Abstract
Background
Middle East respiratory syndrome (MERS) coronavirus causes a highly fatal lower-respiratory tract infection. There are as yet no licensed MERS vaccines or therapeutics. This study (WRAIR-2274) assessed the safety, tolerability, and immunogenicity of the GLS-5300 MERS coronavirus DNA vaccine in healthy adults.
Methods
This study was a phase 1, open-label, single-arm, dose-escalation study of GLS-5300 done at the Walter Reed Army Institute for Research Clinical Trials Center (Silver Spring, MD, USA). We enrolled healthy adults aged 18–50 years; exclusion criteria included previous infection or treatment of MERS. Eligible participants were enrolled sequentially using a dose-escalation protocol to receive 0·67 mg, 2 mg, or 6 mg GLS-5300 administered by trained clinical site staff via a single intramuscular 1 mL injection at each vaccination at baseline, week 4, and week 12 followed immediately by co-localised intramuscular electroporation. Enrolment into the higher dose groups occurred after a safety monitoring committee reviewed the data following vaccination of the first five participants at the previous lower dose in each group. The primary outcome of the study was safety, assessed in all participants who received at least one study treatment and for whom post-dose study data were available, during the vaccination period with follow-up through to 48 weeks after dose 3. Safety was measured by the incidence of adverse events; administration site reactions and pain; and changes in safety laboratory parameters. The secondary outcome was immunogenicity. This trial is registered at ClinicalTrials.gov (number NCT02670187) and is completed.
Findings
Between Feb 17 and July 22, 2016, we enrolled 75 individuals and allocated 25 each to 0·67 mg, 2 mg, or 6 mg GLS-5300. No vaccine-associated serious adverse events were reported. The most common adverse events were injection-site reactions, reported in 70 participants (93%) of 75. Overall, 73 participants (97%) of 75 reported at least one solicited adverse event; the most common systemic symptoms were headache (five [20%] with 0·67 mg, 11 [44%] with 2 mg, and seven [28%] with 6 mg), and malaise or fatigue (five [20%] with 0·67 mg, seven [28%] with 2 mg, and two [8%] with 6 mg). The most common local solicited symptoms were administration site pain (23 [92%] with all three doses) and tenderness (21 [84%] with all three doses). Most solicited symptoms were reported as mild (19 [76%] with 0·67 mg, 20 [80%] with 2 mg, and 17 [68%] with 6 mg) and were self-limiting. Unsolicited symptoms were reported for 56 participants (75%) of 75 and were deemed treatment-related for 26 (35%). The most common unsolicited adverse events were infections, occurring in 27 participants (36%); six (8%) were deemed possibly related to study treatment. There were no laboratory abnormalities of grade 3 or higher that were related to study treatment; laboratory abnormalities were uncommon, except for 15 increases in creatine phosphokinase in 14 participants (three participants in the 0·67 mg group, three in the 2 mg group, and seven in the 6 mg group). Of these 15 increases, five (33%) were deemed possibly related to study treatment (one in the 2 mg group and four in the 6 mg group). Seroconversion measured by S1-ELISA occurred in 59 (86%) of 69 participants and 61 (94%) of 65 participants after two and three vaccinations, respectively. Neutralising antibodies were detected in 34 (50%) of 68 participants. T-cell responses were detected in 47 (71%) of 66 participants after two vaccinations and in 44 (76%) of 58 participants after three vaccinations. There were no differences in immune responses between dose groups after 6 weeks. At week 60, vaccine-induced humoral and cellular responses were detected in 51 (77%) of 66 participants and 42 (64%) of 66, respectively.
Interpretation
The GLS-5300 MERS coronavirus vaccine was well tolerated with no vaccine-associated serious adverse events. Immune responses were dose-independent, detected in more than 85% of participants after two vaccinations, and durable through 1 year of follow-up. The data support further development of the GLS-5300 vaccine, including additional studies to test the efficacy of GLS-5300 in a region endemic for MERS coronavirus.
Funding
US Department of the Army and GeneOne Life Science.
Research in context.
Evidence before this study
There are no licensed vaccines to prevent or therapeutics to treat Middle East respiratory syndrome (MERS) coronavirus infection. The GLS-5300 MERS coronavirus DNA vaccine tested in this phase 1 clinical trial has previously been reported to be immunogenic in mice, camels, and non-human primates, and to protect non-human primates from clinical disease in a challenge model of pulmonary infection. We searched the US National Library of Medicine, ClinicalTrials.Gov, and the European Union Clinical Trials Register databases to identify any clinical trials of MERS coronavirus vaccines using the terms “MERS”, “MERS-CoV”, “Middle East Respiratory Syndrome”, “vaccine”, “phase”, and clinical trial”. To our knowledge, no other data from a human clinical trial of a MERS coronavirus vaccine has been reported to date.
Added value of this study
This study showed that the GLS-5300 MERS coronavirus DNA vaccine was tolerable and immunogenic in humans. The vaccine induced both antibody-based and cellular MERS coronavirus-specific immune responses. The study also compared vaccine-specific responses with those from individuals who had recovered from natural MERS coronavirus infection during the 2015 Korean outbreak. The results showed that the immune responses generated in vaccinated study participants were similar to convalescent responses after natural infection.
Implications of all the available evidence
The unpredictability of zoonotic transmission and a general decrease in the number of cases of MERS coronavirus in the Middle East will make future placebo-controlled trials challenging. Future testing of GLS-5300 is ongoing in a phase 1b/2a trial in South Korea and additional randomised clinical trials are being planned to test the vaccine in endemic regions. The GLS-5300 vaccine might have potential value in the response to any future MERS coronavirus oubreaks. The global public health community should maintain strong interest in the development of a vaccine that is safe and effective to control any potential situation of a MERS coronavirus outbreak.
Introduction
Middle East respiratory syndrome (MERS) coronavirus is a highly fatal cause of lower respiratory tract infection first identified in 2012 in a Saudi Arabian man.1 As of May, 2019, 2442 laboratory-confirmed cases of MERS and 842 (35%) associated deaths were reported across 27 countries.2, 3 Most reported infections have been in Saudi Arabia (1983 cases and 745 [38%] deaths).2, 3
Despite the low transmissibility of MERS coronavirus between humans, the threat of regional or global MERS epidemics is substantial, as exemplified by continued transmission outside the Middle East4, 5, 6 and particularly by a South Korean outbreak in 2015 that emanated from a single traveller and resulted in 186 cases and 38 deaths.7
Correlates of protection from MERS coronavirus infection and disease have not been definitively established. Animal challenge studies have shown protection for several vaccines;8, 9, 10, 11 however, clearly discriminating between binding antibodies, neutralising antibodies, and T-cell responses as the determinant of protection is not possible. Both humoral and cellular immunity probably play a substantial role in viral clearance and mitigation of human clinical disease. MERS coronavirus infection induces neutralising antibodies that persist for several years.12, 13, 14 Passive transfer of either monoclonal antibodies or polyclonal serum samples has the ability to lower viral loads and reduce pathology in the lungs of several animal models.14, 15, 16 However, the concentrations of MERS coronavirus neutralising antibodies do not always correlate with viral clearance, survival, or disease severity.12, 14, 17, 18 A study in Saudi Arabia showed that CD8 T-cell responses directly correlated with shorter stays in hospital intensive care units and lower viral load in the respiratory tract.14 Given the uncertainty in defining a correlate of protection, a vaccine candidate that generates both humoral and cellular immune responses is desirable.
In pre-clinical experiments,10 GLS-5300, a DNA vaccine expressing a full-length MERS coronavirus S-glycoprotein antigen was broadly immunogenic in mice, camels, and non-human primates. GLS-5300 protected non-human primates from radiological and histopathological evidence of pneumonia following MERS coronavirus challenge.11 We therefore did a phase 1 study to assess the safety and immunogenicity of GLS-5300 in humans.
Methods
Study design and participants
We initiated a phase 1, open-label, single-arm, dose-escalation study at the Walter Reed Army Institute for Research Clinical Trials Center (Silver Spring, MD, USA). The study was approved by the Centre's institutional review board. The appendix is available online and contains supplemetnal data figures and tables, the clinical study protocol, and the statistical analysis plan.
Eligible participants were healthy adults aged between 18 and 50 years; able to provide consent to participate and having signed an informed consent form; able and willing to comply with all study procedures; women of childbearing potential agreed to either remain sexually abstinent, use medically effective contraception (oral contraception, barrier methods, or spermicide) or have a partner who was sterile from enrolment to 3 months following the last injection, or had a partner who was unable to induce pregnancy; sexually active men who were considered sexually fertile must have agreed to use either a barrier method of contraception during the study, and agreed to continue the use for at least 3 months following the last injection, or have a partner who was permanently sterile or unable to become pregnant; normal screening electrocardiogram (ECG) or screening ECG with no clinically significant findings; screening laboratory findings must be within normal limits or be grade 0–1 findings; no history of clinically significant immunosuppressive or autoimmune disease; not currently or within the previous 4 weeks taking immuno-suppressive drugs (excluding inhaled, topical skin, or eye drop-containing corticosteroids, low-dose methotrexate, or corticosteroids at a dose <20 mg/day); and willing to allow storage and future use of samples for MERS coronavirus-related research.
Exclusion criteria included previous MERS coronavirus infection or receipt of an experimental treatment or prevention for MERS; serological evidence of HIV, hepatitis B virus, or hepatitis C virus infection; administration of any vaccine within 4 weeks of first dose; pregnancy or breastfeeding; laboratory screening abnormalities above grade 2; body-mass index of 35 kg/m2 or more; administration of any monoclonal or polyclonal antibody product within 4 weeks of the first dose; administration of any blood product within 3 months of first dose; baseline evidence of kidney disease as measured by creatinine greater than 1·5 mg/dL; chronic liver disease or cirrhosis; current or anticipated treatment with TNF-α inhibitors (eg, infliximab, adalimumab, or etanercept); previous major surgery or any radiation therapy within 4 weeks of group assignment; any pre-excitation syndromes (eg, Wolff-Parkinson-White syndrome); metal implants within 20 cm of the planned site(s) of injection; presence of keloid scar formation or hypertrophic scar as a clinically significant medical condition at the planned site(s) of injection; prisoner or participants who are compulsorily detained (involuntary incarceration) for treatment of either a physical or psychiatric illness; active drug or alcohol use or dependence that, in the opinion of the investigator, would interfere with adherence to study requirements or assessment of immunological endpoints; tattoos covering the injection site area; or presence of a cardiac pacemaker or automatic implantable cardioverter defibrillator; or investigator decision related to any condition which might interfere with study requirements. The full protocol is in the appendix (p 17). All participants provided written, informed consent before enrolment.
A separate MERS coronavirus natural-infection sample collection study enrolled participants at the Seoul National University Hospital (SNUH; Seoul, South Korea). Eligible participants were adults previously infected with MERS coronavirus during the outbreak in South Korea in 2015 who had recovered from the illness. Serum was collected from naturally infected participants at the time of acute illness and diagnosis in 2015. Participants were asked to return in 2016–17 for collection of convalescent phase serum and peripheral blood mononuclear cells. This study was reviewed and approved by the SNUH institutional review board and all participants provided written informed consent before enrolment.
Procedures
The study vaccine GLS-5300 was manufactured at VGXI (The Woodlands, TX, USA) according to good manufacturing practices. GLS-5300 contains 6 mg/mL of plasmid pGX9101 in sterile water for injection. Plasmid pGX9101 contains a gene insert designed as an optimised, full-length, microconsensus of the MERS coronavirus S-glycoprotein generated from publicly available clinical sequences up to August 2015.10
Participants were enrolled sequentially using a dose-escalation protocol to receive GLS-5300 at one of three doses: 0·67 mg, 2 mg, or 6 mg per vaccination, with enrolment into higher dose groups occurring after a safety monitoring committee reviewed the data following vaccination of the first five participants at the previous lower dose in each group. GLS-5300 was administered to participants by trained clinical site staff as a single 1 mL intramuscular deltoid injection followed immediately by colocalised intramuscular electroporation to enhance cellular entry of plasmid DNA with the Cellectra-5P Adaptive Constant Current Electroporation device (Inovio Pharmaceuticals, Plymouth Meeting, PA, USA) as described previously19 at the site of inoculation in a three-dose series at baseline, 4 weeks, and 12 weeks. The Cellectra-5P electroporation device applies three pulses at 1 s intervals at strengths of 0·5 A current and voltage of 1–200 V per pulse.
Local and systemic adverse events were recorded at each study visit and graded by the clinical site investigators.20 Additionally, participants were asked to record local and general symptoms for 1 week following each vaccination in a memory aid that was reviewed by study staff at the following visit. Screening and safety laboratory assessments included complete blood counts, comprehensive metabolic panels, measurement of alanine aminotransferase, aspartate aminotransferase, and creatine phosphokinase, and a baseline electrocardiogram.
Serum samples were collected at baseline and weeks 1, 2 or 3, 4, 6, 12, 14, 24, 36, and 60. ELISAs developed in-house (assay details in the appendix, p 9) were done to detect antibodies with binding specificity for the S1 subunit (amino acids 1–725) of MERS coronavirus S (S1-ELISA) and for the full-length S-glycoprotein (full-length-S-ELISA),10 reported as end-point titres; positivity cutoffs were the lowest dilutions tested, 100 for S1 and 10 for full-length S ELISA. Serum samples were also assessed for their ability to neutralise MERS coronavirus strain EMC-2012 infection of Vero cells. (appendix p 9). Samples were tested in triplicate for neutralising antibodies and a sample was scored as positive if two of three replicates showed 50% neutralising activity resulting in a geometric mean titre of at least 7·9.
Whole blood was collected and processed for peripheral blood mononuclear cell isolation at the same timepoints as collection of serum samples, except peripheral blood mononuclear cells, which were collected at only one early timepoint between the first two vaccinations (week 1, 2, 3, or 4). T-cell responses were measured by IFN-γ enzyme-linked immunospot assays (IFN-γ-ELISPOT) after incubation of peripheral blood mononuclear cells with pools of overlapping 15-mer peptides (44–45 peptides per pool) spanning the entire MERS coronavirus S-glycoprotein. IFN-γ-ELISPOT responses were reported as spot-forming units per million peripheral blood mononuclear cells of the total of five MERS S peptide pools. A positive response to vaccination was defined as twice the study mean at baseline: at least 141 spot-forming units per million peripheral blood mononuclear cells. Additionally, multiparameter intracellular cytokine staining flow cytometry to detect CD4+ and CD8+ T cells secreting IFN-γ, tumour necrosis factor (TNF) α, IL-2, or IL-4 following MERS coronavirus S-peptide stimulation was done with evaluable samples at baseline and week 14 (appendix p 9).
Serum and peripheral blood mononuclear cell samples were also collected from individuals who were diagnosed with MERS coronavirus infection during the 2015 Korean outbreak. Convalescent samples were obtained at a mean of 19·8 months (SD 0·7) from MERS diagnosis. Acute phase serum samples from the time of initial admission to hospital, collected a mean of 20·8 days (14·3) from diagnosis and 25·1 days (15·1) from symptom onset, were available in small volumes from each participant. Samples were analysed using the same methods as vaccine sample testing. Serological immune responses for some of the individuals have been previously reported following acute infection18 and 1 year after infection.12
Outcomes
The primary outcome of this study was safety, measured by incidence of adverse events classified by system organ class, preferred term severity, and relationship to study treatment and schedule; administration site reactions (described by frequency and severity grade) and administration site pain; and changes in safety laboratory variables described by frequency and severity grade (eg, liver panel tests and vital signs).
The secondary outcome of this study was immunogenicity. We assessed the cellular and humoral responses to GLS-5300 overall and by dose. Humoral immunogenicity was assessed by qualitative and quantitative ELISAs of binding antibody titres to the S1 glycoprotein subunit and the full-length MERS coronavirus S glycoprotein, and of neutralising antibodies against MERS coronavirus strain EMC2012 on Vero cells. Antigen-specific cellular immune responses to MERS coronavirus S glycoprotein were assessed using IFN-γ-ELISPOT and intracellular cytokine staining assays. Exploratory outcomes were: comparison of S binding antibody and MERS CoV neutralising antibody titres; kinetics and durability of S binding antibody and MERS coronavirus neutralising antibody titres; comparison of IFN-γ ELISPOT and intracellular cytokine staining responses across different vaccine doses; kinetics and durability of IFN-γ ELISPOT; expression of the full length MERS coronavirus S protein in peripheral blood over time; host immune-genotyping when available; epitope mapping of CD4+ and CD8+ T-lymphocyte responses; immunophenotyping and functional characterisation of cellular subsets of interest, including natural killer cells; and isolation, expression, and characterisation of monoclonal antibodies against the MERS coronavirus S protein and assessment of their neutralising and non-neutralising functional activity (study protocol, appendix p 17).
Statistical analysis
This study was designed as an exploratory trial with defined group sizes of 25 participants per dose group, and was not statistically powered to measure any specific outcome. The safety analysis population included all participants who received at least one study treatment and for whom post-dose safety data were available, and were analysed as to the treatment they received. The per-protocol analysis population was used for the clinical study report's primary analysis and included participants who received all vaccine doses, had no major protocol deviations, and had primary endpoint data available. Because ten (13%) participants were excluded from the per-protocol analysis, the more inclusive modified intention-to-treat (ITT) analysis of immunogenicity, comprised all participants who received at least one dose of GLS-5300, is presented to support the main analysis. Participants in this population were grouped according to their original treatment assignment. S1-ELISA, full-length S-ELISA, and EMC-2012 Vero neutralisation assay results are reported as geometric mean endpoint titres with 95% CIs for each dose-group at each timepoint. The Kruskal-Wallis test was used to compare values by dose at each timepoint. Fisher's exact test was used to compare S1 ELISA response rates by group. Two-sided t tests were used to compare mean IFN-γ-ELISPOT values between groups. Intracellular cytokine staining data are reported as the mean frequency with 95% CI of MERS coronavirus S-peptide-stimulated cytokine responses in CD4+ and CD8+ T cells. Statistical significance was defined as a two-sided p-value of less than 0·05 and were nominal as we did not adjust for multiple comparisons. Differences between acute and convalescent samples from natural MERS coronavirus infections and week 14 GLS-5300 samples were assessed by one-way ANOVA with Tukey's multiple comparison. We used GraphPad Prism statistical software (v.7) for analyses. This study is registered on ClinicalTrials.gov (number NCT02670187).
Role of the funding source
The funders of the study had a role in the study design, data collection, data analysis, data interpretation, and writing of the report. The funders had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
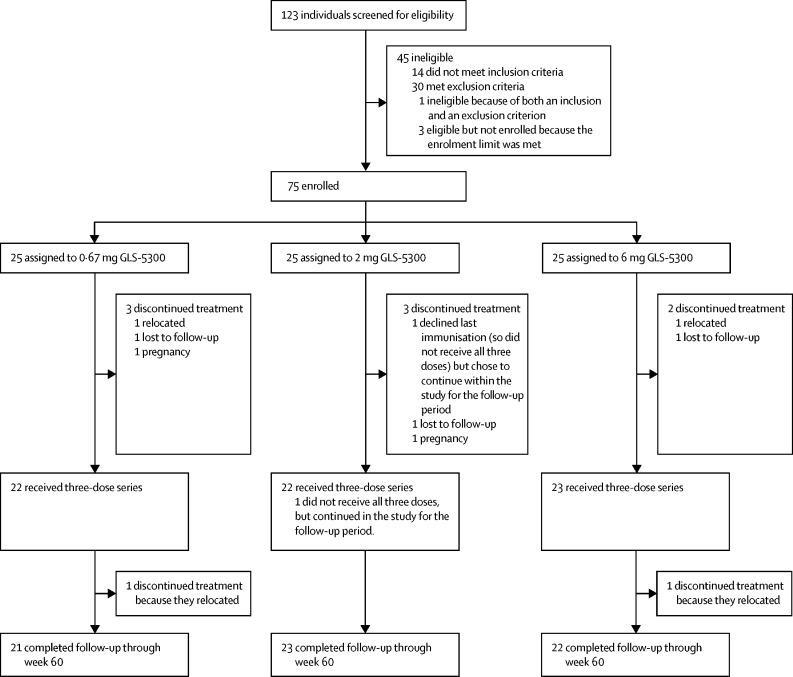
Between Feb 17, and July 22, 2016, we sequentially enrolled 75 participants, 25 assigned to each dose group (figure 1 ). Participant demographic characteristics are in table 1 . Reasons for non-enrolment are in the appendix (p 10), including 15 individuals who were excluded for increases in creatine phosphokinase related to vigorous exercise regimens. Mean age of participants was 32·2 years (SD 9·3). Ten participants were excluded from the per-protocol analysis because they did not complete all study visits (figure 1).
Figure 1.
Trial profile
Ten participants did not complete the vaccination series: four relocated, three were lost-to-follow-up, two became pregnant during the study, and one declined the final vaccination.
Table 1.
Baseline characteristics of study participants
| 0·67 mg GLS-5300 (n=25) | 2 mg GLS-5300 (n=25) | 6 mg GLS-5300 (n=25) | Total (n=75) | ||
|---|---|---|---|---|---|
| Sex | |||||
| Male | 14 (56%) | 14 (56%) | 16 (64%) | 44 (58·7%) | |
| Female | 11 (44%) | 11 (44%) | 9 (36%) | 31 (41·3%) | |
| Ethnicity | |||||
| American Indian or Alaska Native | 0 | 0 | 0 | 0 | |
| Asian | 2 (8%) | 2 (8%) | 2 (8%) | 6 (8%) | |
| Black or African American | 10 (40%) | 11 (44%) | 10 (40%) | 31 (41·3%) | |
| Native Hawaiian or Pacific Islander | 0 | 0 | 0 | 0 | |
| White | 13 (52%) | 12 (48%) | 12 (48%) | 37 (49·3%) | |
| Other | 0 | 0 | 1 (4%) | 1 (1·3%) | |
| Hispanic or Latino | 2 (8%) | 0 | 2 (8%) | 4 (5·3%) | |
| Age, years | 33·2 (10·4) | 33·0 (7·7) | 30·5 (9·8) | 32·2 (9·3) | |
| Age range, years | 19–50 | 19–49 | 19–50 | 19–50 | |
Data are n (%), mean (SD), unless otherwise indicated.
67 (89%) of the enrolled participants completed the entire three-dose vaccination series (figure 1). The most common adverse events were injection-site reactions, reported in 70 participants (93%) of 75. 73 participants (97%) of 75 reported at least one solicited adverse event (table 2 ; appendix p 3). One or more injection-site reactions were reported by 92% or more participants in all dose groups. Systemic reactions were reported by 32% of participants in the 0·67 mg group, 62% in the 2 mg group and 32% in the 6 mg dose group. The most common solicited systemic adverse event was headache, followed by malaise or fatigue, and myalgia; most solicited adverse events were mild in all groups. One participant in the 6 mg dose group reported one episode of severe induration following the third vaccination that resolved within 24 h. One serious adverse event was reported: a second-degree burn reported to have occurred 15 days after the third vaccination. The participant provided no further information and did not return for follow-up. The principal investigator deemed the incident as unrelated to study treatment.
Table 2.
Adverse events
|
0·67 mg GLS-5300 (n=25) |
2 mg GLS-5300 (n=25) |
6 mg GLS-5300 (n=25) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | Mild | Moderate | Severe | Any | Mild | Moderate | Severe | Any | Mild | Moderate | Severe | ||
| Any symptom | 24 (96%) | 18 (72%) | 6 (24%) | 0 | 25 (100%) | 18 (72%) | 7 (28%) | 0 | 24 (96%) | 17 (68%) | 6 (24%) | 1 (4%) | |
| Any systemic symptom | 8 (32%) | 6 (24%) | 2 (8%) | 0 | 13 (52%) | 10 (40%) | 3 (12%) | 0 | 8 (32%) | 6 (24%) | 2 (8%) | 0 | |
| Malaise or fatigue | 5 (20%) | 3 (12%) | 2 (8%) | 0 | 7 (28%) | 6 (25·3%) | 1 (4%) | 0 | 2 (8%) | 2 (8%) | 0 | 0 | |
| Myalgia | 3 (12%) | 2 (8%) | 1 (4%) | 0 | 6 (24%) | 6 (24%) | 0 | 0 | 2 (8%) | 2 (8%) | 0 | 0 | |
| Headache | 5 (20%) | 5 (20%) | 0 | 0 | 11 (44%) | 8 (32%) | 3 (12%) | 0 | 7 (28%) | 6 (24%) | 1 (4%) | 0 | |
| Arthralgia | 2 (8%) | 2 (8%) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (8%) | 2 (8%) | 0 | 0 | |
| Nausea | 2 (8%) | 1 (4%) | 1 (4%) | 0 | 4 (16%) | 3 (12%) | 1 (4%) | 0 | 3 (12%) | 2 (8%) | 1 (4%) | 0 | |
| Any local symptom | 24 (96%) | 19 (76%) | 5 (20%) | 0 | 24 (96%) | 20 (80%) | 4 (16%) | 0 | 23 (92%) | 17 (68%) | 5 (20%) | 1 (4%) | |
| Pain | 23 (92%) | 19 (76%) | 4 (16%) | 0 | 23 (92%) | 20 (80%) | 3 (12%) | 0 | 23 (92%) | 18 (72%) | 5 (20%) | 0 | |
| Tenderness | 21 (84%) | 18 (72%) | 3 (12%) | 0 | 21 (84%) | 21 (84%) | 0 | 0 | 21 (84%) | 17 (68%) | 4 (16%) | 0 | |
| Pruritus | 4 (16%) | 4 (16%) | 0 | 0 | 3 (12%) | 3 (12%) | 0 | 0 | 1 (4%) | 1 (4%) | 0 | 0 | |
| Erythema | 1 (4%) | 1 (4%) | 0 | 0 | 3 (12%) | 2 (8%) | 1 (4%) | 0 | 1 (4%) | 0 | 1 (4%) | 0 | |
| Induration or swelling | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (8%) | 1 (4%) | 0 | 1 (4%) | |
| Bruising | 0 | 0 | 0 | 0 | 1 (4%) | 1 (4%) | 0 | 0 | 3 (12%) | 3 (12%) | 0 | 0 | |
Data are n (%).
There were no grade 3 or higher laboratory abnormalities that were related to study treatment. Laboratory abnormalities were uncommon, except for 15 increases in creatine phosphokinase occurring in 14 participants, of which five (33%) were considered possibly related to study treatment. Unsolicited adverse events were reported by 56 participants (75%) of 75 across all dose groups (appendix p 11). Of unsolicited adverse events, 27 (36%) were infections, of which 24 (89%) were seasonal including 21 upper respiratory tract or viral infections (88%), two occurrences of non-specific or bacterial pharyngitis (8%), and one of otitis (4%). Other infections included single episodes of gastroenteritis, nematode infection, and oral herpetic infection. No infections were considered by the principal investigator as definitely related to study treatment; four upper respiratory tract infections, one oral herpes, and one ear infection were considered as possibly related to study treatment.
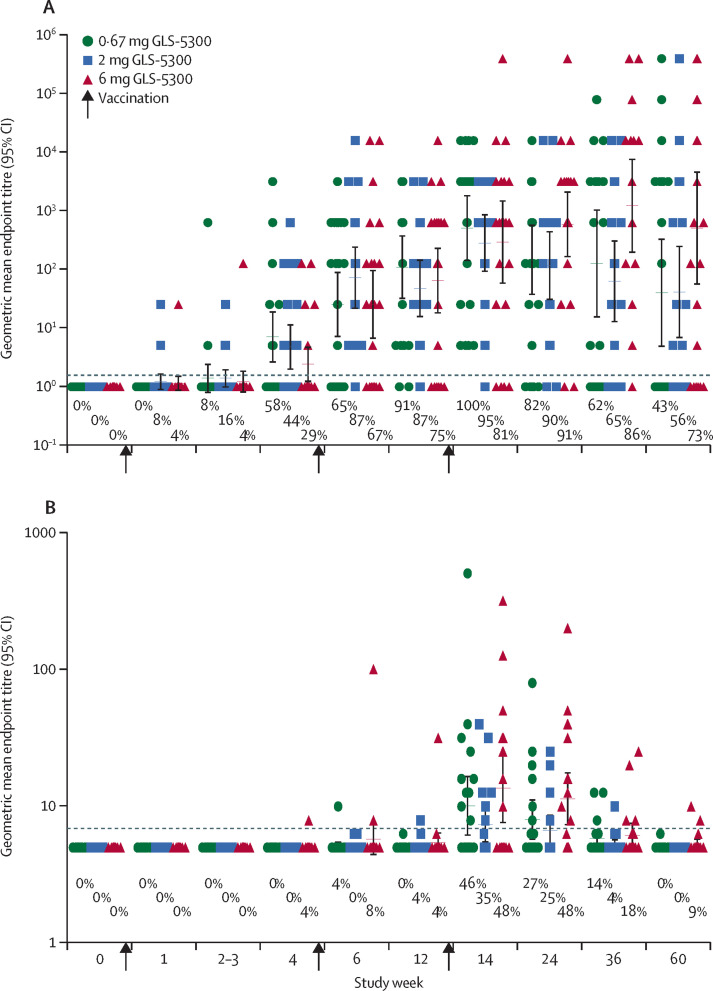
59 (94%) of 63 participants had measurable MERS coronavirus S1 binding antibodies at 2 weeks after the third vaccination at week 14 (figure 2A ). Seroconversion measured by S1-ELISA occurred in 48 participants (66%) of 73 at week 4 just before the second vaccination, and in 59 (86%) of 69 at week 12 just before the third vaccination. There were no differences in S1-ELISA response rates between dose groups from week 4 onward (figure 2, appendix p 12). S1-ELISA seroconversion was also noted in 59 participants (86%) of 69 after two vaccinations and in 61 (94%) of 65 after three vaccinations. S1 ELISA seroreactivity was maintained in 52 participants (79%) of 66 up to study end at week 60. There was a significant difference in S1-ELISA geometric mean endpoint titres between dose groups at week 1, with none thereafter (figure 2, appendix pp 13, 14). A similar pattern of antibody responses was noted when measured by full-length S-ELISA, with 58 vaccinated participants (92%) of 63 seroconverting at week 14 and 38 (58%) of 66 maintaining through week 60 (appendix 4).
Figure 2.
Vaccine-associated antibody responses.
Antibody responses for each dose are shown for available specimens from the modified intention-to-treat population. (A) shows the geometric mean endpoint titre (95% CI error bars) and the proportion of participants who developed antibodies against Middle East respiratory syndrome (MERS) coronavirus determined by S1-ELISA. B shows the geometric mean endpoint titre (95% CI error bars) and the proportion of participants who developed neutralising antibodies against MERS coronavirus determined by EMC-2012 MERS-coronavirus infection of Vero cells.
Neutralising antibodies against MERS coronavirus EMC-2012 infection of Vero cells were detected in 27 participants (43%) of 63 at week 14 and 25 (39%) of 65 at week 24, but only two (3%) of 66 maintained neutralisation activity to end of study. Overall, 34 participants (50%) of 68 had detectable neutralising antibodies at one or more timepoints during the study. Half-maximal neutralising geometric mean endpoint titres peaked at week 14 with a range between 7·9 and 508 (figure 2B). Pearson's correlation of S1-ELISA and neutralising titres was r2=0·8855 and full-length S-ELISA correlation with neutralisation was r2=0·0669 (appendix 4).
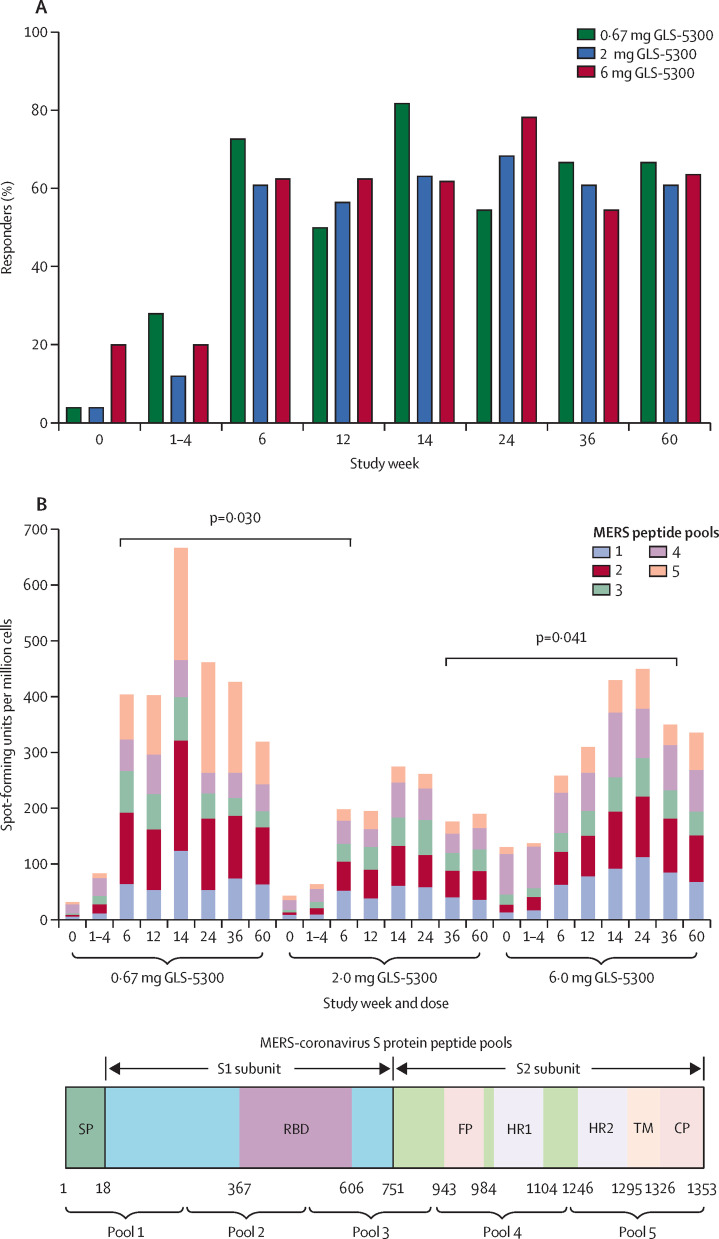
Vaccination with GLS-5300 induced MERS coronavirus S-specific IFN γ-ELISPOT responses in 66 participants overall (88%) of 75, with T-cell responses in 47 participants (71%) of 66 at week 12 after the second vaccination, and in 44 (76%) of 58 at week 14 after the third vaccination. 42 participants (64%) of 66 maintained IFN γ-ELISPOT cellular responses through to the end of the study at week 60 (figure 3 ). Vaccine-induced humoral responses (detectable S1 binding antibodies) were detected in 51 participants (77%) of 66 at week 60. Except for a higher response at week 6 for the 0·67 mg group versus the 2 mg group and at week 36 for the 6 mg group versus the 2 mg group, there were no differences in T-cell responses between dose groups (appendix p 5). IFNγ-ELISPOT responses were detected against all five peptide pools spanning the S-glycoprotein (figure 3). CD8+ and CD4+ T-cell responses were polyfunctional at 2 weeks after the third vaccination (appendix p 5).
Figure 3.
Vaccine-associated cellular responses
Cellular responses to Middle East respiratory syndrome (MERS) coronavirus S peptides were determined by IFNγ-enzyme-linked immunospot assay for available specimens of peripheral blood mononuclear cells at each timepoint in the modified intention-to-treat dataset. (A) shows the proportion of participants with a positive response. (B) shows the total spot-forming units per million peripheral blood mononuclear cells as the sum of average responses to each of the five peptide pools by participants' peripheral blood mononuclear cells in each group at each time, with a representation of the region of the S glycoprotein included in each peptide pool below.
Between Dec 27, 2016, and April 1, 2017, ten adults, previously infected with MERS, were enrolled for the separate MERS coronavirus natural-infection sample collection study (median age 55·5 years, IQR 36·0–56·2). All ten participants (100%) had measurable binding antibodies by S1-ELISA in acute and convalescent samples (appendix p 6) and by full-length S-ELISA in convalescent samples (appendix p 8). Neutralising antibodies persisted from acute to convalescent timepoints in nine individuals (90%), although were very low for three (30%; appendix p 7). Compared with vaccine-induced humoral and cellular responses, antibody titres following natural infection were significantly higher in the acute phase in MERS-infected participants but were not different than vaccine for S1-ELISA or neutralising antibodies in convalescent samples (appendix p 6). Convalescent T-cell responses in naturally infected individuals were broad-based, spanning the S-glycoprotein (appendix p 7) with mean total spot-forming units per million peripheral blood mononuclear cells of 110 (SD 108·0) versus 472 (817·6) induced by GLS-5300 at week 14, although the difference was not significant (p=0·17).
Discussion
In our study, GLS-5300 DNA vaccine against MERS coronavirus was well tolerated, and no vaccine-associated serious adverse events were reported. The most common adverse events were injection site reactions, consistent with findings from other published clinical trial reports of DNA vaccines or placebo co-administered through intramuscular injection and electroporation.21 The vaccine was immunogenic, inducing seroconversion and T-cell responses in most participants. S1-ELISA binding antibody seroconversion and T-cell responses were rapid. Vaccine induced immune responses were durable, as most participants maintained detectable S1 binding antibodies and had cellular immune responses at almost 1 year after the last vaccination. The vaccine-induced antibody and cellular immune responses were similar to those in the convalescent phase from samples in patients who recovered from natural MERS coronavirus infection. Finally, GLS-5300 vaccination induced polyfunctional CD8+ T-cell responses that have been shown to correlate with less severe disease and lower MERS coronavirus shedding.14 Immune responses to GLS-5300 were dose-independent across a nearly 10-fold dose range (0·67 mg to 6 mg per vaccination) suggesting further dose reductions might be possible. A lower dose could extend the vaccine supply in an outbreak situation.
GLS-5300 is the first MERS coronavirus vaccine to advance into human trials. Among vaccine candidates in development,22 four have started or will soon start phase 1 testing, including measles-vectored,9, 23 chimpanzee adenovirus-vectored,24 and modified vaccinia Ankara-vectored vaccines, all expressing full-length S-glycoprotein.25, 26, 27 DNA vaccines and viral-vectored vaccines use recombinant technology that allows for rapid vaccine design in response to emerging infectious diseases. DNA vaccines have additional advantages in rapid manufacture and avoidance of potential toxicities that might occur in live viral-vectored vaccines. Underscoring the potential for rapid deployment of DNA vaccines, GLS-5300 was advanced into the clinic within 9 months of pre-clinical vaccine candidate selection.
Our phase 1 study was not designed to measure GLS-5300 efficacy, as this would require larger randomised studies in a MERS coronavirus-endemic region. Our study had other limitations: as a first-in-man trial primarily assessing vaccine safety, the study was open-label and single-arm rather than a randomised, placebo-controlled, clinical trial (the absence of a placebo group could introduce bias on behalf of investigators or participants). Additionally, the study was not statistically powered to measure any specific outcome, which limits our ability to detect rare events.
In conclusion, this phase 1 clinical trial showed a tolerable safety profile and robust immunogenicity for GLS-5300, a MERS coronavirus DNA vaccine candidate. GLS-5300 vaccination induced similar cellular and antibody responses to those in patients recovered from MERS coronavirus natural infection. An ongoing phase 1/2 a study in South Korea (NCT03721718) will provide additional information on the immunogenicity of GLS-5300 delivered by intradermal injection followed by electroporation and the ability to further reduce the DNA vaccine dose. Additional studies are required to test the efficacy of GLS-5300 in a region endemic for MERS coronavirus. A phase 2 trial is being planned to assess GLS-5300 in the Middle East and South Korea, areas which have been most affected by MERS coronavirus infection. A general decrease in the number of cases of MERS in the Middle East will make future placebo-controlled trials challenging. However, the global public health community should maintain interest in the development of a vaccine that is safe and effective to control any potential future outbreaks.
Acknowledgments
Acknowledgments
The study was co-sponsored by US Department of the Army and GeneOne Life Science, and undertaken at the Walter Reed Army Institute of Research. The GLS-5300 DNA vaccine is being co-developed by GeneOne Life Science and Inovio Pharmaceuticals. This work was supported by a cooperative agreement (W81XWH-07-2-0067) between the Henry M Jackson Foundation for the Advancement of Military Medicine, and the US Department of Defense. Data were reviewed by the Walter Reed Army Institute of Research. We thank Sally Sturm and Jooyeon Oh for technical assistance and Eun Hae Oh for administrative assistance (GeneOne Life Science, Blue Bell, PA, USA). The opinions or assertions contained in this report are the private views of the authors, and are not to be construed as official, or as reflecting true views of the US Department of the Army or the US Department of Defense.
Contributors
The study was designed by KMo, NLM, SJT, CCR, MJ, YKP, CR, and JNM. KMo, KTM, ARC, KP, MLR, JMD, PTS, and APP collected study data and oversaw participant visits. CCR, CR, DK, and JNM provided medical monitoring, regulatory oversight, and project management. Immunogenicity testing was done by TR, CL, GK, KMu, ELR, FIZ, AP, EKD, KYK, HC, and DBW, and was interpreted by KMo, NLM, SJT, CCR, SBK, MJ, YKP, CR, DK, HL, JNM, JB, MCW, SW, and MB. The substudy of naturally infected patients was designed, executed, and analysed by CCR, SBK, MJ, YKP, CR, HL, JNM, and M-dO with immunogenicity testing done as above. Clinical trial data management and statistical analysis were done by JMM. All authors contributed to the writing and editing of the report and approved the final version.
Declaration of interests
CCR, SBK, MJ, CR, DK, HL, and JNM are employees of GeneOne Life Science. MJ reports personal fees from GeneOne Life Science. YKP is an officer of GeneOne Life Science and reports ownership of Inovio Pharmaceuticals stock. JB, MCW, SW, and MB were employees of Inovio Pharmaceuticals during the study. KMu reports grants from GeneOne Life Science and a pending patent for MERS DNA vaccine. ELR reports grants from Wistar and GeneOne Life Science. FIZ and AP report grants from GeneOne Life Science. MLR reports grants from the Henry M Jackson Foundation. TR reports personal fees from GeneOne Life Science. JMM reports support from GeneOne Life Science. NLM reports grants from the Department of the Army. DBW reports grants and personal fees from Inovio, Geneos, and GeneOne Life Science; personal fees from Sanofi and Merck; and has patents for MERS DNA vaccines and for synthetic DNA technology pending to Inovio. SJT reports that during the study he was in the US Army at the Walter Reed Army Institute of Research and his salary and travel costs were paid by the US government; and reports consultative services provided to Coalition for Epidemic Preparedness Innovations as a grant reviewer and to the International Vaccine Institute for consultancy. All other authors declare no competing interests.
Supplementary Material
References
- 1.Corman VM, Eckerle I, Zaki A. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012;17 doi: 10.2807/ese.17.39.20285-en. PII: 20285. [DOI] [PubMed] [Google Scholar]
- 2.WHO MERS situation update. February 2019. http://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-may-2019.html
- 3.WHO Middle East respiratory syndrome coronavirus (MERS-CoV) 2018. http://www.who.int/emergencies/mers-cov/en/
- 4.Bak SL, Jun KI, Jung J. An atypical case of Middle East Respiratory syndrome in a returning traveler to Korea from Kuwait. J Korean Med Sci. 2018;33:e348. doi: 10.3346/jkms.2018.33.e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Middle East respiratory syndrome coronavirus (MERS-CoV)—UK. August 31, 2018. http://www.who.int/csr/don/31-august-2018-mers-united-kingdom/en/
- 6.WHO Middle East respiratory syndrome coronavirus (MERS-CoV) infection—South Korea. Sept 12, 2018. http://www.who.int/csr/don/12-september-2018-mers-republic-of-korea/en/
- 7.Oh MD, Park WB, Park SW. Middle East respiratory syndrome: what we learned from the 2015 outbreak in the Republic of Korea. Korean J Intern Med. 2018;33:233–246. doi: 10.3904/kjim.2018.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman CM, Venkatraman N, Liu T. MERS-CoV spike nanoparticles protect mice from MERS-CoV infection. Vaccine. 2017;35:1586–1589. doi: 10.1016/j.vaccine.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malczyk AH, Kupke A, Prufer S. A highly immunogenic and protective Middle East respiratory syndrome coronavirus vaccine based on a recombinant measles virus vaccine platform. J Virol. 2015;89:11654–11667. doi: 10.1128/JVI.01815-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muthumani K, Falzarano D, Reuschel EL. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aac7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Shi W, Joyce MG. Evaluation of candidate vaccine approaches for MERS-CoV. Nat Commun. 2015;6 doi: 10.1038/ncomms8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choe PG, Perera RA, Park WB. MERS-CoV antibody responses 1 year after symptom onset, South Korea, 2015. Emerg Infect Dis. 2017;23:1079–1084. doi: 10.3201/eid2307.170310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Payne DC, Iblan I, Rha B. Persistence of antibodies against Middle East respiratory coronavirus. Emerg Infect Dis. 2016;22:1824–1826. doi: 10.3201/eid2210.160706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao J, Alshukairi AN, Baharoon SA. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aan5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luke T, Wu H, Zhao J. Human polyclonal immunoglobulin G from transchromosomic bovines inhibits MERS-CoV in vivo. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aaf1061. [DOI] [PubMed] [Google Scholar]
- 16.Pascal KE, Coleman CM, Mujica AO. Pre- and postexposure efficacy of fully immunized antibodies against spike protein in a novel humanized mouse model of MERS-CoV infection. PNAS. 2015;112:8738–8743. doi: 10.1073/pnas.1510830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corman VM, Albarrak AM, Omrani AS. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2016;62:477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park WB, Perera RA, Choe PG. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg Infect Dis. 2015;21:2186–2189. doi: 10.3201/eid2112.151421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diehl MC, Lee JC, Daniels SE. Tolerability of intramuscular and intradermal delivery by CELLECTRA((R)) adaptive constant current electroporation device in healthy volunteers. Hum Vaccin Immunother. 2013;9:2246–2252. doi: 10.4161/hv.24702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services. Food and Drug Administration. Center for Biologics Evaluation and Research Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. September 2007. http://www.fda.gov/cber/guidelines.htm
- 21.Trimble CL, Morrow MP, Kraynyak KA. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386:2078–2088. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maslow JN. Vaccines for emerging infectious diseases: lessons from MERS coronavirus and Zika virus. Hum Vaccin Immunother. 2017;12:2918–2930. doi: 10.1080/21645515.2017.1358325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodmer BS, Fiedler AH, Hanauer JRH, Prüfer S, Mülebach MD. Live-attenuated bivalent measles virus-derived vaccines targeting Middle East respiratory syndrome coronavirus induce robust and multifunctional T cell responses against both viruses in an appropriate mouse model. Virology. 2018;521:99–107. doi: 10.1016/j.virol.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munster VJ, Wells D, Lanmbe T. Protective efficacy of a novel simian adenovirus vaccine against lethal MERS-CoV challenge in a transgenic DPP4 mouse model. NPJ Vaccines. 2017;2:28. doi: 10.1038/s41541-017-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haagmans BL, van den Brand JM, Raj VS. An orthopoxvirus-based vaccine reduces virus excretion after MERS coronavirus infection in dromedary camels. Science. 2016;351:77–81. doi: 10.1126/science.aad1283. [DOI] [PubMed] [Google Scholar]
- 26.Song F, Fux R, Provacia LB. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies. J Virol. 2013;87:11950–11954. doi: 10.1128/JVI.01672-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volz A, Kupke A, Song F. Protective efficacy of recombinant modified vaccinia virus Ankara delivering Middle East Respiratory Syndrome coronavirus spike glycoprotein. J Virol. 2015;89:8651–8656. doi: 10.1128/JVI.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.