Abstract
A series of 4-(1-aryl-2-oxo-1,2-dihydro-indol-3-ylideneamino)-N-substituted benzenesulfonamide derivatives (1–32) was synthesized and evaluated for its in vitro antimicrobial, antiviral and cytotoxic activities. Antimicrobial results indicated that compounds (11) and (18) were found to be the most effective ones. In general, the synthesized compounds were bacteriostatic and fungistatic in their action. The cytotoxic screening results indicated that the compounds were less active than the standard drug 5-fluorouracil (5-FU). None of the compounds inhibited viral replication at subtoxic concentrations. In general, the presence of a pyrimidine ring with electron releasing groups and an ortho- and para-substituted benzoyl moiety favored antimicrobial activities. The results of QSAR studies demonstrated the importance of topological parameters, valence zero order molecular connectivity index (0χv) and valence first order molecular connectivity index (1χv) in describing the antimicrobial activity of synthesized compounds.
Keywords: Isatin, QSAR, Antimicrobial, Antiviral, Anticancer/cytotoxic
1. Introduction
The growing incidence of microbial resistance to currently used antibiotics represents a serious medical problem. Therefore, there is an urgent need to develop new classes of therapeutic agents to treat microbial infections. A combinational therapeutic drug with different mechanisms of action is one of the methods that are being adopted to treat disorders mentioned above. Besides the exploitation of new targets, there is another approach of merging two or more pharmacophores into a single molecule. This approach can also reduce unwanted side effects. The success of this hybridization approach has already been applied in developing novel antibacterial and antimalarial agents to overcome drug resistance (Solomon et al., 2010).
Cancer has become the second cause of mortality in the world and the development of potent and specific anticancer agents is urgently needed because of the problems like severe toxicity as well as resistance to the existing drugs (Kamal et al., 2010). The human immunodeficiency virus (HIV) epidemic continues to have enormous human health consequences. During 2004, around five million adults and children became infected with HIV and by the end of the year; an estimated 39.4 million people worldwide were living with HIV/AIDS. Non-nucleoside reverse transcriptase inhibitors (NNRTIs) have gained a definitive place in the treatment of HIV-1 infections. This makes them less likely to interfere with the normal function of other DNA polymerases and therefore less toxic for the treatment of HIV-infected patients. In this respect, isatin beta-thiosemicarbazone derivatives were found to demonstrate a range of antiviral activities (Bal et al., 2005).
Isatin is a natural product found in a number of plants, possesses antibacterial (Karthikeyan et al., 2010), antifungal (Nandakumar et al., 2010), anticonvulsant (Sridhar et al., 2002), anti-inflammatory (Matheus et al., 2007), anti-HIV (Bal et al., 2005) and anticancer (Sabet et al., 2010) activities. In particular, 1-methylisatin-3-thiosemicarbazone (methisazone) is clinically used as an antiviral agent. Thus isatin is a biologically validated starting point for the design and synthesis of chemical libraries directed at these targets (Adibi et al., 2010).
Quantitative structure–activity relationship (QSAR) is a methodology used to correlate the biological property of molecules with molecular descriptors derived from chemical structures. It is a mathematical model of a statistically validated correlation between the chemical structures and their activity profile (Kumar et al., 2010)
Prompted by the above facts and in continuation of our efforts in developing novel compounds for treatments of antimicrobial, anticancer and antiviral infections (Narang et al., 2012, Judge et al., 2012) we hereby report the synthesis, antimicrobial, cytotoxic and antiviral evaluation and QSAR studies of 4-(1-aryl-2-oxo-1, 2-dihydro-indol-3-ylideneamino)-N-substituted benzene sulfonamides.
2. Results and discussion
2.1. Chemistry
The synthesis of 4-(1-aryl-2-oxo-1, 2-dihydro-indol-3-ylideneamino)-N-substituted benzenesulfonamides (1–32) was accomplished by Scheme 1 . The physicochemical characteristics of the synthesized compounds are presented in Table 1 .
Scheme 1.
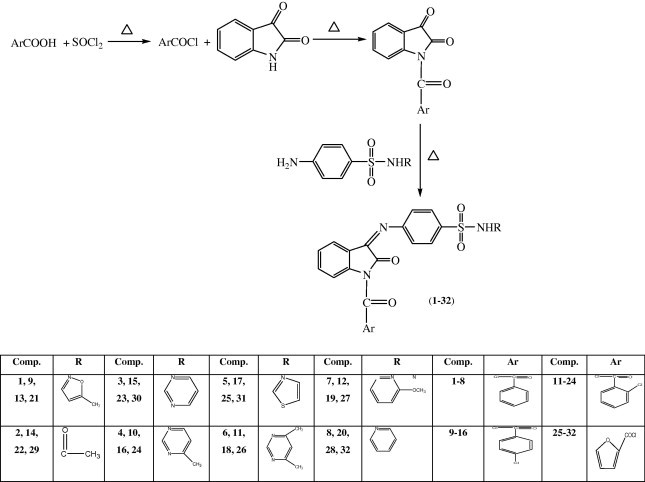
Scheme for the synthesis of 4-(1-aryl-2-oxo-1, 2-dihydro-indol-3-ylideneamino)-N-substituted benzenesulfonamide.
Table 1.
Physicochemical characteristics of the synthesized isatin derivatives.
| Comp. | M. pt. (oC) | M. formula | M. wt. | Rf value | % Yield |
|---|---|---|---|---|---|
| Training set | |||||
| 1 | 246–248 | C25H20N4O5S | 488.12 | 0.72 | 85 |
| 2 | 88–90 | C23H17N3O5S | 447.76 | 0.70 | 80 |
| 3 | 252–254 | C26H20N5O4S | 483.10 | 0.68 | 72 |
| 4 | 268–270 | C29H19N5O4S | 498.53 | 0.64 | 76 |
| 5 | 213–215 | C24H16N4O4S2 | 488.54 | 0.62 | 82 |
| 6 | 124–126 | C27H21N5O4S | 511.55 | 0.70 | 85 |
| 7 | 208–210 | C26H19N5O5S | 513.52 | 0.74 | 74 |
| 8 | 220–222 | C26H18N4O4S | 482.15 | 0.78 | 68 |
| 9 | 203–205 | C25H17ClN4O5S | 520.06 | 0.72 | 76 |
| 10 | 112–114 | C26H18ClN5O4S | 531.97 | 0.72 | 77 |
| 11 | 180–182 | C27H20ClN5O6S | 545.09 | 0.74 | 88 |
| 12 | 223–225 | C26H18ClN5O5S | 547.97 | 0.68 | 66 |
| 13 | 246–248 | C25H17N4O5S | 520.06 | 0.72 | 70 |
| 14 | 120–122 | C23H17ClN3O5S | 482.92 | 0.70 | 82 |
| 15 | 252–254 | C25H16ClN5O4S | 517.06 | 0.68 | 74 |
| 16 | 268–270 | C29H18ClN5O4S | 531.08 | 0.64 | 62 |
| 17 | 213–215 | C24H16ClN4O4S2 | 523.99 | 0.62 | 76 |
| 18 | 124–126 | C27H20ClN5O4S | 545.09 | 0.70 | 82 |
| 19 | 208–210 | C26H18ClN5O5S | 547.97 | 0.74 | 70 |
| 20 | 220–222 | C26H18ClN4O4S | 517.96 | 0.78 | 87 |
| 21 | 108–110 | C23H16ClN4O6S | 476.08 | 0.72 | 58 |
| 22 | 112–114 | C21H16N3O6S | 438.43 | 0.64 | 64 |
| 23 | 100–102 | C23H15N5O5S | 473.46 | 0.68 | 78 |
| 24 | 195–197 | C24H17N5O5S | 487.49 | 0.72 | 84 |
| 25 | 148–150 | C22H15N4O5S2 | 479.51 | 0.70 | 76 |
| 26 | 180–182 | C25H19N5O5S | 501.51 | 0.74 | 60 |
| 27 | 223–225 | C24H17N5O6S | 503.49 | 0.68 | 88 |
| 28 | 228–230 | C24H16N4O5S | 472.47 | 0.62 | 66 |
| Test set | |||||
| 29 | 117–119 | C23H17ClN3O5S | 481.92 | 0.64 | 72 |
| 30 | 120–122 | C25H17ClN5O4S | 518.95 | 0.68 | 64 |
| 31 | 148–150 | C24H16ClN4O4S2 | 523.99 | 0.70 | 82 |
| 32 | 228–230 | C26H17ClN4O4S | 516.96 | 0.62 | 70 |
TLC mobile phase: chloroform.
The synthesized compounds were characterized by IR and 1H NMR spectroscopy and the results are in accordance with the assigned molecular structures. IR stretching band ranging from 1727–1673 cm−1 (C O str., Ar–C O) confirmed the acylation of isatin. IR stretching band at 1674–1618 cm−1 (C N str.) confirm the formation of a Schiff’s base. Further, peak of NH in plane bending at 1582–1488 cm−1, O S O str. at 1182–1143 cm−1 confirmed the presence of sulfonamide moiety in the synthesized compounds.
In the 1H NMR spectra the signals of the respective protons of the synthesized compounds were confirmed based on their chemical shifts, multiplicities and coupling constants. These spectra showed a singlet at 3.23–3.35 ppm, which corresponds to the SO2NH protons and multiplet at 6.11–8.74 ppm, which corresponds to aromatic protons. Specifically, compounds 1, 9, 13, and 21 showed a singlet at 2.31–2.36 ppm which corresponds to CH3 protons on the isoxazole ring.
Similarly compounds 2, 14, 22, and 29 showed a singlet at 1.40–1.95 ppm which corresponds to COCH3 protons, compounds 6, 11, 26, and 28 showed a singlet at 2.33–3.94 ppm which corresponds to pyrimidine-OCH3 protons, and compounds 7, 12, 29, and 27 showed a singlet at 1.40–3.32 ppm, which corresponds to pyridazine-OCH3 protons.
2.2. Antimicrobial activity
The antimicrobial activity of the synthesized compounds was determined by tube dilution method (Cappucino and Sherman 1999) and the results are given in Table 2 . In the case of antimicrobial activity against Staphylococcus aureus, (32) was found to be most active (pMICsa = 1.62). (11) and (18) emerged as most potential candidates against Bacillus subtilis, Escherichia coli and Candida albicans with a pMIC value of 1.67. Against Aspergillus niger, (18) emerged as an effective antifungal agent (pMICan = 1.67).
Table 2.
Antimicrobial activity of synthesized isatin derivatives.
| Comp. | Minimum inhibitory concentration (MIC, μ mol/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|
| pMICsa | pMICbs | pMICec | pMICca | pMICan | pMICab | pMICaf | pMICam | |
| Training set | ||||||||
| 1 | 1.29 | 1.29 | 1.59 | 1.59 | 1.29 | 1.39 | 1.44 | 1.41 |
| 2 | 1.25 | 1.25 | 1.55 | 1.55 | 1.25 | 1.35 | 1.4 | 1.37 |
| 3 | 1.29 | 1.29 | 1.29 | 1.59 | 1.59 | 1.29 | 1.59 | 1.41 |
| 4 | 1.30 | 1.30 | 1.60 | 1.60 | 1.30 | 1.40 | 1.45 | 1.42 |
| 5 | 1.29 | 1.29 | 1.59 | 1.59 | 1.29 | 1.39 | 1.44 | 1.41 |
| 6 | 1.34 | 1.34 | 1.64 | 1.64 | 1.34 | 1.44 | 1.49 | 1.46 |
| 7 | 1.31 | 1.31 | 1.61 | 1.61 | 1.61 | 1.41 | 1.61 | 1.49 |
| 8 | 1.29 | 1.29 | 1.59 | 1.59 | 1.59 | 1.39 | 1.59 | 1.47 |
| 9 | 1.32 | 1.32 | 1.62 | 1.62 | 1.62 | 1.42 | 1.62 | 1.50 |
| 10 | 1.33 | 1.63 | 1.63 | 1.63 | 1.33 | 1.53 | 1.48 | 1.51 |
| 11 | 1.36 | 1.67 | 1.67 | 1.67 | 1.36 | 1.57 | 1.52 | 1.55 |
| 12 | 1.34 | 1.34 | 1.64 | 1.64 | 1.34 | 1.44 | 1.49 | 1.46 |
| 13 | 1.32 | 1.32 | 1.62 | 1.62 | 1.62 | 1.42 | 1.62 | 1.50 |
| 14 | 1.29 | 1.29 | 1.59 | 1.59 | 1.29 | 1.39 | 1.44 | 1.41 |
| 15 | 1.32 | 1.32 | 1.62 | 1.62 | 1.62 | 1.42 | 1.62 | 1.50 |
| 16 | 1.33 | 1.33 | 1.63 | 1.63 | 1.63 | 1.43 | 1.63 | 1.51 |
| 17 | 1.32 | 1.32 | 1.62 | 1.62 | 1.62 | 1.42 | 1.62 | 1.50 |
| 18 | 1.36 | 1.67 | 1.67 | 1.67 | 1.67 | 1.57 | 1.67 | 1.61 |
| 19 | 1.34 | 1.34 | 1.64 | 1.64 | 1.34 | 1.44 | 1.49 | 1.46 |
| 20 | 1.32 | 1.32 | 1.62 | 1.62 | 1.62 | 1.42 | 1.62 | 1.5 |
| 21 | 1.28 | 1.28 | 1.58 | 1.58 | 1.58 | 1.38 | 1.58 | 1.46 |
| 22 | 1.24 | 1.24 | 1.54 | 1.54 | 1.54 | 1.34 | 1.54 | 1.42 |
| 23 | 1.28 | 1.28 | 1.58 | 1.58 | 1.58 | 1.38 | 1.58 | 1.46 |
| 24 | 1.29 | 1.29 | 1.59 | 1.59 | 1.29 | 1.39 | 1.44 | 1.41 |
| 25 | 1.28 | 1.28 | 1.58 | 1.58 | 1.58 | 1.38 | 1.58 | 1.46 |
| 26 | 1.33 | 1.33 | 1.63 | 1.63 | 1.33 | 1.43 | 1.48 | 1.45 |
| 27 | 1.30 | 1.30 | 1.61 | 1.61 | 1.30 | 1.40 | 1.46 | 1.42 |
| 28 | 1.28 | 1.28 | 1.58 | 1.58 | 1.58 | 1.38 | 1.58 | 1.46 |
| Test set | ||||||||
| 29 | 1.29 | 1.59 | 1.59 | 1.59 | 1.29 | 1.49 | 1.44 | 1.47 |
| 30 | 1.32 | 1.62 | 1.62 | 1.62 | 1.32 | 1.52 | 1.47 | 1.50 |
| 31 | 1.32 | 1.32 | 1.62 | 1.32 | 1.32 | 1.42 | 1.32 | 1.38 |
| 32 | 1.62 | 1.32 | 1.32 | 1.32 | 1.32 | 1.42 | 1.32 | 1.38 |
| SD | 0.06 | 0.13 | 0.06 | 0.06 | 0.15 | 0.06 | 0.09 | 0.05 |
| Std | 2.61a | 2.61a | 2.61a | 2.64b | 2.64b | – | – | – |
Norfloxacin.
Fluconazole.
In general, the synthesized compounds were bacteriostatic and fungistatic, as their minimum bactericidal/fungicidal concentration (MFC/MBC) values were 3-fold higher than their MIC values (a drug is considered to be bacteriostatic/fungistatic when its MFC and MBC values are 3-fold higher than its MIC values, Emami et al., 2004).
2.3. Cytotoxic activity
The cytotoxic activity of the synthesized isatin derivatives was determined against breast cancer (MCF 7) and colon cancer (HCT116) cell lines using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Mosmann, 1983), and the results are presented in Table 3 . In general all the synthesized compounds were less active as compared to the standard drug 5-fluorouracil (5-FU). Among the synthesized compounds, 4-[1-(2-chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(4,6-dimethyl-pyrimidin-2-yl)-benzenesulfonamide (18) and 4-[1-(4-chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(5-methyl-isoxazol-3-yl)-benzenesulfonamide (9) were the most effective compounds against the MCF 7 and HCT116 cancer cell lines (IC50 = 90 and 35 μg/mL, respectively), which may be taken as lead compounds for the development of novel cytotoxic agents.
Table 3.
Anticancer activity of synthesized isatin derivatives against HCT 116 and MCF 7 cell linesa.
| Comp. | IC50 (μg/ml) |
|
|---|---|---|
| HCT 116 | MCF 7 | |
| 1 | NA | >1000 |
| 2 | 70 | 400 |
| 3 | 70 | 400 |
| 4 | 45 | 300 |
| 5 | 40 | 400 |
| 6 | >1000 | 250 |
| 7 | 120 | 300 |
| 8 | 50 | 500 |
| 9 | 35 | 500 |
| 10 | 150 | 450 |
| 11 | 50 | 450 |
| 12 | 120 | 500 |
| 13 | 280 | 190 |
| 14 | 400 | 200 |
| 15 | 500 | 150 |
| 16 | 300 | 110 |
| 17 | 200 | 190 |
| 18 | 210 | 90 |
| 19 | 210 | 200 |
| 20 | 480 | 170 |
| 21 | 70 | 100 |
| 22 | 400 | 140 |
| 23 | 110 | 110 |
| 24 | 260 | 100 |
| 25 | 200 | 150 |
| 26 | 120 | 160 |
| 27 | 210 | 100 |
| 28 | 500 | 200 |
| 29 | 70 | 500 |
| 30 | – | >1000 |
| 31 | 90 | 450 |
| 32 | 80 | 500 |
| 5-FU | 0.67 | 6 |
NA – not able to obtain IC50 after three independent tests.
Data represent mean values of three replicates.
2.4. Antiviral activity
The antiviral activity of the synthesized isatin derivatives was determined against different DNA and RNA viruses. None of the derivatives were found to be markedly active against feline corona virus and feline herpes virus in CrFK cell cultures, herpes simplex virus-1 (KOS), herpes simplex virus-2 (G), vaccinia virus, vesicular stomatitis virus, herpes simplex virus-1 and TK- KOS ACVr in HEL cell cultures, vesicular stomatitis virus, Coxsackie virus B4 and respiratory syncytial virus in HeLa cell cultures and Coxsackie virus B4, parainfluenza-3 virus, reovirus-1, Sindbis virus and Punta Toro virus in Vero cell cultures. The anti-HIV activity of synthesized derivatives was studied against wild-type HIV-1 (IIIB) and HIV-2 (ROD) strains in MT-4 cell cultures using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method.
Anti-HIV screening results clearly indicated that none of the synthesized compounds exhibited significant anti-HIV activity at subtoxic concentrations. Only compounds 10 and 11 showed marginal anti-Coxsackie virus activity at ∼45 μg/ml in both (Vero and HeLa) cell cultures.
2.5. Structure–activity relationship
-
a.
The high antibacterial/antifungal activity of compounds 11 and 18 indicated that the presence of electron donating methyl group on the pyrimidine ring increases the antimicrobial activity of synthesized isatin derivatives. The role of electron releasing groups in enhancing the antimicrobial activity of isatin derivatives with pyrimidine is supported by a study of Pandeya et al. (1999).
-
b.
The presence of an ortho- and para chloro-substituted benzoyl moiety increased the antimicrobial activity of synthesized isatin derivatives, whereas an unsubstituted benzoyl moiety leads to a decrease in biological activity which indicates that the presence of a chloro group on ortho or para position of the benzoyl moiety may be useful for its binding to the receptor. The role of the electron withdrawing group in improving antibacterial activity is supported by the studies of Konda et al. (2011).
-
c.
The cytotoxic activity results indicated that the presence of electron releasing groups (methyl) may increase the cytotoxic potential of the synthesized isatin derivatives. The role of electron releasing groups in improving cytotoxic activity is supported by the studies of Yogeeswari et al. (2005) and Mologni et al. (2010).
The above findings are summarized in Fig. 1 .
Figure 1.
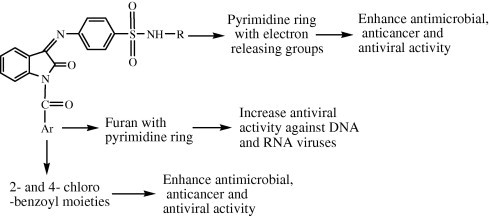
Structure–activity relationship for antimicrobial activity of synthesized isatin derivatives.
2.6. QSAR studies
2.6.1. Development of multi-target QSAR model
In order to identify substituent effect on antimicrobial activity, we established a quantitative structure–activity relationship (QSAR) between in vitro antimicrobial activity and descriptors coding for lipophilic, electronic, steric and topological properties of the molecules under consideration using the linear free energy relationship model (LFER) described by Hansch and Fujita (1964).
Antimicrobial activity data determined as MIC values were first transformed into pMIC values and used as a dependent variable in the QSAR study. The different molecular descriptors (independent variables) like log of octanol–water partition coefficient (log P), molar refractivity (MR), Kier’s molecular connectivity (0 χ, 0 χ v, 1 χ, 1 χ v, 2 χ, 2 χ v) and shape (κα 1) topological indices, Randic topological index (R), Balaban topological index (J), Wiener topological index (W), Total energy (Te), energies of highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), dipole moment (μ) and electronic energy (Ele.E), calculated for synthesized compounds are presented in Table 4 . (Hansch and Fujita, 1964, Hansch et al., 1973, Kier and Hall, 1976, Randic, 1975, Randic, 1993, Balaban, 1982, Wiener, 1947).
Table 4.
Values of selected descriptors used in the regression analysis.
| Comp. | log P | 0χv | 1χv | κα1 | R | J | Te | LUMO | HOMO | μ |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.80 | 19.26 | 12.12 | 23.45 | 16.83 | 1.11 | −6066.58 | −1.43 | −9.40 | 5.03 |
| 2 | 2.65 | 17.73 | 11.13 | 21.83 | 15.28 | 1.30 | −5592.84 | −1.61 | −9.55 | 4.89 |
| 3 | 3.59 | 18.95 | 12.06 | 23.56 | 16.94 | 1.10 | −5941.01 | −1.50 | −9.45 | 6.13 |
| 4 | 3.78 | 19.87 | 12.48 | 24.51 | 17.33 | 1.10 | −6096.83 | −1.48 | −9.44 | 6.46 |
| 5 | 4.18 | 19.22 | 12.64 | 22.87 | 16.44 | 1.11 | −5786.82 | −1.44 | −9.26 | 4.35 |
| 6 | 4.02 | 21.61 | 13.13 | 27.31 | 18.80 | 1.08 | −6892.74 | −1.52 | −9.47 | 4.57 |
| 7 | 3.96 | 20.28 | 12.60 | 25.43 | 17.87 | 1.04 | −6416.38 | −1.24 | −9.31 | 3.63 |
| 8 | 3.97 | 19.08 | 12.20 | 23.24 | 16.94 | 1.10 | −5876.24 | −1.44 | −9.41 | 5.94 |
| 9 | 4.32 | 20.37 | 12.63 | 24.68 | 17.23 | 1.11 | −6426.68 | −1.52 | −9.50 | 5.43 |
| 10 | 4.30 | 20.99 | 12.99 | 25.75 | 17.73 | 1.10 | −6456.92 | −1.53 | −9.50 | 6.26 |
| 11 | 4.54 | 22.73 | 13.64 | 28.55 | 19.20 | 1.08 | −7252.83 | −1.58 | −9.54 | 4.56 |
| 12 | 4.48 | 21.40 | 13.11 | 26.67 | 18.26 | 1.08 | −6776.58 | −1.50 | −9.31 | 4.94 |
| 13 | 4.32 | 20.37 | 12.63 | 24.68 | 17.24 | 1.12 | −6426.62 | −1.46 | −9.43 | 5.87 |
| 14 | 3.17 | 18.85 | 11.65 | 23.07 | 15.69 | 1.32 | −5952.87 | −1.59 | −9.56 | 4.39 |
| 15 | 4.11 | 20.07 | 12.58 | 24.79 | 17.35 | 1.11 | −6301.05 | −1.46 | −9.46 | 5.62 |
| 16 | 4.30 | 20.99 | 13.00 | 25.75 | 17.74 | 1.11 | −6456.86 | −1.46 | −9.44 | 5.91 |
| 17 | 4.70 | 20.33 | 13.15 | 24.10 | 16.85 | 1.13 | −6146.85 | −1.42 | −9.26 | 4.19 |
| 18 | 4.54 | 22.73 | 13.64 | 28.55 | 19.21 | 1.09 | −7252.77 | −1.52 | −9.48 | 4.22 |
| 19 | 4.48 | 21.40 | 13.11 | 26.67 | 18.26 | 1.08 | −6776.49 | −1.45 | −9.23 | 7.97 |
| 20 | 4.48 | 21.40 | 13.11 | 26.67 | 18.26 | 1.08 | −6776.58 | −1.50 | −9.31 | 4.94 |
| 21 | 2.75 | 18.51 | 11.60 | 22.71 | 16.33 | 1.11 | −6102.21 | −1.42 | −9.38 | 4.66 |
| 22 | 2.54 | 18.20 | 11.55 | 22.81 | 16.44 | 1.10 | −5976.63 | −1.49 | −9.43 | 5.76 |
| 23 | 2.54 | 18.20 | 11.55 | 22.81 | 16.44 | 1.10 | −5976.63 | −1.49 | −9.43 | 5.76 |
| 24 | 2.73 | 19.13 | 11.97 | 23.77 | 16.83 | 1.10 | −6132.46 | −1.46 | −9.42 | 6.76 |
| 25 | 3.13 | 18.47 | 12.12 | 22.13 | 15.94 | 1.12 | −5822.44 | −1.43 | −9.27 | 4.07 |
| 26 | 2.97 | 20.87 | 12.61 | 26.56 | 18.30 | 1.08 | −6928.37 | −1.51 | −9.45 | 4.92 |
| 27 | 2.91 | 19.53 | 12.09 | 24.68 | 17.37 | 1.08 | −6452.11 | −1.43 | −9.31 | 5.85 |
| 28 | 2.92 | 18.33 | 11.69 | 22.50 | 16.44 | 1.10 | −5911.86 | −1.44 | −9.39 | 5.67 |
According to ot-QSAR models, one should use five different equations with different errors to predict the activity of a new compound against the five microbial species. The ot-QSAR models, which are present in the whole literature, become impractical to use when we have to predict each compound results for more than one target.
However, very recently the interest has been increased in the development of multi-target QSAR (mt-QSAR) models (Sigroha et al., 2012, Sharma et al., 2012, Kumar et al., 2010). In opposition to ot-QSAR, the mt-QSAR model is a single equation that considers the nature of molecular descriptors which are common and essential for describing the antibacterial and antifungal activities. Based on the facts mentioned above and in continuation of our research in the field of mt-QSAR (Sigroha et al., 2012, Sharma et al., 2012, Kumar et al., 2010), we have attempted to develop three different types of mt-QSAR models viz. mt-QSAR model for describing antibacterial activity of synthesized compounds against S. aureus, B. subtilis and E. coli, mt-QSAR model for describing antifungal activity of synthesized compounds against C. albicans and A. niger as well a common mt-QSAR model for describing the antimicrobial (overall antibacterial and antifungal) activity of isatin derivatives against all the above mentioned microorganisms.
In order to develop mt-QSAR models, initially we calculated the average antibacterial activity, antifungal activity and antimicrobial activity values of substituted isatin derivatives (Table 2). In the present study, a training set consisting of 28 isatin derivatives (1–28) was used for linear regression model generation and a test set consisting of 4 isatin derivatives (29–32) was used for cross validation of the generated models. Preliminary analysis was carried out in terms of a correlation analysis. A correlation matrix constructed for antibacterial activity is presented in Table 5 . The correlations of different molecular descriptors with antibacterial and antifungal activities are presented in Table 6 . The regression models developed using different molecular descriptors are reported in Eqs. (1), (2) together with statistical parameters of regression. The antibacterial activity of synthesized isatin derivatives is explained by the topological parameter, valence zero order molecular connectivity index, 0χv (Eq. (1)).
Table 5.
Correlation matrix for antibacterial activity of synthesized isatin derivatives.
| pMICab | log P | 0χv | 1χv | R | J | LUMO | HOMO | μ | |
|---|---|---|---|---|---|---|---|---|---|
| pMICab | 1 | 0.596 | 0.839 | 0.785 | 0.747 | −0.272 | −0.221 | −0.189 | −0.142 |
| log P | 1 | 0.784 | 0.884 | 0.635 | −0.311 | 0.041 | 0.161 | −0.069 | |
| 0χv | 1 | 0.954 | 0.942 | −0.437 | −0.136 | −0.028 | −0.066 | ||
| 1χv | 1 | 0.869 | −0.496 | −0.009 | 0.147 | −0.103 | |||
| R | 1 | −0.633 | −0.022 | 0.000 | 0.017 | ||||
| J | 1 | −0.542 | −0.429 | −0.156 | |||||
| LUMO | 1 | 0.671 | −0.092 | ||||||
| HOMO | 1 | −0.009 | |||||||
| μ | 1 |
Table 6.
Correlation of molecular descriptors with antimicrobial activity of the isatin derivatives.
| pMICab | pMICaf | pMICam | |
|---|---|---|---|
| log P | 0.596 | 0.339 | 0.634 |
| MR | 0.774 | 0.246 | 0.701 |
| 0χ | 0.795 | 0.178 | 0.674 |
| 0χv | 0.839 | 0.224 | 0.733 |
| 1χ | 0.747 | 0.23 | 0.671 |
| 1χv | 0.785 | 0.291 | 0.737 |
| 2χ | 0.801 | 0.216 | 0.701 |
| 2χv | 0.753 | 0.289 | 0.714 |
| 3χ | 0.767 | 0.023 | 0.56 |
| 3χv | 0.692 | 0.182 | 0.605 |
| κ1 | 0.787 | 0.148 | 0.649 |
| κ2 | 0.717 | 0.146 | 0.598 |
| κ3 | 0.734 | 0.074 | 0.567 |
| κα1 | 0.804 | 0.173 | 0.677 |
| κα2 | 0.752 | 0.184 | 0.646 |
| κα3 | 0.774 | 0.126 | 0.627 |
| R | 0.747 | 0.23 | 0.671 |
| J | −0.272 | −0.36 | −0.412 |
| W | 0.767 | 0.191 | 0.661 |
| Te | −0.795 | −0.201 | −0.687 |
| Ee | −0.797 | −0.191 | −0.683 |
| Ne | 0.796 | 0.19 | 0.681 |
| Ip | 0.189 | −0.108 | 0.071 |
| LUMO | −0.221 | 0.326 | 0.04 |
| HOMO | −0.189 | 0.108 | −0.071 |
| μ | −0.142 | −0.164 | −0.203 |
mt-QSAR model for antibacterial activity
| (1) |
Here and thereafter, n – number of data points, r – correlation coefficient, q 2 – cross validated, r 2 obtained by leave one out method, s – standard error of the estimate and F – Fischer statistics.
As the coefficient of 0χv in Eq. (1) is positive, the antibacterial activity will increase with an increase in the value of 0χv. This is clearly evident from Table 4 that compounds 11 and 18 having highest 0χv value (22.73 each) have highest antibacterial activity value of 1.57 each (Table 2) respectively. Similarly, compound 3 has low 0χv value 18.95, has minimum antibacterial activity (1.29). The value of q2 for Eq. (1) is 0.639, which means that it can predict 63.9% of variance of antibacterial activity.
Topological indices (e.g. 0χv) are numerical quantifier of molecular topology and are sensitive to bonding pattern, symmetry, content of heteroatom as well as degree of complexity of atomic neighborhoods. Since structure of a compound depends on connectivity of its constituent atoms, topological descriptors derived from information based upon connectivity can reveal the role of structural or sub-structural information of a molecule in estimating biological activity, viz. antimicrobial activity (Lather and Madan, 2005). The QSAR model expressed by Eq. (1) was cross validated by its high q2 values (q2 = 0.639) obtained by leave one out (LOO) method. The value of q2 greater than 0.5 is the basic requirement for qualifying a QSAR model to be valid (Golbraikh and Tropsha, 2002).
The predictability of the Eq. (1) is evidenced by the low residual values observed in Table 7 as well by the plot of predicted pMICab against observed pMICab (Fig. 2 ). Further, the plot of observed pMICab versus residual pMICab (Fig. 3 ) indicated that there was no systemic error in the development of linear regression model as the propagation of residuals was observed on both sides of zero (Heravi and Kyani, 2004).
Table 7.
Comparison of observed and predicted antimicrobial activity of the isatin derivatives.
| Comp. | pMICab |
pMICam |
||||
|---|---|---|---|---|---|---|
| Obs. | Pre. | Res. | Obs. | Pre. | Res. | |
| Training set | ||||||
| 1 | 1.39 | 1.39 | 0.00 | 1.41 | 1.44 | −0.03 |
| 2 | 1.35 | 1.33 | 0.02 | 1.37 | 1.39 | −0.02 |
| 3 | 1.29 | 1.38 | −0.09 | 1.41 | 1.44 | −0.03 |
| 4 | 1.40 | 1.41 | −0.01 | 1.42 | 1.47 | −0.05 |
| 5 | 1.39 | 1.39 | 0.00 | 1.41 | 1.47 | −0.06 |
| 6 | 1.44 | 1.48 | −0.04 | 1.46 | 1.50 | −0.04 |
| 7 | 1.41 | 1.43 | −0.02 | 1.49 | 1.47 | 0.02 |
| 8 | 1.39 | 1.38 | 0.01 | 1.47 | 1.45 | 0.02 |
| 9 | 1.42 | 1.43 | −0.01 | 1.50 | 1.47 | 0.03 |
| 10 | 1.53 | 1.45 | 0.08 | 1.51 | 1.49 | 0.02 |
| 11 | 1.57 | 1.52 | 0.05 | 1.55 | 1.53 | 0.02 |
| 12 | 1.44 | 1.47 | −0.03 | 1.46 | 1.50 | −0.04 |
| 13 | 1.42 | 1.43 | −0.01 | 1.50 | 1.47 | 0.03 |
| 14 | 1.39 | 1.37 | 0.02 | 1.41 | 1.42 | −0.01 |
| 15 | 1.42 | 1.42 | 0.00 | 1.50 | 1.47 | 0.03 |
| 16 | 1.43 | 1.45 | −0.02 | 1.51 | 1.49 | 0.02 |
| 17 | 1.42 | 1.43 | −0.01 | 1.5 | 1.50 | 0.00 |
| 18 | 1.57 | 1.52 | 0.05 | 1.61 | 1.53 | 0.08 |
| 19 | 1.44 | 1.47 | −0.03 | 1.46 | 1.50 | −0.04 |
| 20 | 1.42 | 1.47 | −0.05 | 1.50 | 1.50 | 0.00 |
| 21 | 1.38 | 1.36 | 0.02 | 1.46 | 1.42 | 0.04 |
| 22 | 1.34 | 1.35 | −0.01 | 1.42 | 1.41 | 0.01 |
| 23 | 1.38 | 1.35 | 0.03 | 1.46 | 1.41 | 0.05 |
| 24 | 1.39 | 1.38 | 0.01 | 1.41 | 1.44 | −0.03 |
| 25 | 1.38 | 1.36 | 0.02 | 1.46 | 1.45 | 0.01 |
| 26 | 1.43 | 1.45 | −0.02 | 1.45 | 1.47 | −0.02 |
| 27 | 1.40 | 1.40 | 0.00 | 1.42 | 1.44 | −0.02 |
| 28 | 1.38 | 1.35 | 0.03 | 1.46 | 1.42 | 0.04 |
| Test set | ||||||
| 29 | 1.49 | 1.38 | 0.11 | 1.47 | 1.41 | 0.06 |
| 30 | 1.52 | 1.42 | 0.10 | 1.50 | 1.47 | 0.03 |
| 31 | 1.42 | 1.43 | −0.01 | 1.38 | 1.50 | −0.12 |
| 32 | 1.42 | 1.43 | −0.01 | 1.38 | 1.47 | −0.09 |
Figure 2.
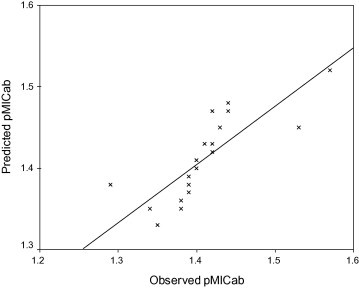
Plot of predicted pMICab values against observed pMICab values for the model developed by Eq. (1).
Figure 3.
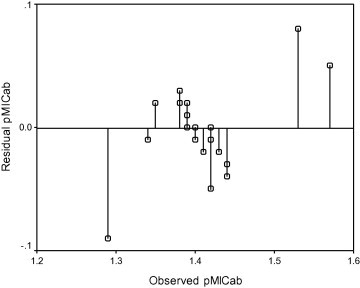
Plot of residual pMICab values against observed pMICab values for the model developed by Eq. (1).
Eq. (2) was developed to predict the antimicrobial activity of isatin derivatives. The antimicrobial activity of synthesized isatin derivatives is governed by the topological parameter, valence first order molecular connectivity index, 1 χ v (Eq. (2)).
True predictive power of a QSAR model is to test their ability to predict accurately the biological activities of compounds from an external test set (compounds which were not used for the model development). The low residual activity values observed in case of test set (29–32) justify the selection of the multiple linear regression models expressed by Eqs. (1), (2). It is important to mention here that no statistically significant correlation was observed in the case of antifungal activity (Table 6).
The cross-validation of the model developed for antimicrobial activity (Eq. (2)) was also done by leave one out (LOO) technique (Schaper, 1999). In case Eq. (2) q 2 value is less than 0.5, the developed model is then considered invalid. But one should not forget the recommendations of Golbraikh and Tropsha (2002) who recently reported that the only way to estimate the true predictive power of a model is to test their ability to predict accurately the biological activities of compounds. The predictive ability of developed model was evidenced by the low residual values observed in Table 7.
mt-QSAR model for antimicrobial activity
| (2) |
The coefficient of 1 χ v is positive, which shows that the antimicrobial activity will increase with the increase in value of 1 χ v of the synthesized compounds, which can be clearly seen from the results of antimicrobial activity (Table 2) and values of 1 χ v are presented in Table 4.
Generally for QSAR studies, the biological activities of compounds should span 2–3 orders of magnitude. But in the present study the range of antibacterial and antifungal activities of the synthesized compounds is within one order of magnitude. But it is important to note that the predictability of the QSAR models developed in the present study is evidenced by their low residual values. This is in accordance with results suggested by earlier studies (Bajaj et al., 2005), according to which the reliability of the QSAR model lies in its predictive ability even though the activity data are in the narrow range. Further, recent literature reveals that the QSAR has been applied to describe the relationship between narrow range of biological activity and physicochemical properties of the molecules (Narasimhan et al., 2007, Sharma et al., 2006, Kumar et al., 2006). When biological activity data lie in a narrow range, the presence of minimum standard deviation of the biological activity justifies its use in QSAR studies (Narasimhan et al., 2007, Kumar et al., 2007). The minimum standard deviation (Table 2) observed in the antimicrobial activity data justifies its use in QSAR studies.
It was observed from mt-QSAR models [Eqs. (1), (2)] that the antibacterial and antimicrobial activity of synthesized isatin derivatives is governed by the topological parameters valence zero order molecular connectivity indices (0 χ v) and valence first order molecular connectivity indices (1 χ v).
3. Experimental
3.1. Chemistry
Starting materials were obtained from commercial sources and were used without further purification. Solvents were dried by standard procedures. Reaction progress was observed by thin layer chromatography. Melting points were determined in open capillary tubes on a Sonar melting point apparatus and are uncorrected.
1H nuclear magnetic resonance (1H NMR) spectra were determined by Bruker 500 MHz NMR spectrometer in appropriate deuterated solvents and are expressed in parts per million (δ, ppm) downfield from tetramethylsilane (internal standard) NMR data are given as multiplicity (s, singlet; d, doublet; t, triplet; m, multiplet) and number of protons. IR spectra were recorded on a Varian Resolutions Pro spectrophotometer in a KBr disk.
3.2. General procedure for the synthesis of 4-(1-aryl-2-oxo-1, 2-dihydro-indol-3-ylideneamino)-N-Substituted benzenesulfonamide (1–32)
Thionyl chloride 32.8 g (0.3 mol) was added to different aromatic acids (0.25 mol) in a round bottomed flask. After addition, the mixture was refluxed for 2 h. The excess of thionyl chloride was removed by distillation. To the solution of acid chloride (1 mol) was added 0.1 mol of isatin and the mixture was refluxed for 1.5 h. Then the reaction mixture was cooled and the resultant precipitate (N-acyl isatin) was collected, washed with hexane and recrystallized from ethyl acetate. A solution of 0.05 mol of different sulfonamides in warm ethanol was added to the solution of corresponding of N-acyl isatins (0.05 mol) in the presence of small amount of glacial acetic acid. The mixture was refluxed for 4–6 h. Then the reaction mixture was allowed to cool at room temperature and the precipitate obtained was filtered, dried and recrystallized from ethanol.
3.2.1. 4-(1-Benzoyl-2-oxo-1,2-dihydro-indol-3-ylideneamino)-N-(5-methyl-4,5-dihydro-isoxazol-3-yl)-benzenesulfonamide (1)
Mp (°C) 246–248; Yield – 85%; IR (KBr pellets) cm−1 1495 (NH in plane bending, sec. amine), 1683 (C O str., Ar–C O), 1650 (C N str.), 1157 syn (O S O str.), 1252 (C–O–N str., isoxazole), 899 (CH out of plane bending, isoxazole), 899–669 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.06–7.87 (14H, m, ArH), 3.35 (1H, s, SO2NH), 2.31 (3H, s, ArCH3).
3.2.2. N-Acetyl-4-(1-benzoyl-2-oxo-1,2-dihydro-indol-3-ylideneamino)- benzenesulfonamide (2)
Mp (°C) 88–90; Yield – 80%; IR (KBr pellets) cm−1 1495 (NH in plane bending, sec. amine), 1686 (C O str., Ar–C O), 1646 (C N str.), 1156 syn (O S O str.), 1447 (CH3 bending vibration, COCH3), 894–669 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 7.33–8.71 (13H, m, ArH), 3.32 (1H, s, SO2NH), 1.40 (3H, s, COCH3).
3.2.3. 4-(1-Benzoyl-2-oxo-1,2-dihydro-indol-3-ylideneamino)-N-pyrimidin-2-yl-benzenesulfonamide (3)
Mp (°C) 252–254; Yield – 72%; IR (KBr pellets) cm−1 1495 (NH in plane bending, sec. amine), 1682 (C O str., Ar–C O), 1648 (C N str.), 1157 syn (O S O str.), 1608 (C N str.,pyrimidine), 768 (CH out of plane bending, 4-sustituted pyrimidine), 900–698 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 7.06–8.52 (16H, m, ArH), 3.35 (1H, s, SO2NH).
3.2.4. 3.2.4.4-(1-Benzoyl-2-oxo-1,2-dihydro-indol-3-ylideneamino)-N-(methyl-pyrimidin-2-yl)-benzene sulfonamide (4)
Mp (°C) 268–270; Yield – 76%; IR (KBr pellets) cm−1 1494 (NH in plane bending, sec. amine), 1678 (C O str., Ar–C O), 1654 (C N str), 1156 syn (O S O str), 1611 (C N str.,pyridine), 752–795 (CH out of plane bending, 4-sustituted pyrimidine), 897–697 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 5.93–7.78 (15H, m, ArH), 3.36 (1H, s, SO2NH), 2.49 (3H, s, ArCH3).
3.2.5. 4-(1-Benzoyl-2-oxoindol-3-ylideneamino)-N-(thiazol-2-yl)-benzenesulfonamide (5)
Mp (°C) 213–215; Yield – 82%; IR (KBr pellets) cm−1 1494 (NH in plane bending, sec. amine), 1687 (C O str., Ar–C O), 1639 (C N str), 1151 syn (O S O str), 1583 (C N str., thiazole), 750 (C–S–C str., thiazole), 895 (CH out of plane bending, thiazole), 895–651 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 7.33–8.71 (15, m, ArH), 3.35 (1H, s, SO2NH).
3.2.6. 3.2.6.4-(1-Benzoyl-2-oxo-1,2-dihydro-indol-3-ylideneamino)-N-(4,6-dimethyl-pyrimidin-2-yl)-benzenesulfonamide (6)
Mp (°C) 124–126; Yield – 85%; IR (KBr pellets) cm−1 1495 (NH in plane bending, sec. amine), 1687 (C O str., Ar–C O), 1646 (C N str), 1156 syn (O S O str), 1277 (C–O–C str.), 1584 (C N str.,pyrimidine), 1609 (C C str.,pyrimidine), 893–669 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 7.33–8.70 (14H, m, ArH), 3.35 (1H, s, SO2NH), 1.58 (6H, s, ArCH3).
3.2.7. 3.2.7.4-(1-Benzoyl-2-oxo-1,2-dihydro-indol-3-ylideneamino)-N-(6-methoxy-pyridazin-3-yl)-benzenesulfonamide (7)
Mp (°C) 208–210; Yield – 74%; IR (KBr pellets) cm−1 1496 (NH in plane bending, sec. amine), 1687 (C O str., Ar–C O), 1646 (C N str), 1150 syn (O S O str), 1286 (C–O–C str.), 1468 (N–N str., pyridazine), 892–676 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 7.35–8.08 (15H, m, ArH), 3.35 (1H, s, SO2NH), 1.46 (3H, s, ArOCH3).
3.2.8. 4-(1-Benzoyl-2-oxo-1,2-dihydro-indol-3-ylideneamino)-N-pyridin-2-yl-benzenesulfonamide (8)
Mp (°C) 220–222; Yield – 68%; IR (KBr pellets) cm−1 1496 (NH in plane bending, sec. amine), 1687 (C O str., Ar–C O), 1645 (C N str), 1164 syn (O S O str), 1583 (C N str.,pyridine), 1609 (C C str.,pyridine), 894–692 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 7.61–8.73 (15H, m, ArH), 3.35 (1H, s, SO2NH).
3.2.9. 4-[1-(4-Chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(5-methyl-isoxazol-3-yl)-benzenesulfonamide (9)
Mp (°C) 203–205; Yield – 76%; IR (KBr pellets) cm−1 1492 (NH in plane bending, sec. amine), 1686 (C O str., Ar–C O), 1644 (C N str), 1143 syn (O S O str), 746 (C–Cl str., Ar–Cl), 893 (CH out of plane bending, isoxazole), 893–695 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.11–8.66 (13H, m, ArH), 3.35 (1H, s, SO2NH), 2.35 (3H, s, ArCH3).
3.2.10. 4-[1-(4-Chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(4-methyl-pyrimidin-2-yl)-benzenesulfonamide (10)
Mp (°C) 112–114; Yield – 77%; IR (KBr pellets) cm−1 1492 (NH in plane bending, sec. amine), 1680 (C O str., Ar–C O), 1643 (C N str), 1152 Syn (O S O str), 715 (C–Cl str., Ar–Cl), 1628 (C N str.,pyrimidine), 760 (CH out of plane bending, 4-sustituted pyrimidine), 890–663 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.64–8.02 (14H, m, ArH), 3.35 (1H, s, SO2NH), 2.37 (3H, s, ArCH3); 13CNMR(DMSO d6, δ ppm):168.41, 166.94, 157.40, 153.40, 138.28, 132.51, 131.29, 130.72, 130.04, 128.43, 113.05.
3.2.11. 3.2.11.4-[1-(4-Chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(4,6-dimethyl-pyrimidi-2-yl)-benzenesulfonamide (11)
Mp (°C) 180–182; Yield – 88%; IR (KBr pellets) cm−1 1492 (NH in plane bending, sec. amine), 1680 (C O str., Ar–C O), 1655 (C N str), 1176 Syn (O S O str), 760 (C–Cl str., Ar–Cl), 1254 (C–O–C str.), 1592 (C N str.,pyrimidine), 851–681 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 7.49–8.02 (13H, m, ArH), 3.35 (1H, s, SO2NH), 2.33 (6H, s, ArOCH3); 13C–NMR (DMSO d6, δ ppm):116.07, 163.03, 139.08, 136.30, 133.95, 132.68, 128.32, 127.02, and 125.56.
3.2.12. 4-[1-(4-Chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(6-methoxy-pyridazin-3-yl)-benzenesulfonamide (12)
Mp (°C) 220–222; Yield – 66%; IR (KBr pellets) cm−1 1492 (NH in plane bending, sec. amine), 1680 (C O str., Ar–C O), 1645 (C N str), 1177 Syn (O S O str), 1254 (C–O–C str.), 1472 (N–N str., pyridazine), 897–669 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 7.49–8.02 (14H, m, ArH), 3.35 (1H, s, SO2NH), 1.40 (3H, s, ArOCH3).
3.2.13. 4-[1-(2-Chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(5-methyl-isoxazol-3-yl)-benzenesulfonamide (13)
Mp (°C) 220–222; Yield – 70%; IR (KBr pellets) cm−1 1522 (NH in plane bending, sec. amine), 1688 (C O str., Ar–C O), 1637 (C N str), 1156 Syn (O S O str), 742 (C-Cl str., Ar–Cl), 893–646 (CH out of plane bending, isoxazole), 893–676 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.11–7.85 (13H, m, ArH), 3.35 (1H, s, SO2NH), 2.36 (3H, s, ArCH3).
3.2.14. N-Acetyl-4-[1-(2-chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-benzene sulfonamide (14)
Mp (°C) 220–222; Yield – 82%; IR (KBr pellets) cm−1 1572 (NH in plane bending, sec. amine), 1683 (C O str., Ar–C O), 1640 (C N str), 1172 Syn (O S O str), 744 (C–Cl str., Ar–Cl), 1376 (CH3 bending vibration, COCH3), 855–680 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.67–7.85 (12H, m, ArH), 3.35 (1H, s, SO2NH), 1.95 (3H, s, COCH3).
3.2.15. 3.2.15.4-[1-(2-Chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-pyrimidin-2-yl-benzenesulfonamide (15)
Mp (°C) 220–222; Yield – 74%; IR (KBr pellets) cm−1 1578 (NH in plane bending, sec. amine), 1684 (C O str., Ar–C O), 1651 (C N str.), 1151 Syn (O S O str.), 742 (C–Cl str., Ar–Cl), (C N str.,pyrimidine), 795 (CH out of plane bending, 4-sustituted pyrimidine), 841–667 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.64–8.44 (15H, m, ArH), 3.35 (1H, s, SO2NH).
3.2.16. 4-[1-(2-Chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(4-methyl-pyrimidin-2-yl)-benzenesulfonamide (16)
Mp (°C) 220–222; Yield – 62%; IR (KBr pellets) cm−1 1492 (NH in plane bending, sec. amine), 1688 (C O str., Ar–C O), 1628 (C N str), 1149 Syn (O S O str), 743 (C–Cl str., Ar–Cl), (C N str.,pyrimidine), 795 (CH out of plane bending, 4-sustituted pyrimidine), 888–663 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.64–8.26 (14H, m, ArH), 3.35 (1H, s, SO2NH), 2.37 (3H, s, ArCH3).
3.2.17. 3.2.17.4-[1-(2-Chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-thiazol-2-yl-benzene sulfonamide (17)
Mp (°C) 220–222; Yield – 76%; IR (KBr pellets) cm−1 1495 (NH in plane bending, sec. amine), 1686 (C O str., Ar–C O), 1629 (C N str), 1174 Syn (O S O str), 743 (C–Cl str., Ar–Cl), 1592 (C N str., thiazole), 743 (C-S-C str., thiazole), 855 (CH out of plane bending, thiazole), 855–680 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.66–7.85 (14, m, ArH), 3.35 (1H, s, SO2NH).
3.2.18. 4-[1-(2-Chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(4,6-dimethyl-pyrimidin-2-yl)-benzenesulfonamide (18)
Mp (°C) 124–126; Yield – 82%; IR (KBr pellets) cm−1 1536 (NH in plane bending, sec. amine), 1684 (C O str., Ar–C O), 1621 (C N str), 1145 Syn (O S O str), 749 (C–Cl str., Ar–Cl), 1284 (C–O–C str.), 1592 (C N str.,pyrimidine), 833–671 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.65–7.83 (13H, m, ArH), 3.94 (1H, s, SO2NH), 3.35 (6H, s, ArCH3); 13C-NMR (DMSO d6, δ ppm):167.53, 160.91, 153.06, 153.00, 152.61, 133.94, 133.47, 132.69, 132.11, 130.49, 129.69, 128.25, 127.85, 126.48, 125.79, 124.17, 122.06.
3.2.19. 4-[1-(4-Chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(6-methoxy-pyridazin-3-yl)-benzenesulfonamide (19)
Mp (°C) 220–222; Yield – 70%; IR (KBr pellets) cm−1 1488 (NH in plane bending, sec. amine), 1665 (C O str., Ar–C O), 1621 (C N str), 1309 anti, 1145 Syn (O S O str), 1283 (C–O–C str.), 1470 (N–N str., pyridazine), 841–671 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.65–7.85 (14H, m, ArH), 3.33 (1H, s, SO2NH), 3.32 (3H, s, ArOCH3).
3.2.20. 3.2.20.4-[1-(2-Chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-pyridin-2-yl-benzene sulfonamide (20)
Mp (°C) 220–222; Yield –87%; IR (KBr pellets) cm−1 1476 (NH in plane bending, sec. amine), 1688 (C O str., Ar–C O), 1635 (C N str), 1167 Syn (O S O str), 744 (C–Cl str., Ar–Cl), 1582 (C N str.,pyridine), 870–679 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.63–8.05 (16H, m, ArH), 3.35 (1H, s, SO2NH).
3.2.21. 4-[1-(Furan-2-carbonyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(5-methyl-isoxazol-3-yl)-benzenesulfonamide (21)
Mp (°C) 220–222; Yield – 58%; IR (KBr pellets) cm−1 1483 (NH in plane bending, sec. amine), 1688 (C O str., Ar–C O), 1652 (C N str.), 1162 Syn (O S O str.), 1070 (C–O srt., furan), 1249 (–C–O–N str., isoxazole), 882–677 (CH out of plane bending, isoxazole), 882–677 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.71–8.73 (12H, m, ArH), 3.35 (1H, s, SO2NH), 2.35 (3H, s, ArCH3).
3.2.22. N-Acetyl-4-[1-(furan-2-carbonyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-benzene sulfonamide (22)
Mp (°C) 220–222; Yield – 64%; IR (KBr pellets) cm−1 1483 (NH in plane bending, sec. amine), 1724 (C O str., Ar–C O), 1657 (C N str.), 1160 Syn (O S O str.), 1069 (C–O srt., furan), 1450 (CH3 bending vibration, COCH3), 901–677 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.71–8.74 (11H, m, ArH), 3.35 (1H, s, SO2NH), 1.43 (3H, s, COCH3).
3.2.23. 3.2.23.4-[1-(Furan-2-carbonyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-pyrimidin-2-yl-benzenesulfonamide (23)
Mp (°C) 220–222; Yield – 78%; IR (KBr pellets) cm−1 1487 (NH in plane bending, sec. amine), 1727 (C O str., Ar–C O), 1654 (C N str.), 1153 Syn (O S O str.), 1072 (C–O srt., furan), 1608 (C N str.,pyrimidine), 752 (CH out of plane bending, 4-sustituted pyrimidine), 882–679 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.64–8.74 (14H, m, ArH), 3.32 (1H, s, SO2NH).
3.2.24. 4-[1-(Furan-2-carbonyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(4-methyl-pyrimidin-2-yl)-benzenesulfonamide (24)
Mp (°C) 220–222; Yield – 84%; IR (KBr pellets) cm−1 1483 (NH in plane bending, sec. amine), 1687 (C O str., Ar–C O), 1656 (C N str), 1161 Syn (O S O str), 1070 (C–O srt., furan), 1608 (C N str.,pyridine), 777 (CH out of plane bending, 4-sustituted pyrimidine), 882–674 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.64–8.74 (13H, m, ArH), 3.35 (1H, s, SO2NH), 1.41 (3H, s, ArCH3).
3.2.25. 3.2.25.4-[1-(Furan-2-carbonyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-thiazol-2-yl-benzene sulfonamide (25)
Mp (°C) 220–222; Yield – 76%; IR (KBr pellets) cm−1 1496 (NH in plane bending, sec. amine), 1687 (C O str., Ar–C O), 1644 (C N str), 1151 Syn (O S O str), 1070 (C–O srt., furan), 1585 (C N str., thiazole), 737 (C–S–C str., thiazole), 881–709 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 7.31–7.92 (12H, m, ArH), 3.35 (1H, s, SO2NH).
3.2.26. 3.2.26.N-(4,6-Dimethyl-pyrimidin-2-yl)-4-[1-(furan-2-carbonyl)-2-oxo-1,2-dihydro-indol-3-ylidene amino]-benzenesulfonamide (26)
Mp (°C) 220–222; Yield – 60%; IR (KBr pellets) cm−1 1527 (NH in plane bending, sec. amine), 1673 (C O str., Ar-C O), 1652 (C N str), 1161 Syn (O S O str), 1243 (C–O–C str.), 1070 (C-O srt., furan), 1607 (C N str.,pyrimidine), 882–667 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.71–8.74 (11H, m, ArH), 3.35 (1H, s, SO2NH), 2.43 (6H, s, ArCH3).
3.2.27. 4-[1-(Furan-2-carbonyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(6-methoxy-pyridazin-3-yl)-benzenesulfonamide (27)
Mp (°C) 220–222; Yield – 88%; IR (KBr pellets) cm−1 1483 (NH in plane bending, sec. amine), 1687 (C O str., Ar–C O), 1674 (C N str), 1161 Syn (O S O str), 1087 (C–O srt., furan), 1242 (C–O–C str.), 1483 (N–N str., pyridazine), 882–672 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.71–8.74 (12H, m, ArH), 3.35 (1H, s, SO2NH), 1.42 (3H, s, ArOCH3).
3.2.28. 3.2.28.4-[1-(Furan-2-carbonyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-pyridin-2-yl-benzenesulfonamide (28)
Mp (°C) 220–222; Yield – 66%; IR (KBr pellets) cm−1 1523 (NH in plane bending, sec. amine), 1685 (C O str., Ar–C O), 1635 (C N str), 1181 Syn (O S O str), 1074 (C-O srt., furan), 1582 (C N str.,pyridine), 870–679 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.63–8.05 (15H, m, ArH), 3.23 (1H, s, SO2NH).
3.2.29. N-Acetyl-4-[1-(4-chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-benzene sulfonamide(29)
Mp (°C) 220–222; Yield – 72%; IR (KBr pellets) cm−1 1491 (NH in plane bending, sec. amine), 1687 (C O str., Ar–C O), 1644 (C N str), 1162 Syn (O S O str), 746 (C–Cl str., Ar–Cl), 1407 (CH3 bending vibration, COCH3), 895–666 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.64–8.66 (12H, m, ArH), 3.35 (1H, s, SO2NH), 1.40 (3H, s, COCH3).
3.2.30. 4-[1-(4-Chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-pyrimidin-2-yl-benzene sulfonamide (30)
Mp (°C) 220–222; Yield – 64%; IR (KBr pellets) cm−1 1492 (NH in plane bending, sec. amine), 1680 (C O str., Ar–C O), 1648 (C N str.), 1177 Syn (O S O str.), 747 (C–Cl str., Ar–Cl), 1611 (C N str.,pyrimidine), 760 (CH out of plane bending, 4-sustituted pyrimidine), 896–666 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 7.44–8.02 (15H, m, ArH), 3.35 (1H, s, SO2NH).
3.2.31. 3.2.31.4-[1-(4-Chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-thiazol-2-yl-benzene sulfonamide (31)
Mp (°C) 220–222; Yield – 82%; IR (KBr pellets) cm−1 1492 (NH in plane bending, sec. amine), 1678 (C O str., Ar–C O), 1645 (C N str), 1177 Syn (O S O str), 759 (C–Cl str., Ar–Cl), 1591 (C N str., thiazole), 759 (C–S–C str., thiazole), 896–654 (CH out of plane bending, thiazole), 896–667 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 7.49–8.02 (14, m, ArH), 3.35 (1H, s, SO2NH).
3.2.32. 4-(1-(4-chlorobenzoyl)-2-oxo-indolin-3-ylideneamino)-N-(pyridin-2-yl)benzenesulfonamide (32)
Mp (°C) 228–230; Yield – 70%; IR (KBr pellets) cm−1 1491 (NH in plane bending, sec. amine), 1686 (C O str., Ar–C O), 1643 (C N str), 1164 Syn (O S O str), 746 (C–Cl str., Ar–Cl), 1587 (C N str.,pyridine), 1610 (C C str.,pyridine), 895–665 (CH out of plane bending, indole). 1H NMR (DMSO) δ; 6.63–8.66 (16H, m, ArH), 3.35 (1H, s, SO2NH); 13CNMR(DMSO d6, δ ppm):165.11, 162.99, 153.21, 152.86, 152.77, 147.83, 145.76, 138.63, 138.40, 138.11, 137.67, 136.10, 132.70, 132.45, 130.90,130.24, 129.00, 128.49, 128.28, 126.14, 126.07, 122.85, 116.41, 113.84, 113.55, 112.46, 112.17, 111.84.
3.3. Antimicrobial assay
The antimicrobial activity was performed against Gram-positive bacteria: S. aureus, B. subtilis, the Gram-negative bacteria E. coli and two fungal strains: C. albicans and A. niger using the tube dilution method (Cappucino and Sherman, 1999). Dilutions of test and standard compounds were prepared in double strength nutrient broth – I.P. (bacteria) or Sabouraud dextrose broth – I.P. (fungi) (Annon, 2007). The samples were incubated at 37 °C for 24 h (bacteria), at 25 °C for 7 h (A. niger) and at 37 °C for 48 h (C. albicans) and the results were recorded in terms of minimum inhibitory concentration (MIC).
3.4. Determination of MBC/MFC
The minimum bactericidal concentration (MBC) and fungicidal concentration (MFC) were determined by subculturing 100 μL of culture from each tube (which remained clear in the MIC determination) on fresh medium. MBC and MFC values represent the lowest concentration of compound that produces a 99.9% end point reduction (Rodriguez-Arguelles et al., 2005).
3.5. Antiviral assays
The antiviral assays [except anti-human immunodeficiency virus (HIV) assays] were based on inhibition of virus-induced cytopathicity in HEL [herpes simplex virus type 1 (HSV-1), HSV-2 (G), vaccinia virus, and vesicular stomatitis virus], Vero (parainfluenza-3, reovirus-1, Coxsackie B4, and Punta Toro virus), HeLa (vesicular stomatitis virus, Coxsackie virus B4, and respiratory syncytial virus), MDCK (influenza A (H1N1; H3N2) and B virus) and CrFK (feline corona virus (FIPV) and feline herpes virus) cell cultures. Confluent cell cultures in microtiter 96-well plates were inoculated with 100 cell culture inhibitory dose-50 (CCID50) of virus (1 CCID50 being the virus dose to infect 50% of the cell cultures) in the presence of varying concentrations (100, 20, 4 μg/ml) of the test compounds. Viral cytopathicity was recorded as soon as it reached completion in the control virus-infected cell cultures that were not treated with the test compounds.
The anti-HIV activity of synthesized derivatives was studied against wild-type HIV-1 (IIIB) and HIV-2 (ROD) strains in MT-4 cell cultures using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method (Pauwels et al. 1988). Briefly, virus stocks were titrated in MT-4 cells and expressed as the 50% cell culture infective dose (CCID50). MT-4 cells were suspended in culture medium at 1 × 105 cells/ml and infected with HIV at a multiplicity of infection of 0.02. Immediately after viral infection, 100 μl of the cell suspension was placed in each well of a flat-bottomed microtiter tray containing various concentrations of test compounds. After 4 days of incubation at 37 °C, the number of viable cells was determined using the MTT method. Compounds were tested in parallel for their cytotoxic effects in uninfected MT-4 cell cultures.
3.6. Anticancer (cytotoxic) evaluation
The cytotoxic activity of the synthesized compounds were determined against breast cancer (MCF 7) and colon cancer (HCT116) cell lines. Cancer cell lines were purchased from the American Type Culture Collection (ATCC), Manassas, VA, USA. All cell lines were cultured in RPMI 1640 (Sigma) supplemented with 10% heat inactivated fetal bovine serum (FBS) (PAA Laboratories) and 1% penicillin/streptomycin (PAA Laboratories). Cultures were maintained in a humidified incubator at 37 °C in an atmosphere of 5% CO2. Cytotoxicity of the synthesized compounds at various concentrations was assessed using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) (Sigma) assay, as described by Mosmann (Mosmann 1983) but with minor modification, following 72 h of incubation. Assay plates were read using a spectrophotometer at 520 nm. Data generated were used to plot a dose–response curve of which the concentration of test compounds required to kill 50% of the cell population (IC50) was determined. Anticancer activity was expressed as the mean IC50 of three independent experiments.
3.7. QSAR studies
The QSAR study was used to find out correlation between antimicrobial activity and physicochemical parameters of synthesized isatin derivatives. The structures of synthesized isatin derivatives were first pre-optimized with the Molecular Mechanics Force Field (MM+) procedure included in Hyperchem (1993) and the resulting geometries were further refined by means of the semiempirical method PM-3 (parametric Method-3). We chose a gradient norm limit of 0.01 kcal/Å for the geometry optimization. The lowest energy structure was used for each molecule to calculate physicochemical properties using TSAR 3.3 software for Windows (TSAR 3D Version 3.3, 2000). Furthermore, the regression analysis was performed using the SPSS software package (SPSS for Windows, 1999). The predictive powers of the equation were validated by determination of cross-validated r 2 (q 2) using leave one out (LOO) cross-validation method (Schaper 1999). The statistical qualities of the equations were judged by the parameters like correlation coefficient (r), standard error of estimate (s) and variance ratio (F) at specified degrees of freedom and some other parameters were employed to confirm their predictability and robustness of the developed models.
4. Conclusion
A series of 4-(1-aryl-2-oxo-1,2-dihydro-indol-3-ylideneamino)-N-substituted benzenesulfonamide derivatives (1–32) were synthesized and evaluated for their in vitro antimicrobial, antiviral and cytotoxic activities. Antimicrobial study results indicated that compounds 4-[1-(4-chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(4,6-dimethy-pyrimidin-2-yl)-benzenesulfonamide (11) and 4-[1-(2-chloro-benzoyl)-2-oxo-1,2-dihydro-indol-3-ylideneamino]-N-(4,6-dimethy-pyrimidin-2-yl)-benzenesulfonamide (18) were found to be the most effective antimicrobial agents. The cytotoxic screening results indicated that the synthesized compounds were less active than the standard drug 5-fluorouracil (5-FU). The antiviral screening results indicated that none of the synthesized compounds markedly inhibited viral replication at subtoxic concentration. In general, the presence of a pyrimidine ring with electron releasing groups and an ortho- and para-substituted benzoyl moiety favored the antimicrobial activities. The results of QSAR studies demonstrated the importance of topological parameters, valence zero order molecular connectivity index (0 χ v) and valence first order molecular connectivity index (1 χ v) in describing the antimicrobial activity.
2nd Cancer Update
Footnotes
Peer review under responsibility of King Saud University.
Available online 13 December 2012
References
- Adibi H., Khodaei M.M., Pakravan P., Abiri R. Synthesis, characterization, and in vitro antimicrobial evaluation of hydrazone and bishydrazone derivatives of isatin. Pharm. Chem. J. 2010;44:219–227. [Google Scholar]
- Annon . Pharmacopoeia of India (vol. I) Controller of Publications, Ministry of Health Department, Govt. of India; New Delhi: 2007. p. 37. [Google Scholar]
- Bajaj S., Sambi S.S., Madan A.K. Prediction of anti-inflammatory activity of N-arylanthranilic acids: computational approach using refined Zagreb indices. Croat. Chem. Acta. 2005;78:165–174. [Google Scholar]
- Bal T.R., Anand B., Yogeeswari P., Sriram D. Synthesis and evaluation of anti-HIV activity of isatin beta-thiosemicarbazone derivatives. Bioorg. Med. Chem. Lett. 2005;15:4451–4455. doi: 10.1016/j.bmcl.2005.07.046. [DOI] [PubMed] [Google Scholar]
- Balaban A.T. Highly discriminating distance based topological indices. Chem. Phys. Lett. 1982;89:399–404. [Google Scholar]
- Cappucino J.G., Sherman N. Addison Wesley; California: 1999. Microbiology-A Laboratory Manual. [Google Scholar]
- Emami S., Falahati M., Banifafemi A., Shafiee A. Stereoselective synthesis and antifungal activity of (Z)-trans-3-azolyl-2-methylchromanone oxime ethers. Bioorgan. Med. Chem. 2004;12:5881–5889. doi: 10.1016/j.bmc.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Golbraikh A., Tropsha A. Beware of q2. J. Mol. Graphics Model. 2002;20:269–276. doi: 10.1016/s1093-3263(01)00123-1. [DOI] [PubMed] [Google Scholar]
- Hansch C., Fujita T. P-σ-π analysis. a method for the correlation of biological activity and chemical structure. J. Am. Chem. Soc. 1964;86:1616–1626. [Google Scholar]
- Hansch C., Leo A., Unger S.H., Kim K.H., Nikaitani D., Lien E.J. “Aromatic” substituent constants for structure-activity correlations. J. Med. Chem. 1973;16:1207–1216. doi: 10.1021/jm00269a003. [DOI] [PubMed] [Google Scholar]
- Heravi M.J., Kyani A. Use of computer-assisted methods for the modeling of the retention time of a variety of volatile organic compounds: a PCA-MLR-ANN approach. J. Chem. Inf. Comput. Sci. 2004;44:1328–1335. doi: 10.1021/ci0342270. [DOI] [PubMed] [Google Scholar]
- Hyperchem, 6.0, 1993. Hypercube, Inc., Florida.
- Judge V., Narasimhan B., Ahuja M., Sriram D., Yogeeswari P., DeClercq E., Pannecouque C., Balzarini J. Synthesis, antimycobacterial, antiviral, antimicrobial activity and QSAR studies of isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazides. Med. Chem. Res. 2012;21(8):1935–1952. [Google Scholar]
- Kamal A., Srikanth Y.V.V., Khan M.N.A., Shaik T.B., Ashraf M. Synthesis of 3,3-diindolyl oxyindoles efficiently catalysed by FeCl3 and their in vitro evaluation for anticancer activity. Bioorg. Med. Chem. Lett. 2010;20:5229–5231. doi: 10.1016/j.bmcl.2010.06.152. [DOI] [PubMed] [Google Scholar]
- Karthikeyan K., Sivakumar P.M., Doble M., Perumal P.T. Synthesis, antibacterial activity evaluation and QSAR studies of novel dispiropyrrolidines. Eur. J. Med. Chem. 2010;45:3446–3452. [PubMed] [Google Scholar]
- Kier L.B., Hall L.H. Academic Press; New York: 1976. Molecular Connectivity in Chemistry and Drug Research. [Google Scholar]
- Konda S.G., Shaikh B.M., Chavan S.A., Dawane B.S. Polyethylene glycol (PEG-400): an efficient and recyclable reaction medium for the synthesis of novel 1,5-benzodiazepines and their antimicrobial activity. Chinese Chem. Lett. 2011;1:65–68. [Google Scholar]
- Kumar A., Narasimhan B., Kumar D. Synthesis, antimicrobial and QSAR studies of substituted benzamides. Bioorgan. Med. Chem. 2007;15:4113–4124. doi: 10.1016/j.bmc.2007.03.074. [DOI] [PubMed] [Google Scholar]
- Kumar A., Sharma P., Gurram V.K., Rane N. Studies on synthesis and evaluation of quantitative structure–activity relationship of phosphorus10-methyl-6-oxo-5-arylazo-6,7-dihydro-5H(1,3) azaphospholo(1,5-d) (1,4) benzodiazepin-2-phospha-3-ethoxycarbonyl-1-phosphorus dichlorides. Bioorg. Med. Chem. Lett. 2006;16:2484–2491. doi: 10.1016/j.bmcl.2006.01.080. [DOI] [PubMed] [Google Scholar]
- Kumar D., Judge V., Narang R., Sangwan S., DeClercq E., Balzarini J., Narasimhan B. Benzylidene/2-chlorobenzylidene hydrazides: synthesis, antimicrobial activity, QSAR studies and antiviral evaluation. Eur. J. Med. Chem. 2010;45:2806–2816. doi: 10.1016/j.ejmech.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lather V., Madan A.K. Topological models for the prediction of anti-HIV activity of dihydro (alkylthio) (naphthylmethyl) oxopyrimidines. Bioorgan. Med. Chem. 2005;13:1599–1604. doi: 10.1016/j.bmc.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Matheus M.E., Violante F.A., Garden S.J., Pinto A.C., Fernandes P.D. Isatins inhibit cyclooxygenase-2 and inducible nitric oxide synthase in a mouse macrophage cell line. Eur. J. Pharmacol. 2007;556:200–206. doi: 10.1016/j.ejphar.2006.10.057. [DOI] [PubMed] [Google Scholar]
- Mologni L., Rostagno R., Brussolo S., Knowles P.P., Kjaer S., Murray-Rust J., Rosso E., Zambon A., Scapozza L., McDonald N.Q., Lucchini V., Gambacorti-Passerini C. Synthesis, structure–activity relationship and crystallographic studies of 3-substituted indolin-2-one RET inhibitors. Bioorgan. Med. Chem. 2010;18:482–1496. doi: 10.1016/j.bmc.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nandakumar A., Thirumurugan P., Perumal P.T., Vembu P., Ponnuswamy M.N., Ramesh P. One-pot multicomponent synthesis and anti-microbial evaluation of 2′-(indol-3-yl)-2-oxospiro(indoline-3,4′-pyran) derivatives. Bioorg. Med. Chem. Lett. 2010;20:4252–4258. doi: 10.1016/j.bmcl.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Narang R., Narasimhan B., Sharma S., Sriram D., Yogeeswari P., DeClercq E., Pannecouque C., Balzarini J. Synthesis, antimycobacterial, antiviral, antimicrobial activity and QSAR studies of nicotinic acid benzylidene hydrazide derivatives. Med. Chem. Res. 2012;21(8):1557–1576. [Google Scholar]
- Narasimhan B., Judge V., Narang R., Ohlan S., Ohlan R. Quantitative structure–activity relationship studies for prediction of antimicrobial activity of synthesized 2, 4-hexadienoic acid derivatives. Bioorg. Med. Chem. Lett. 2007;17:5836–5845. doi: 10.1016/j.bmcl.2007.08.037. [DOI] [PubMed] [Google Scholar]
- Pandeya S.N., Sriram D., Nath G., DeClercq E. Synthesis and antimicrobial activity of schiff and mannich bases of isatin and its derivatives with pyrimidine. Farmaco. 1999;54:624–628. doi: 10.1016/s0014-827x(99)00075-0. [DOI] [PubMed] [Google Scholar]
- Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., DeClercq E. Rapid and automated tetrazolium based colorimetric assay for detection of anti-HIV compounds. J. Virol. Methods. 1988;20:309–322. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- Randic M. On characterization of molecular branching. J. Am. Chem. Soc. 1975;97:6609–6615. [Google Scholar]
- Randic M. Comparative regression analysis: regression based on a single descriptor. Croat. Chem. Acta. 1993;66:289–312. [Google Scholar]
- Rodriguez-Arguelles M.C., Lopez-Silva E.C., Sanmartin J., Pelagatti P., Zani F. Copper complexes of imidazole-2-, pyrrole-2- and indol-3-carbaldehyde thiosemicarbazones: inhibitory activity against fungi and bacteria. J. Inorg. Biochem. 2005;99:2231–2239. doi: 10.1016/j.jinorgbio.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Sabet R., Mohammadpour M., Sadeghi A., Fassihi A. QSAR study of isatin analogues as in vitro anti-cancer agents. Eur. J. Med. Chem. 2010;45:1113–1118. doi: 10.1016/j.ejmech.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Schaper K.J. Free-Wilson-type analysis of non-additive substituent effects on THPB dopamine receptor affinity using artificial neural networks. Quant. Struct. Act. Relat. 1999;18:354–360. [Google Scholar]
- Sharma P., Kumar A., Sharma M. Synthesis and QSAR studies on 5-(2-(2-methylprop-1-enyl)-1H-benzimidazol-1yl)-4,6-diphenyl-pyrimidin-2-(5H)-thione derivatives as antibacterial. Eur. J. Med. Chem. 2006;41:833–840. doi: 10.1016/j.ejmech.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Sharma S.K., Kumar P., Narasimhan B., Ramasamy K., Mani V., Mishra R.K., Majeed A.B.A. Synthesis, antimicrobial, anticancer evaluation and QSAR studies of 6-methyl-4-[1-(2-substituted-phenylamino-acetyl)-1H-indol-3-yl]-2-oxo/thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylic acid ethyl esters. Eur. J. Med. Chem. 2012;48:16–25. doi: 10.1016/j.ejmech.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Sigroha S., Narasimhan B., Kumar P., Khatkar A., Ramasamy K., Mani V., Mishra R.K., Majeed A.B.A. Design, synthesis, antimicrobial, anticancer evaluation, and QSAR studies of 4-(substituted benzylidene-amino)-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-ones. Med. Chem. Res. 2012;21:3863–3875. [Google Scholar]
- Solomon V.R., Hua C., Lee H. Design and synthesis of anti-breast cancer agents from 4-piperazinylquinoline: a hybrid pharmacophore approach. Bioorgan. Med. Chem. 2010;18:1563–1572. doi: 10.1016/j.bmc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- SPSS for windows version (1999) 10.05. SPSS Inc., Bangalore, India.
- Sridhar S.K., Pandeya S.N., Stables J.P., Ramesh A. Anticonvulsant activity of hydrazones, schiff and mannich bases of isatin derivatives. Eur. J. Med. Chem. 2002;16:129–132. doi: 10.1016/s0928-0987(02)00077-5. [DOI] [PubMed] [Google Scholar]
- TSAR 3D Version 3.3 (2000) Oxford Molecular Limited.
- Wiener H. Structural determination of paraffin boiling points. J. Am. Chem. Soc. 1947;69:17–20. doi: 10.1021/ja01193a005. [DOI] [PubMed] [Google Scholar]
- Yogeeswari P., Sriram D., Kavya R., Tiwari S. Synthesis and in-vitro cytotoxicity evaluation of gatifloxacin mannich bases. Biomed. Pharmacother. 2005;59:501–510. doi: 10.1016/j.biopha.2005.06.006. [DOI] [PubMed] [Google Scholar]



