Abstract
Parkinson’s disease (PD) has classically been defined as a movement disorder, in which motor symptoms are explained by the aggregation of alpha-synuclein (α-syn) and subsequent death of dopaminergic neurons of the substantia nigra pars compacta (SNpc). More recently, the multisystem effects of the disease have been investigated, with the immune system being implicated in a number of these processes in the brain, the blood, and the gut. In this review, we highlight the dysfunctional immune system found in both human PD and animal models of the disease, and discuss how genetic risk factors and risk modifiers are associated with pro-inflammatory immune responses. Finally, we emphasize evidence that the immune response drives the pathogenesis and progression of PD, and discuss key questions that remain to be investigated in order to identify immunomodulatory therapies in PD.
Keywords: Immune system, Inflammation, Parkinson’s disease, Alpha-synuclein, Microglia, T cells, Microbiota
1. Introduction
The involvement of the immune system in Parkinson’s disease has been postulated since its initial description by James Parkinson in 1817. In his “An Essay on the Shaking Palsy,” Parkinson suggests that attacks on the nervous system, “considered at the time merely as rheumatic affections, might lay the foundation of this lamentable disease, which might manifest itself at some distant period, when the circumstances in which it had originated, had, perhaps, almost escaped the memory” (Parkinson, 2002). Now, more than 200 years after Parkinson’s initial publication, the evidence has grown substantially to support the idea that inflammatory processes occur long before clinically apparent symptomatology, and initiate and/or drive the progression of PD.
Before addressing the research itself, it is helpful to clearly define what is meant by “inflammation,” as the meaning of this word has expanded greatly in recent years. Classically the process of inflammation can be separated into three different components: (i) Responses from the host immune system that aid in the clearance of invading pathogens such as bacteria or viruses, (ii) responses to aid in the removal of dead or dying cells and wound repair, and (iii) responses that lead to excessive activation or misguided recognition of host antigens and which lead to damage of tissues. This review and others (Prinz and Priller, 2017) use a specific definition of inflammation encompassing all three of these responses. We consider inflammation to be a response that results in the infiltration of hematopoietic cells of a peripheral origin, such as monocytes, T cells, B cells, and neutrophils, into the affected tissue, along with the generation of cytokines and chemokines. Activation of only innate resident cells, such as microglia in “gliosis” or tissue resident macrophages in the absence of peripheral infiltration, should not be considered inflammation, even if this activation leads to cytokine production. While an innate response may result in the clearance of cellular debris, a true inflammatory response will engage the adaptive immune system for response escalation and resolution. Furthermore, the process of innate immune activation without adaptive involvement lacks specificity and memory. It is the interactions between innate (microglia, macrophages, neutrophils, monocytes, etc.) and adaptive cells (T and B cells) that carry out specific and targeted programs based upon the threat, and these interactions can occur at all sites throughout the body. It is with this definition that we propose Parkinson’s disease be considered an inflammatory condition, as there is evidence for both innate and adaptive involvement in the pathogenesis of disease.
Historically, studies of PD have focused on the basal ganglia, where the hallmarks of the disease, including the dramatic death of dopaminergic neurons, the presence of α-syn rich inclusions, and the associated motor circuit dysfunction are most prominent. However, there is long-standing evidence, as well as more recent findings, that highlight early and persistent dysfunction within multiple systems, both within and outside the CNS in patients with PD. These findings of multisystem dysfunction are coupled with a growing awareness of non-motor dysfunction in patients with PD. This evidence supports the notion of PD as a systemic disease that involves multiple tissues and cell types, with the key players including the central nervous system (CNS), gastrointestinal tract, autonomic nervous system, and the innate and adaptive immune systems.
In this review of immune mechanisms in PD, we highlight evidence that the innate and adaptive responses to CNS antigens are involved in disease pathogenesis. We provide a basic overview of innate and adaptive immune system mechanisms, as well as an overview of the clinical and pathological manifestations of inflammation in PD, involving both the CNS and peripheral tissues. This is followed by an overview of the immune system’s role in animal models of the disease, and how these models have identified key immune related pathways in PD pathogenesis. We conclude by identifying and discussing several key questions that we believe will drive future research and therapeutic discovery.
2. PD as a systemic and heterogeneous disease
Parkinson’s disease has classically been considered a movement disorder, as the motor deficits are the most obvious symptoms of disease and are used in part for diagnosing the disease, although a definite diagnosis can only occur postmortem. Cardinal symptoms of PD include tremor, rigidity, akinesia or bradykinesia, and postural instability (Obeso et al., 2017). The symptoms themselves are associated with intraneuronal α-syn rich inclusions throughout the brain and the dramatic loss of dopamine-producing neurons in the substantia nigra pars compacta, leading to a deficit of dopaminergic signaling in the caudate and putamen within the basal ganglia (Obeso et al., 2017). Single-photon emission computerized tomography (SPECT) scans can be used to visualize this loss, through labeling of dopamine transporters (DAT) on the terminals of neurons projecting from the from the SNpc to the caudate and putamen (Benamer et al., 2000; Ichise et al., 1999) although this loss may not be a direct correlate of dopaminergic neuron loss (Honkanen et al., 2019). The loss of uptake is typically asymmetric (Benamer et al., 2000, Ichise et al., 1999), which is reflected in the asymmetric onset of the motor symptoms characteristic of PD.
A description of Parkinson’s disease would be remiss without a brief discussion on the role of α-syn in the disease process. α-syn is typically found at the presynaptic terminals in neurons, but in PD, α-syn rich aggregates are found within the cell soma (Lewy bodies) and within neuronal processes (Lewy neurites) (Burre, 2015;Spillantini et al., 1997; Sulzer and Edwards, 2019). The normal function of α-syn remains somewhat of a mystery, although it has been implicated in processes such as synaptic vesicle release and recycling (Burre, 2015; Sulzer and Edwards, 2019), acting as a molecular chaperone for SNARE complex formation (Burre, 2015; Chandra et al., 2005), binding of dopamine and serotonin transporters (Burre, 2015), and regulating certain forms of synaptic plasticity (Sulzer and Edwards, 2019). Classically, the α-syn inclusions have been thought to be harmful, although there is more recent evidence that soluble, oligomeric forms of α-syn could be the more neurotoxic species (Cremades et al., 2012; Fusco et al., 2017; Luk et al., 2012). Regardless, α-syn pathology has been found throughout the brains of PD patients, and has been suggested to spread in a predictable prion-like manner between interconnected brain regions (Braak et al., 2003). However, it appears that α-syn inclusions alone cannot be blamed for PD, as there are cases of healthy people with high Lewy body load but without symptoms (Bengoa-Vergniory et al., 2017; Frigerio et al., 2011; Parkkinen et al., 2005) and Lewy body load does not necessarily correlate with the severity of symptoms (Bengoa-Vergniory et al., 2017), suggesting that there may be other mechanisms at work that explain the pattern of motor and non-motor symptoms in patients.
James Parkinson himself described many non-motor symptoms of PD including sleep disturbances, autonomic symptoms, and constipation (Parkinson, 2002). 200 years later, we now recognize that PD is a heterogeneous disease with widespread dysfunction and the involvement of multiple systems throughout the body—a disease in which each case has unique aspects. Indeed, it may be best to view PD as a syndrome with some core features, rather than a single disease (De Pablo-Fernandez et al., 2019). Anosmia (loss of smell), constipation, and rapid eye movement (REM) sleep disorder often begin prior to the appearance of motor symptoms, and when present together are highly predictive of later development of PD (Doty, 2012; Postuma et al., 2015). Other non-motor features appear later in the disease: approximately 20–40% of patients experience depression; and 40–56% experience anxiety (Barone et al., 2009). An even larger portion (30–80%) of PD patients will develop dementia, with half of non-demented patients still displaying mild cognitive impairment and cognitive decline over time (Aarsland et al., 2017). A neural correlate to this symptom could be the finding that PD patients have decreased cortical thickness compared to healthy controls (Deng et al., 2016; Sampedro et al., 2019; Uribe et al., 2018). Outside of the CNS, an exciting area of research is exploring the link between CNS and dysfunctions in the gastrointestinal tract, most notably preclinical constipation (Fasano et al., 2015) and associated intestinal inflammation in patients with PD (Devos et al., 2013). These motor and non-motor symptoms of PD and the associated pathology are indicative of a disease of global dysfunction.
3. Innate immune system
In order to understand inflammation as a process, it is important to distinguish between the two branches of the mammalian immune system. Most of our knowledge of the innate and adaptive immune systems comes from studies with mice, other mammals, or in vitro systems. The innate immune system is an ancient and highly conserved system that operates by nonspecific mechanisms and functions as a first line of defense that can later help to activate the adaptive system. Its purpose is to quickly resolve threats to the host, including clearance of invading pathogens such as bacteria or viruses, removal of dead/dying cells, and wound repair. However, under pathological conditions, the immune system can respond with excessive activation or misguided recognition of host antigens, leading to the damage of tissue.
The innate immune system is comprised of tissue resident macrophages that typically originate from the fetal yolk sac or fetal liver and self-renew (Goldmann et al., 2016; Hoeffel and Ginhoux, 2015), dendritic cells, neutrophils, circulating monocytes, granulocytes, and even some non-immune cells that adopt immunological functions as needed. The basic processes of innate immunity that will be discussed in this section are outlined in Fig. 1. Macrophages and dendritic cells, together referred to as antigen presenting cells (APCs), are thought to be the body’s main sensors of danger and initiator of an immune response; they constantly sample their environment, phagocytosing any debris, presenting findings on their surface via major histocompatibility complex (MHC) molecules, and sending out signals or interacting with other immune cells when they encounter danger. The MHC has two classes, MHC class I (MHCI) and MHC class II (MHCII). MHCI is expressed by all nucleated cells whereas MHCII is mainly expressed by the APCs described above. The ability of the innate immune system to present antigen is integral to mounting an effective immune response and communicating with other immune cells. It is important to note that macrophages are generally believed to be less competent at presenting antigens to T cells than dendritic cells, although both are capable of interacting with and activating T cells (Mildner and Jung, 2014). It is generally thought that dendritic cells or macrophages begin any immune response, and lead to its amplification through the recruitment of other cells such as monocytes, granulocytes, and neutrophils.
FIG. 1.
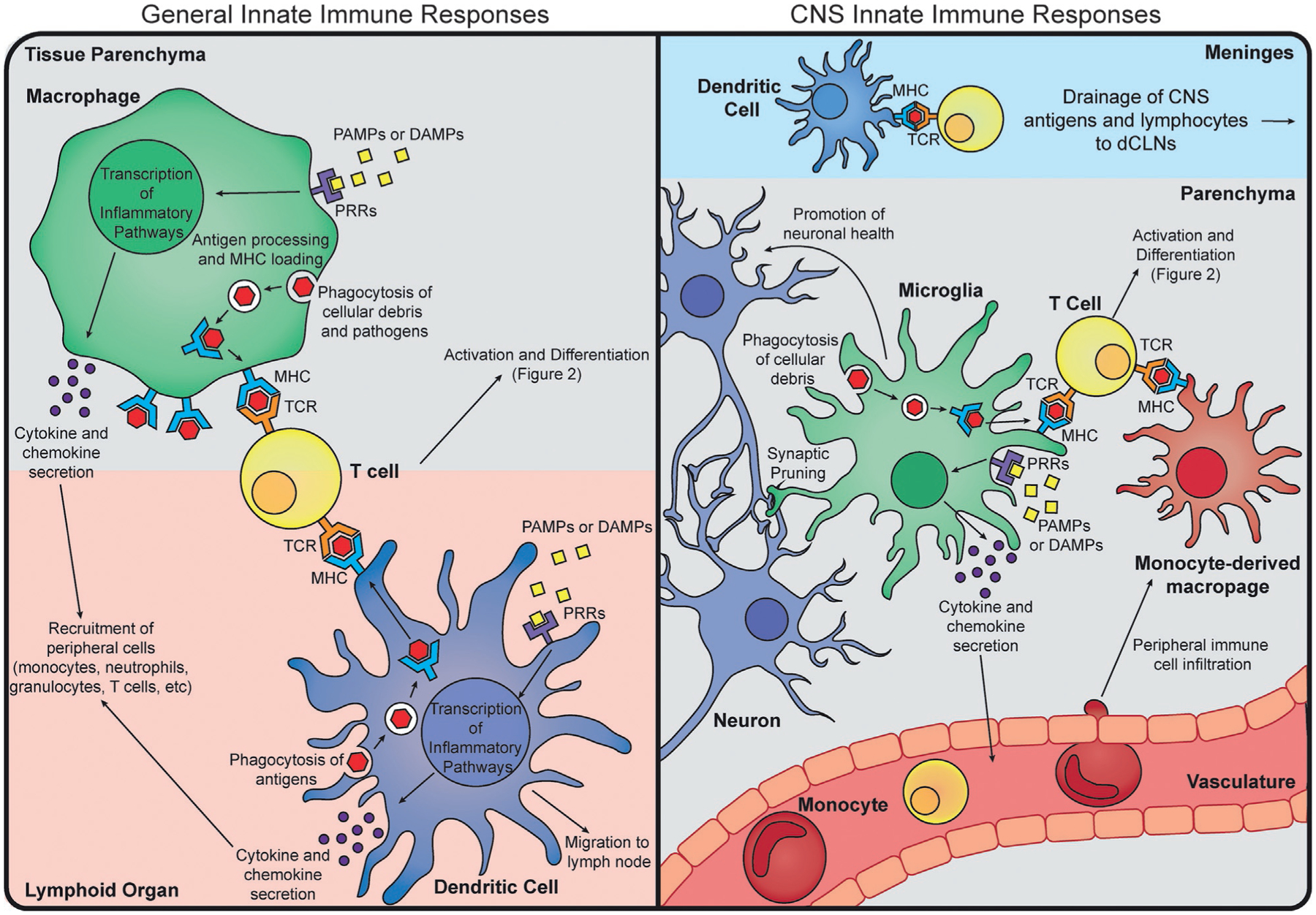
Overview of Innate Immune Responses. General innate immune responses typically involve antigen presenting cell (APC) recognition of pathogen associated molecular patterns (PAMPs, yellow squares) or danger associated molecular patterns (DAMPs) by a pattern recognition receptor (PRR, purple receptor). Upon recognition, the APC will undergo transcription for inflammatory pathways, which will lead to secretion of cytokines and chemokines (purple circles) to recruit more immune cells to the site, and upregulation of surface molecules involved in antigen presentation (MHC and costimulatory molecules, blue receptor). APCs can also phagocytose cellular debris and pathogens, process them, and load the antigenic peptides onto an MHC to be presented to T cells. Macrophages (green) typically carry out these functions within a tissue, whereas dendritic cells (blue) are usually found at tissue boundaries, and may migrate to a lymph node upon antigen uptake. In the CNS, the predominant APCs are microglia (green), although dendritic cells (blue) are found in the leptomeninges. Antigen uptake, pattern recognition, and cytokine secretion are thought to occur similar to general innate responses. Microglia also have tissue-specific homeostatic functions, such as synapse pruning and support of neuronal (blue) health. Infiltrating cells such as monocytes (red) and T cells (yellow), however, can be neuroprotective or neurotoxic, depending on the inflammatory stimulus. While there is some evidence that monocyte-derived macrophages (red) can play a role in antigen presentation during an inflammatory response, there is little known of their longevity in the parenchyma after resolution of the immune response.
Within the brain parenchyma itself, there are few, if any, dendritic cells. Most are found within the leptomeninges, though in very small populations (Mrdjen et al., 2018). Despite their relatively small numbers, their contribution to PD inflammation cannot be ruled out, as meningeal DCs are implicated in antigen presentation and subsequent neuroinflammation in models of multiple sclerosis (MS) (Mundt et al., 2019). However, the role of meningeal immunity and trafficking of immune cells in this space is poorly understood and remains understudied in the context of PD. The largest population of immune cells in the healthy CNS is microglia, the main tissue resident macrophage of the brain (Mrdjen et al., 2018). These cells arise early in development from the fetal yolk sac, and self-renew throughout life without major contribution from peripheral hematopoietic sources (Ginhoux et al., 2010; Goldmann et al., 2016; Nayak et al., 2014). They are thought of as first responders to any injury, modulators of homeostasis, and mediators of neuroinflammation. In homeostatic conditions, microglia express low to undetectable levels of MHCII, and have a generally anti-inflammatory phenotype (Colonna and Butovsky, 2017; Mrdjen et al., 2018; Nayak et al., 2014). However, during inflammation, they can upregulate MHCII and produce cytokines and chemokines. Additionally, peripheral myeloid cells such as monocytes can be recruited to the parenchyma during CNS inflammation, and further contribute to the inflammatory processes (Prinz and Priller, 2017). Whether or not microglia are pathogenic or protective, promoting either damaging inflammation or resolution and tissue repair in disease states is hotly debated, and seems to depend on the exact immune stimulus (Colonna and Butovsky, 2017; Nayak et al., 2014). The differential role between microglia and infiltrating peripheral myeloid cells is also a current area of research; with some hypothesizing that infiltrating cells could prove to be the more pathogenic population.
APCs mount immune responses following the activation of pattern recognition receptors (PRRs) by pathogen associated molecular patterns (PAMPs) or damage associated molecular patterns (DAMPs), activation of the complement cascade, or through antigen presentation to T cells (Fig. 1). Examples of pattern molecules that activate PRRs in these cells are: lipopolysaccharide (LPS) activating toll-like receptor 4 (TLR4) (Qureshi et al., 1999) and UV damaged self-RNA activating TLR3 (Bernard et al., 2012). Recognition of these PAMPs or DAMPs leads to the induction of signaling pathways to produce antimicrobial genes and inflammatory cytokines, depending on the specific immunological challenge (Lamkanfi and Dixit, 2014). Furthermore, these processes can amplify the ability of innate cells to actively present antigen, the process by which peptides (from host cells or from microbes) are loaded and presented on the MHC and interrogated by T cells. Dendritic cells normally take this antigen and migrate to the lymph nodes, where they will then direct T cell activation. Macrophages, on the other hand, typically secrete cytokines and chemokines from their origin tissue to recruit other cells such as monocytes and T cells, to the site of damage (Fig. 1). This initiates an immune response that will be further amplified and then resolved by incoming immune cells (Rankin and Artis, 2018). While innate immune responses are typically nonspecific, they are rapid and therefore key in determining the subsequent immunological response program that is initiated (Rivera et al., 2016). In many cases, the innate immune system alone can ward off pathogens and retain a rudimentary memory of the invader (Netea et al., 2016). However, without the other half of the system, true memory and robust inflammatory reactions would not occur.
4. Adaptive immune system
In contrast to the innate immune system, the adaptive immune system is highly specific and able to remember and effectively mount responses against previously encountered immunological threats. The specificity of the adaptive immune system is achieved due to the ability of T and B cells, collectively called lymphocytes, to rearrange their genomes and create unique antigen specific receptors: T cell receptors (TCRs) and B cell receptors (BCRs) (Bassing et al., 2002). The number of possible unique TCRs or BCRs is estimated to be in the 1013 range (Calis and Rosenberg, 2014; Laydon et al., 2015) and allows recognition of a wide range of bacterial, viral, or fungal antigens in a highly specific manner. Accompanying this vast antigen detection capacity is the ability of a small subset of pathogen-responsive T and B cells to differentiate into a long-lived population that can rapidly respond to re-exposures to the initial pathogen, a process collectively referred to as “memory” (Vitetta et al., 1991). While both T and B cells possess these unique sensing and recall abilities, their downstream immune effector functions are markedly different. The basic processes of the adaptive immunity that will be discussed in this section are outlined in Fig. 2.
FIG. 2.
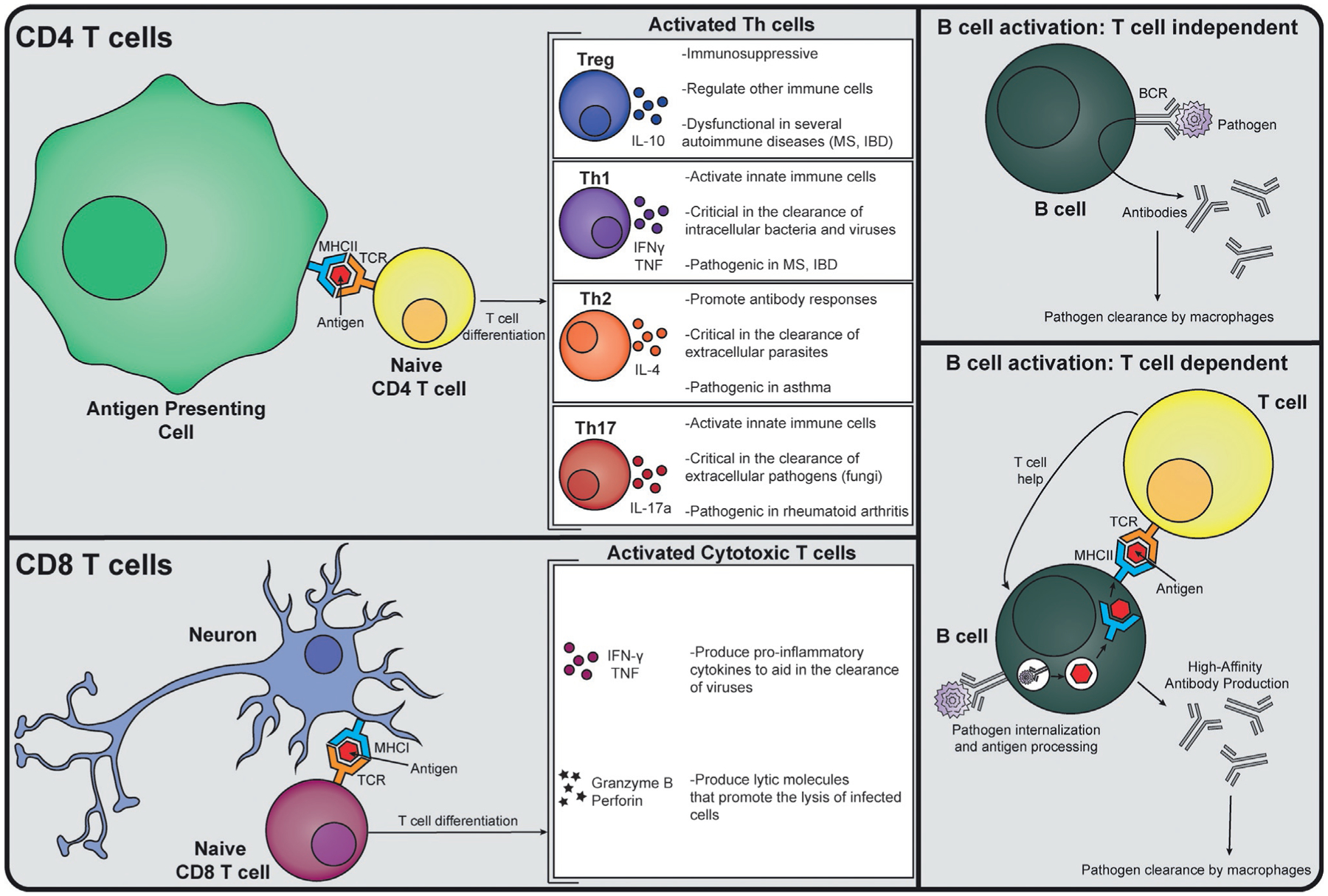
Overview of Adaptive Immune Responses. CD4 T cells (yellow) interact with antigen presenting cells (APCs, green) via an antigen-loaded MHCII molecule, and costimulatory molecules (not shown). Upon antigen recognition by their T cell receptor (TCR), a CD4 T cell can differentiate into a Treg, Th1, Th2, or Th17 cell type, which have different roles in inflammation and produce signature cytokines. A CD8 T cell (maroon) interacts with antigen-loaded onto an MHCI molecule, which can be displayed on the surface of any cell, including neurons (blue). Upon antigen recognition by the TCR, a CD8 T cell will differentiate and produce inflammatory cytokines and lytic molecules. B cells (dark green) can act with or without the help of T cells. The B cell receptor (BCR) can recognize extracellular pathogens (purple), leading to the production and secretion of antibodies targeted toward that pathogen. A B cell can also recognize a pathogen via its BCR, process it, and load an antigenic peptide onto an MHCII molecule, which can then interact with the TCR of a CD4 T cell (yellow). This results in the production of high affinity antibodies targeted toward the pathogen that will promote that pathogen’s clearance.
In a process similar to that in innate immune cells, B cells have the ability to sample soluble antigen directly via their BCR and can differentiate, proliferate, and secrete antibodies specific to that particular antigen (Fig. 2). B cells can also obtain help from T cells to produce high-affinity antibodies (also called immunoglobulins, Igs) that rapidly recognize and clear immunological threats (Chaplin, 2010). However, in the setting of autoimmunity (harmful immune reactions to the body’s own tissues), B cells produce autoantibodies to self-peptides that lead to tissue dysfunction and destruction by the body’s own immune system, such as in the disease systemic lupus erythematosus.
T cells, on the other hand, detect antigens that have been loaded onto the MHC of other cells via their TCR. The MHC has two separate classes that dictate the nature of the antigens as well as the subsequent T cell response. MHC class I is expressed by all nucleated cells in the mammalian body and is responsible for antigen sampling within the intracellular space. CD8 T cells, often referred to as cytotoxic T cells (Tc), are one of the two major subsets of T cells and function mainly via their TCR in recognizing foreign antigens (e.g., viruses) on MHCI. When activated, they proliferate and release cytokines and lytic molecules (e.g., granzyme and perforin) that promote the lysis of the infected cell (Lieberman, 2003) (Fig. 2). In contrast, CD4 T cells recognize antigen-loaded onto MHC class II, which is expressed by antigen presenting cells like macrophages, monocytes, dendritic cells, and B cells. Upon TCR recognition of an MHC-bound antigen, CD4 T cells produce effector cytokines that “help” mediate pathogen clearance by boosting the function of the innate immune system and antibody-producing B cells (Fig. 2).
The cytokines produced by helper CD4 T cells (Th) are pathogen and tissue dependent, and have been generally categorized as having Th1, Th2, Th17, or T regulatory (Treg) type responses (Fig. 2) (Leung et al., 2010). Generally, Th1 immune responses are directed toward intracellular bacteria or viruses and they help clear these immunological threats in part by secreting the cytokine interferon gamma (IFN-γ) which serves to amplify the innate immune system’s antimicrobial and anti-viral capabilities (Leung et al., 2010; Zhu and Paul, 2008) (Fig. 2). However, there are instances where autoimmune Th1 responses occur, as is the case in patients with MS (McFarland and Martin, 2007) or inflammatory bowel disease (IBD) (Neurath, 2014), where they can promote destructive tissue damage through excessive cytokine release and innate immune system activation. Th2 immune responses are typically directed toward extracellular parasites and promote clearance of these pathogens through the secretion of the cytokine IL-4, which helps to support the humoral B cell response (Leung et al., 2010; Zhu and Paul, 2008) (Fig. 2). The overactive allergic responses observed in patients with asthma are an example of aberrant Th2 responses (Lambrecht and Hammad, 2015). Th17 responses are critical in the response to extracellular pathogens such as fungi, in part through the secretion of IL-17a, a cytokine that enhances the effectiveness of the innate immune system— particularly neutrophils (Leung et al., 2010; Zhu and Paul, 2008) (Fig. 2). However, in some autoinflammatory contexts, Th17 responses can become aberrant and promote excessive inflammation and tissue damage, as is the case with rheumatoid arthritis (Leipe et al., 2010). Lastly, Tregs play a regulatory role that serves to facilitate the process of resolving inflammation during pro-inflammatory events as well as preventing misguided host-tissue reactions in part through the innate and adaptive modulating cytokine IL-10 (Vignali et al., 2008). However, breakdowns in the function of Tregs can lead to the loss of regulation of pro-inflammatory immune reactions and can lead to damage of tissues. In fact, nearly all autoimmune diseases have evidence of dysfunctional Tregs that contribute to the break of tolerance and inability to resolve inflammatory events (Dominguez-Villar and Hafler, 2018; Leung et al., 2010) (Fig. 2).
Typically, the innate and adaptive arms of the immune system function in harmony, mounting and resolving effective and protective immune responses. However, as in the examples of autoimmunity cited above, these normal functions can turn aberrant and pathological, and become damaging toward a self-antigen, or something endogenously produced by the body. Typically, self-reactive T cells are deleted during development, but some escape and normally remain inert without the presence of additional signals (Bouneaud et al., 2000; Nemazee, 2017; Theofilopoulos et al., 2017). However, upon failure of central and peripheral tolerance of reactive T cells, an aberrant immune response may be mounted as T cells recognize self-antigens that are presented on MHC molecules of APCs. This response often fails to resolve itself, especially since IL-10 producing Tregs are impaired in either number or function in many autoimmune conditions (Dominguez-Villar and Hafler, 2018; Miyara et al., 2011). This could also be affected by the altered microbiome found in autoimmune diseases, as the gut microbiome strongly influences the development of Tregs (Honda and Littman, 2016; Theofilopoulos et al., 2017). Much of what we know of aberrant, harmful inflammatory processes comes from the study of autoimmune disease, and will likely inform future research in PD. Specifically, the role of APCs and T cells in the pathogenesis of PD should be investigated, as well as the target antigen of the inflammatory response observed.
5. CNS inflammation in human PD
5.1. CNS parenchyma
One of the first findings that connected the immune system to the pathobiology of PD was the observation of an increased number of reactive microglia, originally defined by morphology (Foix and Nicolesco, 1925) and later human leukocyte antigen-DR isotype (HLA-DR, a component of the human MHCII) expression, in the postmortem substantia nigra of PD patients (Mcgeer et al., 1988b). This microgliosis and other immune-PD phenotypes are summarized in Fig. 3. These activated microglia have been observed in direct proximity to Lewy pathology and free melanin in the brain (Mcgeer et al., 1988b). However, that same study acknowledged that the assumption of a microglial identity for these cells was based off their shared reactive morphology to the glioma associated microglia originally described by Penfield (Penfield, 1925). The exact identity of these reactive “microglia” still remains to be determined, as it is possible that some may be peripherally derived macrophages of a monocyte lineage rather than true microglia (Hoeffel and Ginhoux, 2015) (Fig. 1). Regardless of their exact origin, multiple groups have provided further evidence of a pro-inflammatory phenotype (HLA-DR+, CD68+, ICAM-1+) or innate activation (increased TLR2 expression) through histological studies (Croisier et al., 2005; Dzamko et al., 2017; Imamura et al., 2003; Orr et al., 2005; Rozemuller et al., 2000), as well as through the use of positron emission tomography (PET) imaging studies (Gerhard et al., 2006; Iannaccone et al., 2013; Terada et al., 2016). In the PET studies, ligands are specific to the translocator protein, TSPO (previously referred to as the “peripheral benzodiazepine receptor”), a protein that is expressed on the mitochondrial membrane of activated myeloid cells and astrocytes (Cosenza-Nashat et al., 2009; Lavisse et al., 2012). Chronic, increased signal from the TSPO ligands were found in PD patients, suggesting a chronic neuroinflammatory condition of patients that had only previously been observed in postmortem tissue (Fig. 3). This use of noninvasive imaging to detect and track brain inflammation in PD patients could be very useful in determining the effectiveness of immunotherapies for the disease, however, early studies using this method to test anti-inflammatory treatments on reducing TSPO binding (i.e., microglia inflammation) in PD patients have produced mixed results (Bartels et al., 2010; Jucaite et al., 2015). In addition to the evidence of cellular morphology indicative of inflammation in the brains of PD patients, there is also evidence of increased pro-inflammatory cytokine and chemokine molecules in the brain parenchyma. These findings include increased levels of both the pro-inflammatory cytokine tumor necrosis factor (TNF, previously TNF-α) and the T cell associated chemokine CXCL12 in the caudate, putamen, and substantia nigra of PD postmortem brain, respectively (Mogi et al., 1994; Shimoji et al., 2009).
FIG. 3.
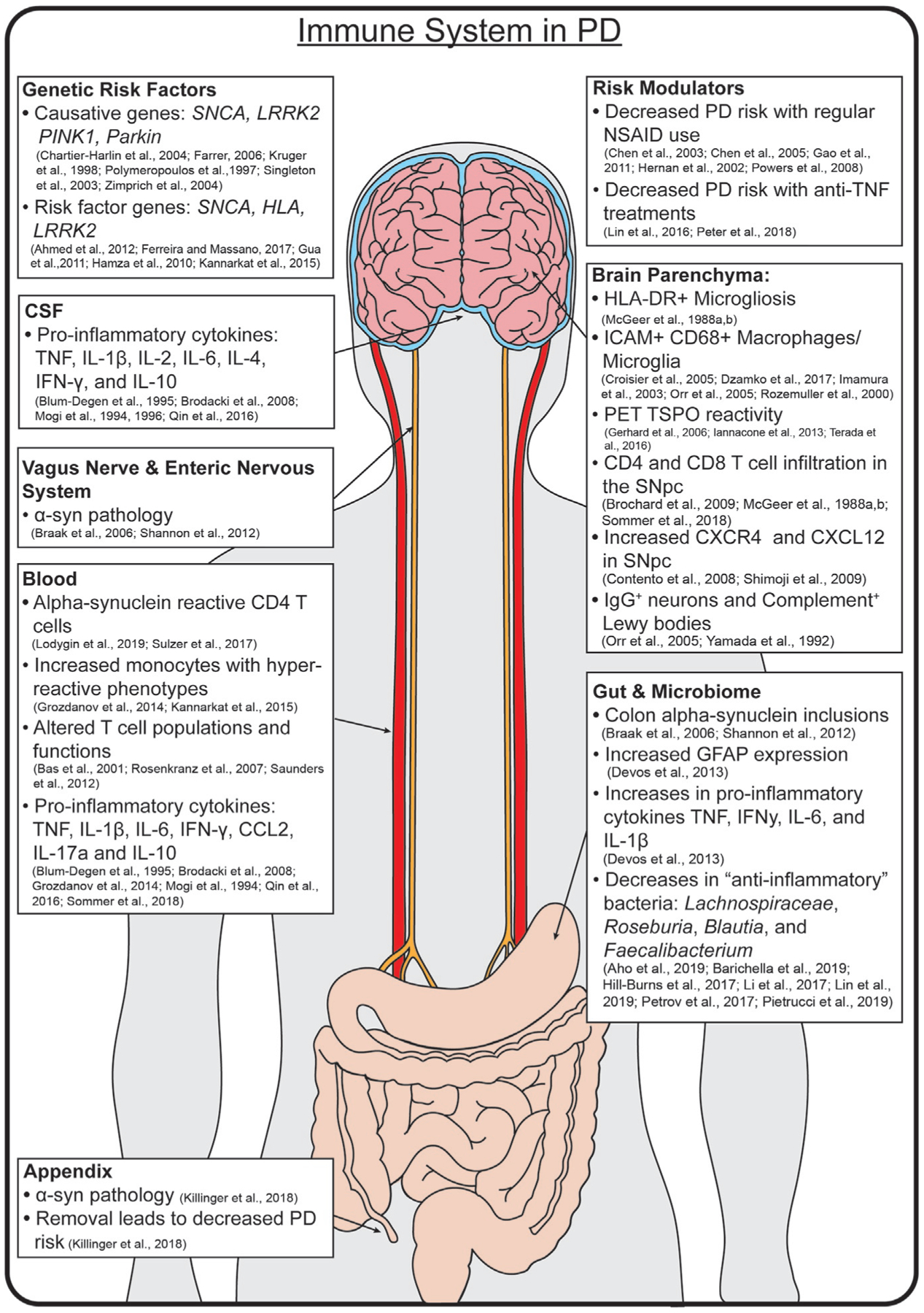
The Immune System in Human PD. PD is a disease of global dysfunction, with inflammation found in multiple tissues. Risk is conferred by specific genes, and modulated by certain anti-inflammatory treatments. Notable inflammatory markers are found throughout the brain, CSF, blood, gut, enteric nervous system, and microbiome.
While earlier findings suggested the presence of Tc (cytotoxic) CD8 T cells in the postmortem substantia nigra of PD patients (Mcgeer et al., 1988a), Brochard’s (Brochard et al., 2009) discovery of increased CD4+ and CD8+ T cells surrounding neuromelanin positive neurons in the PD postmortem brain provided more definitive evidence that the adaptive immune system was involved in disease and has been since replicated (Sommer et al., 2018). This finding is further supported by the observation that levels of CXCR4, a chemokine receptor expressed by T cells (Contento et al., 2008), and its reciprocal ligand CXCL12 are both increased in postmortem brains of PD patients compared to healthy controls (Shimoji et al., 2009) (Fig. 3). While B cells have never been reported to be within patient brain samples directly, there are increases in the number of IgG positive neurons (Orr et al., 2005) and complement positive Lewy bodies (Yamada et al., 1992) in the substantia nigra of patients with PD. These data would suggest that B cells may in fact contribute to the pathobiology of Parkinson’s disease, though likely from a primed secondary lymphoid organ. Taken together, there is a substantial amount of data to suggest that cells of both the innate (microglia or macrophages) and the adaptive immune system (T cells) are in close proximity to each other and neuromelanin containing neurons in the PD brain.
5.2. Cerebrospinal fluid
Cytokine and chemokine molecules are crucial signal transducers in the recruitment, stimulation, and regulation of multiple cell types in an inflammatory response (Fig. 2). In PD, there is substantial evidence that signaling and effector molecules are secreted in both the brain and cerebrospinal fluid (CSF). One of the first results to substantiate this idea was the observation that protein levels of the cytokine TNF are increased in both the caudate and putamen as well as the CSF of PD patients compared to controls (Mogi et al., 1994). Other early observations in the CSF of PD patients showed increased levels of IL-1β, IL-2, IL-6, and IL-4 (Blum-Degen et al., 1995; Mogi et al., 1996; Qin et al., 2016). The combination of IL-1β, IL-2, and IL-6 suggests the presence of a pro-inflammatory process as all three cytokines have been shown to have detrimental effects on neurons and other vulnerable cell types in other inflammatory diseases (Filiano et al., 2017; Neurath, 2014). IL-4, however, has been linked with neuroprotective and neuroregenerative responses in the CNS (Filiano et al., 2017; Walsh et al., 2015) along with non-CNS allergic or autoimmune responses that have heavy humoral influence, such as asthma (Lambrecht and Hammad, 2015). Another more recent study has recapitulated these above findings but also found increased levels of TNF, IFN-γ, and IL-10 in the CSF of PD patients (Brodacki et al., 2008). The presence of TNF and IFN-γ provides further evidence of a pro-inflammatory process, as both are potent immune activators (O’Shea et al., 2002) and detrimental to neuron health (Cebrian et al., 2014; Filiano et al., 2017). Interestingly, IL-10 is typically associated with the regulatory responses (Fig. 2) produced by Tregs to modulate the innate immune system. It is possible that a compensatory and regulatory process occurs alongside the pro-inflammatory response observed in PD.
6. Peripheral inflammation in PD
In addition to the evidence of an activated innate and adaptive immune response in the CNS of PD patients, there is also ample data supporting the idea that a similar inflammatory response occurs throughout the body. This peripheral PD immune phenotype is consistent with the overarching concept that Parkinson’s disease is a systemic, multisystem disease affecting the whole body, and is summarized in Fig. 3.
6.1. Blood
One possible mechanism that can connect the systemic inflammatory phenotypes observed in PD is the bloodstream, the major pathway for immune cell trafficking throughout the body. Furthermore, due to the challenge of detecting inflammation in the brains of living PD patients, the study of blood in individuals with PD has produced much of our understanding of immune responses in the disease. Higher levels of inflammatory cytokines and chemokines are found in the blood and CSF, including: TNF, IL-1β, IL-2, IL-6, IFN-γ, and CCL2 (Blum-Degen et al., 1995; Grozdanov et al., 2014; Mogi et al., 1994, 1996; Qin et al., 2016). IL-17a and IL-10 are also elevated in the blood of PD patients (Brodacki et al., 2008; Sommer et al., 2018). One study correlated higher serum levels of TNF at the start of the study with faster decline of motor function over the following 3 years, and higher IL-1β and IL-2 with faster cognitive decline (Williams-Gray et al., 2016). However, these cytokine measurements are snapshots, taken at one time point, and it is therefore difficult to determine if these inflammatory markers in the bloodstream remain stable over time. Regardless, these findings mirror both the pro-inflammatory and regulatory phenotypes that have been measured in patient brain, indicating that the inflammatory process is potentially one of global coordination and involvement.
Furthermore, there are changes in both the number and activation profiles of immune cells in PD blood. In general, classical monocytes (CD14+CD16−) are enriched in PD patients compared to controls and are hyper-reactive (Grozdanov et al., 2014). Specifically, monocytes derived from patients produce more IL-6 to an LPS stimulus compared to those from healthy controls, and this cytokine production is positively correlated with the Hoehn and Yahr stage of disease severity. Additionally, both healthy controls and PD patients that carry a single nucleotide polymorphism (SNP) in HLA-DR that confers a higher PD risk have higher baseline MHCII expression on blood B cells and monocytes (Kannarkat et al., 2015), indicating that a SNP in HLA-DR could predispose individuals to a more inflammatory phenotype. This could indicate a lower threshold for activation, or a sensitized response to inflammatory stimuli that is present in PD immune cells and predisposes patients to stronger and more damaging immune reactions. PD patients also have lower lymphocyte numbers in their blood compared to controls, an effect which is mostly driven by a decrease in subsets of CD4+ T cells, specifically naı¨ve and memory subsets, whereas numbers of CD8 cytotoxic T cells are unchanged. Although naı¨ve and memory subsets decrease, there is an overall increase in the number of activated, antigen-experienced CD4+ T cells (CD4+ CD25+) (Bas et al., 2001) (Fig. 3). These findings indicate that there is an overall shift to an activated, antigen-experienced T cell phenotype in the blood. Additionally, one study found an increase in T regulatory cells in PD patients, while another reported decreased ability of PD Tregs to suppress the activity of effector T cells in vitro (Rosenkranz et al., 2007; Saunders et al., 2012). These data could be indicative of an attempt to regulate the inflammatory response, but a decreased ability to do so effectively. The idea of T cell driven neuroinflammation is further supported by evidence from (Sulzer et al., 2017), who exposed patient PBMCs to various α-syn peptides, and found cytokine responses from CD4 and CD8 T cells. The predominant responses were IL-5 or IFN-γ, indicating CD4 Th2-MHCII and CD8-MHCI responses to α-syn, with the highest responses to two specific α-syn peptide sequences. One peptide included cleavage sites, and the other included the Ser129 region that is often phosphorylated (pSer129) in abnormal α-syn species. These data have been recapitulated in part by Lodygin et al. (2019), who found that blood circulating α-syn-reactive CD4 T cells were expanded in PD patients. Further studies have implicated a role for Th17 cells in neuron death through IL-17/IL-17R signaling using PD T cells and human iPSC derived midbrain neurons (Sommer et al., 2018). These findings indicate the presence of systemic immune activation, possibly directed toward abnormal α-syn, in human PD.
6.2. Gastrointestinal tract, enteric nervous system, and the microbiome
It has been observed that constipation in PD patients begins long before the onset of motor symptoms, and that fewer bowel movements per day are associated with a higher risk for PD (Abbott et al., 2001). While α-syn pathology is typically discussed in relation to the CNS, inclusions are also found in the colon, in neurons of the enteric nervous system, and within the vagus nerve itself (Braak et al., 2006; Shannon et al., 2012). Additionally, a recent study reported the appendix to be a rich source of α-syn pathology and found an association of reduced PD risk in individuals whose appendix was removed early in life (Killinger et al., 2018). These findings of α-syn GI system are accompanied by increases in markers of inflammation, such as increased glial fibrillary acidic protein (GFAP) and increases in a number of pro-inflammatory cytokines including TNF, IFN-γ, IL-6, and IL-1β (Devos et al., 2013). Interestingly, these cytokines in the gut are higher earlier in disease and decrease with time, perhaps indicating gut inflammation could be an early event in the pathogenesis of Parkinson’s disease. It is difficult to say whether gut inflammation directly contributes to disease pathogenesis, especially since a bona fide innate and adaptive immune response has not yet been reported in PD gut, and additionally it is not known if α-syn inclusions or gut inflammation occurs first. Nevertheless, this hypothesis is supported by findings that a diagnosis of inflammatory bowel disease, in particular Crohn’s disease, increases the risk of developing PD in certain populations (Lin et al., 2016).
Given the observations of both gastrointestinal dysfunction and inflammation in individuals with PD, it is logical to investigate changes in the gut microbiome of these individuals. Not only is the microbiome important in overall gut function, it is also now better appreciated for its role in shaping as well as being shaped by the host’s immune system (Hooper et al., 2012). Therefore, it is not surprising that multiple studies have observed alterations in the microbiomes of diverse cohorts of people with PD across the world (Aho et al., 2019; Barichella et al., 2019; Hill-Burns et al., 2017; Li et al., 2017; Lin et al., 2019; Petrov et al., 2017; Pietrucci et al., 2019; Scheperjans et al., 2015). More specifically, individual bacterial taxa have been implicated in the disease state from the aforementioned studies. Certain bacterial taxa associated with an “anti-inflammatory” environment have consistently found to be reduced in PD stool. These include the bacterial family Lachnospiraceae, and some of its genera Roseburia, Blautia, and Faecalibacterium. Roseburia spp. have been shown experimentally in vitro and in vivo to promote anti-inflammatory processes through a variety of potential mechanisms which include enhancement of gut lining health via increased expression of tight junction proteins (Tan et al., 2019) and modulation of immune responses via downregulation of pro-inflammatory cytokines (e.g., IL-17) (Zhu et al., 2018) and induction of anti-inflammatory cytokines (e.g., IL-10, TGFβ) (Patterson et al., 2017; Shen et al., 2018). Additionally, a species of Faecalibacterium and its cellular byproducts has been shown experimentally to reduce inflammation through multiple potential mechanisms including regulation of Th17 and Treg cell differentiation (Zhou et al., 2018), inhibition of pro-inflammatory pathways and cytokine production (Martin et al., 2014; Sokol et al., 2008), induction of anti-inflammatory molecules (Breyner et al., 2017; Sokol et al., 2008), and promotion of gut barrier health (Carlsson et al., 2013; Martin et al., 2015). With decreased abundance of these species, it is possible that the gut becomes a site predisposed to inflammation.
Overall, the composition of the gut microbiota is clearly altered in PD, as this finding is replicated across numerous studies from multiple geographical populations. How this dysbiosis of the gut microbiota in PD relates to inflammation and immunity in PD is still under investigation. The picture should become clearer as additional research is conducted to investigate how changes in the gut microbiota, at the global and individual microorganisms level, influence, or are affected by, PD pathogenesis.
7. The immuno-genetics of PD
Although a majority of Parkinson’s disease cases appear to be idiopathic, there are multiple gene mutations or SNPs (especially related to the α-syn locus) that can promote familial forms or significantly increase one’s risk to develop Parkinson’s disease. In this section, we will overview some of those PD genes and their links to the inflammatory immune response observed in PD.
7.1. SNCA
Since the initial discovery of genetic variations in SNCA, the gene encoding α-syn, as the cause of certain familial forms of PD (Polymeropoulos et al., 1997), years of research have only strengthened the findings that the hallmark PD protein and the genetic control of its expression are a major determining factor for the development of familial or idiopathic Parkinson’s disease (Chartier-Harlin et al., 2004; Edwards et al., 2010; Kruger et al., 1998; Singleton et al., 2003). These studies and others support the idea that high levels of α-syn can lead to the neurotoxic phenotype observed in human PD. The exact mechanism of how this increased expression leads to neurodegeneration is still unclear, but defects in autophagy, vesicle trafficking, and the generation of toxic, oligomerized species due to α-syn accumulation have been proposed (Lashuel et al., 2013). Another possible mechanism, and one that involves the immune system, is that pathogenic forms of α-syn due to mutation, overaccumulation, or oligomerization can directly activate the immune system. This idea is supported by the finding that pathogenic forms of α-syn can directly induce toll-like receptor 4 (TLR4) mediated microglial cytokine production, ROS production, and phagocytic activity (Fellner et al., 2013). α-syn may also be capable of activating the adaptive immune system, as (Sulzer et al., 2017) identified two antigenic regions of α-syn capable of eliciting inflammatory IFN-γ and IL-5 cytokine responses from PD patient derived CD4 and CD8 T cells. The antigenic Y39 region identified is near several of the PD-associated mutations (A30P, E46K, H50Q, G51D, A53E/T) (Hernandez et al., 2016), while the second antigenic region contains the Ser129 amino acid and requires it’s phosphorylation to activate T cells—further supporting the link of mutated, pathogenic forms of α-syn promoting the inflammatory phenotype observed in PD.
7.2. HLA
HLA is a large, highly polymorphic gene set located on human chromosome 6 and is subdivided into class I and class II regions (MHCI and MHCII, respectively) (Mosaad, 2015). The control of these key immune system genes is essential to the selection of T cells, antigen sampling, and the induction of an immune response. In the context of disease, variations of certain alleles in the HLA region have been associated with the development of a wide range of diseases from rheumatoid arthritis (Stastny, 1978) to narcolepsy (Mignot et al., 1997) and PD (Hamza et al., 2010). The initial findings of (Hamza et al., 2010) which observed associations between SNPs in the HLA-DRA region with late-onset idiopathic Parkinson’s disease, have since been independently replicated (Guo et al., 2011; Kannarkat et al., 2015) and expanded to include the HLA-DRB5 and HLA-DRB1 gene loci as well (Ahmed et al., 2012; International Parkinson Disease Genomics Consortium et al., 2011). Interestingly, in the case of HLA-DRA PD-associated polymorphisms, there is evidence to suggest that this risk may be mediated by higher baseline and induced levels of MHCII proteins on blood monocytes, B cells, and a skew of their phenotype toward inflammatory responses (Kannarkat et al., 2015). Most recently, a class I allele (HLA-A) has also been associated with PD and the IFN-γ response from blood derived CD8 T cells to α-syn (Sulzer et al., 2017), possibly providing an insight to their function in the brain parenchyma of patient. Overall, the exact mechanisms that underlie the PD-associated HLA alleles effect on disease pathology remain unclear, but the most straightforward implication is that they may potentiate the pro-inflammatory microglial and T cell responses observed in PD patients.
7.3. LRRK2
Mutations in leucine-rich repeat kinase (LRRK2) are a genetic cause of PD that appears to act in part by influencing the immune system (Zimprich et al., 2004). Mutations in LRRK2 that cause PD are associated with increased kinase activity, and these increases are also observed in PD patients without a LRRK2 mutation (Di Maio et al., 2018; Ferreira and Massano, 2017). LRRK2 protein is thought to phosphorylate Rab GTPases (Steger et al., 2016) and modulate intracellular vesicle trafficking. Though LRRK2’s expression is mainly thought of in the context of neurons, it is also found to be expressed in high levels by immune cells such as macrophages, monocytes, and B cells (Gardet et al., 2010; Hakimi et al., 2011), where Rab GTPase mediated vesicle trafficking is crucial to the initiation of their immune responses (Prashar et al., 2017). Moreover, LRRK2 alterations are consistently linked to other, classical inflammatory diseases such as leprosy and Crohn’s disease (Bae and Lee, 2015; Hui et al., 2018), and increased kinase activity could amplify the already pro-inflammatory functions of LRRK2 in inflammasome activation (Liu et al., 2017) and nuclear factor of activated T cells (NFAT) activation (Liu et al., 2011). However, further study of LRRK2 and how it functions in the innate and adaptive immune compartments within the context of PD is required to better understand its contribution to potential disease driving neuroinflammation in PD.
7.4. PINK1 and Parkin
Mutations in the genes encoding PTEN-induced kinase 1 (PINK1) and E3 ubiquitin ligase (Parkin) can result in an early onset, slowly progressing, and L-DOPA responsive form of Parkinson’s disease (Farrer, 2006). Furthermore, SNPs in the promoter and coding regions of Parkin have been associated with the development of late-onset PD (Mata et al., 2004). The two proteins have recently been shown to play important roles in the process of mitophagy, or the removal of damaged mitochondria within the cell (Pickrell and Youle, 2015), a process that is important in immune system responses.
It has been shown that individuals with mono or biallelic mutations in parkin, regardless of PD status, have higher amounts of pro-inflammatory cytokines and chemokines (IL-6, IL-1β, CCL2, and CCL4) in their serum compared to controls (Sliter et al., 2018). PINK1 and Parkin knockout mice display a similar inflammatory response in their serum. In both of these knockout mice, the inflammatory response is the result of an accumulation of mitochondrial DNA and its subsequent activation of the innate immune system’s type I interferon response through the stimulator of interferon genes (STING) pathway. Additionally, two recent studies performed in mice reported activation of the adaptive immune system, through the induction of pro-inflammatory cytotoxic CD8 T cells targeting host mitochondrial antigens in PINK1 or Parkin deficient mice subjected to bacterial exposure (Matheoud et al., 2016, 2019). B cells may also play a role in disease as increased amounts of anti-nuclear/dsDNA antibodies can be detected in the serum of oxidatively stressed PINK1 or Parkin deficient mice (Sliter et al., 2018). Broadly, given that the dopamine neurons in the substantia nigra have been shown to have a higher basal rate in mitophagy than other dopamine producing neurons (Guzman et al., 2018), it is logical that a breakdown in the mitophagy machinery, either by dysfunction in PINK1, Parkin, or α-syn pathology could contribute to the neurodegeneration observed in PD. Interestingly, these findings suggest that one consequence of the breakdown of mitophagy machinery is the production of cytosolic DNA and mitochondrial damage that can lead to the activation of both the innate and adaptive immune systems to produce tissue destructive inflammation.
Taken together, there is ample evidence of multiple PD-associated genes having various effects on the immune system. These effects seem to promote a pro-inflammatory response, which coincides with what is observed globally in the immune systems of individuals with PD. Interestingly, this potential PD-variant genetic effect on immune cells may be more direct than we think as (Raj et al., 2014) observed an overrepresentation of immune-specific expression quantitative trait loci (eQTL) overlapping with PD susceptibility loci (e.g., SNCA, LRRK2) from blood monocytes of healthy controls. These data suggest there may be a cell-autonomous effect of PD-associated genes within immune cells themselves (especially innate immune cells) that could contribute to the pathobiology of PD (Harms and Standaert, 2014). However, much more work is needed to better characterize these PD-gene and immune system interactions.
8. Immune modulation and risk of Parkinson’s disease
A number of population studies support the idea that the immune system has an important role in the development of PD. These studies found associations between the chronic use of anti-inflammatory drugs and reduced risk of developing Parkinson’s disease. Nonsteroidal anti-inflammatory drugs (NSAIDs) were originally found to be neuroprotective against dopamine cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD (reviewed in next section) (Aubin et al., 1998; Teismann and Ferger, 2001). Additionally, (Chen et al., 2003) observed that men and women who had reported regular NSAID use had a significantly lower risk of later developing Parkinson’s disease. These same general findings have been observed in subsequent studies using a variety of different cohorts (Chen et al., 2005; Gao et al., 2011; Hernan et al., 2002; Powers et al., 2008). The mechanism by which NSAIDs favor PD protection is unclear, but their ability to inhibit the aberrant COX-2 and NF-κB signaling observed in PD may be relevant (Asanuma and Miyazaki, 2007).
Another anti-inflammatory therapy that is associated with reduced PD risk is the use of anti-TNF therapy (Peter et al., 2018). Inflammatory bowel disease (IBD) patients, a population who are at higher risk of developing PD (Lin et al., 2016; Peter et al., 2018), who were on anti-TNF treatments had a 78% reduction in their PD incidence rate compared to IBD patients not on anti-TNF treatment. Elevated TNF levels in PD brain, CSF, and blood have been reported by several groups (Brodacki et al., 2008; Mogi et al., 1994; Qin et al., 2016), suggesting that TNF may have a key role in promoting the pathophysiology of PD.
9. Inflammation in murine models of PD
It is increasingly clear that findings in human PD have strongly implicated a pro-inflammatory response in the pathogenesis of disease. It has also highlighted that PD is a systemic disease, in which symptoms are found in multiple organ systems. However, it is difficult to parse the initiating events or mechanistic processes in humans, and thus the field has turned to animal models. Models of PD have evolved rapidly over time, beginning with neurotoxin-based models that focused on neuronal death to models that highlight the importance of α-syn in disease processes. Here, we will summarize some of the evidence for inflammation in both neurotoxin and α-syn models of PD.
9.1. Neurotoxin-based animal models of PD
9.1.1. 6-OHDA
The 6-hydroxy-dopamine (6-OHDA) model is a neurotoxin model of PD that has been widely used and characterized since its initial development for studying the death of monoamine neurons in the CNS of rats (Ungerstedt, 1968). The mechanism of 6-OHDA’s neurotoxic effect is through the production of excessive reactive oxygen species in neurons after entering through their dopamine transporter (Soto-Otero et al., 2000). The universal features of the model include the loss of nigrostriatal neurons, the development of L-Dopa responsive bradykinetic motor symptoms, and the marked activation of the innate immune system in the CNS (Bove and Perier, 2012).
In this model, activation of the CNS innate immune system, first described by (Akiyama and Mcgeer, 1989), has been shown to precede overt neuron loss (Cicchetti et al., 2002; Depino et al., 2003; Marinova-Mutafchieva et al., 2009), suggesting that the priming of these inflammatory myeloid cells may help drive this neuron loss via phagocytosis or enhanced inflammatory cytokine production. This notion is supported by the fact that administration of CX3CL1 (a neuron-derived microglial suppressor molecule) (Pabon et al., 2011), iNOS inhibitors (Broom et al., 2011), or dominant negative anti-TNF therapy (Harms et al., 2011) are all neuroprotective in the 6-OHDA model. While the innate immune response in the 6-OHDA model is thought to be neurotoxic, the role of the adaptive immune system is less studied and less clear. T (both CD4 and CD8) and B cells have been found to infiltrate the CNS after 6-OHDA administration at a similar time point as myeloid activation (Theodore and Maragos, 2015). However, these infiltrating lymphocytes may have a net positive neurotrophic effect as one study found that 6-OHDA treated athymic RNU−/− rats lacking T cells displayed exacerbated motor deficits compared to 6-OHDA treated controls (Wheeler et al., 2014). Perhaps the neuroregenerative anti-inflammatory effects of 6-OHDA responding Tregs outweighs any other pro-inflammatory cytokine producing T cells, but no follow-up experiments have been performed to support or reject this hypothesis.
9.1.2. MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) readily crosses the blood brain barrier, where it is metabolized by glial cells to its final neurotoxic form of MPP+ (Meredith and Rademacher, 2011), a potent mitochondrial complex I inhibitor that leads to rapid dopaminergic neuron death and the development of Parkinsonian symptoms in humans (Langston et al., 1983), nonhuman primates (Tetrud and Langston, 1989), and mice (Sonsalla and Heikkila, 1986).
In mice and nonhuman primates, MPTP treatment leads to robust MHCII+ microglial activation (Czlonkowska et al., 1996; Kohutnicka et al., 1998; Mcgeer et al., 2003). Similar MHCII+ microglial activation has been found in human postmortem tissue of individuals who had been exposed to MPTP and developed Parkinsonism (Langston et al., 1999). Similar to the 6-OHDA model of PD, efforts to reduce microglial activation or associated downstream signaling effects (NF-κB, IL-1β, IL-6) via treatment with anti-inflammatory iNOS inhibitors (Du et al., 2001; Wu et al., 2002) or COX-1/2 inhibitors (Teismann and Ferger, 2001) have proven neuroprotective against the effects of MPTP in mice. Additionally, there is ample evidence of an adaptive immune response to MPTP. This includes the infiltration of CD4 and CD8 T cells (Benner et al., 2008; Brochard et al., 2009; Kurkowska-Jastrzebska et al., 1999; Reynolds et al., 2010) as well as B cell mediated IgG deposition (Benner et al., 2008). Additionally, Reynolds et al. (2010) showed that if MPTP intoxication is combined with nitrated α-syn immunizations, α-syn responding CD4 T cells produced multiple pro-inflammatory cytokines including IFN-γ, TNF, IL-17a, and IL-2. Moreover, Th1 (IFN-γ) and Th17 (IL-17a) polarized T cells adoptively transferred into MPTP intoxicated mice exacerbated SN TH+ neuron loss, while Th2 (IL-4), Treg (IL-10), and vasoactive intestinal peptide (VIP, a neuropeptide associated with Treg responses) treated mice did not. Further evidence of the inflammatory role of CD4 T cells in the MPTP model comes from the findings that SCID, Tcrb−/−, Cd4−/−, but not Cd8−/− mice administered MPTP are protected from neurodegeneration (Benner et al., 2008; Brochard et al., 2009). Taken together, these data support the hypothesis that MPTP induces an activation of both the innate and adaptive immune that promotes further pro-inflammatory and neurotoxic effects.
9.1.3. Rotenone and paraquat
Rotenone is a plant derived insecticide and pesticide whose mechanism of action is mainly through binding to complex I in the electron transport chain, and thus disrupting mitochondrial oxidative phosphorylation (Schuler and Casida, 2001). On the other hand, paraquat, a popular herbicide, is thought to be deleterious through promoting excessive ROS production (Day et al., 1999). Similar to 6-OHDA and MPTP, both rotenone and paraquat disrupt mitochondria and promote oxidative stress in the cell.
Both mouse and rat rotenone models of PD display loss of dopaminergic neurons, α-syn pathology, and motor deficits (Bove and Perier, 2012). In one study, rotenone administration in rats recapitulated the hallmark microgliosis that is observed in human PD (Sherer et al., 2003). Another study showed that rotenone administration induced the production of multiple pro-inflammatory signaling mediators including IL-1β, TNF, IL-6, NF-κB, and that these inflammatory molecules, as well as iNOS, could be attenuated with treatment of a heat shock protein inducer (Thakur and Nehru, 2015). However, while there is evidence of an innate response to the rotenone model of PD, to date there is no clear evidence of a role for the adaptive immune system. This absence of data on the adaptive immune system’s role in rotenone models appears to be due to lack of direct study as opposed to any evidence that the PD pathologies produced by the model to be independent of T or B cell responses. Similar to the rotenone model, the paraquat mouse model of PD recapitulates the human hallmarks of disease, including the loss of dopamine neurons in the SN (<25%) (Bove and Perier, 2012) and the presence of α-syn pathology (Manning-Bog et al., 2002). In this model, microglia become activated (Peng et al., 2009; Purisai et al., 2007) and are thought to be crucial in mediating ROS-induced neurodegeneration. Like the rotenone model, there is a clear role for the CNS innate immune system in the paraquat model, but no clear evidence of the involvement of the adaptive arm. Again, this is most likely due to the lack of studies as opposed to the adaptive immune system playing no role in the pathophysiology of the paraquat model.
9.2. α-syn based animal models of PD
9.2.1. Human α-syn transgenics
As previously mentioned, mutations in the α-syn gene (SNCA), or polymorphisms increasing or enhancing α-syn expression in human PD are associated with increased risk for PD (Fuchs et al., 2008; Maraganore et al., 2006). Numerous animal models have been created that express either normal or familial mutations of α-syn. There are many non-murine models that utilize human α-syn, but other reviews have covered this topic extensively (Maulik et al., 2017; Vanhauwaert and Verstreken, 2015; Visanji et al., 2016), and only murine models will be discussed here. Mice that overexpress full length human α-syn under the Thy1, PDGF-β, or even the rat TH promoters develop intracellular inclusions (Fleming et al., 2004, 2011; Masliah et al., 2000; Rockenstein et al., 2002), and some develop decreases in striatal dopamine levels (Lam et al., 2011; Masliah et al., 2000). It should be noted that these models do not present with overt neurodegeneration, and thus often do not display motor deficits (Antony et al., 2011) with the exception of the PDGF-β α-syn mice at 12 months (Masliah et al., 2000) and the Thy1 α-syn mice. Thy1 α-syn mice develop progressive α-syn inclusions, loss of striatal dopamine, and notably, mild motor deficits in the absence of overt cell loss (Chesselet et al., 2012). These mice have been the most extensively characterized α-syn based transgenic model with regards to inflammation. Thy1-α-syn mice develop increased expression of TLR 1, 2, 4, and 8 in the substantia nigra with age, and TNF mRNA increases in both the striatum and substantia nigra (Watson et al., 2012). Furthermore, these same mice have increased nigral MHCII expression at 14 months, and increased CD4 and CD8 T cells in the blood at 22 months. This suggests that the increasing α-syn burden found with age could be related to progressive innate and adaptive immune system involvement in these mice.
Two autosomal dominant mutations, the A53T (Polymeropoulos et al., 1997) that results in an alanine to threonine substitution and A30P (Kruger et al., 1998) that results in an alanine to proline substitution, have been identified in multiple human cohorts. While many of these mutated forms have been made into mouse models, most research has not investigated inflammation. The few studies that have will be discussed here. Mice overexpressing a double mutated form of α-syn at A53T and A30P from the rat TH promoter develop increased ionized calcium binding adaptor molecule 1 (IBA1, which increases on myeloid cells during inflammation) immunostaining in the substantia nigra and striatum, increased numbers of IBA1+ cells (which could represent microglia or infiltrating myeloid cells), increased levels of TNF in both the substantia nigra and striatum, increased cytokine and ROS production, as well as decreased IL-10 levels (Su et al., 2009). The mouse model with PrP (mouse prion protein) driven A53T expression has decreased stool frequency and gastric emptying, although no pure inflammatory markers have been reported (Vidal-Martinez et al., 2016). However, the administration of a drug targeting sphingosine-1-phosphate receptor (fingolimod) attenuates the problems with gut motility, decreases both gut and CNS α-syn pathology, and is neuroprotective (Vidal-Martinez et al., 2016). This is especially interesting, as it strengthens the argument that gut pathology and possibly inflammation contribute to disease progression. Thus, it could be the case that a predisposition to abnormal α-syn, combined with inflammatory processes, leads to further immune system involvement and disease.
9.2.2. Viral mediated α-syn expression
Some PD models utilize adeno-associated viral vectors (AAVs) to induce overexpression of normal or familial forms of mutated human α-syn in neurons. These models are helpful in studying the effect of excess α-syn in neurons of specific brain regions, as they are temporally and spatially restricted depending on where the viral vector is injected. AAVs also preferentially infect postmitotic cells, a mostly neuronal population in the brain, although the type of neuron varies depending on viral serotype. AAV serotype 2 (AAV2) seems to infect dopaminergic neurons of the substantia nigra most effectively, and is used either alone or as a hybrid vector to express α-syn in neurons. These models are especially useful, as they can be used in any transgenic mouse and allow the study of non-cell-autonomous mechanisms of neurodegeneration.
Numerous AAV serotypes have been used to induce human α-syn expression, and lead to intraneuronal α-syn pathology (Lindgren et al., 2012; Volpicelli-Daley, 2017). Just as the case with other α-syn based models of PD, the inflammatory response has not been investigated in depth in a majority of the AAV models. Two exceptions are the AAV2/5 model and AAV2 models. AAV2/5 is used in rats and leads to increased expression of MHCII and CD68, and infiltration CD4 and CD8 T cells in the midbrain (Sanchez-Guajardo et al., 2010). AAV2 based overexpression of human α-syn results in production of pSer129+ intraneuronal α-syn inclusions and death of ~30% of dopaminergic neurons (Harms et al., 2013; Theodore et al., 2008). This model is especially interesting in the context of inflammation, as neuroinflammation is a driving force in the observed neurodegeneration rather than just an effect of the disease process (Harms et al., 2013, 2018; Theodore et al., 2008; Thome et al., 2016; Williams et al., 2018). The neuroinflammatory responses reported in the aforementioned AAV2 studies include: microglial upregulation of MHCII, infiltration of inflammatory monocytes, infiltration of T cells, deposition of IgG in the midbrain, and the production of inflammatory cytokines and chemokines (i.e., IL-6, TNF, and iNOS at early time points). Disruption of many of these processes, such as MHCII knockout (Harms et al., 2013), targeted CIITA (the transcriptional coactivator for inducible MHCII expression) knockdown (Williams et al., 2018), or CCR2 knockout (Harms et al., 2018), which prevents CCR2+ inflammatory monocyte migration to tissues, are protective against neurodegeneration. Additionally, as only a portion of microglia begin expressing MHCII, this could indicate the presence of a group of disease associated microglia (DAMs), as has been identified in other neurodegenerative diseases such as Alzheimer’s disease. Furthermore, increased levels of B cells infiltrate in response to α-syn, and FcyR knockout mice are protected from inflammation and neurodegeneration as well (Cao et al., 2010; Theodore et al., 2008). Viral models have been crucial in delineating the role of an immune response mouse models of PD, and have highlighted a role for inflammation in driving α-syn induced neurodegeneration.
9.2.3. α-syn fibrils
Pathological α-syn can corrupt endogenous protein and lead to the further spread of pathological species (Luk et al., 2009, 2012), a finding that has led to the development of a model of α-syn propagation: the pre-formed fibril (PFF) model. This model uses human or mouse α-syn monomer, which alone is non-pathological (Volpicelli-Daley et al., 2011), to create small fibrils. These fibrils are injected into the brain, and pSer129 positive inclusions appear throughout interconnected regions, depending on the location of injection, and are comprised of both injected and endogenous α-syn protein. In rats and mice, motor deficits appear over time (Luk et al., 2012; Paumier et al., 2015). In vitro, these fibrils are neurotoxic, pro-inflammatory and can activate cultured microglia inducing MHCII and iNOS expression (Cremades et al., 2012; Harms et al., 2013; Williams et al., 2018). Human PFFs injected into M20 human α-syn-expressing mice leads to increased GFAP and IBA1 immunostaining, indicative of astrocytosis and a myeloid response (Sacino et al., 2014; Sorrentino et al., 2017). Additionally, injection of mouse PFFs into WT Sprague Dawley rats led to increased MHCII and IBA1 expression in the midbrain, accompanied by the infiltration of peripheral myeloid cells and CD4 T cells prior to measurable neurodegeneration (Duffy et al., 2018; Harms et al., 2017). However, while there are no reports to date of peripheral cell infiltration in mice given PFFs, robust astrocytosis has been reported. Specifically, the emergence of A1 astrocytes (nomenclature mirrors the M1/M2 phenotypes of macrophages), or astrocytes that are associated with disease states and have a pro-inflammatory skew are found in the fibril model (Yun et al., 2018). However, our picture of the contribution of inflammation to this model remains incomplete, as manipulations aimed at halting an inflammatory response to fibrillar α-syn have not been performed in vivo.
10. Conclusions and key questions
Taken together, the data presented in this chapter indicate that the immune system plays a driving role in both the pathogenesis and progression of PD. As reviewed earlier, these findings harken back to the original description of inflammation in the disease by James Parkinson. The evidence consists of multiple observations of an abnormal innate and adaptive immune system in the blood, gut, and CNS of patients with PD, as well as several genetic and environmental associations linking the immune system and PD risk. This idea has been supported by numerous findings in both humans and animal models. Both genetic familial forms of the disease and genetic risk factors, such as HLA and SNCA, are associated with immune dysfunction. Additionally, PD patients display signatures of global inflammation in the blood, gut, and CNS. This includes elevated blood and CSF cytokine levels, infiltration of peripheral immune cells into the CNS, hyperreactive circulating immune cells, and a dysregulated gut microbiome. There is also evidence that blocking inflammatory events leads to a reduced risk for later developing PD. While there is no evidence to conclude that inflammation alone causes PD itself, many studies in animal models suggest that interrupting the inflammatory response, such as blocking T cell responses, MHCII signaling, CCR2 signaling, use of iNOS inhibitors, or anti-TNF therapy is a neuroprotective strategy, leading us to believe that inflammation is a main driver of disease pathogenesis. Based on the data discussed here, we hypothesize that PD begins and progresses in an autoinflammatory manner, where abnormal α-syn and genetic/environmental risks together lead to disease, as outlined in Fig. 4.
FIG. 4.
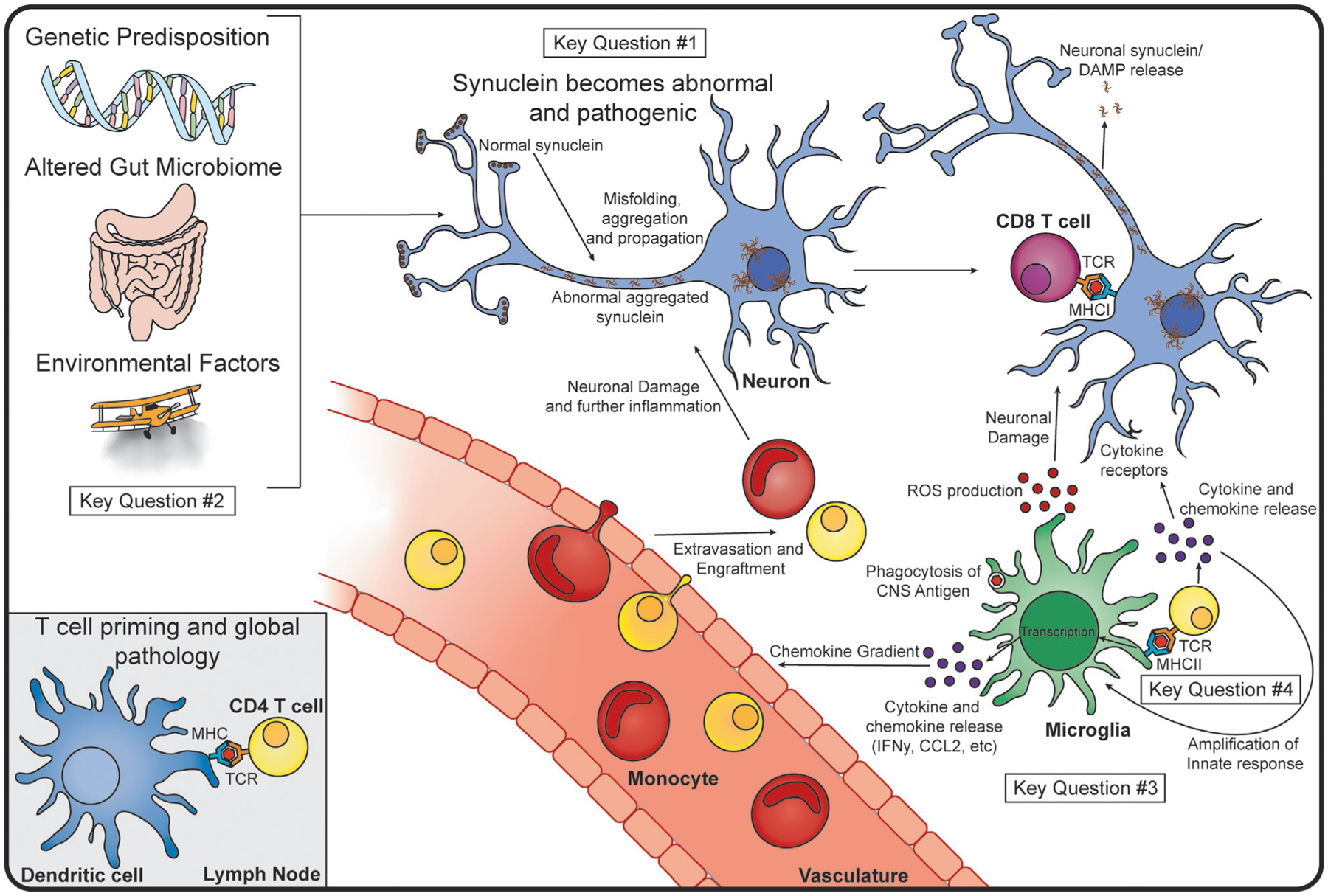
Proposed Mechanism of Immune Involvement in PD and Key Questions. We hypothesize that PD begins due to genetic predispositions, alterations in the gut microbiome, and the influence of external, environmental factors. It is possible that a CNS antigen drains to the lymph node (bottom left), where a dendritic cell (blue) can present it to a CD4 T cell (yellow), priming the T cell for an inflammatory response. Within the CNS, we believe that normal α-syn (brown) becomes misfolded, and begins to propagate and aggregate in neurons (blue). This can lead to the release of toxic α-syn species from neurons, and neuronal presentation of antigen to CD8 T cells (maroon) via MHCI. Microglia take up CNS antigen and present this on their MHCII to CD4 T cells, leading to T cell differentiation and cytokine release. The MHCII-TCR interaction and subsequent cytokines can amplify microglial activation, leading to further production of cytokines and chemokines. Peripheral immune cells, such as monocytes (red) and additional T cells will home to the site of chemokine production, and extravagate from the vasculature or meningeal lymphatics (not shown), and cause further inflammation and neuronal damage in the CNS. As much of this is speculative, and ties together many disparate pieces of data, several key questions remain. These include: #1 “What is the role of α-syn in activating the immune system?” (top middle) #2 “Is Parkinson disease caused by an immune response that originates in the gut?” (left) #3 “What is the role of microglia in Parkinson’s disease?” (bottom right) and #4 “How do T cells contribute to the pathobiology of Parkinson disease?” (bottom right).
While recent research has identified roles for the innate and adaptive immune systems in human disease and animal models, much more work is needed to integrate these findings into a mechanistic understanding of the disease process. Here, we have highlighted four key questions that, if addressed, would provide a clearer picture of how the immune system contributes to the progression of PD (identified in Fig. 4).
10.1. Key question 1: What is the role of α-syn in activating the immune system?
There is ample evidence for immune activation within the CNS in human PD (Fig. 3), although the signal initiating the inflammatory response remains unknown. One likely candidate is the neuronal postsynaptic protein α-syn. α-syn inclusions are found throughout the brain, and are theorized to spread in a prion-like manner through retrograde transport (Henderson et al., 2019; Ma et al., 2019), although this is currently a hotly debated topic. Much of the research on α-syn propagation comes from in vitro experiments, where only monosynaptic transfer is demonstrated (Grozdanov and Danzer, 2018; Mao et al., 2016), or from in vivo models where the extent of spread, and whether it is across only one synapse or multiple, even spreading to neuroanatomical regions that are not directly connected, has been a source of much disagreement within the field (Grozdanov and Danzer, 2018; Kim et al., 2019; Sorrentino et al., 2017). However, in humans, brain derived exosomes have been found to contain elevated levels of α-syn that correlate with disease severity (more α-syn correlated with worse disease measures) (Shi et al., 2014). Therefore, there is evidence of α-syn release from the CNS in human disease, and this α-syn is found throughout the body. The enteric nervous system actually expresses high levels of α-syn as well, and α-syn pathology can be found throughout the enteric neurons in PD (Braak et al., 2006; Shannon et al., 2012). Widespread α-syn pathology, if it is indeed an autoantigen, could help to explain the global symptoms exhibited by PD patients.
Although α-syn pathology is widespread, this does not directly implicate α-syn as an antigen. Multiple experiments have demonstrated that α-syn is able to activate myeloid cells and cultured microglia. Particularly, toll-like receptors on microglia can be activated by α-syn, similar to a PAMP or DAMP (Daniele et al., 2015; Fellner et al., 2013; Kim et al., 2013, 2016; Sanchez-Guajardo et al., 2015; Yun et al., 2018). Interestingly, TLR2 expression is increased in postmortem tissue of PD patients on both neurons and IBA1+ cells, which could represent microglia or other infiltrating myeloid cells (Dzamko et al., 2017). When investigated closely in vitro, neurons secrete oligomeric α-syn, which then activates microglia via TLR2 signaling (Kim et al., 2013, 2016). The secretion of α-syn from neurons has been proposed to occur through the release of exosomes as a way to rid the cell of excess protein (Stefanis et al., 2019). While it is controversial whether neurons in vivo can secrete α-syn, the external application of α-syn seems to reliably activate microglia through TLR1/2 heterodimers, TLR2, or TLR4 (Daniele et al., 2015; Fellner et al., 2013; Kim et al., 2013). In Thy1-asyn mice, TLR 1, 2, 4, and 8 are increased in the substantia nigra, indicating that this process may also occur in vivo (Watson et al., 2012). Activation of TLRs by α-syn can also lead to the production of pro-inflammatory cytokines and chemokines, such as TNF, IL-1β, IL-6, Cox2, IL-1α, and iNOS and reactive oxygen species (Fellner et al., 2013; Russo et al., 2019; Sanchez-Guajardo et al., 2015; Yun et al., 2018). Therefore, in these ways, α-syn acts similar to a foreign antigen, PAMP, or DAMP that would typically activate an APC.
There is also evidence that some T cells in PD patients may be particularly reactive to specific fragments of α-syn. T cells isolated from PD patients contain TCRs that respond to native, fibrillar, and antigenic peptides of α-syn. Additionally, specific alleles of HLA (part of the MHCII complex) bind these peptides with a much higher affinity (Sulzer et al., 2017). It has also been shown in both the pre-formed fibril model in rats and the AAV2 model in mice that abnormal α-syn in the brain can lead to MHCII expression and T cell infiltration (see Sections 9.2.2 and 9.2.3). As α-syn is the common denominator and single manipulation here, it is logical that this response would be directed against some form of α-syn.
This idea of α-syn as the antigen begs the question of: what form of α-syn could be antigenic? There has been much speculation on which form is toxic to neurons (nitrated, phosphorylated, fibrillar, etc.), but much less speculation on which of these species could be antigenic. Proteins present in homeostasis can become post-translationally modified under conditions of cellular stress or inflammation, leading to their recognition by autoreactive T cells that were not deleted in the thymus (Doyle and Mamula, 2012). In the case of α-syn, it seems as if overabundance of α-syn is enough to lead to hyperphosphorylation, ubiquitylation, and aggregation (Chesselet et al., 2012; Fleming et al., 2004; Masliah et al., 2000). As stated above, PD patients have autoreactive T cells to specific fragments of α-syn, including at pSer129, a site that becomes phosphorylated in overabundance and fibril models of PD. Therefore, it could be that cellular stress or genetic predispositions could lead to the generation and recognition of antigenic forms of α-syn, which then lead to inflammation and neurodegeneration. However the question of how native α-syn becomes modified in the first place remains unanswered.
Additionally, there remains the possibility that there is a yet unidentified antigen in disease, as other autoimmune diseases such as multiple sclerosis and rheumatoid arthritis have multiple known autoantigens. It is also possible that α-syn becomes recognized through epitope spreading or molecular mimicry—where a different, but similarly structured antigen becomes recognized first, followed by the recognition of α-syn. α-syn is widespread throughout the body, and this provides many opportunities for interaction with the immune system.
10.2. Key question 2: Is Parkinson’s disease caused by an immune response that originates in the gut?
Gastrointestinal dysfunction was initially remarked upon in the original “An Essay on the Shaking Palsy” and has continued to be an important disease symptom, as most patients with PD suffer from constipation (Fasano et al., 2015). Furthermore, there is evidence of increased intestinal epithelial barrier permeability (Forsyth et al., 2011) and pro-inflammatory cytokines in the GI tract of PD patients (Devos et al., 2013). Abnormal α-syn species have also been detected throughout the whole GI tract, including in the submandibular gland, the colon and the innervating enteric nervous system (Fasano et al., 2015). Braak’s hypothesis attempts to tie these two phenotypes together by suggesting that a pathogen could pass through the leaky intestinal wall of presymptomatic PD patients and promote the formation of abnormal α-syn species across the GI tract and enteric nervous system—a hypothesis that has only grown more feasible with the discovery of direct connections between gut epithelial and vagal neurons (Kaelberer et al., 2018). This reinforces the Braak hypothesis, in which α-syn could be a prion, with the initial seeding event occurring in the enteric nervous system and then propagating up the vagus nerve into the CNS proper. Now, with the better understanding and appreciation of the gut-immune-brain axis (Fung et al., 2017), the evidence of an abnormal microbiota signature in PD patients, and the consistent findings of an overactive immune system in PD, it may be time to update Braak’s hypothesis to reflect this.
The immune system is crucial in directing the composition and location of microbiota in mammals, while microbiota are simultaneously critical in the development and future responses of the immune system in both health and disease (Hooper et al., 2012). In the case of PD, there is ample evidence that the gut microbiota is altered in PD patients, which includes the loss of anti-inflammatory bacteria that could be important in controlling GI inflammation (see Section 6.2). Additionally, there is evidence of innate and adaptive immune activation in the gut, periphery, and central nervous system associated with PD (Fig. 3). Braak initially suggested that a pathogen could be involved in PD, and perhaps the pathogen(s) are particular gut bacteria that elicit damaging, pro-inflammatory responses from the innate and adaptive immune systems, including the production of reactive oxygen species.
It is known that one of the primary biological tools of the inflammatory immune response is the production of ROS to diminish bacterial or viral survival as well as mediate several signaling pathways including the activation of the inflammasome pathway (Yang et al., 2013) which is upregulated in PD (Gordon et al., 2018). Oxidative stress has been shown in multiple PD models to promote the oligomerization and pathogenicity of α-syn (Dias et al., 2013). It is possible then that this pro-inflammatory, ROS rich environment produced by the microbiota and immune system could lead to the formation or propagation of pathogenic α-syn species in the gut and the innervating nervous system. These pathogenic α-syn species, now residing in an inflammatory environment, may also serve as a new target for the immune reaction in the gut, which would help explain the evidence of a α-syn specific cytokine recall response in PD patient derived T cells (Sulzer et al., 2017). Following this, it is possible that the resident and circulating immune system could react to the CNS α-syn pathology in a similar way that it may have in the gut. Whatever the exact mechanisms may be, the idea that the initiation of the pathobiology of PD could be initiated in the gut due to a combination of abnormal bacteria, an overactive immune system, and the propensity of α-syn to oligomerize in oxidative stress conditions is one that warrants consideration in the field of PD.
10.3. Key question 3: What is the role of microglia in Parkinson’s disease?
Microglia have long been the scapegoat for neuroinflammation. They are the tissue resident macrophages of the CNS parenchyma, and are thought of as first responders to any injury, modulators of homeostasis, and mediators of neuroinflammation. Across neurodegenerative diseases, however, there is an ongoing debate of whether the activity of microglia can be harmful or helpful, promoting either damaging inflammation or resolution and tissue repair.
There is extensive data demonstrating that microglia react to abnormal α-syn, as discussed in the Section 9.2 and Key Question 1. These phenotypic changes may be similar to what is seen in other models of neurodegeneration, and microglia may in fact exhibit a mixed phenotype, with signatures characteristic of both damaging inflammation and resolution. There is evidence from models of acute neuron death demonstrating that microglia expand clonally (Tay et al., 2017), suggesting that specific subsets of microglia respondto cell death and general threats to the environment. Recent studies have also identified a subset of disease associated macrophages (DAMs) that contribute to neuroinflammation and cell death (Keren-Shaul et al., 2017). In fact, these DAMs have been found to be consistently present across many models of neurodegeneration, including models of Alzheimer’s disease, ALS, and multiple sclerosis (Song and Colonna, 2018). While no studies have yet investigated whether DAMs are present in models of PD, it is likely that they play a role, as the phenotype can be induced with aging, neurodegeneration, or through acute models that lead to apoptosis and cell death. Interestingly, DAMs express both pro- and anti-inflammatory molecules, making their exact effect on the inflammatory process unclear (Keren-Shaul et al., 2017; Song and Colonna, 2018). Upon exposure to abnormal α-syn, or any robust inflammatory stimulus, a portion of the microglia begins to upregulate MHCII on their surface and become activated. This has been found in the AAV2 overexpression mouse model (Harms et al., 2013, 2018), an α-syn fibril rat model (Harms et al., 2017), as well as neurotoxin models such as paraquat (Peng et al., 2009; Purisai et al., 2007). This would seem to indicate that the portion of microglia upregulating MHCII may indeed be involved in antigen presentation to CD4 T cells and act as DAMs sensing alterations in the local environment. Media analyzed from co-culture of microglia and CD4 T cells exposed to α-syn shows increases in both pro- and anti-inflammatory cytokines, such as TNF, IFN-γ, and Il-10 (Harms et al., 2013). While this is by no means conclusive, it demonstrates that microglia in models of PD may adopt a mixed, DAM-like phenotype.
One caveat to these findings is the issue that, until recently, it has been incredibly difficult to differentiate between infiltrating monocytes and macrophages and tissue resident microglia due to overlap in surface markers between the two populations. Most in vivo studies report all IBA1+ cells as microglia, although many different cell types express this marker promiscuously. This is also a key issue with the TSPO based PET scanning, as this ligand is not specific to microglia or even immune cells—indeed it has been shown to bind to astrocytes (Kuhlmann and Guilarte, 2000; Pannell et al., 2019). In other disease models, such as stroke, it has been shown that infiltrating monocytes are actually protective, and dampen a harmful microglial response (Greenhalgh et al., 2018), and it is therefore integral that the two populations be studied independently. However, based on the current available data, it is clear that there is an inflammatory response ongoing in the brains of mouse models and humans, even if we do not yet know the involved cell types.
10.4. Key question 4: How do t cells contribute to the pathobiology of Parkinson’s disease?
Interaction between antigen presenting cells (whether MHCI or MHCII) and T cells likely accounts for the elevated levels of T cell associated cytokines in the brain, CSF, and blood of PD patients. Sulzer et al. (2017) proposed that “altered degradation of proteins including α-syn could produce antigenic epitopes that trigger immune reactions during aging and Parkinson’s disease.” How exactly are these T cell derived pro-inflammatory cytokines contributing to the α-syn pathology and overt neurodegeneration seen in Parkinson’s disease? Activation of T cells via their TCR can directly lead to the robust production of ROS as well as T cell derived cytokines such as IFN-γ promoting further ROS signaling in macrophages and monocytes (Yang et al., 2013). Additionally, the pro-inflammatory cytokines associated with PD have been shown to be able to skew microglia to be more phagocytic, which could be detrimental to neuron survival (Merson et al., 2010). Another potential mechanism for T cell driven neurodegeneration in PD is through the direct interaction of T cell derived cytokines and their cognate receptors on neurons. It has been recently demonstrated in an iPSC based preclinical model of PD that the Th17 associated cytokine IL-17a could bind to the IL-17R on PD derived midbrain neurons and lead to their apoptosis (Sommer et al., 2018). Lastly, in another preclinical model, it has been suggested that CD8 cytotoxic T cells could directly induce neuronal apoptosis via their TCR interaction with MHCI on dopaminergic neurons in PD (Cebrian et al., 2014). It is likely that a combination of these proposed mechanisms including excess ROS production from the innate and adaptive immune system, direct cytokine signaling on neuronal populations, and T cell directed phagocytosis are contributing to the Parkinson’s disease state (Fig. 4).
10.5. Conclusion
In conclusion, human PD and rodent models of the disease are consistently associated with a pro-inflammatory innate and adaptive immune phenotype. Work in several animal models of the disease have demonstrated the effectiveness of targeting the innate and adaptive immune compartments as a neuroprotective strategy. As the field continues to elucidate the origin and the exact nature of these potentially disease driving immune responses, it will be important to translate these findings into creating and employing much needed disease-modifying immunotherapies.
References
- Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, Ballard C, 2017. Cognitive decline in Parkinson disease. Nat. Rev. Neurol 13, 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, Grandinetti A, Blanchette PL, Popper JS, Ross GW, 2001. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57, 456–462. [DOI] [PubMed] [Google Scholar]
- Ahmed I, Tamouza R, Delord M, Krishnamoorthy R, Tzourio C, Mulot C, Nacfer M, Lambert JC, Beaune P, Laurent-Puig P, Loriot MA, Charron D, Elbaz A, 2012. Association between Parkinson’s disease and the HLA-DRB1 locus. Mov. Disord 27, 1104–1110. [DOI] [PubMed] [Google Scholar]
- Aho VTE, Pereira PAB, Voutilainen S, Paulin L, Pekkonen E, Auvinen P, Scheperjans F, 2019. Gut microbiota in Parkinson’s disease: temporal stability and relations to disease progression. EBioMedicine 44, 691–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Mcgeer PL, 1989. Microglial response to 6-hydroxydopamine-induced substantia nigra lesions. Brain Res. 489, 247–253. [DOI] [PubMed] [Google Scholar]
- Antony PM, Diederich NJ, Balling R, 2011. Parkinson’s disease mouse models in translational research. Mamm. Genome 22, 401–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma M, Miyazaki I, 2007. Common anti-inflammatory drugs are potentially therapeutic for Parkinson’s disease? Exp. Neurol 206, 172–178. [DOI] [PubMed] [Google Scholar]
- Aubin N, Curet O, Deffois A, Carter C, 1998. Aspirin and salicylate protect against MPTP-induced dopamine depletion in mice. J. Neurochem 71, 1635–1642. [DOI] [PubMed] [Google Scholar]
- Bae JR, Lee BD, 2015. Function and dysfunction of leucine-rich repeat kinase 2 (LRRK2): Parkinson’s disease and beyond. BMB Rep. 48, 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barichella M, Severgnini M, Cilia R, Cassani E, Bolliri C, Caronni S, Ferri V, Cancello R, Ceccarani C, Faierman S, Pinelli G, De Bellis G, Zecca L, Cereda E, Consolandi C, Pezzoli G, 2019. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord 34, 396–405. [DOI] [PubMed] [Google Scholar]
- Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, Bottacchi E, Cannas A, Ceravolo G, Ceravolo R, Cicarelli G, Gaglio RM, Giglia RM, Iemolo F, Manfredi M, Meco G, Nicoletti A, Pederzoli M, Petrone A, Pisani A, Pontieri FE, Quatrale R, Ramat S, Scala R, Volpe G, Zappulla S, Bentivoglio AR, Stocchi F, Trianni G, Dotto PD, PRIAMO Study Group, 2009. The Priamo study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov. Disord 24, 1641–1649. [DOI] [PubMed] [Google Scholar]
- Bartels AL, Willemsen AT, Doorduin J, De Vries EF, Dierckx RA, Leenders KL, 2010. [11C]-PK11195 PET: quantification of neuroinflammation and a monitor of anti-inflammatory treatment in Parkinson’s disease? Parkinsonism Relat. Disord 16, 57–59. [DOI] [PubMed] [Google Scholar]
- Bas J, Calopa M, Mestre M, Mollevi DG, Cutillas B, Ambrosio S, Buendia E, 2001. Lymphocyte populations in Parkinson’s disease and in rat models of parkinsonism. J. Neuroimmunol 113, 146–152. [DOI] [PubMed] [Google Scholar]
- Bassing CH, Swat W, Alt FW, 2002. The mechanism and regulation of chromosomal V(D)J recombination. Cell 109 (Suppl), S45–S55. [DOI] [PubMed] [Google Scholar]
- Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG, 2000. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov. Disord 15, 692–698. [DOI] [PubMed] [Google Scholar]
- Bengoa-Vergniory N, Roberts RF, Wade-Martins R, Alegre-Abarrategui J, 2017. Alpha-synuclein oligomers: a new hope. Acta Neuropathol. 134, 819–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, Tsiperson V, Nemachek C, Ciborowski P, Przedborski S, Mosley RL, Gendelman HE, 2008. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS One 3, e1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, Martinez L, Greidinger EL, Yu BD, Gallo RL, 2012. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat. Med 18, 1286–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P, 1995. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci. Lett 202, 17–20. [DOI] [PubMed] [Google Scholar]
- Bouneaud C, Kourilsky P, Bousso P, 2000. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity 13, 829–840. [DOI] [PubMed] [Google Scholar]
- Bove J, Perier C, 2012. Neurotoxin-based models of Parkinson’s disease. Neuroscience 211, 51–76. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, De Vos RA, Jansen Steur EN, Braak E, 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211. [DOI] [PubMed] [Google Scholar]
- Braak H, De Vos RA, Bohl J, Del Tredici K, 2006. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett 396, 67–72. [DOI] [PubMed] [Google Scholar]
- Breyner NM, Michon C, De Sousa CS, Vilas Boas PB, Chain F, Azevedo VA, Langella P, Chatel JM, 2017. Microbial anti-inflammatory molecule (MAM) from Faecalibacterium prausnitzii shows a protective effect on DNBS and DSS-induced colitis model in mice through inhibition of NF-kappaB pathway. Front. Microbiol 8, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S, 2009. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest 119, 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodacki B, Staszewski J, Toczylowska B, Kozlowska E, Drela N, Chalimoniuk M, Stepien A, 2008. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFalpha, and INFgamma concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci. Lett 441, 158–162. [DOI] [PubMed] [Google Scholar]
- Broom L, Marinova-Mutafchieva L, Sadeghian M, Davis JB, Medhurst AD, Dexter DT, 2011. Neuroprotection by the selective iNOS inhibitor GW274150 in a model of Parkinson disease. Free Radic. Biol. Med 50, 633–640. [DOI] [PubMed] [Google Scholar]
- Burre J, 2015. The synaptic function of alpha-synuclein. J. Park. Dis 5, 699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calis JJ, Rosenberg BR, 2014. Characterizing immune repertoires by high throughput sequencing: strategies and applications. Trends Immunol. 35, 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Theodore S, Standaert DG, 2010. Fcgamma receptors are required for NF-kappaB signaling, microglial activation and dopaminergic neurodegeneration in an AAV-synuclein mouse model of Parkinson’s disease. Mol. Neurodegener 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson AH, Yakymenko O, Olivier I, Hakansson F, Postma E, Keita AV, Soderholm JD, 2013. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand. J. Gastroenterol 48, 1136–1144. [DOI] [PubMed] [Google Scholar]
- Cebrian C, Zucca FA, Mauri P, Steinbeck JA, Studer L, Scherzer CR, Kanter E, Budhu S, Mandelbaum J, Vonsattel JP, Zecca L, Loike JD, Sulzer D, 2014. MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat. Commun 5, 3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC, 2005. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 123, 383–396. [DOI] [PubMed] [Google Scholar]
- Chaplin DD, 2010. Overview of the immune response. J. Allergy Clin. Immunol 125, S3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A, 2004. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364, 1167–1169. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A, 2003. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch. Neurol 60, 1059–1064. [DOI] [PubMed] [Google Scholar]
- Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A, 2005. Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann. Neurol 58, 963–967. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR, 2012. A progressive mouse model of Parkinson’s disease: the Thy1-aSyn (“Line 61”) mice. Neurotherapeutics 9, 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, Isacson O, 2002. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur. J. Neurosci 15, 991–998. [DOI] [PubMed] [Google Scholar]
- Colonna M, Butovsky O, 2017. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol 35, 441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contento RL, Molon B, Boularan C, Pozzan T, Manes S, Marullo S, Viola A, 2008. CXCR4-CCR5: a couple modulating T cell functions. Proc. Natl. Acad. Sci. U. S. A 105, 10101–10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S, Lee SC, 2009. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol. Appl. Neurobiol 35, 306–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremades N, Cohen SI, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne P, Aprile FA, Bertoncini CW, Wood NW, Knowles TP, Dobson CM, Klenerman D, 2012. Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. Cell 149, 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB, 2005. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J. Neuroinflammation 2, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czlonkowska A, Kohutnicka M, Kurkowska-Jastrzebska I, Czlonkowski A, 1996. Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson’s disease mice model. Neurodegeneration 5, 137–143. [DOI] [PubMed] [Google Scholar]
- Daniele SG, Beraud D, Davenport C, Cheng K, Yin H, Maguire-Zeiss KA, 2015. Activation of MyD88-dependent TLR1/2 signaling by misfolded alpha-synuclein, a protein linked to neurodegenerative disorders. Sci. Signal 8, ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BJ, Patel M, Calavetta L, Chang LY, Stamler JS, 1999. A mechanism of paraquat toxicity involving nitric oxide synthase. Proc. Natl. Acad. Sci. U. S. A 96, 12760–12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pablo-Fernandez E, Lees AJ, Holton JL, Warner TT, 2019. Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol. 76, 470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Tang CY, Zhang J, Zhu L, Xie ZC, Gong HH, Xiao XZ, Xu RS, 2016. The cortical thickness correlates of clinical manifestations in the mid-stage sporadic Parkinson’s disease. Neurosci. Lett 633, 279–289. [DOI] [PubMed] [Google Scholar]
- Depino AM, Earl C, Kaczmarczyk E, Ferrari C, Besedovsky H, Del Rey A, Pitossi FJ, Oertel WH, 2003. Microglial activation with atypical proinflammatory cytokine expression in a rat model of Parkinson’s disease. Eur. J. Neurosci 18, 2731–2742. [DOI] [PubMed] [Google Scholar]
- Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H, Coron E, Bruley Des Varannes S, Naveilhan P, Nguyen JM, Neunlist M, Derkinderen P, 2013. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis 50, 42–48. [DOI] [PubMed] [Google Scholar]
- Di Maio R, Hoffman EK, Rocha EM, Keeney MT, Sanders LH, De Miranda BR, Zharikov A, Van Laar A, Stepan AF, Lanz TA, Kofler JK, Burton EA, Alessi DR, Hastings TG, Greenamyre JT, 2018. LRRK2 activation in idiopathic Parkinson’s disease. Sci. Transl. Med 10, 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias V, Junn E, Mouradian MM, 2013. The role of oxidative stress in Parkinson’s disease. J. Park. Dis 3, 461–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Villar M, Hafler DA, 2018. Regulatory T cells in autoimmune disease. Nat. Immunol 19, 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, 2012. Olfaction in Parkinson’s disease and related disorders. Neurobiol. Dis 46, 527–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle HA, Mamula MJ, 2012. Autoantigenesis: the evolution of protein modifications in autoimmune disease. Curr. Opin. Immunol 24, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM, 2001. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc. Natl. Acad. Sci. U. S. A 98, 14669–14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MF, Collier TJ, Patterson JR, Kemp CJ, Luk KC, Tansey MG, Paumier KL, Kanaan NM, Fischer DL, Polinski NK, Barth OL, Howe JW, Vaikath NN, Majbour NK, El-Agnaf OMA, Sortwell CE, 2018. Lewy body-like alpha-synuclein inclusions trigger reactive microgliosis prior to nigral degeneration. J. Neuroinflammation 15, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamko N, Gysbers A, Perera G, Bahar A, Shankar A, Gao J, Fu Y, Halliday GM, 2017. Toll-like receptor 2 is increased in neurons in Parkinson’s disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol. 133, 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, Beecham GW, Wang L, Zuchner S, Konidari I, Wang G, Singer C, Nahab F, Scott B, Stajich JM, Pericak-Vance M, Haines J, Vance JM, Martin ER, 2010. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann. Hum. Genet 74, 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer MJ, 2006. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat. Rev. Genet 7, 306–318. [DOI] [PubMed] [Google Scholar]
- Fasano A, Visanji NP, Liu LW, Lang AE, Pfeiffer RF, 2015. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 14, 625–639. [DOI] [PubMed] [Google Scholar]
- Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N, 2013. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia 61, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M, Massano J, 2017. An updated review of Parkinson’s disease genetics and clinicopathological correlations. Acta Neurol. Scand 135, 273–284. [DOI] [PubMed] [Google Scholar]
- Filiano AJ, Gadani SP, Kipnis J, 2017. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat. Rev. Neurosci 18, 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF, 2004. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J. Neurosci 24, 9434–9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Mulligan CK, Richter F, Mortazavi F, Lemesre V, Frias C, Zhu C, Stewart A, Gozes I, Morimoto B, Chesselet MF, 2011. A pilot trial of the microtubule-interacting peptide (NAP) in mice overexpressing alpha-synuclein shows improvement in motor function and reduction of alpha-synuclein inclusions. Mol. Cell. Neurosci 46, 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foix C, Nicolesco J, 1925. Anatomie cérébrale. In: Les noyaux gris centraux et la région mésencéphalo-sous-optique, suivi d’un appendice sur l’anatomie pathologique de la mala-die de Parkinson. Masson et Cie; Paris, pp. 508–539. [Google Scholar]
- Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A, 2011. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6, e28032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio R, Fujishiro H, Ahn TB, Josephs KA, Maraganore DM, Delledonne A, Parisi JE, Klos KJ, Boeve BF, Dickson DW, Ahlskog JE, 2011. Incidental Lewy body disease: do some cases represent a preclinical stage of dementia with Lewy bodies? Neurobiol. Aging 32, 857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J, Tichopad A, Golub Y, Munz M, Schweitzer KJ, Wolf B, Berg D, Mueller JC, Gasser T, 2008. Genetic variability in the SNCA gene influences alpha-synuclein levels in the blood and brain. FASEB J 22, 1327–1334. [DOI] [PubMed] [Google Scholar]
- Fung TC, Olson CA, Hsiao EY, 2017. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci 20, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco G, Chen SW, Williamson PTF, Cascella R, Perni M, Jarvis JA, Cecchi C, Vendruscolo M, Chiti F, Cremades N, Ying L, Dobson CM, De Simone A, 2017. Structural basis of membrane disruption and cellular toxicity by alpha-synuclein oligomers. Science 358, 1440–1443. [DOI] [PubMed] [Google Scholar]
- Gao X, Chen H, Schwarzschild MA, Ascherio A, 2011. Use of ibuprofen and risk of Parkinson disease. Neurology 76, 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardet A, Benita Y, Li C, Sands BE, Ballester I, Stevens C, Korzenik JR, Rioux JD, Daly MJ, Xavier RJ, Podolsky DK, 2010. LRRK2 is involved in the IFN-gamma response and host response to pathogens. J. Immunol 185, 5577–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ, 2006. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis 21, 404–412. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M, 2010. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T, Wieghofer P, Jordao MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FM, Bechmann I, Kerschensteiner M, Linnarsson S, Jung S, Prinz M, 2016. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol 17, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R, Albornoz EA, Christie DC, Langley MR, Kumar V, Mantovani S, Robertson AAB, Butler MS, Rowe DB, O’neill LA, Kanthasamy AG, Schroder K, Cooper MA, Woodruff TM, 2018. Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med 10, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh AD, Zarruk JG, Healy LM, Baskar Jesudasan SJ, Jhelum P, Salmon CK, Formanek A, Russo MV, Antel JP, Mcgavern DB, McColl BW, David S, 2018. Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol. 16, e2005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanov V, Danzer KM, 2018. Release and uptake of pathologic alpha-synuclein. Cell Tissue Res. 373, 175–182. [DOI] [PubMed] [Google Scholar]
- Grozdanov V, Bliederhaeuser C, Ruf WP, Roth V, Fundel-Clemens K, Zondler L, Brenner D, Martin-Villalba A, Hengerer B, Kassubek J, Ludolph AC, Weishaupt JH, Danzer KM, 2014. Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol. 128, 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Deng X, Zheng W, Xu H, Song Z, Liang H, Lei J, Jiang X, Luo Z, Deng H, 2011. HLA rs3129882 variant in Chinese Han patients with late-onset sporadic Parkinson disease. Neurosci. Lett 501, 185–187. [DOI] [PubMed] [Google Scholar]
- Guzman JN, Ilijic E, Yang B, Sanchez-Padilla J, Wokosin D, Galtieri D, Kondapalli J, Schumacker PT, Surmeier DJ, 2018. Systemic isradipine treatment diminishes calcium-dependent mitochondrial oxidant stress. J. Clin. Invest 128, 2266–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi M, Selvanantham T, Swinton E, Padmore RF, Tong Y, Kabbach G, Venderova K, Girardin SE, Bulman DE, Scherzer CR, Lavoie MJ, Gris D, Park DS, Angel JB, Shen J, Philpott DJ, Schlossmacher MG, 2011. Parkinson’s disease-linked LRRK2 is expressed in circulating and tissue immune cells and upregulated following recognition of microbial structures. J. Neural Transm. (Vienna) 118, 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H, 2010. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat. Genet 42, 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Standaert DG, 2014. Monocytes and Parkinson’s disease: invaders from outside? Mov. Disord 29, 1242. [DOI] [PubMed] [Google Scholar]
- Harms AS, Barnum CJ, Ruhn KA, Varghese S, Trevino I, Blesch A, Tansey MG, 2011. Delayed dominant-negative TNF gene therapy halts progressive loss of nigral dopaminergic neurons in a rat model of Parkinson’s disease. Mol. Ther 19, 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, Cron RQ, Shacka JJ, Raman C, Standaert DG, 2013. MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J. Neurosci 33, 9592–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Delic V, Thome AD, Bryant N, Liu Z, Chandra S, Jurkuvenaite A, West AB, 2017. Alpha-synuclein fibrils recruit peripheral immune cells in the rat brain prior to neurodegeneration. Acta Neuropathol. Commun 5, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Thome AD, Yan Z, Schonhoff AM, Williams GP, Li X, Liu Y, Qin H, Benveniste EN, Standaert DG, 2018. Peripheral monocyte entry is required for alpha-synuclein induced inflammation and neurodegeneration in a model of Parkinson disease. Exp. Neurol 300, 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MX, Trojanowski JQ, Lee VM, 2019. alpha-synuclein pathology in Parkinson’s disease and related alpha-synucleinopathies. Neurosci. Lett 709, 134316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ, 2002. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann. Neurol 52, 276–284. [DOI] [PubMed] [Google Scholar]
- Hernandez DG, Reed X, Singleton AB, 2016. Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J. Neurochem 139 (Suppl. 1), 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, Knight R, Payami H, 2017. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord 32, 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffel G, Ginhoux F, 2015. Ontogeny of tissue-resident macrophages. Front. Immunol 6, 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Littman DR, 2016. The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84. [DOI] [PubMed] [Google Scholar]
- Honkanen EA, Saari L, Orte K, Gardberg M, Noponen T, Joutsa J, Kaasinen V, 2019. No link between striatal dopaminergic axons and dopamine transporter imaging in Parkinson’s disease. Mov. Disord 34, 1562–1566. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ, 2012. Interactions between the microbiota and the immune system. Science 336, 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KY, Fernandez-Hernandez H, Hu J, Schaffner A, Pankratz N, Hsu NY, Chuang LS, Carmi S, Villaverde N, Li X, Rivas M, Levine AP, Bao X, Labrias PR, Haritunians T, Ruane D, Gettler K, Chen E, Li D, Schiff ER, Pontikos N, Barzilai N, Brant SR, Bressman S, Cheifetz AS, Clark LN, Daly MJ, Desnick RJ, Duerr RH, Katz S, Lencz T, Myers RH, Ostrer H, Ozelius L, Payami H, Peter Y, Rioux JD, Segal AW, Scott WK, Silverberg MS, Vance JM, Ubarretxena-Belandia I, Foroud T, Atzmon G, Pe’er I, Ioannou Y, McGovern DPB, Yue Z, Schadt EE, Cho JH, Peter I, 2018. Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci. Transl. Med 10, 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannaccone S, Cerami C, Alessio M, Garibotto V, Panzacchi A, Olivieri S, Gelsomino G, Moresco RM, Perani D, 2013. In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Parkinsonism Relat. Disord 19, 47–52. [DOI] [PubMed] [Google Scholar]
- Ichise M, Kim YJ, Ballinger JR, Vines D, Erami SS, Tanaka F, Lang AE, 1999. Spect imaging of pre- and postsynaptic dopaminergic alterations in L-dopa-untreated PD. Neurology 52, 1206–1214. [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y, 2003. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 106, 518–526. [DOI] [PubMed] [Google Scholar]
- International Parkinson Disease Genomics Consortium, Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simon-Sanchez J, Schulte C, Lesage S, Sveinbjornsdottir S, Stefansson K, Martinez M, Hardy J, Heutink P, Brice A, Gasser T, Singleton AB, Wood NW, 2011. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet 377, 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucaite A, Svenningsson P, Rinne JO, Cselenyi Z, Varnas K, Johnstrom P, Amini N, Kirjavainen A, Helin S, Minkwitz M, Kugler AR, Posener JA, Budd S, Halldin C, Varrone A, Farde L, 2015. Effect of the myeloperoxidase inhibitor AZD3241 on microglia: a PET study in Parkinson’s disease. Brain 138, 2687–2700. [DOI] [PubMed] [Google Scholar]
- Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, Bohorquez DV, 2018. A gut-brain neural circuit for nutrient sensory transduction. Science 361, 6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannarkat GT, Cook DA, Lee JK, Chang J, Chung J, Sandy E, Paul KC, Ritz B, Bronstein J, Factor SA, Boss JM, Tansey MG, 2015. Common genetic variant association with altered HLA expression, synergy with pyrethroid exposure, and risk for Parkinson’s disease: an observational and case-control study. NPJ Parkinsons Dis. 1, 15002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I, 2017. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290 e17. [DOI] [PubMed] [Google Scholar]
- Killinger BA, Madaj Z, Sikora JW, Rey N, Haas AJ, Vepa Y, Lindqvist D, Chen H, Thomas PM, Brundin P, Brundin L, Labrie V, 2018. The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci. Transl. Med 10, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ, Lee SJ, 2013. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun 4, 1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Lee HJ, Masliah E, Lee SJ, 2016. Non-cell-autonomous neurotoxicity of alpha-synuclein through microglial toll-like receptor 2. Exp. Neurobiol 25, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, Shen C, Lee H, Kulkarni S, Pasricha PJ, Lee G, Pomper MG, Dawson VL, Dawson TM, Ko HS, 2019. Transneuronal propagation of pathologic alpha-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103, 627–641 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohutnicka M, Lewandowska E, Kurkowska-Jastrzebska I, Czlonkowski A, Czlonkowska A, 1998. Microglial and astrocytic involvement in a murine model of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Immunopharmacology 39, 167–180. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O, 1998. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet 18, 106–108. [DOI] [PubMed] [Google Scholar]
- Kuhlmann AC, Guilarte TR, 2000. Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. J. Neurochem 74, 1694–1704. [DOI] [PubMed] [Google Scholar]
- Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A, 1999. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp. Neurol 156, 50–61. [DOI] [PubMed] [Google Scholar]
- Lam HA, Wu N, Cely I, Kelly RL, Hean S, Richter F, Magen I, Cepeda C, Ackerson LC, Walwyn W, Masliah E, Chesselet MF, Levine MS, Maidment NT, 2011. Elevated tonic extracellular dopamine concentration and altered dopamine modulation of synaptic activity precede dopamine loss in the striatum of mice overexpressing human alpha-synuclein. J. Neurosci. Res 89, 1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H, 2015. The immunology of asthma. Nat. Immunol 16, 45–56. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM, 2014. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I, 1983. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D, 1999. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann. Neurol 46, 598–605. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Overk CR, Oueslati A, Masliah E, 2013. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat. Rev. Neurosci 14, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavisse S, Guillermier M, Herard AS, Petit F, Delahaye M, Van Camp N, Ben Haim L, Lebon V, Remy P, Dolle F, Delzescaux T, Bonvento G, Hantraye P, Escartin C, 2012. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J. Neurosci 32, 10809–10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laydon DJ, Bangham CR, Asquith B, 2015. Estimating T-cell repertoire diversity: limitations of classical estimators and a new approach. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci 370, 1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipe J, Grunke M, Dechant C, Reindl C, Kerzendorf U, Schulze-Koops H, Skapenko A, 2010. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 62, 2876–2885. [DOI] [PubMed] [Google Scholar]
- Leung S, Liu X, Fang L, Chen X, Guo T, Zhang J, 2010. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell. Mol. Immunol 7, 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wu X, Hu X, Wang T, Liang S, Duan Y, Jin F, Qin B, 2017. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci. China Life Sci 60, 1223–1233. [DOI] [PubMed] [Google Scholar]
- Lieberman J, 2003. The ABCS of granule-mediated cytotoxicity: new weapons in the arsenal. Nat. Rev. Immunol 3, 361–370. [DOI] [PubMed] [Google Scholar]
- Lin JC, Lin CS, Hsu CW, Lin CL, Kao CH, 2016. Association between Parkinson’s disease and inflammatory bowel disease: a nationwide Taiwanese retrospective cohort study. Inflamm. Bowel Dis 22, 1049–1055. [DOI] [PubMed] [Google Scholar]
- Lin CH, Chen CC, Chiang HL, Liou JM, Chang CM, Lu TP, Chuang EY, Tai YC, Cheng C, Lin HY, Wu MS, 2019. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflammation 16, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren HS, Lelos MJ, Dunnett SB, 2012. Do alpha-synuclein vector injections provide a better model of Parkinson’s disease than the classic 6-hydroxydopamine model? Exp. Neurol 237, 36–42. [DOI] [PubMed] [Google Scholar]
- Liu Z, Lee J, Krummey S, Lu W, Cai H, Lenardo MJ, 2011. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat. Immunol 12, 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Liu X, Li Y, Zhao J, Liu Z, Hu Z, Wang Y, Yao Y, Miller AW, Su B, Cookson MR, Li X, Kang Z, 2017. LRRK2 promotes the activation of NLRC4 inflammasome during Salmonella typhimurium infection. J. Exp. Med 214, 3051–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodygin D, Hermann M, Schweingruber N, Flugel-Koch C, Watanabe T, Schlosser C, Merlini A, Korner H, Chang HF, Fischer HJ, Reichardt HM, Zagrebelsky M, Mollenhauer B, Kugler S, Fitzner D, Frahm J, Stadelmann C, Haberl M, Odoardi F, Flugel A, 2019. beta-Synuclein-reactive T cells induce autoimmune CNS grey matter degeneration. Nature 566, 503–508. [DOI] [PubMed] [Google Scholar]
- Luk KC, Song C, O’brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM, 2009. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. U. S. A 106, 20051–20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’brien P, Trojanowski JQ, Lee VM, 2012. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Gao J, Wang J, Xie A, 2019. Prion-like mechanisms in Parkinson’s disease. Front. Neurosci 13, 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA, 2002. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J. Biol. Chem 277, 1641–1644. [DOI] [PubMed] [Google Scholar]
- Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin JH, Kang HC, Zhang J, Xu J, Chen R, Park H, Andrabi SA, Kang SU, Goncalves RA, Liang Y, Zhang S, Qi C, Lam S, Keiler JA, Tyson J, Kim D, Panicker N, Yun SP, Workman CJ, Vignali DA, Dawson VL, Ko HS, Dawson TM, 2016. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 353, 6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraganore DM, De Andrade M, Elbaz A, Farrer MJ, Ioannidis JP, Kruger R, Rocca WA, Schneider NK, Lesnick TG, Lincoln SJ, Hulihan MM, Aasly JO, Ashizawa T, Chartier-Harlin MC, Checkoway H, Ferrarese C, Hadjigeorgiou G, Hattori N, Kawakami H, Lambert JC, Lynch T, Mellick GD, Papapetropoulos S, Parsian A, Quattrone A, Riess O, Tan EK, Van Broeckhoven C, Genetic Epidemiology Of Parkinson’S Disease Consortium, 2006. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA 296, 661–670. [DOI] [PubMed] [Google Scholar]
- Marinova-Mutafchieva L, Sadeghian M, Broom L, Davis JB, Medhurst AD, Dexter DT, 2009. Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: a time course study in a 6-hydroxydopamine model of Parkinson’s disease. J. Neurochem 110, 966–975. [DOI] [PubMed] [Google Scholar]
- Martin R, Chain F, Miquel S, Lu J, Gratadoux JJ, Sokol H, Verdu EF, Bercik P, Bermudez-Humaran LG, Langella P, 2014. The commensal bacterium Faecalibacterium prausnitzii is protective in DNBS-induced chronic moderate and severe colitis models. Inflamm. Bowel Dis 20, 417–430. [DOI] [PubMed] [Google Scholar]
- Martin R, Miquel S, Chain F, Natividad JM, Jury J, Lu J, Sokol H, Theodorou V, Bercik P, Verdu EF, Langella P, Bermudez-Humaran LG, 2015. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 15, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L, 2000. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269. [DOI] [PubMed] [Google Scholar]
- Mata IF, Lockhart PJ, Farrer MJ, 2004. Parkin genetics: one model for Parkinson’s disease. Hum. Mol. Genet 13, R127–R133. Spec No 1. [DOI] [PubMed] [Google Scholar]
- Matheoud D, Sugiura A, Bellemare-Pelletier A, Laplante A, Rondeau C, Chemali M, Fazel A, Bergeron JJ, Trudeau LE, Burelle Y, Gagnon E, McBride HM, Desjardins M, 2016. Parkinson’s disease-related proteins PINK1 and parkin repress mitochondrial antigen presentation. Cell 166, 314–327. [DOI] [PubMed] [Google Scholar]
- Matheoud D, Cannon T, Voisin A, Penttinen AM, Ramet L, Fahmy AM, Ducrot C, Laplante A, Bourque MJ, Zhu L, Cayrol R, Le Campion A, McBride HM, Gruenheid S, Trudeau LE, Desjardins M, 2019. Intestinal infection triggers Parkinson’s disease-like symptoms in PINK1(−/−) mice. Nature 571, 565–569. [DOI] [PubMed] [Google Scholar]
- Maulik M, Mitra S, Bult-Ito A, Taylor BE, Vayndorf EM, 2017. Behavioral phenotyping and pathological indicators of Parkinson’s disease in C. elegans models. Front. Genet 8, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland HF, Martin R, 2007. Multiple sclerosis: a complicated picture of autoimmunity. Nat. Immunol 8, 913–919. [DOI] [PubMed] [Google Scholar]
- Mcgeer PL, Itagaki S, Akiyama H, Mcgeer EG, 1988a. Rate of cell death in parkinsonism indicates active neuropathological process. Ann. Neurol 24, 574–576. [DOI] [PubMed] [Google Scholar]
- Mcgeer PL, Itagaki S, Boyes BE, Mcgeer EG, 1988b. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38, 1285–1291. [DOI] [PubMed] [Google Scholar]
- Mcgeer PL, Schwab C, Parent A, Doudet D, 2003. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann. Neurol 54, 599–604. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Rademacher DJ, 2011. MPTP mouse models of Parkinson’s disease: an update. J. Park. Dis 1, 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merson TD, Binder MD, Kilpatrick TJ, 2010. Role of cytokines as mediators and regulators of microglial activity in inflammatory demyelination of the CNS. NeuroMol. Med 12, 99–132. [DOI] [PubMed] [Google Scholar]
- Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C, 1997. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep 20, 1012–1020. [PubMed] [Google Scholar]
- Mildner A, Jung S, 2014. Development and function of dendritic cell subsets. Immunity 40, 642–656. [DOI] [PubMed] [Google Scholar]
- Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z, 2011. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun. Rev 10, 744–755. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T, 1994. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett 165, 208–210. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T, 1996. Interleukin (Il)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci. Lett 211, 13–16. [DOI] [PubMed] [Google Scholar]
- Mosaad YM, 2015. Clinical role of human leukocyte antigen in health and disease. Scand. J. Immunol 82, 283–306. [DOI] [PubMed] [Google Scholar]
- Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, Lelios I, Heppner FL, Kipnis J, Merkler D, Greter M, Becher B, 2018. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395 e6. [DOI] [PubMed] [Google Scholar]
- Mundt S, Mrdjen D, Utz SG, Greter M, Schreiner B, Becher B, 2019. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci. Immunol 4, 31. [DOI] [PubMed] [Google Scholar]
- Nayak D, Roth TL, Mcgavern DB, 2014. Microglia development and function. Annu. Rev. Immunol 32, 367–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemazee D, 2017. Mechanisms of central tolerance for B cells. Nat. Rev. Immunol 17, 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O’neill LA, Xavier RJ, 2016. Trained immunity: a program of innate immune memory in health and disease. Science 352, aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath MF, 2014. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol 14, 329–342. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D, Burn D, Halliday GM, Bezard E, Przedborski S, Lehericy S, Brooks DJ, Rothwell JC, Hallett M, Delong MR, Marras C, Tanner CM, Ross GW, Langston JW, Klein C, Bonifati V, Jankovic J, Lozano AM, Deuschl G, Bergman H, Tolosa E, Rodriguez-Violante M, Fahn S, Postuma RB, Berg D, Marek K, Standaert DG, Surmeier DJ, Olanow CW, Kordower JH, Calabresi P, Schapira AHV, Stoessl AJ, 2017. Past, present, and future of Parkinson’s disease: a special essay on the 200th anniversary of the shaking palsy. Mov. Disord 32, 1264–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr CF, Rowe DB, Mizuno Y, Mori H, Halliday GM, 2005. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain 128, 2665–2674. [DOI] [PubMed] [Google Scholar]
- O’shea JJ, Ma A, Lipsky P, 2002. Cytokines and autoimmunity. Nat. Rev. Immunol 2, 37–45. [DOI] [PubMed] [Google Scholar]
- Pabon MM, Bachstetter AD, Hudson CE, Gemma C, Bickford PC, 2011. CX3CL1 reduces neurotoxicity and microglial activation in a rat model of Parkinson’s disease. J. Neuroinflammation 8, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell M, Economopoulos V, Wilson TC, Kersemans V, Isenegger PG, Larkin JR, Smart S, Gilchrist S, Gouverneur V, Sibson NR, 2019. Imaging of Translocator Protein Upregulation is Selective for Pro-Inflammatory Polarized Astrocytes and Microglia. Glia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J, 2002. An essay on the shaking palsy. 1817. J. Neuropsychiatr. Clin. Neurosci 14, 223–236. discussion 222. [DOI] [PubMed] [Google Scholar]
- Parkkinen L, Pirttila T, Tervahauta M, Alafuzoff I, 2005. Widespread and abundant alpha-synuclein pathology in a neurologically unimpaired subject. Neuropathology 25, 304–314. [DOI] [PubMed] [Google Scholar]
- Patterson AM, Mulder IE, Travis AJ, Lan A, Cerf-Bensussan N, Gaboriau-Routhiau V, Garden K, Logan E, Delday MI, Coutts AGP, Monnais E, Ferraria VC, Inoue R, Grant G, Aminov RI, 2017. Human gut symbiont Roseburia hominis promotes and regulates innate immunity. Front. Immunol 8, 1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumier KL, Luk KC, Manfredsson FP, Kanaan NM, Lipton JW, Collier TJ, Steece-Collier K, Kemp CJ, Celano S, Schulz E, Sandoval IM, Fleming S, Dirr E, Polinski NK, Trojanowski JQ, Lee VM, Sortwell CE, 2015. Intrastriatal injection of pre-formed mouse alpha-synuclein fibrils into rats triggers alpha-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol. Dis 82, 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, 1925. Microglia and the process of phagocytosis in gliomas. Am. J. Pathol 1, 77–90 15. [PMC free article] [PubMed] [Google Scholar]
- Peng J, Stevenson FF, Oo ML, Andersen JK, 2009. Iron-enhanced paraquat-mediated dopaminergic cell death due to increased oxidative stress as a consequence of microglial activation. Free Radic. Biol. Med 46, 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter I, Dubinsky M, Bressman S, Park A, Lu C, Chen N, Wang A, 2018. Anti-tumor necrosis factor therapy and incidence of Parkinson disease among patients with inflammatory bowel disease. JAMA Neurol. 75, 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov VA, Saltykova IV, Zhukova IA, Alifirova VM, Zhukova NG, Dorofeeva YB, Tyakht AV, Kovarsky BA, Alekseev DG, Kostryukova ES, Mironova YS, Izhboldina OP, Nikitina MA, Perevozchikova TV, Fait EA, Babenko VV, Vakhitova MT, Govorun VM, Sazonov AE, 2017. Analysis of gut microbiota in patients with Parkinson’s disease. Bull. Exp. Biol. Med 162, 734–737. [DOI] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ, 2015. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrucci D, Cerroni R, Unida V, Farcomeni A, Pierantozzi M, Mercuri NB, Biocca S, Stefani A, Desideri A, 2019. Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Parkinsonism Relat. Disord 65, 124–130. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL, 1997. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Adler CH, Dugger BN, Hentz JG, Shill HA, Driver-Dunckley E, Sabbagh MN, Jacobson SA, Belden CM, Sue LI, Serrano G, Beach TG, 2015. REM sleep behavior disorder and neuropathology in Parkinson’s disease. Mov. Disord 30, 1413–1417. [DOI] [PubMed] [Google Scholar]
- Powers KM, Kay DM, Factor SA, Zabetian CP, Higgins DS, Samii A, Nutt JG, Griffith A, Leis B, Roberts JW, Martinez ED, Montimurro JS, Checkoway H, Payami H, 2008. Combined effects of smoking, coffee, and NSAIDs on Parkinson’s disease risk. Mov. Disord 23, 88–95. [DOI] [PubMed] [Google Scholar]
- Prashar A, Schnettger L, Bernard EM, Gutierrez MG, 2017. Rab GTPases in immunity and inflammation. Front. Cell. Infect. Microbiol 7, 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Priller J, 2017. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci 20, 136–144. [DOI] [PubMed] [Google Scholar]
- Purisai MG, McCormack AL, Cumine S, Li J, Isla MZ, Di Monte DA, 2007. Microglial activation as a priming event leading to paraquat-induced dopaminergic cell degeneration. Neurobiol. Dis 25, 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XY, Zhang SP, Cao C, Loh YP, Cheng Y, 2016. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol. 73, 1316–1324. [DOI] [PubMed] [Google Scholar]
- Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D, 1999. Endotoxin-tolerant mice have mutations in toll-like receptor 4 (Tlr4). J. Exp. Med 189, 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj T, Rothamel K, Mostafavi S, Ye C, Lee MN, Replogle JM, Feng T, Lee M, Asinovski N, Frohlich I, Imboywa S, Von Korff A, Okada Y, Patsopoulos NA, Davis S, McCabe C, Paik HI, Srivastava GP, Raychaudhuri S, Hafler DA, Koller D, Regev A, Hacohen N, Mathis D, Benoist C, Stranger BE, De Jager PL, 2014. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science 344, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin LC, Artis D, 2018. Beyond host defense: emerging functions of the immune system in regulating complex tissue physiology. Cell 173, 554–567. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE, 2010. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J. Immunol 184, 2261–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A, Siracusa MC, Yap GS, Gause WC, 2016. Innate cell communication kick-starts pathogen-specific immunity. Nat. Immunol 17, 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E, 2002. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J. Neurosci. Res 68, 568–578. [DOI] [PubMed] [Google Scholar]
- Rosenkranz D, Weyer S, Tolosa E, Gaenslen A, Berg D, Leyhe T, Gasser T, Stoltze L, 2007. Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J. Neuroimmunol 188, 117–127. [DOI] [PubMed] [Google Scholar]
- Rozemuller AJ, Eikelenboom P, Theeuwes JW, Jansen Steur EN, De Vos RA, 2000. Activated microglial cells and complement factors are unrelated to cortical Lewy bodies. Acta Neuropathol. 100, 701–708. [DOI] [PubMed] [Google Scholar]
- Russo I, Kaganovich A, Ding J, Landeck N, Mamais A, Varanita T, Biosa A, Tessari I, Bubacco L, Greggio E, Cookson MR, 2019. Transcriptome analysis of LRRK2 knock-out microglia cells reveals alterations of inflammatory- and oxidative stress-related pathways upon treatment with alpha-synuclein fibrils. Neurobiol. Dis 129, 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacino AN, Brooks M, McKinney AB, Thomas MA, Shaw G, Golde TE, Giasson BI, 2014. Brain injection of alpha-synuclein induces multiple proteinopathies, gliosis, and a neuronal injury marker. J. Neurosci 34, 12368–12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro F, Marin-Lahoz J, Martinez-Horta S, Pagonabarraga J, Kulisevsky J, 2019. Dopaminergic degeneration induces early posterior cortical thinning in Parkinson’s disease. Neurobiol. Dis 124, 29–35. [DOI] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M, 2010. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS One 5, e8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Tentillier N, Romero-Ramos M, 2015. The relation between alpha-synuclein and microglia in Parkinson’s disease: recent developments. Neuroscience 302, 47–58. [DOI] [PubMed] [Google Scholar]
- Saunders JA, Estes KA, Kosloski LM, Allen HE, Dempsey KM, Torres-Russotto DR, Meza JL, Santamaria PM, Bertoni JM, Murman DL, Ali HH, Standaert DG, Mosley RL, Gendelman HE, 2012. CD4+ regulatory and effector/ memory T cell subsets profile motor dysfunction in Parkinson’s disease. J. NeuroImmune Pharmacol 7, 927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P, 2015. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord 30, 350–358. [DOI] [PubMed] [Google Scholar]
- Schuler F, Casida JE, 2001. Functional coupling of PSST and ND1 subunits in NADH:ubiquinone oxidoreductase established by photoaffinity labeling. Biochim. Biophys. Acta 1506, 79–87. [DOI] [PubMed] [Google Scholar]
- Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, Kordower JH, 2012. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov. Disord 27, 709–715. [DOI] [PubMed] [Google Scholar]
- Shen Z, Zhu C, Quan Y, Yang J, Yuan W, Yang Z, Wu S, Luo W, Tan B, Wang X, 2018. Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses. J. Gastroenterol. Hepatol 33, 1751–1760. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Kim JH, Greenamyre JT, 2003. Selective microglial activation in the rat rotenone model of Parkinson’s disease. Neurosci. Lett 341, 87–90. [DOI] [PubMed] [Google Scholar]
- Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, Li Y, Aro P, Dator R, He C, Hipp MJ, Zabetian CP, Peskind ER, Hu SC, Quinn JF, Galasko DR, Banks WA, Zhang J, 2014. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 128, 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoji M, Pagan F, Healton EB, Mocchetti I, 2009. CXCR4 and CXCL12 expression is increased in the nigro-striatal system of Parkinson’s disease. Neurotox. Res 16, 318–328. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K, 2003. alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302, 841. [DOI] [PubMed] [Google Scholar]
- Sliter DA, Martinez J, Hao L, Chen X, Sun N, Fischer TD, Burman JL, Li Y, Zhang Z, Narendra DP, Cai H, Borsche M, Klein C, Youle RJ, 2018. Parkin and PINK1 mitigate sting-induced inflammation. Nature 561, 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottiere HM, Dore J, Marteau P, Seksik P, Langella P, 2008. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U. S. A 105, 16731–16736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A, Marxreiter F, Krach F, Fadler T, Grosch J, Maroni M, Graef D, Eberhardt E, Riemenschneider MJ, Yeo GW, Kohl Z, Xiang W, Gage FH, Winkler J, Prots I, Winner B, 2018. Th17 lymphocytes induce neuronal cell death in a human iPSC-based model of Parkinson’s disease. Cell Stem Cell 23, 123–131 e6. [DOI] [PubMed] [Google Scholar]
- Song WM, Colonna M, 2018. The identity and function of microglia in neurodegeneration. Nat. Immunol 19, 1048–1058. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Heikkila RE, 1986. The influence of dose and dosing interval on MPTP-induced dopaminergic neurotoxicity in mice. Eur. J. Pharmacol 129, 339–345. [DOI] [PubMed] [Google Scholar]
- Sorrentino ZA, Brooks MMT, Hudson V 3rd, Rutherford NJ, Golde TE, Giasson BI, Chakrabarty P, 2017. Intrastriatal injection of alpha-synuclein can lead to widespread synucleinopathy independent of neuroanatomic connectivity. Mol. Neurodegener 12, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeiras A, Munoz-Patino AM, Labandeira-Garcia JL, 2000. Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants: potential implication in relation to the pathogenesis of Parkinson’s disease. J. Neurochem 74, 1605–1612. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M, 1997. Alpha-synuclein in Lewy bodies. Nature 388, 839–840. [DOI] [PubMed] [Google Scholar]
- Stastny P, 1978. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N. Engl. J. Med 298, 869–871. [DOI] [PubMed] [Google Scholar]
- Stefanis L, Emmanouilidou E, Pantazopoulou M, Kirik D, Vekrellis K, Tofaris GK, 2019. How is alpha-synuclein cleared from the cell? J. Neurochem 150, 577–590. [DOI] [PubMed] [Google Scholar]
- Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, Wachter S, Lorentzen E, Duddy G, Wilson S, Baptista MA, Fiske BK, Fell MJ, Morrow JA, Reith AD, Alessi DR, Mann M, 2016. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. elife 5, e12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Federoff HJ, Maguire-Zeiss KA, 2009. Mutant alpha-synuclein overexpression mediates early proinflammatory activity. Neurotox. Res 16, 238–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Edwards RH, 2019. The physiological role of alpha-synuclein and its relationship to Parkinson’s disease. J. Neurochem 150, 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, Mcmurtrey C, Hildebrand WH, Mao X, Dawson VL, Dawson TM, Oseroff C, Pham J, Sidney J, Dillon MB, Carpenter C, Weiskopf D, Phillips E, Mallal S, Peters B, Frazier A, Lindestam Arlehamn CS, Sette A, 2017. T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature 546, 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B, Luo W, Shen Z, Xiao M, Wu S, Meng X, Wu X, Yang Z, Tian L, Wang X, 2019. Roseburia intestinalis inhibits oncostatin M and maintains tight junction integrity in a murine model of acute experimental colitis. Scand. J. Gastroenterol 54, 432–440. [DOI] [PubMed] [Google Scholar]
- Tay TL, Mai D, Dautzenberg J, Fernandez-Klett F, Lin G, Sagar, Datta M, Drougard A, Stempfl T, Ardura-Fabregat A, Staszewski O, Margineanu A, Sporbert A, Steinmetz LM, Pospisilik JA, Jung S, Priller J, Grun D, Ronneberger O, Prinz M, 2017. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci 20, 793–803. [DOI] [PubMed] [Google Scholar]
- Teismann P, Ferger B, 2001. Inhibition of the cyclooxygenase isoenzymes COX-1 and COX-2 provide neuroprotection in the MPTP-mouse model of Parkinson’s disease. Synapse 39, 167–174. [DOI] [PubMed] [Google Scholar]
- Terada T, Yokokura M, Yoshikawa E, Futatsubashi M, Kono S, Konishi T, Miyajima H, Hashizume T, Ouchi Y, 2016. Extrastriatal spreading of microglial activation in Parkinson’s disease: a positron emission tomography study. Ann. Nucl. Med 30, 579–587. [DOI] [PubMed] [Google Scholar]
- Tetrud JW, Langston JW, 1989. MPTP-induced parkinsonism as a model for Parkinson’s disease. Acta Neurol. Scand. Suppl 126, 35–40. [DOI] [PubMed] [Google Scholar]
- Thakur P, Nehru B, 2015. Inhibition of neuroinflammation and mitochondrial dysfunctions by carbenoxolone in the rotenone model of Parkinson’s disease. Mol. Neurobiol 51, 209–219. [DOI] [PubMed] [Google Scholar]
- Theodore S, Maragos W, 2015. 6-Hydroxydopamine as a tool to understand adaptive immune system-induced dopamine neurodegeneration in Parkinson’s disease. Immunopharmacol. Immunotoxicol 37, 393–399. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cao S, Mclean PJ, Standaert DG, 2008. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune zresponse in a mouse model of Parkinson disease. J. Neuropathol. Exp. Neurol 67, 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos AN, Kono DH, Baccala R, 2017. The multiple pathways to autoimmunity. Nat. Immunol 18, 716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome AD, Harms AS, Volpicelli-Daley LA, Standaert DG, 2016. microRNA-155 regulates alpha-synuclein-induced inflammatory responses in models of Parkinson disease. J. Neurosci 36, 2383–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U, 1968. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol 5, 107–110. [DOI] [PubMed] [Google Scholar]
- Uribe C, Segura B, Baggio HC, Abos A, Garcia-Diaz AI, Campabadal A, Marti MJ, Valldeoriola F, Compta Y, Bargallo N, Junque C, 2018. Gray/white matter contrast in Parkinson’s disease. Front. Aging Neurosci 10, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhauwaert R, Verstreken P, 2015. Flies with Parkinson’s disease. Exp. Neurol 274, 42–51. [DOI] [PubMed] [Google Scholar]
- Vidal-Martinez G, Vargas-Medrano J, Gil-Tommee C, Medina D, Garza NT, Yang B, Segura-Ulate I, Dominguez SJ, Perez RG, 2016. FTY720/fingolimod reduces synucleinopathy and improves gut motility in A53T Mice: CONTRIBUTIONS OF PRO-BRAIN-DERIVED NEUROTROPHIC FACTOR (PRO-BDNF) AND MATURE BDNF. J. Biol. Chem 291, 20811–20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ, 2008. How regulatory T cells work. Nat. Rev. Immunol 8, 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visanji NP, Brotchie JM, Kalia LV, Koprich JB, Tandon A, Watts JC, Lang AE, 2016. Alpha-Synuclein-based animal models of Parkinson’s disease: challenges and opportunities in a new era. Trends Neurosci. 39, 750–762. [DOI] [PubMed] [Google Scholar]
- Vitetta ES, Berton MT, Burger C, Kepron M, Lee WT, Yin XM, 1991. Memory B and T cells. Annu. Rev. Immunol 9, 193–217. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, 2017. Effects of alpha-synuclein on axonal transport. Neurobiol. Dis 105, 321–327. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM, 2011. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JT, Hendrix S, Boato F, Smirnov I, Zheng J, Lukens JR, Gadani S, Hechler D, Golz G, Rosenberger K, Kammertons T, Vogt J, Vogelaar C, Siffrin V, Radjavi A, Fernandez-Castaneda A, Gaultier A, Gold R, Kanneganti TD, Nitsch R, Zipp F, Kipnis J, 2015. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J. Clin. Invest 125, 699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MB, Richter F, Lee SK, Gabby L, Wu J, Masliah E, Effros RB, Chesselet MF, 2012. Regionally-specific microglial activation in young mice overexpressing human wildtype alpha-synuclein. Exp. Neurol 237, 318–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler CJ, Seksenyan A, Koronyo Y, Rentsendorj A, Sarayba D, Wu H, Gragg A, Siegel E, Thomas D, Espinosa A, Thompson K, Black K, Koronyo-Hamaoui M, Pechnick R, Irvin DK, 2014. T-Lymphocyte deficiency exacerbates behavioral deficits in the 6-OHDA unilateral lesion rat model for Parkinson’s disease. J. Neurol. Neurophysiol 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GP, Schonhoff AM, Jurkuvenaite A, Thome AD, Standaert DG, Harms AS, 2018. Targeting of the class II transactivator attenuates inflammation and neurodegeneration in an alpha-synuclein model of Parkinson’s disease. J. Neuroinflammation 15, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Gray CH, Wijeyekoon R, Yarnall AJ, Lawson RA, Breen DP, Evans JR, Cummins GA, Duncan GW, Khoo TK, Burn DJ, Barker RA, ICICLE-PD Study Group, 2016. Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov. Disord 31, 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S, 2002. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci 22, 1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Mcgeer PL, Mcgeer EG, 1992. Lewy bodies in Parkinson’s disease are recognized by antibodies to complement proteins. Acta Neuropathol. 84, 100–104. [DOI] [PubMed] [Google Scholar]
- Yang Y, Bazhin AV, Werner J, Karakhanova S, 2013. Reactive oxygen species in the immune system. Int. Rev. Immunol 32, 249–270. [DOI] [PubMed] [Google Scholar]
- Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, Kwon SH, Park YJ, Karuppagounder SS, Park H, Kim S, Oh N, Kim NA, Lee S, Brahmachari S, Mao X, Lee JH, Kumar M, An D, Kang SU, Lee Y, Lee KC, Na DH, Kim D, Lee SH, Roschke VV, Liddelow SA, Mari Z, Barres BA, Dawson VL, Lee S, Dawson TM, Ko HS, 2018. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med 24, 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhang M, Wang Y, Dorfman RG, Liu H, Yu T, Chen X, Tang D, Xu L, Yin Y, Pan Y, Zhou Q, Zhou Y, Yu C, 2018. Faecalibacterium prausnitzii produces butyrate to maintain Th17/Treg balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. Inflamm. Bowel Dis 24 (9), 1926–1940. 10.1093/ibd/izy182. [DOI] [PubMed] [Google Scholar]
- Zhu J, Paul WE, 2008. CD4 T cells: fates, functions, and faults. Blood 112, 1557–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Song K, Shen Z, Quan Y, Tan B, Luo W, Wu S, Tang K, Yang Z, Wang X, 2018. Roseburia intestinalis inhibits interleukin17 excretion and promotes regulatory T cells differentiation in colitis. Mol. Med. Rep 17, 7567–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T, 2004. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607. [DOI] [PubMed] [Google Scholar]


