Abstract
By screening a collection of fecal samples from young dogs from different European countries, noroviruses (NoVs) were found in 13/294 (4.4%) animals with signs of enteritis whilst they were not detected in healthy dogs (0/42). An informative portion of the genome (3.4 kb at the 3′ end) was generated for four NoV strains. In the capsid protein VP1 region, strains 63.15/2015/ITA and FD53/2007/ITA were genetically related to the canine GVI.2 strain C33/Viseu/2007/PRT (97.4–98.6% nt and 90.3–98.6% aa). Strain FD210/2007/ITA displayed the highest identity to the GVI.1 canine strain Bari/91/2007/ITA (88.0% nt and 95.0% aa). Strain 5010/2009/ITA displayed only 66.6–67.6% nt and 75.5–81.6% aa identities to the GVI.1 canine strains FD210/2007/ITA and Bari/91/2007/ITA and the GVI feline strain M49-1/2012/JPN. Identity to the other canine/feline NoVs strains in the VP1 was lower than 67.6% nt and 62.7% aa. Based on the full-length VP1 amino acid sequence and the criteria proposed for distinction of NoV genotypes, the canine NoV 5010/2009/ITA could represent the prototype of a third GVI genotype, thus providing further evidence for the genetic heterogeneity of NoVs in carnivores.
Keywords: Norovirus, Dogs, Enteritis, Genogroup VI
Highlights
-
•
Noroviruses are important human pathogens, also found in several animal species.
-
•
Canine noroviruses were detected in 4.4% (13/294) of diarrhoeic dogs.
-
•
Upon genome sequencing, a novel canine norovirus was identified.
-
•
The observed genetic diversity may pose a challenge for diagnostics.
1. Introduction
Noroviruses (NoVs) are members of the Caliciviridae family, and are recognized as one of the leading causes of acute gastroenteritis in humans (Atmar and Estes, 2006, Glass et al., 2009). Epidemics associated with NoVs occur frequently in hospitals, cruise ships and childcare centers (Glass et al., 2009, Hall et al., 2013) and NoV infection has become a major public health concern (Siebenga et al., 2009, Vega et al., 2014).
NoVs have also been identified in several mammalian species, including cows, pigs, lion, cats, dogs, bats, sea lions and harbor porpoises (DeGraaf et al., 2017, Li et al., 2011, Martella et al., 2007, Martella et al., 2008, Pinto et al., 2012, Scipioni et al., 2008, Sugieda et al., 1998, Wu et al., 2016).
NoVs are small, non-enveloped round viruses of approximately 30 to 35 nm in diameter with a positive-sense RNA genome. NoV genome is organized into three open reading frames (ORFs) (Green, 2007). ORF1 encodes a polyprotein that is cleaved by the virus-encoded protease to produce several nonstructural proteins, including the RNA dependent RNA polymerase (RdRp). ORF2 encodes a major capsid protein (VP1) and ORF3 encodes a small basic protein (VP2) that has been associated with capsid stability (Bertolotti-Ciarlet et al., 2003).
Based on the full-length VP1 amino acid sequence, NoVs can be classified into at least seven genogroups (GI to GVII) and > 40 genotypes (Green, 2013, Kroneman et al., 2013, Vinjé, 2015, Zheng et al., 2006). Only GI, GII, and GIV NoVs infect humans, with GII strains being the most prevalent worldwide (Green, 2007). NoVs genetically similar to human NoVs have been recently found in carnivores (Martella et al., 2007, Martella et al., 2008, Pinto et al., 2012, Soma et al., 2014, Summa et al., 2012), raising public health concerns of potential cross-species transmission due to the strict social interactions between humans and pets. Interestingly, significant sequence variation has been found across different canine/feline NoV strains identified to date, allowing the classification of at least 4 genotypes from three distinct genogroups, i.e. GIV.2, GVI.1 and GVI.2 and GVII (Table 1 ).
Table 1.
NoV strains identified in carnivores and proposed classification. The strains analysed in this study are in bold.
| Species | Strain | Cap | Pol | Rec | Accession | Reference |
|---|---|---|---|---|---|---|
| Lion | Pistoia/387/2006/ITA | IV.2 | a | EF450827 | Martella et al., 2007 | |
| Cat | CU081210E/2010/USA | IV.2 | a | JF781268 | Pinto et al., 2012 | |
| Dog | Bari/170/2007/ITA | IV.2 | b | EU224456 | Martella et al., 2008 | |
| Dog | Thessaloniki/30/2008/GRC | IV.2 | b | GU354246 | Ntafis et al., 2010 | |
| Dog | Bari/91/2007/ITA | VI.1 | b | Yes | FJ875027 | Martella et al., 2009 |
| Dog | FD210/2007/ITA | VI.1 | b | Yes | JF939046 | This study |
| Dog | Viseu/C33/2007/PRT | VI.2 | c | GQ443611 | Mesquita et al., 2010 | |
| Dog | FD53/2007/ITA | VI.2 | c | JF930689 | This study | |
| Cat | TE/77-13/ITA | VI.2 | a | Yes | KT245136 | Di Martino et al., 2016 |
| Dog | 63.15/2015/ITA | VI.2 | c | KY486329 | This study | |
| Dog | 5010/2009/ITA | VI.3a | d | KY486328 | This study | |
| Cat | M49-1/2012/JPN | VI.4a | a | Yes | LC011950 | Takano et al., 2015 |
| Dog | HKU_Ca026F/2007/HKG | VII | VII | FJ692500 | Tse et al., 2012 | |
| Dog | HKU_Ca035F/2007/HKG | VII | VII | FJ692501 | Tse et al., 2012 |
Tentative classification as new GVI genotypes based on Zheng's criteria (Zheng et al., 2006).
The first canine NoV was reported in a 2 month-old dog with a four-day history of gastroenteritis and in co-infection with canine parvovirus type-2 (CPV-2) in 2007 in Italy (Martella et al., 2008). Since then, canine NoVs have been reported in several countries in Europe, the Americas and Asia (Azevedo et al., 2012, Mesquita et al., 2010, Mesquita and Nascimento, 2012a, Mesquita and Nascimento, 2012b, Ntafis et al., 2010, Soma et al., 2014, Tse et al., 2012) but the pathogenic role of NoVs in dogs has not been demonstrated firmly. The prevalence of canine NoV in dogs with clinical signs of gastroenteritis has been estimated to vary from 2.1% (Martella et al., 2009) to 40% (Mesquita et al., 2010). A study in the US identified canine NoV at a prevalence of 11% in canine diarrhea samples (Azevedo et al., 2012). Canine NoV has also been detected in healthy dogs (Mesquita et al., 2010), a pattern that is commonly observed in human NoV infections (Ozawa et al., 2007). Serological investigations have confirmed the presence of NoV-specific antibodies in dogs, with various prevalence rates. In a small serological survey in Italy, antibodies to GIV.2 NoV were identified in 5% of the dogs (Di Martino et al., 2010). In a study in UK, antibodies specific for different NoV genotypes were identified in 38.1% of samples collected between 1999 and 2001 and 60.1% of samples collected in 2012–2013 (Caddy et al., 2013). A European-wide serological investigation revealed antibodies to GVI.2 NoV in 36% of the analysed dogs (Mesquita et al., 2014).
In order to draw a more complete picture of NoV molecular epidemiology in dogs, in this study a collection of fecal specimens from diarrheic and healthy animals obtained from different European countries was screened using both broadly-reactive primers for caliciviruses and primers specific for NoVs. The sequence of a large portion of the genome at the 3′ end of two canine NoV strains was determined. The sequence of an additional two canine NoV strains identified in 2007 in Italy was determined and analysed in detail.
2. Materials and methods
2.1. Stools samples
During the years of 2009, 2014, 2015 and 2016, a total of 336 dog fecal samples (n = 294 from dogs with mild to severe gastroenteritis and n = 42 from asymptomatic animals) were collected from domestic animals aged 0–12 months of four European countries (Germany (n = 3), France (n = 26), Spain (n = 26) and Italy (n = 239). The samples were submitted to the laboratory of Animal Infectious Diseases, Department of Veterinary Medicine, University of Bari, Italy, for diagnostic examinations. All the samples were stored at − 80 °C until use. In addition, two stool samples positive for NoV and collected in 2007 in Italy were included in the analysis.
2.2. Nucleic acid preparation
Fecal specimens were diluted 10% w/v in phosphate-buffered saline, pH 7.2, and the debris were removed by centrifugation at 8000 × g for 5 min. Viral nucleic acid was extracted from 200 μl of each clarified stool suspension by the QIAamp cador Pathogen Mini Kit (Qiagen S.p.A., Milan, Italy), following the manufacturer's protocol and the nucleic acid templates were stored at − 80 °C until use.
2.3. Screening by RT-PCR and PCR
To assess the presence of caliciviruses (CVs), the samples were screened using a broadly reactive primer pair, p289–p290, amplifying a band of 315 bp for NoV and a band of 330 bp for vesivirus and sapovirus. The primers are targeted to the highly conserved motifs DYSKWDST and YGDD of the RNA-dependent RNA polymerase (RdRp) region of the polymerase complex (Jiang et al., 1999). In the samples yielding amplicons of the expected sizes, the presence of NoV was confirmed using the NoV-specific primer pair JV12Y–JV13I (Vennema et al., 2002). All the fecal samples collected from dogs with enteritis signs were also tested for the presence of common canine viral pathogens such as CPV-2 and canine coronavirus (CCoV) either by gel-based PCR or by quantitative PCR and RT-PCR (Buonavoglia et al., 2001, Decaro et al., 2005, Pratelli et al., 1999, Vennema et al., 2002).
2.4. Sequence and phylogenetic analysis of the norovirus strains
The amplicons were excised from the gel and purified using the QIAquick gel extraction kit (Qiagen GmbH, Hilden, Germany). The amplicons were subjected to direct sequencing using BigDye Terminator Cycle chemistry and 3730 DNA Analyzer (Applied Biosystems, Foster, CA). Basic Local Alignment Search Tool (BLAST; http://www.ncbi.nlm.nih.gov) and FASTA (http://www.ebi.ac.uk/fasta33) with default values were used to find homologous hits. The sequence of ~ 3.4-kb fragment of the NoV genome (the 3′ end of ORF1, the full-length ORF2, ORF3, and the non-coding region through the poly-A tail of 4 canine NoV strains, FD53/2007/ITA, FD210/2007/ITA, 5010/2009/ITA and 63.15/2015/ITA), was determined by 3′ RACE protocol, as previously described (Scotto-Lavino et al., 2006). cDNA was synthesized by Super Script III First-Strand cDNA synthesis kit (Invitrogen Ltd., Milan, Italy) with primer QT. PCR was then performed with TaKaRa La Taq polymerase (Takara Bio Europe, Saint-Germain-en-Laye, France) with forward primer JV12Y and reverse primer JV13I. The cDNA was purified and cloned by using TOPO® XL Cloning Kit (Invitrogen Ltd., Milan, Italy). Additional primers were designed to determine the complete 3.4-kb sequences by a primer walking strategy (Table 2 ). Sequence editing and multiple alignments were carried out with the Geneious software package version 9.1.6 (Biomatters Ltd., New Zealand). Genome sequences of NoVs strains were retrieved from GenBank and aligned using Clustal W (Larkin et al., 2007). Phylogenetic trees were generated using Bayesian analysis with MrBayes (Huelsenbeck and Ronquist, 2001, Ronquist and Huelsenbeck, 2003).
Table 2.
Oligonucleotides used for cDNA synthesis and PCR amplification in this study.
| Primer | Sequence (5′ to 3′) | Sense | Reference |
|---|---|---|---|
| p289 | TGA CAA TGT AAT CAT CAC CAT A | + | Jiang et al. (1999) |
| p290 | GAT TAC TCC AAG TGG GAC TCC AC | − | Jiang et al. (1999) |
| JV12Y | ATACCACCTATGATGCAGAYTA | + | Vennema et al. (2002) |
| JV13I | TCATCATCACCATAGAAGAG | − | Vennema et al. (2002) |
| QT | CCAGTGAGCAGAGTGACGAGGACTCGAGCTCAAGC(T17) | +/− | Scotto-Lavino et al. (2006) |
| 1492 | TGATGATGAGATCGTATCCA | + | This study |
| 1498 | GAACCAGAGATGGTTGATGG | − | This study |
| 1535 | CTGGCCCAGATGTATAATG | + | This study |
| 1536 | CAGTCGGTAGGATGGTGC | − | This study |
| 1506 | ATCGACCGGCAGCTCTATTG | + | This study |
| 1507 | TGACCAGGTCTCAGAGACTG | − | This study |
3. Results
3.1. Detection of caliciviruses by RT-PCR in stool samples
Using the consensus primer pair p289-p290, CV RNA was found in 37 of 336 samples (11.01%). A total of 13 samples generated amplicons of the expected size for NoV (315 bp) and were subsequently confirmed to contain NoV RNA using NoV-specific primers, either alone (1.4%, 5/336) or in mixed infections with CPV-2 (1.4%, 5/336), CCoV (0.89%, 3/336), canine sapovirus (0.29%, 1/336) and vesivirus (0.29%, 1/336). Seventeen samples (5.05%, 17/336) were found to contain canine sapovirus alone (1.19%, 4/336) or in mixed infection with CPV-2 (0.6%, 2/336), CCoV (2.4%, 8/336), CPV-2/CCoV (0.6%; 2/336) and NoV (0.29%, 1/336). All the NoV and sapovirus positive samples were identified from diarrheic dogs with a prevalence rate of 4.4% (13/294) for NoVs and 5.7% (17/294) for sapovirus, whilst they were not detected from asymptomatic animals (0/42). Eleven NoV-positive samples were identified in the Italian collection of samples (11/239, 4.6%), whilst two NoV-positive samples were identified in the Spanish collection (2/26, 7.6%). NoV RNA was not detected in the German (0/3) and French (0/26) samples.
3.2. Sequencing and phylogenetic analysis
The sequence of ~ 3.4-kb fragment at the 3′ end of the genome, including the partial RdRp and the complete ORF2 and ORF3 of the strains 63.15/2015/ITA, 5010/2009/ITA, FD210/2007/ITA and FD53/2007/ITA was determined and then submitted to GenBank under accession no. JF939046, JF930689, KY486328 and KY486329. The sequences were analysed with cognate sequences of animal and human NoVs reference strains available in the GenBank database.
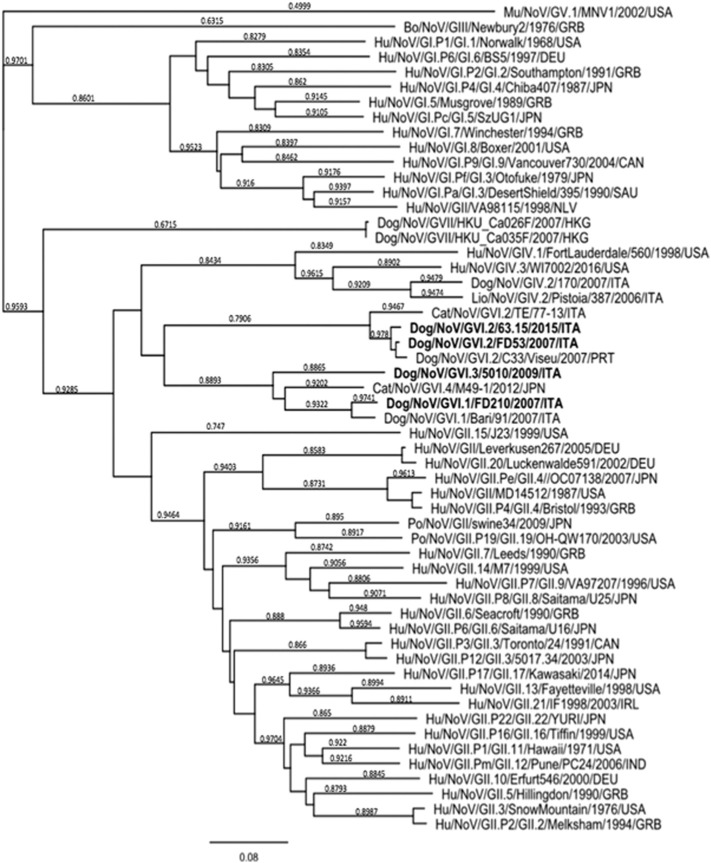
As shown in Table 3 , in the RdRp fragment the four canine strains 63.15/2015/ITA, 5010/2009/ITA, FD210/2007/ITA and FD53/2007/ITA shared 80.1–85.7% nt and 88.8–98.2% aa identities to each other. In the phylogenetic tree based on the 750-nt sequence of the COOH terminus of the polymerase complex, the carnivore NoVs segregated into at least five different genetic clusters (Fig. 1 ). The cluster (pol c) formed by the canine strains 63.15/2015/ITA, FD53/2007/ITA and GVI.2/C33/Viseu/2007/PRT (Mesquita et al., 2010) shared an identity of 85.7–98.8% nt and 97.8–99.6% aa to each other and of 78.2–85.8% nt and 93.8–95.8% aa to the other GIV and GVI canine and feline NoVs strains. Strain FD210/2007/ITA displayed the highest identities (87.6–88.0% nt and 98.5% aa) to the GIV.2 strain Bari/170/2007/ITA and to the GVI.1 strain Bari/91/2007/ITA. This RdRp cluster (pol b) shared 80.1–97.8% nt and 88.8–98.9% aa with the other GIV and GVI canine and feline NoVs strains. Strain 5010/2009/ITA shared 79.9–85.2% nt and 89.6–93.2% aa identity with the other GIV and GVI canine and feline NoVs strains (pol d). A fourth RdRp cluster (pol a) included the feline NoVs GVI.2/TE/77-13/ITA, GIV.2/CU081210E/2010/USA, GVI/2012/M49-1/2012/JPN and the lion strain GIV.2/Pistoia/387/06/ITA (Di Martino et al., 2016, Martella et al., 2007, Pinto et al., 2012, Takano et al., 2015). The identities among NoVs of this cluster ranged between 90.8 and 94.0% nt and 94.3–98.9% aa, whilst the identities to canine/feline NoVs of the other clusters ranged between 78.4 and 85.9% nt and 88.8–99.6% aa. The GVII canine strains Dog/NoV/GVII/Ca_035F/2007/HKG and Dog/NoV/GVII/Ca_026F/2007/HKG (Tse et al., 2012) formed a unique RdRp cluster, distantly related to all the other canine/feline NoVs (64.4–66.9% nt and 69.8–74.4% aa identity).
Table 3.
Nucleotide (nt) and amino acid (aa) sequence identity matrix of partial (750 bp) ORF1 (RdRp). The highest identity values for each strain are in bold. Also, the strains analysed in this study are in bold.
| GVI.2 FD53/2007/ITA |
GVI.1 FD210/2007/ITA |
GVI.2 63.15/2015/ITA |
GVI.3 5010/2009/ITA |
GVI.4 M49-1/2012/JPN |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % nt | % aa | % nt | % aa | % nt | % aa | % nt | % aa | % nt | % aa | |
| Hu/NoV/GI.P2/GI.2/Southampton/1991/ENG | 62.4 | 65.0 | 63.0 | 62.7 | 63.2 | 66.2 | 64.7 | 63.9 | 62.2 | 63.5 |
| Hu/NoV/GII.P2/GII.2/Melksham/1994/GRB | 64.3 | 67.7 | 64.6 | 66.9 | 64.8 | 68.4 | 65.2 | 67.5 | 65.9 | 68.8 |
| Bo/NoV/GIII/Newbury2/1976/ENG | 63.8 | 68.2 | 62.3 | 68.2 | 63.7 | 69.3 | 63.6 | 64.6 | 62.3 | 68.2 |
| Mu/NoV/GV/WU23/2005/USA | 58.4 | 57.0 | 61.3 | 57.1 | 58.9 | 56.2 | 57.8 | 54.9 | 61.0 | 57.6 |
| Hu/NoV/GIV.1/FortLauderdale/560/1998/USA | 69.1 | 73.4 | 67.9 | 72.6 | 68.8 | 73.9 | 68.8 | 72.1 | 69.7 | 73.8 |
| Dog/NoV/GIV.2/170/2007/ITA - pol b | 80.7 | 95.4 | 87.6 | 98.5 | 81.2 | 95.1 | 80.4 | 89.6 | 84.2 | 97.3 |
| Lio/NoV/GIV.2/Pistoia/387/2006/ITA – pol a | 79.6 | 92.8 | 84.9 | 93.2 | 78.4 | 91.7 | 80.9 | 88.8 | 92.7 | 95.8 |
| Hu/NoV/GIV.3/WI7002/2016/USA | 69.1 | 74.2 | 68 | 73 | 69.1 | 75.3 | 68.7 | 73.8 | 70.2 | 74.9 |
| Cat/NoV/GVI/M49-1/2012/JPN - pol a | 78.6 | 95.8 | 85.9 | 96.2 | 79.7 | 95.6 | 81.0 | 90.0 | – | – |
| Dog/NoV/GVI.1/Bari/91/2007/ITA - pol b | 82.6 | 95.4 | 88.0 | 98.5 | 80.7 | 95.1 | 81.7 | 89.6 | 84.7 | 97.0 |
| Dog/NoV/GVI.1/FD210/2007/ITA - pol b | 80.4 | 94.6 | – | – | 81.0 | 93.8 | 80.1 | 88.8 | 85.9 | 96.2 |
| Dog/NoV/GVI.2/FD53/2007/ITA - pol c | – | – | 80.4 | 94.6 | 85.7 | 98.2 | 82.7 | 93.2 | 78.6 | 95.8 |
| Dog/NoV/GVI.2/63.15/2015/ITA - pol c | 85.7 | 98.2 | 81.0 | 93.8 | – | – | 85.2 | 93.2 | 79.7 | 95.6 |
| Dog/NoV/GVI.2/C33/Viseu/2007/PRT - pol c | 98.8 | 99.6 | 80.4 | 94.7 | 85.8 | 97.8 | 83.6 | 92.7 | 78.6 | 96.5 |
| Cat/NoV/GVI.2/TE/77-13/ITA – pol a | 78.5 | 94.2 | 83.4 | 94.7 | 78.7 | 94.2 | 79.9 | 88.8 | 92.4 | 97.7 |
| Dog/NoV/GVI.3/5010/2009/ITA - pol d | 82.7 | 93.2 | 80.1 | 88.8 | 85.2 | 93.2 | – | – | 81.0 | 90.0 |
| Dog/NoV/GVII/HKU_Ca035F/2007/HKG | 66.1 | 73.5 | 64.4 | 72.2 | 67.5 | 74.0 | 66.9 | 69.8 | 66.5 | 73.8 |
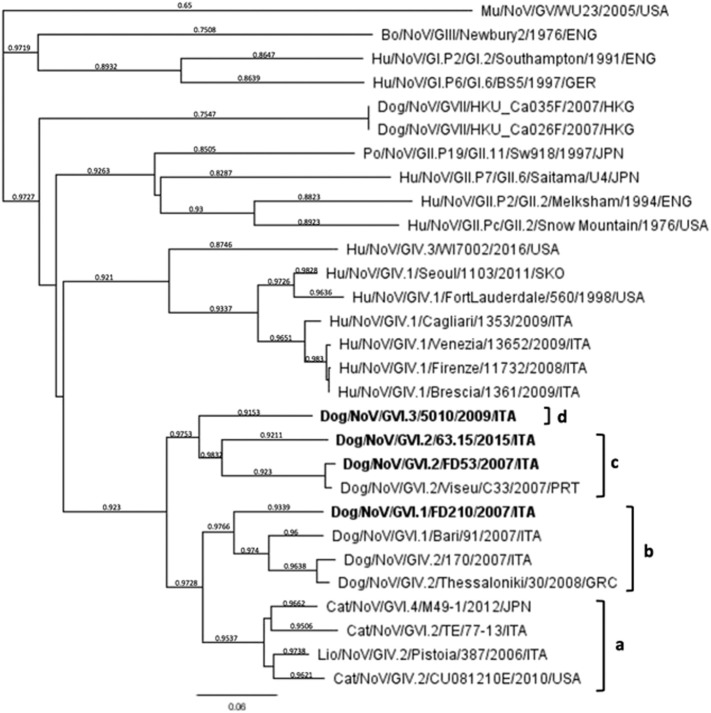
Fig. 1.
Phylogenetic tree based on the 750-nt sequence of the COOH terminus of the polymerase complex. The tree was generated using the Bayesian inference with Generalized Time-Reversible (GTR) model and gamma rate variation and supplying statistical support with subsampling over 200 replicates. Numbers on the tree branches indicate the posterior probability values. The scale bar indicates the number of nt substitutions per site. Strains in bold type indicate the NoV strains detected in this study and letters “a” to “d” indicate the pol type.
The complete ORF2 and ORF3 of the strains 63.15/2015/ITA, 5010/2009/ITA, FD210/2007/ITA and FD53/2007/ITA were also sequenced and the genome organization was determined. The four canine strains from this study shared 56.3–84.1% nt and 62.5–98.3% aa identity to each other in the complete VP1 (Table 4 ). The strains 63.15/2015/ITA and FD53/2007/ITA segregated in the capsid-based phylogenetic tree (Fig. 2 ) with the canine strain C33/Viseu/2007/PRT and the feline strain TE/77-13/ITA into genogroup GVI, genotype 2, whilst strain FD210/2007/ITA segregated with the canine strain Bari/91/2007/ITA into genotype GVI.1 and strain 5010/2009/ITA was grouped with GVI.1 canine NoVs and with feline NoV strain M49-1/2012/JPN. By pair-wise distance comparison, the strains 63.15/2015/ITA and FD53/2007/ITA displayed 84.1% nt and 98.3% aa to each other and shared identity of 84.0% nt (97.4% aa) and 98.0% nt (98.6% aa), respectively, with the GVI.2 prototype strain C33/Viseu/2007/PRT. Identity to the GVI.2 feline strain TE/77-13/ITA was 75.4–83.9% nt and 90.3–90.8% aa. Strain FD210/2007/ITA in the capsid-based phylogenetic tree (Fig. 2) was grouped with GVI.1 canine NoVs and with the feline NoV strain M49-1/2012/JPN. Upon sequence comparison, this canine strain showed the highest identity (86.4% nt and 95.0% aa) to strain Bari/91/2007/ITA. Strain 5010/2009/ITA displayed the highest identity (66.6–67.6% nt and 80.7–81.6% aa) to the canine GVI.1 strains FD210/2007/ITA and Bari/91/2007/ITA, while identities to other GVI.1 canine and feline NoVs ranged between 57.2 and 61.1% nt and 61.9–62.7% aa. The identity displayed between the canine strains from this study and other animal and human GIV NoVs was < 55.7% aa and it was < 45.7% aa to the Chinese GVII canine NoV strains.
Table 4.
Nucleotide (nt) and amino acid (aa) sequence identity matrix in the ORF2 (VP1 capsid protein). The highest identity values for each strain are in bold. Also, the strains analysed in this study are in bold.
| GVI.2 FD53/2007/ITA |
GVI.1 FD210/2007/ITA |
GVI.2 63.15/2015/ITA |
GVI.3 5010/2009/ITA |
GVI.4 M49-1/2012/JPN |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % nt | % aa | % nt | % aa | % nt | % aa | % nt | % aa | % nt | % aa | |
| Hu/NoV/GI.P2/GI.2/Southampton/1991/ENG | 44.5 | 41.9 | 46.5 | 41.7 | 44.4 | 41.8 | 45 | 41.2 | 44.2 | 41.0 |
| Hu/NoV/GII.P2/GII.2/Melksham/1994/GRB | 52.8 | 52.1 | 54.3 | 54.5 | 51.6 | 52.2 | 51.8 | 53.9 | 52.2 | 54.6 |
| Bo/NoV/GIII/Newbury2/1976/ENG | 44.2 | 40.1 | 42.2 | 39.4 | 41.6 | 39.9 | 43.0 | 39.6 | 42.0 | 38.7 |
| Hu/NoV/GIV.1/FortLauderdale/560/1998/USA | 56.2 | 53.9 | 56.3 | 53.3 | 53.7 | 54 | 51.8 | 54.1 | 53.1 | 67.0 |
| Dog/NoV/GIV.2/170/2007/ITA - pol b | 57.5 | 54.6 | 59.1 | 54.6 | 54.5 | 54.6 | 55.6 | 55.2 | 55.0 | 52.3 |
| Lio/NoV/GIV.2/Pistoia/387/2006/ITA – pol a | 58.4 | 54.9 | 59.3 | 54.6 | 54.9 | 54.9 | 56.5 | 55.7 | 56.7 | 52.4 |
| Hu/NoV/GIV.3/WI7002/2016/USA | 55.0 | 55.3 | 55.6 | 53.9 | 52.2 | 55.2 | 52.5 | 54.8 | 52.1 | 52.4 |
| Mu/NoV/GV/WU23/2005/USA | 43.4 | 36.4 | 43.5 | 36.8 | 42.2 | 36.7 | 42.2 | 37.0 | 43.6 | 36.1 |
| Cat/NoV/GVI/M49-1/2012/JPN - pol a | 58.6 | 59.7 | 75.2 | 80.0 | 58.6 | 59.8 | 67.3 | 75.5 | – | – |
| Dog/NoV/GVI.1/Bari/91/2007/ITA - pol b | 60.9 | 62.6 | 86.4 | 95.0 | 55.7 | 62.7 | 67.6 | 81.6 | 75.4 | 80.4 |
| Dog/NoV/GVI.1/FD210/2007/ITA - pol b | 60.9 | 62.6 | – | – | 56.3 | 62.5 | 66.6 | 80.7 | 75.2 | 80.0 |
| Dog/NoV/GVI.2/FD53/2007/ITA - pol c | – | – | 60.9 | 62.3 | 84.1 | 98.3 | 58.9 | 62.6 | 58.6 | 59.7 |
| Dog/NoV/GVI.2/63.15/2015/ITA- pol c | 84.1 | 98.3 | 56.3 | 62.5 | – | – | 61.1 | 62.7 | 58.6 | 59.8 |
| Dog/NoV/GVI.2/C33/Viseu/2007/PRT - pol c | 98.0 | 98.6 | 60.9 | 62.1 | 84.0 | 97.4 | 58.9 | 61.9 | 58.5 | 59.6 |
| Cat/NoV/GVI.2/TE/77-13/ITA – pol a | 83.9 | 90.3 | 61.4 | 61.1 | 75.4 | 90.8 | 57.2 | 61.9 | 58.5 | 58.9 |
| Dog/NoV/GVI.3/5010/2009/ITA - pol d | 58.9 | 62.6 | 66.6 | 80.7 | 61.1 | 62.7 | – | – | 67.3 | 75.5 |
| Dog/NoV/GVII/HKU_Ca035F/2007/HKG | 45.9 | 44.4 | 47.6 | 44.1 | 43.6 | 44.4 | 46.4 | 44.3 | 46.3 | 43.4 |
Fig. 2.
Phylogenetic tree based on the full-length aa sequence of the VP1 protein of NoVs. The tree was generated using the Bayesian inference with GTR model with gamma rate variation and supplying statistical support with subsampling over 200 replicates. Numbers on the tree branches indicate the posterior probability values. The scale bar indicates the number of aa substitutions per site. Bold type indicates the NoV strains detected in this study.
4. Discussion
Screening by RT-PCR of a collection of fecal samples obtained from young dogs (aged 0–12 months) with gastroenteritis identified canine NoVs in 4.4% of the symptomatic dogs, either alone or in mixed infectious with CPV-2, CCoV, canine sapovirus and vesivirus, but not in fecal samples from clinically healthy dogs. In previous molecular investigations canine NoVs have been detected in diarrheic stool samples with a prevalence rate ranging from 2.2% (Martella et al., 2009), to 11.0% (Azevedo et al., 2012) and to 40% (Mesquita et al., 2010). Canine NoVs have also been detected in healthy dogs with a prevalence of 9.0% (Mesquita et al., 2010). Accordingly, the pathogenic role of these viruses in dog enteritis is not firmly established yet. The prevalence rate of 4.4% observed in this study was lower than those reported in the US and Portugal (Azevedo et al., 2012, Mesquita et al., 2010), although different primer sets were used in the various studies. In our investigation, we preferred using broadly reactive primers designed to amplify all the CVs, p289-p290 (Jiang et al., 1999), and subsequently a set of broadly reactive primers able to amplify the majority of NoV strains, JV12Y-JV13I (Vennema et al., 2002). This algorithm, while allowing us to identify genetically diverse NoV strains, was likely less sensitive than other RT-PCR assays based on specific primers. The majority of the NoV-positive samples were identified in the Italian collection of samples (11/239, 4.6%), whilst 2 NoV-positive samples were identified in the Spanish collection (2/26, 7.6%). Mixed infections were identified but they were not unexpected as this eventuality is not infrequent. A limit of our study was surely the non-homogenous size of the sample collections, chiefly the small number of samples analysed for the non-Italian sample collections. Nevertheless, to our knowledge, this is the first report on the presence of NoV in dogs in Spain.
A fragment of about 3.4-kb at the 3′ end of the genome of four samples was used for analysis and comparison with a large selection of NoV strains belonging to all genogroups available on database. In the full-length VP1 capsid gene (ORF1), two strains (FD53/2007/ITA and 63.15/2015/ITA) were characterized as GVI.2, whilst strain FD210/2007/ITA was characterized as GVI.1. A fourth strain, 5010/2009/ITA, although segregating within genogroup GVI, was more diverse genetically in the VP1 and could not be classified into any established GVI genotypes. According to Zheng's classification (Zheng et al., 2006), the canine NoV strains 63.15/2015/ITA and FD53/2007/ITA were classified together with the canine strain C33/Viseu/2007/PRT and the feline strain TE/77-13/ITA, as a genotype 2 (> 85% pairwise aa identity inter genotypes), within the genogroup GVI (> 55% pairwise aa identity inter-genogroups). The canine strain 5010/2009/ITA was also classified into genogroup GVI, but the aa identity to its closest relatives (the canine GVI.1 strains Bari/91/2007/ITA and FD210/2007/ITA) and the GVI feline strain M49-1/2012/JPN was lower (75.5–81.6% aa) than the 85% pairwise aa identity cut-off, proposed to discriminate between different NoV genotypes. Thereby, strain 5010/2009/ITA was tentatively classified as a third genotype (GVI.3) within the genogroup VI. Interestingly, in our analysis the canine GVI.1 strains FD210/2007/ITA, Bari/91/2007/ITA and 5010/2009/ITA appeared equally distant from the feline NoV strain M49-1/2012/JPN (75.5–80.4% aa). This feline strain (GenBank accession number LC011950) is registered in NCBI sequence database as a GVI.1 strain, although it does not fit the criteria for being assigned to this genotype and it was tentatively proposed as a fourth GVI genotype, GVI.4 (Table 1, Table 3; Fig. 1).
NoVs genetically related have been detected in different host species. GII NoV have been found in human and pigs, although porcine NoVs belong to different genotypes (GII.11, GII.18 and GII.19) (Wang et al., 2005). GIII NoV infect ruminants, with genotypes GIII.1 and GIII.2 (Liu et al., 1999, Oliver et al., 2007) being identified in cattle and genotype GIII.3 in sheep (Wolf et al., 2009). However, circulation of NoVs strains belonging to the same genotype in different host species seems rather uncommon. By converse, it seems clear that carnivores (canids and felids) may be infected by NoV strains with the same capsid or polymerase (pol) types (Table 1). Binding of human NoVs to human tissues seems to be mediated by antigens of the histo-blood group antigen (HBGA) family and therefore to be genetically determined (Marionneau et al., 2002). Likewise, binding of GIV.2 and GVI NoVs in dog tissues seems mediated by antigens of the HBGA family (Caddy et al., 2014). Interestingly, virus-like particles (VLPs) of seven different human NoV genotypes (GI.1, GI.2, GI.3, GII.3, GII.4, GII.6, and GII.12) were also found to bind to canine gastrointestinal tissues (Caddy et al., 2015) and human NoV strains of genotypes GII.4 and GII.12 were detected from infected dogs (Summa et al., 2012). Whether dogs and cats share similar HBGAs that can be used as attachment factors by NoV could be hypothesized. Alternatively, carnivore HBGAs could be more “permissive”, i.e. they could allow binding of NoVs of different genotypes. Regardless, this intriguing phenomenon could be a key factor explaining the apparent genetic diversity of carnivore NoVs.
Based on sequence and phylogenetic analysis of the 3′ partial sequence of ORF1 spanning750-nt at the COOH terminus of the RdRp, different pol genetic lineages could be distinguished among canine/feline GIV and GVI NoVs that were indicated, for the limited purpose of this study, with the letters “a” to “d”. The canine strains 63.15/2015/ITA, and FD53/2007/ITA possessed a pol gene of the same lineage as the canine NoV strain GVI.2/C33/Viseu/2007/PRT (pol c), whilst strain FD210/2007/ITA shared the same lineage (pol b) as the canine NoV strains GVI.1/Bari/91/2007/ITA, GIV.2/170/2007/ITA and GIV.2/Thessaloniki/30/2008/GRC (Fig. 1; Table 1). A unique lineage (pol a) was formed by feline NoVs of genogroup GIV and GVI and by the lion NoV strain GIV.2/Pistoia/2006/ITA. Interestingly, inconsistencies were observed between the pol- and capsid-based phylogenies, which are suggestive of potential recombination events (Table 1). This phenomenon has been reported repeatedly when studying NoVs from carnivores (Di Martino et al., 2016, Martella et al., 2009, Takano et al., 2015). The canine NoV strain Bari/91/2007/ITA, resembles GIV.2 NoVs in its pol gene (pol b) whilst it possesses a GVI.1 capsid (Martella et al., 2009). The feline NoV strain, M49-1/2012/JPN possesses a GIV.2 pol (pol a), and a GVI capsid (Takano et al., 2015), whilst the feline strain TE/77-13/2013/ITA possesses a GIV.2 pol (pol a) and a GVI.2 capsid (Di Martino et al., 2016). In all the cases, the recombination site was located within the ORF1/ORF2 overlap, a region highly conserved across all NoV sequences (Bull et al., 2005). RNA recombination is a major force driving viral evolution (Worobey and Holmes, 1999, Lai, 1992). Recombination in viruses may substantially affect phylogenetic groupings, confusing molecular epidemiologic studies, and also can have major implications in vaccine design (Bull et al., 2005). A recombinant NoV can be defined as a strain that clusters with 2 distinct groups of NoV strains when 2 different regions (normally the capsid and polymerase) of the genome are subjected to phylogenetic analysis (Bull et al., 2005). Accordingly, analysis of the diagnostic regions located on the RdRp cannot be used to characterize unequivocally these animal NoVs and a definitive characterization should rely on the ORF2 as proposed for humans NoVs strains (Kroneman et al., 2013).
5. Conclusions
Epidemiological studies are necessary to understand the relevance of newly discovered pathogens. These studies are important as they allow us to obtain information on the genetic diversity of viruses, a step that is propaedeutic to the development of accurate diagnostic tools and necessary for the understanding of viral evolution. Analysis of NoVs identified in carnivores thus far is unveiling a marked genetic diversity and suggests that the evolution of canine and feline NoV is tightly intermingled. This could have implications when enacting prophylaxis measures in animal populations (such as humane shelters, kennels or breeding facilities), as cats and dogs may be infected by a number of common pathogens (Decaro et al., 2010, Di Martino et al., 2009, Di Martino et al., 2016, Martella et al., 2002, Pratelli et al., 2003). Finally, as some animal viruses may be transmitted to humans, generating new threats for human health, defining a baseline of the genetic diversity on animal viruses is important, to understand better and readily identify the origin of novel human viruses.
Conflict of interest statement
All authors declare that there are no financial or other relationships that might lead to a conflict of interest. All authors have seen and approved the manuscript and have contributed significantly to the work.
Acknowledgments
This study was supported by grants from the institution CAPES, Brazil, from the Momentum Program, Hungary, and from the action “Future in Research” of Apulian Region, Italy.
References
- Atmar R.L., Estes M.K. The epidemiologic and clinical importance of norovirus infection. Gastroenterol. Clin. N. Am. 2006;35:275–290. doi: 10.1016/j.gtc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Azevedo M., Mullis L., Vegas E., Britt J., Pereira O., Silva C., Vinjé J. Detection of norovirus in dogs in Arkansas. Am. Soc. Virol. Conf. 2012;23–30 [Google Scholar]
- Bertolotti-Ciarlet A., Crawford S.E., Hutson A.M., Estes M.K. The 3′ end of Norwalk virus mRNA contains determinants that regulate the expression and stability of the viral capsid protein VP1: a novel function for the VP2 protein. J. Virol. 2003;77:11603–11615. doi: 10.1128/JVI.77.21.11603-11615.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R.A., Hansman G.S., Clancy L.E., Tanaka M.M., Rawlinson W.D., White P.A. Norovirus recombination in ORF1/ORF2 overlap. Emerg. Infect. Dis. 2005;11:1079–1085. doi: 10.3201/eid1107.041273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonavoglia C., Martella V., Pratelli A., Tempesta M., Cavalli A., Buonavoglia D., Bozzo G., Elia G., Decaro N., Carmichael L. Evidence for evolution of canine parvovirustype2 in Italy. J. Gen.Virol. 2001;82:3021–3025. doi: 10.1099/0022-1317-82-12-3021. [DOI] [PubMed] [Google Scholar]
- Caddy S., Emmott E., El-Attar L., Mitchell J., de Rougemont A., Brownlie J., Goodfellow I. Serological evidence for multiple strains of canine norovirus in the UK dog population. PLoS One. 2013;5 doi: 10.1371/journal.pone.0081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddy S., Breiman A., le Pendu J., Goodfellow I. Genogroup IV and VI canine noroviruses interact with histo-blood group antigens. J. Virol. 2014;88:10377–10391. doi: 10.1128/JVI.01008-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddy S.L., de Rougemont A., Emmott E., El-Attar L., Mitchell J.A., Hollinshead M., Belliot G., Brownlie J., Le Pendu J., Goodfellow I. Evidence for human norovirus infection of dogs in the United Kingdom. J. Clin. Microbiol. 2015;53:1873–1883. doi: 10.1128/JCM.02778-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Elia G., Campolo M., Desario C., Lucente M.S., Bellacicco A.L., Buonavoglia C. New approaches for the molecular characterization of canine parvovirus type 2 strains. J. Vet. Med. B Infect. Dis Vet. Public Health. 2005;52:316–319. doi: 10.1111/j.1439-0450.2005.00869.x. [DOI] [PubMed] [Google Scholar]
- Decaro N., Buonavoglia D., Desario C., Amorisco F., Colaianni M.L., Parisi A., Terio V., Elia G., Lucente M.S., Cavalli A., Martella V., Buonavoglia C. Characterisation of canine parvovirus strains isolated from cats with feline panleukopenia. Res. Vet. Sci. 2010;89:275–278. doi: 10.1016/j.rvsc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGraaf M., Bodewes R., van Elk C.E., van de Bildt M., Getu S., Aron G.I., Verjans G.M., Osterhaus A.D., van den Brand J.M., Kuiken T., Koopmans M.P. Norovirus infection in harbor porpoises. Emerg. Infect. Dis. 2017;23:87–91. doi: 10.3201/eid2301.161081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino B., Di Rocco C., Ceci C., Marsilio F. Characterization of a strain of feline calicivirus isolated from a dog faecal sample. Vet. Microbiol. 2009;139:52–57. doi: 10.1016/j.vetmic.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino B., Marsilio F., Di Profio F., Lorusso E., Friedrich K.G., Buonavoglia C., Martella V. Detection of antibodies against norovirus genogroup GIV in carnivores. Clin. Vaccine Immunol. 2010;17:180–182. doi: 10.1128/CVI.00312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino B., Di Profio F., Melegari I., Sarchese V., Cafiero M.A., Robetto S., Aste G., Lanave G., Marsilio F., Martella V. A novel feline norovirus in diarrheic cats. Infect. Genet. Evol. 2016;38:132–137. doi: 10.1016/j.meegid.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R.I., Parashar U.D., Estes M.K. Norovirus gastroenteritis. N. Engl. J. Med. 2009;361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K.Y. Caliciviridae: the noroviruses: specific virus families. In: Knipe D.M., Howley P.M., Griffin D.E., Lamb R.A., Martin M.A., Roizman B., Straus S.E., editors. Fields Virology. fifth ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2007. pp. 949–979. [Google Scholar]
- Green K.Y. Caliciviridae: the noroviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. sixth ed. Wolters Kluwer Health/Lippincott Williams and Wilkins; Philadelphia, PA: 2013. pp. 949–979. [Google Scholar]
- Hall A.J., Wikswo M.E., Manikonda K., Roberts V.A., Yoder J.S., Gould L.H. Acute gastroenteritis surveillance through the National Outbreak Reporting System, United States. Emerg. Infect. Dis. 2013;19:1305–1309. doi: 10.3201/eid1908.130482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Jiang X., Huang P.W., Zhong W.M., Farkas T., Cubitt D.W., Matson D.O. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J. Virol. Methods. 1999;83:145–154. doi: 10.1016/s0166-0934(99)00114-7. [DOI] [PubMed] [Google Scholar]
- Kroneman A., Vega E., Vennema H., Vinjé J., White P.A., Hansman G., Green K., Martella V., Katayama K., Koopmans M. Proposal for a unified norovirus nomenclature and genotyping. Arch. Virol. 2013;158:2059–2068. doi: 10.1007/s00705-013-1708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M.C. RNA recombination in animal and plant viruses. Microbiol. Rev. 1992;56:61–79. doi: 10.1128/mr.56.1.61-79.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., Thompson J.D., Gibson T.J., Higgins D.G. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Li L., Shan T., Wang C., Côté C., Kolman J., Onions D., Gulland F.M., Delwart E. The fecal viral flora of California sea lions. J. Virol. 2011;85:9909–9917. doi: 10.1128/JVI.05026-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.L., Lambden P.R., Günther H., Otto P., Elschner M., Clarke I.N. Molecular characterization of a bovine enteric calicivirus: relationship to the Norwalk-like viruses. J. Virol. 1999;73:819–825. doi: 10.1128/jvi.73.1.819-825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marionneau S., Ruvoën N., Le Moullac-Vaidye B., Clement M., Cailleau-Thomas A., Ruiz-Palacoism G., Huang P., Jiang X., Le Pendu J. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology. 2002;122:1967–1977. doi: 10.1053/gast.2002.33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V., Pratelli A., Gentile M., Buonavoglia D., Decaro N., Fiorente P. Analysis of the capsid protein gene of a feline-like calicivirus isolated from a dog. Vet. Microbiol. 2002;85:315–322. doi: 10.1016/s0378-1135(01)00521-1. [DOI] [PubMed] [Google Scholar]
- Martella V., Campolo M., Lorusso E., Cavicchio P., Camero M., Bellacicco A.L., Elia G., Greco G., Corrente M., Desario C., Arista C., Banyaj K., Koopmans M., Buonavoglia C. Norovirus in captive lioncub (Panthera leo) Emerg. Infect. Dis. 2007;13:1071–1073. doi: 10.3201/eid1307.070268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V., Lorusso E., Decaro N., Elia G., Radogna A., D'Abramo M., Desario C., Cavalli A., Corrente M., Germinaro C.A., Banyai K., Di Martino B., Marsilio F., Carmichael L.E., Buonavoglia C. Detection and molecular characterization of a canine norovirus. Emerg. Infect. Dis. 2008;14:1306–1308. doi: 10.3201/eid1408.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V., Decaro N., Lorusso E., Radogna A., Moschidou P., Amorisco F., Lucente M.S., Desario C., Elia G., Banyai K., Carmichael L.E., Buonavoglia C. Genetic heterogeneity and recombination in canine noroviruses. J. Virol. 2009;83:11391–11396. doi: 10.1128/JVI.01385-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita J.R., Nascimento M.S. Molecular epidemiology of canine norovirus in dogs from Portugal, 2007–2011. BMC Vet. Res. 2012;8:107. doi: 10.1186/1746-6148-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita J.R., Nascimento M.S.J. Gastroenteritis outbreak associated with faecal shedding of canine norovirus in a Portuguese kennel following introduction of imported dogs from Russia. Transbound. Emerg. Dis. 2012;59:456–459. doi: 10.1111/j.1865-1682.2011.01284.x. [DOI] [PubMed] [Google Scholar]
- Mesquita J.R., Barclay L., Nascimento M.S.J., Vinjé J. Novel norovirus in dogs with diarrhea. Emerg. Infect. Dis. 2010;16:980–982. doi: 10.3201/eid1606.091861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita J.R., Delgado I., Costantini V., Heenemann K., Vahlenkamp T.W., Vinjé J., Nascimento M.S. Seroprevalence of canine norovirus in 14 European countries. Clin. Vaccine Immunol. 2014;21:898–900. doi: 10.1128/CVI.00048-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntafis V., Xylouri E., Radogna A., Buonavoglia C., Martella V. An outbreak of canine norovirus infection in young dogs. J. Clin. Microbiol. 2010;48:2605–2608. doi: 10.1128/JCM.02528-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S.L., Asobayire E., Charpilienne A., Cohen J., Bridger J.C. Complete genomic characterization and antigenic relatedness of genogroup III, genotype 2 bovine noroviruses. Arch. Virol. 2007;152:257–272. doi: 10.1007/s00705-006-0856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Oka T., Takeda N., Hansman G.S. Norovirus infections in symptomatic and asymptomatic food handlers in Japan. J. Clin. Microbiol. 2007;45:3996–4005. doi: 10.1128/JCM.01516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto P., Wang Q., Chen N., Dubovi E.J., Daniels J.B., Millward L.M., Buonavoglia C., Martella V., Saif L.J. Discovery and genomic characterization of noroviruses from a gastroenteritis outbreak in domestic cats in the US. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Tempesta M., Roperto F.P., Sagazio P., Carmichael L., Buonavoglia C. Fatal coronavirus infection in puppies following canine parvovirus 2b infection. J. Vet. Diagn. Investig. 1999;11:550–553. doi: 10.1177/104063879901100615. [DOI] [PubMed] [Google Scholar]
- Pratelli A., Martella V., Decaro N., Tinelli A., Camero M., Cirone F., Elia G., Cavalli A., Corrente M., Greco G., Buonavoglia D., Gentile M., Tempesta M., Buonavoglia C. Genetic diversity of a canine coronavirus detected in pups with diarrhoea in Italy. J. Virol. Methods. 2003;110:9–17. doi: 10.1016/S0166-0934(03)00081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Scipioni A., Mauroy A., Vinjé J., Thiry E. Animal noroviruses. Vet. J. 2008;178:32–45. doi: 10.1016/j.tvjl.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Scotto-Lavino E., Du G., Frohman M.A. 3′ end cDNA amplification using classic RACE. Nat. Protoc. 2006;1:2742–2745. doi: 10.1038/nprot.2006.481. [DOI] [PubMed] [Google Scholar]
- Siebenga J.J., Vennema H., Zheng D.P., Vinjé J., Lee B.E., Pang X.L., Ho E.C., Lim W., Choudekar A., Broor S., Halperin T., Rasool N.B., Hewitt J., Greening G.E., Jin M., Duan Z.J., Lucero Y., O'Ryan M., Hoehne M., Schreier E., Ratcliff R.M., White P.A., Iritani N., Reuter G., Koopmans M. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001–2007. J. Infect. Dis. 2009;200:802–812. doi: 10.1086/605127. [DOI] [PubMed] [Google Scholar]
- Soma T., Nakagomi O., Nakagomi T., Mochizuki M. Detection of norovirus and sapovirus from diarrheic dogs and cats in Japan. Microbiol. Immunol. 2014;59:123–128. doi: 10.1111/1348-0421.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugieda M., Nagaoka H., Kakishima Y., Ohshita T., Nakamura S., Nakajima S. Detection of Norwalk-like virus genes in the caecum contents of pigs. Arch. Virol. 1998;143:1215–1221. doi: 10.1007/s007050050369. [DOI] [PubMed] [Google Scholar]
- Summa M., von Bonsdorff C.H., Maunula L. Pet dogs — a transmission route for human noroviruses? J. Clin. Virol. 2012;53:244–247. doi: 10.1016/j.jcv.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Takano T., Kusuhara H., Kuroishi A., Takashina M., Doki T., Nishinaka T., Hohdatsu T. Molecular characterization and pathogenicity of a genogroup GVI feline norovirus. Vet. Microbiol. 2015;178:201–217. doi: 10.1016/j.vetmic.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse H., Lau S.K., Chan W.M., Choi G.K., Woo P.C., Yuen K.Y. Complete genome sequences of novel canine noroviruses in Hong Kong. J. Virol. 2012;86:9531–9532. doi: 10.1128/JVI.01312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega E., Barclay L., Gregoricus N., Shirley S.H., Lee D., Vinjé J. Genotypic and epidemiologic trends of norovirus outbreaks in the United States, 2009 to 2013. J. Clin. Microbiol. 2014;52:147–155. doi: 10.1128/JCM.02680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., de Bruin E., Koopmans M. Rational optimization of generic primers used for Norwalk-like virus detection by reverse transcriptase polymerase chain reaction. J. Clin. Virol. 2002;25:233–235. doi: 10.1016/s1386-6532(02)00126-9. [DOI] [PubMed] [Google Scholar]
- Vinjé J. Advances in laboratory methods for detection and typing of norovirus. J. Clin. Microbiol. 2015;53:373–381. doi: 10.1128/JCM.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.H., Han M.G., Cheetham S., Souza M., Funk J.A., Saif L.J. Porcine noroviruses related to human noroviruses. Emerg. Infect. Dis. 2005;11:1874–1881. doi: 10.3201/eid1112.050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S., Williamson W., Hewitt J., Lin S., Rivera-Aban M., Ball A., Scholes P., Savill M., Greening G.E. Molecular detection of norovirus in sheep and pigs in New Zealand farms. Vet. Microbiol. 2009;133:184–189. doi: 10.1016/j.vetmic.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Worobey M., Holmes E.C. Evolutionary aspects of recombination in RNA viruses. J Gen Virol. 1999;80:2535–2543. doi: 10.1099/0022-1317-80-10-2535. [DOI] [PubMed] [Google Scholar]
- Wu Z., Yang L., Ren X., He G., Zhang J., Yang J., Qian Z., Dong J., Sun L., Zhu Y., Du J., Yang F., Zhang S., Jin Q. Deciphering the bat virome catalog to better understand the ecological diversity of bat viruses and the bat origin of emerging infectious diseases. ISME J. 2016;10:609–620. doi: 10.1038/ismej.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D.P., Ando T., Frankhouser R.L., Beard R.S., Glass R.I., Monroe S.S. Norovirus classification and proposed strain nomenclature. Virology. 2006;346:312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]