Abstract
Astroviruses are small, non-enveloped, positive sense, single-stranded RNA viruses first identified in 1975 in children suffering from diarrhea and then described in a wide variety of animals. To date, the list of animal species susceptible to astrovirus infection has expanded to 22 animal species or families, including domestic, synantropic and wild animals, avian, and mammalian species in the terrestrial and aquatic environments. Astrovirus infections are considered among the most common cause of gastroenteritis in children, second only to rotavirus infections, but in animals their association with enteric diseases is not well documented, with the exception of turkey and mink astrovirus infection. Genetic variability has been described in almost all astrovirus species sufficiently examined infecting mammals and birds; however, antigenic variability has been demonstrated for human astroviruses but is far less investigated in animal viruses. Interestingly, there is an increasing evidence of recombination events occurring in astroviruses, which contributes to increase the genetic variability of this group of viruses. A wide variety of species infected, the evident virus genetic diversity and the occurrence of recombination events indicate or imply either cross-species transmission and subsequent virus adaptation to new hosts or the co-infection of the same host with different astroviruses. This can also favor the emergence of novel astroviruses infecting animals or with a zoonotic potential. After more than 30 years from their first description in humans, there are many exciting streams of research to be explored and intriguing questions that remain to be answered about the relatively under-studied Astroviridae family. In the present work, we will review the existing knowledge concerning astrovirus infections in humans and animals, with particular focus on the molecular biology, interspecies transmission and zoonotic potential of this group of viruses.
Keywords: Astrovirus, Taxonomy, Molecular biology, Virus evolution, Cross-species transmission
1. Introduction
Astroviruses are small, non-enveloped RNA viruses first identified in 1975 by electron microscopy (EM) in children suffering from diarrhea (Appleton and Higgins, 1975, Madeley and Cosgrove, 1975b, Matsui and Greenberg, 1996). Since then, enteric infections in humans caused by astrovirus have been reported worldwide mainly in infants and young children. Several studies suggest that astroviruses are the second most common cause of gastroenteritis in children after rotavirus infection (Matsui and Greenberg, 1996).
The name astrovirus derives from the Greek word “astron” (=star) and describes the characteristic five/six pointed star-like projections detectable by negative stained EM of the virions. Although it should be pointed out that the presence of these projections is pH dependent and may only be present in less than 10% of the population (Caul and Appleton, 1982, Koci and Schultz-Cherry, 2002). In fact, in some instances the typical star-like appearance is not easily recognizable in EM preparations and astroviruses could be misidentified as enteroviruses or provisionally named astrovirus-like particles or small round viruses (SRVs) (Guy et al., 2004, Koci and Schultz-Cherry, 2002). This was probably the case of astrovirus infections in ducks, turkeys, and guinea fowl, where picornavirus or enterovirus-like particles were initially described (Cattoli et al., 2005, Guy et al., 2004, Koci and Schultz-Cherry, 2002). For this reason, the advent of more specific tools for virus detection and genome-based identification in the last ten years has allowed the discovery or the confirmation of the presence of astroviruses in a number of different hosts.
Soon after the first description in human beings, astrovirus-like particles were described and reported in domesticated animals. The first reports in animals were from lambs and calves suffering from diarrhea (Snodgrass and Gray, 1977, Woode and Bridger, 1978). Based on clinical and virological observations during acute mortality cases in ducks in the 1980s, the presence of astrovirus was associated with fatal hepatitis (Gough et al., 1984). This was perhaps the first evidence of extra-intestinal localization of astroviruses. Of interest, similar acute duck hepatitis was also reported in the 1960s, one decade before the discovery of astrovirus in humans and 20 years before identification of astroviruses as the cause of hepatitis in ducks (Asplin, 1965a, Asplin, 1965b). At present, the list of animal species susceptible to astrovirus infection has expanded to include domestic, synantropic and wild animals, avian and mammalian species in the terrestrial and aquatic environments (Table 1 ). In the last decade, there has been a dramatic increase in the identification of astroviruses in new animal species due to the advent of better molecular assays and pathogen discovery tools. This list is likely to be far from complete and surely more astrovirus-susceptible hosts are going to be added in the near future.
Table 1.
Chronology of astroviruses discovered, year of first prototype detection and in vitro isolation information.
| Species | Detection | Reference | Disease associated | Isolation | Reference | Substrate for replication |
|---|---|---|---|---|---|---|
| Human (Homo sapiens sapiens) | 1975 | Madeley and Cosgrove (1975a) | Gastroenteritis in children | 1981 | Lee and Kurtz (1981) | Human kidney epithelial (HEK) cells, CaCo2 cell line, baby hamster kidney 21 (BHK-21) |
| Ovine (Ovis aries) | 1977 | Snodgrass and Gray (1977) | Diarrhea in lambs | – | – | – |
| Bovine (Bos taurus) | 1978 | Woode and Bridger (1978)) | Diarrhea in calves, asymptomatic | 1985 | Woode et al. (1985) | Primary bovine embryo kidney cells (EBK), neonatal bovine kidney (NBK) |
| Chicken (Gallus gallus) | 1979 | Yamaguchi et al. (1979)⁎ | Interstitial nephritis in young chicks, enteritis | 1979 | (Yamaguchi et al., 1979) | Primary chicken kidney cells (CK), primary chicken embryo liver (CEL), baby hamster kidney (BHK), chicken hepatoma (LMH) cells |
| Pig (Sus scrofa) | 1980 | Bridger (1980) | Diarrhea in piglets, asymptomatic | 1980 | Shimizu et al. (1990) | Embryonic swine kidney established cell line (ESK), porcine kidney-15 (PK-15) |
| Dog (Canis lupus familiaris) | 1980 | Williams (1980) | Diarrhea in pups, asymptomatic | – | Martella et al. (2011) | Madin-Derby canine kidney cells (MDCK) |
| Cat (Felis catus) | 1981 | Hoshino et al. (1981) | Pyrexia and mild diarrhea, asymptomatic | – | – | – |
| Red deer (Cervus elaphus) | 1981 | Tzipori et al. (1981) | Diarrhea | 1981 | Tzipori et al. (1981) | Primary bovine embryonic kidney cells (EBK) |
| Duck (Anas platyrhynchos domestica) | 1984 | Gough et al. (1984) | Acute hepatitis and mortality in ducklings | 1985 | Gough et al. (1985) | Duck and chicken SPF embrionating eggs: via allantoic, amniotic or yolk-sac routes, LMH cells |
| Mouse (Mus musculus) | 1985 | Kjeldsberg and Hem (1985) | Diarrhea, asymptomatic | – | – | – |
| Turkey (Meleagris meleagris) | 1980 | McNulty et al. (1980) | Poult enteritis complex (PEC), poult enteritis mortality syndrome (PEMS) | 1991 | Guy and Barnes, 1991, Koci et al., 2000) | SPF turkey eggs: 20 day-old via the yolk sac, or 22 day-old via the amniotic cavity |
| Mink (Neovison vison) | 2002 | Englund et al. (2002) | Pre-weaning diarrhea, shaking mink syndrome | – | – | – |
| Guinea fowl (Numida meleagris) | 2005 | Cattoli et al. (2005) | Enteritis | – | – | – |
| Insectivorous bat (⁎⁎) | 2008 | Chu et al. (2008) | – | – | – | – |
| Cheetah (Acinonyx jubatus) | 2009 | Atkins et al. (2009) | Lethargy and anorexia, watery diarrhea | – | – | – |
| California sea lion (Zalophus californianus) | 2010 | Rivera et al. (2010) | Pup with diarrhea adults clinically healthy | – | – | – |
| Steller sea lion (Eumelopias jubatus) | 2010 | Rivera et al. (2010) | Pup without signs of diarrhea | – | – | – |
| Bottlenose dolphin (Tursiops truncatos) | 2010 | Rivera et al. (2010) | Clinically healthy | – | – | – |
| Brown rat (Rattus norvegicus) | 2010 | Chu et al. (2010) | – | – | – | – |
| Roe deer (Capreolus capreolus) | 2010 | Smits et al. (2010) | Diarrhea | – | – | – |
Firstly identified as enterovirus-like, was finally classified as a new member of the family Astroviridae in 2000 (Imada et al., 2000).
Astroviruses were detected in rectal swabs collected from several species insectivorous bats, belonging to different families, namely Rhinolophidae, Vespertilionidae, Emballonuridae, Megadermatidae.
In the present work, we will review the existing knowledge concerning astrovirus infections in humans and animals, with particular focus on molecular biology, interspecies transmission and zoonotic potential of this group of viruses.
2. Etiology and taxonomy
The family Astroviridae comprises non-enveloped, positive sense, single-stranded RNA viruses, typically 28–30 nm in diameter (Matsui and Greenberg, 1996). They have been classified into two genera, namely Mamastroviruses (MAstVs) and Avastroviruses (AAstVs) known to infect mammalian and avian species, respectively (Mendez and Arias, 2007). Few species are officially classified by the International Committee for Taxonomy of Viruses (ICTV). These are: duck astrovirus 1 (DAstV-1), turkey astrovirus 1 and 2 (TAstV-1 and TAstV-2), and avian nephritis virus (ANV) as AAstVs, bovine astrovirus (BoAstV), feline astrovirus (FeAstV), human astroviruses (HAstVs 1–8), ovine astrovirus (OAstV), mink astrovirus (MiAstV), and porcine astrovirus (PoAstV) as MAstrVs.
The genome length is 6.8 to 7.9 kb and includes a 5′ un-translated region (UTR), followed by three open reading frames (ORFs) namely ORF1a, ORF1b, and ORF2, a 3′ UTR and a poly-A tail. There is a frame-shift structure between ORF1a and ORF1b. ORF1 encodes both a protease and an RNA-dependent RNA polymerase. ORF2 is expressed from a subgenomic RNA and encodes for the viral capsid protein (Fig. 1 ). The length of each of these structures varies between species and serotypes. From a genetic point of view, ORF1b appears to be the least divergent and ORF2 the most divergent among the different ORFs (Strain et al., 2008). This is of course an expected event since the region encoding for the viral capsid protein is more subject to selective pressure if compared to regions encoding for non-structural proteins. Unfortunately, only a minimal number of complete genomic astrovirus sequences are available (Table 2 ).
Fig. 1.
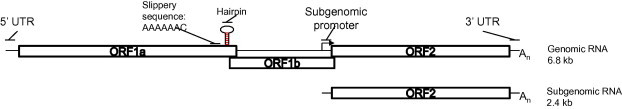
Genome structure of Astroviruses. Astroviruses are single stranded positive sense RNA viruses. Though variable in length among the Astroviridae family, the genome architecture of Astroviruses is similar. For Human Astrovirus-1, the genome is 6.8 kilobases (kb) in length. It contains a 5′ untranslated region (UTR) of 85 nt, a 3′ UTR of 83 nt, and a poly(A) tail. After deposition of the viral genome into the cytoplasm ORF1a is immediately translated to produce the nonstructural proteins. Combination of a slippery (A)6C sequence and downstream structural hairpin contribute to a −1 ribosomal frame shift resulting in translation of the nonstructural polyprotein ORF1ab encoding the RNA dependent RNA polymerase. The subgenomic RNA is 2.4 kb in length and contains a 5′ UTR, 3′ UTR, a poly(A) tail and is transcribed from an internal promoter in the minus strand. ORF2 is translated from the subgenomic RNA to produce the structural proteins.
Table 2.
Summary of the genetic sequences of human and animal astroviruses available in a public database (GenBank – http://www.ncbi.nlm.nih.gov, accessed on 11th May 2011). Prototype sequences are based on ICTV taxonomic proposals 2010.017aV and 2010.018aV or included in the present review (in italics). OAstV: ovine astrovirus; BoAstV: bovine astrovirus; PoAstV: porcine astrovirus; MiAstV: mink astrovirus; FeAstV: feline astrovirus; CaAstvV: canine astrovirus; CcAstV: roe deer astrovirus; BatAstV: bat astrovirus; RatAstV: rat astrovirus; CslAstV: California sea lion astrovirus; BdAstV: bottlenose dolphin astrovirus; HAstV: human astrovirus; Ast-MLB: astrovirus MLB; HMO-AstV: astrovirus human, mink- and ovine-like; Ast-VA: astrovirus VA; DAstV: duck astrovirus; TAstV: turkey astrovirus; CAstV: chicken astrovirus; ANV: Avian Nephritis Virus; ORF: open reading frame.
| Astroviridae | Prototype sequence GenBank accession number | No. of whole genome sequences available | No. of partial sequences available |
|---|---|---|---|
| Mamastrovirus | |||
| OAstV | Y15937⁎–NC002469⁎ | 2 | 0 |
| BoAstV | JF796126 | 0 | 2 (ORF1 and 2) |
| PoAstV | AB037272 | 1 | 62 (ORF2) 12 (ORF1b) |
| GU562296 (PAstV2) | |||
| MiAstV | AY179509 | 3 | 11 (ORF1b) |
| FeAstV | AF056197 | 0 | 2 (ORF2) 1 (ORF1b) |
| CaAstV | FM213330 | 0 | 3 (ORF2) |
| FM213331 | |||
| FM213332 | |||
| CcAstV | HM447045 | 2 | 0 |
| BatAstV | FJ571067 | 0 | 74 (ORF1) 104 (ORF1b) 14 (ORF2) |
| EU847144 | |||
| FJ571066 | |||
| EU847145 | |||
| FJ571068 | |||
| FJ571074 | |||
| EU847155 | |||
| FJ571073 | |||
| FJ571069 | |||
| FJ571072 | |||
| FJ571065 | |||
| FJ571070 | |||
| FJ571071 | |||
| RatAstV | HM450381 | 0 | 6 (ORF1b) 2 (ORF2) |
| CslAstV | FJ890352 | 0 | 4 (ORF1b) 4 (ORF2) |
| FJ890351 | |||
| BdAstV | FJ890355 | 0 | 21 (ORF1) 21 (ORF2) |
| HAstV | DQ070852 | 16 | 1462⁎⁎ |
| AstV-MLB-1; -2 | AY720891 | 4 (MLB-1; -2) | 18 (MLB-1; -2) |
| HMO-AstV -A; -B; -C | DQ344027 | 1 (HMO-A; -B; -C) | 8 (HMO-A; -B; -C) |
| AF260508 | |||
| AstV-VA-1; -2 | DQ028633 | ||
| AB013618 | |||
| L13745 | |||
| AF141381 | |||
| AY720892 | |||
| L23513 | |||
| Z25771 | |||
| FJ402983 (MLB1) | |||
| FJ222451 (MLB1) | |||
| NC013443 (HMO-a) | |||
| GQ415661 (HMO-b) | |||
| GQ415662 (HMO-c) | |||
| FJ973620 (VA-1) | |||
| GQ891990 (HAstV-PS) | |||
| Avastrovirus | |||
| DAstV | FJ434664 | 6 | 4 (ORF1) |
| TAstV | Y15936 (TAstV1) | 12 | 33 (TAstV1) 382⁎⁎⁎ (TAstV2) 1 (TAstV3) |
| EU143843 (TAstV2) | |||
| AF206663 (TAstV2) | |||
| CAstV-1; -2 | NA | 0 | 55 |
| ANV-1; -2 | AB033998 (ANV-1) | 1 | 65 (ORF1) 9 (ORF2) |
| AB046864 (ANV-2) | |||
Sequences obtained from the same virus.
Including “classical” serotypes 1–8.
Including TAstV detected in guinea fowl.
To date, Astroviridae taxonomy takes into account the species of origin. Serotypes have been defined on the basis of twenty-fold, or greater, two-way cross-neutralization titers. Serotypes assigned to the species are given consecutive numbers (Lee and Kurtz, 1982, Mendez and Arias, 2007). Astroviruses do not easily grow in laboratory host systems and for this reason serological classification is difficult and is generally speculated based on percentage of nucleotide and amino acid similarity of the ORF2 encoding for the capsid protein (Chu et al., 2008, Koci et al., 2000, Reuter et al., 2011). It has been reported that if two strains of HAstVs have less than 95% homology at the nucleotide level, they are serologically distinguishable (Walter et al., 2001). Few documented antigenic classifications have been attempted for animal AstVs. Serological assays confirmed by molecular characterization, suggested the existence of a putative novel serotype of turkey astrovirus, TAstV-3 (Tang and Saif, 2004, Tang et al., 2005); similarly the application of cross-virus neutralization assays indicated the presence of more undefined serotypes of bovine AstV (Woode et al., 1985).
Although official classification is currently based on the species of origin of AstV, the discovery of viruses in species never implicated before and of viruses genetically unrelated to those known to infect the same species has highlighted the need to review classification of those viruses following updated guidelines. Several AstVs have recently been detected in new species, namely guinea fowl, insectivorous bats, dogs, cheetahs, marine mammals, and brown rats (Atkins et al., 2009, Cattoli et al., 2005, Chu et al., 2008, Chu et al., 2010, Rivera et al., 2010, Toffan et al., 2009, Zhu et al., 2009). In humans, eight so-called “Classic” serotypes of astroviruses genetically related are known (HAstV1-8), and new species have recently been found in stool samples from patients suffering from gastroenteritis, namely AstV-MLB1 and MLB-2 which are genetically close to rat astroviruses discovered in Hong Kong (Chu et al., 2010, Finkbeiner et al., 2008b, Finkbeiner et al., 2009a, Finkbeiner et al., 2009c), and HAstVs genetically related to MiAstVs and OAstVs, thus called human, mink-, and ovine-like, HMOAstVs A, B, and C and AstV-VA1 (Finkbeiner et al., 2009b, Kapoor et al., 2009). Based on new findings, two proposals for re-classifications of Astroviridae were submitted in 2010 by the Astroviridae Study Group (Bosch et al., 2010a, Bosch et al., 2010b). They both take into consideration genetic criteria based on the full length sequencing of ORF2 (Fig. 2 ). According to the proposals, three new species of Avastroviruses and nineteen new species of Mamastroviruses have ben recognized. These include Avastrovirus GI.A (current TAstV-1), GI.B (current ANV-1 and -2) and GII.A (current TAstV-2, and DAstV). Mamastroviruses GI.A (classical HAstVs 1–8), GI-B (FeAstV), GI.C (PoAstVs), GI.D (California sea lion AstV-2), GI.E (Canine AstV), GI.F (MLB1-HAstV), GI.G (Bottlenose dolphin AstV), and Mamastroviruses GII.A (HMOAstV-A and HastV-VA2), GII.B (HMOAstVs-B and -C, and HastV-Va1), GII.C (MiAstV), GII.D (California sea lion AstV-1), GII.E (Bat AstV), GII.F (OAstV), GII.G to GII.L (both including Bat AstVs exclusively) (see Fig. 2) (Bosch et al., 2010a, Bosch et al., 2010b).
Fig. 2.
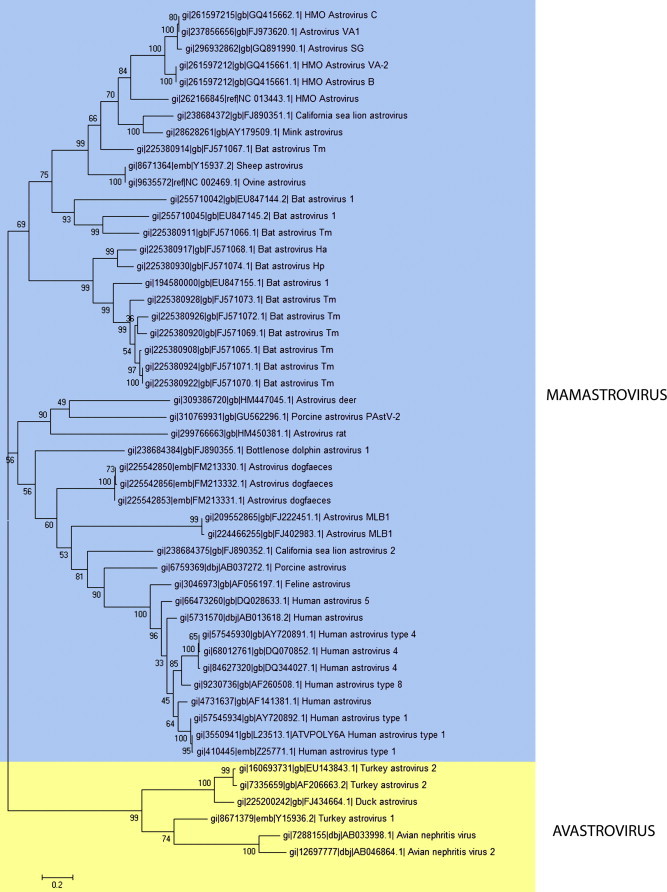
Phylogenetic tree including Mamastrovirus and Avastrovirus prototype ORF2 aminoacid sequences of the viruses listed in Table 2. The phylogenetic tree was obtained using the Neighbor-Joining method and MEGA4 software.
Generally speaking, MAstVs (except HAstV-8) have an overlap of approximately eight nucleotides (nts) between the stop codon of ORF1b and the start codon of ORF2, which is in the same frame of ORF1a. AAstVs differ from this genome structure. The start codon for ORF2 of ANV is 19 nts downstream of the stop codon of ORF1b and ORF2 is maintained at the same frame of ORF1a. For TAstV-1 and TAstV-2, the space between the ORF1b and ORF2 is 18 nts and the ORF2 is in the same frame of ORF1b. In DAstV, ORF2 is placed at a different frame than ORF1a or ORF1b, with a start codon 23 nt downstream of the stop codon of ORF1b. Another difference among astroviruses is placed at the 3′ end of ORF2 and the 3′UTR, where there is a conserved motif of 19 nts, likely encoding for a secondary structure, and which is present in most astroviruses except for TastV-2, PoAstVs belonging to group II according to Luo et al. (2011), BatAstVs and RatAstVs (Chu et al., 2008, Chu et al., 2010, Jonassen et al., 2003).
3. Molecular genetics and biology of astroviruses
The difficulty of astrovirus to grow in common laboratory hosts systems prevented early characterization, but in 1981 Lee and Kurtz demonstrated productive virus growth and passaging with the addition of trypsin in human kidney epithelial (HEK) cells (Lee and Kurtz, 1981). It was not until 1990 that this technique was applied to a more representative cell line; the human colon carcinoma cell line Caco2, which has become standard (Willcocks et al., 1990). The complete genome sequence for Human Astrovirus 1 was determined in 1994 and the first infectious cDNA clone was developed in 1997 (Geigenmuller et al., 1997, Willcocks et al., 1994). Basic research of astrovirus replication and viral protein functions are expanding, but much information is inferred from the large body of work on other positive-strand viruses, including alphaviruses and picornoviruses (Racaniello, 2001, Sondra Schlesinger, 2001, Strauss and Strauss, 1994) or derived from studies of the human astroviruses.
Very little is known about astrovirus attachment and entry. The receptor for virus entry is unknown. The same cell line can be permissive and refractory to different subtypes of HAstV and this suggests different receptor requirements for the various subtypes (Brinker et al., 2000). Caco2 cells support infection by HAstV1–8 subtypes, while another human colon carcinoma cell line only minimally supports infection by HAstV1. When transfected with an infectious in vitro transcribed astrovirus genomic RNA, baby hamster kidney (BHK) cells support virus replication but are refractory to infection by the whole virus, suggesting they lack the appropriate cell surface receptor (Geigenmuller et al., 1997). The variances between virus subtypes and cell lines susceptible to infection suggest there may be multiple receptor requirements.
Early studies with HAstV-1 in Graham 293 cells demonstrated the presence of astrovirus particles in coated pits and later in coated vesicles, suggesting adsorptive endocytosis as a possible mechanism by which astrovirus enters the cells. This was supported by inhibition of viral entry with endocytic inhibitors (Donelli et al., 1992). Once internalized, the plus-strand genome is released into the cytoplasm by unknown mechanisms and ORF1a and 1b are immediately translated by the host machinery. ORF1a is ∼2.8 kb encoding a ∼110 kDa polypeptide (Matsui and Greenberg, 1996) (see Fig. 1). The polypeptide contains a variety of conserved motifs, including several putative transmembrane helices, coiled coil domains, an immunoreactive epitope, putative nuclear localization signal (NLS), and serine protease (Guix et al., 2004, Matsui et al., 1993). The translated polypeptide is cleaved by both cellular protease(s) and the viral protease into at least five peptides (Geigenmuller et al., 2002, Guix et al., 2005, Kiang and Matsui, 2002). The structure of the astrovirus protease has recently been solved and several unique features defined, which differentiate it from other human and viral serine proteases (Speroni et al., 2009). The function of the remaining non-structural proteins (NSP) remains largely unknown. The transmembrane domains may localize nsp1a/2 to the endoplasmic reticulum (ER) membrane to facilitate replication (Guix et al., 2004) similar to other positive-strand RNA viruses (Ahlquist, 2006, Geigenmuller et al., 2002, Guix et al., 2004, Guix et al., 2005, Kiang and Matsui, 2002, Speroni et al., 2009). One peptide, nsp1a/41a, co-localizes with the viral RNA at the ER membrane (Guix et al., 2004); mutations in nsp1a/4 correlate with increased viral titers in vitro and in vivo (Guix et al., 2005) suggesting a role for this protein in viral replication. This peptide was recently shown to contain a hypervariable region that can be phosphorylated, form oligomers and interact with the viral polymerase supporting its importance to productive viral replication (Fuentes et al., 2011). There is relatively little information on the regulation of astrovirus replication by cellular factors. One study demonstrated that the astrovirus activates the cellular extracellular signal-regulated kinase (ERK) 1/2 pathway early in infection independently of replication. Inhibition of ERK1/2 activity resulted in decreased viral replication early in the course of infection (Moser and Schultz-Cherry, 2008).
The second reading frame, ORF1b, which encodes the viral RNA-dependent RNA polymerase (RdRp) (Matsui and Greenberg, 1996), overlaps ORF1a by 70 nucleotides and is translated by a frame-shift into the −1 frame and is a common mechanism, though not unique, among plus-stranded animal RNA viruses (Lewis and Matsui, 1996). It requires a highly conserved heptameric sequence (A6C) and a downstream hairpin structure (Lewis and Matsui, 1995, Lewis and Matsui, 1996, Lewis and Matsui, 1997, Marczinke et al., 1994) (see Fig. 1). This event results in an ORF1a/1b fusion peptide, which is further cleaved near the 1a/1b border and occurs between 7% (in vitro) to 25% (in vivo) of the time (Lewis and Matsui, 1997). Astrovirus polymerase is a supergroup 1 RdRp (Lewis et al., 1994), a group which utilizes a viral-encoded primer attached to the genome (VPg) to initiate transcription (Jang et al., 2010). VPg is believed to exist (Al-Mutairy et al., 2005) although this hypothesis has yet to be confirmed (Carter and Willcocks, 1996). Further, there is no evidence of a viral helicase.
The expression of the RdRp results in production of a minus-strand viral template between 9 and 12 h post-infection (hpi) (Jang et al., 2010), which generates multiple copies of the plus strand genome and a poly-adenylated subgenomic RNA (sgRNA) containing short 5′ and 3′ UTRs and ORF2 (Monroe et al., 1993). ORF2, which encodes the structural or capsid protein, is in the zero frame and overlaps ORF1b slightly (see Fig. 1). Production of the capsid protein from a sgRNA restricts capsid production in the viral replication cycle and allows for an estimated 10-fold excess of capsid to the viral genome by 12 hpi. The sgRNA is about 2.4 kb and encodes the capsid precursor polypeptide of 87–90 kDa (Monroe et al., 1993). The capsid contains at least three domains including a highly conserved 5′ terminal domain, a highly divergent intermediate domain, and the 3′ variable end domain all of which can be distinguished by their sequence identity. Intracellular proteolytic processing by cellular proteases including caspases (Banos-Lara Mdel and Mendez, 2010, Mendez et al., 2002, Mendez et al., 2004) yields an ∼70–80 kDa protein, which is further extracellularly cleaved by trypsin increasing infectivity up to 105 fold, condensing the virion to approximately 28 nm, and transforming the ∼70–80 kDa capsid protein into at least three smaller peptides of approximately 34, 29, and 26 kDa (Bass and Qiu, 2000). Positive sense genomes are packaged into these viral-like particles (VLPs), likely through interactions with the first 70 amino acids of the capsid protein, which is highly basic (Carter and Willcocks, 1996).
The astrovirus capsid protein has several unique biological properties. It has been shown to be a unique inhibitor of the complement pathway by binding to complement C1 and mannose-binding lectin (MBL) inhibiting the activation of the classical and lectin complement pathways, respectively (Bonaparte et al., 2008, Gronemus et al., 2010, Hair et al., 2010;). This was the first description of a non-enveloped icosahedral virus inhibiting complement. However, the importance of this finding during astrovirus infection remains unknown. Another unique feature of the astrovirus capsid protein is its potential action as an enterotoxin. Studies by Moser et al. (2007) demonstrated that astroviruses increase epithelial barrier permeability by disrupting cellular tight junction complexes (Moser et al., 2007). This increase in barrier permeability was independent from viral replication. Recombinant capsid alone was sufficient suggesting that the capsid may contribute to diarrhea in vivo.
Overall, a great deal of work is still needed to understand astrovirus replication. Particularly in relation to the cellular factors required for productive replication, the role of the individual NSPs in replication and controlling cellular functions, and a better understanding of the processing of the capsid protein. One major unanswered question is how the astrovirus is released from the cell without inducing cell death.
4. Human astroviruses
HAstVs predominately affect children under the age of 2, elderly people and immunocompromised individuals and are thought to be involved in up to 20% of the cases of sporadic non-bacterial diarrheas and 0.5–15% of diarrheal outbreaks (Kirkwood et al., 2005, Moser and Schultz-Cherry, 2005). Upon infection, clinical symptoms normally last between 2 to 4 days and consist of watery diarrhea and less commonly vomiting, headache, fever, abdominal pain and anorexia (Glass et al., 1996, Glass et al., 2001, LeBaron et al., 1990, Mitchell et al., 1999). However, there is evidence that HAstV infections can be asymptomatic in children and adults (Mendez-Toss et al., 2004). An interesting study by Quan et al. (2010) suggested that human astroviruses may be able to go systemic when authors detected for the first time HAstV as the causative agent for encephalitis in an immunocompromised child, which has led to important new questions about this HAstV-associated disease, mainly in the immunocompromised patient (Quan et al., 2010).
The prevalence of HAstV depends on the region and test settings (urban hospital versus child care center, etc.). However, most studies report a prevalence rate up to 10% in most areas (range of 2–9%) with rates as high as 30% in some developing countries (De Grazia et al., 2011, Guix et al., 2002). Several extensive epidemiological studies suggested that the maximum detection rate for HAstV was in children from 2 to 4 years of age with the highest rates of infection in the winter months in temperate climates (De Grazia et al., 2011, Guix et al., 2002), although, HAstV can be detected in the spring and summer seasons as well (Herrmann et al., 1991, Noel and Cubitt, 1994). It is not unusual to find HAstV in association with other enteric viruses. Several studies suggest that 33–65% of symptomatic HAstV infection may be associated to other enteric microbes like rotavirus and norovirus (Colomba et al., 2006, Roman et al., 2003). A study in Spain showed a slightly less incidence of co-infections with only 17% of the HAstV positive cases associated with other pathogens (Guix et al., 2002). Regardless, HAstV appears to be a significant cause of gastroenteritis, especially in young children.
Until 2008, human infections were thought to be limited to eight closely related HAstV genotypes now referred to as the “Classic” human astroviruses. In several cases these genotypes were detected and initially differentiated using a set of conserved primers to the ORF1b termed Mon269 (5′ CAACTCAGGAAACAGGGTGT) and Mon270 (5′ TCAGATGCATTGTCATTGGT) (Noel et al., 1995). HAstV-1 appears to be the predominant circulating serotype worldwide followed by types 2–5 and occasionally 8 depending on the region. HAstV-6 and 7 are rarely detected (De Grazia et al., 2011, Gabbay et al., 2007, Guix et al., 2002, Liu et al., 2008, Mendez-Toss et al., 2004, Mustafa et al., 2000, Palombo and Bishop, 1996). There is a great deal of diversity within each of the different genotypes. Two studies of the beginning of year 2000 suggested that a variant could be considered a new subtype if identity was less than 95% nucleotide homology to a reference strain and >0.05 distance by phylogenetic analysis at the 3′ end of ORF2 (Jakab et al., 2003, Walter et al., 2001). Others proposed that strains showing a sequence diversity of at least 7% could be considered as new lineages (Guix et al., 2002, Medina et al., 2000). Based on these suggestions, HAstV-1 has been divided into a least six lineages, HAstV-1a–1f (Gabbay et al., 2007); HAstV-2 into at least three lineages, HAstV-2a–2c (De Grazia et al., 2011, Gabbay et al., 2007); HAstV-3 into two lineages (Belliot et al., 1997a, Belliot et al., 1997b, Liu et al., 2008); and HAstV-4 into two lineages, 4a and 4b. To date there is no information on the association between disease symptoms and HAstV genotype, except for one study which suggests that HAstV-3 may be associated to more severe gastroenteritis and higher fecal viral titers if/when compared to the other genotypes (Caballero et al., 2003). This very interesting work also demonstrated that astroviruses can result in persistent infections, some lasting up to 3 months, and described a quantitative RT-PCR assay to detect the HAstV genomes per gram of feces (Caballero et al., 2003).
The advent of pyrosequencing and pathogen discovery demonstrates that the human stool contains a variety of previously unrecognized astroviruses. A seminal paper by Finkbeiner et al. (2008a) used metagenomic analysis to identify a new genotype of HAstV in the feces of a young boy from Melbourne, Australia with acute diarrhea (Finkbeiner et al., 2008a). They termed the virus MLB1 (AstV-MLB1) and went onto characterize the genome, demonstrating that AstV-MLB1 was highly divergent from any of the known astroviruses (Finkbeiner et al., 2008b). AstV-MLB1 lacks much of the conservation typically seen between HAstV 1–8 in the non-translated regions of the genome, for example in the 5′ and 3′ non-translated regions and the ORF1b/2 junction, suggesting that this is a new genogroup within the Mamastrovirus I family. Further studies have demonstrated that AstV-MLB1 is widely distributed and that, just as in the classic HAstV, there is heterogeneity within the group (Finkbeiner et al., 2009a, Finkbeiner et al., 2009c). Of great interest is the molecular dating analysis on the ORF1b region, which suggests that the newly discovered rat astrovirus may share a common ancestor with AstV-MLB1 with an estimated date of AD 1054 (Chu et al., 2010). Unfortunately, due to the lack of available sequence data, the evolutionary history of astroviruses remains unknown.
By using similar pathogen discovery techniques, the same group discovered and characterized AstV-VA1 during a sporadic diarrheal disease outbreak in Virginia (Finkbeiner et al., 2009b) and demonstrated diversity within the AstV-VA1 genogroup (Finkbeiner et al., 2009c). During this time, a separate group also identified three novel human astrovirus species from adult travellers using pan-astrovirus specific primers to ORF1b that were phylogenetically related to each other with the closest genetic relatives being mink and ovine (Kapoor et al., 2009). Thus, they were named human, mink, and ovine-like HMOAstV types A, B, and C. Intriguingly, AstV-VA1 is closely related to HMOAstV-C. There AstV-VA and HMOAstV viruses are quite distinct from the classic HAstV and AstV-MLB viruses and cluster within the Mamastrovirus II family. Important for surveillance purposes, Finkbeiner et al. (2009c) developed conserved primers to the ORF1b that should detect all human astrovirus genogroups (Finkbeiner et al., 2009c).
It is evident that there are at least three clades of astroviruses co-circulating in humans: Classic, MLB1-like and VA1-like. Co-infection by two different genotypes in one person provides an opportunity for recombination to occur. Indeed, there has been evidence of recombination within the classic HAstV stains (Walter et al., 2001, Wolfaardt et al., 2011).
5. Animal astroviruses
5.1. Mamastroviruses
5.1.1. Ovine astrovirus (OAstV)
Astrovirus infection in lambs probably represents the first report confirming the occurrence of astrovirus infections in animals and was described in 1977 shortly after the description of astroviruses in humans (Snodgrass and Gray, 1977). Studies on pathogenesis of OAstV in lambs demonstrates that it replicates in the absorptive epithelial cells of the small intestine and induces mild diarrhea in gnotobiotic lambs (Gray et al., 1980, Snodgrass et al., 1979). OastV does not seem to play a crucial role in the ovine viral enteritis since no further case reports or studies have followed after the first identification. As a consequence, the epidemiology of OAstV is unknown; the same for the serological or genetic diversity and only the complete genome sequence of the original isolate is available in the public genetic database (GenBank, http://www.ncbi.nlm.nih.gov; Table 2). Nevertheless, this OAstV genome has been extensively analyzed as a prototype Mamastrovirus (Jonassen et al., 2003), and found to harbor, as most of known AstVs, a RNA motif in the 3′ end of the genome common to distinct and genetically different virus families (i.e. Coronaviruses and Picornaviruses) and supposed to be representative of a recombination event between these viruses (Jonassen et al., 1998). Recent findings concerning novel astroviruses detected in human stools and genetically closely related to OAstV and MiAstV, have suggested the possibility for these viruses to have a common ancestor, as assumed for the previously described HAstVs, FeAstVs, and PoAstVs (Finkbeiner et al., 2009b, Jonassen et al., 1998, Kapoor et al., 2009, Quan et al., 2010).
5.1.2. Bovine astrovirus (BoAstV)
The first isolate of bovine astrovirus was reported in England in 1978 (Woode and Bridger, 1978). Astrovirus was considered to be avirulent, as experimentally infected gnotobiotic calves remained clinically normal, although pathological studies on infected calves were not performed. Subsequently, a bovine enteric virus antigenically related to the UK strain of BoAstV was isolated in Florida from a calf with diarrhea (Woode et al., 1984). Although calves experimentally infected with this astrovirus did not develop clinical disease, the virus caused cytopathology of the M cells of the dome epithelium covering the Peyer’s patches (Woode et al., 1984). Similarly to OAstV, the limited clinical significance initially attributed to BoAstV infection in cattle is probably the reason why there has been a lack of reports for more than 25 years, until 2011 (Tse et al., 2011). It was originally concluded that in natural conditions, BoAstV does not seem to be directly associated with a severe diarrheic disease in calves (Bridger et al., 1984, Tse et al., 2011, Woode and Bridger, 1978, Woode et al., 1984) and few controversial data are available on the prevalence of this infection. In 2010, a study conducted in Europe by Smits et al. (2010) described astrovirus infection in European roe deer (Capreolus capreolus) suffering from gastroenteritis (Smits et al., 2010). The virus responsible for the infection was further shown to be genetically related to BoAstV, suggesting that this virus could cross the species barrier to infect both cattle and roe deer (Tse et al., 2011). Although a previous study suggested that BoAstV could be excreted by 60–100% of calves on farms (Bridger et al., 1984), only 5 out of 209 rectal swabs (2.4%) collected from asymptomatic adult cattle were found positive for BoAstV infection (Tse et al., 2011). In bovine, two serotypes have been recognized by serological investigation, namely BoAstV-1 and BoAstV-2 (Woode et al., 1985), and recent phylogenetic analyses support classification of BoAstVs and the newly discovered AstVs in roe deer (CcAstV) under the proposed “genocluster GI” of the Mamastrovirus (Bosch et al., 2010b, Smits et al., 2010, Tse et al., 2011). The antigenic diversity detected in the earlier studies is also reflected in the more recently described genetic diversity for BoAstV. In a recent report, Tse et al. (2011) detected sequences from two different BoAstVs in 2 out of 5 positive specimens (Tse et al., 2011). This finding is in compliance with other reports on the occurrence of co-infections caused by genetically distinct astroviruses infecting the same host, as was described for pigs (Luo et al., 2011) and is a pre-requisite for recombination between different strains. In the same study, at least one recombination event was identified at the ORF2 between BoAstVs and CcAstVs (Tse et al., 2011). However, it should be noted that only two partial BoAstV sequences are available in GenBank (Table 2).
5.1.3. Feline astrovirus (FeAstV)
Feline astrovirus was first described in cats in 1981 (Hoshino et al., 1981). To date, picornavirus-like particles as well as astroviruses from feces of domestic cats have been described in Australia, England, Germany, New Zealand and the USA (Harbour et al., 1987, Herbst and Krauss, 1989, Hoshino et al., 1981, Marshall et al., 1987, Rice et al., 1993). The impact of AstV infection in cats seems to be low as infection of specific-pathogen free kittens caused two episodes of pyrexia and mild diarrhea (Harbour et al., 1987). Similarly, in a 16-month study conducted in Australia, the occurrence of astrovirus infection was not related to the age of cats and to the presence of diarrhea (Marshall et al., 1987). Interestingly, in this study a human serum was found to react against a cat astrovirus by immune electron microscopy, implying similarity in antigenic regions between human and FeAstV (Marshall et al., 1987). To date, only partial sequences of the ORF1b and of the ORF2 have been deposited in public databases (Table 2), and feline astrovirus diversity has not been yet explored. Interestingly, a study on both AstV genome sequences and host species evolution has led to the hypothesis of a cross-species transmission likely occurred from pigs to cats and further from cats to humans, possibly involving intermediate species (Lukashov and Goudsmit, 2002). Recently, a Mamastrovirus was identified in cheetahs (Acinonyx jubatus) raised in captivity showing clinical signs of gastroenteric disease, such as lethargy, anorexia, watery diarrhea and regurgitation (Atkins et al., 2009). The newly discovered virus was shown to be closely related to the FeAstV in ORF2 (88.6%, 97.1% nucleotide and amino acid identity, respectively); although comparison with ORF1b sequences was not possible due to the unavailability of FeAstVs sequences. Cheetah’s AstV clustered relatively more closely to HAstVs at the ORF2 level than at the ORF1b. This was an unexpected finding taking into account that for AstVs, the rate of change is generally higher in the capsid region (van Hemert et al., 2007b) and may indicate that a recombination event occurred between AstVs from different host species (Atkins et al., 2009). Currently it is unclear whether the cheetah AstV had crossed the species barrier from domestic cats or if it had established in cheetahs independently.
5.1.4. Porcine astrovirus (PoAstV)
Astrovirus-like particles were first detected in pigs in the 1980s by EM (Bridger, 1980, Shirai et al., 1985). These reports were followed by the detection PoAstVs in several countries throughout the world; namely South Africa (Geyer et al., 1994), the Czech Republic (Indik et al., 2006), Hungary (Reuter et al., 2011), Quebec (Luo et al., 2011) and Colombia (Ulloa and Gutierrez, 2010), suggesting a wide geographical distribution. Although PoAstV is described as a common finding from fecal samples of apparently healthy pigs (Luo et al., 2011, Reuter et al., 2011), the clinical significance of this infection has not been completely clarified. As a matter of fact, clinical symptoms have been reported mainly in piglets, often in association with rotavirus-, coronavirus- and calicivirus-like infections (Bridger, 1980, Indik et al., 2006, Luo et al., 2011, Shimizu et al., 1990, Shirai et al., 1985). PoAstV prevalence is considered variable from 0% to 83% and 100% based on serological and virological surveillance, respectively (Luo et al., 2011, Shimizu et al., 1990). Though ICTV officially recognizes only one species of PoAstV (PoAstV-1) several reports have demonstrated the existence of multiple serotypes and subtypes. PoAstV-1 was partially characterized in 2006 (Indik et al., 2006), sharing only 86% nucleotide similarity with the previously characterized strain and clearly indicating that multiple serotypes could infect pigs (Indik et al., 2006). A putative PoAstV-2 was detected in Hungary (Reuter et al., 2011), with a low value of similarity (46% and 43% nucleotide and amino acid similarity, respectively) with PoAstV-1 at the ORF2 (Reuter et al., 2011). At the time of analysis, ORF1b sequences of PoAstV-1 were not available and authors found that partial ORF1b sequence of the putative PoAstV-2 shared 67% nucleotide similarity with HAstV-3 (Reuter et al., 2011). A more recent study in Quebec confirmed that pigs could harbor several distinct AstVs most likely derived from different ancestors (Luo et al., 2011). These PoAstVs did not cluster in a monophyletic group and three distinct PoAstV groups were identified, with the group II PoAstVs being the most prevalent (Luo et al., 2011). Group II PoAstVs harbored specific genetic patterns: for instance, they were not genetically related to other animal strains identified to date, they contained a unique insertion of three nucleotides upstream the ORF2 and they did not possess the highly conserved stem-loop-II-like (s2m) motif in the ORF2/3′UTR common to the majority of AstVs and other positive sense RNA viruses (Jonassen et al., 1998, Luo et al., 2011). These findings were similar for the putative PoAstV-2 strain (Reuter et al., 2011).
Recently, there has been evidence of multiple recombination events between distinct PoAstV strains and between PoAstV and HAstV in the variable region of ORF2 (Ulloa and Gutierrez, 2010). Interestingly, authors noticed that all PoAstV-HAstV recombinant viruses emerged from the same geographical area where pigs are raised in promiscuity with human beings. Considering the evident diversity of human strains compared to PoAstVs, the authors suggested that viral transmission likely occurred from humans to pigs and not vice versa, as it had been previously hypothesized (Lukashov and Goudsmit, 2002, Ulloa and Gutierrez, 2010).
5.1.5. Mink astrovirus (MiAstV)
In mink, AstV infection has been considered a significant risk factor for the pre-weaning diarrhea syndrome (Englund et al., 2002) and more recently a novel AstV has been discovered in brain tissue of mink developing the so-called shaking mink syndrome (SMS) (Blomstrom et al., 2010). A pre-weaning syndrome has been well described in mink farms since 1954 in Denmark (Svennekjaer, 1954). Mink farmers refer to this syndrome as “sticky”, “greasy”, or “wet” kits, due to the hyper secretion from apocrine glands in the dorsal neck region thus resulting in soiling of the neck and back (Schneider and Hunter, 1993), together with diarrhea and dehydration at various degrees (Henriksen, 1987). In 2003 the virus associated with this syndrome was entirely sequenced and characterized as a novel astrovirus with less than 67% similarity at the nucleotide level with the closest related OAstV, thus called MiAstV (Mittelholzer et al., 2003a, Mittelholzer et al., 2003b). Interestingly, sequence analysis of MiAstVs from geographically distinct Swedish and Danish farms indicated that they are rather conserved from a genetic point of view; although this observation was mainly based on a short fragment of ORF 1b and geographical clusters were revealed (Mittelholzer et al., 2003a, Mittelholzer et al., 2003b). This appears to be in contrast with the data collected for astroviruses infecting other species, such as swine, bats or turkeys, indicating a high degree of genetic variability (Cattoli et al., 2007, Luo et al., 2011, Pantin-Jackwood et al., 2011, Zhu et al., 2009). Recently, Blomstrom et al. (2010) have identified a unique astrovirus associated with SMS (Table 2), a neurological disorder of unknown etiology (Blomstrom et al., 2010, Gavier-Widen et al., 2004). The AstV associated with SMS and found in the brain tissue of mink, was very similar to MiAstV in ORF1a and ORF1b (88% and 98% homology, respectively) and had a lower identity for ORF2 (67% and 59% homology for nucleotide and amino acid sequences, respectively) (Blomstrom et al., 2010). Intriguingly, there was also low level similarity to a unique AstV associated with a neurological disorder in a child affected by X-linked a gamma-globulinemia (HAstV-PS) (Quan et al., 2010). HastV-PS is also genetically related to AstV-VA1 (Finkbeiner et al., 2009b) and to a lesser extent to MiAstVs and OAstVs, thus falling within the proposed genocluster GII of Mamastroviruses (Bosch et al., 2010b, Quan et al., 2010).
5.1.6. Canine astrovirus (CaAstV)
Since the 1980s astrovirus-like particles have been reported in dogs with and without diarrhea (Williams, 1980, Marshall et al., 1984, Vieler and Herbst, 1995). However, infection in dogs was only recently confirmed by genetic characterization (Toffan et al., 2009, Zhu et al., 2011) and a canine astrovirus has also been recently isolated in cell culture (Martella et al., 2011) (Table 2). To date, canine astroviruses (CaAstV) or astrovirus-like particles in dogs have been reported in the USA, Germany, Austria, Italy, China and France (Grellet et al., submitted, Marshall et al., 1984, Toffan et al., 2009, Vieler and Herbst, 1995, Williams, 1980, Zhu et al., 2011). The clinical significance of CaAstV infections in dogs requires further investigation; however, CaAstV was detected from symptomatic puppies occasionally suffering from diarrhea in association with other enteric viruses, such as rotaviruses (Toffan et al., 2009) and corona- and/or parvo-like viruses (Williams, 1980, Martella et al., 2011). A recent study on the prevalence of CaAstV in Shanghai, China suggested that 12.02% (22/183) of the puppies showing clinical signs of diarrhea were positive for astrovirus by RT-PCR as compared to zero of the 138 healthy controls (Zhu et al., 2011). In a recent study conducted in Italy, 24.5% of 110 stool samples collected from symptomatic dogs and 9.3% of 75 from asymptomatic animals under survey tested positive for the presence of CaAstV RNA (Martella et al., 2011). In the same study, serologic assay indicates that 59% of 54 dogs surveyed in Italy presented specific CaAstV-antibodies, with specific antibodies detected almost exclusively in dogs aged >3 months (Martella et al., 2011). A similar prevalence was found in France where 20.9% (66/316) of the puppies in 42% (14/33) of the breeding kennels surveyed were CaAstV positive by RT-PCR (Grellet et al., submitted). In the same report, the authors noted that puppies less than 7 week-old were especially susceptible to CaAstV infection (Grellet et al., submitted).
Genetic analysis of ORF2 and partial ORF1b identified the virus as a member of the genus Mamastrovirus, falling in the proposed genocluster GI comprising “classic” human, porcine and feline AstVs but genetically well distinguishable from the other Mamastroviruses (Bosch et al., 2010b, Toffan et al., 2009). Among available sequences, the closest similarity was found with HAstV-7 and HAstV-1 (22% and 59.4% nucleotide similarity for ORF2 and partial ORF1b sequences, respectively). Thus, genospecies GI.E has been further proposed as being a new species (Bosch et al., 2010b).
5.1.7. Bat astrovirus (BatAstV)
Novel astroviruses in insectivorous bats were recently discovered in Hong Kong, mainland China and Germany (Chu et al., 2008, Drexler et al., 2011, Zhu et al., 2009). AstV in bats seems to occur as a low pathogenic asymptomatic infection, as it has been observed in relation to the reproductive success of a Myotis myotis colony under study, where AstV was detected together with Coronaviruses and Adenoviruses (Drexler et al., 2011). In this study AstV displayed different patterns of amplification during the observed three-year period (Drexler et al., 2011). In summary, for the first two years of the survey a unique peak occurred only after the formation of a colony with sufficient size and density, and only in the third year a second peak was noticed associated with parturition, similar to what observed for the entire survey period for coronavirus infection in the same colony (Drexler et al., 2011). Interestingly, the second peak was associated with the predominance of a different lineage of AstV in the bat colony, indicating the emergence of a pathogen with enhanced replication efficiency (Drexler et al., 2011). A remarkably high prevalence and genetic diversity were observed for AstVs occurrence in bats (Chu et al., 2008, Drexler et al., 2011, Zhu et al., 2009). The Hong Kong study demonstrated that 7 out of 9 sampled bat species were positive for astrovirus, with a total of 77 BatAstVs found in fecal swabs (Chu et al., 2008). Partial sequence analysis of ORF1a and analysis of the complete ORF1b indicated that bat AstVs clustered alone and fell into two subgroups according to the species of origin (Chu et al., 2008). In particular, this study identified group A and group B BatAstVs, primarily detected in Miniopterus and Myotis spp, without any bat species restriction, and a third group made of five viruses clustering separately (Chu et al., 2008). Full length sequencing of one BatAstV revealed the absence of the s2m motif, similarly to TAstV-2, RatAstV and group II PoAstVs (Chu et al., 2008, Chu et al., 2010, Jonassen et al., 1998, Jonassen et al., 2003, Luo et al., 2011). Viral diversity of BatAstVs was also observed by Zhu (Chu et al., 2008). Seven monophyletic groups (1 to 7) were identified from the ORF1b sequences of known Mamastroviruses. With the exception of group 1 specific for humans, feline and porcine AstVs, BatAstVs fell in all remaining groups, and 5 groups were completely occupied by BatAstVs (Zhu et al., 2009). Five groups revealed a varying degree of host restriction within the bat populations. These findings were confirmed by sequence analysis of the ORF2. Three viral lineages from mammalian hosts were clearly identified, namely “human/feline/porcine”, “bats only”, and a “mixed” group (Zhu et al., 2009). Interestingly, a high degree of viral diversity was confirmed by Zhu et al. (2009). More specifically, viruses belonging to different ORF1b genetic lineages (namely 2, 3 and 6) were identified from a single species in a single cave and sampled in a single day, indicating high level of circulation of different AstVs in the same host (Zhu et al., 2009). On the contrary, viruses detected from a unique species in different sites fell in the same genetic group, confirming a certain degree of host restriction (Zhu et al., 2009). In a recent three-year monitoring of a Myotis Myotis colony in Western Germany, 6 distinct Bat AstVs, as well as a coronavirus and a novel adenovirus, were detected from the fecal samples collected from the sampling site (Drexler et al., 2011). All six BatAstVs found in the German colony fell within the putative genocluster GII and clustered with other previously discovered AstVs from Myotis chinensis and Myotis ricketti in mainland China (aminaocid similarity ranging from 65–86%) (Bosch et al., 2010b, Zhu et al., 2009).
5.1.8. Other astroviruses infecting mammals
In 1981, an astrovirus was associated with an outbreak of gastroenteritis in red deer (Cervus elaphus) raised in captivity. Although genetic characterization was not performed for this virus, antigenic characterization indicated that the new virus was unrelated to OAstV or BoAstV grown in cell cultures (Tzipori et al., 1981). Unfortunately, no further findings have been reported in red deer.
An astrovirus-like infection was associated with an outbreak of gastroenteritis in inbred mice by EM (Kjeldsberg and Hem, 1985). In this report, astrovirus-like particles were detected in the intestinal content of 16/17 nude mice displaying diarrhea and 34/55 symptomless and in 4/10 normal apparently healthy mice (Kjeldsberg and Hem, 1985). The high prevalence in asymptomatic animals might suggest that mice could carry AstV and develop disease when immunocompromised (Kjeldsberg and Hem, 1985). The pathogenicity and genetic patterns of these viruses were not further investigated and to date no further reports in laboratory mice have been documented.
Recently, novel astroviruses were identified in the feces of urban brown rats sampled in Hong Kong (Chu et al., 2010). Sequence analyses revealed a high degree of variability among RatAstVs and the absence of the 2sm motif, similarly to TAstV-2, BatAstVs, and group II PoAstV as described by Luo et al. (2011) (Chu et al., 2010, Jonassen et al., 2003, Luo et al., 2011). These viruses clustered into2 genetically related but distinct clades closely related to the human MLB1 and MLB2 AstVs; leading to the hypothesis of a putative common ancestor (Chu et al., 2010, Finkbeiner et al., 2009a, Jonassen et al., 2003, Luo et al., 2011).
Five genetically distinct AstVs have recently been found in the fecal samples of three marine mammal species: a California sea lion (CslAstV-1, CslAstV-2, CslAstV-3), a Steller’s sea lion (SslAstV) and a bottlenose dolphin (BdAstV) (Rivera et al., 2010). Genetic analyses of ORF1b and ORF2 indicate that these viruses do not cluster in a monophyletic group but across the Mamastrovirus tree, as confirmed by the proposal of re-classification submitted to ICTV. Recombination analyses suggested that a recombination event may have occurred between a human strain (HAstV-4) and a CslAstV-2, possibly resulting in CslAstV-3 (Rivera et al., 2010).
5.2. Avian astroviruses
5.2.1. Duck astrovirus (DAstV)
The first clinical description of a disease syndrome (duck hepatitis) presumably associated with astrovirus infection in poultry dates back to 1965 (Asplin, 1965a, Asplin, 1965b). The astrovirus-associated disease in ducklings was first described in 1984 and was based on morphology of the virions by EM (Gough et al., 1984). This virus was later definitively identified as astrovirus and we are now aware that astrovirus is the causative agent of duck hepatitis, a severe acute disease detectable in 1–2 week-old ducklings which can result up to 50% mortality (Fu et al., 2009). Livers in the affected animals displays typical multiple and widespread hemorrhagic lesions, indicating necrosis of the hepatocyte cytoplasm with the formation of much cavitations or ballooning degeneration at the histopatological investigation (Fu et al., 2009, Gough et al., 1985). Until 1984, the etiologic agent of duck fatal hepatitis was thought to be a Picornavirus. Former classification recognized three picornaviruses as duck hepatitis virus (DHV) I, II and III, based on vaccine protection. Although DHV II was classified as a DAstV, in 2009 Todd et al. (2009) genetically characterized DHV II and III ORF1b and definitely defined them as astroviruses (Todd et al., 2009a). DHV II and DHVIII share a low level of nucleotide and aminoacid identities (64 and 69%) and DHV III appears more closely related to TAstV-2 than DHV II, which is in fact more related to CAstV. However, since these findings were based on sequence analysis of only a small portion of ORF1b (∼390 nt) further characterization is required before phylogenetic and taxonomic relationships can be established. Recently Fu et al. (2009) sequenced the complete genome of a duck astrovirus associated with fatal hepatitis in Chinese ducklings (Fu et al., 2009). Sequence analysis demonstrated that the genome exhibited a classical AstV organization with two unique features; the largest poly-adenylated genome of 7722 nt, and the presence of ORF2 in a different frame than ORF1a or ORF1b, with a start codon 23 nt downstream of the stop codon of ORF1b. From a phylogenetic point of view this virus is more closely related to TAstV-2, which suggests that the duck virus may have originally transmitted from turkeys (Fu et al., 2009).
5.2.2. Turkey astrovirus (TAstVs)
To date, two genetically distinct TAstVs have been recognized: TAstV-1 and TAstV-2. TAstV-1 was firstly detected in diseased turkey flocks in the ’80s (Reynolds and Saif, 1986, Reynolds et al., 1987, Saif et al., 1985, Saif et al., 1990). TAstV-2 was characterized in association with poult enteritis mortality syndrome (PEMS) (Koci et al., 2000) and PEMS-associated TAstV-2 has been shown to cause enteritis, thymic and bursal atrophy, and increased mortality in experimentally infected poults (Behling-Kelly et al., 2002). Field observations indicate that TAstV-2 is currently more prevalent in turkeys than TAstV-1 (Cattoli et al., 2007, Pantin-Jackwood et al., 2006b) and its occurrence is often associated with other enteric viruses (Jindal et al., 2010a, Jindal et al., 2010b, Reynolds et al., 1987). Nevertheless, it is one of the most commonly detected viruses from turkey poults suffering diarrhea. The exact role of TAstV-2 as the etiological agent of poult enteritis complex and PEMS is difficult to estimate since it can be easily detected in apparently healthy adult turkeys (Jindal et al., 2010b, Jindal et al., 2011). Recently, Spackman et al. (2010) have evaluated the effect of TAstV-2, turkey rotavirus and turkey reovirusco-infections in decreased weight gain in broad-breasted white turkey poults. They showed that poults exposed to all three viruses administered in combination had the lowest body weight, although the weights were not significantly different from control birds (Spackman et al., 2010). The prevalence of TAstV varies from 40 to 80% in symptomatic poults (Cattoli et al., 2007, Domanska-Blicharz et al., 2011, Jindal et al., 2010a, Reynolds et al., 1987) to less than 30% to 50% in healthy flocks (Jindal et al., 2010b, Reynolds et al., 1987). Generally speaking TAstVs are widely spread and considered as the most prevalent virus infection in 1–5 week-old poults suffering from diarrhea (Jindal et al., 2010a, Jindal et al., 2010b, Reynolds and Saif, 1986, Reynolds et al., 1987, Saif et al., 1985). Although TAstVs can be detected in adult healthy turkeys (Jindal et al., 2010b), clinical signs of disease usually develop between 1 and 3 weeks of age and generally last up to 2 weeks (Reynolds and Schultz-Cherry, 2003). Severity is usually mild to moderate and is characterized by diarrhea, listlessness, litter eating and nervousness. Although mortality rate is low, morbidity occurring as decreased growth is of great concern (Reynolds and Schultz-Cherry, 2003). Data from full genome sequencing of TAstV-1 and TAstV-2 indicate that they are genetically unrelated and likely to have originated from separate introductions and should therefore be regarded as two subtypes instead of serotypes (Jonassen et al., 2003, Pantin-Jackwood et al., 2011, Strain et al., 2008). Beyond this classification, data from genetic and antigenic analyses of field viruses detected in turkeys indicate the circulation of multiple lineages of TAstV-2. Genetic and antigenic characterization of two turkey isolates related to TAstV-2 (TAstV1987-TAstVT2001 also identified as TAstV-3) suggests that they could be virtually classified as two different serotypes (Pantin-Jackwood et al., 2011, Tang and Saif, 2004, Tang et al., 2005), similarly to strain MN/01 (Strain et al., 2008). Characterization of 23 distinct TAstVs-2 throughout the USA identified different lineages based on ORF1b and ORF2 sequence analysis with no clear geographic neither temporal derives (Pantin-Jackwood et al., 2006a). Molecular characterization of TAstVs revealed an unexpected variability among ORF2, on the basis of what has been reported for human viruses (Pantin-Jackwood et al., 2011, Walter et al., 2001). Such variability was related not only to nucleotide substitutions but also to insertions and deletions at several different positions (Pantin-Jackwood et al., 2011). There were different topologies for ORF1b and ORF2 trees confirmed by SimPlot analysis and episodes of recombination in different isolates collected in different houses of the same farm on the same day (Pantin-Jackwood et al., 2006a, Pantin-Jackwood et al., 2006b, Pantin-Jackwood et al., 2011). Similarly to previous findings, Cattoli et al. (2007) identified different lineages of TAstV-2 circulating in Italian flocks, based on ORF1b or ORF2 sequences, respectively. Importantly, for 29 out of 41 TAstV-2 sequences, the amplification of ORF2 was not achieved, confirming a high rate of variability between the circulating TAstVs-2 (Cattoli et al., 2007). The existence of different sublineages of TAstV-2 in turkey farms has been confirmed by field studies (Domanska-Blicharz et al., 2011, Jindal et al., 2010a, Jindal et al., 2010b, Jindal et al., 2011, Pantin-Jackwood et al., 2006a). Field findings are consistent with further genetic studies (Strain et al., 2008). This analysis suggested that TAstV-1 and TAstV-2 should be reconsidered as subtypes instead of serotypes and that multiple serotypes of TAstV-2 exist (Pantin-Jackwood et al., 2011, Strain et al., 2008). A total of 46 recombination events were identified, with at least one of them for each of ten analyzed TAstVs-2 (Strain et al., 2008). Results from this analysis also pointed out the need for a deep genetic analysis. In fact, a phylogenetic analysis can suggest distinct results if different parts of the genome are analyzed. It is clear that the ORF2 is the most important region to analyze when trying to determine genus and species classification, and the junction across ORF1b and ORF2, as a potential hot spot of recombination (Strain et al., 2008). Whether the currently circulating TAstV-2 sublineages have evolved as immunological escape mutants or circulate as multiple discrete sub-lineages, similar to what happens in HIV infections, is still under debate. In turkeys, antibody response against TAstV-2 is not sufficiently elicited (Koci et al., 2004) and does not seem to play a crucial role in the evolution of TAstVs-2 sublineages, which in fact do not cluster neither geographically nor historically following immunological pressure (Pantin-Jackwood et al., 2006b).
Interestingly, an astrovirus has been found to infect and cause diarrhea in guinea fowl (Cattoli et al., 2005, Cattoli et al., 2007). The origin of this virus remains unknown, although sequence analysis suggests that interspecies transmission might have occurred between turkey and guinea fowl (Cattoli et al., 2007).
5.2.3. Avian nephritis virus (ANV)
Avian nephritis virus (ANV) was first isolated from rectal content of apparently healthy broiler chicks (Yamaguchi et al., 1979) and experimental infections revealed that it mainly results in a sub-clinical disease, although mild growth depression and mortality have been reported for infections with ANV-1 (the strain G-4260) (Imada et al., 1979, Maeda et al., 1979, Yamaguchi et al., 1979). Like many other avian AstVs, ANV was initially classified as a picornavirus based on EM analysis (Maeda et al., 1979, Yamaguchi et al., 1979) and only after complete genome sequencing was it reclassified as a member of the Astroviridae family (Imada et al., 2000). At least two serotypes have been reported to date (Frazier et al., 1990, Imada et al., 1980, Pantin-Jackwood et al., 2011, Shirai et al., 1991). ANV typically causes histological changes in the kidneys although the viral antigen can be detected in other organs, namely liver, spleen, pancreas, jejunum and rectum (Imada et al., 1979, Imada et al., 1983, Shimizu et al., 1990, Shirai et al., 1989, Shirai et al., 1992). Young chicks are the most susceptible and become resistant to disease after the first month of life (Imada et al., 1981). Clinical disease in chicks varies from subclinical to outbreaks of runting stunting syndrome (RSS) and chick nephropathy (Frazier et al., 1990, Imada et al., 1980, Shimizu et al., 1990, Shirai et al., 1992, Takase et al., 1989, Takase et al., 1994, Yamaguchi et al., 1979). However, the impact of ANV infection on RSS is unknown. Although ANV affected body gain in specific-pathogen free chicks (Shirai et al., 1991) an experimental study on the effect of ANV and several unclassified enterovirus-like viruses (ELVs) in commercial chicks demonstrated that only one ELV analyzed among the broad panel produced clinical signs in inoculated chicks (McNulty et al., 1990). Similarly, infection of 1-day-old chicks with three different ELVs and an ANV showed that ELVs are able to induce more severe lesions in the intestine, the kidneys and the pancreas than ANV (Smyth et al., 2007).
Passive and active surveys indicate that ANV is widely distributed worldwide and that serological evidence can be found in turkeys as well (Connor et al., 1987, Decaesstecker et al., 1989, Hewson et al., 2010, Imada et al., 1980, McNulty et al., 1989, Nicholas et al., 1988, Takase et al., 1989, Zhao et al., 2011). Although to date the infection has never been associated with the disease in turkeys, an ANV genetically related to ANV-1 was detected in the feces of turkeys. However, the significance of this finding requires further investigation (Pantin-Jackwood et al., 2006a, Pantin-Jackwood et al., 2011).
5.2.4. Chicken astrovirus (CAstV)
Astrovirus-like virions were first observed in feces of chickens by EM in the 1990s (McNeilly et al., 1994, McNulty et al., 1990), but until recently little was known about their genetic and antigenic characteristics. A preliminary study on a panel of three newly isolated and characterized CAstVs revealed that 1-day-old chicks infected with one CAstV developed mild diarrhea and distension of the small intestine (Baxendale and Mebatsion, 2004). They also showed that those viruses were antigenically and genetically distinct from ANV, the only astrovirus known to infect chickens (Baxendale and Mebatsion, 2004). A small portion of ORF1 was sequenced and showed limited similarity to the non-structural proteins of TAstV-2 (62%), TAstV-1 (58%), and ANV (55%). Serological evidence of CAstV has been demonstrated in broiler flocks in the UK, the Netherlands, Spain, Australia, and the USA (Baxendale and Mebatsion, 2004) and more recently in broiler parent flocks in the UK that spread to European breeder flocks (Todd et al., 2009b), which suggests a worldwide distribution. Recently molecular and antigenic characterization of enterovirus-like viruses (ELVs), previously classified as belonging to the Picornaviridae family, demonstrated that they were distantly related to CAstVs (Todd et al., 2009a). Further studies on CAstV genetic and antigenic characteristics indicate that multiple serotypes exist (Pantin-Jackwood et al., 2006a, Pantin-Jackwood et al., 2011, Todd et al., 2009a, Todd et al., 2009b). In particular, sequencing of ORF2 of 32 avian astroviruses showed the circulation of at least two CAvtVs, namely CAstV-2 to 3 (Pantin-Jackwood et al., 2011). To date, full sequencing of CAstVs has not been done and therefore many questions remain to be answered on viral genome organization and on the origin of this putative new species of AAstV.
6. Genetic variability, interspecies transmission and zoonotic potential of astroviruses
Given the nature of their RNA-dependent RNA polymerase, which is error-prone and switches from copying one RNA molecule to the next without releasing the original strand, RNA viruses have a tendency towards significant genetic variability due to the introduction of mutations and genetic recombination. Astroviruses are not an exception in this term. In fact, genetic variability has been described in almost all mama- and avastrovirus species sufficiently examined (Belliot et al., 1997a, Belliot et al., 1997b, Chu et al., 2010, De Grazia et al., 2011, Finkbeiner et al., 2009a, Finkbeiner et al., 2009b, Finkbeiner et al., 2009c, Gabbay et al., 2007, Kapoor et al., 2009, Liu et al., 2008), PoAstVs (Luo et al., 2011), BoAstVs (Tse et al., 2011), BatAstVs (Chu et al., 2008, Drexler et al., 2011, Zhu et al., 2009), RatAstVs (Chu et al., 2010), TAstVs (Cattoli et al., 2007, Pantin-Jackwood et al., 2006b, Strain et al., 2008) and CAstV (Pantin-Jackwood et al., 2011, Todd et al., 2009b). Genetic variability can be induced by synonymous or non-synonymous nucleotide substitutions, the latter being subjected to stronger positive or negative selection pressure. Data gathered on astroviruses suggest pressures and evolutionary constrains for different selection in different genomic regions. For example, regions of the astrovirus capside protein exposed to the immune pressure and environmental changes, such as predicted epitopes or sites interacting with host cells, are subject to positive selection and codon changes (Lukashov and Goudsmit, 2002, Strain et al., 2008, van Hemert et al., 2007a). Furthermore, there is an increasing evidence of recombination events occurring in astroviruses, which contributes to increased genetic variability. Recombination within the classic HAstV stains was among the first examples described. Walter et al. (2001) characterized a novel recombinant HAstV strain associated with diarrhea in children (Walter et al., 2001). They identified a virus containing an ORF2 region from HAstV-5 and an ORF1b region belonging to HAstV-3 that was widely circulating and stable being isolated from separate geographical regions at least one year apart. This suggested that the recombination even took place at the ORF1b-ORF2 transition region. More recently, a recombinant virus was isolated from a Kenyan child with diarrhea that contained an ORF1a clustered with HAstV-6/7, an ORF1b clustered with HAstV-3, and an ORF2 from HAstV-2 (Wolfaardt et al., 2011). Similar to the study from Walter et al. (2001), the ORF1b-ORF2 junction was identified as a recombination point with a second possible recombination point within the ORF1a region (Walter et al., 2001, Wolfaardt et al., 2011).
Also, recombination events have occurred in astroviruses infecting animals. At least one has occurred during the evolution of BoAstV and CcAstV (Tse et al., 2011) and evidence of recombinations was revealed in distinct studies on TAstV-2 viruses circulating in the USA (Pantin-Jackwood et al., 2006b, Strain et al., 2008).
A major question is whether an animal astrovirus can recombine with a HAstV leading to human disease or facilitate zoonotic transmission and the emergence and spread of novel viruses into the human or the animal population. A recent report suggests that a recombination even may have occurred between a human and a California sea lion astrovirus (Rivera et al., 2010) leading to the hypothesis of a common ancestor or to a zoonotic transmission event. Similarly, a possible recombination event between PoAstV and HAstV3 was described in another recent publication (Ulloa and Gutierrez, 2010).
The wide variety of species infected the evident virus genetic diversity and the occurrence of recombination events indicate or imply the cross-species transmission and subsequent virus adaptation to new hosts and/or the co-infection of the same host with different astroviruses. An interesting study on astroviruses evolution suggests that several cross-species transmissions probably occurred during the evolutionary history of astroviruses (van Hemert et al., 2007a). After the cross-species transmission, the viruses adapted to the new hosts diversifying themselves genetically. Putative common ancestors and ancient interspecies transmissions have been suggested between avian and mammalian species and among mammalians, in more recent time, between sheep and mink, or cheetahs and cats (Atkins et al., 2009, van Hemert et al., 2007a). Cross-species transmission has also been suggested among avian species. For example, the close genetic relatedness between a novel astrovirus causing acute hepatitis in ducks and TAstV-2, and between TAstV-2 and astroviruses infecting guinea fowl suggested transmission of astroviruses between turkey and duck, or guinea fowl, respectively (Cattoli et al., 2007, Fu et al., 2009). Also, ANV-1-like astroviruses were detected in the intestinal contents of healthy turkeys in the USA (Pantin-Jackwood et al., 2006a). Although no documented zoonotic transmission of animal astroviruses to humans is documented, some scientific reports suggested the possibility of cross-species transmission of astroviruses between animals and humans and therefore the zoonotic potential of astrovirus infections. Lukashov and Goudsmit (2002), based on phylogenetic analysis, suggested the transmission of an astrovirus from pigs to cats and then to humans with unidentified intermediate hosts potentially involved (Lukashov and Goudsmit, 2002). Different conclusions were described in a more recent study, suggesting interspecies transmissions of astroviruses from humans to pigs in Canada (Luo et al., 2011). In other recent studies, the suggestion of putative common ancestors of RAstV, MiAstV and OAstV and novel astroviruses found in human stools, namely MLB-like and HMO AstVs, raised questions about the zoonotic transmission of astroviruses (Chu et al., 2010, Kapoor et al., 2009). However, these possibilities remain under investigation.
7. Conclusions and future perspectives
Historically, astroviruses have been considered as species-specific viruses and this was reflected in the past taxonomical criteria for their classification based on the species of origin (Faquet et al., 2005). Considering the almost regular discoveries of novel astroviruses in humans and in animals and the parallel development of genetic and evolutionary studies, not only the capacity of astroviruses to infect a wide variety of hosts in different environments it is now evident but also their potential capability to cross species barriers and become adapted to new hosts. Therefore, the acquired host adaptation presumably does not prevent astroviruses to infect new species. The astonishing genetic diversity of Astroviridae greatly contributes to their successful colonization of/in different environments. It is likely that with increased surveillance and improved diagnostic assays the diversity will only continue to increase. This aspect also imposes alternative nomenclature and taxonomical classification for this virus family, as recently proposed by an ad hoc study group (Bosch et al., 2010a, Bosch et al., 2010b).
After more than 30 years from their first description in clinical samples, there are many exciting avenues of investigation and intriguing questions that remain to be answered as regards the relatively under-studied Astroviridae family, for example those aspects related to biology and epidemiology which still remain unclear. Taking into consideration the molecular biology of astroviruses, a great deal of work is still needed to understand virus replication particularly in relation to cellular factors for productive replication, the role of the individual NSPs in replication and controlling cellular functions and a better understanding of the processing of the capsid protein. Studies on the evolutionary mechanisms of astroviruses and their phylogenetic relationships are at the start and the driving forces governing evolution and adaptation of astroviruses in different host species certainly deserve further research.
In humans, astroviruses are considered among the major viral enteric pathogen; in animals they have been associated with enteric or extra-enteric diseases in some species, such as mink, turkey and duck. However, in many hosts the association between astrovirus infection and disease or clinical syndromes is still debated and under-investigation and the same is for the molecular or phenotypic viral traits potentially associated to pathogenicity and virulence. For example, the pathogenesis and the molecular mechanisms governing virus tissue tropism and the extra-intestinal infections remain largely unknown, particularly for mammalian species.
Furthermore, the virus genetic diversity, the occurrence of multiple infections and recombination events, the ability to cross species barriers and the circulation of astroviruses in animals in close contacts with humans make astroviruses as potential candidates for emerging zoonotic infections.
Acknowledgments
The authors wish to thank Cristian De Battisti and Alice Fusaro, IZSVe-Itay, for their technical support. Francesca Ellero, IZSVe-Italy, is gratefully acknowledged for the revision of the manuscript.
References
- Ahlquist P. Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses. Nat. Rev. Microbiol. 2006;4(5):371–382. doi: 10.1038/nrmicro1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mutairy B., Walter J.E., Pothen A., Mitchell D.K. Genome prediction of putative genome-linked viral protein (VPg) of astroviruses. Virus Genes. 2005;31(1):21–30. doi: 10.1007/s11262-004-2196-1. [DOI] [PubMed] [Google Scholar]
- Appleton H., Higgins P.G. Letter: viruses and gastroenteritis in infants. Lancet. 1975;1(7919):1297. doi: 10.1016/s0140-6736(75)92581-7. [DOI] [PubMed] [Google Scholar]
- Asplin F.D. Duck hepatitis. Vet. Rec. 1965;77:487–488. [PubMed] [Google Scholar]
- Asplin F.D. Duck hepatitis: vaccination against two serological types. Vet. Rec. 1965;77(50):1529–1530. [PubMed] [Google Scholar]
- Atkins A., Wellehan J.F., Jr, Childress A.L., Archer L.L., Fraser W.A., Citino S.B. Characterization of an outbreak of astroviral diarrhea in a group of cheetahs (Acinonyx jubatus) Vet. Microbiol. 2009;136(1–2):160–165. doi: 10.1016/j.vetmic.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banos-Lara Mdel R., Mendez E. Role of individual caspases induced by astrovirus on the processing of its structural protein and its release from the cell through a non-lytic mechanism. Virology. 2010;401(2):322–332. doi: 10.1016/j.virol.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Bass D.M., Qiu S. Proteolytic processing of the astrovirus capsid. J. Virol. 2000;74(4):1810–1814. doi: 10.1128/jvi.74.4.1810-1814.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxendale W., Mebatsion T. The isolation and characterisation of astroviruses from chickens. Avian Pathol. 2004;33(3):364–370. doi: 10.1080/0307945042000220426. [DOI] [PubMed] [Google Scholar]
- Behling-Kelly E., Schultz-Cherry S., Koci M., Kelley L., Larsen D., Brown C. Localization of astrovirus in experimentally infected turkeys as determined by in situ hybridization. Vet. Pathol. 2002;39(5):595–598. doi: 10.1354/vp.39-5-595. [DOI] [PubMed] [Google Scholar]
- Belliot G., Laveran H., Monroe S.S. Detection and genetic differentiation of human astroviruses: phylogenetic grouping varies by coding region. Arch. Virol. 1997;142(7):1323–1334. doi: 10.1007/s007050050163. [DOI] [PubMed] [Google Scholar]
- Belliot G., Laveran H., Monroe S.S. Outbreak of gastroenteritis in military recruits associated with serotype 3 astrovirus infection. J. Med. Virol. 1997;51(2):101–106. doi: 10.1002/(sici)1096-9071(199702)51:2<101::aid-jmv3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Blomstrom A.L., Widen F., Hammer A.S., Belak S., Berg M. Detection of a novel astrovirus in brain tissue of mink suffering from shaking mink syndrome by use of viral metagenomics. J. Clin. Microbiol. 2010;48(12):4392–4396. doi: 10.1128/JCM.01040-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaparte R.S., Hair P.S., Banthia D., Marshall D.M., Cunnion K.M., Krishna N.K. Human astrovirus coat protein inhibits serum complement activation via C1, the first component of the classical pathway. J. Virol. 2008;82(2):817–827. doi: 10.1128/JVI.01847-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch, A., Guix, S., Krishna, N.K., Méndez, E., Monroe, S.S., Pantin-Jackwood, M., Schultz-Cherry, S., 2010a. Three new species in the genus Avastrovirus in the Astroviridae family, ICTV 2010.017abV.
- Bosch, A., Guix, S., Krishna, N.K., Méndez, E., Monroe, S.S., Pantin-Jackwood, M., Schultz-Cherry, S., 2010b. Nineteen new species in the genus Mamastrovirus in the Astroviridae family, ICTV 2010.018.aV.
- Bridger J.C. Detection by electron microscopy of caliciviruses, astroviruses and rotavirus-like particles in the faeces of piglets with diarrhoea. Vet. Rec. 1980;107(23):532–533. [PubMed] [Google Scholar]
- Bridger J.C., Hall G.A., Brown J.F. Characterization of a calici-like virus (Newbury agent) found in association with astrovirus in bovine diarrhea. Infect. Immun. 1984;43(1):133–138. doi: 10.1128/iai.43.1.133-138.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker J.P., Blacklow N.R., Herrmann J.E. Human astrovirus isolation and propagation in multiple cell lines. Arch. Virol. 2000;145(9):1847–1856. doi: 10.1007/s007050070060. [DOI] [PubMed] [Google Scholar]
- Caballero S., Guix S., El-Senousy W.M., Calico I., Pinto R.M., Bosch A. Persistent gastroenteritis in children infected with astrovirus: association with serotype-3 strains. J. Med. Virol. 2003;71(2):245–250. doi: 10.1002/jmv.10476. [DOI] [PubMed] [Google Scholar]
- Carter M.J., Willcocks M.M. The molecular biology of astroviruses. Arch. Virol. Suppl. 1996;12:277–285. doi: 10.1007/978-3-7091-6553-9_30. [DOI] [PubMed] [Google Scholar]
- Cattoli G., Toffan A., De Battisti C., Salviato A., Terregino C., Capua I. Astroviruses found in the intestinal contents of guinea fowl suffering from enteritis. Vet. Rec. 2005;156(7):220. [PubMed] [Google Scholar]
- Cattoli G., De Battisti C., Toffan A., Salviato A., Lavazza A., Cerioli M., Capua I. Co-circulation of distinct genetic lineages of astroviruses in turkeys and guinea fowl. Arch. Virol. 2007;152(3):595–602. doi: 10.1007/s00705-006-0862-4. [DOI] [PubMed] [Google Scholar]
- Caul E.O., Appleton H. The electron microscopical and physical characteristics of small round fecal viruses: an interim scheme for classification. J. Med. Virol. 1982;9(4):257–265. doi: 10.1002/jmv.1890090403. [DOI] [PubMed] [Google Scholar]
- Chu D.K., Chin A.W., Smith G.J., Chan K.H., Guan Y., Peiris J.S., Poon L.L. Detection of novel astroviruses in urban brown rats and previously known astroviruses in humans. J. Gen. Virol. 2010;91(Pt 10):2457–2462. doi: 10.1099/vir.0.022764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K., Poon L.L., Guan Y., Peiris J.S. Novel astroviruses in insectivorous bats. J. Virol. 2008;82(18):9107–9114. doi: 10.1128/JVI.00857-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomba C., De Grazia S., Giammanco G.M., Saporito L., Scarlata F., Titone L., Arista S. Viral gastroenteritis in children hospitalised in Sicily, Italy. Eur. J. Clin. Microbiol. Infect. Dis. 2006;25(9):570–575. doi: 10.1007/s10096-006-0188-x. [DOI] [PubMed] [Google Scholar]
- Connor T.J., McNeilly F., McFerran J.B., McNulty M.S. A survey of avian sera from Northern Ireland for antibody to avian nephritis virus. Avian Pathol. 1987;16(1):15–20. doi: 10.1080/03079458708436348. [DOI] [PubMed] [Google Scholar]
- De Grazia S., Platia M.A., Rotolo V., Colomba C., Martella V., Giammanco G.M. Surveillance of human astrovirus circulation in Italy 2002–2005: emergence of lineage 2c strains. Clin. Microbiol. Infect. 2011;17(1):97–101. doi: 10.1111/j.1469-0691.2010.03207.x. [DOI] [PubMed] [Google Scholar]
- Decaesstecker M., Charlier G., Peeters J., Meulemans G. Pathogenicity of fowl enteroviruses. Avian Pathol. 1989;18(4):697–713. doi: 10.1080/03079458908418643. [DOI] [PubMed] [Google Scholar]
- Domanska-Blicharz, K., Seroka, A., Minta, Z., 2011. One-year molecular survey of astrovirus infection in turkeys in Poland, Arch. Virol. doi:10.1007/s00705-011-0958-3. [DOI] [PMC free article] [PubMed]
- Donelli G., Superti F., Tinari A., Marziano M.L. Mechanism of astrovirus entry into Graham 293 cells. J. Med. Virol. 1992;38(4):271–277. doi: 10.1002/jmv.1890380408. [DOI] [PubMed] [Google Scholar]
- Drexler J.F., Corman V.M., Wegner T., Tateno A.F., Zerbinati R.M., Gloza-Rausch F., Seebens A., Muller M.A., Drosten C. Amplification of emerging viruses in a bat colony. Emerg. Infect. Dis. 2011;17(3):449–456. doi: 10.3201/eid1703.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund L., Chriel M., Dietz H.H., Hedlund K.O. Astrovirus epidemiologically linked to pre-weaning diarrhoea in mink. Vet. Microbiol. 2002;85(1):1–11. doi: 10.1016/s0378-1135(01)00472-2. [DOI] [PubMed] [Google Scholar]
- Faquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A. Elsevier Academic Press; London, UK: 2005. Virus Taxonomy: VIIIth Report of the International Committee on Taxonomy of Viruses. [Google Scholar]
- Finkbeiner S.R., Allred A.F., Tarr P.I., Klein E.J., Kirkwood C.D., Wang D. Metagenomic analysis of human diarrhea: viral detection and discovery. PLoS Pathog. 2008;4(2):e1000011. doi: 10.1371/journal.ppat.1000011. doi:10.1371/journal.ppat.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S.R., Kirkwood C.D., Wang D. Complete genome sequence of a highly divergent astrovirus isolated from a child with acute diarrhea. J. Virol. 2008;5:117. doi: 10.1186/1743-422X-5-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S.R., Le B.M., Holtz L.R., Storch G.A., Wang D. Detection of newly described astrovirus MLB1 in stool samples from children. Emerg. Infect. Dis. 2009;15(3):441–444. doi: 10.3201/1503.081213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S.R., Li Y., Ruone S., Conrardy C., Gregoricus N., Toney D., Virgin H.W., Anderson L.J., Vinje J., Wang D., Tong S. Identification of a novel astrovirus (astrovirus VA1) associated with an outbreak of acute gastroenteritis. J. Virol. 2009;83(20):10836–10839. doi: 10.1128/JVI.00998-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S.R., Holtz L.R., Jiang Y., Rajendran P., Franz C.J., Zhao G., Kang G., Wang D. Human stool contains a previously unrecognized diversity of novel astroviruses. J. Virol. 2009;6:161. doi: 10.1186/1743-422X-6-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier J.A., Howes K., Reece R.L., Kidd A.W., Cavanagh D. Isolation of non-cytopathic viruses implicated in the aetiology of nephritis and baby chick nephropathy and serologically related to avian nephritis virus. Avian Pathol. 1990;19(1):139–160. doi: 10.1080/03079459008418663. [DOI] [PubMed] [Google Scholar]
- Fu Y., Pan M., Wang X., Xu Y., Xie X., Knowles N.J., Yang H., Zhang D. Complete sequence of a duck astrovirus associated with fatal hepatitis in ducklings. J. Gen. Virol. 2009;90(Pt 5):1104–1108. doi: 10.1099/vir.0.008599-0. [DOI] [PubMed] [Google Scholar]
- Fuentes C., Guix S., Bosch A., Pinto R.M. The C-Terminal nsP1a protein of human astrovirus is a phosphoprotein that interacts with the viral polymerase. J. Virol. 2011;85(9):4470–4479. doi: 10.1128/JVI.01515-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay Y.B., Leite J.P., Oliveira D.S., Nakamura L.S., Nunes M.R., Mascarenhas J.D., Heinemann M.B., Linhares A.C. Molecular epidemiology of astrovirus type 1 in Belem, Brazil, as an agent of infantile gastroenteritis, over a period of 18 years (1982–2000): identification of two possible new lineages. Virus Res. 2007;129(2):166–174. doi: 10.1016/j.virusres.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Gavier-Widen D., Brojer C., Dietz H.H., Englund L., Hammer A.S., Hedlund K.O., Hard af Segerstad C., Nilsson K., Nowotny N., Puurula V., Thoren P., Uhlhorn H., Weissenbock H., Agren E., Klingeborn B. Investigations into shaking mink syndrome: an encephalomyelitis of unknown cause in farmed mink (Mustela vison) kits in Scandinavia. J. Vet. Diagn. Invest. 2004;16(4):305–312. doi: 10.1177/104063870401600408. [DOI] [PubMed] [Google Scholar]
- Geigenmuller U., Ginzton N.H., Matsui S.M. Construction of a genome-length cDNA clone for human astrovirus serotype 1 and synthesis of infectious RNA transcripts. J. Virol. 1997;71(2):1713–1717. doi: 10.1128/jvi.71.2.1713-1717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenmuller U., Chew T., Ginzton N., Matsui S.M. Processing of nonstructural protein 1a of human astrovirus. J. Virol. 2002;76(4):2003–2008. doi: 10.1128/JVI.76.4.2003-2008.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer A., Steele A.D., Peenze I., Lecatsas G. Astrovirus-like particles, adenoviruses and rotaviruses associated with diarrhoea in piglets. J. S. Afr. Vet. Assoc. 1994;65(4):164–166. [PubMed] [Google Scholar]
- Glass R.I., Noel J., Mitchell D., Herrmann J.E., Blacklow N.R., Pickering L.K., Dennehy P., Ruiz-Palacios G., de Guerrero M.L., Monroe S.S. The changing epidemiology of astrovirus-associated gastroenteritis: a review. Arch. Virol. Suppl. 1996;12:287–300. doi: 10.1007/978-3-7091-6553-9_31. [DOI] [PubMed] [Google Scholar]
- Glass R.I., Bresee J., Jiang B., Gentsch J., Ando T., Fankhauser R., Noel J., Parashar U., Rosen B., Monroe S.S. Gastroenteritis viruses: an overview. Novartis Found Symp. 2001;238:5–19. doi: 10.1002/0470846534.ch2. (discussion 19–25) [DOI] [PubMed] [Google Scholar]
- Gough R.E., Collins M.S., Borland E., Keymer L.F. Astrovirus-like particles associated with hepatitis in ducklings. Vet. Rec. 1984;114(11):279. doi: 10.1136/vr.114.11.279-a. [DOI] [PubMed] [Google Scholar]
- Gough R.E., Borland E.D., Keymer I.F., Stuart J.C. An outbreak of duck hepatitis type II in commercial ducks. Avian Pathol. 1985;14(2):227–236. doi: 10.1080/03079458508436224. [DOI] [PubMed] [Google Scholar]
- Gray E.W., Angus K.W., Snodgrass D.R. Ultrastructure of the small intestine in astrovirus-infected lambs. J. Gen. Virol. 1980;49(1):71–82. doi: 10.1099/0022-1317-49-1-71. [DOI] [PubMed] [Google Scholar]
- Grellet, A., De Battisti, C., Feugier, A., Pantile, M., Gradjean, D., Cattoli, G., submitted. Prevalence and risk factors of astrovirus infection in puppies from French breeding kennels, Veterinary microbiology. [DOI] [PubMed]
- Gronemus J.Q., Hair P.S., Crawford K.B., Nyalwidhe J.O., Cunnion K.M., Krishna N.K. Potent inhibition of the classical pathway of complement by a novel C1q-binding peptide derived from the human astrovirus coat protein. Mol. Immunol. 2010;48(1–3):305–313. doi: 10.1016/j.molimm.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Guix S., Caballero S., Villena C., Bartolome R., Latorre C., Rabella N., Simo M., Bosch A., Pinto R.M. Molecular epidemiology of astrovirus infection in Barcelona, Spain. J. Clin. Microbiol. 2002;40(1):133–139. doi: 10.1128/JCM.40.1.133-139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guix S., Caballero S., Bosch A., Pinto R.M. C-terminal nsP1a protein of human astrovirus colocalizes with the endoplasmic reticulum and viral RNA. J. Virol. 2004;78(24):13627–13636. doi: 10.1128/JVI.78.24.13627-13636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guix S., Caballero S., Bosch A., Pinto R.M. Human astrovirus C-terminal nsP1a protein is involved in RNA replication. Virology. 2005;333(1):124–131. doi: 10.1016/j.virol.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Guy J.S., Barnes H.J. Partial characterization of a turkey enterovirus-like virus. Avian Dis. 1991;35(1):197–203. [PubMed] [Google Scholar]
- Guy J.S., Miles A.M., Smith L., Fuller F.J., Schultz-Cherry S. Antigenic and genomic characterization of turkey enterovirus-like virus (North Carolina, 1988 isolate): identification of the virus as turkey astrovirus 2. Avian Dis. 2004;48(1):206–211. doi: 10.1637/7077. [DOI] [PubMed] [Google Scholar]
- Hair P.S., Gronemus J.Q., Crawford K.B., Salvi V.P., Cunnion K.M., Thielens N.M., Arlaud G.J., Rawal N., Krishna N.K. Human astrovirus coat protein binds C1q and MBL and inhibits the classical and lectin pathways of complement activation. Mol. Immunol. 2010;47(4):792–798. doi: 10.1016/j.molimm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Harbour D.A., Ashley C.R., Williams P.D., Gruffydd-Jones T.J. Natural and experimental astrovirus infection of cats. Vet. Rec. 1987;120(23):555–557. doi: 10.1136/vr.120.23.555. [DOI] [PubMed] [Google Scholar]
- Henriksen P. En oversigt over syndromet “Fedtede hvalpe” hos mink (A review of “sticky kit” syndrome in mink) Dansk. VetTidskr. 1987;70:580–583. [Google Scholar]
- Herbst W., Krauss H. Electron microscopy in the diagnosis of enteritis in cats. Tijdschr. Diergeneeskd. 1989;114(6):328–333. [PubMed] [Google Scholar]
- Herrmann J.E., Taylor D.N., Echeverria P., Blacklow N.R. Astroviruses as a cause of gastroenteritis in children. N. Engl. J. Med. 1991;324(25):1757–1760. doi: 10.1056/NEJM199106203242501. [DOI] [PubMed] [Google Scholar]
- Hewson K.A., O’Rourke D., Noormohammadi A.H. Detection of avian nephritis virus in Australian chicken flocks. Avian Dis. 2010;54(3):990–993. doi: 10.1637/9230-010610-Reg.1. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Zimmer J.F., Moise N.S., Scott F.W. Detection of astroviruses in feces of a cat with diarrhea. Brief report. Arch. Virol. 1981;70(4):373–376. doi: 10.1007/BF01320252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada T., Yamaguchi S., Kawamura H. Pathogenicity for baby chicks of the G-4260 strain of the picornavirus “avian nephritis virus”. Avian Dis. 1979;23(3):582–588. [PubMed] [Google Scholar]
- Imada T., Yamaguchi S., Miura N., Kawamura H. Antibody survey against avian nephritis virus among chickens in Japan. Natl. Inst. Anim. Health Q (Tokyo) 1980;20(2):79–80. [PubMed] [Google Scholar]
- Imada T., Taniguchi T., Yamaguchi S., Minetoma T., Maeda M., Kawamura H. Susceptibility of chickens to avian nephritis virus at various inoculation routes and ages. Avian Dis. 1981;25(2):294–302. [PubMed] [Google Scholar]
- Imada T., Maeda M., Furuta K., Yamaguchi S., Kawamura H. Pathogenicity and distribution of avian nephritis virus (G-4260 strain) in inoculated laying hens. Natl. Inst. Anim. Health Q (Tokyo) 1983;23(2):43–48. [PubMed] [Google Scholar]
- Imada T., Yamaguchi S., Mase M., Tsukamoto K., Kubo M., Morooka A. Avian nephritis virus (ANV) as a new member of the family Astroviridae and construction of infectious ANV cDNA. J. Virol. 2000;74(18):8487–8493. doi: 10.1128/jvi.74.18.8487-8493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indik S., Valicek L., Smid B., Dvorakova H., Rodak L. Isolation and partial characterization of a novel porcine astrovirus. Vet. Microbiol. 2006;117(2–4):276–283. doi: 10.1016/j.vetmic.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Jakab F., Walter J.E., Berke T., Matson D.O., Mitchell D.K., Szucs G. Molecular characterization and sequence analysis of human astroviruses circulating in Hungary. FEMS Immunol. Med. Microbiol. 2003;39(2):97–102. doi: 10.1016/S0928-8244(03)00236-0. [DOI] [PubMed] [Google Scholar]
- Jang S.Y., Jeong W.H., Kim M.S., Lee Y.M., Lee J.I., Lee G.C., Paik S.Y., Koh G.P., Kim J.M., Lee C.H. Detection of replicating negative-sense RNAs in CaCo-2 cells infected with human astrovirus. Arch. Virol. 2010;155(9):1383–1389. doi: 10.1007/s00705-010-0718-9. [DOI] [PubMed] [Google Scholar]
- Jindal N., Patnayak D.P., Chander Y., Ziegler A.F., Goyal S.M. Detection and molecular characterization of enteric viruses from poult enteritis syndrome in turkeys. Poult. Sci. 2010;89(2):217–226. doi: 10.3382/ps.2009-00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal N., Patnayak D.P., Chander Y., Ziegler A.F., Goyal S.M. Detection and molecular characterization of enteric viruses in breeder turkeys. Avian Pathol. 2010;39(1):53–61. doi: 10.1080/03079450903490289. [DOI] [PubMed] [Google Scholar]
- Jindal, N., Patnayak, D.P., Chander, Y., Ziegler, A.F., Goyal, S.M., 2011. Comparison of capsid gene sequences of turkey astrovirus-2 from poult-enteritis-syndrome-affected and apparently healthy turkeys, Arch. Virol. doi:10.1007/s00705-011-0931-1. [DOI] [PubMed]
- Jonassen C.M., Jonassen T.O., Grinde B. A common RNA motif in the 3′ end of the genomes of astroviruses, avian infectious bronchitis virus and an equine rhinovirus. J. Gen. Virol. 1998;79:715–718. doi: 10.1099/0022-1317-79-4-715. [DOI] [PubMed] [Google Scholar]
- Jonassen C.M., Jonassen T.T.O., Sveen T.M., Grinde B. Complete genomic sequences of astroviruses from sheep and turkey: comparison with related viruses. Virus Res. 2003;91(2):195–201. doi: 10.1016/s0168-1702(02)00269-1. [DOI] [PubMed] [Google Scholar]
- Kapoor A., Li L., Victoria J., Oderinde B., Mason C., Pandey P., Zaidi S.Z., Delwart E. Multiple novel astrovirus species in human stool. J. Gen. Virol. 2009;90(Pt 12):2965–2972. doi: 10.1099/vir.0.014449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang D., Matsui S.M. Proteolytic processing of a human astrovirus nonstructural protein. J. Gen. Virol. 2002;83(Pt 1):25–34. doi: 10.1099/0022-1317-83-1-25. [DOI] [PubMed] [Google Scholar]
- Kirkwood C.D., Clark R., Bogdanovic-Sakran N., Bishop R.F. A 5-year study of the prevalence and genetic diversity of human caliciviruses associated with sporadic cases of acute gastroenteritis in young children admitted to hospital in Melbourne, Australia (1998–2002) J. Med. Virol. 2005;77(1):96–101. doi: 10.1002/jmv.20419. [DOI] [PubMed] [Google Scholar]
- Kjeldsberg E., Hem A. Detection of astroviruses in gut contents of nude and normal mice. Brief report. Arch. Virol. 1985;84(1–2):135–140. doi: 10.1007/BF01310560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koci M.D., Seal B.S., Schultz-Cherry S. Molecular characterization of an avian astrovirus. J. Virol. 2000;74(13):6173–6177. doi: 10.1128/jvi.74.13.6173-6177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koci M.D., Schultz-Cherry S. Avian astroviruses. Avian Pathol. 2002;31(3):213–227. doi: 10.1080/03079450220136521. [DOI] [PubMed] [Google Scholar]
- Koci M.D., Kelley L.A., Larsen D., Schultz-Cherry S. Astrovirus-induced synthesis of nitric oxide contributes to virus control during infection. J. Virol. 2004;78(3):1564–1574. doi: 10.1128/JVI.78.3.1564-1574.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBaron, C.W., Furutan, N.P., Lew, J.F., Allen, J.R., Gouvea, V., Moe, C., Monroe, S.S., 1990. Viral agents of gastroenteritis. Public health importance and outbreak management, MMWR Recomm. Rep. 39 (RR-5), pp. 1–24. [PubMed]
- Lee T.W., Kurtz J.B. Serial propagation of astrovirus in tissue culture with the aid of trypsin. J. Gen. Virol. 1981;57(Pt 2):421–424. doi: 10.1099/0022-1317-57-2-421. [DOI] [PubMed] [Google Scholar]
- Lee T.W., Kurtz J.B. Human astrovirus serotypes. J. Hyg. 1982;89:539–540. doi: 10.1017/s0022172400071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T.L., Greenberg H.B., Herrmann J.E., Smith L.S., Matsui S.M. Analysis of astrovirus serotype 1 RNA, identification of the viral RNA-dependent RNA polymerase motif, and expression of a viral structural protein. J. Virol. 1994;68(1):77–83. doi: 10.1128/jvi.68.1.77-83.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T.L., Matsui S.M. An astrovirus frameshift signal induces ribosomal frameshifting in vitro. Arch. Virol. 1995;140(6):1127–1135. doi: 10.1007/BF01315421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T.L., Matsui S.M. Astrovirus ribosomal frameshifting in an infection-transfection transient expression system. J. Virol. 1996;70(5):2869–2875. doi: 10.1128/jvi.70.5.2869-2875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T.L., Matsui S.M. Studies of the astrovirus signal that induces (-1) ribosomal frameshifting. Adv. Exp. Med. Biol. 1997;412:323–330. doi: 10.1007/978-1-4899-1828-4_53. [DOI] [PubMed] [Google Scholar]
- Liu M.Q., Peng J.S., Tang L., Zhou Y., Yang B.F., Wang Y.H., Wang B., Zhou D.J., Huang H.J., Ho W.Z. Identification of new subtype of astrovirus type 3 from an infant with diarrhea in Wuhan, China. Virology. 2008;375(1):301–306. doi: 10.1016/j.virol.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Lukashov V.V., Goudsmit J. Evolutionary relationships among Astroviridae. J. Gen. Virol. 2002;83(Pt 6):1397–1405. doi: 10.1099/0022-1317-83-6-1397. [DOI] [PubMed] [Google Scholar]
- Luo Z., Roi S., Dastor M., Gallice E., Laurin M.A., L’homme Y. Multiple novel and prevalent astroviruses in pigs. Vet. Microbiol. 2011;149(3–4):316–323. doi: 10.1016/j.vetmic.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeley C.R., Cosgrove B.P. Letter: 28 nm particles in faeces in infantile gastroenteritis. Lancet. 1975;2(7932):451–452. doi: 10.1016/s0140-6736(75)90858-2. [DOI] [PubMed] [Google Scholar]
- Madeley C.R., Cosgrove B.P. Letter: viruses in infantile gastroenteritis. Lancet. 1975;2(7925):124. doi: 10.1016/s0140-6736(75)90020-3. [DOI] [PubMed] [Google Scholar]
- Maeda M., Imada T., Taniguchi T., Horiuchi T. Pathological changes in chicks inoculated with the picornavirus “avian nephritis virus”. Avian Dis. 1979;23(3):589–596. [PubMed] [Google Scholar]
- Marczinke B., Bloys A.J., Brown T.D., Willcocks M.M., Carter M.J., Brierley I. The human astrovirus RNA-dependent RNA polymerase coding region is expressed by ribosomal frameshifting. J. Virol. 1994;68(9):5588–5595. doi: 10.1128/jvi.68.9.5588-5595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J.A., Healey D.S., Studdert M.J., Scott P.C., Kennett M.L., Ward B.K., Gust I.D. Viruses and virus-like particles in the faeces of dogs with and without diarrhoea. Aust. Vet. J. 1984;61(2):33–38. doi: 10.1111/j.1751-0813.1984.tb07186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J.A., Kennett M.L., Rodger S.M., Studdert M.J., Thompson W.L., Gust I.D. Virus and virus-like particles in the faeces of cats with and without diarrhoea. Aust. Vet. J. 1987;64(4):100–105. doi: 10.1111/j.1751-0813.1987.tb09638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V., Moschidou P., Lorusso E., Mari V., Camero M., Bellacicco A., Losurdo M., Pinto P., Desario C., Banyai K., Elia G., Decaro N., Buonavoglia C. Detection and characterization of canine astroviruses. J. Gen. Virol. 2011;92(Pt 8):1880–1887. doi: 10.1099/vir.0.029025-0. [DOI] [PubMed] [Google Scholar]
- Matsui S.M., Kim J.P., Greenberg H.B., Young L.M., Smith L.S., Lewis T.L., Herrmann J.E., Blacklow N.R., Dupuis K., Reyes G.R. Cloning and characterization of human astrovirus immunoreactive epitopes. J. Virol. 1993;67(3):1712–1715. doi: 10.1128/jvi.67.3.1712-1715.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S.M., Greenberg H.B. Astroviruses. In: David P.M.H., Knipe M., editors. Anonymous. Lippincott Williams and Wilkins; Philadelphia: 1996. pp. 811–824. [Google Scholar]
- McNeilly F., Connor T.J., Calvert V.M., Smyth J.A., Curran W.L., Morley A.J., Thompson D., Singh S., McFerran J.B., Adair B.M., McNulty M.S. Studies on a new enterovirus-like virus isolated from chickens. Avian Pathol. 1994;23(2):313–327. doi: 10.1080/03079459408418999. [DOI] [PubMed] [Google Scholar]
- McNulty M.S., Curran W.L., McFerran J.B. Detection of astroviruses in turkey faeces by direct electron microscopy. Vet. Rec. 1980;106(26):561. doi: 10.1136/vr.106.26.561. [DOI] [PubMed] [Google Scholar]
- McNulty M.S., Connor T.J., McNeilly F. A survey of specific pathogen-free chicken flocks for antibodies to chicken anaemia agent, avian nephritis virus and group a rotavirus. Avian Pathol. 1989;18(2):215–220. doi: 10.1080/03079458908418596. [DOI] [PubMed] [Google Scholar]
- McNulty M.S., Connor T.J., McNeilly F., McFerran J.B. Biological characterisation of avian enteroviruses and enterovirus-like viruses. Avian Pathol. 1990;19(1):75–87. doi: 10.1080/03079459008418658. [DOI] [PubMed] [Google Scholar]
- Medina S.M., Gutierrez M.F., Liprandi F., Ludert J.E. Identification and type distribution of astroviruses among children with gastroenteritis in Colombia and Venezuela. J. Clin. Microbiol. 2000;38(9):3481–3483. doi: 10.1128/jcm.38.9.3481-3483.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez E., Fernandez-Luna T., Lopez S., Mendez-Toss M., Arias C.F. Proteolytic processing of a serotype 8 human astrovirus ORF2 polyprotein. J. Virol. 2002;76(16):7996–8002. doi: 10.1128/JVI.76.16.7996-8002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez E., Salas-Ocampo E., Arias C.F. Caspases mediate processing of the capsid precursor and cell release of human astroviruses. J. Virol. 2004;78(16):8601–8608. doi: 10.1128/JVI.78.16.8601-8608.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Toss M., Griffin D.D., Calva J., Contreras J.F., Puerto F.I., Mota F., Guiscafre H., Cedillo R., Munoz O., Herrera I., Lopez S., Arias C.F. Prevalence and genetic diversity of human astroviruses in Mexican children with symptomatic and asymptomatic infections. J. Clin. Microbiol. 2004;42(1):151–157. doi: 10.1128/JCM.42.1.151-157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez E., Arias C.F. Astroviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 981–1000. [Google Scholar]
- Mitchell D.K., Matson D.O., Jiang X., Berke T., Monroe S.S., Carter M.J., Willcocks M.M., Pickering L.K. Molecular epidemiology of childhood astrovirus infection in child care centers. J. Infect. Dis. 1999;180(2):514–517. doi: 10.1086/314863. [DOI] [PubMed] [Google Scholar]
- Mittelholzer C., Englund L., Hedlund K.O., Dietz H.H., Svensson L. Detection and sequence analysis of Danish and Swedish strains of mink astrovirus. J. Clin. Microbiol. 2003;41(11):5192–5194. doi: 10.1128/JCM.41.11.5192-5194.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelholzer C., Hedlund K.O., Englund L., Dietz H.H., Svensson L. Molecular characterization of a novel astrovirus associated with disease in mink. J. Gen. Virol. 2003;84(Pt 11):3087–3094. doi: 10.1099/vir.0.19267-0. [DOI] [PubMed] [Google Scholar]
- Monroe S.S., Jiang B., Stine S.E., Koopmans M., Glass R.I. Subgenomic RNA sequence of human astrovirus supports classification of Astroviridae as a new family of RNA viruses. J. Virol. 1993;67(6):3611–3614. doi: 10.1128/jvi.67.6.3611-3614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser L.A., Schultz-Cherry S. Pathogenesis of astrovirus infection. Viral Immunol. 2005;18(1):4–10. doi: 10.1089/vim.2005.18.4. [DOI] [PubMed] [Google Scholar]
- Moser L.A., Carter M., Schultz-Cherry S. Astrovirus increases epithelial barrier permeability independently of viral replication. J. Virol. 2007;81(21):11937–11945. doi: 10.1128/JVI.00942-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser L.A., Schultz-Cherry S. Suppression of astrovirus replication by an ERK1/2 inhibitor. J. Virol. 2008;82(15):7475–7482. doi: 10.1128/JVI.02193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa H., Palombo E.A., Bishop R.F. Epidemiology of astrovirus infection in young children hospitalized with acute gastroenteritis in Melbourne, Australia, over a period of four consecutive years, 1995 to 1998. J. Clin. Microbiol. 2000;38(3):1058–1062. doi: 10.1128/jcm.38.3.1058-1062.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas R.A., Goddard R.D., Luff P.R. Prevalence of avain nephritis virus in England. Vet. Rec. 1988;123(15):398. doi: 10.1136/vr.123.15.398. [DOI] [PubMed] [Google Scholar]
- Noel J., Cubitt D. Identification of astrovirus serotypes from children treated at the Hospitals for Sick Children, London 1981–93. Epidemiol. Infect. 1994;113(1):153–159. doi: 10.1017/s0950268800051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel J.S., Lee T.W., Kurtz J.B., Glass R.I., Monroe S.S. Typing of human astroviruses from clinical isolates by enzyme immunoassay and nucleotide sequencing. J. Clin. Microbiol. 1995;33(4):797–801. doi: 10.1128/jcm.33.4.797-801.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo E.A., Bishop R.F. Annual incidence, serotype distribution, and genetic diversity of human astrovirus isolates from hospitalized children in Melbourne, Australia. J. Clin. Microbiol. 1996;34(7):1750–1753. doi: 10.1128/jcm.34.7.1750-1753.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin-Jackwood M.J., Spackman E., Woolcock P.R. Molecular characterization and typing of chicken and turkey astroviruses circulating in the United States: implications for diagnostics. Avian Dis. 2006;50(3):397–404. doi: 10.1637/7512-020606R.1. [DOI] [PubMed] [Google Scholar]
- Pantin-Jackwood M.J., Spackman E., Woolcock P.R. Phylogenetic analysis of Turkey astroviruses reveals evidence of recombination. Virus Genes. 2006;32(2):187–192. doi: 10.1007/s11262-005-6875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin-Jackwood M.J., Strother K.O., Mundt E., Zsak L., Day J.M., Spackman E. Molecular characterization of avian astroviruses. Arch. Virol. 2011;156(2):235–244. doi: 10.1007/s00705-010-0849-z. [DOI] [PubMed] [Google Scholar]
- Quan P.L., Wagner T.A., Briese T., Torgerson T.R., Hornig M., Tashmukhamedova A., Firth C., Palacios G., Baisre-De-Leon A., Paddock C.D., Hutchison S.K., Egholm M., Zaki S.R., Goldman J.E., Ochs H.D., Lipkin W.I. Astrovirus encephalitis in boy with X-linked agammaglobulinemia. Emerg. Infect. Dis. 2010;16(6):918–925. doi: 10.3201/eid1606.091536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. Picornaviridae. In: David P.M.H., Knipe M., editors. Anonymous. Lippincott Williams and Wilkins; Philadelphia: 2001. p. 722. [Google Scholar]
- Reuter G., Pankovics P., Boros A. Identification of a novel astrovirus in a domestic pig in Hungary. Arch. Virol. 2011;156(1):125–128. doi: 10.1007/s00705-010-0827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds D.L., Saif Y.M. Astrovirus: a cause of an enteric disease in turkey poults. Avian Dis. 1986;30(4):728–735. [PubMed] [Google Scholar]
- Reynolds D.L., Saif Y.M., Theil K.W. A survey of enteric viruses of turkey poults. Avian Dis. 1987;31(1):89–98. [PubMed] [Google Scholar]
- Reynolds D.L., Schultz-Cherry S. Astrovirus infections. In: Saif Y.M., editor. Anonymous. 11th ed. Yowa State Press, A Blackwell Publishing; 2003. pp. 320–326. [Google Scholar]
- Rice M., Wilks C.R., Jones B.R., Beck K.E., Jones J.M. Detection of astrovirus in the faeces of cats with diarrhoea. N Z Vet. J. 1993;41(2):96–97. doi: 10.1080/00480169.1993.35743. [DOI] [PubMed] [Google Scholar]
- Rivera R., Nollens H.H., Venn-Watson S., Gulland F.M., Wellehan J.F., Jr Characterization of phylogenetically diverse astroviruses of marine mammals. J. Gen. Virol. 2010;91(Pt 1):166–173. doi: 10.1099/vir.0.015222-0. [DOI] [PubMed] [Google Scholar]
- Roman E., Wilhelmi I., Colomina J., Villar J., Cilleruelo M.L., Nebreda V., Del Alamo M., Sanchez-Fauquier A. Acute viral gastroenteritis: proportion and clinical relevance of multiple infections in Spanish children. J. Med. Microbiol. 2003;52(Pt 5):435–440. doi: 10.1099/jmm.0.05079-0. [DOI] [PubMed] [Google Scholar]
- Saif L.J., Saif Y.M., Theil K.W. Enteric viruses in diarrheic turkey poults. Avian Dis. 1985;29(3):798–811. [PubMed] [Google Scholar]
- Saif Y.M., Saif L.J., Hofacre C.L., Hayhow C., Swayne D.E., Dearth R.N. A small round virus associated with enteritis in turkey poults. Avian Dis. 1990;34(3):762–764. [PubMed] [Google Scholar]
- Schneider R.R., Hunter D.B. Mortality in mink kits from birth to weaning. Can. Vet. J. 1993;34(3):159–163. [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Shirai J., Narita M., Yamane T. Cytopathic astrovirus isolated from porcine acute gastroenteritis in an established cell line derived from porcine embryonic kidney. J. Clin. Microbiol. 1990;28(2):201–206. doi: 10.1128/jcm.28.2.201-206.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai J., Shimizu M., Fukusho A. Coronavirus-, calicivirus-, and astrovirus-like particles associated with acute porcine gastroenteritis. Nippon Juigaku Zasshi. 1985;47(6):1023–1026. doi: 10.1292/jvms1939.47.1023. [DOI] [PubMed] [Google Scholar]
- Shirai J., Nakamura K., Narita M., Furuta K., Hihara H., Kawamura H. Visceral urate deposits in chicks inoculated with avian nephritis virus. Vet. Rec. 1989;124(25):658–661. doi: 10.1136/vr.124.25.658. [DOI] [PubMed] [Google Scholar]
- Shirai J., Nakamura K., Shinohara K., Kawamura H. Pathogenicity and antigenicity of avian nephritis isolates. Avian Dis. 1991;35(1):49–54. [PubMed] [Google Scholar]
- Shirai J., Tanimura N., Uramoto K., Narita M., Nakamura K., Kawamura H. Pathologically and serologically different avian nephritis virus isolates implicated in etiology of baby chick nephropathy. Avian Dis. 1992;36(2):369–377. [PubMed] [Google Scholar]
- Smits S.L., van Leeuwen M., Kuiken T., Hammer A.S., Simon J.H., Osterhaus A.D. Identification and characterization of deer astroviruses. J. Gen. Virol. 2010;91(Pt 11):2719–2722. doi: 10.1099/vir.0.024067-0. [DOI] [PubMed] [Google Scholar]
- Smyth J.A., Connor T.J., McNeilly F., Moffet D.A., Calvert V.M., McNulty M.S. Studies on the pathogenicity of enterovirus-like viruses in chickens. Avian Pathol. 2007;36(2):119–126. doi: 10.1080/03079450601161398. [DOI] [PubMed] [Google Scholar]
- Snodgrass D.R., Gray E.W. Detection and transmission of 30 nm virus particles (astroviruses) in faeces of lambs with diarrhoea. Arch. Virol. 1977;55(4):287–291. doi: 10.1007/BF01315050. [DOI] [PubMed] [Google Scholar]
- Snodgrass D.R., Angus K.W., Gray E.W., Menzies J.D., Paul G. Pathogenesis of diarrhoea caused by astrovirus infections in lambs. Arch. Virol. 1979;60(3–4):217–226. doi: 10.1007/BF01317493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondra Schlesinger M.S. Togaviridae: The viruses and their replication. In: David P.M.H., Knipe M., editors. Anonymous. Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 895–916. [Google Scholar]
- Spackman E., Day J.M., Pantin-Jackwood M.J. Astrovirus, reovirus, and rotavirus concomitant infection causes decreased weight gain in broad-breasted white poults. Avian Dis. 2010;54(1):16–21. doi: 10.1637/8986-070909-Reg.1. [DOI] [PubMed] [Google Scholar]
- Speroni S., Rohayem J., Nenci S., Bonivento D., Robel I., Barthel J., Luzhkov V.B., Coutard B., Canard B., Mattevi A. Structural and biochemical analysis of human pathogenic astrovirus serine protease at 2.0 A resolution. J. Mol. Biol. 2009;387(5):1137–1152. doi: 10.1016/j.jmb.2009.02.044. [DOI] [PubMed] [Google Scholar]
- Strain E., Kelley L.A., Schultz-Cherry S., Muse S.V., Koci M.D. Genomic analysis of closely related astroviruses. J. Virol. 2008;82(10):5099–5103. doi: 10.1128/JVI.01993-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J.H., Strauss E.G. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 1994;58(3):491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennekjaer N.C. Minksyngdomme og aktuelle problemer i minkavlen (Mink diseases and current problems in mink farming) Dansk. Pelsdyravl. 1954;12:384–404. [Google Scholar]
- Takase K., Shinohara K., Tsuneyoshi M., Yamamoto M., Yamada S. Isolation and characterisation of cytopathic avian enteroviruses from broiler chicks. Avian Pathol. 1989;18(4):631–642. doi: 10.1080/03079458908418638. [DOI] [PubMed] [Google Scholar]
- Takase K., Uchimura T., Yamamoto M., Yamada S. Susceptibility of embryos and chicks, derived from immunized breeding hens, to avian nephritis virus. Avian Pathol. 1994;23(1):117–125. doi: 10.1080/03079459408418979. [DOI] [PubMed] [Google Scholar]
- Tang Y., Saif Y.M. Antigenicity of two turkey astrovirus isolates. Avian Dis. 2004;48(4):896–901. doi: 10.1637/7169-021904R. [DOI] [PubMed] [Google Scholar]
- Tang Y., Murgia A.M., Saif Y.M. Molecular characterization of the capsid gene of two serotypes of turkey astroviruses. Avian Dis. 2005;49(4):514–519. doi: 10.1637/7353-030305R.1. [DOI] [PubMed] [Google Scholar]
- Todd D., Smyth V.J., Ball N.W., Donnelly B.M., Wylie M., Knowles N.J., Adair B.M. Identification of chicken enterovirus-like viruses, duck hepatitis virus type 2 and duck hepatitis virus type 3 as astroviruses. Avian Pathol. 2009;38(1):21–30. doi: 10.1080/03079450802632056. [DOI] [PubMed] [Google Scholar]
- Todd D., Wilkinson D.S., Jewhurst H.L., Wylie M., Gordon A.W., Adair B.M. A seroprevalence investigation of chicken astrovirus infections. Avian Pathol. 2009;38(4):301–309. doi: 10.1080/03079450903055421. [DOI] [PubMed] [Google Scholar]
- Toffan A., Jonassen C.M., De Battisti C., Schiavon E., Kofstad T., Capua I., Cattoli G. Genetic characterization of a new astrovirus detected in dogs suffering from diarrhoea. Vet. Microbiol. 2009;139(1–2):147–152. doi: 10.1016/j.vetmic.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse, H., Chan, W.M., Tsoi, H.W., Fan, R.Y., Lau, C.C., Lau, S.K., Woo, P.C., Yuen, K.Y., 2011. Re-discovery and genomic characterization of bovine astroviruses, J. Gen. Virol. doi:10.1099/vir.0.030817-0. [DOI] [PubMed]
- Tzipori S., Menzies J.D., Gray E.W. Detection of astrovirus in the faeces of red deer. Vet. Rec. 1981;108(13):286. doi: 10.1136/vr.108.13.286. [DOI] [PubMed] [Google Scholar]
- Ulloa J.C., Gutierrez M.F. Genomic analysis of two ORF2 segments of new porcine astrovirus isolates and their close relationship with human astroviruses. Can. J. Microbiol. 2010;56(7):569–577. doi: 10.1139/w10-042. [DOI] [PubMed] [Google Scholar]
- van Hemert F.J., Berkhout B., Lukashov V.V. Host-related nucleotide composition and codon usage as driving forces in the recent evolution of the Astroviridae. Virology. 2007;361(2):447–454. doi: 10.1016/j.virol.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hemert F.J., Lukashov V.V., Berkhout B. Different rates of (non-)synonymous mutations in astrovirus genes; correlation with gene function. J. Virol. 2007;4:25. doi: 10.1186/1743-422X-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieler E., Herbst W. Electron microscopic demonstration of viruses in feces of dogs with diarrhea. Tierarztl. Prax. 1995;23(1):66–69. [PubMed] [Google Scholar]
- Walter J.E., Briggs J., Guerrero M.L., Matson D.O., Pickering L.K., Ruiz-Palacios G., Berke T., Mitchell D.K. Molecular characterization of a novel recombinant strain of human astrovirus associated with gastroenteritis in children. Arch. Virol. 2001;146(12):2357–2367. doi: 10.1007/s007050170008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks M.M., Carter M.J., Laidler F.R., Madeley C.R. Growth and characterisation of human faecal astrovirus in a continuous cell line. Arch. Virol. 1990;113(1–2):73–81. doi: 10.1007/BF01318354. [DOI] [PubMed] [Google Scholar]
- Willcocks M.M., Brown T.D., Madeley C.R., Carter M.J. The complete sequence of a human astrovirus. J. Gen. Virol. 1994;75(Pt 7):1785–1788. doi: 10.1099/0022-1317-75-7-1785. [DOI] [PubMed] [Google Scholar]
- Williams F.P., Jr Astrovirus-like, coronavirus-like, and parvovirus-like particles detected in the diarrheal stools of beagle pups. Arch. Virol. 1980;66(3):215–226. doi: 10.1007/BF01314735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfaardt M., Kiulia N.M., Mwenda J.M., Taylor M.B. Evidence of a recombinant wild-type human astrovirus strain from a Kenyan child with gastroenteritis. J. Clin. Microbiol. 2011;49(2):728–731. doi: 10.1128/JCM.01093-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G.N., Bridger J.C. Isolation of small viruses resembling astroviruses and caliciviruses from acute enteritis of calves. J. Med. Microbiol. 1978;11(4):441–452. doi: 10.1099/00222615-11-4-441. [DOI] [PubMed] [Google Scholar]
- Woode G.N., Pohlenz J.F., Gourley N.E., Fagerland J.A. Astrovirus and Breda virus infections of dome cell epithelium of bovine ileum. J. Clin. Microbiol. 1984;19(5):623–630. doi: 10.1128/jcm.19.5.623-630.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G.N., Gourley N.E., Pohlenz J.F., Liebler E.M., Mathews S.L., Hutchinson M.P. Serotypes of bovine astrovirus. J. Clin. Microbiol. 1985;22(4):668–670. doi: 10.1128/jcm.22.4.668-670.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Imada T., Kawamura H. Characterization of a picornavirus isolated from broiler chicks. Avian Dis. 1979;23(3):571–581. [PubMed] [Google Scholar]
- Zhao W., Hua X.G., Yuan L., Cui L., Shan T.L., Dai X.Q., Zhu A.L., Yu Y., Zhu C.X., Yang Z.B. Sequence analyses of the representative Chinese-prevalent strain of avian nephritis virus in healthy chicken flocks. Avian Dis. 2011;55(1):65–69. doi: 10.1637/9506-081810-Reg.1. [DOI] [PubMed] [Google Scholar]
- Zhu H.C., Chu D.K., Liu W., Dong B.Q., Zhang S.Y., Zhang J.X., Li L.F., Vijaykrishna D., Smith G.J., Chen H.L., Poon L.L., Peiris J.S., Guan Y. Detection of diverse astroviruses from bats in China. J. Gen. Virol. 2009;90(Pt 4):883–887. doi: 10.1099/vir.0.007732-0. [DOI] [PubMed] [Google Scholar]
- Zhu, A.L., Zhao, W., Yin, H., Shan, T.L., Zhu, C.X., Yang, X., Hua, X.G., Cui, L., 2011. Isolation and characterization of canine astrovirus in China, Arch. Virol. doi:10.1007/s00705-011-1022-z. [DOI] [PubMed]


