Abstract
Peptide-induced permeabilization of lipid vesicles has been measured for decades and has provided many insights into the sequence-structure-function relationships of membrane-active peptides. However, researchers in the field have noted that many experiments show transient permeabilization, in which a burst of leakage occurs immediately after peptide addition, followed by a slowdown or cessation of leakage before all contents have been released. This widely observed, but rarely studied, phenomenon is not explained by standard equilibrium pore models that are commonly invoked in both experimental and computational studies. Here we discuss observations of transient permeabilization, and we outline a pathway towards understanding this enigmatic phenomenon.
Introduction
Membrane permeabilizing peptides are ubiquitous in nature, where they have evolved for offensive or defensive use as antimicrobials, toxins, or venoms. Examples include eukaryotic antibacterial and antiviral peptides that are part of host innate immune systems. Such antimicrobial peptides (AMPs) disrupt bacterial membranes(1–4) as well as viral envelopes(5). There are also bacterial and fungal peptides that act either on other microbe membranes(6, 7) or on eukaryotic host cell membranes(8). There are viral peptides that can act on bacterial membranes(9) or eukaryotic host membranes(10, 11). Finally, insects, arachnids, and some vertebrates produce complex venoms or toxin delivery systems that can include membrane permeabilizing peptides(12). The most well-known example of this class is the archetypal peptide, melittin, which is the principal component of Honey Bee venom(4, 13, 14).
Numerous potential applications of membrane active peptides have been discussed in the literature. Antibacterial and antiviral activities, which depend on selective disruption of pathogen membranes over host cell membranes, are being pursued as solutions to the growing problems of drug resistant bacterial infections(15) and outbreaks of viral diseases(16). A few anti-infective peptides have reached the clinic, notably daptomycin for bacterial infections (17) and enfuvirtide for HIV (18). However, vigorous efforts which have continued in this direction for many years have not brought many successes to date. Other applications of membrane active peptides are even further from the clinic. For example, cytolytic peptides can be used as anticancer agents if the selective cell lysis can be fine-tuned(19). Membrane active peptides can also be used for delivery of drugs and other cargo to cells, which can occur through spontaneous translocation(20, 21), transient plasma membrane disruption(22), or endosomal membrane permeabilization(23). Despite their significant translational potential, membrane-active peptides have mostly not met the expectations of the scientific community.
Further engineering, re-design, and optimization will be required to bring these various membrane active peptide-based technologies to fruition. Yet these processes are impeded by an incomplete knowledge of peptide sequence-structure-function relationships(24). Towards improved understanding of peptides in membranes, many studies have been carried out using synthetic lipid bilayer systems. Such systems complement cell-based or biological studies because synthetic membranes have defined and controllable composition (lipids, peptide, buffers etc.) and because synthetic systems enable both functional and structural assays to be done simultaneously. However, significant questions remain about the mechanistic insights gained from vesicle permeabilization studies. In this review we discuss the enigma of the transient permeabilization of synthetic lipid vesicles by peptides. This is a commonly observed mechanism that has not been fully explained or comprehensively studied and has not been properly modeled with molecular dynamics simulations. Until we better understand transient membrane permeabilization, progress will be impeded by a ubiquitous mechanism of action that our models nearly always fail to capture.
Experimental Protocols in Membrane Permeabilization
The primary function of every biological membrane is to serve as a permeability barrier. Membranes are thus a critical point of vulnerability, which explains why membrane permeabilizing peptides and proteins are so common in nature. To develop membrane-active peptide applications, membrane vulnerability needs to be exploited against pathogens or diseased or targeted cells but protected against normal or non-targeted host cells. To better understand the fundamental principles of membrane permeabilization, synthetic vesicle leakage studies are used in which probe molecules are entrapped in the internal volume of lipid vesicles and excess probe is removed from the external space. Peptide-induced leakage of the probe from the vesicle interior into the external volume is inferred from a change in a measurable property. Fluorescent probes are commonly used, including probes that are quenched by a co-encapsulated quencher, such as ANTS/DPX, or those that are self-quenched by high entrapped concentration, such as calcein or carboxyfluorescein(25). Binary systems such as Terbium (Tb3+) and dipicolinic acid (DPA) in which one probe is inside and one is outside the vesicles have also been used extensively(26).
The size dependence of vesicle leakage has been studied by leakage of macromolecule-sized probes, such as dextrans(27, 28). A recently described assay uses TAMRA and biotin-labelled dextrans that form binary complexes with external streptavidin that is labelled with Alexafluor488, a FRET donor for TAMRA(28–30). Release of the dextran enables complex formation with streptavidin resulting in FRET quenching of AF488. Size dependence has also been probed with other macromolecules, such as enzyme(28).
In a typical vesicle leakage experiment, a membrane active peptide is added to a solution of vesicles with entrapped probes. The measurement reports on the total accumulated release of probe. Upon addition to a solution of vesicles, membrane active peptides will partition into the bilayers quickly, usually in less than one minute(31), driven by hydrophobic and electrostatic interactions. Peptides often gain secondary structure in or on the bilayer(32) which also takes place quickly. Leakage is reported as a function of peptide concentration, or peptide to lipid ratio (P:L). Some papers report time courses of leakage, while others report fractional leakage at a fixed time point (often 30–60 min).
Equilibrium pore formation
The equilibrium pore model is the simplest mechanism one can envision to describe vesicle permeabilization. In this case, some of the peptides that have partitioned into a vesicle will self-assemble into “pores” or otherwise create defects in the bilayer. They will thus create pathways through which entrapped probes can escape(4, 33–35). It can be assumed that there is an equilibrium between bound peptides in active or “open” pores in the membrane, bound peptides in inactive structures or in “closed” pores, and peptides that remain in solution. These pools of peptide can be in rapid equilibria, resulting in dynamic or short-lived pores, or they can exchange more slowly, resulting in longer-lived pores. But in either case, the average number of pores per vesicle, and the leakage rates from each vesicle will fluctuate only a little around a mean determined by the overall number of peptides bound to each vesicle.
To model equilibrium pore formation, the following reasonable assumptions can be made: i) the average number of pores per vesicle is constant after a short initial assembly phase, ii) the active secondary structure and tertiary structure, if any, of the membrane-bound peptides are constant, i.e. they are equilibrium states, iii) the probe molecules diffuse out of the vesicle independently. The last assumption means that a constant fraction of the probes remaining entrapped in the vesicle will diffuse out per unit time. Vesicle leakage curves measure total accumulated (integrated) leakage of vesicle contents. Therefore, like a nuclear decay process, the scenario described will give rise to a simple exponential approach toward 100% release, a process that can be described by the equation
| (1) |
where t is time and k is a rate constant that will depend on peptide concentration. More specifically, k depends on the number of peptides bound to each vesicle(36).
To report concentration in a vesicle leakage experiment, it would be ideal to know the number of peptides bound to each vesicle, but this would require knowing the number of lipids per vesicle for each lipid composition used. Although experiments are usually performed with vesicles of nominally uniform size, the number of lipids per vesicle is very difficult to measure, so it is usually not known precisely. It is much more convenient to express concentration in terms of overall peptide and lipid concentrations, i.e. peptide to lipid ratio (P:L), which are known accurately for any experiment. If possible, a more useful expression of effective concentration can be obtained by taking into account the partition coefficient of the peptide, which can be measured separately(37). If this information is known, the potentially active concentration of peptide is best expressed as bound peptide per lipid (Pbound:L)(36).
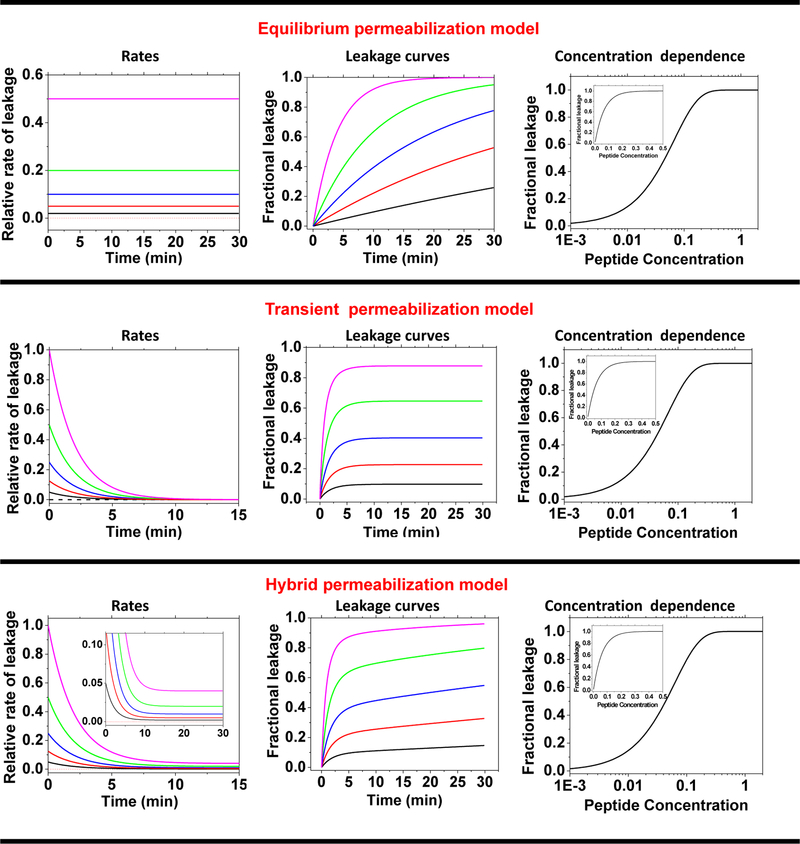
In Figure 1, top row, we show a simulation of equilibrium pore formation. For each curve, the rate (k in equation 1) is constant with time, reflecting the equilibrium state of the pores or the pathway across the bilayer. The different rates in the left panel are assumed to scale linearly with the peptide concentration. By using equation 1, the set of constant rates in the left panel are converted to the predicted family of exponential kinetic curves, shown in the middle panel. By noting the extent of leakage after 30 minutes, as is often done experimentally, we obtain the concentration dependence of leakage in the simulation. This is shown in the right-most panel of the top row as a semi-log plot (inset is on the linear scale).
Figure 1.
Simple models can recreate the observed behaviors of equilibrium and transient permeabilization. For all leakage models, the left panel shows the rate versus time for a series of peptide concentrations, assuming that rate or initial rate is proportional to peptide concentration. The colors from black to purple reflect increasing peptide concentration. The middle panel shows the predicted kinetic curves for the rates shown at the left, calculated with equation 1. The right panel shows the concentration dependence of the accumulated leakage at 30 min, on semi-log and linear scales (inset). Top row: Equilibrium permeabilization is simulated using a constant rate of leakage which results in an asymptotic approach toward 100% release of contents. Middle row: Transient permeabilization can be simulated by leakage rates that start at levels that are proportional to peptide concentration, and then rapidly decay at a constant exponential rate toward zero. Bottom row: Hybrid permeabilization model. Rates decay as in the transient pore model, but asymptotically approach a residual nonzero rate that is proportional to peptide concentration.
Transient Permeabilization
Vesicle leakage experiments have been performed for decades and there are now many papers in the literature that show leakage kinetic curves. Interestingly, only a few published leakage curves show the expected behavior for equilibrium pore formation across all peptide concentrations. Instead, what is often observed is a burst of leakage that occurs immediately after peptide is added, followed by a slowing or cessation of leakage that occurs over the subsequent 5–30 minutes(38–40). In such transient leakage processes, most leakage occurs immediately after addition of peptides to vesicles, or vesicles to peptides. After that, leakage rapidly slows or stops, ending at a plateau with incomplete leakage. In such experiments, peptide concentration appears to determine the plateau level, rather than the rate of approach towards 100% as it would in the equilibrium pore model. Only at the highest peptide concentration do the leakage kinetics approach the expected equilibrium pore behavior.
In the middle row of Figure 1, we model transient leakage. The initial rate of leakage is dependent on peptide concentration, left panel. However, in the transient leakage model, the instantaneous rate of leakage decreases exponentially from its initial value toward an asymptote of zero. The kinetic leakage curves in the middle panel are obtained by numerically integrating equation 1 using the time-dependent instantaneous rates in the left panel. The concentration dependence, created from the leakage at 30 minutes, is shown in the right panel.
In the bottom row of Figure 1, we simulate a hybrid leakage model. In this scenario, the rate decreases exponentially, like the transient leakage model, but the plateau level is not zero. Instead, in the hybrid model, there is a small residual rate of leakage that is proportional to peptide concentration creating a continuous slope of leakage after the transient phase. The hybrid kinetic curves show a burst of leakage followed by a slow background rate of leakage. This is the most commonly observed behavior in the vesicle leakage literature. In the right panel we show the extent of leakage at 30 min. for the hybrid model. Note that the three concentration dependence curves, based on leakage at 30 minutes (shown in the right column of Figure 1) for the three models, are essentially identical in shape. The difference between the three models can only be seen in the kinetic curves.
Experimental examples
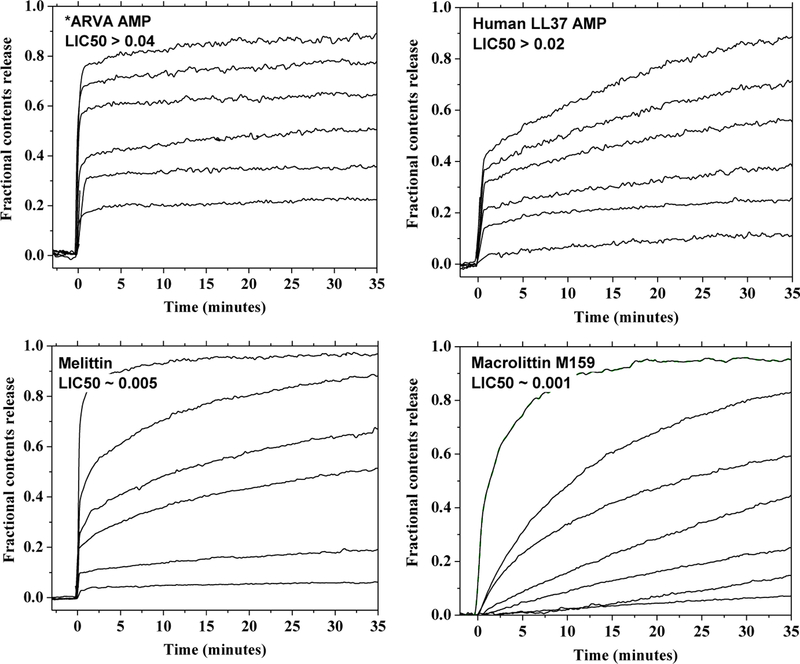
To demonstrate these types of kinetic leakage curves, we show in Figure 2 curves for four example membrane permeabilizing peptides. To indicate the range of potencies studied, on each plot we show the 50% “leakage inducing concentration” LIC50, which is the total peptide to lipid ratio that induces 50% leakage of vesicle contents at 30 minutes. The first peptide is a 12-residue synthetic antimicrobial peptide called *ARVA which was selected in a high-throughput screen for lipid vesicle permeabilization by peptides with β-sheet structure(41). Second, we studied the human AMP LL37, which has α-helical secondary structure(42). LL37 has been proposed to form equilibrium membrane-spanning pores in bilayers(43) under some conditions and to be a transient pore former under others(44). Third, we studied the Honey Bee venom peptide melittin, the most widely studied membrane permeabilizing peptide in the literature(4, 14, 45). Melittin permeabilizes bacterial, viral and eukaryotic membranes indiscriminately. Lastly, we studied the synthetically evolved melittin analog Macrolittin 159 (M159). M159 is evolved(29) from a melittin analog called MelP5 which itself was specifically selected in a screen for its ability to form equilibrium pores in bilayers(46). M159 forms macromolecule-sized pores in bilayers at extremely low concentrations (P:L ≤ 1:1000) and is one of the most potent membrane-active peptides known(29).
Figure 2.
Experimental leakage curves for four peptides. *ARVA and LL37 are antimicrobial peptides that show the characteristics of transient permeabilization; a rapid burst of leakage followed by a dramatic slowing of leakage over a short period of time. Melittin is a bee venom peptide that behaves as a transient pore former at low concentration. M159 is a highly potent pore forming peptide that behaves as an equilibrium pore former at all concentrations. LIC50, the peptide to lipid ratio that causes 50% leakage at 30 minutes, is shown for each peptide.
The two AMPs, *ARVA and LL37, have relatively low activity against PC vesicles (LIC50 ≥ 0.02) and show the type of transient pore formation behavior that is observed for many AMPs and other membrane permeabilizing peptides across the literature. Addition of peptide causes an immediate burst of leakage that quickly slows or stops before all contents are released. The final leakage level, but not the rate of leakage, is determined by the amount of peptide added. For *ARVA the residual background leakage quickly approaches zero, while for LL37 there is slightly higher background leakage. This behavior is essentially the one predicted by the hybrid model of Figure 1, bottom row. In either case, the curves approach plateaus that are different for each peptide concentration and do not approach 100% except for the highest peptide concentration studied. Thus, despite the simplicity of the models in Figure 1, the simulated kinetic curves for transient and hybrid leakage effectively recapitulate the types of experimental curves observed in many published leakage studies.
The bee venom lytic peptide melittin demonstrates a change in behavior across the concentration range of its activity, as we have reported previously(44). At concentrations lower than about P:L 1:200, melittin is a transient pore former in PC vesicles, with a burst of leakage that slows to a plateau that is less than 100% leakage and that depends on peptide concentration. Again, this is similar to the simulated data in Figure 1, bottom. At higher concentrations melittin begins to act more like an equilibrium pore former, probably because it disrupts the integrity of the vesicles through detergent-like effects. The P:L at which this transition occurs is likely dependent on lipid composition(27). Interestingly, melittin has also been reported to cause transient permeabilization of both the outer membrane and inner membrane of E. coli bacteria(47).
The fourth peptide studied, Macrolittin 159, is a rare example of a peptide that appears to behave as an equilibrium pore former across all concentrations studied. At low concentrations, M159 causes a slow steady leakage of vesicle contents that does not have a burst phase and does not appear to slow down with time. At higher concentrations, the approach to 100% release is more rapid. But at all concentrations, the behavior is close to that expected for equilibrium pore formation. Importantly, the LIC50 of M159 is so low that detergent-like vesicle destabilization cannot be occurring.
In previous studies we have verified that equilibrium and transient permeabilization are distinct mechanisms by probing vesicle permeabilization using an alternate, “two-step” leakage assay(44). In this assay, vesicles contain entrapped Tb3+ and external DPA as in a typical leakage experiment. However, the vesicles also contain a small fraction of lipids labelled with the dye NBD which can be quenched by the membrane-impermeant quencher dithionite, S2O42−. To probe whether leakage occurs via a transient or an equilibrium process, total accumulated leakage of Tb3+/DPA is first measured after equilibrium is reached (at least 8 hours after peptide addition). Then, the reducing agent dithionite is added. Upon contact with the stable free radical of NBD, dithionite rapidly eliminates its fluorescence by chemical reduction. However, dithionite is not membrane permeant and will only quench the NBD-lipids on the inner monolayer of the vesicles if the vesicles remain permeable at equilibrium due to equilibrium pores, or of the peptide, at equilibrium, induces induced rapid translocation of lipids between monolayers. In other words, dithionite will quench 100% of the NBD only if there are equilibrium pores. Transient pores will allow some Tb3+ to leak out initially but the vesicles will not be permeable to dithionite at equilibrium. In this case, dithionite will quench only the external NBD, or about 55% of the total.
Using this two-step assay, we have previously shown that transient pore formation is common in AMPs, including LL37. The two-step assay also showed that melittin is a transient pore former at P:L ≤ 1:200 and gradually approached equilibrium pore forming behavior at P:L ≥ 1:100. In the same experiments, equilibrium pore formation was observed for several other peptides, in addition to melittin at P:L ≥ 1:100. These include the α-helical fungal pore-former alamethicin, and two lentivirus lytic peptide sequences (LLP1 and LLP2) from the GP41 fusion protein of HIV. Except for melittin, these equilibrium pore formers were the peptides that were highly active at very low concentration, P:L ≤ 1:1000, where there are ≤ 100 peptides per vesicle. The transient pore formers in this study, like most AMPs, were active only at much higher concentrations (P:L ≥ 1:100) when there are many thousands of peptides per vesicle.
Graded, all-or-none, and stochastic permeabilization
The equilibrium pore model predicts graded release, in which all individual vesicles release roughly equal fractions of their contents as a function of time. Graded release has been observed experimentally for membrane permeabilizing peptides, including transient pore formers(48, 49). However, vesicle permeabilization also sometimes occurs by an all-or-none process in which individual vesicles have either released all their contents or have released none(34, 41, 50, 51). All-or-none permeabilization is not consistent with the equilibrium pore model. Even more inexplicable, examples of transient, all-or-none permeabilization have been reported, as in the case of the peptide *ARVA(41). This mechanism means that leakage is the result of catastrophic, stochastic events that enable release of all the contents of some vesicles, and that these events have a transient, decreasing probability of occurrence.
Ensemble measurements of vesicle permeabilization do not reveal individual events. However, some experimental approaches, including tethered vesicle platforms and giant unilamellar vesicles have enabled individual events to be observed in a few publications(52, 53). In some of these experiments, stochastic all-or-none release is observed in which individual vesicles remain stable and unpermeabilized for a stochastically determined lag time, followed by a sudden, catastrophic loss of all vesicle contents(52, 53). Once again, this is a behavior that cannot be explained by equilibrium pore formation.
Hoernke and colleagues have developed a useful quantitative mathematical model that effectively describes leakage from vesicles caused by detergents, peptides, and other amphiphiles(54). They have modeled leakage as a series of discreet events. When leakage occurs in a few, large events, it leads to all-or-none behavior. When leakage occurs in many, smaller events, it leads to graded leakage. Interestingly, these authors have also shown that even detergents can cause transient permeabilization of lipid vesicles(55, 56).
What causes transient permeabilization?
Dissipation of transbilayer asymmetry.
One model, perhaps first described in 2001 for detergents(56), hypothesizes that leakage occurs concomitantly with the dissipation of the initial asymmetry across the bilayer. When a membrane-active peptide or other amphiphile is added to a solution containing lipid vesicles, or visa-versa, the peptide partitions into the membrane, usually in less than one minute. But the lipid bilayer has two monolayers that are not equivalent in this scenario; the outer monolayer is exposed to the external solution and receives all the peptide, at least initially, while the inner monolayer receives none of it. Most peptides do not rapidly translocate across lipid bilayers, so an imbalance in mass, charge and surface pressure across the bilayer will quickly arise. Perhaps the most widely accepted explanation for transient permeabilization of lipid vesicles is that leakage occurs i) only while the transbilayer asymmetry exists, or ii) only during the dissipation of the transbilayer asymmetry. To explain transient permeabilization, dissipation of asymmetry must occur, and permeability must be greatly reduced once the peptides have become equally distributed between the monolayers of the bilayer. Otherwise, the behavior would be indistinguishable from equilibrium pore formation.
For dissipation-dependent transient leakage, it is possible to envision mechanistic models for either graded or for all-or-none vesicle leakage. Transient, graded leakage can be explained if the transbilayer asymmetry dissipates continuously, or in many small steps(54); leakage will occur until the asymmetry is dissipated. As the peptide concentration is increased, the dissipation may continue longer, or the membrane perturbation that enables leakage may be greater. In either case, more leakage will occur as more peptide is initially present on the outer monolayer.
Transient, all-or-none leakage can occur if the dissipation of asymmetry is catastrophic, leading to individual vesicle destruction, or if leakage occurs in a small number of large events(54). For transient, all-or-none leakage some fraction of the vesicles will not release their contents during the leakage phase, except at the highest peptide concentration. Thus, there must be an alternate pathway to equilibrium that does not cause permeabilization. This may include slow non-catastrophic equilibration by membrane translocation(57). In an experiment, perhaps each vesicle has a specific probability of being permeabilized during the dissipation, and this probability (which is equal to the fractional leakage) will increase with increasing peptide concentration.
Vesicle aggregation and fusion.
Many transient leakage curves in the literature report on the action of cationic peptides such as AMPs that have been added to anionic lipid vesicles, which mimic bacterial membranes. However, most anionic vesicle leakage experiments with AMPs (or with cell penetrating peptides) are characterized by an immediate, dramatic increase in solution turbidity upon peptide addition. This is the result of electrostatically driven vesicle aggregation, and perhaps fusion, which can lead to loss of membrane integrity and thus can cause leakage. These processes are likely to be transient and they will occur rapidly after addition of peptide to vesicles and will not continue indefinitely, thereby potentially giving rise to transient permeabilization. We note, however, that aggregation and fusion, while common, are artifacts that are probably not relevant for the biological activity of AMPs.
Either of the two processes: vesicle aggregation/fusion, or dissipation of transbilayer asymmetry, may dominate in the action of a particular peptide, or the two processes may operate in parallel. Next we discuss approaches that can be used to distinguish between these two mechanisms.
Experimental tests of transient permeabilization mechanisms.
These proposed mechanisms of transient permeabilization can be tested. For example, to test the effect of aggregation and fusion on leakage mechanism one can suppress these processes by using neutral lipids such as phosphatidylcholine or by adding 5 mol% PEG-lipids(58) to anionic bilayers. This concentration of PEG lipids creates a steric barrier that prevents bilayer contact(59, 60) but does not otherwise inhibit peptide-induced permeabilization. This is an experimental modification that should be used by all researchers who study anionic vesicle permeabilization by AMPs and CPPs because it reduces likely artifacts of vesicle aggregation which are severe when cationic peptides are added to anionic bilayers. We note that the experiments in Figure 2 that show transient permeabilization caused by ARVA and LL37 were performed with 100% PC vesicles. In this case, strong electrostatic attractions between peptides and zwitterionic vesicles were absent and so aggregation was minimal. We can thus conclude that, for these peptides, fusion and aggregation are not responsible for transient leakage.
The asymmetry dissipation model can be tested directly, as it leads to a prediction that peptide, lipid or both peptide and lipid will translocate during the permeabilization. Methods have been published for measuring translocation of peptides(57) and lipids(61). At equilibrium, the peptides should be equally distributed between the inner and outer monolayer of the vesicles, a prediction that is testable. It should also be possible to test this idea by creating a sudden inside out asymmetry of peptide and measuring the leakage that follows. Vesicles with entrapped probe could be made initially in the presence of peptide such that peptide is both inside and outside the vesicle and at equilibrium. Sudden removal of the external peptide (for example by an ion exchange resin, by charged vesicles, or by binding moieties such as biotin) will create an inside-out asymmetry that will lead to leakage if the mechanism involves the dissipation of transbilayer asymmetry. This model also predicts that the time period over which the peptides are added to vesicles could affect the leakage, with slower addition leading to less leakage because asymmetry dissipation could take place slowly without much leakage. On the other hand, a behavior following the equilibrium pore model should not be affected by the time over which a peptide is added.
Finally, it is possible that time-resolved experimental techniques, perhaps aided by single vesicle experiments, could be used to directly observe subtle changes in peptide structure, lipid structure, or peptide-lipid interactions that might take place during the transient leakage phase of a vesicle permeabilization experiment.
Molecular dynamics simulations to test hypotheses
While experimentalists have considered transient permeabilization for some time, the molecular dynamics community, by necessity, usually models only equilibrium processes when they explore structure function relationships in membrane permeabilizing peptides. In fact, equilibrium is a necessary requirement for a simulation to be considered high quality. We urge MD researchers to make it a goal to work towards simulating transient permeabilization events in bilayers. Peptide and lipid translocation may currently be considered too slow to simulate on atomistic MD time scales. However, great technical advances have been made in recent years towards the use of modern supercomputers for membrane simulations, and toward the application of course-grained and hybrid models that enable long time-scale simulations(62). We thus hope that important insights about molecular events will be gained through such computational studies of transient membrane permeabilization.
Acknowledgement.
We dedicate this manuscript to our friend and colleague Frances Separovic, who has inspired us and many others with her boundless devotion to exceptional science, teaching and service. We thank our many students, postdocs, and collaborators who have contributed to our knowledge of peptide-membrane interactions. Funded by NSF DMR 1709892 (K.H.) and NIH R01 GM111824 and NSF DMR 1710053 (WCW).
Footnotes
Conflict of Interest. The authors declare no conflict of interest.
References
- 1.Henderson JM, Waring AJ, Separovic F, Lee KYC, Antimicrobial Peptides Share a Common Interaction Driven by Membrane Line Tension Reduction. Biophys J 111, 2176–2189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li W, Tailhades J, O’Brien-Simpson NM, Separovic F, Otvos L Jr., Hossain MA, Wade JD, Proline-rich antimicrobial peptides: potential therapeutics against antibiotic-resistant bacteria. Amino. Acids 46, 2287–2294 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Sani MA, Whitwell TC, Gehman JD, Robins-Browne RM, Pantarat N, Attard TJ, Reynolds EC, O’Brien-Simpson NM, Separovic F, Maculatin 1.1 disrupts Staphylococcus aureus lipid membranes via a pore mechanism. Antimicrob. Agents Chemother 57, 3593–3600 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith R, Separovic F, Milne TJ, Whittaker A, Bennett FM, Cornell BA, Makriyannis A, Structure and orientation of the pore-forming peptide, melittin, in lipid bilayers. J. Mol. Biol 241, 456–466 (1994). [DOI] [PubMed] [Google Scholar]
- 5.Badani H, Garry RF, Wimley WC, Peptide entry inhibitors of enveloped viruses: the importance of interfacial hydrophobicity. Biochim. Biophys Acta 1838, 2180–2197 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leitgeb B, Szekeres A, Manczinger L, Vagvolgyi C, Kredics L, The history of alamethicin: a review of the most extensively studied peptaibol. Chem. Biodivers 4, 1027–1051 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Andersen OS, Koeppe RE, Roux B, Gramicidin channels. IEEE Trans. Nanobioscience 4, 10–20 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Pokorny A, Kilelee EM, Wu D, Almeida PF, The activity of the amphipathic peptide delta-lysin correlates with phospholipid acyl chain structure and bilayer elastic properties. Biophys. J 95, 4748–4755 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saier MH Jr., Reddy BL, Holins in bacteria, eukaryotes, and archaea: multifunctional xenologues with potential biotechnological and biomedical applications. J Bacteriol 197, 7–17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He J, Melnik LI, Komin A, Wiedman G, Fuselier T, Morris CF, Starr CG, Searson PC, Gallaher WR, Hristova K, Garry RF, Wimley WC, Ebola Virus Delta Peptide is a Viroporin. J Virol, (2017). [DOI] [PMC free article] [PubMed]
- 11.Costin JM, Rausch JM, Garry RF, Wimley WC, Viroporin potential of the lentivirus lytic peptide (LLP) domains of the HIV-1 gp41 protein. Virol. J 4, 123 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raaymakers C, Verbrugghe E, Hernot S, Hellebuyck T, Betti C, Peleman C, Claeys M, Bert W, Caveliers V, Ballet S, Martel A, Pasmans F, Roelants K, Antimicrobial peptides in frog poisons constitute a molecular toxin delivery system against predators. Nat. Commun 8, 1495 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habermann E, Bee and wasp venoms. Science 177, 314–322 (1972). [DOI] [PubMed] [Google Scholar]
- 14.Smith R, Separovic F, Bennett FC, Cornell BA, Melittin-induced changes in lipid multilayers - a solid- state NMR study. Biophys. J 63, 469–474 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa F, Teixeira C, Gomes P, Martins MCL, Clinical Application of AMPs. Adv Exp Med Biol 1117, 281–298 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Jackman JA, Costa VV, Park S, Real ALCV, Park JH, Cardozo PL, Ferhan AR, Olmo IG, Moreira TP, Bambirra JL, Queiroz VF, Queiroz-Junior CM, Foureaux G, Souza DG, Ribeiro FM, Yoon BK, Wynendaele E, De SB, Teixeira MM, Cho NJ, Therapeutic treatment of Zika virus infection using a brain-penetrating antiviral peptide. Nat. Mater 17, 971–977 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Straus SK, Hancock RE, Mode of action of the new antibiotic for Gram-positive pathogens daptomycin: comparison with cationic antimicrobial peptides and lipopeptides. Biochim. Biophys. Acta 1758, 1215–1223 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Poveda E, Briz V, Soriano V, Enfuvirtide, the first fusion inhibitor to treat HIV infection. AIDS Rev 7, 139–147 (2005). [PubMed] [Google Scholar]
- 19.Pan H, Soman NR, Schlesinger PH, Lanza GM, Wickline SA, Cytolytic peptide nanoparticles (‘NanoBees’) for cancer therapy. Wiley. Interdiscip. Rev. Nanomed. Nanobiotechnol 3, 318–327 (2011). [DOI] [PubMed] [Google Scholar]
- 20.He J, Kauffman WB, Fuselier T, Naveen SK, Voss TG, Hristova K, Wimley WC, Direct Cytosolic Delivery of Polar Cargo to Cells by Spontaneous Membrane-translocating Peptides. J Biol. Chem 288, 29974–29986 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marks JR, Placone J, Hristova K, Wimley WC, Spontaneous membrane-translocating peptides by orthogonal high-throughput screening. J. Am. Chem. Soc 133, 8995–9004 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauffman WB, Guha S, Wimley WC, Synthetic molecular evolution of hybrid cell penetrating peptides. Nat. Commun 9, 2568 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bechinger B, Towards membrane protein design: pH-sensitive topology of histidine-containing polypeptides. J. Mol. Biol 263, 768–775 (1996). [DOI] [PubMed] [Google Scholar]
- 24.Wimley WC, Hristova K, Antimicrobial Peptides: Successes, Challenges and Unanswered Questions. J. Membr. Biol 239, 27–34 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ambroggio EE, Separovic F, Bowie JH, Fidelio GD, Bagatolli LA, Direct visualization of membrane leakage induced by the antibiotic peptides: maculatin, citropin, and aurein. Biophys J 89, 1874–1881 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duzgunes N, Wilschut J, Fusion assays monitoring intermixing of aqueous contents. Methods Enzymol 220, 3–14 (1993). [DOI] [PubMed] [Google Scholar]
- 27.Ladokhin AS, White SH, ‘Detergent-like’ permeabilization of anionic lipid vesicles by melittin. Biochim. Biophys. Acta 1514, 253–260 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Wiedman G, Fuselier T, He J, Searson PC, Hristova K, Wimley WC, Highly efficient macromolecule-sized poration of lipid bilayers by a synthetically evolved peptide. J Am. Chem. Soc 136, 4724–4731 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S, Kim SY, Pittman AE, King GM, Wimley WC, Hristova K, Potent Macromolecule-Sized Poration of Lipid Bilayers by the Macrolittins, A Synthetically Evolved Family of Pore-Forming Peptides. J Am. Chem. Soc 140, 6441–6447 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Wiedman G, Kim SY, Zapata-Mercado E, Wimley WC, Hristova K, PH-Triggered, Macromolecule-Sized Poration of Lipid Bilayers by Synthetically Evolved Peptides. J. Am. Chem. Soc 139, 937–945 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pokorny A, Almeida PF, Kinetics of dye efflux and lipid flip-flop induced by delta-lysin in phosphatidylcholine vesicles and the mechanism of graded release by amphipathic, alpha-helical peptides. Biochemistry 43, 8846–8857 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Sani MA, Whitwell TC, Separovic F, Lipid composition regulates the conformation and insertion of the antimicrobial peptide maculatin 1.1. Biochim. Biophys Acta 1818, 205–211 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Anderluh G, Razpotnik A, Podlesek Z, Macek P, Separovic F, Norton RS, Interaction of the eukaryotic pore-forming cytolysin equinatoxin II with model membranes: 19F NMR studies. J Mol Biol 347, 27–39 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Schwarz G, Zong RT, Popescu T, Kinetics of melittin induced pore formation in the membrane of lipid vesicles. Biochim. Biophys. Acta 1110, 97–104 (1992). [DOI] [PubMed] [Google Scholar]
- 35.Schwarz G, Robert CH, Kinetics of pore-mediated release of marker molecules from liposomes or cells. Biophys. Chem 42, 291–296 (1992). [DOI] [PubMed] [Google Scholar]
- 36.Kim SY, Pittman AE, Zapata-Mercado E, King GM, Wimley WC, Hristova K, Mechanism of Action of Peptides That Cause the pH-Triggered Macromolecular Poration of Lipid Bilayers. J Am Chem Soc 141, 6706–6718 (2019). [DOI] [PubMed] [Google Scholar]
- 37.White SH, Wimley WC, Ladokhin AS, Hristova K, Protein folding in membranes: Determining the energetics of peptide-bilayer interactions. Methods Enzymol 295, 62–87 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Hristova K, Selsted ME, White SH, Critical role of lipid composition in membrane permeabilization by rabbit neutrophil defensins. J. Biol. Chem 272, 24224–24233 (1997). [DOI] [PubMed] [Google Scholar]
- 39.Wimley WC, Selsted ME, White SH, Interactions between human defensins and lipid bilayers: Evidence for the formation of multimeric pores. Protein Sci 3, 1362–1373 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lau QY, Li J, Sani MA, Sinha S, Li Y, Ng FM, Kang C, Bhattacharjya S, Separovic F, Verma C, Chia CSB, Elucidating the bactericidal mechanism of action of the linear antimicrobial tetrapeptide BRBR-NH2. Biochim Biophys Acta Biomembr 1860, 1517–1527 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Rathinakumar R, Wimley WC, Biomolecular engineering by combinatorial design and high-throughput screening: small, soluble peptides that permeabilize membranes. J. Am. Chem. Soc 130, 9849–9858 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y, Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem. J 341, 501–513 (1999). [PMC free article] [PubMed] [Google Scholar]
- 43.Lee CC, Sun Y, Qian S, Huang HW, Transmembrane pores formed by human antimicrobial peptide LL-37. Biophys. J 100, 1688–1696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krauson AJ, He J, Wimley WC, Determining the mechanism of membrane permeabilizing peptides: Identification of potent, equilibrium pore-formers. Biochim. Biophys. Acta 1818, 1625–1632 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wimley WC, How Does Melittin Permeabilize Membranes? Biophys J 114, 251–253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krauson AJ, He J, Wimley WC, Gain-of-Function Analogues of the Pore-Forming Peptide Melittin Selected by Orthogonal High-Throughput Screening. J. Am. Chem. Soc 134, 12732–12741 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z, Choi H, Weisshaar JC, Melittin-induced Permeabilization, Re-sealing, and Re-permeabilization of E. coli Membranes. Biophys J XXX, ZZZ-Z (2017). [DOI] [PMC free article] [PubMed]
- 48.Fiser R, Konopasek I, Different modes of membrane permeabilization by two RTX toxins: HlyA from Escherichia coli and CyaA from Bordetella pertussis. Biochim. Biophys Acta 1788, 1249–1254 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Rausch JM, Marks JR, Rathinakumar R, Wimley WC, Beta-sheet pore-forming peptides selected from a rational combinatorial library: mechanism of pore formation in lipid vesicles and activity in biological membranes. Biochemistry 46, 12124–12139 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gregory SM, Pokorny A, Almeida PF, Magainin 2 Revisited: A Test of the Quantitative Model for the All-or-None Permeabilization of Phospholipid Vesicles. Biophys. J 96, 116–131 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gregory SM, Cavenaugh A, Journigan V, Pokorny A, Almeida PF, A quantitative model for the all-or-none permeabilization of phospholipid vesicles by the antimicrobial peptide cecropin A. Biophys. J 94, 1667–1680 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wheaten SA, Lakshmanan A, Almeida PF, Statistical analysis of peptide-induced graded and all-or-none fluxes in giant vesicles. Biophys J 105, 432–443 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park S, Jackman JA, Cho NJ, Comparing the Membrane-Interaction Profiles of Two Antiviral Peptides: Insights into Structure-Function Relationship. Langmuir 35, 9934–9943 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Braun S, Pokorna S, Sachl R, Hof M, Heerklotz H, Hoernke M, Biomembrane Permeabilization: Statistics of Individual Leakage Events Harmonize the Interpretation of Vesicle Leakage. ACS Nano 12, 813–819 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Hovakeemian SG, Liu R, Gellman SH, Heerklotz H, Correlating antimicrobial activity and model membrane leakage induced by nylon-3 polymers and detergents. Soft Matter 11, 6840–6851 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heerklotz H, Membrane stress and permeabilization induced by asymmetric incorporation of compounds. Biophys J 81, 184–195 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fuselier T, Wimley WC, Spontaneous Membrane Translocating Peptides: The Role of Leucine-Arginine Consensus Motifs. Biophys J 113, 835–846 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hristova K, Kenworthy AK, McIntosh TJ, Effect of bilayer composition on the phase behavior of liposomal suspensions containing poly(ethylene glycol)-lipids. Macromolecules 28, 7693–7699 (1995). [Google Scholar]
- 59.Kenworthy AK, Hristova K, Needham D, McIntosh TJ, Range and magnitude of the steric pressure between bilayers containing phospholipids with covalently attached poly(ethylene glycol). Biophys. J 68, 1921–1936 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wimley WC, Determining the Effects of Membrane-Interacting Peptides on Membrane Integrity. Methods Mol. Biol 1324, 89–106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Devaux PF, Lipid transmembrane asymmetry and flip-flop in biological membranes and in lipid bilayers. Cur. Opinion Struc. Biol 3, 489–494 (1993). [Google Scholar]
- 62.Wang Y, Chen CH, Hu D, Ulmschneider MB, Ulmschneider JP, Spontaneous formation of structurally diverse membrane channel architectures from a single antimicrobial peptide. Nat Commun 7, 13535 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]