Abstract
Context
Complex relationships between aldosterone and calcium homeostasis have been proposed.
Objective
To disentangle the influence of aldosterone and intravascular volume on calcium physiology.
Design
Patient-oriented and epidemiology studies.
Setting
Clinical research center and nationwide cohorts.
Participants/Interventions
Patient-oriented study (n = 18): Participants were evaluated after completing a sodium-restricted (RES) diet to contract intravascular volume and after a liberalized-sodium (LIB) diet to expand intravascular volume. Cross-sectional studies (n = 3755): the association between 24h urinary sodium and calcium excretion and risk for kidney stones was assessed.
Results
Patient-oriented study: compared to a RES-diet, a LIB-diet suppressed renin activity (LIB: 0.3 [0.1, 0.4] vs. RES: 3.1 [1.7, 5.3] ng/mL/h; P < 0.001) and plasma aldosterone (LIB: 2.0 [2.0, 2.7] vs. RES: 20.0 [16.1, 31.0] vs. ng/dL; P < 0.001), but increased calciuria (LIB: 238.4 ± 112.3 vs. RES: 112.9 ± 60.8 mg/24hr; P < 0.0001) and decreased serum calcium (LIB: 8.9 ± 0.3 vs. RES: 9.8 ± 0.4 mg/dL; P < 0.0001). Epidemiology study: mean urinary calcium excretion was higher with greater urinary sodium excretion. Compared to a urinary sodium excretion of < 120 mEq/day, a urinary sodium excretion of ≥220 mEq/day was associated with a higher risk for having kidney stones in women (risk ratio = 1.79 [95% confidence interval 1.05, 3.04]) and men (risk ratio = 2.06 [95% confidence interval 1.27, 3.32]).
Conclusions
High dietary sodium intake suppresses aldosterone, decreases serum calcium, and increases calciuria and the risk for developing kidney stones. Our findings help disentangle the influences of volume from aldosterone on calcium homeostasis and provide support for the recommendation to restrict dietary sodium for kidney stone prevention.
Keywords: aldosterone, kidney stone, parathyroid hormone, calcium, renin, angiotensin
High dietary sodium intake and states of high sodium reabsorption, such as primary aldosteronism, induce intravascular volume expansion (1). Prior studies have shown that high sodium intake can increase calciuria (2–6) and that individuals with primary aldosteronism have hypercalciuria and hyperparathyroidism, both of which normalize when primary aldosteronism is treated (7–11). The mechanisms leading to hypercalciuria and hyperparathyroidism in primary aldosteronism have generally been attributed to a potential direct effect of aldosterone on the kidney and parathyroid gland (12) rather than the influence of aldosterone on intravascular volume.
A better understanding of these relationships is important since both high dietary sodium intake (2) and primary aldosteronism are common (13). In addition, excess aldosterone production is associated with higher parathyroid hormone (PTH) levels (7,8,11,14) and a higher risk for cardiovascular (15–17), skeletal (12,18–20), and kidney disease (21–23).
Our objective was to better understand, and attempt to disentangle, the interrelationships between dietary sodium intake, intravascular volume, and renin–angiotensin–aldosterone system (RAAS) activity and how these may influence calcium–parathyroid physiology. We conducted 2 complementary studies to evaluate these physiologic systems: a patient-oriented study and an epidemiology study. The patient-oriented study employed dietary sodium interventions to modulate intravascular volume and RAAS activity to disentangle the influence of volume and aldosterone on calcium homeostasis and calcium-regulatory hormones. The epidemiology study included 3 large nationwide cohorts to investigate the association between urinary sodium and calcium excretion and the risk for developing kidney stones.
Materials and Methods
We describe the methodology for 2 complementary studies: a patient-oriented study and an observational study that included 3 nationwide cohorts.
Study population: patient-oriented study
The patient-oriented study was a post-hoc analysis of pre-randomization data from a previously published clinical trial that employed dietary sodium modulation (n = 18) (24). As previously described (24), participants were included if they were between the ages of 18 and 70 years, had well-controlled type 2 diabetes mellitus but no history of cardiovascular disease, normal complete blood count, liver function testing, urinalysis, and electrocardiogram. Exclusion criteria included presence of chronic kidney disease (estimated glomerular filtration (eGFR) rate <60 ml/min/1.73 m2), poorly controlled diabetes (defined as hemoglobin A1C ≥ 8.5% or use of >1 daily insulin injection) or microvascular complications of diabetes (prior documented retinopathy, nephropathy, or peripheral neuropathy requiring medical intervention), stage 2 hypertension or use of >1 antihypertensive medication, history of kidney stones, parathyroid or granulomatous disorders, liver failure, congestive heart failure, coronary artery disease or cerebrovascular accident, and/or active tobacco or recreational drug use. Brigham and Women’s Hospital Institutional Review Board approved the protocol, and all participants provided informed consent.
Study protocol: patient-oriented study
Study participants underwent 3 study visits. The screening evaluation included a detailed history, physical examination, and laboratory assessment. After inclusion in the study, participants underwent an antihypertensive medication washout for 2 to 12 weeks, if applicable, as previously described (24) such that all study measurements were performed without the influence of any antihypertensive medication.
Participants were then asked to complete one week of dietary sodium restriction (RES) to evaluate the impact of sodium restriction, and resultant intravascular volume contraction and RAAS stimulation, on calcium and parathyroid physiology. The RES diet contained 10 mmol of sodium per day for 5–7 days, which approximates the presumed sodium intake of ancestral human diets (25). Participants arrived following the RES diet for visit 2 where study measurements were made (see the following discussion). Participants were then asked to complete one week of a liberalized sodium intake (LIB) to evaluate the impact of a high sodium intake, and resultant intravascular volume expansion and RAAS suppression, on calcium and parathyroid physiology. The LIB diet contained at least 200 mmol of sodium per day for 5 to 7 days, which approximates the average sodium intake in contemporary human societies (2). Participants arrived following the LIB diet for visit 3 where study measurements were repeated (see the following discussion). Both diets also contained 100 mEq/day of potassium and 600 mg/day of calcium carbonate to standardize the intakes of these electrolytes that have known effects on the RAAS and calcium-regulatory hormones. All meals were prepared by professional dietary staff in our institutional Clinical Research Center.
Each study visit took place at Brigham and Women’s Hospital Clinical Research Center. Participants at visits 2 and 3 underwent the following measurements: basic serum electrolytes including calcium, PTH, 25-hydroxyvitamin D3 [25(OH)D], 1,25-dihydroxyvitamin D3 [1,25(OH) 2D], plasma renin activity (PRA) and aldosterone when upright and following 60 minutes of supine posture, and 24-h urine collections to evaluate sodium and calcium excretion. During visit 3, participants also received a 150-min infusion of para-aminohippurate (PAH), the clearance of which was used to measure renal plasma flow (RPF), and an infusion of exogenous angiotensin II with escalating doses (first 45 min: 0.3 ng/kg/min; second 45 min: 1.0 ng/kg/min), as previously described (24). The angiotensin II infusion permitted the dynamic and in vivo measurements of stimulated aldosterone and renovascular responses to angiotensin II.
Study population: cohort study
Data from 3 large longitudinal cohorts—Nurses’ Health Study I (NHS I) established in 1976 consisting of female nurses; Nurses’ Health Study II (NHS II) established in 1989 consisting of female nurses; and the Health Professionals Follow-up Study (HPFS) established in 1986 consisting of male health professionals—were analyzed, as previously described (26). These cohorts are followed biennially via questionnaire with the retention rate for all 3 cohorts exceeding 90% (26). Participants were included in the current analyses if they did not have a history of cancer or cardiovascular disease and met the age inclusion criteria, as described (26).
Study protocol: cohort study
As previously described (26), the biennial questionnaire explored whether participants had been diagnosed with a kidney stone (HPFS: since 1986; NHS I: since 1992; NHS II: since 1991). Supplementary questionnaires were sent to those reporting a first diagnosis of kidney stones for additional information as well as randomly selected controls. In all 3 cohorts, a validation study using review of medical records found that self-reported nephrolithiasis was confirmed in >90% of cases.
Individuals with a history of kidney stone disease confirmed by review as well as randomly selected controls had 24-h urine samples collected. The specifics of urine collection and sampling as well as exclusion criteria are previously discussed in detail (26). For participants with known stone disease, 24-h urine samples were collected after the incident nephrolithiasis event.
Laboratory measurements
Patient-oriented study.
Serum and urine values were measured as previously described (24). RPF was measured using PAH clearance (24,27). During angiotensin II infusions, ionized calcium, PTH (Beckman Coulter, Fullerton, CA, US), serum aldosterone (Siemens, Los Angeles, CA, US), and PAH values were measured in duplicate at each time point and averaged for analysis. Participants submitted 24-h urine samples from the 24 hours leading up to arrival for visits 2 and 3, which were used to assess for urinary sodium and calcium (LabCorp, Burlington, NC, US).
Cohort study.
Twenty-four-hour urine samples (performed using the system provided by Mission Pharmacal; San Antonio, TX, US), collected from participants confirmed to have kidney stones and appropriate controls, were assessed for calcium (via atomic absorption spectrophotometer), creatinine (via Cobas centrifugal analyzer), and sodium (determined directly via flame emission photometry), as well as oxalate, uric acid, citrate, magnesium, potassium, phosphorus, and pH, as previously described (26).
Statistical analysis
Data are presented as mean ± standard deviation, except for non-normally distributed variables (PRA, aldosterone), which are presented as median [interquartile range].
For the patient-oriented study, paired t-tests were used to compare mean differences between the RES and LIB diets, except for nonnormally distributed variables where Wilcoxon signed-rank tests were used. Repeated measures analysis of variance with interaction was used to determine the effect of escalating doses of angiotensin II infusion on aldosterone, ionized calcium, PTH, and RPF.
For the cohort study, as previously described (26), a single 24-h urine collection was used. Categorical variables were compared using the chi-squared test, and continuous variables were compared with the t-test. Participants were categorized by 24-h urinary sodium excretion to avoid assuming linearity (<120, 120–139, 140–159, 160–179, 180–199, 200–219, ≥220 mEq/day), and the relative risk for having a kidney stone was assessed for each category of sodium excretion via adjusted logistic regression models. Models were adjusted for age, body mass index, history of hypertension, and numerous urinary parameters (volume, creatinine, oxalate, uric acid, citrate, magnesium, potassium, phosphorus, and pH). Adjustment for urinary calcium was performed to evaluate the role of calciuria in modifying the relation between urinary sodium excretion and kidney stones.
A two-tailed P value of <0.05 was deemed statistically significant. Data analysis was performed using STATA statistical software (version 15) (College Station, TX, US) and SAS 9.4 (Cary, NC, US).
Results
Study participants
Baseline screening characteristics for the 18 participants in the patient-oriented study are shown in Table 1, and the characteristics for the cohort participants sorted by case and control status in each cohort are shown in Table 2. By design, participants from NHS II were substantially younger than the original participants in HPFS and NHS I since NHS II participants were recruited to be in the subsequent generation (28).
Table 1.
Baseline characteristics of participants in the patient-oriented study
| N | 18 |
| Age, years | 50.8 (7.9) |
| Female, n (%) | 7 (39) |
| White, n (%) | 13 (72) |
| BMI, kg/m2 | 31.5 (5.3) |
| Systolic blood pressure, mmHg | 124.9 (16.2) |
| Diastolic blood pressure, mmHg | 80.0 (10.6) |
| MAP, mmHg | 95.0 (11.8) |
| Duration of diabetes, years | 6.6 (4.3) |
| HbA1c, % | 6.9 (0.8) |
| eGFR, mL/min/1.73m2 | 101.1 (14.8) |
| Serum creatinine, mg/dL | 0.81 (0.10) |
| Serum potassium, mmol/L | 4.4 (0.3) |
| 25(OH)D, ng/mL | 30.3 (17.1) |
| PTH, pg/mL | 23.9 (9.7) |
| Calcium, mg/dL | 9.6 (0.4) |
Mean (standard deviation) for continuous variables and number (percentage) for categorical variables.
Table 2.
Characteristics of participants in the nationwide cohort studies
| HPFS | NHS I | NHS II | ||||
|---|---|---|---|---|---|---|
| Case (n = 728) | Control (n = 417) | Case (n = 947) | Control (n = 407) | Case (n = 833) | Control (n = 443) | |
| Age, years | 63.4 (8.8)b | 64.6 (6.3) | 66.5 (7.8)b | 65.5 (5.7) | 48.4 (6.2)a | 51.0 (5.1) |
| Body mass index, kg/m2 | 26.2 (3.6) | 26.2 (3.6) | 27.4 (5.7)a | 25.8 (5.1) | 27.8 (6.6) | 26.6 (6.0) |
| Family history of kidney stones, % | 20.6 | 13.0 | NA | NA | 31.5 | 14.5 |
| 24h Urinary Sodium Excretion, mEq | 186 (70)b | 177 (71) | 142 (60) | 136 (56) | 153 (63) | 151 (64) |
| 24h Urinary Calcium Excretion, mg | 212 (111)a | 171 (92) | 198 (109)b | 183 (94) | 218 (100)a | 187 (87) |
Values are means (standard deviation) for continuous varaibles and number (percentage) for categorical variables.
a P ≤ 0.01 for case compared with control within the cohort.
b P < 0.05 for case compared with control within the cohort.
Effect of dietary sodium intake on the RAAS and calcium and calcium-regulatory hormones
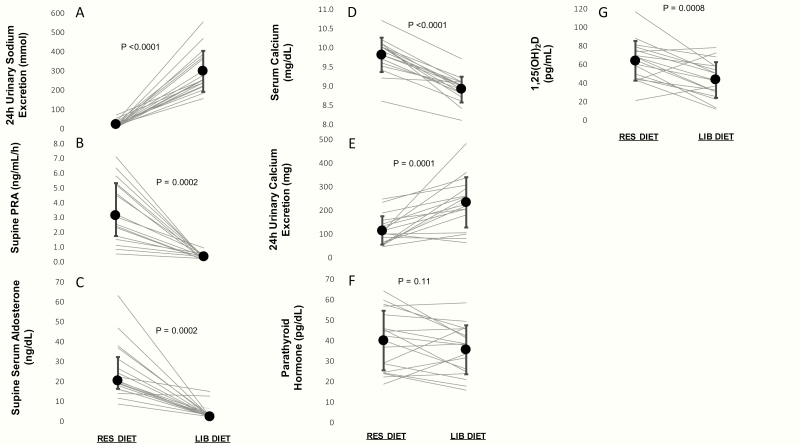
As expected based on the design of our dietary intervention, 24-h urinary sodium excretion was very low on a RES diet and increased substantially on a LIB diet (RES: 20.0 ± 16.9 vs. LIB: 294.9 ± 106.8 mmol/24 h; P < 0.0001). Accordingly, renin and aldosterone were maximally stimulated on a RES diet and maximally suppressed on a LIB diet (Table 3). These changes in urinary sodium excretion, PRA, and aldosterone were uniform and consistent in all 18 participants (100%) (Fig. 1A–1C).
Table 3.
Effects of dietary sodium intake on the RAAS, calcium, and calcium-regulatory hormones in the patient-oriented study
| RES | LIB | Delta | P–value | |
|---|---|---|---|---|
| 24-hour urinary sodium, mmol/24 h | 20.0 (16.9) | 294.9 (106.8) | +274.9 | <0.0001 |
| 24-hour urinary creatinine, mg/24 h | 1388.1 (369.1) | 1668.5 (469.5) | +280.4 | 0.07 |
| 24-hour urinary potassium, mmol/24 h | 66.0 (21.2) | 119.8 (27.6) | 53.8 | <0.0001 |
| Upright PRA, ng/mL/h | 6.5 [4.9, 12.0] | – | – | – |
| Supine PRA, ng/mL/h | 3.1 [1.7, 5.3] | 0.3 [0.1, 0.4] | –2.8 | <0.001 |
| Upright Aldosterone, ng/dL | 45.6 [31.1, 69.5] | – | – | – |
| Supine Aldosterone, ng/dL | 20.0 [16.1, 31.0] | 2.0 [2.0, 2.7] | –18.0 | <0.001 |
| Supine Systolic BP, mmHg | 123.3 (15.3) | 128.1 (15.4) | +4.8 | 0.29 |
| Supine Diastolic BP, mmHg | 78.5 (10.7) | 75.6 (10.1) | –2.9 | 0.46 |
| Supine MAP, mmHg | 93.4 (11.7) | 93.1 (11.0) | –0.3 | 0.94 |
| Serum Calcium, mg/dL | 9.8 (0.4) | 8.9 (0.3) | –0.9 | <0.0001 |
| 24-hour urinary calcium, mg/24 h | 112.9 (60.8) | 238.4 (112.3) | +125.5 | <0.001 |
| Serum phosphorus, mg/dL | 4.0 (0.7) | 3.8 (0.6) | –0.2 | 0.21 |
| Parathyroid Hormone, pg/mL | 39.7 (14.5) | 35.2 (11.9) | –4.4 | 0.11 |
| 1,25(OH)2D, pg/mL | 63.3 (21.5) | 42.8 (19.4) | –20.5 | <0.001 |
| 25(OH)D, ng/mL | 33.2 (15.8)a | 25.1 (10.0)a | –8.1 | <0.001 |
Data displayed as mean (SD) for normally distributed variables and median [25th percentile,75th percentile] for nonnormally distributed variables.
Abbreviation: PRA, plasma renin activity.
a N=16—two participants did not have 25(OH)D measurements available.
Figure 1.
Effects of restricted and liberalized dietary sodium intake on sodium, the RAAS, calcium, and calcium-regulatory hormones.
In parallel with these expected physiologic changes, transitioning from the RES diet to the LIB diet also significantly increased calciuria (LIB: 238.4 ± 112.3 vs. RES: 112.9 ± 60.8 mg/24 h; P < 0.001) and significantly decreased total serum calcium (LIB: 8.9 ± 0.3 vs. RES: 9.8 ± 0.4 mg/dL; P < 0.0001) (Table 3). Serum calcium decreased in all 18 participants (100%) while an increase in calciuria was seen in 15/18 participants (83%) (Fig. 1D and 1E). To assess the influence of dilutionary effects that may accompany volume changes induced by dietary sodium modulation, albumin-corrected calcium was also calculated; the decrease in total serum calcium on LIB persisted after correcting for serum albumin (LIB: 8.6 ± 0.4 vs. RES: 9.2 ± 0.5 mg/dL; P = 0.0001). In parallel to the changes in serum calcium and calciuria, there was a nonstatistically significant decrease in PTH when participants transitioned from the RES to LIB diet (LIB: 35.2 ± 11.9 vs. RES: 39.7 ± 14.5 pg/mL; P = 0.11) seen in 11/18 participants (61%) and a significant decrease in 1,25(OH)2D (LIB: 42.8 ± 19.4 vs. RES: 63.3 ± 21.5 pg/mL; P < 0.001) (Table 3) seen in 15/18 participants (83%) (Fig. 1F and 1G).
Effects of angiotensin II infusion on aldosterone, calcium, PTH, and renal plasma flow
When participants received infusions of escalating doses of angiotensin II over 90 minutes, there was an acute and dose-dependent increase in serum aldosterone and circulating PTH (Table 4) similar to previous reports (29). Importantly, these increases in PTH and aldosterone were independent of any changes in ionized calcium. As expected, renal plasma flow declined by 15% during the infusion of angiotensin II (Table 4).
Table 4.
Effect of angiotension II on aldosterone, ionized calcium, parathyroid hormone, and renal plasma flow
| Baseline | AngII 0.3 ng/kg/min | AngII 1.0 ng/kg/min | Delta | P | |
|---|---|---|---|---|---|
| Aldosterone, ng/dL | 3.8 (3.9) | 6.7 (5.2) | 11.2 (6.0) | +7.4 | <0.001* |
| Ionized calcium, mmol/L | 1.14 (0.03) | 1.14 (0.04) | 1.14 (0.03) | 0.0 | 0.90 |
| Parathyroid hormone, pg/mL | 35.1 (12.3) | 36.2 (17.3) | 41.3 (20.6) | +6.3 | 0.03 |
| Renal plasma flow, mL/min/1.73 m2 | 628.4 (182.2) | 587.3 (150.3) | 548.9 (152.2) | –79.5 | <0.001* |
| Relative change in Renal Plasma Flow, % | 0 | –6.4 | –14.8 | — | — |
One participant did not receive angiotensin II because of isolated elevated blood pressures immediately prior to administration, which later normalized without intervention. Thus, only 17 of 18 participants completed the angiotensin II infusion.
Association between urinary sodium excretion and kidney stones
Higher urinary sodium excretion was associated with higher adjusted mean urinary calcium excretion (Table 5). When compared with urinary sodium excretion of <120 mEq/day, urinary sodium excretion of ≥220 mEq/day was associated with a higher risk for kidney stones in the male HPFS cohort (risk ratio (RR) = 2.06, 95% confidence interval [CI] 1.27, 3.32) and in the female NHS I cohort (RR = 1.79, 95% CI 1.05, 3.04), but not in the substantially younger female NHS II cohort (RR = 1.01, 95% CI 0.63, 1.63) (Table 5). Importantly, these associations were markedly attenuated and, in NHS I, no longer statistically significant after adjusting for urinary calcium excretion (HPFS RR 1.69, 95% CI 1.03–2.76; NHS I RR 1.46, 95% CI 0.85–2.51).
Table 5.
Association between urinary sodium and calcium excretion and incident kidney stones
| HPFS | NHS I | NHS II | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Urinary Sodium Excretion (mEq/day) | Mean Adjusted Urinary Calcium Excretion (mg/day) | RR | (95% CI) | Mean Adjusted Urinary Calcium Excretion (mg/day) | RR | (95% CI) | Mean Adjusted Urinary Calcium Excretion (mg/day) | RR | (95% CI) |
| <120 | 178 | 1.00 | (reference) | 161 | 1.00 | (reference) | 189 | 1.00 | (reference) |
| 120–139 | 186 | 1.10 | (0.68, 1.77) | 179 | 1.33 | (0.91, 1.93) | 206 | 0.93 | (0.63, 1.39) |
| 140–159 | 192 | 0.90 | (0.56, 1.43) | 174 | 1.37 | (0.91, 2.07) | 196 | 1.10 | (0.74, 1.64) |
| 160–179 | 201 | 1.10 | (0.68, 1.77) | 193 | 1.44 | (0.90, 2.29) | 214 | 1.17 | (0.75, 1.84) |
| 180–199 | 205 | 1.49 | (0.88, 2.50) | 188 | 1.05 | (0.64, 1.71) | 206 | 0.94 | (0.54, 1.63) |
| 200–219 | 203 | 1.61 | (0.93, 2.81) | 170 | 0.93 | (0.51, 1.69) | 234 | 1.14 | (0.64, 2.01) |
| 220+ | 228 | 2.06 | (1.27, 3.32) | 208 | 1.79 | (1.05, 3.04) | 229 | 1.01 | (0.63, 1.63) |
Risk adjusted for age, history of hypertension, body mass index and urinary factors—total volume, creatinine, oxalate, uric acid, citrate, magnesium, potassium, phosphosphorus and pH.
HPFS: Health Professionals Follow-up Study—728 cases and 417 controls (total 1145).
NHS I: Nurses’ Health Study I—947 cases and 407 controls (total 1354).
NHS II: Nurses’ Health Study II—833 cases and 443 controls (1276).
Discussion
In these patient-oriented and epidemiology studies, we found that a high dietary sodium intake that induced volume expansion and suppression of the RAAS also significantly increased calciuria and decreased serum calcium and was associated with a higher risk for developing kidney stones. Conversely, the data may be interpreted to indicate that dietary sodium restriction that induced volume contraction and stimulation of the RAAS also significantly decreased calciuria, increased serum calcium, and lowered the risk for incident kidney stones. Although changes in intravascular volume can induce dilutionary effects on metabolite concentrations, the results of the angiotensin II infusion and albumin-corrected calcium levels, and parallel findings in large epidemiologic populations, suggest that our findings may represent unique physiology beyond the effects of hemodilution or hemoconcentration. Collectively, these findings implicate the high dietary sodium intake that is prevalent worldwide as a modifiable risk factor for increased calciuria and kidney stones. Moreover, the results of this study may provide mechanistic insights to support dietary sodium restriction for kidney stone prevention and potential explanations for the hypercalciuria seen in primary aldosteronism.
An interesting finding of our patient-oriented study was that, despite significant and uniform decreases in serum calcium, PTH did not uniformly increase. In fact, there was a nonsignificant decrease in PTH that paralleled the suppression of aldosterone when participants were loaded with sodium, and this was accompanied by a statistically significant decline in 1,25(OH)2D levels (a biomarker of PTH activity). 25(OH)D levels also declined with high dietary sodium intake, suggesting a potential hemodilutional effect with volume expansion; however, the albumin-corrected calcium levels were consistent, suggesting that, despite hemodilutional effects of volume expansion, there appeared to be true changes in calcium homeostasis. In support of this phenomenon, prior studies have demonstrated physiologic relationships between the RAAS and calcium-regulatory systems (7,8,11,14,29–38). Increasing angiotensin II and aldosterone levels can increase PTH, while suppressing (or blocking) angiotensin II and aldosterone levels with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker can lower PTH (8,14,24,29,30,39). Our current study replicated some of these phenomena by demonstrating acute and dose-dependent increases in aldosterone and PTH with exogenous angiotensin II administration. Importantly, this significant change in PTH with angiotensin II infusion was independent of ionized calcium and paralleled a significant reduction in renal plasma flow. Since both angiotensin type I receptors and mineralocorticoid receptors are expressed in normal and adenomatous human parathyroid tissue (29,36), one possible explanation for the findings of our study is that the suppression of angiotensin II and aldosterone by a high sodium diet can blunt PTH secretion even when serum calcium declines.
Our population-based studies provide complementary results to our patient-oriented study. In large cohorts of men and women, higher urinary sodium excretion (a proxy for dietary sodium intake) was associated with greater calciuria and a higher risk of developing kidney stones. This was further supported in our statistical models; when adjustment for urinary calcium excretion was included, the association between urinary sodium excretion and incident kidney stones was attenuated and no longer statistically significant, suggesting that developing kidney stones was mediated, in part, through an increase in urinary calcium excretion when urinary sodium excretion was higher. The reason this finding was not observed in the NHS II cohort is not clear; however, one potential explanation is the substantially younger age of the NHS II population and consequently shorter duration to develop kidney stone events when compared to the much older NHS I and HPFS cohorts. It is worth noting that the associations we observed were only evident when comparing the extremes of dietary sodium excretion and that we used categorical comparisons to account for the observational distribution of sodium excretion and non-linearity between exposure and outcome. Although this approach permitted relatively similar sodium excretion differences when compared to the patient-oriented intervention we performed, it was unable to describe a dose-dependent relationship.
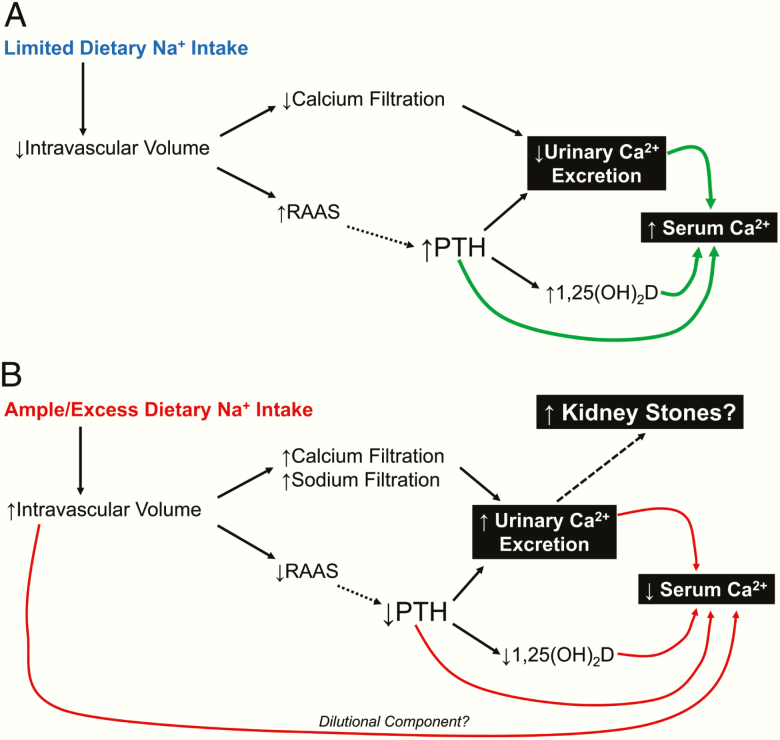
We hypothesize a teleological explanation (Fig. 2) for the phenomena demonstrated in our studies. In a state of restricted sodium intake (Fig. 2A) that approximates presumed ancestral human sodium intake (25), intravascular volume is contracted, leading to reductions in glomerular filtration and urinary calcium excretion. In parallel, RAAS activity is increased to counter the low intravascular volume by increasing renal sodium reabsorption. Several studies have now proposed that RAAS activation may also stimulate PTH secretion via the direct effects of angiotensin II (as also seen in the current study) and/or aldosterone on parathyroid tissue (8,14,24,29–31,39). These physiologic increases in PTH can further decrease urinary calcium excretion (by increasing reabsorption of filtered calcium) and will also increase synthesis of 1,25(OH)2D. The net effect of these pathways would facilitate a maintenance of or an increase in serum calcium. Thus, from an evolutionary perspective, the RAAS may have played an additional physiologic role: beyond maintaining volume homeostasis, it may have also played a role in calcium homeostasis.
Figure 2.
Teleologic proposals linking dietary sodium intake, RAAS activity, and calcium homeostasis. A: Limited dietary sodium intake. B: Ample/excess dietary sodium intake. Solid lines indicate established physiologic relationships. Dashed lines indicate proposed interactions suggested by recent evidence.
In contrast, states of dietary sodium excess (Fig. 2B), as is typical in most modern industrialized human societies (2), cause intravascular volume expansion, increased glomerular filtration, decreased RAAS activity, and increased urinary excretion of sodium and calcium (and potassium). Intravascular volume expansion may contribute to hemodilutional decreases in serum calcium, but, in addition, several studies have proposed that the suppression (or blocking) of angiotensin II and/or aldosterone may relatively blunt PTH secretion (8,24,29–31,39) and, consequently, 1,25(OH)2D synthesis. The net effect of these pathways would facilitate a decrease in serum calcium and an increase in calciuria and risk for kidney stones. Thus, in contrast to the evolutionary model in Fig. 2A, the modern high dietary sodium intake may introduce risks for hypertension (and other well-established sodium-related cardiovascular diseases) as well as a negative calcium balance and heightened risk for kidney stones.
It is worth speculating how our findings may inform our understanding of clinical scenarios such as primary aldosteronism and the use of loop diuretics. Prior studies have shown that individuals with primary aldosteronism have hypercalciuria (10), low-normal serum calcium levels, and secondary hyperparathyroidism (7–11) that normalize following targeted treatment of primary aldosteronism. The implicated mechanism for this hypercalciuria in primary aldosteronism has been presumed to be the excess interactions between aldosterone and the mineralocorticoid receptor in the kidney and/or the parathyroid glands (12). However, primary aldosteronism is a state of excess aldosterone and intravascular volume expansion; in this regard, our study findings show that calciuria can be substantially increased by expanding volume when dietary sodium intake is high, even though aldosterone levels are suppressed. Thus, the calciuria in primary aldosteronism may be influenced by aldosterone levels; however, the influence of intravascular volume expansion in primary aldosteronism may be substantial or even dominant. In contrast, loop diuretics can contract intravascular volume, stimulate the RAAS, increase calciuria, and are associated with higher PTH levels (40,41) and a higher risk for developing primary hyperparathyroidism (42). To what extent this heightened RAAS activity plays a role in PTH secretion when loop diuretics are used is uncertain but warrants further investigation since loop diuretics may increase the risk for adverse skeletal outcomes (43–45), and many patients use a combination of loop diuretics and RAAS inhibitors. Although our studies did not specifically investigate patients with primary aldosteronism or the influence of loop diuretics, these clinical scenarios pose interesting and complex variations of volume/sodium status, RAAS activity, and calcium–PTH physiology that need further evaluation in dedicated studies.
There are several limitations that warrant mentioning. The sample size of the patient-oriented study was modest; however, the study was well-controlled for most confounders, and as seen in Fig. 1, for most of the measured parameters, participants exhibited similar and consistent responses suggesting reproducibility and validation of the observed physiology. Participants in the patient-oriented study had diabetes; therefore, the results of this study may not be directly generalizable to other populations. However, for both of the latter 2 limitations, it is important to note that our current findings reproduced those of prior studies in nondiabetic participants (3–6,29), thereby suggesting that the demographic characteristics of the current study population may not be the only determinants of the results. Future studies should evaluate these complex interrelationships in other populations, especially those with primary aldosteronism and/or those using loop diuretics, since understanding the interactions between volume, RAAS, and calcium homeostasis in these scenarios may be unique and of clinical relevance. Although none of the participants in the patient-oriented study had a diagnosis of nephropathy or kidney disease, we did not measure urinary protein or circulating insulin levels and thus cannot comment on how these factors may have influenced the results. However, participants in the patient-oriented study had relatively mild diabetes and normal glomerular filtration rates. Further, there are many other factors (such as magnesium, fibroblast growth factor-23, leptin, and others) that we did not measure that may also play a role in the hormonal interactions we observed. We did not have ionized calcium values in the RES diet portion of our study, although correcting serum calcium for albumin is a reasonable and well-accepted alternative. The patient-oriented study protocol evaluated extreme and subacute (~1 week) dietary sodium modulations; the downstream changes induced by these sodium diets may not be as dramatic chronically. However, our epidemiology studies reflect population level associations between dietary sodium and calcium excretion (as a proxy for chronic sodium intake) and kidney stones and support prior work that showed dietary sodium restriction reduces the incidence of kidney stones (46). The patient-oriented study was not a true balance study; sequestering participants in a clinical research center for weeks to ensure complete balance in sodium, potassium, and calcium intake and output was not feasible. Instead, dietary instructions, as described, were provided to each participant by a professional research dietician. It is thus theoretically possible that some ad libitum intake by certain participants could have contributed to variability in urinary calcium excretion; however, as seen in Fig. 1, almost every participant responded similarly in terms of urinary and serum calcium changes, and greater urinary calcium excretion paralleled lower serum calcium. Further, we observed consistent relationships between higher urinary sodium and calcium excretion in both the patient-oriented and epidemiologic studies. While we cannot exclude the possibility of some variability in dietary intake beyond what we prescribed and/or some variability in the adequacy of urine collections, it is unlikely that variability in either of these factors were the sole determinant of our findings and more likely that changes in dietary sodium, volume, and other parameters were involved.
Summary
By using dietary sodium interventions, our findings help to disentangle the influences of volume and aldosterone on calcium and parathyroid homeostasis. We found that increased dietary sodium intake, which induces volume expansion and suppression of the RAAS, decreases serum calcium and increases calciuria. The consequences of this increased calciuria may be a substantially higher risk of developing kidney stones. Our findings provide mechanistic, evolutionary, and epidemiologic support for the recommendation to restrict dietary sodium intake for the prevention of kidney stones. Whether RAAS inhibition may represent another protective mechanism against nephrolithiasis warrants further study.
Acknowledgments
Financial Support: This work was supported in part by the following grants from the National Institutes of Health: R01 DK091417 (GC), R01 DK107407 (AV), R01 DK115392 (AV).
Additional Information
Disclosure Summary: AV reports consulting fees unrelated to the contents of this work from Corcept Therapeutics, HRA Pharma, Orphagen Pharmaceuticals, Selenity Therapeutics.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr Rev. 2018;39(6):1057–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heaney RP. Role of dietary sodium in osteoporosis. J Am Coll Nutr. 2006;25(3 Suppl):271S–276S. [DOI] [PubMed] [Google Scholar]
- 3. Muldowney FP, Freaney R, Moloney MF. Importance of dietary sodium in the hypercalciuria syndrome. Kidney Int. 1982;22(3):292–296. [DOI] [PubMed] [Google Scholar]
- 4. Kleeman CR, Bohannan J, Bernstein D, Ling S, Maxwell MH. Effect of variations in sodium intake on calcium excretion in normal humans. Proc Soc Exp Biol Med. 1964;115:29–32. [PubMed] [Google Scholar]
- 5. Massey LK, Whiting SJ. Dietary salt, urinary calcium, and kidney stone risk. Nutr Rev. 1995;53(5):131–139. [DOI] [PubMed] [Google Scholar]
- 6. Breslau NA, McGuire JL, Zerwekh JE, Pak CY. The role of dietary sodium on renal excretion and intestinal absorption of calcium and on vitamin D metabolism. J Clin Endocrinol Metab. 1982;55(2):369–373. [DOI] [PubMed] [Google Scholar]
- 7. Maniero C, Fassina A, Seccia TM, et al. . Mild hyperparathyroidism: a novel surgically correctable feature of primary aldosteronism. J Hypertens. 2012;30(2):390–395. [DOI] [PubMed] [Google Scholar]
- 8. Pilz S, Kienreich K, Drechsler C, et al. . Hyperparathyroidism in patients with primary aldosteronism: cross-sectional and interventional data from the GECOH study. J Clin Endocrinol Metab. 2012;97(1):E75–E79. [DOI] [PubMed] [Google Scholar]
- 9. Rossi E, Sani C, Perazzoli F, Casoli MC, Negro A, Dotti C. Alterations of calcium metabolism and of parathyroid function in primary aldosteronism, and their reversal by spironolactone or by surgical removal of aldosterone-producing adenomas. Am J Hypertens. 1995;8(9):884–893. [DOI] [PubMed] [Google Scholar]
- 10. Rossi GP, Ragazzo F, Seccia TM, et al. . Hyperparathyroidism can be useful in the identification of primary aldosteronism due to aldosterone-producing adenoma. Hypertension. 2012;60(2): 431–436. [DOI] [PubMed] [Google Scholar]
- 11. Asbach E, Bekeran M, König A, et al. . Primary and secondary hyperparathyroidism in patients with primary aldosteronism - findings from the German Conn’s registry. Exp Clin Endocrinol Diabetes. 2019. doi: 10.1055/a-1027-6472 [DOI] [PubMed] [Google Scholar]
- 12. Petramala L, Zinnamosca L, Settevendemmie A, et al. . Bone and mineral metabolism in patients with primary aldosteronism. Int J Endocrinol. 2014;2014:836529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fagugli RM, Taglioni C. Changes in the perceived epidemiology of primary hyperaldosteronism. Int J Hypertens. 2011;2011:162804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fischer E, Hannemann A, Rettig R, et al. . A high aldosterone to renin ratio is associated with high serum parathyroid hormone concentrations in the general population. J Clin Endocrinol Metab. 2014;99(3):965–971. [DOI] [PubMed] [Google Scholar]
- 15. Hagström E, Hellman P, Larsson TE, et al. . Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119(21):2765–2771. [DOI] [PubMed] [Google Scholar]
- 16. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8): 1243–1248. [DOI] [PubMed] [Google Scholar]
- 17. Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, März W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Eur Heart J. 2010;31(10):1237–1247. [DOI] [PubMed] [Google Scholar]
- 18. Ceccoli L, Ronconi V, Giovannini L, et al. . Bone health and aldosterone excess. Osteoporos Int. 2013;24(11):2801–2807. [DOI] [PubMed] [Google Scholar]
- 19. Salcuni AS, Palmieri S, Carnevale V, et al. . Bone involvement in aldosteronism. J Bone Miner Res. 2012;27(10):2217–2222. [DOI] [PubMed] [Google Scholar]
- 20. Salcuni AS, Carnevale V, Battista C, et al. . Primary aldosteronism as a cause of secondary osteoporosis. Eur J Endocrinol. 2017;177(5):431–437. [DOI] [PubMed] [Google Scholar]
- 21. Hostetter TH, Ibrahim HN. Aldosterone in chronic kidney and cardiac disease. J Am Soc Nephrol. 2003;14(9):2395– 2401. [DOI] [PubMed] [Google Scholar]
- 22. Ibrahim HN, Hostetter TH. Aldosterone in renal disease. Curr Opin Nephrol Hypertens. 2003;12(2):159–164. [DOI] [PubMed] [Google Scholar]
- 23. Wenzel U. Aldosterone and progression of renal disease. Curr Opin Nephrol Hypertens. 2008;17(1):44–50. [DOI] [PubMed] [Google Scholar]
- 24. Zaheer S, Taquechel K, Brown JM, Adler GK, Williams JS, Vaidya A. A randomized intervention study to evaluate the effect of calcitriol therapy on the renin-angiotensin system in diabetes. J Renin Angiotensin Aldosterone Syst. 2018;19(1): 1470320317754178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campbell NR, Correa-Rotter R, Cappuccio FP, et al. . Proposed nomenclature for salt intake and for reductions in dietary salt. J Clin Hypertens (Greenwich). 2015;17(4):247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int. 2008;73(4):489–496. [DOI] [PubMed] [Google Scholar]
- 27. Vaidya A, Sun B, Larson C, Forman JP, Williams JS. Vitamin D3 therapy corrects the tissue sensitivity to angiotensin ii akin to the action of a converting enzyme inhibitor in obese hypertensives: an interventional study. J Clin Endocrinol Metab. 2012;97(7):2456–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bao Y, Bertoia ML, Lenart EB, et al. . Origin, methods, and evolution of the three nurses’ health studies. Am J Public Health. 2016;106(9):1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown JM, Williams JS, Luther JM, et al. . Human interventions to characterize novel relationships between the renin-angiotensin-aldosterone system and parathyroid hormone. Hypertension. 2014;63(2):273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown J, de Boer IH, Robinson-Cohen C, et al. . Aldosterone, parathyroid hormone, and the use of renin-angiotensin-aldosterone system inhibitors: the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2015;100(2):490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown JM, Vaidya A. Interactions between adrenal-regulatory and calcium-regulatory hormones in human health. Curr Opin Endocrinol Diabetes Obes. 2014;21(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rossi GP. Hyperparathyroidism, arterial hypertension and aortic stiffness: a possible bidirectional link between the adrenal cortex and the parathyroid glands that causes vascular damage? Hypertens Res. 2011;34(3):286–288. [DOI] [PubMed] [Google Scholar]
- 33. Rossi GP, Prisco S. Does angiotensin II regulate parathyroid hormone secretion or not? Clin Endocrinol (Oxf). 2018;89(5):568–569. [DOI] [PubMed] [Google Scholar]
- 34. Tomaschitz A, Ritz E, Pieske B, et al. . Aldosterone and parathyroid hormone: a precarious couple for cardiovascular disease. Cardiovasc Res. 2012;94(1):10–19. [DOI] [PubMed] [Google Scholar]
- 35. Vaidya A, Brown JM, Williams JS. The renin-angiotensin-aldosterone system and calcium-regulatory hormones. J Hum Hypertens. 2015;29(9):515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maniero C, Fassina A, Guzzardo V, et al. . Primary hyperparathyroidism with concurrent primary aldosteronism. Hypertension. 2011;58(3):341–346. [DOI] [PubMed] [Google Scholar]
- 37. Kitazawa S, Fukase M, Kitazawa R, et al. . Immunohistologic evaluation of parathyroid hormone-related protein in human lung cancer and normal tissue with newly developed monoclonal antibody. Cancer. 1991;67(4):984–989. [DOI] [PubMed] [Google Scholar]
- 38. Gao X, Yamazaki Y, Tezuka Y, et al. . The crosstalk between aldosterone and calcium metabolism in primary aldosteronism: a possible calcium metabolism-associated aberrant “neoplastic” steroidogenesis in adrenals. J Steroid Biochem Mol Biol. 2019;193:105434. [DOI] [PubMed] [Google Scholar]
- 39. Lenzini L, Prisco S, Vanderriele PE, et al. . PTH modulation by aldosterone and angiotensin II is blunted in hyperaldosteronism and rescued by adrenalectomy. J Clin Endocrinol Metab. 2019;104(9):3726–3734. [DOI] [PubMed] [Google Scholar]
- 40. Zaheer S, de Boer I, Allison M, et al. . Parathyroid hormone and the use of diuretics and calcium-channel blockers: the multi-ethnic study of atherosclerosis. J Bone Miner Res. 2016;31(6):1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Isakova T, Anderson CA, Leonard MB, et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Group Diuretics, calciuria and secondary hyperparathyroidism in the Chronic Renal Insufficiency Cohort. Nephrol Dial Transplant. 2011;26(4):1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vaidya A, Curhan GC, Paik JM, Kronenberg H, Taylor EN. Hypertension, antihypertensive medications, and risk of incident primary hyperparathyroidism. J Clin Endocrinol Metab. 2015;100(6):2396–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carbone LD, Johnson KC, Bush AJ, et al. . Loop diuretic use and fracture in postmenopausal women: findings from the Women’s Health Initiative. Arch Intern Med. 2009;169(2):132–140. [DOI] [PubMed] [Google Scholar]
- 44. Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L. Loop diuretics increase bone turnover and decrease BMD in osteopenic postmenopausal women: results from a randomized controlled study with bumetanide. J Bone Miner Res. 2006;21(1):163–170. [DOI] [PubMed] [Google Scholar]
- 45. Bokrantz T, Schiöler L, Boström KB, et al. . Antihypertensive drug classes and the risk of hip fracture: results from the Swedish primary care cardiovascular database. J Hypertens. 2020;38(1):167–175. [DOI] [PubMed] [Google Scholar]
- 46. Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126(7):497–504. [DOI] [PubMed] [Google Scholar]