Highlights
-
•
Rhinoviruses (RV) frequently cause respiratory tract infections in young children.
-
•
We evaluated characteristics of RV infections in relation to clinical outcome.
-
•
In young children clinical outcome was not related to RV species or types.
-
•
Outcome of RV disease is more likely influenced by multiple (host-specific) factors.
Keywords: Rhinovirus, Respiratory tract infection, Epidemiology, Clinical outcome
Abstract
Background
Several studies have been published regarding the epidemiology and clinical significance of the different rhinovirus (RV) species (-A, -B and -C). However, data on RV types and the associations with clinical outcome in young children are limited. Here, we investigated the clinical, virological and epidemiological characteristics of RV infections in young children with mild or asymptomatic infection (non-hospitalised children) and in symptomatic young children admitted to the hospital.
Objectives
The aim of this study was to evaluate associations between different characteristics of RV infections and clinical outcome in young children.
Study design
RV-infected children were retrospectively selected from a Dutch birth cohort (EUROPA-study) and from hospitalised children admitted to the hospital because of respiratory symptoms. In total 120 RV-typed samples could be selected from 65 non-hospitalised and 49 hospitalised children between November 2009 and December 2012.
Results
RV-A was the predominant species in both study populations, followed closely by RV-C. RV-B was observed only sporadically. The distribution of the RV species was comparable in non-hospitalised and hospitalised children. In children with respiratory distress who required ICU-admission the distribution of RV species did not differ significantly from the non-hospitalised children. No predominant RV type was present in non-hospitalised nor hospitalised children. However, hospitalised children were younger, had more often an underlying illness, a higher RV load and more frequently a bacterial co-infection.
Conclusions
Clinical outcome of RV infected young children was not related to RV species or types, but may more likely be influenced by multiple (host-specific) factors.
1. Introduction
Rhinoviruses (RVs) are causative agents of frequently and recurrently occurring respiratory tract infections in young children. Symptoms range from a mild common cold to severe life-threatening pneumonia and about 1/3 of RV infections are asymptomatic [1]. RVs are classified phylogenetically into three species: RV-A, RV-B, and RV-C, and are further divided into >150 types [2].
Recently, several studies have been published regarding the molecular epidemiology and clinical significance of RVs, suggesting the infecting species might influence the clinical outcome [3], [4]. RV-A and RV-B have been associated with mild disease, whereas RV-C may cause more severe disease [5]. Particularly in children, RV-C seems to be associated with asthma exacerbations, acute lower respiratory tract infections, bronchiolitis, and pneumonia [6], [7] .
However, RV species may not be the sole determinant of clinical outcome of an RV infection. Given the wide diversity of RV types within RV species, the RV type rather than the species may relate to severity of disease [8]. Together with host susceptibility, the RV type and the cellular tropism, as partly determined by the virus receptor used, may be important factors contributing to disease and clinical outcome in young children [9]. Up to date, data on the relation between RV types and clinical symptoms combined with the occurrence of RV types during various seasons in one year and circulation patterns are limited and often available either for hospitalised or asymptomatic children.
2. Objectives
This study describes the clinical, virological and epidemiological characteristics of RV infections in young children with mild or asymptomatic infection who do not need hospitalisation for their respiratory tract symptoms, as compared to young children in need of admittance to the hospital because of their respiratory tract disease. By comparing these groups associations between the different characteristics of RV infections, i.e. the infecting species and types, viral load, viral and bacterial co-infections, and seasonality, in relation to clinical outcome are studied.
3. Study design
3.1. Study population and sample selection
This retrospective study consisted of successfully sequenced RV-positive respiratory samples (naso- or oropharyngeal swabs and washes) from young children with mild or asymptomatic RV infection (non-hospitalised children) and from hospitalised children. For this study we selected children that met the following criteria: (i) date of birth between May 2008 and May 2010, (ii) sampling date between November 2009 and December 2012, (iii) a respiratory sample positive for RV in the 5′UTR multiplex PCR and successfully VP4/VP2 sequenced.
Non-hospitalised children were selected from the EUROPA-trial (Early Unbiased Risk Assessment of Paediatric Asthma), a prospective cohort study in Amsterdam, the Netherlands, which focused on the prediction of early signs of asthma [10]. In this study, samples were obtained from children visiting the family physician with respiratory tract symptoms and from asymptomatic children. The population of hospitalised children consisted of retrospectively selected samples from children admitted to the Academic Medical Center (AMC), Amsterdam, because of respiratory tract disease (defined as a combination of one or more of the following symptoms: fever, rhinitis, cough, dyspnoea, tachypnoea, and/or wheezing). To investigate circulation of RV species and types in children with more severe illness we performed a subgroup analysis within the hospitalised population of children admitted to the Intensive Care Unit (ICU) due to respiratory distress.
Information about the children from the EUROPA-study was obtained from study report forms and all children with mild or asymptomatic infection were monitored for the development of severe disease that required hospitalisation at a later time point. The EUROPA-study was approved by the Medical Ethical Committee of the AMC (09/066) and the parents gave written informed consent. The EUROPA study is registered in the Dutch Trial Register (NTR-1955). The samples taken from the hospitalised children were part of routine diagnostic tests and were processed according to hospital ethical guidelines and the Dutch code of conduct for responsible use of human tissue and medical research in 2011. A standardized record form was designed to obtain the patients’ information from medical records, including demographic and clinical characteristics.
3.2. Virological and bacterial assessments
All respiratory samples were assessed within 24 h after collection at the Laboratory of Clinical Virology of the AMC according to standard operating procedures for the presence of respiratory tract associated viruses (enterovirus (EV), human parechovirus (HPeV), influenzavirus A and B, para-influenzavirus 1–4, human bocavirus (HBoV), human coronavirus, respiratory syncytial virus (RSV), adenovirus and human metapneumovirus), using a multiplex polymerase chain reaction (PCR) as described previously by Jansen et al. [11] with adapted RV primers published elsewhere [12]. A Ct-value of 40 or more was considered to be negative. The complete protocol for RV typing is provided as supplementary data (see Supplementary file). All RVs were divided in (i) the major group (which bind to the intercellular adhesion molecule 1 (ICAM-1)), (ii) the minor group (which use the low-density lipoprotein receptor (LDL-R) as a receptor), and (iii) the RV-C group which most probably uses the human cadherin-related family member 3 (CDHR3) receptor [13], [14]. Information on bacterial co-infection was included in case a bacteriological culture was performed on the same day, or one day before or after virological sampling.
3.3. Data analysis
An infection was classified as a new infection in the event of a first RV-positive sample from a patient. If multiple samples were available from the same patient, infections were defined as a new infection when the sample yielded a different type than the previous sample, and/or when the time between the two samples was at least three months.
For data analysis, all infections were considered statistically independent. To avoid overrepresentation of data only the first RNA positive sample from a new infection was included. Data were analysed using SPSS statistical software (version 21.0, SPPS Inc., Chicago, IL). Categorical variables were compared by means of chi-squared test. Differences between continuous variables were determined using Student’s t test or ANOVA if normally distributed and non-parametric tests if not normally distributed. A two-sided p-value <0.05 was considered statistically significant.
4. Results
4.1. Patient characteristics
Following our selection criteria we were able to include 120 RV-positive respiratory tract samples. A total of 68 RV-positive samples were included from children with mild or asymptomatic infection (non-hospitalised children) and 52 RV-positive samples were included from children who needed hospitalisation because of respiratory symptoms. Patient and virological characteristics are summarized in Table 1 .
Table 1.
Patient and virological characteristics at time of infection.
| New infections in non-hospitalised children (n = 68), n (%) | New infections in all hospitalised children (n = 52), n (%) | New infections in ICU admitted children (n = 26), n (%) | |
|---|---|---|---|
| Non-hospitalised children (n = 65) | Hospitalised children (n = 49) | RV infected hospitalised children (ICU) (n = 26) | |
| Median age in years (IQR) | 1.5 (1.0–2.2) | 0.8 (0.5–1.8) | 0.7 (0.3–1.9) |
| Gender | |||
| Male | 40 (58.8) | 30 (57.7) | 16 (61.5) |
| Female | 28 (41.2) | 22 (42.3) | 10 (38.5) |
| Underlying illness | |||
| No underlying illness | 68 (100.0) | 24 (46.2) | 11 (42.3) |
| >1 Underlying illness | – | 7 (13.5) | 3 (11.5) |
| Prematurity | – | 14 (26.9) | 7 (26.9) |
| Immunodeficiency | – | – | – |
| Malignancy | – | 2 (3.8) | 1 (3.8) |
| Cardial | – | 2 (3.8) | 1 (3.8) |
| Metabolic | – | – | – |
| Pulmonary | – | 2 (3.8) | 1 (3.8) |
| Renal | – | 2 (3.8) | 1 (3.8) |
| Genetic disorder | – | 8 (15.4) | 6 (23.1) |
| Viral coinfection | 34 (50.0) | 23 (44.2) | 9 (34.6) |
| Bacterial coinfection (32 samples unknown) | 1 (1.5) | 14 (70.0) | 12 (66.7) |
| Mean Ct-value (SD) | 28.3 (2.3) | 26.0 (2.9) | 25.7 (3.3) |
RV, rhinovirus; ICU, Intensive Care Unit; IQR, interquartile range.
In the hospitalised group, 26 children were admitted to a general ward and 26 to the Intensive Care Unit (ICU) due to respiratory distress. Of the ICU-admitted children, 57.7% (n = 15) had an underlying illness, most frequently prematurity (n = 7), which is comparable with the hospitalised children who were not ICU-admitted (underlying illness: 46.2% (n = 12)), of which seven were premature. No manifest underlying illness was present in the non-hospitalised children at inclusion. At the time of RV infection the hospitalised children were younger (median 0.8 years, interquartile range (IQR) 0.5–1.8) than the non-hospitalised children (median 1.5 years, IQR 1.0–2.2) (p < 0.001).
4.2. RV species and types
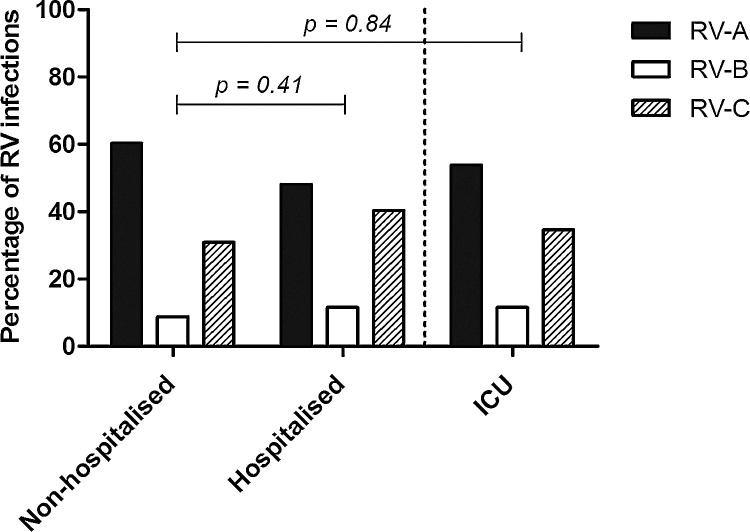
The prevalence of RV species, stratified by study group, is illustrated in Fig. 1 . In both groups the most dominant species was RV-A, followed by RV-C and RV-B. The distribution of RV species was comparable between non-hospitalised and hospitalised children (p = 0.41). Also, the distribution of RV species in the ICU-admitted children from the hospitalised group did not differ significantly from the non-hospitalised children (p = 0.84). When dividing the RVs according to their receptor usage (ICAM-1, LDL-R and most probably CDHR3), Table 2 shows that the receptor use was comparable among the non-hospitalised and the hospitalised children, as well as among the ICU-admitted children.
Fig. 1.
Distribution of RV species in non-hospitalised, hospitalised and ICU-admitted children.
Distribution of RV species was comparable in all populations.
Table 2.
RV receptor.
| New infections in non-hospitalised children (n = 68), n (%) | New infections in all hospitalised children (n = 52), n (%) | New infections in ICU admitted children (n = 26), n (%) | |
|---|---|---|---|
| ICAM-1a (%) | 38 (55.9) | 24 (46.2) | 13 (50.0) |
| LDL-Rb (%) | 2 (2.9) | 1 (1.9) | 0 (0.0) |
| CDHR3c (%) | 22 (32.4) | 21 (40.4) | 9 (34.6) |
| Receptor to be determined | 6 (10.3) | 6 (11.5) | 4 (15.4) |
ICAM, intercellular adhesion molecule 1.
LDL-R, low-density lipoprotein receptor.
CDHR3, human cadherin-related family member 3.
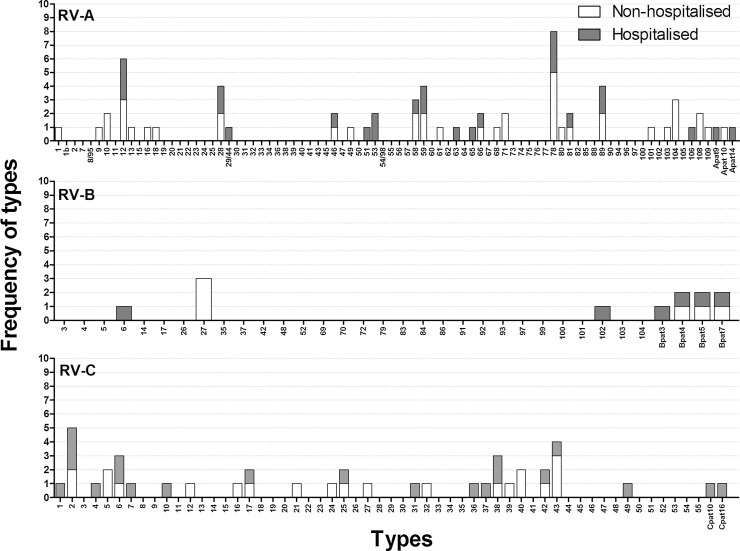
When we further analysed the study groups for the occurrence of RV types, we did not observe skewing towards one type in either the hospitalised or non-hospitalised group (Fig. 2 ). We observed 19 RV types in both populations, while the other 48 RV types were detected in either hospitalised or non-hospitalised children. RV-A78 was the most prevalent type in non-hospitalised children, whereas in the hospitalised children these were RV-A78 and RV-A12. Within the RV-B species, RV-B6 and RV-102 were only seen in hospitalised children and RV-B27 solely in non-hospitalised children. Provisionally assigned type B4 (Bpat4), B5 (Bpat5) and B7 (Bpat7) were detected in both populations. For RV-C, the most prevalent type was RV-C2 in hospitalised children and RV-C43 in non-hospitalised children. The most commonly detected types in ICU-admitted children (RV-A12, RV-A28, RV-A89, and RV-C6) were all detected in non-hospitalised children as well.
Fig. 2.
Frequency of RV types.
For each species the prevalence of detected types in both populations is illustrated. White bars indicate non-hospitalised children and shaded bars indicate hospitalised children.
4.3. Viral load
Ct value is a well-established semi-quantitative measurement of viral load [15]. The mean Ct-value of RV infections in children was significantly lower than the mean Ct-value of the samples from the non-hospitalised children (Ct-value 26.0 versus 28.3, p < 0.001) (Table 1), implying a higher viral load in symptomatic children.
4.4. Viral and bacterial co-infections
In the non-hospitalised and hospitalised children, a viral co- or triple-infection was detected in 50.0% (n = 34) and 44.2% (n = 23) of the samples respectively. The most frequently detected co-infecting viruses in the non-hospitalised group were HBoV (30.9%, n = 21), adenovirus (13.2%, n = 9), and RSV (13.2%, n = 9). In the hospitalised children, RSV was the most common viral co-pathogen (19.2%, n = 10). A viral co-infection was detected in 34.6% (n = 9) of the ICU-samples, five of which were with RSV. There were no significant differences between the study groups regarding the number of viral co-infections and there was no significant difference in the number of viral co-infections detected in children infected with RV-A, -B, or -C.
A bacterial co-infection (as diagnosed by a positive bacterial throat or sputum culture) was present in only one of the non-hospitalised children, while positive bacterial cultures were detected in 14 (70.0%) of the 20 hospitalised children. From the remaining hospitalised children there was no information available on bacterial co-infections. The most frequently detected pathogens were Moraxella catarrhalis (n = 6) and Haemophilus influenzae (n = 5). Although the numbers of children with bacterial co-infections were small there was no skewing towards one RV species (p = 0.26). In ICU children a bacterial co-infection was present in at least 66.7% of the samples. The most frequently detected pathogens were M. catarrhalis (n = 5) and H. influenzae (n = 5).
4.5. Seasonal distribution of RV species and types
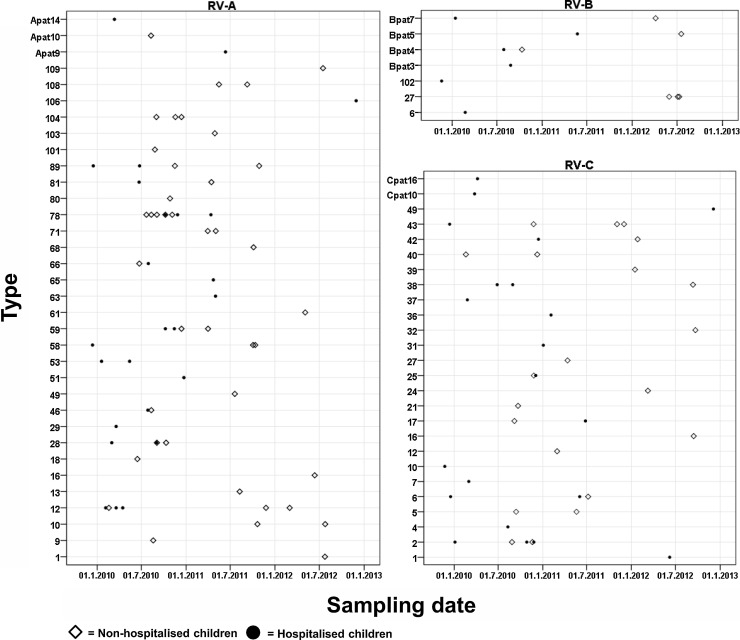
Combining both populations, RV infections were seen all year round with a peak in incidence in the autumn and winter of 2010 (Fig. 3 ). Fig. 4 shows that there is no distinct circulation pattern for the different RV types in time. Some trends could be observed. Type RV-A78, which was the most prevalent strain in the hospitalised population, was found only between July 2010 and July 2011. The second most prevalent type, RV-A12, was detected in the spring of 2010 and was sporadically detected again, but only in non-hospitalised children in the winter of 2011–2012. RV-C2, the most prevalent RV-C, was mainly observed during the autumn and the beginning of winter 2010–2011. In conclusion, despite small numbers no season-specific types could be observed.
Fig. 3.
Seasonal distribution of RV species.
Stacked bars indicate the seasonal distribution of RV species.
Fig. 4.
Circulation of RV types in non-hospitalised and hospitalised children.
The x-axis indicates the study period and the y-axis indicates the detected types from corresponding species detected during study period. White diamonds indicate non-hospitalised patients and black circles indicate hospitalised children.
5. Discussion
Here we described for the first time the clinical, virological and epidemiological characteristics of RV infections in young children with mild or no symptoms compared to symptomatic children hospitalised due to respiratory tract disease.
RV-A was the predominant species in both study populations, followed closely by RV-C. RV-B was observed only sporadically. These findings are in accordance with other studies [9], [16], [17] in both non-hospitalised and hospitalised children and suggest that our populations are representative for larger populations.
The distribution of the RV species was comparable in non-hospitalised and hospitalised children. Also in children with respiratory distress who required ICU-admission the distribution of RV species did not differ significantly from the non-hospitalised children. RV-C was not overrepresented in hospitalised children nor in ICU-admitted children suggesting that in our study populations an infection with RV-C alone might not be associated with more severe disease. Previous studies on disease severity showed that infection with RV-C was associated with more severe respiratory symptoms in children. However, these studies were mainly conducted in hospitalised patients where specific risk groups, such as immunocompromised patients, were overrepresented which might bias the results [5], [18]. More recent studies are consistent with our findings [3], [19], [20]. In addition, several studies in adults were in line with our results [21], [22].
We detected a large number of RV types by using the VP4/VP2 fragment for typing. A recent study by Martin et al. [8] found that specific RV-A and RV-C types were associated with lower respiratory tract infections. We found several types in both populations and no predominant RV type could be found in severely ill children. However, due to the relatively small study population no definite conclusions can be made regarding specific RV types and clinical outcome.
When comparing the mild or asymptomatic RV infected children with the hospitalised children several differences between the study groups that might be important determinants for clinical outcome emerged. When evaluating the patient characteristics we found a high percentage of the hospitalised children having an underlying illness. Prematurity was the most common and a probable risk factor for more serious RV disease. Secondly, the median age in the hospitalised children was significantly lower than the median age at sampling for non-hospitalised children. As younger children are more vulnerable for hospitalisation this was not an unexpected finding. However, as our study is not a matched case-control study we cannot exclude an age-specific distribution of RV species and types. Ct-values differed significantly between non-hospitalised and hospitalised children. We found a higher viral load (indicated by lower Ct values) in hospitalised children, suggesting that a higher viral load might lead to more severe disease [15], [23]. This has been described in other studies as well [24] . Another influencing factor might be infection with co-pathogens. The frequency of viral co-infections was comparable in both groups, but a bacterial co-infection was detected more often in severely ill children. Our study indicates that bacterial co-infection, and especially co-infection with respiratory pathogens such as M. catarrhalis and H. influenzae, might be an important contribution to disease severity.
Some limitations of our study need to be considered. First, because of the retrospective study design in combination with the relatively small sample size a matched case-control study was not possible. By selecting children of comparable age and period of sample collection we made the study groups as comparable as possible. A post hoc power analysis showed that with our sample size we would be able to detect large differences between the study groups indicating that our sample size was sufficient to support our main finding that RV species were equally distributed among all study groups. Information on bacterial co-infections was not available for all patients. Previous studies describing relations between molecular epidemiology of RV infections and clinical outcome did not take bacterial co-infections into account [8], [25]. Based on the results from our study bacterial co-pathogens might be an important contributing factor to disease severity and justify the inclusion and analyses of influences of these bacterial co-pathogens in future studies.
In conclusion, our results support previous findings suggesting that clinical outcome is not related to RV species or types alone, but may more likely be influenced by multiple (host-specific) factors, such as age, chronic underlying illness, viral load and the presence of a bacterial co-infection. A more detailed investigation of host-specific genetic factors, such as polymorphisms in susceptibility genes (e.g. CDHR3) and alterations in chemokine genes would contribute to an even better understanding in the relation between RV and clinical outcome. To further elucidate this association, large longitudinal cohorts of RV-infected patients and samples from both the acute period of infection as well as follow-up samples are needed.
Conflict of interest
All authors approved the final manuscript and they have no conflict of interest to declare.
Sources of funding
X.T. and L.L. were completely and A.B. was partially funded by a grant from Crucell Vaccine Institute (CVI). The institution of P.S. has received a charity grant by the Dutch Lung Foundation for the Europa Cohort Study. K.W. and A.B. are partially funded by the Seventh Framework Programme of the European Union IAPP under contract PIAPP-GA-2013-612308 (AIROPico, www.airopico.eu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship contribution
M.S., P.S., M.D., K.W. and D.P. contributed substantially to the conception of the work. A.B., X.T., L.L., J.W., R.J., and R.M. analysed and interpreted the data. A.B. and X.T. drafted the manuscript and all authors revised it critically for important intellectual content. All authors approved the final version of the manuscript for publication and agreed to be accountable for all aspects of the work.
Acknowledgements
We owe our gratitude to all the parents and children contributing to this study from the Emma Children's hospital and the EUROPA-study. Furthermore we wish to acknowledge the significant contribution of Janke Schinkel, Simone Hashimoto, Aline Sprikkelman, Wim van Aalderen and Eric Haarman, without whom this study would not have been possible. Part of this work is presented at the Scientific spring Meeting of the Dutch Society of Medical Microbiology and the European Society for Pediatric Infectious Diseases.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jcv.2015.10.024.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Jacobs S.E., Lamson D.M., St George K., Walsh T.J. Human rhinoviruses. Clin. Microbiol. Rev. 2013;26(1):135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McIntyre C.L., Knowles N.J., Simmonds P. Proposals for the classification of human rhinovirus species A, B and C into genotypically assigned types. J. Gen. Virol. 2013;94(Pt. 8):1791–1806. doi: 10.1099/vir.0.053686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W.J., Arnold J.C., Fairchok M.P., Danaher P.J., McDonough E.A., Blair P.J. Epidemiologic, clinical, and virologic characteristics of human rhinovirus infection among otherwise healthy children and adults: rhinovirus among adults and children. J. Clin. Virol. 2015;64:74–82. doi: 10.1016/j.jcv.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peltola V., Jartti T., Putto-Laurila A., Mertsola J., Vainionpaa R., Waris M. Rhinovirus infections in children: a retrospective and prospective hospital-based study. J. Med. Virol. 2009;81(10):1831–1838. doi: 10.1002/jmv.21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauinger I.L., Bible J.M., Halligan E.P., Bangalore H., Tosas O., Aarons E.J. Patient characteristics and severity of human rhinovirus infections in children. J. Clin. Virol. 2013;58(1):216–220. doi: 10.1016/j.jcv.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bizzintino J., Lee W.M., Laing I.A., Vang F., Pappas T., Zhang G. Association between human rhinovirus C and severity of acute asthma in children. Eur. Respir. J. 2011;37(5):1037–1042. doi: 10.1183/09031936.00092410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu Q.B., Wo Y., Wang L.Y., Wang H.Y., Huang D.D., Zhang X.A. Molecular epidemiology of human rhinovirus in children with acute respiratory diseases in Chongqing, China. Sci. Rep. 2014;4:6686. doi: 10.1038/srep06686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin E.K., Kuypers J., Chu H.Y., Lacombe K., Qin X., Strelitz B. Molecular epidemiology of human rhinovirus infections in the pediatric emergency department. J. Clin. Virol. 2015;62:25–31. doi: 10.1016/j.jcv.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee W.M., Lemanske R.F., Jr., Evans M.D., Vang F., Pappas T., Gangnon R. Human rhinovirus species and season of infection determine illness severity. Am. J. Respir. Crit. Care Med. 2012;186(9):886–891. doi: 10.1164/rccm.201202-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Schee M.P., Hashimoto S., Schuurman A.C., van Driel J.S., Adriaens N., van Amelsfoort R.M. Altered exhaled biomarker profiles in children during and after rhinovirus-induced wheeze. Eur. Respir. J. 2015;45(2):440–448. doi: 10.1183/09031936.00044414. [DOI] [PubMed] [Google Scholar]
- 11.Jansen R.R., Schinkel J., Koekkoek S., Pajkrt D., Beld M., de Jong M.D. Development and evaluation of a four-tube real time multiplex PCR assay covering fourteen respiratory viruses, and comparison to its corresponding single target counterparts. J. Clin. Virol. 2011;51(3):179–185. doi: 10.1016/j.jcv.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaramillo-Gutierrez G., Benschop K.S., Claas E.C., de Jong A.S., van Loon A.M., Pas S.D. September through October 2010 multi-centre study in the Netherlands examining laboratory ability to detect enterovirus 68, an emerging respiratory pathogen. J. Virol. Methods. 2013;190(1–2):53–62. doi: 10.1016/j.jviromet.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Schuler B.A., Schreiber M.T., Li L., Mokry M., Kingdon M.L., Raugi D.N. Major and minor group rhinoviruses elicit differential signaling and cytokine responses as a function of receptor-mediated signal transduction. PLoS One. 2014;9(4):e93897. doi: 10.1371/journal.pone.0093897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bochkov Y.A., Watters K., Ashraf S., Griggs T.F., Devries M.K., Jackson D.J. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc. Natl. Acad. Sci. U. S. A. 2015;112(17):5485–5490. doi: 10.1073/pnas.1421178112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller J.A., Njenga M.K., Bigogo G., Aura B., Ope M.O., Nderitu L. Association of the CT values of real-time PCR of viral upper respiratory tract infection with clinical severity, Kenya. J. Med. Virol. 2013;85(5):924–932. doi: 10.1002/jmv.23455. [DOI] [PubMed] [Google Scholar]
- 16.Linder J.E., Kraft D.C., Mohamed Y., Lu Z., Heil L., Tollefson S. Human rhinovirus C: age, season, and lower respiratory illness over the past 3 decades. J. Allergy Clin. Immunol. 2013;131(1):69–77.e6. doi: 10.1016/j.jaci.2012.09.033. e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piralla A., Lilleri D., Sarasini A., Marchi A., Zecca M., Stronati M. Human rhinovirus and human respiratory enterovirus (EV68 and EV104) infections in hospitalized patients in Italy, 2008–2009. Diagn. Microbiol. Infect. Dis. 2012;73(2):162–167. doi: 10.1016/j.diagmicrobio.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Piralla A., Rovida F., Campanini G., Rognoni V., Marchi A., Locatelli F. Clinical severity and molecular typing of human rhinovirus C strains during a fall outbreak affecting hospitalized patients. J. Clin. Virol. 2009;45(4):311–317. doi: 10.1016/j.jcv.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Launes C., Armero G., Anton A., Hernandez L., Gimferrer L., Cisneros C. Molecular epidemiology of severe respiratory disease by human rhinoviruses and enteroviruses at a tertiary paediatric hospital in Barcelona, Spain. Clin. Microbiol. Infect. 2015;21(8):799. doi: 10.1016/j.cmi.2015.04.021. e5–7. [DOI] [PubMed] [Google Scholar]
- 20.Tovey E.R., Stelzer-Braid S., Toelle B.G., Oliver B.G., Reddel H.K., Willenborg C.M. Rhinoviruses significantly affect day-to-day respiratory symptoms of children with asthma. J. Allergy Clin. Immunol. 2015;135(3):663–669. doi: 10.1016/j.jaci.2014.10.020. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCulloch D.J., Sears M.H., Jacob J.T., Lyon G.M., Burd E.M., Caliendo A.M. Severity of rhinovirus infection in hospitalized adults is unrelated to genotype. Am. J. Clin. Pathol. 2014;142(2):165–172. doi: 10.1309/AJCPHIKRJC67AAZJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi S.H., Hong S.B., Kim T., Kim S.H., Huh J.W., Do K.H. Clinical and molecular characterization of rhinoviruses A, B, and C in adult patients with pneumonia. J. Clin. Virol. 2015;63:70–75. doi: 10.1016/j.jcv.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Jansen R.R., Schinkel J., Dek I., Koekkoek S.M., Visser C.E., de Jong M.D. Quantitation of respiratory viruses in relation to clinical course in children with acute respiratory tract infections. Pediatr. Infect. Dis. J. 2010;29(1):82–84. doi: 10.1097/INF.0b013e3181b6de8a. [DOI] [PubMed] [Google Scholar]
- 24.Ambrosioni J., Bridevaux P.O., Aubert J.D., Soccal P., Wagner G., Kaiser L. Role of rhinovirus load in the upper respiratory tract and severity of symptoms in lung transplant recipients. J. Clin. Virol. 2015;64:1–5. doi: 10.1016/j.jcv.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Principi N., Zampiero A., Gambino M., Scala A., Senatore L., Lelii M. Prospective evaluation of rhinovirus infection in healthy young children. J. Clin. Virol. 2015;66:83–89. doi: 10.1016/j.jcv.2015.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.