Abstract
We have previously shown that Notch1 plays a critical role in modulating melanoma tumor cell growth and survival. Here we show that Notch1 also contributes to an immune-suppressive tumor microenvironment (TME). Notch1 inhibition reduces immune suppressive cells (i.e. MDSCs and Tregs) while allowing the recruitment of functional CD8(+) T cells, leading to a decrease in the Tregs/CD8(+) ratio, a key parameter in assessing positive responses to immune-checkpoint inhibitors. Inhibition of Notch1 improves the antitumor activity of nivolumab and ipilimumab, particularly when given in combination. Mechanistically, tumor-associated Notch1 regulates the expression of several chemokines involved in MDSCs and Tregs recruitment. Among them, CCL5, IL6 and IL8, or MIP2 in mouse, were consistently reduced by Notch1 depletion in several human and mouse melanoma cell lines. Notch1 controls the transcription of IL8 and IL6; and the secretion of CCL5 likely by inhibiting the expression of SNAP23, a member of the SNAREs family of proteins involved in cell exocytosis. Inhibition of SNAP23 decreases CCL5 secretion similarly to Notch1 inhibition. Hence, targeting Notch1 would affect both melanoma intrinsic growth/survival properties, and provide an immune-responsive TME, thus improving immune therapy efficacy.
Keywords: melanoma, Notch1, immunotherapy, immunecheckpoint inhibitors (ICIs)
Introduction
Notch proteins are single-pass trans-membrane receptors involved in embryogenesis and self renewal in adult tissues, but are often re-activated in several cancers. Notch1, one of the four members of the Notch family, has been associated with a number of malignancies, including prostate, lung, ovarian and renal cancers and T-cell acute lymphoblastic leukemia (T-ALL) where it is found frequently mutated[1-6].
Notch1 plays crucial roles in the maintenance of melanocyte stem and precursor cell homeostasis[7]. Lack of Notch1 in the melanocytic lineage results in premature hair graying in the mouse caused by loss of melanoblasts in the embryo and premature differentiation of melanocyte stem cells in the adult mouse[7]. In mature melanocytes, Notch1 expression is reduced; however, melanomas show increased expression and activity of Notch1 compared to normal melanocytes[8, 9]. Our previous work demonstrated that Notch1 is elevated in up to 60% of melanomas and that Notch1 depletion in melanoma cells causes cell death and inhibits proliferation, leading to reduced melanoma growth. Accordingly, forced expression of active Notch1 (NIC) transforms primary human melanocytes in vitro and confers metastatic properties to primary melanoma cells[8-13], overall underpinning a critical role of Notch1 signaling in controlling several aspects of melanoma tumorigenesis.
A role of cancer associated Notch in modulating the immune component of the tumor microenvironment has started to emerge. For example, inhibition of Notch signaling by a γ-secretase inhibitor decreased tumor burden in a mouse model of head and neck squamous cell carcinoma, and importantly was associated with decreases in myeloid-derived suppressor cells (MDSCs), tumour-associated macrophages (TAMs) and regulatory T cells (Tregs), as well as immune checkpoint molecules (PD1, CTLA4, TIM3 and LAG3) in the tumor[14]. Similarly, in a murine model of melanoma, reduced tumor Notch1 resulted in reduced infiltration of immune suppressive cells (e.g. Tregs, MDSCs) and an increase in cytotoxic T cells [15].
MDSCs are a heterogeneous population of cells that are defined by their myeloid origin, immature state and ability to potently suppress T cell responses. Therefore, high numbers of tumor-infiltrating MDSCs are often associated with higher tumor burden and metastatic disease, leading to poor survival in cancer patients[16]. In melanoma, circulating MDSCs have a negative impact on survival and inversely correlate with the presence of functional antigen-specific T cells[17]. Also, patients with higher circulating MDSCs respond less to immunetherapies such as ipilimumab (anti CTLA4)[18] and nivolumab (anti PD1)[19]. Tregs, in particular peripheral Tregs, are induced from naïve CD4(+)T cells and are defined by the expression of the Forkhead Box P3 (FoxP3) transcription factor[20]. They also contribute to an immune suppressive TME. It has been shown that an accumulation of FoxP3+ Tregs and, in particular, a higher abundance of Tregs over CD8(+)T cells within tumor tissue is associated with worse prognosis in several cancers including melanoma[20, 21].
Given the promise of immune checkpoint inhibitor therapies in improving the life expectancy of patients[22, 23], it is important to understand the underlying mechanisms of resistance toward these treatments. Here we show that Notch1 controls the types of immune cells that infiltrate melanoma tumors. Notch1 depletion results in a decrease in MDSCs and Tregs and an increase in functional cytotoxic effector T cells (CD8), leading to an overall decrease in the Tregs/CD8 ratio. Importantly, Notch1 inhibition significantly improves the anti tumor effects of nivolumab (anti-PD1) and ipilimumab (anti-CTLA4). Mechanistically, we propose that melanoma cell expression of Notch1 controls the expression and secretion of several chemokines involved in MDSCs and Tregs recruitment to the tumor site. Thus, inhibiting Notch1 can improve the effectiveness of checkpoint inhibitors, while simultaneously affecting the growth and survival of cancer cells.
Materials and methods
Cell lines, mice, and tumor models
Human melanoma cell lines WM266-4, K457, SKMel2, S2842, MeWo, FEMX, and mouse lines B16F10, Yumm1.1 were purchased from ATCC or were kindly provided by Dr. Marianne Powell (Stanford University) and Dr. Marcus Bosenberg (Yale University). Cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100U/ml penicillin and 100μg/ml streptomycin at 37 °C in a 5% CO2 atmosphere.
Eight-weeks old female C57BL/6 mice were purchased from The Jackson Laboratory and maintained under pathogen free conditions. 1.5×106 B16F10 cells or 1×106 YUMM1.1 cells expressing shGFP or shNotch1 were subcutaneously injected into the flanks of 5 mice for a total of 10 tumors per group per experimental repeat. At least two experimental repeats were performed. Tumor size was measured by caliper every 3 days using the formula: (LxW2/2). Tumors and spleens were harvested at day 17 and day 25 for shGFP expressing B16F10 tumors and Yumm1.1 tumors, respectively and at day 21 and day 29 for shNotch1 expressing B16F10 tumors and Yumm1.1 tumors, respectively. For the treatment with immune checkpoints ipilimumab (anti-CTLA4, BioXcell, West Lebanon, NH) and nivolumab (anti-PD1, BioXcell, West Lebanon, NH), B16F10 cells expressing either shGFP or shNotch1 were inoculated subcutaneously into the flank of 8 weeks old C57BL/6 mice. At day 8 post-inoculation, i.e. when tumors in all animals were approximately 150-200 mm3, mice carrying either shGFP or shNotch1 expressing tumors, were divided into four groups of treatments: isotype IgG (Ctrl) anti-PD1 (200 μg/dose), anti-CTLA4 (200 μg/dose) or a combination of anti-PD1 and anti-CTLA4, given by intraperitoneal injection every other day for the duration of the experiment, as previously reported in [24, 25] Tumors were measured as indicated above and tumors and spleen were collected at the end time point for flow cytometry analysis.
shRNA, and antibodies for Western Blotting
TRCN0000003359 shNotch1 was used against human Notch1 as previously described[8, 9,13]. TRCN0000025935 (1) and TRCN0000025895 (2) shNotch1 were used for mouse Notch1 knockdown. The shRNAs used for SNAP23 knockdown were: TRCN0000218715 (1), TRCN0000229795 (2), TRCN0000144789 (3) (Sigma). Antibodies used for WB were: anti-Notch1 (C20, Santa Cruz Biotechnology), anti-α-Tubulin (DM1A, Sigma), anti-SNAP23 (ab3340, Abcam) and anti-SNAP23 (D-11, Santa Cruz Biotechnology).
Flow cytometry analysis
Spleens were dissociated mechanically into single cell suspensions by 40μm strainers in ice-cold 2% FBS HBSS buffer. Tumors were digested with collagenase D dissociation buffer, and single cell suspensions were collected from discontinuous percoll gradients as described previously[26]. Dead cells discrimination was performed by Viobility 405/452 Fixable Dye (Miltenyi Biotec). Cell surface antigens were probed by incubating cells at 4°C for 30 minutes. Intracellular staining of Foxp3 was done using the fixation/Permeabilization buffer according to the manufactured recommendations (Miltenyi Biotec). The antibodies used for flow cytometry were: anti-CD11b-PE (M1/70), anti-Ly6C-FITC (HK1.4), anti-Ly6G-APC (1A8), anti-CD8-APC (53.6.7), anti-CD4-FITC (GK1.5), anti-CD69-PE (H1.2F3) (BioLegend) and MACS Treg detection kit (Miltenyi Biotec). Cells were analyzed with Attune NxT flow cytometer and followed by flow-Jo software analysis. MDSCs were phenotyped as CD11b+ Ly6G− Ly6Chi (Mono-MDSCs) and CD11b+ Ly6G+ Ly6Clo (PMN-MDSCs). Treg cells were phenotyped as CD4+Foxp3+.
MDSCs migration assay
MDSCs were isolated from spleens of tumor free C57BL/6 mice by sorting Gr1+-APC/CD11b+-FITC cells. Purity was determined by FACS to be at 90% (Suppl. Fig. 1). 500μl 2×106 MDSCs were plated into 24-transwell inserts, and 750 μl conditioned media from B16F10 cells expressing either shGFP or shNotch1 were added to the bottom chambers. After 5 hours’ incubation, the numbers of cells in insert and bottom chambers were counted.
Quantitiative RT-PCR
Total RNA was isolated by High Pure RNA Isolation Kit (Roche) from cultured cells or tumor cell suspensions, and synthesis to cDNA by iScript™ cDNA Synthesis Kit (Biorad). Primers used are listed in Suppl. Table 1.
Chemokines C-arrays and ELISA assays
2 ×106 cells expressing either shGFP or shNotch1 were seeded in 10 cm plates with 10% FBS DMEM complete media, and cultured for one day. Cells were then washed three times with PBS before addition of serum free media. After three days in culture, supernatants were collected and the volumes were adjusted by total protein amount in cell lysates. Chemokines were quantified as pg/μg total protein in whole cell lysates. C-arrays and ELISA assays were performed according to manufacturer’s instructions. Human Cytokine Array C5, Mouse Cytokine Array C3, Human IL-8 ELISA Kit (ELH-IL8-1), Human RANTES (CCL5) ELISA Kit (ELH-RANTES-1), Mouse RANTES ELISA Kit (ELM-RANTES-1), Human IL-6 ELISA Kit (ELH-IL6-1), Mouse IL-6 ELISA Kit (ELM-IL6-1), Mouse MIP-2 ELISA Kit (ELM-MIP2-1) were all from Raybiotech.
Results
Notch1 affects myeloid derived suppressor cells (MDSCs).
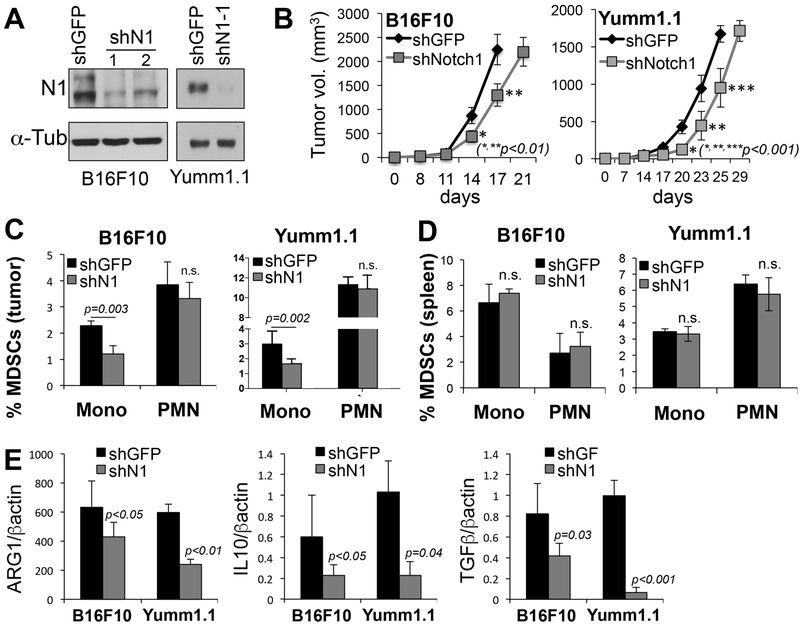
We have previously shown that Notch1 plays a critical role in human melanoma tumor growth[8]. Similarly, inoculation of two syngeneic murine melanoma cell lines B16F10 and Yumm1.1[27] into immune competent C57BL/6 mice showed that depletion of Notch1 (fig. 1A) is sufficient to delay tumor growth also in an immune competent context (Fig. 1B). Of note, active Notch1 (Notch1-NICD) appears to be mostly nuclear in both cell lines (Suppl. Fig. 2). The levels of Notch2, which has been shown to have redundant functions with Notch1, were undetected in Yumm1.1 and were unchanged in B16F10 cells depleted of Notch1. Hence, we have excluded that the effects observed could have been in part due to Notch2. To specifically address the question as to whether the expression of Notch1 in melanoma tumors may influence the immune component of the tumor microenvironment, we measured the presence of several immune cells in both B16F10 and Yumm1.1 tumors. The analysis was done in tumors of comparable sizes, i.e. at 17 and 25 days for shGFP expressing tumors, and at 21 and 29 days for the shNotch1 expressing tumors, respectively (Fig. 1B). Of note, Notch1 did not affect the expression of PDL1 (not shown), which is overexpressed in several tumors including melanoma, and contributes to cancer immune escape[28].
Figure 1. Inhibition of Notch1 inhibits melanoma growth and affects the tumor microenvironment.
A) Expression of mouse specific shNotch1 in B16F10 (left) and Yumm1.1 (right). shN1 #1 was used for the in vivo growth. B) Tumor growth of B16F10 and Yumm1.1 mouse melanoma cells. C) % MDSCs (CD11b+; Ly6Chi; ly6G− = monocytic; and CD11b+; Ly6Clo; Ly6G+ = polymorphonuclear) in B16F10 and Yumm1.1 tumors expressing shGFP or shNotch1 (n=20). D) %MDSCs in spleen of mice bearing B16F10 and Yumm1.1 tumors expressing shGFP or shNotch1 (n=20). E) Arginase-1, IL10 and TGFβ expression detected by quantitative PCR in B16F10 and Yumm1.1 tumor lysates, normalized to β-actin.
A higher percentage of MDSCs in melanoma tumors has been shown to be associated with lower patient survival and lower response to immune checkpoint inhibitors[17-19]. Hence, we first assessed whether Notch1 could affect MDSCs in the TME. We detected both the monocytic (Mo) (CD11b+Ly6ChiLy6G−) and polymorphonuclear (PMN) (CD11b+Ly6CloLy6G+) MDSC populations (Suppl. Fig. 3) in the TME and found that Notch1 inhibition associated with a decrease in the number of Mo-MDSCs, while the PMN population did not change (fig. 1C). This is intriguing, as it has been shown that tumor associated Mo-MDSCs have a more potent suppressive activity than PMN-MDSCs[29]. Importantly, tumor expression of Notch1 did not appear to influence MDSCs in peripheral organs such as the spleen in either tumor type (Fig. 1D), indicating a local effect on the tumor microenvironment.
MDSCs use several mechanisms to induce immunosuppression, including the production of arginase 1 (Arg1) and inducible nitric oxide synthase (iNOS), leading to CD4(+) and CD8(+) T-cell inhibition[30]; recruitment of Tregs by elevated production of IL-10 and TGFb; and are able to inhibit the migration of CD8(+)T cells to the TME[29]. Interestingly, inhibition of Notch1 caused a significant reduction of mediators such as ARG1, IL10 and TGFβ in the tumor milieu (Fig. 1E), suggesting inhibition of Notch1 in the tumor could stimulate the recruitment of cytotoxic T cells while inhibiting that of inhibitory Tregs.
Tumor associated Notch1 affects CD8(+) T cells, Tregs and the Tregs/CD8 ratio.
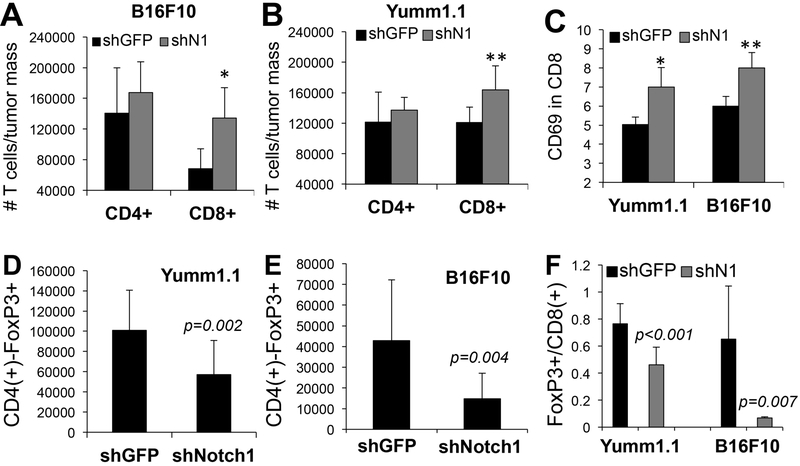
Given the decrease in factors associated with CD4(+) and CD8(+) T cell inhibition, we sought to determine whether Notch1 inhibition affected these cell populations. Interestingly, we found that in tumors expressing shNotch1, the number of CD8(+) T cells, normalized by tumor mass, was 50% and 30% higher than in shGFP expressing tumors in B16F10 and Yumm1.1, respectively (Fig. 2A, B, Suppl. Fig. 4). These CD8(+) T cells were active, as demonstrated by an increase in CD69 positivity (Fig. 2C), a marker of CD8 activation[31, 32]. However, no significant differences were observed in the CD4(+) population in the TME (Fig. 2A, B) and in both cell types in the spleen (not shown).
Figure 2. Notch1 affects CD8(+) T cells and Tregs in the TME.
A-B) Absolute number of CD4(+) and CD8(+) T cells in B16F10 (A) and Yumm1.1 (B) tumors (*p=0.01; **p=0.02, n=20). C) % of CD69 positive CD8(+) T cells (***p<0.01, n=20). D, E). Absolute number of Tregs (CD4+/Foxp3+) cells in Yumm1.1 (A) and B16F10 (B) tumors originated from cells expressnig either shGFP or shNotch1. n=20. F) Tregs/CD8 ratio in the TME. The ratio was calculated by dividing the absolute number of CD4+/foxp3+ and CD8+ T cells in tumors. Absolute numbers were obtained by normalizing the number of cells detected by Flow cytometry to the tumor mass. Values are the avarage between two experimental repeats.
A decrease in IL10 and TGFβ in the TME led us to hypothesize that inhibition of Notch1 could affect the abundance of regulatory T cells (Tregs) as well. Tregs were detected by sorting Foxp3+ cells among the CD4(+) T cell population. Indeed, knock down of Notch1 in both B16F10 and Yumm1.1 tumors led to a decrease in the number of regulatory T cells detected in the TME (fig. 2D, E). Again, no change was observed in Tregs in the spleens (not shown), reiterating a potential role of tumor associated Notch1 in recruiting immune cells to the tumor microenvironment.
Studies have shown that a higher abundance of Tregs over CD8(+)T effector cells within tumor tissue is associated with worse prognosis in several cancers including melanoma[20, 21]. The decrease in Tregs associated with an increase in the number of CD8(+) T cells, resulted in a consequent significant reduction in the Tregs/CD8 ratio (Fig. 2F). Together, the data suggest Notch1 promotes an immune suppressive TME by recruiting MDSCs and Tregs and by decreasing the overall number of cytotoxic T cells at the tumor site. Given that responses to immune checkpoints inhibitors have been associated to an increase in the CD8 population over Tregs[33], inhibition of Notch1 can potentially improve the efficacy of immune therapies by tipping the scale toward a more immune responsive TME.
Notch1 inhibition improves tumor responses to immune checkpoint inhibitors.
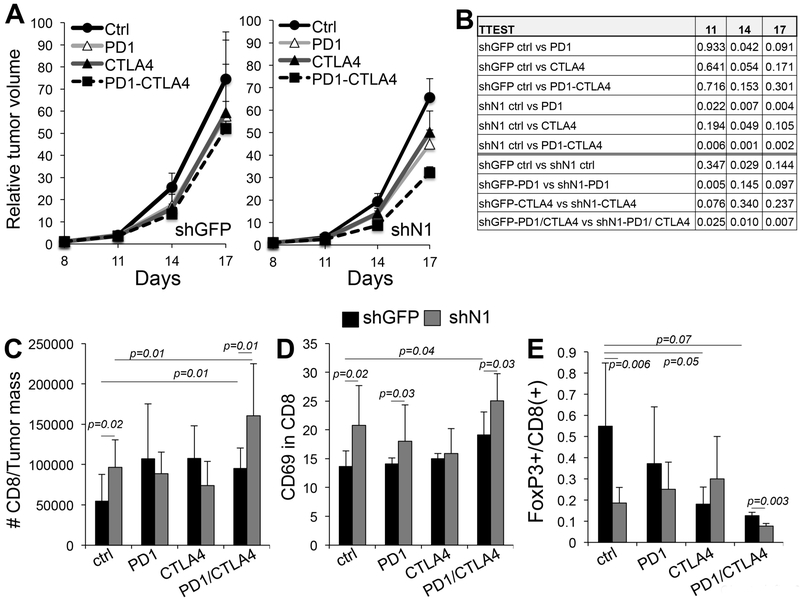
To test whether inhibiting Notch1 in the tumor would improve the efficacy of immune checkpoint inhibitors (ICIs), B16F10 cells expressing either shGFP or shNotch1 were inoculated subcutaneously into the flank of C57BL/6 mice. Yumm1.1 derived tumors were previously shown to be unresponsive to anti-PD1 therapy and were therefore excluded from this analysis[34]. Mice were then treated as describe in material and methods. Tumors from cells expressing shNotch1 grew slower as expected; and, importantly, where further suppressed by the ICIs treatments with respect to the shGFP group, averaging a 26, 36 and 40% reduced tumor growth over shGFP, at days 11, 14 and 17 of treatment with the combination therapy (Fig. 3A, B). Analysis of immune cells in the tumors at the end time point confirmed that inhibition of Notch1 leads to an increase in functional (CD69 positive) CD8(+) T cells, as well as the combination anti-PD1/anti-CTLA4 (fig. 3C, D). The number of functional CD8(+) T cells was even greater when the combination treatment was associated with Notch1 inhibition, reiterating the notion that ablation of Notch1 signaling can improve responses to ICIs. With the exception of the combination therapy, ICIs did not affect the tumor associated Treg population (Suppl. Fig. 4B); however, Notch1 inhibition did reduce this cell type population, confirming our previous results. Importantly, likely, as a consequence of an increase in CD8(+) T cells due to both Notch1 inhibition and ICI treatment, and a Notch1 dependent decrease in Tregs, we observed an overall decrease in the Tregs/CD8 ratio, particularly in tumors that were treated with both inhibitors and that also expressed shNotch1 (Fig. 3E). As for MDSCs, we observed a reduction of both the monocytic and polymorphonuclear MDSCs in the tumor particularly with ipilimumab (anti-CTLA4) (Suppl. Fig. 5A). Again, Notch1 inhibition reduced tumor associated Mo-MDSCs as previously shown. Interestingly, both antibodies, particularly when given in combination, led to a reduction of MDSCs and Tregs and a concomitant increase of both the CD4(+) and CD8(+) T cell populations in the spleens (Suppl. Fig. 6), suggesting a systemic affect of the inhibitors. Interestingly, melanoma patients treated with ipilimumab (anti-CTLA4) a similar systemic effect was observed[35].
Figure 3: Notch1 inhibition improves the efficacy of immune checkpoint inhibitors.
A) growth rates of B16F10 cells expressing either shGFP or shNotch1 treated with anti PD1, anti-CTLA4 or a combination of the two (n=10 per group). B) statistical analysis of growth difference between groups at all time points. C) Absolute number of CD8(+) T cells in the tumors in A. D) % of CD69 positive CD8(+) T cells. E) Tregs/CD8 ratio in the tumors in A. The ratio was calculated by dividing the absolute number of CD4+/foxp3+ and CD8+ T cells in tumors. Absolute numbers were obtained by normalizing the number of cells detected by Flow cytometry to the tumor mass. Values are the avarage between two experimental repeats.
Together, these findings suggest that associating inhibition of Notch1 to immune checkpoint inhibitor treatment could significantly improve the efficacy of the latter.
Tumor associated Notch1 controls the expression and secretion of chemokines.
The observation that tumor associated Notch1 did not influence MDSCs (and other cells) in peripheral organs such as the spleen, suggests that Notch1 may promote an immune suppressive tumor microenvironment by recruiting immune suppressive cells such as MDSCs to the TME. To test this possibility, we isolated MDSCs from the spleens of C57BL/6 mice and stimulated them with conditioned media from B16F10 expressing either shGFP or shNotch1. A 30% reduction in migration was observed in cells stimulated with shNotch1 conditioned media (Fig.4A). These data imply Notch1 modulates the recruitment of MDSCs to the TME via alterations in the secretion of chemo-attractants.
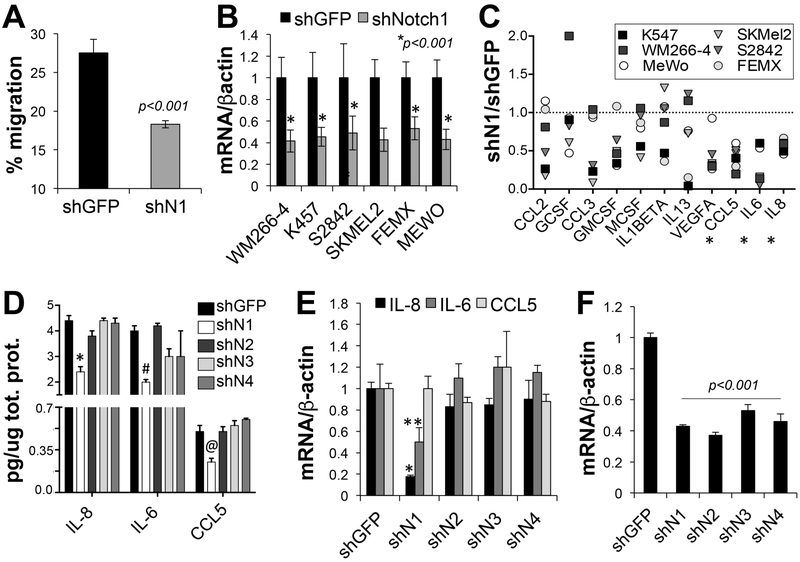
Figure 4: Notch1 affects MDSCs recruitment by regulating the expression and secretion of chemokines.
A) In vitro migration of MDSCs stimulated with serum free condition media of B16F10 cells expressing shGFP or shNotch1. B) Notch1 mRNA levels in all cells expressing shNotch1. Values are normalized to shGFP for each cell line. C) Protein levels of chemokines associated with MDSCs function, secreted in media of the cells in B expressing shGFP and shNotch1. The values are the ratio between shNotch1 and shGFP (set at 1 – dotted line). Serum free Media was collected after three days incubation and volumes normalized to adjust for cell number differences, evaluated as the total protein content of cell lysates. D) ELISAs for IL-8, IL-6 and CCL5. Conditioned media from WM266-4 cells depleted of all Notch receptors was used. Treatment was done as in C and chemokine content reported as pg/ug total protein in lysates as a relative measure of cell density. E) mRNA levels of the three chemokines in WM266-4 cells expressing shRNAs against all Notch receptors. Values are the ratio between Notch mRNA and β-actin mRNA, used as loading control. F) Notch mRNA levels in WM266-4 cells expressing all Notch shRNAs. All experiments were run in triplicate and values are the mean of three independent experiments.
MDSC are actively recruited to primary and metastatic tumor sites by several chemokines produced by the tumor. CCL2, CCL5, IL6 and IL8, are among the main chemokines implicated in MDSC migration to tumors[29, 30, 36-39]. To determine whether Notch1 controlled the production of chemokines, we knocked down Notch1 in six human melanoma cell lines encompassing common oncogenic drivers: WM266-4, K457 (mutated BRAF); SKMel2, S2842 (mutated RAS), and MeWo, FEMX (WT, WT) (Fig. 4B) and used condition media from shGFP and shNotch1 cells to hybridized a chemokine array containing 80 chemokines (Fig. 4C). Several chemokines implicated in MDSCs expansion, activation and infiltration into tumors were shown to be decreased in the media of cells depleted of Notch1. However only IL6, IL8 and CCL5 were consistently inhibited in all six cell lines. A similar array was conducted in B16F10 and Yumm1.1 conditioned media (Suppl. Fig. 7A). IL6, CCL5 and the IL8 mouse homologue MIP2 were inhibited by Notch1 knock down in both mouse cell lines and in tumor homogenates (Suppl. Fig. 7B).
To further corroborate the observation from the array, we conducted ELISA assays (Fig. 4D). These confirmed the reduced secretion of IL6, IL8 and CCL5 in media of cells depleted of Notch1 but not of the other Notch receptors. Additionally, while Notch1 modulated IL6 and IL8 at the transcriptional level, it did not affect the transcription of CCL5 (Fig. 4E), suggesting Notch1 may control its secretion.
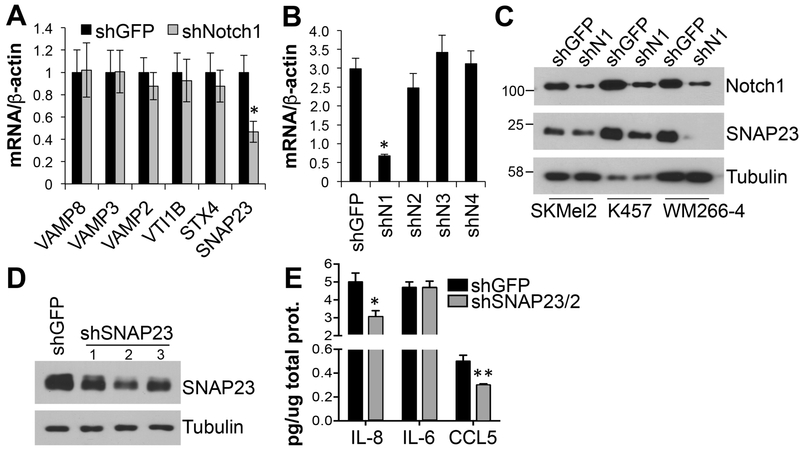
The secretion of CCL5 involves the fusion of vescicles and/or granules loaded with the chemokine with the plasma membrane through the interaction of specific SNAREs (soluble N-ethylmaleimide–sensitive factor attachment protein [SNAP] receptors). SNAREs include vesicle-associated membrane proteins (VAMPs) on the granules, and syntaxins and SNAP23 on the plasma membrane. Once in contact they form a complex allowing the fusion and consequent release of the chemokines[40]. Interestingly, inhibition of Notch1, but not that of the other Notch receptors, reduced the expression of SNAP23 (Fig. 5A, B, C). Additionally, SNAP23 inhibition (Fig. 5D) resulted in diminished secretion of CCL5 and IL-8, though no effects were seen on the secretion of IL-6 (Fig. 5E). These data indicate Notch1 regulates both the expression and secretion of these chemokines by modulating the expression of the fusion/secretion machinery.
Figure 5. Notch1 controls the expression of SNAP23.
A) mRNA expression levels of several components of the SNAREs (soluble N-ethylmaleimide–sensitive factor attachment protein [SNAP] receptors) family of membrane fusion proteins in WM266-4 cells depleted of Notch1. B) mRNA expression of SNAP23 in cells depleted of all Notch receptors, normalized to β-actin. C) SNAP23 protein expression in several human melanoma cell lines expressing shGFP or shNotch1. D) SNAP23 shRNA knock down levels. E) ELISAs for IL-8, IL-6 and CCL5. Conditioned media from WM266-4 cells depleted of SNAP23 was used. shSNAP23/2 was used in the experiment. Treatment was done as in fig. 3C and chemokine content reported as pg/ug total protein in lysates as a relative measure of cell density. *,**p<0.01. Values are the mean of three independent experiments run in triplicate.
Discussion
Here we show that tumor associated Notch1 not only affects melanoma growth and survival properties, as we have previously shown[8, 9,13], but it also contributes to an immune suppressive tumor microenvironment, by promoting the expression and secretion of key chemokines involved in the recruitment of MDSCs and Tregs. Inhibition of Notch1 results in a decrease of MDSCs and Tregs and an increase in active CD8(+) T cells. Since the presence of tumor-infiltrating CD8(+) T cells generally correlates with a favorable prognosis[41-43] and improved responses to immune checkpoint inhibitor therapies[44], inhibiting Notch1 in conjunction with immune checkpoint inhibitors could improve their efficacy. Indeed, inhibition of Notch1 resulted in an improve response to anti-PD1 and anti-CTLA4 therapies, particularly when given in combination.
Immune checkpoints inhibitors targeting CTLA4, PD1 and PDL1 have shown significant clinical benefits particularly in melanoma. 20% of melanoma patients treated with anti CTLA4 show evidence of continued durable disease control or even response 5-10 years after starting therapy. 33% patients respond to anti PD1 therapy, with 70-80% patients maintaining the response from the initial treatment. Combination immunotherapy has recently shown up to 58% response in metastatic patients, however it is still early to determine whether combination therapy provides superior survival benefits than monotherapy[45]. These partial, albeit significant, responses can be ascribed to intrinsic and acquired resistance to these therapies. Indeed, approximately one fourth to one third of patients with metastatic melanoma will relapse over time, despite receiving continued therapy[46]. Several mechanisms of resistance have been identified, both intrinsic to the tumor and associated to the tumor microenvironment. The most relevant intrinsic mechanisms include the absence of antigenic proteins, for example due to mutations in HLA factors; or genetic T cell exclusion, e.g. by expression of PDL1[23, 45]. TME associated mechanisms include the absence of antigenic T cells, and the accumulation of suppressor cells such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs)[23, 45].
Our data indicate Notch1 favors the recruitment of MDSCs and Tregs via modulation of the expression and secretion of several chemokines. Inhibition of Notch1 leads to the reduction of MDSCs and Tregs populations infiltrating the tumor and a consequent increase in the number of activated CD8(+) T cells, hence producing a more immune responsive TME. Importantly, when inhibition of Notch1 was complemented to immune checkpoint inhibitors, we observed a further enhancement of activated CD8(+) T cells, particularly in the combination therapy, and a consequent further reduction of the Treg/CD8 ratio observed with Notch1 knock down alone or ICIs only. In support of a role of Notch signaling in modulating the immune component of the TME, a recent report showed that inhibition of Jagged1-induced Notch signaling by a Jagged1 neutralizing antibody reduced MDSCs and increased CD8(+) T cells infiltrating tumors overall leading to decreased tumor burden in mice[47]. Additionally, our findings are in line with a recent work by Yang et al[15] in which reduced tumor associated Notch1 in B16 melanomas resulted in reduced infiltration of immune suppressive cells (e.g. Tregs, MDSCs) and an increase in cytotoxic T cells.
Hence, combination therapy involving Notch1 inhibition and immune checkpoint inhibitors (ICIs) would likely potentiate the efficacy of immunomodulators. Indeed, our data would point in that direction as we observe a significant improvement of anti-tumor effects of both nivolumab (anti-PD1) and ipilimumab (anti-CTLA4), especially when given in combination. In support of our approach, other studies have already shown the efficacy of combining immune checkpoint inhibitors (ICIs) with other therapies. For example, combination of anti-PD1 with BRAF and MEK inhibitors improves the antitumor activity of the ICI and of the targeted therapies alone[48]. Additionally, there is growing evidence of synergistic combinations with immunostimulatory agents, such as the agonistic anti-CD137 (4-1BB) or anti-CD134 (OX40), that can potentiate CD8+ T-cell activation[49]. Blockade of Notch1 would therefore have several advantages: i) it would affect cancer cells directly by inhibiting their growth and survival; ii) it would decrease the infiltration of immune suppressive cells; iii) and increase that of cytotoxic CD8(+) T cells.
Yet, caution should be considered when inhibiting Notch signaling. It has been shown that Notch1 and Notch2 act redundantly in controlling CD8(+) T cell priming and effector T cell differentiation[50, 51]. Hence, a better approach should target Notch1 specifically by for example a neutralizing antibody, in order to maintain Notch2 function and therefore ensure inhibition of immune suppressive cell populations and maintenance of functional effector T cells. Together, novel approaches targeting tumor-associated cancer drivers and TME modulators hold promise to improve overall patient outcomes.
Supplementary Material
Highlights.
Melanoma associated Notch1 induces an immune suppressive Tumor Microenvironment by recruiting MDSCs and Tregs to the tumor.
MDSCs and Tregs recruitment depends on the secretion of several chemokines whose expression and/or secretion is controlled by tumor associated Notch1.
Inhibition of melanoma associated Notch1 results in a decrease in immune suppressive cells and an increase in cytotoxic effector T cells overall improving the efficacy of immune checkpoint inhibitors (ICIs).
Hence, targeting Notch1 in conjunction with ICIs holds the promise of improving overall patient outcomes.
Acknowledgment:
*This work was supported by NIH grants R01 CA177652 and R21 CA187695 to BB.
Footnotes
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J, TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms, Cell, 66 (1991) 649–661. [DOI] [PubMed] [Google Scholar]
- [2].Konishi J, Kawaguchi KS, Vo H, Haruki N, Gonzalez A, Carbone DP, Dang TP, Gamma-secretase inhibitor prevents Notch3 activation and reduces proliferation in human lung cancers, Cancer Res, 67 (2007) 8051–8057. [DOI] [PubMed] [Google Scholar]
- [3].Pahlman S, Stockhausen MT, Fredlund E, Axelson H, Notch signaling in neuroblastoma, Semin Cancer Biol, 14 (2004) 365–373. [DOI] [PubMed] [Google Scholar]
- [4].Rangarajan A, Syal R, Selvarajah S, Chakrabarti O, Sarin A, Krishna S, Activated Notch1 signaling cooperates with papillomavirus oncogenes in transformation and generates resistance to apoptosis on matrix withdrawal through PKB/Akt, Virology, 286 (2001) 23–30. [DOI] [PubMed] [Google Scholar]
- [5].Santagata S, Demichelis F, Riva A, Varambally S, Hofer MD, Kutok JL, Kim R, Tang J, Montie JE, Chinnaiyan AM, Rubin MA, Aster JC, JAGGED1 expression is associated with prostate cancer metastasis and recurrence, Cancer Res, 64 (2004) 6854–6857. [DOI] [PubMed] [Google Scholar]
- [6].Sjolund J, Johansson M, Manna S, Norin C, Pietras A, Beckman S, Nilsson E, Ljungberg B, Axelson H, Suppression of renal cell carcinoma growth by inhibition of Notch signaling in vitro and in vivo, J Clin Invest, 118 (2008) 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Osawa M, Fisher DE, Notch and melanocytes: diverse outcomes from a single signal, J Invest Dermatol, 128 (2008) 2571–2574. [DOI] [PubMed] [Google Scholar]
- [8].Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ, Powell MB, Notch1 is an effector of Akt and hypoxia in melanoma development, J Clin Invest, 118 (2008) 3660–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang K, Wong P, Zhang L, Jacobs B, Borden EC, Aster JC, Bedogni B, A Notch1-neuregulin1 autocrine signaling loop contributes to melanoma growth, Oncogene, 31 (2012) 4609–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I, Brown EJ, Capobianco AJ, Herlyn M, Liu ZJ, Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression, J Clin Invest, 115 (2005) 3166–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu ZJ, Xiao M, Balint K, Smalley KS, Brafford P, Qiu R, Pinnix CC, Li X, Herlyn M, Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression, Cancer Res, 66 (2006) 4182–4190. [DOI] [PubMed] [Google Scholar]
- [12].Pinnix CC, Lee JT, Liu ZJ, McDaid R, Balint K, Beverly LJ, Brafford PA, Xiao M, Himes B, Zabierowski SE, Yashiro-Ohtani Y, Nathanson KL, Bengston A, Pollock PM, Weeraratna AT, Nickoloff BJ, Pear WS, Capobianco AJ, Herlyn M, Active Notch1 confers a transformed phenotype to primary human melanocytes, Cancer Res, 69 (2009) 5312–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang K, Wong P, Salvaggio C, Salhi A, Osman I, Bedogni B, Synchronized Targeting of Notch and ERBB Signaling Suppresses Melanoma Tumor Growth through Inhibition of Notch1 and ERBB3, J Invest Dermatol, 136 (2016) 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mao L, Zhao ZL, Yu GT, Wu L, Deng WW, Li YC, Liu JF, Bu LL, Liu B, Kulkarni AB, Zhang WF, Zhang L, Sun ZJ, gamma-Secretase inhibitor reduces immunosuppressive cells and enhances tumour immunity in head and neck squamous cell carcinoma, Int J Cancer, 142 (2018) 999–1009. [DOI] [PubMed] [Google Scholar]
- [15].Yang Z, Qi Y, Lai N, Zhang J, Chen Z, Liu M, Zhang W, Luo R, Kang S, Notch1 signaling in melanoma cells promoted tumor-induced immunosuppression via upregulation of TGF-beta1, J Exp Clin Cancer Res, 37 (2018) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ, Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy, Cancer Immunol Immunother, 58 (2009) 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, Maio M, Sucker A, Schilling B, Schadendorf D, Buttner P, Garbe C, Pawelec G, Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells, Clin Cancer Res, 20 (2014) 1601–1609. [DOI] [PubMed] [Google Scholar]
- [18].Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, Michielin O, Romano E, Speiser DE, Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab, Cancer Immunol Immunother, 63 (2014) 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gibney GT, Kudchadkar RR, DeConti RC, Thebeau MS, Czupryn MP, Tetteh L, Eysmans C, Richards A, Schell MJ, Fisher KJ, Horak CE, Inzunza HD, Yu B, Martinez AJ, Younos I, Weber JS, Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma, Clin Cancer Res, 21 (2015) 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chaudhary B, Elkord E, Regulatory T Cells in the Tumor Microenvironment and Cancer Progression: Role and Therapeutic Targeting, Vaccines (Basel), 4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shang B, Liu Y, Jiang SJ, Liu Y, Prognostic value of tumor-infiltrating Foxp3+ regulatory T cells in cancers: a systematic review and meta-analysis, Sci Rep, 5 (2015) 15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Marzuka A, Huang L, Theodosakis N, Bosenberg M, Melanoma Treatments: Advances and Mechanisms, J Cell Physiol, (2015). [DOI] [PubMed] [Google Scholar]
- [23].Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A, Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy, Cell, 168 (2017) 707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kleffel S, Posch C, Barthel SR, Mueller H, Schlapbach C, Guenova E, Elco CP, Lee N, Juneja VR, Zhan Q, Lian CG, Thomi R, Hoetzenecker W, Cozzio A, Dummer R, Mihm MC Jr., Flaherty KT, Frank MH, Murphy GF, Sharpe AH, Kupper TS, Schatton T, Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth, Cell, 162 (2015) 1242–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF, Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment, J Immunother Cancer, 2 (2014) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cardona AE, Huang D, Sasse ME, Ransohoff RM, Isolation of murine microglial cells for RNA analysis or flow cytometry, Nat Protoc, 1 (2006) 1947–1951. [DOI] [PubMed] [Google Scholar]
- [27].Meeth K, Wang JX, Micevic G, Damsky W, Bosenberg MW, The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations, Pigment Cell Melanoma Res, 29 (2016) 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Spranger S, Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment, Int Immunol, 28 (2016) 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kumar V, Patel S, Tcyganov E, Gabrilovich DI, The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment, Trends Immunol, 37 (2016) 208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gabrilovich DI, Nagaraj S, Myeloid-derived suppressor cells as regulators of the immune system, Nat Rev Immunol, 9 (2009) 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Alari-Pahissa E, Notario L, Lorente E, Vega-Ramos J, Justel A, Lopez D, Villadangos JA, Lauzurica P, CD69 does not affect the extent of T cell priming, PLoS One, 7 (2012) e48593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Caruso A, Licenziati S, Corulli M, Canaris AD, De Francesco MA, Fiorentini S, Peroni L, Fallacara F, Dima F, Balsari A, Turano A, Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation, Cytometry, 27 (1997) 71–76. [DOI] [PubMed] [Google Scholar]
- [33].Quezada SA, Peggs KS, Curran MA, Allison JP, CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells, J Clin Invest, 116 (2006) 1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Homet Moreno B, Zaretsky JM, Garcia-Diaz A, Tsoi J, Parisi G, Robert L, Meeth K, Ndoye A, Bosenberg M, Weeraratna AT, Graeber TG, Comin-Anduix B, Hu-Lieskovan S, Ribas A, Response to Programmed Cell Death-1 Blockade in a Murine Melanoma Syngeneic Model Requires Costimulation, CD4, and CD8 T Cells, Cancer Immunol Res, 4 (2016) 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].de Coana YP, Wolodarski M, Poschke I, Yoshimoto Y, Yang Y, Nystrom M, Edback U, Brage SE, Lundqvist A, Masucci GV, Hansson J, Kiessling R, Ipilimumab treatment decreases monocytic MDSCs and increases CD8 effector memory T cells in long-term survivors with advanced melanoma, Oncotarget, 8 (2017) 21539–21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang Y, Lv D, Kim HJ, Kurt RA, Bu W, Li Y, Ma X, A novel role of hematopoietic CCL5 in promoting triple-negative mammary tumor progression by regulating generation of myeloid-derived suppressor cells, Cell Res, 23 (2013) 394–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen MF, Kuan FC, Yen TC, Lu MS, Lin PY, Chung YH, Chen WC, Lee KD, IL-6-stimulated CD11b+ CD14+ HLA-DR- myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus, Oncotarget, 5 (2014) 8716–8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Alfaro C, Teijeira A, Onate C, Perez G, Sanmamed MF, Andueza MP, Alignani D, Labiano S, Azpilikueta A, Rodriguez-Paulete A, Garasa S, Fusco JP, Aznar A, Inoges S, De Pizzol M, Allegretti M, Medina-Echeverz J, Berraondo P, Perez-Gracia JL, Melero I, Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs), Clin Cancer Res, 22 (2016) 3924–3936. [DOI] [PubMed] [Google Scholar]
- [39].Katoh H, Watanabe M, Myeloid-Derived Suppressor Cells and Therapeutic Strategies in Cancer, Mediators Inflamm, 2015 (2015) 159269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lacy P, Stow JL, Cytokine release from innate immune cells: association with diverse membrane trafficking pathways, Blood, 118 (2011) 9–18. [DOI] [PubMed] [Google Scholar]
- [41].Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K, Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer, Proc Natl Acad Sci U S A, 102 (2005) 18538–18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, Kudoh S, Ochiai A, Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer, Cancer, 113 (2008) 1387–1395. [DOI] [PubMed] [Google Scholar]
- [43].Yamada N, Oizumi S, Kikuchi E, Shinagawa N, Konishi-Sakakibara J, Ishimine A, Aoe K, Gemba K, Kishimoto T, Torigoe T, Nishimura M, CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection, Cancer Immunol Immunother, 59 (2010) 1543–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y, Lin YJ, Wojtkiewicz G, Iwamoto Y, Mino-Kenudson M, Huynh TG, Hynes RO, Freeman GJ, Kroemer G, Zitvogel L, Weissleder R, Pittet MJ, Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy, Immunity, 44 (2016) 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jenkins RW, Barbie DA, Flaherty KT, Mechanisms of resistance to immune checkpoint inhibitors, Br J Cancer, 118 (2018) 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, K.-. investigators, Pembrolizumab versus Ipilimumab in Advanced Melanoma, N Engl J Med, 372 (2015) 2521–2532. [DOI] [PubMed] [Google Scholar]
- [47].Sierra RA, Trillo-Tinoco J, Mohamed E, Yu L, Achyut BR, Arbab A, Bradford JW, Osborne BA, Miele L, Rodriguez PC, Anti-Jagged Immunotherapy Inhibits MDSCs and Overcomes Tumor-Induced Tolerance, Cancer Res, 77 (2017) 5628–5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, Pinheiro EM, Koya RC, Graeber TG, Comin-Anduix B, Ribas A, Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma, Sci Transl Med, 7 (2015) 279ra241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA, Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination, Clin Cancer Res, 19 (2013) 997–1008. [DOI] [PubMed] [Google Scholar]
- [50].Backer RA, Helbig C, Gentek R, Kent A, Laidlaw BJ, Dominguez CX, de Souza YS, van Trierum SE, van Beek R, Rimmelzwaan GF, ten Brinke A, Willemsen AM, van Kampen AH, Kaech SM, Blander JM, van Gisbergen K, Amsen D, A central role for Notch in effector CD8(+) T cell differentiation, Nat Immunol, 15 (2014) 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kuijk LM, Verstege MI, Rekers NV, Bruijns SC, Hooijberg E, Roep BO, de Gruijl TD, van Kooyk Y, Unger WW, Notch controls generation and function of human effector CD8+ T cells, Blood, 121 (2013) 2638–2646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.