The “Membrane Trafficking: Vesicle Formation, Cargo Sorting and Fusion” Minisymposium covered a diversity of powerful techniques and approaches to gain exciting mechanistic and structural insights into the regulation of how vesicles form, how cargoes get sorted, and how vesicles fuse. New intracellular nanovesicles were revealed, as well as higher-resolution views of tubular structures at ER exit sites (ERES). Other talks demonstrated new ways to block endocytosis in vivo as well as a promising new way to target a subset of cancers.
Vesicle formation and fission are critical for specific cargo capture and transport. Biophysical, biochemical, and structural studies are making critical progress in teasing out molecular details of fundamental protein–protein interactions and their roles. Kasey Day (Stachowiak lab, University of Texas, Austin) examined the molecular role of the endocytic adaptor proteins Eps15 and Fcho1, which form multivalent liquid droplets to control the dynamic formation of clathrin-coated pits. Using blue light–activated Cry2 fusion proteins to drive the levels of oligomerization, she demonstrated that the more fluid complexes were better catalysts of clathrin-mediated endocytosis than more solid assemblies that led to stalled pits. Catherine Deatherage (Burd lab, Yale University) reported the reconstitution of the retromer components (Vps26, Vps29, and Vps35) on supported lipid bilayers, along with the retromer-binding WASH subunit Fam21, sorting nexin Snx3, Rab7, and cargo. Unexpectedly, the retromer did not substantially oligomerize on a membrane even in the presence of cargo or auxiliary proteins. These data speak against a prevailing model assuming that the retromer complex oligomerizes and thereby concentrates retrograde cargo in sorting vesicles, acting as typical coat proteins do. Marijn Ford (University of Pittsburgh School of Medicine) used cryo-EM to determine the structure of a different complex, that of the yeast SNX-BAR protein Mvp1, which is critical for retromer-dependent sorting in endocytosis. Unlike other dimeric BAR domain–containing proteins, Mvp1 forms an autoinhibited tetramer that sequesters the membrane-binding faces inside. Importantly, Mvp1 tetramerization depends on the presence of its low-complexity N-terminus (∼120 residues). This tetrameric conformation is likely a key regulatory step of SNX-BAR proteins in endocytic trafficking (Sun et al., 2020). High-resolution structures are also being used to develop novel inhibitors: Zhiming Chen (Schmid lab, UT Southwestern) presented a new membrane-penetrating peptide mimic, called CMEpi, that acutely and selectively inhibits clathrin-mediated endocytosis.
Further improvements in the dynamics and resolution of imaging methods, as well as the use of creative cell biology approaches, have led to critical advances in studies of the secretory and endocytic pathways. Jason Casler (Glick lab, University of Chicago) reported that cargoes targeted to the vacuole or to the secretory pathway exit the Golgi at different kinetic stages of TGN maturation in yeast. Vacuolar cargo leaves TGN earlier via the Gga2 clathrin adaptor, while at a later step the AP-1 adaptor mediates intra-Golgi recycling and secretion of exocytic cargo. The former cargo is first transported from the TGN to the late endosome from where it is delivered into the vacuole via a “kiss-and-run” transfer, directly visualized in the presented study. Aubrey Weigel (HHMI–Janelia Research Campus) used cryo-correlative light electron microscopy (structural illumination microscopy, SIM, combined with focused ion beam scanning electron microscopy, FIB-SEM) for higher-resolution visualization of the ultrastructure and dynamics of ERES in mammalian cells. ERES is formed by an assembly of highly interlaced and constricted tubules of ∼30-nm diameter; when cells are overloaded with secretory cargo, individual tubules and the complete ERES assembly expand. Upon cargo exit, COPII markers remain at ERES, while COPI components move away together with the exported cargo in extended tubular transport intermediates formed along microtubules. Next, using mitochondrial targeting of tumor protein D54 (TPD54) knockdown experiments and imaging, Stephen Royle (University of Warwick, UK) discovered a new class of transport vesicles (30 nm) in mammalian cells, termed intracellular nanovesicles (INVs). These TDP54 vesicles appeared to be rather heterogeneous, containing various cargoes as well as different sets of Rab and SNARE proteins. Consequently, they likely function at multiple intracellular transport steps, in both secretory and endocytic pathways (Larocque et al., 2020).
Intracellular vesicles fuse through the action of SNARE proteins, but other mechanistic aspects of the regulation of fusion are still being uncovered. Using biochemical binding assays and single-particle negative stain EM, Dante Lepore (Munson lab, University of Massachusetts Medical School, Worcester) analyzed dramatic conformational changes in the yeast exocyst complex that activate the exocyst for interactions with vesicles, SNAREs, and the SNARE regulator Sec1 (Rossi et al., 2020). Less well understood is a challenging fusion event–-how do giant vesicles fuse? Tom Biton (Avinoam and Shilo labs, Weizmann Institute of Science, Israel) examined the fusion of Drosophila melanogaster salivary gland giant (5–8 µm!) vesicles. Superresolution imaging and EM indicate that, surprisingly, the vesicles do not undergo full fusion or kiss-and-run, but rather open a wide fusion pore (up to 3 µm; Figure 1) and then constrict again, using several important BAR domain–containing proteins.
FUGURE 1:
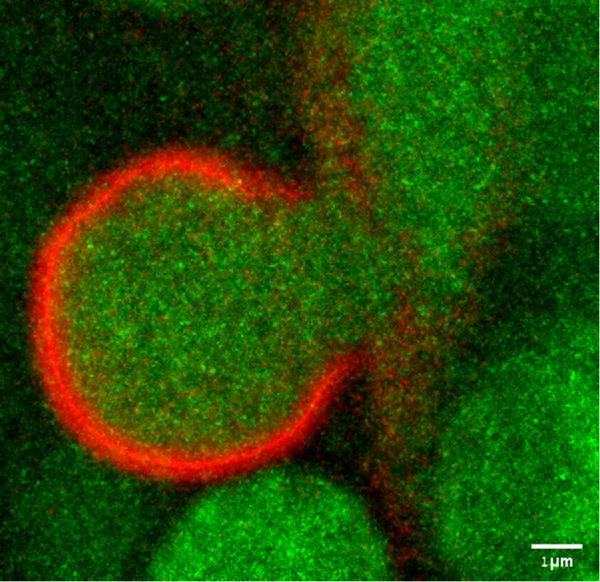
Snapshot of a fusing giant secretory vesicle from the Drosophila larval salivary gland showing a fully expanded fusion pore before constriction, imaged using SRRF (Gustafsson et al., 2016). Content is labeled by Sgs3-GFP (green) and F-actin by Lifeact-Ruby (red). Scale bar: 1 μm.
Finally, Marta Miaczynska (International Institute of Molecular and Cell Biology, Warsaw, Poland) presented how genetic lesions affecting components of the trafficking machinery in cancer could represent a therapeutic target. She described the mechanisms behind synthetic lethality between VPS4A and VPS4B ATPases involved in membrane remodeling, of which the latter is frequently deleted in different cancer types. Lack of both VPS4 paralogues induces immunogenic cell death, with release of immunomodulatory damage-associated molecular patterns (Szyman´ska et al., 2020).
Footnotes
Molecular Biology of the Cell Volume 31 Page 399
MBoC is pleased to publish this summary of the Minisymposium on “Vesicle Formation, Cargo Sorting and Fusion,” held at the 2019 ASCB EMBO Meeting, Washington, D.C., December 8, 2019.
REFERENCES
- Gustafsson N, Culley S, Ashdown G, Owen DM, Pereira PM, Henriques R. (2016). Fast live-cell conventional fluorophore nanoscopy with ImageJ through super-resolution radial fluctuations. Nat Commun. , 12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocque G, La-Borde PJ, Clarke NI, Carter NJ, Royle SJ. (2020). Tumor protein D54 defines a new class of intracellular transport vesicles. J Cell Biol , jcb.201812044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G, Lepore D, Kenner L, Czuchra AB, Plooster M, Frost A, Munson M, Brennwald P. (2020). Exocyst structural changes associated with activation of tethering downstream of Rho/Cdc42 GTPases. J Cell Biol , jcb.201904161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Varlakhanova NV, Tornabene BA, Ramachandran R, Zhang P, Ford MGJ. (2020). The cryo-EM structure of the SNX-BAR Mvp1 tetramer. Nat Commun, 10.1038/s41467-020-15110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyman´ska M, Nowak P, Kolmus K, Cybulska M, Goryca K, Derezin´ska-Wołek M, Szumera-Ciec´kiewicz A, Brewin´ska-Olchowik M, Grochowska A, Piwocka K, et al (2020). Synthetic lethality between VPS4A and VPS4B triggers an inflammatory response in colorectal cancer. EMBO Mol Med , e10812. [DOI] [PMC free article] [PubMed] [Google Scholar]


