Abstract
A 48 kDa, chitin-binding lectin with antifungal, antiviral and apoptosis-inducing activities was isolated from the rhizomes of Setcreasea purpurea Boom, a member of family Commelinaceae. Setcreasea purpurea lectin (designated as SPL) is a homotetrameric protein consisting of 12031.9 Da subunits linked by non-covalent bonds as determined by SDS-PAGE, gel filtration and MS. The N-terminal 25 amino-acid sequence of SPL, NVLGRDAYCGSQNPGATCPGLCCSK was determined and homology analysis suggested that SPL belongs to the family of chitin-binding plant lectins composed of hevein domains. The lectin exhibited strong hemagglutinating activity towards rabbit erythrocytes at 0.95 μg/ml and the activity could be reversed exclusively by chitin hydrolysate (oligomers of GlcNAc). Its hemagglutinating activity was stable in pH range of 2.0–9.0 and it showed excellent thermal tolerance. SPL showed antifungal activity against Rhizoctonia solani, Sclerotinia sclerotiorum, Penicillium italicum and Helminthosporiun maydis. It also exhibited inhibitory effect on HIV-1 (IIIB) and HIV-2 (ROD), with an EC50 of 13.8 ± 1.3 and 57.1 ± 15 μg/ml, respectively. Subsequently, MTT method, cell morphological analysis and LDH activity-based cytotoxicity assays demonstrated that SPL was highly cytotoxic to CNE-1 cells and induced apoptosis in a dose-dependent manner. Moreover, due to the caspase inhibitors analyses, caspase was also found to play an important role in the potential apoptotic mechanism of SPL.
Keywords: Setcreasea purpurea lectin, Chitin-binding specificity, Thermostability, Antifungal activity, Antiviral activity, Apoptosis-inducing activity
1. Introduction
Lectins are carbohydrate-binding proteins that bind carbohydrates reversibly and possess the ability to agglutinate cells or precipitate polysaccharides and glycoconjugates, occurring in plants, animals, bacteria, viruses and fungi [1], [2]. According to their molecular structure and evolutionary relationships, plant lectins can be subdivided into seven different families, which include the legume lectins, type-2 ribosome-inactivating proteins, the amaranthin family, the Cucurbitaceae phloem lectins, monocot mannose-binding lectins, jacalin-like lectins and chitin-binding lectins [3]. And they have attracted great interest on account of their various biological activities, such as cell agglutination, antiviral [4], antifungal [5], antineoplastic [6] and anti-insect activities [7].
Chitin is a naturally occurring polysaccharide composed of β (1 → 4) linked N-acetylglucosamine (GlcNAc) monomers that form a complex supermolecular structure [8]. The family of chitin-binding lectins comprises all lectins possessing chitin-binding and/or GlcNAc/GlcNAc oligomer activities and containing at least one hevein domain. The term ‘hevein domain’ refers to hevein, a small 43 amino-acid protein from the latex of the rubber tree [3]. It should be noted that there are also chitin-binding lectins without hevein domain(s). For example, the Cucurbitaceae phloem lectins are a small family of chitin-binding agglutinins only found in the phloem exudate of Cucurbitaceae species and show no sequence similarity to the hevein-like chitin-binding lectins [3]. Since the first hevein-like chitin-binding lectin, WGA (wheat germ agglutinin) from Triticum aestivum L. was reported in 1972 [9], [10], more chitin-binding lectins have been found. The lectins are widespread in the Gramineae and Solanaceae species and also found in roots and leaves of Phytolacca sp. seeds and rhizomes of Urtica dioica, seeds of Chelidonium majus, and in green tissues of Viscum album [3].
Many of the lectins have shown antifungal activity against phytopathogenic species, as chitin is the key component of the cell wall of these microorganisms. They also have shown to affect fungal growth and development, disturbing the synthesis and/or deposition of chitin in the cell wall [11], [12]. The chitin-binding lectins have been reported to possess antiviral and antineoplastic activities as well, such as the anti-HIV activity of UDA (Urtica dioica lectin), the antitumor activity of WGA (Wheat germ agglutinin) [13], [14].
Setcreasea purpurea Boom is a member of Commelinaceae family which is mainly distributed in tropical countries. Many plants of Commelinaceae family have been used as traditional medicines. Setcreasea purpurea, a native of Mexico, is an ornamental plant that widely used in hanging baskets and container gardens for its beautiful flowers and purple leaves. However, there is a dearth of information about the bioactive proteins of Setcreasea purpurea. Herein, we purified a chitin-binding lectin (SPL) from rhizomes of Setcreasea purpurea and characterized some significantly biological characteristics, especially its chitin-specificity, antifungal, antiviral and antitumor activities. These results would provide new evidence for understanding more significant biological implications of SPL in further investigations.
2. Materials and methods
2.1. Plant materials
The rhizomes of Setcreasea purpurea were collected from the campus of Sichuan University (Chengdu, China).
2.2. Chemicals and reagents
CNE-1 cell lines were provided by Chengdu Kanghong Pharmaceuticals Group. l-Glutamine, penicillin and streptomycin were supplied by GIBCO (Grand Island, NY). The host cell lines and the tested viruses were obtained from Rega Institute for Medical Research, Katholieke Universiteit Leuven. DEAE-Sepharose, CM-Sepharose, Sephacryl S-100 and standard molecular weight markers were procured from Pharmacia (Pharmacia, Uppsala, Sweden). d-Galactose, d-glucose, maltose, fetuin, lactose, arabinose, sucrose, d-fructose, chitin, d-mannose, mannan, 2-methyl-d-glucoside, N-acetygalactosamine (GalNAc), N-acetylglucosamine (GlcNAc), N-acetyllactosamine, thyroglobulin, ovomucoid, z-DEVD-fmk, z-IETD-fmk, z-VAD-fmk and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (St. Louis, USA). ABO blood group erythrocytes were obtained from healthy donors while fresh rabbit blood cells were obtained from the local market. The tested fungi Sclerotinia sclerotiorum, Aspergillus niger, Penicillium italicum, Helminthosporiun maydis, Candida albicans, Trichoderma reesei and Rhizoctonia solani were obtained from the microbiology laboratory of Sichuan University. They were cultured at 28 °C according to the China catalogue of cultures [15].
2.3. Purification of SPL
Three hundred grams of fresh Setcreasea purpurea rhizomes were crushed and soaked in 600 ml of 0.145 M NaCl buffer overnight at 4 °C before filtered through muslin cloth. The filtrate was centrifuged at 9,000 × g for 30 min and the resulting supernatant obtained was dialyzed against the 0.02 M Tris–HCl buffer (pH 8.0) at 4 °C for 24 h. Then the crude extract was loaded on a column of DEAE-Sepharose (1.6 cm × 20 cm) preliminarily equilibrated with the buffer (0.02 M Tris–HCl, pH 8.0) and eluted with a linear gradient of NaCl of 0–0.5 M in the same buffer. Fractions showing hemagglutinating activity were pooled and dialyzed against 0.02 M sodium acetate buffer (pH 4.6) before applied to a CM-Sepharose (1.6 cm × 20 cm) column and eluted with a linear NaCl gradient (0–0.5 M) in the same buffer. Then active fractions were concentrated to 3 mg/ml and loaded on a Sephacyl S-100 column (2.0 cm × 100 cm) equilibrated with 0.02 M PBS (pH 7.0). The purified lectin, designated as SPL, was obtained by elution from the column with 0.02 M PBS (pH 7.0) before dialyzed against water and lyophilized.
2.4. Determination of molecular mass
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out using 15% (W/V) acrylamide in gels as described by Laemmli and Favre [16]. The samples were dissolved in electrophoresis buffer containing SDS with or without 5% β-mercaptoethanol. Protein bands were visualized by Coomassie Brilliant Blue R-250. Native molecular mass of the lectin was determined by gel-filtration chromatography on the same Sephacryl S-100 column according to the method of Whitakar [17] and Andrews [18].
Matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrum was obtained using a Voyager-RP mass spectrometer (PerSeptive Bio-systems) according to the method of Woo et al. [19].
2.5. In-gel tryptic digestion
In-gel digestion of SPL was carried out using mass spectrometry grade Trypsin Gold (Promega, Madison, WI) according to the manufacturer's instructions. Briefly band of SPL was excised out of the SDS-PAGE gel stained with Coomassie Brilliant Blue R-250 (Merck) and cut into small pieces, and then destained twice with 100 mM NH4HCO3, 50% acetonitrile (ACN) at 37 °C for 45 min. After dehydration with 100% ACN and drying, the gel was preincubated in 10–20 μl of trypsin solution (10 ng/μl) for 1 h. Sufficient digestion buffer (40 mM NH4HCO3, 10% ACN) was then added to cover the gel, which was incubated and mildly shaken overnight at 37 °C (12–14 h). Tryptic digests were extracted using Milli-Q water followed by double extraction with 50% ACN, 5% TFA for 1 h each time. The combined extracts were dried in a SpeedVac concentrator (Thermo Scientific) at 4 °C. The samples were then subjected to mass spectrometry analysis.
2.6. MALDI-TOF MS analysis and protein identification
Mass spectra were performed by using a Q-TOF mass spectrometer (Micromass, Manchester, UK) fitted with a MALDI source (Micromass). Matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-Q-TOF) analysis the tryptic fragments of SPL was performed as described previously [20]. The Peptide mass fingerprintings of SPL was acquired and processed using MassLynx V 4.1 software (Micromass) and was converted to PKL files by the ProteinLynx 2.2.5 software (Waters). The PKL file was analyzed using the MASCOT search engine (http://www.matrixscience.com). The search parameters were defined as follows: Database, Swiss-Prot; taxonomy, Green plants; enzyme, trypsin; allowance of one missed cleavage. Carbamidomethylation was selected as a fixed modification and oxidation of methionine was allowed to be variable. The peptide and fragment mass tolerance was set at 0.1 for MS.
2.7. The N-terminal amino-acid sequence analysis
Following tricine-SDS-PAGE, the lectin was transferred to a polyvinylidene difluoride (PVDF) membrane (BIO-RAD) stained with coomassie brilliant blue R-250. Band corresponding to this lectin was excised from the membrane. Then N-terminal amino-acid sequence was determined by using a Hewlett-Packard HP G1000A Edman degradation unit and an HP 1000 HPLC System [21]. BLASTp method from NCBI and manually data mining were both used to search its homology sequence [22].
2.8. Protein determination
Protein concentration was determined as described by Lowry et al. [23], using crystalline bovine serum albumin (BSA) as a standard.
2.9. Assay for hemagglutinating activity
Red blood cells were prepared from either rabbit or human blood and treated as described previously [24]. Hemagglutinating activity of SPL was determined in 96-well microtiter U plates by the method of serial double dilution method using 2% suspension of rabbit erythrocytes. Lectin (25 μl) was serially diluted twofold in 0.15 M NaCl, and an equal volume of erythrocytes in suspension was added to the microtiter plates. The mixture was incubated for 1 h at room temperature before the plate read. The hemagglutination titer, defined as the reciprocal of the highest dilution exhibiting hemagglutination, was defined as one hemagglutination unit. The specific hemagglutinating activity was defined as unit/mg protein [25]. Specificity of the lectin to human (groups A, B and O) erythrocytes was performed in a similar manner.
2.10. Preparation of chitin hydrolysate
Chitin hydrolysis was carried out as decribed by Rupley [26] and the partially purified chitin hydrolysate was tested with SPL.
2.11. Carbohydrates-binding specificity
For hemagglutinating inhibition tests, serial twofold dilutions of sugar samples were prepared in phosphate-buffered saline. All dilutions were mixed with 25 μl of SPL with two units. The mixture was allowed to stand for 30 min at room temperature and mixed with an equal volume of 2% rabbit erythrocyte suspension. Sugars or their derivatives were tested at concentration of 160 mM while polysaccharides and glycoproteins at concentration of 4 mg/ml. All the experimentally tested simple sugars, oligosaccharides, polysaccharides or glycoproteins were d-galactose, d-glucose, maltose, fetuin, lactose, arabinose, sucrose, d-fructose, chitin, d-mannose, mannan, 2-methyl-d-glucoside, N-acetygalactosamine (N-GalNAc), N-acetylglucosamine (N-GlcNAc), N-acetylactosamine, thyroglobulin, and ovomucoid.
2.12. Effect of temperature and pH on hemagglutinating activity of SPL
To determine the thermal stability, SPL was dissolved in phosphate-buffered saline (PBS, 0.15 M, pH 7.0) at 1 mg/l and treated at 30–100 °C for 30 min. The samples were then cooled down to room temperature immediately in ice water.
In order to determine the pH stability of SPL, buffers with pH ranging from 2.0 to 12.0 were used as follows: sodium citrate buffer for pH 2.0–5.0, monobasic phosphate buffer for pH 6.0–8.0, carbonate/bicarbonate buffer for pH 9.0–11.0 and KCl/NaOH buffer for pH 12.0 (all 0.1 M). A volume of 50 μl of lectin solution (100 μg) was incubated with 50 μl of buffer for 1 h at room temperature. The samples were adjusted to pH 7.0 and the residual hemagglutinating activity was measured.
2.13. Metal ion requirement
Demetallization of purified lectin was performed by the method of Paulova et al. [27]. The activity in normal and demetallized samples was compared by the hemagglutination assay.
2.14. Assay of antifungal activity of SPL
The assay for antifungal activity was performed by adding SPL (1.51 mg/ml, PBS, 0.02 M, pH 7.0) to a solution with approximately 1 × 105 spores/ml in 24-well microplate and PDA medium (final volume of 500 μl), followed by a twofold serially dilution. The microplate was incubated for 72 h at 28 °C and the mycelia growth inhibition was observed visually and compared with control microcultures containing only spores of fungi and medium [28]. The antifungal activity was also evaluated using Oxford cups (6 cm × 10 mm) on PDA plates, where fungal mycelia were placed over the solid PDA [29]. The cups were put uprightly on the upper layer agar and filled with solutions of SPL (approx 1.51 mg/ml, PBS, 0.02 M, pH 7.0) treated with different temperatures (room temperature 25, 40, 60, 75, and 95 °C, respectively, 20 min). The PBS buffer was chosen as control. Incubation of the plates was carried out at 28 °C for 72 h. Seven fungal species, Sclerotinia sclerotiorum, Aspergillus niger, Penicillium italicum, Helminthosporiun maydis, Candida albicans, Trichoderma reesei and Rhizoctonia solani were examined.
2.15. Antiviral assays
Using previously established procedures [30], the in vitro antiviral activities of SPL were determined against a variety of DNA and RNA viruses, and their cytotoxicities for the host cell lines were assayed in parallel with those of standard drugs with known antiviral activities. The viruses and cells used were herpes simplex virus type 1 (HSV-1) (strain KOS), herpes simplex virus type 2 (HSV-2) (strain G), vesicular stomatitis virus and thymidine-kinase-deficient HSV-1 (TK−) (strain KOS) in HEL cells; Vesicular stomatitis virus, respiratory syncytial virus in HeLa cells; influenza A (H1N1 and H3N2 subtypes) and influenza B virus in Madin Darby canine kidney (MDCK) cells; para-influenza virus type 3, reovirus type 1, sindbis virus, coxsackie B4 virus and Punta Toro virus in Vero cells; HIV-1 (IIIB) and HIV-2 (strain ROD) in human T-lymphocyte (CEM) cells (at compound concentrations up to 100 μg/ml). The antiviral activities were determined as percent inhibition of microscopically visible virus-induced cytopathicity.
2.16. Inhibition of CNE-1 cell growth
Assays were performed as reported by Mosmann [31]. CNE-1 cells (1 × 105 cells/ml) in exponential growth phase were seeded independently into each well of a 96-well culture plate with final volume 100 μl containing 1 × 104 cells per well. These plates were incubated at 37 °C for 24 h. And then various concentrations of SPL were added. After another 24 h, 0.05 mg (10 μl of 5 mg/ml) MTT was added to each well and incubated at 37 °C for 4 h. The absorbance of the samples was measured at 570 nm with a spectrophotometer [Model 3550 Microplate Reader (BIO-RAD)] [32]. The percentage of cell growth inhibition was calculated as follows:
2.17. Lactate dehydrogenase (LDH) activity-based cytotoxicity assays
LDH activity was assessed using a standardized kinetic determination kit (Zhongsheng LDH kit, Beijing, China). LDH activity was measured in both floating dead cells and viable adherent cells. The floating cells were collected from the culture medium by centrifugation (240 × g, 4 °C, 5 min) and the LDH content from the pellets was used as an index of apoptotic cell death (LDHp) [33]. The LDH released in the culture supernatant (extracellular LDH, or LDHe), was used as an index of necrotic death, and the LDH present in the adherent viable cells as intracellular LDH (LDHi). The percentage of apoptotic and necrotic cell death was calculated as follows:
2.18. Observations of cell morphological changes
The CNE-1 cells seeded into 96-well culture plates were incubated for 24 h. The cells were treated with 0.05% DMSO and SPL (0.5 pM) was added to the cells. Then the cellular morphology was observed using phase contrast microscopy (Leica, Wetzlar, Germany). Apoptotic nuclear morphology was assessed using PI staining. The cells being treated with or without SPL were fixed with 3.7% paraformaldehyde for 30 min at room temperature before washed and stained with 50 μg/ml PI at 37 °C for 30 min. All the cells were washed and suspended again, respectively, in PBS for morphological observation by a fluorescent microscope (Olympus, Tokyo, Japan).
2.19. The effect of inhibitors on SPL-induced CNE-1 cell death
CNE-1 cells were seeded at a density of 1 × 105 cell/well into 96-well culture plate. After 24 h incubation, the cells were treated with or without general caspase inhibitor (z-VAD-fmk), caspase-3 inhibitor (z-DEVD-fmk), and caspase-8 inhibitor (z-IETD-fmk) at given concentrations for 1 h, and then treated with SPL for 24 h. MTT assay was determined as described above.
2.20. Statistical analysis
All the presented data and results were confirmed in at least three independent experiments. The data are expressed as means ± SD. Statistical comparisons were made by Student's t-test. P < 0.05 was considered statistically significant.
3. Results
3.1. Purification of Setcreasea purpurea lectin
SPL was purified by a combination of ion exchange and gel chromatography. The crude protein extract was applied to a diethylaminoethyl-Sepharose column which was preliminarily equilibrated with 20 mM Tris–HCl (pH 8.0) after dialysis against the same buffer. Four adsorbed peaks were eluted with a linear 0–0.5 M NaCl gradient from the column, but only A3 was detected with hemagglutinating activity (Fig. 1A). A3 was then loaded on the carboxymethyl-Sepharose column and three peaks were eluted with a linear 0–0.5 M NaCl gradient from the column (Fig. 1B). B2 showed strong agglutinating activity, however, low hemagglutinating activity was also detected in B3. The mixture of B2 and B3 was resolved into two peaks, C1 and C2, on gel-filtration Sephacryl S-100. The activity was observed only on C2 that was collected and represented purified lectin (Fig. 1C). As shown in Table 1 , there was approximately a 12.7-fold increase in specific hemagglutinating activity from the crude extract to the purified lectin.
Fig. 1.
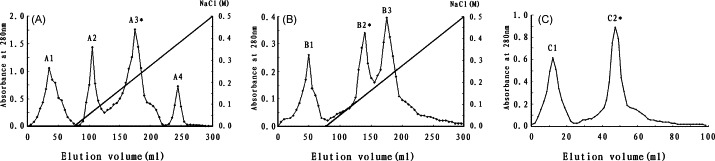
(A) Anion exchange chromatography of the crude extract. The adsorbed (A3) fraction with hemagglutinating activity was eluted with 0–0.5 M NaCl gradient at a flow rate of 2 ml/min. (B) Cation exchange chromatography of the active fraction A3 from the DEAE-Sepharose column on the CM-Sepharose column. The adsorbed (B2,B3) fraction with hemagglutinating activity was eluted with 0–0.5 M NaCl gradient at a flow rate of 2 ml/min. (C) Gel filtration of fraction B2 and B3 on Sephacryl S-100 column pre-equilibrated with 0.02 M PBS, pH 7.0 (a flow rate of 45 ml/h). The elution profiles were monitored at 280 nm.
Table 1.
Specific hemagglutinating activities and yields of chromatographic fractions obtained at different steps of purification of lectin.
| Steps | Total protein (mg) | Specific hemagglutinating activity (HU/mg)a | Total hemagglutination activity (HU) | Recovery of activity (%) | Purification fold |
|---|---|---|---|---|---|
| Crude extract | 420 | 24 | 10080 | 100 | 1 |
| DEAE-Sepharose | 155 | 58 | 8990 | 89.0 | 2.4 |
| CM-Sepharose | 33 | 221 | 7293 | 72.4 | 9.2 |
| SephacrylS-100 | 21 | 305 | 6405 | 63.5 | 12.7 |
The calculations represent the data for 300 g of rhizomes.
Specific activity is defined as the hemagglutinating unit (HU) divided by the protein concentration (mg/ml) of the assay solution. 2% rabbit erythrocytes were used for the assay.
3.2. Determination of molecular mass of SPL
SPL showed a single band of approximately 12 kDa on SDS-PAGE with the presence or absence of 2-mercaptoethanol (Fig. 2A) and gel-filtration chromatography of the lectin gave a symmetrical single peak corresponding to an apparent molecular mass of 48 kDa (Fig. 2B). The mass spectrometry analysis appeared as a peak corresponding to m/z 12031.9 Da (Fig. 2C). These results suggested that SPL is a tetramer consisted of four identical subunits of 12031.9 Da, which are not held by disulphide but non-covalent bonds.
Fig. 2.
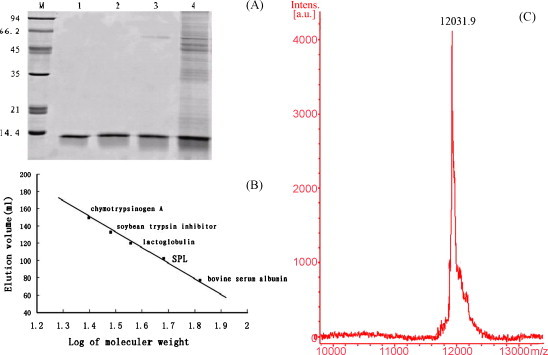
(A) SDS-PAGE pattern of SPL. Lane M: molecular mass markers, from top to bottom: Phosphorylase b (94 kDa); albumin bovine (66.2 kDa); ovalbumin (45 kDa); carbonic anhydrase (35 kDa); trypsin inhibitor (21 kDa) and alactalbumin (14.4 kDa): (lane 1) in the absence of 2-mercaptoethanol; (lane 2) in the presence of 2-mercaptoethanol; (lane 3) fraction B2 and B3 from CM-Sepharose; (lane 4) fraction A3 from DEAE-Sepharose. (B) Native molecular mass estimation of SPL on Sephacryl S-100 gel-filtration chromatography column. Standards used for gel-filtration analysis were bovine serum albumin (68 kDa), lactoglobulin (36 kDa), soybean trypsin inhibitor (30.2 kDa) and chymotrypsinogen A (25 kDa). (C) Molecular mass determination by MALDI-TOF.
3.3. Hemagglutinating activity
SPL exhibited a high specific agglutination activity when assayed with rabbit erythrocytes. Indeed, the minimal concentration required for agglutination rabbit blood cells was as low as 0.95 μg/ml, possessing a stronger hemagglutinating activity when compared with some typical chitin-binding lectins, e.g. UDA (Urtica dioica lectin) and CMA (Chelidonium majus lectin). However, human A-, B-, and O-types of erythrocytes were poorly agglutinated by SPL, even at lectin concentrations as high as 500 μg/ml (Table 2 ).
Table 2.
Comparison of SPL with other chitin-binding lectins on the hemagglutinating activity and carbohydrate-binding specificity.
| Lectin | Specific agglutination activity towards rabbit erythrocytes (μg/ml) | Carbohydrate-binding specificity |
|---|---|---|
| SPL | 0.95 | (GlcNAc)n |
| WGA | 0.4 | GlcNAc/(GlcNAc)n |
| UDA | 2.5 | (GlcNAc)n |
| Hevein | N | (GlcNAc)n |
| VisalbCBA | 10 | GlcNAc/(GlcNAc)n |
| CMA | 7.5 | (GlcNAc)n |
3.4. Carbohydrate-binding specificity
The carbohydrate-binding specificity of SPL was determined in details by inhibition assays of the agglutination. Of all the simple sugars, oligosaccharides, polysaccharides or glycoproteins, which were used for the assays, only the chitin hydrolysate (oligomers of GlcNAc) had a strong inhibitory effect on the agglutinating activity of SPL, with a minimum sugar concentrations of 0.3125 mg/ml for complete inhibition of the activity (4 hemagglutination units). However, unlike some chitin-binding lectins, SPL exhibited no specificity towards GlcNAc (Table 2).
3.5. The identification of SPL by mass spectrometry and database search
The peptide mass fingerprinting of SPL was obtained by MALDI-Q-TOF MS analysis (Fig. 3A). Then, MS profile of SPL was analyzed by using the MASCOT search engine against the Swiss-Prot protein database. For all the queries, no value of MOWSE score was higher than that indicates homology (data not shown). These results showed that SPL matched with no protein (or lectin) from database searching.
Fig. 3.
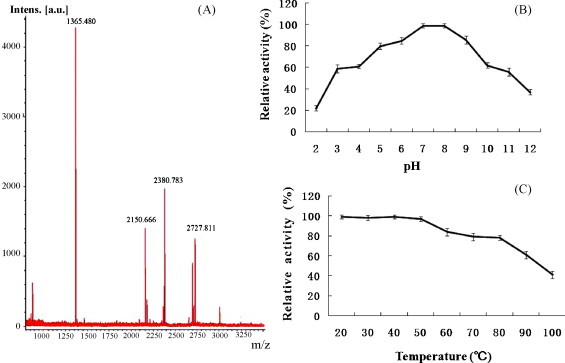
(A) Peptide mass fingerprint of the tryptic digests of SPL. (B) Thermal stability of SPL on its hemagglutinating activity. (C) pH stability of SPL on its hemagglutinating activity.
3.6. The N-terminal amino-acid sequence analysis
To further identify SPL, the N-terminal amino-acid sequence analysis was performed. The N-terminal 25 amino-acid sequence of SPL was NVLGRDAYCGSQNPGATCPGLCCSK. A comparison of the N-terminus of SPL to some typical hevein-like chitin-binding lectins showed that SPL, from residues 9 to 25, exhibited a homology to these chitin-binding lectins both in terms of amino-acid sequences and highly conserved cysteine residues (Fig. 4 ). Although a few gaps have to be created between these lectins, the resemblances revealed by the alignment indicated that SPL belongs to the chitin-binding plant lectin family.
Fig. 4.

The alignment was refined manually to maximize sequence conservation for each individual sequence. Alignment of selected amino-acid sequences of the chitin-binding lectins: UDA (Urtica dioica lectin) [38]; hevein: a chitin-binding protein [36]; WGA (Wheat germ agglutinin) [39]; VisalbCBA (Viscum album lectin) [36]. Gaps are represented by dashes. The identical or conserved residues in all sequences in the alignment were boxed. “:” indicates semi-conserved substitutions.
3.7. Effect of temperature, acid, and alkali on lectin-induced hemagglutination
The effects of pH and temperature on hemagglutinating activity and stability are shown in Fig. 3B and C. The hemagglutinating activity of SPL was stable up to 80 °C. However, only 40% hemagglutination activity was lost at 90 °C for 30 min. Even after boiling the lectin solution for 30 min, SPL still showed activity at 62.5 μg/ml. SPL was quite stable from pH 5.0 to 9.0, and still retains 20% and 40% agglutination activity even at pH 2 and 12, respectively. The optimal pH and thermostability were quite similar to other chitin-binding proteins [28]. The hemagglutinating activity of SPL was not affected by EDTA or by the addition of 1 mM Na+, K+, Ca2+, Cu2+, Mg2+, Mn2+ or Fe3+ in the agglutination assay after demetallization.
3.8. Assay of antifungal activity
In the inhibition assays performed in 24-well microplate, SPL was in contact with approx 1 × 105 spores/ml of each fungi and the lectin was devoid of antifungal activity against Candida albicans, Aspergillus niger and Trichoderma reesei even up to1.51 mg/ml. But this lectin inhibited the germination of Rhizoctonia solani, Penicillium italicum, Sclerotinia sclerotiorum and Helminthosporiun maydis at a minimum concentration of 48.1, 48.1, 96.2 and 96.2 μg/ml, respectively.
In the inhibition assays performed on agar plates, each SPL solution was treated with different temperatures. The result showed the treated SPL also inhitited normal growth of hyphaes of Rhizoctonia solani, Penicillium italicum, Sclerotinia sclerotiorum and Helminthosporiun maydis to varying degrees, by preventing the mycelias from producing spores, resulting in sterile fungus around the Oxford cups (Fig. 5 ). The antifungal activity of SPL appeared to be heat-resistant, as its inhibitory effects on the four fungal species were not significantly affected when SPL was treated with 60 °C for 20 min and they were also not eradicated by a treatment at 75 °C, especially for Rhizoctonia solani and Penicillium italicum, which was further indicating that SPL was a steady protein with thermal tolerance (Fig. 5).
Fig. 5.
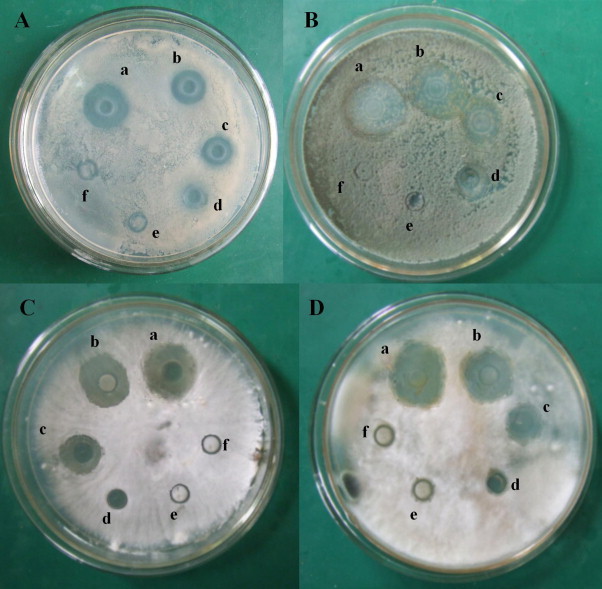
Inhibitory activity of SPL towards four fungi (A) Rhizoctonia solani, (B) Penicillium italicum, (C) Sclerotinia sclerotiorum, and (D) Helminthosporiun maydis: (a) 1.51 mg/ml of SPL in 0.02 M PBS buffer (pH 7.0), room temperature (25 °C); (b) 1.51 mg/ml of SPL in 0.02 M PBS buffer (pH 7.0), treated with 40 °C, 20 min; (c) 1.51 mg/ml of SPL in 0.01 M PBS buffer (pH 7.0), treated with 60 °C, 20 min; (d) 1.51 mg/ml of SPL in 0.02 M PBS buffer (pH 7.0), treated with 75 °C, 20 min; (e) 1.51 mg/ml of SPL in 0.02 M PBS buffer (pH 7.0), treated with 95 °C, 20 min; (f) 0.02 M PBS buffer (pH7.0), room temperature (25 °C).
3.9. Antiviral activity of SPL in vitro
To measure the inhibitory effect on the viral cytopathogenic effects (CPE) in cell culture, SPL was exposed to different cell cultures that were infected by a variety of DNA and RNA viruses. However, none of the viruses were inhibited at subtoxic concentrations. Only the replication of HIV-1 (IIIB) and HIV-2 (ROD) were inhibited by SPL in CEM cell cultures at EC50 (μg/ml) values of 13.8 ± 1.3 and 57.1 ± 15, respectively. It was also found that SPL showed a relatively low cytotoxicity to the uninfected control cells (Table 3 ).
Table 3.
Anti-HIV-1 and -HIV-2 activity and cytostatic properties of SPL in human T-lymphocyte (CEM) cells.
| Compound | EC50 (μg/ml) |
CC50 (μg/ml) | |
|---|---|---|---|
| HIV-1 | HIV-2 | ||
| SPL | 13.8 ± 1.3 | 57.1 ± 15 | >100 |
| UDA | 1.0 | – | 50 |
| PCL | 0.05 | 0.10 | 74 |
| CML | 11 ± 3.9 | 71 ± 41 | >100 |
| GNA | 0.92 | 0.78 | 6.9 |
| LRA | 0.79 | 0.59 | 7.1 |
PCL, Polygonatum cyrtonema Hua lectin [40]; UDA, Urtica dioica agglutinin [41]; CML, Clematis montana lectin [42]; GNA, Galanthus nivalis agglutinin [42]; LRA, Lycoris radiate agglutinin [42]. EC50 = effective concentration or concentration required to protect CEM cells against the cytopathogenicity of HIV by 50%. CC50 = cytotoxic concentration or concentration required to reduce CEM cell viability by 50%.
3.10. Growth inhibition of CNE-1 cells
Inhibition of cell proliferation by SPL was measured by MTT assay. It was found that SPL-induced CNE-1 cell (a well differentiated human nasopharyngeal carcinoma cells) death in a dose-dependent manner and the lectin from 0.5 × 10−4 to 0.5 pM showed a potent inhibitory effect on the growth of CNE-1 cells. The inhibition ratio reached 50% when CNE-1 cells were incubated with SPL (0.5 pM) for 24 h (Fig. 6A). Therefore, 24 h incubation with 0.5 pM SPL is sufficient for half inhibition of cell growth (IC50).
Fig. 6.
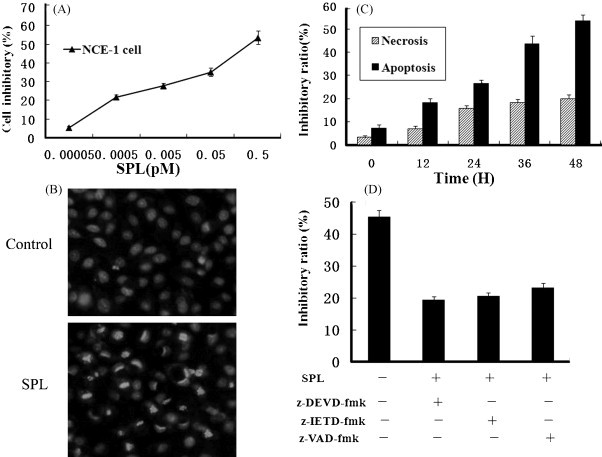
In vitro anti-proliferative effect of SPL in CNE-1 cell: (A) the cells were cultured for different concentrations of SPL. SPL-induced CNE-1 cell apoptosis. The cell death rate was measured by MTT assay. (B) Morphological observation of CNE-1 cells. The cellular morphologic changes were observed after the cells were incubated without SPL, or cultured with 0.5 pM SPL for 24 h. (C) Induction of CNE-1 cell death was characterized by apoptosis and necrosis. Cells treated with various doses of SPL for 24 h were measured by LDH activity-based assay. (D) Effects of inhibitors on SPL-induced CNE-1 cell death. CNE-1 cells were cultured in the absence or presence of z-VAD-fmk (20 μmol/l), z-DEVD-fmk (20 μmol/l) or z-IETD-fmk (20 μmol/l) 1 h prior to the addition of SPL, then incubated for 24 h. n = 5; mean ± S.D.
3.11. Morphological observation
After stained by PI, CNE-1 cells were observed under the fluorescent microscopy. As shown in Fig. 5B, in the control group, the CNE-1 cells were normal as observed. While among SPL (0.5 pM) treated cells, dense nucleolus, condensed chromatin, and fragmented nuclei were observed. Also, there was a reduction in nuclear volume. Some of the nucleus degenerated into discretely spherical fragments of highly condensed chromatin (Fig. 6B). These results suggested that SPL could induce CNE-1 cells apoptosis.
3.12. The balance between apoptosis and necrosis
To evaluate the lectin-induced CNE-1 cell death, the ratio of LDH released from viable cells, floating dead cells and the culture medium were compared. The number of apoptotic and necrotic cells was shown in Fig. 6C, respectively, suggesting that necrosis was also triggered by SPL in CNE-1 cells, whereas the number of apoptotic cells was much higher than necrotic cells. With progressively increasing concentration of SPL, more and more apoptotic and necrotic cells showed up. It demonstrated that the major reason of SPL-induced CNE-1 cell death was apoptosis rather than necrocytosis.
3.13. Participation of caspase-8 and caspase-3 in apoptosis
To evaluate the involvement of caspases in SPL-induced cell death, the inhibitors for caspase-8 (z-IETD-fmk, 20 mM), caspase-3 (z-DEVD-fmk, 20 mM) and pan-caspase inhibitor (z-VAD-fmk, 20 mM) were applied. Incubation with SPL for 24 h in the presence of the inhibitors of caspase-3, -8, and pan-caspase showed inhibitory effects on CNE-1 cell death. In particular, the effects of caspase-3 and caspase-8 inhibitors were more significant than others (Fig. 6D).
4. Discussion
The present article describes the isolation and partial characterization of a lectin from Setcreasea purpurea Boom and based on its source of species, N-terminal amino-acid sequence, carbohydrate-binding specificity, excellent stability and other qualities, this lectin could be classified to the family of chitin-binding plant proteins composed of hevein domains. In recent years, few of the researches on Setcreasea purpurea were carried out except karyotypes [43] and there were no reports on the bioactive proteins. This lectin, designated as SPL, might represent the first isolated proteinaceous constituent of Setcreasea purpurea.
The purification was achieved by a combined procedure including ion exchange and gel-filtration chromatography. The results of SDS-PAGE, gel filtration and MS analysis indicated that SPL is a homotetramer of 48kDa in which subunits are not held by disulphide linkages but joined together by non-covalent bonds.
According to the results of peptide mass fingerprinting analysis and database searching, SPL exhibited no sequence similarity with other lectins. However, there are very few mass fingerprinting peptides from lectins in databanks and the successful protein identification of peptide mass fingerprintings depends on several factors, such as the sequence coverage obtained, protein/genomic sequence database size, the choice of proteolytic enzyme [44], so peptide mass fingerprintings analysis is not remarkable enough to identify this lectin. To further identify this lectin, the N-terminal sequence of 25 amino-acid residues was determined and compared with several typical hevein-like chitin-binding lectins. The homology both in terms of amino-acid sequences and highly conserved cysteine residues suggested that SPL belongs to the family of chitin-binding plant lectins composed of hevein domains. Additionally, hevein-like chitin-binding lectins are widespread in the Gramineae family and typical lectins have been characterized from all species of the tribe Triticeae, the genera Brachypodium and Oryza, Agropyrum repens (couch grass) and Phragmites australis [3]. Setcreasea purpurea Boom is not a member of Gramineae family but a member of Commelinaceae family. However, it should be noted that both Commelinaceae and Gramineae family belong to the same Commelinidae subclassis, so Setcreasea purpurea may be considered a distant relative of the plants in Gramineae family. Thus, this chitin-binding lectin obtained from Setcreasea purpurea Boom may further reveal that the hevein-like chitin-binding lectins are relatively distributed in some species.
SPL possessed remarkable hemagglutinating activity towards rabbit erythrocytes and this ability was quite stable in pH range of 2.0–9.0 and it showed amazing thermal tolerance, with activity even at 100 °C for 30 min. The excellent stability of SPL was in good agreement with most chitin-binding lectins. It was previously found that Jackin, a chitin-binding lectin from jackfruit, preserved its secondary structure as its native structural features even up to 80 °C [28]. These chitin-binding lectins are very stable proteins and it is said that the high content of conserved cysteines was involved in several intrachain disulfide bonds, giving them rigidity and stability over a wide range of pH and temperature [28].
The assay of carbohydrate-binding specificity indicated that the hemagglutination activity of SPL could be preferentially inhibited by chitin hydrolysate (oligomers of GlcNAc). Chitin is a naturally occurring polysaccharide composed of β (1 → 4) linked N-acetylglucosamine (GlcNAc) monomers that form a complex supermolecular structure [8]. Since GlcNAc is the basic element of chitin, some chitin-binding lectins showed specificity for GlcNAc, such as Gramineae lectins and tomato lectins [3]. However, GlcNAc was not specific to SPL, which could only be inhibited by chitin hydrolysate (oligomers of GlcNAc). This feature also appears to UDA (Urtica dioica lectin) [34], CBL3 (Cyphomandra betacea lectin) [45] and some other chitin-binding lectins. It is worth noting that the chitin-binding lectins, such as VisalbCBA (Viscum album lectin) and WGA (Wheat germ agglutinin) [3], [36], though can be inhibited by both glcNAc and GlcNAc oligomers, the results showed that the GlcNAc oligomers were inhibitorier than glcNAc. It is confirmed that the sugar-binding activity and specificity of the chitin-binding lectins are determined exclusively by their hevein domains and the carbohydrate-binding sites of them have a complex structure that is most complementary to the trimer or tetramer of GlcNAc[3]. So this is capable of explaining why SPL was not inhibited by GlcNAc but could be strongly inhibited by chitin hydrolysate (oligomers of GlcNAc).
Some chitin-binding lectins have been reported to possess antifungal activity. UDA (Urtica dioica lectin), one of these antifungal lectins, inhibits growth of several phytopathogenic and saprophytic chitin-containing fungi in vitro [34]. Another chitin-binding protein, Hevein, also inhibits several chitin-containing fungi and is a more potent inhibitor than chitinase for several fungi [35]. In this present, SPL exhibited antifungal activity towards four phytopathogenic harmful fungi with quantitatively tested results, though to a somewhat lesser extent than UDA (Urtica dioica lectin) [35]. It is widely acknowledged that chitin or chitin oligomers and some other saccharides are important components of most fungal cell walls. Since SPL is a chitin-binding lectin, the antifungal mechanism of it may relate to its carbohydrate-binding property. The antifungal activity of SPL also appeared to be thermal tolerance, still possessing some activity by treatments of high temperatures, which was analogous with the hevein [35]. In view of the antifungal activity and excellent stability of SPL, it can be a potent candidate in plant genetic engineering for breeding antifungal plants.
Many carbohydrate-specific plant lectins have shown antiviral activity and on the basis of carbohydrate-binding specificities, the antiviral activity of plant lectins varies considerably. The mannose-specific plant lectins are highly effective against viruses, such as the anti-HIV activities of Polygonatum cyrtonema lectin [40] and Clematis montana lectin [42], the anti-HSV activity of Ophiopogon japonicus lectin [46]. A number of galactose-, N-acetylgalactosamine- (N-GalNAc-), N-acetylglucosamine- (N-GlcNAc-) and glucose-specific plant agglutinines exhibited anti-coronaviral activity as well [14], [47]. It is confirmed that the chitin-binding lectins isolated from Urtica dioica and from the tobacco plant are markedly active against the SARS-CoV [14]. In this study, SPL exhibited a suppressive action on HIV-1, HIV-2 and reproducible antiviral effect was observed. It is indicated that though the mannose-specific plant lectins are highly effective against HIV, some chitin-binding lectins, such as UDA (Urtica dioica lectin), also possesses anti-HIV activity [14], [48]. It should be emphasized that SPL is another chitin-binding lectin that is against HIV, although the anti-HIV activity of SPL is not so remarkable when compared with other known lectins, such as Polygonatum cyrtonema lectin [40]. It is possible that plant lectins with different specificity interfere with different targets necessary for viral entry, depending on the place of the glycans that are targeted [49]. As to HIV, it was recently indicated that plant lectins with different carbohydrate specificities (mannose-specific or N-GlcNAc-specific), represent carbohydrate-binding agents with a similar mode of antiviral action, but with different genetic barriers [49]. Therefore, the antiviral activity against HIV of SPL may be well associated with its N-GlcNAc-specificity.
Recently, the remarkable antineoplastic activity of plant lectins has driven much attention to cancer studies [50] and many studies have further presented the anti-tumour activities of plant lectins on a variety of malignant cells [51], [52]. The antineoplastic activity is also known in chitin-binding lectins, such as STL (Solanum tuberosum lectin) and WGA (Wheat germ agglutinin) [13]. WGA, a representative chitin-binding lectin, was highly toxic to human pancreatic carcinoma cells in vitro, with high levels of membrane binding to sialic acid residues and lectin internalization. Studies also reported the effect of WGA on lymphoma cell survival and found that it restricted tumour growth when compared with the control [50], [53]. Our investigation confirmed that SPL, a new member of the chitin-binding lectin family, also possessed an obvious cytotoxic effect on CNE-1 cells (a well differentiated human nasopharyngeal carcinoma cells) and induced apoptosis in a time- and dose-dependent manner.
The apoptosis is an evolutionary conserved process that plays a crucial role in metazoan development [50], [54]. Therefore, if the molecules or pathways of apoptosis required to suppress the tumorigenesis are identified, apoptosis modulation can emerge as a new target for cancer therapy [50]. It is suggested that the sugar-binding part as well as the other part of the lectin molecule is responsible for the apoptosis-inducing activity [13]. ConA, the firstly reported legume lectin, is cytotoxic or strongly inhibitory to some tumor cells, which has been found to induce A375 cell death in a caspase-dependent manner as well as through a mitochondrial apoptotic pathway [55]. Polygonatum cyrtonema lectin and Polygonatum odoratum lectin, typical mannose-binding lectins, has also been found to induce apoptosis and autophagy in tumor cells through mitochondria-mediated pathways [56], [57]. As to SPL, we found a typical caspase-dependent apoptosis through participation of three caspase inhibitors, expecially the effects of caspase-3 and caspase-8 inhibitors. However, the apoptotic mechanisms of actions of chitin-binding lectins are not as clear-cut as some mannose-binding lectins or legume lectins, so the further research ought to be focused on elucidating the details of apoptotic mechanisms of this lectin.
In summary, a new lectin has been isolated from the rhizomes of Setcreasea purpurea for the first time. We have also concentrated our efforts on its biological activities and pleasantly discovered that SPL is a chitin-binding lectin with antitumor, antiviral and antifungal activities. Future studies will be aimed at the clone and sequence analysis of SPL to obtain more information of the relationship between molecular basis and biological characteristics of this protein. It may provide more promising insights into pharmaceutical exploitation in treatment of diseases or other biotechnological applications in future.
Acknowledgements
We thank Dr. Ying Gu for her critical review on this manuscript and We are also grateful to Professor Jan Balzarini (Rega Institute for Medical Research, Katholieke University Leuven, Belgium) for his kind help to provide the antiviral tests. This work was supported by grants from the National Natural Science Foundation of China (General Programs: Nos. 30670469 and 30970643).
References
- 1.Liener I.E., Sharon N., Goldstein I.J. Academic Press; London: 1986. The lectins: properties, functions, and application in biology and medicine. [Google Scholar]
- 2.Sharon N., Lis H. Lectins as cell recognition molecules. Science. 1989;246:227–234. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- 3.Van Damme E., Peumans W.J., Barre A., Rouge P. Plant lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit Rev Plant Sci. 1998;7:575–662. [Google Scholar]
- 4.Li Y.R., Liu Q.H., Wang H.X., Ng T.B. A novel lectin with potent antitumor, mitogenic and HIV-1 reverse transcriptase inhibitory activities from the edible mushroom Pleurotus citrinopileatus. Biochim Biophys Acta. 2008;1780:51–57. doi: 10.1016/j.bbagen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Herre J., Willment J.A., Gordon S., Brown G.D. The role of Dectin-1 in antifungal immunity. Crit Rev Immunol. 2004;24:193–203. doi: 10.1615/critrevimmunol.v24.i3.30. [DOI] [PubMed] [Google Scholar]
- 6.Abdullaev F.I., de Mejia E.G. Antitumor effect of plant lectins. Nat Toxins. 1997;5:157–163. doi: 10.1002/1522-7189(1997)5:4<157::AID-NT6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Macedo M.L., Damico D.C., Freire M.G., Toyama M.H., Marangoni S., Novello J.C. Purification and characterization of an N-acetylglucosamine-binding lectin from Koelreuteria paniculata seeds and its effect on the larval development of Callosobruchus maculatus (Coleoptera: Bruchidae) and Anagasta kuehniella (Lepidoptera: Pyralidae) J Agric Food Chem. 2003;51:2980–2986. doi: 10.1021/jf034013i. [DOI] [PubMed] [Google Scholar]
- 8.Blackwell J. Physical methods for the determination of chitin structure and conformation. Methods Enzyme. 1988;161:435–442. [Google Scholar]
- 9.Nagata Y., Burger M.M. Wheat germ agglutinin. Isolation and crystallization. J Biol Chem. 1972;247:2248–2250. [PubMed] [Google Scholar]
- 10.LeVine D., Kaplan M.J., Greenaway P.J. The purification and characterization of wheat-germ agglutinin. Biochem J. 1972;129:847–856. doi: 10.1042/bj1290847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selitrennikoff C.P. Antifungal proteins. Appl Environ Microbial. 2001;67:2883–2894. doi: 10.1128/AEM.67.7.2883-2894.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng T.B. Antifungal proteins and peptides of leguminous and nonleguminous origins. Peptides. 2004;25:1215–1222. doi: 10.1016/j.peptides.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Wang H.X., Ng T.B., Vincent E.C.O., Liu W.K. Effects of lectins with deferent carbohydrate-binding specificities on hepatoma, choriocarcinoma, melanoma and osteosarcoma cell lines. Int J Biochem Cell Biol. 2000;32:365–372. doi: 10.1016/s1357-2725(99)00130-2. [DOI] [PubMed] [Google Scholar]
- 14.Keyaerts E., Vijgen L., Pannecouque C., Damme E.V., Peumans W., Egberink H., Balzarini J., Ranst M.V. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75:179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.China committee for culture collections of microorganisms . China Machine Press; Beijing: 1992. China catalogue of cultures. p. 249. [Google Scholar]
- 16.Laemmli U.K., Favre M. A method of protein separation. J Mol Biol. 1973;80:575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- 17.Whitakar J.R. Determination of molecular weights of proteins by gel filtration on Sephadex. Anal Chem. 1963;35:1950–1953. [Google Scholar]
- 18.Andrews P. Estimation of molecular weights of proteins by Sephadex gelfiltration. Biochem J. 1964;91:222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo B.H., Lee J.T., Na D.H., Lee K.C. Sepharose-unbinding ricin E as a source for ricin A chain immunotoxin. J Immunol Methods. 2001;249:91–98. doi: 10.1016/s0022-1759(00)00330-6. [DOI] [PubMed] [Google Scholar]
- 20.Tong A.P., Wu L.H., Lin Q.S., Lau Q.C., Zhao X., Li J., Chen P. Proteomic analysis of cellular protein alterations using a hepatitis B virus-producing cellular model. Proteomics. 2008;8:2012–2023. doi: 10.1002/pmic.200700849. [DOI] [PubMed] [Google Scholar]
- 21.Lam S.S., Wang H., Ng T.B. Purification and characterization of novel ribosome inactivating proteins, alpha- and beta-pisavins, from seeds of the garden pea Pisum sativum. Biochem Biophys Res Commun. 1998;253:135–142. doi: 10.1006/bbrc.1998.9764. [DOI] [PubMed] [Google Scholar]
- 22.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 23.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with follin pheol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Bruno C., Hetty M.S., Willy J.P. A new type of cereal lectin from leaves of couch grass (Agropyrum repens) Eur J Biochem. 1985;148:315–322. doi: 10.1111/j.1432-1033.1985.tb08841.x. [DOI] [PubMed] [Google Scholar]
- 25.Yan Q.J., Jiang Z.Q., Yang S.Q., Deng W., Han L. A novel homodimeric lectin from Astragalus mongholicus with antifungal activity. Arch Biochem Biophys. 2005;442:72–81. doi: 10.1016/j.abb.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Rupley J.A. The hydrolysis of chitin by concentrated hydrochloric acid, and the preparation of low-molecular-weight substrates for lusozyme. Biochim Biophys Acta. 1964;83:245–255. doi: 10.1016/0926-6526(64)90001-1. [DOI] [PubMed] [Google Scholar]
- 27.Paulova M., Entlicher G., Ticha M., Kostir J.V., Kocourek J. Studies on phytohemagglutinins. VII. Effect of Mn2+ and Ca2+ on hemagglutination and polysaccharide precipitation by phytohemagglutinin of Pisum sativum L. Biochim Biophys Acta. 1971;237:513–518. doi: 10.1016/0304-4165(71)90271-6. [DOI] [PubMed] [Google Scholar]
- 28.Trindade M.B., Lopes J.L., Soares-Costa A., Monteiro-Moreira A.C., Moreira R.A., Oliva M.L., Beltramini L.M. Structural characterization of novel chitin-binding lectins from the genus Artocarpus and their antifungal activity. Biochim Biophys Acta. 2005;1764:146–152. doi: 10.1016/j.bbapap.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Cao J., Jiang W., He H. Induced resistance in Yali Pear (Pyrus bretschneideri Rehd.) fruit against infection by Penicillium expansum by postharvest infiltration of acibenzolar-s-methy. J Phytopathol. 2005;153:640–647. [Google Scholar]
- 30.Balzarini J., Van Laethem K., Hatse S., Vermeire K., De Clercq E., Peumans W., Van Damme E. Profile of resistance of human immunodeficiency virus to mannose-specific plant lectins. J Virol. 2004;78:10617–10627. doi: 10.1128/JVI.78.19.10617-10627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 32.Gu Q.L., Shen B.H., Li L.N. A preliminary study on arsenic trioxide induced apoptosis of gastric cancer lines. Chin J Digest. 1998;18:69–71. [Google Scholar]
- 33.Cheng Y., Qiu F., Huang J., Tashiro S.-i., Onodera S., Ikejima T. Apoptosis-suppressing and autophagypromoting effects of Calpain on Oridonin-induced L929 cell death. Arch Biochem Biophys. 2008;475:148–155. doi: 10.1016/j.abb.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 34.Broekaert W.F., Peumans W.J., Parijs J.V., Leyns F. A chitin-binding lectin from stinging nettle rhizomes with antifungal properties. Science. 1989;245:1100–1102. doi: 10.1126/science.245.4922.1100. [DOI] [PubMed] [Google Scholar]
- 35.Parijs J.V., Broekaert W.F., Goldstein I.J., Peumans W.J. Hevein: an antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta. 1991;183:258–264. doi: 10.1007/BF00197797. [DOI] [PubMed] [Google Scholar]
- 36.Peumans W.J., Verhaert P., Pffiller U., Van Damme E. Isolation and partial characterization of a small chitin-binding lectin from mistletoe (Viscum album) FEBS Lett. 1996;396:261–265. doi: 10.1016/0014-5793(96)01108-8. [DOI] [PubMed] [Google Scholar]
- 37.Willy J.P., Marc D.L., Herry M.S., Willem F.B. Isolation and partial characterization of a new lectin from seeds of the greater celandine (Chelidonium majus) Plant Physiol. 1985;78:379–383. doi: 10.1104/pp.78.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beintema J.J., Peumans W.J. The primary structure of stinging nettle (Urtica dioica) agglutinin A two-domain member of the hevein family. FEBS Lett. 1992;299:131–134. doi: 10.1016/0014-5793(92)80231-5. [DOI] [PubMed] [Google Scholar]
- 39.Natasha V.R., Thea A.W. Isolation and characterization of a cDNA clone encoding wheat germ agglutinin. Proc Natl Acad Sci. 1987;84:6745–6749. doi: 10.1073/pnas.84.19.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.An J., Liu J.Z., Wu C.F., Li J., Dai L., Van Damme E., Balzarini J., De Clercq E., Chen F., Bao J.K. Anti-HIV I/II activity and molecular cloning of a novel mannose/sialic acid-binding lectin from rhizome of Polygonatum cyrtonema Hua. Acta Biochim Biophys Sin. 2006;38:70–78. doi: 10.1111/j.1745-7270.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 41.Lv H., Yang K., Yao Q., Zhang B., Leng F.W., Bian H.J., Balzarini J., Van Damme E., Bao J.K. Nebrodeolysin, a novel hemolytic protein from mushroom Pleurotus nebrodensis with apoptosis-inducing and anti-HIV-1 effects. Phytomedicine. 2009;16:198–205. doi: 10.1016/j.phymed.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Peng H., Lv H., Wang Y., Liu Y.H., Li C.Y., Meng L., Chen F., Bao J.K. Clematis montana lectin, a novel mannose-binding lectin from traditional Chinese medicine with antiviral and apoptosis-inducing activities. Peptides. 2009;30:1805–1815. doi: 10.1016/j.peptides.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakurai T., Ichikawa S. Karyotypes, Giemsa, C-banding patterns of Zebrina pendula, Z-purpusii and Setcreasea purpurea, compared with those of Tradescantia ohiensis. Genes Genet Syst. 2001;76:235–242. doi: 10.1266/ggs.76.235. [DOI] [PubMed] [Google Scholar]
- 44.Horn D.M., Peters E.C., Klock H., Meyers A., Brock A. Improved protein identification using automated high mass measurement accuracy MALDI FT-ICR MS peptide mass fingerprinting. Int J Mass Spectrom. 2004;238:189–196. [Google Scholar]
- 45.Sampietro A.R., Isla M.I., Quiroga E.N., Vattuone M.A. An N-acetylglucosamine oligomer binding agglutinin (lectin) from ripe Cyphomandra betacea Sendt. Fruits. Plant Sci. 2001;160:659–667. doi: 10.1016/s0168-9452(00)00442-8. [DOI] [PubMed] [Google Scholar]
- 46.Tian Q., Wang W., Miao C., Peng H., Liu B., Leng F., Dai L., Chen F., Bao J.K. Purification, characterization and molecular cloning of a novel mannose-binding lectin from rhizomes of Ophiopogon japonicus with antiviral and antifungal activities. Plant Sci. 2008;175:877–884. [Google Scholar]
- 47.Balzarini J., Schols D., Neyts J., Van Damme E., Peumans W., De Clercq E. Alpha-(1-3)- and alpha-(1-6)-d-mannose-specific plant lectins are markedly inhibitory to human immunodeficiency virus and cytomegalovirus infections in vitro. Antimicrob Agents Chemother. 1991;35:410–416. doi: 10.1128/aac.35.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balzarini J., Neyts J., Schols D., Hosoya M., Van Damme E., Peumans W., De Clercq E. The mannose-specific plant lectins from cymbidium hybrid and epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antiviral Res. 1992;18:191–207. doi: 10.1016/0166-3542(92)90038-7. [DOI] [PubMed] [Google Scholar]
- 49.Balzarini J., Van Laethem K., Hatse S., Froeyen M., Peumans W., VanDamme E., Schols D. Carbohydrate-binding agents cause deletions of highly conserved glycosylation sites in HIV GP120: a new therapeutic concept to hit the achilles heel of HIV. J Biol Chem. 2005;280:41005–41014. doi: 10.1074/jbc.M508801200. [DOI] [PubMed] [Google Scholar]
- 50.Liu B., Bian H.J., Bao J.K. Plant lectins: potential antineoplastic drugs from bench to clinic. Cancer Lett. 2010;287:1–12. doi: 10.1016/j.canlet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 51.De Mejía E.G., Prisecaru V. Lectins as bioactive plant proteins: a potential in cancer treatment. Crit Rev Food Sci Nutr. 2005;45:425–445. doi: 10.1080/10408390591034445. [DOI] [PubMed] [Google Scholar]
- 52.Choi S.H., Lyu S.Y., Park W.B. Mistletoe lectin induces apoptosis and telomerase inhibition in human A253 cancer cells through dephosphorylation of Akt. Arch Pharm Res. 2004;27:68–76. doi: 10.1007/BF02980049. [DOI] [PubMed] [Google Scholar]
- 53.Chiara D.P., Omar P., Maria T.S., Carlo T., Chiara Z., Gianni Z., Marina F., Angelo P., Corrado R., Roberto C. Effects of wheat germ agglutinin on human gastrointestinal epithelium: insights from an experimental model of immune/epithelial cells interaction. Toxicol Appl Pharmacol. 2009;237:146–153. doi: 10.1016/j.taap.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Andrew T. Apoptosis and autophagy: regulatory connections between two supposedly different processes. Apoptosis. 2008;13:1–9. doi: 10.1007/s10495-007-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu B., Li C.Y., Bian H.J., Min M.W., Chen L.F., Bao J.K. Antiproliferative activity and apoptosis-inducing mechanism of Concanavalin A on human melanoma A375 cells. Arch Biochem Biophys. 2009;482:1–6. doi: 10.1016/j.abb.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Liu B., Zhang B., Min M.W., Bian H.J., Chen L.F., Liu Q., Bao J.K. Induction of apoptosis by Polygonatum odoratum lectin and its molecular mechanisms in murine fibrosarcoma L929 cells. Biochim Biophys Acta: Gen Subjects. 2009;1790:840–844. doi: 10.1016/j.bbagen.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 57.Liu B., Cheng Y., Zhang B., Bian H.J., Bao J.K. Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria-mediated ROS–p38–p53 pathway. Cancer Lett. 2009;275:54–60. doi: 10.1016/j.canlet.2008.09.042. [DOI] [PubMed] [Google Scholar]


