Abstract
There is currently an outbreak of respiratory disease caused by a novel coronavirus. The virus has been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the disease it causes has been named coronavirus disease 2019 (COVID-19). More than 16% of patients developed acute respiratory distress syndrome, and the fatality ratio was 1%–2%. No specific treatment has been reported. Herein, we examined the effects of favipiravir (FPV) versus lopinavir (LPV)/ritonavir (RTV) for the treatment of COVID-19. Patients with laboratory-confirmed COVID-19 who received oral FPV (Day 1: 1600 mg twice daily; Days 2–14: 600 mg twice daily) plus interferon (IFN)-α by aerosol inhalation (5 million international unit (IU) twice daily) were included in the FPV arm of this study, whereas patients who were treated with LPV/RTV (Days 1–14: 400 mg/100 mg twice daily) plus IFN-α by aerosol inhalation (5 million IU twice daily) were included in the control arm. Changes in chest computed tomography (CT), viral clearance, and drug safety were compared between the two groups. For the 35 patients enrolled in the FPV arm and the 45 patients in the control arm, all baseline characteristics were comparable between the two arms. A shorter viral clearance median time was found for the FPV arm versus the control arm (4 d (interquartile range (IQR): 2.5–9) versus 11 d (IQR: 8–13), P < 0.001). The FPV arm also showed significant improvement in chest CT compared with the control arm, with an improvement rate of 91.43% versus 62.22% (P = 0.004). After adjustment for potential confounders, the FPV arm also showed a significantly higher improvement rate in chest CT. Multivariable Cox regression showed that FPV was independently associated with faster viral clearance. In addition, fewer adverse events were found in the FPV arm than in the control arm. In this open-label before-after controlled study, FPV showed better therapeutic responses on COVID-19 in terms of disease progression and viral clearance. These preliminary clinical results provide useful information of treatments for SARS-CoV-2 infection.
Keywords: Favipiravir, COVID-19, SARS-CoV-2, Antiviral therapy, Open-label nonrandomized control study
1. Introduction
A recent outbreak of coronavirus disease 2019 (COVID-19) caused by the novel coronavirus designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) started at the end of 2019. The clinical characteristics of COVID-19 include respiratory symptoms, fever, cough, dyspnea, and pneumonia [1], [2], [3], [4]. As of February 25, 2020, at least 77 785 cases and 2666 deaths had been identified across China [5] and in other countries; in particular, 977 and 861 cases were identified in Republic of Korea and Japan, respectively. The outbreak has already caused global alarm. On January 30, 2020, the World Health Organization (WHO) declared that the outbreak of SARS-CoV-2 constituted a Public Health Emergency of International Concern (PHEIC), and issued advice in the form of temporary recommendations under the International Health Regulations (IHR).
It has been revealed that SARS-CoV-2 has a genome sequence that is 75%–80% identical to that of SARS-CoV, and has more similarities to several bat coronaviruses [6]. SARS-CoV-2 is the seventh reported human-infecting member of the family Coronaviridae, which also includes SARS-CoV and the Middle East respiratory syndrome (MERS)-CoV. It has been identified as the causative agent of COVID-19. Both the clinical and the epidemiological features of COVID-19 patients demonstrate that SARS-CoV-2 infection can lead to intensive care unit (ICU) admission and high mortality. About 16%–21% of people with the virus in China have become severely ill, with a 2%–3% mortality rate [1], [4]. However, there is no specific treatment against the new virus. Therefore, it is urgently necessary to identify effective antiviral agents to combat the disease and explore the clinical effect of antiviral drugs.
One efficient approach to discover effective drugs is to test whether the existing antiviral drugs are effective in treating other related viral infections. Several drugs, such as ribavirin, interferon (IFN), favipiravir (FPV), and lopinavir (LPV)/ritonavir (RTV), have been used in patients with SARS or MERS, although the efficacy of some drugs remains controversial. It has recently been demonstrated that, as a prodrug, FPV (half maximal effective concentration (EC50) = 61.88 μmol·L−1, half-maximal cytotoxic concentration (CC50) > 400 μmol·L−1, selectivity index (SI) > 6.46) effectively inhibits the SARS-CoV-2 infection in Vero E6 cells (ATCC-1586) [7]. Furthermore, other reports show that FPV is effective in protecting mice against Ebola virus challenge, although its EC50 value in Vero E6 cells was as high as 67 μmol·L−1 [8]. Therefore, clinical studies are urgently needed to evaluate the efficacy and safety of this antiviral nucleoside for COVID-19 treatment.
In this study, we performed a comprehensive evaluation of the clinical efficacy of treatment for COVID-19 patients at the Third People’s Hospital of Shenzhen. We aimed to compare the clinical outcomes between patients who treated with FPV and patients treated with LPV/RTV. These findings will provide useful information for treatment of the SARS-CoV-2 infection.
2. Methods
2.1. Study design
Regarding the emergency epidemic situation of COVID-19, we conducted an open-label, nonrandomized, before-after controlled study in an isolation ward of the National Clinical Research Center for Infectious Diseases (the Third People’s Hospital of Shenzhen), Shenzhen, China. From January 30 to February 14, 2020, laboratory-confirmed patients with COVID-19 were consecutively screened, and eligible patients were included in the FPV arm of the study. Patients who had initially been treated with antiviral therapy with LPV/RTV from January 24 to January 30, 2020 were screened, and eligible patients were included in the control arm of the study. The study was conducted according to the guidelines of the Declaration of Helsinki and the principles of Good Clinical Practice, and was approved by the Ethics Committee of the third People’s Hospital of Shenzhen (No. 2020-002-02). Written informed consent was obtained from all patients. The study was reported according to the Consolidated Standards of Reporting Trials guidelines and was registered on the Chinese Clinical Trial Registry (ID: ChiCTR2000029600).
2.2. Eligibility criteria
All patients admitted to both the FPV and the control arms of the study were assessed for eligibility criteria. The inclusion criteria included: aged 16–75 years old; nasopharyngeal swabs samples tested positive for the novel coronavirus RNA; duration from disease onset to enrolment was less than 7 d; willing to take contraception during the study and within 7 d after treatment; and no difficulty in swallowing the pills. The exclusion criteria included the following: severe clinical condition (meeting one of the following criteria: a resting respiratory rate greater than 30 per minute, oxygen saturation below 93%, oxygenation index (OI) < 300 mmHg (1 mmHg = 133.3 Pa), respiratory failure, shock, and/or combined failure of other organs that required ICU monitoring and treatment); chronic liver and kidney disease and reaching end stage; previous history of allergic reactions to FPV or LPV/RTV; pregnant or lactating women; women of a childbearing age with a positive pregnancy test, breastfeeding, miscarriage, or within 2 weeks after delivery; and participated in another clinical trial against SARS-CoV-2 treatment currently or in the past 28 d.
2.3. Trial treatment
FPV (Zhejiang Hisun Pharmaceutical Co., Ltd., China; 200 mg per tablet) was given orally. The dose was 1600 mg twice daily on Day 1 and 600 mg twice daily on Days 2–14. LPV/RTV (AbbVie Inc., USA; 200 mg/50 mg per tablet) were given orally. The dose was LPV 400 mg/RTV 100 mg twice daily. Both FPV and LPV/RTV were continued until the viral clearance was confirmed or until 14 d had passed. In addition, all participants received IFN-α1b 60 µg (Beijing Tri-Prime Gene Pharmaceutical Co., China; 30 μg per ampule) twice daily by aerosol inhalation. Standard care included oxygen inhalation, oral or intravenous rehydration, electrolyte correction, antipyretics, analgesics, and antiemetic drugs.
2.4. Efficacy measures
The efficacy of the treatment was assessed by the time of viral clearance and the improvement rate of chest computed tomography (CT) scans on Day 14 after treatment. Chest CT scans were conducted on Days 4, 9, and 14 after treatment, with a fluctuation of 2 d. The CT findings were graded and scored using the method described previously [9], [10] by two medical diagnostic radiographers who were blind to grouping. The CT findings were graded on a three-point scale: 1 as normal attenuation, 2 as ground-glass attenuation, and 3 as consolidation. Each lung zone—with a total of six lung zones in each patient—was assigned a score on the following scale, according to the distribution of the affected lung parenchyma, using a method modified from a previously described protocol [10]: 0 as normal, 1 as 25% abnormality, 2 as 25%–50% abnormality, 3 as 50%–75% abnormality, and 4 as 75% abnormality. The five-point scale of the lung parenchyma distribution was then multiplied by the radiologic scale described above. Points from all zones were added for a final total cumulative score, with a value ranging from 0 to 72 (Fig. 1 ). A change of “improved” in the chest CT was defined as the total cumulative score being lower than before medication; a change of “worse” was defined as the total cumulative score being higher than before medication; and a change of “constant” was defined as the total cumulative score being the same as before treatment (Fig. 1).
Fig. 1.
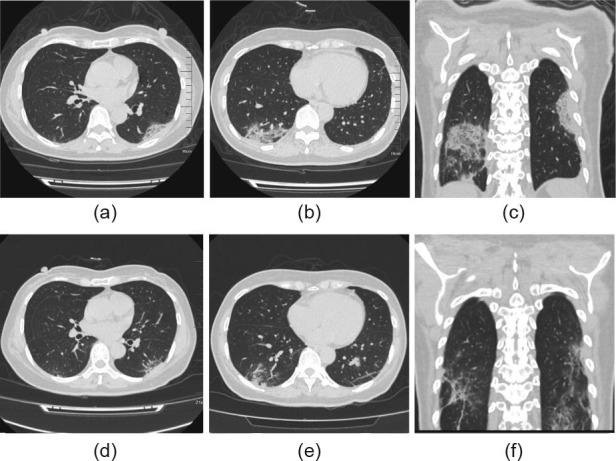
Score of chest CT scan for a 56-year-old female patient with COVID-19 from the FPV arm. (a–c) show parts of the CT images obtained prior to the FPV treatment, which were scored as 15 according to the scoring method. (d–f) show parts of the CT images obtained on Day 12 after the FPV treatment, which were scored as 6.
The presence of SARS-CoV-2 was detected by the real-time quantitative polymerase chain reaction (qPCR) method, as previously reported [5]. Viral ribonucleic acids (RNAs) were extracted from the samples using the QIAamp RNA viral kit (Qiagen, Germany), and quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed using a commercial kit specific for SARS-CoV-2 detection (GeneoDX Co., Ltd., China), which was approved by the China Food and Drug Administration (CFDA) (rebranded and restructured as the National Medical Products Administration of the State Administration for Market Regulation of the People's Republic of China since 2018). “Viral clearance” was defined as the presence of two consecutive negative results with qPCR detection over an interval of 24 h.
2.5. Safety analysis
Safety was assessed by a standardized questionnaire for adverse events and by laboratory tests.
2.6. Statistics analysis
The quantitative data were described as the mean ± standard deviation, or as the median (interquartile range (IQR)). The qualitative data were described by number of cases (proportion, %). Patient characteristics were compared using the χ2 test or Fisher’s exact test for categorical data, and the Wilcoxon rank-sum test or Student’s t-test for continuous data. The factors affecting the changes in chest CT were analyzed using binary logistic regression. The analysis of viral clearance time was calculated using the Kaplan–Meier method and the difference analysis of the viral clearance time under different treatments was calculated using the log-rank test. Potential influencing factors of viral clearance were analyzed by univariate and multivariate Cox regression models. A P value lower than 0.05 was required for statistical significance. All of the analysis was performed using SPSS Version 22.0 and GraphPad Prism 7.0.
3. Results
3.1. Patients and baseline analysis
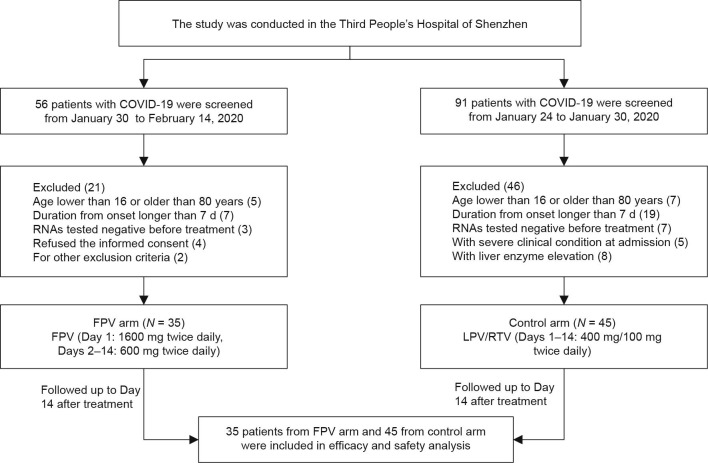
From January 30, 56 patients with laboratory-confirmed COVID-19 were screened, of which 35 were eligible for the FPV arm of the study. A total of 91 laboratory-confirmed COVID-19 patients who had started treatment with LPV/RTV between January 24 and January 30, 2020 were screened, of which 45 were eligible for the control arm of this study. All enrolled patients finished the therapy and were followed up for 14 d after the treatment began (Fig. 2 ). All the baseline characteristics were compared between the FPV and the control arms. As shown in Table 1 , there were no significant differences between the baseline characteristics of the two arms. All patients were moderate cases as defined by the National Health Commission of the People's Republic of China.
Fig 2.
Flowchart for the present trial. (FPV: LPV/RTV).
Table 1.
Baseline characteristics of patients with SARS-CoV-2 infection.
| Characteristic | COVID-19 patients |
|||
|---|---|---|---|---|
| Total (N = 80) | FPV (N = 35) | LPV/RTV (N = 45) | P value | |
| Age, median (IQR) | 47 (35.75–61) | 43 (35.5–59) | 49 (36–61) | 0.61 |
| Age subgroup | ||||
| 15–44 | 36 (45.0%) | 18 (51.4%) | 18 (40.0%) | — |
| 45–64 | 33 (41.3%) | 13 (37.1%) | 20 (44.4%) | — |
| ≥ 65 | 11 (13.7%) | 4 (11.4%) | 7 (15.6%) | 0.47 |
| Male | 35 (43.8%) | 14 (40.0%) | 21 (46.7%) | 0.55 |
| BMI, median (IQR) | 22.9 (16.2–31.6) | 22.7 (16.2–31.6) | 23.1 (16.4–28.4) | 0.51 |
| Epidemiology | ||||
| History of visiting Wuhan City | 46 (57.5%) | 20 (57.1%) | 26 (57.8%) | — |
| Not been to Wuhan City | 34 (42.5%) | 15 (20.0%) | 19 (17.8%) | 0.95 |
| Onset symptoms | ||||
| Fever | 59 (73.8%) | 22 (62.9%) | 37 (82.2%) | 0.11 |
| Cough | 22 (27.5%) | 12 (34.3%) | 10 (22.2%) | 0.23 |
| Headache/myalgia | 8 (10.0%) | 3 (8.6%) | 5 (11.1%) | 1.00 |
| Chill | 1 (1.3%) | 0 (0%) | 1 (2.2%) | 1.00 |
| Diarrhea | 1 (1.3%) | 1 (2.9%) | 0 (0%) | 0.44 |
| Stuffy nose/sore throat | 8 (10.0%) | 6 (17.1%) | 2 (4.4%) | 0.13 |
| Laboratory test, median (IQR) | ||||
| WBC (× 109 L−1) | 6.0 (3.5–5.2) | 8.1 (3.8–6.6) | 4.3 (3.4–4.9) | 0.21 |
| Neutrophils (× 109 L−1) | 2.8 (2.1–3.4) | 3.0 (2.1–3.7) | 2.6 (2.1–3.1) | 0.43 |
| Lymphocyte (× 109 L−1) | 1.3 (0.9–1.6) | 1.5 (1.0–1.8) | 1.2 (0.9–1.4) | 0.06 |
| ALT (U·L−1) | 22.2 (15.0–26.3) | 21.6 (15.0–24.0) | 22.6 (15.5–27.0) | 0.54 |
| AST (U·L−1) | 25.1 (18.0–28.0) | 24.1 (18.0–26.0) | 25.8 (19.0–31.0) | 0.47 |
| GGT (U·L−1) | 25.5 (14–31.1) | 26.9 (14.0–33.0) | 24.4 (14.4–31.1) | 0.48 |
| CRP (mg·dL−1) | 18.6 (5.0–20.0) | 15.0 (3.0–19.2) | 21.4 (5.0–23.2) | 0.33 |
| IL-6 (ng·L−1) | 13.4 (4.4–16.2) | 14.0 (3.5–11.0) | 12.9 (5.3–16.8) | 0.77 |
| T lymphocyte count | 973.8 (594.3–1227.0) | 1046.7 (600.8–1314.8) | 925.2 (572.8–1211.5) | 0.40 |
| CD4+ T lymphocyte count | 562.3 (382.5–733) | 593.3 (369.0–802.75) | 542.3 (388.0–689.0) | 0.54 |
| CD8+ T lymphocyte count | 354.4 (206.5–496.5) | 397.8 (212.3–528.5) | 326.4 (207.5–423) | 0.76 |
| Ct values, median (IQR) | 30.0 (26.5–33.8) | 30.7 (28.0–33.3) | 29 (26.0–34.0) | 0.38 |
| Chest CT score, median (IQR) | 9.5 (4.0–14.0) | 12 (4.0–14.0) | 9 (4.5–14.0) | 0.78 |
BMI: body mass index; WBC: white blood cell; ALT: alanine aminotransferase; AST: aspartate aminotransferases; GGT: γ-glutamyl transpeptidase; CRP: c-reactive protein; IL: interleukin; Ct: cycle threshold.
3.2. Viral response to the antiviral therapy
The Kaplan–Meier survival curves for the length of time until viral clearance for both kinds of antiviral therapy were presented in Fig. 3 . The median time of viral clearance for the patients treated with FPV was estimated to be 4 d (IQR: 2.5–9), which was significantly shorter than the time for patients in the control arm, which was 11 d (IQR: 8–13) (P < 0.001). Two patients in the FPV arm turned negative for viral RNA detection in nasopharyngeal swabs at Days 18 and 21, respectively. For patients in the control arm, the viral RNA detection all turned negative within 27 d.
Fig 3.
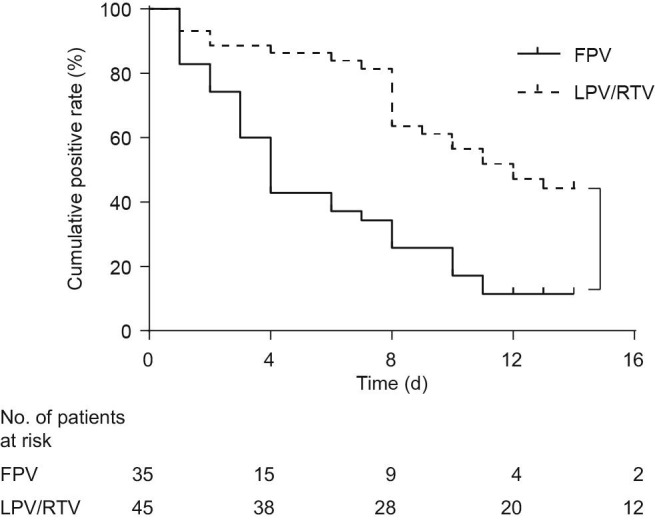
Kaplan–Meier survival curves for the length of time until viral clearance for both kinds of antiviral therapy (P < 0.001).
3.3. Chest CT changes in COVID-19 patients’ response to treatment
The non-parametric Mann–Whitney U test was used to determine the significance of the difference between the chest CT changes in response to the two different treatments (Table 2 ). Meanwhile, the improvement rates of the chest CT changes for the two arms of the study were compared on Days 4, 9, and 14 after treatment. No significant difference in the improvement rates was found between the two arms on Days 4 and 8 (P > 0.05). However, on Day 14 after treatment, the improvement rates of the chest CT changes in the FPV arm were significantly higher than those in the control arm (91.4% versus 62.2 %, 32/35 versus 28/45, P = 0.004).
Table 2.
Chest CT changes in patients with COVID-19 after treatment.
| Chest CT changes | COVID-19 patients (N = 80) |
||
|---|---|---|---|
| FPV (N = 35) | LPV/RTV (N = 45) | P value | |
| Day 4 after treatment | |||
| Improved | 8 (22.86%) | 8 (17.78%) | — |
| Worse | 9 (25.71%) | 15 (33.33%) | — |
| Constant | 18 (51.43%) | 22 (48.89%) | 0.42 |
| Day 9 after treatmenta | — | ||
| Improved | 18 (56.25%) | 16 (35.55%) | — |
| Worse | 8 (25.00%) | 16 (35.55%) | — |
| Constant | 6 (18.75%) | 13 (28.90%) | 0.11 |
| Day 14 after treatment | — | ||
| Improved | 32 (91.43%) | 28 (62.22%) | — |
| Worse | 1 (3.23%) | 9 (20.00%) | — |
| Constant | 2 (6.45%) | 8 (17.78%) | 0.004 |
For three patients in the FPV arm, the chest CT scan on Days 6–9 after medication was not carried out.
Furthermore, the patients were divided into two groups based on the time of viral clearance. On Day 14 after treatment, the improvement rates of the chest CT changes in the group with viral clearance within 7 d of treatment were higher than those of the patients with viral clearance after 7 d of treatment (Fig. 4 ).
Fig 4.
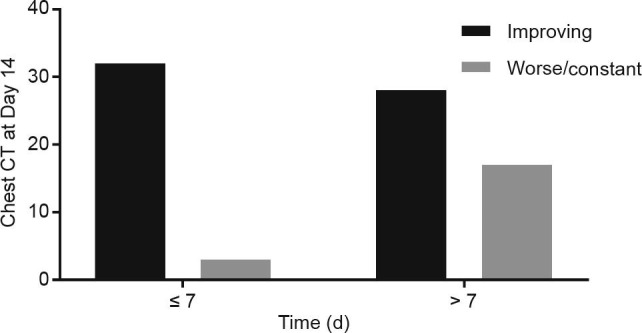
Time of viral clearance and improving chest CT scan on Day 14 after treatment.
3.4. Multivariate analysis of the changes in chest CT
Univariate analysis using χ 2 test, t-test, or Wilcoxon rank-sum test was conducted before multivariate analysis; the significant variables (P < 0.10) in the univariate analysis were as follows: Antiviral therapy and whether or not fever was present. A multivariate logistic regression analysis was conducted to identify the independent factors affecting the changes in chest CT. We chose the change in chest CT (0 = no change or worse, 1 = improved) as the dependent variable, and the variables that were significant in the univariate analysis or were professionally significant (including age, underlying disease, and severity of disease in baseline) as the independent variables. The result showed that there were two statistically significant factors in the model: antiviral therapy (odds ratio (OR) = 3.190, 95% confidence interval (CI) = 1.041–9.780) and fever (OR = 3.622, 95%CI = 1.054–12.442). This means that antiviral therapy and fever were independent factors that affected the chest CT after we had controlled the confounding factors. The patients who were treated with FPV had greater improvement in chest CT (Table 3 ).
Table 3.
Logistic regression of changes in chest CT.
| Factors | Partial regression coefficient | Standard error | Wald | P value | OR | OR 95%CI |
|---|---|---|---|---|---|---|
| Age | −0.019 | 0.018 | 1.167 | 0.280 | 0.981 | 0.947–1.016 |
| Antiviral therapy | 1.160 | 0.572 | 4.121 | 0.042 | 3.190 | 1.041–9.780 |
| Fever | 1.287 | 0.630 | 4.177 | 0.041 | 3.622 | 1.054–12.442 |
| Underlying diseases | 0.279 | 0.961 | 0.084 | 0.771 | 1.322 | 0.201–8.693 |
| Severity in baseline | 21.080 | 40192.970 | 0 | 1.000 | 1.43 × 109 | 0−∞ |
| Constant | 0.036 | 1.040 | 0.001 | 0.973 | 1.036 | — |
3.5. Multivariate analysis of viral clearance
Univariate analysis using the log-rank test and univariate Cox regression was conducted before the multivariate analysis; the significant variables (P < 0.10) in the univariate analysis were as follows: antiviral therapy, platelet (PLT), T lymphocyte count, and time from onset to treatment. A multivariate Cox regression model was used to explore the independent factors affecting viral clearance. The time of viral clearance was set as the TIME variable, viral clearance (0 = no, 1 = yes) was set as the status, and the variables that were significant (P < 0.10) in the univariate Cox regression analysis or were professionally significant (including age, and whether underlying diseases were present or not) were set as independent variables. The result showed that the model was significant (P = 0.003). The significant factors were as follows: T lymphocyte count (hazard ratio (HR) = 1.002, 95%CI = 1.000–1.005) and antiviral therapy (HR = 3.434, 95%CI = 1.162–10.148). This means that the treatment and T lymphocyte count were independent factors that affected the viral clearance after we controlled the other confounding factors. As the result shows, compared with LPV/RTV, FPV has a greater effect on viral clearance (Table 4 ).
Table 4.
Cox regression of viral clearance.
| Factors | Partial regression coefficient | Standard error | Wald | P value | HR | HR 95%CI |
|---|---|---|---|---|---|---|
| WBC | −0.866 | 0.602 | 2.072 | 0.150 | 0.421 | 0.129–1.368 |
| Hb | 0.002 | 0.017 | 0.011 | 0.917 | 1.002 | 0.969–1.036 |
| PLT | 0.011 | 0.006 | 2.818 | 0.093 | 1.011 | 0.998–1.024 |
| Neutrophils | 0.805 | 0.657 | 1.500 | 0.221 | 2.236 | 0.617–8.105 |
| T lymphocyte count | 0.002 | 0.001 | 5.165 | 0.023 | 1.002 | 1.000–1.005 |
| CD8+ T lymphocyte | −0.003 | 0.002 | 1.557 | 0.212 | 0.997 | 0.993–1.002 |
| Time from onset to treatment | 0.196 | 0.102 | 3.675 | 0.055 | 1.217 | 0.996–1.486 |
| FPV versus LPV/RTV | 1.234 | 0.553 | 4.980 | 0.026 | 3.434 | 1.162–10.148 |
| Age | 0.015 | 0.988 | 1.000 | 0.971–1.029 | ||
| Underlying diseases | −0.785 | 1.006 | 0.609 | 0.435 | 0.456 | 0.064–3.275 |
3.6. Adverse events after medication
The total number of adverse events in the FPV arm of the study was four (11.43%), which was significantly fewer than the 25 adverse events (55.56%) in the control arm (P < 0.001). Two patients had diarrhea, one had a liver injury, and one had a poor diet in the FPV arm. Meanwhile, there were five patients with diarrhea, five with vomiting, six with nausea, four with rash, three with liver injury, and two with chest tightness and palpitations in the control arm (Table 5 ).
Table 5.
Statistics of adverse reactions after medication.
| Characteristic | Treatment |
||
|---|---|---|---|
| FPV (N = 35) | LPV/RTV (N = 45) | P value | |
| Total number of adverse reactions | 4 (11.43%) | 25 (55.56%) | < 0.001 |
| Diarrhea | 2 (5.71%) | 5 (11.11%) | 0.46 |
| Vomiting | 0 (0%) | 5 (11.11%) | 0.06 |
| Nausea | 0 (0%) | 6 (13.33%) | 0.03 |
| Rash | 0 (0%) | 4 (8.89%) | 0.13 |
| Liver and kidney injury | 1 (2.86%) | 3 (6.67%) | 0.63 |
| Others | 1 (2.86%) | 2 (4.44%) | 1.00 |
4. Discussion
In this open-label comparative controlled study of patients with COVID-19, those treated with FPV appeared to have faster viral clearance and better chest CT changes than patients treated with LPV/RTV. As this is not a randomized, double-blind, parallel trial, further well-designed and large-scale confirmatory trials are warranted. However, given the huge influence caused by the spread of COVID-19 worldwide, our results may provide useful information of treatments for this emerging disease.
FPV, which is known as a prodrug, is a novel RNA-dependent RNA polymerase (RdRp) inhibitor, which has been shown to be effective in the treatment of influenza and Ebola virus [8], [11], [12], [13], [14], [15]. Recently, a report from Wang et al. [7] showed that both FPV and remdesivir were effective in reducing the SARS-CoV-2 infection in vitro (EC50 = 61.88 μmol·L−1, CC50 > 400 μmol·L−1, SI > 6.46). This study highlighted FPV as a potential clinical intervention for COVID-19.
The quasi-experimental design of the present study might have been open to selection bias in patient recruitment. However, given the large number of patients presenting simultaneously and the very high infectivity of the disease, it was ethically unacceptable to allocate patients to receive different experimental drugs, and a randomization process was infeasible. Furthermore, in the context of rumors and distrust of hospital isolation, using a randomized design at the outset might have led even more patients to refuse being isolated. Therefore, we chose to conduct a before-after designed trial, in which patients consecutively admitted to the hospital during two separate periods were included in two groups, respectively. As the baseline characteristics of the two groups were comparable and the results remained after adjustment for potential confounders, the influence due to confounding bias, if any, should not be a major concern.
The current study also found that early viral clearance contributed to the improvement of chest CT on Day 14. This finding suggests that improvement of the disease may depend on inhibition of the SARS-CoV-2, and that FPV controls the disease progression of COVID-19 by inhibiting the SARS-CoV-2. Until recently, the pathogenesis of COVID-19 had not been well clarified. Since the infection of SARS-CoV-2 was thought to be self-limited and characterized by systemic inflammation reaction, symptomatic and supportive treatment was mainly recommended by the WHO and the National Health Commission of the People's Republic of China. This description is similar to MERS-CoV, for which nonspecific therapeutic interventions are often introduced to prevent severe morbidity and mortality [16]. How antivirals would contribute to control of the disease is controversial. Although there have been many registered clinical trials focusing on antiviral drugs for COVID-19, the timing, duration of treatment, and study endpoints have not been unified. In the current study, the time of viral clearance was introduced as a primary endpoint to evaluate the antiviral effect of FPV on the SARS-CoV-2 and successfully identify the priority of FPV. The relationship between the time of viral clearance and the improvement in chest CT indicates that viral clearance is an ideal surrogate for the clinical endpoint. A limitation of the present study was that the relationship between the viral titer and the clinical prognosis was not well clarified. Future research could pay more attention to this point.
More adverse events occurred in the control arm than those in the FPV arm, and the adverse event rate was similar to previous studies of LPV/RTV in patients with acquired immune deficiency syndrome (AIDS). It is worth mentioning that the treatment duration of FPV in the present study was twice as long as that used for treating influenza. However, the adverse events in the experimental arm were rare and tolerable, and none of the patients needed to discontinue FPV treatment. These results suggest that the treatment duration of FPV may be prolonged if necessary. All the patients were discharged with 2 consecutive negative RNA detection (interval above 24 h) and clinical improvement, and were isolated at designated isolation location and followed for another 14 d after discharge.
SARS-CoV-2 infection has been spreading quickly all over the world; while specific drugs have not yet been consolidated for the time being. The task at hand was to run a well-designed trial to identify effective treatments based on a high level of evidence. However, at the beginning of this study, certain conditions did not allow the randomization of patients to receive either standard care or an experimental drug. In this pilot study of a before-after controlled trial, we found that FPV showed better treatment outcomes in COVID-19 patients in terms of their disease progression and viral clearance. Our results provided preliminary evidence for treatment of the SARS-CoV-2 infection. Furthermore, we introduced the time of viral clearance, which can be used as a primary endpoint for trials on antiviral treatment, and might be a useful surrogate outcome for designing protocols investigating COVID-19 related treatments as well.
Acknowledgements
The authors are very grateful to Professor Li Song for his guidance in the study design and clinical research. This work was supported by the National Science and Technology Major Project (2017ZX10204401, 2018ZX10711001, 2017ZX10103011, 2018ZX09711003, and 2020YFC0841700), Sanming Project of Medicine in Shenzhen (SZSM201412003 and SZSM201512005), Shenzhen Science and Technology Research and Development Project (202002073000001), China Postdoctoral Science Foundation (2019M660836), Guangdong Special Fund for Science and Technology Innovation Strategy in 2020, and the Science and Technology Emergency Project for the prevention and control of the novel coronavirus by the Department of Science and Technology of Guangdong Province (2020B111105001).
5. Authors’ contribution
Lei Liu, Yingxia Liu, Qingxian Cai, Minghui Yang, Wu Zhong, and Jun Chen contributed to the study design. Qingxian Cai, Minghui Yang, Dan Shu, Junxia Xia, Xuejiao Liao, Dongjing Liu, Yuanbo Gu, Qiue Cai, Xiaohe Li, Jiaye Liu,Ling Peng, Deliang Huang, and Jing Zhang contributed to the collection of clinical data. Qingxian Cai, Minghui Yang, Shurong Zhang, Fuxiang Wang, Li Chen, Shuyan Chen, Zhaoqin Wang, and Zheng Zhang contributed to the data analysis. Qingxian Cai, Minghui Yang, Jun Chen, Yang Yang, Chenguang Shen, Ruiyuan Cao, and Wu Zhong contributed to the manuscript preparation. All the authors have read and approved the manuscript.
Compliance with ethics guidelines
Qingxian Cai, Minghui Yang, Dongjing Liu, Jun Chen, Dan Shu, Junxia Xia, Xuejiao Liao, Yuanbo Gu, Qiue Cai, Yang Yang, Chenguang Shen, Xiaohe Li, Ling Peng, Deliang Huang, Jing Zhang, Shurong Zhang, Fuxiang Wang, Jiaye Liu, Li Chen, Shuyan Chen, Zhaoqin Wang, Zheng Zhang, Ruiyuan Cao, Wu Zhong, Yingxia Liu, and Lei Liu declare that they have no conflict of interest or financial conflicts to disclose.
Footnotes
Author’s note to “Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study.” The work by Cai et al., 2020, had been published its initial version online on 23rd March 2020 and was temporarily retracted on 2nd April 2020 upon the editor’s request to prevent any possible dispute on some expressions. The authors did not modify the data and the conclusion. The corrected version with language editing and format-changes in figures is now available online from 16th April 2020. The authors apologize for any inconvenience caused.
References
- 1.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Health Commission of the People’s Republic of China. Daily briefing on novel coronavirus cases in China [Internet]. Beijing: National Health Commission of the People’s Republic of China; c2020 [updated 2020 Mar 12; cited 2020; c2020 Feb 25]. Available form: http://en.nhc.gov.cn/DailyBriefing.html.
- 6.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oestereich L., Lüdtke A., Wurr S., Rieger T., Muñoz-Fontela C., Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res. 2014;105:17–21. doi: 10.1016/j.antiviral.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Grieser C., Goldmann A., Steffen I.G., Kastrup M., Fernández C.M., Engert U. Computed tomography findings from patients with ARDS due to Influenza A (H1N1) virus-associated pneumonia. Eur J Radiol. 2012;81(2):389–394. doi: 10.1016/j.ejrad.2010.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang Y., Yu C., Chang S., Galvin J.R., Liu H., Hsiao C. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology. 2005;236(3):1067–1075. doi: 10.1148/radiol.2363040958. [DOI] [PubMed] [Google Scholar]
- 11.Madelain V., Oestereich L., Graw F., Nguyen T.H., De Lamballerie X., Mentré F. Ebola virus dynamics in mice treated with favipiravir. Antiviral Res. 2015;123:70–77. doi: 10.1016/j.antiviral.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Sissoko D., Laouenan C., Folkesson E., M’Lebing A.B., Beavogui A.H., Baize S. Experimental treatment with favipiravir for Ebola virus disease (the JIKI trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 2016;13(3) doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouazza N., Treluyer J.M., Foissac F., Mentré F., Taburet A.M., Guedj J. Favipiravir for children with Ebola. Lancet. 2015;385(9968):603–604. doi: 10.1016/S0140-6736(15)60232-X. [DOI] [PubMed] [Google Scholar]
- 15.MDVI, LLC. Phase 3 efficacy and safety study of favipiravir for treatment of uncomplicated influenza in adults [Internet]. Bethesda: National Library of Medicine; [update 2015 Nov 11; cited 2020 Mar 7]. Available from: https://clinicaltrials.gov/ct2/show/NCT02008344.
- 16.Chafekar A., Fielding B.C. MERS-CoV: understanding the latest human coronavirus threat. Viruses. 2018;10(2):E93. doi: 10.3390/v10020093. [DOI] [PMC free article] [PubMed] [Google Scholar]