Highlights
-
•
Respiratory viruses are potentially implicated in one quarter to one-third of adult cases of community-acquired pneumonia.
-
•
In such cases influenza is the most frequently detected viral pathogen.
-
•
Failure to test for respiratory viruses in hospitalised patients may lead to missed opportunities for early therapeutic intervention.
Keywords: Community, Acquired, Pneumonia, Virus, Aetiology, Pathogen
Abstract
Community-acquired pneumonia (CAP) is an important respiratory disease and the fifth leading cause of mortality in Europe. The development of molecular diagnostic tests has highlighted the contributions of respiratory viruses to the aetiology of CAP, suggesting the incidence of viral pneumonia may have been previously underestimated. We performed a systematic review and meta-analysis to describe the overall identification of respiratory viruses in adult patients with CAP in Europe, following PRISMA guidelines (PROSPERO; CRD42016037233). We searched EMBASE, MEDLINE, CINAHL, WHOLIS, COCHRANE library and grey literature sources for relevant studies, and screened these against protocol eligibility criteria. Two researchers performed data extraction and risk of bias assessments, independently, using a piloted form. Results were synthesised narratively, and random effects meta-analyses performed to calculate pooled estimates of effect; heterogeneity was quantified using I2. Twenty-eight studies met inclusion criteria of which 21 were included in the primary meta-analysis. The pooled proportion of patients with identified respiratory viruses was 22.0% (95% CI: 18.0%–27.0%), rising to 29.0% (25.0%–34.0%) in studies where polymerase chain reaction (PCR) diagnostics were performed. Influenza virus was the most frequently detected virus in 9% (7%–12%) of adults with CAP. Respiratory viruses make a substantial contribution to the aetiology of CAP in adult patients in Europe; one or more respiratory viruses are detected in about one quarter of all cases.
1. Introduction
Community-acquired pneumonia (CAP) is a principal cause of excess hospitalisation and mortality worldwide [1], [2], [3]. Historically, the overriding clinical approach to the management of CAP has been to focus on bacterial aetiologies, with Streptococcus pneumoniae the dominant pathogen [4], [5], [6], [7], [8]. More recently, coupled to the increasing availability of polymerase chain reaction (PCR) tests, the identification of viral pathogens in the aetiology of CAP has increased. Contemporary studies identify that viruses may be implicated in 15%-30% of all CAP [9], [10], [11]; in turn this heightens the possibility that empirical antibiotic treatment of CAP in the absence of adequate testing for viral pathogens may contribute to inappropriate antibiotic usage [12], [13].
Given the considerable variation across individual studies in estimating the contribution of respiratory viruses to CAP aetiology, reliable summaries of relevant data are necessary to inform future research and policy initiatives, particularly as new respiratory virus vaccines and antiviral drugs are anticipated in the short to medium term [11], [14], [15], [16], [17].
Two recent systematic reviews of studies investigating the proportions of viral pathogens in patients with CAP focussed on studies that only used polymerase chain reaction (PCR)-based assays to detect viral pathogens and pooled results from studies conducted across the world. [18], [19] We report an additional systematic review of studies conducted within the World Health Organization European Region, which offers additional granularity according to setting, timing of study, viral diagnostic techniques and study quality.
2. Methods
The study protocol was registered on the National Institute for Health Research International Prospective Register of Systematic Reviews (PROSPERO; CRD42016037233; available at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016037233) and conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [20]
2.1. Eligibility criteria
We identified studies which investigated the aetiology of CAP in adults in Europe (defined as those countries covered by the WHO Regional Office for Europe http://www.euro.who.int/en/countries) and reported quantitative data on the identification of respiratory viruses. We searched for original articles describing longitudinal studies or case series, in English, which investigated adults aged ≥16 years diagnosed with CAP. All other study designs were excluded. We included studies that performed either PCR or non-PCR detection techniques.
We excluded studies of paediatric populations and patients residing in nursing homes, residential care homes or rehabilitation facilities. Studies of adults diagnosed with CAP based on clinical signs but without radiologic confirmation, and studies focused on CAP in adults with severe immunosuppression through disease and/or drug treatment were also excluded.
2.2. Search strategy and screening
The following electronic databases were systematically searched: EMBASE, MEDLINE, CINAHL, WHOLIS, and Web of Science from January 1999 to April 2016. A comprehensive search strategy was developed for EMBASE (Supplementary Appendix A) and subsequently adjusted as required to suit other databases. The reference lists of all eligible articles were manually searched to identify other eligible studies.
All identified articles were imported to ENDNOTE software X4 (Thomson Reuters, Toronto, CA, USA) and duplicates removed. Two review authors (YA and JSN-V-T) independently screened the retained articles against protocol eligibility criteria, in three stages: by title, abstract and full text. Any disagreements were resolved through discussion between YA and JSN-V-T; and a third author (WSL) adjudicated over any outstanding discrepancies.
2.3. Data extraction and risk of bias assessment
Data extraction for each eligible study was also performed independently by YA and JSN-V-T using a pre-piloted data extraction form using Microsoft® Office Excel® 2010 (Microsoft Corporation, Richmond, VA, USA). For all included studies, information was extracted on: author(s); year of publication; country; healthcare setting; number of evaluable patients; viral diagnostic techniques employed; samples collected for virus detection; number of respiratory virus pathogens tested for; and number and proportion of respiratory viruses detected. YA and JSN-V-T independently assessed the quality of all included studies, using criteria adapted from the Newcastle − Ottawa scale for observational studies [21], focusing on three key domains: representativeness of patient population; ascertainment of CAP diagnosis; and ascertainment of virus aetiology. We awarded zero or one star in each domain; for representativeness, one star was awarded for studies sampling from the general community (as opposed to more specialised patient subgroups); for ascertainment of CAP diagnosis we awarded one star for independent radiographic confirmation of diagnosis; and for virus aetiology, one star for use of ‘gold standard’ PCR diagnostic techniques.
2.4. Summary measures, and analysis
The proportion of respiratory viruses identified in evaluable CAP patients was pooled using the generic inverse variance approach, based on a random effects model (DerSimonian- Laird weights method) [22], stabilising the variances using the Freeman-Tukey double arcsine transformation so that studies with proportions close to 0% or 100% were appropriately estimated [23]. Exact binomial confidence intervals were computed for outcomes. The primary outcome was the overall contribution of respiratory viruses in the aetiology of CAP, calculated as the total number of patients with respiratory viruses identified (numerator) as a proportion of the total number of evaluable patients (denominator). We report, as secondary outcomes, the contribution of individual viruses calculated as the total number of patients with individual respiratory viruses identified as a proportion of all evaluable patients for each specific pathogen.
Heterogeneity between studies was quantified using the I2 statistic [24]. We investigated potential sources of heterogeneity by performing subgroup analyses; by study setting (inpatient vs. outpatient), study quality, viral diagnostic methods used (PCR diagnostic techniques vs non-PCR methods) and mixed infections (bacterial and viral infections). All analyses were conducted using the metaprop commands within Stata (V.13, Stata Corp, College Station, Texas, USA).
3. Results
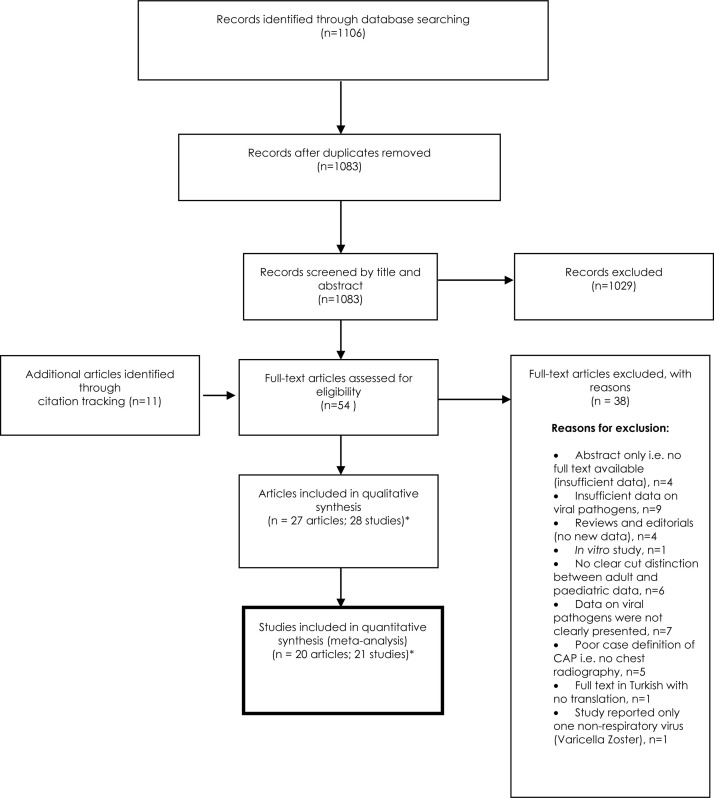
We identified a total of 1106 articles from database searches, reducing to 1083 after the removal of duplicates. Eleven additional papers were identified via citation tracking. After screening, 27 articles remained within protocol eligibility criteria (Fig. 1 1 ); one of the included articles [25] presented two separate studies and data from both were extracted and presented separately. Thus, 28 studies from 27 articles were included in the systematic review [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51]., and 21 from 20 in the primary meta-analysis [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]. When examined as full-text articles, seven studies did not present sufficient quantitative data for inclusion in the primary meta-analysis [45], [46], [47], [48], [49], [50], [51]. (Fig. 1).
Fig. 1.
PRISMA flowchart.
3.1. Study characteristics
All 28 studies included in the systematic review were prospective or retrospective longitudinal studies or case-series. The patient population size in each ranged from 71 to 1356 (total = 8777). The earliest publications were in 2001 [37], [40], and the most recent article was published in October 2015 [26].
Studies from 11 different European countries were included of which Spain was most frequently represented (9 studies; 32.1%) [27], [28], [31], [33], [41], [44], [47], [50], [51].. Nineteen studies2 (67.9%) [25], [26], [29], [30], [31], [32], [35], [36], [39], [40], [41], [42], [43], [44], [47], [48], [49], [50] were carried out among inpatient populations (n = 5515 patients), three [34], [38], [46] (10.7%) in outpatient/community populations (n = 524 patients) and six (21.4%)[27], [28], [33], [37], [45], [51]. in mixed populations (n = 2738 patients). Details of the characteristics of the included studies are summarised in Table 1 . Sixteen studies (57.1%) [26], [29], [30], [32], [34], [35], [36], [39], [41], [42], [43], [44], [45], [47], [49], [50] had used PCR techniques for the detection of respiratory viruses, alone or in combination with other diagnostic methods. 14 studies (50%) obtained upper respiratory samples [26], [28], [30], [35], [36], [38], [39], [41], [42], [43], [44], [46], [49], [50], 16 (57.1%) lower respiratory [25], [31], [32], [33], [34], [38], [42], [43], [45], [46], [47], [48], [49], [50], [51]., and six (21.4%) both [38], [42], [43], [46], [49], [50]. In 10 (35.7%) studies (9 publications) respiratory tract sampling was combined with assessment of paired serology [25], [31], [32], [33], [45], [46], [49], [50], [51].; and in four (14.3%) studies, serology alone was performed [27], [29], [37], [40].
Table 1.
Characteristics of included studies.
| First author | Study setting | Study design | Patient characteristics | Total number of patients with CAP | Number of viruses tested for | Male% | Diagnostic methods | Principal study focus | Specimen sites* |
|---|---|---|---|---|---|---|---|---|---|
| Le Bel [26] | France, inpatients | Prospective cohort | Patients aged >18 years presented to Emergency dept. | 319 | 8 | 101 (31.7%) | PCR | Inflammatory biomarkers in CAP patients | UR |
| Capelastegui [27] | Spain, inpatients and outpatients | Prospective cohort | Patients aged >18 years in the community and hospital | 700 | 5 | Incomplete: 239 of 390 (61.3%) | Blood cultures, urinary antigen tests, serology, direct immunofluorescence antibody assay | Aetiology of CAP | S |
| Cilloniz [28] | Spain, inpatients and outpatients | Prospective cohort | Patients aged >16 years admitted to the Emergency wards and outpatients. | 568 | 5 | 301 (53.0%) | Serology, blood culture, antigen tests. | Aetiology of CAP | UR |
| Clark [29] | UK, inpatients | Prospective cohort | Patients aged >18 years admitted to hospital with acute respiratory infection but subset with CAP patients | 166 | 9 | 87(52.4%) | Blood and Sputum culture, PCR | Aetiology of ARI in adults | S |
| Das [30] | France, inpatients | Prospective cohort | Patients aged >18 years admitted to the Emergency dept. | 125 | 7 | Not reported | PCR | Aetiology of CAP | UR |
| de Roux [31] | Spain, inpatients | Prospective cohort | Patients aged >18 years admitted to hospital | 1356 | 5 | 893(65.8%) | Serology, complement fixation kit tests for viruses. | Viral CAP in non-immunocompromised adults | LR, S |
| Diederen [32] | Netherlands, Inpatients | Prospective cohort | Patients aged >18 years admitted to the hospital | 242 | 8 | 7(2.9%) | PCR, serology, ELISA | Detection of respiratory pathogens using PCR | LR, S |
| Guiterrez [33] | Spain, inpatients and outpatients | Prospective cohort | Patients aged >15 years admitted to the hospital | 493 | 5 | 308(62.5%) | Blood and sputum cultures, complement fixation tests. | Investigating the influence of age and gender on the incidence of CAP | LR, S |
| Holm [34] | Denmark, outpatients | Prospective cohort | Patients aged >18 years with CAP presenting to the GP | 48 | 6 | 28(58.3%) | PCR | Aetiology of CAP | LR |
| Holter [35] | Norway, inpatients | Prospective cohort | Patients aged >18 years admitted to the hospital | 267 | 8 | 140(52.4%) | Culture, serology, PCR | Aetiology of CAP in Norway | UR |
| Howard-a [25] | UK, inpatients | Prospective cohort | Patients aged >15 years | 69 | Uncertain | 6(8.7%) | Complement fixation tests, blood culture | Not reported | LR, S |
| Howard-b [25] | UK, inpatients | Prospective cohort | Patients aged >16 years | 99 | Uncertain | 6(6.1%) | Complement fixation tests, blood culture | Aetiology of CAP | LR,S |
| Johansson [36] | Sweden, inpatients | Prospective cohort | Patients aged >18 years admitted to the hospital | 184 | 9 | 94(51.1%) | Culture, PCR, Serology | Aetiology of CAP | UR |
| Joikinen [37] | Finland, inpatients and outpatients | Prospective cohort | Patients aged >15 years admitted to the hospital and patients in the community | 345 | 7 | 176(51.0%) | Serology | Aetiology of CAP in adults in Eastern Finland | S |
| Koksal [38] | Turkey, outpatients | Cross −sectional | Patients aged >17 years with CAP in outpatient settings | 292 | 6 | 147(50.3%) | Culture, direct immunofluorescence, serology | Aetiology of CAP in adults in Turkey | UR, LR |
| Liberman [39] | Israel, inpatients | Prospective cohort | Patients aged >18 years admitted to the hospital | 183 | 8 | 105(57.4%) | PCR | Evaluate the role of respiratory viruses in adult CAP | UR |
| Lim [40] | UK, inpatients | Prospective cohort | Patients aged >16 years admitted to the hospital | 267 | 5 | 135(50.6%) | Other conventional methods | Investigate the aetiology of CAP and implication for CAP management | S |
| Sangil [41] | Spain, inpatients | Prospective cohort | Patients aged >18 years admitted to the hospital | 169 | 9 | Not reported | PCR, serology | Aetiology of CAP using PCR and other conventional methods. | UR |
| Shibli [42] | Israel, inpatients | Prospective cohort | Patients aged >18 years admitted to the hospital | 127 | 6 | 73(57.5%) | PCR, DNA & RNA extraction, Serology | Investigate the aetiology of CAP in hospitalised patients . | UR, LR |
| van Gageldonk-Lafeber [43] | Netherland, inpatients | Prospective cohort | Patients aged >18 years presented to the Emergency dept. | 339 | 9 | 212(62.5%) | Culture, serology, antigen tests, PCR | Aetiology of CAP | UR, LR |
| Viasus [44] | Spain, inpatients | Prospective cohort | Adult patients admitted to the hospital | 747 | 8 | 423(56.8%) | PCR, Serology | Aetiology of CAP | UR |
| Templeton [45] | Netherlands, inpatients and outpatients | Prospective cohort | Patients aged >18 years admitted to the hospital | 136 | 7 | 75(55.1%) | Culture, PCR, Serology | Aetiology of CAP | LR, S |
| Bochud [46] | Switzerland, outpatients | Prospective cohort | Patients aged >15 years | 184 | 4 | 82(44.6%) | Serology | Aetiology of CAP in outpatients | UR, LR, S |
| Marcos [47] | Spain, inpatients | Prospective cohort | Patients aged >14 years admitted to the hospital | 198 | 7 | 115(58.1%) | PCR, immunofluorescence and culture | Aetiology of CAP | LR |
| Hohenthal [48] | Finland, inpatients | Prospective cohort | Patients aged >18 years admitted to the hospital | 71 | 7 | 48(67.6%) | Culture | Diagnostic value of BAL | LR |
| Huijskens [49] | Netherland, inpatients | Prospective | Patients aged >18 years presented to the emergency dept. | 408 | 11 | 250(61.3%) | PCR, Culture and serology | to differentiate pure bacterial, pure viral and mixed viral and bacterial aetiologies based on clinical signs admission | UR, LR, S |
| Cilloniz [50] | Spain, Inpatients | Prospective cohort | >18 years with CAP admitted to ICU within 24 h | 362 | 5 | 232(64.1%) | Immunofluorescence, PCR | polymicrobial pneumonia | UR, LR, S |
| Almirall [51] | Spain, inpatients and outpatients | Prospective cohorts | >14 years, 216 patients were managed at home and 280 patients were admitted to hosp. | 496 | 7 | 302(60.9%) | Culture, serology, Immunofluorescence | Differences in aetiology of CAP | LR, S |
Specimen sites: UR = upper respiratory tract; LR = lower respiratory tract; S = serological assessment (using paired sera). *In studies which sampled from >1 site, not all patients will have undergone sampling at all sites.
3.2. Risk of bias assessment
Study population representativeness, diagnostic accuracy of CAP and ascertainment of virus aetiology were assessed with a maximum of three stars per study. Eleven studies [26], [30], [32], [34], [35], [36], [39], [41], [42], [43], [45](39.3%) were assessed as being at low risk of bias (three stars; one star per domain), 143 studies [25], [26], [29], [33], [37], [38], [40], [44], [46], [47], [49], [50], [51]. (53.6%) at moderate risk of bias (2 out of 3 stars), and three [28], [31], [48] (7.1%) were at high risk of bias (one or zero stars). Six studies3 (21.4%) reported difficulty in obtaining adequate samples for microbiological testing [25], [27], [32], [37], [41]. Within-study variation in viral diagnostic methods across different study years was reported in ten studies (35.7%) [26], [29], [30], [35], [36], [39], [41], [42], [43], [44].
3.3. Overall identification of respiratory viruses
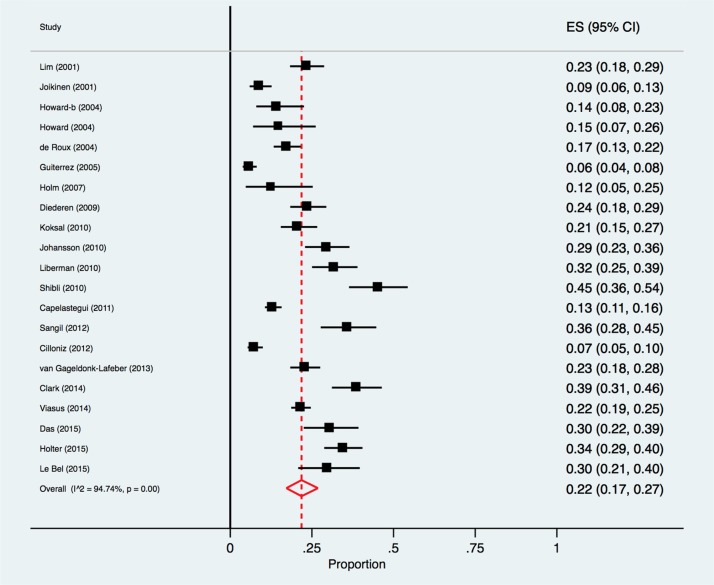
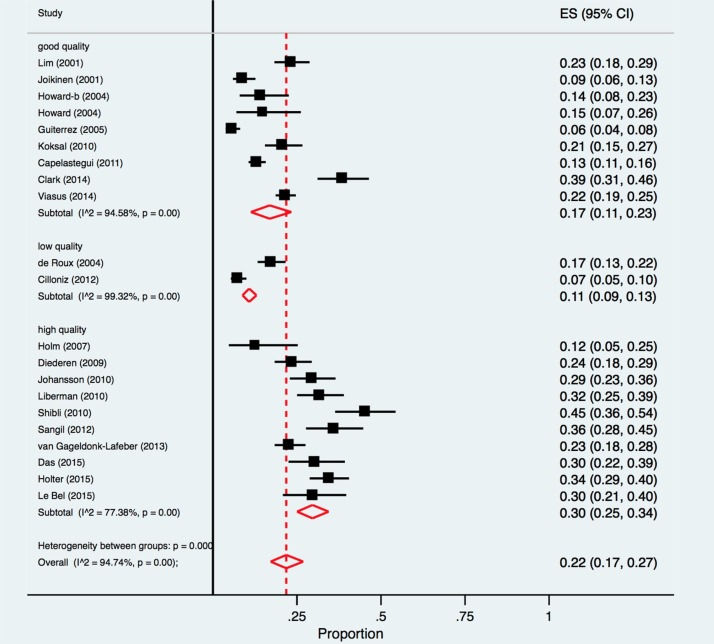
The percentage of respiratory viruses detected in CAP patients ranged from 6% in a Spanish study comprising both inpatients and outpatients [33], to 45% in a study of hospitalised patients in Israel [42]. By meta-analysis, the pooled proportion of respiratory viruses detected in CAP patients was 22.0% (95% CI 17.0%-27.0%; I2 = 94.7%) (Fig. 2 ).
Fig. 2.
Forest plot: overall identification of respiratory viruses in European adult patients with CAP.
ES = effect size for pooled identification of respiratory viruses.
There was a significant trend for the identification of respiratory virus pathogens to be lower in studies (n = 8)3 published from 2001 to 2009 [25], [31], [32], [33], [34], [37], [40], (pooled estimate = 14.0% (95%CI 9.0%–21.0%)) compared with more recent studies (n = 13) published after 2010 [26], [27], [28], [29], [30], [35], [36], [38], [39], [41], [42], [43], [44] (pooled estimate = 27.0% (95%CI 20.0%–33.0%)), test for subgroup differences, p = 0.007 (Supplementary Appendix B).
3.4. Sub-group analyses
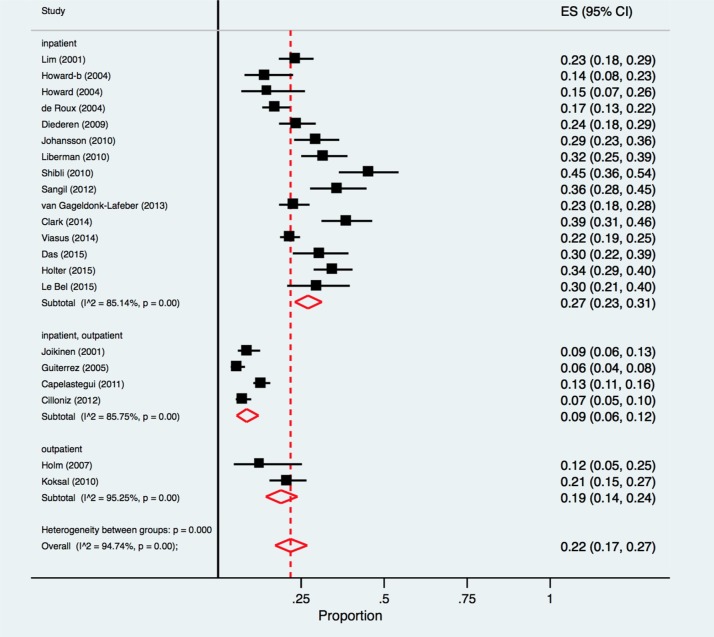
The pooled proportion of respiratory viruses identified among patients with CAP in inpatient studies (n = 15) [25], [26], [29], [30], [31], [32], [35], [36], [39], [40], [41], [42], [43], [44] was 27.0% (95% CI 23.0%–31.0%; I2 = 85.1%); compared with 19.0% (95% CI 14.0%–24.0%; I2 = 95.3%) for outpatient studies (n = 2) [34], [38], and 9.0% (95% CI 6.0%–12.0%; I2 = 85.8%) in studies with mixed populations (n = 4) [27], [28], [33], [37] (Fig. 3 ). Each of these populations revealed results that were statistically significantly different from each other (test for subgroup differences, p < 0.01). Studies with mixed populations [27], [28], [33], [37], relied exclusively on non-PCR diagnostic methods and were of lower quality compared to other studies.
Fig. 3.
Forest plot: overall identification of respiratory viruses in European patients with CAP, stratified by study setting.
ES = effect size for pooled identification of respiratory viruses.
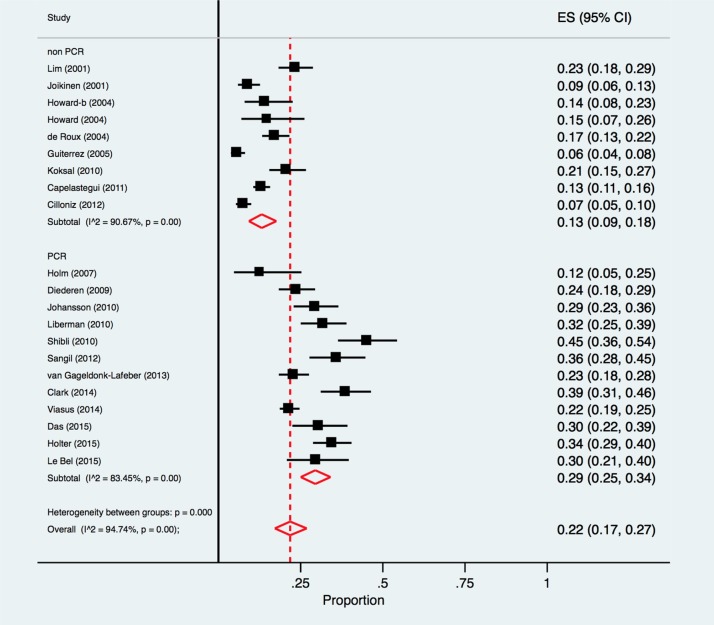
The pooled proportion of respiratory viral pathogens identified in 12 studies [26], [29], [30], [32], [34], [35], [36], [38], [41], [42], [43], [44] using PCR (with or without additional testing methods) was 29.0% (95% CI 25.0%–34.0%, I2 = 83.5%) compared with 13.0% (95% CI 9.0%–18.0%, I2 = 90.7%) in nine studies3 using other non-PCR methods [25], [27], [28], [31], [33], [37], [38], [40], with a significant difference between the two groups, p < 0.001 (Fig. 4 ).
Fig. 4.
Forest Plot: overall identification of respiratory viruses in European patients with CAP, by diagnostic method employed.
ES = effect size for pooled identification of respiratory viruses.
In lower risk of bias studies (NOS score = 3 stars) [26], [30], [32], [34], [35], [36], [39], [41], [42], [43]the pooled proportion for total respiratory viral pathogens was 30%, (95% CI 25%–34%, I2 = 77.4%), compared with 11% (95% CI 9%–13%, I2 = 99.3%) in higher risk of bias studies (NOS score = 1 star)[28], [31], explaining the observed overall heterogeneity between studies, p < 0.001 (Fig. 5 ).
Fig. 5.
Forest plot: overall identification of respiratory viruses in European patients with CAP, according to study quality.
ES = effect size for pooled identification of respiratory viruses.
3.5. Mixed infections
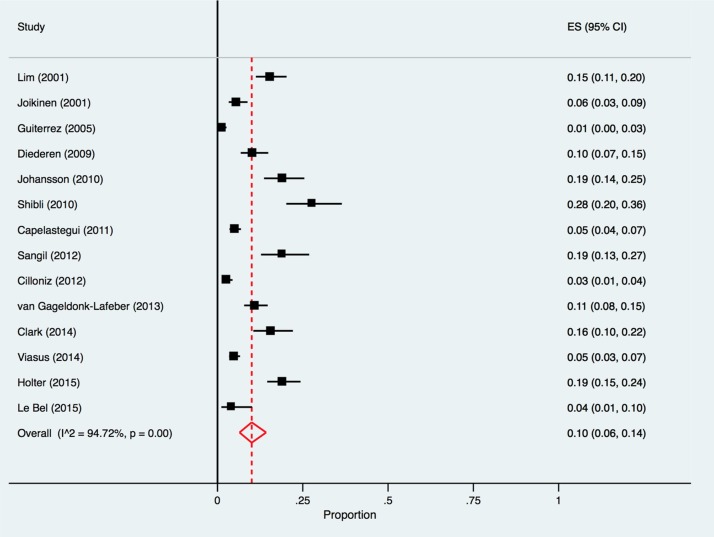
The pooled proportion of mixed respiratory viruses and bacterial co-infections detected in CAP patients was 10% (95% CI 6%–14%, I2 = 94.7%) reported across 14 studies [26], [27], [28], [29], [32], [33], [35], [36], [37], [40], [41], [42], [43], [44] (Fig. 6 ).
Fig. 6.
Forest plot: mixed respiratory virus and bacterial co-infections in European patients with CAP.
ES = effect size for pooled identification of respiratory viruses.
3.6. Individual viruses
Data on the seven most common respiratory viruses identified are presented in Table 2 . Influenza viruses were most frequently detected (9%), followed by rhinoviruses (5%) and coronaviruses (4%); together accounting for the majority of respiratory viruses detected (Table 2).
Table 2.
Summary of individual pathogen-specific meta-analyses for respiratory viruses most commonly identified in European adult patients with CAP.
| Virus type | Pooled% | 95% CI | No. of studies (and patients) included in pathogen-specific meta-analysis | I2(%) |
|---|---|---|---|---|
| Influenza (A or B) | 9 | 7–12 | 17 (6487) | 93.45 |
| Rhinovirus | 5 | 4–7 | 12 (3324) | 88.22 |
| Coronavirus | 4 | 2–7 | 7 (1343) | 80.37 |
| Parainfluenza | 3 | 2–5 | 14 (5600) | 88.35 |
| Human metapneumovirus (hMPV) | 2 | 1–2 | 10 (1779) | 7.49 |
| Respiratory syncytial virus (RSV) | 2. | 1–3 | 17 (5968) | 82.42 |
| Adenovirus | 1 | 0−1 | 13 (3166) | 32.88 |
Enterovirus, poliovirus, cytomegalovirus, coxsackie virus, varicella-zoster virus, human bocavirus and herpes simplex virus were detected in <1% of adult patients with CAP.
4. Discussion
This review updates evidence on the microbiological identification of respiratory viral pathogens in adult patients with radiographically confirmed CAP in Europe. Overall our data suggest that respiratory viruses are detectable in at least 22% of radiologically confirmed CAP cases, mostly hospitalised cases. However significantly higher proportions of respiratory viruses were evident in studies conducted after 2010 (27%), studies that included viral PCR techniques (29%), and studies assessed to be at lower risk of bias (29%), suggesting that the true proportion of CAP associated with respiratory viruses is at least one quarter (25%). Our findings accord with recent major studies or reviews conducted in Asia and North America [11], [14], [52], [53]. In the CDC EPIC study [11], viruses were detected in 27.0% of adult patients with CAP, while Qu et al. detected viruses in 27.5% of Asian patients with CAP [51].
Our review suggests that in Europe, as in other parts of the world, a relatively large burden of CAP disease may be attributable to viral infections. However, the clinico-pathological significance of virus detection in patients with CAP remains uncertain. A clear limitation of our approach (and of each of the included studies) is that no proof is offered that the virus or viruses identified were of pathological significance in all cases. There was also heterogeneity between studies in terms of the respiratory sites sampled and/or use of serology. Viruses recovered from upper respiratory tract (URT) sites might have less pathological significance than those recovered from lower respiratory tract (LRT) sites; nevertheless, in the absence of concomitant sampling from URT and LRT it is not possible to disregard viruses identified from URT sites which may have been replicated in the LRT if it had also been sampled. Whilst respiratory viruses are undoubtedly implicated in the aetiology of a substantial proportion of the cases in which they are detected, asymptomatic illness associated with virus shedding is well recognised, especially in children who experience longer periods of shedding than adults [54]. In addition, modern PCR diagnostic techniques are comparatively more sensitive than methods for the detection of bacteria, and capable of detecting small quantities of nucleic acid which may not in all cases represent culturable virus; therefore, some patients with ‘viral-only’ pathogens identified may also have a microbiologically unrecognised bacterial infection; and some detections of viral pathogens may represent previous or resolved virus infection. In a recent study, Gadsby et al. employed PCR techniques to identify bacteria as well as viruses from lower respiratory tract samples, viruses were detected in 30% of 323 adults admitted to hospital with CAP and a co-bacterial pathogen was detected in 82% of these [55]. In contrast, we noted only 10% of cases with a bacterial co-pathogen; this might reflect the use of PCR testing for bacteria by Gadsby and colleagues, whereas the studies we included used standard approaches for the identification of bacteria. The detection of respiratory viruses in healthy asymptomatic individuals is not as extensively described as in symptomatic patients; nevertheless Jartti and colleagues summarised data from 51 studies, noting maximum baseline prevalences of respiratory viruses in asymptomatic subjects as follows: rhinoviruses, 15%; adenoviruses, 5.3%; influenza, 4.3%; RSV, 2.6%; coronaviruses, 2.5%; eneteroviruses 1.2%; human bocavirus, 1.1%; parainfluenza, 0.9%; and hMPV, 0.6% [54]. Jansen and colleagues have observed that rhinovirus is extremely common in asymptomatic children (28%), but that if other viruses are identified, notably RSV, adenoviruses and hMPV, these are much more likely to be clinically relevant56; this may be different in adults. We lacked direct comparison with any such ‘asymptomatic control’ group in the included studies, nor did we have access to data on the host response to viruses in individual subjects. However separate studies in asymptomatic patients [54], [56] offer important contextualization for our findings; and inclusion of an asymptomatic comparator group would be likely to add granularity in future studies.
Since previous work identified high heterogeneity in the extant literature from other parts of the world, [18], [19] we expected this and decided, a priori, that high heterogeneity would not preclude meta-analysis. We were unable to identify a single clear reason for the observed high heterogeneity which we attribute to multiple factors including study quality (Fig. 5), variable settings, patient populations, sampling sites, and diagnostic methods (Fig. 4); disease severity and co-infections with other pathogens. Since rhinovirus and Respiratory syncytial virus (RSV) RSV infections have a predilection for asthmatic patients [57], [58], underlying comorbidities may also have influenced our findings.
Influenza (9%) viruses, rhinoviruses (5%) and coronaviruses (4%) accounted for the majority of virus detections; these proportions are similar to the estimates reported previously by Burk et al. and Wu et al [18], [19]. However, RSV was identified in only 2% of adult CAP which may be relevant to the potential role of future RSV vaccines targeted at the elderly.
These findings highlight the importance of respiratory viruses in the aetiology of adult CAP, and the potential relevance of our findings towards improving clinical outcomes, and reducing inappropriate antibiotic use. Influenza appears to be the most significant virus pathogen, followed by rhinoviruses and coronaviruses. Notwithstanding, different included studies tested for between 4 and 11 separate respiratory viruses (Table 1); if all included studies had tested for all 11 viruses the overall proportion of virus detection may well have been considerably higher, although, as discussed above, not all detections necessarily have clinical relevance to CAP. This potential source of bias will not have affected the estimates for individual viruses (Table 2) because these analyses were organism-specific and based on all available data by organism. Viral diagnostic evaluation of CAP facilitates greater precision in the assessment of illness severity, and the tailoring of therapy, in particular the rapid use of neuraminidase inhibitors for cases of influenza and more judicious use of antibiotics. Since there are realistic near-term prospects for novel antiviral treatments for several respiratory virus infections and RSV vaccines [59], [60], [61], there is a need to establish baseline data on the incidence of viral CAP and develop a wider culture of testing for respiratory virus pathogens without which it will be difficult to assess the impact of advances in therapy.
We included only articles reported in English. An analysis including country-specific data reported in other languages may reveal regional variations in the contribution of respiratory viruses to the microbiology of CAP. Although, the effect of age was considered as an important source of heterogeneity, a sub-analysis by age could not be performed due to the lack of detailed reporting of study results by age groups; this may have influenced our results. Similarly, subgroup analyses could not be performed according to patient illness severity, patient comorbidities, type of respiratory sample or the presence of specific bacterial co-pathogens due to lack of data. Publication bias applies when studies reporting ‘positive’ findings are more likely to be published than those reporting ‘negative’ findings and is an important consideration in meta-analyses evaluating treatment effects. However, in the context of studies examining the proportion of CAP patients in whom viruses were detected, well-conducted ‘negative’ studies are as ‘surprising’ as ‘positive’ studies and both would be expected to be published. The first study to examine the use of standard publication bias tests for proportional meta-analyses (such as this one) found that funnel plots and statistical tests potentially yield misleading results, especially where the proportions within the studies are either very high or very low [62]. These researchers describe an alternative method that can be used to explore the potential for publication bias, where the sample size is used instead of the standard error for each study; however, the reliability and accuracy of this method has yet to be fully explored and independently validated. Therefore, we elected not to analyses publication bias.
5. Conclusion
This systematic review suggests that, in Europe, respiratory viruses are identifiable in at about one quarter of all adults presenting with CAP. Of these, the most frequently identified pathogens are influenza viruses, rhinoviruses and coronaviruses, accounting for over one half of all identified viral pathogens. Further study to determine the importance of identifying viral pathogens in relation to treatment with antibiotics or antivirals is warranted.
Contributorship
All of the authors designed and contributed to the systematic review. Y.A. and J.S. N-V-T. performed study selection independently. Y.A. and JS. N-V-T. performed paired data extraction, data synthesis and quantitative analyses. Y.A. and JS. N-V-T drafted the article, and all other authors critically reviewed the article before submission.
Financial support
This study was undertaken by Y.A. as a Master of Public Health (International Health) dissertation at the University of Nottingham. There was no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Potential conflicts of interest
J.S.N-V-T. reports: research grants from the World Health Organization, F. Hoffman-La Roche and GlaxoSmithKline, unconnected to the submitted work; and honoraria from Novavax and Prep Biopharm. Also, non-financial support from European Scientific Working Group on Influenza (ESWI) to support the delivery of a plenary lecture at a scientific conference. W.S.L. reports that his institution has received unrestricted investigator initiated research funding from Pfizer for a pneumonia cohort study. All other authors declare no conflicts.
Acknowledgments
We thank Sharon Figgens (University of Nottingham) and Hongxin Zhao (Public Health England) for assistance with literature searches.
Footnotes
One article presented data on two separate studies [25].
Citation #25 describes two studies.
Citation #25 describes two studies.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jcv.2017.07.019.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Murray C.J.L., Lopez A., Mathers C., Stein C. Global Programme on Evidence for Health Policy, Discussion Paper No. 36. World Heal Organ; Geneva: 2001. The Global Burden of Disease 2000 Project: Aims, Methods and Data Sources. [Google Scholar]
- 2.Quan T.P., Fawcett N.J., Wrightson J.M., Finney J., Wyllie D., Jeffery K. Increasing burden of community-acquired pneumonia leading to hospitalisation. Thorax. 2016;17:1998–2014. doi: 10.1136/thoraxjnl-2015-207688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trotter C.L., Stuart J.M., George R., Miller E. Increasing hospital admissions for pneumonia, England. Emerg. Infect. Dis. 2008;14(5):727–733. doi: 10.3201/eid1405.071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lode H.M. Managing community-acquired pneumonia: a European perspective. Respir. Med. 2007:1864–1873. doi: 10.1016/j.rmed.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Torres A., Peetermans W.E., Viegi G., Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68(11):1057–1065. doi: 10.1136/thoraxjnl-2013-204282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welte T., Torres A., Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 7.Rozenbaum M.H., Pechlivanoglou P., van der Werf T.S., Lo-Ten-Foe J.R., Postma M.J., Hak E. The role of Streptococcus pneumoniae in community-acquired pneumonia among adults in Europe: a meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32(3):305–316. doi: 10.1007/s10096-012-1778-4. [DOI] [PubMed] [Google Scholar]
- 8.Lim W.S., Baudouin S.V., George R.C., Hill A.T., Jamieson C., Le Jeune I., Macfarlane J.T., Read R.C., Roberts H.J., Levy M.L., Wani M. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(October (Suppl. 3)):iii1–55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 9.Johnstone J., Majumdar S.R., Fox J.D., Marrie T.J. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest. 2008;134(6):1141–1148. doi: 10.1378/chest.08-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavia A.T. What is the role of respiratory viruses in community-acquired pneumonia? What is the best therapy for influenza and other viral causes of community-acquired pneumonia? Infect. Dis. Clin. 2013;27(1):157–175. doi: 10.1016/j.idc.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain S., Self W.H., Wunderink R.G., Fakhran S., Balk R., Bramley A.M. Community-acquired pneumonia requiring hospitalization among U.S. adults. N. Engl. J. Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.E. Polverino, A. Torres Marti, Community-acquired Pneumonia. Minerva Anestesiol. 77 (2011) 196–211. [PubMed]
- 13.Woodhead M. Community-acquired pneumonia in Europe: causative pathogens and resistance patterns. Eur. Respir. J. 2002;20(July (36 Suppl.)):20s–27s. doi: 10.1183/09031936.02.00702002. [DOI] [PubMed] [Google Scholar]
- 14.Self W.H., Williams D.J., Zhu Y., Ampofo K., Pavia A.T., Chappell J.D. Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J. Infect. Dis. 2015;213(4):584–591. doi: 10.1093/infdis/jiv323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Itzstein M., Wu W.Y., Kok G.B., Pegg M.S., Dyason J.C., Jin B. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363(6428):418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 16.Muthuri S.G., Venkatesan S., Myles P.R., Leonardi-Bee J., Lim W.S., Al Mamun A. Impact of neuraminidase inhibitors on influenza A(H1N1)pdm09-related pneumonia: an IPD meta-analysis. Influenza Other Respir. Viruses. 2016;10(3):192–204. doi: 10.1111/irv.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson L.J., Dormitzer P.R., Nokes D.J., Rappuoli R., Roca A., Graham B.S. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;2(April (31 Suppl.)):B209–B215. doi: 10.1016/j.vaccine.2012.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burk M., El-Kersh K., Saad M., Wiemken T., Ramirez J., Cavallazzi R. Viral infection in community-acquired pneumonia: a systematic review and meta-analysis. Eur. Respir. Rev. 2016;25(140):178–188. doi: 10.1183/16000617.0076-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X., Wang Q., Wang M., Su X., Xing Z., Zhang W. Incidence of respiratory viral infections detected by PCR and real-time PCR in adult patients with community-acquired pneumonia: a meta-analysis. Respiration. 2015;89(4):343–352. doi: 10.1159/000369561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. BMJ. 2009;339 b2535. [PMC free article] [PubMed] [Google Scholar]
- 21.Wells G.A., Shea B., O'Connell D., Peterson J., Welch V. 2017. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (last Accessed: 23 February 2017) [Google Scholar]
- 22.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Freeman M.F., Tukey J.W. Transformations related to the angular and the square root. Ann. Math Stat. 1950;21:607–611. [Google Scholar]
- 24.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard L.S.G.E., Sillis M., Pasteur M.C., Kamath A.V., Harrison B.D.W. Microbiological profile of community-acquired pneumonia in adults over the last 20 years. J. Infect. 2005;50(2):107–113. doi: 10.1016/j.jinf.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Bel J.L., Hausfater P., Chenevier-Gobeaux C., Blanc F.-X., Benjoar M., Ficko C. Diagnostic accuracy of C-reactive protein and procalcitonin in suspected community-acquired pneumonia adults visiting emergency department and having a systematic thoracic CT scan. Crit. Care. 2015;19:1–12. doi: 10.1186/s13054-015-1083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capelastegui A., Espana P.P., Bilbao A., Gamazo J., Medel F., Salgado J. Etiology of community-acquired pneumonia in a population-based study: link between etiology and patients characteristics, process-of-care, clinical evolution and outcomes. BMC Infect. Dis. 2012;12:134. doi: 10.1186/1471-2334-12-134. (no pagination) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cilloniz C., Ewig S., Polverino E., Angeles Marcos M., Prina E., Sellares J. Community-acquired pneumonia in outpatients: aetiology and outcomes. Eur. Respir. J. 2012;40(4):931–938. doi: 10.1183/09031936.00168811. [DOI] [PubMed] [Google Scholar]
- 29.Clark T.W., Medina M.J., Batham S., Curran M.D., Parmar S., Nicholson K.G. Adults hospitalised with acute respiratory illness rarely have detectable bacteria in the absence of COPD or pneumonia; viral infection predominates in a large prospective UK sample. J. Infect. 2014;69(5):507–515. doi: 10.1016/j.jinf.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das D., Claessens Y.E., Mayaud C., Leport C., Bouvard E., Houhou N. Viruses detected by systematic multiplex polymerase chain reaction in adults with suspected community-acquired pneumonia attending emergency departments in France. Clin. Microbiol. Infect. 2015;21(6):e1–e8. doi: 10.1016/j.cmi.2015.02.014. 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Roux A., Marcos M.A., Garcia E., Mensa J., Ewig S., Lode H. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest. 2004;125(4):1343–1351. doi: 10.1378/chest.125.4.1343. [DOI] [PubMed] [Google Scholar]
- 32.Diederen B.M., Van Der Eerden M.M., Vlaspolder F., Boersma W.G., Kluytmans J.A., Peeters M.F. Detection of respiratory viruses and Legionella spp. by real-time polymerase chain reaction in patients with community acquired pneumonia. Scand. J. Infect. Dis. 2009;41(1):45–50. doi: 10.1080/00365540802448799. [DOI] [PubMed] [Google Scholar]
- 33.Gutierrez F., Masia M., Mirete C., Soldan B., Carlos Rodriguez J., Padilla S. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. J. Infect. 2006;53(3):166–174. doi: 10.1016/j.jinf.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Holm A., Nexoe J., Bistrup L.A., Pedersen S.S., Obel N., Nielsen L.P. Aetiology and prediction of pneumonia in lower respiratory tract infection in primary care. Br. J. Gen. Pract. 2007;57(540):547–554. [PMC free article] [PubMed] [Google Scholar]
- 35.Holter J.C., Muller F., Bjorang O., Samdal H.H., Marthinsen J.B., Jenum P.A. Etiology of community-acquired pneumonia and diagnostic yields of microbiological methods: a 3-year prospective study in Norway. BMC Infect Dis. 2015;15(1):64. doi: 10.1186/s12879-015-0803-5. (no pagination) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson N., Kalin M., Tiveljung-Lindell A., Giske C.G., Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin. Infect. Dis. 2010;50(2):202–209. doi: 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jokinen C., Heiskanen L., Juvonen H., Kallinen S., Kleemola M., Koskela M. Microbial etiology of community-acquired pneumonia in the adult population of 4 municipalities in eastern Finland. Clin. Infect. Dis. 2001;32(8):1141–1154. doi: 10.1086/319746. [DOI] [PubMed] [Google Scholar]
- 38.Koksal I., Ozlu T., Bayraktar O., Yilmaz G., Bulbul Y., Oztuna F. Etiological agents of community-acquired pneumonia in adult patients in Turkey; a multicentric, cross-sectional study. Tuberkuloz ve Toraks. 2010;58(2):119–127. [PubMed] [Google Scholar]
- 39.Lieberman D., Shimoni A., Shemer-Avni Y., Keren-Naos A., Shtainberg R. Respiratory viruses in adults with community-acquired pneumonia. Chest. 2010;138(4):811–816. doi: 10.1378/chest.09-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim W.S., Macfarlane J.T., Boswell T.C.J., Harrison T.G., Rose D., Leinonen M. Study of community acquired pneumonia aetiology (SCAPA) in adults admitted to hospital: implications for management guidelines. Thorax. 2001;56(4):296–301. doi: 10.1136/thorax.56.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sangil A., Calbo E., Robles A., Benet S., Viladot M., Pascual V. Aetiology of community-acquired pneumonia among adults in an H1N1 pandemic year: the role of respiratory viruses. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31(10):2765–2772. doi: 10.1007/s10096-012-1626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibli F., Chazan B., Nitzan O., Flatau E., Edelstein H., Blondheim O. Etiology of community-acquired pneumonia in hospitalized patients in Northern Israel. Isr. Med. Assoc. J. 2010;12(8):477–482. [PubMed] [Google Scholar]
- 43.Van Gageldonk-Lafeber A.B., Wever P.C., Van Der Lubben I.M., De Jager C.P.C., Meijer A., De Vries M.C. The aetiology of community-acquired pneumonia and implications for patient management. Neth. J. Med. 2013;71(8):418–425. [PubMed] [Google Scholar]
- 44.Viasus D., Marinescu C., Villoslada A., Cordero E., Galvez-Acebal J., Farinas M.C. Community-acquired pneumonia during the first post-pandemic influenza season: a prospective, multicentre cohort study. J. Infect. 2013;67(3):185–193. doi: 10.1016/j.jinf.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Templeton K.E., Scheltinga S.A., van den Eeden W.C., Graffelman W.A., van den Broek P.J., Claas E.C. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin. Infect. Dis. 2005;41(3):345–351. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bochud P.Y., Moser F., Erard P., Verdon F., Studer J.P., Villard G. Community-acquired pneumonia. A prospective outpatient study. Medicine. 2001;80(2):75–87. doi: 10.1097/00005792-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Marcos M.A., Camps M., Pumarola T., Martinez J.A., Martinez E., Mensa J. The role of viruses in the aetiology of community-acquired pneumonia in adults. Antivir. Ther. 2006;11(3):351–359. [PubMed] [Google Scholar]
- 48.Hohenthal U., Sipilä J., Vainionpää R., Meurman O., Rantakokko-jalava K., Nikoskelainen J. Diagnostic value of bronchoalveolar lavage in community-acquired pneumonia in a routine setting: a study on patients treated in a Finnish university hospital. Scand. J. Infect. Dis. 2004;36(3):198–203. doi: 10.1080/00365540410019183. [DOI] [PubMed] [Google Scholar]
- 49.Huijskens E.G., Koopmans M., Palmen F.M., van Erkel A.J., Mulder P.G., Rossen J.W. The value of signs and symptoms in differentiating between bacterial, viral and mixed aetiology in patients with community-acquired pneumonia. J. Med. Microbiol. 2014;63(3):441–452. doi: 10.1099/jmm.0.067108-0. [DOI] [PubMed] [Google Scholar]
- 50.Cillóniz C., Ewig S., Ferrer M., Polverino E., Gabarrús A., Puig de la Bellacasa J. Community-acquired polymicrobial pneumonia in the intensive care unit: aetiology and prognosis. Crit. Care. 2011;15(5):R209. doi: 10.1186/cc10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almirall J., Boixeda R., Bolibar I., Bassa J., Sauca G., Vidal J. Differences in the etiology of community-acquired pneumonia according to site of care: a population-based study. Respir. Med. 2007;101(10):2168–2175. doi: 10.1016/j.rmed.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Farida H., Gasem M.H., Suryanto A., Keuter M., Zulkarnain N., Satoto B. Viruses and gram-negative bacilli dominate the etiology of community-acquired pneumonia in Indonesia, a cohort study. Int. J. Infect. Dis. 2015;38:101–107. doi: 10.1016/j.ijid.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qu J.-X., Gu L., Pu Z.-H., Yu X.-M., Liu Y.-M., Li R. Viral etiology of community-acquired pneumonia among adolescents and adults with mild or moderate severity and its relation to age and severity. BMC Infect. Dis. 2015;15(1):89. doi: 10.1186/s12879-015-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jartti T., Jartti L., Peltola V. Identi cation of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. Pediatr. Infect. Dis. J. 2008;27:1103–1107. doi: 10.1097/INF.0b013e31817e695d. [DOI] [PubMed] [Google Scholar]
- 55.Gadsby N.J., Russell C.D., McHugh M.P., Mark H., Conway Morris A., Laurenson I.F. Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin. Infect. Dis. 2016;62(7):817–823. doi: 10.1093/cid/civ1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jansen R.R., Wieringa J., Koekkoek S.M., Visser C.E., Pajkrt D., Molenkamp R. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J. Clin. Microbiol. 2011;49(7):2631–2636. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomsen S.F., vd Sluis S., Stensballe L.G., Posthuma D., Skytthe A., Kyvik K.O., Duffy D.L., Backer V., Bisgaard H. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am. J. Respir. Crit. Care Med. 2009;179:1091–1097. doi: 10.1164/rccm.200809-1471OC. [DOI] [PubMed] [Google Scholar]
- 58.Gern J.E., Busse W.W. Association of rhinovirus infections with asthma. Clin. Microbiol. Rev. 1999;12(1):9–18. doi: 10.1128/cmr.12.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayden F.G. Advances in antivirals for non-influenza respiratory virus infections. Influenza Other Respir. Viruses. 2013;7:36–43. doi: 10.1111/irv.12173. (45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hayden F.G. Newer influenza antivirals, biotherapeutics and combinations. Influenza Other Respir. Viruses. 2013;7:63–75. doi: 10.1111/irv.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jaberolansar N., Toth I., Young P.R., Skwarczynski M. Recent advances in the development of subunit-based RSV vaccines. Expert Rev. Vaccines. 2016;15(1):53–68. doi: 10.1586/14760584.2016.1105134. [DOI] [PubMed] [Google Scholar]
- 62.Hunter J.P., Saratzis A., Sutton A.J., Boucher R.H., Sayers R.D., Bown M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J. Clin. Epidemiol. 2014;67(8):897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.