with 12 mg of elderberry extract, containing 720 mg anthocyanins consisting mainly of cyanidin-3-sambubioside and cyanidin-3-glucoside. The blood plasma samples, which were collected before and between 10 min and 24 h after the consumption of the extract exhibited at least five additional anthocyanin-like compounds, primarily cyanidin-3-sambubioside and cyanidin-3-glucoside. The levels of all detected compounds decreased after 1 h, disappearing after 24 h. Their maximum concentration in blood plasma was reached within 72 min after the intake, and its average value was 97.4 nmol/l of total anthocyanins. In the urine samples, collected before and between 0 and 24 h after the intake, at least six compounds were detected at 520 nm after extract consumption, and the major anthocyanins were also cyanidin-3-sambubioside and cyanidin-3-glucoside. Most anthocyanin-like compounds appeared in urinary excretion during the first 4 h. The total amount of the anthocyanins excreted during 24 h after the intake of the elderberry extract was 397.0 µg of cyanidin-3-glucoside equivalents, while the average excretion rate of these compounds during the first 4 h was 77.2 µg/h and during the second 4 h–13.4 µg/h (Milbury, Cao, Prior, & Blumberg, 2002). In a study by Bitsch et al., cyanidin glucosides and glucuronides were detected in urinary excretion. Seven healthy volunteers consumed 150 mL of elderberry juice concentrate which contained 3.57 g of total anthocyanins. Within 5 h after the ingestion of the juice dose, the urinary excretion of total cyanidin glucosides (cyanidin-3,5-diglucoside, cyanidin-3-sambubioside, cyanidin-3-glucoside) and their glucuronide conjugates was 1.876 mg of cyanidin-3,5-diglucoside, which constituted 0.053% of the administered dose. The percentage of the excreted glucuronides was only 0.003%, while the share of the glucuronides in relation to all excreted anthocyanins was 6.2%. The maximum excretion rate of anthocyanins was reached after one hour of intake, followed by a sharp drop (Bitsch et al., 2004). In another study, the urinary excretion, the plasma concentration of elderberry anthocyanins as well as other antioxidant parameters were determined. Eight participants received doses of elderberry juice (200, 300 and 400 ml) in separate treatments. The total anthocyanin content, including cyanidin-3-sambubioside and cyanidin-3-glucoside, in urinary excretion increased with the increase in the juice dose taken. The lowest elderberry juice dose (200 ml), which contained 361 mg of total anthocyanins, caused the excretion of, on average, 41.4 µg/h of anthocyanins, while after the highest dose (400 ml) containing 722 mg of total anthocyanins, the average urinary excretion of these compounds was 113.2 µg/h. Within 7 h, the values of the fraction of orally ingested total anthocyanins in urinary excretion were 0.033, 0.038 and 0.040% after the intake of 200, 300 and 400 ml of juice, respectively. The aim of the second treatment was to investigate the effect of the intake of 400 ml elderberry juice or water (control) on the content and antioxidant capacity of total phenolics and anthocyanins and of ascorbic and uric acid in blood plasma. Both the content and the antioxidant activity of total phenolics and anthocyanins increased after juice ingestion, reaching the highest level 1 h after the intake, whereas the values of these parameters did not change in the control group. At the same time, the concentrations of ascorbic and uric acid were unaffected by the elderberry juice or water intake (Netzel et al., 2005). Czank et al. found that the bioavailability of anthocyanins was higher and the diversity of their metabolites was greater than previously thought. Eight healthy male participants received 500 mg bolus dose of isotopically labelled cyanidin-3-glucoside (6,8,10,3′,5′-13C5-cyanidin-3-glucoside) and their samples of blood, urine, breath and faeces were examined within 48 h after the ingestion. The average recovery of 13C in breath, urine and faeces was 43.9% and the relative bioavailability from urine and breath was 12.38%. The 13C labelled metabolites consisted of 24 compounds and included phase II conjugates of C3G and cyanidin, degradants, phase II conjugates of protocatechuic acid, phenylacetic acids, phenylpropenoic acids and hippuric acid. The metabolites reached a 42-fold peak of serum concentration relative to 13C5-C3G, and were detected much later than the parent anthocyanin (Czank et al., 2013). The results of the above-mentioned studies on the bioavailability of elderberry antioxidants are summarized in Table 2 .
Table 2.
Bioavailability of elderberry antioxidants.
| Human subjects | Elderberry preparation | Dose | Duration | Urinary excretion | Cmax | tmax | Metabolites | References |
|---|---|---|---|---|---|---|---|---|
| 7 volunteers (3 female, 4 male) | 4 g of spray-dried elderberry juice (50 ml of fresh juice equivalent), containing 12.5% of anthocyanins | 500 mg of anthocyanins | 6 h | 0.04% of cyanidin-3-sambubioside, 0.01% of cyanidin-3-glucoside | – | – | Probable but not identified | Murkovic et al. (2001) |
| 4 female subjects | 12 mg of elderberry extract dissolved in 500 ml of water | 720 mg of anthocyanins | 24 h | 0.055% | 97.4 nmol/l | 72 min | – | Milbury et al. (2002) |
| 7 volunteers (6 female, 1 male) | 150 ml of concentrated elderberry juice | 3569 mg of anthocyanins | 5 h | 0.053% | – | – | Glucuronide conjugates (0.003%) | Bitsch et al. (2004) |
| 8 volunteers (4 female, 4 male) | 200 ml of elderberry juice | 361 mg | 7 h | 0.033% | – | Netzel et al. (2005) | ||
| 300 ml of elderberry juice | 541 mg | 7 h | 0.038% | |||||
| 400 ml of elderberry juice | 722 mg of anthocyanins | 7 h | 0.040% | |||||
| 400 ml of elderberry juice | 2240 mg of total phenolics | 4 h | – | 16.1 mg/l | 1 h | |||
| 710 mg of anthocyanins | – | 52.6 µg/l | 1 h | |||||
| 8 male subjects | 500 mg of isotopically labelled cyanidin-3-glucoside (6,8,10,3′,5′-13C5-C3G) | 500 mg (as two 250 mg capsules) | 48 h | 5.37% | 0.14 µmol/l | 1.81 h | Phase II conjugates of C3G and cyanidin, degradants, phase II conjugates of protocatechuic acid, phenylacetic acids, phenylpropenoic acids, hippuric acid | Czank et al. (2013) |
*abbreviation: Cmax – maximum plasma concentration, tmax – time to reach Cmax.
8. Medicinal potential
Flowers and berries of Sambucus nigra are known as a traditional remedy for various kinds of ailments and diseases and both are commonly used in folk medicine (Kohlmünzer, 1998). They are primarily used to treat common symptoms related to cold, feverish conditions, coughing, nasal congestion, mucous discharge and influenza, as well as a preventively to strengthen the immune system (Elliman, 1994, Grieve, 1971, Hoffmann, 1990, The ABC Clinical Guide to Elder Berry, 2003). Due to the presence of flavonoids, elderflowers demonstrate primarily diaphoretic, antipyretic and diuretic properties. They obturate the capillary walls, improve their flexibility and prevent infiltration of red blood cells and plasma outside the vessels thanks to the content of compounds (rutin) having the properties of vitamin P. Moreover, elderflowers show anti-inflammatory and antibacterial properties and therefore are used for gargling to treat sore throats or as compresses to treat conjunctivitis. They are most often used as infusions of dried flowers for internal or external application. The fruit of Sambucus nigra, similarly to flowers, exhibits diaphoretic, antipyretic and diuretic properties, but apart from that it act as a laxative and detoxifier, hence elderberries are often a component of herbal mixtures used as a remedy for constipation or to help slimming. In addition, elder fruit demonstrates a moderate analgesic effect and can be used as an adjuvant painkiller against migraine, sciatica and neuralgic pains (Beaux et al., 1999, Bown, 1995, Grieve, 1984, Ożarowski and Jaroniewski, 1987, Schmersahl, 1964). Not only elder flowers and berries are beneficial to health, but also bark, root, stem and leaves of Sambucus nigra have been used especially by the rural population as medicine or food. For instance, elder bark has been known for its diuretic and slimming properties, while elder leaves may help strengthen resistance to infectious diseases (Baytop, 1984, Wójcik, 2002). Nowadays remedies against cold, flu and other infectious illnesses are being developed using Sambucus nigra fruit or flowers. They are used as components of dietary supplements in the form of syrups, drops, tablets, capsules, lozenges, aerosols, emulsions or suspensions. Frequently reported in the literature is the antiviral activity of Sambucus nigra fruit. A very popular supplement with such properties that is sold as a prophylactic agent in many countries is the Sambucol preparation, patented in Israel by Dr. Madeleine Mumcuoglu (Kinoshita et al., 2012, Martens, 2008, Wierzbicki, 2002, Zakay-Rones et al., 2004). The healing properties of elderberry are mostly associated with the presence of phenolic compounds, which are characterized by a strong antioxidant activity and therefore are able to eliminate free radicals and counteract the oxidative stress, a factor causing the degradation of the human body, thus contributing to the development of a number of diseases (Gollucke, Peres, Odair, & Ribeiro, 2013). Thole et al. used mass spectrometry and liquid chromatography-mass spectrometry to demonstrate the chemopreventive potential of elderberry. It was found that elderberry fruit extract, or rather an extract fraction that in addition to the phenolic compounds included e.g. iridoid monoterpene glycosides, phytosterols and sesquiterpenes, caused a strong induction of quinone reductase and inhibition of cyclooxygenase-2. This indicates antitumor activity of Sambucus nigra fruit, especially that preventing the initiation and promotion of cancerogenesis stages (Thole et al., 2006). The results of the studies on the health-promoting properties of elderberry are shown in Table 3 (in vitro) and in Table 4 (in vivo).
Table 3.
Medicinal potential of elderberry (Sambucus nigra) preparations – in vitro studies.
| Medicinal potential | Elderberry preparation | Results | References |
|---|---|---|---|
| Antibacterial activity | Ethanol extract of elder flowers and berries | Inhibitory activity for most of the 13 nosocomial pathogens (e.g. Staphylococcus sp., Bacillus cereus, Salmonella poona, Staphylococcus aureus, Pseudomonas aeruginosa) | Hearst et al. (2010) |
| Aqueous extract of elder leaves | Moderate activity against Bacillus cereus and Serratia marcescens | ||
| Standardized liquid extract of elderberry (Rubini®) | Reduction in the growth of Streptococcus pyogenes, Streptococcus Group C and G, Branhamella catarrhalis | Krawitz et al. (2011) | |
| Antiviral activity | Standardized liquid extract of elderberry (Rubini®) | Reduction in the spread of the foci size of influenza B human virus, reduction in the foci of influenza A (KAN-1) human virus in Madin-Darby canine kidney cell culture (MDCK) | Krawitz et al. (2011) |
| Elderberry extract: | Inhibition of influenza A (H1N1) human virus in MDCK | Roschek et al. (2009) | |
| concentration of 252 µg/ml | IC50 for H1N1 | ||
| concentration of 1000 µg/ml | 100% inhibition of H1N1 | ||
| Concentrated elderberry juice: | Inhibition of H1N1 in MDCK | Kinoshita et al. (2012) | |
| extract concentration of 720 µg/ml | IC50 for H1N1 (samples were given during infection) | ||
| extract concentration of 3600 µg/ml | IC50 for H1N1 (samples were given immediately after infection) | ||
| Elderberry extract | Inhibition of infectious bronchitis virus (IBV) – a pathogenic chicken coronavirus. Reduction in IBV titers by several orders of magnitude, in dependence of the dose applied | Chen et al. (2014) | |
| Elder bark extract | High activity against feline immunodeficiency virus (FIV) – common domestic cats virus | Uncini Manganelli et al. (2005) | |
| Extract concentration of 500 µg/ml | 100% inhibition of syncytia formation | ||
| Diabetes treatment | Elder flowers extract (1 g/L) | Increase in glucose uptake, glucose oxidation and glycogenesis in mice abdominal muscles without added insulin | Gray et al. (2000) |
| Impact on urinary parameters | Elder flower extract in the form of capsules | No apparent inhibitory effect on uridine diphospho-glucuronosyltransferase (UGTs). IC50 values were higher than 500 µg/mL for UGTs enzymes (UGT1A1, UGT1A4, UGT1A6, UGT1A9 and UGT2B7) | Choi et al. (2014) |
| Antitumour activity | Elderberry extract (extract fraction including phenolic compounds, iridoid monoterpene glycosides, phytosterols and sesquiterpenes) | Prevention of the initiation and promotion of cancerogenesis stages: strong induction of quinone reductase and inhibition of cyclooxygenase-2 | Thole et al. (2006) |
Table 4.
Medicinal potential of elderberry (Sambucus nigra) preparations – in vivo studies.
| Medicinal potential | Study design | Elderberry preparation | Dosage | Duration | Results | References |
|---|---|---|---|---|---|---|
| Antiviral activity | Female mice (6 weeks old) infected with influenza A virus | Concentrated elderberry juice separated in three fractions (low, medium and high molecular weight) | 2 times/day | 14 days | Suppression of the viral yield in the bronchoalveolar lavage fluids (BALFs) and lungs; increase in the level of IFV specific neutralizing antibody in the BALFs and serum and in the level of the secretory IgA in the BALFs and feces | Kinoshita et al. (2012) |
| R, D-B, P-C, 60 patients (18–54 years) presenting influenza A and B symptoms | Standardized elderberry extract (Sambucol® syrup) | 15 ml, 4 times/day | 5 days | Symptoms of influenza A and B virus ebbed four days earlier in the elderberry group compared to the placebo group | Zakay-Rones et al. (2004) | |
| R, D-B, P-C, 64 patients (16–60 years) presenting flu symptoms | Proprietary elderberry extract as lozenge (175 mg of extract in each lozenge) | 4 lozenges/day | 48 h | 28% of patients in the elderberry group were void of all flu symptoms and 60% of patients experienced a relief of some symptoms, whereas the placebo group demonstrated no improvement | Kong (2009) | |
| Diabetes treatment | Male rats (28 weeks old) divided into four groups including diabetic rats | Polyphenolic elderberry extract | 0.040 g/kg body every 2 days | 16 weeks | Improvement of the bone mineral density and of the antioxidative capacity of serum; reduction in the body fat in diabetic rats; decrease in the lipid peroxidation level in serum; improvement of the osteoporosis status | Badescu et al. (2012) |
| Male rats (11 weeks old) divided into four groups including type 2 diabetic rats fed a high-fat diet | Elderberry polar extract | 350 mg/kg body/day | 4 weeks | The polar extract lowered fasting blood glucose, the lipophilic extract decreased insulin secretion. Both extracts reduced insulin resistance | Salvador et al. (2017) | |
| Elderberry lipophilic extract | 190 mg/kg body/day | |||||
| Effect on metabolic dysfunctions in obesity | Male mice (8 weeks old) divided into four groups including diet-induced obese mice | Elderberry extract (13% of anthocyanins) | 0.25% of extract (20–40 mg anthocyanins/kg body1.25% of extract (100–200 mg/kg body | 16 weeks | Both elderberry groups had a lower liver weight and serum TAG concentration, and lower serum inflammatory markers, insulin resistance and hepatic lipids compared to the control obese group; the addition of 1.25% of extract reduced liver cholesterol and PPARγ2 mRNA compared to both other obese groups | Farrel, Norris, Ryan et al. (2015) |
| Effect on cholesterol and HDL dysfunctions | Male mice (10 weeks old) in a mouse model of hyperlipidemia and HDL dysfunction | Elderberry extract (13% of anthocyanins) | 1.25% of extract (100–200 mg anthocyanins/kg body | 6 weeks | No significant differences in serum lipids between groups; reduction in aspartate transaminase and fasting glucose in the elderberry group; changes in hepatic and intestinal mRNA with an improvement in HDL function and a reduction in hepatic cholesterol levels; increase in serum paraxonase-1 arylesterase activity in the elderberry group | Farrel, Norris, Lee et al. (2015) |
| Effect on lipid and antioxidant status | R, P-C, D-B, 34 healthy volunteers (20 males, 24 females) | 400 mg spray-dried elderberry powder in gelatinous capsules (10% of anthocyanins in each capsule) | 3 times/day | 2 and 3 weeks | No significant differences in the changes in serum lipids and vitamin A, E and β-carotene in the elderberry group were observed compared to the placebo group; a decrease in the level of vitamin C in both groups; | Murkovic et al. (2004) |
| 6 volunteers | The same | A single dose | 1 day | An increase in total anthocyanins in serum; | ||
| Effect on weight reduction | 80 participants | Elderberry juice with flower extract; tablets containing berry powder and flower extract (1 mg anthocyanins, 370 mg flavonol glycosides, 150 mg hydroxycinnamates); Tablets containing Asparagus officinalis powder (19 mg saponins) | At least 3 L of elderberry juice, 3 Sambucus nigra tablets and 9 Asparagus officinalis tablets/day | 15 days | The BMI index dropped by approx. 3%, the weight fell by an average of 3.2 kg, systolic blood pressure decreased by an average of over 5% and diastolic blood pressure by 2.5% | Chrubasik et al. (2008) |
| Effect on weight reduction and urinary parameters | 11 volunteers | Diluted (1:5) concentrate of elderberries (from 120 g of berries) and flowers (flower juice and extract from 3.9 g of dried flowers) | 200 ml divided into up 6 portions | 7 days | An average weight reduction was 2.6 kg; no effect on pH, hydrogen ion concentration and 24 h hydrogen excretion in urine; no effect on the solubility of stone-inducing ions | Walz and Chrubasik (2008) |
| Antidepressant potential | Male mice divided into six groups | S. nigra extract and S. ebulus extract; | 200–1200 mg/kg | – | S. nigra showed better activity than S. ebulus; reduction in the immobility time and increase in the activity in the Sambucus groups compared to the control group (measured by the forced swimming test and tail suspension test); a dose of 1200 mg/kg of extract significantly increased the activity compared to the imipramine group | Mahmoudi et al. (2014) |
| Imipramine | 10 mg/kg |
*Abbreviation: R – randomized, D-B – double-blind, P-C – placebo-controlled.
9. Antibacterial and antiviral properties
The antibacterial activity of elder flowers, berries and leaves (aqueous or ethanol extracts of freeze-dried elder concentrates) was studied against 13 nosocomial pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), which is known as a clinically significant pathogen connected with infections of skin and soft tissue. Despite a 100-fold dilution, ethanol extracts of both elder flowers and berries exhibited inhibitory activity for most of the analysed bacteria, both Gram-positive, such as Staphylococcus sp. or Bacillus cereus, and Gram-negative, such as Salmonella poona or Pseudomonas aeruginosa. Elderflower extracts displayed a higher antimicrobial efficacy and larger zones of inhibition against a broad range of bacteria, particularly MRSA (17 mm) or Pseudomonas aeruginosa (9 mm), than other extracts. A ten-fold diluted aqueous extract of elder leaves showed moderate activity against the development of Bacillus cereus and Serratia marcescens (6 mm), but was not able to inhibit the growth of any crucial nosocomial pathogens (Hearst et al., 2010). Sambucus nigra fruit possesses antimicrobial activity against human pathogenic bacteria that cause infections of the upper respiratory tract. The addition of (Rubini®), a standardized liquid extract of elderberry, at a concentration of 10%, to bacterial liquid cultures decreased the growth of Gram-positive bacteria strains of Streptococcus pyogenes and Streptococcus Group C and G, as well as Gram-negative bacteria Branhamella catarrhalis, by more than 70% compared to the control samples. A concentration of 20% of the elderberry extract inhibited the bacterial development by 99% (Krawitz et al., 2011).
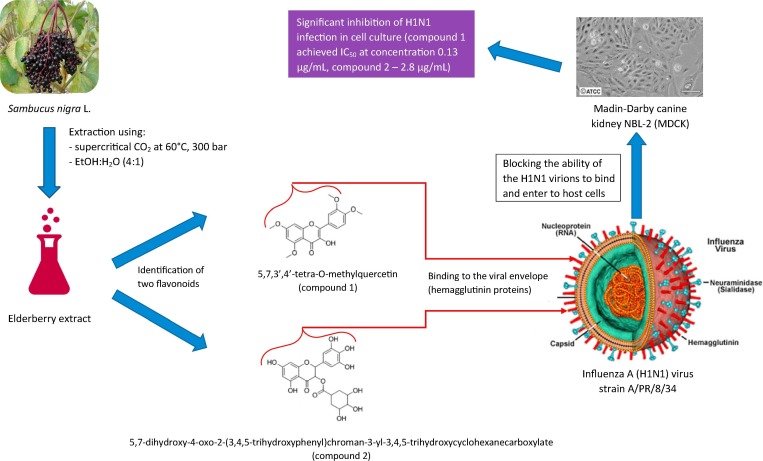
The antiviral activity of Rubini® was investigated against two different human pathogenic virus strains, such as influenza A (KAN-1, H5N1) and B (B/Mass), in the Madin-Darby canine kidney cell culture (Fig. 1 ). Treatment with elderberry extract distinctly reduced the spread of the foci size of influenza B virus, whereas the foci size of KAN-1 was enlarged, but its number was reduced in comparison to the untreated control (Krawitz et al., 2011). It was found that the elderberry extract at a concentration of 252 µg/ml caused a 50% inhibition (IC50) of human influenza A (H1N1) virus applied to infect the Madin-Darby canine kidney (MDCK) NBL-2 cells, while a concentration of 1000 µg/ml allowed a 100% inhibition of this virus infection. In addition, it was shown that phenolic compounds from the elderberry fruit extract bind to H1N1 virions, thus blocking their ability to infect host cells. Two compounds which bind to H1N1 were identified: 5,7,3′,4′-tetra-O-methylquercetin and 5,7-dihydroxy-4-oxo-2-(3,4,5-trihydroxyphenyl)chroman-3-yl-3,4,5-trihydroxycyclohexanecarboxylate (Fig. 1). Both compounds were synthesized and it was demonstrated that compound 1 achieved an IC50 of 0.13 µg/ml for inhibition of H1N1 infection, and dihydromyricetin corresponding compound 2 gave an IC50 of 2.8 µg/ml. The mechanism of action was shown in Fig. 2 . Taking into account these parameters, the effect of elderberry flavonoids related to inhibition of H1N1 is comparable to that of the known anti-influenza medicines, such as Oseltamivir (Tamiflu®) and Amantadine (Roschek, Fink, McMichael, Li, & Alberte, 2009). In another study in which MDCK was used as well, it was observed that concentrated elderberry juice could affect H1N1 in the early stages of viral replication: IC50 was achieved with an extract concentration of 720 µg/ml when the samples were given during infections, and with an extract concentration of 3600 µg/ml when the samples were applied immediately after infection. Additionally, in vivo experiments showed that elderberry effectively suppressed viral replication and could stimulate the immune response. Concentrated elderberry juice was separated in three fractions (low, medium and high molecular weight) that were administered to infected mice. The most effective fraction was the high-molecular-weight fraction (HM), which suppressed the viral yield in the bronchoalveolar lavage fluids (BALFs) and lungs. Fraction II, which was isolated from HM and contained mainly acidic polysaccharides, caused an increase in the level of IFV specific neutralizing antibody in the BALFs and serum and also the level of the secretory IgA in the BALFs and feces. The medium-molecular-weight fraction, which was rich in polyphenols, showed a lower activity (Fig. 1) (Kinoshita et al., 2012). Furthermore, elderberry extracts demonstrate an inhibitory effect on infectious bronchitis virus (IBV), a pathogenic chicken coronavirus, at an early stage of the virus infection. Elderberry extract reduced IBV titers by several orders of magnitude, in dependence of the dose applied. The increase in the elderberry extract concentration caused a decrease in virus titers and vice versa. In addition, electron microscopy of virions that were treated with elderberry extract demonstrated compromised envelopes and the presence of membrane vesicles. There are data that indicate that the pre-treatment with elderberry extract of IBV infection leads to damage to the virus extensive membrane and probably renders the virus non-infectious (Chen et al., 2014). Antiviral activity of elder bark was evaluated in relation to feline immunodeficiency virus (FIV), which frequently occurs in domestic cats and from the point of view of biological and pathogenic functions is very similar to the human immunodeficiency virus (HIV). It was shown that elder bark extract had a high activity against FIV because it inhibited syncytia formation, with the inhibition level being dependent on extract concentration. Syncytia inhibition level of 100% was achieved when the extract concentration was 500 µg/ml (Uncini Manganelli, Zaccaro, & Tomei, 2005).
Fig. 1.
Antiviral (influenza A and B) properties of elderberry fruit (Kinoshita et al., 2012, Kong, 2009, Krawitz et al., 2011, Roschek et al., 2009, Zakay-Rones et al., 2004).
Fig. 2.
Mechanism of action of the bioactive compounds of Sambucus nigra on influenza A virus (Roschek et al., 2009).
Considering clinical trials randomized, double-blind and placebo-controlled research concerning influenza A and B virus infections was carried out in the autumn/winter season 1999/2000 in Norway (Fig. 1). The study was attended by humans suffering from at least two symptoms of flu including fever ≥38 °C and one or more respiratory influenza symptoms. The study used a standardized elderberry extract, the Sambucol® syrup. The participants, who were randomly divided into two groups, received either 15 ml of the elderberry syrup or placebo four times a day during five days. The effects of the treatment were monitored by a visual analogue scale. It turned out that in the group treated with the elderberry syrup, symptoms ebbed on average four days earlier than in the placebo group (Zakay-Rones et al., 2004). In 2009, during the spring flu season, a randomized, short-term, double-blind and placebo-controlled clinical trial was conducted with participants who showed three or more flu symptoms, such as fever, coughing, headache, muscle aches, nasal mucus discharge or nasal congestion, for less than 24 h (Fig. 1). The patients were divided at random into two groups. The first group received 4 slow-dissolve lozenges of a proprietary elderberry extract a day (each lozenge contained 175 mg of the proprietary elderberry extract) and the second group received placebo lozenges. After 48 h of treatment, patients from the group treated by elderberry extract demonstrated significant reduction in most of the flu-like symptoms – 28% of patients were void of all symptoms and 60% of patients received relief of some symptoms and had one or two mild symptoms, whereas the placebo group demonstrated either no improvement or even aggravated symptoms (Kong, 2009). Selected antiviral properties of elderberry fruit (regarding to influenza A and B virus) are illustrated in Fig. 1.
10. Diabetes, obesity and metabolic dysfunctions
Elder may be an effective traditional remedy for diabetes, and as such, it could be used as a dietary adjunct in diabetes treatment. It was found that water-soluble compounds included in elder flowers were able to directly stimulate glucose metabolism and promote insulin secretion through clonal pancreatic β-cells. An extract of elder flowers (1 g/L) significantly increased glucose uptake, glucose oxidation and glycogenesis in vitro in mice abdominal muscles without added insulin (Gray, Abdel-Wahab, & Flatt, 2000). The fruit of Sambucus nigra positively influences the treatment of diabetic osteoporosis. A polyphenolic extract of elderberry improved the bone mineral density and reduced the body fat of diabetic rats. Also, the antioxidative capacity of serum improved in rats treated with the polyphenolic extract as the concentration of reduced glutathione (GSH) was restored to normal. The concentration of malondialdehyde, which is an index of the lipid peroxidation level, decreased in the serum, thus resulting in an improved osteoporosis status (Badescu, Badulescu, Badescu, & Ciocoiu, 2012). Salvador et al. investigated the effect of elderberry lipophilic and polar extract supplementation on diabetes management indices in rats. Type 2 diabetic rats (induced by streptozotocin) were fed a high-fat diet and received for 4 weeks 190 and 350 mg/kg body weight/day doses of lipophilic and polar elderberry extract, respectively. The polar extract lowered fasting blood glucose, whereas the lipophilic extract decreased secretion. Both extracts reduced insulin resistance and no significant alterations in the hematological indices, sera lipids and trace elements homeostasis from sera and tissues were observed (Salvador et al., 2017). Farrel et al. studied the effect of anthocyanin-rich elderberry extract on metabolism in a diet-induced obese mouse model. Four types of diet were used for 16 weeks: low-fat diet (LFD), high-fat lard-based diet (HFD), HFD with 0.25% of elderberry extract and HFD with 1.25% of elderberry extract. It was found that both groups of mice which received elderberry extract were characterized by a significantly lower liver weight and serum TAG concentration, and by significantly lower serum inflammatory markers, insulin resistance and hepatic fatty acid synthase mRNA, in comparison to HFD. In addition, HFD with 1.25% of elderberry extract reduced liver cholesterol and PPARγ2 mRNA compared to HFD and HFD with 0.25% of elderberry extract (Farrel, Norris, Ryan et al., 2015). Furthermore, supplementation with 1.25% of elderberry extract of hyperlipidemic mice was conducted in order to determination the effect on atherosclerosis and HDL dysfunctions. It was not observed significantly differences in serum lipids between elderberry and control group, however, aspartate transaminase and fasting glucose were decreased in elderberry group of mice. Hepatic and intestinal mRNA changes with an improvement in HDL function and a decrease in hepatic cholesterol levels were also reported in elderberry-fed mice. Additionally, this group showed significantly higher activity of serum paraxonase-1 arylesterase. The results indicate the reduction of elderberry in aorta total cholesterol content and then less atherosclerosis progression (Farrel, Norris, Lee, Chun, & Blesso, 2015).
A randomized, placebo-controlled and double-blind study was conducted to determine the impact of anthocyanins obtained from elderberry juice on the lipid and antioxidant status. 34 healthy volunteers received 400 mg of spray-dried elderberry powder in gelatinous capsules or placebo three times a day during 2 and 3 weeks. Each capsule contained 10% anthocyanins and represented the equivalent of 5 ml of elderberry juice. Furthermore, an additional trial was carried out to study the short-term effect of elderberry anthocyanins on serum lipid concentration, where six volunteers received a single dose of 10 capsules, which was the equivalent of 50 ml of elderberry juice. In the placebo-controlled study, the elderberry group did not show statistically significant differences in changes in serum lipids as well as vitamin A, E and β-carotene, compared to the placebo group, and even, a decrease in the level of vitamin C was observed in both groups. In a single-dose experiment conducted as part of the same study, an increase in total anthocyanins in serum was found during the postprandial phase. The serum lipids concentration was lower in the elderberry group, but differences were not significant (Murkovic et al., 2004). In a study in which patients took, among others, dietary supplements from the fruit and flowers of Sambucus nigra, the BMI index dropped by approx. 3%, the weight fell by an average of 3.2 kg, systolic blood pressure decreased by an average of over 5% and diastolic blood pressure by 2.5%. Moreover, physical and mental well-being as well as the quality of life of the patients significantly improved (Chrubasik et al., 2008). A diluted concentrate from elder berries and flowers, used among others in hypocaloric diet in Switzerland, resulted in average weight loss of 2.6 kg in 11 volunteers during 7 days of diet (Walz & Chrubasik, 2008).
11. Antidepressant potential
Mahmoudi et al. (2014) evaluated the antidepressant activity of elderberry extracts by exposing mice to the forced swimming test (FST) and the tail suspension test (TST). The tests demonstrated very good antidepressant properties of the extract tested: it shortened the immobility time and increased the activity of mice which were given the extract as compared to the control group, with the immobility time and activity level being dependent on the extract dose. Moreover, elderberry extract at a dose of 1200 mg/kg caused a far higher activity of mice in the FST than imipramine (10 mg/kg), a strong and effective antidepressant drug (Mahmoudi et al., 2014).
12. Impact on urinary parameters
Research on the impact of some proprietary elder concentrate on the urinary electrolyte balance showed that 200 ml of concentrate made from fresh berries, flower juice and dried flower extract, did not influence pH, hydrogen ion concentration and 24 h hydrogen excretion in urine collected from 11 participants. Elderberry concentrate also had no impact on the solubility of stone-inducing ions, which suggests that it can be used in patients with idiopathic nephrolithiasis without any risk of harm (Walz & Chrubasik, 2008). Elderberry as a dietary supplement in the form of capsules containing flower extract had no apparent inhibitory effect on uridine diphospho-glucuronosyltransferase (UGTs). IC50 values were higher than 500 µg/mL for all the five studied UGTs enzymes in vitro, such as UGT1A1, UGT1A4, UGT1A6, UGT1A9 and UGT2B7 (Choi, Park, Yoon, & Bae, 2014).
13. Industrial uses of elderberry
Being rich in anthocyanins, elderberry belongs to several berry species that are used as natural colorants in different industries. Particularly, elderberry fruit is used as a food colorant, an ingredient in pharmaceuticals, concentrates, juices, syrups, jams, jellies, powders and other food preserves, a filling in pies, cakes or desserts, and also for the production of alcoholic beverages, such as wine (Bermúdez-Soto and Thomás-Barberán, 2004, Inami et al., 1996, Kaack et al., 2005). Elderberry pomace, which is a waste by-product of juice production, is an important raw material used in the production of anthocyanin extracts and lyophilized dyes. It is also used as animal feed and organic fertilizer (Kaack, 1990, Seabra et al., 2010).
Elderflowers, similarly to berries, are also widely used in food industry. They are natural flavouring components in alcoholic and non-alcoholic beverages, sparkling, bitter and white wine, fruit brandies and various spirits, as well as in tea and products like yoghurt or ice cream (Fazio et al., 2013, Kaack et al., 2006).
According to a 2010 report by the European Herb Growers Association, Sambucus nigra (flowers and berries) was the most harvested medicinal plants intended for export trade and for tea and phytopharmaceutical production in Bulgaria and Romania (European Herb Growers Association (Europam), 2010). Elderberry ranked as the 18th best-selling herbal dietary supplement on the medicine, food and mass market in the USA in 2011 (Engels & Brinckmann, 2013).
14. Conclusion
Numerous studies indicate that the fruit and flowers of Sambucus nigra are highly nutritious and rich in bioactive compounds such as polyphenols and anthocyanins. Thanks to these compounds, elderberry is characterized by high antioxidant activity, which significantly affects its health-promoting properties. It was shown that elderberry has mainly antibacterial and antiviral properties, can reduce sugar and lipid concentration, and even exhibit antidepressant and antitumour properties. Most studies have focused on the antiviral properties of elderberry fruit, so there is a high need for further research into the other properties of this valuable plant. Considering the growing fashion for natural, organic and health-promoting food, it can be concluded that elderberry as a natural component of food products fits perfectly into this trend and has a good chance to increase its role as a beneficial component of a healthy diet.
References
- Akbulut M., Ercisli S., Tosun M. Physico-chemical characteristics of some wild grown European elderberry (Sambucus nigra L.) genotypes. Pharmacognosy Magazine. 2009;5(20):320–323. [Google Scholar]
- Atkinson M.D., Atkinson E. Sambucus nigra L. Biological flora of the British Isles. Journal of Ecology. 2002;90:895–923. doi: 10.1111/1365-2745.12566. [DOI] [Google Scholar]
- Badescu L., Badulescu O., Badescu M., Ciocoiu M. Mechanism by Sambucus nigra extract improves bone mineral density in experimental diabetes. Evid Based Complement Alternat Medicine. 2012 doi: 10.1155/2012/848269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baytop T. Istanbul Üniversitesi Yayınları No: 3255, Eczacılık Fakültesi No: 40; Istanbul: 1984. Türkiye Bitkiler ile Tedavi (Geçmişte ve Bugün) p. 407. [Google Scholar]
- Beaux D., Fleurentin J., Mortier F. Effect of extracts of Orthosiphon stamineus Benth, Hieracium pilosella L., Sambucus nigra L. and Arctostaphylos uva-ursi (L.) Spreng. in rats. Phytotherapy Research. 1999;13(3):222–225. doi: 10.1002/(SICI)1099-1573(199905)13:3<222::AID-PTR447>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Soto M.J., Thomás-Barberán F.A. Evaluation of commercial red fruit juice concentrates as ingredients for antioxidant functional juices. European Food Research and Technology. 2004;219:133–141. doi: 10.07/s00217-004-0940-3. [Google Scholar]
- Bitsch R., Netzel M., Sonntag S., Strass G., Frank T., Bitsch I. Urinary excretion of cyanidin glucosides and glucuronides in healthy humans after elderberry juice ingestion. Journal of Biomedicine and Biotechnology. 2004;5:343–345. doi: 10.1155/S111072430440309X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowska J., Zadernowski R., Waźbińska J., Puczel U. Charakterystyka chemiczna owoców bzu czarnego (Sambucus nigra L.) Roczniki Akademii Rolniczej w Poznaniu. 2000;31:13–17. [Google Scholar]
- Bown D. Dorling Kindersley; London: 1995. Encyclopaedia of herbs and their uses. [Google Scholar]
- Bridle P., Timberlake C. Anthocyanins as natural food colours-selected aspects. Food Chemistry. 1997;58:103–109. [Google Scholar]
- Bromley J., Hughes B.G.M., Leong D.C.S., Buckley N.A. Life-threatening interaction between complementary medicines: Cyanide toxicity following ingestion of amygdalin and vitamin C. Annals of Pharmacotherapy. 2005;39:1566–1569. doi: 10.1345/aph.1E634. [DOI] [PubMed] [Google Scholar]
- Brønnum-Hansen K., Jacobsen F., Flink J.M. Anthocyanin colourants from elderberry (Sambucus nigra L.). 1. Process considerations for production of the liquid extract. Journal of Food Technology. 1985;20(6):703–711. [Google Scholar]
- Byers P.L., Thomas A.L., Gold M.A. Agroforestry in Action University of Missouri Center for Agroforestry AF1016; 2014. Growing and marketing elderberries in missouri; pp. 1–12. [Google Scholar]
- Charlebois D., Byers P., Finn C.E., Thomas A. Elderberry: Botany, Horticulture, Potential. Horticultural Reviews. 2010;37(4):214–280. [Google Scholar]
- Chen C., Zuckerman D.M., Brantley S., Sharpe M., Childress K., Hoiczyk E. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Veterinary Research. 2014;10:24. doi: 10.1186/1746-6148-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E.J., Park J.B., Yoon K.D., Bae S.K. Evaluation of the in vitro/in vivo potential of five berries (bilberry, blueberry, cranberry, elderberry and raspberry ketones) commonly used as a herbal supplements to inhibit uridine diphospho-glucuronosyltransferase. Food and Chemical Toxicology. 2014;72:13–19. doi: 10.1016/j.fct.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Christensen L.P., Kaack K., Fretté X.C. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of elderflower extracts rich in flavonoids and phenolic acids. European Food Research and Technology. 2008;227:293–305. [Google Scholar]
- Chrubasik C., Maier T., Dawid C., Torda T., Schieber A., Hofmann T. An observational study and quantification of the actives in a supplement with Sambucus nigra and Asparagus officinalis used for weight reduction. Phytotherapy Research. 2008;22:913–918. doi: 10.1002/ptr.2415. [DOI] [PubMed] [Google Scholar]
- Czank C., Cassidy A., Zhang Q., Morrison D.J., Preston T., Kroon P.A. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study1-3. American Journal of Clinical Nutrition. 2013;97:995–1003. doi: 10.3945/ajcn.112.049247. [DOI] [PubMed] [Google Scholar]
- Dawidowicz A.L., Wianowska D., Baraniak B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts) LWT – Food Science and Technology. 2006;39:308–315. [Google Scholar]
- DellaGreca M., Fiorentino A., Monaco P., Previtera L., Simonet A.M. Cyanogenic glycosides from Sambucus nigra. Natural Product Letters. 2000;14:175–182. doi: 10.1080/1057563021000040772. [DOI] [PubMed] [Google Scholar]
- Diviš P., Pořízka J., Vespalcová M., Matějíček A., Kaplan J. Elemental composition of fruits from different black elder (Sambucus nigra L.) cultivars grown in The Czech Republic. Journal of Elementology. 2015;20(3):549–557. [Google Scholar]
- DStatis . Statistisches Bundesamt; Wiesbaden: 2016. Obst, gemüse, gartenbau.https://www.destatis.de/DE/ZahlenFakten/Wirtschaftsbereiche/LandForstwirtschaftFischerei/ObstGemueseGartenbau/Tabellen/Strauchbeerenanbau2014.html;jsessionid=DD0917AC3D7C894F88E47E2683E59CCF.cae4 Accesed 2016-11-26 at: [Google Scholar]
- Dulf F.V., Oroian I., Vodnar D.C., Socaciu C., Pintea A. Lipid classes and fatty acid regiodistribution in triacylglycerols of seed oils of two Sambucus species (S. nigra L. and S. ebulus L.) Molecules. 2013;18:11768–11782. doi: 10.3390/molecules181011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duymuş H.G., Göger F., Hüsnü Can Başer K. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chemistry. 2014;155:112–119. doi: 10.1016/j.foodchem.2014.01.028. [DOI] [PubMed] [Google Scholar]
- Ellenberg H. 4th ed. Cambridge University Press; Cambridge, UK: 1988. Vegetation ecology of Central Europe. [Google Scholar]
- Elliman W. Elderberry, flu contrary. Hadassah Magazine. 1994;12:40–41. [Google Scholar]
- Engels G., Brinckmann J. European elder. Herbal Gram. 2013;97:1–7. [Google Scholar]
- Espín J.K., Soler-Rivas C., Wichers H.J., García-Viguera C. Anthocyanin-based natural colorants: A new source of antiradical activity for foodstuff. Journal of Agriculture and Food Chemistry. 2000;48(5):1588–1592. doi: 10.1021/jf9911390. [DOI] [PubMed] [Google Scholar]
- European Herb Growers Association (Europam) Europam; Vienna, Austria: 2010. Production of medicinal and aromatic plants in Europe status 2010.www.europam.net [Google Scholar]
- Farrel N., Norris G., Lee S.G., Chun O.K., Blesso C.N. Anhocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food & Function. 2015;6(4):1278–1287. doi: 10.1039/c4fo01036a. [DOI] [PubMed] [Google Scholar]
- Farrel N.J., Norris G.H., Ryan J., Porter C.M., Jiang C., Blesso C.N. Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. British Journal of Nutrition. 2015;114:1123–1131. doi: 10.1017/S0007114515002962. [DOI] [PubMed] [Google Scholar]
- Fazio A., Plastina P., Meijerink J., Witkamp R.F., Gabriele B. Comparative analyses of seeds of wild fruits of Rubus and Sambucus species from Southern Italy: Fatty acids composition of the oil, total phenolic content, antioxidant and anti-inflammatory properties of the methanolic extracts. Food Chemistry. 2013;140:817–824. doi: 10.1016/j.foodchem.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Finn C.E., Thomas A.L., Byers P.L., Kemal M. Evaluation of American (Sambucus canadensis) and European (S. nigra) elderberry genotypes grown in diverse environments and implications for cultivar development. HortScience. 2008;43(5):1385–1391. [Google Scholar]
- Fiorini M. Preparative high-performance liquid chromatography for the purification of natural anthocyanins. Journal of Chromatography A. 1995;692:213–219. [Google Scholar]
- Förster-Waldl E., Marchetti M., Schöll I., Focke M., Radauer C., Kinaciyan T. Type I allergy to elderberry (Sambucus nigra) is elicited by a 33.2 kDa allergen with significant homology to ribosomal inactivating proteins. Clinical and Experimental Allergy. 2003;33:1703–1710. doi: 10.1111/j.1365-2222.2003.01811.x. [DOI] [PubMed] [Google Scholar]
- Gollucke A.P., Peres R.C., Odair A., Ribeiro D.A. Polyphenols: A nutraceutical approach against diseases. Recent Patents on Food, Nutrition & Agriculture. 2013;5(3):214–219. doi: 10.2174/2212798405666131129153239. [DOI] [PubMed] [Google Scholar]
- Gray A.M., Abdel-Wahab Y.H.A., Flatt P.R. The traditional plant treatment, Sambucus nigra (elder), exhibits insulin-like and insulin-releasing actions in vitro. Journal of Nutrition. 2000;130(1):15–20. doi: 10.1093/jn/130.1.15. [DOI] [PubMed] [Google Scholar]
- Grieve M. Dover Publications; New York: 1971. A modern herbal. [Google Scholar]
- Grieve M. Penguin; 1984. A modern herbal. [Google Scholar]
- Hearst C., McCollum G., Nelson D., Ballard L.M., Millar B.C., Goldsmith C.E. Antibacterial activity of elder (Sambucus nigra L.) flower or berry against hospital pathogens. Journal of Medicinal Plants Research. 2010;4(17):1805–1809. [Google Scholar]
- Hoffmann D. Element Books LTD; Dorset, England: 1990. The new holistic herbal. [Google Scholar]
- Höhne F. Holunderanbau – Was kann wie erreicht werden. Beerenobst. 2014;08:219–227. [Google Scholar]
- Hultén E., Fries M. Koeltz Scientific Books; Königstein, Germany: 1986. Atlas of North European vascular plants north of the tropic of cancer. [Google Scholar]
- Inami O., Tamura I., Kikuzaki H., Nakatani N. Stability of anthocyanins of Sambucus canadensis and Sambucus nigra. Journal of Agriculture and Food Chemistry. 1996;44:3090–3096. [Google Scholar]
- ITIS . Taxonomy and Nomenclature; 2016. Sambucus L. Taxonomic serial no.: 35315. Integrated taxonomic information system.http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=35315 Accesed: 2016-11-17 at: [Google Scholar]
- Jensen S.R., Nielsen B.J. Cyanogenic glucosides in Sambucus nigra L. Acta Chemica Scandinavica. 1973;27:2661–2662. doi: 10.3891/acta.chem.scand.27-2661. [DOI] [PubMed] [Google Scholar]
- Jimenez P., Cabrero P., Basterrechea J.E., Tejero J., Cordoba-Diaz D., Girbes T. Isolation and molecular characterization of two lectins from dwarf elder (Sambucus ebulus L.) blossoms related to the Sam n1 allergen. Toxins. 2013;5:1767–1779. doi: 10.3390/toxins5101767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez P., Cabrero P., Cordoba-Diaz D., Cordoba-Diaz M., Garrosa M., Girbés T. Lectin digestibility and stability of elderberry antioxidants to heat treatment in vitro. Molecules. 2017;22:95. doi: 10.3390/molecules22010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez P., Tejero J., Cabrero P., Cordoba-Diaz D., Girbes T. Differential sensitivity of D-galactose-binding lectins from fruits of dwarf elder (Sambucus ebulus L.) to a stimulated gastric fluid. Food Chemistry. 2013;136:794–902. doi: 10.1016/j.foodchem.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Kaack K. New varieties of elderberry (Sambucus nigra L.) Tidsskrift for Planteavl. 1984;93:59–65. [Google Scholar]
- Kaack K. Processing of anthocyanin colourant from elderberry (Sambucus nigra L.) pomace. Tidsskrift for Planteavl. 1990;94:423–429. [Google Scholar]
- Kaack K. ‘Sampo’ and ‘Samdal’, elderberry cultivars for juice concentrates. Fruit Varieties Journal. 1997;51(1):28–31. [Google Scholar]
- Kaack K. Aroma composition and sensory quality of fruit juices processed from cultivars of elderberry (Sambucus nigra L.) European Food Research and Technology. 2008;227:45–56. [Google Scholar]
- Kaack K., Austed T. Interaction of vitamin C and flavonoids in elderberry (Sambucus nigra L.) during juice processing. Plant Foods for Human Nutrition. 1998;52:187–198. doi: 10.1023/a:1008069422202. [DOI] [PubMed] [Google Scholar]
- Kaack K., Christensen L. Effect of packing materials and storage time on volatile compounds in tea processed from flowers of black elder (Sambucus nigra L.) European Food Research and Technology. 2008;227:1259–1273. [Google Scholar]
- Kaack K., Christensen L.P., Hughes M., Eder R. The relationship between sensory quality and volatile compounds in raw juice processed from elderberries (Sambucus nigra L.) European Food Research and Technology. 2005;221:244–254. [Google Scholar]
- Kaack K., Christensen L.P., Hughes M., Eder R. Relationship between sensory quality and volatile compounds of elderflower (Sambucus nigra L.) extracts. European Food Research and Technology. 2006;223:57–70. [Google Scholar]
- Kaack K., Fretté X.C., Christensen L.P., Landbo A.K., Meyer A.S. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of juice. European Food Research and Technology. 2008;226:843–855. [Google Scholar]
- Kinoshita E., Hayashi K., Katayama H., Hayashi T., Obata A. Anti-Influenza virus effects of elderberry juice and its fractions. Bioscience, Biotechnology, and Biochemistry. 2012;76(9):1633–1638. doi: 10.1271/bbb.120112. [DOI] [PubMed] [Google Scholar]
- Kislichenko V.S., Vel’ma V.V. Amino-acid composition of flowers, leaves and extract of Sambucus nigra flowers. Chemistry of Natural Compounds. 2006;42(1):125–126. [Google Scholar]
- Kohlmünzer S. Wydawnictwo Lekarskie PZWL; Warszawa: 1998. Farmakognozja. Podręcznik dla studentów farmacji; pp. 163–176. [Google Scholar]
- Kollanyi I., Kollanyi G., Hajdu B. Special edition. Horticulture [Kertgazdasag]; 2005. Varieties and candidate varieties for enlarging the assortment of elderberry; pp. 83–88. [Google Scholar]
- Kołodziej B., Drożdżal K. Właściwości przeciwutleniające kwiatów i owoców bzu czarnego pozyskiwanego ze stanu naturalnego. Żywność Nauka Technologia Jakość. 2011;4(77):36–44. [Google Scholar]
- Kołodziej B., Maksymiec N., Drożdżal K., Antonkiewicz J. Effect of traffic pollution on chemical composition of raw elderberry (Sambucus nigra L.) Journal of Elementology. 2012;17:67–78. [Google Scholar]
- Kong F. Pilot clinical study on a proprietary elderberry extract: Efficacy in addressing influenza symptoms. Online J Pharmacol Pharmacokin. 2009;5:32–43. [Google Scholar]
- Krawitz C., Mraheil M.A., Stein M., Imirzalioglu C., Domann E., Pleschka S. Inhibitory activity of a standardized elderberry liquid extract against clinically-relevant human respiratory bacterial pathogens and influenza A and B viruses. BMC Complementary and Alternative Medicine. 2011;11:16. doi: 10.1186/1472-6882-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Finn C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. Journal of the Science of Food and Agriculture. 2007;87:2665–2675. doi: 10.1002/jsfa.3029. [DOI] [PubMed] [Google Scholar]
- Leja M., Mareczek A., Nanaszko B. Antyoksydacyjne właściwości owoców wybranych gatunków dziko rosnących drzew i krzewów. Roczniki Akademii Rolniczej w Poznaniu. 2007;41:327–331. [Google Scholar]
- Lennon J.J., Turner J.R.G. Predicting the spatial distribution of climate: Temperature in Great Britain. Journal of Animal Ecology. 1995;64:370–392. [Google Scholar]
- Mahmoudi M., Ebrahimzadeh M.A., Dooshan A., Arimi A., Ghasemi N., Fathiazad F. Antidepressant activities of Sambucuc ebulus and Sambucus nigra. European Review for Medical and Pharmacological Sciences. 2014;18(22):3350–3353. [PubMed] [Google Scholar]
- Martens A. Wydawnictwo RM; Warszawa: 2008. Czarny bez. Zdrowie z natury czarny bez. [Google Scholar]
- McKay S.A. Demand increasing for aronia and elderberry in North America. NY Fruit Quality. 2001;9:2–3. [Google Scholar]
- Mikulic-Petkovsek M., Samoticha J., Eler K., Stampar F., Veberic R. Traditional elderflower beverages: A rich source of phenolic compounds with high antioxidant activity. Journal of Agriculture and Food Chemistry. 2015;63:1477–1487. doi: 10.1021/jf506005b. [DOI] [PubMed] [Google Scholar]
- Milbury P.E., Cao G., Prior R.L., Blumberg J. Bioavailability of elderberry anthocyanins. Mechanisms of Ageing and Development. 2002;123:997–1006. doi: 10.1016/s0047-6374(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Mohebalian P.M., Cernusca M.M., Aguilar F.X. Discovering niche markets for elderberry juice in the United States. HortTechnology. 2012;22(4):556–566. [Google Scholar]
- Mratinić E., Fotirić M. Selection of black elderberry (Sambucus nigra L.) and evaluation of its fruits usability as biologically valuable food. Genetica. 2007;3(39):305–314. [Google Scholar]
- Murkovic M., Abuja P.M., Bergmann A.R., Zirngast A., Adam U., Winklhofer-Roob B.M. Effects of elderberry juice on fasting and postprandial serum lipids and low-density lipoprotein oxidation in healthy volunteers: A randomized, double-blind, placebo-controlled study. European Journal of Clinical Nutrition. 2004;58(2):244–249. doi: 10.1038/sj.ejcn.1601773. [DOI] [PubMed] [Google Scholar]
- Murkovic M., Mülleder U., Adam U., Pfannhauser W. Detection of anthocyanins from elderberry juice in human urine. Journal of the Science of Food and Agriculture. 2001;81:934–937. [Google Scholar]
- Netzel M., Strass G., Herbst M., Dietrich H., Bitsch R., Bitsch I. The excretion and biological antioxidant activity of elderberry antioxidants in healthy humans. Food Research International. 2005;38:905–910. [Google Scholar]
- NPGS National Plant Germplasm System . Germplasm Resource Information Network (GRIN); 2016. Species of sambucus.https://npgsweb.ars-grin.gov/gringlobal/taxonomylist.aspx?category=species&type=genus&value=Sambucus&id=10679 Accesed 2016-11-17. [Google Scholar]
- NRCS Natural Resources Conservation Service Classification for Kingdom Plantae Down to Family Caprifoliaceae. 2016. http://plants.usda.gov/java/ClassificationServlet?source=display&classid=Caprifoliaceae date:2016-11-17 Accesed at:
- NRCS Natural Resources Conservation Service . The United States Department of Agriculture; 2016. PLANTS database. Classification for kingdom plantae down to genus sambucus L.http://plants.usda.gov/java/ClassificationServlet?source=display&classid=SAMBU date:2016-11-17 Accesed at: [Google Scholar]
- Ognik K., Rusinek E., Sembratowicz I., Truchliński J. Zawartość metali ciężkich oraz azotanów (V) i azotanów (III) w owocach bzu czarnego i aronii czarnoowocowej w zależności od miejsca pozyskania i okresu wegetacyjnego. Rocz PZH. 2006;57(3):235–241. [PubMed] [Google Scholar]
- Olejnik A., Olkowicz M., Kowalska K., Rychlik J., Dembczyński M., Myszka K. Gastrointestinal digested Sambucus nigra L. fruit extract protects in vitro cultured human colon cells against oxidative stress. Food Chemistry. 2016;197:648–657. doi: 10.1016/j.foodchem.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Oszmiański J., Moutounet M. Tannins in some anthocyanin rich fruits (in Polish). Zeszyty Naukowe Akademii Rolniczej we Wrocławiu. Technologia Żywności VIII. 1995;273:47–54. [Google Scholar]
- Ożarowski A., Jaroniewski W. Instytut Wydawniczy Związków Zawodowych; Warszawa: 1987. Rośliny lecznicze i ich praktyczne zastosowanie; pp. 93–96. [Google Scholar]
- Pietta P., Bruno A. Separation of flavonol-2-O-glycosides from Calendula officinalis and Sambucus nigra by high-performance liquid and micellar electrokinetic capillary chromatography. Journal of Chromatography. 1992;593:165–170. doi: 10.1016/0021-9673(92)80282-y. [DOI] [PubMed] [Google Scholar]
- Pliszka B., Waźbińska J., Puczel U., Huszcza-Ciołkowska G. Biologicznie czynne związki polifenolowe zawarte w owocach różnych odmian hodowlanych i dziko rosnącego bzu czarnego. Zeszyty Problemowe Postępów Nauk Rolniczych. 2005;507:443–449. [Google Scholar]
- Prior R.L. Fruits and vegetables in the prevention of cellular oxidative damage. American Journal of Clinical Nutrition. 2003;78(Suppl.):570S–578S. doi: 10.1093/ajcn/78.3.570S. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C.A., Miller N.J., Paganga G. Structure – antioxidant activity relationships of flavonoids and phenolic acid. Free Radical Biology & Medicine. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Roper TR, Mahrd DL, McManus PS (1998) Growing currants, gooseberries & elderberries in Wisconsin. University of Wisconsin-Extension, Cooperative Ext. Publ.
- Roschek B., Jr, Fink R.C., McMichael M.D., Li D., Alberte R.S. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry. 2009;70(10):1255–1261. doi: 10.1016/j.phytochem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Salvador A.C., Król E., Lemos V.C., Santos S.A.O., Bento F.P.M.S., Costa C.P. Effect of elderberry (Sambucus nigra L.) extract supplementation in STZ-induced diabetic rats fed with a high-fat diet. International Journal of Molecular Sciences. 2017;18:13. doi: 10.3390/ijms18010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmersahl K.J. Über die Wirkstoffe der diaphoretischen Drogen des DAB 6. Naturwissenschaften. 1964;51:361. [Google Scholar]
- Schmitzer V., Veberic R., Slatnar A., Stampar F. Elderberry (Sambucus nigra L.) wine: A product reach in health promoting compounds. Journal of Agriculture and Food Chemistry. 2010;58:10143–10146. doi: 10.1021/jf102083s. [DOI] [PubMed] [Google Scholar]
- Seabra I.J., Braga M.E.M., Batista M.T.P., de Sousa H.C. Fractioned high pressure extraction of anthocyanins from elderberry (Sambucus nigra L.) pomace. Food and Bioprocess Technology. 2010;3:674–683. [Google Scholar]
- Senica M., Stampar F., Veberic R., Mikulic-Petkovsek M. The higher the better? Differences in phenolics and cyanogenic glycosides in Sambucus nigra leaves, flowers and berries from different altitudes. Journal of the Science of Food and Agriculture. 2016 doi: 10.1002/jsfa.8085. [DOI] [PubMed] [Google Scholar]
- Statistic Austria . STATISTIK AUSTRIA Bundesanstalt Statistik Österreich; 2016. Obsternte 2015. Erhebung der erwerbsobstanlagen.http://www.statistik.at/web_de/statistiken/wirtschaft/land_und_forstwirtschaft/agrarstruktur_flaechen_ertraege/obst/index.html Accesed 2016-02-26 at: [Google Scholar]
- Stintzing F.C., Stintzing A.S., Carle R., Frei B., Wrolstad R.E. Color and antioxidant properties of cyanidin-based anthocyanin pigments. Journal of Agriculture and Food Chemistry. 2002;50:6172–6181. doi: 10.1021/jf0204811. [DOI] [PubMed] [Google Scholar]
- Stoilova I., Wilker M., Stoyanova A., Krastanov A., Stanchev V. Antioxidant activity of extract from elder flower (Sambucus nigra L.) Herba Polonica. 2007;53(1):45–54. [Google Scholar]
- Stripe F. Ribosome-inactivating proteins. Toxicon. 2004;44:371–383. doi: 10.1016/j.toxicon.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Tejero J., Jiménez P., Quinto E.J., Cordoba-Diaz D., Garrosa M., Cordoba-Diaz M. Elderberries: A source of ribosome0inactivating proteins with lectin activity. Molecules. 2015;20:2364–2387. doi: 10.3390/molecules20022364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ABC Clinical Guide to Elder Berry (2003) The American Botanical Council.
- Thole J.M., Burns Kraft T.F., Sueiro L.A., Kang Y.H., Gills J.J., Cuendet M. A comparative evaluation of the anticancer properties of European and American elderberry fruits. Journal of Medicinal Food. 2006;9(4):498–504. doi: 10.1089/jmf.2006.9.498. [DOI] [PubMed] [Google Scholar]
- Uncini Manganelli R.E., Zaccaro L., Tomei P.E. Antiviral activity In vitro of Urtica dioica L., Parietaria diffusa M. et K. and Sambucus nigra L. Journal of Ethnopharmacology. 2005;98(3):323–327. doi: 10.1016/j.jep.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Veberic R., Jakopic J., Stampar F., Schmitzer V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chemistry. 2009;114:511–515. [Google Scholar]
- Vítová E., Divišová R., Sůkalová K., Matějíček A. Determination and quantification of volatile compounds in fruits of selected elderberry cultivars grown in Czech Republic. Journal of Food and Nutrition Research. 2013;52(1):1–11. [Google Scholar]
- Vulić J.J., Vračar L.O., Šumić Z.M. Chemical characteristics of cultivated elderberry fruit. Acta Periodica Technologica. 2008;39:85–90. [Google Scholar]
- Walkowiak-Tomczak D., Czapski J., Młynarczyk K. Assessment of colour changes during storage of elderberry juice concentrate solutions using the optimization method. Acta Scientiarum Polonorum Technologia Alimentaria. 2016;15(3):299–309. doi: 10.17306/J.AFS.2016.3.29. [DOI] [PubMed] [Google Scholar]
- Walz B., Chrubasik S. Impact of a proprietary concentrate of Sambucus nigra L. on urinary pH. Phytotherapy Research. 2008;22:977–978. doi: 10.1002/ptr.2407. [DOI] [PubMed] [Google Scholar]
- Way, R.D. (1981) Elderberry Culture in New York State. New York State Agricultural Experiment Station, Geneva, a Division of the New York State College of Agriculture and Life Sciences, a Statutory College of the State University, at Cornell University, Ithaca 91:1–4.
- Waźbińska J., Puczel U. Fruit characteristics of elderberry (Sambucus nigra L.) grown on two different soils. Journal of Fruit and Ornamental Plant Research. 2002;10:111–121. [Google Scholar]
- Waźbińska J., Puczel U., Senderowska J. Yield in elderberry cultivars grown on two different soils in 1997–2003. Journal of Fruit and Ornamental Plant Research. 2004;12:175–181. [Google Scholar]
- Wierzbicki A. Dziki bez czarny – pozyskiwanie surowca i jego zastosowanie. Wiadomości Zielarskie. 2002;4:8–10. [Google Scholar]
- Williamson E., Driver S., Baxter K. Pharmaceutical Press; London: 2009. Stockley’s herbal medicines interactions. A guide to the interactions of herbal medicines, dietary supplements and nutraceuticals with conventional medicines. [Google Scholar]
- Wójcik J. Nowe wskazania lecznicze Sambucus nigra L. Wiadomości Zielarskie. 2002;4:11–12. [Google Scholar]
- Youdim K.A., Martin A., Joseph J.A. Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress. Free Radical Biology & Medicine. 2000;29:51–60. doi: 10.1016/s0891-5849(00)00329-4. [DOI] [PubMed] [Google Scholar]
- Zakay-Rones Z., Thom E., Wollan T., Wadstein J. Randomized study of the efficacy and safety of oral elderberry extract in the treatment of influenza A and B virus infections. Journal of International Medical Research. 2004;32(2):132–140. doi: 10.1177/147323000403200205. [DOI] [PubMed] [Google Scholar]
- Zheng W., Wang S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries and lingonberries. Journal of Agriculture and Food Chemistry. 2003;51(2):502–509. doi: 10.1021/jf020728u. [DOI] [PubMed] [Google Scholar]