Summary
Background
Acute stroke patients with large artery occlusive disease (LAOD) have a distinct pathophysiology and may respond differently to anticoagulation treatments. We compared the efficacy of a low-molecular-weight heparin (LMWH), nadroparin calcium, with aspirin in Asian acute stroke patients with LAOD.
Methods
Acute ischaemic stroke patients with onset of symptoms less than 48 h and LAOD (diagnosed by transcranial doppler imaging, carotid duplex scan, or magnetic resonance angiography) were recruited. Patients were randomly assigned to receive either subcutaneous nadroparin calcium 3800 anti-factor Xa IU/0·4 mL twice daily or oral aspirin 160 mg daily for 10 days, and then all received aspirin 80–300 mg once daily for 6 months. This study is registered at www.strokecenter.org/trials (number 493).
Findings
Among 603 patients recruited, 353 (180 LMWH, 173 aspirin) had LAOD (300 had intracranial LAOD only, 42 had both intracranial and extracranial disease, and 11 had extracranial disease only). The proportion of patients with good outcomes at 6 months (Barthel index ≥85) was 73% in the LMWH group and 69% in the aspirin group (absolute risk reduction 4%; 95% CI −5 to 13). Analysis of prespecified secondary outcome measures showed a benefit in outcome for LMWH versus aspirin on the modified Rankin scale dichotomised at 0–1 (odds ratio 1·55, 95% CI 1·02–2·35). Haemorrhagic transformation of infarct and severe adverse events were similar in both groups. Post-hoc analyses of patients without LAOD, and all treated patients, showed similar proportions with a good outcome in aspirin and LMWH groups (78% vs 79% and 73% vs 75%, respectively).
Interpretation
Overall, the results do not support a significant benefit of LMWH over aspirin in patients with LAOD. The benefits indicated in most outcome measures warrant further investigation into the use of anticoagulation for acute stroke in patients with large artery atherosclerosis, particularly in intracranial atherosclerosis.
Introduction
Stroke is the third most common cause of death worldwide and the leading cause of disability in adults. The burden of stroke is particularly heavy in Asia, where it accounts for more than half of worldwide mortality from stroke. Acute intervention for ischaemic stroke is currently the subject of intensive clinical research. Among medical treatments, aspirin1, 2 and tissue plasminogen activator3, 4, 5 have been shown to have a beneficial effect on outcome. However, the treatment effect of aspirin is small, and use of tissue plasminogen activator is limited by its narrow therapeutic window.6 Antithrombotic agents are used in acute ischaemic stroke to inhibit clot propagation, prevent re-embolisation, and facilitate clot lysis.7, 8 However, randomised controlled trials have not found unfractionated heparin to be effective in improving functional outcome.2
Several medium-sized randomised placebo-controlled trials using low-molecular-weight heparin (LMWH) or heparinoid for the treatment of acute ischaemic stroke have been reported since 1995.9, 10, 11, 12 Only the Fraxiparine in Ischaemic Stroke (FISS) study of nadroparin calcium in 312 Chinese patients was positive in its primary outcome,9 but this result was not reproduced in the larger FISS-bis study,10 the Trial of ORG 10172 in Acute Stroke Treatment (TOAST),11 nor the Tinzaparin in Acute Ischemic Stroke Trial (TAIST) study.12 Recent systematic reviews and meta-analyses of LMWH and heparinoids in acute ischaemic stroke have concluded that immediate full-dose anticoagulant therapy is not associated with net short-term or long-term benefit, and the routine use of any type of anticoagulant is not supported.13, 14 However, debate has continued in Asia on whether the results of the FISS study, the only positive trial of LMWH, were due to chance or related to ethnic differences in the underlying pathophysiological mechanism of ischaemic stroke.
Extracranial atherosclerotic stenosis is a well-known risk factor for stroke worldwide. By contrast, intracranial large artery occlusive disease (LAOD) is an important cause of stroke among Asians, Hispanics, and African Americans.15, 16, 17 Asian data have suggested that LAOD is the cause of ischaemic stroke in about a third to a half of patients.18, 19 The predominance of intracranial LAOD in Asian stroke patients might explain why the beneficial effect of LMWH in Chinese patients was not reproduced in studies of white people. In concordance with this hypothesis, a post-hoc analysis of the TOAST study has shown that heparinoid treatment could increase the odds of a favourable outcome in patients with stroke secondary to LAOD.20
With this background of a positive trial and definite differences in stroke subtype, especially intracranial atherosclerosis in Asians, uncertainty remains about the benefit of LMWH for LAOD. The study objective was to test the hypothesis that subcutaneous nadroparin calcium is superior to aspirin in improving stroke outcome at 6 months in patients with acute ischaemic stroke and LAOD.
Methods
This study was an academically funded, investigator-initiated, multicentre, randomised controlled trial with blinded outcome assessment, done at multiple trial sites in Hong Kong and Singapore between April 25, 2001, and Sept 11, 2004. The study protocol received ethics committee approval at all participating centres. All participants or their legally acceptable representatives provided written informed consent.
Participants
Patients who were diagnosed with acute ischaemic stroke were randomly assigned to receive either nadroparin calcium 3800 anti-factor Xa IU/0·4 mL subcutaneously twice daily (LMWH group) or aspirin 160 mg once daily (aspirin group) for 10 days, and then all received aspirin 80–300 mg once daily for 6 months. Inclusion criteria were as follows: age 18–90 years; clinical diagnosis of acute ischaemic stroke; symptoms of stroke less than 48 h before receiving the first dose of trial medication (counted from time last known to be symptom free); presence of motor deficit as a result of acute stroke, brain CT scan excluding intracerebral haemorrhage; women of non-childbearing potential (ie, physiologically incapable of becoming pregnant, including any woman who was postmenopausal) or of childbearing potential but with a negative urine pregnancy test immediately before randomisation. If vascular imaging was done before randomisation, it had to show moderate or greater stenosis in the internal carotid, vertebrobasilar, middle cerebral, anterior cerebral, and posterior cerebral arteries as confirmed by carotid duplex scan, transcranial doppler imaging, or magnetic resonance angiography, according to previously published criteria.18, 21 All randomised patients, except for two who were ineligible and two who withdrew their consent, received the allocated treatment and 588 completed the study at 6 months. If neuroimaging was not done before randomisation, results of the neuroimaging were interpreted without knowledge of the treatment allocation. Only patients with LAOD confirmed by neuroimaging were included in the primary analysis.
Exclusion criteria were as follows: prestroke modified Rankin scale (mRS) score greater than 1; National Institutes of Health stroke scale (NIHSS) score greater than 22; history of intracerebral haemorrhage; known contraindication for the use of LMWH or aspirin (including haemorrhagic diathesis); patient on anticoagulation therapy (excluding aspirin) before the onset of stroke or definite indication for anticoagulation; sustained hypertension (blood pressure >220/>120 mm Hg) immediately before randomisation; coexisting systemic diseases such as terminal carcinoma, renal failure (creatinine >200 μmol/L, if known), cirrhosis, severe dementia or psychosis, brain tumour or other significant non-ischaemic brain lesion on brain CT scan, atrial fibrillation on ECG (past or present); chronic rheumatic heart disease or metallic heart valve; thrombocytopenia (platelet count <100×109/L, if known); or participation in another clinical trial.
Procedures
Baseline data and measurements collected included demographics, medical history, and prestroke mRS and NIHSS scores. At day 10, or earlier if discharged, outcome was assessed by NIHSS, Barthel index, mRS, and mini-mental state examination (MMSE). At 6 months after randomisation, NIHSS, Barthel index, mRS, MMSE, and International Stroke Trial (IST) questions were assessed by a clinician or nurse without knowledge of the treatment allocation. Haemorrhage, thromboembolic events, adverse events, and overall mortality during treatment and follow-up were documented.
The primary outcome was a combined endpoint at 6 months, defined as survival with a Barthel index of at least 85 (good outcome), as used in the FISS-bis study.10 Secondary outcomes included: NIHSS score at day 10 and month 6; change in NIHSS score between baseline and day 10, and between baseline and month 6; mRS score at day 10 and month 6 (favourable outcomes were defined as survival with mRS score of 0–1 and also 0–2), MMSE scores at day 10 and month 6, IST questions at month 6 (good outcome or independent defined as IST outcome “indifferent or good”, and bad outcome [dependent/dead] defined as IST outcome “dead or bad”);2 overall mortality at day 10 and month 6; and thromboembolic events during the study period (recurrent stroke, coronary syndrome, deep vein thrombosis, and pulmonary embolus).
CT scans of the brain at randomisation and at day 10 were read independently by radiologists who were unaware of the treatment allocation. The safety measures included haemorrhagic episodes occurring between days 1 and 10, defined as the presence of any of the following: symptomatic haemorrhagic transformation of the cerebral infarct or symptomatic intracerebral haemorrhage not associated with cerebral infarction; asymptomatic haemorrhagic transformation of the cerebral infarct or asymptomatic intracerebral haemorrhage not associated with cerebral infarction; serious extracranial haemorrhages (eg, gastrointestinal bleeding, haematoma, haematuria); thromboembolic events during the study period (recurrent stroke, coronary syndrome, deep vein thrombosis, pulmonary embolus); death from any cause; and death related to haemorrhagic complications.
All neurological events were assessed by an events evaluation committee. Progressing stroke was defined as neurological deterioration from day 2 to day 9 after onset of symptoms, but excluding haemorrhagic transformation of infarct or infarct in another vascular territory. Recurrent stroke was defined as neurological deterioration from day 10 to month 6 or infarct in another vascular territory.
Randomisation into the trial was done through the central randomisation office at the Clinical Trials and Epidemiology Research Unit in Singapore by means of sealed envelopes or allocation via the internet. Block randomisation was used (block sizes of 4 and 6), stratified by regions (Hong Kong, Kowloon, New Territories, Singapore), time from onset of stroke (0–24 h, 24–48 h), NIHSS score (0–8, ≥9), and whether neurovascular investigations were done before randomisation (vascular lesion present, vascular lesion status unknown), with a one-to-one treatment allocation. The treatment assignment was generated by computer.
Statistical analysis
Data management and statistical analysis were done at the Clinical Trials and Epidemiology Research Unit in Singapore independently of the investigators. In the TOAST study, 53% of the placebo group and 68% of the treatment group in the LAOD subgroup had a good outcome. For the purposes of sample size calculation, we assumed that the outcomes of our patients would be the same as in the TOAST study. With a two-sided test size of 5% and power of 80%, the required sample size planned for the trial was at least 330 patients with LAOD. Analyses were done on an intention-to-treat basis. When month 6 efficacy outcomes were not obtained, the last available outcome was used in its place (last observation carried forward method). The associations between treatment groups and the primary endpoints (combined outcomes) were determined using chi-squared tests. Odds ratios (ORs) with 95% CIs were calculated. The two-sample t test was used to assess the quantitative secondary endpoints, and mean differences between treatment groups were presented with 95% CI. Logistic regression and multiple regression analyses were done for binary and continuous outcomes, respectively, and adjusted for the stratification variables: time from onset of stroke (0–24 h, 24–48 h), NIHSS score (0–8, ≥9), whether neurovascular investigations were done before randomisation (vascular lesion present, vascular lesion status unknown). In addition to the primary analysis of the LAOD subgroup, similar analyses were also done for all randomised and treated patients and the non-LAOD subgroup.
The trial data were collected on printed forms, and subsequently entered on to computer by use of CLINTRIAL software (Clinsoft Corporation Release 4.4, Lexington, MA, USA). Statistical analyses were generated using SAS version 9.1 (SAS Institute Inc, Cary, NC, USA). Reporting of this study was done in accordance with the CONSORT statement.22 For the purpose of this report, values of ORs greater than 1·0 and positive values for risk reductions indicate an advantage of LMWH over aspirin.
Role of the funding source
The funding sources had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We enrolled 603 patients in 11 participating hospitals from April, 2001, to September, 2004. In hospitals where screening logs were available, we estimated that about 3·5% of acute stroke patients were eligible as per the study protocol. Common barriers for recruitment included late presentation, refusal, and the severe acute respiratory syndrome epidemic. Four patients did not receive any trial medications and thus no data were recorded: two withdrew consent and two were ineligible due to presence of exclusion criteria (figure 1 ). 246 patients who were randomised (125 LWMH, 121 aspirin) were excluded from the primary analysis according to the protocol after vascular imaging investigations did not show any LAOD (15 with vascular imaging done before randomisation, 226 patients with vascular imaging done after randomisation) or because no vascular imaging could be done (n=5). Our primary study population comprised the 353 patients with confirmed LAOD: 164 patients with vascular imaging done before randomisation and 189 patients with vascular imaging done after randomisation. The location of LAOD was solely intracranial in 300 (86%) patients, solely extracranial in 11 (3%), and both intracranial and extracranial in 42 (12%). All patients underwent transcranial doppler examination, 46% underwent carotid duplex doppler examination, and less than 1% underwent magnetic resonance angiography. The baseline clinical and stroke characteristics of patients and baseline variables between the two groups were similar (table 1 ), although there were slightly more patients with diabetes mellitus in the aspirin group.
Figure 1.
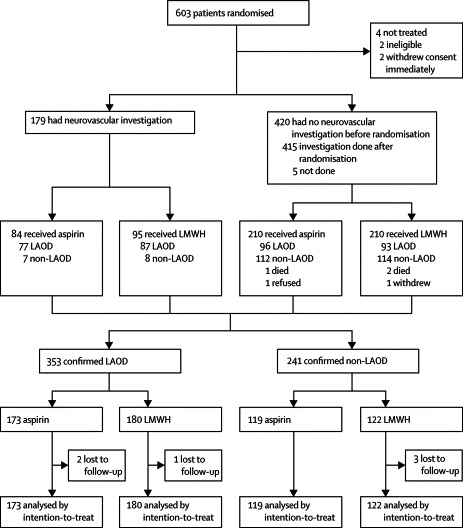
Trial profile
LAOD=Large artery occlusive disease; LMWH=low-molecular-weight heparin.
Table 1.
Baseline characteristics of patients with large artery occlusive disease (n=353)
| Aspirin (n=173) | LMWH (n=180) | ||
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 67·2 (10·5) | 66·7 (9·8) | |
| Median (range) | 68 (35–89) | 69 (37–88) | |
| Sex | |||
| Male | 100 (58%) | 106 (59%) | |
| Female | 73 (42%) | 74 (41%) | |
| Time to treatment (h) | |||
| Mean (SD) | 28 (12) | 30 (11) | |
| 0–24 h | 73 (42%) | 66 (37%) | |
| >24–48 h | 100 (58%) | 114 (63%) | |
| NIHSS at baseline | |||
| Mean (SD) | 7 (4) | 7 (4) | |
| Median (range) | 6 (1–21) | 6 (1–22) | |
| Score 0–8 | 123 (71%) | 129 (72%) | |
| Score ≥9 | 50 (29%) | 51 (28%) | |
| Prestroke modified Rankin scale | |||
| Score 0 | 145 (84%) | 150 (83%) | |
| Score 1 | 28 (16%) | 30 (17%) | |
| Known risk factor | |||
| History of stroke/transient ischaemic attack | 33 (19%) | 39 (22%) | |
| Ischaemic heart disease | 15 (9%) | 20 (11%) | |
| Hypertension | 136 (79%) | 141 (78%) | |
| Diabetes mellitus | 87 (50%) | 73 (41%) | |
| Insulin dependent | 2 | 1 | |
| Non-insulin dependent | 85 | 72 | |
| Hyperlipidaemia | 82 (47%) | 86 (48%) | |
| Smoking history (including current and ex-smoker) | 76 (44%) | 82 (46%) | |
| Self-reported habitual drinking | 29 (17%) | 25 (14%) | |
| Peripheral vascular disease | 7 (4%) | 3 (2%) | |
Data are numbers (%), unless otherwise indicated. LMWH=Low-molecular-weight heparin; NIHSS=National Institutes of Health stroke scale.
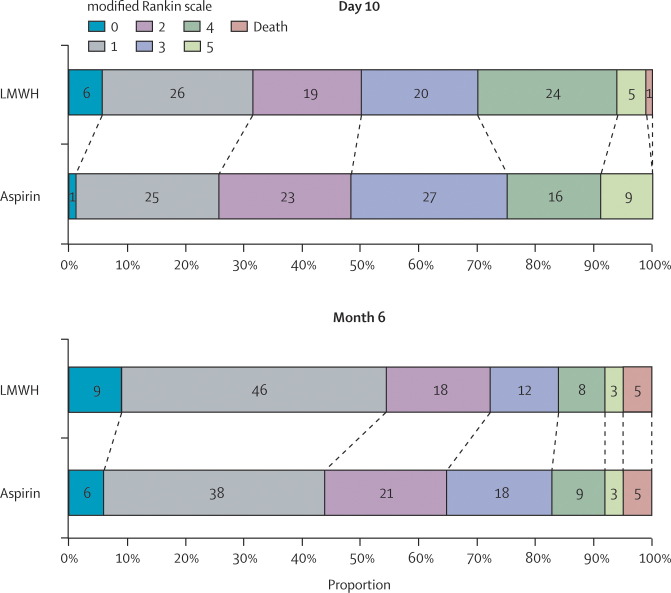
In the primary outcome analysis at 6 months, the proportion of patients with good outcomes (Barthel index of at least 85) was 73% (131 of 180) in the LMWH group and 69% (119 of 173) in the aspirin group (absolute risk reduction [ARR] 4%; 95% CI −5 to 13). The efficacy outcomes in terms of patients' independence are shown in figure 2 . Secondary outcomes analysis showed a significant effect on favourable outcomes with mRS score 0–1 versus ≥2 (LMWH 54% vs aspirin 44%; ARR 10%; OR 1·55, 1·02–2·35), whereas a non-significant benefit was found for mRS score 0–2 versus ≥3 (LMWH 72% vs aspirin 65%; ARR 7%; OR 1·39, 0·89–2·19; figure 3 ) and IST outcome (LMWH 62% vs aspirin 54%; ARR 8%; OR 1·37, 0·89–2·10; figure 3). Mean MMSE scores were 24·7 (SD 5·5) for LMWH and 23·3 (SD 7·0) for aspirin (p=0·055). Mean NIHSS scores were 3·8 (SD 4·7) for LMWH and 3·9 (SD 4·3) for aspirin (p=0·89).
Figure 2.
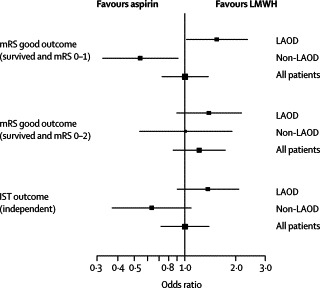
Forest plot of secondary outcomes for independent living at 6 months
LAOD=Large artery occlusive disease; LMWH=low-molecular-weight heparin; mRS=modified Rankin scale; IST=International Stroke Trial.
Figure 3.
Modified Rankin scale scores of aspirin and low-molecular-weight heparin (LMWH) groups at day 10 and month 6 for patients with large artery occlusive disease
Total percentages might not add up to 100 due to rounding.
The OR for the primary outcome remained unaltered after adjusting by the stratification variables: time from onset of stroke, NIHSS score at baseline, and whether neurovascular investigation was done before randomisation (table 2 ). After adjustment, patients on LMWH still had a significant advantage with a mRS score of 0–1 (adjusted OR 1·64, 95% CI 1·04–2·60). The MMSE score of patients allocated LMWH became significantly higher compared to those allocated aspirin (adjusted estimate of difference 1·44, 0·15–2·73). However, an adjusted analysis that accounted for stratification factors had little effect on the ORs or estimates of the remaining secondary outcomes.
Table 2.
Primary outcome using Barthel index at 6 months
| Aspirin | LMWH | Unadjusted OR (95% CI) | Adjusted OR*(95% CI) | |
|---|---|---|---|---|
| All treated patients | ||||
| Good outcome | 213 (73%) | 226 (75%) | 1·10 (0·77–1·59) | 1·18 (0·79–1·75)† |
| Bad outcome | 80 (27%) | 77 (25%) | ||
| LAOD patients | ||||
| Good outcome | 119 (69%) | 131 (73%) | 1·19 (0·75–1·89) | 1·21 (0·74–1·99) |
| Bad outcome | 53 (31%) | 49 (27%) | ||
| Non-LAOD patients | ||||
| Good outcome | 93 (78%) | 95 (79%) | 1·02 (0·55–1·89) | 1·21 (0·61–2·39) |
| Bad outcome | 26 (22%) | 26 (21%) | ||
Good outcome is defined as survival with Barthel index ≥85; bad outcome is defined as died or Barthel index ≤80.
Odds ratio (OR) after adjustment for the stratification variables: time from onset of stroke (0–24 h, 24–48 h), National Institutes of Health stroke scale score at baseline (0–8, ≥9), and neurovascular investigations at randomisation (vascular lesion present, vascular lesion status unknown). OR >1 indicate advantage of low-molecular-weight heparin (LMWH).
OR after adjustment for large artery occlusive disease (LAOD) status in addition to the three stratification variables.
There was no significant difference in the occurrence of haemorrhagic transformation of infarct (symptomatic or asymptomatic), adverse events, or serious adverse events (haemorrhagic or non-haemorrhagic) between the two groups (table 3 ).
Table 3.
Neurological deterioration, mortality, recurrent vascular events, haemorrhagic transformation, and adverse events in patients with large artery occlusive disease
| Aspirin (n=173) | LMWH (n=180) | p | ||
|---|---|---|---|---|
| Neurological deterioration | ||||
| Day 2–9 | 8 (5%) | 10 (6%) | 0·69 | |
| Progressing stroke | 8 | 9 | ||
| Non-stroke neurological manifestation | 0 | 1 | ||
| Day 10 to month 6 | ||||
| Recurrent stroke | 9 (5%) | 8 (4%) | 0·74 | |
| Mortality | ||||
| Day 10 | 0 (0%) | 1 (1%) | 1·00 | |
| Month 6 [total patients assessed] | 8 (5%) [171] | 9 (5%) [179] | 0·88 | |
| Other vascular events by 6 months | ||||
| Acute coronary syndrome | 2 (1%) | 6 (3%) | 0·28 | |
| Deep vein thrombosis | 2 (1%) | 1 (1%) | 0·62 | |
| Pulmonary embolus | 0 (0%) | 0 (0%) | .. | |
| Haemorrhagic transformation on CT scan | 7 (4%) | 6 (3%) | 0·72 | |
| Symptomatic | 2 | 1 | ||
| Asymptomatic | 5 | 5 | ||
| Adverse event or severe adverse events | 83 (48%) | 87 (48%) | 0·95 | |
| Haemorrhagic adverse events | 15 (9%) | 25 (14%) | 0·12 | |
Data are numbers (%), unless otherwise indicated. LMWH=Low-molecular-weight heparin.
In the post-hoc analysis of patients excluded because of the absence of LAOD, the proportion of patients with good outcomes at 6 months with Barthel index of at least 85 was 79% (95 of 121) in the LMWH group and 78% (93 of 119) in the aspirin group (ARR 0·4%, 95% CI −10 to 11). The efficacy outcomes in terms of independence in these patients are shown in figure 2. Secondary outcomes showed a significant risk for LMWH versus aspirin in outcomes with mRS score 0–1 versus ≥2 (LMWH 51% vs aspirin 66%; ARR −15%; OR 0·54, 0·32–0·91), but not for mRS 0–2 versus ≥3 (LMWH 80% vs aspirin 80%; ARR 0·2%; OR 1·01, 0·54–1·90) and IST outcome (LMWH 61% vs aspirin 71%; ARR −10%; OR 0·63, 0·37–1·09). The mean MMSE score was 25·7 (SD 4·8) for LMWH versus 25·9 (SD 6·0) for aspirin (p=0·77). Mean NIHSS scores were 2·8 (SD 3·0) for LMWH and 3·1 (SD 4·2) for aspirin (p=0·51). The adjusted ORs or estimates of the primary and secondary outcomes remained unchanged. No significant differences in safety measures between the two groups were found (table 3).
In the post-hoc analysis of all randomised and treated patients, the proportion of patients with good outcomes at 6 months with a Barthel index of at least 85 was 75% (226 of 303) in the LMWH group and 73% (213 of 293) in the aspirin group (ARR 1·9%; 95% CI −5 to 9). The efficacy outcomes in terms of patients' independence are shown in figure 2. Secondary outcomes showed no significant benefit for LMWH over aspirin in outcomes with mRS score 0–1 versus ≥2 (LMWH 53% vs aspirin 53%; ARR −0·1%; OR 1·00, 0·73–1·38) and for mRS score 0–2 versus ≥3 (LMWH 75% vs aspirin 71%; ARR 3·8%; OR 1·22, 0·85–1·75), and IST outcome (LMWH 61% vs aspirin 61%; ARR 0·03%; OR 1·00, 0·72–1·39). The mean MMSE scores were 25·1 (SD 5·2) for LMWH and 24·4 (SD 6·7) for aspirin (p=0·17). The mean NIHSS scores were 3·4 (SD 4·1) for LMWH and 3·6 (SD 4·3) for aspirin (p=0·62). There was no change in the adjusted OR or estimates of the primary and secondary outcomes. The safety measures in the aspirin and LWMH groups were similar, but significantly more patients had adverse events or serious adverse events with haemorrhage in the LWMH group (14% vs 9% aspirin; OR 1·77, 1·05–2·97, p=0·031).
Discussion
This trial was designed to assess the effects of LMWH versus aspirin on acute stroke patients with LAOD (predominantly due to intracranial atherosclerosis). Ischaemic stroke is a heterogeneous disease with three main stroke subtypes: cardioembolic, LAOD, and small vessel disease. Because of the diversity of stroke mechanisms, the best acute treatment of individual stroke subtypes may be different. Previous acute trials of anticoagulation have not systematically targeted underlying arterial lesions.23 In patients with LAOD, atherothrombosis is a common mechanism and thus anticoagulation may be particularly useful in this stroke subtype. A previous post-hoc analysis suggested that anticoagulation may be beneficial among patients with internal carotid occlusive disease.20 Among acute stroke patients with intracranial atherosclerosis, microemboli were detected in 30% of patients by use of transcranial doppler monitoring, and 50% of patients had multiple acute infarcts on diffusion-weighted MRI, suggesting artery-to-artery embolism was an important stroke mechanism in patients with middle cerebral artery stenosis.24 Although our data did not show a definitive benefit of LMWH over aspirin, the results were compatible with an earlier study on Asian patients who were at high risk of intracranial atherosclerosis.9 Moreover, the results also suggested that LMWH might be hazardous in patients without LAOD. Future large studies of anticoagulation on acute stroke patients with LAOD should be done to clarify the role of anticoagulation in different stroke subtypes.
The choice of primary outcome measure is crucial in the case of acute stroke. The Barthel index was used in this study and is the most commonly used disability measure in acute stroke trials.25, 26 However, the Barthel index is a not sensitive measure among patients with relatively mild stroke.27 The stroke severity of our patients was relatively mild: the median Barthel index score was 100 and 71% of patients had a score of at least 85 at 6 months. Several recent studies have used a dichotomised mRS score or combined several outcome measures in a global statistical test.28 The choice of primary outcome measure may determine whether a trial is positive or negative.5 Future studies on patients with intracranial atherosclerosis may consider using a dichotomised mRS score as the primary outcome measure.
We found that the LMWH group had slightly better cognitive function in terms of MMSE scores. Cognitive function plays a vital role for independent living. Patients without physical impairment but who are cognitively impaired may need help for their daily functions. The difference in cognitive outcome might explain the differences in disability status, although there was no difference in the NIHSS scores (a measure of physical impairment). However, the differences could also indicate small differences in the baseline MMSE in the groups. Our data suggest that future acute stroke trials should measure cognitive function as an outcome indicator.
This academically funded study was done without any substantial support from industry. Moreover, the data analyses were done independently of the investigators. The use of (predominantly) ultrasound to delineate vascular lesion also strengthens the methodology of this study. However, use of open-label trial medication might have caused bias, even though the assessors at month 6 were unaware of treatment allocation. The use of the last observation carried forward method could introduce substantial bias. In addition, the relatively small sample size could provide misleading conclusions on possible benefit or hazard. However, the effect would be small in our analysis because there were only about 2% of missing data in our study. Nevertheless, the results of this study should resurrect interest in large clinical trials on anticoagulation in acute ischaemic stroke patients with LAOD, especially intracranial disease.
Contributors
K S Wong, C Chen, P W Ng, T H Tsoi, H L Li, W C Fong, J Yeung, C K Wong, and K K Yip participated in the design of the protocol, recruited patients, and wrote the draft of the manuscript. H Gao and H B Wong collected the case record forms, did the statistical analysis, and wrote the statistical results of the manuscript. All authors approved of the final version of the manuscript.
FISS-tris investigators and participating hospitals
Trial coordination—Clinical Effectiveness Unit (CEU), Professional Services and Medical Development Division, Hospital Authority Head Office, Hong Kong: Dr S P Lim (manager), Simon Lee (study coordinator), Jenny Heung, Irene Shek, Andrew Wong (research nurses). Clinical Trials and Epidemiology Research Unit (CTERU), Singapore: Hong Gao (clinical project coordinator).
Data management—Clinical Trials and Epidemiology Research Unit (CTERU), Singapore: Wong Hwee Bee (senior biostatistician).
Singapore—Singapore General Hospital (114 patients): Christopher Chen (investigator), Hui-Meng Chang, Meng-Cheong Wong.
Hong Kong—United Christian Hospital (99 patients): Ping Wing Ng (investigator), Man-Kei Choi, Kwok-Fai Hui, Patrick Kwan, Yee-Man Lam, Kwok-Man Lo, Kwok-Yiu Sha, T C Sim. Prince of Wales Hospital (97 patients): Ka Sing Wong (principal investigator), Richard Kay, Andrew Hui, Michael Fu, Vincent Mok, Thomas Leung. Pamela Youde Nethersole Eastern Hospital (90 patients): Tak Hong Tsoi (investigator), Mandy Au-Yeung, Chun-Ming Cheung, Sonny Hon, Ka-Lock Shiu. Princess Margaret Hospital (45 patients): Kwok-Kwong Lau (investigator), Terrence Li, Eric Chan. Queen Elizabeth Hospital (41 patients): Patrick Li (investigator), John Chan, Yuk-Fai Cheung, Wing Chi Fong, Man-Wai Lo, Hiu-Tung Lui, Winnie Wong. Alice Ho Miu Ling Nethersole Hospital (32 patients): Jonas Yeung (investigator), Bun-Hey Fung. Northern District Hospital (32 patients): Chi-Keung Wong (investigator), Chi Keung Sung, Siu-Hung Li. Ruttonjee Hospital (27 patients): Chee My Chang (investigator), Kin-Ying Mok, Kin Keung Yip, Ming-Hung Fu. Kwong Wah Hospital (11 patients): Kwai Fu Ko (investigator), Wing-Keung Cheng. Queen Mary Hospital (8 patients): Raymond Cheung (investigator), Koon-Ho Chan, Gardian Fong, Windsor Mak. Tseung Kwan O Hospital (3 patients): Kin-Lun Tsang.
Conflicts of interest
We have no conflicts of interest.
Acknowledgments
Acknowledgments
This study was supported by the Clinical Effectiveness Unit, Hospital Authority in Hong Kong, the National Medical Research Council of Singapore (NMRC/0691/2002), and the Clinical Trials and Epidemiology Research Unit, Singapore. We thank the drug manufacturer (Sanofi Aventis Singapore, GlaxoSmithKline) for providing free samples of nadroparin for patients in Singapore. We thank Prof Philip Bath (University of Nottingham, UK) for his comments on the draft manuscript.
Contributors
K S Wong, C Chen, P W Ng, T H Tsoi, H L Li, W C Fong, J Yeung, C K Wong, and K K Yip participated in the design of the protocol, recruited patients, and wrote the draft of the manuscript. H Gao and H B Wong collected the case record forms, did the statistical analysis, and wrote the statistical results of the manuscript. All authors approved of the final version of the manuscript.
FISS-tris investigators and participating hospitals
Trial coordination—Clinical Effectiveness Unit (CEU), Professional Services and Medical Development Division, Hospital Authority Head Office, Hong Kong: Dr S P Lim (manager), Simon Lee (study coordinator), Jenny Heung, Irene Shek, Andrew Wong (research nurses). Clinical Trials and Epidemiology Research Unit (CTERU), Singapore: Hong Gao (clinical project coordinator).
Data management—Clinical Trials and Epidemiology Research Unit (CTERU), Singapore: Wong Hwee Bee (senior biostatistician).
Singapore—Singapore General Hospital (114 patients): Christopher Chen (investigator), Hui-Meng Chang, Meng-Cheong Wong.
Hong Kong—United Christian Hospital (99 patients): Ping Wing Ng (investigator), Man-Kei Choi, Kwok-Fai Hui, Patrick Kwan, Yee-Man Lam, Kwok-Man Lo, Kwok-Yiu Sha, T C Sim. Prince of Wales Hospital (97 patients): Ka Sing Wong (principal investigator), Richard Kay, Andrew Hui, Michael Fu, Vincent Mok, Thomas Leung. Pamela Youde Nethersole Eastern Hospital (90 patients): Tak Hong Tsoi (investigator), Mandy Au-Yeung, Chun-Ming Cheung, Sonny Hon, Ka-Lock Shiu. Princess Margaret Hospital (45 patients): Kwok-Kwong Lau (investigator), Terrence Li, Eric Chan. Queen Elizabeth Hospital (41 patients): Patrick Li (investigator), John Chan, Yuk-Fai Cheung, Wing Chi Fong, Man-Wai Lo, Hiu-Tung Lui, Winnie Wong. Alice Ho Miu Ling Nethersole Hospital (32 patients): Jonas Yeung (investigator), Bun-Hey Fung. Northern District Hospital (32 patients): Chi-Keung Wong (investigator), Chi Keung Sung, Siu-Hung Li. Ruttonjee Hospital (27 patients): Chee My Chang (investigator), Kin-Ying Mok, Kin Keung Yip, Ming-Hung Fu. Kwong Wah Hospital (11 patients): Kwai Fu Ko (investigator), Wing-Keung Cheng. Queen Mary Hospital (8 patients): Raymond Cheung (investigator), Koon-Ho Chan, Gardian Fong, Windsor Mak. Tseung Kwan O Hospital (3 patients): Kin-Lun Tsang.
Conflicts of interest
We have no conflicts of interest.
References
- 1.CAST (Chinese Acute Stroke Trial) Collaborative Group CAST: randomised placebo-controlled trial of early aspirin use in 20 000 patients with acute ischaemic stroke. Lancet. 1997;349:1641–1649. [PubMed] [Google Scholar]
- 2.International Stroke Trial Collaborative Group The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19 435 patients with acute ischaemic stroke. Lancet. 1997;349:1569–1581. [PubMed] [Google Scholar]
- 3.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 4.Bath PM. Low molecular weight heparin in acute stroke. Expert Opin Investig Drugs. 1998;7:1323–1330. doi: 10.1517/13543784.7.8.1323. [DOI] [PubMed] [Google Scholar]
- 5.Hacke W, Kaste M, Fieschi C. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II) Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 6.Zivin JA. Thrombolytic stroke therapy: past, present, and future. Neurology. 1999;53:14–19. doi: 10.1212/wnl.53.1.14. [DOI] [PubMed] [Google Scholar]
- 7.del Zoppo GJ. Antithrombotic treatments in acute ischemic stroke. Thromb Haemost. 1999;82:938–946. [PubMed] [Google Scholar]
- 8.Samama MM, Desnoyers PC, Conard J, Bousser MG. Acute ischemic stroke and heparin treatments. Thromb Haemost. 1997;78:173–179. [PubMed] [Google Scholar]
- 9.Kay R, Wong KS, Yu YL. Low-molecular-weight heparin for the treatment of acute ischemic stroke. N Engl J Med. 1995;333:1588–1593. doi: 10.1056/NEJM199512143332402. [DOI] [PubMed] [Google Scholar]
- 10.Hommel M, FISS-bis Investigators Group Fraxiparine in Ischemic Stroke Study (FISS bis) Cerebrovasc Dis. 1998;8(suppl 4):19. [Google Scholar]
- 11.Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. JAMA. 1998;279:1265–1272. [PubMed] [Google Scholar]
- 12.Bath PM, Lindenstrom E, Boysen G. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet. 2001;358:702–710. doi: 10.1016/s0140-6736(01)05837-8. [DOI] [PubMed] [Google Scholar]
- 13.Gubitz G, Sandercock P, Counsell C. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2004;3 doi: 10.1002/14651858.CD000024.pub2. CD000024. [DOI] [PubMed] [Google Scholar]
- 14.Bath PM, Iddenden R, Bath FJ. Low-molecular-weight heparins and heparinoids in acute ischemic stroke: a meta-analysis of randomized controlled trials. Stroke. 2000;31:1770–1778. doi: 10.1161/01.str.31.7.1770. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Wong KS. Racial distribution of intracranial and extracranial atherosclerosis. J Clin Neurosci. 2003;10:30–34. doi: 10.1016/s0967-5868(02)00264-3. [DOI] [PubMed] [Google Scholar]
- 16.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 17.Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke. 1986;17:648–655. doi: 10.1161/01.str.17.4.648. [DOI] [PubMed] [Google Scholar]
- 18.Wong KS, Li H, Chan YL. Use of transcranial Doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease. Stroke. 2000;31:2641–2647. doi: 10.1161/01.str.31.11.2641. [DOI] [PubMed] [Google Scholar]
- 19.Wong KS, Huang YN, Gao S, Lam WW, Chan YL, Kay R. Intracranial stenosis in Chinese patients with acute stroke. Neurology. 1998;50:812–813. doi: 10.1212/wnl.50.3.812. [DOI] [PubMed] [Google Scholar]
- 20.Adams HPJ, Bendixen BH, Leira E. Antithrombotic treatment of ischemic stroke among patients with occlusion or severe stenosis of the internal carotid artery: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) Neurology. 1999;53:122–125. doi: 10.1212/wnl.53.1.122. [DOI] [PubMed] [Google Scholar]
- 21.Wong KS, Lam WW, Liang E, Huang YN, Chan YL, Kay R. Variability of magnetic resonance angiography and computed tomography angiography in grading middle cerebral artery stenosis. Stroke. 1996;27:1084–1087. doi: 10.1161/01.str.27.6.1084. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- 23.Caplan LR. Resolved: heparin may be useful in selected patients with brain ischemia. Stroke. 2003;34:230–231. doi: 10.1161/01.str.0000047036.77466.e8. [DOI] [PubMed] [Google Scholar]
- 24.Wong KS, Gao S, Chan YL. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study. Ann Neurol. 2002;52:74–81. doi: 10.1002/ana.10250. [DOI] [PubMed] [Google Scholar]
- 25.Lees KR, Asplund K, Carolei A. Glycine antagonist (gavestinel) in neuroprotection (GAIN International) in patients with acute stroke: a randomised controlled trial. Lancet. 2000;355:1949–1954. doi: 10.1016/s0140-6736(00)02326-6. [DOI] [PubMed] [Google Scholar]
- 26.Fisher M, Albers GW, Donnan GA. Enhancing the development and approval of acute stroke therapies: stroke therapy academic industry roundtable. Stroke. 2005;36:1808–1813. doi: 10.1161/01.STR.0000173403.60553.27. [DOI] [PubMed] [Google Scholar]
- 27.Duncan PW, Samsa GP, Weinberger M. Health status of individuals with mild stroke. Stroke. 1997;28:740–745. doi: 10.1161/01.str.28.4.740. [DOI] [PubMed] [Google Scholar]
- 28.Lees KR, Hankey GJ, Hacke W. Design of future acute-stroke treatment trials. Lancet Neurol. 2003;2:54–61. doi: 10.1016/s1474-4422(03)00267-9. [DOI] [PubMed] [Google Scholar]