Summary
Background
A re-emergence of scarlet fever has been noted in Hong Kong, South Korea, and England, UK, since 2008. China also had a sudden increase in the incidence of the disease in 2011. In this study, we aimed to assess the epidemiological changes before and after the upsurge. We also aimed to explore the reasons for the upsurge in disease in 2011, the epidemiological factors that contributed to it, and assess how these could be managed to prevent future epidemics.
Methods
In this observational study, we extracted the epidemiological data for all cases of scarlet fever between 2004 and 2016 in China from the Chinese Public Health Science Data Center, the official website of National Health Commission of the People's Republic of China, and the National Notifiable Infectious Disease Surveillance System. These data had been collected from 31 provinces and regions in China and included geographical, seasonal, and patient demographic information. We used descriptive statistical methods and joinpoint regression to examine the spatiotemporal patterns and annual percentage change in incidence of the upsurge of disease across China.
Findings
Between Jan 1, 2004, and Dec 31, 2016, 502 723 cases of scarlet fever, with ten fatalities, were reported in China, resulting in an annualised average incidence of 2·8807 per 100 000 people. The annual average incidence increased from 1·457 per 100 000 people in 2004 to 4·7638 per 100 000 people in 2011 (incidence rate ratio [IRR] 3·27, 95% CI 3·22–3·32; p<0·0001), peaking in 2015 (5·0092 per 100 000 people). The annual incidence after the 2011 upsurge of scarlet fever, between 2011 and 2016, was twice the average annual incidence reported between 2004 and 2010 (4·0125 vs 1·9105 per 100 000 people; IRR 2·07, 95% CI 2·06–2·09; p<0·0001). Most cases were distributed in the north, northeast, and northwest of the country. Semi-annual patterns were observed in May–June and November–December. The median age at onset of disease was 6 years, with the annual highest incidence observed in children aged 6 years (49·4675 per 100 000 people). The incidence among boys and men was 1·54 greater than that among girls and women before the upsurge, and 1·51 times greater after the upsurge (p<0·0001 for both). The median time from disease onset to reporting of the disease was shorter after the upsurge in disease than before (3 days vs 4 days; p=0·001).
Interpretation
To our knowledge, this is the largest epidemiological study of scarlet fever worldwide. The patterns of infection across the country were similar before and after the 2011 upsurge, but the incidence of disease was substantially higher after 2011. Prevention and control strategies being implemented in response to this threat include improving disease surveillance and emergency response systems. In particular, the school absenteeism and symptom monitoring and early-warning system will contribute to the early diagnosis and report of the scarlet fever. This approach will help combat scarlet fever and other childhood infectious diseases in China.
Funding
National Key R&D Plan of China Science and key epidemiological disciplines of Zhejiang Provincial Health of China.
Introduction
Scarlet fever is a bacterial infection caused by Streptococcus pyogenes (group A streptococcus)1 that usually affects children aged 5–15 years. Scarlet fever was a common childhood disease in the 18th and 19th centuries throughout Europe and the USA.2 However, the incidence of scarlet fever declined in the 20th century with the use of effective antibiotics, along with improvements in hygiene and nutrition.3, 4 In the past decade, there was a sudden increase in the incidence of the disease in several countries and areas, including in Asia (eg, Vietnam in 2009,2 Hong Kong in 2011,5 and South Korea in 20156) and Europe (eg, the UK in 20141, 7). To date, the studies of these outbreaks have been small, but overall they have shown that children younger than school age (ie, younger than 10 years) have the greatest risk of infection, and that seasonal patterns and group A streptococcus emm gene types are varied.
After implementation of the expanded programme on immunisation in 1978 and the enlarged national immunisation programme in 2008, the morbidities of all childhood vaccine-preventable infectious diseases in China decreased.8, 9 By contrast, reports of scarlet fever, which does not have a vaccine, have shown a substantial increase in the past 7 years in China.10 The reasons for this increase could include microbial, host, and meteorological factors. Unlike other countries that have also had an upsurge, China had a sudden increase in the number of susceptible children as a result of the partial two-child policy in 2011 and the universal two-child policy in 2015.11, 12 These changes resulted in a substantial increase of 1 million births from 2015 to 2016.12 As such, scarlet fever is an increasing threat to the growing child population in China.
Research in context.
Evidence before this study
We searched PubMed with the keywords “scarlet fever”, “epidemiology”, “surveillance”, “outbreak”, “re-emergence”, and “Group A streptococcus” for all journal articles written in English before Jan 31, 2018. Scarlet fever epidemics have been increasingly reported in east Asia (ie, Vietnam, Hong Kong, South Korea, and China) and Europe (England, UK) since 2008. One study indicated that England was having a sudden and widespread increase of scarlet fever, with the highest incidence of the disease in nearly 50 years. However, these reports have assessed epidemiological parameters in a small population, and so have insufficient power to compare changes in the incidence of the disease after its re-emergence.
Added value of this study
To the best of our knowledge, there have been no complete, systematic, multiregional, and comprehensive investigations of the increasing pattern of scarlet fever across China or epidemiological changes after the 2011 upsurge completed to date. This childhood disease started to surge in 2011 and to occur at increased incidence for 6 consecutive years, with an annual percentage change of 9% (95% CI 4·1–14·1) in the whole of China. The incidence of scarlet fever was twice as high after the upsurge. Increased incidence was identified throughout China, and the fastest increases were seen in Hainan, Guangdong, and Hunan Provinces in the south of China.
Implications of all the available evidence
The re-emergence of scarlet fever has become a great challenge for the Chinese Government. Since partial relaxation of the one-child policy in 2011 and then the universal two-child policy in 2015, the child population, which is the most susceptible to this disease, increased by 1 million in 2016. Considering the findings of this study, effective prevention and control strategies should be expanded for childhood diseases that cannot be prevented with a vaccine—eg, scarlet fever, infectious diarrhoea, and acute haemorrhagic conjunctivitis. The Chinese Government now has the ability to enforce school-based absenteeism and symptom surveillance and early-warning systems for these infectious diseases, and to prepare additional medical resources to respond to this potential threat in the future.
In response to the 2003 severe acute respiratory syndrome (SARS) outbreak, China established the National Notifiable Infectious Disease Surveillance System (NNIDSS) for 39 infectious diseases.9 Scarlet fever is the only group A streptococcal disease recorded in the NNIDSS to date. In this Article, we describe the epidemiological patterns of scarlet fever in China from 2004 to 2016, focusing on the changes in the disease patterns before and after the upsurge in 2011. To our knowledge, this is the largest epidemiological study that has been done on scarlet fever worldwide.
Methods
Case definitions and data sources
The Chinese Government established an internet-based NNIDSS in 2003. To date, this surveillance system covers a population of 1·3 billion people from 31 provinces and regions in China.9 39 notifiable infectious diseases are monitored by use of this surveillance system and they are divided into three categories—classes A, B, and C—all of which must be reported within a specified timeframe. All class A infectious diseases and the class B diseases pulmonary anthrax and SARS should be reported to the surveillance system within 2 h of diagnosis, whereas the other class B and the class C infectious diseases should be reported within 24 h. Scarlet fever is a class B notifiable infectious disease in China. The definitions for probable, clinical, and laboratory-confirmed infections of scarlet fever should meet the criteria issued by the Ministry of Health of the People's Republic of China (appendix pp 1–4).
According to the 2004 Chinese Infectious Diseases Law, clinicians must complete a standardised infectious diseases card and report to the NNIDSS when they identify any probable, clinical, or laboratory-confirmed case of scarlet fever within 24 h of diagnosis. The local epidemiologist will do a field investigation once they have received the disease card using a standardised form, which includes basic demographic information (sex, date of birth, occupation, and living address); case classification; date of symptom onset, diagnosis, and death (if applicable); and clinical outcome. The epidemiologist then records their investigational data in the NNIDSS once they have finished their field investigation.
Data extraction
In this observational study, we extracted data from the Chinese open access notifiable infectious disease report database (available from the Chinese Public Health Science Data Center and the official website of National Health Commission of the People's Republic of China13) and from the NNIDSS covering the period from Jan 1, 2004, to Dec 31, 2016.
This study was done according to the principles and guidelines of the Declaration of Helsinki, and was approved by the Research Ethics Committee of the Zhejiang Provincial Center for Disease Control and Prevention. All initial information identifying patients was anonymised in this study.
We extracted data on scarlet fever, including the number of cases and deaths, the incidence and mortality for scarlet fever, and patient data on age, sex, and date of disease onset, diagnosis, and death (if applicable). We collected all data available for the study period and no exclusion criteria were used. We stratified the data by 31 provinces and areas and, to assess the epidemiological features of the disease, we substratified the study period into timeframes before (2004–10) and after (2011–2016) the upsurge of disease. Population data are from the National Bureau of Statistics of the People's Republic of China and are updated at the end of every year.
Statistical analysis
We defined the incidence (per 100 000 people) as the number of annual cases divided by the population size. We defined mortality (per 100 000 people) as the number of fatalities per year divided by the total population. The case–fatality rate (per 100 people) was the number of annual deaths divided by the number of annual cases.
We estimated the incidence rate ratio (IRR) as the mean incidence for the period 2011–16 divided by the mean incidence for the period 2004–10, and their 95% CIs were estimated by use of the method proposed by Armitage and Berry.14 To analyse the spatiotemporal pattern of the incidence of scarlet fever, we used the ring map toolbox in ArcGIS 10.2 (Esri Inc, Redlands, CA, USA). To observe the seasonality in each province or area, we first standardised the monthly incidence of scarlet fever for different areas and then applied a radar chart to display the z score of incidence in each area by using R package fmsb.15 Each radar chart has 12 spikes representing the 12 months of the year, and different colours indicate different years.9
We used Joinpoint regression software (version 4.5.0.1; appendix p 5), developed by the National Cancer Institute, to examine the annual percentage change of the crude incidence rate from 2004 to 2016.9 We used the t test to assess whether an annual percentage change was significantly different from zero; p values were from a two-sided test, with a value of 0·05 indicating significance. We used a parametric method as joinpoint regression (parametric and empirical quantile types) to calculate the 95% CIs. In describing patterns, an increase or decrease is proven when the slope of annual percentage change is significant (p<0·05). A stable trend refers to a non-significant annual percentage change (p≥0·05). We used the χ2 test to compare the different proportions of infected people by sex, year, and occupation. We used a Mann–Whitney U test to compare the time intervals between the two epidemic periods (2004–10 and 2011–16).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
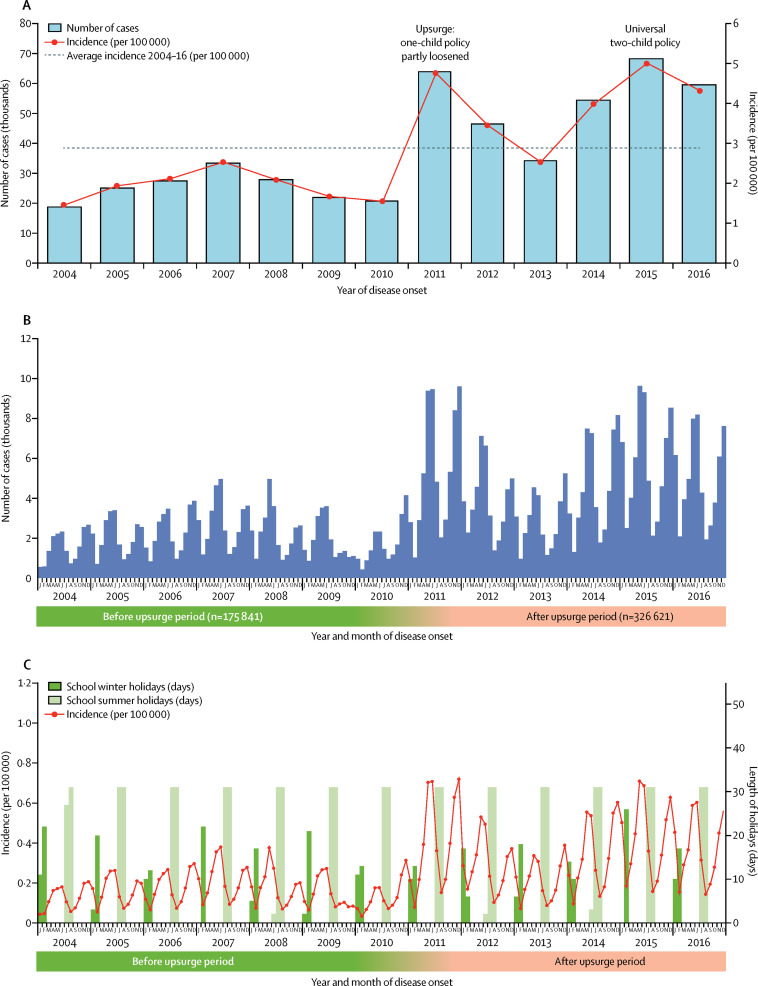
From Jan 1, 2004, to Dec 31, 2016, 502 723 cases of scarlet fever were reported, resulting in an annualised average incidence of 2·8807 per 100 000 people (table ). In 2004, when national surveillance started, the average incidence of scarlet fever in the 31 provinces and areas monitored was 1·457 per 100 000 people and the first upsurge in cases occurred in 2011, with a significantly higher incidence of 4·7638 per 100 000 people (IRR 3·27, 95% CI 3·22–3·32; p<0·0001; figure 1A ). After the upsurge in 2011, the annual incidence of scarlet fever remained higher than average (calculated across the whole period 2004–16), except for in 2013 (figure 1A). The highest incidence of scarlet fever was observed in 2015 (5·0092 per 100 000 people), which then decreased slightly in 2016 (4·3247 per 100 000 people; figure 1A). In the period after the upsurge of the disease, 2011–16, the average incidence per year was twice that of the period 2004–10 (4·0125 vs 1·9105 per 100 000 people; IRR 2·07, 95% CI 2·06–2·09; p<0·0001; table).
Table.
Annual incidence of scarlet fever by 31 surveillance provinces or areas in China, 2004–16
| Number of cases 2004–16 | Number of deaths 2004–16 |
Annualised mean incidence (per 100 000 people) |
Incidence rate ratio (95% CI) | Annual percentage change (95% CI); p value | |||
|---|---|---|---|---|---|---|---|
| 2004–16 | 2004–10 | 2011–16 | |||||
| Beijing | 34 044 | 0 | 14·0412 | 10·9994 | 17·5901 | 1·60 (1·57 to 1·64) | 3·1% (−4·3 to 11·1); 0·40 |
| Tianjin | 12 311 | 0 | 7·2411 | 5·152 | 9·6784 | 1·89 (1·82 to 1·96) | 8·8% (3·9 to 13·8); <0·001 |
| Hebei | 30 472 | 0 | 3·2687 | 2·1514 | 4·5723 | 2·13 (2·08 to 2·18) | 8·5% (2·2 to 15·3); 0·013 |
| Shanxi | 23 466 | 0 | 5·1059 | 3·2866 | 7·2283 | 2·20 (2·14 to 2·26) | 8·3% (2·3 to 14·6); 0·0102 |
| Inner Mongolia | 25 973 | 0 | 8·1087 | 5·4855 | 11·1690 | 2·04 (1·98 to 2·09) | 8·4% (3·1 to 13·9); 0·0044 |
| Liaoning | 54 680 | 1 | 9·7239 | 8·696 | 10·9230 | 1·26 (1·24 to 1·28) | 2·3% (−3·1 to 8); 0·38 |
| Jilin | 26 486 | 0 | 7·4120 | 4·7225 | 10·5497 | 2·23 (2·17 to 2·29) | 8·4% (−1·1 to 18·8); 0·08 |
| Heilongjiang | 43 555 | 4 | 8·7377 | 7·5102 | 10·1698 | 1·35 (1·33 to 1·38) | 2·7% (−4·4 to 10·3); 0·43 |
| Shanghai | 22 610 | 0 | 7·7537 | 3·3433 | 12·8991 | 3·86 (3·73 to 3·99) | 15·9% (6·9 to 25·7); <0·001 |
| Jiangsu | 18 901 | 1 | 1·8620 | 1·2228 | 2·6077 | 2·14 (2·07 to 2·20) | 11·0% (5·6 to 16·7); <0·001 |
| Zhejiang | 17 577 | 0 | 2·5342 | 1·4084 | 3·8477 | 2·74 (2·65 to 2·83) | 13·9% (7·7 to 20·5); <0·001 |
| Anhui | 5556 | 0 | 0·7033 | 0·413 | 1·0420 | 2·53 (2·39 to 2·68) | 13·1% (7 to 19·5); <0·001 |
| Fujian | 3636 | 0 | 0·7533 | 0·4355 | 1·1240 | 2·59 (2·41 to 2·78) | 13·1% (7·2 to 19·4); <0·001 |
| Jiangxi* | 511 | 0 | 0·0876 | 0·0377 | 0·1458 | 3·86 (3·13 to 4·75) | 28·4% (15·5 to 42·7); <0·001 |
| Shandong | 37 992 | 1 | 3·0323 | 1·3841 | 4·9552 | 3·59 (3·50 to 3·67) | 17·9% (11·7 to 24·4); <0·001 |
| Henan | 13 013 | 0 | 1·0594 | 0·7186 | 1·4571 | 2·03 (1·96 to 2·10) | 8·8% (3·2 to 14·8); 0·0051 |
| Hubei | 5852 | 0 | 0·7787 | 0·3876 | 1·2351 | 3·20 (3·02 to 3·39) | 17·3% (11·1 to 23·8); <0·001 |
| Hunan | 4865 | 1 | 0·5649 | 0·1918 | 1·0001 | 5·24 (4·87 to 5·64) | 22·6% (13·5 to 32·4); <0·001 |
| Guangdong | 13 853 | 0 | 1·0269 | 0·3537 | 1·8123 | 5·16 (4·93 to 5·40) | 22·3% (15·9 to 29·1); <0·001 |
| Guangxi | 3877 | 0 | 0·6306 | 0·4725 | 0·8150 | 1·73 (1·62 to 1·85) | 4·6% (−1·4 to 10·9); 0·12 |
| Hainan | 39 | 0 | 0·0337 | 0·0052 | 0·0670 | 13·34 (4·11 to 43·33) | 17·9% (5·9 to 31·3); 0·0063 |
| Chongqing | 4813 | 0 | 1·2721 | 0·9406 | 1·6588 | 1·78 (1·68 to 1·88) | 7·0% (1·4 to 12·9); 0·019 |
| Sichuan | 18 461 | 0 | 1·7356 | 1·4671 | 2·0488 | 1·40 (1·36 to 1·44) | 2·3% (−2·1 to 6·8); 0·28 |
| Guizhou | 6021 | 0 | 1·2892 | 0·8476 | 1·8044 | 2·13 (2·02 to 2·24) | 10·1% (6·4 to 14); <0·001 |
| Yunnan | 13 611 | 1 | 2·2786 | 1·6027 | 3·0671 | 1·92 (1·85 to 1·99) | 7·7% (3·3 to 12·2); <0·001 |
| Tibet | 791 | 0 | 2·0485 | 1·658 | 2·5040 | 1·51 (1·31 to 1·74) | 2·8% (−4·6 to 10·7); 0·43 |
| Shaanxi† | 15 550 | 0 | 3·1821 | 2·0742 | 4·4746 | 2·16 (2·09 to 2·24) | 62·4% (−0·2 to 164·2); 0·050 |
| Gansu | 10 785 | 0 | 3·2003 | 2·2733 | 4·2819 | 1·89 (1·81 to 1·96) | 8·3% (4·3 to 12·5); <0·001 |
| Qinghai | 3252 | 1 | 4·4469 | 4·0906 | 4·8627 | 1·19 (1·12 to 1·28) | 2·6% (−3·4 to 8·9); 0·38 |
| Ningxia Hui Autonomous Region | 7885 | 0 | 9·5393 | 5·4613 | 14·2970 | 2·61 (2·48 to 2·74) | 12·4% (6·8 to 18·3); <0·001 |
| Xinjiang Uygur Autonomous Region | 22 285 | 0 | 7·8001 | 5·1676 | 10·8713 | 2·09 (2·04 to 2·15) | 10·2% (6 to 14·7); <0·001 |
| Overall | 502 723 | 10 | 2·8807 | 1·9105 | 4·0125 | 2·07 (2·06 to 2·09) | 9·0% (4·1 to 14·1); 0·002 |
Annual percentage change was significant during 2004–14.
Annual percentage change was significant during 2014–16.
Figure 1.
The incidence and number of scarlet fever cases reported in China
(A) Number of cases and incidence by year. (B) Number of cases by month. (C) Incidence in school holidays.
Nationally, people became infected with scarlet fever throughout the year, with semi-annual seasonal peaks (figure 1B). The first seasonal pattern started in February, at the time when the spring semester began in schools. The number of cases then peaked in May and June, and decreased in July and August. The second seasonal pattern was observed beginning in early September, when the autumn semester in schools started. Disease activity then increased to a second peak in November and December. The incidence of scarlet fever clearly decreased during the school holidays in spring (January–February) and summer (July–August; figure 1C).
We divided the 31 provinces into seven geographical areas to identify the different seasonal patterns by use of radar charts (appendix pp 6–12). In northern China, the two periods in which the incidence of scarlet fever clearly increased were November–December and May–June. In central and eastern China, two clear peaks in incidence still existed, but the peak in winter was slightly later in the year, in December–January. In southwest China, there was only one increase in the incidence, in the period of May–June. And in south China, Guangdong and Guangxi provinces had a clearer two-peak pattern during 2014–16 than did southwest China. Hainan province, also in south China, had few cases of scarlet fever, and subsequently the seasonality of the disease was not clear. We used a heat map to show time series of cases standardised by the proportion of annual cases. The seasonal pattern in the heat map was similar to that in the radar chart (appendix p 13).
Seasonal trends were similar in the periods before and after the upsurge of scarlet fever incidence. However, the incidence was much higher in the spring than in the winter in 2005, 2007, 2008, and 2009. The magnitude of seasonal rises were similar after the upsurge. During the periods both before and after the upsurge, the highest incidence of the year was seen in January, while the smallest increase in IRR was seen in April compared with all other months (appendix p 14).
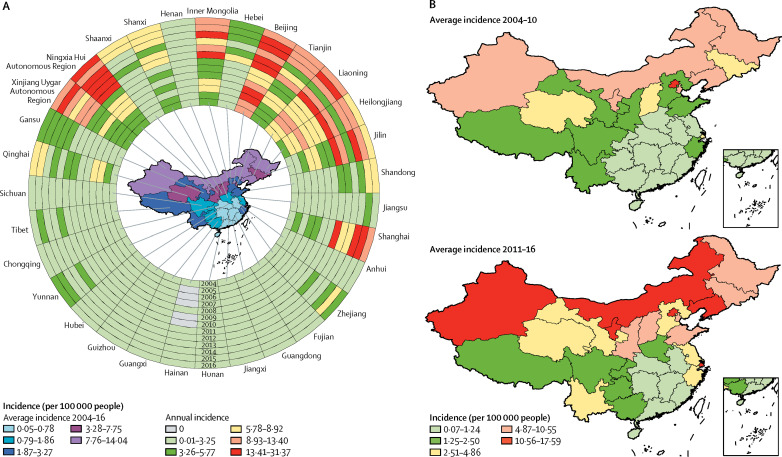
Looking at the geographical distribution of scarlet fever across China in 2004–16, cases were predominantly reported in the north, northeast, and northwest of the country. In the ring map (figure 2A ), the northern areas from the Jilin province to the Xinjiang Uygur Autonomous Region (in a counter-clockwise direction) had a pattern of steadily increasing scarlet fever incidence from the innermost ring in 2004 to the outermost ring in 2016 (apart from the expected peaks for 2011 and 2015, and in the Henan province where the annual incidence remained steady at 0·01–3·25). Across the whole study period, the provinces with a latitude higher than 33·4 degrees north had a higher annual incidence than those at lower latitudes. The provinces or areas with the highest average annual incidence between 2004 and 2016 were Beijing (14·0412 per 100 000 people), Liaoning province (9·7239 per 100 000 people), and Ningxia Hui Autonomous Region (9·5393 per 100 000 people; table). By contrast, the provinces and areas in the south and southeast maintained a low annual incidence. The areas and provinces with the lowest recorded average annual incidence between 2004 and 2016 were Hunan province (0·5649 per 100 000 people), Jiangxi province (0·0876 per 100 000 people), and Hainan province (0·0337 per 100 000 people).
Figure 2.
Spatiotemporal distribution of scarlet fever cases in China, 2004–16
(A) Annual incidence of scarlet fever per 100 000 people in the 31 Chinese provinces investigated. The 13 rings contain data for each year studied, with the innermost ring bearing data for 2004, and moving outwards through the years to the outermost ring bearing data for 2016. (B) Choropleth maps of the average annual incidence of scarlet fever, by region, based on the annual incidence per 100 000 people in China before (2004–10) and after (2011–16) the upsurge in the incidence of infections.
The geographical distribution of scarlet fever was similar before and after the 2011 upsurge, during which scarlet fever was predominantly distributed in the north, northeast, and northwest of the country (figure 2B). The annual percentage change across the whole of China was 9·0% (95% CI 4·1–14·1; table). The most substantial increases in incidence were in the southern regions, in the Hunan (22·6%, 13·5–32·4), Guangdong (22·3%, 15·9–29·1), and Hainan provinces (17·9%, 5·9–31·3). Comparing the incidence before and after the upsurge, the IRRs for all 31 provinces are greater than 1, and range from 1·19 (95% CI 1·12–1·28) in the Qinghai province to 13·34 (4·11–43·33) in the Hainan province. The variation in incidence in the Hainan province is quite high because the region did not report any cases of the disease for some years before the upsurge, resulting in an unstable estimation of IRR over the period we were investigating (table).
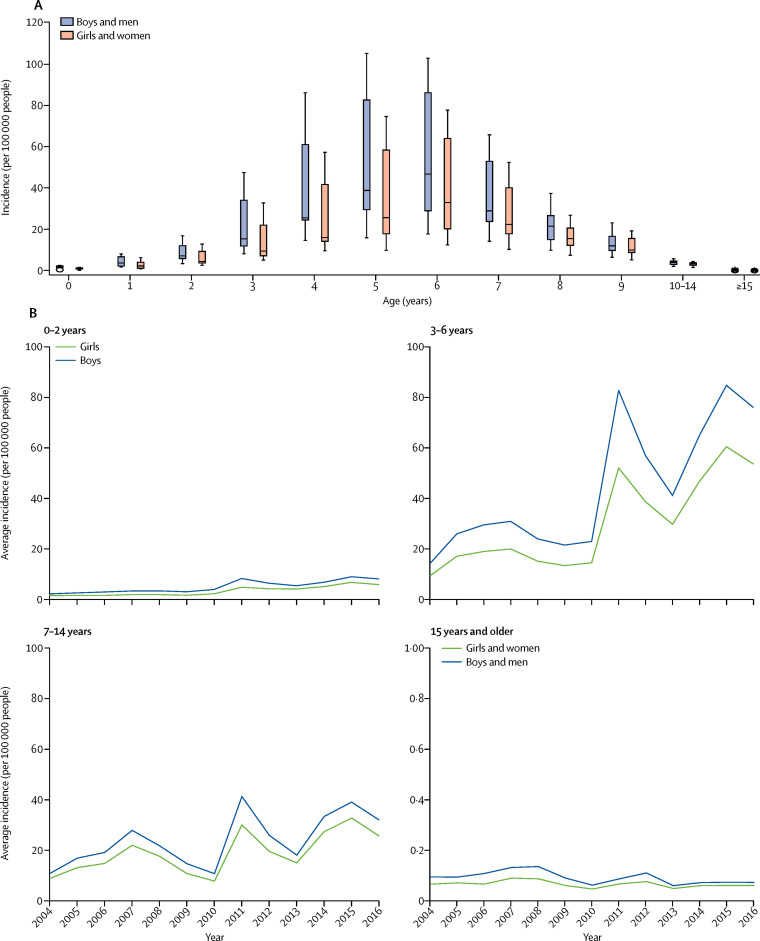
We observed that people of all ages had been infected with scarlet fever. The median age at onset of disease was 6 years (ranging from 4 days to 83 years; data not shown). The average annual incidence was highest among children aged 6 years (49·4675 per 100 000 people) and was lowest among people aged 80–85 years (0·0096 per 100 000 people; appendix pp 15–16).
We analysed the pattern of reported scarlet fever cases by the age and sex of the patients. Overall, the annual incidence was higher among men and boys than among women and girls in all age groups (male-to-female ratio 1·54 for before the upsurge and 1·51 after the upsurge, p<0·0001 for both; figure 3A , appendix p 17–19). The annual incidence among patients aged 0–2 years and 3–6 years peaked in 2011 and 2015 (figure 3B), whereas the incidence among those aged 7–14 years peaked at three timepoints, in 2007, 2011, and 2015 (figure 3B). By contrast, the annual incidence among those aged 15 years and older peaked in 2008 for men and in 2007 for women and for both sexes in 2012, but the incidence after 2008 remained substantially lower for this age group than for the other age groups (figure 3B).
Figure 3.
Sex-specific distribution of scarlet fever cases by age in China, 2004–16
(A) Age and sex distribution of the number of cases of scarlet fever over the entire study period. The boxes represent the 50% of cases distribution and the lines indicate the IQR. (B) The annualised average incidence, by patient age group and sex. The scale for those aged 15 years and older is from 0–1 because the average incidence is so low.
At the time of the 2011 upsurge, the biggest increase in the incidence of scarlet fever appeared to be among children aged 7 years and younger. The most substantial increase in incidence after the upsurge was among children aged 3 years (IRR 3·25, 95% CI 3·18–3·33) and 4 years (3·25, 3·19–3·30; appendix pp 15–16). For children of school age (ie, aged 7–14 years), the average annual incidence in 2011–16 was double that in 2004–10 (figure 3B). In this age group, comparing the incidence before and after the upsurge in disease, the greatest increase in incidence was seen among children aged 7 years (IRR 2·25) and the smallest increase was seen among those aged 10–14 years (IRR 1·38; appendix pp 15–16). The incidence after 2011 was two times higher in men than in women (appendix pp 17–19). However, the male-to-female ratio between the two periods was similar (1·54 vs 1·51; p=0·053; appendix pp 17–19).
Occupational data were available for 18 911 patients with scarlet fever. We divided these patients into two groups: the child group (<15 years) and the adult group (≥15 years). 18 663 patients were in the child group, of whom 14 761 (79·1%) attended kindergarten and school. 248 patients were in the adult group, of whom 63 (25·4%) were students and 54 (21·8%) were workers. The occupational profile of patients who were reported to have scarlet fever was similar before and after the upsurge; although, the proportion of children in kindergarten with the disease in the period was slightly higher after the upsurge than before the upsurge (45·63% vs 40·14%; p<0·0001), whereas the opposite was true for pre-nursery-school age children (19·53% vs 25·21%; p<0·0001; appendix p 20).
During the study period, 502 723 cases of scarlet fever were reported in China, but only ten reported cases resulted in death. There were no significant differences in overall mortality (0·0001 vs 0·000112 per 100 000 people per year; p=0·953) and case–fatality rate (0·0023% vs 0·0018%; p=0·748) in the periods before and after the 2011 upsurge in the whole of China (appendix pp 21–22).
Of the ten fatal cases of scarlet fever, nine patients had demographic information available. All nine patients were aged 4–15 years, with a mean age of 8·5 years (SD 4), and the group comprised six boys and three girls (appendix p 23). The median number of days from onset to diagnosis was 4 days (range 0–5), the median number of days from onset to death was 7 days (range 1–28), and the median number of days from diagnosis to death was 4 days (range 0–25). The median number of days from onset to diagnosis, onset to report date, and diagnosis to report date in the period after the upsurge of disease was significantly shorter than it had been before the upsurge (all p<0·05; appendix p 24). For clinically confirmed cases, the median time from disease onset to reporting the disease decreased from 4 days in 2004–10 to 3 days in 2011–16 (p=0·001; appendix p 24).
Discussion
Using a 13-year national surveillance dataset, we found that the resurgence of scarlet fever in China started in 2011. To the best of our knowledge, this study has the largest and most comprehensive population coverage of any reported to date. Our study clearly shows patterns in disease incidence across the years, including changes in spatial and seasonal patterns, age-specific incidence, and fatalities before and after the resurgence of the disease.
A substantial increase in the incidence of scarlet fever has been seen in east Asia and the UK since 2008.1, 3, 5, 6, 7 This study has shown that China also had a striking increase in the incidence of scarlet fever in 2011 that coincided with a resurgence of the disease in Hong Kong and South Korea, whereas England did not have an increase in incidence until 2014. In China, the average annual incidence of scarlet fever in the period after the upsurge, between 2011 and 2016, was double that reported before the upsurge (2004–11). However, the highest annual incidence in China, which was in 2015 (5·0092 per 100 000 people; figure 1A), is much smaller than the incidence seen in China during the scarlet fever epidemic in 1958 (27·51 per 100 000 people per year).16 Additionally, the highest incidence in China in the current resurge of scarlet fever was much lower than the incidences reported in the other countries (eg, 33·2 per 100 000 people in the UK;1, 7 18·1 per 100 000 people in Hong Kong;3 and 13·7 per 100 000 people in South Korea6).
Although the predominant group A streptococcus emm gene types are varied in China (emm12, emm1),16, 17, 18 South Korea (emm3, emm28),6 and England (emm3, emm12),1, 19 all these countries had the same challenges of scarlet fever re-emergence. Scientists have found that one of the possible causes of the scarlet fever upsurge is that S pyogenes is expanding from a single clonal lineage to multiclonal lineages.1, 16, 20 In China, a report16 has indicated that the emm12 gene had highly diversified clones with a high macrolide resistance. Furthermore, the emm gene types and mobile elements were found to be more dispersed geographically and annually than previously thought.16, 21 The diversity in emm gene types resulted in no cross-immunity to the new strains or linkages circulating in China.
A second important reason for the upsurge in scarlet fever in China was a natural cyclical pattern of scarlet fever. In the mid-19th century, scarlet fever epidemics were found to follow 4-year pattern, but this pattern disappeared during the 20th century with the widespread application of antibiotics. In China, over the past 66 years, scarlet fever epidemics have had four stages, including high incidence (1950–79), decrease (1980–94), low incidence (1995–2010), and resurgence (2011–16).16 Through these four stages, the disease cycle has been approximately every 6 years.16 The 2011 upsurge in the incidence of scarlet fever could signal a return to a high-incidence stage of the disease.16 A third reason might be an increased number of susceptible children in China after the loosening of the one-child policy, which started in 2011.11, 12 Consistent with this idea, the incidence of childhood infectious diseases that are not preventable by vaccine (eg, diarrhoea) has increased in the past 5 years. Finally, improvements in early surveillance and warning could have been contributed to by increased reporting, as has been seen with the increased diagnosis of infectious diseases in China since the NNIDSS was created.9, 22 However, the reasons for resurgence of scarlet fever in Hong Kong, South Korea, and England cannot be fully explained by these same factors, and further studies are needed.
The annual incidence of scarlet fever in China varied across the 31 provinces investigated, with the highest incidences in the north, and the lowest incidences in the south. This difference is due to several factors. First, differences in meteorological effects could partly explain the variation.23 In northern China, people stay indoors more with closed windows and doors during the cold season (October–April), which leads to bad ventilation and increases the chance of infection. Second, site-to-site differences in risk factors might have been present, such as long-term exposure to severe air pollutants in northern regions, which could have been a risk factor for infection.24 Finally, group A streptococcus epidemiological surveillance has shown that the proportion of group A streptococcus emm12 gene type strains was gradually replaced by emm1 gene types in some areas in the north of China from 2011 to 2014, leading to minimal cross-immunity.16, 25
Our age profiling of scarlet fever infections is consistent with those from other countries, with scarlet fever commonly affecting children younger than 10 years.1, 3, 5, 16 The paucity of infections among children younger than 6 months was most likely due to protection through maternal antibodies. The highest incidence of scarlet fever was found among children aged 6 years and the pattern of increasing incidence was apparent in children aged 3–4 years. This pattern could be partly attributable to the paucity of herd immunity among children aged 3–6 years to group A streptococcus infection;16 another cause could be restricted protection conferred by antibodies toward the M protein, with little cross-immunity toward other types of M protein.2, 26, 27, 28 Our findings indicate that boys and men have a higher risk of infection than girls and women, in all age groups, which could be attributable to more physical interactions or poorer personal hygiene among boys than among girls.29 Thus, in the future, specific strategies and measures should target preschool aged children and boys.28
Scarlet fever was observed to have a dual seasonal pattern in China, with different patterns in some regions. Areas in the north and south of China had semi-annual seasonal patterns peaking in May–June and November–December, whereas areas in southwest China had only one peak in May–June. Geographical differences in seasonal patterns could have been associated with climatic and demographic factors. Our study indicated that the incidence of scarlet fever decreased substantially during school holidays in China. The seasonal patterns in China were similar to those in Hong Kong and South Korea, but different from those in Poland (which peaked in January and March) and England (which peaked in February–March).1, 3, 6, 30 The length and timing of the school holidays might have contributed to the different seasonal patterns in east Asia and Europe.
Scarlet fever was a major cause of death for children in the 18th and 19th centuries, when mortality from scarlet fever was as high as 30%,31, 32 but since this period scarlet fever has become a milder disease, and rarely results in death.30, 33 Possible reasons for this reduction in mortality are the introduction of effective antibiotics and improve-ments in diagnosis and treatment.1, 2 Fatalities due to scarlet fever in China have become quite rare, with case–fatality rates of 0·0023% in the period 2004–10 and 0·0018% in the period 2011–16. For those patients who died, older age (mean 8·5 years) and delayed diagnosis (median 4 days since disease onset) could have contributed to the mortality risk. However, co-occurring diseases could also be a contributing factor to mortality risk, as discussed by Lau and colleagues' in their report of the scarlet fever outbreak in Hong Kong in 2011,33 in which two children with scarlet fever died who had underlying medical illnesses (co-infected with chickenpox). Generally, in the current phase of the disease in China, although the number of scarlet fever cases has increased, the disease severity was mild and did not change from before to after the upsurge.
This study has several limitations. First, the national surveillance system is not complete, and most patients with subclinical and asymptomatic cases of scarlet fever were not included because they did not seek medical consultation or were underdiagnosed. Second, data that were collected during the first year of roll-out of the national surveillance system are probably less reliable than those from the later years. Third, we could not calculate the directed age-standardised incidence of scarlet fever in China because the number of age-specific cases from the 31 provinces and areas were unavailable. Finally, we did not have data for emm gene typing of group A streptococcus and so were unable to monitor the antigen shift throughout China.
In conclusion, the overall incidence of scarlet fever has substantially increased in China since 2011. We analysed the epidemic and disease patterns from 31 different provinces and areas and compared them with patterns in other countries, before and after the 2011 upsurge. Although the demographic distribution and fatalities in China did not vary substantially between the periods before and after the upsurge, the seasonal patterns are similar to those in other Asian countries, but different from those in European countries.
In response to the prevalent risk of scarlet fever, the Chinese Government has drafted a series of technical projects to help health-care professionals improve the diagnosis and treatment of childhood diseases. Additionally, the government has improved infectious disease surveillance systems, including school absenteeism, symptom monitoring, and early-warning systems to enable prompt recognition of school outbreaks. Finally, national and regional emergency response systems have been established to rapidly carry out medical relief and epidemic control. All these measures are crucial to combat the threat of this re-emerging disease and other childhood infectious diseases that do not have vaccines in China.
Acknowledgments
Acknowledgments
We thank the hospitals, local health departments, and prefecture Center for Disease Control and Prevention in China for assistance in coordinating data collection. The views expressed are those of the authors and do not necessarily represent the policy of the Chinese Government. We would like to express our sincere gratitude to Kent M Suárez for his English editing. Requests for study data will be reviewed and considered by the corresponding authors. This study was supported by the National Key R&D Plan of China (2016YFC0202005) and key epidemiological disciplines of Zhejiang Provincial Health of China.
Contributors
YoL did the literature search and drafted the manuscript. L-WY contributed to data preparation and statistical analyses. YiL and WX collected and analysed data and generated the figures. SQ, NZ, ZY, and XG did the field investigation. S-LL and T-CC contributed equally and designed the study, did the statistical analyses, data interpretations, and revised the manuscript. All authors contributed to the development of the manuscript and approved the final draft.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Lamagni T, Guy R, Chand M. Resurgence of scarlet fever in England, 2014–16: a population-based surveillance study. Lancet Infect Dis. 2017;18:180–187. doi: 10.1016/S1473-3099(17)30693-X. [DOI] [PubMed] [Google Scholar]
- 2.Andrey DO, Posfay-Barbe KM. Re-emergence of scarlet fever: old players return? Expert Rev Anti Infect Ther. 2016;14:687–689. doi: 10.1080/14787210.2016.1195684. [DOI] [PubMed] [Google Scholar]
- 3.Lee CF, Cowling BJ, Lau EHY. Epidemiology of reemerging scarlet fever, Hong Kong, 2005–2015. Emerg Infect Dis. 2017;23:1707–1710. doi: 10.3201/eid2310.161456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gale AH. A century of changes in the mortality and incidence of the principal infections of childhood. Arch Dis Child. 1945;20:2–21. doi: 10.1136/adc.20.101.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh YC, Huang YC. Scarlet fever outbreak in Hong Kong, 2011. J Microbiol Immunol Infect. 2011;44:409–411. doi: 10.1016/j.jmii.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Park DW, Kim SH, Park JW. Incidence and characteristics of scarlet fever, South Korea, 2008–2015. Emerg Infect Dis. 2017;23:658–661. doi: 10.3201/eid2304.160773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guy R, Williams C, Irvine N. Increase in scarlet fever notifications in the United Kingdom, 2013/2014. Euro Surveill. 2014;19:20749. doi: 10.2807/1560-7917.es2014.19.12.20749. [DOI] [PubMed] [Google Scholar]
- 8.Cui F, Shen L, Li L. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China. Emerg Infect Dis. 2017;23:765–772. doi: 10.3201/eid2305.161477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang S, Wu J, Ding C. Epidemiological features of and changes in incidence of infectious diseases in China in the first decade after the SARS outbreak: an observational trend study. Lancet Infect Dis. 2017;17:716–725. doi: 10.1016/S1473-3099(17)30227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahara G, Chhetri JK, Guo X. Increasing prevalence of scarlet fever in China. BMJ. 2016;353:i2689. doi: 10.1136/bmj.i2689. [DOI] [PubMed] [Google Scholar]
- 11.Zeng Y, Hesketh T. The effects of China's universal two-child policy. Lancet. 2016;388:1930–1938. doi: 10.1016/S0140-6736(16)31405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouyang Y. China relaxes its one-child policy. Lancet. 2013;382:e28. doi: 10.1016/s0140-6736(13)62544-1. [DOI] [PubMed] [Google Scholar]
- 13.National Health Commission of the People's Republic of China The month report of the legal infectious diseases in China. National Health Commission of the People's Republic of China. http://www.nhfpc.gov.cn/jkj/s3578/201304/60a94504eb64415abdc26fe85adc6364.shtml (in Chinese).
- 14.Armitage P, Berry G. 3rd edn. Blackwell; London: 1994. Statistical methods in medical research; p. 131. [Google Scholar]
- 15.Nakazawa M. Package ‘fmsb’. 2018. https://cran.r-project.org/web/packages/fmsb/index.html
- 16.You Y, Davies MR, Protani M, McIntyre L, Walker MJ, Zhang J. Scarlet fever epidemic in China caused by Streptococcus pyogenes serotype M12: epidemiologic and molecular analysis. EBioMedicine. 2018;28:128–135. doi: 10.1016/j.ebiom.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang P, Peng X, Zhang D. Characteristics of group A streptococcus strains circulating during scarlet fever epidemic, Beijing, China, 2011. Emerg Infect Dis. 2013;19:909–915. doi: 10.3201/eid1906.121020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies MR, Holden MT, Coupland P. Emergence of scarlet fever Streptococcus pyogenes emm12 clones in Hong Kong is associated with toxin acquisition and multidrug resistance. Nat Genet. 2015;47:84–87. doi: 10.1038/ng.3147. [DOI] [PubMed] [Google Scholar]
- 19.Chalker V, Jironkin A, Coelho J. Genome analysis following a national increase in scarlet fever in England 2014. BMC Genomics. 2017;18:224. doi: 10.1186/s12864-017-3603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.You YH, Song YY, Yan XM. Molecular epidemiological characteristics of Streptococcus pyogenes strains involved in an outbreak of scarlet fever in China, 2011. Biomed Environ Sci. 2013;26:877–885. doi: 10.3967/bes2013.016. [DOI] [PubMed] [Google Scholar]
- 21.Peng X, Yang P, Wu S. emm types of mutation in scarlet-fever-related group A streptococcal, among children in Beijing, 2011–2014. Zhonghua Liu Xing Bing Xue Za Zhi. 2015;36:1397–1400. (in Chinese). [PubMed] [Google Scholar]
- 22.Zhang H, Wang L, Lai S, Li Z, Sun Q, Zhang P. Surveillance and early warning systems of infectious disease in China: from 2012 to 2014. Int J Health Plann Manage. 2017;32:329–338. doi: 10.1002/hpm.2434. [DOI] [PubMed] [Google Scholar]
- 23.Duan Y, Yang LJ, Zhang YJ, Hunag XL, Pan GX, Wang J. Effects of meteorological factors on incidence of scarlet fever during different periods in different districts of China. Sci Total Environ. 2017;581–582:19–24. doi: 10.1016/j.scitotenv.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Zhou M, He G, Liu Y. The associations between ambient air pollution and adult respiratory mortality in 32 major Chinese cities, 2006–2010. Environ Res. 2015;137:278–286. doi: 10.1016/j.envres.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Ben Zakour NL, Davies MR, You Y. Transfer of scarlet fever-associated elements into the group A streptococcus M1T1 clone. Sci Rep. 2015;5:15877. doi: 10.1038/srep15877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Malmanche SA, Martin DR. Protective immunity to the group A streptococcus may be only strain specific. Med Microbiol Immunol. 1994;183:299–306. doi: 10.1007/BF00196680. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson BK, Villasenor-Sierra A, Norgren M, Stevens DL. Opsonization of T1M1 group A streptococcus: dynamics of antibody production and strain specificity. Clin Infect Dis. 2001;32:E24–E30. doi: 10.1086/318448. [DOI] [PubMed] [Google Scholar]
- 28.Wong SSY, Yuen K-Y. Streptococcus pyogenes and re-emergence of scarlet fever as a public health problem. Emerg Microbes Infect. 2012;1:e2. doi: 10.1038/emi.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mhaske MS, Khismatrao DS, Kevin F, Pandve HT, Kundap RP. Morbidity pattern and personal hygiene in children among private primary school in urban area: are the trends changing? J Family Med Prim Care. 2013;2:266–269. doi: 10.4103/2249-4863.120753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staszewska-Jakubik E, Czarkowski MP, Kondej B. Scarlet fever in Poland in 2014. Przegl Epidemiol. 2016;70:195–202. [PubMed] [Google Scholar]
- 31.Stevens DL, Tanner MH, Winship J. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989;321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 32.Cannaday P, McNitt T, Horn K, Goodpasture H, Gentry LO. A family outbreak of serious streptococcal infection. JAMA. 1976;236:585–586. [PubMed] [Google Scholar]
- 33.Lau EH, Nishiura H, Cowling BJ, Ip DK, Wu JT. Scarlet fever outbreak, Hong Kong, 2011. Emerg Infect Dis. 2012;18:1700–1702. doi: 10.3201/eid1810.120062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.