Abstract
Human rhinovirus (HRV) is an emerging viral pathogen.
Aim
To characterize a group of patients admitted due to infection by this agent in a general hospital in Chile.
Methods
Cases were identified by RT-PCR for 1 year through active surveillance of patients admitted with severe respiratory illness. Diagnosis was not available during hospitalization. Thirty-two cases were identified, 90% were ≥60 years old or had co-morbid conditions. Human rhinovirus-related admissions represented 23.7% of hospitalization due to severe acute respiratory infections among adults and ranked second to influenza (37.8%). Patients presented with pneumonia (68.8%), decompensated chronic lung conditions (21.9%), heart failure or influenza-like illness (6.3% each). Admission to intensive or intermediate care units was required by 31.2% and in-hospital mortality reached 12.5%. A CURB-65 score ≥3 was significantly associated to in-hospital mortality (p < 0.05). Most patients received antibiotics (90%).
Conclusions
Human rhinovirus infections in elderly patients with co-morbid conditions are associated with hospitalizations, requiring critical or semi-critical antibiotics use. A high CURB-65 score was associated to in-hospital mortality.
Keywords: Rhinovirus, Respiratory tract infections, Viral pneumonia, Reverse transcriptase polymerase chain reaction, Adult, Hospitalization
Introduction
Upper and lower viral respiratory infections represent an important global health burden for adult patients due to their morbidity, absenteeism, outpatient medical visits, hospitalizations, mortality, and inappropriate antibiotic use.1, 2, 3, 4, 5, 6, 7, 8, 9 Human rhinovirus is one of the etiological agents involved in acute respiratory infections and has been associated with crisis of asthma, exacerbated chronic bronchitis, bronchiolitis and pneumonia in children.10 Nonetheless, its role in adult patients is less accepted despite progressive evidence of its importance in outpatient clinical settings4, 11, 12 or in hospitalized or immunosuppressed patients.13, 14 This unrecognized role is changing after the recent availability of molecular diagnostic testing that has enhanced our knowledge on this viral agent.2, 3, 15, 16
In this work we report seasonality, clinical features and outcomes of adult patients hospitalized during 1 year for severe respiratory acute infection secondary to human rhinovirus infection in a general hospital.
Patients and methods
Study design and setting
The Hospital Militar of Santiago is one of the six active surveillance centers for acute severe respiratory infections requiring admission, a system launched in July 2011 by the Pan American Health Organization (PAHO) and the Chilean Ministry of Health after the influenza A (H1N1) pandemic to recognize new respiratory viral threats. It attends active and retired military personnel and their relatives. The average age of admitted patients is 76 years.
This was a prospective descriptive study that included all adult patients ≥18 years admitted due to severe acute respiratory infection (SARI) associated with a confirmed human rhinovirus detection obtained from respiratory samples during the year 2012 (see below). SARI was defined by the presence of fever and respiratory symptoms associated either with tachypnea (≥30 min–1) or a low digital pulse oxymetry (<90%) at room air.17 All patients fulfilling these criteria were reported on-line to a centralized surveillance system and nasopharyngeal swab samples are assessed, at local level, for the presence of influenza A-B, respiratory syncytial virus, parainfluenza 1, 2, and 3, adenovirus and human metapneumovirus by indirect immunofluorescence using commercial reagents. Influenza A or B positive samples as well as all negative samples were referred to a reference laboratory (Instituto de Salud Pública: ISP) for further analyses using molecular techniques. Influenza virus was tested and sub typed with the molecular kit FluRUO-01, and non-influenza respiratory viruses using kit KK0812, provided by the CDC. The latter allows detection by PCR of adenovirus, respiratory syncytial virus, human metapneumovirus, parainfluenza 1, 2, and 3, as well as rhinovirus (HRV), not included in the initial immunofluorescence panel.18 For nucleic acid extraction we used the automated Easy Mag system from Biomerieux. Amplification was performed using Ag Path –ID kits from Ambion and a real time thermo cycler (Applied Biosystems). The results of a positive HRV detection were not available during hospitalization.
Cases were analyzed according to demographic information, seasonality, comorbidities, clinical presentation including chest X-ray, PaFiO2, and outcome at the moment of discharge. Some topics were collected prospectively (demographics, comorbidities, vaccine history, tobacco smoking, place of hospitalization) and other retrospectively (clinical presentation and symptoms, laboratory data, management, severity score and outcome). Respiratory failure was defined as a PaFiO2 < 300 and graded.19, 20
Statistical analysis
Results are shown is a descriptive manner. Factors associated to mortality were identified calculating odds ratios (OR) with their respective confidence intervals. Two severity scores (CURB-65 and CAP-PIRO) were calculated and compared to predict in-hospital mortality.21, 22
Results
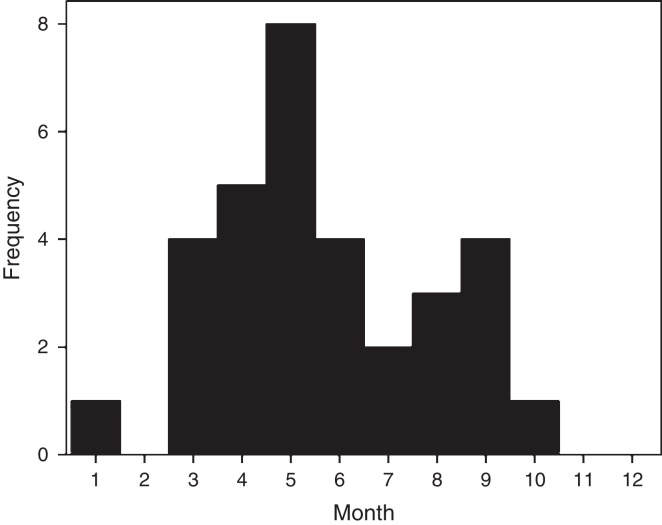
Thirty-two confirmed patients, each with a single severe episode of HRV infection were admitted during 2012. Cases presented a seasonal pattern along the year but were detected continuously during 8 months from March to October (summer to spring in southern hemisphere) (Fig. 1). Of 135 adult patients admitted with SARI during year 2012 with a confirmed viral etiology, HRV ranked second only to influenza infections (23.7% versus 37.8% for influenza).
Fig. 1.
Monthly distribution of 32 patients admitted with human rhinovirus-associated severe acute respiratory infection during one year at a general hospital in Chile.
Mean age was elevated (79.5 years) and 90% were older than 60 years (Table 1). Only two patients lived in a long-term care facility, but near 20% were bedridden. Over 50% of the patients had two or more comorbidities, mainly chronic pulmonary conditions, heart disease, diabetes mellitus, or neurologic diseases. Cancer, morbid obesity, or kidney disease were not common. No case was associated with chronic liver disease and only one patient was immunosuppressed. Previous tobacco smoking affected near a third of this population and current smoking was still admitted by two out of 32 patients. Almost one-third of patients with chronic lung disease were domiciliary oxygen users (Table 1).
Table 1.
Features of 32 patients admitted with SARI associated with human rhinovirus.
| Variable | Results |
|---|---|
| Age in years, mean (range) | 79.5 (49–95) |
| Age ≥60 years, n (%) | 31 (90%) |
| Female, n (%) | 17 (53.1%) |
| Bedridden, n (%) | 6 (18.8%) |
| Long-term care facility, n (%) | 2 (6.3%) |
| Previous tobacco smoking, n (%) | 9 (28.1%) |
| Current tobacco smoking, n (%) | 2 (6.3%) |
| Asthma/COPD/lung fibrosis, n (%) | 17 (53.1%) |
| Domiciliary oxygen user, n (%) | 5 (15.6%) |
| Cerebrovascular disease/dementia n (%) | 10 (31.3%) |
| Diabetes mellitus n (%) | 10 (31.3%) |
| Heart disease n (%) | 11 (34.4%) |
| Kidney disease n (%) | 3 (9.4%) |
| Morbid obesity n (%) | 2 (6.3%) |
| Malignancies, n (%) | 3 (9.4%) |
| Immunosuppresion, n (%) | 1 (3.1%) |
| Any comorbidity, n (%) | 30 (93.8%) |
| One comorbidity n (%) | 12 (37.5%) |
| ≥ 2 comorbidities, n (%) | 18 (56.3%) |
Clinical manifestations
Most patients were admitted under the diagnosis of community-acquired pneumonia, followed by chronic obstructive pulmonary disease (COPD) exacerbations or other conditions (Table 2). Symptoms in descending order of frequency were cough and sputum, fever in nearly half of cases, and confusion/disorientation (Table 2). The latter manifestation was strongly associated with previous neurological disease. Seven out of 10 patients with a neurological condition presented disorientation or confusion compared with two out of 22 without this comorbidity (OR 23.2; 95% CI 3.2–169, p < 0.01). Classic upper respiratory symptoms such as headache, rhinorrhea, and odynophagia were less frequent (<25%). No patients presented diarrhea, and only two developed nauseas or abdominal pain. Bronchial obstructive signs were common (∼60%), a finding associated with chronic lung disease (OR 20.7; 95% CI 3.2–133, p < 0.01), or previous or current tobacco consumption (OR 24.4; 95% CI 1.3–469, p < 0.01). Hypotension was present in one fifth of patients at admission.
Table 2.
Clinical manifestations among 32 patients admitted with human rhinovirus-associated severe respiratory infection during year 2012.
| Variable, n (%) | Results |
|---|---|
| Clinical presentation | |
| Community-acquired pneumonia | 22 (68.8%) |
| COPD exacerbation | 5 (15.6%) |
| Decompensated lung fibrosis | 2 (6.3%) |
| Decompensated chronic heart failure | 2 (6.3%) |
| Influenza-like illness | 1 (3.1%) |
| CURB-65 score | |
| < 2 | 14 (43.8) |
| ≥ 2 | 18 (56.3%) |
| Cough | 32 (100%) |
| Sputum | 26 (81.3%) |
| Fever | 14 (43.8%) |
| Confusion/disorientation | 9 (28.1%) |
| Rhinorrhea | 7 (21.9%) |
| Myalgia | 4 (12.5%) |
| Odynophagia | 2 (6.3%) |
| Headache | 2 (6.3%) |
| Nausea/abdominal pain | 2 (6.3%) |
| Hypotension | 7 (21.9%) |
| Bronchial obstructive signs | 19 (59.4%) |
Symptoms evolved between one and 21 days before admission (average five, mean three days) and 90% presented symptoms for less than seven days before hospitalization. Nearly two-thirds of these patients were hospitalized at first consultation (Table 2).
Some physical and laboratory findings are shown in Table 3. As part of the severe respiratory infection definition used in this work, most patients presented with tachypnea, low O2 saturation, or respiratory failure (PaFiO2 < 300; 25% with a PaFiO2 < 200) but only half had fever. Leukocytosis, neutrophilia, elevated erythrocyte sedimentation rate, plasmatic LDH or BUN were not remarkable and hyponatremia was anecdotal (Table 3). Of interest is that lymphopenia and C-reactive protein (CRP) ≥ 100 mg/L were frequent findings.
Table 3.
Medical consultations and physical and laboratory parameters at admission among 32 patients with human rhinovirus-associated respiratory infection during year 2012.
| Variable | Results |
|---|---|
| Consultations before admission | |
| 0–1 | 29 (90.6%) |
| >1 | 3 (9.4%) |
| Days with symptoms before hospitalization mean (range) | 3 (1–21) |
| Temperature °C, mean (range) | 37.2 (36.0–40.0) |
| Temperature < 37 °C, n (%) | 16 (50%) |
| Respiratory rate/min, mean (range) | 27.1 (18–40) |
| Respiratory rate ≥30/min, n (%) | 13 (40.6%) |
| Heart rate/min, mean (range) | 98.3 (55–131) |
| O2 pulse digital oxymetry %, mean (range) | 85.1 (67–95) |
| O2 Saturation <90%, n (%) | 25 (78.1%) |
| PaO2 at admission mmHg, mean (range) | 58.8 (39.5–78.3) |
| PaO2 <60 mmHg, n (%) | 16 (50.0%) |
| PaFiO2, mean (range) | 239.4 (78–367) |
| PaFiO2 <300, n (%) | 22 (78.6%)* |
| PaFiO2 200–299, n (%) | 15 (53.6%)* |
| PaFiO2 100–199, n (%) | 6 (21.4%)* |
| PaFiO2 <100, n (%) | 1 (3.6%)* |
| Blood leukocyte count/μL, mean (range) | 12012 (4800–27200) |
| Leucocytosis >12,000/μL | 13 (40.6%) |
| Leucocytosis >15,000/μL | 8 (25.0%) |
| Blood polymorphonuclear count/μL, mean (range) | 9984 (3860–25910) |
| Neutrophilia >8000/μL | 19 (59.4%) |
| Neutrophilia >12,000/μL | 12 (37.5%) |
| Lymphocyte count/μL, mean (range) | 1225.8 (180–7750) |
| Lymphocyte count <1000/μL, n (%) | 19 (59.4%) |
| Erythrocyte sedimentation rate mm/h, mean (range) | 42.6 (2–106) |
| Plasma sodium concentration mEq/L, mean (range) | 138.4 (120–150) |
| Hyponatremia (plasma sodium <135 mEq/L) | 4 (12.5%) |
| LDH in plasma U/L mean (range) | 232.1 (117–468) |
| Blood urea nitrogen mg/dL mean (range) | 25 (7–77) |
| C-reactive protein mg/L, mean (range) | 122.8 (7.2–494) |
| C-reactive protein ≥100 mg/L, n (%) | 15 (46.9%) |
of 28 patients with available information.
No other viral agent was detected in any of these patients by RT-PCR. Blood cultures were taken in nine patients (28.1%) without demonstrating pathogenic microorganisms and two cases presented urinary pneumococcal antigen detection (6.2%), one of them with hypotension and a CURB-65 score of 4.
Imaging studies
Interstitial (n = 8; 25%), consolidated (n = 6; 18.8%), and mixed (n = 8; 25%) patterns were observed in chest X-ray and one-third had normal imaging (n = 10; 31.3%). Pleural effusion was also recorded (n = 8; 25%).
Management and outcome
Ten patients (31.2%) were transferred to an intermediate care unit (n = 9) or coronary unit (n = 1) (Table 4). Six patients required non-invasive mechanical support. In addition, one patient evolved with acute lung edema secondary to heart failure. Antibiotic and oseltamivir use were frequent (Table 4). Four patients died (in-hospital mortality 12.5%). Mean length of stay (LOS) was 8.3 days (range 1 to 24) and over 60% remained in the hospital more than a week (Table 4).
Table 4.
Management and outcomes among 32 patients with human rhinovirus-associated respiratory infection during year 2012.
| Variable | Results |
|---|---|
| Place of hospitalization, n (%) | |
| General ward | 22 (68.8%) |
| Intermediate care | 9 (28.1%) |
| Intensive care | 1 (3.1%) |
| Ventilatory support | |
| Non-invasive mechanical ventilation, n (%) | 6 (18.8%) |
| Treatment, n (%) | |
| Systemic corticoids | 19 (59.4%) |
| Inhaled corticoids | 11 (34.4%) |
| Antibiotics | 29 (90.6%) |
| Antivirals (oseltamivir) | 21 (65.6%) |
| Hospital length | |
| Mean (range) in days | 11.8 (2–49) |
| ≥7 days, n (%) | 20 (62.5%) |
| Deceased, n (%) | 4 (12.5%) |
Severity scores and risk factors for mortality
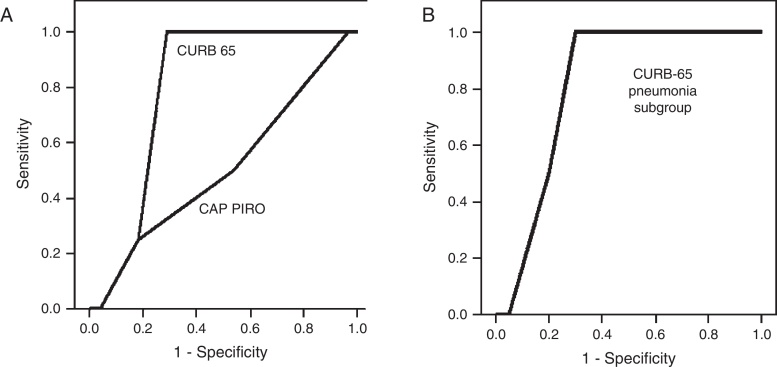
A CURB-65 score < 2 points was present in 43.7% of the population (n = 14), a value considered as cutoff for ambulatory management. For the whole group, the only factor associated to mortality was a CURB 65 score ≥3 (OR 23.4; 95% CI 1.11–489, p < 0.05 by bilateral Fisher's test) but was not significant among the 22 patients with pneumonia. The area under the curve (AUC) obtained by the Receiver Operator Curve (ROC) to predict in-hospital mortality by the CURB-65 score was 0.799 (95% CI 0.650–0.948) (Fig. 2A) and 0.813 (95% CI 0.626–0.999) for the subgroup with pneumonia, a value that was not significantly different (Fig. 2B). Using a cutoff score ≥3 points to predict mortality, sensitivity was 100% and specificity 71.4%. A cutoff score for the subgroup with pneumonia was not possible to determinate due to the small number of patients deceased with this condition (n = 2). The AUC for the CAP-PIRO score was 0.509 demonstrating futility to predict mortality (Fig. 2A). Deceased patients were not associated with pneumococcal infection and two of them did not have pneumonia on the chest X-ray.
Fig. 2.
(A) ROC analysis for CURB-65 and CAP-PIRO score to predict in-hospital mortality. All patients were included. (B) ROC analysis for the subgroup with pneumonia.
Discussion
HRV has been progressively recognized as one of the leading causes of acute respiratory infections among adults patients in ambulatory11, 12, 23, 24 or in-patient settings.3, 25, 26, 27 Its frequency may surpass other viral agents as a cause of respiratory infections.12 Admissions by HRV infection are associated with asthmatic crisis, chronic medical conditions, malignancies, or immunosuppression,11, 28, 29, 30, 31, 32 and occurs during the influenza season as beyond this timeframe.16 In addition, HRV has been associated to outbreaks with an increased case-fatality ratio.33, 34 The spectrum of clinical illness in adults includes asthma, exacerbations of chronic pulmonary obstructive, heart failure and pneumonia.29
Detailed descriptions of adult patients affected by HRV are scarce as the expansion of molecular techniques is recent, besides case-mixing with other etiologies or bias toward severely ill patients.3, 13, 16, 23, 25, 27, 35 In this work we aimed to characterize seasonality and clinical features of adult patients admitted by HRV infection during one year of active surveillance. Several findings in this case series deserve comments.
First, HRV infections had a prolonged seasonality that encompassed three quarters of the year, and represented nearly a quarter of adult hospitalizations by SARI of a known viral cause, underscoring its medical and pathogenic relevance. Only influenza remained more important in our prospective study, as described previously.36 Some authors have shown that HRV infections have the most prolonged seasonality among viral causes of pneumonia in adults, surpassing influenza, adenovirus, coronavirus, respiratory syncytial virus, or parainfluenza.3 Second, as indicated by others, HRV-associated hospitalization affected elderly and frail patients with chronic comorbidities and with a past or current history of smoking.12, 33, 35 Third, admissions were relevant because they were prolonged, required intermediate or intensive care resources in nearly 30% of cases, and had a case fatality rate over 10%. This profile has been described in the literature and underscores the relevance of this viral agent.3, 33, 34 As a way of comparison, mortality by seasonal or pandemic influenza A is similar.3, 5 The proportion of HRV infected patients that requires hospitalization (∼2–10%) depends on the population studied and intensity of search.3, 13, 25, 27, 35 In addition, HRV is more frequently encountered in symptomatic adult patients than in controls demonstrating its pathogenic relevance.1, 2, 26 All of these facts indicate that HRV infections can no longer be considered a minor nuisance and its diagnostic search is justified.
Fourth, clinical manifestations were variable and modulated by underlying conditions such as cardiac, neurologic, or pulmonary diseases, and tobacco smoking. Some cases present as community-acquired pneumonia or as a decompensated chronic condition. Fever was not universal and, if present, was of low grade; upper respiratory illness was not a hallmark. Fifth, to the best of our knowledge, mortality risk factors have not been described for HRV infection in adults,3, 28, 30, 33, 34 although a high severity score has been linked to pneumococcal co-infection.3 The higher mortality registered for those with a CURB 65 ≥ 3 is in line with the original report of this scoring system22 and provides evidence that it is a suitable approach for HRV infections even when patients do not have pneumonia. In fact, the AUC of the CURB-65 ROC analysis for the whole group and the pneumonia subgroup did not reveal differences, suggesting that the distinction between patients with and without respiratory infiltrates is perhaps not necessary in patients affected by HRV infections. On the other side, despite their severity at admission, nearly 40% of patients had a low score (<2) of CURB-65, and the specificity of a higher score was low to predict mortality (∼70%) indicating a limited utility as a surrogate marker of risk. CAP-PIRO score demonstrated to be uselessness and no cutoff calculation was attempted. The limitations observed with CURB-65 to recognize patients in need of admission were probably related to the infrequent presence of hypotension in most viral respiratory illness and to the fact that a low digital pulse oxymetry is not considered for risk evaluation. In this sense, the current criteria adopted by the Chilean Ministry of Health together with the Pan American Health Organization appears to be better adapted to recognize severely ill patients affected by respiratory viral diseases.17
HRV infection appears associated to a wide spectrum of disease and is able to rapidly decompensate vulnerable patients as reflected by the relatively short symptomatic period before admission and the high rate of hospitalization at the first medical visit.
There are probably few distinctive clinical differences between viral and bacterial pneumonia in adults. Lobar condensation, leukocytosis and hyponatremia are more frequently described in patients with bacterial disease, but overlapping is common.25, 37 In accordance with these reports, our patients were characterized by minor changes in these parameters and with a low frequency of consolidation. On the other hand, neutrophilia, lymphopenia and elevated C-reactive protein (≥100 mg/L) were frequent findings in this work. Neutrophilia has been commonly found in adult patients hospitalized with different agents of viral pneumonia but less frequently in influenza cases3; lymphopenia has been described in patients with pandemic A (H1N1) influenza, SARS and metapneumovirus infection,5, 38, 39, 40 and CRP is increased in a fraction of patients admitted with influenza.5
Unfortunately, the results of viral diagnoses were not available at the time of the study and could not be useful for de-escalation of antibiotic therapy or to suspend antiviral influenza therapy. Knowing of a specific viral etiology has been on one hand associated to a reduction in antibiotic consumption in patients with respiratory viral illness, but on the other hand, the presence of abnormal lung findings has prompted antibiotic continuation despite a recognized viral infection.9 Moreover, the probability of viral-bacterial co-infection is another modulating factor that can influence antibiotic therapeutic decisions. Bacterial-viral co-infection rates are variable among patients with respiratory infections (range 4–17%)3, 25, 27 and before a full array of rapid diagnostic techniques for etiological agents are available,15 it would be difficult to curtail or de-escalate antibiotic consumption. An imperative effort on medical education will be necessary because a viral diagnosis, even with normal chest X-ray, is not considered by clinicians to be sufficient to stop antibiotics.7 Emergence of antibiotic resistance and Clostridium difficile are on the verge of this diagnostic shortcoming.
Our work has some limitations that require comments. HRV can be detected some weeks after infection and can be amplified from respiratory samples generating a suboptimal specificity.18, 41 However, these are universal limitations present across all studies, and do not invalidate the scientific evidence showing similar results in different scenarios. In addition, a systematic search for respiratory bacterial pathogens was not carried out in our study, making possible that part of the results be explained by co-infections as it was the case in at least two patients. We believe that this factor is rather secondary because some patients had negative blood cultures and the reported frequency of viral-bacterial co-infection is not high in the literature (<20%).36 On the other hand, no viral-viral co-infection could be demonstrated in our patients making our findings unbiased by the contribution of other viral respiratory agents. The frequency of viral-viral co-infection appears to be low in adults with respiratory infections (<10%).36 Finally, the results of this work were derived from stringent criteria for severity and they do not necessarily represent cases with mild disease.
Conclusions
This work provides further evidence that HRV is an important respiratory pathogen among patients admitted with SARI not only due to its relatively high frequency but also because HRV infections are able to tumble vulnerable adult patients associated to a wide spectrum of illness, using up intermediate or critical care resources, and sometimes associated with prolonged hospitalization or death. In addition, the absence of a timely diagnosis for HRV and other viral respiratory pathogens is contributing to an inappropriate antibiotic use.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Graat J.M., Schouten E.G., Heijnen M.L.A., et al. A prospective, community-based study on virologic assessment among elderly people with and without symptoms of acute respiratory infection. J Clin Epidemiol. 2003;56:1218–1223. doi: 10.1016/S0895-4356(03)00171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Gageldonk-Lafeber A.B., Heijnen M.L.A., Bartelds A.I.M., Peters M.F., van der Plas M.S., Wilbrink B. A case-control study of acute respiratory tract infection in general practice patients in the Netherlands. Clin Infect Dis. 2005;41:490–497. doi: 10.1086/431982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jennings L.C., Anderson T.P., Beynon K.A., et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008;63:42–48. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 4.Asner S., Peci A., Marchand-Austin A., et al. Respiratory viral infection in institutions from late stage of the first and second waves of pandemic influenza A (H1N1) 2009, Ontario, Canada. Influenza Other Respi Viruses. 2012;6:e11–e15. doi: 10.1111/j.1750-2659.2012.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong M., Fica A., Dabanch J., Olivares F., Fasce R., Triantafilo V. Morbidity and mortality associated to influenza A (H1N1) 2009 admissions in two hospitals of the Metropolitan area and analysis of its economic impact. Rev Chilena Infectol. 2012;29:664–671. doi: 10.4067/S0716-10182012000700014. [DOI] [PubMed] [Google Scholar]
- 6.Naghipour M., Hart C.A., Dove W., Leatherbarrow A.J.H., Cuevas L.E. Adenovirus infections within a family cohort in Iran. Pediatr Pulmonol. 2009;44:749–753. doi: 10.1002/ppul.20785. [DOI] [PubMed] [Google Scholar]
- 7.Shiley K.T., Lautenbach E., Lee I. The use of antimicrobial agents after diagnosis of viral respiratory tract infections in hospitalized adults: antibiotics or anxiolytics? Infect Control Hosp Epidemiol. 2010;31:1177–1183. doi: 10.1086/656596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhavnani D., Phatinawin L., Chantra S., Olsen S.J., Simmerman J.M. The influence or rapid influenza diagnostic testing on antibiotic prescribing patterns in rural Thailandia. Int J Infect Dis. 2007;11:355–359. doi: 10.1016/j.ijid.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Falsey A.R., Murata Y., Walsh E.E. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch Intern Med. 2007;167:354–360. doi: 10.1001/archinte.167.4.ioi60207. [DOI] [PubMed] [Google Scholar]
- 10.Papadopoulos N.G., Moustaki M., Tsolia M., et al. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002;165:1285–1289. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
- 11.Teichtahl H., Buckmaster N., Pertnikovs E. The incidence of respiratory tract infection in adults requiring hospitalization for asthma. Chest. 1997;112:591–596. doi: 10.1378/chest.112.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholson K.G., Kent J., Hammersley V., Cancio E. Risk factors for lower respiratory complications of rhinovirus infections in elderly people living in the community: prospective cohort study. BMJ. 1996;313:1119–1123. doi: 10.1136/bmj.313.7065.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara K., Yahara K., Gotoh K., et al. Clinical study concerning the relationship between community-acquired pneumonia and viral infection in northern Thailand. Intern Med. 2011;50:991–998. doi: 10.2169/internalmedicine.50.4738. [DOI] [PubMed] [Google Scholar]
- 14.Gutman J.A., Peck A.J., Kuypers J., Boeckh M. Rhinovirus as a cause of fatal lower respiratory tract infection in adult stem cell transplantation patients: a report of two cases. Bone Marrow Transplant. 2007;40:809–811. doi: 10.1038/sj.bmt.1705827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caliendo A.M. Multiplex PCR and emerging technologies for the detection of respiratory pathogens. Clin Infect Dis. 2011;52(Suppl. 4):S326–S330. doi: 10.1093/cid/cir047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J.K., Jeon J.S., Kim J.W., Rheem I. Epidemiology of respiratory virus infection using multiplex RT-PCR in Cheonan. Korea. J Microbiol Biotechnol. 2013;23:267–273. doi: 10.4014/jmb.1212.12050. [DOI] [PubMed] [Google Scholar]
- 17.Ministerio de Salud de Chile. [Guide of intensified surveillance of severe acute respiratory infections. July 2011]. Available at http://epi.minsal.cl/epi/html/bolets/reportes/Influenza/Guia_de_Vigilancia_Intensificaca_de_las_IRA_junio2011.pdf.
- 18.Lu X., Holloway B., Dare R.K., et al. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinovirus. J Clin Microbiol. 2008;46:533–539. doi: 10.1128/JCM.01739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The American–European consensus conference on ARDS: definitions, mechanism, relevant outcomes and clinical coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 20.Cornejo R. In: Fundamentos del Cuidado Quirúrgico. Burdiles P., Brunet L., Papapietro K., Cabané P., editors. Editorial Mediterráneo; Santiago: 2011. Acute respiratory insufficiency in the surgical pacient; pp. 231–241. Available at http://www.mediterraneo.cl/documentos/catalogo/extracto_978-956-220-327-2.pdf. [Google Scholar]
- 21.Rello J., Rodriguez A., Lisboa T., Gallego M., Lujan M., Wunderink R. PIRO score for community-acquired pneumonia: A new prediction rule for assessment of severity in intensive care unit patients with community-acquired pneumonia. Crit Care Med. 2009;37:456–462. doi: 10.1097/CCM.0b013e318194b021. [DOI] [PubMed] [Google Scholar]
- 22.Lim W.S., van der Eerden M.M., Laing R., et al. Defining community acquired pneumonia severity on presentation at hospital: An international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe A.S., Carraro E., Candeias J.M., et al. Viral etiology among the elderly presenting acute respiratory infection during the influenza season. Rev Soc Bras Med Trop. 2011;44:18–21. doi: 10.1590/s0037-86822011000100005. [DOI] [PubMed] [Google Scholar]
- 24.Ren L., Gonzalez R., Wang Z., et al. Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005-2007. Clin Microbiol Infect. 2009;15:1146–1153. doi: 10.1111/j.1469-0691.2009.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnstone J., Majumdar S.R., Fox J.D., Marrie J.M. Viral infections in adults hospitalized with community-acquired pneumonia. Chest. 2008;134:1141–1148. doi: 10.1378/chest.08-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieberman D., Shimoni A., Shemer-Avni Y., Keren-Naos A., Shtainberg R., Lieberman D. Respiratory viruses in adults with community-acquired pneumonia. Chest. 2010;138:811–816. doi: 10.1378/chest.09-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luchsinger V., Ruiz M., Zunino E., et al. Community-acquired pneumonia in Chile: the clinical relevance in the detection of viruses and atypical bacteria. Thorax. 2013;68:1000–1006. doi: 10.1136/thoraxjnl-2013-203551. [DOI] [PubMed] [Google Scholar]
- 28.Malcolm E., Arruda E., Hayden F.G., Kaiser L. Clinical features of patients with acute respiratory illness and rhinovirus in their bronchoalveolar lavages. J Clin Virol. 2001;21:9–16. doi: 10.1016/s1386-6532(00)00180-3. [DOI] [PubMed] [Google Scholar]
- 29.El-Sahly H.M., Atmar R.L., Glezen W.P., Greenberg S.B. Spectrum of clinical illness in hospitalized patients with “common cold” virus infection. Clin Infect Dis. 2000;31:96–100. doi: 10.1086/313937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camps Serra M., Cervera C., Pumarola T., et al. Virological diagnosis in community acquired pneumonia in immunocompromised patients. Eur Respir J. 2008;31:618–624. doi: 10.1183/09031936.00073807. [DOI] [PubMed] [Google Scholar]
- 31.Costa C., Bergallo M., Astegiano S., et al. Detection of human rhinoviruses in the lower respiratory tract of lung transplant recipients. Arch Virol. 2011;156:1439–1443. doi: 10.1007/s00705-011-0986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parody R., Rabella N., Martino R., et al. Upper and lower respiratory tract infections by human enterovirus and rinovirus in adult patients with hematological malignancies. Am J Hematol. 2007;82:807–811. doi: 10.1002/ajh.20974. [DOI] [PubMed] [Google Scholar]
- 33.Louie J.K., Yagi S., Nelson F.A., et al. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis. 2005;41:262–265. doi: 10.1086/430915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hicks L., Shepard C.W., Britz P.H., et al. Two outbreaks of severe respiratory disease in nursing homes associated with rhinovirus. J Am Geriatr Soc. 2006;54:284–289. doi: 10.1111/j.1532-5415.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 35.Falsey A.R., Walsh E.E., Hayden F.G. Rhinovirus and Coronavirus infection-associated hospitalizations among older adults. J Infect Dis. 2002;185:1338–1342. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo Y.B., Song J.Y., Choi M.J., et al. Etiology and clinical outcomes of acute respiratory virus infection in hospitalized adults. Infect Chemother. 2014;46:67–76. doi: 10.3947/ic.2014.46.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiumefreddo R., Zaborsky R., Haeuptle J., et al. Clinical predictors for Legionella in patients presenting with community-acquired pneumonia to the emergency department. BMC Pulmonary Medicine. 2009;9:4. doi: 10.1186/1471-2466-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xi X., Xu Y., Jiang L., et al. Hospitalized adult patients with 2009 influenza A (H1N1) in Beijing, China: risk factors for hospital mortality. BMC Infect Dis. 2010;10:256. doi: 10.1186/1471-2334-10-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fica A., Hernández L., Porte L., Castro M., Weitzel T. Respiratory infections caused by metapneumovirus in elderly patients. Rev Chilena Infectol. 2011;28:174–178. [PubMed] [Google Scholar]
- 40.Lee N., Rainer T.H., Ip M., et al. Role of laboratory variables in differentiating SARS-coronavirus from other causes of community-acquired pneumonia within the first 72 h of hospitalization. Eur J Clin Microbiol Infect Dis. 2006;25:765–772. doi: 10.1007/s10096-006-0222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention Clusters of Acute Respiratory Illness Associated with Human Enterovirus 68 – Asia, Europe, and United States, 2008–2010. MMWR. 2011;60:1301–1304. [PubMed] [Google Scholar]