Highlights
-
•
A plethora of systems biology datasets on virus–host interactions are available.
-
•
These include transcriptomic, proteomic and RNAi screening approaches.
-
•
The studies revealed exciting new aspects and opened up new areas of research.
-
•
Current limitations: use of artificial assay systems, limited perturbation and difficult comparison of datasets.
-
•
Future directions: platform for primary data, tools to compare datasets, CRISPR/cas9 technology.
Abstract
Viruses are completely dependent on their host cells for the successful production of progeny viruses. At each stage of the viral life cycle an intricate interplay between virus and host takes place with the virus aiming to usurp the host cell for its purposes and the host cell trying to block the intruder from propagation. In recent years these interactions have been studied on a global level by systems biology approaches, such as RNA interference screens, transcriptomic or proteomic methodologies, and exciting new insights into the pathogen–host relationship have been revealed. In this review, we summarize the available data, give examples for important findings from such studies and point out current limitations and potential future directions.
Current Opinion in Microbiology 2015, 26:79–88
This review comes from a themed issue on Host-microbe interactions: viruses
Edited by Ivan Marazzi and Adolfo Garcia-Sastre
For a complete overview see the Issue and the Editorial
Available online 23rd June 2015
http://dx.doi.org/10.1016/j.mib.2015.06.001
1369-5274/© 2015 Elsevier Ltd. All rights reserved.
Introduction
Human viruses intrinsically depend on cellular factors to successfully infect and replicate inside their host. Viruses hijack the cellular machinery, exploit it and often reprogram it for their own needs, both to utilize it for replication and to evade antiviral innate immune defenses. Here, in this review, we summarize systematic approaches that have been developed to identify these key cellular determinants for viral infections. First of all, the knowledge of these critical host–pathogen interactions is crucial to understand the viral life cycle and moreover, will provide the basis for the development of therapeutic interventions. We will highlight novel concepts that arose from the comprehensive understanding of the host-viral circuitry.
A biological system, such as the evolutionary interplay between a virus and its host, functions through the combined regulatory mechanisms and entangled engagement of various classes of molecules and states (DNA, RNA, regulatory RNA, protein interactions, protein abundance, post-translational modifications, epigenetic modifications, metabolites, etc.) in a spatial and temporal order [1]. Systems-based ‘omics’ approaches such as genomics, proteomics, transcriptomics, metabolomics and other approaches are instrumental to capture pieces of this host–pathogen map in a comprehensive and unbiased survey (summarized in Table 1 ). Perturbation studies, RNA interference (RNAi) or cDNA overexpression, have facilitated the establishment of systematic cell-based loss-of-function or gain-of-function screening platforms, respectively. These functional genomics approaches are a powerful tool informing us about the immediate relevance of the identified factors for viral replication. In this review we focus on the genome-wide siRNA screening approaches deciphering the host cellular repertoire affecting HIV and influenza A virus (IAV) replication providing a global cellular map of the viral–host relationship (six global RNAi screens in human cells for IAV and five for HIV) [2, 3••, 4, 5, 6•, 7, 8••, 9, 10, 11, 12]. Proteomics data can provide global insight into host proteins hijacked by the virus. Recent studies have comprehensively documented the protein complexes directly interacting with viral proteins encoded by the retrovirus HIV-1 and the herpesvirus KSHV by systematically affinity tagging and purifying all viral proteins followed by mass spectrometry [13••, 14]. Combining the genome-wide siRNA approaches above with this kind of proteome-wide strategies would add another level of confidence in selecting the relevant host factors responsible for replication that serve as immediate interconnections of viral components with the host proteome. Studies integrating proteomics and hit validation by RNAi are a starting point in achieving this goal (exemplified by [15, 16, 17]).
Table 1.
Screening approaches to identify novel cellular factors affecting viral replication (selected publications)
| Global viral screens |
Influenza |
Genomics | [2, 3••, 4, 5, 6•, 7, 81, 82] |
| Proteomics and genomics |
[15, 16] |
||
| HIV |
Genomics | [9, 8••, 83, 10, 11, 12, 84, 85] | |
| Phosphoproteomics cellular reprogramming through HIV | [26•] | ||
| Proteomics |
[13••] |
||
| Other viruses |
Genomics | HCV [86, 87] Dengue [88] WNV [89] |
|
| Proteomics | KSHV [14] Herpesvirus [90] |
||
| Proteomics and genomics |
HCV [17] |
||
| Effectors of antiviral response |
Genomics | HCV, YFV, WNV, chikungunya virus, Venezuelan equine encephalitis virus, HIV [63••] various ds DNA, (+)ssRNA, (−)ssRNA viruses [19] VSV, MHV-68 [91] |
|
| Genomics combined with IFN stimulation | HCV [92] HCV [93] |
||
| Proteomics and genomics |
Many different RNA and DNA virus ORFs [18] |
||
| Cellular reprogramming/rewiring through viruses |
Phospho-proteomics | HIV [26•] | |
| Proteomics |
HPV, EBV, Ad5 and PyV [94] |
||
| Innate immune response pathway members affecting viral replication |
Cytosolic DNA pathway |
Proteomics and genomics |
HIV [20] |
| Type I interferon regulation |
Proteomics and genomics |
VSV, Sendai, HSV [23] |
|
| TLR pathway |
Genomics | Sendai [22] | |
| Transcriptmomics, genetic perturbations, phosphoproteomics |
EMCV, NDV, VSV, Influenza [21] |
||
| In vivo screening approach | Genomics | [75] | |
Recent approaches surveyed key molecules playing roles in common antiviral strategies (summarized in Table 1). For instance, Pichlmair et al. investigated the proteome of 70 viral proteins from 30 different viruses targeting virus-specific and pan-viral cellular processes as an immune escape strategy suggesting certain conserved cellular alert mechanisms [18]. Intriguingly, a pan-viral cellular immune response strategy implying an unexpected role for the DNA sensor cGAS in controlling RNA viruses was uncovered by Schoggins et al. [19]. They demonstrated a gain-of-function approach by screening a library of interferon-stimulated genes (ISGs) on antiviral activity against 14 different viruses. To complete the picture of the innate response sensing viral pathogens, several studies interrogated regulators of pathways affecting viral replication (see Table 1). Lee et al. integrated various complementary ‘omics’ approaches to identify new components of the innate response to cytosolic DNA affecting retroviral infections in mouse embryonic fibroblasts (MEFs) [20], whereas two other studies covered the TLR interactome [21, 22]. To understand the cellular complexes formed after pathogen recognition, Li et al. systematically explored the human IFN interactome responsible for regulating cellular antiviral defense and IFN production [23].
These recent novel systems-biology strategies have started to uncover the important framework that orchestrates the cellular defense against viral pathogens, however, important questions and answers are still outstanding. Non-structural viral proteins indeed protect viruses against unknown cellular proteins [24], however none of the above mentioned studies systematically investigated how non-structural proteins influence the results of genomics screens, for example, by comparing global screens that were performed with viruses mutated in one gene at a time. Viral proteins are known to influence innate factors by either directly leading them to proteasomal degradation [24] or changing the activation status of the restricting factors through post-translational modifications [25]. A future systematic survey on the alteration of the global cellular protein abundance/phospho-proteome/ubiquitinome through viral modulators would significantly add to the cooperative picture of viral replication. A first global study on cellular rewiring successfully uncovered novel cellular factors that are differentially phosphorylated upon HIV-1 infection [26•].
Given the gigantic wealth of information collected by various ‘omics’ studies, it becomes extremely important to gather the data in a user-friendly format, and provide novel analysis tools to comb the plethora of data to be able to compare them to own lab-generated datasets. Many global initiatives (as summarized in Table 2 ) have started to develop such tools and compendia. An all-encompassing tool integrating various datasets in a comprehensive and unbiased model of viral-host interaction, a so-called meta-analysis, will be a big challenge but promising direction for the future.
Table 2.
Global Initiatives and web resources for viral–host interaction research
| Name | Web-resource | Description | Reference |
|---|---|---|---|
| Host–pathogen interactions | |||
| Systems Biology Program for infectious diseases | http://www.niaid.nih.gov/labsandresources/resources/dmid/sb/Pages/default.aspx | Data and reagents that result from the research conducted, such as VIPR — the virus pathogen resource (all virus families) | [95] |
| Virus Human Interactome Network Map | http://interactome.dfci.harvard.edu/V_hostome | Host–viral interactome network of several polyomaviruses, human papillomaviruses, Epstein–Barr Virus and Adenovirus 5 | [94] |
| Pathogen-Portal | http://www.pathogenportal.org/portal/portal/PathPort/Home | Bioinformatics resource searching available data and tools to analyze host-pathogen interactions for a variety of different viruses | |
| Virusmint | http://mint.bio.uniroma2.it/virusmint/Welcome.do | Resource for protein interactions between viral and human proteins reported in the literature | [96] |
| Systemsbiology-Metaanalysis | http://hivsystemsbiology.org/ | Collection of various ‘omics’ datasets related to HIV, DENV, HCV, HPV, Influenza, VSV, WNV, Lists of genes induced by innate responses, list of gene knockout in mice without an abnormal phenotype, list of druggable genes | [97] |
| HIV | |||
| HIV Systems Biology | http://hivsystemsbiology.org/ | Collection of ‘omics’ HIV data, genes connected to viral association studies, interactive HIV replication cycle and a browser for HIV-protein interactions (GPS-Prot) | [97, 98] |
| Specialized Centers for HIV/AIDS-Related Structural Biology (HARC, HIVE, PCHPI, CHEETAH, CRNA) | http://www.nigms.nih.gov/Research/SpecificAreas/AIDSStructuralBiology/Pages/HIVspecializedcenters.aspx | Structural information on interactions between HIV viral proteins and cellular factors | [98] |
| HIV-1-human interaction database | http://www.ncbi.nlm.nih.gov/genome/viruses/retroviruses/hiv-1/interactions/ | Literature collection of published reports of protein interactions, and human gene knockdowns that affect virus replication and infectivity | [99, 100, 101] |
| Influenza | |||
| Systems-Virology Center | https://www.systemsvirology.org | Data and resources on Influenza and SARS-Coronavirus | [102] |
| The Influenza Research Database (IRD) | http://www.fludb.org | User interface for searching and analyzing comparative genomics data | [103] |
| Systems influenza program | http://www.systemsinfluenza.org/#viralhost | Collection of transcriptomics, proteomics, and lipidomics datasets | [102] |
| Flumap | http://www.influenza-x.org/flumap | Literature-based and manually curated map of Influenza-host interactions | [104] |
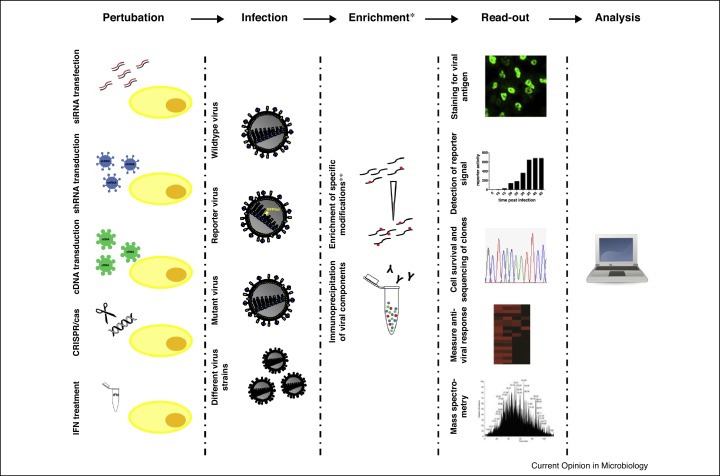
Many of the studies mentioned above revealed important novel insights into virus–host interactions and opened up new research avenues. In this review we can only highlight a few selected examples for which we illustrate how screening approaches have led to an advanced understanding of the complex virus–host interplay (Figure 1 ).
Figure 1.
Overview of screening approaches. Graphical representation of the most common screening approaches used to study virus interactions. *The enrichment step is only used for certain types of screens, such as phospho-proteomic analysis or screens for viral interactors. **The enrichment of specific posttranslational modifications includes phosphorylation, ubiquitination, etc.
Advances in the field of influenza virus entry based on RNAi screens
In the field of IAV entry several novel host factors have been identified and characterized for their role in the early steps of virus infection as a result of RNAi screens for host factors of influenza viruses. For example, the cytoplasmic dipeptidase prolidase has been found to be involved in early endosomal trafficking of the virus [27]. Interestingly, also the tetraspanin CD81 which had previously been detected in influenza virions was revealed to be required for early events in virus infection, most likely at the level of fusion of viral and endosomal membranes [28, 29]. Exciting progress has also been made at the stage of viral uncoating: Su and colleagues performed an shRNA screen for host factors of IAV and focused on one particular hit found to be required for early steps of viral infection, the E3 ubiquitin ligase ITCH [6•]. They could show that the virus gets trapped in the endosome upon knockdown of ITCH. Moreover, ITCH was found to be phosphorylated upon infection, thereby recruited to the endosome where it interacts with the viral matrix protein M1 and ubiquitinates M1. These results gave the first insight into cellular factors involved in the uncoating process of IAV and this was further elucidated by a recent study by Banerjee et al. [30•]. On the basis of the results of an siRNA screen for IAV host factors the class I HDACs (histone deacetylases) had emerged as regulators of IAV entry and therefore the authors followed up on HDAC6 [30•, 31]. They found that the incoming virus carries unanchored ubiquitin chains that become recognized by an HDAC6-dependent pathway of the aggresome machinery. This leads to the recruitment of microtubules and associated motor proteins. Their model suggests that the motor proteins can generate physical force to disrupt the virions and thereby enable complete uncoating. Currently, it is unclear how the function of ITCH and the aggresome pathway are linked but certainly these studies have uncovered exciting new insights into the uncoating process of IAV.
Besides the progress on cellular factors with a proviral function in IAV entry the screening approaches have also advanced the area of antiviral factors: One of the genome-wide RNAi screens for IAV identified the IFITM (IFN-inducible transmembrane) proteins with the family members IFITM1, IFITM2, IFITM3 and IFITM5 in humans as novel antiviral restriction factors and two other screens confirmed this result [3••, 5, 15]. Brass and colleagues observed that knockdown of IFITM3 led to increased viral replication, whereas overexpression of the IFITMs efficiently blocked the virus. It soon became clear that the antiviral potential of IFITM3 was not limited to influenza viruses, but also included members of flaviviruses, filoviruses, bunyaviruses, coronaviruses, reoviruses and HIV-1 [3••, 32, 33, 34, 35]. Moreover, it was found that the IFITM proteins localize to endosomes and/or the plasma membrane and block at the stage of viral entry [32, 36]. Since then, a whole field of research on the antiviral potential, the mechanism of viral inhibition and the in vivo role of the IFITM proteins has opened up and flourished. We have learnt that besides humans, other species, including pigs, chicken and mice, encode for IFITM proteins with powerful antiviral activity [37, 38, 39, 40]. The mechanism of antiviral action is not completely understood yet but it is assumed that fusion between viral and endosomal membranes is targeted by the IFITM proteins [36, 41]. Current models suggest that the IFITMs either impact the composition or activity of components of endosomal vesicles to which they localize and thereby hinder fusion [42]. Alternatively, the IFITMs could affect the membrane properties of endosomes resulting in unfavorable conditions for fusion between cellular and viral membranes [43]. In line with both models most of the viruses that are sensitive to IFITM restriction are enveloped viruses that fuse in late endosomal compartments. However, also the non-enveloped reoviruses are blocked efficiently by IFITMs, whereas arenaviruses which fuse at membranes of acidic endosomal compartments are resistant to IFITM restriction [3••, 32, 35]. This highlights that we do not yet fully understand the antiviral mechanism of IFITM proteins. Interestingly, two new studies reported the incorporation of IFITM proteins into HIV-1 virions and inhibition of viral fusion as a result of IFITM packaging [44••, 45••]. It will be important to see if other viruses incorporate IFITMs as well and if this can explain some of the open questions on the mechanism of action.
Regarding the in vivo role of IFITMs it could be shown that ifitm3 −/− mice are more susceptible to IAV infection than wildtype mice as evidenced by increased weight loss and mortality [37]. Furthermore, recent findings in human patients highlighted the importance of IFITM3 in the host defense of humans against IAV [37]. It was observed that patients homozygous for a polymorphism in the IFITM3 gene that results in an inactive variant of IFITM3 had an approximately 20-fold higher incidence of severe influenza than those with intact IFITM3. Another study came to a similar conclusion but a third one could not confirm this result [46, 47]. Moreover, a recent study reported that the inactive version of IFITM3 described in the susceptible patients has potent antiviral activity in their experimental system [48]. In conclusion, more studies are required to resolve some of the contradictions but there is promising evidence for an important role in antiviral defense of IFITM3 in humans.
Advances in the field of early events in HIV replication based on RNAi screens
Details of the early steps of lentiviral replication including uncoating, nuclear import and integration have long been incompletely understood. Recent genome-wide RNAi studies have started to shed some light and instigated new intriguing research concepts to this enigmatic field of research. The first two genome-wide siRNA screens discovered members of the nucleo-cytoplasmic transport machinery as important host factors, such as the nuclear pore proteins Nup153 and Nup358 and Transportin-SR2/TNPO3 [8••, 9]. The latter was identified independently in a yeast-to-hybrid screen as an HIV-interacting protein [49]. The precise step at which these cellular factors act on viral replication had been a matter of discussion in the field (reviewed in [50]). However, their involvement in nuclear import of the pre-integration complex (PIC) has been heavily supported by various reports, starting with the initial mapping to the nuclear import step [8••] up to mapping of TNPO3 by De Iaco et al. [51]. An intriguing model of import-coupled integration had been suggested based on the observations, that (i) two more members of the nucleo-cytoplasmic transport machinery, amongst them Nup98 confirmed in two screens [8••, 12], affected unexpectedly HIV-1 integration [8••, 52], and that (ii) integration of HIV is dependent on the nuclear transport machinery even in cycling cells where post-nuclear envelope breakdown would otherwise make the nucleus accessible to the HIV PIC [8••]. Follow-up studies extended the finding that correct trafficking through the pore facilitates integration steps such that preferred regions of the genome for integration are targeted [53••, 54]. Moreover, blocking the interaction between cyclophilin A, a long-known cellular factor relevant for HIV-1 infection [55], and viral capsid relieves the dependence on TNPO3 and Nup358 and subsequently alters integration targeting [54]. Interestingly, a truncated version of the cleavage and polyadenylation specificity factor-6 (CPSF6) identified in a murine cDNA expression screen as a HIV-1 restricting gene had been shown to prevent nuclear entry [56]. On the basis of mutational studies, a model was proposed that binding of CPSF6 to the HIV-1 capsid mediates the dependence for TNPO3 and Nup358 [57]. An indirect role for TNPO3 promoting HIV-1 infection has been proposed: TNPO3, responsible for import of cargo proteins containing RS domains [58], transports CSPF6 possessing such a domain into the nucleus [51]. In the absence of TNPO3, CPSF6 accumulates in the cytoplasm causing a delay in nuclear import of the viral DNA [51]. Recently, the study by Rasaiyaah et al. went a step ahead and described for the first time that cofactor usage is linked to evasion of innate sensors [59••]. The suggested model posits that host factors, such as Cyclophilin A together with CPSF6 and Nup358, prevent premature uncoating and improper timing of reverse transcription, thus avoiding sensing of viral DNA by the DNA sensor cGAS [60]. Together with the engagement by other host factors, such as TNPO3 and Nup153, transport of the PICs through the nuclear pore can take place and will lead to proper integration site selection (reviewed in [61, 62]). Recently, an overexpression screening approach to discover restriction factors of viral replication identified Myxovirus resistance protein 2 (MxB) as an ISG inhibiting HIV-1 replication [63••]. Subsequently, three reports described MxB as a potent inhibitor of early events in the life cycle [64, 65, 66], though the precise step of inhibition still needs to be determined (summarized in [67]). Intriguingly, the expression of MxB, a protein accumulating at the nuclear rim [68], was shown to influence the integration site selection of the provirus, similar to cellular import factors such as TNPO3 or Nup358 [69•]. These observations further point to the fact that HIV PIC nuclear import and integration may be functionally linked. Though much progress has been made by identification of the above mentioned host factors, still many questions are unanswered, such as the role of these factors and not yet identified co-factors in the timing and spatial model of the coupled processes of uncoating — nuclear import — integration.
Current limitations and future directions
As evidenced by the examples described above, follow-up studies from RNAi screen have led to exciting new findings and opened up new areas of research, for example, the IFITM restriction factors, or led to novel concepts, for example, the coupled processes for viral nuclear import and integration site selection. However, when looking at the numbers of host factors identified by various approaches, only very few hits have been confirmed and further characterized for their proviral or antiviral role. In order to fully exploit the potential of the screens many more follow-up studies are required.
It is often difficult to choose promising host factors from hit lists that contain several hundred factors. This has been further complicated by the observation that the overlap in hits is surprisingly small between different screens for the same virus [70, 71, 72]. This discrepancy can be partially explained by differences in experimental systems, such as virus strain, cell line or screening assay or limitations of high-throughput screenings, such as off-target effects, false positives and false negatives [70, 73], but a major contributing factor for variation between screens seems to be the different filtering criteria that are employed to rank the best genes [71]. The hit identification process will automatically introduce a bias into the otherwise unbiased genome-wide datasets. Despite the divergence in gene sets, a greater overlap was identified in shared overrepresented functional groups, pathways [71, 72] or shared protein complexes [71]. Thus, an analysis of multiple datasets will be more effective in identifying intriguing new candidates and pathways exploited by viruses. However, a re-analysis of the primary datasets from multiple screens would be most useful. Unfortunately, for most screens the primary data are not published and therefore not accessible for other researchers. In order to overcome this current limitation a public database for the deposit of primary screening datasets would be desirable. Such a repository could also help to make the integration of datasets from different methodological approaches, for example, proteomic, transcriptomic and RNAi data, possible. Single ‘omics’ datasets can only capture a part of the whole and thus, it will be necessary to look from various angles to define the parameters affecting virus replication.
An additional limitation of the currently available datasets is the need to use convenient experimental systems for high-throughput assays. First, in most screens transformed cell lines that are easy to transfect have been used and it is unclear how results from these systems can be extrapolated to primary cells. For instance, cGAMP responses differ in mouse versus human cells [74] and pattern recognition receptors and other pathway members are not expressed equally in all cells (e.g. STING and cGAS lack expression in 293Tcells [60]), and thus primary sentinel cells of the immune system would be more appropriate to screen for antiviral effects than cell lines. Improved methods to perturb experimental systems based on primary cells or tissues are expected to be developed and solve such technical problems. Moreover, in vivo screens are already being established to address the important question which aspects of the virus–host interplay hold true in animal models [75].
Second, current approaches to modulate gene expression by siRNA or shRNA come with off-target effects and usually do not block the expression of a gene completely. Future screening approaches using CRISPR/cas9 methodology will overcome some of the limitations [76••, 77••, 78••]. CRISPR provides an elegant way of introducing targeted loss-of-function mutations at specific sites in the genome. The advantages include high screening sensitivity by homozygous knockouts, which is especially important when partial knockdown retains gene function. Moreover, whereas RNAi is limited to transcripts, CRISPR can target different elements across the genome, such as promoters, introns and intergenic regions. Catalytically inactive mutants of Cas9 linked to different functional domains can furthermore provide a means for genome-scale gain-of-function screening approaches using Cas9 activators, epigenetic modifiers or GFP-tagged Cas9 proteins to visualize genomic sequences [79, 80]. Specifically, the latter could provide a promising platform for imaging of viral sequences embedded within the native chromatin organization and dynamics in living human cells. Current limitations of the technology include the inability to target genes essential for cell survival, for example, main regulators of the cell cycle. Moreover, also for CRISPR/cas9 off-target effects have been described. Novel strategies to address this problem, such as the concerted action of paired nickases, are being pursued [79]. In sum, the CRISPR/cas9 methodology will be complementary to RNAi and can broaden the repertoire of perturbation modalities.
Conclusions
In recent years massive efforts have been undertaken to shed light on virus-host interactions via unbiased high-throughput screening approaches. Such genomics, proteomics, transcriptomics and other methodologies have already revealed exciting new insights into the intricate interplay between viruses and their hosts but the available datasets have even more potential. Currently, the integration of different datasets is difficult and often restricted by the availability of primary data. Future efforts should focus on providing a framework for primary datasets and comparative and integrated analysis to uncover the full potential of the screens. Ultimately, such a meta-analysis will shed light on the three-dimensional map of host-pathogen interactions, and support the development of novel antivirals, adjuvants, and vaccines.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
SSt is supported by grants from the Swiss National Science Foundation (31003A_156805) and the Novartis Research Foundation. RK is supported by grants from the German Center for Infection Research (DZIF). We thank Nina Hein-Fuchs for assisting in designing the tables and together with Carsten Münk for critical reading of the manuscript.
Contributor Information
Renate König, Email: Renate.Koenig@pei.de.
Silke Stertz, Email: stertz.silke@virology.uzh.ch.
References
- 1.Nurse P., Hayles J. The cell in an era of systems biology. Cell. 2011;144:850–854. doi: 10.1016/j.cell.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 2.Konig R., Stertz S., Zhou Y., Inoue A., Hoffmann H.H., Bhattacharyya S., Alamares J.G., Tscherne D.M., Ortigoza M.B., Liang Y. Human host factors required for influenza virus replication. Nature. 2010;463:813–817. doi: 10.1038/nature08699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Brass A.L., Huang I.C., Benita Y., John S.P., Krishnan M.N., Feeley E.M., Ryan B.J., Weyer J.L., van der Weyden L., Fikrig E. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell. 2009;139:1243–1254. doi: 10.1016/j.cell.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study revealed the antiviral potential of the IFITM proteins as a result of a genome-wide RNAi screen.
- 4.Karlas A., Machuy N., Shin Y., Pleissner K.P., Artarini A., Heuer D., Becker D., Khalil H., Ogilvie L.A., Hess S. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature. 2010;463:818–822. doi: 10.1038/nature08760. [DOI] [PubMed] [Google Scholar]
- 5.Ward S.E., Kim H.S., Komurov K., Mendiratta S., Tsai P.L., Schmolke M., Satterly N., Manicassamy B., Forst C.V., Roth M.G. Host modulators of H1N1 cytopathogenicity. PLoS ONE. 2012;7:e39284. doi: 10.1371/journal.pone.0039284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Su W.C., Chen Y.C., Tseng C.H., Hsu P.W., Tung K.F., Jeng K.S., Lai M.M. Pooled RNAi screen identifies ubiquitin ligase Itch as crucial for influenza A virus release from the endosome during virus entry. Proc Natl Acad Sci U S A. 2013;110:17516–17521. doi: 10.1073/pnas.1312374110. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an important follow-up study from RNAi screens for IAV host factors providing mechanistic insight into IAV uncoating.
- 7.Tran A.T., Rahim M.N., Ranadheera C., Kroeker A., Cortens J.P., Opanubi K.J., Wilkins J.A., Coombs K.M. Knockdown of specific host factors protects against influenza virus-induced cell death. Cell Death Dis. 2013;4:e769. doi: 10.1038/cddis.2013.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Konig R., Zhou Y., Elleder D., Diamond T.L., Bonamy G.M., Irelan J.T., Chiang C.Y., Tu B.P., De Jesus P.D., Lilley C.E. Global analysis of host–pathogen interactions that regulate early-stage HIV-1 replication. Cell. 2008;135:49–60. doi: 10.1016/j.cell.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study proposes for the first time a molecular coupling between nuclear import of the viral PIC and viral DNA integration processes.
- 9.Brass A.L., Dykxhoorn D.M., Benita Y., Yan N., Engelman A., Xavier R.J., Lieberman J., Elledge S.J. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H., Xu M., Huang Q., Gates A.T., Zhang X.D., Castle J.C., Stec E., Ferrer M., Strulovici B., Hazuda D.J. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe. 2008;4:495–504. doi: 10.1016/j.chom.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Genovesio A., Kwon Y.J., Windisch M.P., Kim N.Y., Choi S.Y., Kim H.C., Jung S., Mammano F., Perrin V., Boese A.S. Automated genome-wide visual profiling of cellular proteins involved in HIV infection. J Biomol Screen. 2011;16:945–958. doi: 10.1177/1087057111415521. [DOI] [PubMed] [Google Scholar]
- 12.Yeung M.L., Houzet L., Yedavalli V.S., Jeang K.T. A genome-wide short hairpin RNA screening of jurkat T-cells for human proteins contributing to productive HIV-1 replication. J Biol Chem. 2009;284:19463–19473. doi: 10.1074/jbc.M109.010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Jager S., Cimermancic P., Gulbahce N., Johnson J.R., McGovern K.E., Clarke S.C., Shales M., Mercenne G., Pache L., Li K. Global landscape of HIV–human protein complexes. Nature. 2012;481:365–370. doi: 10.1038/nature10719. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first global proteomics approach on viral proteins interacting with the host proteome.
- 14.Davis Z.H., Verschueren E., Jang G.M., Kleffman K., Johnson J.R., Park J., Von Dollen J., Maher M.C., Johnson T., Newton W. Global mapping of herpesvirus–host protein complexes reveals a transcription strategy for late genes. Mol Cell. 2015;57:349–360. doi: 10.1016/j.molcel.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapira S.D., Gat-Viks I., Shum B.O., Dricot A., de Grace M.M., Wu L., Gupta P.B., Hao T., Silver S.J., Root D.E. A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infection. Cell. 2009;139:1255–1267. doi: 10.1016/j.cell.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe T., Kawakami E., Shoemaker J.E., Lopes T.J., Matsuoka Y., Tomita Y., Kozuka-Hata H., Gorai T., Kuwahara T., Takeda E. Influenza virus–host interactome screen as a platform for antiviral drug development. Cell Host Microbe. 2014;16:795–805. doi: 10.1016/j.chom.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramage H.R., Kumar G.R., Verschueren E., Johnson J.R., Von Dollen J., Johnson T., Newton B., Shah P., Horner J., Krogan N.J. A combined proteomics/genomics approach links hepatitis C virus infection with nonsense-mediated mRNA decay. Mol Cell. 2015;57:329–340. doi: 10.1016/j.molcel.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pichlmair A., Kandasamy K., Alvisi G., Mulhern O., Sacco R., Habjan M., Binder M., Stefanovic A., Eberle C.A., Goncalves A. Viral immune modulators perturb the human molecular network by common and unique strategies. Nature. 2012;487:486–490. doi: 10.1038/nature11289. [DOI] [PubMed] [Google Scholar]
- 19.Schoggins J.W., MacDuff D.A., Imanaka N., Gainey M.D., Shrestha B., Eitson J.L., Mar K.B., Richardson R.B., Ratushny A.V., Litvak V. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M.N., Roy M., Ong S.E., Mertins P., Villani A.C., Li W., Dotiwala F., Sen J., Doench J.G., Orzalli M.H. Identification of regulators of the innate immune response to cytosolic DNA and retroviral infection by an integrative approach. Nat Immunol. 2013;14:179–185. doi: 10.1038/ni.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chevrier N., Mertins P., Artyomov M.N., Shalek A.K., Iannacone M., Ciaccio M.F., Gat-Viks I., Tonti E., DeGrace M.M., Clauser K.R. Systematic discovery of TLR signaling components delineates viral-sensing circuits. Cell. 2011;147:853–867. doi: 10.1016/j.cell.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang C.Y., Engel A., Opaluch A.M., Ramos I., Maestre A.M., Secundino I., De Jesus P.D., Nguyen Q.T., Welch G., Bonamy G.M. Cofactors required for TLR7- and TLR9-dependent innate immune responses. Cell Host Microbe. 2012;11:306–318. doi: 10.1016/j.chom.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S., Wang L., Berman M., Kong Y.Y., Dorf M.E. Mapping a dynamic innate immunity protein interaction network regulating type I interferon production. Immunity. 2011;35:426–440. doi: 10.1016/j.immuni.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahon C., Krogan N.J., Craik C.S., Pick E. Cullin E3 ligases and their rewiring by viral factors. Biomolecules. 2014;4:897–930. doi: 10.3390/biom4040897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salomon D., Orth K. What pathogens have taught us about posttranslational modifications. Cell Host Microbe. 2013;14:269–279. doi: 10.1016/j.chom.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Wojcechowskyj J.A., Didigu C.A., Lee J.Y., Parrish N.F., Sinha R., Hahn B.H., Bushman F.D., Jensen S.T., Seeholzer S.H., Doms R.W. Quantitative phosphoproteomics reveals extensive cellular reprogramming during HIV-1 entry. Cell Host Microbe. 2013;13:613–623. doi: 10.1016/j.chom.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first description of global phospho-proteomics upon entry of HIV-1 into target cells.
- 27.Pohl M.O., Edinger T.O., Stertz S. Prolidase is required for early trafficking events during influenza A virus entry. J Virol. 2014;88:11271–11283. doi: 10.1128/JVI.00800-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He J., Sun E., Bujny M.V., Kim D., Davidson M.W., Zhuang X. Dual function of CD81 in influenza virus uncoating and budding. PLoS Pathog. 2013;9:e1003701. doi: 10.1371/journal.ppat.1003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw M.L., Stone K.L., Colangelo C.M., Gulcicek E.E., Palese P. Cellular proteins in influenza virus particles. PLoS Pathog. 2008;4:e1000085. doi: 10.1371/journal.ppat.1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Banerjee I., Miyake Y., Nobs S.P., Schneider C., Horvath P., Kopf M., Matthias P., Helenius A., Yamauchi Y. Influenza: a virus uses the aggresome processing machinery for host cell entry. Science. 2014;346:473–477. doi: 10.1126/science.1257037. [DOI] [PubMed] [Google Scholar]; This is an important follow-up study from RNAi screens for IAV host factors providing mechanistic insight into IAV uncoating.
- 31.Yamauchi Y., Boukari H., Banerjee I., Sbalzarini I.F., Horvath P., Helenius A. Histone deacetylase 8 is required for centrosome cohesion and influenza A virus entry. PLoS Pathog. 2011;7:e1002316. doi: 10.1371/journal.ppat.1002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang I.C., Bailey C.C., Weyer J.L., Radoshitzky S.R., Becker M.M., Chiang J.J., Brass A.L., Ahmed A.A., Chi X., Dong L. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 2011;7:e1001258. doi: 10.1371/journal.ppat.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mudhasani R., Tran J.P., Retterer C., Radoshitzky S.R., Kota K.P., Altamura L.A., Smith J.M., Packard B.Z., Kuhn J.H., Costantino J. IFITM-2 and IFITM-3 but not IFITM-1 restrict Rift Valley fever virus. J Virol. 2013;87:8451–8464. doi: 10.1128/JVI.03382-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu J., Pan Q., Rong L., He W., Liu S.L., Liang C. The IFITM proteins inhibit HIV-1 infection. J Virol. 2011;85:2126–2137. doi: 10.1128/JVI.01531-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anafu A.A., Bowen C.H., Chin C.R., Brass A.L., Holm G.H. Interferon-inducible transmembrane protein 3 (IFITM3) restricts reovirus cell entry. J Biol Chem. 2013;288:17261–17271. doi: 10.1074/jbc.M112.438515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feeley E.M., Sims J.S., John S.P., Chin C.R., Pertel T., Chen L.M., Gaiha G.D., Ryan B.J., Donis R.O., Elledge S.J. IFITM3 inhibits influenza A virus infection by preventing cytosolic entry. PLoS Pathog. 2011;7:e1002337. doi: 10.1371/journal.ppat.1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Everitt A.R., Clare S., Pertel T., John S.P., Wash R.S., Smith S.E., Chin C.R., Feeley E.M., Sims J.S., Adams D.J. IFITM3 restricts the morbidity and mortality associated with influenza. Nature. 2012;484:519–523. doi: 10.1038/nature10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benfield C., Smith S.E., Wright E., Wash R.S., Ferrara F., Temperton N.J., Kellam P. Bat and pig interferon-induced transmembrane protein 3 restrict cell entry by influenza virus and lyssaviruses. J Gen Virol. 2015 doi: 10.1099/vir.0.000058. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith S.E., Gibson M.S., Wash R.S., Ferrara F., Wright E., Temperton N., Kellam P., Fife M. Chicken interferon-inducible transmembrane protein 3 restricts influenza viruses and lyssaviruses in vitro. J Virol. 2013;87:12957–12966. doi: 10.1128/JVI.01443-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanz C., Yanguez E., Andenmatten D., Stertz S. Swine interferon-inducible transmembrane proteins potently inhibit influenza A virus replication. J Virol. 2015;89:863–869. doi: 10.1128/JVI.02516-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desai T.M., Marin M., Chin C.R., Savidis G., Brass A.L., Melikyan G.B. IFITM3 restricts influenza A virus entry by blocking the formation of fusion pores following virus-endosome hemifusion. PLoS Pathog. 2014;10:e1004048. doi: 10.1371/journal.ppat.1004048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey C.C., Zhong G., Huang I.C., Farzan M. IFITM-family proteins: the cell's first line of antiviral defense. Annu Rev Virol. 2014;1:261–283. doi: 10.1146/annurev-virology-031413-085537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amini-Bavil-Olyaee S., Choi Y.J., Lee J.H., Shi M., Huang I.C., Farzan M., Jung J.U. The antiviral effector IFITM3 disrupts intracellular cholesterol homeostasis to block viral entry. Cell Host Microbe. 2013;13:452–464. doi: 10.1016/j.chom.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Compton A.A., Bruel T., Porrot F., Mallet A., Sachse M., Euvrard M., Liang C., Casartelli N., Schwartz O. IFITM proteins incorporated into HIV-1 virions impair viral fusion and spread. Cell Host Microbe. 2014;16:736–747. doi: 10.1016/j.chom.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study described for the first time that IFITM proteins get incorporated into HIV virions.
- 45••.Tartour K., Appourchaux R., Gaillard J., Nguyen X.N., Durand S., Turpin J., Beaumont E., Roch E., Berger G., Mahieux R. IFITM proteins are incorporated onto HIV-1 virion particles and negatively imprint their infectivity. Retrovirology. 2014;11:103. doi: 10.1186/s12977-014-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; See annotation to Ref. [44••].
- 46.Zhang Y.H., Zhao Y., Li N., Peng Y.C., Giannoulatou E., Jin R.H., Yan H.P., Wu H., Liu J.H., Liu N. Interferon-induced transmembrane protein-3 genetic variant rs12252-C is associated with severe influenza in Chinese individuals. Nat Commun. 2013;4:1418. doi: 10.1038/ncomms2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mills T.C., Rautanen A., Elliott K.S., Parks T., Naranbhai V., Ieven M.M., Butler C.C., Little P., Verheij T., Garrard C.S. IFITM3 and susceptibility to respiratory viral infections in the community. J Infect Dis. 2014;209:1028–1031. doi: 10.1093/infdis/jit468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams D.E., Wu W.L., Grotefend C.R., Radic V., Chung C., Chung Y.H., Farzan M., Huang I.C. IFITM3 polymorphism rs12252-C restricts influenza A viruses. PLOS ONE. 2014;9:e110096. doi: 10.1371/journal.pone.0110096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christ F., Thys W., De Rijck J., Gijsbers R., Albanese A., Arosio D., Emiliani S., Rain J.C., Benarous R., Cereseto A. Transportin-SR2 imports HIV into the nucleus. Curr Biol. 2008;18:1192–1202. doi: 10.1016/j.cub.2008.07.079. [DOI] [PubMed] [Google Scholar]
- 50.Hilditch L., Towers G.J. A model for cofactor use during HIV-1 reverse transcription and nuclear entry. Curr Opin Virol. 2014;4:32–36. doi: 10.1016/j.coviro.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Iaco A., Santoni F., Vannier A., Guipponi M., Antonarakis S., Luban J. TNPO3 protects HIV-1 replication from CPSF6-mediated capsid stabilization in the host cell cytoplasm. Retrovirology. 2013;10:20. doi: 10.1186/1742-4690-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Nunzio F., Fricke T., Miccio A., Valle-Casuso J.C., Perez P., Souque P., Rizzi E., Severgnini M., Mavilio F., Charneau P. Nup153 and Nup98 bind the HIV-1 core and contribute to the early steps of HIV-1 replication. Virology. 2013;440:8–18. doi: 10.1016/j.virol.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Ocwieja K.E., Brady T.L., Ronen K., Huegel A., Roth S.L., Schaller T., James L.C., Towers G.J., Young J.A., Chanda S.K. HIV integration targeting: a pathway involving Transportin-3 and the nuclear pore protein RanBP2. PLoS Pathog. 2011;7:e1001313. doi: 10.1371/journal.ppat.1001313. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first demonstration of altered integration targeting of HIV after depletion of nuclear import factors.
- 54.Schaller T., Ocwieja K.E., Rasaiyaah J., Price A.J., Brady T.L., Roth S.L., Hue S., Fletcher A.J., Lee K., KewalRamani V.N. HIV-1 capsid-cyclophilin interactions determine nuclear import pathway, integration targeting and replication efficiency. PLoS Pathog. 2011;7:e1002439. doi: 10.1371/journal.ppat.1002439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luban J., Bossolt K.L., Franke E.K., Kalpana G.V., Goff S.P. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell. 1993;73:1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- 56.Lee K., Ambrose Z., Martin T.D., Oztop I., Mulky A., Julias J.G., Vandegraaff N., Baumann J.G., Wang R., Yuen W. Flexible use of nuclear import pathways by HIV-1. Cell Host Microbe. 2010;7:221–233. doi: 10.1016/j.chom.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price A.J., Fletcher A.J., Schaller T., Elliott T., Lee K., KewalRamani V.N., Chin J.W., Towers G.J., James L.C. CPSF6 defines a conserved capsid interface that modulates HIV-1 replication. PLoS Pathog. 2012;8:e1002896. doi: 10.1371/journal.ppat.1002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kataoka N., Bachorik J.L., Dreyfuss G. Transportin-SR: a nuclear import receptor for SR proteins. J Cell Biol. 1999;145:1145–1152. doi: 10.1083/jcb.145.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59••.Rasaiyaah J., Tan C.P., Fletcher A.J., Price A.J., Blondeau C., Hilditch L., Jacques D.A., Selwood D.L., James L.C., Noursadeghi M. HIV-1 evades innate immune recognition through specific cofactor recruitment. Nature. 2013;503:402–405. doi: 10.1038/nature12769. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes for the first time a link between cofactor usage and evasion of innate sensors.
- 60.Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goff S.P. HIV: slipping under the radar. Nature. 2013;503:352–353. doi: 10.1038/nature12707. [DOI] [PubMed] [Google Scholar]
- 62.Ambrose Z., Aiken C. HIV-1 uncoating: connection to nuclear entry and regulation by host proteins. Virology. 2014;454–455:371–379. doi: 10.1016/j.virol.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Schoggins J.W., Wilson S.J., Panis M., Murphy M.Y., Jones C.T., Bieniasz P., Rice C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study using an over-expression screening approach testing more than 300 ISGs on the inhibition of replication of a variety of viruses. Identified MxB as an inhibitor for HIV-1.
- 64.Goujon C., Moncorge O., Bauby H., Doyle T., Ward C.C., Schaller T., Hue S., Barclay W.S., Schulz R., Malim M.H. Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature. 2013;502:559–562. doi: 10.1038/nature12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Z., Pan Q., Ding S., Qian J., Xu F., Zhou J., Cen S., Guo F., Liang C. The interferon-inducible MxB protein inhibits HIV-1 infection. Cell Host Microbe. 2013;14:398–410. doi: 10.1016/j.chom.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 66.Kane M., Yadav S.S., Bitzegeio J., Kutluay S.B., Zang T., Wilson S.J., Schoggins J.W., Rice C.M., Yamashita M., Hatziioannou T. MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature. 2013;502:563–566. doi: 10.1038/nature12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fackler O.T., Keppler O.T. MxB/Mx2: the latest piece in HIV's interferon puzzle. EMBO Rep. 2013;14:1028–1029. doi: 10.1038/embor.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melen K., Keskinen P., Ronni T., Saveneva T., Lounatmaa K., Julkunen I. Human MxB protein, an interferon-a-inducible GTPase, contains a nuclear targeting signal and is localized in the heterochromatin region beneath the nuclear envelope. J Biol Chem. 1996;271:23478–23486. doi: 10.1074/jbc.271.38.23478. [DOI] [PubMed] [Google Scholar]
- 69•.Matreyek K.A., Wang W., Serrao E., Singh P., Levin H.L., Engelman A. Host and viral determinants for MxB restriction of HIV-1 infection. Retrovirology. 2014;11:90. doi: 10.1186/s12977-014-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study linking MxB to integration site selection.
- 70.Stertz S., Shaw M.L. Uncovering the global host cell requirements for influenza virus replication via RNAi screening. Microbes Infect. 2011;13:516–525. doi: 10.1016/j.micinf.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bushman F.D., Malani N., Fernandes J., D’Orso I., Cagney G., Diamond T.L., Zhou H., Hazuda D.J., Espeseth A.S., Konig R. Host cell factors in HIV replication: meta-analysis of genome-wide studies. PLoS Pathog. 2009;5:e1000437. doi: 10.1371/journal.ppat.1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watanabe T., Watanabe S., Kawaoka Y. Cellular networks involved in the influenza virus life cycle. Cell Host Microbe. 2010;7:427–439. doi: 10.1016/j.chom.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pache L., Konig R., Chanda S.K. Identifying HIV-1 host cell factors by genome-scale RNAi screening. Methods. 2011;53:3–12. doi: 10.1016/j.ymeth.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 74.Conlon J., Burdette D.L., Sharma S., Bhat N., Thompson M., Jiang Z., Rathinam V.A., Monks B., Jin T., Xiao T.S. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J Immunol. 2013;190:5216–5225. doi: 10.4049/jimmunol.1300097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Varble A., Benitez A.A., Schmid S., Sachs D., Shim J.V., Rodriguez-Barrueco R., Panis M., Crumiller M., Silva J.M., Sachidanandam R. An in vivo RNAi screening approach to identify host determinants of virus replication. Cell Host Microbe. 2013;14:346–356. doi: 10.1016/j.chom.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 76••.Wang T., Wei J.J., Sabatini D.M., Lander E.S. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014;343:80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first descriptions of genome-wide screening using CRISPR/cas9.
- 77••.Shalem O., Sanjana N.E., Hartenian E., Shi X., Scott D.A., Mikkelsen T.S., Heckl D., Ebert B.L., Root D.E., Doench J.G. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]; See annotation to Ref. [76••].
- 78••.Zhou Y., Zhu S., Cai C., Yuan P., Li C., Huang Y., Wei W. High-throughput screening of a CRISPR/Cas9 library for functional genomics in human cells. Nature. 2014;509:487–491. doi: 10.1038/nature13166. [DOI] [PubMed] [Google Scholar]; See annotation to Ref. [76••].
- 79.Sander J.D., Joung J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen B., Gilbert L.A., Cimini B.A., Schnitzbauer J., Zhang W., Li G.W., Park J., Blackburn E.H., Weissman J.S., Qi L.S. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155:1479–1491. doi: 10.1016/j.cell.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hao L., Sakurai A., Watanabe T., Sorensen E., Nidom C.A., Newton M.A., Ahlquist P., Kawaoka Y. Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature. 2008;454:890–893. doi: 10.1038/nature07151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sui B., Bamba D., Weng K., Ung H., Chang S., Van Dyke J., Goldblatt M., Duan R., Kinch M.S., Li W.B. The use of random homozygous gene perturbation to identify novel host-oriented targets for influenza. Virology. 2009;387:473–481. doi: 10.1016/j.virol.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nguyen D.G., Wolff K.C., Yin H., Caldwell J.S., Kuhen K.L. “UnPAKing” human immunodeficiency virus (HIV) replication: using small interfering RNA screening to identify novel cofactors and elucidate the role of group I PAKs in HIV infection. J Virol. 2006;80:130–137. doi: 10.1128/JVI.80.1.130-137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu L., Oliveira N.M., Cheney K.M., Pade C., Dreja H., Bergin A.M., Borgdorff V., Beach D.H., Bishop C.L., Dittmar M.T. A whole genome screen for HIV restriction factors. Retrovirology. 2011;8:94. doi: 10.1186/1742-4690-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nguyen D.G., Yin H., Zhou Y., Wolff K.C., Kuhen K.L., Caldwell J.S. Identification of novel therapeutic targets for HIV infection through functional genomic cDNA screening. Virology. 2007;362:16–25. doi: 10.1016/j.virol.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 86.Tai A.W., Benita Y., Peng L.F., Kim S.S., Sakamoto N., Xavier R.J., Chung R.T. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe. 2009;5:298–307. doi: 10.1016/j.chom.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Q., Brass A.L., Ng A., Hu Z., Xavier R.J., Liang T.J., Elledge S.J. A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc Natl Acad Sci U S A. 2009;106:16410–16415. doi: 10.1073/pnas.0907439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sessions O.M., Barrows N.J., Souza-Neto J.A., Robinson T.J., Hershey C.L., Rodgers M.A., Ramirez J.L., Dimopoulos G., Yang P.L., Pearson J.L. Discovery of insect and human dengue virus host factors. Nature. 2009;458:1047–1050. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krishnan M.N., Ng A., Sukumaran B., Gilfoy F.D., Uchil P.D., Sultana H., Brass A.L., Adametz R., Tsui M., Qian F. RNA interference screen for human genes associated with West Nile virus infection. Nature. 2008;455:242–245. doi: 10.1038/nature07207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Uetz P., Dong Y.A., Zeretzke C., Atzler C., Baiker A., Berger B., Rajagopala S.V., Roupelieva M., Rose D., Fossum E. Herpesviral protein networks and their interaction with the human proteome. Science. 2006;311:239–242. doi: 10.1126/science.1116804. [DOI] [PubMed] [Google Scholar]
- 91.Liu S.Y., Sanchez D.J., Aliyari R., Lu S., Cheng G. Systematic identification of type I and type II interferon-induced antiviral factors. Proc Natl Acad Sci U S A. 2012;109:4239–4244. doi: 10.1073/pnas.1114981109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fusco D.N., Brisac C., John S.P., Huang Y.W., Chin C.R., Xie T., Zhao H., Jilg N., Zhang L., Chevaliez S. A genetic screen identifies interferon-alpha effector genes required to suppress hepatitis C virus replication. Gastroenterology. 2013;144 doi: 10.1053/j.gastro.2013.02.026. 1438–1449, 1449, 1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao H., Lin W., Kumthip K., Cheng D., Fusco D.N., Hofmann O., Jilg N., Tai A.W., Goto K., Zhang L. A functional genomic screen reveals novel host genes that mediate interferon-alpha's effects against hepatitis C virus. J Hepatol. 2012;56:326–333. doi: 10.1016/j.jhep.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rozenblatt-Rosen O., Deo R.C., Padi M., Adelmant G., Calderwood M.A., Rolland T., Grace M., Dricot A., Askenazi M., Tavares M. Interpreting cancer genomes using systematic host network perturbations by tumour virus proteins. Nature. 2012;487:491–495. doi: 10.1038/nature11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pickett B.E., Sadat E.L., Zhang Y., Noronha J.M., Squires R.B., Hunt V., Liu M., Kumar S., Zaremba S., Gu Z. ViPR: an open bioinformatics database and analysis resource for virology research. Nucleic Acids Res. 2012;40:D593–D598. doi: 10.1093/nar/gkr859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chatr-aryamontri A., Ceol A., Peluso D., Nardozza A., Panni S., Sacco F., Tinti M., Smolyar A., Castagnoli L., Vidal M. VirusMINT: a viral protein interaction database. Nucleic Acids Res. 2009;37:D669–D673. doi: 10.1093/nar/gkn739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bushman F.D., Barton S., Bailey A., Greig C., Malani N., Bandyopadhyay S., Young J., Chanda S., Krogan N. Bringing it all together: big data and HIV research. AIDS. 2013;27:835–838. doi: 10.1097/QAD.0b013e32835cb785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fahey M.E., Bennett M.J., Mahon C., Jager S., Pache L., Kumar D., Shapiro A., Rao K., Chanda S.K., Craik C.S. GPS-Prot: a web-based visualization platform for integrating host–pathogen interaction data. BMC Bioinformatics. 2011;12:298. doi: 10.1186/1471-2105-12-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fu W., Sanders-Beer B.E., Katz K.S., Maglott D.R., Pruitt K.D., Ptak R.G. Human immunodeficiency virus type 1, human protein interaction database at NCBI. Nucleic Acids Res. 2009;37:D417–D422. doi: 10.1093/nar/gkn708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ptak R.G., Fu W., Sanders-Beer B.E., Dickerson J.E., Pinney J.W., Robertson D.L., Rozanov M.N., Katz K.S., Maglott D.R., Pruitt K.D. Cataloguing the HIV type 1 human protein interaction network. AIDS Res Hum Retroviruses. 2008;24:1497–1502. doi: 10.1089/aid.2008.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pinney J.W., Dickerson J.E., Fu W., Sanders-Beer B.E., Ptak R.G., Robertson D.L. HIV–host interactions: a map of viral perturbation of the host system. AIDS. 2009;23:549–554. doi: 10.1097/QAD.0b013e328325a495. [DOI] [PubMed] [Google Scholar]
- 102.Aderem A., Adkins J.N., Ansong C., Galagan J., Kaiser S., Korth M.J., Law G.L., McDermott J.G., Proll S.C., Rosenberger C. A systems biology approach to infectious disease research: innovating the pathogen–host research paradigm. mBio. 2011;2:e00310–e00325. doi: 10.1128/mBio.00325-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Squires R.B., Noronha J., Hunt V., Garcia-Sastre A., Macken C., Baumgarth N., Suarez D., Pickett B.E., Zhang Y., Larsen C.N. Influenza research database: an integrated bioinformatics resource for influenza research and surveillance. Influenza Other Respir Viruses. 2012;6:404–416. doi: 10.1111/j.1750-2659.2011.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matsuoka Y., Matsumae H., Katoh M., Eisfeld A.J., Neumann G., Hase T., Ghosh S., Shoemaker J.E., Lopes T.J., Watanabe T. A comprehensive map of the influenza A virus replication cycle. BMC Syst Biol. 2013;7:97. doi: 10.1186/1752-0509-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]