Abstract
Bioaerosols exposure can lead to many adverse health effects and even result in death if highly infectious agents involved. Apparently, there is a great need for rapid detection of bioaerosols, for which air sampling often is the first critical step. However, currently available samplers often either require an external power and/or with low sampling flow rate, thus falling short of providing a practical solution when response time is of great concern.
Here, we have designed and evaluated a new portable high volume bioaerosol sampler named as HighBioTrap through optimizing its operating parameters. The sampler was operated at a sampling flow rate of 1200 L/min, with an impaction velocity of about 10.2 m/s (S/W = 1.5, T/W = 1), while the weight of the sampler is about 1.9 kg. The performances of the HighBioTrap sampler were tested both in lab controlled and natural environments (outdoor and indoor environments in a university building) along with the reference sampler-the BioStage impactor using aerosolized Polystyrene (PS) uniform microspheres of various sizes, aerosolized bacteria and also ambient air particles. The microbial community structures of collected culturable bacterial aerosol particles both by the HighBioTrap and the BioStage impactor in the natural environments were analyzed using gene sequence method.
Experimental results with PS particles showed the HighBioTrap has a cutoff size of ~ 2 µm. The widely used impactor design equation was found to be not applicable for predicting the performance of the HighBioTrap due to its large Reynolds number. When sampling aerosolized individual Pseudomonas fluorescens and Bacillus subtilis bacterial particles, the HighBioTrap had physical collection efficiencies of 10% and 20%, respectively. Despite the higher desiccation effects introduced by higher flow rate, the HighBioTrap was shown to obtain a higher microbial diversity than the BioStage impactor for both in outdoor and indoor environments given the same sampling time (p < 0.01). Our data also showed that most of the desiccation effects might have occurred between 3 and 5 min of the sampling and an impaction velocity of around 10 m/s might be a close-to-optimal impaction velocity for collecting most environmental bacterial aerosols while maximally preserving their culturability. This work contributes to our understanding of microbial sampling stress (impaction velocity and sampling time), while developing a portable high volume sampler. The HighBioTrap sampler could find its great efficiencies in qualitative microbial aerosol detection and analysis, such as investigation of microbial aerosol diversity for a particular environment, or when the low level of pathogens is present and detection time is of great concern.
Keywords: Bioaerosol, HighBioTrap sampler, High flow-rate, Portable
Highlights
-
•
A portable 1200 L/min bioaerosol sampler (HighBioTrap) with cutoff size of 2 µm was designed.
-
•
Most of the sampling stress might have occurred within 3–5 min of the sampling.
-
•
The HighBioTrap was shown to recover more species than the BioStage per unit of time.
1. Introduction
Bioaerosols are particles of biological origins which are suspended in air, including viruses, bacteria, fungi, pollen, plant debris, and their derivatives. Exposure to bioaerosols can lead to many respiratory and other adverse health effects, such as infections, hypersensitivity pneumonitis and toxic reactions (Douwes, Thorne, Pearce, & Heederik, 2003). The generation and transmission of some pathogenic microbial species can even cause severe casualties, such as the 2003 severe acute respiratory syndrome (SARS) (Ksiazek et al., 2003), the H1N1 flu in 2009 (Bautista et al., 2010), H7N9 avian influenza in 2013 (Li et al., 2014), and Middle East respiratory syndrome (MERS) in 2015 (Zumla, Hui, & Perlman, 2015). For certain highly infectious agents, even very low dose inhalation exposure could constitute a serious threat, such as influenza A virus (Blachere et al., 2009), Francisella tularensis and Mycobacterium tuberculosis (Cole & Cook, 1998). In addition, the potential bioterrorism attack using highly infectious microbial agents is posing an increasing threat to the public (Jernigan et al., 2002). For detecting these agents, we need to collect as much as possible amount of air. On the other hand, for "detect-to-warn" objective, the ideal time was described to be 1 min by National Research Council of the United States of America (National Research Council, 2005), thus the time for air sampling is very limited for certain urgent circumstances. Accordingly, it is of critical importance to develop a detection protocol that is capable of capturing low counts within a very short time period to allow a proper counter-measure to be mounted.
Use of microbial aerosol samplers is usually the first step to assess the presence of bioaerosols and their exposure risks (Yao and Mainelis, 2006aa, Yao and Mainelis, 2006bb). The currently available microbial aerosol samplers can be generally classified into four major types: impaction, impingement, filtration, and electrostatic precipitation (Willeke et al., 1998, Yao and Mainelis, 2006c, Yao and Mainelis, 2007). The impaction-based samplers are widely used in bioaerosol investigations (Xu & Yao, 2011). Previously, Yao and Mainelis (2007) investigated the performances of several commonly used microbial aerosol samplers in both laboratory settings and field tests. One of the important parameter describing the performance of the sampler is the cut-off size, at which the sampler has a collection efficiency of at least 50%. This parameter can be derived experimentally from the sampler's physical collection efficiency curve. For the bioaerosol detection, the cut-off size should be generally below the particle size range of interest (in single particle form) to ensure ideal collection efficiency. Such a particle size is currently assumed to be either 2.5–10 µm aerodynamic diameter (AD), as used in the U.S. Government Biological Integrated Detection System, or 1–10 µm AD, as used in the U.S. Government Joint Biological Point Detection System (Haglund & Mcfarland, 2004). For a typical impactor design, a number of important parameters such as Reynolds number (Re), impactor jet diameter (W), jet-to-late distance (S), jet throat length (T), sampling flow rate (Q) and the number of impactor jets should be considered (Marple & Willeke, 1976).
The widely used bioaerosol collection device BioStage (SKC Inc., Eighty Four, PA) is a standard impactor with a cut-off size of 0.65 µm, 400 impactor jets (W = 0.25 mm), an impaction velocity of 24 m/s, and a Reynolds number of about 400, which provides high collection efficiency of most microbial particles present in the air. Therefore, it is recognized as the gold standard bioaerosol sampler (Brachman et al., 1964) and used as the reference sampler in many studies evaluating microbial samplers (Yao & Mainelis, 2006a). However, the flowrate of the BioStage sampler is only 28.3 L/min and it takes at least half an hour to obtain 1 m3 air in addition to requiring an external power source. Previous studies have shown that longer sampling time would introduce more desiccation and particle bounce effects (He and Yao, 2011, Xu and Yao, 2011, Zhen et al., 2009). Also the performance of samplers with low flowrate is easily affected by wind velocity and atmospheric movement in outdoor environments (Xu & Yao, 2013). Although the BioStage impactor has higher physical collection efficiency, it causes damages to the cells being collected due to its high impaction velocity (24 m/s). In certain scenarios, the target microorganisms appear to be in low counts, thus much larger sampling flowrates are required to concentrate several orders of magnitude within the response time (Haglund & Mcfarland, 2004), especially in an emergency situation. Flowrates of 100–1000 L/min are generally thought to be practical for the reliable detection of low-concentration biological agents that could be expected from an accidental or intentional release (Haglund & Mcfarland, 2004). For example, a cyclone type sampler with flowrate of 1000 L/min was chosen to detect Legionella in the air during the Legionnaires disease outbreak (Mathieu et al., 2006). In addition, another added feature for a microbial aerosol sampler is to be more portable (An, Mainelis, & Yao, 2004). Compared to the conventional samplers which require vacuum pumps and external power supply, portable samplers, such as the Reuters Centrifugal Sampler (RCS) (Biotest Diagnostics Corp., Denvile, NJ, USA), has the capability to be used in various conditions, for example, in remote areas where the electricity is not available or restricted. Nonetheless, most of these existing battery-powered samplers have sampling flow rates of less than 300 L/min.
In this work, we designed and evaluated a light (about 1.9 kg) and lithium battery-powered bioaerosol sampler (HighBioTrap) which samples air at a flow rate of 1200 l per minute. The HighBioTrap was designed based on the particle inertia principle. The physical and biological collecting performances were evaluated using aerosolized Polystyrene particles, Bacillus subtilis var. niger vegetative cells, Pseudomonas fluorescens and also environmental aerosol particles. The cutoff size of the HighBioTrap was experimentally derived using the results from polystyrene particles. The HighBioTrap was compared with the BioStage sampler when sampling bacterial and fungal aerosols both in outdoor and indoor environments. The culturable bacteria collected both by the HighBioTrap and the BioStage sampler were gene-sequenced and compared. This work contributes to the efforts of detecting low level bioaerosol particles and also bio-defense, while improving our understanding of microbial sampling stress (impaction velocity and sampling time).
2. Materials and methods
2.1. Sampler design
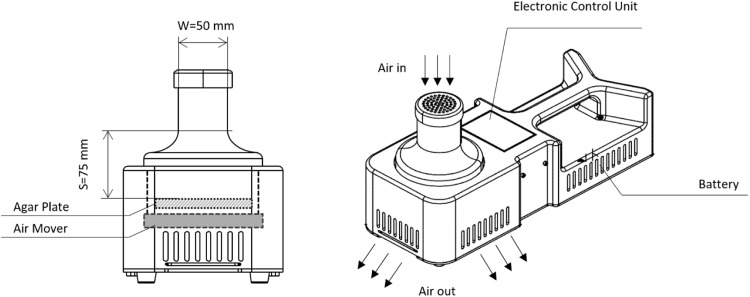
The three-dimensional design sketch of the HighBioTrap impactor is shown in the Fig. 1. To minimize the variations of the physical collection efficiency as mentioned in previous studies (Therkorn and Mainelis, 2013, Xu and Yao, 2011), a 90-mm(diameter) petri dish filled with 30 mL agar (achieving a jet-to-plate distance of 75 mm) was used for the sampling both for the HighBioTrap and the BioStage. The HighBioTrap sampler, about 1.9 kg, was designed based on particle inertia with a single “nozzle” (its physical characteristics along with other commercially available ones are listed in Table 1). The HighBioTrap's operating parameters are: U = 10.2 m/s, S/W = 1. 5, T/W = 1, Reynolds number = 33953, Q = 1200 L/min, S = 75 mm, nozzle diameter = 50 mm. Previously, the impactor design method using stokes number was proposed by Marple and Willeke (1976) has been widely used. For a standard impactor, its performance can be characterized using the following equation (Whyte, Green, & Albisu, 2007)
| (1) |
Where U is impaction speed (m/s), r is the radius of curvature of the streamline, which can be assumed to the radius or half width of the “nozzle”, is the density of the particles, d is the aerodynamic diameter of the microbial particle, C is the Cunningham correction factor, which is estimated by 1+0.165/d under the normal condition (293 K, 101.3 kPa), is the viscosity of the air under room temperature . Here, the HighBioTrap was designed to be a high volume and portable sampling device with a single impaction nozzle.
Fig. 1.
Three-dimensional sketch of the HighBioTrap and also relevant dimensions; the sampler is battery-powered and operates at a sampling flow rate of 1200 L/min with an impaction velocity of about 10.2 m/s. The sampler weighs about 1.9 kg. Its physical characteristics are listed in Table 1.
Table 1.
Physical characteristics of HighBioTrap and other portable microbial samplers.
| sampler | Sampling flow rate, L/min | Jet (nozzle) velocity, m/s | Jet (nozzle) diameter, W, mm | jet-to-plate distance, S, mm | S/W ratio | theoretical d 50, μm | experimental d 50, μm | Reynolds number |
|---|---|---|---|---|---|---|---|---|
| HighBioTrap | 1200 | 10.2 | 50 | 75 | 1.25 | N/A | 2 | 33953 |
| SAS super 180 | 180 | 15 | 0.8 | 4.7 | 6 | 1.3 | 2.1 | 791 |
| MAS−100 | 100 | 10.8 | 0.7 | 2.8 | 4 | 1.47 | 1.7 | 503 |
| RCS High Flow | 100 | 8 | N/A | N/A | N/A | 1.7 | 1.2 | N/A |
| Millipore Air Tester | 140 | 14 | 0.46 | 5.84 | 12.7 | 1 | 2.3 | 423 |
| SMA MicroPortable | 141.5 | 6.3 | 6.3 | 5 | 0.8 | 6 | 4.8 | 2638 |
| BioCulture | 120 | 1.27 | 1.25 | 1.7 | 0.75 | 8.13 | 7 | 193 |
| Microflow | 120 | 1 | 2.5 | 1.89 | 0.84 | 8.7 | > 10 | 45–180 |
Using the Eq. (1), we obtained a theoretical cutoff size of 15.5 µm, which turns out to be far different from the later experimentally determined cutoff point. Compared to other commercially available impactors as listed in Table 1, the HighBioTrap has a very large nozzle size, which is about 10 times of those of other impactors. The HighBioTrap also has a very high Reynolds number. If the Eq. (1) were used for designing the impactor to achieve a sampling flow rate of 1200 L/min with a cutoff size of about 2 µm (the experimentally determined value for the HighBioTrap), we need at least 50 impactor jets with a diameter of 4 mm each. This discrepancy might be largely due to the large nozzle size and higher Reynolds number. Accordingly, the Eq. (1) might not be applicable or efficient for the prediction of its theoretical performance.
The Reynolds number is an important parameter to consider when designing an impactor. The Reynolds number can be calculated using the Eq. (2) below,
| (2) |
where ρ (1.205 kg/m3) and η ) is the density and viscosity of the air under room temperature, respectively. v is the jet velocity (10.2 m/s), D is the hydraulic diameter (for the round tube, D is equal to the tube diameter), and Re is the Reynolds number. Using the Eq. (2), the Reynolds number of our sampler with a round nozzle of 50 mm width is about 33953. This high Reynolds number indicates that the air flow is in turbulent status. Therefore, the jet-to-plate distance was designed to be 75 mm to minimize the effect of the whirlpool, which correspondingly results in a S/W ratio of 1.5 for the HighBioTrap. Nonetheless, the high flow rate and reasonable impaction velocity is our top priority consideration for the design of the HighBioTrap sampler. Here, we wanted to design a high volume and portable sampler, which requires low pressure drop. Accordingly, the jet nozzle cannot be small otherwise it is rather difficult to make it portable because of the power needed. In general, for Reynolds number between 500 and 3000, the collection efficiency curve is very sharp, like typical “S”. Here, the Reynolds number for our sampler is very large, i.e., 33953, and according to reference (Marple & Willeke, 1976) for large Reynolds number the collection curve tends to be more flat.
During the operation of the sampler, particles in the air are moved by the high volume fan to be impacted onto the agar plate. The fan was used to create a vacuum such as the air can be sucked in and directed toward the collection surface. The sampler power is provided by the lithium battery which can operate continuously for more than 5 h. In this work, jet velocity is the most important parameter considered in the design process. As mentioned above, U directly influenced the collection efficiency. Besides, impaction stress caused by high jet velocity affected the ability to maintain microorganism culturability during and after the collection. According to previous work done by Yao and Mainelis (2006a), when sampling aerosolized bacteria and fungi, portable samplers of good relative overall efficiency (taking BioStage as a reference, relative overall efficiency of 15% or higher), such as SAS super 180, MAS-100, RCS High Flow, Millipore Air Tester were found to have jet velocities ranging from 8 to 15 m/s, as presented in the Table 1. The HighBioTrap sampler is designed to have a flow rate of 1200 l per minute, correspondingly achieving a jet velocity of about 10.2 m/s.
2.2. Laboratory test of the sampler
2.2.1. Test particles
During the operation of the sampler, a φ90 mm culture dish filled with 30 mL agar is used for the sampling. Polystyrene (PS) uniform microspheres (Bangs Laboratories, Inc., Fishers, IN) were used as the test particles to evaluate the physical collection efficiencies and cut-off sizes of the HighBioTrap sampler. The test particles had mean aerodynamic sizes of 0.5, 0.8, 2.07, 3.11, and 5.15 µm. The selected size range includes majority of the airborne bacteria and fungi. The particles suspensions were diluted in the deionized water to the final concentration of C ≈ 108 mL−1.
Besides, two types of bacterial suspensions (B. subtilis var. niger and P. fluorescens) were used to test the sampler's biological collection performance. B. subtilis are Gram positive bacteria and P. fluorescens are Gram negative bacteria, both of which are commonly found environmental microbes. B. subtilis are often used as a surrogate for B. anthracis because of similarity in physical and biological aspects (Hill et al., 1999), while P. fluorescens represent the sensitive organisms (Neidhardt, Ingraham, & Schaechter, 1990). Bacterial suspensions were prepared following the procedure described in a previous study (Yao & Mainelis, 2006a). Before preparing their suspensions, these two bacterial strains were cultured at 37 °C for 24 h on Luria-Bertani (LB) agar plates. After the cultivation, bacterial colonies were washed off and harvested with 20 mL sterile DI-water. Stock bacterial suspensions were washed 3 times using a centrifuge (Eppendorf Centrifuge 5804 R, Eppendorf, Hamburg, Germany) at 7000 rpm for 7 min and a vortex to re-suspended (Vortex Genie 2, Scientific Industries Co., Ltd.). Prior to the aerosolization, the freshly prepared bacterial suspension was diluted using DI water to achieve desired airborne bioaerosol concentration. Here, we measured the aerosolized bacterial aerosol concentration size distributions as shown in Fig S1 (Supporting information). In our work, we used vegetative cells of B. subtilis, however it is possible that some of them could form spores (they should be a small fraction, but we did not measure it). The reported aerodynamic diameter should largely represent those of vegetative cells. In addition to three aerosolized particles, the physical collection efficiency was also determined by indoor air particles. The test site was in a laboratory of about 40 m2 suited on the 4th floor of a university building.
2.2.2. Experimental setup
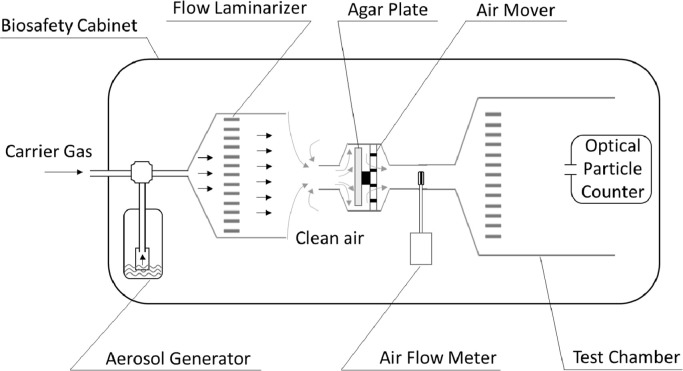
The experimental setup used in this research is shown in Fig. 2. The Collison nebulizer (BGI, Inc.) was used to aerosolize test particles discussed above at a regulated flow rate of 2.5 L/min to ensure a stable aerosolization process. The aerosol was being generated by flowing N2 into the particle suspension. The test particles were then carried by nitrogen into the test chamber and diluted with the clean air before passing the HighBioTrap sampler. Because the HighBioTrap has a very high sampling flow rate, the aerosol generating N2 flow (2.5 L/min) was not enough to supply the demand. Accordingly, the clean air inside the BioSafety hood was used to compensate. The speed controller was used to adjust the fan speed to keep the working flow rate of the HighBioTrap sampler remaining constantly at 1200 L/min no matter whether there is an agar plate in place or not. In our experimental setup, the fan not only served as a motor to move the air particles to impact onto the plate, but also a mixer to dilute the aerosol with the clean air. Before every sampling process, the sampler is wiped using 75% ethanol and then put the sampler under the ultraviolet radiation for half an hour.
Fig. 2.
A schematic representation of the experimental setup for investigating its physical collection efficiencies using PS particles (mean aerodynamic size: 0.5, 0.8, 2.07, 3.11, and 5.15 µm) and an Optical Particle Counter (GRIMM).
2.2.3. Determination of collection efficiencies
Traditionally, the overall collection efficiency of a sampler is determined by the comparison of particle number concentration entering and leaving the sampler. However, the difference of the particle concentrations between upstream and downstream of the investigated sampler is caused by collection onto the agar media, as well as the motor (fan), thus possibly leading to an overestimate of the actual collection efficiency of the sampler. For the tests, the downstream concentration and size distribution of particles with agar plate in the sampler, Cwith, and without agar plate in the sampler, C without, were monitored using an optical particle counter (model 1.108; Grimm Technologies Inc., Douglasville, GA, USA) operating at QOPC = 1.2 L/min. The HighBioTrap sampler's physical collection efficiency, E COLL, was determined using the following:
| (3) |
2.3. Environmental sampling and evaluation
Besides the laboratory test, the HighBioTrap sampler was also evaluated when sampling bacterial and fungal aerosols both in outdoor and indoor environments.
2.3.1. Environmental sampling process
In our work, the field tests were conducted in two different indoor environments but in the same building. Among them, the indoor environment in the 4th floor laboratory (close to window for sampling outdoor air) was used for the physical collection efficiency determination. For the storage room (60 m2) on the second basement floor, it was used for determining the biological collection efficiency. The BioStage sampler was used as a reference sampler to evaluate the HighBioTrap sampler's performance during the field testing. The two samplers are supplied with LB and Sabouraud's Dextrose Agar for collecting culturable bacteria and fungi, respectively. One indoor and one outdoor location were selected to evaluate the performance of the HighBioTrap. The storage room is equipped with central air conditioning system but without any open window. The HighBioTrap sampler and the BioStage sampler were placed on a table at a height of 1 m above the floor and spaced at an interval of 1.5 m from each other. To avoid artificial effects caused by air turbulence, minimal activity occurred during sample collection indoors. To avoid effects from ground source and human activities, an area outside at 4th floor of a midsize building was used for outdoor testing. The samplers were placed 1 m above the floor close to the window (for sampling outdoor air) and spaced at an interval of 1.5 m from each other in the 4th floor room. These particular sites were selected to represent two very different sampling environments.
The measurements were performed by sampling for the same amount of time as well as collecting the same amount of air volume by the test and reference samplers. For the test by sampling for the same time, the samplers were set to sample for 1, 2, 3, 4 and 5 min to collect bacterial aerosols, while operating 1, 3, 5, 7 and 10 min to collect fungal aerosols, simultaneously. For the measurement by sampling the same volume of air, the BioStage sampler sampling time was set to be tBioStage = 17.7 min (500 L of air at QBioStage = 28.3 L/min). During the sampling process of BioStage, the HighBioTrap was operated for tHighBioTrap = 25 s (500 L of air at QHighBioTrap = 1200 L/min), and repeat the sampling process 8 times to collect 8 agar plate samples. For sampling the same amount of air, we were testing the performance of each sampler for sampling the same amount of airborne particles in the same environment. This is based on the assumption that given a very short time period the particle concentration won’t change greatly. For each sampling location, the sampling experiments for both samplers were repeated three times within 1 h. For the HighBioTrap sampler, it takes about 3 s to achieve the final flow rate (1200 L/min) and also 3 s to stop the fan when the sampler is turned off. The sampling time of the HighBioTrap is controlled by an integrated electric control unit and the sampling time was counted from the start of the sampler to the time at which the power is being turned. During the first 3 s, the sampler samples at a gradually increasing flow rate, but counted as 1200 L/min, and after turning off the power the fan continues working for another 3 s but with a gradually reducing flow rate. Therefore, the first and last three seconds' sampling compensate each other for the total sampling air volume for the given sampling time The bioaerosol concentration levels were not obtained for the environment during the sampling. There could be some concentration variations during the sampling, however they have occurred to both samplers and could be also averaged out among the three repeats. Here, the conditions of temperature and relative humidity (RH) were not measured at the time of experiments. However, if the conditions have impacts, they will occur to both samplers since the samplers were operated simultaneously. Nonetheless, both of these two environments are indoor environments in the campus building which typically remain at a room temperature of about 25 °C and a RH value of about 40%.
2.3.2. Culturing detection and high-throughput gene sequencing
After the incubation for 1–2 days at 37 °C, all bacterial and fungal colonies formed on the agar plate, NCFU, HighBioTrap and NCFU, BioStage were manually counted. The number of CFUs obtained from each sampler was used to calculate the airborne concentrations of culturable organisms, C (CFU/m3):
| (4) |
For the BioStage impactor, the culturable concentrations were statistically corrected according to the equation described by (Feller, 1968). Compared to the BioStage which has 400 holes, the HighBioTrap just has one nozzle. The BioStage impactor has more focused air stream, e.g., 400 air streams, while the HighBioTrap has only air stream which is not focused, thus particles in the air stream have low probability of hitting on the same spot on the agar surface. This is evidenced by a typical agar plate image with 400 visible agar spots after sampling with the BioStage impactor, however not by the HighBioTrap.
The relative collection efficiency of HighBioTrap was calculated by the following equation:
| (5) |
In addition to the CFU count method, the culturable bacterial aerosol community structures were determined by High-throughput sequencing. The bacterial colonies were scraped from the agar surfaces using an inoculation loop and transferred to the 1.5 mL tube for the Illumina sequencing (Sangon Biotech, Inc., Shanghai, China). Briefly, microbial DNA was extracted from bacteria colonies samples using the E.Z.N.A. Soil DNA Kit (Omega Bio-tek, Norcross, GA, U.S.) according to the manufacturer's protocols. The V3–V4 region of the bacteria 16 S ribosomal RNA genes were amplified by polymerase chain reaction (94 °C for 3 min, followed by 5 cycles at 94 °C for 30 s, 45 °C for 30 s, and 62 °C for 30 s, and 20 cycles at 94 °C for 20 s, 55 °C for 20 s, and 72 °C for 30 s, and a final extension at 72 °C for 5 min) using primers 341 F′-(CCCTACACGACGCTCTTCCGATCTG(barcode)CCTACGGGNGGCWGCAG)−3′ and 805 R 5′-(GACTGGAGTTCCTTGGCACCCGAGAATTCCAGACTACHVGGGTATCTAATCC)−3′. PCR reactions were performed in a 30 μL mixture containing 15 μL of 2 × Taq master Mix, 1 μL of each primer (10 μM), and 10–20 ng of template DNA. Then the second round of polymerase chain reaction was carried out in the same 30 μl mixture (95 °C for 3 min, followed by 5 cycles at 94 °C for 20 s, 55 °C for 20 s, and 72 °C for 30 s, and a final extension at 72 °C for 5 min). After purification using the Agencourt AMPure XP (Beckman Instruments, Inc., USA) and quantification using Qubit2.0 DNA detection kits (Life Technologies, Inc., USA), a mixture of amplicons was used for sequencing on an Illumina platform by Sangon Biotech, Inc., Shanghai, China. In this work, we did not perform gene sequence for fungal species analysis, but in our previous work it was found that the dominant fungal species include Aspergillus, Alternaria, Cladsporium, and Penicillium in the environment (Li et al., 2013) where we also tested the HighBioTrap in this work.
2.4. Statistical analysis
In this work, during the bacterial aerosol sampling we did not use nystatin to inhibit the fungal growth as we did in a previous work (Wei, Zou, & Yao, 2014). In the future, the fungal inhibitor can be added during an extended sampling in a highly contaminated environment. The differences in the culturable microbial aerosol concentrations obtained by the HighBioTrap sampler and Biostge were analyzed by paired t-test via the statistical component of SigmaPlot 16 (Systat Software, Inc.) and analysis of variance (ANOVA). All the data were tested for the normality using Shapiro-Wilk test. A p value of less than 0.05 indicated a statistically significant difference at a confidence level of 95%.
3. Results and discussion
This study designed and evaluated a high volume (1200 L per minute) portable microbial sampler by impacting biological particles directly onto the agar plate for sampling bioaerosols. The physical performances of the HighBioTrap sampler were characterized by using aerosolized PS particles and further tested by two different aerosolized bacterial particles and indoor air particles. The biological performances of the HighBioTrap sampler were tested when sampling both culturable bacterial and fungal aerosols in outdoor and indoor environments. The cultural counts were compared with those that were obtained using the BioStage sampler. In addition, the bacterial community diversities were investigated by using high throughout sequencing to identify the impaction stress of two samplers and their ability of preserving the microbial aerosol culturability.
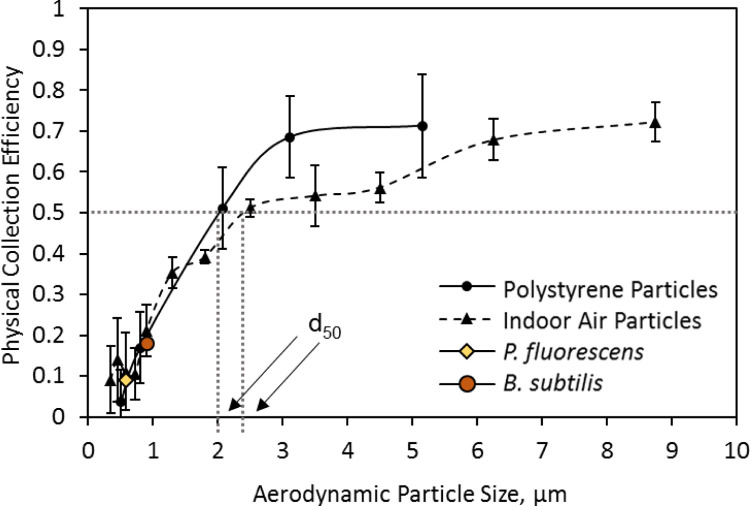
Fig. 3 showed physical collection efficiency determined by laboratory tests when sampling aerosolized PS particles, bacterial aerosols (B. subtilis var. niger and P. fluorescens) as well as indoor air particles. As observed in Fig. 3, the cut-off size when sampling aerosolized PS particles is about 2 µm, which is slightly bigger than the SAS super 180, MAS-100, RCS High Flow, Millipore Air Tester while much smaller than SMA MicroPortable, BioCulture, and Microflow as shown in the Table 1. The HighBioTrap was proposed to collect the most of airborne biological particles, which covers a larger size range from 0.3 µm to several tens of micrometers. Also, the HighBioTrap sampler was designed to have a high flow rate (> 1000 L/min) to substantially reduce the sampling time. The wider nozzle diameter and longer impaction distance (as shown in Table 1) were adopted in the sampler design in achieving portable and high flow rate sampling strategy. This, however, resulted in a relatively large cutoff size of about 2 µm compared to 0.65 µm for the BioStage impactor. But for most of natural bacteria, the aerodynamic size was larger than 3 µm (Shaffer & Lighthart, 1997) due to aggregation or adhesion to large non-biological particles, which implies acceptable collection efficiency of the HighBioTrap for environmental sampling. As the particle size increased, the collection efficiency increased up to 70% at about 3 µm but remained the same at 5 µm. The variances of collection efficiencies were getting larger as the particle size getting lager. When preparing PS suspension used for aerosolization, the particle suspension of each size is diluted to the same concentration of about 108 particles/mL and mixed well, while the lager particles are harder to be aerosolized by the nebulizer. Therefore, the concentration of large particles was lower than the smaller particles as identified by the OPC (not listed here), which resulted in the different variances of collection efficiencies of different particle sizes. The curve seems not to be very sharp as that of the Andersen type impactors discussed by Ranz and Wong (1952), and this also implies poorer cut-off characteristics. The relative flat physical collection efficiency curve was mainly caused by the higher Reynolds number of the air flow in the jet. Previously, the impactors are designed to have a low volume of about several tens of liters per minute and maintain laminar motion as the Reynolds numbers were recommended to be kept from 500 to 3000 (Marple & Willeke, 1976). While the HighBioTrap is designed to have an extreme high flow rate and the air flow is at fierce turbulence with a Reynolds number of 33,953, which would lead to some loss of bigger particles. The Reynolds number effect was previously studied on the basis of potential flow theory (Davies & Aylward, 1951). However, when sampling indoor aerosol particles, the collection efficiencies were observed to be slightly lower as shown in Fig. 3 compared to outdoor air sampling.
Fig. 3.
Physical collection efficiencies of the HighBioTrap sampler when collecting different sized PSL particles (mean aerodynamic size: 0.5, 0.8, 2.07, 3.11, and 5.15 µm), room air particles and also bacteria: P. fluorescence, B. subtilis. The data represent averages from at least three repeats and error bars stand for standard deviation.
The physical performances of sampling B. subtilis and P. fluorescens were also determined by aerosolizing their pure bacteria suspensions as shown in Fig. 3. For these two bacterial aerosols, the collection efficiencies are about 20% and 10%, respectively, which are lower than the performances of the RCS High Flow and MAS-100 but comparable to other portable samplers (such as Microflow, SMA MicroPortable, Millipore Air Tester, SAS Super 180, BioCulture; these samplers have low sampling flow rates of less than 200 L/min) as investigated previously by Yao and Mainelis (2007). Nonetheless, as a result of the 10 times higher flow rate than that of RCS High Flow and 50 times than that of the BioStage, the HighBioTrap can collect much more bacteria when sampling for the same time.
The physical collection efficiency is related to the sampler's ability to obtain a representative aerosol sample, while the biological efficiency reveals its ability to preserve the culturability of the collected microbes. Besides, the interplay of mechanical characteristics and environmental factors determines the overall collection performance of a sampler (Xu & Yao, 2011). The biological collection efficiencies of the HighBioTrap sampler was assessed by field tests in this study by sampling culturable bacterial and fungal aerosols in both indoor and outdoor environments.
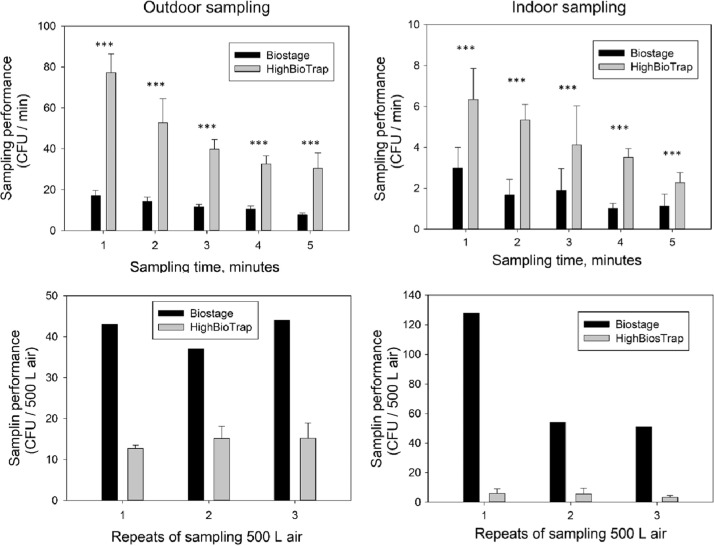
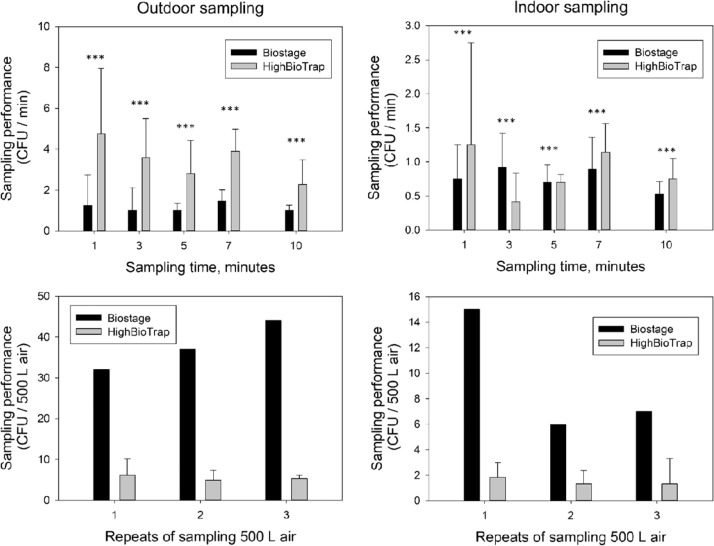
The number of airborne culturable bacteria and fungi determined with the test and reference sampler in indoor and outdoor locations are presented in Fig. 4, Fig. 5, respectively. For the outdoor sampling site, the mean count of airborne culturable bacteria as determined by the HighBioTrap was 77 CFU (77 CFU per minute) by sampling 1 min to 153 CFU (31 CFU per minute) by sampling 5 min, which is significantly higher than that determined by the reference sampler, 17–37 CFU (17 to 8 CFU per minute) from sampling 1–5 min (p < 0.001). Although the cut-off size of the HighBioTrap is larger than that of BioStage as described above, the 42 times higher flow rate of the HighBioTrap, compared to the BioStage, ensures more bacteria collected by the same sampling time and the CFU count ratio is about 300–450%. However, the biological efficiency of the HighBioTrap identified by sampling the same volume air is lower than that of the reference sampler. As shown in Fig. 4, when sampling 500 L air in outdoor sampling site (the 4th floor lab room window site), the HighBioTrap sampler presented about 14 CFU on average, which is significantly lower than 41 CFU identified by the BioStage (p < 0.001). This result also can be obtained from the sampling tests of the same time by converting the CFU count to the bacteria concentration using the Eq. (4) described in “Materials and Methods” section (for the BioStage, the concentration result is factor-corrected, while not for the HighBioTrap). The BioStage impactor consistently reported higher concentrations than the HighBioTrap when sampling airborne bacteria. The differences between the bacterial concentrations measured by the samplers were also analyzed by the paired t-tests. All of their p-values were below 0.001, indicating that there was a statistically significant difference between the concentrations measured by the BioStage and the HighBioTrap under all sampling time settings. The relative collection efficiency identified by the Eq. (5) described in “Materials and Methods” section ranged from 7.2% to 10.7%.
Fig. 4.
Comparison of bacterial aerosol collection performances between the HighBioTrap and the BioStage in different environments with different sampling times and volumes. The data represent averages from no fewer than three repeats and error bars stand for the standard deviation. “***” indicates a statistically significant difference between the measurements by two samplers.
Fig. 5.
Comparison of fungal aerosol collection performance between the HighBioTrap and the BioStage Sampler in different environments with different sampling times and volumes. The data represent averages from three repeats and error bars stand for the standard deviation. “***” indicates a statistically significant difference between the measurements by two samplers.
Based on the measurements of the both samplers, the sampling time was found to have a significant influence on sampling performance (CFU captured per minute) for both methods (ANVOA test, p < 0.001). The shortest sampling time was found to present highest bacterial CFU count about 17 CFU/minute by the BioStage and 77 CFU/minute by the HighBioTrap, while the longest sampling time reported the lowest CFU count, about 8 CFU/minute by the BioStage and 31 CFU/minute by the HighBioTrap. An increase in sampling time would introduce a stronger desiccation effect on the microorganisms already collected. The amount of desiccation can be quantified as the volume of air received over per unit area of collection surface per unit time by following equation as presented previously by Zhen et al. (2009):
Two samplers used the same ager plate but operated at different flow rates. As a result, when sampling for the same time, the HighBioTrap was estimated to have a 42 times higher desiccation factor than that of BioStage impactor. In addition, the longer sampling time will introduce severe particle bounce, because the desiccation made the collection surface drier and harder during the sampling process. The impaction velocity of the BioStage is higher than that of the HighBioTrap, which caused microbial aerosol embedding. In addition, the relative collection efficiency determined by 500 L air sampling test was about 35.0%, significantly improved compared to that obtained using the same time sampling test (ANOVA test, p = 0.036). This is due to the fact that when sampling 500 L, the BioStage took 17.6 min which posed sustained desiccation stress on the agar plate, while the HighBioTrap sampler used only 25 s which thus caused quick desiccation (because of shorter time) and less particle bounce. Nonetheless, the desiccation and particle bounce effects might partly contribute to the lower collection efficiency of the HighBioTrap sampler.
Similar results were obtained when sampling indoor bacterial aerosols. The HighBioTrap reported higher bacteria count but lower bacterial concentration than the BioStage at every sampling time setting, and the sampling time played an important role in collection efficiency for both methods. The relative collection efficiencies were 4.72–8.25% determined by equal time sampling, and 7.14% determined by equal volume sampling test. The results were lower than those when sampling outdoor bacterial aerosols (paired t-test, p = 0.0111 and 0.000839). This result indicated that the HighBioTrap had relative higher efficiency when sampling particles in outdoor environments. On one hand, the microorganisms in outdoor environment appear in aggregation or adhere to large non-biological particles as discussed above, which improved the actual collection efficiencies. On the other hand, the HighBioTrap sampler is designed to have a flow rate of 1200 l per minute, corresponding to impaction velocity of ∼10.2 m/s, which makes it more tolerant to the outdoor air conditions, e.g., wind speed and atmospheric movement speed in vertical direction. While the BioStage with the standard inlet flow of ∼0.9 m/s, which can be easily affected by the environmental air, with a typical wind velocity of 100–300 cm/s. When sampling indoor bacterial aerosols, 4 min sampling yielded the highest CFU count for the HighBioTrap, and 3 min sampling reported the highest CFU count for the BioStage, which indicated that the desiccation and particle bounce significantly affected the collection of indoor bacteria. This might be due to the fact that indoor microorganisms presented smaller size distribution and also high humidity levels leading to less desiccation.
Similar to the bacterial sampling performance, the fungal sampling performances under different conditions were measured by the HighBioTrap and the BioStage impactor, and the results are presented in Fig. 5. The HighBioTrap sampler reported higher fungal count per minute but lower fungal concentration than the BioStage both in indoor and outdoor environments, except sampling 3 and 5 min indoors (paired t-test, p < 0.001). The sampling time had a great influence on collection efficiency for both methods (ANOVA t-test, p < 0.001) for that the particle bounce and desiccation effect increased with the prolonged sampling time. The relative fungal collection efficiencies were 5.4–8.4% (equal-time-test) and 14.8% (equal-volume-test) at outdoor sampling site, and 1.1–5.4% (equal-time-test) and 17.8% (equal-volume- test) at indoor sampling site. The paired t-test showed that the collection efficiencies between outdoor and indoor determined by equal-time-test is statistically significantly different (paired t-test, p = 0.00114) but the difference of collection efficiency between outdoor and indoor determined by equal-volume-test is not statistically significant (ANOVA test, p=0.46). This might be due to the fact that the indoor fungi are less resistant to the desiccation stress (Xu & Yao, 2011) and the HighBioTrap posed higher desiccation effects to the sample than the BioStage in a same-time sampling process.
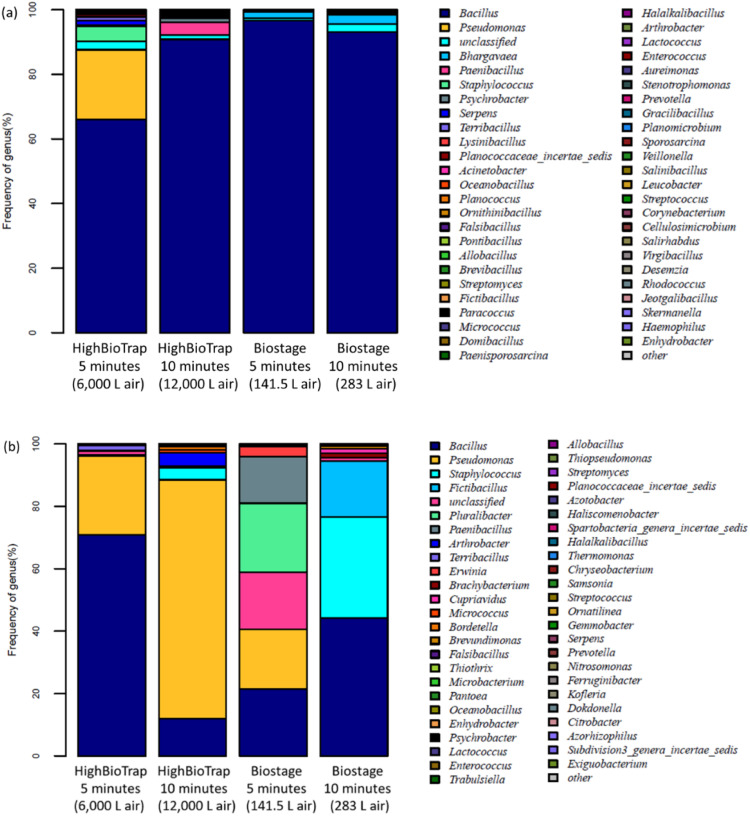
As described above, the collection efficiency of a sampler is determined by its physical performance as well as other factors, such as impaction stress, embedding, and desiccation. These factors together affect the culturable bioaerosol diversity obtained by the sampler. The sampler of higher biological collection efficiency might obtain air sample of lower species diversity, because it may collect most of the dominant species while caused stronger culturability damages (Xu & Yao, 2011). Also, the environmental factors such as temperature, humidity, solar radiation et al., will affect the concentration and species composition of bioaerosols. In terms of our experiments, the comparison tests of two samplers were performed in the same time and environment, thus in any case the influences posed to two samplers were accordingly the same. In our study, the culturable bacterial diversities obtained by the HighBioTrap and reference sampler were determined by the high-throughput gene sequencings. The culturable bacterial species distributions in genus level of samples collected by the HighBioTrap and BioStage at outdoor and indoor environment were presented in Fig. 6.
Fig. 6.
Culturable bacterial species distribution in genus level of samples collected by the HighBioTrap and the BioStage in (a) outdoor and (b) indoor environment. Different colors in the plot represent different species names corresponding to the text on the right side, and the length of the color block represents the relative abundance of the specie. (Indoor environment has different humidity level, bacterial aerosol size distributions and also species structures compared to outdoor environments.) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
The results from Fig. 6 indicate that sampler type and sampling time could result in different culturable bacterial aerosol diversity. Shannon index was widely used for estimating biological species diversity, and higher Shannon index presents higher species diversity. The calculation of the Shannon index can be accessed through the online database (http://www.mothur.org/wiki/Shannon). The Shannon index of the sample collected by the HighBioTrap sampler (5 min for 6 m3 air, and 10 min for 12 m3 air) and the BioStage (5 min for 141.5 L air and 10 min for 283 L air) are 3.35, 3.04, 1.60, and 1.52, respectively. For the HighBioTrap sampler, the culturable bacterial aerosol diversity obtained was higher than those obtained by the BioStage. Although, the higher desiccation effect limited the collection efficiency of the HighBioTrap for an extended sampling, high flow rate would lead to the collection of more microorganisms at the same time, which correspondingly increased the probability of the collection of less frequently occurring species. While for the BioStage, due to its higher sampling stress, e.g., higher impaction velocity (24 m/s) and higher embedding, the culturable bacterial diversity is relative lower, and some species lost the culturability or viability after the sampling process. As shown in the Fig. 6, the predominant species of the sample collected by the HighBioTrap for 5 min are Bacillus, Pseudomonas, Staphylococcus, while for the BioStage, the predominant species was only Bacillus (the top 15 species in genus level were listed in Table S1), the species which is more resistant to the environmental stress or impaction stress. Similar to the results obtained by the culture method, the sampling time is an important factor influencing the collection of bacterial aerosols for both methods. Despite that increasing collection time would result in more microorganisms collected by the samplers, an increase in sampling time would introduce a stronger desiccation and particle bounce effect on the microorganisms, which unfortunately reduced the diversity of the sample due to damages to those sensitive species.
When sampling at the indoor site, the bacterial species obtained are different by two samplers. The Shannon index of the sample collected by the HighBioTrap (5 min for 6 m3 air, and 10 for 12 m3 air) and the BioStage (5 min for 141.5 L air and 10 min for 283 L air) are 2.24, 1.24, 2.59, and 2.68, respectively. This indicates when sampling indoor bacterial aerosols, the BioStage can collect more microorganisms and obtain higher bacterial diversity, which might be due to the increasing collection efficiency of the BioStage in the indoor environments as discussed above. This is also due to the dominance of smaller bacteria in indoor environments (Reponen, Willeke, Grinshpun, & Nevalainen, 2011). As seen from Fig. 6(b), when increasing the sampling time, Pseudomonas was shown to be collected more, which indicates the Pseudomonas species was dominant in the environment (the top 15 species in genus level were listed in Table S2). While for the BioStage, increasing sampling time caused the loss of Pseudomonas, which indicated the high sampling stress resulting from the long time sampling, e.g., the impaction injuries, of the BioStage. Although the BioStage impactor has higher physical collection efficiency, it causes damages to the cells being collected due to its high impaction velocity (24 m/s). The biological collection efficiency is a function of the samplers' physical collection efficiency, sampling stress, collection medium surface characteristics and also the target species. The sampler's physical collection efficiency is largely determined by the cutoff size, while the sampling stress includes impaction and desiccation. The collection medium surface determines the particle bounce and the microbial particle embedding, e.g., the agar plate. The target species is also an important factor to consider when selecting a sampler as sensitive microbes are more vulnerable to the sampling stress as discussed. The developed HighBioTrap has a very high sampling flow rate (1200 L/min) compared to the BioStage impactor (28.3 L/min), thus given the same amount of time the HighBioTrap can recover more less-frequently microbial species from the air than the BioStage impactor, thus resulting in higher microbial aerosol diversity. On the other hand, the HighBioTrap also has lower impaction velocity (10.2 m/s) compared to that of the BioStage impactor (it is 24 m/s). Overall, the impaction stress difference between the two samplers might play a lesser role in the culturable diversity difference recovered by two different samplers given the same sampling time compared to the difference in their air volumes collected. Nonetheless, increased sampling time led to more desiccation effects for the HighBioTrap than the BioStage impactor. The HighBioTrap sampler was designed to be mostly used for field sampling in natural environments where microbial aerosol particles often appear to be agglomerates or attach to large non-biological particles. These possibilities certainly allow the HighBioTrap to collect those small biological aerosol particles in nature. In terms of species detection for qualitative analysis, the HighBioTrap has obvious advantages compared to the BioStage impactor.
4. Conclusions
As part of our continued effort to developing efficient bioaerosol sampling protocols, this study is focused on the development of high flow rate portable sampler. The experimental tests showed that the HighBioTrap has a cutoff size of 2 µm, and had reasonable biological and physical collection efficiencies compared to the reference sampler, the BioStage impactor. Similar to other studies, the results from this study indicated that sampling time, sampling environment, desiccation and impaction stress are important factors influencing the overall performance of a microbial aerosol sampler. The HighBioTrap developed in this work was shown to report the higher outdoor bacterial aerosol diversity compared to the reference sampler given the same sampling time, which is largely resulting from higher flow rate. High flow rate resulted in collecting less frequently occurring species in short sampling time, and low impaction velocity (~10.2 m/s) preserves more on microbial culturability. In addition to developing the high volume sampler, our data also showed that strong desiccation effects might occur between 3 and 5 min after the sampling. Based on our data and existing literature, we believe that an impaction velocity of around 10 m/s might be a close-to-optimal collection velocity for collecting most environmental bacterial aerosols while maximally preserving their culturability. The developed HighBioTrap sampler could be extremely useful when low level of pathogens is present and also detection response is of great concern. The sampler could be also very useful when analyzing microbial aerosol diversity for a particular environment. In couple with a biosensor, the HighBioTrap is capable of providing rapid detection of target microbial agents in the environment.
Acknowledgements
This study was supported by the NSFC Distinguished Young Scholars Award (21725701), and the National Natural Science Foundation of China (Grants 91543126, 21611130103, 21477003, 41121004), the Ministry of Science and Technology (grants 2016YFC0207102, 2015CB553401, 2015DFG92040). The HighBioTrap sampler has been licensed to Beijing dBlueTech Co., Ltd, Beijing China. Here, all the authors declare no conflicting interests exist.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jaerosci.2017.11.012.
Appendix A. Supplementary material
Supplementary material
References
- An H., Mainelis G., Yao M. Evaluation of a high-volume portable bioaerosol sampler in laboratory and field environments. Indoor Air. 2004;14(6):385–393. doi: 10.1111/j.1600-0668.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- Bautista, E., Chotpitayasunondh, T., Gao, Z., Harper, S. A., Shaw, M., Uyeki, T. M., … Hui, D. (2010). Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. [DOI] [PubMed]
- Blachere F.M., Lindsley W.G., Pearce T.A., Anderson S.E., Fisher M., Khakoo R., Thewlis R.E. Measurement of airborne influenza virus in a hospital emergency department. Clinical Infectious Diseases. 2009;48(4):438. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- Brachman P.S., Ehrlich R., Eichenwald H.F., Gabelli V.J., Kethley T.W., Madin S.H., Silver I.H. Standard sampler for assay of airborne microorganisms. Science. 1964;144(3624):1295. [Google Scholar]
- Cole E.C., Cook C.E. Characterization of infectious aerosols in health care facilities: An aid to effective engineering controls and preventive strategies. American Journal of Infection Control. 1998;26(4):453–464. doi: 10.1016/S0196-6553(98)70046-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council N.R. National Academies Press; 2005. Sensor systems for biological agent attacks: Protecting buildings and military bases. [Google Scholar]
- Davies C.N., Aylward M. The trajectories of heavy, solid particles in a two-dimensional jet of ideal fluid impinging normally upon a plate. Proceedings of the Physical Society. 1951;64(10):889. [Google Scholar]
- Douwes J., Thorne P., Pearce N., Heederik D. Bioaerosol health effects and exposure assessment: Progress and prospects. Annals of Occupational Hygiene. 2003;47(3):187–200. doi: 10.1093/annhyg/meg032. [DOI] [PubMed] [Google Scholar]
- Feller W. I(3) John Wiley & Sons; New York: 1968. An introduction to probability theory and its applications. [Google Scholar]
- Haglund J., Mcfarland A. A circumferential slot virtual impactor. Aerosol Science & Technology. 2004;38(38):664–674. [Google Scholar]
- He Q., Yao M. Integration of high volume portable aerosol-to-hydrosol sampling and qPCR in monitoring bioaerosols. Journal of Environmental Monitoring. 2011;13(3):706–712. doi: 10.1039/c0em00559b. [DOI] [PubMed] [Google Scholar]
- Hill S.C., Pinnick R.G., Niles S., Pan Y.L., Holler S., Chang R.K., Feather G. Real‐time measurement of fluorescence spectra from single airborne biological particles. Field Analytical Chemistry & Technology. 1999;3(4–5):221–239. [Google Scholar]
- Jernigan D.B., Raghunathan P.L., Bell B.P., Brechner R., Bresnitz E.A., Butler J.C., Fischer M. Investigation of bioterrorism-related anthrax, United States, 2001: Epidemiologic findings. Emerging Infectious Diseases. 2002;8(10):1019–1028. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Lim W. A novel coronavirus associated with severe acute respiratory syndrome. New England Journal of Medicine. 2003;348(20):1953. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Li J., Li M., Shen F., Zou Z., Yao M., Wu C.Y. Characterization of biological aerosol exposure risks from automobile air conditioning system. Environmental Science & Technology. 2013;47(18):10660–10666. doi: 10.1021/es402848d. [DOI] [PubMed] [Google Scholar]
- Li Q., Zhou L., Zhou M., Chen Z., Li F., Wu H., Wang D. Epidemiology of human infections with avian influenza A(H7N9) virus in China. New England Journal of Medicine. 2014;370(6):520. doi: 10.1056/NEJMoa1304617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marple V.A., Willeke K. Impactor design. Atmospheric Environment. 1976;10(10):891–896. [Google Scholar]
- Mathieu L., Robine E., Deloge-Abarkan M., Ritoux S., Pauly D., Hartemann P., Zmirou-Navier D. Legionella bacteria in aerosols: Sampling and analytical approaches used during the legionnaires disease outbreak in Pas-de-Calais. Journal of Infectious Diseases. 2006;193(9):1333–1335. doi: 10.1086/503115. [DOI] [PubMed] [Google Scholar]
- Neidhardt, F. C., Ingraham, J. L., & Schaechter, M. (1990). Physiology of the Bacterial Cell.
- Ranz W.E., Wong J.B. Jet impactors for determining the particle-size distributions of aerosols. AMA Archives of Industrial Hygiene & Occupational Medicine. 1952;5(5):464. [PubMed] [Google Scholar]
- Reponen T., Willeke K., Grinshpun S., Nevalainen A. Biological Particle Sampling. 2011 [Google Scholar]
- Shaffer B.T., Lighthart B. Survey of culturable airborne bacteria at four diverse locations in oregon: Urban, rural, forest, and coastal. Microbial Ecology. 1997;34(3):167. doi: 10.1007/s002489900046. [DOI] [PubMed] [Google Scholar]
- Therkorn J.H., Mainelis G. Effect of agar plate volume on accuracy of culturable bioaerosol impactors. Aerosol Science & Technology. 2013;47(12):1353–1362. [Google Scholar]
- Wei K., Zou Z., Yao M. Charge levels and Gram (±) fractions of environmental bacterial aerosols. Journal of Aerosol Science. 2014;74(4):52–62. [Google Scholar]
- Whyte W., Green G., Albisu A. Collection efficiency and design of microbial air samplers. Journal of Aerosol Science. 2007;38(1):97–110. [Google Scholar]
- Willeke K., Lin X., Grinshpun S.A. Improved aerosol collection by combined impaction and centrifugal motion. Aerosol Science and Technology. 1998;28(5):439–456. [Google Scholar]
- Xu Z., Xu H., Yao M. Applicability of a modified MCE filter method with Button Inhalable Sampler for monitoring personal bioaerosol inhalation exposure. Environmental Science and Pollution Research. 2013;20(5):2963–2972. doi: 10.1007/s11356-012-1204-6. [DOI] [PubMed] [Google Scholar]
- Xu Z., Yao M. Analysis of culturable bacterial and fungal aerosol diversity obtained using different samplers and culturing methods. Aerosol Science and Technology. 2011;45(9):1143–1153. [Google Scholar]
- Yao M., Mainelis G. Effect of physical and biological parameters on enumeration of bioaerosols by portable microbial impactors. Journal of Aerosol Science. 2006;37(11):1467–1483. [Google Scholar]
- Yao M., Mainelis G. Investigation of cut-off sizes and collection efficiencies of portable microbial samplers. Aerosol Science and Technology. 2006;40(8):595–606. [Google Scholar]
- Yao M., Mainelis G. Utilization of natural electrical charges on airborne microorganisms for their collection by electrostatic means. Journal of Aerosol Science. 2006;37(4):513–527. [Google Scholar]
- Yao M., Mainelis G. Use of portable microbial samplers for estimating inhalation exposure to viable biological agents. Journal of Exposure Science and Environmental Epidemiology. 2007;17(1):31–38. doi: 10.1038/sj.jes.7500517. [DOI] [PubMed] [Google Scholar]
- Zhen S., Li K., Yin L., Yao M., Zhang H., Chen L., Chen X. A comparison of the efficiencies of a portable BioStage impactor and a Reuter centrifugal sampler (RCS) High Flow for measuring airborne bacteria and fungi concentrations. Journal of Aerosol Science. 2009;40(6):503–513. [Google Scholar]
- Zumla A., Hui D.S., Perlman S. Middle east respiratory syndrome. Lancet. 2015;40(7):1015–1017. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material