Summary
Dramatically rising costs in drug development are in large part because of the high failure rates in clinical phase trials. The poor correlation of animal studies to human toxicity and efficacy have led many developers to question the value of requiring animal studies in determining which drugs should enter in-human trials. Part 1 of this 2-part series examined some of the data regarding the lack of concordance between animal toxicity studies and human trials, as well as some of the potential reasons behind it. This second part of the series focuses on some alternatives to animal trials (hereafter referred to as animal research) as well as current regulatory discussions and developments regarding such alternatives.
Key Words: animal research, drug development, toxicity, translational research
Abbreviations and Acronyms: 3D, 3-dimensional; BC, body-on-chip; BM, bone marrow; CV, cardiovascular; FDA, Food and Drug Administration; NIH, National Institutes of Health; OC, organ-on-chip; TE, tissue engineering
Increasingly, toxicologic testing in animals and preclinical animal studies in drug development have been questioned because of poor correlation with in-human results (1). In addition, public opinion plays a key role in determining how animal research is funded and regulated, and public opinion polls both in the United States and Europe demonstrate a steadily growing unease among the public sector for use of animals in industrial and pharmaceutical toxicity testing. Trends in public opinion regarding animal research in Great Britain, for example, demonstrated relatively steady approval (75%) of animal research between 1999 and 2010, so long as there was no alternative. However, public opinion began to shift significantly around 2012, with approval of animal research in the face of no alternative dropping to 66%, and less than one-half of the public agreeing that scientists could be trusted to be truthful about the experiments they were running (2). Annual Gallup polls in the United States demonstrated a steady decline in public opinion about the “moral acceptability” of animal research, from 65% approval in 2001 to 51% in 2017 (3). Opposition to animal research grew during the same period from 25% to 44%. Less than one-half of Americans 18 to 34 years of age felt that medical testing on animals was acceptable (4).
In 2003, the seventh amendment to the European Union’s Cosmetics Directive (5) stipulated an end to animal testing in the European Union for cosmetic purposes by 2009, regardless of the availability of alternative models by that time. In 2013, the National Institutes of Health (NIH) announced a program to phase out all research involving chimpanzees, and in 2015, to end all funding for invasive research involving chimpanzees (6,7). In 2016, Congress called on the NIH to review its policies related to research involving all nonhuman primates, and in 2017, a spending bill was passed by Congress and signed into law that required the Department of Veterans Affairs to suspend all dog research not specifically approved by the agency’s secretary (8). In other parts of the globe, similar trends are demonstrated across Europe (9) and in New Zealand, Australia, and Japan (2). Animal research will not be entirely replaced by other methods anytime soon, but the potential for faster and ultimately less expensive commercialization of therapeutics, together with the reduction of animal use in the early phases of drug development, has helped to increase research funding for alternatives.
Overall, the use of alternative methods for product testing and preclinical testing of medical drugs and devices has increased dramatically over the last quarter of a century. Between 1990 and 2015, the number of published papers using “alternative animals” (e.g., insects, fish, worms and shrimp) and in silico analysis increased over 900%. In 2015, more than 88,000 studies were published using in silico modeling, versus just 7,405 studies in 1990. Over the same period, testing and research use of guinea pigs and rabbits, previous favorites in the cosmetic industry, fell by 68% and 40%, respectively, although the use of rats and dogs remained stable, and the use of mice increased (10).
Alternatives to Animal Research
Predicting negative side effects of putative therapeutic agents is an important goal in drug development: prohibitive side effects or toxicity are a major reason why drugs that reach clinical trials fail to progress to market (lack of efficacy and lack of commercial interest are others). Animal toxicity testing fails to predict toxicity in almost 50% of drugs in the pipeline between Phase I trials and early post-market withdrawals (1), well after significant time and resources have been spent on what will prove to be a “failed” therapeutic. Testing that predicts probable late and expensive “drop outs” during drug development—termed a “fail early” strategy—would not only reduce costs, but also allow development resources to be redirected toward agents more likely to pass clinical trials. Alternative methods of drug testing that provide more consistent, rapid, and translatable results will also increase human safety.
In silico modeling
Early prediction of human toxicity is critical in decreasing the costs of drug development, and in silico testing has recently been promoted as an important, human-based tool for preclinical evaluation (11). In silico, or computational, modeling refers to experiments or elements of experiments that are performed on computer computational models or via computer simulation. An in silico method is not simply a statistical analysis of known experimental data, but rather is a modeling approach using known characteristics of a chemical or chemical similar to it, information about the underlying chemical or biological system in which it will be used, and, when available, known preclinical and clinical data to predict untested system-level behaviors of a given chemical. Mayourian et al. (12) provide a detailed review of various in silico techniques particularly relevant to cardiovascular (CV) research.
One of the most important advantages of in silico testing involves scale—in the number of chemicals that can be tested quickly (throughput), the types of endpoints and biological pathways covered, and the range of conditions that can be rapidly simulated. Computational research does not yet provide complete replacement of animal experimentation in drug development but can significantly increase the scale and speed of preclinical drug development, thus reducing expense in animal testing phases, leveraging information from fewer experimental animals to increase drug development, decreasing the timeline for new drugs to enter the market by reducing the time for preclinical testing, reducing overall costs of drug development, and improving access to new and novel therapies for patients in need. Today, practically all toxicological research already includes in silico elements (13).
Another potentially important advantage of in silico testing is its potential to identify areas for rescue or repurposing of existing drugs (14). Rescue refers to redirecting drugs that have already failed efficacy trials for one application, but then can be successfully reintroduced into clinical trials for another indication. Repurposing of a drug (also called “repositioning” or “reprofiling”) involves reapplying drugs that are either in clinical use already, or might have been retired from further development or marketing—because of production of a more efficacious drug, risks of adverse effects, or commercialization considerations—to new indications. In both cases, preclinical phases and animal testing can often be bypassed altogether, and if human clinical phase trials are needed, they may be abbreviated to late-phase studies for the new indication, saving years of clinical testing (15). Costs of drug approval for repurposing or rescue are estimated by some authors to be around $40 to $80 million, compared with $1 to $2 billion for the development of an entirely new entity (16). Repurposing of approved drugs may even be accomplished without going through reapproval at all, as once the U.S. Food and Drug Administration (FDA) has issued a marketing approval for any purpose, off-label use for other purposes is at the discretion of the physician, so long as the physician feels it is “medically appropriate for their patient” (17). A contemporary example of repurposing an established drug is the proposed use of chloroquine—an approved treatment for malaria, long used to treat some autoimmune diseases such as lupus—for treatment of coronavirus infection. Chloroquine has the advantage of already being approved (which means that animal toxicity studies do not need to be repeated) and it has a human side effect profile that is largely understood, at least in its current usages. It is an FDA-approved drug that could therefore be used off label without FDA-approved human clinical trials, and is immediately available. However, it is critical to point out that, as of this writing, it is not known to be efficacious against coronaviruses, and it has significant potential human toxicity. It is vital that reasonable human data be generated before it can be determined whether or not it should be recommended for coronavirus patients—to avoid unknown toxicities and to avoid creating a shortage of drug that would not only be of minimal benefit to pandemic victims, but also prevent patients who are known to benefit from it from being treated.
While computer models alone would seem to be an unlikely predictor of drug behavior within complex physiologic systems or human beings, such modeling is nevertheless proving to be both effective and clinically relevant. In 2012, University of California, San Francisco researchers were able to successfully “predict” side effects (i.e., not merely the adverse side effects) of 656 drugs on the market, based on a computer model that compared the similarity of the drugs’ chemical structures to other molecules known to cause the same side effects (18), showing that such models could potentially be used early in drug development to prioritize which agents to pursue or repurpose. Of note, 26% of the side-effect targets identified by the computer model were entirely unrelated to the previously known targets of the drugs (39 of 151), suggesting that previously unrecognized therapeutic effects might be found for existing drugs, allowing them to be repurposed for new diseases and conditions. Despite these exciting findings, however, the model suffered from similar problems of poor specificity to that of animal studies: almost one-half (46%) of its predictions of adverse drug activity proved to be false. Luechtefeld et al. (19) demonstrated that a computer comparison algorithm predicted toxicity for thousands of chemicals across 9 types of tests—from inhalational injury to hazards to aquatic environments—with accuracy similar to that of animal models, and with better reproducibility.
CV toxicity, specifically related to QT prolongation and arrhythmogenicity, is a major cause of drug relabeling or market withdrawal, second only to hepatotoxicity; from 1990 to 2006, it constituted 26% of drug withdrawals from the market (20). As a result, guidelines for new drug development include clinical testing for electrophysiologic changes that might predict QT changes specifically (21). In vitro assays relevant to this change are relatively easy to perform but demonstrate marginal likelihood ratios for predicting toxicity (22). When combined with computational simulations, Lancaster and Sobie (23) were able to use solely in vitro preclinical data to correctly classify 86 drugs as torsadogenic or nontorsadogenic approximately 90% of the time. In 2017, Passini et al. (24) found that a computer simulation of human heart cells was better able to predict risk of arrhythmogenicity of 62 drugs—including analgesics, antihistamines, and antibiotics—than animal testing (accuracy of 89% to 96% vs. 75% to 85%, respectively). In 2019, Moreno et al. (25) used computational modeling to design a novel in silico mexiletine “booster” that may improve the efficacy of mexiletine in suppressing arrhythmias.
There are a number of problems yet to solve regarding computational methods. As with animal research, lack of specificity could prompt unnecessary testing during drug development, or alternatively stop a safe and efficacious compound from progressing to further drug development because of false positive toxicity findings (26). Currently there is lack of widespread understanding of computational model construction, creating a “black box effect” that limits trust and acceptance of in silico data in preference to the familiarity of animal research, which may be less reliable. Additionally, a major challenge to computer modeling is achieving the computational power necessary to sufficiently simulate complex mechanical and physiological systems.
The CompBiomed Project, initially funded by the European Union in 2016 and re-funded in 2020 for another 4-year period, has the goal of creating an entire in silico human organism for use in drug testing, disease modeling, and even personalized approaches to individual patient therapy based on patient-specific modeling and simulations (27).
While the FDA has expressed interest in in silico testing for drug research (28), as of 2020, there are no guidances that allow in silico tests to replace preclinical animal testing in drug development, although in silico data can certainly be submitted in support of animal findings, and may reduce the number of animal experiments that are required.
The FDA supports modeling and simulation research to help predict clinical outcomes, inform clinical trial design, support evidence of efficacy, identify the most relevant patients to study, and predict product safety (29). The FDA has published guidances on the formats for submitting in silico data in support of regulatory advancement of devices on its website (30). However, development of drug and biologicals and toxicological evaluation of metabolites of these entities is still vested in animal studies (31), which can be particularly problematic for testing metabolites that occur in human subjects but cannot be replicated in an animal model.
Animal-free recombinant antibodies
Not only are antibodies used in a variety of research, diagnostic, and regulatory applications, but also antibodies and other biologicals are increasingly becoming mainstream therapeutic agents themselves. Traditionally, the development of antibodies has required the expensive process of immunizing animals, and then sometimes fusing antibody-producing B cells with “immortalized” cell cultures to increase supply. More than 300 companies now supply antibodies for such research and development, with an estimated market value in 2011 of $1.6 billion (32). However, antibodies produced from immunized animals exhibit variability in DNA sequencing and variability in target binding. The lack of reproducibility in research using animal-based antibodies has been deemed a “crisis,” with some scientists claiming that over one-half of all commercially available antibodies are unreliable in binding their intended targets (32). One proposed way to tackle the problems of variability in animal-produced antibody structure and targeting is the use of antibodies manufactured in engineered “recombinant” cells, and the use of antibodies from “human antibody libraries” (33). Advantages include faster production, more consistency in quality, and less biological variation with better potential for research and therapeutic reproducibility. Overall, costs would be eventually be reduced, but only after initial increased expenses of changing to recombinant antibody production from current methods.
Tissue Engineering
Perhaps one of the most “futuristic” approaches to drug and device development is represented by the emerging field of tissue engineering (TE). Unlike cell suspensions and “tissue culture” cellular monolayers, TE constructs have 3-dimensional (3D) structure. TE can more closely mimic the considerable influence that 3D cell-to-cell and cell-to-matrix interactions have over cell behavior in actual tissue and organ systems; something that cell and tissue cultures cannot. In addition, although a considerable body of TE research utilizes animal cells and tissues, TE can allow the creation of 3D tissue structures utilizing human cells—the actual therapeutic target—and likely increase the probability that activity in the engineered human tissue will more accurately reflect or predict the outcomes in human patients. Research regarding TE is burgeoning: the average annual number of published papers more than doubled between 1991 and 2010 (34). The largest body of publications presented various 3D constructions of human skin, made from human karyocytes—derived from neonatal foreskin and mammary tissue discarded after plastic surgery—that vary in complexity from more simple epidermal layers on collagen substrates, to tissue that included epidermis, dermis, and immune cells, and even vascularized skin equivalents with a vascular network (35). TE models have been created that mimic human corneal epithelium and stroma, urothelium, and human oral and vaginal mucosa. Emerging TE studies include engineered human liver tissue, and human neurospheres, as well as models for corneal innervation and the interaction between metastatic tumor cells and bone (34).
TE and the human heart
CV medicine, and the heart in particular, affords many challenges for reducing animal research, as it involves not merely the pharmacologic effects of drugs on the CV cells and systems, but also the effects of topographical properties, motion, and forces in cardiac and vascular shape and mechanical function. As a result, some of the most complex and dynamic alternative research is evolving in this field. The combination of TE with the methodology of 3D printing has resulted in some startling innovations with promise to revolutionize CV bioprosthetic interventions as well as pharmacologic testing and drug development.
For more than 2 decades, biologists have been able to turn embryonic stem cells into beating heart muscle cells in a dish (36). Cardiac cells have also long been known to have intrinsic capacity to self-assemble into spontaneously beating spheroids, or syncytia (37)—making them particularly interesting with regard to 3D TE. Furthermore, cardiac constructs from primary cardiac myocytes develop a primitive vascular network with or without addition of endothelial cells (38).
Engineered 3D constructs of heart muscle permit measurement of virtually all parameters of heart function, including twitch force, kinetics, beating rate, rhythm, diastolic tension, and intracellular calcium movement (39, 40, 41, 42, 43). Engineered cardiac tissue has the potential for use as a research tool, as a replacement for animal toxicity testing of therapeutic agents, and as a therapy unto itself. The models are easy manipulated genetically, and they behave quantitatively much like native muscle, making them reasonable platforms for disease modeling and toxicity testing. In 2018, for example, Truitt et al. (44), used an engineered 3D cardiac microtissue model to characterize mechanisms of human toxicity of sunitibnib, a drug with considerable cardiotoxicity that is used widely to treat renal cell, gastrointestinal, and neuroendodrine tumors.
However, the pharmaceutical industry has been slow to adopt such models as drug development tools, and regulatory agencies have not yet put forth guidances for how these technologies can or should be integrated into preclinical and clinical safety trials, nor whether they can begin to replace animal models in either capacity. The process of defining how engineered tissue might be approved and introduced as a therapy, in contrast, has quickly developed (45, 46, 47, 48), but even the expedited processes available at the FDA for such therapeutics generally still follow the pathway of preclinical animal studies followed by human trials.
Human tissue-on-chip engineering
Cell-based toxicity assays (via traditional “tissue culture” approach) are problematic in predicting drug toxicity in preclinical testing because cultivated cells often do not retain their original organ functions and morphologies when taken out of the context of intraorgan connection and interactions. In tissue culture, for example, it is difficult to maintain cellular functions for sustained periods of time. Tissue culture cells receive nutrients, oxygen and other substances almost solely by diffusion. However, in vivo, cells obtain oxygen, nutrients, and other substances that regulate their function via blood flow, and they experience and respond to physical stimulation such as stretching and sheer forces within their complex environments. Such differences may account for rapid deactivation, senescence, and cellular loss in many in vitro cultures. In addition, interactions between organs cannot be directly tested in tissue culture.
Microfabrication techniques and microfluid technology combined with computer technology has led to a new type of in vitro organ model: the so-called organ-on-chip (OC), which has been further combined into multiorgan chip interactions to mimic whole-body responses, or “body-on-chip” (BC). Creating a hybrid of human tissue on a computer chip that can replicate the structure and function of human organs may seem like the realm of science fiction; however, this new technology not only is a current reality, but also is quickly entering the regulatory framework for assessing new therapeutic compounds.
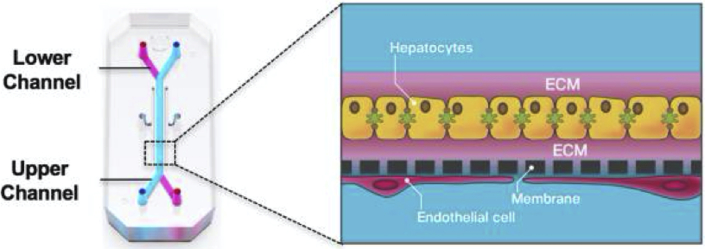
In OCs, transparent chips about the size of an AA battery contain microchannels that are lined with cultured human cells and have microsensor capabilities (e.g., photolithography). These channels allow microfluid (or air) flow that mimic breathing motions, muscle contractions, and other physiologic stressors (Figure 1). The chips are then placed into a research system similar to a computer, in which toxins, chemicals, and medicines can be introduced to test the OC’s response and behavior. In recent developments, at the Wyss Institute at Harvard University, OCs have been connected together to mimic multiorgan interactions within a body (49). Software within the research system allows the investigator to manipulate cell architecture, tissue-to-tissue interfaces, mechanical forces in the environment, and biochemical changes within the environment of the OCs or BCs.
Figure 1.
Liver-on-Chip: Example of Organ-on-Chip Technology
On the left is a chip about the size of an AA battery. Primary hepatocytes are grown in the upper parenchymal channel within an ECM sandwich, on top of an extracellular matrix (ECM)–coated, porous membrane that separates the 2 parallel microchannels. Relevant species-specific liver sinusoidal endothelial cells with or without liver Kupffer cells or stellate cells are cultured on the opposite side of the membrane in the lower vascular channel.
Reprinted with permission from Jang KJ, Otieno MA, Ronxhi J, et al. Reproducing human and cross-species drug toxicities using a liver-chip. Sci Transl Med 2019; eaas5516 (68). Reprinted with permission from AAAS.
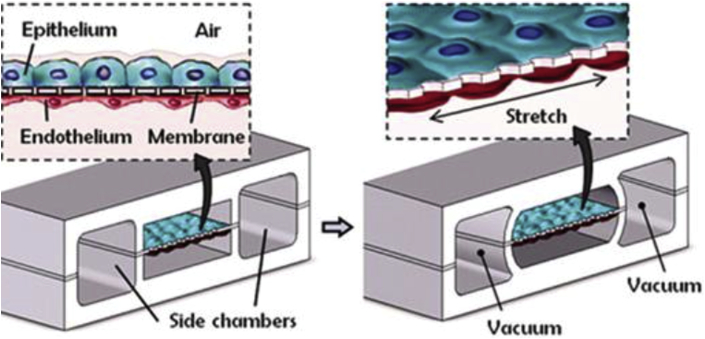
Such OCs and BCs have undergone rapid advancement in the last 5 years. They include the “lung-on-chip” or “breathing lung” chip developed at Harvard University (48), in which the 2-layer channel structure of the microchip is separated by a microporous, stretchable silicone membrane on which alveolar cells and vascular endothelial cells are cultured. The chip mimics the physiological expansion and contraction of alveolar movement by altering pressure on both sides of the membrane via vacuum chambers (Figure 2). Researchers were able to reproduce inflammatory reactions, and even to allow neutrophils to enter via a side channel to respond to bacterial invasion. Other organs mimicked by OCs include the liver, kidney, and gut (50).
Figure 2.
Lung-on-Chip With Breathing Motion
Alveolar-capillary barrier is created using flexible matrix coated with extracellular matrix (ECM). The device recreates breathing motions by applying a vacuum to the side chambers, causing alternating mechanical stretching and releasing of the membrane, and mimicking alveolar distension and relaxation.
Reprinted with permission from Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science 2010;328:662–8.
In 2017, the FDA announced a multiyear research and development agreement with Emulate, Inc. (Cambridge, Massachusetts), a biotech spinout from the Wyss Institute specializing in OCs (51). Earlier this year, researchers from Wake Forest School of Medicine demonstrated that multiorganoid BC systems were both stable and capable of detecting hepatic and cardiotoxicity at human-relevant doses in almost all members of a panel of drugs they tested that had to be recalled by the FDA for hepatic or cardiotoxicity (52). Bear in mind that for all of these drugs, preclinical animal studies and all phases of human clinical trials had failed to detect significant toxicity.
Heart-on-a-chip
Despite being the most frequent cause of death in the United States and a cause of almost 18 million deaths annually worldwide (53), the number of novel drug approvals for CV disease has undergone progressive decline (54). In 2019, just 2 drugs were approved under the classification of CV disease, both of which were for treatment of a rare form of cardiomyopathy—transthyretin-mediated amyloidosis (55). There were no new drug therapies approved for coronary artery disease and hypertensive cardiac disease, which is responsible for 60% of CV deaths (56). CV toxicity is a major cause of drug withdrawals from the market (16%), second only to hepatic toxicity (57).
The ability to mimic physiologic and mechanical effects in the microchip environment has vastly extended the CV research applications of OC technology in the last 10 years (38,58,59). In 2011, Grosberg et al. (60) developed a “heart-on-chip” platform with anisotropically organized cardiomyocytes, which could be stimulated to mimic in vivo production of electrical impulses by pacemaker cells--demonstrating that the heart-on-chip could be used to study and measure contractile behavior, cellular alignment, and functionality of cardiac cells, and suggesting that chip technology could be used to study and evaluate pharmacologic interventions on cardiac contractile function. A similar approach demonstrated that in vitro “chip” testing of pharmacologic effects of isoproterenol was comparable to results of in vivo studies in rats (61). McCain et al. (62) have successfully created a “failing heart-on-chip” platform, further suggesting that chip technology can be used to create disease models, and not merely as a high-throughput method of testing drugs. Ren et al. (63) fabricated a heart-on-chip to mimic hypoxic myocardial injury. Researchers are exploring chip technology as a means of evaluating the use of stem cells to assess therapies for myocardial repair, as well as to generate in vitro models of cardiac disease. In one case, an in vitro disease model of Barth syndrome was developed, and then used to test potential pharmacological and genetic therapeutic options (64).
“Accuracy” of OCs in predicting human drug toxicity and modeling disease
Before the FDA and other regulatory bodies will agree to allow OC technology to replace any phases of animal testing, the technology will have to be proven at least as accurate, if not more so than current testing. Early data are scarce but promising.
In a pulmonary edema model created in an OC, the response to a low-molecular-weight pulmonary edema therapeutic was shown to be similar in the OC to results obtained in animal models (65). InSpheroAG (Schlieren, Switzerland), a commercial developer of a liver chip assay, claims that the sensitivity of the test (in predicting human toxicity) over animal testing is increased by a factor of 2 (66). During development of a vascularized human bone marrow (BM) OC, researches demonstrated that it reproduced aspects of BM injury, including myeloerythroid toxicity after clinically relevant exposures to chemotherapeutic drugs and ionizing radiation, and myeloid recovery after drug-induced myelosuppression, suggesting that a BM OC system may be useful for predicting human toxicity and designing in-human trials. When the BM OC was constructed using cells from patients with a rare genetic disorder, it not only reproduced key hemopoietic defects of the disease, but also led to the discovery of a previously undescribed neutrophil maturation abnormality (67). Thus, the BM OC may be useful in discovering new, human-relevant therapeutic targets.
In late 2019, Jang et al. (68) developed a multispecies liver OC that was able to differentiate species-specific toxicities of test compounds in human, canine, and rat species. In one case, a proprietary test compound from Janssen Pharmaceuticals (Raritan, New Jersey), which had shown liver inflammation in rats and was subsequently discontinued from further development, showed replication of the rat findings but demonstrated a lack of toxicity in human cells. With a second proprietary compound, the canine portion of the canine chip was able to replicate the liver inflammation that had been found in dogs, and also suggested changes in human cells, suggesting a high likelihood of human toxicity. In addition, the authors tested their chip against drugs that had survived preclinical animal tests, only to be discontinued during human trials due to liver toxicity, and found that the chip accurately predicted human toxicity (Table 1). This is particularly intriguing news, as liver injury is the most frequent reason for post market withdrawal of drugs (69). Cross-species chips may be able to hone animal testing to only those species whose toxicity has been shown by chip analysis to be relevant to humans, sparing animals, time, and expense while preserving human safety.
Table 1.
Correlation of Liver Chip Results for Drugs Halted in Clinical Trials (i.e., Drugs That Had Passed Animal Toxicity Studies)
| Drug | Clinical Trial Results | Possible Mechanism of Liver Injury | Liver Chip Results |
|---|---|---|---|
| Fialuridine (antiviral) | Discontinued in phase II: liver failure and deaths in 5 of 15 patients due to microvesicular steatosis. Animal toxicity studies did not predict severe hepatic injury in humans. | Drug-induced mitochondrial injury | Significant hepatocyte lipid accumulation, increased liver injury markers |
| TAK-875 (G-protein-coupled receptor 40 agonist) | Discontinued in phase III clinical trials due to treatment-related elevations in transaminases and several instances of drug-induced liver injury. In vivo and in vitro studies had detected formation of active metabolites. | Formation of reactive metabolites, suppression of mitochondrial respiration, inhibition of hepatic transporters | Reactive metabolites, hepatic transporter inhibition, mitochondrial dysfunction, lipid accumulation, markers of oxidative stress, release of inflammatory cytokines |
| Janssen proprietary compound JNJ-1 (colony-stimulating factor receptor kinase inhibitor). | Discontinued in phase I clinical trial due to very high elevations in liver transaminases in 2 subjects. Although minimal elevations were seen in rats and dogs, no microscopic liver changes were found in the animal models. | Kupffer cell depletion | Kupffer cell depletion, decreased IL-6 and MCP-1 in clinically significant concentrations |
For reference, please see Jang et al. (68).
IL = interleukin; MCP-1 = monocyte chemoattractant protein-1.
Despite academic enthusiasm for OC technology, industry has been cautious in adopting it for preclinical drug screening. In 2018, only about 20% of the top 50 pharmaceutical companies routinely used OC screening (66). OC technology is currently most widely applied in screening research for therapeutic targets, in preclinical testing in which no regulatory submissions are in question, than for toxicity testing, which at this time carries considerable regulatory uncertainty. A significant impediment is lack of standards for validation parameters for compounds, endpoints, exposure times, and thresholds for sensitivity and specificity, which contribute to the hesitance about acceptance/rejection criteria from the FDA and other regulators, including whether OC technology can at least partially replace animal testing in preclinical phases.
In order to overcome some of these obstacles, the NIH National Center for Advancing Translational Sciences has collaborated with the FDA in the Tissue Chip for Drug Screening program to develop human tissue chips that accurately model the structure and function of human organs and predict drug safety in humans more rapidly and effectively. Updated information about funding opportunities for chip research can be found on their website (70).
Additional Regulatory Steps
In December of 2017, the Toxicology Working Group at the U.S. FDA released its Predictive Technology Roadmap, which was formulated with a mission to strengthen the FDA’s commitment to promoting the development and use of new technologies to better predict human, animal and environmental responses to a wide range of substances relevant to FDA’s regulatory mission (71). In a September 2018, public hearing for stakeholder feedback on the roadmap, Commissioner Scott Gottlieb expressed a desire to apply OC technology across the life cycle of regulated product development, observing that “results in animals are not always predictive of results in humans ” (66). Although the FDA has emphasized support for alternative research methods, to date most therapeutics require animal testing in the preclinical phases, and do not allow substitution of alternative methods. Under the 21st Century Cures Act (72), certain therapeutics wholly or partially including human cells, tissues, and “therapeutic tissue engineering products” may achieve status as “regenerative medicine advanced therapies” and be eligible for certain expedited pathways at the FDA for approval, including focused and expedited FDA review, acceptance of retrospective data in lieu of some clinical study requirements, and other measures (48). However, these programs do not yet generally allow substitution of alternative research for preclinical animal studies.
In 2006, the European Union passed REACH (Registration, Evaluation, Authorization, and restriction of Chemicals) legislation that set a goal of reducing animal testing, and REACH does embrace some in silico methods (48,73). The European Medicines Agency has also put forth guidelines to eliminate or significantly restrict animal testing for certain human medicine products in order to reduce animal use in drug regulatory approvals (74, 75, 76).
Summary
Goals of reducing the time and cost of drug development, together with reduced public support for animal research, are driving attempts to find alternatives to animal testing, which does not sufficiently identify human safety and toxicity for therapeutics. Reducing pursuit of drugs that prove in late phase of development to have intolerable human toxicity is an important step in meeting those goals. In addition, accurately identifying agents that are human safe but currently fail animal testing will likely increase potential effective therapeutics in human disease. Although alternatives to animal research, such as cell and tissue platforms, computational in silico modeling, 3D tissue platforms and OC research have shown great promise in facilitating drug development while decreasing time and expense, they have yet to make significant inroads as replacements for preclinical animal testing. Although both the United States and European Union have recognized the value of pursuing alternative methods of research, such methods still await wider regulatory acceptance to replace animal testing in drug approval processes.
Footnotes
Dr. Van Norman has received financial support from the Journal of the American College of Cardiology.
The author attests they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Van Norman G. Limitations of animal studies for predicting toxicity in clinical trials: is it time to rethink our current approach? J Am Coll Cardiol Basic Trans Science. 2019;4:845–854. doi: 10.1016/j.jacbts.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacArthur Clark J., Clifford P., Jarrett W., Pekow C. Communicating about animal research with the public. ILAR J. 2020;60:34–42. doi: 10.1093/ilar/ilz007. [DOI] [PubMed] [Google Scholar]
- 3.Jones J.M. Americans hold record liberal views on most moral issues. https://news.gallup.com/poll/210542/americans-hold-record-liberal-views-moral-issues.aspx Gallup.com. Published May 11, 2017. Available at:
- 4.Wilke J., Saad L. Older Americans’ moral attitudes changing. https://news.gallup.com/poll/162881/older-americans-moral-attitudes-changing.aspx Gallup.com. Published June 3, 2013. Available at:
- 5.EUR-Lex Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32003L0015 Available at:
- 6.National Institutes of Health NIH to reduce significantly the use of chimpanzees in research (news release). June 26, 2018. https://www.nih.gov/news-events/news-releases/nih-reduce-significantly-use-chimpanzees-research Available at:
- 7.National Institutes of Health . November 17, 2015. NIH will no longer support biomedical research on chimpanzees.https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-will-no-longer-support-biomedical-research-chimpanzees Available at: [Google Scholar]
- 8.Grimm D. Dog research at U.S. Department of Veterans Affairs gets formal review. Science. December 10, 2018 https://www.sciencemag.org/news/2018/12/dog-research-us-department-veterans-affairs-gets-formal-review Available at: [Google Scholar]
- 9.von Roten F.C. Public perceptions of animal experimentation across Europe. Public Underst Sci. 2013;22:691–703. doi: 10.1177/0963662511428045. [DOI] [PubMed] [Google Scholar]
- 10.Freires I.A., de Cassia Orlandi Sardi J., Dias de Castro R., Rosalen P.L. Alternative animal and non-animal models for drug discovery and development: bonus or burden? Pharmaceut Res. 2017;34:681–686. doi: 10.1007/s11095-016-2069-z. [DOI] [PubMed] [Google Scholar]
- 11.Loiodice S., Nogueira de Costa A., Atienzar F. Current trends in in silico, in vitro toxicology and safety biomarkers in early drug development. Drug Chem Tox. 2019;42:113–121. doi: 10.1080/01480545.2017.1400044. [DOI] [PubMed] [Google Scholar]
- 12.Mayourian J., Sobie E.A., Costa K.D. An introduction to computational modeling of cardiac electrophysiology and arrhythmogenicity. In: Ishikawa K., editor. Experimental Models of Cardiovascular Diseases: Methods and Protocols. Springer, Humana Press; New York, NY: 2018. pp. 17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raunio H. In silico toxicology—non-testing methods. Front Pharmacol. 2011;2:33. doi: 10.3389/fphar.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cha Y., Erez T., Reynolds I.J. Drug repurposing from the perspective of pharmaceutical companies. Br J Pharmacol. 2018;175:168–180. doi: 10.1111/bph.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2014;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 16.Papapetropoulos A., Szabo C. Inventing new therapies without reinventing the wheel: the power of drug repurposing. Br J Pharmacol. 2018;175:165–167. doi: 10.1111/bph.14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Food and Drug Administration Understanding unapproved use of approved drugs “off label”. https://www.fda.gov/patients/learn-about-expanded-access-and-other-treatment-options/understanding-unapproved-use-approved-drugs-label Available at:
- 18.Lounkine E., Keiser M.J., Whitebread S. Large-scale prediction and testing of drug activity on side-effect targets. Nature. 2012;486:361–368. doi: 10.1038/nature11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luechtefeld T., Marsh D., Rowlands C., Hartung T. Machine learning of toxicological big data enables read-across structure activity relationship (RASAR) outperforming animal test reproducibility. Toxicol Sci. 2018;165:198–212. doi: 10.1093/toxsci/kfy152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah R.R. Drug-induced QT interval prolongation: does ethnicity of the thorough QT study population matter? Br J Clin Pharacol. 2013;75:347–358. doi: 10.1111/j.1365-2125.2012.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration Guidance for industry: E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. 2005. https://www.fda.gov/media/71372/download Available at: [PubMed]
- 22.Gintant G. An evaluation of hERG current assay performance: translating preclinical safety studies of clinical QT prolongation. Pharmacol Ther. 2011;129:109–119. doi: 10.1016/j.pharmthera.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Lancaster M.C., Sobie E.A. Improved prediction of drug-induced torsades de pointes through simulations of dynamics and machine learning algorithms. Clin Pharm Therapeut. 2016;100:371–379. doi: 10.1002/cpt.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Passini E., Britton O.J., Lu H.R. Human in silico drug trials demonstrate higher accuracy than animal models in predicting clinical pro-arrhythmic cardiotoxicity. Front Physiol. 2017;8:668. doi: 10.3389/fphys.2017.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolaja K. Computer model predicts side effects. Nature. 2012;486:326–327. doi: 10.1038/nature11198. [DOI] [PubMed] [Google Scholar]
- 26.Moreno J.D., Zhu W., Mangold K., Chung W., Silva J.R. A molecularly detailed Nav1.5 model reveals a new class I antiarrhythmic target. J Am Coll Cardiol Basic Trans Science. 2019;4:736–751. doi: 10.1016/j.jacbts.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CompBiomed https://www.compbiomed.eu Available at:
- 28.Mattes W.B. 2015 Science Writers Symposium. New drug, old drug, no drug; uses of spectral data-activity relationships (SDAR) modeling. U.S. Food and Drug Administration: 2015. https://www.fda.gov/media/94571/download Available at:
- 29.U.S. Food and Drug Administration How simulation can transform regulatory pathways. https://www.fda.gov/science-research/about-science-research-fda/how-simulation-can-transform-regulatory-pathways Available at:
- 30.U.S. Food and Drug Administration . September 21, 2016. Reporting of computational modeling studies in medical device submissions: guidance for industry and Food and Drug Administration staff.https://www.regulations.gov/document?D=FDA-2013-D-1530-0013 Available at: [Google Scholar]
- 31.U.S. Food and Drug Administration Safety testing of drug metabolites: guidance for industry. 2020. https://www.fda.gov/media/72279/download Available at:
- 32.Baker M. Blame it on the antibodies. Nature. 2015;521:274–276. doi: 10.1038/521274a. [DOI] [PubMed] [Google Scholar]
- 33.Groff K., Allen D., Casey W., Clippinger A. Increasing the use of animal-free recombinant antibodies. ALTEX. 2020 Jan 8 doi: 10.14573/altex.2001071. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 34.de Vries R.B.M., Leenaars M., Tra J. The potential of tissue engineering or developing alternatives to animal experiments: a systematic review. J Tissue Regen Med. 2015;9:771–778. doi: 10.1002/term.1703. [DOI] [PubMed] [Google Scholar]
- 35.Groeber F., Englehardt L., Lange J. A first vascularized skin equivalent as an alternative to animal experimentation. ALTEX. 2016;33:415–422. doi: 10.14573/altex.1604041. [DOI] [PubMed] [Google Scholar]
- 36.Eschenhagen T, Wakatsuki T, Elson EL. A new method to measure isometric force of contraction in embryonic cardiac myocytes. Paper presented at: Second International Conference on Cellular Engineering (no. 96-17); August 29–22, 1995; La Jolla, California.
- 37.Moscona A.A. Tissues from dissociated cells. Sci Am. 1959;200:132–134. doi: 10.1038/scientificamerican0559-132. [DOI] [PubMed] [Google Scholar]
- 38.Hirt M.N., Hansen A., Echenhagen T. Cardiac tissue engineering. State of the art. Circ Res. 2014;2:354–367. doi: 10.1161/CIRCRESAHA.114.300522. [DOI] [PubMed] [Google Scholar]
- 39.Ribas J., Sadeghi H., Manbaci A. Cardiovascular organ-on-a-chip platforms for drug discovery and development. Appl In Vitro Tech. 2016;2:82–96. doi: 10.1089/aivt.2016.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li R.A., Keung W., Cashman T.J. Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials. 2018;163:116–127. doi: 10.1016/j.biomaterials.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nugraha B., Buiono M., von Boehmenr L., Hoerstrup S.P., Emmert M.Y. Human cardiac organoids for disease modeling. Clin Pharmacol Ther. 2019;206:79–85. doi: 10.1002/cpt.1286. [DOI] [PubMed] [Google Scholar]
- 42.Novakovi G.V., Eschenhagen T., Mummery C. Myocardial tissue engineering: in vitro models. Cold Spring Harb Perspect Med. 2014;4:a014076. doi: 10.1101/cshperspect.a014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathur A., Ma Z., Loskill P., Jeeawoody S., Healy K.E. In vitro cardiac tissue models: current status and future prospects. Adv Drug Deliv Rev. 2016;96:203–213. doi: 10.1016/j.addr.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Truitt R., Mu A., Corbin E.A. Increased afterload augments sunitinib-induced cardiotoxicity in an engineered cardiac mouse microtissue model. J Am Coll Cardiol Basic Trans Science. 2018;3:265–276. doi: 10.1016/j.jacbts.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.U.S. Food and Drug Administration Guidance document: regulatory considerations for human cells, tissues, and cellular and tissue-based products: minimal manipulation and homologous use. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-human-cells-tissues-and-cellular-and-tissue-based-products-minimal Available at:
- 46.U.S. Food and Drug Administration Evaluation of devices used with regenerative medicine advanced therapies: guidance for industry. February 2019. https://www.fda.gov/media/120266/download Available at:
- 47.U.S. Food and Drug Administration Regenerative medicine advanced therapy designation. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/regenerative-medicine-advanced-therapy-designation Available at:
- 48.U.S. Food and Drug Administration Expedited programs for regenerative medicine therapies for serious conditions: guidance for industry. February 2019. https://www.fda.gov/media/120267/download Available at:
- 49.Wysse Institute for Biologically Inspired Engineering, Harvard University. https://wyss.harvard.edu Available at: Accessed March 22, 2020.
- 50.Kimura H., Sakai Y., Fujii T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab Pharmacokin. 2018;33:43–48. doi: 10.1016/j.dmpk.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 51.U.S. Food and Drug Administration FDA researchers to evaluate ‘organs-on-chips’ technology. Constituent Update. April 11, 2017 https://www.fda.gov/food/cfsan-constituent-updates/fda-researchers-evaluate-organs-chips-technology Available at: [Google Scholar]
- 52.Skardal A., Aleman J., Forsythe S. Drug compound screening in single and integrated multi-organoid body-on-a-chip systems. Biofabrication. 2020;12 doi: 10.1088/1758-5090/ab6d36. [DOI] [PubMed] [Google Scholar]
- 53.Virani S.S., Alonso A., Benjamin E. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 54.Hwang T.J., Lauffenburger J.C., Franklin J.M., Kesselheim A.S. Temporal trends and factors associated with cardiovascular drug development, 1990-2012. J Am Coll Cardiol Basic Trans Science. 2016;1:301–308. doi: 10.1016/j.jacbts.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.U.S. Food and Drug Administration New drug therapy approvals 2019. Advancing health through innovation. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2019 Available at:
- 56.Mozaffarian D., Benjamin E.J., Go A.S. Heart disease and stroke statistics-2016 Update: a report from the American Heart Association. Circulation. 2016;133 doi: 10.1161/CIR.0000000000000350. e38-360. [DOI] [PubMed] [Google Scholar]
- 57.Siramshetty B.V., Nickel J., Omieczynski C. WITHDRAWN—a resource for withdrawn and discontinued drugs. Nucl Acids Res. 2016;44:D1080–D1086. doi: 10.1093/nar/gkv1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benam K.H., Dauth S., Hassell B. Engineered in vitro disease models. Annu Rev Pathol. 2015;10:195–262. doi: 10.1146/annurev-pathol-012414-040418. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Y., Rafatian N., Wang E.Y. Towards chamber specific heart-on-a-ship for drug testing applications. Adv Drug Deliv Rev. 2020 Jan 7 doi: 10.1016/j.addr.2019.12.002. (E-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grosberg A., Alford P.W., McCain M.L. Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip. 2011;11:4165. doi: 10.1039/c1lc20557a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Agarwal A., Goss J.A., Cho A. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip. 2013;13:3599–3608. doi: 10.1039/c3lc50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCain M.L., Sheehy S.P., Grosberg A. Recapitulating maladaptive, multiscale remodeling of failing myocardium on a chip. Proc Natl Acad Sci U S A. 2013;110:9770–9775. doi: 10.1073/pnas.1304913110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ren L., Liu W., Wang Y. Investigation of hypoxia-induced myocardial injury dynamics in a tissue interface mimicking microfluidic device. Anal Chem. 2013;85:235–244. doi: 10.1021/ac3025812. [DOI] [PubMed] [Google Scholar]
- 64.Wang G., McCain M.L., Yang L. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20:616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huh D., Leslie C.D., Matthews B.D. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med. 2012;4:159ra47. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rein J. Organs on a chip! FDA’s predictive toxicology roadmap. Update Magazine. Oct/Nov 2018. https://www.fdli.org/2018/10/organs-on-a-chip-fdas-predictive-toxicology-roadmap/ Available at:
- 67.Chou D.B., Frismantas V., Milton Y. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat Biomed Eng. 2020 Jan 27 doi: 10.1038/s41551-019-0495-z. (E-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jang K.J., Otieno M.A., Ronxhi J. Reproducing human and cross-species drug toxicities using a liver-chip. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aax5516. eaax5516. [DOI] [PubMed] [Google Scholar]
- 69.Lasser K.E., Allen P.D., Whoolhandler S.J., Himmelstein D.U., Wolfe S.M., Bor D.H. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287:2215–2220. doi: 10.1001/jama.287.17.2215. [DOI] [PubMed] [Google Scholar]
- 70.National Institutes of Health National Center for Advancing Translational Sciences Tissue chip funding information. https://ncats.nih.gov/tissuechip/funding Available at:
- 71.U.S. Food and Drug Administration FDA’s predictive technology roadmap. https://www.fda.gov/media/109634/download Available at:
- 72.Rein J. Food and Drug Law Institute: Organs on chip! FDA’s predictive toxicology roadmap. Update Magazine. October/November 2018. https://www.fdli.org/2018/10/organs-on-a-chip-fdas-predictive-toxicology-roadmap/ Available at:
- 73.21st Century Cures Act. Public Law 114-255. 114th Congress. December 13, 2016. https://www.congress.gov/114/plaws/publ255/PLAW-114publ255.pdf Available at:
- 74.European Commission Regulation (EC) No 1907/2006 – Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) https://osha.europa.eu/en/legislation/directives/regulation-ec-no-1907-2006-of-the-european-parliament-and-of-the-council Available at:
- 75.European Commission Regulation (EC) No. 440/2008 – laying down test methods pursuant to Regulation (EC) No. 1907/2006 of the European Parliament and the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) https://www.ecolex.org/details/legislation/council-regulation-ec-no-4402008-laying-down-test-methods-pursuant-to-regulation-ec-no-19072006-of-the-european-parliament-and-of-the-council-on-the-registration-evaluation-authorisation-and-restriction-of-chemicals-reach-lex-faoc114509/ Available at:
- 76.European Medicines Agency Committee for Medicinal Products for Human Use Reflection paper providing an overview of the current regulatory testing requirements for medicinal products for human use and opportunities for implementation of the 3Rs. October 18, 2018. https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-providing-overview-current-regulatory-testing-requirements-medicinal-products-human_en.pdf Available at: