Abstract
Apoptotic cells form membrane blebs, but little is known about how the formation and dynamics of membrane blebs are regulated. The size of blebs gradually increases during the progression of apoptosis, eventually forming large extracellular vesicles called apoptotic bodies that have immune-modulating activities. In this study, we investigated the molecular mechanism involved in the differentiation of blebs into apoptotic blebs by comparing the dynamics of the bleb formed during cell migration and the bleb formed during apoptosis. We revealed that the enhanced activity of ROCK1 is required for the formation of small blebs in the early phase of apoptosis, which leads to the physical disruption of nuclear membrane and the degradation of Lamin A. In the late phase of apoptosis, the loss of asymmetry in phospholipids distribution caused the enlargement of blebs, which enabled translocation of damage-associated molecular patterns to the bleb cytoplasm and maturation of functional apoptotic blebs. Thus, changes in cell membrane dynamics are closely linked to cytoplasmic changes during apoptotic bleb formation.
INTRODUCTION
Various intracellular changes occur over the course of apoptosis. Molecular mechanisms of nuclear condensation, genome fragmentation, and exposure of phosphatidylserine (PS) to the outer leaflet of plasma membrane during apoptosis have been studied intensively (Nagata and Tanaka, 2017). The formation of plasma membrane blebs is an invariable characteristic of apoptosis but the knowledge of its molecular mechanism is limited (Charras, 2008). In the case of blebs that form during programmed necrosis, proteins that open pores in the cell membrane translocate to the plasma membrane where they enhance the permeability of the plasma membrane and cause the cell to rupture (Shi et al., 2015). However, it remains unclear how the interaction between the plasma membrane and the actin cytoskeleton is regulated to form apoptotic membrane blebs. In this study, we investigated the molecular mechanisms of bleb formed during apoptosis (apoptotic bleb).
In previous studies, caspase-3 was shown to be essential in the formation of blebs during apoptosis, as caspase–3-deficient cells cannot form blebs (Janicke et al., 1998; Zheng et al., 1998). Subsequently, it was shown that ROCK1 is cleaved and activated by caspase-3 during apoptosis (Coleman et al., 2001; Sebbagh et al., 2001). Activation of ROCK1 by caspase-3 is Rho-independent and induces bleb formation by up-regulating contractility of the actomyosin cortex. The up-regulation of the contractile force exerted by the actomyosin cortex increases the pressure inside the cell and leads to the detachment of the plasma membrane from the underlying actin cortex. The plasma membrane continues to expand as it detaches from the actin cortex, but the actin cortex is rapidly reassembled below the plasma membrane to halt bleb expansion and eventually cause it to contract. Such expansion and contraction of blebs facilitate the packaging of cellular debris into apoptotic blebs, which function as damage-associated molecular patterns (DAMPs) and promote the conversion of apoptotic blebs to apoptotic bodies (Caruso and Poon, 2018).
Membrane blebs are observed not only in apoptosis but also in cytokinesis and cell movement (Paluch and Raz, 2013). We previously identified molecular networks involved in the regulation of membrane blebs during cell migration (Aoki et al., 2016). In particular, we revealed that two small G proteins, Rnd3 and RhoA, are involved in the regulation of the actin cytoskeleton during bleb formation and regression (Ikenouchi and Aoki, 2017).
However, it remains unclear whether apoptotic membrane blebs are controlled by the same molecular mechanism as blebs associated with cell migration. There are clear differences in the dynamics of blebs that form during cell migration and during apoptosis. The blebs of freely moving cells are generally of constant size, whereas those that form during apoptosis progressively increase in size with the maturation of apoptosis and eventually develop into apoptotic blebs containing DAMPs (Tixeira et al., 2019). However, how bleb dynamics are altered during apoptosis and contribute to the progression of apoptosis remains unclear. In the present study, we addressed this issue by combining experimental observations and mathematical simulation.
RESULTS AND DISCUSSION
The size of apoptotic blebs changes with the progression of apoptosis
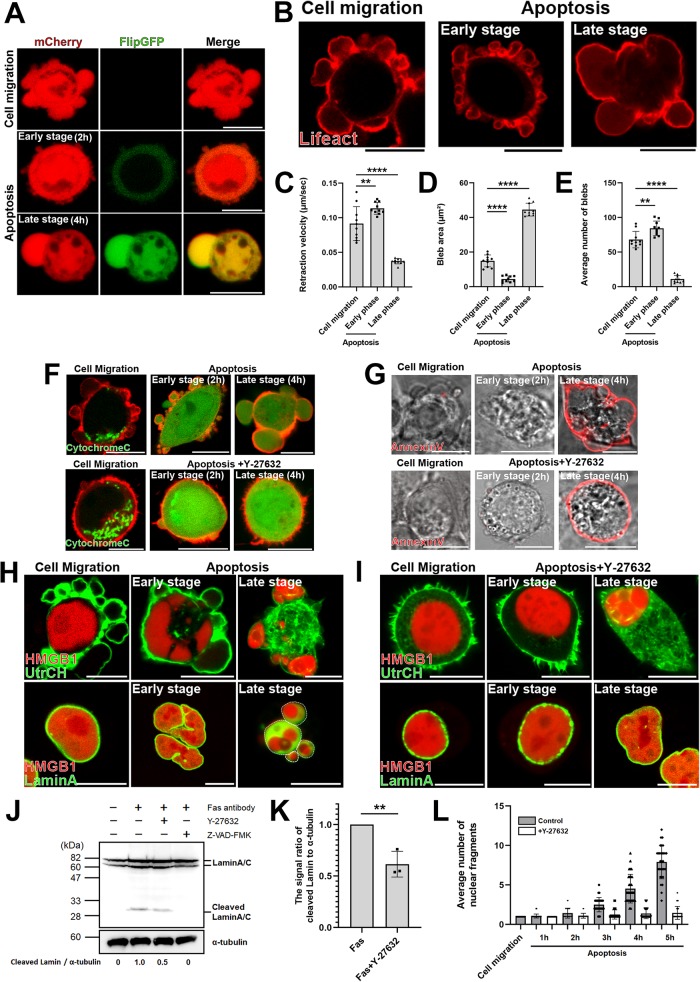
We previously reported that the human colon cancer-derived DLD1 cells form blebs and migrate freely when seeded on collagen gel. We first established DLD1 cells stably expressing GFP-based fluorogenic caspase-3 reporter, FlipGFP(casp3)-T2A-mCherry (Zhang et al., 2019), in order to demonstrate that treatment with anti-Fas antibody induces apoptosis of DLD1 cells. We confirmed that the activity of FlipGFP-based caspase-3 reporter was up-regulated only during apoptosis and not during cell migration (Figure 1A).
FIGURE 1:
The dynamic behavior of membrane blebs changes with the progression of apoptosis. (A) Migratory blebs and apoptotic blebs of DLD1 cells stably expressing FlipGFP(casp3)-T2A-mCherry, a GFP-based fluorogenic caspase-3 reporter. When DLD1 cells were seeded on Type I Collagen gel, they actively formed blebs and moved around freely (top). DLD1 cells were treated with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 2 h (Early stage) or 4 h (Late stage) to induce apoptosis (bottom). Results shown are representative of three independent experiments. Scale bar, 10 μm. (B) DLD1 cells were transfected with Lifeact-RFP. DLD1 cells actively formed blebs and moved around freely (left). DLD1 cells were treated with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 2 h (Early stage) or 4 h (Late stage) to induce apoptosis (right). Results shown are representative of five independent experiments. Scale bar, 10 μm. (C) The retraction velocities of membrane blebs in freely moving DLD1 cells and apoptotic DLD1 cells were quantified. The speed of the retraction phase of membrane blebs of apoptotic cells in the late stage was slower than that in early stage. Error bars indicate the SD calculated based on the values from N = 10 independent blebs. **P < 0.01, ****P < 0.0001 (one-way analysis of variance [ANOVA]). (D) The sizes of membrane blebs in freely moving DLD1 cells and apoptotic DLD1 cells were quantified. The size of membrane blebs of apoptotic cells in the late stage was significantly larger than that in early stage. Error bars are SD of N = 10 independent blebs. ****P < 0.0001 (one-way ANOVA). (E) The frequencies of membrane blebs in freely moving DLD1 cells and apoptotic DLD1 cells during 10 min were quantified. The number of blebs formed during 10 min in apoptotic cells in the late stage was significantly fewer than that in early stage. Error bars are SD of N = 10 independent blebs. **P < 0.01, ****P < 0.0001 (one-way ANOVA). (F) (Top panel) Membrane blebbing of DLD1 cells transfected with Cytochrome C-GFP and Lifeact–RFP from the early stage to the late stage of apoptosis. Cells were treated with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 2 h (Early stage) and 4 h (Late stage). Bottom panel: membrane blebbing of DLD1 cells transfected with Cytochrome C-GFP and Lifeact–RFP from the early stage to the late stage of apoptosis under ROCK inhibition. Cells were treated with 250 ng/ml anti-Fas antibody, 10 mg/ml cycloheximide, and 10 µM Y-27632 for 2 h (Early stage) and 4 h (Late stage). Results shown are representative of three independent experiments. Scale bar, 10 μm. (G) Top panel: Membrane blebbing of DLD1 cells stained with AnnexinV-Cy3 from the early stage to the late stage of apoptosis. Cells were treated with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 2 h (Early stage) and 4 h (Late stage). Bottom panel: membrane blebbing of DLD1 cells stained with AnnexinV-Cy3 from the early stage to the late stage of apoptosis under ROCK inhibition. Cells were treated with 250 ng/ml anti-Fas antibody, 10 mg/ml cycloheximide and 10 µM Y-27632 for 2 h (Early stage) and 4 h (Late stage). Results shown are representative of three independent experiments. Scale bar, 10 μm. (H) Top panel: membrane blebbing of DLD1 cells transfected with the calponin homology domain of utrophin (UtrCH)-GFP, a filamentous actin marker, and HMGB1-mScarlet from the early stage to the late stage of apoptosis. Cells were treated with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 2 h (Early stage) and 4 h (Late stage). Bottom panel: membrane blebbing of DLD1 cells transfected with LaminA–GFP and HMGB1-mScarlet from the early stage to the late stage of apoptosis. Cells were treated with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 2 h (Early stage) and 4 h (Late stage). White broken lines indicate margin of large blebs formed during the late phase of apoptosis. Results shown are representative of three independent experiments. Scale bar, 10 μm. (I) Top panel: membrane blebbing of DLD1 cells transfected with UtrCH-GFP and HMGB1-mScarlet from the early stage to the late stage of apoptosis under ROCK inhibition. Cells were treated with 250 ng/ml anti-Fas antibody, 10 mg/ml cycloheximide, and 10 µM Y-27632 for 2 h (Early stage) and 4 h (Late stage). Scale bar, 10 μm. Bottom panel: membrane blebbing of DLD1 cells transfected with LaminA-GFP and HMGB1-mScarlet from the early stage to the late stage of apoptosis under ROCK inhibition. Cells were treated with 250 ng/ml anti-Fas antibody, 10 mg/ml cycloheximide, and 10 µM Y-27632 for 2 h (Early stage) and 4 h (Late stage). Results shown are representative of three independent experiments. Scale bar, 10 μm. (J) DLD1 cells were left untreated or treated with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 4 h to induce apoptosis, either in the absence or in the presence of 10 µM Y-27632 or 50µM Z-VAD-FMK as indicated. Lysates were run on 10% SDS–PAGE and immunoblotted with anti-LaminA/C and α-tubulin antibodies. Cleaved LaminA/C and α-tubulin signals were quantified by densitometry to derive the ratio of cleaved LaminA/C to α-tubulin. The signal ratio of DLD1 cells treated with anti-Fas antibody and cycloheximide only (lane 2) was set to 1. Results shown are representative of three independent experiments. (K) The quantified means from N = 3 independent experiments of the signal ratio of cleaved LaminA/C to α-tubulin shown in J. Error bars indicate the SD. **P < 0.01 (Student’s t test). (L) Quantitative analysis of changes in the number of nuclear fragments labeled with HMGB1-mScarlet from the early stage to the late stage of apoptosis. Cells were treated with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide with or without 10 µM Y-27632 for 5 h. The number of HMGB1-mScarlet fragments were counted every hour. The SD was calculated based on the values from N = 30 independent cells.
Next, we compared the morphology of blebs between migrating cells and apoptotic cells. While blebs associated with cell movement form at a constant speed and size apoptosis, the bleb dynamics and bleb size of apoptosis-associated blebs change over time (Figure 1, B–D; Supplemental Figure S1A; Supplemental Movies S1–S3). Specifically, in early stage apoptosis, small blebs with rapid regression speed are formed, and in late stage apoptosis, large blebs with slow regression speed are formed (Figure 1, B and D). As a result, the number of blebs formed during the defined time period (10 min) is greater in the early stage than in the later stage of apoptosis (Figure 1E).
Movie S1.
Membrane blebbing in freely-moving DLD1 cells. DLD1 cells expressing Lifeact-RFP were imaged. Frames were taken every 5 s. (Scale bar, 10 μm.).
Movie S2.
Membrane blebbing in early-phase apoptotic DLD1 cells. DLD1 cells expressing Lifeact-RFP were treated with anti-Fas antibody (250 ng /mL) and cycloheximide (10 mg/mL) for 2 hours and imaged. The cells formed smaller blebs as compared to the freely-moving DLD1 cells. Frames were taken every 5 s. (Scale bar, 10 μm.).
Movie S3.
Membrane blebbing in late-phase apoptotic DLD1 cells. DLD1 cells expressing Lifeact-RFP were treated with anti-Fas antibody (250 ng /mL) and cycloheximide (10 mg/mL) for 4 hours and imaged. The retraction speed of membrane blebs was retarded in late-stage apoptotic cells. Frames were taken every 5 s. (Scale bar, 10 μm.).
Cells exhibit various changes over the course of apoptosis, such as the release of cytochrome c from mitochondria to the cytoplasm, the exposure of PS to the outer leaflet of plasma membrane, and the cytoplasmic release of nuclear proteins including High Mobility Group Box 1 (HMGB1) (Wickman et al., 2013). We first sought to determine whether the morphological changes of blebs are associated with specific intracellular changes. Bleb formation and cytochrome c release occur from the early stage of apoptosis (Figure 1F), whereas exposure of PS and release of HMGB1 into the cytoplasm are observed only in the late stage of apoptosis (Figure 1, G and H). Furthermore, in the late stage of apoptosis, the nuclear membrane disappears by the cleavage of Lamin A by caspases (Figure 1H) (Rao et al., 1996).These cellular changes are characteristic of apoptosis and are never observed in freely moving DLD1 cells (Figure 1, F and H).
What is the physiological significance of membrane blebbing in apoptosis? The addition of the ROCK inhibitor, Y-27632, to cells undergoing apoptosis suppresses the formation of membrane blebs (Tixeira et al., 2019). We found that suppression of bleb formation during apoptosis by treatment with the ROCK inhibitor did not affect the exposure of PS to the outer leaflet of plasma membrane (Figure 1G). However, interestingly, translocation of HMGB1 to the cytoplasm and formation of apoptotic body were completely suppressed (Figure 1, H and I).
Lamin A, one of the bona fide constituents of the nuclear envelope, is degraded by caspase during apoptosis. Therefore, we examined whether the suppression of bleb formation by ROCK1 inhibition during apoptosis affected the degradation of Lamin A. Lamin A was not degraded in migrating cells with blebs but was degraded in apoptotic cells as expected. Importantly, Y-27632 treatment significantly reduced the degradation of Lamin A compared with control apoptotic cells. This finding indicates that the caspase-mediated degradation of Lamin A and subsequent collapse of the nuclear envelope are enhanced as a direct consequence of increased acto-myosin contractility and membrane blebbing. (Figure 1, J and K). Thus, repeated expansion and retraction of blebs are required to package cytoplasmic components into cell membrane protrusions. In particular, in order for Lamin A present inside of the nuclear membrane to be degraded by caspase activated in the cytoplasm, the nuclear membrane needs to be physically disrupted. Considering that nuclear membrane disruption did not occur when treated with Y-27632 (Figure 1F), the formation of blebs in the early stages of apoptosis plays essential roles in the degradation of Lamin A and the formation of apoptotic blebs in the later stages of apoptosis by physically disrupting the nuclear membrane.
Localization of Rnd3 and RhoA in apoptotic blebs
The blebs that form at the onset of apoptosis is smaller than those that form during migration but increases as apoptosis progresses. The enlarged bleb contains substances such as HMGB1 and develops into apoptotic body. Then, how does the change in bleb dynamics mechanistically contribute to the intracellular changes during apoptosis?
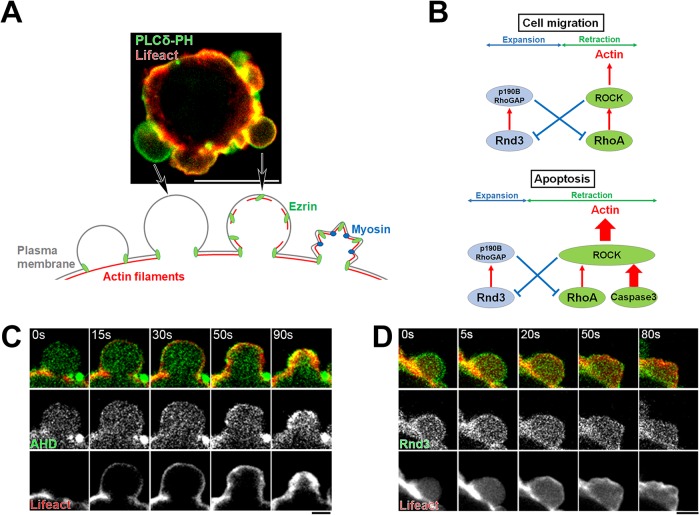
We previously analyzed the mechanism of bleb formation during cell migration and identified the involvement of two small GTPases, Rnd3 and RhoA (Figure 2A). On the one hand, Rnd3 localizes at the plasma membrane only during the expansion phase of bleb dynamics, where it inhibits RhoA through activation of p190-Rho-GAP (Wennerberg et al., 2003) (Supplemental Figure S1, B and D). On the other hand, RhoA localizes at the plasma membrane only during bleb retraction where it activates ROCK1 (Aoki et al., 2016) (Supplemental Figure S1, C and E). ROCK1 phosphorylates Rnd3, which results in the sequestration of Rnd3 in the cytoplasm via its binding to 14-3-3 protein (Riou et al., 2013). RhoA also plays a central role in the regulation of the actin cytoskeleton in bleb during retraction. First, during the retraction of membrane blebs, RhoA activates mDia-1, a member of formin family proteins, and induces massive regrowth of actin filaments (Bovellan et al., 2014). In addition, ROCK1 activated by RhoA phosphorylates Ezrin and phosphorylated Ezrin promotes the reassembly of the actin cortex by recruiting Eps8 to the plasma membrane (Aoki et al., 2016). Thus, RhoA mediates the rapid recovery of the actin cytoskeleton during the retraction phase. Taken together, we proposed that the reciprocal negative feedback mechanism between Rnd3 and RhoA acts as a master regulator of the reversal of membrane expansion to retraction during blebbing (Ikenouchi, 2016) (Figure 2B).
FIGURE 2:
Dynamics of membrane bleb during early stage apoptosis. (A) Membrane blebbing of DLD1 cells transfected with Lifeact–RFP and GFP-tagged PLCδ-PH domain. Arrows indicate representative examples of the expanding bleb and the retracting bleb. Scale bar, 10 μm. (B) Models describing the regulation of actin cortex by the interplay between Rnd3 and RhoA during membrane blebbing of migrating cells and apoptotic cells. (C) Membrane blebbing of DLD1 cells transfected with Lifeact–RFP and Anillin homology domain (AHD)-GFP, a biosensor for the detection of active RhoA, and treated with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 2 h. AHD-GFP was recruited to retracting membrane blebs where reassembly of the actin cortex occurred. Results shown are representative of three independent experiments. Scale bar, 2 μm. (D) Membrane blebbing of DLD1 cells transfected with Lifeact–RFP and Rnd3-GFP and treated with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 2 h. Rnd3 was recruited to expanding membrane blebs; however, this membrane localization was gradually lost on the initiation of retraction of bleb (t = 20 s). Results shown are representative of three independent experiments. Scale bar, 2 μm.
Therefore, we explored next whether the double-negative feedback mechanism between Rnd3 and RhoA is also involved in apoptotic blebbing. We examined the distribution of Rnd3 and RhoA in membrane blebs of apoptosis. We found that the changes in localization of Rnd3 and RhoA during the bleb cycle are common in both apoptotic blebs and blebs formed during cell migration, such that Rnd3 localizes to the plasma membrane in the expanding phase and active RhoA localizes to the plasma membrane in the retraction phase during apoptosis (Figure 2, C and D).
RhoA-independent constitutive activation of ROCK1 reduces bleb size
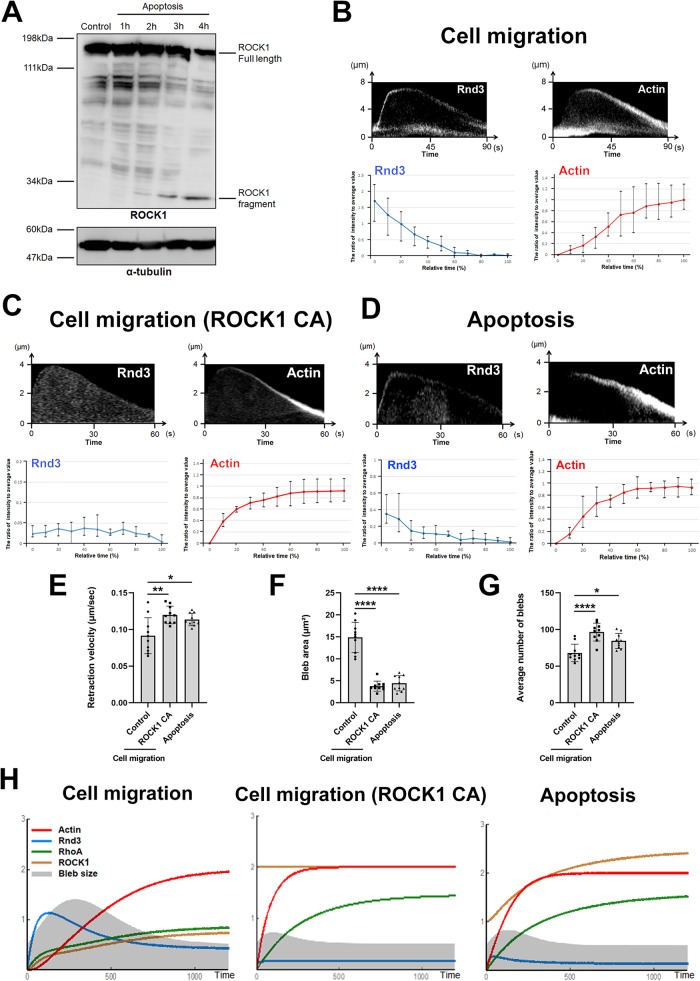
Then, what accounts for the difference in the dynamics of membrane blebs between cell migration and apoptosis? It was demonstrated that ROCK1 is cleaved by activated caspase-3 after induction of apoptosis and becomes constitutively active throughout the course of apoptosis (Coleman et al., 2001; Sebbagh et al., 2001). We confirmed that ROCK1 is cleaved at 2 h after induction of apoptosis by treatment with an anti-Fas antibody (Figure 3A). Thus, forced activation of ROCK1 by caspase-3 may account for the characteristics of bleb dynamics in the early stage of apoptosis.
FIGURE 3:
The mathematical simulation of bleb dynamics in the early phase of apoptosis. (A) Total cell lysates of DLD1 cells treated with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 1, 2, 3, and 4 h were resolved by SDS–PAGE and immunoblotted with anti-ROCK1 and α-tubulin antibodies. The 30-kDa band corresponding to the cleaved ROCK1 fragment was increased with the progression of apoptosis. Results shown are representative of three independent experiments. (B) (Top panel) Kymographs showing the localization of Rnd3 (left) and actin filaments (right) during bleb retraction in migrating DLD1 cells (Cell migration). Bleb extension is shown on the vertical axis, and time (sec) is shown on the horizontal axis. Results shown are representative of five independent experiments. Rnd3 was recruited to the plasma membrane of expanding membrane blebs where reassembly of the actin cortex did not occur. (Bottom panel) Quantitative analysis of changes in fluorescence intensity of GFP-Rnd3 or RFP-Lifeact in membrane blebs. For each of five independent cells, a single bleb was selected from a single cell, and the fluorescence intensity during bleb retraction was measured in a total of five independent blebs. The value obtained by subtracting the minimum fluorescence intensity (background) from the fluorescence intensity at each time point was divided by the average fluorescence intensity value to obtain the relative fluorescence intensity value at each time. (C) (Top panel) Kymographs showing the localization of Rnd3 (left) and actin filaments (right) during bleb retraction in freely moving DLD1 cells expressing ROCK1ΔC (constitutive active: CA). Bleb extension is shown on the vertical axis, and time (sec) is shown on the horizontal axis. Results shown are representative of five independent experiments. The localization of Rnd3 at the plasma membrane was impaired in DLD1 cells expressing ROCK1 CA. (Bottom panel) Quantitative analysis of changes in fluorescence intensity of GFP-Rnd3 or RFP-Lifeact in membrane blebs of freely moving DLD1 cells expressing ROCK1 CA. For each of five independent cells, a single bleb was selected from a single cell, and the fluorescence intensity during bleb retraction was measured in a total of five independent blebs. The value obtained by subtracting the minimum fluorescence intensity (background) from the fluorescence intensity at each time point was divided by the average fluorescence intensity value to obtain the relative fluorescence intensity value at each time. (D) (Top panel) Kymographs showing the localization of Rnd3 (left) and actin filaments (right) during bleb retraction in early phase apoptotic DLD1 cells during the early stage of apoptosis. Bleb extension is shown on the vertical axis, and time (sec) is shown on the horizontal axis. Results shown are representative of five independent experiments. The localization of Rnd3 at the plasma membrane was impaired in DLD1 cells in the early stage of apoptosis. (Bottom panel) Quantitative analysis of changes in fluorescence intensity of GFP-Rnd3 or RFP-Lifeact in membrane blebs during the early stage of apoptosis. For each of five independent cells, a single bleb was selected from a single cell, and the fluorescence intensity during bleb retraction was measured in a total of five independent blebs. The value obtained by subtracting the minimum fluorescence intensity (background) from the fluorescence intensity at each time point was divided by the average fluorescence intensity value to obtain the relative fluorescence intensity value at each time. (E) The retraction velocities of membrane blebs in control DLD1 cells, ROCK1 CA expressing DLD1 cells, and early phase apoptotic DLD1 cells were quantified. The SD was calculated based on the values from N = 10 independent blebs. *P < 0.05, **P < 0.01 (one-way ANOVA). (F) The sizes of membrane blebs in control DLD1 cells, ROCK1 CA overexpressing DLD1 cells, and early phase apoptotic DLD1 cells were quantified. The SD was calculated based on the values from N = 10 independent blebs. ****P < 0.0001 (one-way ANOVA). (G) The frequencies of membrane blebs in control DLD1 cells, ROCK1 CA expressing DLD1 cells, and early phase apoptotic DLD1 cells during 10 min were quantified. The SD was calculated based on the values from N = 10 independent cells. *P < 0.05, ****P < 0.0001 (one-way ANOVA). (H) Results of mathematical simulation of bleb dynamics in migrating DLD1 cell (left), ROCK1 CA expressing cell (middle), and early phase apoptotic cell (right). For the details of mathematical simulations, please see Supplemental Figure S2. The figures show the results of mathematical simulations for the cycle of expansion and retraction of bleb in each condition. The gray shape shows the change in size of the bleb over time. The red line shows the amount of actin in the plasma membrane, the blue line shows the amount of Rnd3 in the plasma membrane, the green line shows the amount of activated RhoA in the plasma membrane, and the yellow line shows the amount of activated ROCK1 in the plasma membrane. The unit of time axis (horizontal axis) and vertical axis is arbitrary.
We examined whether expression of the constitutively active form of ROCK1 is responsible for reduced bleb size at the early stage of apoptosis by experiments and mathematical simulations. The expression of a constitutive active ROCK1 mutant in nonapoptotic DLD1 cells caused the displacement of Rnd3 from the plasma membrane from the beginning of the expansion phase and accelerated the accumulation of actin filaments (Figure 3, B and C). As a result, bleb size and bleb retraction time decreased in DLD1 cells expressing constitutively active ROCK1 relative to control DLD1 cells, and bleb dynamics were instead similar to that in the early stage of apoptosis (Figure 3, D–G).
Next, we examined the mechanism by which ROCK1 activation caused the reduction of bleb size by mathematical simulation. We constructed a mathematical model of membrane blebs based on the experimentally identified molecular networks of membrane blebs in freely moving DLD1 cells, specifically focusing on the double-negative feedback loop between Rnd3 and RhoA (Supplemental Figure S2A). Parameters in the mathematical simulation were optimized based on measurement values obtained from experimental data (Supplemental Figure S2). We simulated the case where ROCK1 is active regardless of the Rnd3-RhoGAP pathway to reflect constitutive ROCK1 activation by caspase-3 during early stage apoptosis. Postulating ROCK1 activity at a consistently high level promotes actin reassembly, decreases the amount of Rnd3 at the plasma membrane, and causes the formation of consistently smaller blebs compared with the control simulation, in agreement with the experimental observation (Figure 3H).
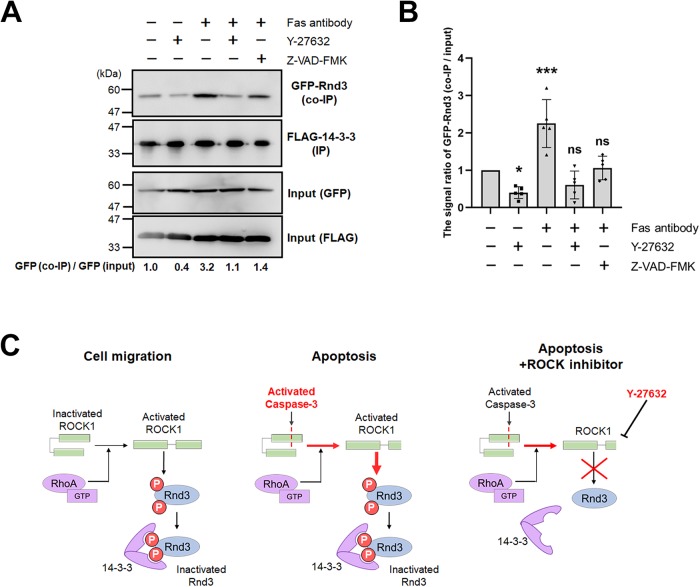
We next examined whether apoptosis-associated ROCK1 activation increases Rnd3 phosphorylation. It was previously reported that ROCK1-phosphorylated Rnd3 tightly binds to 14-3-3, resulting in the sequestration of Rnd3 in the cytoplasm. Given this background, we found that the amount of Rnd3 bound to 14-3-3 was higher during apoptosis than during cell motility and that the amount of Rnd3 bound to 14-3-3 during apoptosis was significantly reduced by Y-27632 treatment, indicating that Rnd3 was phosphorylated by ROCK1 during apoptosis (Figure 4, A and B). In summary, the feedback loop of Rnd3-RhoA also functions in the bleb dynamics during apoptosis just as with bleb-driven cell migration. However, parallel, RhoA-independent activation of ROCK1 by caspase-3 increased the phosphorylation level of Rnd3 in apoptotic cells relative to migratory cells, resulting in the enhanced removal of Rnd3 from the plasma membrane and the formation of small blebs (Figure 4C).
FIGURE 4:
ROCK1 regulates Rnd3 interaction with 14-3-3 during apoptosis. (A) DLD1 cells coexpressing FLAG-14-3-3β and GFP-Rnd3 were treated with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 3 h to induce apoptosis in conjunction with either 10 µM Y-27632 or 50 µM Z-VAD-FMK. Lysates were prepared and immunoprecipitated with anti-FLAG antibody-conjugated beads and the precipitates were probed with anti-GFP or anti-FLAG antibodies. GFP-Rnd3 (co-IP) and GFP-Rnd3 (input) signals were quantified by densitometry to obtain the ratio co-IP to input. The signal ratio of control cells was set to 1. Results shown are representative of five independent experiments. (B) The means of N = 5 independent experiments represented by A are shown with the SD. *P < 0.05, ***P < 0.001 (one-way ANOVA). (C) A model describing the regulation of Rnd3 activation by ROCK1 and 14-3-3 during apoptosis.
On the basis of these results, we conclude that the decrease in bleb size observed in the early phase of apoptosis is caused by constitutive activation of ROCK1 by caspase-3.
Bleb expansion is triggered by loss of plasma membrane phospholipid asymmetry
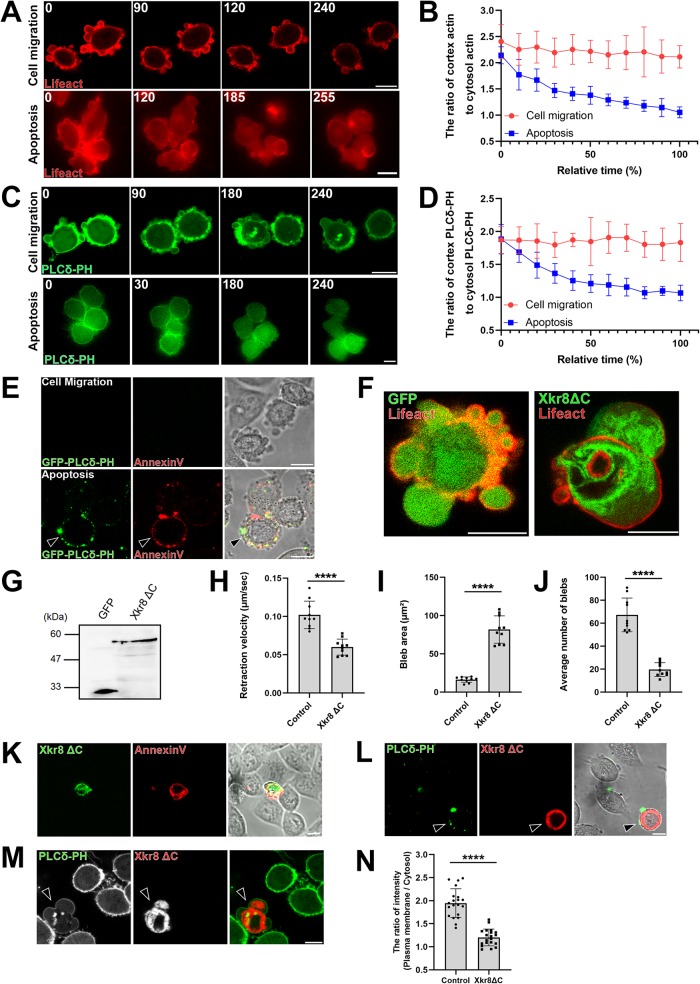
Blebs begin to incorporate proteins expelled from the nucleus, such as HMGB-1, at 4 h after the induction of apoptosis, and their size gradually increases. What accounts for the change in bleb size—and presumably the shift in bias from bleb retraction to bleb expansion—between the early and late stages of apoptosis? We quantified the fluorescence intensities of RFP-Lifeact and GFP-PLCδ-PH in blebs formed during cell migration and during apoptosis and analyzed change in the ratio between the two over time (Figure 5, A–D). The actin cortex and the level of phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) in the inner leaflet of plasma membrane decreased as apoptosis progressed.
FIGURE 5:
Bleb expansion is triggered by loss of plasma membrane phospholipid asymmetry. (A, C) Membrane blebbing of migrating (top panels) and apoptotic (bottom panels) DLD1 cells expressing either Lifeact–RFP (A) or PLCδ-PH-GFP (C). Apoptosis was induced by simultaneous treatment with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 3 h. Times indicated are relative to an arbitrary starting point for migratory cells and to the first image after treatment for apoptotic cells (min). Results shown are representative of five independent experiments. Scale bar, 10 μm. (B, D) Quantification of experiments depicted in A and C. Fluorescence intensities of either Lifeact-RFP (B) or PLCδ-PH-GFP (D) were measured at the cell cortex and the cytosol and their ratio were plotted against time. The SD was calculated based on the values from N = 5 independent experiments. (E) PtdIns(4,5)P2 and PS exposure to the outer leaflet of the plasma membrane during apoptosis. Cells were treated with 250 ng/ml anti-Fas antibody and 10 mg/ml cycloheximide for 4 h. Migrating and late-phase apoptotic cells were stained with purified GFP-tagged PLCδ-PH protein and AnnexinV-Cy3. Arrowhead shows the apoptotic cell stained with PLCδ-PH and AnnexinV. Of note, the attachment of lipid binding proteins such as PLCδ-PH or Annexin V to the outer leaflet of plasma membranes inhibits bleb expansion. Therefore, the morphology of blebs of cells stained with these proteins are different from that of untreated cells. Result shown is representative of three independent experiments. Scale bar, 10 μm. (F) Membrane blebbing of DLD1 cells transfected with GFP tag only and Lifeact- RFP (left) and membrane blebbing of DLD1 cells transfected with GFP-tagged Xkr8ΔC, a constitutively active form of Xkr8, and Lifeact-RFP (right). Result shown is representative of three independent experiments. Scale bar, 10 μm. (G) Total cell lysates of DLD1 cells expressing GFP tag only and GFP-tagged Xkr8ΔC separated by SDS–PAGE and immunoblotted with an anti-GFP mAb. (H) The retraction velocities of membrane blebs in freely moving DLD1 cells (Control) and freely moving DLD1 cells expressing Xkr8ΔC-GFP were quantified. The SD was calculated based on the values from N = 10 independent blebs. ****P < 0.0001 (Student’s t test). Expression of Xkr8ΔC-GFP led to the reduction in the speed of the retraction phase of membrane blebs. (I) The sizes of membrane blebs in freely moving DLD1 cells (Control) and freely moving DLD1 cells expressing Xkr8ΔC-GFP were quantified. The SD was calculated based on the values from N = 10 independent blebs. ****P < 0.0001 (Student’s t test). Expression of Xkr8ΔC-GFP led to the enlargement of bleb size. (J) The frequencies of membrane blebs in freely moving DLD1 cells (Control) and freely moving DLD1 cells expressing Xkr8ΔC-GFP during 10 min were quantified. The SD was calculated based on the values from N = 10 independent cells. ****P < 0.0001 (Student’s t test). (K) PS exposure in Xkr8ΔC expressing DLD1 cell. Cells were stained with AnnexinV-Cy3. Result shown is representative of three independent experiments. Scale bar, 10 μm. (L) DLD1 cells expressing RFP-tagged Xkr8ΔC were stained with purified GFP-tagged PLCδ-PH protein to detect PtdIns(4,5)P2 exposure to the outer leaflet of the plasma membrane (arrowheads). Result shown is representative of five independent experiments. Scale bar, 10 μm. (M) PtdIns(4,5)P2 localization at the inner leaflet of the plasma membrane was observed by stably expressing PLCδ-PH-GFP in DLD1 cells. Arrowhead indicates the Xkr8ΔC-RFP-coexpressing cell. Result shown is representative of five independent experiments. Scale bar, 10 μm. (N) The fluorescence intensity of the PLCδ-PH-GFP (inner leaflet) signal was quantified in wild-type DLD1 cells and Xkr8ΔC-RFP-expressing DLD1 cells. The SD was calculated based on the values from N = 20 independent cells. ****P < 0.0001 (Student’s t test).
The exposure of PS to the outer leaflet of the plasma membrane by lipid scramblases during late stage apoptosis contributes to recognition and clearance of apoptotic cells by phagocyte. Recently, it was revealed that activation of the lipid scramblase Xkr8 by caspase-3 is responsible for PS translocation during apoptosis (Suzuki et al., 2013).The activation of lipid scramblase causes the exposure not only of PS but also other phospholipid species present in the inner leaflet of the plasma membrane. PtdIns(4,5)P2 is an important component of the plasma membrane inner leaflet where it is essential for the polymerization of actin cytoskeleton and the proteins such as Ezrin that link the plasma membrane to the actin cytoskeleton (Janmey et al., 2018). Therefore, we considered the possibility that the decrease in actin cytoskeleton observed in the late stage of apoptosis may be caused by the decrease in PtdIns(4,5)P2 at the inner leaflet of the plasma membrane. Actually, live imaging of DLD1 cells expressing GFP-PLCδ-PH, which is known to bind to specifically (Varnai and Balla, 1998), indicated that the amount of PtdIns(4,5)P2 at the inner leaflet of plasma membrane was significantly reduced in the late stage of apoptosis as compared with the early stage (Figure 5, C and D).
We stained apoptotic DLD1 cells with the purified PtdIns(4,5)P2 probe GFP-PLCδ-PH to examine whether PtdIns(4,5)P2 is translocated to the outer leaflet of the plasma membrane during apoptosis. Phosphatidylserine and PtdIns(4,5)P2 are exposed at the outer leaflet of the plasma membrane in apoptotic cells. Accordingly, cells undergoing apoptosis, but not migratory cells, were prominently stained by Annexin-V and GFP-tagged PLCδ-PH domain protein and Annexin-V, specific probes for PS and PtdIns(4,5)P2, respectively (Figure 5E).
We next examined whether artificially decreasing PtdIns(4,5)P2 from the inner leaflet of plasma membrane could increase bleb size similar to late stage apoptosis. We overexpressed a constitutively active mutant of Xkr8 (Sakuragi et al., 2019) in freely moving DLD1 cells and examined the changes of bleb dynamics (Figure 5, F–K). In cells expressing the constitutively active form of Xkr8, the size of bleb was significantly enlarged (Figure 5I). We next examined whether activation of Xkr-8 by caspase-3 cleavage can expose PtdIns(4,5)P2 to the outer leaflet of the plasma membrane and decrease PtdIns(4,5)P2 in the inner leaflet of the plasma membrane. Cells overexpressing the dominant active form of Xkr-8 were broadly stained with GFP-PLCδ-PH, indicating that PtdIns(4,5)P2 is exposed to the outer leaflet of the plasma membrane in these cells (Figure 5L). Next, we established DLD1 cells stably expressing GFP-PLCδ-PH to visualize PtdIns(4,5)P2 in the inner leaflet of the plasma membrane. In these cells, expression of dominant active Xkr-8 reduced the signal derived from GFP-PLCδ-PH, indicating that PtdIns(4,5)P2 was reduced in the inner leaflet of the plasma membrane by expression of dominant active Xkr-8 (Figure 5, M and N). On the basis of these findings, we concluded that at the later stage of apoptosis, scrambling of phospholipids leads to the reduction of the amount of PtdIns(4,5)P2 present in the inner leaflet of plasma membrane, which reduces actin polymerization and the plasma membrane cross-linking activity of Ezrin during retracting phase, resulting in the formation of larger blebs. On the other hand, it was reported that actin molecules are degraded by caspases during apoptosis (Mashima et al., 1999). Taken together, the reduction of PtdIns(4,5)P2 in the inner leaflet of plasma membrane by scramblase activation and the degradation of actin by caspases cooperatively contribute to the destruction of the actin cortex and the enlargement of blebs during late stage apoptosis.
Concluding remarks
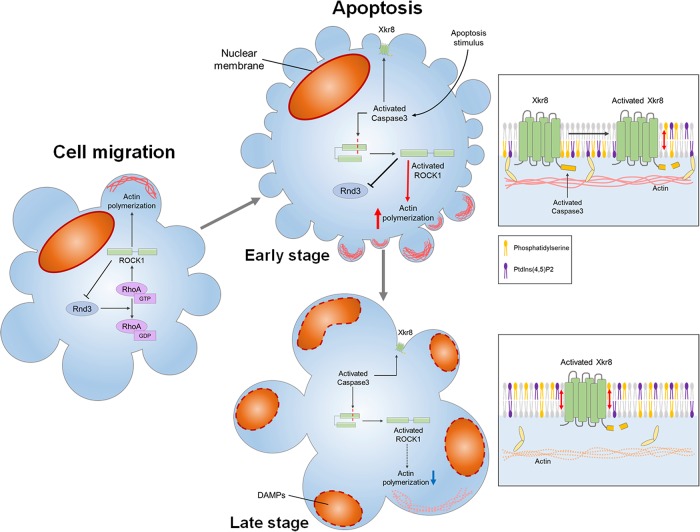
In this study, we analyzed the molecular mechanism of changes in bleb dynamics during apoptosis. We showed that Rnd3 and RhoA are localized to the apoptotic bleb, indicating that the double-negative feedback loop between Rnd3 and RhoA is a core machinery of bleb cycle in the apoptosis similar to the case of cell migration. Furthermore, we demonstrated that the changes of apoptotic blebs in the dynamics of bleb expansion and retraction are caused by constitutive activation of ROCK1 and Xkr8 by caspase-3.
In the apoptosis, membrane blebs function as concentration sites for phagocytic markers and help apoptotic cells to be detected and phagocytosed by phagocytes and/or neighboring cells (Segundo et al., 1999). By packing the cytoplasmic contents into apoptotic bodies, it prevents the dissemination of intracellular degradation products that can become autoantigens and avoids unnecessary immune responses (Schiller et al., 2008; Zirngibl et al., 2015). Furthermore, interleukin-1α or DAMPs such as HMGB1 in the apoptotic body are recently reported to modulate immune cell responses (Berda-Haddad et al., 2011; Schiller et al., 2013; Tucher et al., 2018). Thus, the importance of apoptotic blebs has become widely recognized (Caruso and Poon, 2018).
Apoptotic bleb contains substances necessary for communication with surrounding cells, not just cellular debris or by-products of apoptosis. We found that HMGB1 translocation to the cytoplasm of blebs was impaired in apoptotic cells in which bleb formation is suppressed by the treatment with Y-27632. We demonstrated that ROCK1 activation by caspase-3 was required for the induction of small blebs at the early phase of apoptosis and large bleb enough to contain substances such as HMGB1 was formed by the loss of asymmetric distribution of PtdIns(4,5)P2 during maturation of apoptotic body (Figure 6). Future studies need to clarify how changes in the cytoplasm and changes in membrane dynamics are coordinated in order to form functional apoptotic bleb containing various immune-modulating substances.
FIGURE 6:
The size of apoptotic blebs changes with the progression of apoptosis. In freely moving cells, bleb cycle is regulated by the double-negative feedback loop between Rnd3-p190RhoGAP and RhoA-ROCK1 (left). Rnd3-p190RhoGAP helps bleb growth by suppressing RhoA activation and actin polymerization during the expansion phase. On the other hand, RhoA-ROCK1 promotes actin reassembly during the retraction phase and also removes Rnd3 from the plasma membrane by phosphorylating Rnd3. During apoptosis, bleb cycle is also regulated this double-negative feedback loop between Rnd3-p190 RhoGAP and RhoA-ROCK1, but in the early stage of apoptosis, ROCK1 is activated by caspase-3 in a RhoA independent manner. Therefore, ROCK1 excludes Rnd3 from the plasma membrane and RhoA is more likely to be activated in the early stage of apoptosis, resulting in the acceleration of retraction of bleb and the reduction in the size of blebs. Caspase 3 also cleaves the C-terminal region of Xkr8, leading to constitutive activation of Xkr8. Owing to the phospholipid scrambling activity of Xkr8, PtdIns(4,5)P2 together with PS in the inner leaflet of the plasma membrane translocates to the outer leaflet of the plasma membrane. At the late stage of apoptosis, due to the phospholipid scrambling by Xkr8, the amount of PtdIns(4,5)P2 in the inner leaflet decreases. Reduction of the amount of PtdIns(4,5)P2 prevents the formation of the actin cortex and inhibits cross-linking activity of Ezrin, leading to the formation of larger bleb. Fragmented cellular components and substances such as DAMPs now can translocate to the bleb to form a functional apoptotic body.
MATERIALS AND METHODS
Reagents
DLD1 cells were purchased from the American Type Culture Collection. DLD1 cells were grown in DMEM supplemented with 10% (vol/vol) fetal calf serum. The following primary antibodies were used for immunoblotting: mouse anti-ROCK1 antibody mAb (Abcam), mouse anti–α-tubulin mAb (Sigma), and rabbit anti-Lamin A/C antibody pAb (Cell Signaling Technology). cDNAs encoding full-length human Rnd3, human Anillin C terminus, human Xkr8ΔC, human Lamin A, and human 14-3-3β were amplified by RT-PCR, fused to the sequence encoding enhanced GFP or FLAG tag, and ligated into the pCAGGS-neo vector. pEGFP-CytochromeC and pcDNA3-FlipGFP (Casp3 cleavage seq) T2A mCherry were obtained from Addgene (No. 41181 and No. 124428).
Live imaging
All fluorescence imaging was performed using a 63× oil-immersion objective on an inverted microscope (LSM700; Carl Zeiss Micro Imaging) interfaced to a laser-scanning confocal microscope equipped with a heating stage heated to 37°C. Phosphatidylserine exposed on the outer leaflet of the plasma membrane is stained by using Annexin V-Cy3 Apoptosis Detection Kit (Biovision). Images were captured on a device camera and acquired on a PC using ZEN2012 software (LSM700; Carl Zeiss MicroImaging). Images were acquired at 488 nm for GFP-tagged proteins or at 555 nm for RFP-tagged proteins. Each imaging video frame is an 8-bit grayscale image, and the frame interval is indicated in the figure legends. The movie captures a single cell.
Immunoblotting
Cells were treated with anti-Fas antibody (SY-001) purchased from MBL (Nagoya, Japan) (250 ng/ml) and cycloheximide was purchased from Sigma (St Louis, MO) (10 mg/ml) to induce apoptosis. To inhibit ROCK or pan-caspase activity during apoptosis, cells were also treated with ROCK inhibitor Y-27632 (10 µM) or caspase inhibitor Z-VAD-FMK (50 µM) purchased from MBL (Nagoya, Japan). Cells were washed with phosphate-buffered saline (PBS) and dissolved in SDS sample buffer. Aliquots of the cell lysate were immunoblotted with anti-ROCK1 antibody, anti-Lamin A/C antibody, and anti–α-tubulin antibody. The signal intensity was quantified by using ImageJ.
Immunoprecipitation
Cells were treated with 250 ng/ml anti-Fas antibody (SY-001) purchased from MBL (Nagoya, Japan) and 10 mg/ml cycloheximide purchased from Sigma (St Louis, MO) to induce apoptosis. To inhibit ROCK or pan-caspase activity during apoptosis, cells were also treated with 10 µM Y-27632 or 50 µM Z-VAD-FMK purchased from MBL (Nagoya, Japan). Cells were washed with PBS and lysed with IP buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, and protease inhibitors). Lysates were incubated with 10 μl of anti-DYKDDDDK mAb beads (Wako Pure Chemical Industries) for 2 h. Beads were washed with IP buffer and bound proteins were eluted in SDS sample buffer. Aliquots of the lysate and eluate were immunoblotted with anti-FLAG antibody and anti-GFP antibody. The signal intensity was quantified by using ImageJ.
Visualization and quantitative analysis of membrane bleb dynamics
We previously described the method of image analysis for visualization of membrane bleb dynamics in detail (Aoki et al., 2016). A tricolor map visualizes the membrane bleb dynamics more directly (Supplemental Figure S1). It is a (θ, t)-visualization and the angle θ corresponds to the cell contour point in the direction θ from the center of the gravity of the cell. If the contour point lies on an expanding (retracting) bleb at t, the pixel (θ, t) is printed in red (blue) in the tricolor map. Otherwise, that is, if the contour point is rather stationary, the pixel is printed in white.
The intensity of fluorescent protein was quantified using Fiji/ImageJ.
Supplementary Material
Acknowledgments
We thank all members of the Ikenouchi laboratory and Iwasa laboratory (Department of Biology, Faculty of Sciences, Kyushu University) for helpful discussions. This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (JP17H06012 [J.I.], JP16H06280 [S.U.], 17K19402 [S.U.], 17J00242 [K.A.], 16J01030 [S.S.], and 15H004423 [Y.I.]), AMED-PRIME (15664862), grants from the MSD Life Science Foundation and Takeda Science Foundation, and a JSPS Research Fellowship for Young Scientists (DC1) (K.A. and S.S.).
Abbreviations used:
- DAMP
damage-associated molecular pattern
- HMGB1
High Mobility Group Box 1
- PBS
phosphate-buffered saline
- PS
phosphatidylserine
- PtdIns(4,5)P2
phosphatidylinositol 4,5-bisphosphate.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E19-12-0691) on February 12, 2020.
REFERENCE
- Aoki K, Maeda F, Nagasako T, Mochizuki Y, Uchida S, Ikenouchi J. (2016). A RhoA and Rnd3 cycle regulates actin reassembly during membrane blebbing. Proc Natl Acad Sci USA , E1863–E1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berda-Haddad Y, Robert S, Salers P, Zekraoui L, Farnarier C, Dinarello CA, Dignat-George F, Kaplanski G. (2011). Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1alpha. Proc Natl Acad Sci USA , 20684–20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovellan M, Romeo Y, Biro M, Boden A, Chugh P, Yonis A, Vaghela M, Fritzsche M, Moulding D, Thorogate R, et al (2014). Cellular control of cortical actin nucleation. Curr Biol , 1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso S, Poon IKH. (2018). Apoptotic Cell-derived extracellular vesicles: more than just debris. Front Immunol , 1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charras GT. (2008). A short history of blebbing. J Microsc , 466–478. [DOI] [PubMed] [Google Scholar]
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. (2001). Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol , 339–345. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J. (2016). How do cells sense actin cortex-free membrane? Cell Cycle , 2687–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J, Aoki K. (2017). Membrane bleb: A seesaw game of two small GTPases. Small GTPases , 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke RU, Ng P, Sprengart ML, Porter AG. (1998). Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J Biol Chem , 15540–15545. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Bucki R, Radhakrishnan R. (2018). Regulation of actin assembly by PI(4,5)P2 and other inositol phospholipids: An update on possible mechanisms. Biochem Biophys Res Commun , 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima T, Naito M, Tsuruo T. (1999). Caspase-mediated cleavage of cytoskeletal actin plays a positive role in the process of morphological apoptosis. Oncogene , 2423–2430. [DOI] [PubMed] [Google Scholar]
- Nagata S, Tanaka M. (2017). Programmed cell death and the immune system. Nat Rev Immunol , 333–340. [DOI] [PubMed] [Google Scholar]
- Paluch EK, Raz E. (2013). The role and regulation of blebs in cell migration. Curr Opin Cell Biol , 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L, Perez D, White E. (1996). Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol , 1441–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou P, Kjaer S, Garg R, Purkiss A, George R, Cain RJ, Bineva G, Reymond N, McColl B, Thompson AJ, et al (2013). 14-3-3 proteins interact with a hybrid prenyl-phosphorylation motif to inhibit G proteins. Cell , 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuragi T, Kosako H, Nagata S. (2019). Phosphorylation-mediated activation of mouse Xkr8 scramblase for phosphatidylserine exposure. Proc Natl Acad Sci USA , 2907–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller M, Bekeredjian-Ding I, Heyder P, Blank N, Ho AD, Lorenz HM. (2008). Autoantigens are translocated into small apoptotic bodies during early stages of apoptosis. Cell Death Differ , 183–191. [DOI] [PubMed] [Google Scholar]
- Schiller M, Heyder P, Ziegler S, Niessen A, Classen L, Lauffer A, Lorenz HM. (2013). During apoptosis HMGB1 is translocated into apoptotic cell-derived membranous vesicles. Autoimmunity , 342–346. [DOI] [PubMed] [Google Scholar]
- Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. (2001). Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol , 346–352. [DOI] [PubMed] [Google Scholar]
- Segundo C, Medina F, Rodriguez C, Martinez-Palencia R, Leyva-Cobian F, Brieva JA. (1999). Surface molecule loss and bleb formation by human germinal center B cells undergoing apoptosis: role of apoptotic blebs in monocyte chemotaxis. Blood , 1012–1020. [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature , 660–665. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S. (2013). Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science , 403–406. [DOI] [PubMed] [Google Scholar]
- Tixeira R, Phan TK, Caruso S, Shi B, Atkin-Smith GK, Nedeva C, Chow JDY, Puthalakath H, Hulett MD, Herold MJ, Poon IKH. (2019). ROCK1 but not LIMK1 or PAK2 is a key regulator of apoptotic membrane blebbing and cell disassembly. Cell Death Differ , 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucher C, Bode K, Schiller P, Classen L, Birr C, Souto-Carneiro MM, Blank N, Lorenz HM, Schiller M. (2018). Extracellular vesicle subtypes released from activated or apoptotic T-lymphocytes carry a specific and stimulus-dependent protein cargo. Front Immunol , 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P, Balla T. (1998). Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol , 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg K, Forget MA, Ellerbroek SM, Arthur WT, Burridge K, Settleman J, Der CJ, Hansen SH. (2003). Rnd proteins function as RhoA antagonists by activating p190 RhoGAP. Curr Biol , 1106–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman GR, Julian L, Mardilovich K, Schumacher S, Munro J, Rath N, Zander SA, Mleczak A, Sumpton D, Morrice N, et al (2013). Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ , 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Schepis A, Huang H, Yang J, Ma W, Torra J, Zhang SQ, Yang L, Wu H, Nonell S, et al (2019). Designing a green fluorogenic protease reporter by flipping a beta strand of GFP for imaging apoptosis in animals. J Am Chem Soc , 4526–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng TS, Schlosser SF, Dao T, Hingorani R, Crispe IN, Boyer JL, Flavell RA. (1998). Caspase-3 controls both cytoplasmic and nuclear events associated with Fas-mediated apoptosis in vivo. Proc Natl Acad Sci USA , 13618–13623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirngibl M, Furnrohr BG, Janko C, Munoz LE, Voll RE, Gregory CD, Schett G, Herrmann M. (2015). Loading of nuclear autoantigens prototypically recognized by systemic lupus erythematosus sera into late apoptotic vesicles requires intact microtubules and myosin light chain kinase activity. Clin Exp Immunol , 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.