Abstract
Background:
Older adults ≥75 years of age carry an increased risk of mortality after ST-elevation myocardial infarction (STEMI) complicated by cardiogenic shock.
Objectives:
To examine the use of percutaneous coronary intervention (PCI) in older adults with STEMI and shock and its influence on in-hospital mortality.
Methods:
We used a large publicly available all-payer inpatient health care database sponsored by AHRQ between 1999 to 2013. The primary outcome was in-hospital mortality. The influence of PCI on in-hospital mortality was assessed by quintiles of propensity score (PS).
Results:
Of the 317,728 encounters with STEMI and shock in the U.S., 111,901 (35%) were adults ≥75 years. Of these, 53% were females and 83% were Caucasians. The median [IQR] number of chronic conditions was 8 [6,10]. The diagnosis of STEMI and cardiogenic shock in older patients decreased significantly over time (proportion of older adults with STEMI and shock: 1999: 42% vs. 2013: 29%). Concomitantly, the rate of PCI utilization in older adults increased (1999: 27% vs 2013: 56%, p <0.001), with declining in-hospital mortality rates (1999: 64% vs. 2013: 46%, p <0.001). Utilizing PS matching methods, PCI was associated with lower risk of in-hospital mortality across quintiles of propensity score (Mantel-Haenszel OR: 0.48, 95% CI 0.45–0.51). This reduction in hospital mortality risk was seen across the 4 different United States census bureau regions (Adjusted OR: Northeast: 0.41, 95% CI 0.36–0.47; Midwest: 0.49, 95% CI 0.42–0.57; South: 0.51, 95% CI 0.46–0.56; West: 0.46, 95% CI 0.41–0.53).
Conclusions:
This large and contemporary analysis shows that utilization of PCI in older adults with STEMI and cardiogenic shock is increasing and paralleled by a substantial reduction in mortality. While clinical judgement is critical, older adults should not be excluded from early revascularization based on age in the absence of absolute contraindications.
Keywords: Older adults, Cardiogenic shock, ST Elevation Myocardial Infarction, Percutaneous Coronary Intervention, Mortality
Condensed Abstract
The influence of percutaneous coronary intervention (PCI) in adults ≥ 75 years of age with STEMI and cardiogenic shock was examined since the publication of the SHOCK trial. Using propensity score methods, treatment with PCI appears to be associated with significant improvement in hospital mortality in older patients, but the risk is higher in patients with multiple coexisting conditions or high weight of disease burden. From a public health perspective, early revascularization should be considered for older adults in the absence of absolute contraindications (i.e. active bleeding, severe neurocognitive decline, and very limited life expectancy with end-stage disease processes).
Introduction
The population of older adults is rapidly expanding and is in fact the fastest growing sector of the United States census (1,2). Although life expectancy is increasing, and there have been significant therapeutic advances, the burden of cardiovascular disease among older adults remains high. Older patients ≥75 years of age constitute 14–28% of all patients with ST-segment-elevation myocardial infarction (STEMI)—an illness that is among those most associated with death and disability (3–6). Among older adults with STEMI, >10% will develop cardiogenic shock, defined as the presence of hypotension and hypoperfusion—a condition associated with a mortality rate as high as 79% (3).
The two reports from “The SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK?” (SHOCK) Trial failed to find a significant benefit of early revascularization for older patients ≥75 years of age, contrasting with the situation in younger adults (7,8). However, a follow-up study from the SHOCK TRIAL Registry, using a larger sample size than the original clinical trial, revealed that PCI in older patients was indeed associated with improved survival, reducing the estimated hospital mortality to 48% (9). Following these publications, the 2013 American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) Guideline for the Management of STEMI did not exclude older adults with cardiogenic shock from early revascularization. Consideration of risk factors (including the presence of frailty, multimorbidity, and life expectancy) was advised in decision making about this treatment (10).
Older age is associated with lower rates of early surgical and percutaneous revascularization rates, and higher in-hospital mortality, when compared with younger patients. These figures are based on small-sized studies: National estimates on the use of percutaneous coronary intervention (PCI) during hospital admission in older patients with STEMI and cardiogenic shock have not been examined. Furthermore, the influence of PCI on in-hospital mortality in older adults with STEMI and cardiogenic shock remains controversial (11). In our present study, we aimed to exploit nationally representative data to: (1) evaluate the rate of utilization of PCI in older adult patients ≥75 years of age with STEMI and cardiogenic shock; and (2) examine the influence of PCI on in-hospital mortality in older patients since the publication of the SHOCK Trial.
Methods
The National Inpatient Sample (NIS) contains all discharge data from an approximate 20% stratified sample of hospitals in the United States, excluding rehabilitation and long-term acute care facilities. It is part of the Healthcare Quality and Utilization Project (HCUP), which is sponsored by the Agency for Healthcare Research and Quality (AHRQ) (12). Data are used to identify, track, and analyze national trends in healthcare utilization, access and disparity, and develop measures to improve quality of care. Each individual hospitalization is de-identified and maintained as a unique entry with one primary discharge diagnosis and ≤25 secondary diagnoses. Annual data quality assessments are performed to assure the internal validity of the database. Estimates from the NIS are compared to the American Hospital Association Annual Survey Database, the National Hospital Discharge Survey from the National Center for Health Statistics, and the MedPAR inpatient database from Centers of Medicare and Medicaid.(13) Reports describing these comparisons are published in the NIS website(14) and show that, in most respects, the data from the NIS resemble the data from typical hospitals in the American Hospital Association. All NIS and National Hospital Discharge Survey estimates agree in overall and regional comparisons, and NIS Medicare measures are consistent with MedPar statistics (15) These reports strengthen the external validity of NIS database. Data on baseline demographics, insurance status, comorbidities, hospital outcome, and length of stay were available in the reports for analysis. Ascertainment for the primary disposition was performed for each encounter and reported as either discharged routinely, against medical advice, transferred to another health care facility, or died. To ensure that the results were accurate, we verified the data against the HCUP standards, available from the HCUP website.
All adult patients ≥75 years of age who experienced STEMI and cardiogenic shock during an index hospitalization occurring between 1999 and 2013 were included in our analysis. In order to minimize bias as a result of referral to a PCI-site from non-PCI capable hospital, we only included patients who were admitted from the Emergency Department. Patients who were not admitted to the hospital in whom a decision was made to go for PCI but died on the way to the catheterization laboratory or died while waiting for an available catheterization laboratory in the emergency department were excluded by design of the NIS. STEMI was identified using International Classification of Diseases, 9th Revision, codes (ICD-9) diagnoses codes 410.1x, 410.2x, 410.3x, 410.4x, 410.5x, 410.6x, and 410.8x as a primary or as any secondary diagnosis. Cardiogenic shock was identified as an event occurring during the same hospitalization as STEMI, using the ICD-9 of 785.51, and PCI was identified using ICD-9-CM procedure codes of 36.01 to 36.07, 36.09, or 0.66 at any time during the hospitalization (13). As such, PCI could have been performed as either early or late (semi-elective) in relation to hospital admission date.(13) Bleeding was identified using ICD-9-CM diagnosis codes: 430 to 432, 578.X, 719.1X, 423.0, 599.7, 626.2, 626.6, 626.8, 627.0, 627.1, 786.3, 784.7, or 459.0.(16) Death during index hospitalization was the primary outcome of this study. Hospitals enrolled in the program report mortality data as a quality indicator to AHRQ in order to measure and track clinical performance and outcomes. Ascertainment for the primary disposition was performed for each encounter and reported as either discharged routinely, against medical advice, transferred to another health care facility, or died. Comorbid diagnoses such as hypertension, diabetes mellitus with or without complications, obesity, valvular heart disease, peripheral vascular disease, pulmonary circulation disease, chronic obstructive pulmonary disease, renal failure, liver failure, coagulopathy, weight loss, fluid and electrolyte disorder, chronic blood loss anemia and alcohol abuse were pre-identified according to AHRQ definitions. (14).
Statistical analysis
Baseline demographics, comorbidities, hospital characteristics, and inpatient outcomes were compared for older adults ≥ 75 years of age who were treated with versus without PCI. For years 1999 to 2013, we used NIS discharge weights to produce discharge-level estimates for the diagnosis of STEMI and cardiogenic shock.(14) We did not use hospital-level estimates in this analysis because of the new NIS data structure redesign beginning in 2012 thereafter. For years prior to 2012, trend weights were used to create national estimates that are consistent with the data after 2012.(14) Weighted frequencies and percentages were calculated for categorical variables, and weighted means and standard deviations for continuous variables. Annual trends in PCI and mortality rates were assessed, based on discharge weights, using linear regression with calendar year as an independent variable.
To address confounding by comorbidity, we used methods introduced by Rosenbaum and Rubin utilizing propensity scores in observational studies.(17) The propensity score is the probability of treatment (PCI) given in terms of potentially confounding variables (here denoted by X2−Xp):
Rosenbaum and Rubin showed that, when comparing persons with the same propensity score, the distributions of confounding variables are the same among all treatment groups. This is the “balancing” property: It implies, in an idealized sense, that analyses that compare persons with like propensity scores eliminate confounding by the variables used to construct the scores. Using logistic regression, propensity scores were estimated by modeling the associations of the following covariates with treatment by PCI: gender, race, diabetes mellitus, hypertension, obesity, perivascular vascular disease, pulmonary circulation disorder, chronic lung disease, renal failure, liver disease, coagulopathy, weight loss, electrolyte and fluid disorder, blood loss, and alcohol abuse.
Propensity score distributions were similar between the PCI vs. non-PCI groups (Figure 1), indicating control for confounding to be possible across the entire range of the propensity score. The balancing properties of the covariates distribution between the PCI and non-PCI group were assessed within each subclassification of the propensity score, and the balancing properties were satisfied.(18) We observed the log-transformed probability of death to decrease sharply after an estimated propensity score of 0.4 for being exposed to treatment with PCI, however (Online Figure 1). For these reasons, we chose to model the propensity score in two ways. First, as described by Rubin and colleagues(19), the propensity score for receiving treatment with PCI was subclassified into five groups (i.e. quintiles), and analyses comparing mortality by treatment status were performed within each group. Second, the propensity score was treated as continuous variable in a regression of mortality on treatment, with a spline term at a propensity score of 0.4 (Online Figure 1).
Figure 1. Overlap plot for propensity score.
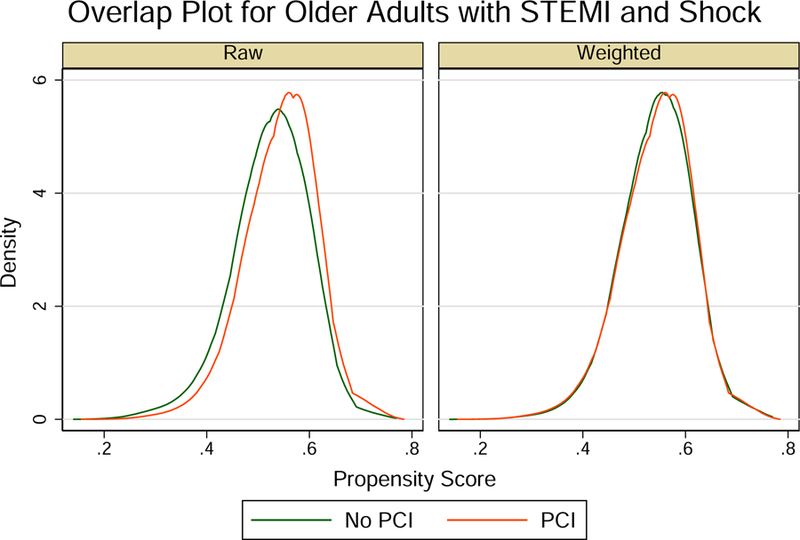
Overlap plot for the estimated density of the propensity scores among older adults presenting with ST-elevation myocardial infarction (STEMI) and cardiogenic shock treated with versus without percutaneous coronary intervention (PCI) in (A) raw data (B) weighted data.
Online Figure 1.

Log-transformed probability of death by the propensity score. The graph illustrates a decrease in the risk of death at a propensity score [Pr(PCI) = 0.4]. As such a spline term at Pr(PCI) = 0.4 was chosen.
To compare older adults with STEMI and cardiogenic shock by treatment with PCI, we performed a logistic regression to examine the association of hospital mortality with treatment by PCI (versus non-PCI) within each of the five quintiles of the propensity score. We generated the Mantel-Haenszel odds ratio estimate for in hospital mortality controlling for propensity block for those treated with versus without PCI. We failed to reject the test for homogeneity of odds ratio, indicating a good model fit (p = 0.508). Mindful of tradeoffs between accuracy and precision when applying weighting in regression analyses, we estimated the association between treatment with PCI and in hospital mortality within propensity score subclassifications using both unweighted and discharge-weighted analyses. Because the parameters between the unweighted and the weighted data were similar, we have elected to present the estimates derived from the unweighted sample as they are generally more efficient.(20) Because ischemic and bleeding risk coexist in this high-risk cohort, and it might be influenced by PCI and consequent antithrombotic therapies, we performed a sensitivity analysis including bleeding events during hospital admission in the propensity score models. In order to address immortal-time bias that could favor survival for the PCI group, we performed 2 additional sensitivity analyses. First, we repeated the propensity score analysis after excluding patients who died within the first 48 hours of hospital admission. Second, we repeated the propensity score analysis of PCI on hospital mortality after excluding patients who died within the first week of hospital admission.
We then aimed to estimate the association between treatment with PCI and in-hospital mortality by United States census bureau regions. Accordingly, we performed region-specific logistic regression analyses. We presented the estimated treatment effects in older adults with STEMI and cardiogenic shock using survey analysis methods. We reproduced the results using inverse probability treatment weighting, which is defined as the inverse of the estimated propensity score for PCI patients and the inverse of one minus the estimated propensity score for non-PCI patients (18). All statistical analyses were performed using STATA version 15 MP (Stata-Corp, College Station, Texas). We considered p value of <0.05 as significant, and all tests were two sided. To avoid common errors in the study-design, we followed a checklist that was developed to highlight best research practices and appropriate use of this large administrative database (Online Table 2) (21,22). This study was exempt from full IRB review because it is publicly available and without patient identifiers.
Online Table 2.
Estimated treatment effects in older adults comparing percutaneous coronary intervention (PCI) to no PCI on the risk of in hospital mortality after ST-elevation myocardial infarction and cardiogenic shock by propensity score subclass excluding patients who died within the first 48 hours of hospital admission.
| Propensity Score Subclass1 | Treatment | N (%) | Events (%) | OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| 1 | PCI | 835 (39) | 285 (34) | 0.54 | 0.46 – 0.66 | <0.001 |
| No PCI | 1,316 (61) | 638 (49) | – | – | – | |
| 2 | PCI | 1,372 (45) | 438 (32) | 0.61 | 0.52 – 0.71 | <0.001 |
| No PCI | 1,645 (55) | 715 (44) | – | – | – | |
| 3 | PCI | 740 (47) | 248 (34) | 0.66 | 0.54 – 0.81 | <0.001 |
| No PCI | 850 (53) | 368 (43) | - | – | – | |
| 4 | PCI | 1,664 (47) | 431 (26) | 0.52 | 0.45 – 0.60 | <0.001 |
| No PCI | 1,866 (53) | 751 (40) | – | – | – | |
| 5 | PCI | 2,934 (50) | 688 (23) | 0.47 | 0.42 – 0.52 | <0.001 |
| No PCI | 2,952 (50) | 1,167 (40) | – | – | – |
Abbreviations: OR = Odds Ratio; CI = confidence interval
Propensity Score: Using logistic regression, the propensity score was estimated by modeling the associations of the following covariates with treatment (percutaneous coronary intervention) given the covariates: gender, race, diabetes mellitus, hypertension, obesity, peripheral vascular disease, pulmonary circulation disorder, chronic lung disease, renal failure, liver disorder, coagulopathy, weight loss, electrolyte imbalance, blood loss, and alcoholism.
Results
We identified 64,766 unique encounters who were admitted with STEMI and cardiogenic shock from 1999 to 2013. Using survey analysis methods, the number of weighted hospital admissions nationally over the same period was estimated as 317,728 encounters, of whom 111,901 (35%) were for patients age 75 years or older. The baseline characteristics for older adults with STEMI and cardiogenic shock were derived from weighted data and presented in Table 1. Among these older adults, those who did not receive PCI were older in age and more likely to be female and belong to an underrepresented minority. The differences in the prevalence of most cardiovascular risk factors and non-cardiovascular diagnoses achieved statistical significance, commensurate with the very large sample size, but a difference of ~ 20% or more was observed for obesity (higher prevalence for those PCI-treated) and valvular heart disease and congestive heart failure (lower prevalence for those PCI-treated). Older patients who received PCI had significantly lower disease burden compared to those who did not receive PCI as estimated by the Charlson comorbidity index. Medicare was the primary payer for >90% of older patients. Most patients were cared for by large hospital systems in urban locations (Table 1). Older adults who were not treated with PCI had shorter mean hospital length of stay, lower mean total hospital charges, and higher crude mortality rate (Table 1). The proportion of older patients admitted with STEMI and cardiogenic shock decreased over time following the SHOCK trial, and moreover, this decline in hospital admissions was observed in all regions of United States (Figure 2). While there is a consistent decline in rates of STEMI and cardiogenic shock in older patients, the rates of utilization of PCI in STEMI and cardiogenic shock since the publication of the SHOCK trial results increased progressively during the study period, and this was paralleled by a substantial reduction in the crude unadjusted mortality rates (Central Illustration).
Table 1.
Baseline characteristics for older adults with ST elevation myocardial infarction and cardiogenic shock.
| Variable | Overall | No PCI | PCI | p-value* |
|---|---|---|---|---|
| Unweight No. (%) | 22,774 (100) | 13,165 (57.8) | 9,609 (42.2) | - |
| Weighted No. (%) | 111,901 (100) | 64,682 (57.8) | 47,213 (42.2) | - |
| Age, mean ±SE | 82.0 ± 0.04 | 82.6 ± 0.6 | 81.2 ± 0.05 | <0.001 |
| Female (%) | 53.5 | 54.5 | 52.2 | <0.001 |
| Minority (non-white) (%) | 16.1 | 16.2 | 16.0 | 0.739 |
| Cardiovascular Risk Factors (%) | ||||
| Hypertension | 45.8 | 43.9 | 46.9 | <0.001 |
| Diabetes without Chronic Complications | 18.8 | 19.2 | 18.2 | 0.047 |
| Obesity | 2.0 | 1.7 | 2.5 | <0.001 |
| Valvular Heart Disease | 13.0 | 15.4 | 9.7 | <0.001 |
| Peripheral Vascular Disease | 8.7 | 8.9 | 8.7 | 0.308 |
| Congestive Heart Failure | 37.7 | 43.7 | 29.6 | <0.001 |
| Non-Cardiovascular Diagnoses (%) | ||||
| Chronic Pulmonary Disease | 17.2 | 17.7 | 16.4 | 0.014 |
| Renal Failure | 13.3 | 13.7 | 12.8 | 0.045 |
| Coagulopathy | 9.1 | 8.4 | 9.9 | <0.001 |
| Weight Loss | 4.4 | 4.5 | 4.3 | 0.556 |
| Fluid and Electrolytes Disorder | 35.2 | 36.1 | 33.9 | 0.001 |
| Chronic Blood Loss Anemia | 1.7 | 1.5 | 2.0 | 0.004 |
| Alcohol Abuse | 0.7 | 0.7 | 0.8 | 0.446 |
| No. of Chronic Conditions, mean ±SE | 7.7 ± 0.05 | 7.7 ± 0.07 | 7.7 ± 0.07 | 0.729 |
| Charlson comorbidity index, mean ±SE | 2.8 (1.6) | 2.9 (1.6) | 2.7 (1.6) | <0.001 |
| Expected Primary Payer, (%) | 0.076 | |||
| Medicare | 91 | 91 | 91 | |
| Medicaid | 1.3 | 1.3 | 1.3 | |
| Private | 6 | 6 | 7 | |
| Self-pay, no charge, other | 1.7 | 1.7 | 0.7 | |
| Hospital Characteristics | ||||
| Hospital Size (%) | <0.001 | |||
| Small | 9 | 11 | 6 | |
| Medium | 23 | 25 | 20 | |
| Large | 68 | 65 | 73 | |
| Hospital Location (%) | <0.001 | |||
| Rural | 10 | 13 | 6 | |
| Urban | 9 | 9 | 9 | |
| Hospital Region, (%) | <0.001 | |||
| Northeast | 22 | 25 | 18 | |
| Midwest/North Central | 23 | 21 | 26 | |
| South | 34 | 33 | 35 | |
| West | 20 | 20 | 22 | |
| Length of stay, mean ±SE | 8.25 ± 0.11 | 7.77 ± 0.14 | 8.91 ± 0.12 | <0.001 |
| Total Charges, dollars, mean ±SE | 88,211± 1,997 | 69,844 ± 1,971 | 114,990 ± 2,639 | <0.001 |
| Disposition of Patient (%) | <0.001 | |||
| Home | 12 | 6 | 19 | |
| Facility/other | 34 | 32 | 38 | |
| Died | 54 | 62 | 43 |
Abbreviations: CHF = congestive heart failure; No = number.
% may not add to 100 because of rounding
Significance defined as p < 0.05.
Figure 2. Older adults with ST-elevation myocardial infarct (STEMI) and cardiogenic shock by regions of the United States over the study period (1999–2013).
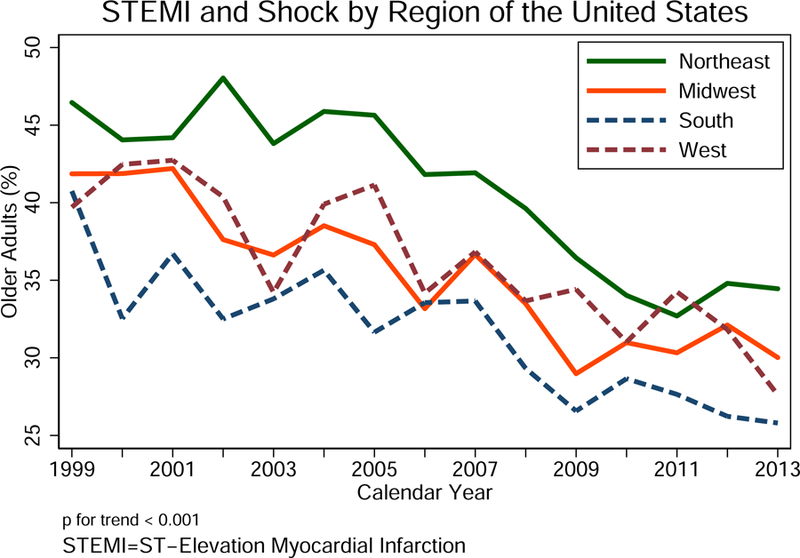
Note that the percent older adults (y-axis) is calculated as the number of admissions for older patients divided by the total number of admissions for STEMI and cardiogenic shock by region of the U.S. overtime.
Central Illustration. Percutaneous coronary intervention in older adults with ST-elevation myocardial infarction and cardiogenic shock: 1999–2013.
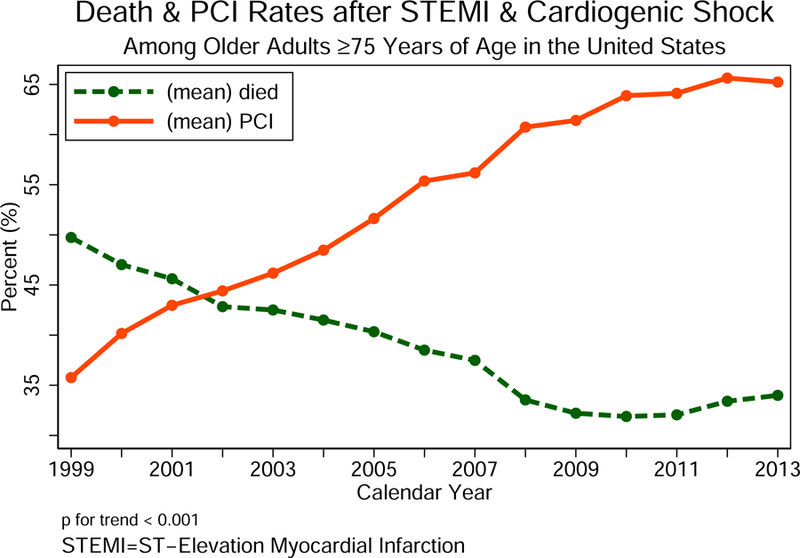
The rates of utilization of percutaneous coronary intervention (PCI) in older patients with ST-elevation myocardial infarction (STEMI) and cardiogenic shock since the publication of the SHOCK trial results between 1999 and 2013.
The association of PCI with mortality in older adults with STEMI and cardiogenic shock is presented in Table 2, by propensity score subclass for unweighted analyses. In both unweighted and discharge-weighted analyses there was consistent survival benefit of roughly 50% decreased mortality odds associated with treatment by PCI in all subclassification of the propensity score (unweighted Mantel-Haenszel OR: 0.48, 95% CI 0.45–0.51; no weighted estimate differed from its unweighted counterpart by >0.02 in any propensity score stratum). When the propensity score was treated as continuous variable with a spline term at 0.4, there remained significant improvement in mortality in the PCI group adjusting for the propensity score (OR 0.47, CI 0.44–0.50). The improvement in mortality in the PCI group was also observed when the propensity score was treated a continuous variable without a spline term. The overall mean probability of death in older patients after STEMI and cardiogenic shock by treatment status with PCI adjusted for the propensity score is presented in Figure 3. When discharge weights were applied to the logistic regression, there was a consistent survival benefit associated with treatment by PCI in the 5-propensity score subclassification (Table 3). Of the total study population, 5,455 (8.4%) patients had a bleeding event during the hospital encounter. The overall mortality rate in the PCI group was higher if the patient had a bleeding event (unadjusted mortality in PCI group: no-bleeding 29% vs. bleeding 34%, p-value <0.001). In order to account for the excess death observed in the bleeding group, an additional sensitivity analysis was performed to include bleeding in the model. PCI remained significant associated with survival within each quintile of the propensity score. To address immortal-time bias that could favor survival for the PCI group, we excluded patients who died within the first 48 hours of hospital admission. After the exclusion, there was a similar and consistent survival benefit associated with treatment with PCI within each subclassification of the propensity score (Online Table 2). Similar effects were observed if patients who died within the first week of admission were excluded (Online Table 3). Similar survival benefits associated with PCI were observed across the four regions of the United States census bureau (Table 4; unweighted analyses adjusting for propensity score with spline).
Table 2.
Estimated treatment effects in older adults comparing percutaneous coronary intervention (PCI) to no PCI on the risk of in hospital mortality after ST-elevation myocardial infarction and cardiogenic shock by propensity score subclass.
| Propensity Score Subclass1 | Treatment | N (%) | Events (%) | OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| 1 | PCI | 1,263 (36) | 560 (44) | 0.44 | 0.38 – 0.50 | <0.001 |
| No PCI | 2,283 (64) | 1,473 (65) | – | – | – | |
| 2 | PCI | 958 (40) | 444 (46) | 0.53 | 0.45 – 0.62 | <0.001 |
| No PCI | 1,441 (60) | 894 (62) | – | – | – | |
| 3 | PCI | 1,627 (41) | 748 (46) | 0.50 | 0.44 – 0.57 | <0.001 |
| No PCI | 2,374 (59) | 1,489 (63) | - | – | – | |
| 4 | PCI | 2,034 (45) | 868 (43) | 0.47 | 0.42 – 0.53 | <0.001 |
| No PCI | 2,542 (55) | 1,557 (61) | – | – | – | |
| 5 | PCI | 4,525 (45) | 1,532 (41) | 0.47 | 0.41 – 0.49 | <0.001 |
| No PCI | 3,727 (55) | 2762 (61) | – | – | – |
Abbreviations: OR = Odds Ratio; CI = confidence interval
Propensity Score: Using logistic regression, the propensity score was estimated by modeling the associations of the following covariates with treatment (percutaneous coronary intervention) given the covariates: gender, race, diabetes mellitus, hypertension, obesity, peripheral vascular disease, pulmonary circulation disorder, chronic lung disease, renal failure, liver disorder, coagulopathy, weight loss, electrolyte imbalance, blood loss, and alcoholism.
Figure 3. Adjusted probability of death in older patients with ST-elevation myocardial infarction (STEMI) and cardiogenic shock by treatment with percutaneous coronary intervention (PCI).

The probability of death was adjusted for hypertension, diabetes mellitus with or without complications, obesity, valvular heart disease, peripheral vascular disease, pulmonary circulation disease, chronic obstructive pulmonary disease, renal failure, liver failure, coagulopathy, weight loss, fluid and electrolyte disorder, chronic blood loss anemia and alcohol abuse.
Table 3.
Estimated treatment effects in older adults comparing percutaneous coronary intervention (PCI) to no PCI on the risk of in hospital mortality after ST-elevation myocardial infarction and cardiogenic shock adjusting for propensity score subclass using survey analysis method.
| Propensity Score Subclass1 | Treatment | Subpopulation Size N (%) | Events (%) | OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| 1 | PCI | 21,953 (32) | 9,992 (46) | 0.45 | 0.38 – 0.54 | <0.001 |
| No PCI | 46,505 (68) | 30,064 (65) | – | – | – | |
| 2 | PCI | 25,026 (37) | 10,924 (44) | 0.50 | 0.42 – 0.59 | <0.001 |
| No PCI | 43,332 (63) | 26,379 (61) | – | – | – | |
| 3 | PCI | 27,406 (40) | 12,199 (45) | 0.51 | 0.43 – 0.60 | <0.001 |
| No PCI | 40,633 (60) | 24,889 (61) | - | – | – | |
| 4 | PCI | 28,063 (41) | 11,971 (43) | 0.45 | 0.37 – 0.55 | <0.001 |
| No PCI | 39,952 (59) | 24,955 (62) | – | – | – | |
| 5 | PCI | 32,346 (48) | 12,371 (38) | 0.45 | 0.38 – 0.54 | <0.001 |
| No PCI | 35,664 (52) | 20,566 (58) | – | – | – |
Abbreviations: OR = Odds Ratio; CI = confidence interval
Propensity Score: Using logistic regression, the propensity score was estimated by modeling the associations of the following covariates with treatment (percutaneous coronary intervention) given the covariates: gender, race, diabetes mellitus, hypertension, obesity, peripheral vascular disease, pulmonary circulation disorder, chronic lung disease, renal failure, liver disorder, coagulopathy, weight loss, electrolyte imbalance, blood loss, and alcoholism. Survey analysis was performed using a self-weighted, stratified systematic, random sample of discharges from all hospitals in the sampling frame (see Methods section).
Online Table 3.
Estimated treatment effects in older adults comparing percutaneous coronary intervention (PCI) to no PCI on the risk of in hospital mortality after ST-elevation myocardial infarction and cardiogenic shock by propensity score subclass excluding patients who died within the first week of hospital admission.
| Propensity Score Subclass1 | Treatment | N (%) | Events (%) | OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| 1 | PCI | 768 (43) | 187 (24) | 0.71 | 0.57 – 087 | 0.001 |
| No PCI | 1,019 (57) | 318 (31) | – | – | – | |
| 2 | PCI | 1,065 (47) | 214 (20) | 0.69 | 0.57 – 0.85 | <0.001 |
| No PCI | 1,195 (53) | 315 (27) | – | – | – | |
| 3 | PCI | 654 (50) | 116 (18) | 0.76 | 0.57 – 0.99 | 0.047 |
| No PCI | 660 (50) | 146 (22) | - | – | – | |
| 4 | PCI | 1,456 (51) | 187 (13) | 0.60 | 0.49 – 0.74 | <0.001 |
| No PCI | 1,415 (49) | 278 (20) | – | – | – | |
| 5 | PCI | 2,491 (54) | 275 (11) | 0.55 | 0.46 – 0.65 | <0.001 |
| No PCI | 2,157 (46) | 399 (19) | – | – | – |
Abbreviations: OR = Odds Ratio; CI = confidence interval
Propensity Score: Using logistic regression, the propensity score was estimated by modeling the associations of the following covariates with treatment (percutaneous coronary intervention) given the covariates: gender, race, diabetes mellitus, hypertension, obesity, peripheral vascular disease, pulmonary circulation disorder, chronic lung disease, renal failure, liver disorder, coagulopathy, weight loss, electrolyte imbalance, blood loss, and alcoholism.
Table 4.
Estimated treatment effects in older adults comparing percutaneous coronary intervention (PCI) to no PCI on the risk of in hospital mortality after ST-elevation myocardial infarction and cardiogenic shock by region of the United States adjusting for propensity score.
| United States Regionα | Treatment | Sample Size N (%) | Events (%) | OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Northeast | PCI | 1,616 (34) | 675 (42) | 0.41 | 0.36 – 0.47 | <0.001 |
| No PCI | 3,203 (67) | 2,026 (63) | – | – | – | |
| Midwest | PCI | 2,441 (47) | 1,055 (43) | 0.49 | 0.42 – 0.57 | <0.001 |
| No PCI | 2,753 (53) | 1,727 (63) | – | – | – | |
| South | PCI | 3,389 (43) | 1,520 (45) | 0.51 | 0.46 – 0.56 | <0.001 |
| No PCI | 4,456 (57) | 2,758 (62) | - | – | – | |
| West | PCI | 2,163 (44) | 904 (42) | 0.46 | 0.41 – 0.53 | <0.001 |
| No PCI | 2,753 (56) | 1,664 (61) | – | – | – |
Abbreviations: OR = Odds Ratio; CI = confidence interval
Propensity Score: Using logistic regression, the propensity score was estimated by modeling the associations of the following covariates with treatment (percutaneous coronary intervention) given the covariates: gender, race, diabetes mellitus, hypertension, obesity, peripheral vascular disease, pulmonary circulation disorder, chronic lung disease, renal failure, liver disorder, coagulopathy, weight loss, electrolyte imbalance, blood loss, and alcoholism.
United States region was categorized according to the census bureau region into 4 group: (1) Region 1 (Northeast): (A) Division 1 (New England): Maine, New Hampshire, Vermont, Massachusetts, Rhode Island, Connecticut; (B) Division 2 (Mid Atlantic) New York, Pennsylvania, New Jersey; (2) Region 2 (Midwest) (Prior to June 1984, the Midwest Region was designated as the North Central Region); (A) Division 3 (East North Central) Wisconsin, Michigan, Illinois, Indiana, Ohio; (B) Division 4 (West North Central) Missouri, North Dakota, South Dakota, Nebraska, Kansas, Minnesota, Iowa; (3) Region 3 (South); (A) Division 5 (South Atlantic) Delaware, Maryland, District of Columbia, Virginia, West Virginia, North Carolina, South Carolina, Georgia, Florida; (B) Division 6 (East South Central) Kentucky, Tennessee, Mississippi, Alabama; (C) Division 7 (West South Central) Oklahoma, Texas, Arkansas, Louisiana; (4) Region 4 (West); (A) Division 8 (Mountain) Idaho, Montana, Wyoming, Nevada, Utah, Colorado, Arizona, New Mexico; (B) Division 9 (Pacific) Alaska, Washington, Oregon, California, Hawaii.
Discussion
This large and contemporary observational study described the current utilization of early revascularizations with PCI in older adults with STEMI and cardiogenic shock in the United States. To our knowledge, this study is the largest to examine clinical outcomes for this older population. We evaluated the treatment effect associated with PCI for patients aged 75 years or older admitted to the hospital with STEMI and cardiogenic shock from 1999 to 2013. The major findings of this study are: (1) Older adults who were not treated with PCI had worse comorbidity burden, shorter hospital length of stay, and higher crude mortality rates after STEMI and cardiogenic shock than those treated with PCI; (2) In the United States, the rate of utilization of PCI for older adults increased significantly since the publication of the SHOCK trial; (3) The crude mortality rates after STEMI and cardiogenic shock for older adults improved over the past decade; (4) Using propensity score methods, treatment with PCI appears to be associated with significant improvement in hospital mortality in older patients.
Recent evidence suggests that there has been an increase in utilization of PCI in older adults with ACS, including those with ST-elevation myocardial infarction. (23,24) For example, in a large registry, the proportion of adults 80 years or older with STEMI who underwent PCI increased from 9.2% in 2001 to 31.2% in 2010. (25) These trends were similar in the United States and Europe. (26–28) The Acute Myocardial Infarction in Switzerland Plus Registry examined hospital admission in older adults (≥75 years of age) with STEMI and shock from 1997 to 2006. (29) The rate of utilization of PCI or thrombolysis was significantly lower in older adults (older adults: 15.1% vs. younger adults 39.7%) but there was a parallel decrease in mortality rate between 1997 and 2006 from 82.8% to 75.6%. Dzavik and colleagues reported results from the SHOCK registry and showed that early revascularization resulted in a decrease in in-hospital mortality in older adults with STEMI and cardiogenic shock (early revascularization: 48% vs. late or no revascularization: 79%; relative risk 0.45, p=0.001). (30) While the SHOCK trial showed little or no benefit of early revascularization on mortality in adults age 75 years or older, our study shows that the rates of utilization of PCI in older patients with STEMI and cardiogenic shock is rising. This rise has been paralleled by an equivalent decline in unadjusted mortality rates over the study period. While it is plausible that older adults in more recent years are healthier in later years than those in earlier years, improvements in optimal medical therapies and revascularization strategies can explain higher longevity after cardiovascular illness in more recent years.
Despite the improvement in survival associated with early revascularization as reported by these studies(29,30), many older adults with multiple chronic conditions, worse disease burden, and possibly limited life expectancy as assessed by interventional cardiologists, do not receive early revascularization with PCI.(31) This study was aimed to address this important selection bias by implementing different methods of propensity matching to understand the influence of early revascularization adjusting for demographic, clinical, and hospital characteristics between older adults with early revascularization versus those without revascularization. Independent of the propensity methods used, early revascularization in the setting of cardiogenic shock and STEMI was associated with a reduction of in-hospital mortality risk (Absolute Risk Reduction = 21%; Relative Risk Reduction = 41%).
While the mortality rate in older patients is improving since the publication of the SHOCK trial, complexities in management of older adults with STEMI and cardiogenic shock represent a major challenge. The aging process is associated with worse kidney function, presence of comorbidities, frailty, and predilection to medication induced adverse events. These complexities result in delays in care, and often many older adults with STEMI are not offered early revascularization when their presentations are complicated by cardiogenic shock.(31) In 2007, Alexander and colleagues reviewed the scientific evidence and published an AHA Scientific Statement on Acute Coronary Care in the Elderly focusing on STEMI. Based on the available evidence at the time, it was recommended that older adults with shock selected based on clinical judgement may derive benefit from early revascularization. (3) Clinical judgement remains a critical aspect of acute cardiovascular care in older patients. However, we also believe that the result of this study provides additional important evidence that PCI with early revascularization may be associated with improved mortality risk in older patients, but the risk is higher in older patients with underlying multiple coexisting conditions or high weight of disease burden.
Strengths and Limitations
There are important strengths to this study. First, this study utilized a large nationally representative sample of older patients with STEMI and cardiogenic shock since the publication of the SHOCK trial results. Second, this study used a rigorous application of a state-of-the-art methodology to address measured confounding associated with invasive vs. non-invasive therapies. Third, this study fills an important gap in knowledge regarding the benefit of invasive therapy among older adults presenting with STEMI and cardiogenic shock with varying degree of multimorbidity.
There are several limitations for this study. First, NIS is an administrative dataset with comorbidities identified as ICD-9-CM codes. Such datasets are prone to coding errors by providers or institutions (e.g., over coding of cardiogenic shock by providers and coders). While this is an important inherent limitation associated with research using administrative datasets, this method in identifying patients with STEMI and shock has been used in prior research. (32) Further, it allows studying large populations with a specific diagnosis or a procedure with the aim to (1) improve healthcare delivery and utilization; (2) identify and eventually eliminate inequalities of care in vulnerable subgroups of the community; (3) maximize benefit associated with pharmacotherapy or invasive care for patients. Second, older adults with STEMI and cardiogenic shock are a high-risk patient population for short term mortality. Operators may identify older patients who are not suitable for early revascularization at the time of STEMI and shock (i.e., active bleeding, very limited life expectancy with end-stage disease process, severe neurocognitive decline), and include patients’ preferences or advance directives in decision making process. While these variables are difficult to capture in administrative database and represent a limitation in this study, the large sample size along with the application of different propensity score methods attempt to make the two groups of older adults with and without PCI more comparable. In fact, a comparison between propensity score in older patients treated with and without PCI showed significant overlap in propensity to treatment with PCI (Figure 1). Third, the temporal relationship between the occurrence of STEMI and cardiogenic shock and the decision to attempt PCI was not available in this claims-based dataset. Although this is an important limitation, most cases of cardiogenic shock in older adults occur secondary to STEMI and not as a result of PCI. As such, the bias of cardiogenic shock secondary to failed PCI procedure is minimal, but the key temporal relationship lies between the occurrence of STEMI and cardiogenic shock on the one hand and the decision to perform PCI. The influence of “immortal-time” bias occurs at the beginning of the trajectory and at the end. Patients who don’t make it to the cardiac catheterization laboratory because they die while waiting for PCI can potentially be counted in the no-PCI group. The patients who receive late (semi-elective) PCI (i.e. > 12 hours of presentation) after they have survived their medically treated episode of cardiogenic shock could have been counted in the PCI group. These patients, by definition, are associated with survival, because it can only be performed in patients who have already survived the episode of cardiogenic shock. In randomized trials, survivors with late PCI could be counted as survivors in the ‘no-PCI’ group (i.e. intention to treat analysis). In observational studies, these fatalities can be misclassified as having occurred in the PCI group. However, we tried to mitigate this problem by the sensitivity analysis excluding early (< 48 hours & < 7 days) fatalities. While this bias may not have been completely eliminated, most patients with STEMI complicated by cardiogenic shock receive PCI early after diagnosis to adhere with national quality metrics of door-to-balloon time as set by the ACC/AHA/ESC. For example, in the National Cardiovascular Data Registry’s (NCDR’s) Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With The Guidelines (ACTION Registry-GWTG) national database, 11,406 patients presenting with STEMI and cardiogenic shock were studied. (33) Of the 92.3% who underwent coronary angiography, 79% underwent PCI (median [IQR] age of population was 65 [56–76] years). The median time from hospital arrival to PCI for STEMI patients with cardiogenic shock was 1.2 hours (~72 minutes). In another cohort from Europe of 1,333 consecutive patients with STEMI complicated by cardiogenic shock (mean age = 63.9 ± 12.6 years), the mean (SD) time between symptom onset to start of PCI was 272 ± 267 mins (~4.5 ± 4.45 hours) (34). Two standard deviations of the time between symptom onset to the start of PCI were <12 hours (i.e., >95 % of the cohort receive PCI in < 12 hours from onset of symptoms). While this limitation is important, the design of the NIS excluded patients who did not survive into hospital admission from both the PCI and no-PCI groups. Because of this issue of immortal-time bias, the observed benefit of ‘PCI during hospitalization’ (Odds Ratio = 0.49) could potentially be exaggerated. Although the design of the NIS and the exclusion of early fatalities did not eliminate the bias completely, the benefit of PCI cannot be explained only by misclassification of fatalities that occurred in patients waiting for PCI, especially that most patients in “real world” clinical practice receive PCI early after diagnosis.
While other analytic methods were proposed to address “immortal-time” bias in observational studies, they are not free of limitations.(35–37) Randomization to early vs. late PCI can address “immortal-time” bias, but in the context of STEMI and cardiogenic shock, a condition with high mortality rate, randomization can be challenged by ethical consideration.(38) Because we used a claims-based dataset to identify diagnoses and procedures, the optimal timing of intervention (early versus late), measures of severity of shock, and burden of illness, revascularization technology and related pharmacotherapy were not available to us for analysis. However, the results of this study remain informative from public health perspective to highlight the influence of PCI on hospital mortality in geriatric population with STEMI and cardiogenic shock.
Conclusions
Given that most older adults have atypical symptoms, varying degrees of coexisting disease burden, and higher risks of mechanical complications, they are more likely to be denied early revascularization or have delays in reperfusion therapy.(31) In a landmark manuscript endorsed by ACC, AHA, American Geriatric Society, Rich and colleagues identified critical knowledge gaps in cardiovascular care of the older adult populations. (39) The panel of experts pointed out that studies are needed to evaluate the benefits of invasive care in older patients with ACS (i.e. STEMI and non-STEMI) in the setting of multimorbidity and varying degrees of disease burden. (39) We believe that this study fills important gaps in addressing the influence of invasive care for older adults with multimorbidity. From a public health perspective, early revascularization should not be denied for older adults in the absence of absolute contraindications (i.e., active bleeding, severe neurocognitive decline, and very limited life expectancy with end-stage disease processes).
Online Table 1.
Study design checklist to ensure appropriate use of National Inpatient Sample.
| Section A: Research Design |
| • Does the study consider it can only detect disease conditions, procedure, and diagnostic tests in hospital settings? |
| Yes, this study focused on outcomes for patients with ST-elevation myocardial infarction and cardiogenic shock in hospitalized patients. |
| • Does the study acknowledge that it includes encounters, not individual patients? |
| Yes, the Results section clearly indicated that the study population included 64,766 unique encounters. |
| • Does the study avoid diagnosis/procedure-specific volume assessments for units that are not a part of the sampling frame of the NIS, and are therefore not representatively sampled? E.g. (a) geographic units, like US states, (b) healthcare facilities (after 2011), and (c) individual healthcare providers. |
| This study examined the proportion of older adults with STEMI and cardiogenic shock out of the total number of STEMI and cardiogenic shock cases that were identified. This proportion was stratified by U.S. region. No specific volume assessments were made. |
| Section B: Data Interpretation |
| • Does the study attempt to identify disease conditions or procedures of interest using administrative codes or their combinations that have been previously validated? |
| Yes, these ICD-9-CM codes were obtained from AHRQ data dictionary. * |
| • Does the study limit its assessment to only in-hospital outcomes, rather than those occurring after discharge? |
| Yes, only in-hospital mortality was included as an outcome variable. No post-discharge outcomes were available for this analysis. |
| • Does the study distinguish complications from comorbidities or clearly note where it cannot? |
| We could not distinguish some complications from comorbidities related to the outcome of in hospital mortality. |
| Section C: Data Analysis |
| • Does the study clearly account for the survey design of the NIS and its components-clustering, stratification, and weighting? |
| Yes, see Methods section. |
| • Does the study adequately address the changes in data structure over time (for trends analyses)? |
| Yes. In 2012, the data structure for the HCUP National Inpatient Sample was redesigned. We used trend weight to create national estimates in the years prior |
CLINICAL PERSPECTIVE.
Competency in Practice-Based Learning and Improvement: Following publication of the results of the SHOCK trial, utilization of PCI for older patients presenting with STEMI and cardiogenic shock in the United States increased, and this trend has been associated with improved in-hospital survival.
Translational Outlook: Further studies are needed to explore ways to enhance access to early revascularization for older adults with STEMI and cardiogenic shock.
Acknowledgement:
The authors would like to acknowledge (1) Jane and Stanley F. Rodbell family for their generous research support aimed at improving outcomes for older Americans living with cardiovascular disease; (2) Dr. James Tonascia, Curtis L. Meinert Professor in Clinical Trials, Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, for his valuable input on the application of statistical methods for this analysis; (3) Dr. Jodi B. Segal, Professor of Medicine, Health Policy, and Management, and co-Director of the Center for Health Services and Outcomes Research in Johns Hopkins Bloomberg School of Public Health and School of Medicine, for her insightful comments on the methods and design of this study.
Disclosure: Dr. Damluji receives research funding from the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center (OAIC) Funded by the National Institute on Aging P30-AG021334. This study was also funded in part by a research grant from the Jane and Stanley F. Rodbell family in support of geriatric cardiology research at Sinai Hospital of Baltimore. Drs. Bandeen-Roche and Walston are co-principle investigators of the OAIC and they are supported by funding from NIA P30-AG021334.
Dr. Moscucci received book royalties from Wolters Kluwer Lippincott Williams & Wilkins and has stock ownership in Gilead Sciences, Inc.
Presentation: This work is part of a Ph.D. thesis for Dr. Damluji at Johns Hopkins University’ GTPCI program. It was presented as an oral presentation at the American College of Cardiology (Geriatric Cardiology Section) 66th Annual Scientific Session, Washington, D.C. The study received best clinical research award at the National Claude D. Pepper Older Americans Independence Centers funded by the National Institute on Aging, National Institutes of Health.
Abbreviations
- AHRQ
Agency for Healthcare Research and Quality
- CABG
Coronary artery bypass grafting
- CCI
Charlson Comorbidity Index
- HCUP
Healthcare Quality and Utilization Project
- ICD-9
International Classification of Diseases, 9th Revision
- NIS
National Inpatient Sample
- PCI
Percutaneous coronary intervention
- STEMI
ST-elevation myocardial infarction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare that they have no other competing interests.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS et al. Executive Summary: Heart Disease and Stroke Statistics−-2016 Update: A Report From the American Heart Association. Circulation 2016;133:447–54. [DOI] [PubMed] [Google Scholar]
- 2.Damluji AA, Ramireddy A, Forman DE. Management and Care of Older Cardiac Patients. In: Vasan RS, Sawyer DB, editors. Encyclopedia of Cardiovascular Research and Medicine Oxford: Elsevier, 2018:245–265. [Google Scholar]
- 3.Alexander KP, Newby LK, Armstrong PW et al. Acute coronary care in the elderly, part II: ST-segment-elevation myocardial infarction: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation 2007;115:2570–89. [DOI] [PubMed] [Google Scholar]
- 4.Thompson CR, Buller CE, Sleeper LA et al. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: a report from the SHOCK Trial Registry. SHould we use emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol 2000;36:1104–9. [DOI] [PubMed] [Google Scholar]
- 5.Menon V, Webb JG, Hillis LD et al. Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol 2000;36:1110–6. [DOI] [PubMed] [Google Scholar]
- 6.Bueno H, Martinez-Selles M, Perez-David E, Lopez-Palop R. Effect of thrombolytic therapy on the risk of cardiac rupture and mortality in older patients with first acute myocardial infarction. Eur Heart J 2005;26:1705–11. [DOI] [PubMed] [Google Scholar]
- 7.Dzavik V, Sleeper LA, Picard MH et al. Outcome of patients aged >or=75 years in the SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK (SHOCK) trial: do elderly patients with acute myocardial infarction complicated by cardiogenic shock respond differently to emergent revascularization? Am Heart J 2005;149:1128–34. [DOI] [PubMed] [Google Scholar]
- 8.Hochman JS, Sleeper LA, Webb JG et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med 1999;341:625–34. [DOI] [PubMed] [Google Scholar]
- 9.Prasad A, Lennon RJ, Rihal CS, Berger PB, Holmes DR Jr. Outcomes of elderly patients with cardiogenic shock treated with early percutaneous revascularization. Am Heart J 2004;147:1066–70. [DOI] [PubMed] [Google Scholar]
- 10.O’Gara PT, Kushner FG, Ascheim DD et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2013;61(4):485–510. [DOI] [PubMed] [Google Scholar]
- 11.van Diepen S, Katz JN, Albert NM et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017;136:e232–e268. [DOI] [PubMed] [Google Scholar]
- 12.Houchens RL RD, Setodji CM, Uscher-Pines L, Roderick J.A. Little. Nationwide Inpatient Sample Redesign Final Report. Deliverable #182303 2012;Agency for Healthcare Quality and Research, Rockville, MD. [Google Scholar]
- 13.Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001–2008. JAMA 2011;305:1769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NIS Related Reports Available at: http://www.hcup-us.ahrq.gov/db/nation/nis/nisrelatedreports.jsp. Accessed October 2018.
- 15.Shahul S, Hacker MR, Novack V et al. The effect of hospital volume on mortality in patients admitted with severe sepsis. PLoS One 2014;9:e108754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao SV, Dai D, Subherwal S et al. Association between periprocedural bleeding and long-term outcomes following percutaneous coronary intervention in older patients. JACC Cardiovasc Interv 2012;5:958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 18.Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 2013;6:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997;127:757–63. [DOI] [PubMed] [Google Scholar]
- 20.WINSHIP C, RADBILL L. Sampling Weights and Regression Analysis. Sociological Methods & Research 1994;23:230–257. [Google Scholar]
- 21.Khera R, Krumholz HM. With Great Power Comes Great Responsibility: Big Data Research From the National Inpatient Sample. Circ Cardiovasc Qual Outcomes 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nallamothu BK. Better-Not Just Bigger-Data Analytics. Circ Cardiovasc Qual Outcomes 2017;10. [DOI] [PubMed]
- 23.Cantor WJ, Goodman SG, Cannon CP et al. Early cardiac catheterization is associated with lower mortality only among high-risk patients with ST- and non-ST-elevation acute coronary syndromes: observations from the OPUS-TIMI 16 trial. Am Heart J 2005;149:275–83. [DOI] [PubMed] [Google Scholar]
- 24.Fox KA, Steg PG, Eagle KA et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA 2007;297:1892–900. [DOI] [PubMed] [Google Scholar]
- 25.Velders MA, James SK, Libungan B et al. Prognosis of elderly patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention in 2001 to 2011: A report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) registry. Am Heart J 2014;167:666–73. [DOI] [PubMed] [Google Scholar]
- 26.Johnman C, Oldroyd KG, Mackay DF et al. Percutaneous coronary intervention in the elderly: changes in case-mix and periprocedural outcomes in 31,758 patients treated between 2000 and 2007. Circ Cardiovasc Interv 2010;3:341–5. [DOI] [PubMed] [Google Scholar]
- 27.Tisminetzky M, Erskine N, Chen HY et al. Changing Trends in, and Characteristics Associated with, Not Undergoing Cardiac Catheterization in Elderly Adults Hospitalized with ST-Segment Elevation Acute Myocardial Infarction. J Am Geriatr Soc 2015;63:925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonsen L, Jensen LO, Thayssen P et al. Comparison of outcomes of patients >/= 80 years of age having percutaneous coronary intervention according to presentation (stable vs unstable angina pectoris/non-ST-segment elevation myocardial infarction vs ST-segment elevation myocardial infarction). Am J Cardiol 2011;108:1395–400. [DOI] [PubMed] [Google Scholar]
- 29.Jeger RV, Radovanovic D, Hunziker PR et al. Ten-year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med 2008;149:618–26. [DOI] [PubMed] [Google Scholar]
- 30.Dzavik V, Sleeper LA, Cocke TP et al. Early revascularization is associated with improved survival in elderly patients with acute myocardial infarction complicated by cardiogenic shock: a report from the SHOCK Trial Registry. Eur Heart J 2003;24:828–37. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Qadir HM, Ivanov J, Austin PC, Tu JV, Dzavik V. Temporal trends in cardiogenic shock treatment and outcomes among ontario patients with myocardial infarction between 1992 and 2008. Circ Cardiovasc Qual Outcomes 2011;4:440–7. [DOI] [PubMed] [Google Scholar]
- 32.Kolte D, Khera S, Aronow WS et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc 2014;3:e000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson ML, Peterson ED, Peng SA et al. Differences in the profile, treatment, and prognosis of patients with cardiogenic shock by myocardial infarction classification: A report from NCDR. Circ Cardiovasc Qual Outcomes 2013;6:708–15. [DOI] [PubMed] [Google Scholar]
- 34.Zeymer U, Vogt A, Zahn R et al. Predictors of in-hospital mortality in 1333 patients with acute myocardial infarction complicated by cardiogenic shock treated with primary percutaneous coronary intervention (PCI); Results of the primary PCI registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK). Eur Heart J 2004;25:322–8. [DOI] [PubMed] [Google Scholar]
- 35.Boulware LE, Tangri N, Ephraim PL et al. Comparative effectiveness studies to improve clinical outcomes in end stage renal disease: the DEcIDE patient outcomes in end stage renal disease study. BMC Nephrol 2012;13:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crews DC, Scialla JJ, Boulware LE et al. Comparative effectiveness of early versus conventional timing of dialysis initiation in advanced CKD. Am J Kidney Dis 2014;63:806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sjolander A, Nyren O, Bellocco R, Evans M. Comparing different strategies for timing of dialysis initiation through inverse probability weighting. Am J Epidemiol 2011;174:1204–10. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein CE, Weijer C, Brehaut JC et al. Ethical issues in pragmatic randomized controlled trials: a review of the recent literature identifies gaps in ethical argumentation. BMC Med Ethics 2018;19:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rich MW, Chyun DA, Skolnick AH et al. Knowledge Gaps in Cardiovascular Care of the Older Adult Population: A Scientific Statement From the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation 2016;133:2103–22. [DOI] [PubMed] [Google Scholar]


