Highlights
-
•
Respiratory viral coinfections are common in children.
-
•
Overall pediatric respiratory viral coinfections have no impact on severity.
-
•
Prognostic role of specific viral interactions remains unclear.
Abbreviations: ARI, acute respiratory infections; EPHPP, Effective Public Health Practice Project; PICU, pediatric intensive care unit; RSV, respiratory syncytial virus; OR, odds ratio; CI, confidence interval; MD, mean difference; SD, standard deviation; IQ, interquartile range
Keywords: Coinfection, Respiratory tract infections, Child, Viruses, Prognosis, Respiratory insufficiency
Abstract
Background
With advent of molecular diagnostic technologies, studies have reported detection of two or more respiratory viruses in about 30% of children with respiratory infections. However, prognostic role of coinfection remains unclear.
Objective
Evaluate relation between respiratory viral confection and illness severity in children.
Study design
MEDLINE (through PUBMED), EMBASE, EBSCO, LILACS databases were searched up to March 2015 by two independent reviewers. Studies assessing severity of viral coinfection in patients aged less than 18 years were included. Standardized forms were used for data extraction of population, study design, clinical syndromes, virus combinations compared and severity outcomes. Risk of bias and quality of evidence were assessed through EPHPP and GRADE. Subgroup analysis was performed according to age and viral combinations.
Results
Of 5218 records screened, 43 were included in analysis. Viral coinfection did not influence risks of all outcomes assessed: length of stay (mean difference in days in coinfection, −0.10 [95% confidence interval: −0.51 to 0.31]), length of supplemental oxygen (−0.42 [−1.05 to 0.20]), need of hospitalization (odds ratio of coinfection, 0.96 [95% confidence interval: 0.61–1.51]), supplemental oxygen (0.94 [0.66 to 1.34]), need of intensive care (0.99 [0.64 to 1.54]), mechanical ventilation (0.81 [0.33 to 2.01]) and death (2.22 [0.83 to 5.95]). Sub-analyses according to age and viral combinations have not shown influence of these factors in outcomes.
Conclusions
Respiratory viral coinfection did not increase severity in all outcomes assessed. Further studies are necessary to confirm this finding, especially regarding role of specific viral interactions.
1. Background
Acute respiratory infections (ARI) are a major cause of hospital admission in young children and viruses are the most frequent etiological agents involved in such cases [1], [2]. Viral detection techniques have greatly improved in recent years, as the use of molecular diagnostic tests has importantly increased the ability to identify respiratory viruses in children with ARI [3]. Until recently, infection by two or more viral agents concomitantly, in infants and toddlers, was considered an unusual event. However, as these new diagnostic techniques became more readily available in clinical settings, studies have been showing a much higher prevalence of respiratory coinfection [4]. In most of reports, detection of two or more respiratory virus simultaneously ranges from 10 to 30% in pediatric patients [5], [6], [7]. In reports that analyzed respiratory infections by nucleic acid amplification techniques assessing a large number of viruses, such prevalence is higher than 40% [8], [9], [10].
The relationship between detection of multiple respiratory viral infections and severity of disease in children has not been well established. Several studies have reported longer length of stay in hospital, an increased risk of hospitalizations, of admission to pediatric intensive care unit (PICU), of need for mechanical ventilation and even higher mortality, when two or more respiratory viruses were detected [11], [12], [13], [14], [15], [16], [17].
On the other hand, other reports have not found an association between viral coinfection and such outcomes, even in centers with high prevalence of respiratory viral coinfection [4], [8], [18], [19]. Furthermore, an Italian study found that coinfection of respiratory syncytial virus (RSV) and metapneumovirus was a protection factor for length of hospital stay and hypoxia, when compared to RSV infection alone [20]. A French study also found shorter length of hospitalization in infants with concomitant RSV and rhinovirus coinfection comparing to single RSV infection [21].
2. Objectives
Due to the lack of consensus regarding whether mixed viral infection in children with ARI contributes to the severity of the disease, the aim of this study is to evaluate the prognostic role of respiratory viral coinfection in children.
3. Study design
The protocol of this systematic review was registered a priori in International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42014007250 (http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014007250).
3.1. Eligibility criteria
Observational studies involving patients aged less than 18 years or with a subgroup analysis of patients within this aged group with ARI diagnosed by biology molecular assays in whose comparison of severity between those with one and two or more virus detected was possible. The following severity outcomes were selected for inclusion: need and length of hospitalization, use of supplemental oxygen, admission to PICU, mechanical ventilation and death. Only studies including the following viruses through biology molecular assays: RSV, influenza, adenovirus, parainfluenza, rhinovirus and metapneumovirus were included in main meta-analysis. Studies reporting only patients with specific comorbidities were excluded, as well as studies, which included only outpatients.
3.2. Information sources
Literature search was done through subject headings and words throughout the text related to respiratory viral coinfection and severity outcomes in the following databases: MEDLINE (through PUBMED), EMBASE, EBSCO, LILACS up to 24 March 2015. Search was performed from reference lists from selected articles, printed journals, abstracts and citations of selected articles from ISI Web of Science. Attempt to contact study authors for additional information was done whenever necessary. There were no language restrictions. When reviewers considered potential for inclusion in screened studies published in languages other than English, Spanish or Portuguese, a specific technical translation was asked.
MEDLINE search strategy: (coinfection*) OR “co-infection” OR co-detection* OR codetection*)) OR coinfection[MeSH Terms]) OR “dual infection*”) OR ‘mixed infections’ AND (((((sever* OR death*) OR “mechanical ventilation”) OR “respiratory insufficiency”) OR “oxygen therapy”) OR hospitalization*)) OR artificial respiration[MeSH Terms]) OR oxygen inhalation therapy[MeSH Terms]) OR respiratory insufficiency[MeSH Terms]) OR death[MeSH Terms]) AND ((((neonate*) OR newborn*) OR infant*) OR child*)) AND virus*))).
3.3. Study selection
Two independent reviewers assessed titles and abstracts. Studies which potentially met inclusion criteria were selected for full text reading and eligibility evaluation. A third reviewer assessed eligibility when discrepancies occurred.
3.4. Data collection process and data items
Data were extracted in duplicate from each eligible study to an Excel table according to a standardized template, specific for this review. It comprised the following items: first author, title, year of publication, country, design, patients age, number of viruses search using biology molecular assays, place of hospitalization (ward/PICU), level of quality, total number of included patients, number of positive samples, specific viral combinations compared, number of samples with coinfection, outcome(s), odds ratio (OR) or relative risk, statistics tests, confounding factors, and significant factors.
3.5. Risk of bias in individual studies and quality of evidence
Two authors independently assessed risk of bias and quality of evidence of included studies. Risk of bias was assessed using Quality Assessment Tool for Quantitative Studies of Effective Public Health Practice Project (EPHPP). According to this tool, studies are classified into three categories of quality: Strong, Moderate and Weak. Main aspects considered for classification are selection bias, study design, confounders, blinding, data collection methods and withdrawals and drop-outs. Overall quality of evidence for all outcomes assessed was done according to GRADE guidelines (Grading of Recommendations Assessment, Development and Evaluation) [22]. As interventional studies to evaluate severity of viral coinfection are not possible, observational studies were considered the highest level of evidence for all outcomes. The overall levels were downgraded according detection of risk of bias, inconsistency, indirectness and imprecision. Inconsistency was considered serious when substantial heterogeneity was detected (I 2 greater than 50% or P < 0.01). Serious indirectness was detected when most of studies compared a specific viral combination rather than all coinfections versus all single infections. Imprecision was considered when optimal information size was not met and/or a wide 95% confidence interval (CI) was detected. Disagreements between the review authors over the quality of evidence and risk of bias were resolved by a third reviewer.
3.6. Summary measures and synthesis of results
Statistical analysis was performed using Review Manager 5.3. The contribution of coinfection to severity was assessed using risk ratio and 95% (CI) for categorical variables and mean difference (MD) and 95% CI for continuous variables. For studies reporting multiple comparisons of virus combinations, all patients and events were joined if such procedure did not carry risk of including the same patients twice. For situations in which such risk was detected and for continuous outcomes, only combination with the largest number of patients was included in meta-analysis. Statistical heterogeneity was measured using I 2 test. Although serious heterogeneity was regarded as a sign of low quality of evidence, additional sub-groups analysis was considered necessary a priori regardless of heterogeneity. Random effect model were used for pooled OR and MD calculations. If basic required data on results were not presented (e.g.: measures of central tendency) and attempt to contact authors were not successful, the study was excluded from analysis. For articles that presented continuous variable as median, the standard deviation (SD) was calculated from interquartile range (IQ) dividing it by 1.35 if the sample size was considered large and the distribution of the outcome was similar to the normal distribution. In conditions above were not met, study was excluded from meta-analysis [23]. After preliminary analysis considering all included articles, main analysis was performed with studies including minimal set of viruses mentioned above. Analysis was stratified by age in order to assess possible differences in severity in studies including only infants (0–23 months), preschool children (0–59 months) and all children (0–18 years).
3.7. Risk of bias across studies
Publication bias was assessed by visual inspection of funnel plot graphic.
3.8. Additional analysis
A sensitivity analysis excluding weak quality studies according to EPHPP tool was also performed. Specific combinations of viruses were analyzed if there was sufficient data to permit performing statistical analysis. A post hoc analysis was performed excluding studies based mainly on bocavirus and adenovirus combinations, as single or coinfection, due to possibility of persistent asymptomatic shedding of these viruses.
4. Results
4.1. Study selection
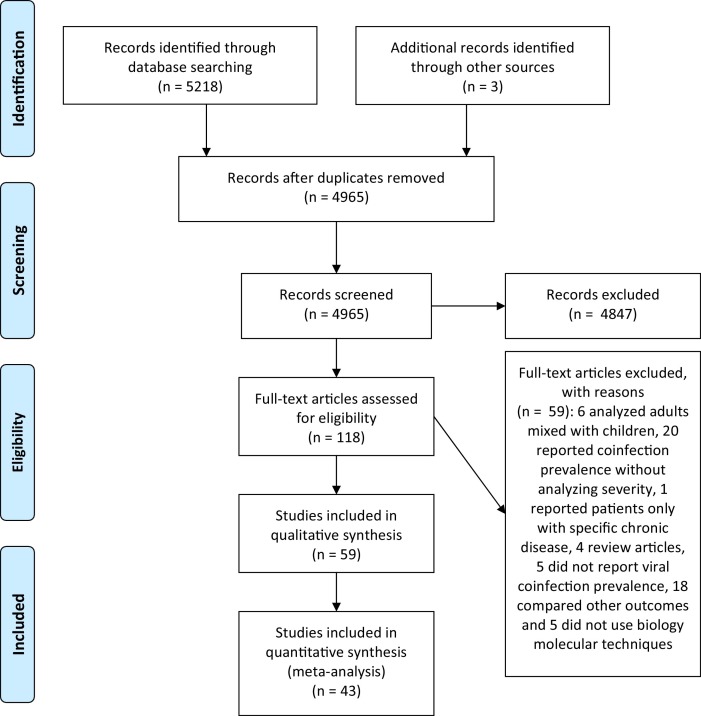
As shown in Fig. 1 , 5218 articles were identified and the title and abstracts were screened and other 3 articles were identified through grey literature. After removing duplicates, 4965 articles were assessed for eligibility. Of those, 118 studies were included for full-text reading (three translated from Chinese), 59 were included for preliminary analysis and 43 of those selected for main analysis according to criteria described above. Among these, 41 were published in English and two in Spanish. An attempt of contact was done with 33 authors, of which 16 provided additional information.
Fig. 1.
Flow diagram of study selection process.
4.2. Study characteristics
Table 1 summarizes the characteristics of the studies included in the main analysis, such as age, prevalence of coinfection, setting of enrollment, viruses tested through molecular biology techniques, specific combinations of viruses compared and outcomes assessed. Forty-three studies with 17234 patients were included [6], [8], [9], [10], [12], [13], [14], [15], [16], [17], [18], [19], [21], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53]. Final analyses included the following outcomes: length of stay (24 studies, 3548 patients), need of hospitalization (11 studies, 9637 patients), need of supplemental oxygen (12 studies, 2285 patients) and length of supplemental oxygen (5 studies, 674 patients), need of PICU (11 studies, 2630 patients) and mechanical ventilation (3 studies, 492 patients, after sensitivity analysis) and death (7 studies, 2296 patients); none study analyzing length of mechanical ventilation and length of PICU stay was included because none searched all viruses required.
Table 1.
Characteristics of studies included in main analysis.
| First author | Year | Country | Number of patients compared | Prevalence of coinfection (%) | Age | Design | Clinical Syndrome/Comorbitidies (%) | Setting | Viruses tested with biology molecular assays | Comparison | Outcome included |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahn | 2013 | South Korea | 187 | 57.2 | <18 y | Prospective | ARI/No | ER | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV | HBoV coinfection vs HBoV single infection | LOS |
| Aramburo | 2011 | USA | 80 | 15 | <9 y | Prospective | LRTI/76.3 | PICU | RSV, Flu, PIV, AdV, HRV, hMPV, EV | Coinfection vs single infection | Death |
| Arruda | 2014 | Brazil | 34 | 50 | <2 y | Prospective | LRTI/100 (Preterm) | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV | RSV coinfection vs RSV single infection | LOS |
| Asner | 2015 | Canada | 472 | 17.1 | <18 y | Retrospective | ARI/33 | ER, PW, PICU | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | Coinfection vs single infection | NH, NPICU and Death |
| Brand | 2011 | Netherlands | 104 | 41.3 | <2 y | Prospective | Bronchiolitis/19 | ER, PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | RSV coinfection vs RSV single infection | NMV |
| Calvo | 2008 | Spain | 172 | 86 | <2 y | Prospective | LRTI/15 | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, EV | Coinfection vs RSV single infection | LOS |
| Cilla | 2008 | Spain | 226 | 26.9 | <3 y | Prospective | Pneumonia/4 | ER | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV | Coinfection vs single infection | NH |
| Costa | 2013 | Brazil | 337 | 31.4 | <5 y | Retrospective | ARI/16.3 | ER | RSV, Flu, PIV, AdV, HRV, hMPV | RSV + HRV vs RSV HRV coinfection vs HRV |
LOS |
| da Silva | 2013 | Brazil | 215 | 10.2 | <3 y | Prospective | LRTI/24 | ER | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV | RSV + HRV vs. HRV and RSV single infection | LOS and LO2 |
| Do | 2011 | Vietnam | 222 | 27.2 | <15 y | Prospective | LRTI/Not mentioned | PW, PICU | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | Coinfection vs single infection | LOS, NO2 and NPICU |
| Franz | 2010 | Germany | 303 | 32 | <16 y | Prospective | LRTI/48 | ER | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | RSV coinfection vs RSV, HRV coinfection vs HRV, HBoV coinfection vs HBoV, AdV coinfection vs AdV | LOS, NO2 |
| Frobert | 2011 | France | 37 | 37.8 | <2 y | Retrospective | Bhonchiolitis/pneumonia/58 | PICU | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | RSV coinfection vs RSV | NMV |
| Gagliardi | 2013 | Brazil | 70 | 25.7 | <5 y | Prospective | ARI/Not mentioned | ER | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | RSV coinfection vs RSV | LOS, NMV |
| García-García | 2007 | Spain | 52 | 75 | <14 y | Prospective | ARI/21 | ER, PW | RSV, Flu, PIV, AdV, HRV, hMPV, HBoV | HBoV coinfection vs HBoV single infection | LOS, NO2 |
| Gerna | 2008 | Italy | 47 | 31.9 | <3 y | Prospective | LRTI/Not mentioned | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | RSV coinfection vs RSV | LOS |
| Goka | 2012 | United Kingdom | 2157 | 6.7 | <5 y | Retrospective | ARI/Not mentioned | OUT, ER, PW | RSV, Flu, PIV, AdV, HRV, hMPV | Flu A (H1N1) and A (H3N2) single infections vs coinfection between both and Flu B, RSV, AdV, HRV (vs (H1N1 only), hMPV (vs H3N2 only) | NH |
| Goka | 2014 | United Kingdom | 6065 | 16.7 | <5 y | Retrospective | ARI/Not mentioned | OUT, ER, PW | RSV, Flu, PIV, AdV, HRV, hMPV | Coinfection vs single infection | NH |
| Guerrier | 2013 | Cambodja | 551 | 10.8 | <5 y | Prospective | LRTI/2 | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | Coinfection vs single infection | LOS and Death |
| Huguenin | 2012 | France | 126 | 67.4 | <1 y | Prospective | Bronchiolitis/Not mentioned | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | Coinfection vs single infection | LOS, LO2, NO2 and NPICU |
| Kouni | 2013 | Greece | 397 | 42.5 | <14 y | Prospective | ARI/15.2 | ER | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV | Coinfection vs single infection | NH |
| Kristoffersen | 2011 | Norway | 130 | 10.7 | <16 y | Prospective | ARI/33 | ER | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | RSV + HCoV vs RSV, HCoV OC43 and HCoV NL63 | LOS |
| Lees | 2014 | United Kingdom | 448 | 10.4 | <16 y | Retrospective | ARI/65.5 | ER,PW, PICU | RSV, Flu, PIV, AdV, HRV, hMPV | Coinfection vs single infection | NO2 and NPICU |
| Marguet | 2009 | France | 141 | 21.2 | <1 y | Prospective | Bronchiolitis/No | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, EV | RSV + HRV vs RSV and HRV | LOS, LO2 and NO2 |
| Martinez | 2012 | Chile | 110 | 37.2 | <18 y | Prospective | ARI/No | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | Coinfection vs single infection | LOS and LO2 |
| Martinez-Roig | 2014 | Spain | 385 | 61.8 | <15 y | Prospective | ARI/No | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | Coinfection vs single infection | NO2, NPICU and NMV |
| Midulla | 2009 | Italy | 98 | 15.3 | <1 y | Prospective | Bronchiolitis/ No |
PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV | RSV + HBoV vs RSV, HRV and HBoV | LOS |
| Miyaji | 2013 | Japan | 151 | 12.5 | <18 y | Prospective | ARI/Not mentioned | ER | RSV, Flu, PIV, AdV, HRV, hMPV, HBoV, EV | Coinfection vs single infection | LOS, LO2, NH and NO2 |
| Nascimento | 2010 | Brazil | 72 | 47.2 | <2 y | Prospective | Bronchiolitis/19% | ER | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | Coinfection vs single infection, RSV coinfection vs RSV | NPICU and NH |
| Papenburg | 2012 | Canada | 918 | 17.1 | <3 y | Prospective | ARI/14 | Out, ER, PW, PICU | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, EV | Coinfection vs single infection, RSV coinfection vs RSV, hMPV coinfection vs Hmpv | NH |
| Rajatonirina | 2013 | Madagascar | 273 | 70.3 | <5 y | Prospective | LRTI/17.8 | ER | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV | Coinfection vs single infection | Death |
| Rhedin | 2012 | Sweden | 83 | 14.4 | <17 y | Retrospective | ARI/48.5 | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | Flu A (H1N1) coinfections vs Flu A (H1N1) | LOS, NPICU and Death |
| Richard | 2008 | France | 180 | 24.4 | <1 y | Retrospective | Bronchiolitis/43.9 | PW, PICU | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, EV | Coinfection vs single infection | NPICU |
| Spaeder | 2015 | USA | 511 | 12.7 | <18 y | Retrospective | ARI/26.2 | PICU | RSV, Flu, PIV, AdV, HRV, hMPV, EV | HRV coinfection vs HRV | Death |
| Suryadevara | 2011 | USA | 187 | 27.8 | <2 y | Prospective | ARI/17.9 | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, EV | Coinfection vs single infection | LOS, NO2 and NPICU |
| Tran | 2013 | Vietnam | 257 | 22.9 | <14 y | Prospective | ARI/No | ER | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV | RSV coinfection vs RSV | LOS |
| Tran | 2014 | Vietnam | 78 | 66.6 | <14 y | Prospective | ARI/No | ER | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV | HBoV coinfection vs HBoV | LOS |
| Venter | 2011 | South Africa | 510 | 54.7 | <5 y | Prospective | ARI/61.1 | ER, PW, PICU | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV | Coinfection vs single infections of RSV, PIV-3, Flu A, AdV, HRV, hMPV, HBoV, HCoV | NH, NPICU and Death |
| Xiang | 2010 | China | 69 | 46.3 | <13 y | Prospective | Pneumonia/32.3 | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | RSV + HRV A vs HRV A, RSV + HRV B vs HRV B | LOS and NO2 |
| Xiao | 2010 | China | 45 | 55.5 | <14 y | Prospective | ARI/2.2 | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV | hMPV coinfection vs hMPV | NH |
| Xiao | 2013 | China | 76 | 57.9 | <13 y | Prospective | LRTI/18.4 | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV | hMPV vs hMPV coinfection, hMPV + HBoV and hMPV + RSV | LOS |
| Zeng | 2014 | China | 202 | 52.9 | <13 y | Prospective | LRTI/3.9 | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV | HRV coinfection vs HRV | NO2 |
| Zhang | 2010 | China | 327 | 40 | <14 y | Prospective | ARI/7.9 | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV | RSV coinfection vs RSV | LOS and NH |
| Zhang | 2012 | China | 129 | 37.9 | <3 y | Prospective | ARI/No | PW | RSV, Flu, PIV, AdV, HRV, hMPV, HCoV, HBoV, EV | Coinfection vs single infection | NPICU |
Abbreviations: AdV = Adenovirus, ARI = Acute respiratory infections, EV = Enterovirus, ER = Emergency room, Flu = Influenza, HBoV = Human Bocavirus, HCoV = Human Coronavirus, hMPV = Human Metapneumovirus, HRV = Human Rhinovirus, LOS = Length of stay, LO2 = Length of supplemental oxygen, LRTI = Lower respiratory tract infection, NH = Need of Hospitalization, NMV = Need of mechanical ventilation, NO2 = Need of supplemental oxygen, NPICU = Need of pediatric intensive care unit, OUT = outpatient, PIV = Parainfluenza virus, PW = Pediatric ward, RSV = Respiratory Syncytial Virus.
4.3. Risk of bias within studies and quality of evidence
As shown in Supplemental Table 1, of 43 studies included, quality according to EPHPP tool was classified as strong in 13, moderate in 22 and weak in 8. The overall quality of evidence found was very low for risk of death, low for need of supplemental oxygen and need of mechanical ventilation and moderate for the remaining outcomes, as described in Table 2 .
Table 2.
Summary of findings and quality of evidence for severity of viral coinfections versus single infections in children.
| Outcomes | No of Participants (studies) | Quality of the evidence (GRADE) |
Relative effect (95% CI) |
Anticipated absolute effects |
|
|---|---|---|---|---|---|
| Risk with Single infection | Risk difference with viral coinfection (95% CI) | ||||
| Length of stay | 3548 (24 studies) | ⊕⊕⊕⊝ MODERATEa,b,c,d,e due to indirectness |
The mean length of stay in viral coinfection group was 0.1 lower (0.51 lower to 0.31 higher) | ||
| Death | 2296 (7 studies) | ⊕⊝⊝⊝ VERY LOWe,f,g,h,i due to risk of bias, inconsistency, imprecision |
OR 2.22 (0.83–5.95) |
26 per 1000 | 30 more per 1000 (from 4 fewer to 111 more) |
| Need of hospitalization | 9637 (11 studies) | ⊕⊕⊕⊝ MODERATEe,g,j,k,ldue to inconsistency |
OR 0.96 (0.61–1.51) |
749 per 1000 | 8 fewer per 1000 (from 104 fewer to 69 more) |
| Need of mechanical ventilation | 492 (3 studies) | ⊕⊕⊝⊝ LOWb,e,i,m,n due to indirectness, imprecision |
OR 0.81 (0.33–2.01) |
63 per 1000 | 11 fewer per 1000 (from 41 fewer to 56 more) |
| Length of supplemental oxygen | 674 (5 studies) | ⊕⊕⊕⊝ MODERATEc,d,e,o,pdue to risk of bias |
The mean length of supplemental oxygen in viral coinfection group was 0.42 lower (1.05 lower to 0.2 higher) | ||
| Need of supplemental oxygen | 2285 (12 studies) | ⊕⊕⊝⊝ LOWe,g,l,q,r due to inconsistency, indirectness |
OR 0.94 (0.66–1.34) | 512 per 1000 | 15 fewer per 1000 (from 103 fewer to 72 more) |
| Need of PICU | 2630 (11 studies) | ⊕⊕⊕⊝ MODERATEe,g,l,s,t due to inconsistency |
OR 0.99 (0.64–1.54) | 220 per 1000 | 2 fewer per 1000 (from 67 fewer to 83 more) |
Note: CI: Confidence interval; OR: Odds ratio;
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Sixteen of twenty four included studies have no substantial risk of bias. The remaining eight have problems due to potential selection bias and/or failures to report or control of confounders.
No serious inconsistency was found.
In sixteen included studies, comparison was based upon specific viral combinations.
Optimal sample size for detecting a difference of 1 day (alpha 0.05 and power of 80%) was met and null hypothesis, which was considered the most plausible, was met.
No substantial publications bias was detected.
Four of included studies have failures to report or control of confounders.
High statistical heterogeneity (p < 0.01 and/or I2 > 50%) was found.
In four included studies, comparison was based upon all viral coinfections and all single infections.
Optimal information size was not achieved and 95% confidence interval was wide.
Eight included studies have no substantial risk of bias. The remaining three have failure to report or control of confounders.
In seven included studies, comparison was based upon all viral coinfections and all single infections.
Optimal sample size was met and 95% confidence interval was narrow and included null effect, which was considered most plausible hypothesis.
No serious risk of bias was found in most of bias domains of included studies.
In two included studies, comparison was based upon specific viral combinations.
Three included studies have a substantial risk of bias due to selection bias and/or failure to report or control of confounders.
In three included studies, comparison was based upon all viral coinfections and all single infections.
Eight studies have no substantial risk of bias. The remaining four have failure to report or control of confounders.
In half of included studies, comparison was based upon specific viral combinations.
Six included studies have no substantial risk of bias. The remaining five have failure to report or control of confounders.
In nine included studies, comparison was based upon all viral coinfections and all single infections.
4.4. Results of individual studies and synthesis of results
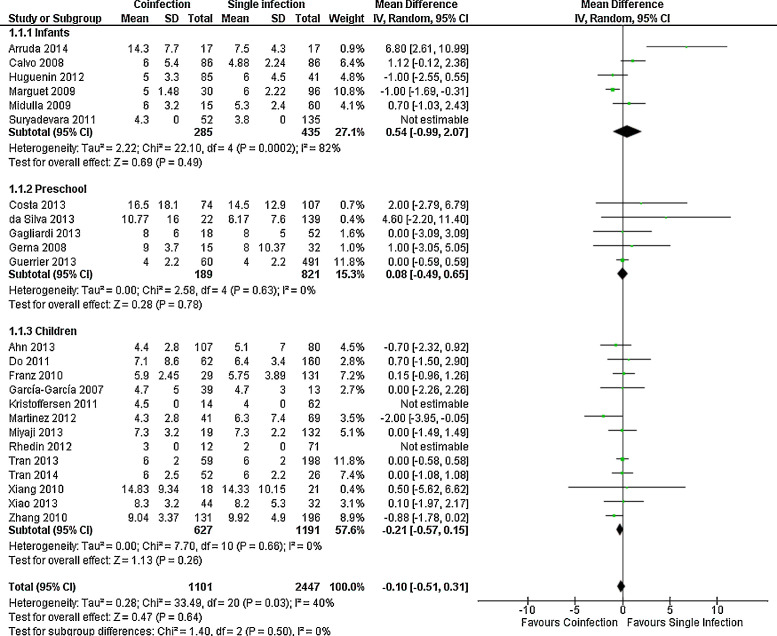
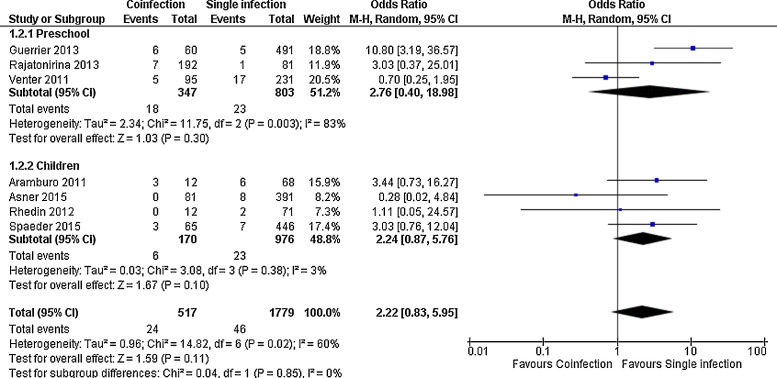
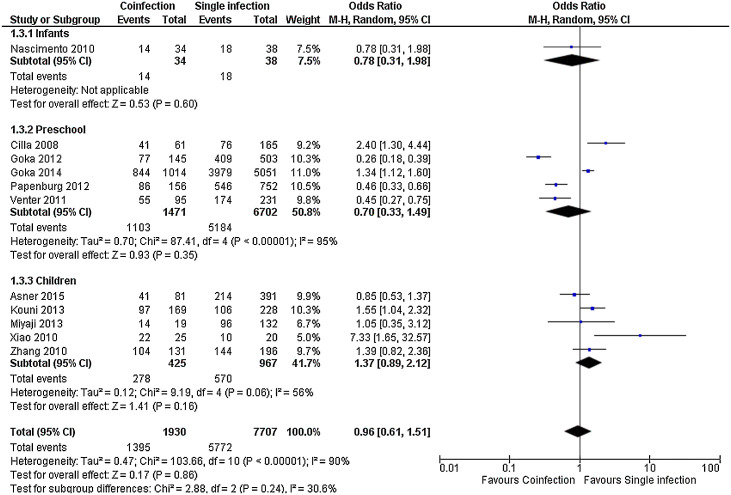
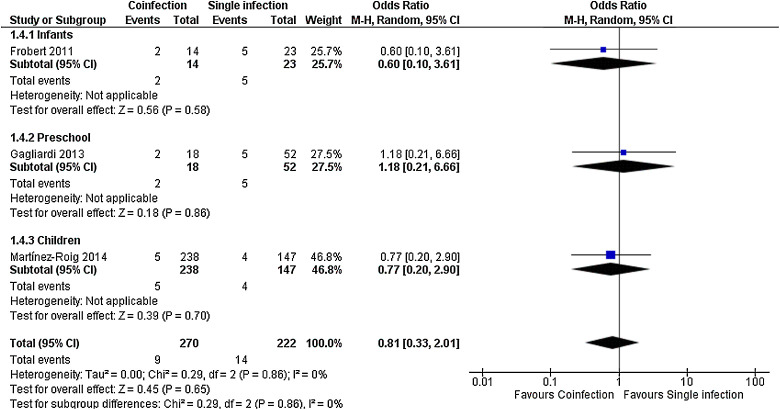
For most outcomes assessed, as shown in Fig. 2, Fig. 3, Fig. 4 and Supplemental Figs. 1, 2 and 3, the severity of single and multiple infections were not different, both in the whole group and in the age subgroups, as for length of stay, MD in days −0.10 (−0.51, 0.31), death, OR 2.22 (0.83, 5.95), need of hospitalization, OR 0.96 (0.61, 1.51), length of supplemental oxygen, MD −0.42 (−1.05, 0.20), need of supplemental oxygen, OR 0.94 (0.66, 1.34) and need of PICU, OR 0.99 (0.64, 1.54). Need of mechanical ventilation was not significant in the overall analysis, but had a trend to occur more often in children with single infection (OR 0.51 (0.26, 1.00)); in infants, however, it was significant (OR 0.34 (0.14, 0.83)) (Supplemental Fig. 4). Preliminary analysis for all outcomes including studies with less complete viral panels had similar results but with higher heterogeneity (data not shown).
Fig. 2.
Meta-analysis for length of stay comparing patients with viral coinfections and single infections in three age groups. Negative value indicates shorter time in patients with coinfections.
Fig. 3.
Meta-analysis for risk of death comparing patients with viral coinfections and single infections in three age groups. Value higher than one indicates higher risk in patients with coinfections.
Fig. 4.
Meta-analysis for risk of hospitalization comparing patients with viral coinfections and single infections in three age groups. Value less than one indicates less risk in patients with coinfections.
Risk of bias across studies and additional analysis: Higher risk of mechanical ventilation found in studies including only infants was not sustained once a sensitivity analysis excluding weak quality studies was done (Fig. 5 ). In studies analyzing all other outcomes, findings were not changed after exclusion of weak quality studies (data not shown). None of seven outcomes was changed after exclusion of studies based on bocavirus and adenovirus and after comparing only studies with the same combinations, such as RSV plus others versus only RSV, for example (data not shown). Funnel plot of all outcomes is shown in Supplemental Fig. 5 and was not considered suggestive of an important publication bias for all outcomes, excepting need of mechanical ventilation before sensitivity analysis, which is changed after exclusion of weak quality studies.
Fig. 5.
Meta-analysis for risk of mechanical ventilation comparing patients with viral coinfections and single infections in three age groups. Value less than one indicates less risk in patients with coinfections.
5. Discussion
Viral coinfection did not increase severity of any outcome assessed in this systematic review and meta-analysis. From an empirical point of view, it might be logical to expect that the detection of two or more viruses could enhance illness severity; however, our results did not confirm this.
A few reasons might contribute to our findings. First, the use of molecular biology techniques has demonstrated that viruses may be excreted for a prolonged period, with few or no symptoms [54], [55]. Therefore, some patients labeled as having coinfection may actually be subject of co-detection. Studies assessing viral load in attempt to make such distinction have not shown consistent differences between groups for most viruses [6], [30], [56]. On the other hand, some of these viruses, excreted in otherwise asymptomatic hosts, could enhance overall virulence if another viral infection was to occur. Consequently, it is difficult to rule out the role of even a co-detection in disease severity. Second, the limited possibilities of confirming bacterial etiology in lower respiratory tract infections and, consequently, bacterial-virus coinfection, is still an important challenge in clinical practice [57], [58]. Third, the prognosis of respiratory infections may be modified, to better or worse, by specific viral interactions and virus combinations, as some studies have suggested [9]. Nonetheless, sub-analyses with viruses that may have persistent shedding, bocavirus and adenovirus, and attempt to assess role of especific viral combinations did not change our findings. This analysis was particularly difficult not only because report and comparison of several different combinations across studies. As shown in Table 1, since many combinations are reported as, for example, “RSV coinfections”, including all different viruses detected with RSV into a single group, evaluation of specific viral interactions was hampered for most outcomes.
There are two previous systematic review and meta-analyses on this topic [59], [60]. Both included a mixed population of adults and children, included less studies and had a smaller number of outcomes. The first one included 21 studies and subjects from different age groups and included a subgroup analysis in children; risk of hospitalization was not assessed. They found no association between viral coinfection and length of stay, oxygen requirements, admission to PICU and need of mechanical ventilation [59]. They found a higher mortality risk in preschool children, which was not consistent with our results and previous studies [41], [46]. The second systematic review with mixed adult-children population included 19 studies, and reported an overall increase in risk of hospitalization in patients with two or more viruses detected, but there was not a subgroup analysis only with children for this outcome [60]. Besides of a search that ended more than two years later, some methodological reasons seem to account for the differences of those two studies with ours. First, they used less strict criteria for inclusion in meta-analysis and analysis was done including studies with a smaller number of viruses than those we assessed. We pooled studies including at least RSV, influenza, adenovirus, parainfluenza, rhinovirus and metapneumovirus, due to their virulence and prevalence, in an attempt to avoid a measurement bias.
Our study has limitations. Many studies did not measure, report or adjust severity for other confounding factors and interventions that could affect outcomes, such as how fast supportive actions are initiated and presence of comorbidities. Still, this is a broad systematic review and meta-analysis with a considerable number of studies and subjects, and with no language restriction; we also included additional sensitivity analyses excluding weak quality studies and stratified analysis of age groups. We believe that these measures rendered consistent results that can contribute to what is known on this important issue.
The results of this systematic review and meta-analysis suggest that the overall detection of two or more viruses has no impact on disease severity in children with respiratory infections for most clinically important outcomes, despite a relatively high prevalence of codetection or coinfection of respiratory viruses. Further studies are necessary, particularly to clarify the prognostic role of viral combinations.
Authors’ contribution
Dr. Scotta conceptualized and designed the study, acted as third reviewer when necessary, extracted data from studies, made initial interpretation of data, drafted the initial manuscript, and approved the final manuscript as submitted.
Drs. Chakr, de Souza, Jones, Pinto, Pitrez and Stein acted in study’s conception and design, analysis and interpretation of data, critically revised the manuscript, and approved the final manuscript as submitted.
Drs. de Moura and Becker acted in study’s conception and design, acquisition of data as two independent reviewers and interpretation of data, critically revised the manuscript, and approved the final manuscript as submitted.
Dr. Mattiello and Dr Sarria conceptualized and designed the study, supervised data extraction data from studies, performed statistical analysis and interpretation of data, critically revised the manuscript and approved the final manuscript as submitted.
Funding
There was no specific funding for this study. First author was supported by Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) as scholarship student.
Competing interests
None declared.
Ethical approval
Not required.
Acknowledgements
We thank to all contacted authors who kindly provided additional data and greatly contributed to improve the quality of this review.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jcv.2016.04.019.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Bourgeois F.T., Valim C., Wei J.C., McAdam A.J., Mandl K.D. Influenza and other respiratory virus-related emergency department visits among young children. Pediatrics. 2006;118:e1–8. doi: 10.1542/peds.2005-2248. [DOI] [PubMed] [Google Scholar]
- 2.Yorita K.L., Holman R.C., Sejvar J.J., Steiner C.A., Schonberger L.B. Infectious disease hospitalizations among infants in the United States. Pediatrics. 2008;121(2):244–252. doi: 10.1542/peds.2007-1392. [DOI] [PubMed] [Google Scholar]
- 3.Gharabaghi F., Hawan A., Drews S.J., Richardson S.E. Evaluation of multiple commercial molecular and conventional diagnostic assays for the detection of respiratory viruses in children. Clin. Microbiol. Infect. 2011;17(December (12)):1900–1906. doi: 10.1111/j.1469-0691.2011.03529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Paulis M., Gilio A.E., Ferraro A.A. Severity of viral coinfection in hospitalized infants with respiratory syncytial virus infection. J. Pediatr. (Rio. J) 2011;87(4):307–313. doi: 10.2223/JPED.2100. [DOI] [PubMed] [Google Scholar]
- 5.Macao P., Dias A., Azevedo L., Jorge A., Rodrigues C. Acute bronchiolitis: a prospective study. Acta Med. Port. 2011;2(Suppl. 24):407–412. [PubMed] [Google Scholar]
- 6.Franz A., Adams O., Willems R. Correlation of viral load of respiratory pathogens and co-infections with disease severity in children hospitalized for lower respiratory tract infection. J. Clin. Virol. 2010;48(4):239–245. doi: 10.1016/j.jcv.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain S., Williams D.J., Arnold S.R. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J. Med. 2015;372(9):835–845. doi: 10.1056/NEJMoa1405870. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nascimento M.S., Souza A.V., Ferreira A.V., Rodrigues J.C., Abramovici S., Silva Filho L.V. High rate of viral identification and coinfections in infants with acute bronchiolitis. Clinics (Sao Paulo) 2010;65(11):1133–1137. doi: 10.1590/S1807-59322010001100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Silva E.R., Pitrez M.C., Arruda E. Severe lower respiratory tract infection in infants and toddlers from a non-affluent population: viral etiology and co-detection as risk factors. BMC Infect. Dis. 2013;13:41. doi: 10.1186/1471-2334-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Garcia M.L., Calvo Rey C., Pozo Sanchez F. Human bocavirus infections in Spanish 0-14 year-old: clinical and epidemiological characteristics of an emerging respiratory virus. An. Pediatr. (Barc.) 2007;67(3):212–219. doi: 10.1016/S1695-4033(07)70609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scotta M.C., Mattiello R., Marostica P.J., Jones M.H., Martins L.G., Fischer G.B. Risk factors for need of mechanical ventilation in children with influenza A(H1N1)pdm09. J. Pediatr. (Rio. J.) 2013;89(5):444–449. doi: 10.1016/j.jped.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Guerrier G., Goyet S., Chheng E.T. Acute viral lower respiratory tract infections in Cambodian children: clinical and epidemiologic characteristics. Pediatr. Infect. Dis. J. 2013;32(1):e8–13. doi: 10.1097/INF.0b013e31826fd40d. [DOI] [PubMed] [Google Scholar]
- 13.Richard N., Komurian-Pradel F., Javouhey E. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr. Infect. Dis. J. 2008;27(3):213–217. doi: 10.1097/INF.0b013e31815b4935. [DOI] [PubMed] [Google Scholar]
- 14.Zhang G., Hu Y., Wang H., Zhang L., Bao Y., Zhou X. High incidence of multiple viral infections identified in upper respiratory tract infected children under three years of age in Shanghai, China. PloS One. 2012;7(9):e44568. doi: 10.1371/journal.pone.0044568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cilla G., Onate E., Perez-Yarza E.G., Montes M., Vicente D., Perez-Trallero E. Viruses in community-acquired pneumonia in children aged less than 3 years old: high rate of viral coinfection. J. Med. Virol. 2008;80(10):1843–1849. doi: 10.1002/jmv.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao N.G., Xie Z.P., Zhang B. Prevalence and clinical and molecular characterization of human metapneumovirus in children with acute respiratory infection in China. Pediatr. Infect. Dis. J. 2010;29(2):131–134. doi: 10.1097/inf.0b013e3181b56009. [DOI] [PubMed] [Google Scholar]
- 17.Arruda E., Jones M.H., Escremim de Paula F. The burden of single virus and viral coinfections on severe lower respiratory tract infections among preterm infants: a prospective birth cohort study in Brazil. Pediatr. Infect. Dis. J. 2014;33(10):997–1003. doi: 10.1097/INF.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 18.Costa L.F., Queiroz D.A., Lopes da Silveira H. Human rhinovirus and disease severity in children. Pediatrics. 2014;133(2):e312–321. doi: 10.1542/peds.2013-2216. [DOI] [PubMed] [Google Scholar]
- 19.Gagliardi T.B., Paula F.E., Iwamoto M.A. Concurrent detection of other respiratory viruses in children shedding viable human respiratory syncytial virus. J. Med. Virol. 2013;85(10):1852–1859. doi: 10.1002/jmv.23648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canducci F., Debiaggi M., Sampaolo M. Two-year prospective study of single infections and co-infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease. J. Med. Virol. 2008;80(4):716–723. doi: 10.1002/jmv.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marguet C., Lubrano M., Gueudin M. In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS One. 2009;4(2):e4596. doi: 10.1371/journal.pone.0004596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balshem H., Helfand M., Schunemann H.J., Oxman A.D., Kunz R., Brozek J. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P.T., Deeks J.J., editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Chapter 7: selecting studies and collecting data.www.cochrane-handbook.org Version 5.1.0 (updated March 2011) Available from. [Google Scholar]
- 24.Ahn J.G., Choi S.Y., Kim D.S., Kim K.H. Human bocavirus isolated from children with acute respiratory tract infections in Korea, 2010–2011. J. Med. Virol. 2014;86(12):2011–2018. doi: 10.1002/jmv.23880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aramburo A., van Schaik S., Louie J. Role of real-time reverse transcription polymerase chain reaction for detection of respiratory viruses in critically ill children with respiratory disease: is it time for a change in algorithm? Pediatr. Crit. Care Med. 2011;12(July (4)):e160–165. doi: 10.1097/PCC.0b013e3181f36e86. [DOI] [PubMed] [Google Scholar]
- 26.Brand H.K., de Groot R., Galama J.M. Infection with multiple viruses is not associated with increased disease severity in children with bronchiolitis. Pediatr. Pulmonol. 2012;47(4):393–400. doi: 10.1002/ppul.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calvo C., Garcia-Garcia M.L., Blanco C. Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J. Clin. Virol. 2008;42(3):268–272. doi: 10.1016/j.jcv.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Do A.H., van Doorn H.R., Nghiem M.N. Viral etiologies of acute respiratory infections among hospitalized Vietnamese children in Ho Chi Minh City, 2004–2008. PLoS One. 2011;6(3):e18176. doi: 10.1371/journal.pone.0018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frobert E., Escuret V., Javouhey E. Respiratory viruses in children admitted to hospital intensive care units: evaluating the CLART(R) Pneumovir DNA array. J. Med. Virol. 2011;83(1):150–155. doi: 10.1002/jmv.21932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerna G., Campanini G., Rognoni V. Correlation of viral load as determined by real-time RT-PCR and clinical characteristics of respiratory syncytial virus lower respiratory tract infections in early infancy. J. Clin. Virol. 2008;41(1):45–48. doi: 10.1016/j.jcv.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Goka E., Vallely P., Mutton K., Klapper P., Influenza A. viruses dual and multiple infections with other respiratory viruses and risk of hospitalisation and mortality. Influenza Other Respir Viruses. 2013;7(November (6)):1079–1087. doi: 10.1111/irv.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goka E.A., Vallely P.J., Mutton K.J., Klapper P.E. Single, dual and multiple respiratory virus infections and risk of hospitalization and mortality. Epidemiol. Infect. 2015;143(1):37–47. doi: 10.1017/S0950268814000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huguenin A., Moutte L., Renois F. Broad respiratory virus detection in infants hospitalized for bronchiolitis by use of a multiplex RT-PCR DNA microarray system. J. Med. Virol. 2012;84(6):979–985. doi: 10.1002/jmv.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kouni S., Karakitsos P., Chranioti A., Theodoridou M., Chrousos G., Michos A. Evaluation of viral co-infections in hospitalized and non-hospitalized children with respiratory infections using microarrays. Clin. Microbiol. Infect. 2013;19(August (8)):772–777. doi: 10.1111/1469-0691.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristoffersen A.W., Nordbo S.A., Rognlien A.G., Christensen A., Dollner H. Coronavirus causes lower respiratory tract infections less frequently than RSV in hospitalized Norwegian children. Pediatr. Infect. Dis. J. 2011;30(4):279–283. doi: 10.1097/INF.0b013e3181fcb159. [DOI] [PubMed] [Google Scholar]
- 36.Martínez P., Cordero J., Valverde C. Co-infección viral respiratoria en niños hospitalizados por infección respiratoria aguda y su impacto en la gravedad clínica. Rev. Chilena Infectol. 2012;29(2):169–174. doi: 10.4067/S0716-10182012000200008. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Roig A., Salvado M., Caballero-Rabasco M.A., Sanchez-Buenavida A., Lopez-Segura N., Bonet-Alcaina M. Viral coinfection in childhood respiratory tract infections. Arch. Bronconeumol. 2015;51(1):5–9. doi: 10.1016/j.arbr.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Midulla F., Scagnolari C., Bonci E. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch. Dis. Child. 2010;95(1):35–41. doi: 10.1136/adc.2008.153361. [DOI] [PubMed] [Google Scholar]
- 39.Miyaji Y., Kobayashi M., Sugai K. Severity of respiratory signs and symptoms and virus profiles in Japanese children with acute respiratory illness. Microbiol. Immunol. 2013;57(12):811–821. doi: 10.1111/1348-0421.12102. [DOI] [PubMed] [Google Scholar]
- 40.Papenburg J., Hamelin M.E., Ouhoummane N. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J. Infect. Dis. 2012;206(2):178–189. doi: 10.1093/infdis/jis333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajatonirina S., Razanajatovo N.H., Ratsima E.H. Outcome risk factors during respiratory infections in a paediatric ward in Antananarivo, Madagascar 2010–2012. PLoS One. 2013;8(9):e72839. doi: 10.1371/journal.pone.0072839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhedin S., Hamrin J., Naucler P. Respiratory viruses in hospitalized children with influenza-like illness during the h1n1 2009 pandemic in Sweden. PLoS One. 2012;7(12):e51491. doi: 10.1371/journal.pone.0051491. [corrected] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suryadevara M., Cummings E., Bonville C.A. Viral etiology of acute febrile respiratory illnesses in hospitalized children younger than 24 months. Clin. Pediatr. (Phila.) 2011;50(6):513–517. doi: 10.1177/0009922810394834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tran D.N., Nguyen T.Q., Nguyen T.A., Hayakawa S., Mizuguchi M., Ushijima H. Human bocavirus in children with acute respiratory infections in Vietnam. J. Med. Virol. 2014;86(6):988–994. doi: 10.1002/jmv.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tran D.N., Pham T.M., Ha M.T. Molecular epidemiology and disease severity of human respiratory syncytial virus in Vietnam. PLoS One. 2013;8(1):e45436. doi: 10.1371/journal.pone.0045436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venter M., Lassauniere R., Kresfelder T.L., Westerberg Y., Visser A. Contribution of common and recently described respiratory viruses to annual hospitalizations in children in South Africa. J. Med. Virol. 2011;83(8):1458–1468. doi: 10.1002/jmv.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiang Z., Gonzalez R., Xie Z. Human rhinovirus C infections mirror those of human rhinovirus A in children with community-acquired pneumonia. J. Clin. Virol. 2010;49(2):94–99. doi: 10.1016/j.jcv.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao N.G., Zhang B., Xie Z.P. Prevalence of human metapneumovirus in children with acute lower respiratory infection in Changsha, China. J. Med. Virol. 2013;85(3):546–553. doi: 10.1002/jmv.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng S.Z., Xiao N.G., Xie Z.P. Prevalence of human rhinovirus in children admitted to hospital with acute lower respiratory tract infections in Changsha, China. J. Med. Virol. 2014;86(11):1983–1989. doi: 10.1002/jmv.23861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang R.F., Jin Y., Xie Z.P. Human respiratory syncytial virus in children with acute respiratory tract infections in China. J. Clin. Microbiol. 2010;48(11):4193–4199. doi: 10.1128/JCM.00179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lees E.A., Carrol E.D., Gerrard C. Characterisation of acute respiratory infections at a United Kingdom paediatric teaching hospital: observational study assessing the impact of influenza A (2009 pdmH1N1) on predominant viral pathogens. BMC Infect. Dis. 2014;14:343. doi: 10.1186/1471-2334-14-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spaeder M.C., Custer J.W., Miles A.H. A multicenter outcomes analysis of children with severe rhino/enteroviral respiratory infection. Pediatr. Crit. Care Med. 2015;16(February (2)):119–123. doi: 10.1097/PCC.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 53.Asner S., Rose W., Petrich A., Richardson S., Tran D. Is virus coinfection a predictor of severity in children with viral respiratory infections? Clin. Microbiol. Infect. 2015;21(3) doi: 10.1016/j.cmi.2014.08.024. 264. e261–264. e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rhedin S., Lindstrand A., Rotzen-Ostlund M. Clinical utility of PCR for common viruses in acute respiratory illness. Pediatrics. 2014;133(3):e538–545. doi: 10.1542/peds.2013-3042. [DOI] [PubMed] [Google Scholar]
- 55.Martin E.T., Kuypers J., McRoberts J.P., Englund J.A., Zerr D.M. Human bocavirus 1 primary infection and shedding in infants. J. Infect. Dis. 2015;212(4):516–524. doi: 10.1093/infdis/jiv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin E.T., Kuypers J., Wald A., Englund J.A. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respir Viruses. 2012;6(January (1)):71–77. doi: 10.1111/j.1750-2659.2011.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rambaud-Althaus C., Althaus F., Genton B., D'Acremont V. Clinical features for diagnosis of pneumonia in children younger than 5 years: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15(April (4)):439–450. doi: 10.1016/S1473-3099(15)70017-4. [DOI] [PubMed] [Google Scholar]
- 58.Hammitt L.L., Murdoch D.R., Scott J.A. Specimen collection for the diagnosis of pediatric pneumonia. Clin. Infect. Dis. 2012;54(Suppl. 2):S132–139. doi: 10.1093/cid/cir1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asner S.A., Science M.E., Tran D., Smieja M., Merglen A., Mertz D. Clinical disease severity of respiratory viral co-infection versus single viral infection: a systematic review and meta-analysis. PLoS One. 2014;9(6):e99392. doi: 10.1371/journal.pone.0099392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goka E.A., Vallely P.J., Mutton K.J., Klapper P.E. Single and multiple respiratory virus infections and severity of respiratory disease: a systematic review. Paediatr. Respir. Rev. 2014;15(December (4)):363–370. doi: 10.1016/j.prrv.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.