Highlights
-
•
Evaluate the use of multiplex real-time PCR for diagnosing respiratory infections.
-
•
857/966 samples from 914 children were positive for one or multiple viruses.
-
•
Respiratory syncytial virus and rhinovirus were the most prevalent.
-
•
Co-infections were associated with severe respiratory symptoms.
-
•
The spread of respiratory viruses returned to the one it was before the flu outbreak.
Abbreviations: ARI, acute respiratory infections; NAT, nucleic acid tests; MPLA, multiplex ligation-dependent probe amplification; IV, influenza viruses; PiV, parainfluenza viruses; RSV, respiratory syncytial viruses; RV, rhinovirus; CoV, coronaviruses; MPV, human metapneumovirus; ADV, adenovirus
Keywords: Respiratory, Viruses, Children, Multiplex-PCR, Spread, Symptoms
Abstract
Background
The use of a multiplex molecular technique to identify the etiological pathogen of respiratory viral infections might be a support as clinical signs are not characteristic.
Objectives
The aim of the study was to evaluate a multiplex molecular real-time assay for the routine diagnosis of respiratory viruses, to analyze the symptoms associated with the pathogens detected and to determine the spread of virus during the period.
Study design
Respiratory samples were collected from children presenting with respiratory symptoms and attending the emergency unit during the 2010–2011 winter seasons. Samples were tested with the multiplex RespiFinder® 15 assay (PathoFinder™) which potentially detects 15 viruses.
Results
857 (88.7%) of the 966 samples collected from 914 children were positive for one (683 samples) or multiple viruses (174 samples). The most prevalent were the respiratory syncytial virus (39.5%) and the rhinovirus (24.4%). Influenza viruses were detected in 139 (14.4%) samples. Adenovirus was detected in 93 (9.6%) samples, coronaviruses in 88 (9.1%), metapneumovirus in 51 (5.3%) and parainfluenzae in 47 (4.9%). Rhinovirus (40%) was the most prevalent pathogen in upper respiratory tract infections while respiratory syncytial virus (49.9%) was the most prevalent in lower respiratory tract infections. Co-infections were associated with severe respiratory symptoms.
Conclusion
The multiplex assay detected clinically important viruses in a single genomic test and thus will be useful for detecting several viruses causing respiratory tract disorders.
1. Background
Acute respiratory infections (ARIs) are more prevalent than any other form of infectious disease in children. They range from mild upper respiratory tract problems to serious lower respiratory infections such as bronchiolitis and pneumonia. Viruses are the main pathogens and they account for many emergency hospital admissions [1], [2]. Clinical signs and symptoms overlap between different viruses, but also between viruses and bacteria, making etiological diagnosis based on clinical presentation alone difficult and sometimes leading to overuse of antibiotics.
Techniques involving culture, fluorescent detection of antigens or immunochromatography have been replaced by nucleic acid tests (NATs). Due to numerous viruses that might be involved, many monoplex nucleic acid tests are necessary to identify the pathogen(s) responsible for a respiratory disorder. This strategy is thus expensive and time consuming. The use of multiplex assays should significantly reduce hands-on time and cost, and rapidly provide reliable results. The multiplex ligation-dependent probe amplification technology (MPLA)-RespiFinder® Respiratory assay [3] recently became commercially available. This assay is approved for in vitro diagnosis in Europe and Canada and can detect up to 15 respiratory viruses.
2. Objectives
This prospective study was done to evaluate this multiplex technique for use in clinical diagnosis. All the samples taken from children attending the emergency unit of the Toulouse University Hospital suffering from ARIs were collected prospectively and analyzed. Clinical data related to the viruses detected were also analyzed, as was the spread of seasonal respiratory viruses for the winter following the influenza A H1N1pdm09 epidemic (October 2010 to March 2011).
3. Study design
3.1. Samples
Nasopharyngeal swabs (Virocult® Kitnia, Labarthe Inard, France), aspirates or nasal washes were prospectively collected from children under 15 with symptoms of ARIs (see below) who attended the emergency unit of the Toulouse University Hospital between October 1, 2010 and March 31, 2011 and sent to the Virology Department for analysis.
Paediatricians completed a specific questionnaire related to the symptoms, including fever and upper respiratory manifestations (rhinitis, pharyngitis, otitis, sore throat, cough) and presence of symptoms of lower respiratory infections (bronchiolitis, pneumonia, acute flu and flu syndrome). Whether or not a child had been vaccinated against influenza was also recorded.
3.2. Detection of respiratory viruses
3.2.1. Nucleic acid extraction
The collected samples were diluted in 1 ml Minimum Essential Medium (Gibco® – Life Technologies, Rockville, MD, USA) and nucleic acids were extracted with the MagNA Pure 96™ instrument using the MagNA Pure 96 DNA and viral NA small volume kit® (Roche Diagnostics, Meylan, France) according to the manufacturer's instructions (extracted volume: 200 μL, elution volume: 100 μL).
3.2.2. Multiplex PCR method
Extracts were analyzed using the RespiFinder® 15 assay (PathoFinder™, Maastricht, Netherlands), a multiplex ligation-dependent probe amplification (MLPA) technology [3]. This assay can detect 15 viruses: influenza viruses (IV) types A and B, parainfluenza viruses 1 to 4 (PiV), respiratory syncytial viruses A and B (RSV), rhinovirus (RV), coronaviruses 229E, OC43 and NL63 (CoV), human metapneumovirus (MPV) and adenovirus (ADV). The test also includes a probe for detecting the avian Influenza virus A H5N1. An internal control for PCR inhibitors detection was included in each test. A positive Flu A sample and a negative sample were used as controls.
3.2.3. Influenza A virus subtyping
Samples that were positive for influenza A were subtyped with the RealTime Ready Inf A/H1N1 Detection Set (Roche Diagnostics, Meylan, France) on the Light Cycler 480™ system (Roche Diagnostics, Meylan, France).
3.3. Statistical analysis
Data were analyzed using Stata™ v9.0 software (StataCorp, Texas, USA). Qualitative variables were analyzed with the Chi-squared test. p values of less than 0.05 were considered significant. Logistic regression was used to determine the odds ratios (OR) of age and co-infections linked to severe respiratory symptoms.
4. Results
4.1. Patient population and clinical features
A total of 914 children, of whom 509/914 (55.7%) were male were enrolled in the study between October 1, 2010 and March 31, 2011 and provided 966 samples. The mean age was 1.6 ± 2.6 years and the median age was 7.3 months [range: 0.2–186], but 572/914 (62.6%) children were under 1 year old.
The 914 children in the study group included 232/914 (25.4%) with upper respiratory tract infections (243 samples) defined as rhino-pharyngitis or sore throat, with or without otitis media. 682 children (723 samples) suffered from lower respiratory infections defined as bronchiolitis (338/914; 37%), pneumonia or broncho-pneumonia (109/914; 11.9%), acute asthma (78/914; 8.5%) and flu or flu-like syndrome (158/914; 17.3%). 76 (8.3%) children were suffering from a chronic (n = 10) or a congenital disorder (n = 66): respiratory, haematological, neurological, cardiac disorders. 25/914 (2.7%) children had been vaccinated in the fall against flu, and 16/25 (64%) of them were suffering from a chronic or congenital disorder.
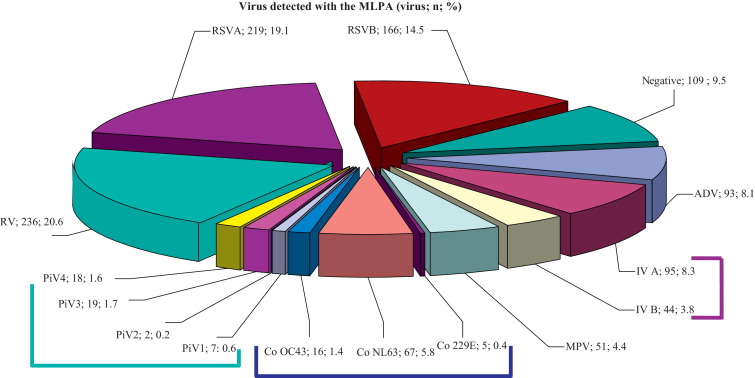
4.2. Virus detected with the MLPA in single or multiple infections (Fig. 1)
Fig. 1.
Results obtained on the 966 samples that had been tested with the MLPA. Lines group viruses of the same family. (RSV: respiratory syncytial virus, RV: rhinovirus, IV: influenza A and B viruses, ADV: adenovirus, CoV: coronaviruses, MPV: metapneumovirus, PiV: parainfluenza viruses).
At least one virus was detected in 857/966 specimens (88.7%). The most prevalent pathogen was RSV, detected in 382/966 samples (39.5%) of which 219/382 (57.3%) were type A. Three samples contained both A and B. RV was detected in 236/966 (24.4%) samples and IV in 139/966 (14.4%) samples which were mainly type A (95/139 samples; 68.3%). A subsample of 37 IVA-positive samples was subtyped: 33/37 (89.2%) were influenza AH1N1pdm09, the others were seasonal H3N2 IV.
ADV was detected in 93 (9.6%) samples and CoV in 88 (9.1%) samples (CoV NL63, OC43 and 229E in 67, 16 and 5 samples respectively). MPV were detected in 51 samples (5.3%) and PiV in 47 (4.9%) samples (type 1 in 7 (14.9%) samples, type 2 in 3 (6.4%) samples, type 3 in 19 (40.4%) samples and type 4 in 18 (38.3%) samples).
More than one virus was detected in 174 (18%) samples. Of the multiple infections, 32.6% involved rhinovirus, 25.3% RSV, 26.9% IV, 48.4% ADV, 14% MPV, 57.1% CoV and 37% PIV. Two viruses were involved in 162 (16.8%) samples (Table 1 ), three viruses in 10 samples (1%) and four viruses in 2 samples (0.2%).
Table 1.
Virus associations and their prevalence among double-infections (Bi-Is).
| Virus | RV | RSV A | RSV B | IVA | IVB | ADV | CoV 229E | CoV OC43 | CoV NL63 | MPV | PiV 1 | PiV 2 | PiV 3 | PiV 4 | No. of cases where Bi-Is were observed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total detections | 236 | 382 |
139 |
93 | 88 |
51 | 47 |
||||||||
| No. of double infections | 75 | 44 |
48 |
18 |
8 |
45 | 2 |
4 |
38 |
11 | 1 |
0 |
6 |
10 |
|
| % isolated in double infections | 31.8 | 24.1 | 18.7 | 48.4 | 50 | 21.6 | 36.2 | ||||||||
| ♦ | ♦ | 14 | |||||||||||||
| ♦ | ♦ | 23 | |||||||||||||
| ♦ | ♦ | 2 | |||||||||||||
| ♦ | ♦ | 1 | |||||||||||||
| ♦ | ♦ | 14 | |||||||||||||
| ♦ | ♦ | 1 | |||||||||||||
| ♦ | ♦ | 2 | |||||||||||||
| ♦ | ♦ | 6 | |||||||||||||
| ♦ | ♦ | 4 | |||||||||||||
| ♦ | ♦ | 1 | |||||||||||||
| ♦ | ♦ | 2 | |||||||||||||
| ♦ | ♦ | 5 | |||||||||||||
| ♦ | ♦ | 2 | |||||||||||||
| ♦ | ♦ | 3 | |||||||||||||
| ♦ | ♦ | 3 | |||||||||||||
| ♦ | ♦ | 9 | |||||||||||||
| ♦ | ♦ | 1 | |||||||||||||
| ♦ | ♦ | 10 | |||||||||||||
| ♦ | ♦ | 1 | |||||||||||||
| ♦ | ♦ | 1 | |||||||||||||
| ♦ | ♦ | 4 | |||||||||||||
| ♦ | ♦ | 2 | |||||||||||||
| ♦ | ♦ | 11 | |||||||||||||
| ♦ | ♦ | 4 | |||||||||||||
| ♦ | ♦ | 2 | |||||||||||||
| ♦ | ♦ | 2 | |||||||||||||
| ♦ | ♦ | 2 | |||||||||||||
| ♦ | ♦ | 2 | |||||||||||||
| ♦ | ♦ | 3 | |||||||||||||
| ♦ | ♦ | 1 | |||||||||||||
| ♦ | ♦ | 2 | |||||||||||||
| ♦ | ♦ | 1 | |||||||||||||
| ♦ | ♦ | 7 | |||||||||||||
| ♦ | ♦ | 1 | |||||||||||||
| ♦ | ♦ | 1 | |||||||||||||
| ♦ | ♦ | 4 | |||||||||||||
| ♦ | ♦ | 1 | |||||||||||||
| ♦ | ♦ | 1 | |||||||||||||
RV: rhinovirus; RSV A: respiratory syncytial virus type A; RSV B: type B; IVA: influenza A; IVB: influenza B; ADV: adenovirus; CoV 229: coronavirus 229E; CoV OC43: OC43; CoV NL63: NL63; MPV: human metapneumovirus; P1: parainfluenza virus type 1; P2: type 2; P3: type 3; P4: type 4.
4.3. Viruses detected in upper respiratory infections
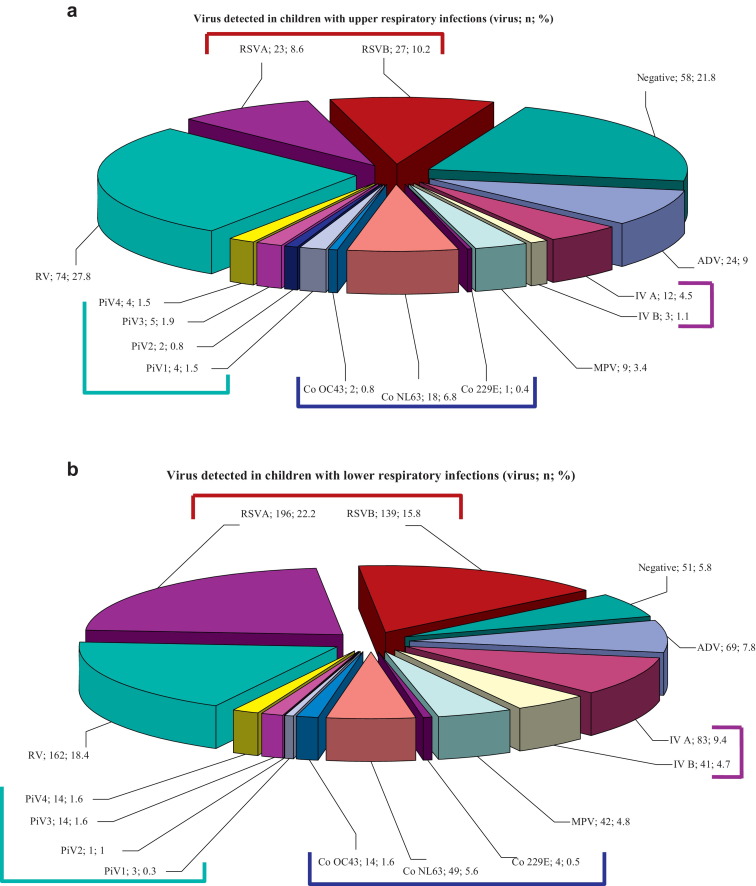
185/243 (76.1%) samples collected among children with upper respiratory tract infections were positive. RV was the virus most frequently detected in 74/185 (40%) samples, followed by RSV in 49/185 (26.5%) samples (Fig. 2a).
Fig. 2.
(a) Results obtained from the 243 samples that had been collected among 232 children presenting with upper respiratory infections. Lines group viruses of the same family. (b) Results obtained from the 723 samples that had been collected among 682 children presenting with lower respiratory infections. Lines group viruses of the same family. (RSV: respiratory syncytial virus, RV: rhinovirus, IV: influenza A and B viruses, ADV: adenovirus, CoV: coronaviruses, MPV: metapneumovirus, PiV: parainfluenza viruses).
4.4. Viruses detected in lower respiratory infections
672/723 (92.9%) samples collected among children with lower respiratory tract infections were positive. RSV was the most frequently detected in 335/672 (49.9%) samples. 195/335 (58.2%) were type A while 139/335 (41.5%) were type B. The second most involved virus was RV in 162/672 (24.1%) samples (Fig. 2b).
Viruses were detected in 336/360 (93.3%) samples collected among children with bronchiolitis and in 96/112 (85.7%) samples collected among children with pneumonia, RSV being the most frequently detected. Almost all cases of acute asthma (80/85; 94.1%) were positive for at least one virus, with RV being the most frequent.
The 156 patients suffering from flu or a flu-like syndrome provided 166 samples, of these 106 (64.6%) were IV positive (69 IVA and 37 IVB). Thirty of the 758 remaining patients were IV positive (p < 0.0001). Six (24%) children who had been vaccinated against flu developed an influenza infection, including five suffering from a chronic or congenital disorder. Flu-like symptoms were associated with the older age group: mean age of children presenting with IV was 38 months (95% CI, 32–44) vs 15 months (95% CI, 13–17) (p < 0.0001).
Individual virus detection rate in relation to specific symptoms are shown in Table 2 .
Table 2.
Clinical presentation at admission and virus identified.
| Syndrome (samples: n) |
||||||
|---|---|---|---|---|---|---|
| Upper respiratory infections (243) | Lower respiratory infections (723) |
Total samples (966) | ||||
| Rhinopharyngitidis and ORL syndrome (243) | Bronchiolitis (360) | Pneumonia (112) | Flu and flu syndrome (166) | Asthma (85) | ||
| RSV | 40 | 173 | 31 | 15 | 22 | 281 |
| RV | 64 | 43 | 18 | 5 | 26 | 156 |
| IV | 11 | 3 | 3 | 91 | 3 | 111 |
| ADV | 15 | 5 | 7 | 10 | 3 | 40 |
| CoV | 12 | 8 | 8 | 6 | 1 | 35 |
| MPV | 8 | 19 | 5 | 3 | 4 | 39 |
| PiV | 12 | 8 | 2 | 2 | 3 | 27 |
| Number of co-infectionsa | 23 | 77 | 22 | 28 | 18 | 168 |
| Total positive | 185 | 336 | 96 | 160 | 80 | 857 |
RV: rhinoviruses, RSV: respiratory syncytial virus, IV: influenza A and B viruses, ADV: adenovirus, MPV: human metapneumovirus, CoV: coronaviruses, PiV: parainfluenzae viruses.
Bold numbers represent the most prevalent pathogen linked to a syndrome.
Co-infections involved either one virus or the other.
4.5. Co-infection and clinical presentations
We found co-infections in 24 (9.96%) samples from children with upper respiratory tract symptoms and in 144 (19.9%) samples from children with lower respiratory infections (p < 0.01). The most frequent co-infections in cases of severe respiratory symptoms involved RSV (associated with RV, ADV, or CoV) and RV associated with ADV. 25% of the cases of bronchiolitis involving RSV were co-infections.
4.6. Viruses and demography
610 samples were collected among 572 children who were under 1 year old. 549/610 (90%) samples were positive. 356 samples were collected among the 294 older children: 307/356 (86.2%) samples tested positive (ns).
RSV was more prevalent in children under 1 year old (48.2% vs 24.8%; p < 0.0001), while RV was not (26.4% vs 21.1%; ns). ADV (14.8% vs 3.4%) and IV (24.4% vs 9.2%; p < 0.0001) were more frequent in children older than 6 months.
Co-infections were more frequent in younger children (1.3 years vs 1.7 years; p < 0.05).
Four parameters were included in the multivariate analysis: VRS infection, presence of co-infection, RV infection and age under 1 year. Independent factors associated with severe respiratory symptoms were VRS infection (adjusted OR, 3.8; 95% CI, 2.64–5.68), co-infections (adjusted OR, 2.5; 95% CI, 1.55–3.94) and RV infection (adjusted OR, 1.2; 95% CI, 0.85–1.83). Age under 1 year was not associated with such symptoms.
4.7. Seasonality
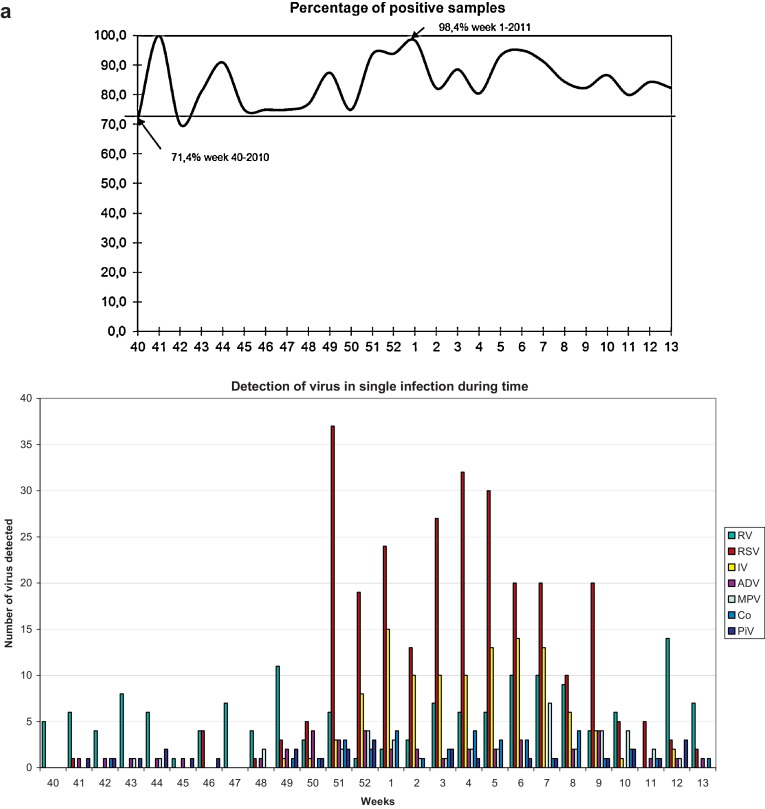
The frequency of positive samples varied from 71.4% (5/7 positive samples; week 40, 2010) to 98.4% (60/61 positive samples; weeks 1, 2011; p < 0.05) (Fig. 3a).
Fig. 3.
(a) Percentage of positive samples (Y-axis) detected during the study period (weeks 40–52/2010 and 1–13/2011; X-axis). (b) Number of viruses (Y-axis) detected during the study period (weeks 40–52/2010 and 1–13/2011; X-axis).
All the viruses in the panel were detected in our patients, but their distributions varied (Fig. 3b). RV was the first of the three main viruses to appear and was detected throughout the study. RSV appeared in October and reached epidemic peak in week 51 of 2010; IV did not appear until December and reached a plateau between weeks 1 and 7 of 2011. IVA was predominant until week 7 of 2011, while IVB was the most prevalent thereafter, decreasing slowly until the end of March. ADV, MPV and CoV were most frequently detected during the winter months, although PiV was detected throughout the study.
5. Discussion
The availability of molecular assays has made laboratory diagnosis more efficient and has led to the improved detection of a broad spectrum of respiratory viruses [4], [5].
This study evaluates the use of a new multiplex molecular technique for detecting up to 15 respiratory viruses in routine practice. We collected respiratory samples from a group of children suffering from acute respiratory infections and the results obtained were analyzed in comparison to the clinical manifestations. The spread of the viruses was also analyzed during this winter period.
A very high percentage of the samples were positive giving a virus signature in nearly 90% of cases, and all the viruses in the test panel were detected. These results are similar to those for young children and infants obtained by others (88–92%) [6], [7], [8], whereas the rate of detection in adults was lower (43%) [9]. RV, RSV, and IV were the three most prominent pathogens detected, while ADV, CoV, MPV and PiV were less frequent (<10% of cases).
As expected, RV was the most frequently pathogen detected in upper respiratory infections [10], but it was also found in lower respiratory infections, as was described recently by O’Callaghan-Gordo [11]. RSV remained however the most prominent agent in lower respiratory infections as published previously in bronchiolitis [12] and pneumonia [13]. ADV, CoV, MPV and PiV also accounted for lower respiratory tract ARIs as shown previously: ADV infection was shown to lead to severe chronic disease and to the increase in the mortality rate in children [14], whereas CoV [15], MPV [16] and PiV [17] can be responsible for severe lower respiratory tract infections requiring hospitalization, especially among the youngest.
Flu and flu syndromes were preferentially linked to influenza viruses and older children seemed to be more susceptible to these viruses. We found a relationship between an influenza virus infection and severe infection due to hyperthermia, but not with more severe respiratory symptoms, in contrast to the situation in adults [18].
The use of multiplex assays has demonstrated that infections with multiple viruses are common [4]; the frequency of such infections can be as high as 27%, 30% or 46% [5], [19], [20]. This was exactly the case for our children: all the viruses in the panel were detected in co-infections, not only RSV, but also ADV and CoVs. Martin et al. [21] described similar results. They detected ADV in association with other viruses in 52% of samples and CoV in 50%. They also showed that multiple virus infections were correlated with less severe disease. The relationship between co-infections and illness severity remains uncertain and may depend upon the virus detected. Dual infections involving RSV and RV [22] or RSV and MPV can lead to severe bronchiolitis [23] whereas co-infections without RSV do not [24].
We also analyzed the way viral infections spread during the winter. RSV and IV were epidemic while the frequency of the other viruses changed little. Our study carried out in the winter following the influenza AH1N1pdm09 pandemia shows that IV and RV infections had returned to their pattern. This has also been described in Germany by Gröndahl et al. [25] who showed that the AH1N1pdm09 outbreak had a great impact on the spread of other respiratory viruses, but that the virus infections had return to their usual epidemiologic characteristics the year after.
This new multiplex technique dramatically shortens hands-on time. The results for 15 viral pathogens can be obtained in about one day. It is also much simpler than conventional routine techniques, which need many and various steps and a wide range of technical competence (culture, PCR, immunofluorescence). While multiplex assays are more expensive than monoplex PCRs, laboratories can still choose to perform single PCRs in a strategic stepwise fashion. This strategy may appear to be less expensive but it needs regular revision to take into account the spread of virus(es). Moreover, if several monoplex PCRs are needed, this then can become more expensive than multiplex PCRs. It must also be remembered that diagnosis of co-infections is more uncertain due to a smaller number of viruses tested.
In conclusion, our results indicate that multiplex PCR techniques can be used in the routine etiological diagnosis of respiratory infections. The MLPA-Respifinder® 15 assay revealed that a high percentage of samples from children attending the emergency unit with ARIs during the autumn and winter of 2010/2011contained at least one virus, and that co-infections were very common. RSV and RV were the most prevalent pathogens, particularly in the youngest children, and co-infections were associated with more severe respiratory symptoms. The epidemiology of viruses responsible for ARIs had returned to its usual characteristics one year after the AH1N1pdm09 pandemic.
Funding
None.
Competing interests
None of the authors of this manuscript has any commercial or other association that might pose a conflict of interest (e.g., pharmaceutical stock ownership, consultancy).
Ethical approval
Not required.
Acknowledgement
The English text was checked by Dr Owen Parkes.
References
- 1.Nicholson K.G., McNally T., Silverman M., Simons P., Stockton J.D., Zambon M.C. Rates of hospitalisation for influenza, respiratory syncytial virus and human metapneumovirus among infants and young children. Vaccine. 2006;24:102–108. doi: 10.1016/j.vaccine.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Massin M.M., Montesanti J., Gerard P., Lepage P. Spectrum and frequency of illness presenting to a pediatric emergency department. Acta Clin Belg. 2006;61:161–165. doi: 10.1179/acb.2006.027. [DOI] [PubMed] [Google Scholar]
- 3.Schouten J.P., McElgunn C.J., Waaijer R., Zwijnenburg D., Diepvens F., Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:e57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansuy J.M., Mengelle C., Da Silva I., Grog I., Saune K., Izopet J. Performance of a rapid molecular multiplex assay for the detection of influenza and picornaviruses. Scand J Infect Dis. 2012;44:963–968. doi: 10.3109/00365548.2012.704150. [DOI] [PubMed] [Google Scholar]
- 5.Simoes E.A., Patel C., Sung W.K., Lee C.W., Loh K.H., Lucero M. Pathogen chip for respiratory tract infections. J Clin Microbiol. 2013;51:945–953. doi: 10.1128/JCM.02317-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhat N., Tokarz R., Jain K., Haq S., Weatherholtz R., Chandran A. A Prospective Study of Agents associated with acute respiratory infection among young American Indian children. Pediatr Infect Dis J. 2013;32:e324–e333. doi: 10.1097/INF.0b013e31828ff4bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freymuth F., Vabret A., Cuvillon-Nimal D., Simon S., Dina J., Legrand L. Comparison of multiplex PCR assays and conventional techniques for the diagnostic of respiratory virus infections in children admitted to hospital with an acute respiratory illness. J Med Virol. 2006;78:1498–1504. doi: 10.1002/jmv.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruohola A., Waris M., Allander T., Ziegler T., Heikkinen T., Ruuskanen O. Viral etiology of common cold in children, Finland. Emerg Infect Dis. 2009;15:344–346. doi: 10.3201/eid1502.081468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brittain-Long R., Westin J., Olofsson S., Lindh M., Andersson L.M. Prospective evaluation of a novel multiplex real-time PCR assay for detection of fifteen respiratory pathogens-duration of symptoms significantly affects detection rate. J Clin Virol. 2010;47:263–267. doi: 10.1016/j.jcv.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papadopoulos N.G. Do rhinoviruses cause pneumonia in children? Paediatr Respir Rev. 2004;5:S191–S195. doi: 10.1016/s1526-0542(04)90036-x. [DOI] [PubMed] [Google Scholar]
- 11.O’Callaghan-Gordo C., Bassat Q., Diez-Padrisa N., Morais L., Machevo S., Nhampossa T. Lower respiratory tract infections associated with rhinovirus during infancy and increased risk of wheezing during childhood. A cohort study. PLoS ONE. 2013;8:e69370. doi: 10.1371/journal.pone.0069370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stempel H.E., Martin E.T., Kuypers J., Englund J.A., Zerr D.M. Multiple viral respiratory pathogens in children with bronchiolitis. Acta Paediatr. 2009;98:123–126. doi: 10.1111/j.1651-2227.2008.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murtagh P., Giubergia V., Viale D., Bauer G., Pena H.G. Lower respiratory infections by adenovirus in children. Clinical features and risk factors for bronchiolitis obliterans and mortality. Pediatr Pulmonol. 2009;44:450–456. doi: 10.1002/ppul.20984. [DOI] [PubMed] [Google Scholar]
- 15.Jean A., Quach C., Yung A., Semret M. Severity and outcome associated with human coronavirus OC43 infections among children. Pediatr Infect Dis J. 2013;32:325–329. doi: 10.1097/INF.0b013e3182812787. [DOI] [PubMed] [Google Scholar]
- 16.Edwards K.M., Zhu Y., Griffin M.R., Weinberg G.A., Hall C.B., Szilagyi P.G. Burden of human metapneumovirus infection in young children. N Engl J Med. 2013;368:633–643. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cilla G., Onate E., Perez-Yarza E.G., Montes M., Vicente D., Perez-Trallero E. Viruses in community-acquired pneumonia in children aged less than 3 years old: high rate of viral coinfection. J Med Virol. 2008;80:1843–1849. doi: 10.1002/jmv.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dao C.N., Kamimoto L., Nowell M., Reingold A., Gershman K., Meek J. Adult hospitalizations for laboratory-positive influenza during the 2005–2006 through 2007–2008 seasons in the United States. J Infect Dis. 2010;202:881–888. doi: 10.1086/655904. [DOI] [PubMed] [Google Scholar]
- 19.Pabbaraju K., Tokaryk K.L., Wong S., Fox J.D. Comparison of the Luminex xTAG respiratory viral panel with in-house nucleic acid amplification tests for diagnosis of respiratory virus infections. J Clin Microbiol. 2008;46:3056–3062. doi: 10.1128/JCM.00878-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fairchok M.P., Martin E.T., Chambers S., Kuypers J., Behrens M., Braun L.E. Epidemiology of viral respiratory tract infections in a prospective cohort of infants and toddlers attending daycare. J Clin Virol. 2010;49:16–20. doi: 10.1016/j.jcv.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin E.T., Kuypers J., Wald A., Englund J.A. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respir Viruses. 2012;6:71–77. doi: 10.1111/j.1750-2659.2011.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Silva E.R., Pitrez P.M., Arruda E., Mattiello R., Sarria E.E., de Paula F.E. Severe lower respiratory tract infection in infants and toddlers from a non-affluent population: viral etiology and co-detection as risk factors. BMC Infect Dis. 2013;13:41. doi: 10.1186/1471-2334-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semple M.G., Cowell A., Dove W., Greensill J., McNamara P.S., Halfhide C. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382–386. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aberle J.H., Aberle S.W., Pracher E., Hutter H.P., Kundi M., Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatr Infect Dis J. 2005;24:605–610. doi: 10.1097/01.inf.0000168741.59747.2d. [DOI] [PubMed] [Google Scholar]
- 25.Grondahl B., Ankermann T., von Bismarck P., Rockahr S., Kowalzik F., Gehring S. The 2009 pandemic influenza A(H1N1) coincides with changes in the epidemiology of other viral pathogens causing acute respiratory tract infections in children. Infection. 2013;42:303–308. doi: 10.1007/s15010-013-0545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]