Abstract
Bovine noroviruses are enteric pathogens detected in fecal samples of both diarrheic and non-diarrheic calves from several countries worldwide. However, epidemiological information regarding bovine noroviruses is still lacking for many important cattle producing countries from South America. In this study, three bovine norovirus genogroup III sequences were determined by conventional RT-PCR and Sanger sequencing in feces from diarrheic dairy calves from Argentina (B4836, B4848, and B4881, all collected in 2012). Phylogenetic studies based on a partial coding region for the RNA-dependent RNA polymerase (RdRp, 503 nucleotides) of these three samples suggested that two of them (B4836 and B4881) belong to genotype 2 (GIII.2) while the third one (B4848) was more closely related to genotype 1 (GIII.1) strains. By deep sequencing, the capsid region from two of these strains could be determined. This confirmed the circulation of genotype 1 (B4848) together with the presence of another sequence (B4881) sharing its highest genetic relatedness with genotype 1, but sufficiently distant to constitute a new genotype. This latter strain was shown in silico to be a recombinant: phylogenetic divergence was detected between its RNA-dependent RNA polymerase coding sequence (genotype GIII.2) and its capsid protein coding sequence (genotype GIII.1 or a potential norovirus genotype). According to this data, this strain could be the second genotype GIII.2_GIII.1 bovine norovirus recombinant described in literature worldwide. Further analysis suggested that this strain could even be a potential norovirus GIII genotype, tentatively named GIII.4. The data provides important epidemiological and evolutionary information on bovine noroviruses circulating in South America.
Keywords: Norovirus, Diarrhea, Bovine, Genotype, Argentina, Recombination
Highlights
-
•
Molecular prevalence of bovine Noroviruses in Argentina is reported.
-
•
Newborn calves positive to Norovirus presented diarrhea.
-
•
Phylogenetic inferences of the strains detected were performed and genotype–genogroups were determined for each strain.
-
•
A tentative new genotype is reported.
-
•
This is the first report of bovine Noroviruses from Argentina, one of the main meat and dairy farming countries worldwide.
1. Introduction
Noroviruses are a major cause of epidemic and sporadic gastroenteritis in humans (Green, 2013). They were also identified in cattle and other animal species with reports of lesions and clinical signs of gastroenteritis (Bridger et al., 1984, Hsu et al., 2007, Martella et al., 2007, Ntafis et al., 2010, Pinto et al., 2012, Scipioni et al., 2008a, Sugieda and Nakajima, 2002, Wang et al., 2007, Wolf et al., 2009). Noroviruses belong to the genus Norovirus, within the Caliciviridae family. They are non-enveloped viruses with a diameter of approximately 27–40 nm and possess a positive-sense, single stranded RNA genome of around 7.5 kb composed of three open reading frames (ORF) (Green, 2013). The ORF1 encodes a large polyprotein that is further cleaved into at least six non-structural proteins by the viral 3C-like protease, including the RNA-dependent RNA polymerase (RdRp, P). ORF2 and ORF3 encode the major (VP1) and minor (VP2) capsid proteins, respectively (Green, 2013). The VP1 capsid protein is organized into two domains: a shell domain (S domain, N-terminal part) and a protruding domain (P domain, C-terminal part, exhibiting two sub-domains: P1 and P2) (Prasad et al., 1999). The P2 is a hypervariable domain and has an external localization, compatible with its function of both cell receptor ligand and immunogenic determinant (Tan et al., 2004).
Noroviruses are genetically and antigenically diverse (Green, 2013, Zheng et al., 2006). As no cell culture system has been established for growing Norovirus (with the exception of murine strains), currently reverse transcription-polymerase chain reaction (RT-PCR) and genomic sequencing are used for characterizing viruses and understanding the phylogenetic relationships among strains detected in animals and humans worldwide. Routinely, Noroviruses are genotyped by analysis of partial capsid (VP1) or polymerase (RdRp) gene sequences. However, genotyping based exclusively on one region of Norovirus genome is not an accurate representation of viral epidemiology, especially due to frequent recombination within Noroviruses (Bull et al., 2007). Recombination commonly occurs at the ORF1–ORF2 junction although other recombination sites have been reported (Bull et al., 2005, Bull et al., 2007, Chhabra et al., 2010, Eden et al., 2013). To address the inconsistencies in Norovirus genotyping, a nomenclature system which incorporates both polymerase and capsid regions has been proposed (Kroneman et al., 2013). At least six genogroups (GI to GVI) have been described in the genus Norovirus and a tentative genogroup VII has been proposed (Green, 2013, Vinjé, 2015).
Currently, bovine Norovirus sequences described so far cluster within GIII. This genogroup is further subdivided into genotypes: GIII.1 and GIII.2 (Di Martino et al., 2014, Green, 2013, Mauroy et al., 2009a, Oliver et al., 2003) including all bovine Norovirus strains and a proposed GIII.3 containing ovine Noroviruses (Wolf et al., 2009). The bovine Norovirus strains Bo/DE/1980/GIII.1/Jena (Gunther and Otto, 1987, Liu et al., 1999, Otto et al., 2011) and Bo/UK/1976/GIII.2/Newbury2 (Dastjerdi et al., 1999, Woode and Bridger, 1978) are the prototypes for GIII.1 and GIII.2, respectively, while Ov/NZL/2007/GIII.3/Norsewood30 is the prototype strain for GIII.3 (Wolf et al., 2009). Genetic studies on bovine Noroviruses have been hampered by the small number of partial sequences available and, more importantly, the paucity of complete genome sequences (Di Martino et al., 2014, Mauroy et al., 2012, Mauroy et al., 2014).
Viral RNA recombination is a major evolutionary mechanism for viruses. It can affect phylogenetic grouping, virulence, prophylactic and diagnostic approaches. Bull et al. defined recombinant Norovirus as those that cluster with two distinct groups of Norovirus strains when two different regions (normally, the capsid and the RdRp) of the genome are phylogenetically analyzed (Bull et al., 2005). Studying all available sequences in 2007, Bull et al. reported the existence of two recombinant prototypes for bovine Noroviruses: GIII.P1_GIII.2 and GIII.P2_GIII.1 with Bo/UK/2000/GIII.P1_GIII.2/Thirsk10 and Bo/US/2003/GIII.P2_GIII.1/B-1SVD as reference strains, respectively (Bull et al., 2007, Oliver et al., 2004). Since then, molecular characterization of bovine Noroviruses from diverse geographical settings have revealed the circulation of a number of recombinant strains genetically related to Bo/UK/2000/GIII.P1_GIII.2/Thirsk10 (Di Martino et al., 2014, Han et al., 2004, Jor et al., 2010, Mauroy et al., 2009b). However, to our knowledge no other bovine Norovirus strains related to Bo/US/2003/GIII.P2_GIII.1/B-1SVD have been reported.
Livestock industry is a major economic activity in Argentina. Among cattle infectious diseases, neonatal calf diarrhea is considered an important sanitary problem of both beef and dairy cattle (Virtala et al., 1996). Molecular detection of bovine Norovirus sequences has been frequently associated with calf diarrhea (Scipioni et al., 2008b). Their pathogenicity is believed to be low or moderate (Jung et al., 2014), although studies analyzing their economic impact (weight loss, health status, veterinary treatment cost) as co-infecting agent in combination with other enteric pathogens have not yet been conducted. Molecular characterizations of bovine Noroviruses circulating in cattle from different regions of the world have been performed but still there is scarce data regarding South American countries, where large cattle populations are living, such as Argentina or Brazil. The aim of this study was to investigate the presence and genetic diversity of bovine Noroviruses in fecal samples from Argentinean diarrheic dairy calves.
2. Materials and methods
2.1. Fecal samples
Between 2008 and 2012, a total of 90 fecal samples from dairy calves suffering from diarrhea were collected from herds belonging to the major dairy regions of Argentina. The provinces included were Buenos Aires, Córdoba, Santa Fe and Entre Ríos. The ages of the sampled calves ranged from 1 to 90 days old. From these, 69 samples were received at the Diagnosis Service of the Virology Institute of the National Institute of Agriculture Technology (INTA) between 2008 and 2012. These 69 samples tested negative for Group A Rotavirus (RVA) and Bovine Coronavirus (BCoV). Screening for bovine RVA was performed using an antigen capture ELISA (Cornaglia et al., 1989) while screening for BCoV was done using an ELISA based on monoclonal antibodies previously described (Smith et al., 1996). These results were reported elsewhere (Badaracco et al., 2012, Bok et al., 2015).
The other 21 samples were selected from 11 dairy herds included in a statistically designed survey carried out in Southern Buenos Aires province to study the microbiology of calf diarrhea in dairy herds from the dairy region named “Cuenca Lechera Mar y Sierras” between 2008 and 2009, These 21 samples tested negative for RVA, BCoV, Salmonella ssp. and Cryptosporidium ssp.
2.2. RNA extraction and bovine Norovirus one step RT-PCR
For the viral assays, fecal suspensions of samples were prepared by diluting feces 1:4 in distilled water. The suspensions were then vortexed for 15 s, centrifuged at 2500g for 5 min, and supernatants were collected and stored at − 80 °C for further testing. The RNA was extracted using the QIAamp Viral RNA Mini Kit (Qiagen) following manufacturer's instructions from a 200 μl starting volume of the centrifuged fecal suspensions. The recovered RNA was eluted in 40 μl of elution buffer and stored at − 80 °C until further use.
For the amplification of the partial RdRp coding region, a One Step RT-PCR kit was used (Qiagen) according to manufacturer's instructions. Primers used were CBECu-F (5′-AGTTAYTTTTCCTTYTAYGGBGA-3′) and CBECu-R (5′-AGTGTCTCTGTCAGTCATCTTCAT-3′), with an expected amplicon size of 532 nt (Smiley et al., 2003b). The mixture was incubated for 30 min at 50 °C for reverse transcription, followed by 15 min at 95 °C for initial PCR activation. Then, 35 amplification cycles (1 min at 94 °C, 1 min at 46 °C and 2 min at 72 °C) were performed followed by a final extension step at 72 °C for 10 min. The amplification products were analyzed by 2% agarose gel electrophoresis and visualized under UV light. Agarose gel purification was performed on RT-PCR amplicons of the expected size using the QIAquick Gel Extraction kit (Qiagen). The RT-PCR products were Sanger sequenced directly in both directions obtaining a region spanning the primer binding areas.
2.3. Sample preparation for next generation sequencing
Fecal suspensions were homogenized for 1 min at 3000 rpm with a MINILYS homogenizer (Bertin Technologies, France) and filtered consecutively through 100 μm, 10 μm and 0.8 μm membrane filters (Millipore). The filtrate was then treated with a cocktail of Benzonase (Norovirusagen) and Micrococcal Nuclease (New England Biolabs.) at 37 °C for 2 h in a homemade buffer (1 M Tris, 100 mM CaCl2 and 30 mM MgCl2) to digest free-floating nucleic acids. RNA and DNA were extracted using the QIAamp Viral RNA Mini Kit (Qiagen) according to the manufacturer's instructions, but without carrier RNA in the lysis buffer. First and second strand synthesis and random PCR amplification for 20 cycles were performed using a slightly modified Whole Transcriptome Amplification (WTA) Kit procedure (Sigma-Aldrich), with a denaturation temperature of 95 °C instead of 72 °C to allow for the denaturation of dsDNA and dsRNA. This modification leads to the amplification of both DNA and RNA. WTA products were purified with MSB Spin PCRapace spin columns (Stratec) and were prepared for Illumina sequencing using the KAPA Library Preparation Kit (Kapa Biosystems). Libraries were quantified with the KAPA Library Quantification kit (Kapa Biosystems) and sequencing of the samples was performed on a HiSeq 2000 platform (Illumina) for 201 cycles (100 bp paired ends). Each sample was attributed a total of approximately 1 million paired end reads.
2.4. Bioinformatics analysis
Raw reads were trimmed for quality and adapters using Trimmomatic (Bolger et al., 2014) and were de novo assembled into contigs using SPAdes (Bankevich et al., 2012). Scaffolds were classified using a tBLASTx search against all complete viral genomes in GenBank using an e-value cut-off of 10− 10. Scaffolds with a significant tBLASTx hit were retained and used for a second tBlastx search against the GenBank nucleotide database using an e-value of 10− 4 (Altschul et al., 1990). The trimmed reads were mapped onto the obtained Noroviruses consensus sequences for sequence validation using BWA (Li and Durbin, 2010).
2.5. Phylogenetic analysis
Sequences were edited with the BioEdit 7.0.5.3 sequence Alignment Editor (Hall, 1999). Sequences with the highest nucleotide similarity within GenBank were retrieved using the BLAST tool (available at http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). Alignments were performed using the MEGA version 6 software package (Tamura et al., 2011). Phylogenetic relationships were inferred using the same software.
Nucleotide identity plots of partial RdRp coding region for (3′-end), single capsid protein and small basic protein coding regions compared to bovine Norovirus reference strains Bo/DE/1980/GIII.1/Jena and Bo/UK/1976/GIII.2/Newbury2 were performed using the Simplot software version 3.5.1 (available at http://sray.med.som.jhmi.edu/SCRoftware).
Three-dimensional modeling was achieved with the SWISS-MODEL tool (Arnold et al., 2006) and the Chimera software (available at http://www.cgl.ucsf.edu/chimera) on the basis of amino acid alignments with the Bo/DE/1980/GIII.1/Jena reference strain.
3. Results
3.1. Detection and phylogenetic relationships of Argentinean bovine Norovirus strains
A total of 14 samples (14/90, 15.5%) presented an amplification product of the expected size after One-Step RT-PCR using CBECu-F/R primers targeting the RdRp genomic region of the bovine Norovirus genome. Only seven of these samples presented a strong enough signal to be sequenced. Furthermore, Sanger sequence analysis confirmed that only three of these seven amplicons belonged to bovine Noroviruses. This indicates an apparent detection rate of 3.3% (3/90) in samples negative for RVA and BCoV. It is important to highlight that phylogenetic analysis of the remaining four amplicons indicated that those sequences were closely related to a ruminant bacteria named Prevotella sp. (see Supplementary Material). In particular, Prevotella ruminicola is commonly found in the rumen and hindgut of cattle and sheep (Nathan et al., 2015).
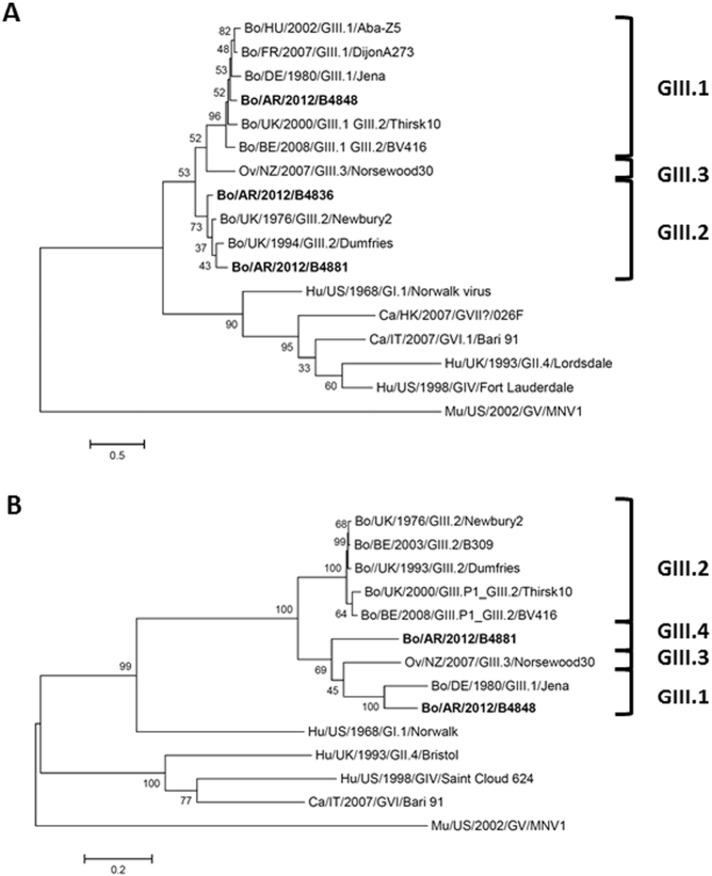
Positive samples for bovine Norovirus were identified as Bo/AR/2012/B4836 (B4836), Bo/AR/2012/B4848 (B4848) and Bo/AR/2012/B4881 (B4881). These three samples came from young calves with diarrhea from different provinces of Argentina. Sample B4836 was collected from a 9-day-old calf from a dairy herd in Santa Fe province. Sample B4848 came from a 2-day-old calf from Buenos Aires province and B4881 was taken from a 3-day-old animal from Córdoba province. All Norovirus positive samples were collected in 2012. Using the BLAST tool, the three partial sequences obtained from RdRp gene showed to be genetically most closely related to GIII bovine Norovirus sequences. The sequence of B4848 was most closely related with GIII.1 viruses, whereas both B4836 and B4881 were most closely related to GIII.2 viruses (Fig. 1A). Considering the fact that recombination commonly occurs at the ORF1-ORF2 junction, we attempted to get the complete VP1 coding sequences by next generation sequencing, which was successful for the B4848 and B4881 strains. By phylogenetic analyses, these two sequences were more closely related to GIII.1 viruses (Fig. 1B). This indicates contradictory phylogenetic inferences for B4881, grouping the polymerase (ORF1, RdRp) and capsid regions (ORF2, VP1) of this strain into different genotypes, which may suggest recombination between the RdRp and VP1 coding regions at the genotype level.
Fig. 1.
Phylogenetic trees constructed by the Maximum Likelihood method. A. Phylogenetic tree based on a partial coding region for the RNA-dependent RNA polymerase (RdRp, 532 nucleotides) of Norovirus sequences identified in this study (B4836, B4848 and B4881) and other bovine Norovirus reference sequences. The substitution model (Tamura 3-parameter + γ distribution) was selected on the basis of both lowest Akaike and Bayesian information criterion scores. The scale bar represents the phylogenetic distances expressed as units of expected nucleotide substitutions per site. B. Phylogenetic tree based on complete capsid protein (VP1) sequence of two of the bovine Norovirus sequences identified in this study (B4848 and B4881), and other bovine Norovirus reference sequences. The substitution model (LG model + γ distribution + assuming that a certain fraction of sites are evolutionarily invariable) was selected on the basis of both lowest Akaike and Bayesian information criterion scores. The scale bar represents the phylogenetic distances expressed as units of expected amino acid substitutions per site. The numbers at the nodes indicate the frequencies of occurrence for 1000 bootstrap replicate trees. The sequences detected in this study are repaired in bold case. GenBank submission numbers: 1866865 for partial RdRp sequences and 1866066 for VP1/VP2 coding sequences.
3.2. Recombination analysis
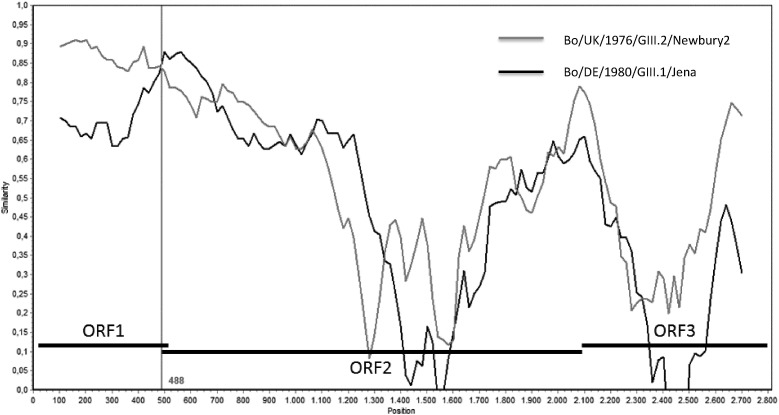
In order to clarify if strain B4881 could be a recombinant genotype, a nucleotide identity plot –Simplot- analysis was performed on the sequence encompassing the end of the RdRp gene (ORF1, 3′ region, 498 nt), and both the entire ORF2 and ORF3 regions. For this analysis both reference strains for genotypes GIII.1 (GIII.1 Jena) and GIII.2 (GIII.2 Newbury2) were included. For ORF1, the percentage of nucleotide identity between B4881 and GIII.1 Jena was between 83% and 91%, while the similarity to GIII.2 Newbury2 was between 63% and 82% (Fig. 2 ). However, Fig. 2 shows a putative recombination breakpoint located within the ORF1/ORF2 overlap, from where B4881 nucleotide sequence seems to be more similar to GIII.2 Newbury2 sequence than to GIII.1 Jena. Furthermore, for the ORF2/3 region, the similarity between B4881 and both GIII.1 Jena and GIII.2 Newbury2 strains is particularly low, especially in the more variable region of the capsid coding sequence (P domain). These results suggest that B4881 sequence could be a representative of a potential new genotype within GIII, tentatively named GIII.4.
Fig. 2.
Nucleotide identity plot of a partial coding region for the RdRp (3ʹend of ORF1, 498 nt), the ORF2 and the ORF3 nucleotide sequences of the Bo/AR/2012/B4881 genome compared with bovine Norovirus reference strains GIII.1 Jena and GIII.2 Newbury2. Nucleotide positions are reported on the X-axis and similarity score on Y-axis following a sliding window of 200 bp. The vertical line indicates localization of the putative recombination breakpoint.
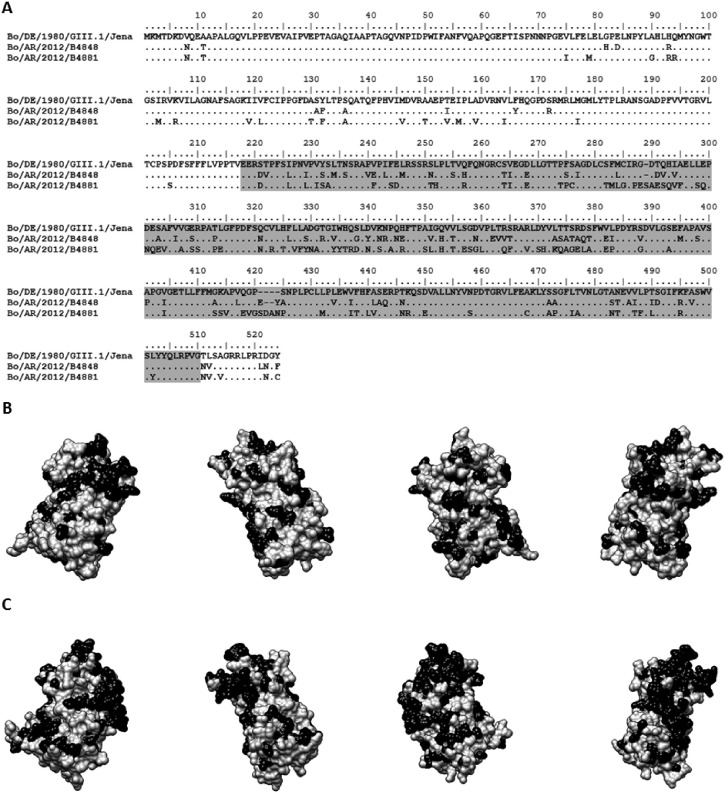
To further explore the positions of the amino acid changes observed in B4848 and B4881, their VP1 sequences were compared to reference strain GIII.1 Jena, using three-dimensional modeling (Fig. 3 ). Because VP1 is the major capsid protein, it is important to evaluate if the amino acid changes are mainly in the P domain or the S domain, especially because the P domain has both cell receptor ligand and immunogenic determinant activities. As expected, B4881 showed a higher number of modifications than B4848 compared to GIII.1 Jena reference sequence. It seems also clear that most of the amino acid changes observed in B4881 are located at the P domain.
Fig. 3.
Amino acid characterization of the putative recombinant bovine Norovirus strain B4848. A. Amino acid alignment between the capsid protein (VP1) sequences of reference GIII.1 Jena strain and Argentinean bovine Norovirus sequences obtained in this study (B4848 and B4881). The sequence coding the P domain is highlighted in gray. B and C. P domain three-dimensional modeling of B4848 (B) and B4881 (C) strains. The structures were solved using SWISS-MODEL workspace (Arnold et al., 2006) based on homology modeling with the 3sldC and 3astA pdb templates for B4848 and B4881, respectively. Amino acid changes compared to the sequence of the reference strain GIII.1 Jena are indicated in black. Four positions in an axial rotating view are showed.
4. Discussion
The circulation of bovine Noroviruses has been poorly studied in South America, with only a single report of a partial RdRp sequences from bovine enteric caliciviruses in Venezuela (Alcala et al., 2003). In the present work, a screening was conducted aiming at the detection of Norovirus in Argentinean cattle herds belonging to the main dairy regions of the country. Both genotype GIII.1- and GIII.2-related sequences were found to circulate in Argentinean dairy calves. Moreover, according to the genetic analyses, one of the sequences (B4881) may also constitute a potential new genotype in the bovine Norovirus GIII, tentatively named GIII.4.
The detection rate of Norovirus reported in this study (3.3%) is in agreement with studies in Belgium (7.5%, 10 out of 133 fecal samples using a CBECu-F/R primer set) on both dairy and beef cattle and in South Korea (2.8%, 18 out of 645 stool samples from two to 90 days old calves using the same primers) (Mauroy et al., 2009a, Park et al., 2007). In contrast, the prevalence of bovine Noroviruses was higher in studies conducted in the USA (72%, 54 out of 75 fecal samples from diarrheic and healthy veal calves using primers for human Norwalk like Viruses or Bovine Enteric Caliciviruses) and Norway (49.6%, 208 out of 419 fecal samples using a Real Time RT-PCR) (Jor et al., 2010, Smiley et al., 2003a, Smiley et al., 2003b). Thus, detection rate results by RT-PCR seem to depend on the continent, the country, the sampling strategy, the test strategy (conventional RT-PCR or qPCR), and the test conditions, such as annealing temperature and primers. Furthermore, sequencing of amplification products should be conducted to confirm bovine Norovirus identity, as we identified several sequences that could be attributed to Prevotella sp.
The phylogenetic analysis performed provides evidences for the circulation of rare viruses in South America. It has been shown that B4881 would represent a new genotype, where partial ORF1 sequence is more conserved and still close to GIII.2 genotype but ORF2/3 sequences are too divergent from previously described genotypes, and therefore it has been tentatively named GIII.4. From an evolutionary point of view, the ability to exchange VP1 sequences from different genotypes could give bovine Noroviruses another alternative for herd immunity evasion and a potential evolutionary strategy. Even if the genetic diversity within GIII seems to be lower than in some other genogroups (Mauroy et al., 2012, Mauroy et al., 2014, Zheng et al., 2006) and in the absence of biologically relevant criteria's (such as virus neutralization analysis), the definition of clear cut-off in term of pairwise divergence for those sequences will be soon needed. Epidemiologically, this data is also important as it indicates that most of the changes at the amino acid sequence level are located in the P domain of the major capsid protein, VP1. This may have major implications in the immunogenic characteristics of this novel genotype. More studies should be carried out in other to determine this matter as well as the relative prevalence of this potentially new genotype in this and other parts of the world. Further epidemiological analysis of sequences from this specific region of the world would clarify the hypothesis of topotypes for bovine Noroviruses and determine the genetic divergence of the viruses circulating in the region.
5. Conclusion
In conclusion, this work allowed us to report the circulation of both GIII.1 and GIII.2 bovine Noroviruses in Argentinean dairy calves, increasing the geographical boundaries of this virus. It also provides new genetic data for GIII.1 strains, which seem to be less prevalent worldwide. Furthermore, the presence of a potentially new genotype within the GIII genogroup is also reported. Additional studies are needed to clarify the situation in the Argentinean herds as well as in other South American countries.
Acknowledgments
We gratefully acknowledge the technical assistance and helpful advices from Dr. Jan Vinjé, Dr. João Mesquita and Dr. Fernando Fernández.
This study was supported by INTA-BID PICT No. 0325, ANPCyT—Argentina and INTA PNLEC1602 and the Scientific-Technological Cooperation Programme between the MINCYT—Argentina and the FWO—Flanders, Belgium. Project No. 02/13. “Identification of novel viral agents responsible for diarrhea in foals, piglets and South American Camelids from Argentina, using advanced metagenomic”.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.meegid.2016.02.034.
Appendix A. Supplementary data
Alignment of primers used for RdRp amplification by RT-PCR together with Prevotella sp. consensus sequence obtained. Consensus amplicon obtained using CBECu-F and CBECu-R primers set in four out of seven samples was identified as Prevotella sp. using Blast tool. CBCu-R primer is as a reverse complement.
References
- Alcala A.C., Hidalgo M.A., Obando C., Vizzi E., Liprandi F., Ludert J.E. Molecular identification of bovine enteric caliciviruses in Venezuela. Acta Cient. Venez. 2003;54:148–152. [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arnold K., Bordoli L., Kopp J., Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Badaracco A., Garaicoechea L., Rodríguez D., Uriarte E.L., Odeón A., Bilbao G., Galarza R., Abdala A., Fernández F., Parreño V. Bovine rotavirus strains circulating in beef and dairy herds in Argentina from 2004 to 2010. Vet. Microbiol. 2012;158(3-4):394–399. doi: 10.1016/j.vetmic.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., Pyshkin A.V., Sirotkin A.V., Vyahni N., Tesler G., Alekseyev M.A., Pevzner P.A. SPAdes: a new genome assembly algorithm and its applications to singe-cell sequencing. J. Comput. Biol. 2012;19(5):455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Uradel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok M., Miño S., Rodriguez D., Badaracco A., Nuñes I., Souza S.P., Bilbao G., Louge Uriarte E., Galarza R., Vega C., Odeon A., Saif L.J., Parreño V. Molecular and antigenic characterization of bovine Coronavirus circulating in Argentinean dairy cattle during 1994–2010. Vet. Microbiol. 2015;181(3-4):221–229. doi: 10.1016/j.vetmic.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J.C., Hall G.A., Brown J.F. Characterization of a calici-like virus (Newbury agent) found in association with astrovirus in bovine diarrhea. Infect. Immun. 1984;43:133–138. doi: 10.1128/iai.43.1.133-138.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R.A., Hansman G.S., Clancy L.E., Tanaka M.M., Rawlinson W.D., White P.A. Norovirus Recombination in ORF1/ORF2 overlap. Emerg. Infect. Dis. 2005;11(7):1079–1085. doi: 10.3201/eid1107.041273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R.A., Tanaka M.M., White P.A. Norovirus recombination. J. Gen. Virol. 2007;88:3347–3359. doi: 10.1099/vir.0.83321-0. [DOI] [PubMed] [Google Scholar]
- Chhabra P., Walimbe A.M., Chitambar S.D. Complete genome characterization of Genogroup II norovirus strains from India: Evidence of recombination in ORF2/3 overlap. Infect. Genet. Evol. 2010;10(7):1101–1109. doi: 10.1016/j.meegid.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Cornaglia E.M.B.M., Fitjman N., Schudel A.A. Enzyme linked immunosorbent assay, immunofluorescent test and electrophoresis analysis of rotaviral RNA in the diagnosis and characterization of the bovine rotavirus. Rev. Latinoam. Microbiol. 1989;31:4. [Google Scholar]
- Dastjerdi A.M., Green J., Gallimore C.I., Brown D.W., Bridger J.C. The bovine Newbury agent-2 is genetically more closely related to human SRSVs than to animal caliciviruses. Virology. 1999;254:1–5. doi: 10.1006/viro.1998.9514. [DOI] [PubMed] [Google Scholar]
- Di Martino B., Di Profio F., Di Felice E., Melegari I., Ceci C., Mauroy A., Thiry E., Martella V., Marsilio F. Genetic heterogeneity of bovine noroviruses in Italy. Arch. Virol. 2014 doi: 10.1007/s00705-014-2109-0. [DOI] [PubMed] [Google Scholar]
- Eden J.S., Tanaka M.M., Boni M.F., Rawlinson W.D., White P.A. Recombination whitin the pandemic norovirus GII.4 linage. J. Virol. 2013;87(11):6270–6282. doi: 10.1128/JVI.03464-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K.Y. Caliciviridae: The Noroviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. Lippincott, Williams and Wilkins; Philadelphia: 2013. pp. 582–608. [Google Scholar]
- Gunther H., Otto P. Diarrhea in young calves. 7. "Zackenvirus" (Jena agent 117/80)—a new diarrhea pathogen in calves. Arch. Exp. Vet. 1987;41:934–938. [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999:95–98. [Google Scholar]
- Han M.G., Smiley J.R., Thomas C., Saif L.J. Genetic recombination between two genotypes of genogroup III bovine noroviruses (BoNVs) and capsid sequence diversity among BoNVs and Nebraska-like bovine enteric caliciviruses. J. Clin. Microbiol. 2004;42:5214–5224. doi: 10.1128/JCM.42.11.5214-5224.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C.C., Riley L.K., Livingston R.S. Molecular characterization of three novel murine noroviruses. Virus Genes. 2007;34:147–155. doi: 10.1007/s11262-006-0060-1. [DOI] [PubMed] [Google Scholar]
- Jor E., Myrmel M., Jonassen C.M. SYBR Green based real-time RT-PCR assay for detection and genotype prediction of bovine noroviruses and assessment of clinical significance in Norway. J. Virol. Methods. 2010;169:1–7. doi: 10.1016/j.jviromet.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Scheuer K.A., Zhang Z., Wang Q., Saif L.J. Pathogenesis of GIII.2 bovine norovirus, CV186-OH/00/US strain in gnotobiotic calves. Vet. Microbiol. 2014;168:202–207. doi: 10.1016/j.vetmic.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroneman A., Vega E., Vennema H., Vinje J., White P.A., Hansman G., Green K., Martella V., Katayama K., Koopmans M. Proposal for a unified norovirus nomenclature and genotyping. Arch. Virol. 2013;158:2059–2068. doi: 10.1007/s00705-013-1708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26(5):589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.L., Lambden P.R., Gunther H., Otto P., Elschner M., Clarke I.N. Molecular characterization of a bovine enteric calicivirus: relationship to the Norwalk-like viruses. J. Virol. 1999;73:819–825. doi: 10.1128/jvi.73.1.819-825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V., Campolo M., Lorusso E., Cavicchio P., Camero M., Bellacicco A.L., Decaro N., Elia G., Greco G., Corrente M., Desario C., Arista S., Banyai K., Koopmans M., Buonavoglia C. Norovirus in captive lion cub (Panthera leo) Emerg. Infect. Dis. 2007;13:1071–1073. doi: 10.3201/eid1307.070268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauroy A., Scipioni A., Mathijs E., Saegerman C., Mast J., Bridger J.C., Ziant D., Thys C., Thiry E. Epidemiological study of bovine norovirus infection by RT-PCR and a VLP-based antibody ELISA. Vet. Microbiol. 2009;137:243–251. doi: 10.1016/j.vetmic.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauroy A., Scipioni A., Mathijs E., Thys C., Thiry E. Molecular detection of kobuviruses and recombinant noroviruses in cattle in continental Europe. Arch. Virol. 2009;154:1841–1845. doi: 10.1007/s00705-009-0518-2. [DOI] [PubMed] [Google Scholar]
- Mauroy A., Scipioni A., Mathijs E., Ziant D., Daube G., Thiry E. Complete genome sequence of a novel bovine norovirus: evidence for slow genetic evolution in genogroup III genotype 2 noroviruses. J. Virol. 2012;86:12449–12450. doi: 10.1128/JVI.02251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauroy A., Scipioni A., Mathijs E., Ziant D., Daube G., Thiry E. Genetic and evolutionary perspectives on genogroup III, genotype 2 bovine noroviruses. Arch. Virol. 2014;159:39–49. doi: 10.1007/s00705-013-1791-7. [DOI] [PubMed] [Google Scholar]
- Nathan N.M., Kothari R.K., Patel A.K., Joshi C.G. Functional Characterization Reveals Novel Putative Coding Sequences in Prevotella ruminicola Genome Extracted from Rumen Metagenomic Studies. J. Mol. Microbiol. Biotechnol. 2015;25(4):292–299. doi: 10.1159/000437265. [DOI] [PubMed] [Google Scholar]
- Ntafis V., Xylouri E., Radogna A., Buonavoglia C., Martella V. Outbreak of canine norovirus infection in young dogs. J. Clin. Microbiol. 2010;48:2605–2608. doi: 10.1128/JCM.02528-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S.L., Dastjerdi A.M., Wong S., El-Attar L., Gallimore C., Brown D.W., Green J., Bridger J.C. Molecular characterization of bovine enteric caliciviruses: a distinct third genogroup of noroviruses (Norwalk-like viruses) unlikely to be of risk to humans. J. Virol. 2003;77:2789–2798. doi: 10.1128/JVI.77.4.2789-2798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S.L., Brown D.W., Green J., Bridger J.C. A chimeric bovine enteric calicivirus: evidence for genomic recombination in genogroup III of the Norovirus genus of the Caliciviridae. Virology. 2004;326:231–239. doi: 10.1016/j.virol.2004.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto P.H., Clarke I.N., Lambden P.R., Salim O., Reetz J., Liebler-Tenorio E.M. Infection of calves with bovine norovirus GIII.1 strain Jena virus: an experimental model to study the pathogenesis of norovirus infection. J. Virol. 2011;85:12013–12021. doi: 10.1128/JVI.05342-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.I., Jeong C., Kim H.H., Park S.H., Park S.J., Hyun B.H., Yang D.K., Kim S.K., Kang M.I., Cho K.O. Molecular epidemiology of bovine noroviruses in South Korea. Vet. Microbiol. 2007;124:125–133. doi: 10.1016/j.vetmic.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto P., Wang Q., Chen N., Dubovi E.J., Daniels J.B., Millward L.M., Buonavoglia C., Martella V., Saif L.J. Discovery and genomic characterization of noroviruses from a gastroenteritis outbreak in domestic cats in the US. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B.V., Hardy M.E., Dokland T., Bella J., Rossmann M.G., Estes M.K. X-ray crystallographic structure of the Norwalk virus capsid. Science (New York, N.Y.) 1999;286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- Scipioni A., Mauroy A., Vinje J., Thiry E. Animal noroviruses. Vet. J. 2008;178:32–45. doi: 10.1016/j.tvjl.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Scipioni A., Mauroy A., Ziant D., Saegerman C., Thiry E. A SYBR Green RT-PCR assay in single tube to detect human and bovine noroviruses and control for inhibition. Virol. J. 2008;5:94. doi: 10.1186/1743-422X-5-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J.R., Hoet A.E., Traven M., Tsunemitsu H., Saif L.J. Reverse transcription-PCR assays for detection of bovine enteric caliciviruses (BEC) and analysis of the genetic relationships among BEC and human caliciviruses. J. Clin. Microbiol. 2003;41:3089–3099. doi: 10.1128/JCM.41.7.3089-3099.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J.R., Hoet A.E., Traven M., Tsunemitsu H., Saif L.J. Reverse transcription-PCR assays for detection of bovine enteric caliciviruses (BEC) and analysis of the genetic relationships among BEC and human caliciviruses. J. Clin. Microbiol. 2003;41:3089–3099. doi: 10.1128/JCM.41.7.3089-3099.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.R., Tsunemitsu H., Heckert R.A., Saif L.J. Evaluation of two antigen-capture ELISAs using polyclonal or monoclonal antibodies for the detection of bovine coronavirus. J. Vet. Diagn. Invest. 1996;8(1):99–105. doi: 10.1177/104063879600800116. [DOI] [PubMed] [Google Scholar]
- Sugieda M., Nakajima S. Viruses detected in the caecum contents of healthy pigs representing a new genetic cluster in genogroup II of the genus “Norwalk-like viruses”. Virus Res. 2002;87:165–172. doi: 10.1016/s0168-1702(02)00107-7. [DOI] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M., Hegde R.S., Jiang X. The P domain of norovirus capsid protein forms dimer and binds to histo-blood group antigen receptors. J. Virol. 2004;78:6233–6242. doi: 10.1128/JVI.78.12.6233-6242.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinjé J. Advances in laboratory methods for detection and typing of norovirus. J. Clin. Microbiol. 2015;53(2):373–381. doi: 10.1128/JCM.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtala A.M., Mechor G.D., Grohn Y.T., Erb H.N. Morbidity from nonrespiratory diseases and mortality in dairy heifers during the first three months of life. J. Am. Vet. Med. Assoc. 1996;208:2043–2046. [PubMed] [Google Scholar]
- Wang Q.H., Costantini V., Saif L.J. Porcine enteric caliciviruses: genetic and antigenic relatedness to human caliciviruses, diagnosis and epidemiology. Vaccine. 2007;25:5453–5466. doi: 10.1016/j.vaccine.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S., Williamson W., Hewitt J., Lin S., Rivera-Aban M., Ball A., Scholes P., Savill M., Greening G.E. Molecular detection of norovirus in sheep and pigs in New Zealand farms. Vet. Microbiol. 2009;133:184–189. doi: 10.1016/j.vetmic.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Woode G.N., Bridger J.C. Isolation of small viruses resembling astroviruses and caliciviruses from acute enteritis of calves. J. Med. Microbiol. 1978;11:441–452. doi: 10.1099/00222615-11-4-441. [DOI] [PubMed] [Google Scholar]
- Zheng D.P., Ando T., Fankhauser R.L., Beard R.S., Glass R.I., Monroe S.S. Norovirus classification and proposed strain nomenclature. Virology. 2006;346:312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of primers used for RdRp amplification by RT-PCR together with Prevotella sp. consensus sequence obtained. Consensus amplicon obtained using CBECu-F and CBECu-R primers set in four out of seven samples was identified as Prevotella sp. using Blast tool. CBCu-R primer is as a reverse complement.