Summary
Highly virulent strains of Clostridium difficile have emerged since 2003, causing large outbreaks of severe, often fatal, colitis in North America and Europe. In 2008–10, virulent strains spread between continents, with the first reported cases of fluoroquinolone-resistant C difficile PCR ribotype 027 in three Asia-Pacific countries and Central America. We present a risk assessment framework for assessing risks of further worldwide spread of this pathogen. This framework first requires identification of potential vehicles of introduction, including international transfers of hospital patients, international tourism and migration, and trade in livestock, associated commodities, and foodstuffs. It then calls for assessment of the risks of pathogen release, of exposure of individuals if release happens, and of resulting outbreaks. Health departments in countries unaffected by outbreaks should assess the risk of introduction or reintroduction of C difficile PCR ribotype 027 using a structured risk-assessment approach.
Introduction
Clostridium difficile is a major cause of diarrhoea in patients in hospital and long-term care facilities.1, 2 C difficile infection is through exposure to the organism or its spores via the faecal-oral route; the spores can persist in the environment for many months. Once infected, the person might remain asymptomatic or progress to C difficile disease. Infection and progression to disease are facilitated by use of antibiotics, which disrupt the normal flora and permit proliferation of the toxin-producing C difficile. A wide range of antibiotics have been implicated historically, including cephalosporins, penicillins, and clindamycin.3, 4, 5 Infection ranges in severity; in its most severe form it can cause toxic megacolon with subsequent colonic perforation, peritonitis, shock, and death. Colectomy might be needed to avert perforation but is associated with high risk given the age and medical comorbidities of typical patients infected with C difficile. Infection is associated with hospitals and long-term care facilities because of frequent antibiotic use, widespread contamination of the patient's environment with spores, and the density of people at high risk (ie, elderly people with several comorbidities).
In the past two decades, the incidence of C difficile infection has increased in many industrialised countries,6, 7, 8, 9, 10, 11, 12, 13 particularly among elderly people.12 Groups previously thought to be low risk, including otherwise healthy people in the community without prior exposure to antibiotics,14, 15 children,16 and peripartum women, have also been affected.17, 18, 19 Concurrent with an increase in incidence, an alarming increase in severity of infection has been reported, starting with reports of hospital outbreaks in Quebec, Canada, from 2003. These outbreaks were associated with high case-fatality rates20, 21 and were estimated to have caused about 2000 deaths.22 This happened at the same time as an increase in severity of infection in the USA, characterised by increases in reported C difficile infection-related admission to hospital, colectomies, and deaths, dated retrospectively from the mid-1990s.13, 23, 24, 25, 26 Muto and colleagues8 noted a four-times increase in severe cases (resulting in colectomy or death) from 1999 to 2001 in a hospital outbreak in Pittsburgh, PA. A similar pattern of emergence has developed in Europe since 2005, with increasing incidence of infection10, 27 and some hospital outbreaks with more severe cases and higher case-fatality rates than previously experienced.28, 29 Outbreaks have been particularly severe in the UK, with a six-times increase in C difficile infection-related mortality from 1999 to 2006,30, 31 which preceded a stabilisation and substantial decline in incidence and mortality from 2007 to 2009.32, 33
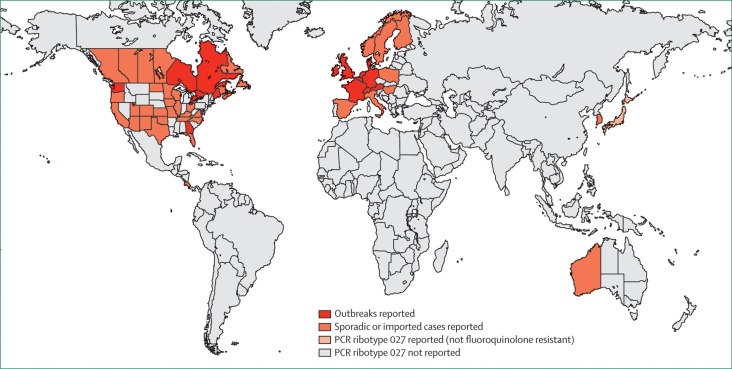
These North American and European outbreaks (figure 1 ) coincided with the emergence of a hypervirulent strain of C difficile, PCR ribotype 027/North American pulse-field type 1,34, 35 that caused more severe colitis and higher mortality than other strains.37, 63, 64, 65 Increased virulence might be due to genetic mutations in a toxin regulator gene (tcdC) that cause hyperproduction of toxins A and B.66, 67 The strain also produces a binary toxin associated with severe diarrhoea.68, 69 Unlike isolates of C difficile PCR ribotype 027 obtained before 2001, isolates obtained during the North American and European epidemics were resistant to fluoroquinolones and genetically closely related.34, 35, 36, 64, 70
Figure 1.
Countries* where Clostridium difficile PCR ribotype 027 has been reported
Sources of information include reports from the USA8, 34 and Canada,35 reporting hospital outbreaks since 2001; the UK,29, 36 the Netherlands,37, 38, 39 Belgium,40 and Ireland,41 reporting hospital outbreaks since 2005; France,28, 42 Switzerland,43 and Luxembourg,44 reporting hospital outbreaks since 2006; Germany,45, 46, 47 reporting hospital outbreaks since 2007; and Austria27, 48 and Denmark,44, 45, 49 reporting hospital outbreaks since 2008. Sporadic or imported cases of infection caused by C difficile PCR ribotype 027 have also been reported from Costa Rica,50 Finland,51, 52 Hungary,45, 53 Italy,54 Norway,55 Poland,44, 45 Spain,45 Sweden,56 Western Australia,57 South Korea,58 and Hong Kong.59 The earliest known isolate from the Netherlands was collected in 2002,37 but—like earlier strains of PCR ribotype 027 in North America, isolates from Japan,60, 61 and the majority of recent isolates from Sweden56, 62—it was susceptible to fluoroquinolones and is thought a historic strain not associated with the recent international epidemic. *And states or provinces of the USA, Canada, and Australia.
So far, continents other than North America and Europe have been spared outbreaks of C difficile PCR ribotype 027 infection, and evidence suggests a decline in the incidence of infection associated with this ribotype in the UK32 and the Netherlands.71 However, alarmingly in 2008–10, the first cases of C difficile PCR ribotype 027 infections were reported in Western Australia,57 South Korea,58 Hong Kong,59 and Costa Rica,50 showing the potential of this organism to spread between continents beyond its current north Atlantic domain.
The emergence of C difficile PCR ribotype 027 is of worldwide concern, both for affected and unaffected countries. It is uncertain if the failure to detect the strain in many countries means it is truly absent, because molecular genotyping is rarely used. Many industrialised countries now rely on enzyme-linked immunoassays for diagnosis, which do not identify specific strains,72 whereas most developing countries have no C difficile surveillance or do routine diagnostic investigation. However, these countries must identify this strain if, where, and when it emerges to avoid the large epidemics of North America and parts of Europe. We review the types and sources of evidence that need to be assembled to understand the risks of further worldwide emergence of this and other international epidemic strains of C difficile.
Risk assessment frameworks
Risk analysis, comprising risk management, assessment, and communication, is an emerging branch of epidemiology that provides a structured, evidence-based, approach to addressing health risks. Approaches are framed by international standards such as those of the Food and Agriculture Organization/World Health Organization Codex Alimentarius for microbiological risk assessment73 or the Office International des Epizooties (OIE) system,74 primarily aimed at international movement of veterinary and zoonotic pathogens. Risk assessment (the technical component of risk analysis) is fundamentally based on the clear definition of a risk question. Using the OIE framework as an example, risk assessment comprises four consecutive analytic components: release assessment, exposure assessment, consequence assessment, and risk estimation.74 During each of the first three, all available evidence from published work and expert opinion is reviewed and organised on the basis of a diagrammatic construction of risk pathways. The final risk estimation involves the combination of the results of the preceding three stages to produce an overall estimate of risk.
Quantitative risk assessment uses a probabilistic risk model to derive numerical expressions of risk and associated uncertainties, whereas qualitative risk assessment permits ranking or categorisation of risk. There are many reported applications of quantitative risk assessment to veterinary, zoonotic, and food-borne diseases, including Alban and colleagues75 who investigated risk of human salmonellosis and campylobacteriosis associated with consumption of pork products in Denmark, Bemrah and colleagues76 who investigated risk of listeriosis from consumption of unpasteurised cheese, and Bronsvoort and colleagues77 who investigated risk of importation of classic swine fever into Denmark. However, there are few examples for infectious diseases primarily involving transmission between people, and none reported for C difficile infection.
For C difficile infection, risk is likely to depend on the infection history of the country of interest. If C difficile PCR ribotype 027 is not known to be present (as in most countries in the Asia-Pacific region, Africa, and Latin America), the risk question could focus on the likelihood of introduction through specified, hazardous, movements across boundaries. Alternatively, the risk question could consider endogenous emergence through a set of hazardous biological mechanisms, such as patterns of use of specific antibiotics. If C difficile PCR ribotype 027 has been detected but has not caused outbreaks (as in Australia, Korea, Hong Kong, and parts of Europe), the risk question could focus on the likelihood of reintroduction or an outbreak due to endogenous transmission. If C difficile PCR ribotype 027 is present and outbreaks have happened (as in North America and parts of western Europe), the assessment of risk could focus on the likelihood of recurrence, the effect of a particular intervention (or no intervention) on the size or frequency of outbreaks, or of previously unaffected groups in the population becoming affected. We focus on the risk of introduction, or reintroduction, of C difficile PCR ribotype 027 to countries that have not experienced epidemics associated with this strain.
Vehicles of introduction
The movements of people, animals, vectors, and inanimate objects across international boundaries has spread many infectious diseases, including influenza,78 severe acute respiratory syndrome,79 dengue,80 chikungunya,81, 82 and malaria.83 However, identification of imported C difficile PCR ribotype 027 is probably much more difficult than for these infections because screening of travellers is not done and outbreaks associated with importation of this strain are likely to happen months or years after importation, leading to delayed identification of the incursion.
For importation of the C difficile epidemic strain, a major hazard of interest is international transfer of hospital patients. There is evidence that asymptomatic carriage is common among patients admitted to hospital, and these carriers can act as a source of transmission in settings where C difficile PCR ribotype 027 is present.84, 85 Theoretically, either a diarrhoeic or asymptomatically infected patient transferred between health-care institutions (including those in different countries) could act as a source of this strain. The first documented case of infection due to PCR ribotype 027 in Ireland was in a patient transferred from a hospital in the UK,41 and the only reported case in Western Australia was in a patient transferred from the USA.57 Other anecdotal reports also suggest this as a possible (although unproven) means of international spread. For example, transfer of patients from Belgium was investigated as a potential source of introduction of C difficile PCR ribotype 027 to France in 200642 and the index case of infection with this strain in Switzerland had previously been admitted to hospital in Spain.43
Another potential hazard is movement of people from the community across boundaries (eg, tourists, business travellers, international migrants, and military personnel). A tourist from the UK was reported to have had pseudomembranous colitis caused by C difficile PCR ribotype 027 while on holiday in Austria; endogenous cases were subsequently reported in that country.45 A person with community-acquired, fluoroquinolone-susceptible, C difficile PCR ribotype 027 infection in Sweden had previously travelled to Italy,56 suggesting (but not confirming) recreational travel as a potential source. However, asymptomatic carriage is less studied in the community than in hospital patients and the role of asymptomatic carriers in the movement of C difficile strains between jurisdictions is unknown. The often quoted prevalence of asymptomatic C difficile carriage in healthy adults, 2–3%, comes from a 1981 report;86 however, recent evidence of an increase in incidence of community-acquired cases in some countries warrants studies to update estimates of prevalence of asymptomatic carriage in the community. The role of antibiotic use in the community as a factor promoting carriage of C difficile is also poorly understood. In a French study of people prescribed antibiotics in the community,87 one of 262 had detectable pretreatment colonisation and seven had detectable post-treatment infection with toxigenic strains of C difficile.
Although animals have not previously been implicated in any known events of C difficile crossing boundaries, they can be symptomatically or asymptomatically infected. C difficile is recognised as an important cause of enteric disease in piglets88, 89, 90, 91 and horses.92, 93 Identical strains of C difficile have been isolated in people and dogs, horses, and pigs.94, 95, 96, 97, 98 These findings also pertain to C difficile PCR ribotype 027: studies in North America isolated this strain from calves,99 a dog,98, 100 and a horse with colitis.101
Toxigenic strains of C difficile, including PCR ribotype 027, have been isolated from commercially available meat.102, 103 Recent studies in Arizona, USA, and four provinces of Canada, found a high proportion of retail meat samples containing C difficile PCR ribotype 027.104, 105 An unspecified toxigenic C difficile strain has been isolated from meat for consumption by pets.106 Isolation of toxigenic strains of C difficile from salad in the UK107 suggests that the bacterium could be widely distributed in a range of human foodstuffs.108 These studies combined show a potential for transmission between animals and people (either directly or via the food chain), and international spread of toxigenic strains of C difficile via movement or trade in livestock, companion animals, meat, and other foodstuffs is therefore possible.
Risk of pathogen release in new areas
Assuming individuals with known infection are treated in the country of origin or prevented from entering the destination country, the probability of release of the pathogen is dependent on the disease status of the country of origin, the proportion of individuals screened as part of disease surveillance in the country of origin, the performance (ie, sensitivity and specificity) of diagnostic tests used in screening, the frequency of movement between countries, and the proportion of individuals that are screened (and performance of screening tests) on arrival in the destination country.
Several countries have reported infections with C difficile PCR ribotype 027 (figure 1), but the disease status of all other countries is unknown because of a lack of routine surveillance and, in many countries, a lack of capacity or resources for diagnostic testing, including genotyping. Clearly, risk of release depends on surveillance practices in both the country of origin and the importing country because the effectiveness of surveillance establishes the probability of infected individuals moving between the originating and importing countries without being detected, and the bacterium being contained.
Information on C difficile surveillance can be obtained for most countries affected by outbreaks of C difficile PCR ribotype 027 from published questionnaire studies of hospital laboratory practices. A study in eight European countries found that the reasons for C difficile testing (eg, physician request vs routine screening for samples fulfilling preset criteria such as age of patient and stool consistency) and the tests used, varied widely between laboratories and countries.109 Findings were similar in studies in Australia,110 the UK,11, 111 Ireland,112 Canada,113 and the USA.7, 114 In some European countries, national laboratory surveillance has been instigated in response to emergence of C difficile PCR ribotype 027. A national laboratory network was set up in France to characterise strains of C difficile.28 In Belgium, laboratory-based surveillance of clusters of cases of C difficile infection and prospective hospital-based surveillance have been set up, finding PCR ribotype 027 strains in 150 (52·1%) of 288 isolates.115 A national laboratory surveillance system was also set up in the Netherlands, finding that 218 (25·3%) of 863 cases were caused by PCR ribotype 027 in 2005–06.37
Additionally, laws and mandates relating to diagnosis and reporting of C difficile can be reviewed to understand surveillance practices. In the UK, laboratory surveillance was made mandatory by the Health Protection Agency (HPA) in January, 2004.111, 116 All stool specimens from diarrhoeic patients older than 65 years were tested for C difficile toxins A and B, with the number of C difficile infections in each hospital required to be reported to the HPA;117 in 2007, mandatory reporting was extended to include individuals older than 2 years.118 Isolates from hospital outbreaks and a sample of other isolates are sent to a national reference laboratory for genetic typing.119 Some provinces in Canada have mandatory reporting of C difficile infections,120, 121 as do a few US states, and some countries of the European Union.45 Continent-wide surveillance studies in Europe are under development,45 but surveillance in the USA and Canada is hampered by the lack of a nationwide approach.
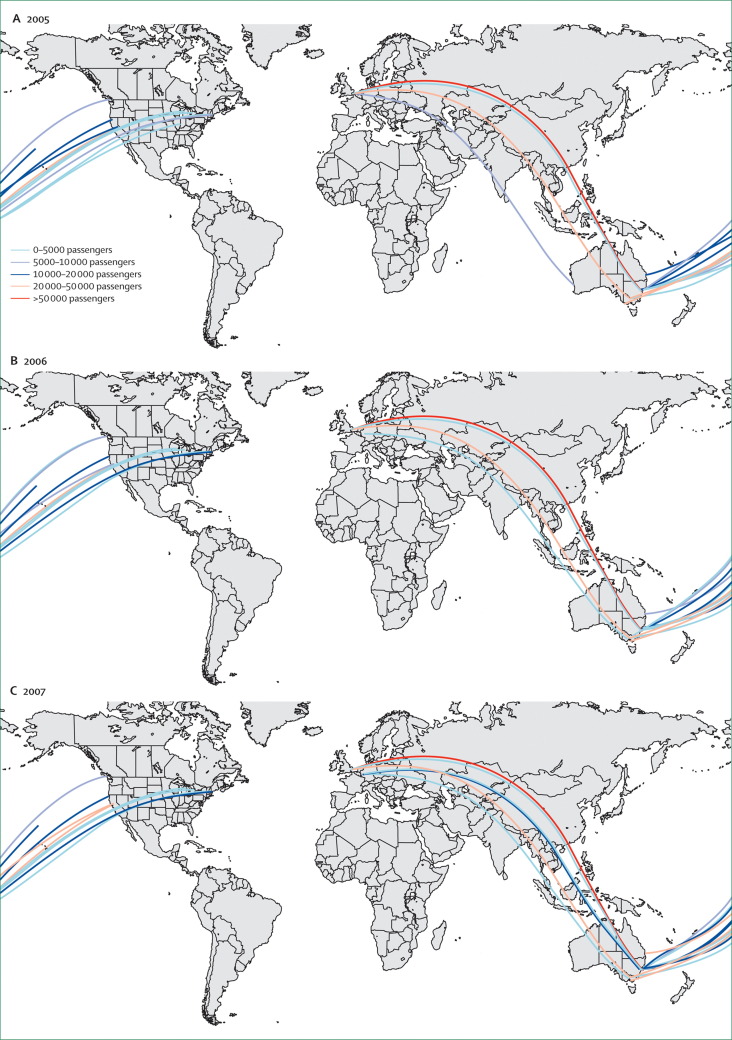
Risk of release is proportional to the frequency of movement of individuals between countries. This can be estimated from various sources that are illustrated here by examples from Australia. All people visiting Australia, apart from citizens of Australia and New Zealand, require an entry visa from the Department of Immigration and Citizenship and, for an assessment of risk focusing on international spread via tourists or business visitors, annual numbers of visitors from specific countries of origin can be estimated by the number of offshore visas granted (table 1 ). Alternatively, the frequency of movement of individuals can be estimated by the carrying capacity of international airlines on specific international routes (figure 2 ). For the assessment of risk focusing on international transfers of patients, numbers of patients entering from affected countries can be obtained from medical retrieval companies. Australia has strict regulations regarding the species of domestic animals entering and countries from which animals can be imported, and a risk assessment focusing on animal sources could make use of quarantine records to estimate the number of domestic animals entering by country of origin (table 2 ). Importation of livestock is highly regulated in Australia and this is an unlikely source of C difficile PCR ribotype 027, but for other countries livestock trade data can be used to establish the number of animals entering. Similarly, for Australia and other countries, data on trade in meat products could also be obtained (eg, from the UN Commodity Trade Statistics Database).
Table 1.
Number of offshore visitor visas granted for travel to Australia
| Number of visas, 2006–07 | Number of visas, 2007–08 | |
|---|---|---|
| UK | 674 771 | 631 900 |
| USA | 385 384 | 400 906 |
| Germany | 138 230 | 144 852 |
| Canada | 101 276 | 114 457 |
| France | 101 505 | 112 143 |
Visas granted to citizens of selected countries from which fluoroquinolone-resistant Clostridium difficile PCR ribotype 027 has been reported, 2006–07 and 2007–08, from the Department of Immigration and Citizenship annual reports.122
Figure 2.
Annual passenger-carrying capacity of international air-transport links between countries affected by fluoroquinolone-resistant Clostridium difficile PCR ribotype 027 and Australia
Frequency of indirect travel between affected countries and Australia via international hubs can also be estimated. Data from the International Air Transport Association.123
Table 2.
Number of dogs, cats, and horses entering Australia each year
| Number of animals, 2005 | Number of animals, 2006 | Number of animals, 2007 | Number of animals, 2008 | |
|---|---|---|---|---|
| UK | 2594 | 3028 | 3113 | 2610 |
| USA | 1022 | 1160 | 1153 | 1213 |
| Canada | 231 | 270 | 332 | 320 |
| Netherlands | 129 | 118 | 121 | 115 |
| Germany | 89 | 116 | 111 | 134 |
| France | 75 | 87 | 93 | 62 |
Number entering from selected countries from which fluoroquinolone-resistant Clostridium difficile PCR ribotype 027 has been reported, 2005–08. Estimated on the basis of travel permits granted by the Australian Quarantine and Inspection Service (Lam G, Australian Quarantine and Inspection Service, Canberra, ACT, Australia, personal communication).
Risk of exposure in new areas
Exposure to C difficile, leading to transmission, can be via several pathways. In hospitals, workers' hands and the environment are major modes of transmission;85, 124, 125, 126 aerial dissemination is also possible.127 Transmission is also increasing in the community and recent studies have raised the possibility of additional means of transmission, such as via the food chain102 or contact with companion animals or livestock.
Establishing the risk of exposure to C difficile requires information on contact rates between infected carriers and individuals free of infection; the contact pattern, either via direct contact or indirect contact with a health-care worker or contaminated environment; the duration of shedding by asymptomatic individuals with the infection; the amount of environmental contamination (numbers of spores shed) by asymptomatic individuals with the infection; duration of survival of the pathogen in the environment; and the effect of infection-control interventions.
Observational studies to establish values for variables such as contact rates between infected and susceptible individuals, duration of shedding and survival of pathogens, and effect of intervention strategies are difficult and expensive to do, and suffer from issues such as poor generalisability, inadequate statistical power, and many types of inherent bias. Therefore, many investigations of infectious-disease transmission use mathematical models, which are built using multiple sources of available evidence to estimate plausible values for many of these parameters. Uncertainties surrounding these estimates are often captured by use of a stochastic Monte Carlo simulation approach. Only two publications,128, 129 both by the same group, present mathematical models for C difficile transmission and focus exclusively on the health-care setting, using a single ward-based model. Exposure assessment is likely to be the most challenging aspect of C difficile risk assessment and substantial future research is needed to understand the transmission dynamics of this organism.
Risk of infections and outbreaks
The consequences of transmission, including infections and outbreaks, depend on the risk profile of the population into which the pathogen has been introduced. Antibiotic use is the main factor determining susceptibility to infection in an individual exposed to C difficile and data on levels of use of different types of antibiotic will probably provide the most important source of information for assessing the risk of infections and outbreaks. Availability of antimicrobial drugs varies widely between countries, with those countries that do not have good systems for regulating the use of antimicrobial drugs (particularly developing countries) also being the countries with the poorest systems for monitoring use and associated disease risks.
Studies have shown variation in levels of antibiotic prescribing between countries130 and over time.131 Goossens and colleagues132 investigated levels of antibiotic prescribing in the community in 26 European countries, with estimates ranging from 10·0 defined daily doses per 1000 people in the Netherlands to 32·2 defined daily doses per 1000 people in France (although different assessment methods were used in each country), and varying by season, with a marked winter peak. Patrick and colleagues130 also showed seasonal variation (characterised by a winter peak) in antibiotic prescribing in Denmark and British Columbia, Canada. Interestingly, seasonal variation in rates of C difficile infection, with a winter peak, have also been reported in the USA.6
Fluoroquinolone use has been identified as a major risk factor for infection in settings where C difficile PCR ribotype 027 is present,8, 21, 35, 65, 133, 134 and increasing fluoroquinolone use in hospitals has been shown to precede outbreaks of infection associated with this strain,8 possibly because fluoroquinolone resistance gives the strain a selective advantage.34 Linder and colleagues131 reported on fluoroquinolone prescribing patterns in the USA, using data from national surveys of emergency department and outpatient clinic visits. They found a rapid (three times) increase in fluoroquinolone prescriptions from 1995 to 2002, and that fluoroquinolones had become the most common class of antibiotics prescribed to adults in 2002. Patrick and colleagues130 also showed an increase in fluoroquinolone prescribing in British Columbia from 1997 to 2000. Clindamycin was the predominant risk factor for infection in a study by McFarland and colleagues135 and some recent isolates of C difficile PCR ribotype 027 are resistant to this antibiotic.43, 136
Monitoring of veterinary antibiotic use, potentially including manufacturing, sales, distribution, prescribing, and administration data, has been highlighted as an area of importance for risk assessment of emerging antibiotic resistance in human pathogens.137, 138 In livestock, prolonged oral administration of broad-spectrum antibiotics and inadvertent under dosing increase the risk of emergence of antibiotic resistance that can then be passed to human pathogens.139 Comprehensive veterinary prescription surveillance systems exist in the Netherlands and Nordic countries (Denmark, Finland, Norway, and Sweden). However, there is a lack of comprehensive, systematically collected, or readily available veterinary prescribing data in the USA137 and other countries138 and a lack of consensus on the optimum approach to surveillance of antibiotic use in animals.140
Other established risk factors for C difficile infection are advanced age and presence of comorbidities,4, 8, 21, 135, 141, 142, 143, 144, 145 current or previous admission to hospital,4, 21, 133, 135, 141, 146, 147 and sharing an environment with other people infected with C difficile.4, 141, 144 Risk-based surveillance, whereby specific high-risk groups (eg, patients who are elderly, are receiving antibiotics, have a previous history of hospital admission, or are in international transfer) are systematically targeted by hospital preadmission screening programmes, should be investigated in future risk assessment studies as a potential means of reducing the risk of incursion of epidemic strains.
Conclusions
Pépin and colleagues21, 148 give a compelling theory of the evolution of the international C difficile PCR ribotype 027: a new, fluoroquinolone-resistant, hyper-virulent strain149 causing more severe diarrhoea and, therefore, more intense environmental contamination, was circulating at low levels across a wide geographical region150 until widespread, increasing, use of fluoroquinolones131 in highly susceptible populations (characterised by increasing age and frequency of comorbidities, located in under-resourced, overcrowded, health-care facilities) precipitated a rapidly emerging epidemic that spread internationally. Many of the factors that precipitated the epidemics in North America and Europe are present in countries without known circulation of C difficile PCR ribotype 027, such as hospital overcrowding and understaffing, high levels of antibiotic (particularly fluoroquinolone) use, and an ageing population of hospital patients with increasing numbers and severity of comorbidities. Additionally, international travel is increasing and there is a high volume of international travel between affected and unaffected countries. It is highly probable that C difficile PCR ribotype 027 already has been or will be introduced undetected into countries not affected at present, via hospital transfers, asymptomatic carriers, or other vehicles, due to a lack of screening and the frequent movement of people and commodities across boundaries.
If C difficile PCR ribotype 027 is introduced into an unaffected country or if a highly pathogenic strain emerges, it is improbable with current surveillance that they would be identified until a large outbreak of severe C difficile infection happens, leading to otherwise preventable illness, colectomies, deaths, and huge costs to health services. Now is the time to act to assemble an evidence base for reducing the risk and consequences of future outbreaks in unaffected countries.
Search strategy and selection criteria
Data for this Review were obtained from publications identified by a systematic search of PubMed, focusing on those published from January, 2001, to March, 2010. Search terms included “Clostridium difficile”, “ribotype 027”, “NAP 1”, “international epidemic strain”, “risk”, and “surveillance”. Abstracts of English, French, and Spanish language papers were read and considered for inclusion, although only English language papers were selected for the final review. Secondary, manual, searches of the cited references of these articles were done and relevant articles were included, some of which were published before 2001. The last search of published work was done on March 17, 2010.
Acknowledgments
Acknowledgments
ACAC is funded by an Australian National Health and Medical Research Council Career Development Award (#631619). RJSM is funded by a University of Queensland Research Scholarship and an International Postgraduate Research Award (#41795457). AJT is supported by a grant from the Bill and Melinda Gates Foundation (#49446).
Contributors
ACAC had the original idea for the Review, searched the published work, created figures and tables, and drafted the paper. RJSM oversaw the risk assessment component and contributed to the drafts. AJT provided figures and contributed to the drafts. DLP oversaw the epidemiological description of Clostridium difficile infection and contributed to the drafts. TVR oversaw the microbiological description of C difficile and contributed to the drafts. All authors approved the final version.
Conflict of interests
We declare that we have no conflicts of interest.
References
- 1.Larson HE, Price AB, Honour P, Borriello SP. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet. 1978;1:1063–1066. doi: 10.1016/s0140-6736(78)90912-1. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett JG, Moon N, Chang TW, Taylor N, Onderdonk AB. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75:778–782. [PubMed] [Google Scholar]
- 3.Thomas C, Stevenson M, Riley TV. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review. J Antimicrob Chemother. 2003;51:1339–1350. doi: 10.1093/jac/dkg254. [DOI] [PubMed] [Google Scholar]
- 4.Chang VT, Nelson K. The role of physical proximity in nosocomial diarrhea. Clin Infect Dis. 2000;31:717–722. doi: 10.1086/314030. [DOI] [PubMed] [Google Scholar]
- 5.McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium difficile carriage and C difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis. 1990;162:678–684. doi: 10.1093/infdis/162.3.678. [DOI] [PubMed] [Google Scholar]
- 6.Archibald LK, Banerjee SN, Jarvis WR. Secular trends in hospital-acquired Clostridium difficile disease in the United States, 1987–2001. J Infect Dis. 2004;189:1585–1589. doi: 10.1086/383045. [DOI] [PubMed] [Google Scholar]
- 7.Chandler RE, Hedberg K, Cieslak PR. Clostridium difficile-associated disease in Oregon: increasing incidence and hospital-level risk factors. Infect Control Hosp Epidemiol. 2007;28:116–122. doi: 10.1086/511795. [DOI] [PubMed] [Google Scholar]
- 8.Muto CA, Pokrywka M, Shutt K. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005;26:273–280. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- 9.Polk RE, Oinonen M, Pakyz A. Epidemic Clostridium difficile. N Engl J Med. 2006;354:1199–1203. [PubMed] [Google Scholar]
- 10.Vonberg RP, Schwab F, Gastmeier P. Clostridium difficile in discharged inpatients, Germany. Emerg Infect Dis. 2007;13:179–180. doi: 10.3201/eid1301.060611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilcox MH, Smyth ET. Incidence and impact of Clostridium difficile infection in the UK, 1993–1996. J Hosp Infect. 1998;39:181–187. doi: 10.1016/s0195-6701(98)90256-0. [DOI] [PubMed] [Google Scholar]
- 12.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis. 2006;12:409–415. doi: 10.3201/eid1203.051064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg. 2007;142:624–631. doi: 10.1001/archsurg.142.7.624. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Severe Clostridium difficile-associated disease in populations previously at low risk—four states, 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1201–1205. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Surveillance for community-associated Clostridium difficile—Connecticut, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:340–343. [PubMed] [Google Scholar]
- 16.Benson L, Song X, Campos J, Singh N. Changing epidemiology of Clostridium difficile-associated disease in children. Infect Control Hosp Epidemiol. 2007;28:1233–1235. doi: 10.1086/520732. [DOI] [PubMed] [Google Scholar]
- 17.Garey KW, Jiang ZD, Yadav Y. Peripartum Clostridium difficile infection: case series and review of the literature. Am J Obstet Gynecol. 2008;199:332–337. doi: 10.1016/j.ajog.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Hecker MT, Riggs MM, Hoyen CK, Lancioni C, Donskey CJ. Recurrent infection with epidemic Clostridium difficile in a peripartum woman whose infant was asymptomatically colonized with the same strain. Clin Infect Dis. 2008;46:956–957. doi: 10.1086/527568. [DOI] [PubMed] [Google Scholar]
- 19.Rouphael NG, O'Donnell JA, Bhatnagar J. Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008;198 doi: 10.1016/j.ajog.2008.01.062. 635e1-e6. [DOI] [PubMed] [Google Scholar]
- 20.Pepin J, Valiquette L, Alary ME. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171:466–472. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pépin J, Saheb N, Coulombe MA. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41:1254–1260. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 22.Eggertson L. C difficile may have killed 2000 in Quebec: study. CMAJ. 2005;173:1020–1021. doi: 10.1503/cmaj.051226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris AM, Jobe BA, Stoney M. Clostridium difficile colitis: an increasingly aggressive iatrogenic disease? Arch Surg. 2002;137:1096–1100. doi: 10.1001/archsurg.137.10.1096. [DOI] [PubMed] [Google Scholar]
- 24.Wysowski DK. Increase in deaths related to enterocolitis due to Clostridium difficile in the United States, 1999–2002. Public Health Rep. 2006;121:361–362. [PMC free article] [PubMed] [Google Scholar]
- 25.Zilberberg MD, Shorr AF, Kollef MH. Increase in adult Clostridium difficile-related hospitalizations and case-fatality rate, United States, 2000–2005. Emerg Infect Dis. 2008;14:929–931. doi: 10.3201/eid1406.071447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999–2004. Emerg Infect Dis. 2007;13:1417–1419. doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Indra A, Huhulescu S, Kernbichler S. First cases of Clostridium difficile PCR ribotype 027 acquired in Austria. Euro Surveill. 2008;13:18875. [PubMed] [Google Scholar]
- 28.Coignard B, Barbut F, Blanckaert K. Emergence of Clostridium difficile toxinotype III, PCR-ribotype 027-associated disease, France, 2006. Euro Surveill. 2006;11:E060914. doi: 10.2807/esw.11.37.03044-en. 1. [DOI] [PubMed] [Google Scholar]
- 29.Health Protection Agency Outbreak of Clostridium difficile infection in a hospital in south east England. Commun Dis Rep Wkly. 2005;15:2–3. [Google Scholar]
- 30.Office for National Statistics Deaths involving Clostridium difficile: England and Wales, 2001–2005. Health Stat Q. 2007;33:71–75. [PubMed] [Google Scholar]
- 31.Office for National Statistics Deaths involving Clostridium difficile: England and Wales, 1999 and 2001–06. Health Stat Q. 2008;37:52–56. [PubMed] [Google Scholar]
- 32.Wilcox M. Clostridium difficile ribotyping network for England and Northern Ireland 2008/09 report. Health Protection Agency; London: 2009. [Google Scholar]
- 33.Centre for Infections Mandatory Surveillance Team . Summary points and commentary on quarterly (January 2006 to June 2008), calendar year (2004–2007) and financial year (2007/2008) Clostridium difficile data derived from Mandatory Surveillance, October 2008. Health Protection Agency; London: 2008. [Google Scholar]
- 34.McDonald LC, Killgore GE, Thompson A. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 35.Loo VG, Poirier L, Miller MA. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 36.Smith A. Outbreak of Clostridium difficile infection in an English hospital linked to hypertoxin-producing strains in Canada and the US. Euro Surveill. 2005;10:E050630. doi: 10.2807/esw.10.26.02735-en. 2. [DOI] [PubMed] [Google Scholar]
- 37.Goorhuis A, Van der Kooi T, Vaessen N. Spread and epidemiology of Clostridium difficile polymerase chain reaction ribotype 027/toxinotype III in The Netherlands. Clin Infect Dis. 2007;45:695–703. doi: 10.1086/520984. [DOI] [PubMed] [Google Scholar]
- 38.van Steenbergen J, Debast S, van Kregten E. Isolation of Clostridium difficile ribotype 027, toxinotype III in the Netherlands after increase in C difficile-associated diarrhoea. Euro Surveill. 2005;10:E050714. 1. [PubMed] [Google Scholar]
- 39.Kuijper EJ, van den Berg RJ, Debast S. Clostridium difficile ribotype 027, toxinotype III, the Netherlands. Emerg Infect Dis. 2006;12:827–830. doi: 10.3201/eid1205.051350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joseph R, Demeyer D, Vanrenterghem D. First isolation of Clostridium difficile PCR ribotype 027, toxinotype III in Belgium. Euro Surveill. 2005;10:E051020. doi: 10.2807/esw.10.42.02815-en. 4. [DOI] [PubMed] [Google Scholar]
- 41.Long S, Fenelon L, Fitzgerald S. First isolation and report of clusters of Clostridium difficile PCR 027 cases in Ireland. Euro Surveill. 2007;12:E070426. doi: 10.2807/esw.12.17.03183-en. 3. [DOI] [PubMed] [Google Scholar]
- 42.Tachon M, Cattoen C, Blanckaert K. First cluster of C difficile toxinotype III, PCR-ribotype 027 associated disease in France: preliminary report. Euro Surveill. 2006;11:E060504. doi: 10.2807/esw.11.18.02951-en. 1. [DOI] [PubMed] [Google Scholar]
- 43.Fenner L, Widmer AF, Stranden A. First cluster of clindamycin-resistant Clostridium difficile PCR ribotype 027 in Switzerland. Clin Microbiol Infect. 2008;14:514–515. doi: 10.1111/j.1469-0691.2008.01989.x. [DOI] [PubMed] [Google Scholar]
- 44.Kuijper EJ, Coignard B, Brazier JS. Update of Clostridium difficile-associated disease due to PCR ribotype 027 in Europe. Euro Surveill. 2007;12:E1–E2. doi: 10.2807/esm.12.06.00714-en. [DOI] [PubMed] [Google Scholar]
- 45.Kuijper EJ, Barbut F, Brazier JS. Update of Clostridium difficile infection due to PCR ribotype 027 in Europe, 2008. Euro Surveill. 2008;13:pii 18942. [PubMed] [Google Scholar]
- 46.Kleinkauf N, Weiss B, Jansen A. Confirmed cases and report of clusters of severe infections due to Clostridium difficile PCR ribotype 027 in Germany. Euro Surveill. 2007;12:E071115. doi: 10.2807/esw.12.46.03307-en. 2. [DOI] [PubMed] [Google Scholar]
- 47.Zaiss NH, Weile J, Ackermann G. A case of Clostridium difficile-associated disease due to the highly virulent clone of Clostridium difficile PCR ribotype 027, March 2007 in Germany. Euro Surveill. 2007;12:E071115. doi: 10.2807/esw.12.46.03306-en. 1. [DOI] [PubMed] [Google Scholar]
- 48.Indra A, Huhulescu S, Fiedler A. Outbreak of Clostridium difficile 027 infection in Vienna, Austria 2008–2009. Euro Surveill. 2009;14:pii 19186. [PubMed] [Google Scholar]
- 49.Bacci S, St-Martin G, Olesen B. Outbreak of Clostridium difficile 027 in North Zealand, Denmark, 2008–2009. Euro Surveill. 2009;14:pii 19186. [PubMed] [Google Scholar]
- 50.Quesada-Gomez C, Rodriguez C, Mdel M Gamboa-Coronado. Emergence of Clostridium difficile NAP1 in Latin America. J Clin Microbiol. 2010;48:669–670. doi: 10.1128/JCM.02196-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lukkarinen H, Eerola E, Ruohola A. Clostridium difficile ribotype 027-associated disease in children with norovirus infection. Pediatr Infect Dis J. 2009;28:847–848. doi: 10.1097/INF.0b013e31819d1cd9. [DOI] [PubMed] [Google Scholar]
- 52.Lyytikainen O, Mentula S, Kononen E. First isolation of Clostridium difficile PCR ribotype 027 in Finland. Euro Surveill. 2007;12:E071108. doi: 10.2807/esw.12.45.03303-en. 2. [DOI] [PubMed] [Google Scholar]
- 53.Terhes G, Urban E, Konkoly-Thege M. First isolation of Clostridium difficile PCR ribotype 027 from a patient with severe persistent diarrhoea in Hungary. Clin Microbiol Infect. 2009;15:885–886. doi: 10.1111/j.1469-0691.2009.02807.x. [DOI] [PubMed] [Google Scholar]
- 54.Baldan R, Cavallerio P, Tuscano A. First report of hypervirulent strains polymerase chain reaction ribotypes 027 and 078 causing severe Clostridium difficile infection in Italy. Clin Infect Dis. 2010;50:126–127. doi: 10.1086/649011. [DOI] [PubMed] [Google Scholar]
- 55.Ingebretsen A, Hansen G, Harmanus C, Kuijper EJ. First confirmed cases of Clostridium difficile PCR ribotype 027 in Norway. Euro Surveill. 2008;13:pii 8011. [PubMed] [Google Scholar]
- 56.Huang H, Weintraub A, Fang H, Nord CE. Community acquired Clostridium difficile infection due to a moxifloxacin susceptible ribotype 027 strain. Scand J Infect Dis. 2009;41:158–159. doi: 10.1080/00365540802484836. [DOI] [PubMed] [Google Scholar]
- 57.Riley TV, Thean S, Hool G, Golledge CL. First Australian isolation of epidemic Clostridium difficile PCR ribotype 027. Med J Aust. 2009;190:706–708. doi: 10.5694/j.1326-5377.2009.tb02644.x. [DOI] [PubMed] [Google Scholar]
- 58.Tae CH, Jung SA, Song HJ. The first case of antibiotic-associated colitis by Clostridium difficile PCR ribotype 027 in Korea. J Korean Med Sci. 2009;24:520–524. doi: 10.3346/jkms.2009.24.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng VC, Yam WC, Chan JF. Clostridium difficile ribotype 027 arrives in Hong Kong. Int J Antimicrob Agents. 2009;34:492–493. doi: 10.1016/j.ijantimicag.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Kato H, Ito Y, van den Berg RJ, Kuijper EJ, Arakawa Y. First isolation of Clostridium difficile 027 in Japan. Euro Surveill. 2007;12:E070111. doi: 10.2807/esw.12.02.03110-en. 3. [DOI] [PubMed] [Google Scholar]
- 61.Sawabe E, Kato H, Osawa K. Molecular analysis of Clostridium difficile at a university teaching hospital in Japan: a shift in the predominant type over a five-year period. Eur J Clin Microbiol Infect Dis. 2007;26:695–703. doi: 10.1007/s10096-007-0355-8. [DOI] [PubMed] [Google Scholar]
- 62.Huang H, Weintraub A, Fang H, Nord CE. Community acquired Clostridium difficile infection due to a moxifloxacin susceptible ribotype 027 strain. Scand J Infect Dis. 2009;41:158–159. doi: 10.1080/00365540802484836. [DOI] [PubMed] [Google Scholar]
- 63.Miller M, Gravel D, Mulvey M. Health care-associated Clostridium difficile infection in Canada: patient age and infecting strain type are highly predictive of severe outcome and mortality. Clin Infect Dis. 2010;50:194–201. doi: 10.1086/649213. [DOI] [PubMed] [Google Scholar]
- 64.Barbut F, Mastrantonio P, Delmee M. Prospective study of Clostridium difficile infections in Europe with phenotypic and genotypic characterisation of the isolates. Clin Microbiol Infect. 2007;13:1048–1057. doi: 10.1111/j.1469-0691.2007.01824.x. [DOI] [PubMed] [Google Scholar]
- 65.Labbe AC, Poirier L, Maccannell D. Clostridium difficile infections in a Canadian tertiary care hospital before and during a regional epidemic associated with the BI/NAP1/027 strain. Antimicrob Agents Chemother. 2008;52:3180–3187. doi: 10.1128/AAC.00146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akerlund T, Persson I, Unemo M. Increased sporulation rate of epidemic Clostridium difficile Type 027/NAP1. J Clin Microbiol. 2008;46:1530–1533. doi: 10.1128/JCM.01964-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warny M, Pepin J, Fang A. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 68.Barbut F, Decre D, Lalande V. Clinical features of Clostridium difficile-associated diarrhoea due to binary toxin (actin-specific ADP-ribosyltransferase)-producing strains. J Med Microbiol. 2005;54:181–185. doi: 10.1099/jmm.0.45804-0. [DOI] [PubMed] [Google Scholar]
- 69.Barbut F, Gariazzo B, Bonne L. Clinical features of Clostridium difficile-associated infections and molecular characterization of strains: results of a retrospective study, 2000–2004. Infect Control Hosp Epidemiol. 2007;28:131–139. doi: 10.1086/511794. [DOI] [PubMed] [Google Scholar]
- 70.Bourgault AM, Lamothe F, Loo VG, Poirier L. In vitro susceptibility of Clostridium difficile clinical isolates from a multi-institutional outbreak in Southern Quebec, Canada. Antimicrob Agents Chemother. 2006;50:3473–3475. doi: 10.1128/AAC.00479-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hensgens MP, Goorhuis A, Notermans DW, van Benthem BH, Kuijper EJ. Decrease of hypervirulent Clostridium difficile PCR ribotype 027 in the Netherlands. Euro Surveill. 2009;14:pii 19402. doi: 10.2807/ese.14.45.19402-en. [DOI] [PubMed] [Google Scholar]
- 72.Riley TV. Epidemic Clostridium difficile. Med J Aust. 2006;185:133–134. doi: 10.5694/j.1326-5377.2006.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 73.WHO . Principles and guidelines for the conduct of microbiological risk assessment. World Health Organization; Geneva: 1999. http://www.who.int/foodsafety/publications/micro/cac1999/en/index.html (accessed April 22, 2010). [Google Scholar]
- 74.Vose D, Acar J, Anthony F. Antimicrobial resistance: risk analysis methodology for the potential impact on public health of antimicrobial resistant bacteria of animal origin. Rev Sci Tech. 2001;20:811–827. doi: 10.20506/rst.20.3.1319. [DOI] [PubMed] [Google Scholar]
- 75.Alban L, Nielsen EO, Dahl J. A human health risk assessment for macrolide-resistant Campylobacter associated with the use of macrolides in Danish pig production. Prev Vet Med. 2008;83:115–129. doi: 10.1016/j.prevetmed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 76.Bemrah N, Sanaa M, Cassin MH, Griffiths MW, Cerf O. Quantitative risk assessment of human listeriosis from consumption of soft cheese made from raw milk. Prev Vet Med. 1998;37:129–145. doi: 10.1016/s0167-5877(98)00112-3. [DOI] [PubMed] [Google Scholar]
- 77.Bronsvoort BM, Alban L, Greiner M. Quantitative assessment of the likelihood of the introduction of classical swine fever virus into the Danish swine population. Prev Vet Med. 2008;85:226–240. doi: 10.1016/j.prevetmed.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 78.Follin P, Lindqvist A, Nystrom K, Lindh M. A variety of respiratory viruses found in symptomatic travellers returning from countries with ongoing spread of the new influenza A(H1N1)v virus strain. Euro Surveill. 2009;14:pii 19242. doi: 10.2807/ese.14.24.19242-en. [DOI] [PubMed] [Google Scholar]
- 79.Wilder-Smith A, Paton NI, Goh KT. Experience of severe acute respiratory syndrome in singapore: importation of cases, and defense strategies at the airport. J Travel Med. 2003;10:259–262. doi: 10.2310/7060.2003.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanna JN, Ritchie SA, Richards AR. Multiple outbreaks of dengue serotype 2 in north Queensland, 2003/04. Aust N Z J Public Health. 2006;30:220–225. doi: 10.1111/j.1467-842x.2006.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 81.Johnson DF, Druce JD, Chapman S. Chikungunya virus infection in travellers to Australia. Med J Aust. 2008;188:41–43. doi: 10.5694/j.1326-5377.2008.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 82.Receveur M, Ezzedine K, Pistone T, Malvy D. Chikungunya infection in a French traveller returning from the Maldives, October, 2009. Euro Surveill. 2010;15:19494. doi: 10.2807/ese.15.08.19494-en. [DOI] [PubMed] [Google Scholar]
- 83.Hanna JN, Ritchie SA, Eisen DP. An outbreak of Plasmodium vivax malaria in Far North Queensland, 2002. Med J Aust. 2004;180:24–28. doi: 10.5694/j.1326-5377.2004.tb05769.x. [DOI] [PubMed] [Google Scholar]
- 84.Riggs MM, Sethi AK, Zabarsky TF. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis. 2007;45:992–998. doi: 10.1086/521854. [DOI] [PubMed] [Google Scholar]
- 85.McCoubrey J, Starr J, Martin H, Poxton IR. Clostridium difficile in a geriatric unit: a prospective epidemiological study employing a novel S-layer typing method. J Med Microbiol. 2003;52:573–578. doi: 10.1099/jmm.0.05179-0. [DOI] [PubMed] [Google Scholar]
- 86.Viscidi R, Willey S, Bartlett JG. Isolation rates and toxigenic potential of Clostridium difficile isolates from various patient populations. Gastroenterology. 1981;81:5–9. [PubMed] [Google Scholar]
- 87.Beaugerie L, Flahault A, Barbut F. Antibiotic-associated diarrhoea and Clostridium difficile in the community. Aliment Pharmacol Ther. 2003;17:905–912. doi: 10.1046/j.1365-2036.2003.01531.x. [DOI] [PubMed] [Google Scholar]
- 88.Songer JG, Post KW, Larson DJ, Jost BH, Glock RD. Infection of neonatal swine with Clostridium difficile. Swine Health Prod. 2000;8:185–189. [Google Scholar]
- 89.Songer JG. The emergence of Clostridium difficile as a pathogen of food animals. Anim Health Res Rev. 2004;5:321–326. doi: 10.1079/ahr200492. [DOI] [PubMed] [Google Scholar]
- 90.Waters EH, Orr JP, Clark EG, Schaufele CM. Typhlocolitis caused by Clostridium difficile in suckling piglets. J Vet Diagn Invest. 1998;10:104–108. doi: 10.1177/104063879801000122. [DOI] [PubMed] [Google Scholar]
- 91.Yaeger M, Funk N, Hoffman L. A survey of agents associated with neonatal diarrhea in Iowa swine including Clostridium difficile and porcine reproductive and respiratory syndrome virus. J Vet Diagn Invest. 2002;14:281–287. doi: 10.1177/104063870201400402. [DOI] [PubMed] [Google Scholar]
- 92.Baverud V, Gustafsson A, Franklin A, Aspan A, Gunnarsson A. Clostridium difficile: prevalence in horses and environment, and antimicrobial susceptibility. Equine Vet J. 2003;35:465–471. doi: 10.2746/042516403775600505. [DOI] [PubMed] [Google Scholar]
- 93.Arroyo LG, Staempfli H, Weese JS. Molecular analysis of Clostridium difficile isolates recovered from horses with diarrhea. Vet Microbiol. 2007;120:179–183. doi: 10.1016/j.vetmic.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 94.Weese JS, Finley R, Reid-Smith RR, Janecko N, Rousseau J. Evaluation of Clostridium difficile in dogs and the household environment. Epidemiol Infect. 2009 doi: 10.1017/S0950268809991312. published online Dec 2. [DOI] [PubMed] [Google Scholar]
- 95.Goorhuis A, Bakker D, Corver J. Emergence of Clostridium difficile infection due to a new hypervirulent strain, polymerase chain reaction ribotype 078. Clin Infect Dis. 2008;47:1162–1170. doi: 10.1086/592257. [DOI] [PubMed] [Google Scholar]
- 96.Arroyo LG, Kruth SA, Willey BM. PCR ribotyping of Clostridium difficile isolates originating from human and animal sources. J Med Microbiol. 2005;54:163–166. doi: 10.1099/jmm.0.45805-0. [DOI] [PubMed] [Google Scholar]
- 97.Keel K, Brazier JS, Post KW, Weese S, Songer JG. Prevalence of PCR ribotypes among Clostridium difficile isolates from pigs, calves, and other species. J Clin Microbiol. 2007;45:1963–1964. doi: 10.1128/JCM.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lefebvre SL, Waltner-Toews D, Peregrine AS. Prevalence of zoonotic agents in dogs visiting hospitalized people in Ontario: implications for infection control. J Hosp Infect. 2006;62:458–466. doi: 10.1016/j.jhin.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 99.Rodriguez-Palacios A, Stampfli HR, Duffield T. Clostridium difficile PCR ribotypes in calves, Canada. Emerg Infect Dis. 2006;12:1730–1736. doi: 10.3201/eid1211.051581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lefebvre SL, Arroyo LG, Weese JS. Epidemic Clostridium difficile strain in hospital visitation dog. Emerg Infect Dis. 2006;12:1036–1037. doi: 10.3201/eid1206.060115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Songer JG, Trinh HT, Dial SM, Brazier JS, Glock RD. Equine colitis X associated with infection by Clostridium difficile NAP1/027. J Vet Diagn Invest. 2009;21:377–380. doi: 10.1177/104063870902100314. [DOI] [PubMed] [Google Scholar]
- 102.Rodriguez-Palacios A, Staempfli HR, Duffield T, Weese JS. Clostridium difficile in retail ground meat, Canada. Emerg Infect Dis. 2007;13:485–487. doi: 10.3201/eid1303.060988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rodriguez-Palacios A, Reid-Smith RJ, Staempfli HR. Possible seasonality of Clostridium difficile in retail meat, Canada. Emerg Infect Dis. 2009;15:802–805. doi: 10.3201/eid1505.081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Songer JG, Trinh HT, Killgore GE. Clostridium difficile in retail meat products, USA, 2007. Emerg Infect Dis. 2009;15:819–821. doi: 10.3201/eid1505.081071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weese JS, Avery BP, Rousseau J, Reid-Smith RJ. Detection and enumeration of Clostridium difficile spores in retail beef and pork. Appl Environ Microbiol. 2009;75:5009–5011. doi: 10.1128/AEM.00480-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weese JS, Rousseau J, Arroyo L. Bacteriological evaluation of commercial canine and feline raw diets. Can Vet J. 2005;46:513–516. [PMC free article] [PubMed] [Google Scholar]
- 107.Bakri MM, Brown DJ, Butcher JP, Sutherland AD. Clostridium difficile in ready-to-eat salads, Scotland. Emerg Infect Dis. 2009;15:817–818. doi: 10.3201/eid1505.081186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weese JS. Clostridium difficile in food—innocent bystander or serious threat? Clin Microbiol Infect. 2010;16:3–10. doi: 10.1111/j.1469-0691.2009.03108.x. [DOI] [PubMed] [Google Scholar]
- 109.Barbut F, Delmee M, Brazier JS. A European survey of diagnostic methods and testing protocols for Clostridium difficile. Clin Microbiol Infect. 2003;9:989–996. doi: 10.1046/j.1469-0691.2003.00683.x. [DOI] [PubMed] [Google Scholar]
- 110.Chen F, Chakera A, Seow C. More on Clostridium diffcile-associated diarrhoea in Australia. Anaerobe. 1999;5:205–207. [Google Scholar]
- 111.Taylor J, Foster K, Berrington A. Clostridium difficile: a questionnaire survey of laboratory practice in England, Wales, and Northern Ireland. Commun Dis Public Health. 2004;7:322–327. [PubMed] [Google Scholar]
- 112.Fitzpatrick F, Oza A, Gilleece A, O'Byrne AM, Drudy D. Laboratory diagnosis of Clostridium difficile-associated disease in the Republic of Ireland: a survey of Irish microbiology laboratories. J Hosp Infect. 2008;68:315–321. doi: 10.1016/j.jhin.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 113.Alfa MJ, Du T, Beda G. Survey of incidence of Clostridium difficile infection in Canadian hospitals and diagnostic approaches. J Clin Microbiol. 1998;36:2076–2080. doi: 10.1128/jcm.36.7.2076-2080.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sohn S, Climo M, Diekema D. Varying rates of Clostridium difficile-associated diarrhea at prevention epicenter hospitals. Infect Control Hosp Epidemiol. 2005;26:676–679. doi: 10.1086/502601. [DOI] [PubMed] [Google Scholar]
- 115.Delmee M, Ramboer I, Van Broeck J, Suetens C. Epidemiology of Clostridium difficile toxinotype III, PCR-ribotype 027 associated disease in Belgium, 2006. Euro Surveill. 2006;11:E060914. doi: 10.2807/esw.11.37.03045-en. 2. [DOI] [PubMed] [Google Scholar]
- 116.Health Protection Agency Surveillance of Clostridium difficile associated disease: report of the national standards group. CDR Weekly. 2003;40:5–6. [Google Scholar]
- 117.National Clostridium difficile Standards Group National Clostridium difficile Standards Group: Report to the Department of Health. J Hosp Infect. 2004;56(suppl 1):1–38. doi: 10.1016/j.jhin.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 118.Donaldson L, Beasley C. Letter from the Chief Medical Officer and Chief Nursing Officer: infection caused by Clostridium difficile. Department of Health; London: 2005. [Google Scholar]
- 119.Durai R. Epidemiology, pathogenesis, and management of Clostridium difficile infection. Dig Dis Sci. 2007;52:2958–2962. doi: 10.1007/s10620-006-9626-y. [DOI] [PubMed] [Google Scholar]
- 120.Silversides A. Ontario's hospitals surpass those of Quebec in C difficile rates. CMAJ. 2008;178:1649. doi: 10.1503/cmaj.080804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eggertson L, Sibbald B. Need for national surveillance for hospital infections. CMAJ. 2004;171:22. doi: 10.1503/cmaj.1040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Department of Immigration and Citizenship . Annual Report 2007–08. Australian Government; Canberra: 2008. http://www.immi.gov.au/about/reports/annual/2007-08/ (accessed April 22, 2010). [Google Scholar]
- 123.Tatem AJ, Hay SI. Climatic similarity and biological exchange in the worldwide airline transportation network. Proc Biol Sci. 2007;274:1489–1496. doi: 10.1098/rspb.2007.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fawley WN, Parnell P, Verity P, Freeman J, Wilcox MH. Molecular epidemiology of endemic Clostridium difficile infection and the significance of subtypes of the United Kingdom epidemic strain (PCR ribotype 1) J Clin Microbiol. 2005;43:2685–2696. doi: 10.1128/JCM.43.6.2685-2696.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Verity P, Wilcox MH, Fawley W, Parnell P. Prospective evaluation of environmental contamination by Clostridium difficile in isolation side rooms. J Hosp Infect. 2001;49:204–209. doi: 10.1053/jhin.2001.1078. [DOI] [PubMed] [Google Scholar]
- 126.Samore MH, Venkataraman L, DeGirolami PC, Arbeit RD, Karchmer AW. Clinical and molecular epidemiology of sporadic and clustered cases of nosocomial Clostridium difficile diarrhea. Am J Med. 1996;100:32–40. doi: 10.1016/s0002-9343(96)90008-x. [DOI] [PubMed] [Google Scholar]
- 127.Roberts K, Smith CF, Snelling AM. Aerial dissemination of Clostridium difficile spores. BMC Infect Dis. 2008;8:7. doi: 10.1186/1471-2334-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Starr JM, Campbell A. Mathematical modeling of Clostridium difficile infection. Clin Microbiol Infect. 2001;7:432–437. doi: 10.1046/j.1198-743x.2001.00291.x. [DOI] [PubMed] [Google Scholar]
- 129.Starr JM, Campbell A, Renshaw E, Poxton IR, Gibson GJ. Spatio-temporal stochastic modelling of Clostridium difficile. J Hosp Infect. 2009;71:49–56. doi: 10.1016/j.jhin.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 130.Patrick DM, Marra F, Hutchinson J. Per capita antibiotic consumption: how does a North American jurisdiction compare with Europe? Clin Infect Dis. 2004;39:11–17. doi: 10.1086/420825. [DOI] [PubMed] [Google Scholar]
- 131.Linder JA, Huang ES, Steinman MA, Gonzales R, Stafford RS. Fluoroquinolone prescribing in the United States: 1995 to 2002. Am J Med. 2005;118:259–268. doi: 10.1016/j.amjmed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 132.Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 133.Kazakova SV, Ware K, Baughman B. A hospital outbreak of diarrhea due to an emerging epidemic strain of Clostridium difficile. Arch Intern Med. 2006;166:2518–2524. doi: 10.1001/archinte.166.22.2518. [DOI] [PubMed] [Google Scholar]
- 134.McCusker ME, Harris AD, Perencevich E, Roghmann MC. Fluoroquinolone use and Clostridium difficile-associated diarrhea. Emerg Infect Dis. 2003;9:730–733. doi: 10.3201/eid0906.020385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McFarland LV, Clarridge JE, Beneda HW, Raugi GJ. Fluoroquinolone use and risk factors for Clostridium difficile-associated disease within a Veterans Administration health care system. Clin Infect Dis. 2007;45:1141–1151. doi: 10.1086/522187. [DOI] [PubMed] [Google Scholar]
- 136.Drudy D, Goorhuis B, Bakker D. Clindamycin-resistant clone of Clostridium difficile PCR Ribotype 027, Europe. Emerg Infect Dis. 2008;14:1485–1487. doi: 10.3201/eid1409.071346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.DeVincent SJ, Viola C. Introduction to animal antimicrobial use data collection in the United States: methodological options. Prev Vet Med. 2006;73:105–109. doi: 10.1016/j.prevetmed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 138.Viola C, DeVincent SJ. Overview of issues pertaining to the manufacture, distribution, and use of antimicrobials in animals and other information relevant to animal antimicrobial use data collection in the United States. Prev Vet Med. 2006;73:111–131. doi: 10.1016/j.prevetmed.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 139.Ungemach FR, Muller-Bahrdt D, Abraham G. Guidelines for prudent use of antimicrobials and their implications on antibiotic usage in veterinary medicine. Int J Med Microbiol. 2006;296(suppl 41):33–38. doi: 10.1016/j.ijmm.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 140.DeVincent SJ, Viola C. Deliberations of an Advisory Committee regarding priorities, sources, and methods for collecting animal antimicrobial use data in the United States. Prev Vet Med. 2006;73:133–151. doi: 10.1016/j.prevetmed.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 141.Dubberke ER, Reske KA, Yan Y. Clostridium difficile—associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis. 2007;45:1543–1549. doi: 10.1086/523582. [DOI] [PubMed] [Google Scholar]
- 142.Biller P, Shank B, Lind L. Moxifloxacin therapy as a risk factor for Clostridium difficile-associated disease during an outbreak: attempts to control a new epidemic strain. Infect Control Hosp Epidemiol. 2007;28:198–201. doi: 10.1086/511789. [DOI] [PubMed] [Google Scholar]
- 143.Changela U, Cannon JP, Aneziokoro C. Risk factors and mortality associated with Clostridium difficile-associated diarrhoea at a VA hospital. Int J Antimicrob Agents. 2004;24:562–566. doi: 10.1016/j.ijantimicag.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 144.Dubberke ER, Reske KA, Olsen MA. Evaluation of Clostridium difficile-associated disease pressure as a risk factor for C difficile-associated disease. Arch Intern Med. 2007;167:1092–1097. doi: 10.1001/archinte.167.10.1092. [DOI] [PubMed] [Google Scholar]
- 145.Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol. 2002;23:653–659. doi: 10.1086/501989. [DOI] [PubMed] [Google Scholar]
- 146.Modena S, Bearelly D, Swartz K, Friedenberg FK. Clostridium difficile among hospitalized patients receiving antibiotics: a case-control study. Infect Control Hosp Epidemiol. 2005;26:685–690. doi: 10.1086/502603. [DOI] [PubMed] [Google Scholar]
- 147.Palmore TN, Sohn S, Malak SF, Eagan J, Sepkowitz KA. Risk factors for acquisition of Clostridium difficile-associated diarrhea among outpatients at a cancer hospital. Infect Control Hosp Epidemiol. 2005;26:680–684. doi: 10.1086/502602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037–1042. doi: 10.1503/cmaj.050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Blossom DB, McDonald LC. The challenges posed by reemerging Clostridium difficile infection. Clin Infect Dis. 2007;45:222–227. doi: 10.1086/518874. [DOI] [PubMed] [Google Scholar]
- 150.MacCannell DR, Louie TJ, Gregson DB. Molecular analysis of Clostridium difficile PCR ribotype 027 isolates from eastern and western Canada. J Clin Microbiol. 2006;44:2147–2152. doi: 10.1128/JCM.02563-05. [DOI] [PMC free article] [PubMed] [Google Scholar]