Abstract
Purpose
The usual first-line strategy of wild-type EGFR (wtEGFR) non-small cell lung cancer (NSCLC) remains cisplatin-based chemotherapy. However, cisplatin often loses effectiveness because most tumors acquire drug resistance over time. As EGFR is the most important pro-survival/proliferation signal receptor in NSCLC cells, we aimed at investigating whether cisplatin resistance is related to EGFR activation and further evaluating the combined effects of cisplatin/gefitinib (EGFR-tyrosine kinase inhibitor, EGFR-TKI) on cisplatin-resistant wtEGFR NSCLC cells.
Materials and methods
EGFR activation was analysed in parental and cisplatin-resistant wtEGFR NSCLC cell lines (H358 and H358R, A549 and A549R). Cellular proliferation and apoptosis of H358R/A549R cells treated with cisplatin or gefitinib, alone or in combination were investigated, and the related effector protein was detected by western blot analysis. Anti-tumor effect of two drugs combined was evaluated in animal models of H358R xenografts in vivo.
Results
EGFR was significantly phosphorylated in cisplatin-resistant wtEGFR NSCLC cells H358R and A549R than their parental cells. In H358R and A549R cells, anti-proliferative ability of gefitinib was further improved, and gefitinib combined with cisplatin enhanced inhibition of cellular survive/proliferation, and promotion of apoptosis in vitro. The combined effects were also associated with the inhibition of EGFR downstream effector proteins. Similarly, in vivo, gefitinib and cisplatin in combination significantly inhibited tumor growth of H358R xenografts.
Conclusion
Abnormal activation of EGFR may induce wtEGFR NSCLC cell resistance to cisplatin. The combined effects of cisplatin/gefitinib suggest that gefitinib, as a combination therapy for patients with cisplatin-resistant wtEGFR NSCLC should be considered.
Keywords: Gefitinib, Cisplatin, Resistance, wtEGFR NSCLC
Introduction
Non-small cell lung cancer (NSCLC) accounts for about 85% of lung cancers and is the leading cause of cancer- related deaths worldwide. More than 65% NSCLC patients present with locally advanced or metastatic disease when diagnosed (Reck et al. 2013). Despite much effort was made to find out new therapeutic strategies in NSCLC, cisplatin-based chemotherapy remains the backbone therapy in wild-type EGFR NSCLC (wtEGFR NSCLC). Unfortunately, less than 15% of patients with lung cancer survive more than 5 years.
The main reason for such low survival rate of wtEGFR NSCLC is that most patients develop resistance after several cycles of cisplatin-based chemotherapy. Researches have discovered the mechanism of cisplatin resistance mainly includes: pre-target resistance (Chen et al. 2012; Kuo et al. 2012; Ishida et al. 2010); on-target resistance (Friboulet et al. 2013; Kamal et al. 2010; Olaussen et al. 2006); post-target resistance (Goloudina et al. 2012; Motte et al. 2007; Michaud et al. 2009) and off-target resistance (Ren et al. 2010; Shen et al. 2010; Yu et al. 2011). The susceptibility of wtEGFR NSCLC cells to cisplatin can be limited by off-target mechanisms, that is, molecular circuitries that deliver compensatory pro-survival signals even though they are not directly activated by cisplatin (Galluzzi et al. 2012).
EGFR is the most important pro-survival signal receptor for EGF and belongs to tyrosine kinase receptor of wtEGFR NSCLC cells. The abnormal activation of EGFR downstream signal pathways, such as Ras/Raf/MAPK, PI3K/AKT/mTOR, and Jak/stat, induces tumor cells anti-apoptosis, proliferation, angiogenesis and drug resistance (Leon et al. 2016). There are reports also revealed a EGF-independent activation of EGFR in epithelial and non-epithelial cells (Lu et al. 2014; Hardbower et al. 2016; Guo et al. 2015). Therefore, we wondered whether the off-target resistance of cisplatin is related to ligand-independent activation of EGFR.
If cisplatin resistance is related to EGFR activation, inhibiting EGFR activation should restore the cisplatin sensitivity of cisplatin-resistant wtEGFR NSCLC cells. The commonly used EGFR inhibitor in clinical is EGFR tyrosine kinase inhibitor (EGFR-TKI). Gefitinib, as the first generation of EGFR-TKI, has small side effects and significant anti-tumor activity, especially for EGFR-mutant NSCLC. However, the indication of gefitinib in patients with wtEGFR NSCLC is more debated (Zhao et al. 2014).
In our study, we investigated the activation of EGFR in wtEGFR NSCLC parental cell lines and cisplatin-resistant cell lines, further assessed the effects of gefitinib in combination with cisplatin on cisplatin-resistant cell lines. Our results showed gefitinib restored most sensitivity of cisplatin-resistant wtEGFR NSCLC cells to cisplatin and support the view that EGFR-TKI may become a combined treatment strategy for patients with cisplatin-resistant wtEGFR NSCLC.
Materials and methods
Cell lines, chemicals and antibodies
Human wtEGFR NSCLC cell lines H358 and A549 were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). Cisplatin-resistant cell lines, H358R and A549R, were induced by constant exposure to cisplatin (2 μmol/L) to imitate acquired resistance. Both cell lines were cultured in 10% FBS-containing medium (RPMI1640, Gibco, Thermo Fisher Scientific) and maintained in a 5% CO2 incubator at 37 °C. Cisplatin (A8321) was purchased from APExBIO Technology LLC (Houston, Texas, USA); Gefitinib (ZD1839) was obtained from Med Chem Express (Monmouth, Junction, USA). (3, 4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolyl carbocyanine iodide JC-1 were provided by Sigma-Aldrich (St. Louis, MO, USA). Antibodies used were following: total EGFR (#4267), phospho-EGFR (#3777), Phospho-AKT Antibody Sampler Kit (#9916), Phospho-Erk1/2 Antibody Sampler Kit (#9911), Apoptosis Antibody Sampler Kit (#9915) and other antibody sampler kits were obtained from Cell Signaling Technology (Danvers, MA, USA).
IC50 measurements
Cells were plated in 96-well plates over night and treated by the indicated drugs for 48 h. Then the cells were incubated with MTT 4 h at 37 °C, formazan cristae were solubilized in dimethyl sulfoxide (DMSO) and optical density (OD) was read at 570 nm. IC50 was calculated as described by Chou and colleagues (Chou 2006).
Phosphorylated EGFR detection
Flow cytometry was performed for the detection of intracellular phosphorylated EGFR with BD FACSCalibur. Cells were fixed by 4% paraformaldehyde, permeabilized by 90% methanol and saturated by 5% BSA. Followed incubated in the p-EGFR antibody diluted in 1% BSA and coupled to fluorescent (Alexa Fluor 488) secondary antibody at room temperature. Non-specific binding of secondary antibody was measured by omitting primary antibody and the corresponding mean fluorescence was subtracted from the signal measured with each primary antibody.
Western blot analysis
Total protein was extracted from cells lysed with radioimmunoprecipitation buffer (RIPA, Beyotime Biotechnology, Shanghai, China) containing a protease inhibitor cocktail (Beyotime Biotechnology, Shanghai, China) and centrifuged. The protein concentration of the supernatant was measured with BCA200 protein assay kit (Biosharp Life Science, Hefei, China) and equalized before loading to the gel. Target proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto a polyvinylidene fluoride membrane (Millipore, USA). After blocking with Tris-buffered saline/Tween 20 containing 5% fat-free milk, detecting antibodies were applied. Protein bands were visualized with a chemiluminescence detection kit (Thermo), and images were captured with a scanner using Quality One software (Bio‐Rad).
Viability assay
Cells were seeded into 96-well plates and cultured for 24 h in a 5% CO2 incubator at 37 °C. Then the cells were treated by the indicated drugs for 48 h. Carrier dimethyl sulfoxide was used as a control. Cell viability was measured with MTT assay in accordance with the manufacturer’s instructions. Ten microliters of MTT (5 mg/mL) were added to each well and incubated for 4 h. After aspiration, 100 μl of DMSO were added. Cell proliferation/inhibition rate was calculated with Graphpad Prism Version 5.0 software according to ODλ=570 nm by use of a microplate reader (Bio-Rad Model 680; Bio-Rad Laboratories Inc., Hertfordshire, UK).
Clone formation assay
Cells were seeded in six-well culture plates at a density of 1000 cells per well and cultured for 24 h. The cells were treated with the drugs for another 24 h. Then the medium containing the reagents was removed and replaced with fresh medium. The cells were further cultured for about 10 days until colonies were visible. Colonies were fixed with 4% paraformaldehyde and stained with 0.1% (w/v) crystal violet. Cell colonies were counted, and the number cultured with drugs was compared with the control.
Cell-cycle analysis
The cell cycle was analyzed with flow cytometry. Cells were planted in 12-well plates and incubated for 24 h followed by treatment with drugs for additional 48 h. The cells were dissociated and fixed with cold 75% ethanol, then incubated with RNase (100 ng/mL) and stained with propidium iodide (PI, 50 ng/mL). Dye was removed, and the cells were resuspended with PBS for cell-cycle analysis by flow cytometry (BD FACSCalibur, USA).
Apoptosis detection
Cell apoptosis was analyzed with an Annexin V-FITC/propidium iodide (PI) staining protocol. Cells were seeded in 24-well plates and incubated for 24 h, followed by drug treatment for an additional 48 h. Cells were collected after digestion and centrifugation, then incubated with Annexin V-FITC/PI at room temperature for 15 min in the dark. Flow cytometry was performed after cells were incubated with fluorescence staining.
Mitochondrial membrane potential (ΔΨm) measurement
JC-1 (5,5′,6,6′‐tetrachloro‐1,1′,3,3′‐tetraethyl‐benzimidazolyl carbocyanine iodide) is a sensitive probe for measuring mitochondrial membrane potential (Zhdanov et al. 2017). The high/low mitochondrial membrane potential of cells treated with dugs determined red/green fluorescence intensity excited by polymer/monomer of JC-1, from which the mitochondrial apoptosis level of the cells was analyzed. After the cells were incubated with drugs for 48 h, they were stained with 10 µg/mL JC-1 for a half hour at room temperature. Fluorescence was visualized by fluorescence microscope.
Animal experiments
All animal experiments were carried out in accordance with the principles and procedures approved by the Animal Experimental Ethics Committee of Anhui University of Science and Technology. Approximately 5–6 weeks of age female BALB/c nude mice were purchased from Nanjing Junke Biotechnology Co. Ltd. 100 µl H358R cell suspension (5 × 107/mL) was subcutaneously injected into the right shoulders of the mice. When tumors reached a size of about 100 mm3, mice were randomly divided into four groups. The “control” group has no treatment; the “cisplatin” group was treated by cisplatin of intraperitoneal injection at day 0 and day 7 (2 mg/kg); the “gefitinib” group was received gefitinib (150 mg/kg) daily orally by gavage; the “cisplatin/gefitinib” group was treated by both drugs as described above. Tumor volume was measured with a caliper two perpendicular tumor diameters twice a week, and the formula [(length) × (width)2/2] was used to estimate the tumor growth. Body weight was measured to assess systemic toxicity.
Statistical analysis
All experiments were repeated three times independently and analyzed by Student t-test or two-way ANOVA using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). P < 0.05 was taken as statistically significant.
Results
IC50 to cisplatin of H358R and A549R cells
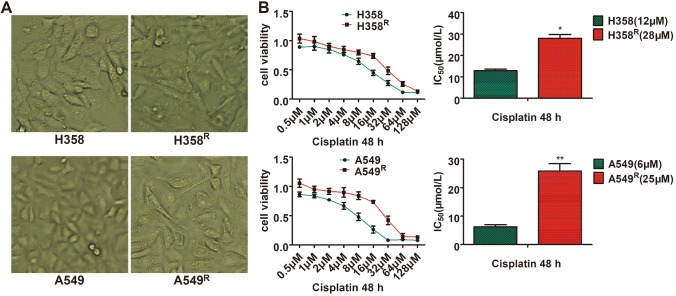
We induced two cisplatin-resistant wtEGFR NSCLC cell lines, named H358R and A549R, derivated from H358 and A549 cells by continuous treatment with 2 μmol/L cisplatin. Two months later, cells could proliferate and subcultured in the presence of cisplatin. The cell morphology also changes significantly compared to their parental cells (Fig. 1a). To determine their resistance, we measured their IC50 to cisplatin. The IC50 to cisplatin of H358R and A549R cells was 28 μM and 25 μM, respectively (Fig. 1b).
Fig. 1.
Morphology and resistance of cisplatin-resistant cell lines H358R and A549R. a H358R and A549R cell lines showed irregular long spindles, flat, and blurred borders compared to their parental cell lines. b Cells were treated by a range of increasing cisplatin concentrations for 48 h and IC50 was calculated as described by Chou and colleagues with MTT assay (*P < 0.05, **P < 0.01)
Significant phosphorylation of EGFR and downstream AKT/ERK in H358R and A549R cells
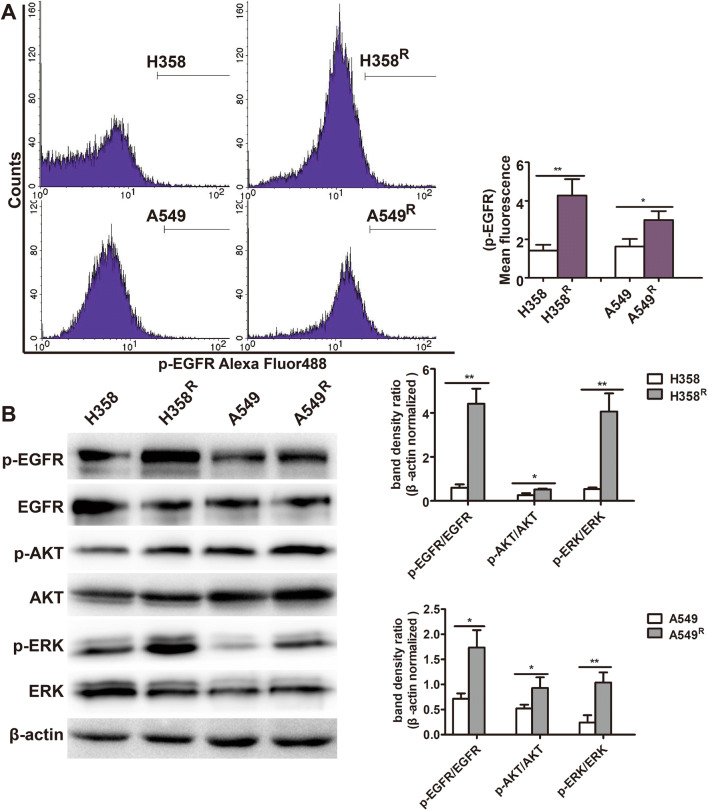
To determine whether the resistance to cisplatin of wtEGFR NSCLC cells is related to activation of EGFR, we detected EGFR phosphorylation in H358R and A549R cells using flow cytometry. As shown in Fig. 2a, EGFR phosphorylation was significantly increased compared with their parental H358 and A549 cells. We further detected phosphorylation level of EGFR and its downstream AKT/ERK in H358R and A549R cells, using western blot assay. The results also confirmed that phosphorylation of EGFR and its downstream molecules AKT/ERK in H358R and A549R cells was significantly increased (Fig. 2b), suggesting abnormal activation of EGFR signal in cisplatin-resistant cell lines H358R and A549R.
Fig. 2.
Significant phosphorylation of EGFR, AKT, ERK in H358R and A549R cell lines. a Cells were fixed and incubated with p-EGFR antibody. Fluorescence was measured by flow cytometry. b Expression of p-EGFR, p-AKT, p-ERK in H358R and A549R cells by western blotting analysis (*P < 0.05, **P < 0.01)
Gefitinib increases inhibition of proliferation in H358R and A549R cells
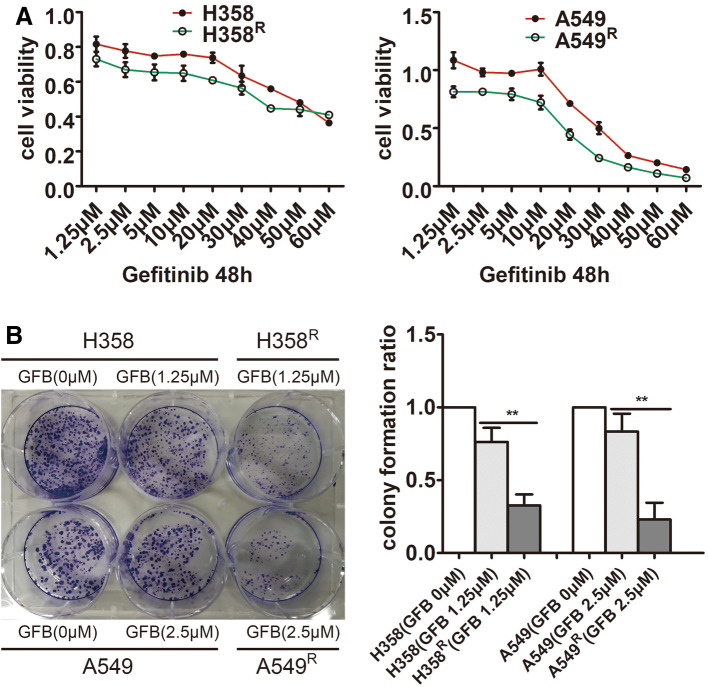
Gefitinib is an EGFR tyrosine kinase inhibitor that effectively inhibits EGFR phosphorylation and cell survival/proliferation. To further test whether EGFR phosphorylation is effective in H358R and A549R cells, we measured the proliferation activity of H358R/A549R cells and their parental cells after gefitinib treatment separately by MTT (Fig. 3a) and clone formation assay (Fig. 3b). The results showed that the inhibitory effect of gefitinib on H358R and A549R cells is further enhanced, which demonstrated the effective activation of EGFR in H358R and A549R cells at the cytological level.
Fig. 3.
Increased sensitivity of H358R and A549R cell lines to gefitinib (GFB). a Cells were treated by a range of increasing gefitinib concentrations for 48 h and cell viability was calculated by MTT assay. b Cells were incubated with gefitinib (GFB) for 48 h and the inhibitory effects of gefitinib (GFB) on single cell proliferation in H358R/A549R and their parental cells were assessed by clone formation assay (**P < 0.01)
Gefitinib inhibits EGFR/AKT/ERK phosphorylation in H358R and A549R cells
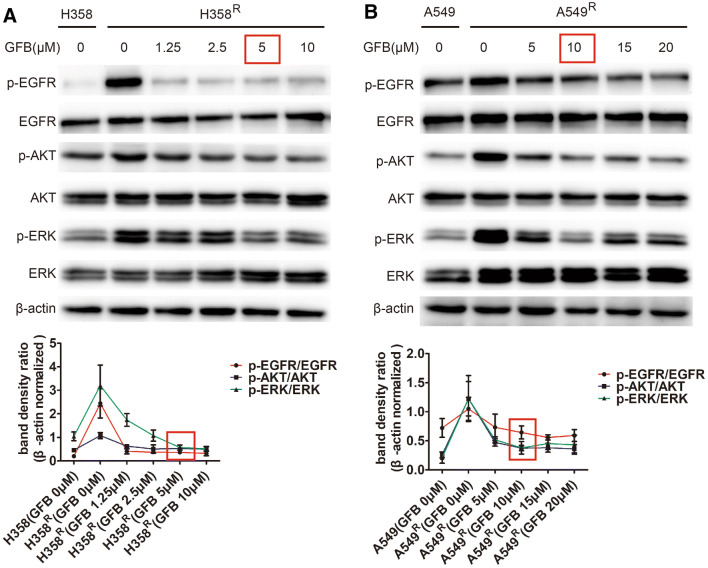
To determine the minimum concentration of gefitinib inhibiting phosphorylation of EGFR and its downstream molecules AKT/ERK in H358R and A549R cells, we used western blot to detect EGFR/AKT/ERK phosphorylation levels after 48 h of gefitinib at different intervention concentrations. As shown in Fig. 4a, 5 μM gefitinib could simultaneously inhibit EGFR/AKT/ERK phosphorylation in H358R cells. For A549R cells, the minimum concentration of gefitinib inhibiting EGFR/AKT/ERK phosphorylation is 10 μM (Fig. 4b). 5 μM and 10 μM of gefitinib will be used as a subsequent combination concentration.
Fig. 4.
Gefitinib (GFB) inhibited EGFR/AKT/ERK phosphorylation in H358R and A549R cells. a Detected EGFR/AKT/ERK phosphorylation levels after 48 h of gefitinib (GFB) at different intervention concentrations by western blot. The minimum concentration of gefitinib (GFB) inhibiting p-EGFR, p-AKT, p-ERK in H358R is 5 μM. b Similarly, 10 μM gefitinib (GFB) could simultaneously inhibit p-EGFR, p-AKT, p-ERK in A549R cells
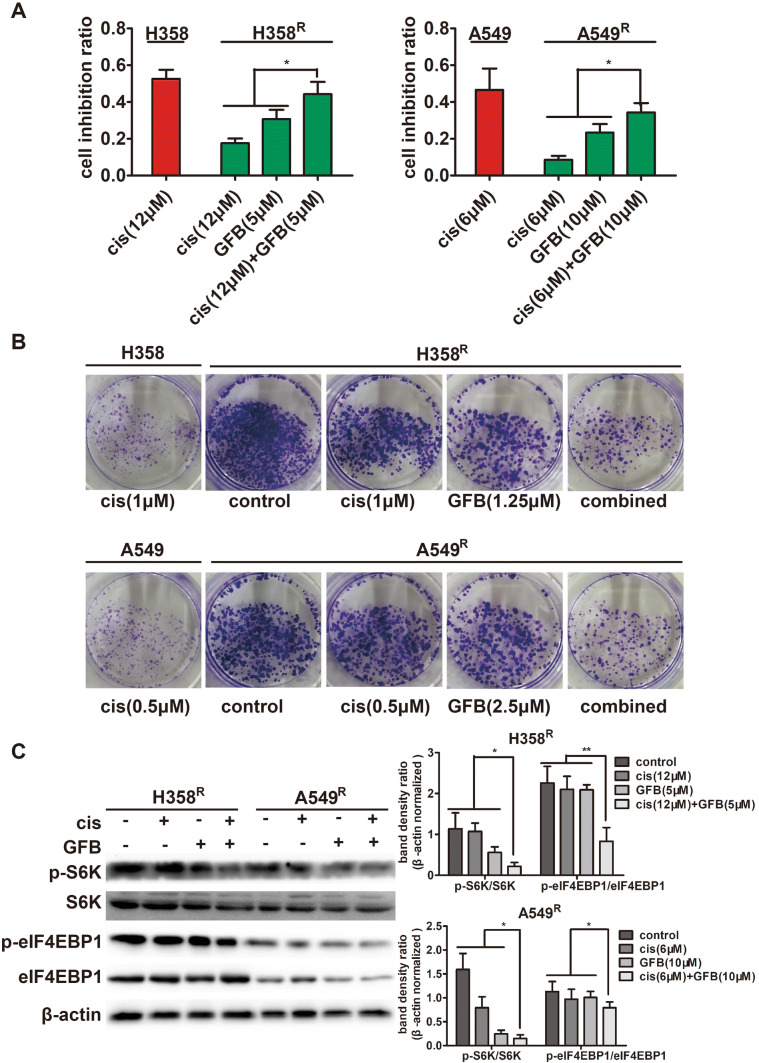
Gefitinib combined with cisplatin significantly inhibits proliferation of H358R and A549R cells
After determining the concentration of gefitinib, we observed the effect of gefitinib combined with cisplatin on the proliferation and viability of H358R and A549R cells. We treated H358R/A549R cells and their parental cells with the IC50 to cisplatin 48 h of the parental cell lines, respectively. MTT assay showed the cell growth inhibition ratio of cisplatin on H358R/A549R cells was significantly lower than that of their parental cells. However, after combining with gefitinib, the inhibition ratio was significantly improved (Fig. 5a). In addition, clone formation experiments showed that gefitinib combined with cisplatin can effectively inhibit the proliferation of H358R/A549R cells (Fig. 5b). And the above experiments showed that compared with the parental cell lines, the sensitivity of cisplatin on H358R/A549R cells has been largely restored when the two drugs were in combination.
Fig. 5.
Gefitinib (GFB) combined with cisplatin (cis) significantly inhibits proliferation of H358R and A549R cells. a Inhibition ratio of cells incubated with cisplatin (cis), gefitinib (GFB), or a combination of two drugs for 48 h, was measured with MTT assay. b Proliferation inhibition of cells incubated with cisplatin (cis), gefitinib (GFB), or a combination of two drugs for 24 h, was measured with clone formation assay. c Expression of related proliferation regulation protein in H358R and A549R cells incubated with cisplatin (cis), gefitinib (GFB), or a combination of two drugs for 48 h, was detected by western blot (*P < 0.05, **P < 0.01)
S6K and eIF4EBP1 are the most important molecules downstream of EGFR/AKT/ERK that directly regulate protein synthesis and promote cell proliferation (Jhanwar-Uniyal et al. 2017). We tested the phosphorylation levels of these molecules in H358R/A549R cells after gefitinib combined with cisplatin treatment. The results showed that the combination of two drugs can significantly reduce phosphorylation level of S6K and eIF4EBP1 (Fig. 5c), which explains the anti-proliferative effect of the combination of gefitinib and cisplatin.
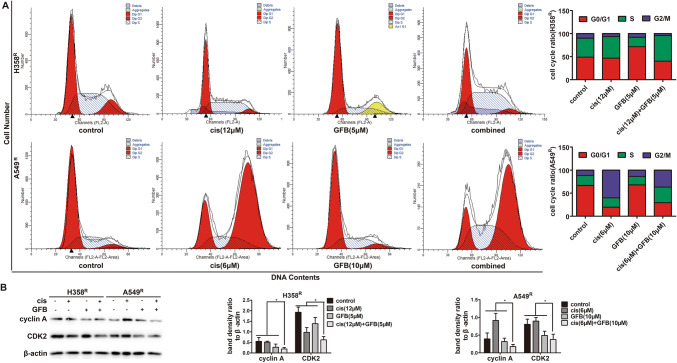
Gefitinib combined with cisplatin promotes S arrest in H358R and A549R cells
To investigate whether gefitinib combined with cisplatin-induced inhibition of cell proliferation was associated with cell-cycle dysregulation, we analyzed the effect of gefitinib on cell-cycle progression in cisplatin-treated H358R/A549R cells based on DNA content by propidium iodide staining of flow cytometry analysis. With H358R cells treated with cisplatin and gefitinib individually, there was an increase in the percentage of cells in the S and G0/G1 phase compared with the percentage in controls, respectively. With combined cispaltin and gefitinib treatment, the percentages of H358R cells in the S phase were further improved, compared with the values of their monotherapy group (Fig. 6a). The percentage of A549R cells in the G2/M and G0/G1 phase was increased after cisplatin and gefitinib treatment and similar results were obtained when gefitinib combined with cispaltin was used simultaneously (Fig. 6b). Collectively, these results suggest gefitinib combined with cisplatin promotes S arrest and blocks cell-cycle progression in H358R and A549R cells.
Fig. 6.
Gefitinib (GFB) combined with cisplatin (cis) promotes S arrest in H358R and A549R cells. a The percentages of H358R and A549R cells in the S phase were improved when incubated with a combination of cisplatin (cis) and gefitinib (GFB) for 48 h, was analyzed by flow cytometry. b Cyclin A and CDK2 expression of S phase regulatory proteins in H358R and A549R cells incubated with drugs for 48 h, was detected by western blot (*P < 0.05)
The proteins cyclin A and CDK2 govern S-to-G2/M phase progression, and down-regulation of them leads to S phase arrest (Oakes et al. 2014). Thus, we examined the expression of endogenous cyclin A, CDK2. As indicated in Fig. 6c, cyclin A and CDK2 values were further reduced in the H358R/A549R cell lines when treated with cisplatin plus gefitinib. These data indicate that cisplatin and gefitinib in combination enhanced cells arrest at S phase mainly through the down-regulation of the key S phase regulatory proteins cyclin A and CDK2.
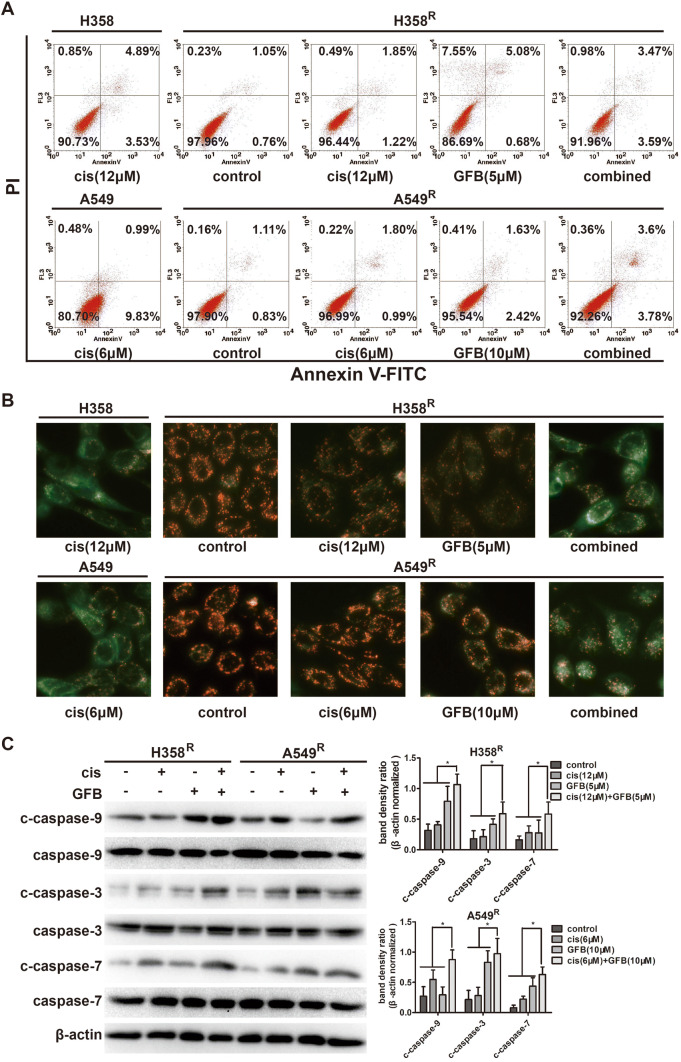
Gefitinib combined with cisplatin enhances the mitochondrial apoptosis in H358R and A549R cells
To evaluate the apoptotic effect of gefitinib combined with cisplatin in H358R and A549R cells, Annexin V-FITC/PI staining, cell mitochondrial membrane potential measurement (JC-1), and western blot were performed. As shown in Fig. 7a, flow cytometry analysis of AnnexinV-FITC/PI staining displayed that gefitinib combined with cisplatin had a significantly higher apoptosis ratio than cisplatin alone in H358R/A549R cells. In Fig. 7b, JC-1 staining showed the green fluorescence intensity of H358R/A549R cells treated with cisplatin monotherapy increased only slightly. However, when combining with gefitinib, the green fluorescence intensity was significantly enhanced. And the green fluorescence intensity of the combined group was very close to that of the parent cell lines treated with cisplatin. Gefitinib combined with cisplatin reduced mitochondrial membrane potential also indicates the effect of enhanced mitochondrial apoptosis in H358R/A549R cells.
Fig. 7.
Gefitinib (GFB) combined with cisplatin (cis) enhances the mitochondrial apoptosis in H358R and A549R cells. a Apoptosis ratio of cells incubated with cisplatin (cis), gefitinib (GFB), or a combination of two drugs for 48 h, was analyzed by flow cytometry. b Fluorescence microscope viewed mitochondrial membrane potential of cells after drugs treatment 48 h by JC-1 staining. c Expression of mitochondrial apoptosis-related protein in H358R and A549R cells when cisplatin (cis), gefitinib (GFB), or a combination of two drugs treatment 48 h, was examined by western blot (*P < 0.05, **P < 0.01)
Correspondingly, western blot experiments (Fig. 7c) showed that after gefitinib combined with cisplatin treatment, cleaved caspase-9, cleaved caspase-3, cleaved caspase-7 levels detected in H358R/A549R cells were more higher than that in cisplatin treatment group. These results prove that gefitinib/cisplatin combined can promote the apoptosis of cisplatin-resistant H358R/A549R cells and restore most of the sensitivity of these cells to cisplatin.
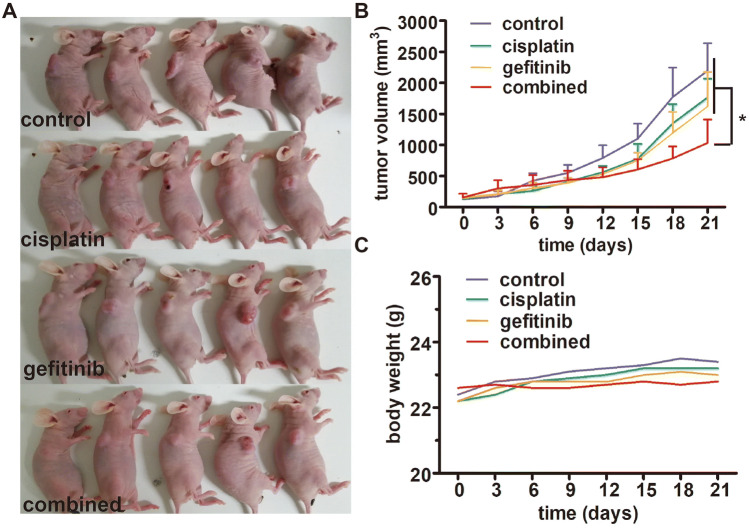
Gefitinib enhances the anti-tumor effect of cisplatin in H358R xenograft in vivo
The anti-tumor effect of gefitinib combined with cisplatin was evaluated in the H358R tumor-bearing nude mouse model. Tumor growth was measured twice a week and represented on the graph in Fig. 8a, b. As expected, cisplatin had moderate anti-tumor activity at a dose of 2 mg/kg because of its resistance. However, when combined with gefitinib, the tumor growth was significantly suppressed, and there was a steady trend of reduced tumor volume. The weight of the tumor-bearing mice had a stable trend, and weights of the treated mice were slightly lower than those of the tumor-bearing control mice (Fig. 8c). Thus, these in vivo findings are evidence of the anti-tumor effect of cisplatin enhanced by gefitinib in cisplatin-resistant H358R xenograft.
Fig. 8.
Gefitinib enhances the anti-tumor effect of cisplatin in H358R xenograft in vivo.a Tumor volume of xenograft tumors (H358R) treated with cisplatin, gefitinib, and cisplatin + gefitinib, respectively. b Growth curves of xenograft tumors (H358R) in each treatment group. c Body weight changes of model mouses in each treatment group during the treatment period
Discussion
wtEGFR NSCLC is the major histological form of epithelial lung cancer, and it remains the principle cause of cancer-related death. Although advances in molecular diagnosis and targeted therapies have been providing personalized therapies that translate into clinically meaningful benefits, for patients with advanced wtEGFR NSCLC, cisplatin-based chemotherapy is still the first-line treatment option (Griesinger et al. 2019). However, drug resistances are often inevitable accompanied by the long-term use of cisplatin, significantly hampering its therapeutic efficacy and clinical outcomes (Lin et al. 2019). The purpose of our research is to explore the mechanism of cisplatin resistance and clinical strategies to release or reduce the resistance.
In the study, we developed two cisplatin-resistant wtEGFR NSCLC cell lines H358R/A549R and found that EGFR was significantly activated in these cells. Moreover, they were more sensitive to EGFR inhibitor gefitinib than their parental cells, suggesting that cisplatin resistance is associated with abnormal activation of EGFR. EGFR is a transmembrane receptor protein and the complex cross-talk with several membrane receptors, including the other members of the erbB/HER-family (Roskoski 2014) involved in the fine regulation of epithelial cell proliferation, anti-apoptosis, angiogenesis and drug resistance (Leon et al. 2016). Its complicated downstream signaling pathways, especially PI3K/AKT/mTOR and Ras/Raf/MAPK, achieve the multiplicity of biological effects induced by EGFR activation (Cao et al. 2019). In this work, we found that the EGFR and downstream node molecules AKT/ERK in PI3K/AKT/mTOR and Ras/Raf/MAPK pathways were indeed abnormally activated, and their activities were significantly reduced when application of gefitinib in H358R/A549R cell lines.
It is noteworthy that these two cell lines sensitized to gefitinib by cisplatin induced express mutated KRAS. The KRAS mutation has been used as a predictive marker of low sensitivity to EGFR-TKI in lung cancer cells harboring EGFR activating mutations (Timar 2014). However, whether the KRAS mutation affects the response of wtEGFR NSCLC to EGFR-TKI is still controversial (Ulivi et al. 2014). It has shown KRAS status does not affect the cell survival/growth and phosphorylation of AKT/ERK kinases in wtEGFR NSCLC (Ihle et al. 2012), the EGFR, AKT and ERK phosphorylation induced by cisplatin in our KRAS-mutated cell lines would thus not involve KRAS. And KRAS does not appear as a major determinant for the mechanism of gefitinib sensitization described in our study.
Gefitinib is an aniline quinazoline derivative. It blocks tumor growth, metastasis, angiogenesis, and promotes tumor cell apoptosis by inhibiting EGFR-tyrosine kinase and blocking the EGFR signaling pathway (Zhang et al. 2017; Wang et al. 2019). The benefit of gefitinib has been demonstrated in mutant EGFR NSCLC as first-line treatment, but in wtEGFR NSCLC, gefitinib is restricted to maintenance therapy in pretreated patients (Choi et al. 2015; Landi and Cappuzzo 2015). From the analysis of our research results, sensitized gefitinib can not only be used as maintenance treatment for cisplatin-resistant patients, but also be used in combination with cisplatin to improve drug sensitivity, enhancing anti-tumor efficacy in patients with cisplatin-resistant wtEGFR NSCLC.
At the cytological level, we described the effect of gefitinib in combination with cisplatin in H358R/A549R cell lines. The anti-proliferative and pro-apoptotic activities of gefitinib and cisplatin in combination were increased compared with that of them monotherapy. In particular, the combined effect of the two drugs was close to that of cisplatin on parental cells. Similarly, in vivo, geifitinib and cisplatin combined treatment had a greater inhibitory effect on H358R xenograft growth than did either drug alone. The fact of anti-tumor effect significantly enhanced when gefitinib and cisplatin combined administration suggests a novel combination treatment strategy for cisplatin-resistant wtEGFR NSCLC that deserves clinical study.
By detecting EGFR/AKT/ERK downstream molecules, gefitinib combined with cisplatin significantly inhibited regulatory proteins associated with cell proliferation, cell cycle and mitochondrial apoptosis, which was consistent with anti-proliferative and pro-apoptotic effects. Considering the intricate signal networks and the frequent crosstalk between the pathways in the cell, we have not studied the upstream regulatory mechanism of these molecules. The combined dose of cisplatin also needs to be further explored to achieve the optimal ratio with gefitinib and exert the greatest anti-tumor effect, even if IC50 to cisplatin of sensitive cell lines was used in our experiment. In addition, whether this combination therapy strategy is suitable for other types of cisplatin-resistant wtEGFR NSCLC cells also requires further research, as we have only tested this two cell lines H358 and A549. These questions and the molecular mechanism of cisplatin to regulate EGFR activation will be the content of our subsequent research.
Conclusion
EGFR is abnormally activated in our induced cisplatin-resistant cell lines, and these cells have increased sensitivity to gefitinib. Gefitinib combined with cisplatin significantly enhances anti-tumor effects in vitro and in vivo. These results suggest that gefitinib and cisplatin in combination is a promising strategy for treating cisplatin-resistant wtEGFR NSCLC.
Acknowledgements
We thank Professor Emeritus, William R. Brown, M.D. (University of Colorado School of Medicine Denver CO USA) for revising the manuscript, and we would also like to acknowledge our families for their support and understanding, even during the outbreak of a new coronavirus in China. In addition, we very thank the experimental mice for their dedication to our research.
Author contributions
XT and AL conceived and designed the study. WC, XL and YZ performed the biological methodology. YM and RX performed molecular analysis and acquisition of data: RZ, XL and SZ were the data manager for the study. AL drafted the manuscript; RW and JL carried out a critical revision of the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Funding
None.
Data availability
Yes.
Compliance with ethical standards
Conflict of interest
None.
Ethical approval
All animal experiments were carried out in accordance with the principles and procedures approved by the Committee on the Ethics of Animal Experiments of Anhui University of Science and Technology.
Consent to participate
Yes.
Consent for publication
Yes.
Availability of data and material
Yes.
Code availability
Yes.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Amin Li, Weiya Cao, Xueke Liu and Yinci Zhang contributed equally to this work.
References
- Cao Q, You X, Xu L, Wang L, Chen Y. PAQR3 suppresses the growth of non-small cell lung cancer cells via modulation of EGFR-mediated autophagy. Autophagy. 2019;30:1–12. doi: 10.1080/15548627.2019.1659654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Yan JJ, Chen WC, Kuo MT, Lai YH, Lai WW, Liu HS, Su WC. Predictive and prognostic value of human copper transporter 1 (hCtr1) in patients with stage III non-small-cell lung cancer receiving first-line platinum-based doublet chemotherapy. Lung Cancer. 2012;75:228–234. doi: 10.1016/j.lungcan.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MK, Hong JY, Chang WJ, Kim MJ, Kim SM, Jung HA, Do IG, Choi YL, Sun JM, Ahn JS, Park K, Ahn MJ. A phase II trial of gefitinib monotherapy in pretreated patients with advanced non-small cell lungcancer not harboring activating EGFR mutations: implications of sensitive EGFR mutation test. Cancer Chemother Pharmacol. 2015;75:1229–1236. doi: 10.1007/s00280-015-2740-9. [DOI] [PubMed] [Google Scholar]
- Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- de La Motte RT, Galluzzi L, Olaussen KA, Zermati Y, Tasdemir E, Robert T, Ripoche H, Lazar V, Dessen P, Harper F, Pierron G, Pinna G, Araujo N, Harel-Belan A, Armand JP, Wong TW, Soria JC, Kroemer G. A novel epidermal growth factor receptor inhibitor promotes apoptosis in non-small cell lung cancer cells resistant to erlotinib. Cancer Res. 2007;67:6253–6262. doi: 10.1158/0008-5472.CAN-07-0538. [DOI] [PubMed] [Google Scholar]
- Friboulet L, Olaussen KA, Pignon JP, Shepherd FA, Tsao MS, Graziano S, Kratzke R, Douillard JY, Seymour L, Pirker R, Filipits M, André F, Solary E, Ponsonnailles F, Robin A, Stoclin A, Dorvault N, Commo F, Adam J, Vanhecke E, Saulnier P, Thomale J, Le Chevalier T, Dunant A, Rousseau V, Le Teuff G, Brambilla E, Soria JC. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med. 2013;368:1101–1110. doi: 10.1056/NEJMoa1214271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- Goloudina AR, Tanoue K, Hammann A, Fourmaux E, Le Guezennec X, Bulavin DV, Mazur SJ, Appella E, Garrido C, Demidov ON. Wip1 promotes RUNX2-dependent apoptosis in p53-negative tumors and protects normal tissues during treatment with anticancer agents. Proc Natl Acad Sci USA. 2012;109:E68–E75. doi: 10.1073/pnas.1107017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesinger F, Korol EE, Kayaniyil S, Varol N, Ebner T, Goring SM. Efficacy and safety of first-line carboplatin-versus cisplatin-based chemotherapy for non-small cell lung cancer: a meta-analysis. Lung Cancer. 2019;135:196–204. doi: 10.1016/j.lungcan.2019.07.010. [DOI] [PubMed] [Google Scholar]
- Guo G, Gong K, Wohlfeld B, Hatanpaa KJ, Zhao D, Habib AA. Ligand-independent EGFR signaling. Cancer Res. 2015;75:3436–3441. doi: 10.1158/0008-5472.CAN-15-0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardbower DM, Singh K, Asim M, Verriere TG, Olivares-Villagómez D, Barry DP, Allaman MM, Washington MK, Peek RM, Jr, Piazuelo MB, Wilson KT. EGFR regulates macrophage activation and function in bacterial infection. J Clin Investig. 2016;126:3296–3312. doi: 10.1172/JCI83585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle NT, Byers LA, Kim ES, Saintigny P, Lee JJ, Blumenschein GR, Tsao A, Liu S, Larsen JE, Wang J, Diao L, Coombes KR, Chen L, Zhang S, Abdelmelek MF, Tang X, Papadimitrakopoulou V, Minna JD, Lippman SM, Hong WK, Herbst RS, Wistuba II, Heymach JV, Powis G. Effect of KRAS oncogene substitutions on protein behavior: implications for signaling and clinical outcome. J Nat Cancer Inst. 2012;104:228–239. doi: 10.1093/jnci/djr523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, McCormick F, Smith-McCune K, Hanahan D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell. 2010;17:574–583. doi: 10.1016/j.ccr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhanwar-Uniyal M, Amin AG, Cooper JB, Das K, Schmidt MH, Murali R. Discrete signaling mechanisms of mTORC1 and mTORC2: connected yet apart in cellular and molecular aspects. Adv Biol Regul. 2017;64:39–48. doi: 10.1016/j.jbior.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Kamal NS, Soria JC, Mendiboure J, Planchard D, Olaussen KA, Rousseau V, Popper H, Pirker R, Bertrand P, Dunant A, Le Chevalier T, Filipits M, Fouret P. International adjuvant lung trial-bio investigators. MutS homologue 2 and the long-term benefit of adjuvant chemotherapy in lung cancer. Clin Cancer Res. 2010;16:1206–1215. doi: 10.1158/1078-0432.CCR-09-2204. [DOI] [PubMed] [Google Scholar]
- Kuo MT, Fu S, Savaraj N, Chen HH. Role of the human high-affinity copper transporter in copper homeostasis regulation and cisplatin sensitivity in cancer chemotherapy. Cancer Res. 2012;72:4616–4621. doi: 10.1158/0008-5472.CAN-12-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi L, Cappuzzo F. Experience with erlotinib in the treatment of non-small cell lung cancer. Ther Adv Respir Dis. 2015;9:146–163. doi: 10.1177/1753465815588053. [DOI] [PubMed] [Google Scholar]
- Leon G, MacDonagh L, Finn SP, Cuffe S, Barr MP. Cancer stem cells in drug resistant lung cancer: targeting cell surface markers and signaling pathways. Pharmacol Ther. 2016;158:71–90. doi: 10.1016/j.pharmthera.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Lin YX, Wang Y, An HW, Qi B, Wang J, Wang L, Shi J, Mei L, Wang H. Peptide-based autophagic gene and cisplatin co-delivery systems enable improved chemotherapy resistance. Nano Lett. 2019;19:2968–2978. doi: 10.1021/acs.nanolett.9b00083. [DOI] [PubMed] [Google Scholar]
- Lu N, Wang L, Cao H, Liu L, Van Kaer L, Washington MK, Rosen MJ, Dubé PE, Wilson KT, Ren X, Hao X, Polk DB, Yan F. Activation of the epidermal growth factor receptor in macrophages regulates cytokine production and experimental colitis. J Immunol. 2014;192:1013–1023. doi: 10.4049/jimmunol.1300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud WA, Nichols AC, Mroz EA, Faquin WC, Clark JR, Begum S, Westra WH, Wada H, Busse PM, Ellisen LW, Rocco JW. Bcl-2 blocks cisplatin-induced apoptosis and predicts poor outcome following chemoradiation treatment in advanced oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2009;15:1645–1654. doi: 10.1158/1078-0432.CCR-08-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes V, Wang W, Harrington B, Lee WJ, Beamish H, Chia KM, Pinder A, Goto H, Inagaki M, Pavey S, Gabrielli B. Cyclin A/Cdk2 regulates Cdh1 and claspin during late S/G2 phase of the cell cycle. Cell Cycle. 2014;13:3302–3311. doi: 10.4161/15384101.2014.949111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaussen KA, Dunant A, Fouret P, Brambilla E, André F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH, Stahel R, Sabatier L, Pignon JP, Tursz T, Le Chevalier T, Soria JC. IALT bio investigators. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382:709–719. doi: 10.1016/S0140-6736(13)61502-0. [DOI] [PubMed] [Google Scholar]
- Ren JH, He WS, Nong L, Zhu QY, Hu K, Zhang RG, Huang LL, Zhu F, Wu G. Acquired cisplatin resistance in human lung adenocarcinoma cells is associated with enhanced autophagy. Cancer Biother Radiopharm. 2010;25:75–80. doi: 10.1089/cbr.2009.0701. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014;79:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Shen DW, Ma J, Okabe M, Zhang G, Xia D, Gottesman MM. Elevated expression of TMEM205, a hypothetical membrane protein, is associated with cisplatin resistance. J Cell Physiol. 2010;225:822–828. doi: 10.1002/jcp.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timar J. The clinical relevance of KRAS gene mutation in non-small-cell lung cancer. Curr Opin Oncol. 2014;26:138–144. doi: 10.1097/CCO.0000000000000051. [DOI] [PubMed] [Google Scholar]
- Ulivi P, Delmonte A, Chiadini E, Calistri D, Papi M, Mariotti M, Verlicchi A, Ragazzini A, Capelli L, Gamboni A, Puccetti M, Dubini A, Burgio MA, Casanova C, Crinò L, Amadori D, Dazzi C. Gene mutation analysis in EGFR wild type NSCLC responsive to erlotinib: are there features to guide patient selection? Int J Mol Sci. 2014;16:747–757. doi: 10.3390/ijms16010747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu J, Liu J, Chen X, Chang M, Li J, Zhou J, Bai C, Song Y. GLRX inhibition enhances the effects of geftinib in EGFR-TKI-resistant NSCLC cells through FoxM1 signaling pathway. J Cancer Res Clin Oncol. 2019;145:861–872. doi: 10.1007/s00432-019-02845-y. [DOI] [PubMed] [Google Scholar]
- Yu H, Su J, Xu Y, Kang J, Li H, Zhang L, Yi H, Xiang X, Liu F, Sun L. p62/SQSTM1 involved in cisplatin resistance in human ovarian cancer cells by clearing ubiquitinated proteins. Eur J Cancer. 2011;47:1585–1594. doi: 10.1016/j.ejca.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li J, Hu J, Li D, Wang X, Zhang R, Zhang H, Shi M, Chen H. Cigarette smoke extract induces EGFR-TKI resistance via promoting EGFR signaling pathwayand ROS generation in NSCLC cell lines. Lung Cancer. 2017;109:109–116. doi: 10.1016/j.lungcan.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Zhao N, Zhang XC, Yan HH, Yang JJ, Wu YL. Effificacy of epidermal growth factor receptor inhibitors versus chemotherapy as second-line treatment in advanced non-small-cell lung cancer with wild-type EGFR: a meta-analysis of randomized controlled clinical trials. Lung Cancer. 2014;85:66–73. doi: 10.1016/j.lungcan.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Zhdanov AV, Aviello G, Knaus UG, Papkovsky DB. Cellular ROS imaging with hydro-Cy3 dye is strongly influenced by mitochondrial membrane potential. Biochim Biophys Acta Gen Subj. 2017;1861:198–204. doi: 10.1016/j.bbagen.2016.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Yes.