Abstract
Background
Human rhinoviruses (HRVs) are among the most common causes of community-acquired pneumonia (CAP) in children. However, the differential roles of the three HRV species HRV-A, HRV-B, and HRV-C in pediatric CAP are not fully understood.
Objective
To determine the distribution of HRV species and their roles in children hospitalized with CAP in Beijing, China.
Study design
Nasopharyngeal aspirates were collected between April 2007 and March 2008 from 554 children with a primary diagnosis of CAP. HRVs in the clinical samples were detected by RT-PCR and by sequencing. Infections with other respiratory viruses were identified by PCR.
Results
HRVs were detected in 99 patients (17.87%). Among these patients, 51.52% tested positive for HRV-A, 38.38% for HRV-C, and 10.10% for HRV-B. HRVs were detected throughout the study period. The monthly distribution of HRV infections varied with HRV species. Median age, gender, symptoms, severity, and duration of hospitalization for single HRV-C infections were similar to those observed for single HRV-A infections. Co-infections with other respiratory viruses were detected in 57.58% of the HRV-positive children. HRV/RSV dual infections were correlated with a higher frequency of shortness of breath (HRV-A group, P2tail = 0.01; HRV-C group, P2tail = 0.015) and lower median ages (HRV-A group, P2tail = 0.049; HRV-C group, P2tail = 0.009).
Conclusion
Our study shows that HRV-C strains circulate at a prevalence intermediate between HRV-A and HRV-B. The severity of clinical manifestations for HRV-C is comparable to that for HRV-A in children with CAP. These findings point to an important role of both HRV-A and HRV-C in pediatric CAP.
Keywords: Rhinovirus, Clinical manifestation, Etiology, Community-acquired pneumonia, Children
1. Background
Community-acquired pneumonia (CAP) causes a high percentage of morbidity and mortality among children worldwide.1 Viruses account for 14–35% of pediatric CAP.2 Next to respiratory syncytial viruses (RSV), human rhinoviruses (HRVs) are the most common viral pathogens responsible for CAP3, 4 and the most frequently detected viruses in upper respiratory tract infections (RTIs).5, 6
HRVs are non-enveloped, positive single-stranded RNA viruses of the Picornaviridae family. Based on nucleotide sequence homologies, HRVs can be divided into three genetic species, HRV-A, HRV-B,7 and the recently identified HRV-C.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21
Studies of HRV-C in pediatric RTIs have focused on the molecular identification of HRV-C from clinical samples. Few studies have evaluated the clinical significance of HRV-C infections in association with those of HRV-A and HRV-B within the same cohort. We previously reported the presence of HRV-C in hospitalized children with lower RTIs in Beijing, China.21 However, to determine the differential roles of these HRV species in pediatric CAP, all HRV species need to be analyzed.
2. Objective
To determine the distribution of HRV species and their roles in hospitalized children with CAP in Beijing, China.
3. Study design
3.1. Subjects and clinical samples
From April 2007 through March 2008, nasopharyngeal aspirates were collected from 554 pediatric patients (male: female ratio, 1.7; median age, 8.95 months; age range, 0.6–190 months) with a primary diagnosis of viral CAP upon admission to the Beijing Children's Hospital. CAP was clinically defined as the presence of signs and symptoms of pneumonia due to an infection acquired outside the hospital.2 Individual CAP cases were classified as mild or severe according to the guidelines recommended by the Chinese Medical Association (Table 1 ).22 Clinical information was collected with each sample. Samples were stored at −80 °C prior to use.
Table 1.
Disease severity assessment of community-acquired pneumonia (CAP)a.
| Mild | Severe | |
|---|---|---|
| Infants | 1. Axillary temperature <38.5 °C | 1. Axillary temperature ≥38.5 °C |
| 2. Increased respiratory rate but <70 breaths/min | 2. Respiratory rate ≥70 breaths/min | |
| Chest wall inspiratory pitting | ||
| Nasal flaring | ||
| Cyanosis | ||
| Intermittent apnea | ||
| Grunting respiration | ||
| 3. Taking full feeds | 3. Not feeding | |
| Older children | 1.Axillary temperature <38.5 °C | 1. Axillary temperature ≥38.5 °C |
| 2.Increased respiratory rate but <50 breaths/min | 2. Respiratory rate ≥50 breaths/min | |
| Nasal flaring | ||
| Cyanosis | ||
| Grunting respiration | ||
| 3. No signs of dehydration | 3. Signs of dehydration | |
Adopted from Ref. [22].
3.2. HRV detection by specific RT-PCR
To detect HRVs, RT-PCR was performed after nucleic acid extraction using the NucliSens easyMAG™ platform (bioMérieux, Marcy l’Etoile, France). Because one primer pair is not sufficient to detect all HRV strains due to the high degree of viral genome diversity, we used three sets of primers to detect and sequence HRVs. Two independent primer pairs were employed to amplify the HRV VP4/VP2 gene junction.7, 21 Primers 9895F (5′-GGGACCAACTACTTTGGGTGTCCGTGT-3′) and 9565R (5′-GCATCIGGYARYTTCCACCACCANCC-3′) were used to amplify the VP4/VP2 region in most of the known HRV species. Primers RVC556F (5′-ACTACTTTGGGTGTCCGTGTTTC-3′) and RVC886R (5′-TTTCCRATAGTGATTTGCTTKAGCC-3′) were used to identify the VP4/VP2 region of HRV-C. Samples negative for these screenings were subjected to a third PCR using primers that amplify the 5′-UTR of HRVs15: primer P1-1 (5′-CAAGCACTTCTGTYWCCCC-3′), P3-1 (5′-ACGGACACCCAAAGTAG-3′), P2-1 (5′-TTAGCCACATTCAGGGGC-3′), P2-2 (5′-TTAGCCACATTCAGGAGCC-3′), and P2-3 (5′-TTAGCCGCATTCAGGGG-3′). All PCR products were cloned into a pMD-18T vector and were verified by sequencing (Takara, Dalian, China).
3.3. Multiple virus detection
All samples were simultaneously screened for other viral infections, including for human parainfluenza virus, influenza virus, respiratory syncytial virus (RSV), enterovirus, human coronaviruses (229E, NL63, HKU1, and OC43), metapneumovirus, adenovirus, and bocavirus. Screens were performed using multiplex RT-PCR, or single RT-PCR assays.23
3.4. Statistical analysis
The association of demographic and clinical symptoms was compared between different patient groups using the chi-square test (χ 2 test). The Fisher exact test was used to analyze demographic and clinical data of patients with single or dual viral infections. Student's t-tests were used to compare the duration of hospitalization in patients with or without HRV/RSV co-infections, and in patients with or without co-morbidity. P-values < 0.05 were considered statistically significant.
3.5. Accession numbers
Nucleotide sequences generated by this work are available at GenBank (accession numbers GU568038–GU568122).
4. Results
4.1. HRV detection in clinical specimens
RT-PCR analysis indicated that HRV infections were found in 99 (17.87%) CAP patients (73 males and 26 females). HRV-positive patients ranged in age from 1 month to 13 years (median age: 6 months; average age: 20.2 months). Of the HRV-infected patients, 51 (51.52%) tested positive for HRV-A, 38 (38.38%) for HRV-C, and only 10 (10.10%) for HRV-B (Table 2 ).
Table 2.
Incidences of HRV infections in different age groups.
| Age (months) | No. of samples | Number of positive samples (%) |
|||
|---|---|---|---|---|---|
| HRV-A | HRV-B | HRV-C | HRV+ | ||
| ≤6 | 246 | 31 (12.60%) | 5 (2.03%) | 18 (7.32%) | 54 (21.95%) |
| 6.1 to ≤12 | 81 | 9 (11.11%) | 0 | 8 (9.88%) | 17 (20.99%) |
| 12.1 to ≤24 | 34 | 3 (8.82%) | 2 (5.88%) | 4 (11.76%) | 9 (26.47%) |
| 24.1 to ≤36 | 23 | 3 (13.04%) | 0 | 2 (8.70%) | 5 (21.74%) |
| 36.1 to ≤72 | 46 | 2 (4.35%) | 0 | 3 (6.52%) | 5 (10.87%) |
| >72.1 | 124 | 3 (2.42%)a | 3 (2.42%) | 3 (2.42%) | 9 (7.26%)b |
| Total | 554 | 51 (9.21%) | 10 (1.81%) | 38 (6.86%) | 99 (17.87%) |
Incidence of this age group is significantly lower than that of other age groups: χ2 = 12.29, P < 0.05.
Incidence of this age group is significantly lower than that of other age groups: χ2 = 16.33, P < 0.01.
Demographic analysis indicated that the overall HRV-positive rate of the older age group (>72.1 months; 7.26%) was about 2.9 times lower than that of the younger age group (≤72 months; 20.93%) (χ 2 = 16.33, P < 0.01) (Table 2). The older group represented 22.38% of all the subjects studied but only accounted for 9.09% of the HRV-positive patients. This difference may be attributed to the lower incidence of HRV-A in the older age group (χ 2 = 12.29, P < 0.05). HRVs were identified primarily in males (73.74%), indicating that gender may be a risk factor for pediatric HRV infection.
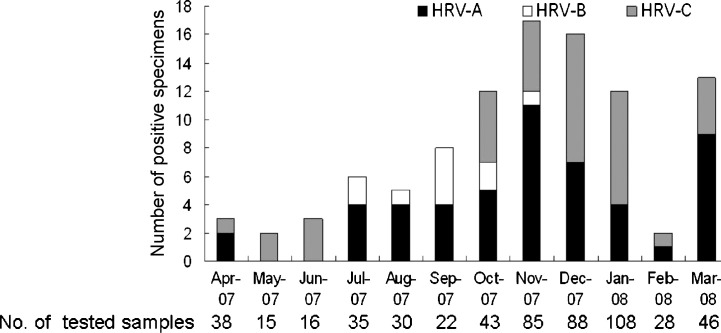
HRVs were detected throughout the study period. The monthly distribution of HRV infections in the children assessed varied with HRV species (Fig. 1 ). HRV-A was not detected in May and June of 2007. HRV-B was only detected between July and November of 2007. HRV-C was not detected from July to September 2007. HRV-C was the only HRV group detected in May and June 2007.
Fig. 1.
Seasonal distribution of the different HRV species in children with CAP. Detection numbers of HRV-A, HRV-B, and HRV-C positive samples are shown for the indicated month. The number of samples analyzed during each month is indicated under the corresponding column.
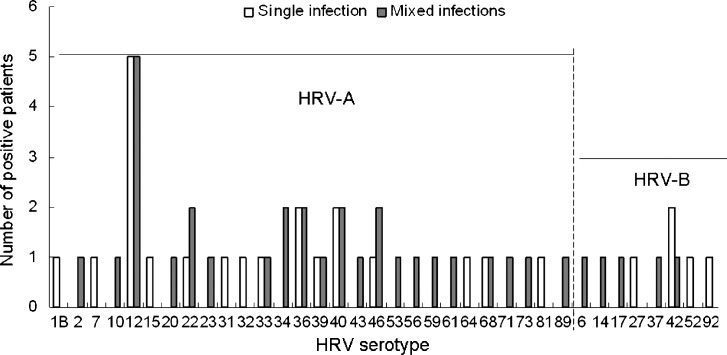
Multiple HRV-A and -B serotypes co-circulated in patients during the study period (Fig. 2 ). We detected 61 HRV strains closely related to prototype strains of 36 HRV-A or -B serotypes. The most frequently detected, closest prototype strain for HRV-A was HRV12 (n = 10); while that for HRV-B was HRV42 (n = 3). Overall, the pattern of major HRV serotypes circulating in children differs from that reported in adults.24 Furthermore, in one 2-month-old male, we found a recurrent HRV-A infection with two different serotypes, HRV-39 and HRV-12, within a 10-day interval.
Fig. 2.
Serotype distribution in single infections or mixed infections with HRV-A or HRV-B. All HRV serotypes of HRV-A and HRV-B detected in this study are shown, including 28 HRV-A serotypes and 8 HRV-B serotypes.
4.2. Co-infection with other respiratory tract viruses
Co-infections with other respiratory viruses were frequently detected in HRV-positive patients (n = 57, 57.58%) (Table 3 ). The virus most frequently detected with HRV was RSV (n = 39, 39.39%). Other co-infections included human parainfluenza virus (n = 10, 10.10%), adenovirus (n = 6, 6.06%), bocavirus (n = 5, 5.05%), influenza virus B (n = 3, 3.03%), human coronavirus (n = 2, 2.02%), enterovirus (n = 1, 1.01%), and metapneumovirus (n = 1, 1.01%).
Table 3.
Mixed viral infections detected in HRV infected children.
| HRV-A (N = 51) |
HRV-B (N = 10) |
HRV-C (N = 38) |
Total (N = 99) |
|
|---|---|---|---|---|
| None | 21 | 5 | 16 | 42 |
| RSV | 19a | 1 | 19b,c,d,e | 39 |
| PIV3 | 5b | 1 | 1 | 7 |
| PIV4 | 1 | 1 | 1 | 3 |
| HBoV | 4 | 1f | 0 | 5 |
| AdV | 2 | 1 | 3 | 6 |
| HCoV NL63 | 0 | 0 | 1 | 1 |
| HCoV HKU1 | 1 | 0 | 0 | 1 |
| Influenza virus B | 1 | 0 | 2 | 3 |
| Enterovirus | 0 | 1 | 0 | 1 |
| hMPV | 0 | 0 | 1 | 1 |
Notes: More than two (three or four) viruses were co-detected in some patients. The additional viruses detected are indicated with superscripts to show the names of such viruses and number of patients.
PIV: human parainfluenza virus; RSV: respiratory syncytial virus; HCoV: human coronaviruses; hMPV: human metapneumovirus; AdV: adenovirus; HBoV: human bocavirus.
In one patient, PIV4 was co-detected.
In two patients, AdV was co-detected.
In one patient, AdV and hMPV were co-detected.
In one patient, HCoV NL63 was co-detected.
In one patient, influenza virus B was co-detected.
In this patient, enterovirus was co-detected.
4.3. Clinical manifestations of children with HRV-A or HRV-C infection
To determine differences in clinical manifestations between HRV-A and HRV-C infections, we compared the demographic data and symptomatology between patients infected with HRV-A and patients infected with HRV-C (Table 4 ). HRV-B was excluded due to the small number of positive patients. Cough, shortness of breath, and fever were the major symptoms observed in pediatric HRV-A or HRV-C infections. Abnormal breath sounds on auscultation were the main sign reported for children infected with HRV-A or HRV-C. Chest radiographies often showed increased lung markings or patchy shadows in infected patients. No statistically significant difference was observed between patients infected with HRV-A and HRV-C in regards to a mixed infection ratio or co-detected virus type.
Table 4.
Demographic data and clinical symptoms observed in HRV-A or HRV-C infected children.
| HRV-A | HRV-A/RSV | HRV-C | HRV-C/RSV | |
|---|---|---|---|---|
| Single infection | Co-infections | Single infection | Co-infections | |
| (N = 21) | (N = 18) | (N = 16) | (N = 14) | |
| Age (months) | ||||
| ≤6a | 8 (38.10%) | 14 (77.78%) | 4 (25%) | 10 (71.43%) |
| 6.1 to ≤12 | 4 (19.05%) | 2 (11.11%) | 2 (12.5%) | 4 (28.57) |
| 12.1 to ≤24 | 2 (9.52%) | 1 (5.56%) | 2 (12.5%) | 0 |
| 24.1 to ≤36 | 2 (9.52%) | 1 (5.56%) | 2 (12.5%) | 0 |
| 36.1 to ≤72 | 2 (9.52%) | 0 | 3 (18.75%) | 0 |
| >72.1 | 3 (14.29%) | 0 | 3 (18.75%) | 0 |
| Range | 1.2–141.2 | 1–26.3 | 1.2–159.8 | 1–11 |
| Medianb | 12 | 4.55 | 24.45 | 4 |
| Male (%) | 17 (80.95%) | 13 (72.22%) | 12 (75%) | 10 (71.43%) |
| Symptoms and signs | ||||
| Cough | 20 (95.24%) | 18 (100%) | 15 (93.75%) | 14 (100%) |
| Shortness of breathc | 6 (28.57%) | 13 (72.22%) | 6 (37.5%) | 11 (78.57%) |
| Fever | 11 (52.38%) | 5 (27.78%) | 7 (43.75%) | 5 (35.71%) |
| Swelling of tonsils | 2 (9.52%) | 0 | 1 (6.25%) | 1 (7.14%) |
| Expectoration | 10 (47.62%) | 4 (22.22%) | 4 (25%) | 4 (28.57%) |
| Rhinorrhea | 3 (14.29%) | 3 (16.67%) | 3 (18.75%) | 1 (7.14%) |
| Sneezing | 1 (4.76%) | 0 | 1 (6.25%) | 0 |
| Abnormal breath sounds on auscultation | 16 (76.19%) | 16 (88.89%) | 11 (68.75%) | 13 (92.86%) |
| Duration of hospitalization (days) | ||||
| Range | 3–42 | 5–37 | 3–29 | 5–11 |
| Median | 10 | 12.5 | 10 | 9 |
| Mean | 14.33 ± 10.15 | 14.83 ± 9.34 | 11.6 ± 6.51 | 8.71 ± 1.38 |
| Severity | ||||
| Mild | 4 (19.05%) | 2 (11.11%) | 2 (12.5%) | 1 (7.14%) |
| Severe (I)d | 9 (42.86%) | 6 (33.33%) | 8 (50%) | 5 (35.71%) |
| Severe (II)d | 7 (33.33%) | 5 (27.78%) | 4 (25%) | 8 (57.14%) |
| Severe (III)d | 1 (4.76%) | 5 (27.78%) | 2 (12.5%) | 0 |
| Respiratory failure | 4 (19.05%) | 9 (50%) | 2 (12.5%) | 1 (7.14%) |
| Nasal continuous positive airway pressure | 4 (19.05%) | 6 (33.33%) | 0 | 1 (7.14%) |
Fisher exact test: HRV-A single infection vs HRV-A/RSV dual infection, P2tail = 0.02; HRV-C single infection vs HRV-C/RSV dual infection, P2tail = 0.02.
Fisher exact test: HRV-A single infection vs HRV-A/RSV dual infection, P2tail = 0.049; HRV-C single infection vs HRV-C/RSV dual infection, P2tail = 0.009.
Fisher exact test: HRV-A single infection vs HRV-A/RSV dual infection, P2tail = 0.01; HRV-C single infection vs HRV-C/RSV dual infection, P2tail = 0.015.
In the disease severity assessment, children who had one of the three severe indices in Table 1 were labeled severe (I), children who had two of three severe indices were labeled severe (II) , and children who had all the three severe indices were labeled severe (III).
To avoid overrepresentation of HRV infections in patients with multiple viral infections, we compared the demographic and clinical data between patients with HRV-A single infections (n = 21) and patients with HRV-C single infections (n = 16). However, we found no statistically significant differences when median age, gender, symptomatology, and time hospitalized were compared between these subgroups. These findings indicate that children with HRV-A infection and children with HRV-C infection show similar clinical manifestations (Table 4).
Given the high ratio of HRV/RSV co-infections (39/99), we investigated whether differences exist between patients with HRV/RSV co-infections and patients with HRV single infections (Table 4). In both the HRV-A group and the HRV-C group, the median ages of patients co-infected with RSV were lower than those of patients with single infections (HRV-A group, P 2tail = 0.049; HRV-C group, P 2tail = 0.009). Notably, children ≤6 months old were significantly more affected by HRV/RSV co-infections than by single HRV infections (HRV-A group, P 2tail = 0.02; HRV-C group, P 2tail = 0.02).
HRV/RSV co-infections were correlated with increased frequency in shortness of breath (HRV-A/RSV group, P 2tail = 0.01; HRV-C/RSV group, P 2tail = 0.015). In the HRV-A group, more patients with co-infections exhibited three severe indices (5:1, P 2tail = 0.07) (Table 1) and respiratory failure (9:4, P 2tail = 0.09), although no statistically significant differences were found.
Co-morbidities were observed in 32.32% of HRV-positive patients. Congenital cardiac disease including patent foramen ovale (n = 13, 13.13%), respiratory failure (n = 9, 9.09%) and heart failure (n = 5, 5.05%) were the most frequently observed co-morbidities. Aside from increased prevalence in males versus females (χ 2 = 4.62, P < 0.05), we found no significant differences in demographic data and clinical manifestations between HRV-positive patients with co-morbidities (87.50%) and without co-morbidities (67.16%) (Table 5 ).
Table 5.
Disease severity and viral co-infections in HRV-positive patients with or without co-morbidities.
| Patients with no co-morbidity | Patients with co-morbidities | |
|---|---|---|
| (N = 67) | (N = 32) | |
| Age (months) | ||
| ≤6 | 34 (50.75%) | 20 (62.50%) |
| 6.1 to ≤12 | 13 (19.40%) | 4 (12.50%) |
| 12.1 to ≤24 | 6 (8.96%) | 3 (9.38%) |
| 24.1 to ≤36 | 3 (4.48%) | 2 (6.25%) |
| 36.1 to ≤72 | 5 (7.46%) | 0 |
| >72.1 | 6 (8.96%) | 3 (9.38%) |
| Range | 1–159.8 | 1.2–141.2 |
| Median | 6 | 5 |
| Male (%)a | 45 (67.16%) | 28 (87.50%) |
| Symptoms and signs | ||
| Cough | 65 (97.01%) | 28 (87.50%) |
| Shortness of breath | 33 (49.25%) | 17 (53.13%) |
| Fever | 32 (47.76%) | 14 (43.75%) |
| Swelling of tonsils | 3 (4.48%) | 2 (6.25%) |
| Expectoration | 24 (35.82%) | 8 (25%) |
| Rhinorrhea | 10 (14.93%) | 6 (18.75%) |
| Sneezing | 2 (2.99%) | 2 (6.25%) |
| Abnormal breath sounds on auscultation | 54 (80.60%) | 22 (68.75%) |
| Duration of hospitalization (days) | ||
| Range | 3–42 | 3–29 |
| Median | 10 | 10.5 |
| Mean | 12.53 ± 7.79 | 12.25 ± 6.55 |
| Severity | ||
| Mild | 13 (19.40%) | 4 (12.50%) |
| Severe (I) | 22 (32.84%) | 13 (40.63%) |
| Severe (II) | 23 (34.33%) | 5 (15.63%) |
| Severe (III) | 9 (13.43%) | 9 (28.13%) |
| Respiratory failure | 11 (16.42%) | 9 (28.13%) |
| Nasal continuous positive airway pressure | 7 (10.45%) | 8 (25%) |
| Co-infection | 40 (59.70%) | 17 (53.13%) |
| RSV | 27 (40.30%) | 11 (34.38%) |
| PIV | 8 (11.94%) | 2 (6.25%) |
| HBoV | 3 (4.48%) | 2 (6.25%) |
| AdV | 4 (5.97%) | 2 (6.25%) |
| Influenza virus B | 1 (1.49%) | 2 (6.25%) |
| hMPV | 1 (1.49%) | 0 |
| HCoV HKU1 | 1 (1.49%) | 0 |
| HCoV NL63 | 0 | 1 (3.13%) |
| Enterovirus | 0 | 1 (3.13%) |
Chi-square test: χ2 = 4.62, P < 0.05.
5. Discussion
Here we report the detection of HRV-A, -B, and -C and their relative clinical manifestations in hospitalized children with CAP in Beijing, China. Our work shows that the majority of the HRV-positive patients were infected by HRV-A (51.52%) and HRV-C (38.38%). HRV-B accounted for only 10.10% of the cases of HRV infections. Our findings are similar to results found in pediatric asthma cases.19, 20
Although HRV infections occurred throughout the study period, HRV species showed different monthly distributions. Other studies in Asia also documented fluctuations in HRV infections over time.25, 26 Linsuwanon et al. identified different temporal distributions of HRV species in children suffering from lower respiratory diseases in Thailand.26 Furthermore, HRV-A was detected in hospitalized adults and children in Hong Kong throughout the study period, while HRV-B infections appeared limited during that same period.25
In our study, the clinical manifestations for single infections of HRV-A and HRV-C were very similar. A study performed in Thailand also found that patients infected with different HRV species exhibited comparable initial body temperature and median length of hospitalization.26 We did not observe statistical significance in the median age of patients with HRV-A or HRV-C single infections, as reported elsewhere.20 The disparity between this latter study and ours could be attributed to different patient selection criteria.
Consistent with previous reports,4, 27 we found that more than half of HRV infections were associated with other respiratory viral infections. Because we detected a high percentage (39.39%) of HRV/RSV co-infections, we compared the clinical symptoms and severity between patients with HRV-A or HRV-C single infections to those of patients with HRV/RSV co-infections. The median age of patients co-infected with HRV/RSV was significantly lower than that of patients with single HRV-A or HRV-C infection. Furthermore, more children with dual infections had shortness of breath than those with single infections. Common causes of shortness of breath are asthma, chronic obstructive pulmonary disease, pulmonary edema, and pneumonia.28 Because the prevalence of asthma is low in Chinese children,29 shortness of breath can be assumed to be an indication of CAP severity. In addition, in the HRV-A positive group, more children with co-infections had three severe indices and respiratory failure than those with single HRV infection (5:1 and 9:4, respectively). These differences between HRV single infection and HRV/RSV co-infections may be attributed to the RSV infection. RSV seems to be a more virulent virus, infecting younger age groups, causing shortness of breath and other symptoms. For instance, Midulla et al. reported that infants with RSV bronchiolitis were younger and had a significantly higher clinical severity score upon hospital admission than infants with HRV bronchiolitis.30 Further investigations of such co-infections are necessary to evaluate their specific role in disease severity.
We observed no significant difference in clinical severity and presence of co-infections between patients with co-morbidities and patients without co-morbidities (Table 5). Our findings differ from those of Louie et al., who found an increased likelihood of lower respiratory tract complications from HRV infection in adults with chronic underlying illness.31
A recurrent infection of HRV-A with two different serotypes, HRV-39 and HRV-12, was detected in a two-month old boy. Recurrent infections due to different HRV strains in individual patients have been reported previously.20, 25 However, for all these cases, infection routes by HRVs are unclear and need to be investigated further.
In summary, this study shows that HRV-A and HRV-C play important roles in the pathogenesis of pediatric CAP. Although HRV-A and HRV-C are two distinct HRV species, their clinical manifestations (such as median age, gender, symptoms, severity, and duration of hospitalization) are similar.
6. Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We are grateful to the clinicians of the Beijing Children's Hospital for their assistance in obtaining samples. We also thank Dr. Tapani Hovi [National Institute for Health and Welfare (THL), Helsinki, Finland] for his critical reading of the manuscript. This study was supported in part by grants from the International Scientific and Technological Cooperative Project of the Chinese Ministry of Science and Technology (S2010KR0788), an intramural grant of the Institute of Pathogen Biology, Chinese Academy of Medical Sciences (2009IPB106) and the Fondation Mérieux.
Contributor Information
Kunling Shen, Email: kunlingshen@hotmail.com.
Jianwei Wang, Email: wangjw28@163.com.
References
- 1.McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429–437. doi: 10.1056/NEJMra011994. [DOI] [PubMed] [Google Scholar]
- 2.British Thoracic Society of Standards of Care Committee BTS guidelines for the management of community acquired pneumonia in childhood. Thorax. 2002;57:i1–i24. doi: 10.1136/thorax.57.90001.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamano-Hasegawa K., Morozumi M., Nakayama E., Chiba N., Murayama S.Y., Takayanagi R. Comprehensive detection of causative pathogens using real-time PCR to diagnose pediatric community-acquired pneumonia. J Infect Chemother. 2008;14:424–432. doi: 10.1007/s10156-008-0648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juvén T., Mertsola J., Waris M., Leinonen M., Meurman O., Roivainen M. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19:292–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Lahti E., Peltola V., Waris M., Virkki R., Rantakokko-Jalava K., Jalava J. Induced sputum in the diagnosis of childhood community-acquired pneumonia. Thorax. 2009;64:252–257. doi: 10.1136/thx.2008.099051. [DOI] [PubMed] [Google Scholar]
- 6.Nascimento-Carvalho C.M., Ribeiro C.T., Cardoso M.R., Barral A., Araújo-Neto C.A., Oliveira J.R. The role of respiratory viral infections among children hospitalized for community-acquired pneumonia in a developing country. Pediatr Infect Dis J. 2008;27:939–941. doi: 10.1097/INF.0b013e3181723751. [DOI] [PubMed] [Google Scholar]
- 7.Savolainen C., Blomqvist S., Mulders M.N., Hovi T. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J Gen Virol. 2002;83:333–340. doi: 10.1099/0022-1317-83-2-333. [DOI] [PubMed] [Google Scholar]
- 8.Palmenberg A.C., Spiro D., Kuzmickas R., Wang S., Djikeng A., Rathe J.A. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science. 2009;324:55–59. doi: 10.1126/science.1165557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisdom A., Leitch E.C., Gaunt E., Harvala H., Simmonds P. Screening respiratory samples for detection of human rhinoviruses (HRVs) and enteroviruses: comprehensive VP4–VP2 typing reveals high incidence and genetic diversity of HRV species C. J Clin Microbiol. 2009;47:3958–3967. doi: 10.1128/JCM.00993-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang T., Wang W., Bessaud M., Ren P., Sheng J., Yan H. Evidence of recombination and genetic diversity in human rhinoviruses in children with acute respiratory infection. PLoS ONE. 2009;4(7):e6355. doi: 10.1371/journal.pone.0006355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamson D., Renwick N., Kapoor V., Liu Z., Palacios G., Ju J. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004–2005. J Infect Dis. 2006;194:1398–1402. doi: 10.1086/508551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arden K.E., McErlean P., Nissen M.D., Sloots T.P., Mackay I.M. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78:1232–1240. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McErlean P., Shackelton L.A., Lambert S.B., Nissen M.D., Sloots T.P., Mackay I.M. Characterisation of a newly identified human rhinovirus, HRV-QPM, discovered in infants with bronchiolitis. J Clin Virol. 2007;39:67–75. doi: 10.1016/j.jcv.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kistler A., Avila P.C., Rouskin S., Wang D., Ward T., Yagi S. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis. 2007;196:817–825. doi: 10.1086/520816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee W.M., Kiesner C., Pappas T., Lee I., Grindle K., Jartti T. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2(10):e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau S.K., Yip C.C., Tsoi H.W., Lee R.A., So L.Y., Lau Y.L. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45:3655–3664. doi: 10.1128/JCM.01254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renwick N., Schweiger B., Kapoor V., Liu Z., Villari J., Bullmann R. A recently identified rhinovirus genotype is associated with severe respiratory tract infection in children in Germany. J Infect Dis. 2007;196:1754–1760. doi: 10.1086/524312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briese T., Renwick N., Venter M., Jarman R.G., Ghosh D., Köndgen S. Global distribution of novel rhinovirus genotype. Emerg Infect Dis. 2008;14:944–947. doi: 10.3201/eid1406.080271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khetsuriani N., Lu X., Teague W.G., Kazerouni N., Anderson L.J., Erdman D.D. Novel human rhinoviruses and exacerbation of asthma in children. Emerg Infect Dis. 2008;14:1793–1796. doi: 10.3201/eid1411.080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller E.K., Edwards K.M., Weinberg G.A., Iwane M.K., Griffin M.R., Hall C.B. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123:98–104e1. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang Z., Gonzalez R., Xie Z., Xiao Y., Chen L., Li Y. Human rhinovirus group C infection in children with lower respiratory tract infection. Emerg Infect Dis. 2008;14:1665–1667. doi: 10.3201/eid1410.080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Subspecialty Group of Respiratory Diseases of Pediatric Society, Chinese Medical Association, and The Editorial Board, Chinese Journal of Pediatrics Guidelines for management of childhood community acquired pneumonia (for trial implementation) (I) Chin J Pediatr. 2007;45:83–90. [in Chinese] [PubMed] [Google Scholar]
- 23.Ren L., Gonzalez R., Wang Z., Xiang Z., Wang Y., Zhou H. Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005–2007. Clin Microbiol Infect. 2009;15:1146–1153. doi: 10.1111/j.1469-0691.2009.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang Z, Gonzalez R, Wang Z, Xiao Y, Chen L, Li T, et al. Human rhinovirus group C infection in Chinese adults with acute respiratory tract infection. J Infect; in press. [DOI] [PMC free article] [PubMed]
- 25.Lau S.K., Yip C.C., Lin A.W., Lee R.A., So L.Y., Lau Y.L. Clinical and molecular epidemiology of human rhinovirus C in children and adults in Hong Kong reveals a possible distinct human rhinovirus C subgroup. J Infect Dis. 2009;200:1096–1103. doi: 10.1086/605697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linsuwanon P., Payungporn S., Samransamruajkit R., Posuwan N., Makkoch J., Theanboonlers A. High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J Infect. 2009;59:115–121. doi: 10.1016/j.jinf.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cilla G., Oñate E., Perez-Yarza E.G., Montes M., Vicente D., Perez-Trallero E. Viruses in community-acquired pneumonia in children aged less than 3 years old: high rate of viral coinfection. J Med Virol. 2008;80:1843–1849. doi: 10.1002/jmv.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woollard M., Greaves I. 4 shortness of breath. Emerg Med J. 2004;21:341–350. doi: 10.1136/emj.2004.014878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee Worldwide variations in the prevalence of asthma symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC) Eur Respir J. 1998;12:315–335. doi: 10.1183/09031936.98.12020315. [DOI] [PubMed] [Google Scholar]
- 30.Midulla F., Scagnolari C., Bonci E., Pierangeli A., Antonelli G., De Angelis D. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child. 2010;95:35–41. doi: 10.1136/adc.2008.153361. [DOI] [PubMed] [Google Scholar]
- 31.Louie J.K., Yagi S., Nelson F.A., Kiang D., Glaser C.A., Rosenberg J. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis. 2005;41:262–265. doi: 10.1086/430915. [DOI] [PMC free article] [PubMed] [Google Scholar]