In this report, we describe a novel model of progressive T-cell-driven colitis in mice with deficient TGFβ signaling in dendritic cells, which requires both CD4+ and CD8+ lymphocytes and is dependent on CD40L expression by CD4+ T cells.
Keywords: dendritic cells, inflammation, autoimmunity, CD40L, IFNγ, adoptive transfer
Abstract
Background
Inflammatory bowel disease (IBD) is a multifactorial disorder, with the innate and adaptive immune cells contributing to disease initiation and progression. However, the intricate cross-talk between immune cell lineages remains incompletely understood. The role of CD8+ T cells in IBD pathogenesis has been understudied, largely due to the lack of appropriate models.
Methods
We previously reported spontaneous colitis in mice with impaired TGFβ signaling due to dendritic cell–specific knockout of TGFbR2 (TGFβR2ΔDC). Here, we demonstrate that crossing TGFβR2ΔDC mice with a Rag1-/- background eliminates all symptoms of colitis and that adoptive transfer of unfractionated CD3+ splenocytes is sufficient to induce progressive colitis in Rag1-/-TGFβR2ΔDC mice.
Results
Both CD4+ and CD8+ T cells are required for the induction of colitis accompanied by activation of both T-cell lineages and DCs, increased expression of mucosal IFNγ, TNFα, IL6, IL1β, and IL12, and decreased frequencies of CD4+FoxP3+ regulatory T cells. Development of colitis required CD40L expression in CD4+ T cells, and the disease was partially ameliorated by IFNγ neutralization.
Conclusions
This novel model provides an important tool for studying IBD pathogenesis, in particular the complex interactions among innate and adaptive immune cells in a controlled fashion, and represents a valuable tool for preclinical evaluation of novel therapeutics.
Introduction
Inflammatory bowel disease (IBD) is an inflammatory disorder with a multifactorial etiology and is believed to arise from complex interactions among genetic, immune, and microbial factors.1 Adaptive immune cells are one of the central players, as they set the tone for the induction and regulation of inflammation and the establishment of memory and chronicity that culminates in the characteristic pathology. Many of the >50 existing animal models of IBD offer unique advantages and disadvantages, from ease of induction to controlled timing or site involvement.2 Although frequently used in combination, these models can be classified into 4 groups: chemically induced models (eg, dextran sulfate sodium [DSS] or trinitrobenzene sulfonic acid [TNBS]), cell transfer models, congenic/spontaneous models, genetically engineered models, and infectious models. A model most closely approximating IBD would be characterized by the progressive nature of symptoms (not self-limiting), require gut microbiota as a triggering factor, and require intact innate and adaptive immune responses. Shortcomings of many of the existing models are blamed for the significant failure to translate therapeutic efficacy from bench to bedside. Much of the current understanding of the IBD-related T-cell-related immunopathology has been based on the CD4+ CD45RBhigh-naïve T-cell transfer colitis model, which depends on the clonal expansion of autoreactive CD4+ T cells in a lymphopenic host.3 Although it is a valuable model for understanding the contribution of pathogenic and regulatory CD4+ T cells to IBD and for preclinical studies, it fails to appreciate the involvement and interaction with other adaptive immune cells, in particular CD8+ T cells. In mice lacking both IL10 and TNFR2, exacerbated spontaneous colitis was characterized by an expansion of colonic CD8+ T cells and could be ameliorated by CD8+ T-cell depletion.4 SAMP1 and SAMP1/Yit mice develop ileitis characterized by local expansion of CD8+ effector and central memory (TEF and TCM) T cells.5 In mice sensitized and challenged with 2,4-dinitrobenzene sulfonic acid (DNBS), CD8+ T cells (Tc1) specific to hapten-modified self-proteins were rapidly recruited upon challenge into the colonic lamina propria (cLP), and when transferred to naïve recipients, they were sufficient to induce colitis.6 In IBD patients, both CD4+ and CD8+ circulating T cells are activated,7 and the transcriptional profile of activated circulating CD8+ T cells can be predictive of a more active disease course.8 Similarly, a markedly increased population of activated CD8+ T cells could be found in the gut lamina propria of both CD and UC patients.9 Importantly, CD8+ T cells represent an extremely diverse population, both phenotypically and functionally, including both pathogenic and immunosuppressive subsets; compared with the roles of CD4+ T cells, they have been relatively understudied. Thus, a model that can allow precise dissection of the complexities of the interactions among cells of the adaptive and innate immune systems is needed.
We have previously described a conditional knockout mouse model with dendritic cell–specific deletion TGFβ signaling (TGFβR2ΔDC), a strain characterized by spontaneous multi-organ autoimmunity, including progressive colitis, and activation of both CD4+ and CD8+ T cells.10 Using the same genetic approach, another group showed that TGFβR2ΔDC mice develop dysbiosis associated with the relative expansion of Enterobacteriaceae and that transfer of the dysbiotic community to wild-type mice conferred susceptibility to DSS-induced colitis.11 In the present study, we crossed TGFβR2ΔDC with the Rag1-/- background, which entirely eliminated the spontaneous pathology and demonstrated that an adoptive transfer of total CD3+ splenocytes from naïve mice is capable of inducing chronic colitis. Both CD4+ and CD8+ T cells are required for the disease development associated with elevated expression of IFNγ, TNFα, IL6, IL1β, and IL12. The development of colitis required the expression of CD40L on CD4+ T cells and could be partially attenuated by neutralization of IFNγ. This novel model provides an excellent venue for the dissection of the complex network of interactions between the adaptive and innate immune cells in the pathogenesis of IBD.
METHODS
Mice
All mice used in the study were on the C57BL/6J genetic background and were bred and maintained with unrestricted access to food and drinking water in the specific pathogen-free animal facility at the University of Arizona BIO5 Institute. Wild-type (WT) C57BL/6J mice, C.Cg-Foxp3tm2Tch/J (FoxP3IRES-GFP),12 CD40L-/- (CD154-/-; B6; 129S-Cd40lgtm1Imx/J), and Rag1-/- mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Conditional knockout TGFβR2ΔDC mice were generated in our laboratory10 and maintained in the same BIO5 animal environment. Newborn pups were co-fostered with the breeding pair and were weaned at 4 weeks of age, after which they were co-housed as Cre+ and Cre- males or females (separated by sex only). Cre+ TGFβR2ΔDC or Cre- TGFβR2ΔDC were crossed with Rag1-/- mice (B6.129S7-Rag1tm1Mom/J from the Jackson Laboratory) to develop Rag1-/-×Cre+ TGFβR2ΔDC or Rag1-/-×Cre- TGFβR2ΔDC recipient mice (throughout the manuscript referred to as Rag1-/- TGFβR2ΔDC and Rag1-/- Cre-, respectively). All recipient mice were used at 6–8 weeks of age. In some experiments, male Foxp3IRES-GFP or CD40L-/- mice were used as donors. The animal facility is routinely monitored and consistently determined free from common murine pathogens (MHV, MPV, MVM, TMEV, Mycoplasma pulmonis, Sendai, EDIM, MNV, and ecto- and endoparasites). All animals used in the experiments were handled in accordance with University of Arizona University Animal Care guidelines and with an approved Institutional Animal Care and Use Committee (IACUC) protocol (Kiela, 07-126).
Induction of Experimental Colitis Using Naïve CD4+CD45RBHigh T-Cell Transfer
Adoptive naïve T-cell transfer in Rag1-/- mice was conducted as described previously.13 Briefly, animals were injected intraperitoneally with 5×105 naïve CD4+ CD45RBhi T cells. Naïve T cells were first magnetically enriched from the spleens of naïve WT C57BL/6 mice using negative selection LS columns (Miltenyi; Bergisch Gladbach, Germany) and subsequently flow-sorted (CD4 PE, CD45RB FITC; FACSAria II, Beckton-Dickinson, Franklin Lakes, NJ, USA). Donor T cells were sex-matched to recipients. Animals were weighed weekly to monitor health status, along with a weekly fecal collection. No animals were allowed to drop below a critical weight loss of 20% of their starting weight.
Induction of Experimental Colitis Using Total T-Cell Transfer
WT C57BL/6J, Foxp3IRES-GFP, or CD40L-/- donor mice were killed, and spleens were harvested. The spleens were gently homogenized to collect single cells, treated with red blood cell (RBC) lysis buffer to remove RBCs. For all T-cell transfer experiments, splenocytes were subjected to CD3+ T-, CD4+ T-, or CD8+ T-cell isolation by negative selection kits and magnetic columns (Miltenyi Biotec). Purity of cells >95% was considered adequate for each experiment. Cells suspended in sterile united states pharmacopeial convention (USP)-grade phosphate buffered saline (PBS) were intraperitoneally injected into Rag1-/- Cre- or Rag1-/-Tgfbr2ΔDC recipient mice (106 cells/mouse). A subset of mice was injected with PBS only. Body weights were recorded at the time of T-cell injection and monitored weekly. Mice were regularly observed for symptoms of colitis such as weight loss, diarrhea, loss of grooming, and rough fur.
Flow Cytometry
Single-cell suspensions were prepared from spleens and mesenteric lymph nodes. Colonic lamina propria cells from mice were isolated using a method described previously.14 The cells were first stained for viability (Zombie Aqua, Biolegend, San Diego, CA, USA), followed by blocking with anti-FcγIII/II receptor antibody (2.4G2). Incubations with antibodies for cell surface markers were conducted in the dark for 30 minutes at 4°C.
The following fluorochrome-conjugated antimouse antibodies were used: CD4, CD44, CD62L, CD25, CD11c, PDCA1, MHCII, F4/80, CD80, CD86, CD40, E-cadherin, CD45RB, and pSMAD2/3 (Supplementary Table 1). The gating strategy used to identify dendritic cells is described in Supplementary Figure 1. For pSMAD2/3 staining, the cells from colonic lamina propria were fixed with equal volumes of prewarmed fixation buffer (BDBioscience 554655) for 10 minutes at 37°C, washed twice with PBS, and permeabilized with prechilled Perm Buffer III (BDPhosflow 558050) for 30 minutes on ice. Cells were washed 3 times with cold PBS and incubated with Fc block in staining buffer, after which anti-CD11c, anti-PDCA1, and anti-pSmad2/3 were added and incubated for 60 minutes in the dark. The cells were then washed and analyzed by flow cytometry. All data were acquired using the LSR Fortessa flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and analyzed using FlowJo 10.0.06 (Tree Star Inc, Ashland, OR, USA).
Histological Examination
At the end of the experiments, tissue sections were excised from the proximal and distal ends of colon, liver, pancreas, and stomach, fixed in 10% buffered formalin (Fisher Scientific, Waltham, MA), transferred to 70% ethanol, and embedded in paraffin. Samples were cut into 5-μm-thick sections, and hematoxylin and eosin (H&E) staining was performed using the standard procedure. H&E-stained slides were blindly assessed by a veterinary pathologist based on lesion scoring criteria for mouse intestinal lesions modified from Burich et al.15
Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was isolated from mouse proximal and distal colon using TRIzol reagent (ThermoFisher, Waltham, MA), followed by reverse transcription polymerase chain reaction using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). Real-time quantitative polymerase chain reaction (qPCR) was performed to evaluate mucosal expression of relevant cytokines and targets using LightCycler96 thermocycler (Roche). Ct values were obtained using LightCycler96 software (version 1.1.0.1320) and were analyzed by using the comparative Ct method as the means of relative quantification, normalized to TBP as the housekeeping gene and relative to a calibrator (normalized Ct value obtained from PBS-injected Rag1-/- Cre- mice), and expressed as 2-ΔΔCt (Applied Biosystems User Bulletin #2: Rev B “Relative Quantification of Gene Expression”).
Fecal Lipocalin 2 Detection
The fecal samples were weighed and suspended in 1 mL of PBS, followed by vigorous vortexing (400 rpm). The samples were then centrifuged to obtain the fecal pellet and a clear supernatant. The supernatant was separated and stored at –20oC until use. Fecal lipocalin 2 (LCN2) was detected by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Bio-Techne Corporation, Minneapolis, MN, USA) according to the manufacturer’s instructions.
In vivo IFNγ Neutralization
Four weeks after adoptive CD3+ T-cell transfer in Rag1-/-Tgfbr2ΔDC mice, mice were injected intraperitoneally (i.p.) with 1 mg of purified rat antimouse IFN-γ antibody (XMG1.2, BioXcell, West Lebanon, NH) or isotype control monoclonal antibody TNP647 (BioXcell) in 0.5 mL of InvivoPure dilution buffer (BioXcell) once a week for 4 consecutive weeks. In weekly intervals, body weights were recorded, and fecal samples were collected for evaluation of LCN2 analysis.
Statistical Analysis
Data are expressed as mean ± SD. Statistical analysis was performed using Graph Pad Prism, version 7.0 for Windows (Graph Pad Software, San Diego, CA, USA). One-way ANOVA, the unpaired 2-tailed Student t test, or the Mann-Whitney t test was applied, depending on the data set and data distribution (as verified by Shapiro-Wilk test). The Bonferroni multiple-comparisons test was used where applicable.
RESULTS
Total T Cells Are Sufficient to Cause Colitis in Rag1-/-Tgfbr2ΔDC Mice
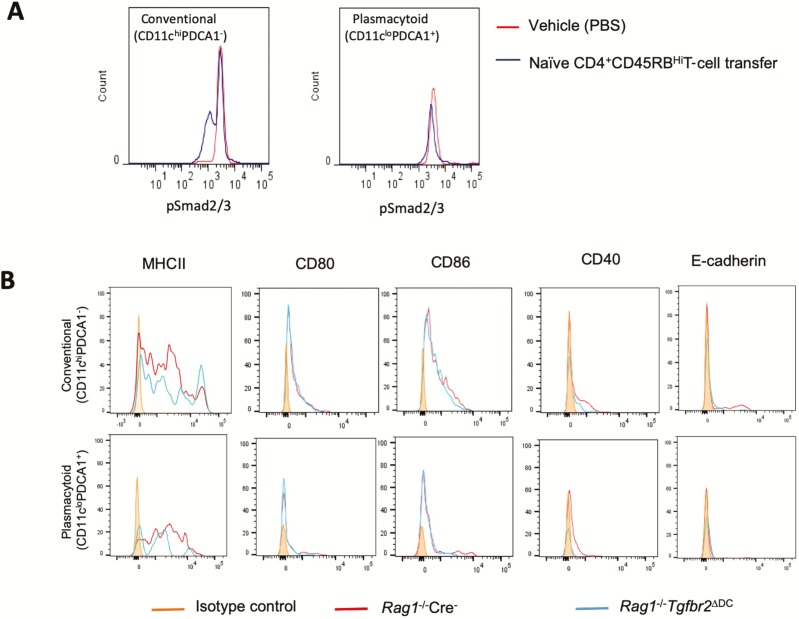
TGFβ signaling in DCs is imperative for the control of autoimmunity, and Tgfbr2ΔDC (CD11c-Cre × Tgfbr2fl/fl) mice develop a spontaneous autoinflammatory phenotype mainly involving the gastrointestinal tract.10 This observation is of physiological relevance as T-cell-mediated colitis in the CD4+ CD45RBHigh adoptive transfer model is associated with decreased TGFβ signaling in colonic cDCs (but not pDCs), as determined by flow cytometric analysis of pSmad2/3 (Fig. 1A). Crossing of Tgfbr2ΔDC mice into a T- and B-cell-deficient Rag1-/- background entirely eliminated all described symptoms, and Rag1-/-Tgfbr2ΔDC mice maintained a normal and healthy phenotype with no overall or histological evidence of autoimmunity (data not shown). We evaluated mesenteric lymph node (MLN) dendritic cells for the expression of various activation markers such as E-cadherin, MHCII, CD40, CD80, and CD86 in both conventional and plasmacytoid dendritic cells (cDC and pDC, respectively) and found no difference in their expression between DCs from Rag1-/- Cre- and Rag1-/-Tgfbr2ΔDC mice (Fig. 1B).
Figure 1.
A, TGFβ signaling in DCs is reduced in T-cell-mediated colitis. CD4+ CD45RBhi-naïve T cells were adoptively transferred into Rag1-/- mice to induce colitis. PBS-injected mice served as the control group. At the end of the experiment, mice were killed, and single cells from the colonic lamina propria were subjected to flow cytometry to evaluate pSmad2/3 expression in conventional (CD11chi PDCA1-) and plasmacytoid DCs (CD11clo PDCA1+). Histograms show decreases in pSmad2/3 expression in conventional but not plasmacytoid DCs. B, Dendritic cells from Rag-/-Cre+Tgfbr2ΔDC mice are not activated at steady state. Surface expression of MHCII, CD80, CD86, CD40, and E-cadherin on the conventional and plasmacytoid DCs from MLNs of Rag1-/- Cre- and Rag1-/-Tgfbr2ΔDC mice. Three to 4 mice from each genotype were pooled to get a sufficient number of cells. The histograms are representative of 2 independent experiments.
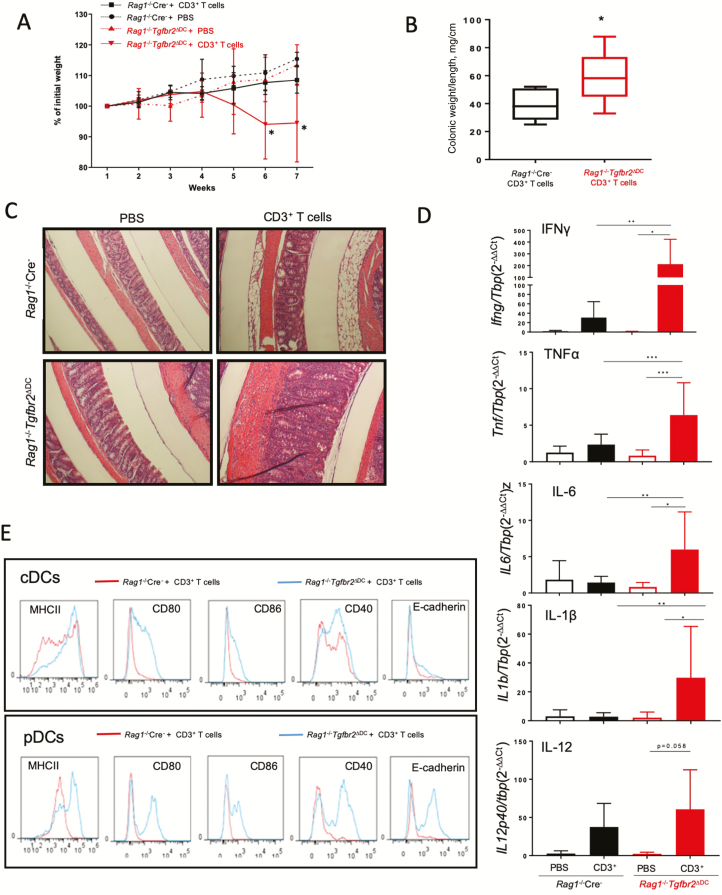
After establishing that DCs from Rag1-/-Tgfbr2ΔDC mice display no evidence of phenotypic activation, we tested if restitution of the total CD3+ T cells would be sufficient to re-establish colitis in Rag1-/-Tgfbr2ΔDC mice. We adoptively transferred 106 of the total CD3+ T cells isolated from the spleens of naïve WT mice into Rag1-/- Cre- and Rag1-/-Tgfbr2ΔDC mice. Rag1-/-Tgfbr2ΔDC mice transferred with CD3+ T cells started losing weight by week 4 and developed visible symptoms of colitis. By weeks 6–7, Rag1-/-Tgfbr2ΔDC mice reconstituted with CD3+ T cells had lost significantly more weight as compared with Rag1-/- Cre- controls, who continued gaining weight and maintained a healthy appearance (Fig. 2A). After death, Rag1-/-Tgfbr2ΔDC mice demonstrated significant colonic hyperplasia, as defined by the higher colonic weight-to-length ratio compared with that of Rag1-/- Cre- mice (Fig. 2B). The histopathological examination also showed immune cell infiltration of the colonic mucosa and thickening of the smooth muscle layers in the colons of Rag1-/-Tgfbr2ΔDC mice as compared with those of Rag1-/-Cre- littermates (Fig. 2C). In addition to the colitis, inflammation also extended to the liver and pancreas, as reported in the parental Tgfbr2ΔDC strain (Supplementary Fig. 2).10 Contrary to the original observations, we did not observe significant histological differences in the forestomach or glandular stomach (Supplementary Fig. 2).
Figure 2.
Adoptive transfer of CD3+ T cells is sufficient to induce colitis in Rag1-/-Tgfbr2ΔDC mice. Rag1-/- Cre- and Rag1-/-Tgfbr2ΔDC mice were injected i.p. with 106 CD3+ splenocytes per mouse and monitored for 7 weeks for weight loss. PBS-injected mice served as controls. A, Weight loss curve in Rag1-/-Tgfbr2ΔDC mice as compared with Rag1-/- Cre- mice after CD3+ T-cell transfer (n = 8–10 mice per genotype; *P < 0.05 between adoptively transferred Rag1-/-Tgfbr2ΔDC mice and other groups). B, Colonic weight-to-length ratio (mg/cm) in T-cell-transferred mice (*P < 0.05, unpaired 2-tailed t test). C, Representative H&E-stained colons from mice from the 4 experimental groups. D, Expression of mucosal cytokines evaluated by qPCR, analyzed by the 2-∆∆CT method against a TBP housekeeping gene. One-way ANOVA with Bonferroni multiple comparison test was applied. *P < 0.05; **P < 0.005, ***P < 0.0005 for post hoc test. E, Activation status of dendritic cells from mice after adoptive transfer, as evaluated by flow cytometry. Representative histograms show surface expression of activation markers: MHCII, CD80, CD86, CD40, and E-cadherin on conventional (CD11chiPDCA1-) and plasmacytoid (CD11clo PDCA1+) DCs.
Rag1 -/- Tgfbr2 ΔDC mice adoptively transferred with total T cells had significantly elevated expression of several IBD-associated cytokines in the colonic mucosa, including IFNγ, TNFα, IL12b, IL6, IL1β, and IL12p40 (the latter at a borderline P value of 0.058) (Fig. 2D). None of these transcripts were statistically significantly elevated in T-cell-transferred Rag1-/- Cre- mice (Fig. 2D). We observed no significant change in the expression of IL17A and IL10 (data not shown). To determine whether the inflammation was associated with markers of immune cell activation, we analyzed dendritic cells and T cells from Rag1-/- Cre- and Rag1-/-Tgfbr2ΔDC mice transferred with CD3+ T cells by flow cytometry. Only the Rag1-/-Tgfbr2ΔD mice transferred with T cells had elevated expression of MHCII, along with all the activation/co-stimulatory molecules (Fig. 2E). However, there was no difference in the frequency of activated CD4+ CD62Llo CD44hi T cells in MLNs between Rag1-/-Cre- and Rag1-/-Tgfbr2ΔDC mice after T-cell transfer (94.8% ± 0.97% vs 94.2% ± 2.0%). Interestingly, adoptively transferred Rag1-/-Tgfbr2ΔDC mice showed a trend of increased CD8+ CD62Llo CD44hi T cells in the MLNs (59.76% ± 8.05% vs 71.34% ± 15.9%; n.s.). Overall, our results indicate that activated T cells lead to enhanced activation/maturation of TGFβR2-deficient DCs, which subsequently feeds forward the ensuing inflammatory response and colitis.
Colitis in Rag1-/-Tgfbr2ΔDC Mice Requires Both CD4+ and CD8+ Cells
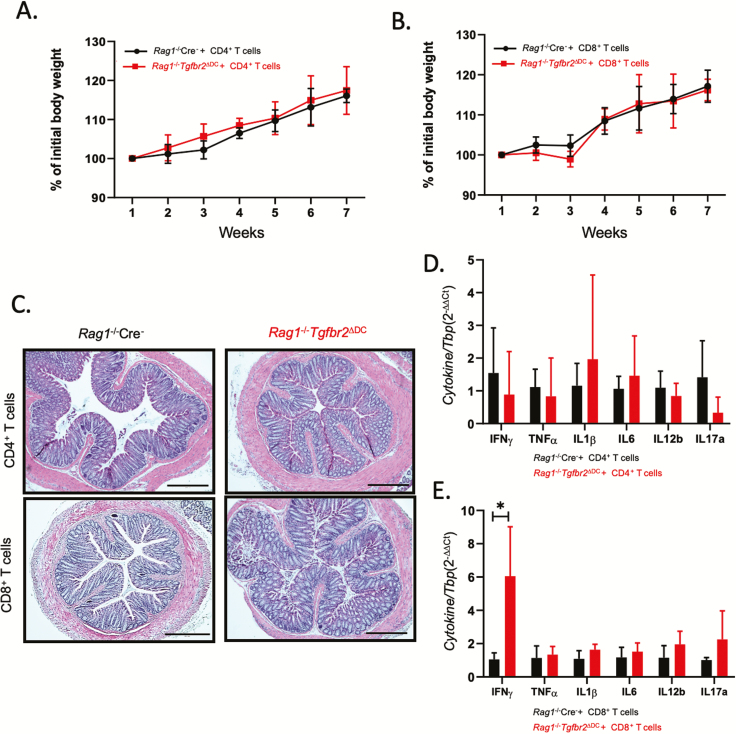
We then assessed whether colitis is driven primarily by CD4+ or CD8+ T cells or if both subsets are essential. We transferred Rag1-/- Cre- and Rag1-/-Tgfbr2ΔDC mice with either 5×105 CD4+ or 5×105 CD8+ T cells and monitored them for 8 weeks. The mice transferred exclusively with CD4+ or CD8+ T cells did not lose body weight (Fig. 3A, B) and did not exhibit any symptoms of disease. Histological analysis revealed no significant inflammation in any of the organs affected by total CD3+ T-cell transfer, that is, colon (Fig. 3C) or liver and pancreas (data not shown). There were also no significant differences in the mucosal expression of pro-inflammatory cytokines between Rag1-/- Cre- and Rag1-/-Tgfbr2ΔDC mice transferred with either CD4+ or CD8+T cells, with the exception of IFNγ, which was significantly increased in Rag1-/-Tgfbr2ΔDC mice transferred with CD8+ T cells, consistent with the homing of IFNγ-producing CD8+ T cells to the colonic mucosa (Fig. 3D, E). Contrary to the effect of total T-cell transfer, we observed no differences in the T-cell (Supplementary Fig. 3) or DC activation markers (Supplementary Fig. 4), suggesting that CD4+ and CD8+ cells act synergistically to promote hyperactivation of TGFbR2-deficient DCs.
Figure 3.
Both CD4+ and CD8+ T cells are necessary to induce colitis in Rag1-/-Tgfbr2ΔDC mice. Colitis did not develop in Rag1-/-Tgfbr2ΔDC mice adoptively transferred with CD4+ or CD8+ T cells alone (n = 5–8 mice per genotype). A–B, Weight loss curves for mice transferred with CD4+ or CD8+ T cells. C, H&E staining of colonic segments representative of each experimental group. D–E, qPCR analysis of colonic mucosal expression of selected cytokines in mice transferred with CD4+ or CD8+ T cells. Data were analyzed using the 2-∆∆CT method with TBP used as a housekeeping gene. The unpaired 2-tailed t test was used to analyze the data. *P < 0.05.
CD40-CD40L Interaction Is Critical for Driving Inflammatory Responses in Adoptively Transferred Rag1-/-Tgfbr2ΔDC Mice
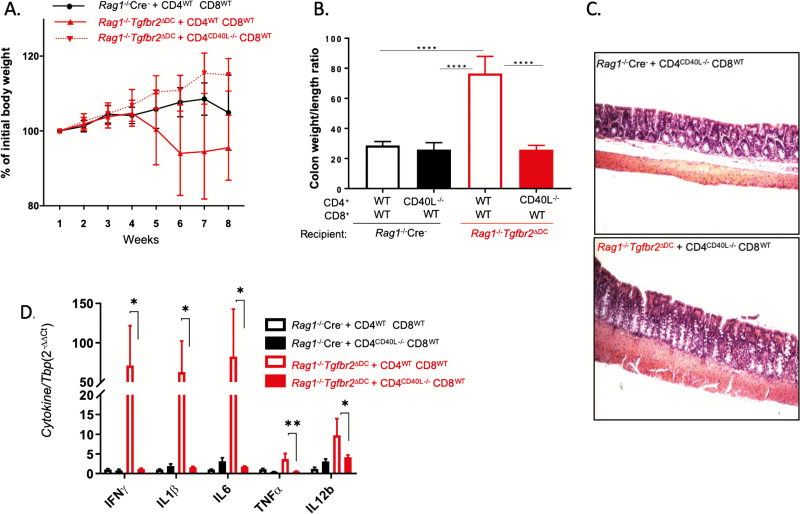
The role of CD40-CD40L interaction is essential for evoking humoral and cell-mediated immunity and the development of T-cell-dependent autoimmune diseases. Interaction of CD40 on DCs with CD40L on CD4+ T cells induces maturation (upregulation of co-stimulatory molecules) of DCs, which in turn stimulates T cells for a productive immune response. To determine whether this interaction was also required for driving inflammation in our model, Rag1-/- Cre- and Rag1-/-Tgfbr2ΔDC mice were transferred with a combination of WT CD4+ (CD4WT) and WT CD8+ T cells (CD8WT; 5×105 each) or CD40L-/- CD4+ T cells (CD4CD40L-/-) and CD8WT T cells (5×105 each) and monitored for 8 weeks. Although in this study the deviation was too high to show a statistically significant difference in weight loss, there was an appreciable difference in the course of the average body weights between Rag1-/-Tgfbr2ΔDC mice that received WT T cells and those that received CD4CD40L-/- and CD8WT T cells (Fig. 4A).
Figure 4.
CD40L expression on CD4 cells is necessary for CD3+ T-cell-mediated colitis. Rag1-/- Cre- and Rag1-/-Tgfbr2ΔDC mice (4–7 mice per genotype) were adoptively transferred with a 1:1 mix of CD4WT and CD8WT cells (5×105 each) or CD4CD40L-/- and CD8WT cells. The mice were followed up for 8 weeks and killed. A, Weight loss curves. B, Colonic hyperplasia expressed as the weight-to-length ratio. One-way ANOVA with a Bonferroni multiple-comparisons post hoc test was used for data analysis. ****P < 0.0001 for post hoc test. C, Representative H&E images of colonic segments of Rag1-/-Cre- and Rag1-/-Tgfbr2ΔDC mice adoptively transferred with CD4CD40L-/- and CD8WT cells demonstrating similar lack of colonic pathology. D, qPCR analysis of colonic mucosal expression of selected cytokines in the 4 experimental groups. Data were analyzed using the 2-∆∆CT method with TBP used as a housekeeping gene. The unpaired 2-tailed t test was used to analyze the data. *P < 0.05; **P < 0.005.
Rag1 -/- Tgfbr2 ΔDC mice that received CD4CD40L-/- and CD8WT T cells showed no colonic hyperplasia (colon weight/length ratio) (Fig. 4B), and histological analysis of the colon also revealed a very mild or no inflammation in the colon of Rag1-/-Tgfbr2ΔDC mice transferred with CD4CD40L-/- and CD8WT T cells as compared with mice transferred with WT CD3+ T cells (Fig. 4C). The observations were consistent with mucosal cytokine expression (Fig. 4D). These results demonstrated that expression of CD40L on CD4+ T cells is required for driving colitis in adoptively transferred Rag1-/-Tgfbr2ΔDC mice.
Total T-Cell Transfer Colitis Is Characterized by Reduction in FoxP3+ Tregs T Cells
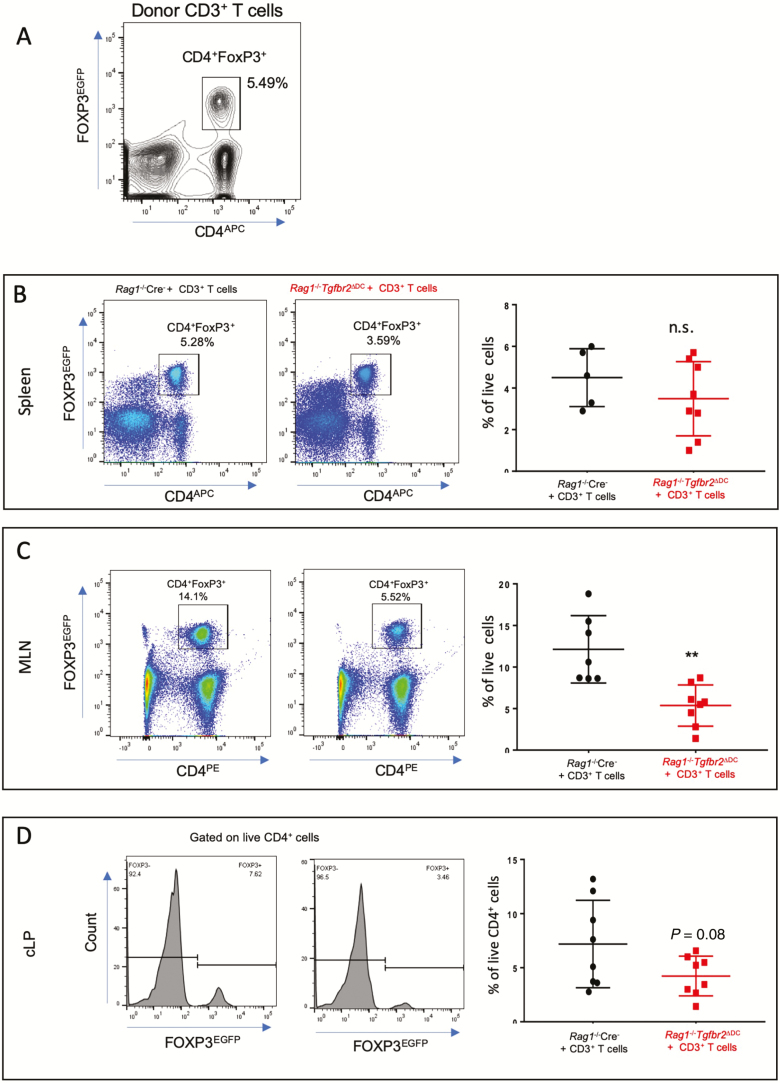
Circulating numbers of CD4+CD25+FoxP3+ regulatory T cells (Tregs) are decreased in patients with active IBD and are normalized (both in numbers and immunosuppressive function) in remission.16 CD4+ Foxp3+ Treg cells are capable of ameliorating experimental colitis in vivo.17 We have previously demonstrated that TGFβ signaling in dendritic cells is important for the maintenance of peripheral Foxp3+ T regulatory cells.10 To understand the fate of these cells in adoptively transferred Rag1-/-Tgfbr2ΔDC mice, we used FoxP3IRES-GFP mice as T-cell donors to induce colitis in Rag1-/- Cre- and Rag1-/-Tgfbr2ΔDC mice and followed them for 8 weeks. Among donor CD3+ T cells, CD4+ FoxP3+ cells represented 5.49% of total live cells (Fig. 5A). As anticipated, Rag1-/-Tgfbr2ΔDC mice developed colitis within 8 weeks of adoptive transfer of CD3+ T cells (data not shown). The spleens, MLNs, and colonic lamina propria were harvested, and the frequency of CD4+ FoxP3+ T cells was determined using flow cytometry. There was no difference in the frequency of CD4+ FoxP3+ T cells in the spleens in both recipient strains (Fig. 5B). However, Treg frequency was significantly reduced in the MLNs and colonic lamina propria of Rag1-/-Tgfbr2ΔDC mice when compared with Rag1-/- Cre- mice (Fig. 5C, D).
Figure 5.
Decrease of CD4+ FOXP3+ Tregs is associated with colitis in adoptively transferred Rag1-/-Tgfbr2ΔDC mice. FoxP3IRES-GFP mice were used as donors for the CD3+ T-cell adoptive transfer into Rag1-/- Cre- and Rag1-/-Tgfbr2ΔDC mice (n = 7–8 mice per genotype). A, Flow cytometry analysis of the initial number of CD4+FOXP3+ Tregs among the donor CD3+ T cells before adoptive transfer. Single-cell suspensions were prepared from spleens, MLNs, and cLP, stained, and analyzed by flow cytometry. B, Representative cytograms and a summary graph of CD4+ FOXP3+ Tregs from the spleens of recipient mice 7 weeks after adoptive transfer. C, Representative cytograms and the summary graph of CD4+ FOXP3+ Tregs from the MLNs of recipient mice. D, Representative histograms and a summary graph of CD4+ FOXP3+ Tregs from the cLP of recipient mice. The unpaired t test was used to analyze data. **P < 0.005.
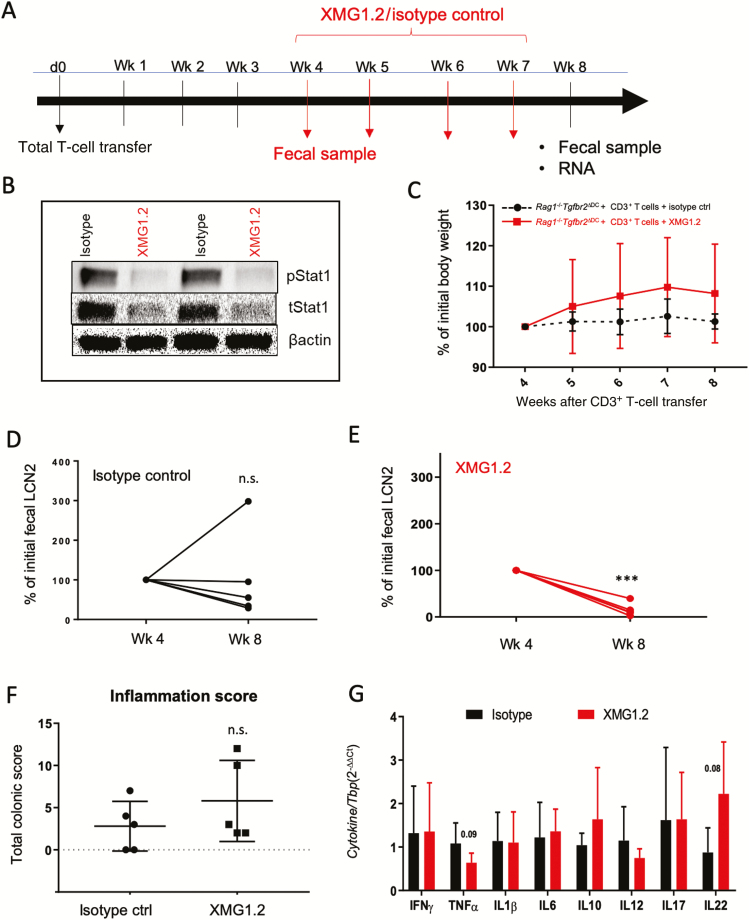
Modulatory Role of IFNγ in Rag1-/-Tgfbr2ΔDC With Adoptive T-Cell Transfer Colitis
The role of IFNγ in mouse models of colitis has not been consistently demonstrated, with contrary evidence published,18, 19 whereas clinical trials with neutralizing humanized anti-IFNγ antibody (fontolizumab) have failed to reach a clinical end point despite lowering C-reactive protein levels.20 The magnitude of increase in the expression of mucosal IFNγ upon CD3+ T-cell transfer prompted us to ask whether the severe inflammation and pathology caused in this model is mediated by this cytokine. To this end, we examined the effects of neutralizing anti-IFNγ antibody (XMG1.2) in our CD3+ T-cell transfer model of colitis. Rag1-/-Tgfbr2ΔDC mice were adoptively transferred with CD3+ T cells as before, allowed 4 weeks for the development of colitis, and then injected with XMG1.2 (or isotype control antibody) once a week for the next 4 weeks (Fig. 6A). The neutralization of IFNγ signaling was confirmed by complete abrogation of pSTAT1 protein expression in the colonic mucosa of mice injected with XMG1.2 as compared with isotype control (Fig. 6B). In this study, T-cell-transferred mice injected with the isotype control antibody failed to accrue body weight, whereas those injected with XMG1.2 continued to gain weight, albeit without demonstrable statistical significance (Fig. 6C). Adoptively transferred Rag1-/-Tgfbr2ΔDC mice that received the isotype control did not show a significant change in the fecal LCN2 concentration (Fig. 6D), whereas the mice injected with XMG1.2 showed a highly significant decrease in fecal concentration of the inflammatory marker after 4 injections (Fig. 6E). Paradoxically, Rag1-/-Tgfbr2ΔDC mice injected with XMG1.2 had higher colonic inflammation scores than the isotype control mice, albeit without reaching statistical significance (Fig. 6F). At the study’s conclusion, IFNγ neutralization had only a modest effect on mucosal TNFα expression (P = 0.08), led to increased IL22 mRNA (P = 0.08), and did not affect the expression of other cytokines tested (Fig. 6G). Overall, the data demonstrate that blockade of IFNγ in established total T-cell colitis modulates disease severity but does not abolish it completely, consistent with the clinical trial of fontolizumab.20
Figure 6.
IFNγ modulates inflammatory response in adoptively transferred Rag1-/-Tgfbr2ΔDC mice. A, Cartoon depiction of the experimental design with the timing of adoptive T-cell transfer, antibody injection, death, and tissue collection. B, Western blot analysis of colonic pSTAT1 and total STAT1 expression demonstrated the effectiveness of IFNγ neutralization. Representative blot from 2 mice in each experimental group. C, Weight loss curve in adoptively transferred Rag1-/-Tgfbr2ΔDC mice treated with isotype control or XMG1.2 antibody. D–E, Differences in fecal LCN2 concentration between the fourth and seventh weeks of the study as determined by ELISA (ng/mL/g). Due to varying inflammatory responses in mice at 4 weeks post-transfer, data were expressed as change relative to the initial LCN2 level at the onset of treatment. The paired t test was used to analyze data: ***P < 0.0005. F, Histological inflammation scores were assigned to the proximal and distal colon, and their sum was used as a total colonic score. G, qPCR analysis of colonic mucosal expression of selected cytokines in the 4 experimental groups. Data were analyzed using the 2-∆∆CT method with TBP used as a housekeeping gene. The unpaired 2-tailed t test was used to analyze the data. Only trends were observed without reaching statistical significance.
Discussion
Several animal models of IBD approximating the pathology of IBD have been described in the literature, with the accepted limitation that none of them completely recapitulates the immunopathology of human IBD.21 Although chemically induced models are most widely used, one of the most accepted models has been the CD4+ CD45RBHigh naïve T-cell transfer into syngeneic recipients. In the absence of nTregs, CD4+ T cells into a lymphopenic host with intact gut microbiota are thought to undergo autoreactive clonal expansion and lead to a progressive Th1/Th17-mediated colitis.22 Rag1-/- mice reconstituted with naïve T cells develop chronic hepatitis (also observed in our model), reminiscent of the sclerosing cholangitis observed in IBD patients. This model has been considered excellent for studying the role of immunity and the microbiota, along with a model for preclinical evaluation of potential novel therapeutics for CD. However, the pathology in human IBD is most likely not exclusively dependent on CD4+ T cells, as there are other immune cells, both innate and adaptive, that are also implicated in disease onset and progression. Many recent studies have described the increase in circulating activated CD8+ T cells in patients with IBD.7, 9, 23 Also, the increase in CD8+ T cells was correlated with plasma IL6 and C-reactive protein.7 Despite these studies, it is not clear how CD8+ T cells contribute to disease pathogenesis.
TGFβ has been recognized as a master adaptive immune regulator for its capability to block Th1 and Th2 responses while promoting Treg and Th17 cell differentiation.24, 25 TGFβ also has multiple effects on DCs, including downregulation of cell surface MHC and co-stimulatory molecules, chemokine receptors, and regulation of chemotaxis.26 We and others have studied the role of TGFβ signaling in DCs in maintaining and regulating DC tolerance in experimental models of autoimmune diseases, such as multiple sclerosis, autoimmune pancreatitis, and colitis.10, 27, 28 All these studies have described the presence of activated DCs and substantial infiltration of activated T cells at the site of inflammation. These data have been extensively corroborated by showing that abrogation of TGFβ signaling in DCs increases their migration toward local lymph nodes,29 thus increasing their interaction with the cells of the adaptive immune system. In this report, we show that chronic T-cell-mediated colitis is associated with decreased TGFβ signaling in MLN cDCs, which validates our previous findings that such a mechanism contributes to the pathogenesis of colitis and other autoinflammatory symptoms in mice with conditional DC-specific deletion of Tgfbr2.10 In these mice, inflammatory symptoms were associated with a highly pro-inflammatory dendritic cell phenotype and an increased number of activated CD4+ and CD8+ T cells in the affected organs.10 In the current report, crossing the DC-specific conditional Tgfbr2 knockout into Rag1-/- entirely eliminated the spontaneous pathology, thus demonstrating the requirements for the adaptive immune responses. Moreover, DCs from the Rag1-/-Tgfbr2ΔDC mice did not show the inflammatory phenotype characteristic of their parental strain, suggesting that activation of DCs in the absence of TGFβ signaling is not intrinsic, but is contingent upon interaction with the cells of the adaptive immune system. Indeed, all the inflammatory features were restored in Rag1-/-Tgfbr2ΔDC mice upon transfer with unfractionated WT CD3+ splenocytes, including the induction of MHCII, CD80, CD86, CD40, and E-cadherin. This was associated with colonic inflammation, as evidenced by macroscopic and histological examination, and the elevated expression of mucosal IFNγ, TNFα, IL6, IL1β, and IL12.
It is important to consider the complexity of DC-T-cell cross-talk, which results in mutual stimulation. Recently, Bain et al.30 induced colitis by the adoptive transfer of CD45.1+ splenocytes into Rag1-/- mice with conditional deletion of Tgfbr1 in dendritic cells. However, unlike in our study, where we found an increase in activated CD4+ and CD8+ cells in the mucosa of Rag1-/-Tgfbr2ΔDC mice upon T-cell transfer, Bain et al. demonstrated homing and infiltration of only CD4+ cells and not CD8+ cells in the colon.30 We demonstrated that neither CD4+ nor CD8+ T cells transferred alone into Rag1-/-Tgfbr2ΔDC mice were sufficient to induce inflammation or result in T-cell or DC activation, thus providing evidence that both lineages are required for the manifestation of disease in our model. Activated T cells trigger DCs via CD40L, resulting in an enhanced T-cell stimulatory capacity, increased IL-12 production, and extended lifespan.31 CD40/CD40L engagement played a critical role in our model, as a co-transfer of CD4CD40L-/- CD8WT cells did not result in measurable inflammation. One of the ways that the CD40/CD40L dyad contributes to autoimmune diseases is T-lymphocyte priming in the secondary lymphoid organs by APCs overexpressing CD40, which favor the activation of autoreactive T lymphocytes and production of pro-inflammatory cytokines by APCs.32 It is plausible that CD40/CD40L engagement results in the initial activation of DCs; however, this will require further studies. Studies using different experimental systems showed that CD4+ and CD8+ T cells can interact directly with one another.33, 34 Bourgeois et al.34 showed that CD4+ T-cell help to CD8+ responses does not require CD40 expression by the APCs but relies instead on the expression of CD40 by CD8+ T cells. In a study with intravital 2-photon laser-scanning microscopy imaging, Barinov et al.35 demonstrated the direct binding of CD8+ to CD4+ T cells and showed that most of the T/DC complexes were ternary in nature, harboring both T/DC and CD4/CD8 direct interactions. Although we did not explore this aspect in our study, it is plausible that direct CD4/CD8 interaction, perhaps facilitated by CD40L, contributes to the disease pathogenesis. Indeed, IBD patients show an increased expression of CD40 and CD40L in the gut mucosa36, 37 and increased circulating levels of soluble CD40L.38 CD40L overexpression in T cells in mice resulted in multi-organ autoimmunity and early mortality due to intestinal inflammation, with colonic infiltration of CD40L+CD4+ and CD8+ T cells and high numbers of CD40+ APCs.39 Blocking CD40L antibody or CD40 antisense oligonucleotide was effective in murine models of IBD,40–42 and a small (18 patients) single-dose open-label study with CD patients treated with anti-CD40 monoclonal antibody showed 72% and 22% response and remission rates, respectively.43 Thus, although perhaps not as robust as the classical CD4+ CD45RBhigh T-cell transfer model, our novel model may be more physiologically and clinically relevant as it highlights the contribution of CD8 cells and the complex cross-talk between various innate and adaptive immune cells. It can also be utilized for controlled mechanical studies to test novel therapeutics targeting the CD40/CD40L dyad, and possibly other novel therapeutic approaches targeting more complex inflammatory pathways.
CD4+ FoxP3+ Treg cells, in their numbers and immunosuppressive functions, contribute to the complex pathogenesis of IBD and are capable of preventing or reversing experimental colitis.44 In our model, colitis was accompanied by decreased frequencies of mucosal and MLN (but not splenic) CD4+ FoxP3+ Tregs, consistent with our original observations in Tgfbr2ΔDC mice.10 The limitation of the model is that a majority of the splenic Tregs are natural (nTreg) cells of thymic origin, and it is not immediately possible to discern if the mucosal/MLN FoxP3+ T cells are the same cells or their descendants homed to those sites, or de novo differentiated inducible Tregs (iTregs). The decreased frequency of Tregs in our model of colitis is likely the effect of deficient TGFβ signaling in DCs. In a collaborative study, we previously reported that CD4+ Foxp3+ Treg cells induced ex vivo with TGFβ are capable of suppressing a lupus-like chronic graft-vs-host disease by preventing the expansion of immunogenic DCs and inducing tolerogenic DCs that generate a new wave of Tregs, a phenomenon dependent on intact TGFβ signaling in DCs.45 Similar mechanisms may exist in the gut and could explain the reduced numbers of Tregs in the colonic LP and MLNs. It is also plausible that the inflammatory mediators associated with the disease per se could affect Treg numbers or FoxP3 expression, as reported for TNFα,46 IL6,47 IL1β,48 or IFNγ.10
The latter cytokine (IFNγ) is highly induced in the classical CD4+ CD45RBhigh T-cell transfer model, and its neutralization prevents development of colitis.3 It is believed to drive Th1 responses and may influence the disease location, as IFNγ -/--naïve T cells fail to induce colitis and promote small intestinal involvement.49 In our previous work, neutralization of IFNγ reversed the negative effect of Tgfbr2-deficient DCs on in vitro Treg conversion.10 A high increase in the expression of IFNγ in our model prompted us to test the involvement of this cytokine in the pathogenesis of colitis. Interestingly, upon neutralizing IFNγ beginning in the early stages of colitis, we were only able to partially ameliorate the disease and not completely prevent or reverse it, with the only observable effect on fecal LCN2. This finding is consistent with very modest effects of Fontolizumab in Crohn’s disease clinical trials, where the clinical end point was not achieved despite the lowering of systemic CRP levels.20
In summary, our novel model of colitis provides not only a clinically relevant tool for studying the pathogenesis of IBD but also evidence for the pathogenic involvement of both CD4+ and CD8+ T-cell lineage and the requirement of CD40/CD40L interaction(s) in disease development. Further studies with this model may provide a new understanding of the factors involved in the genesis of IBD and an improved venue for the preclinical testing of novel pharmacological interventions.
Supplementary Material
Notes
Supported by: the National Institute of Diabetes and Digestive and Kidney Diseases Grant 5R01DK109711 (to P.R. Kiela and F.K. Ghishan). The founders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest: All authors declare that there is no conflict of interest to disclose.
Author contributions: D.R.J. designed and performed experiments, analyzed results, and wrote the paper; R.R. performed experiments, analyzed results, and edited the paper; C.A.H. performed qPCR analysis; M.T.M.K. was responsible for most experimental animals and performed experiments; V.F.D.P. performed experiments and edited the paper; D.G.B. analyzed histology and scored samples; F.K.G. supervised experiments, provided funding, edited the manuscript; P.R.K. supervised experiments, designed the experiments, analyzed results, provided funding, edited the manuscript.
References
- 1. Hall AB, Tolonen AC, Xavier RJ. Human genetic variation and the gut microbiome in disease. Nat Rev Genet. 2017;18:690–699. [DOI] [PubMed] [Google Scholar]
- 2. Koboziev I, Karlsson F, Zhang S, et al. Pharmacological intervention studies using mouse models of the inflammatory bowel diseases: translating preclinical data into new drug therapies. Inflamm Bowel Dis. 2011;17:1229–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Powrie F, Leach MW, Mauze S, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. [DOI] [PubMed] [Google Scholar]
- 4. Punit S, Dubé PE, Liu CY, et al. Tumor necrosis factor receptor 2 restricts the pathogenicity of CD8(+) T cells in mice with colitis. Gastroenterology. 2015;149:993–1005.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McNamee EN, Wermers JD, Masterson JC, et al. Novel model of TH2-polarized chronic ileitis: the SAMP1 mouse. Inflamm Bowel Dis. 2010;16:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nancey S, Holvöet S, Graber I, et al. CD8+ cytotoxic T cells induce relapsing colitis in normal mice. Gastroenterology. 2006;131:485–496. [DOI] [PubMed] [Google Scholar]
- 7. Funderburg NT, Stubblefield Park SR, Sung HC, et al. Circulating CD4(+) and CD8(+) T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology. 2013;140:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JC, Lyons PA, McKinney EF, et al. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn disease and ulcerative colitis. J Clin Invest. 2011;121:4170–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schreiber S, MacDermott RP, Raedler A, et al. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991;101:1020–1030. [DOI] [PubMed] [Google Scholar]
- 10. Ramalingam R, Larmonier CB, Thurston RD, et al. Dendritic cell-specific disruption of TGF-β receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. J Immunol. 2012;189:3878–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ihara S, Hirata Y, Serizawa T, et al. TGF-β signaling in dendritic cells governs colonic homeostasis by controlling epithelial differentiation and the luminal microbiota. J Immunol. 2016;196:4603–4613. [DOI] [PubMed] [Google Scholar]
- 12. Lin W, Haribhai D, Relland LM, et al. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. [DOI] [PubMed] [Google Scholar]
- 13. Harrison CA, Laubitz D, Ohland CL, et al. Microbial dysbiosis associated with impaired intestinal Na+/H+ exchange accelerates and exacerbates colitis in ex-germ free mice. Mucosal Immunol. 2018;11:1329–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larmonier CB, Shehab KW, Laubitz D, et al. Transcriptional reprogramming and resistance to colonic mucosal injury in poly(ADP-ribose) polymerase 1 (PARP1)-deficient mice. J Biol Chem. 2016;291:8918–8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burich A, Hershberg R, Waggie K, et al. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281:G764–G778. [DOI] [PubMed] [Google Scholar]
- 16. Boschetti G, Nancey S, Sardi F, et al. Therapy with anti-TNFα antibody enhances number and function of Foxp3(+) regulatory T cells in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:160–170. [DOI] [PubMed] [Google Scholar]
- 17. Huber S, Schramm C, Lehr HA, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173:6526–6531. [DOI] [PubMed] [Google Scholar]
- 18. Ito R, Shin-Ya M, Kishida T, et al. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol. 2006;146:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davidson NJ, Hudak SA, Lesley RE, et al. IL-12, but not IFN-gamma, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J Immunol. 1998;161:3143–3149. [PubMed] [Google Scholar]
- 20. Reinisch W, Hommes DW, Van Assche G, et al. A dose escalating, placebo controlled, double blind, single dose and multidose, safety and tolerability study of fontolizumab, a humanised anti-interferon gamma antibody, in patients with moderate to severe Crohn’s disease. Gut. 2006;55:1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones-Hall YL, Grisham MB. Immunopathological characterization of selected mouse models of inflammatory bowel disease: comparison to human disease. Pathophysiology. 2014;21:267–288. [DOI] [PubMed] [Google Scholar]
- 22. Powrie F, Leach MW. Genetic and spontaneous models of inflammatory bowel disease in rodents: evidence for abnormalities in mucosal immune regulation. Ther Immunol. 1995;2:115–123. [PubMed] [Google Scholar]
- 23. Probert CS, Chott A, Saubermann LJ, et al. Prevalence of an ulcerative colitis-associated CD8+ T cell receptor beta-chain CDR3-region motif and its association with disease activity. J Clin Immunol. 2001;21:126–134. [DOI] [PubMed] [Google Scholar]
- 24. Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. [DOI] [PubMed] [Google Scholar]
- 26. Ogata M, Zhang Y, Wang Y, et al. Chemotactic response toward chemokines and its regulation by transforming growth factor-beta1 of murine bone marrow hematopoietic progenitor cell-derived different subset of dendritic cells. Blood. 1999;93:3225–3232. [PubMed] [Google Scholar]
- 27. Boomershine CS, Chamberlain A, Kendall P, et al. Autoimmune pancreatitis results from loss of TGFbeta signalling in S100A4-positive dendritic cells. Gut. 2009;58:1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laouar Y, Town T, Jeng D, et al. TGF-beta signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008;105:10865–10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Imai K, Minamiya Y, Koyota S, et al. Inhibition of dendritic cell migration by transforming growth factor-β1 increases tumor-draining lymph node metastasis. J Exp Clin Cancer Res. 2012;31:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bain CC, Montgomery J, Scott CL, et al. TGFβR signalling controls CD103+CD11b+ dendritic cell development in the intestine. Nat Commun. 2017;8:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sallusto F, Lanzavecchia A. The instructive role of dendritic cells on T-cell responses. Arthritis Res. 2002;4(Suppl 3):S127–S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iezzi G, Sonderegger I, Ampenberger F, et al. CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc Natl Acad Sci U S A. 2009;106:876–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ahmed KA, Wang L, Munegowda MA, et al. Direct in vivo evidence of CD4+ T cell requirement for CTL response and memory via pMHC-I targeting and CD40L signaling. J Leukoc Biol. 2012;92:289–300. [DOI] [PubMed] [Google Scholar]
- 34. Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. [DOI] [PubMed] [Google Scholar]
- 35. Barinov A, Galgano A, Krenn G, et al. CD4/CD8/dendritic cell complexes in the spleen: CD8+ T cells can directly bind CD4+ T cells and modulate their response. PLoS One. 2017;12:e0180644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu Z, Colpaert S, D’Haens GR, et al. Hyperexpression of CD40 ligand (CD154) in inflammatory bowel disease and its contribution to pathogenic cytokine production. J Immunol. 1999;163:4049–4057. [PubMed] [Google Scholar]
- 37. Battaglia E, Biancone L, Resegotti A, et al. Expression of CD40 and its ligand, CD40L, in intestinal lesions of Crohn’s disease. Am J Gastroenterol. 1999;94:3279–3284. [DOI] [PubMed] [Google Scholar]
- 38. Ludwiczek O, Kaser A, Tilg H. Plasma levels of soluble CD40 ligand are elevated in inflammatory bowel diseases. Int J Colorectal Dis. 2003;18:142–147. [DOI] [PubMed] [Google Scholar]
- 39. Clegg CH, Rulffes JT, Haugen HS, et al. Thymus dysfunction and chronic inflammatory disease in gp39 transgenic mice. Int Immunol. 1997;9:1111–1122. [DOI] [PubMed] [Google Scholar]
- 40. Liu Z, Geboes K, Colpaert S, et al. Prevention of experimental colitis in SCID mice reconstituted with CD45RBhigh CD4+ T cells by blocking the CD40-CD154 interactions. J Immunol. 2000;164:6005–6014. [DOI] [PubMed] [Google Scholar]
- 41. Stuber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arranz A, Reinsch C, Papadakis KA, et al. Treatment of experimental murine colitis with CD40 antisense oligonucleotides delivered in amphoteric liposomes. J Control Release. 2013;165:163–172. [DOI] [PubMed] [Google Scholar]
- 43. Kasran A, Boon L, Wortel CH, et al. Safety and tolerability of antagonist anti-human CD40 Mab ch5D12 in patients with moderate to severe Crohn’s disease. Aliment Pharmacol Ther. 2005;22:111–122. [DOI] [PubMed] [Google Scholar]
- 44. Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. [DOI] [PubMed] [Google Scholar]
- 45. Lan Q, Zhou X, Fan H, et al. Polyclonal CD4+Foxp3+ Treg cells induce TGFβ-dependent tolerogenic dendritic cells that suppress the murine lupus-like syndrome. J Mol Cell Biol. 2012;4:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang Q, Cui F, Fang L, et al. TNF-α impairs differentiation and function of TGF-β-induced Treg cells in autoimmune diseases through Akt and Smad3 signaling pathway. J Mol Cell Biol. 2013;5:85–98. [DOI] [PubMed] [Google Scholar]
- 47. Yamada A, Arakaki R, Saito M, et al. Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2016;22:2195–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O’Sullivan BJ, Thomas HE, Pai S, et al. IL-1 beta breaks tolerance through expansion of CD25+ effector T cells. J Immunol. 2006;176:7278–7287. [DOI] [PubMed] [Google Scholar]
- 49. Dohi T, Fujihashi K, Koga T, et al. T helper type-2 cells induce ileal villus atrophy, goblet cell metaplasia, and wasting disease in T cell-deficient mice. Gastroenterology. 2003;124:672–682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.