Abstract
Centrioles must be eliminated or inactivated from the oocyte to ensure that only the two functional centrioles contributed by the sperm are present in the zygote. Such removal can occur during oogenesis, as in Drosophila, where departure of Polo kinase from centrosomes leads to loss of microtubule nucleating activity and centriole removal. In other species, oocyte-derived centrioles are removed around the time of fertilization through incompletely understood mechanisms. Here, we use confocal imaging of live starfish oocytes and zygotes expressing markers of microtubule nucleating activity and centrioles to investigate this question. We first assay the role of Polo-like kinase 1 (Plk1) in centriole elimination. We find that although Plk1 localizes around oocyte-derived centrioles, kinase impairment with BI-2536 does not protect centrioles from removal in the bat star Patiria miniata. Moreover, we uncover that all four oocyte-derived centrioles lose microtubule nucleating activity when retained experimentally in the zygote of the radiate star Asterias forbesi. Interestingly, two such centrioles nevertheless retain the centriolar markers mEGFP::PACT and pmPoc1::mEGFP. Together, these findings indicate that centrioles can persist when Plk1 activity is impaired, as well as when microtubule nucleating activity is lacking, uncovering further diversity in the mechanisms governing centriole removal.
INTRODUCTION
Centrioles are cylindrical microtubule-based organelles fundamental notably for recruiting the pericentriolar material (PCM), and thus for forming the centrosome, an important microtubule-organizing center (MTOC) of animal cells (reviewed in Bornens, 2012; Gönczy and Hatzopoulos, 2019). Control of centriole inheritance at fertilization is critical to ensure bipolar spindle formation and faithful chromosome segregation during the first cell division of metazoan organisms. In most species, maternal centrioles are eliminated or inactivated from the oocyte, and two paternal centrioles are contributed to the zygote by the sperm (reviewed in Delattre and Gönczy, 2004; Manandhar et al., 2005). The mechanisms by which maternal centrioles are removed are incompletely understood.
Most proliferating animal cells are born with two centrioles, each of which then seeds assembly of one procentriole that remains engaged in the vicinity of the centriole until mitosis. At that time, the two centrosomes, each containing a centriole/procentriole pair, act as MTOCs and direct bipolar spindle formation. The centriole and procentriole within each centrosome then disengage from one another during mitosis, so that each resulting cell will be endowed with two centrioles. These two centrioles are structurally distinct, with the older centriole, also called the mother centriole, harboring appendages, which are absent from the younger, or daughter, centriole (reviewed in Firat-Karalar and Stearns, 2014; Loncarek and Bettencourt-Dias, 2018; Gönczy and Hatzopoulos, 2019).
There are exceptions to the above canonical cycle of centriole inheritance. One such interesting case is encountered at fertilization (reviewed in Delattre and Gönczy, 2004; Manandhar et al., 2005). If each gamete contributed a pair of centrioles, then the zygote would be endowed with four centrioles, which would each duplicate during the first cell cycle, leading to the presence of four centrosomes and potentially tetrapolar spindle formation. In most metazoan species, including Drosophila and human beings, this problem is solved through the removal of centrioles during oogenesis (Mahowald and Strassheim, 1970; Szollosi et al., 1972; Gard, 1994; Sathananthan et al., 2001; Mikeladze-Dvali et al., 2012). In other organisms, such as starfish, centrioles remain in the oocyte and direct assembly of the two meiotic spindles before being removed (Nakashima and Kato, 2001; Shirato et al., 2006; Crowder et al., 2015). In either scenario, the zygote inherits only the two functional centrioles contributed by the sperm. While this is a fundamental feature of metazoan development, how centrioles are removed from the female gamete remains generally poorly understood.
One exception to such lack of understanding is in Drosophila. Here, regulation of the PCM by the kinase Polo is key for eliminating centrioles during oogenesis (Pimenta-Marques et al., 2016). Polo localizes to centrosomes during early oogenesis but is no longer detectable at that location as oocytes mature; this is followed by PCM disassembly, loss of microtubule nucleating activity, and then centriole elimination. Moreover, RNAi-mediated depletion of Polo results in precocious PCM disassembly and centriole elimination. Conversely, targeting excess Polo to centrioles enables PCM maintenance and prevents centriole elimination. Such persisting centrioles act as MTOCs and result in abnormal meiosis and abortive zygotic development (Pimenta-Marques et al., 2016). The extent to which the contribution of Polo uncovered in flies will prove general is unclear, including in systems where maternal centrioles serve to assemble the two meiotic spindles and are eliminated or inactivated solely thereafter.
Starfish constitute an attractive model to analyze centriole removal from the oocyte, since in this system the process takes place in a stereotyped manner after oocyte maturation. In the bat star Patiria miniata, two of the four centrioles present in the mature oocyte are shed in the first polar body, whereas a further one is expelled with the second polar body, leaving a single centriole in the zygote (Figure 1, A and B). This remaining centriole is eliminated shortly after meiosis II completion. Monitoring of fluorescent fusion proteins specific for either mother or daughter centriole established that this last remaining unit is a daughter centriole (Borrego-Pinto et al., 2016a). Moreover, mother and daughter centrioles show distinct behavior when experimentally retained in the P. miniata zygote through inhibition of cytokinesis with an actin-depolymerizing drug. The two oocyte-derived mother centrioles nucleate microtubules and persist, which, together with the two sperm-derived centrioles, leads to the formation of a tetrapolar spindle during the first mitosis. In contrast, daughter centrioles do not act as MTOCs and are eliminated. Intriguingly, an analysis in the radiate star Asterias forbesi suggested an apparent difference from P. miniata, since all four centrioles experimentally retained in the A. forbesi zygote fail to sustain microtubule nucleation, as observed by polarization microscopy (Sluder et al., 1989, 1993). In the absence of molecular markers, however, it is not clear how this apparent difference relates to the fate of centrioles.
FIGURE 1:
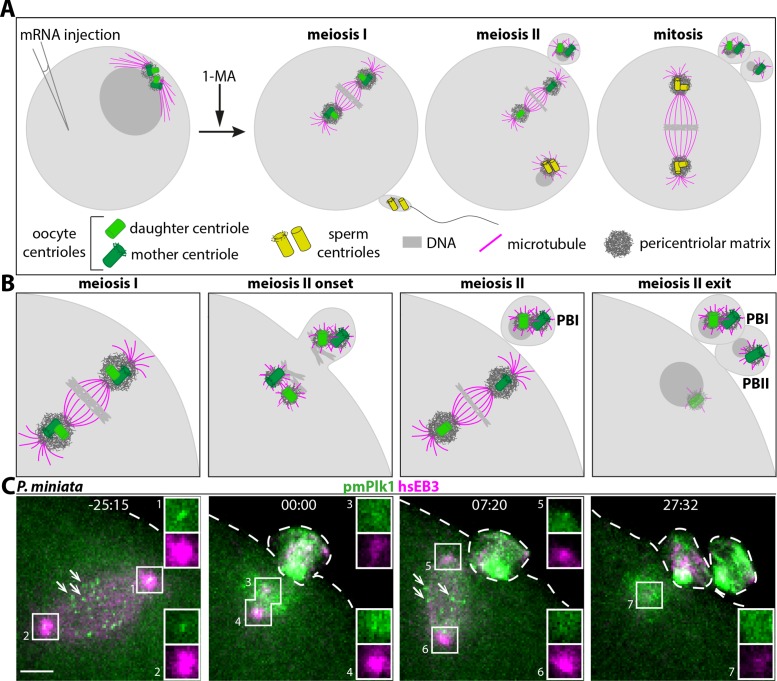
Centriole behavior and Plk1 distribution in P. miniata oocytes. (A, B) Schematic of centriole fate during meiotic divisions of P. miniata starfish oocyte, together with overview of experimental procedure utilized in this work, not to scale; B shows higher magnification views of the region with meiotic spindles. Arrested oocytes are injected with mRNA(s) coding for the protein(s) of interest; meiotic resumption is induced by 1-methyladenine (1-MA), followed by fertilization, depending on the experiment, and then by confocal time-lapse microscopy. During the first meiotic division, pairs of centrioles, each containing a mother centriole (dark green, bearing appendages) and a daughter centriole (light green), are present at the poles of the spindle (B, meiosis I, metaphase represented). First polar body (PBI) extrusion results in the removal of 2n DNA and of a pair of centrioles from the oocyte (B, meiosis II onset). The two remaining centrioles then disengage from one another and their surrounding PCM drives the formation of the meiosis II spindle (B, meiosis II, metaphase represented). The mother centriole is invariably positioned toward the plasma membrane and thus is extruded in the second polar body (PBII), together with 1n DNA (B, meiosis II exit). The remaining daughter centriole then loses MTOC activity and is eliminated (depicted as fading away in meiosis II exit panel). Fertilization results in the sperm contributing 1n DNA and a pair of centrioles (yellow) to the zygote. Sperm-derived centrioles then duplicate, leading to two centriole pairs that recruit PCM (dark gray) and govern bipolar spindle formation during the first mitosis (A, mitosis). (C) Still images from dual-color time-lapse confocal microscopy of P. miniata oocyte expressing mRNAs encoding the microtubule marker hsEB3::mCherry3 (in magenta throughout the paper) and mEGFP::pmPlk1 (green), which localizes at centrioles (insets) and kinetochores (arrows point to three of them at metaphase of meiosis I and II). Here and in other figures, images are maximum-intensity projections of selected z-planes spanning the region of interest, and insets are 1.4×-magnified single z-plane of the boxed regions; the oocyte plasma membrane is indicated with a dashed line. Moreover, unless stated otherwise, time is indicated in minutes:seconds starting from centriole disengagement at meiosis II onset and scale bars are 5 µm.
RESULTS AND DISCUSSION
Plk1 localizes at centrioles in starfish oocytes
We sought to investigate whether Polo-like kinase 1 (Plk1) can protect centrioles from elimination in starfish, in a manner analogous to the role played by its homologue Polo in Drosophila. First, we determined whether Plk1 localizes to centrioles in P. miniata oocytes. To this end, we generated mRNAs coding for mEGFP tagged P. miniata and H. sapiens Plk1 proteins. Here and thereafter, oocytes were injected with in vitro transcribed mRNAs, in this case encoding mEGFP::pmPlk1, as well as the microtubule-associated protein hsEB3::mCherry3 to monitor growing microtubules and thus MTOC activity. After overnight incubation to allow translation of the injected mRNAs, oocytes were matured with 1-methyladenine (1-MA; Kanatani et al., 1969), leading to resumption of cell cycle progression, nuclear envelope breakdown (NEBD) and then execution of the two meiotic divisions, which were filmed using time-lapse confocal microscopy (Figure 1, A and B). The number of oocytes analyzed for each experiment and a summary of the results are provided in Supplemental Table S1.
As shown in Figure 1C, we found that mEGFP::pmPlk1 localizes to the two poles of the meiosis I spindle in P. miniata oocytes (-25:15, insets 1 and 2). At the onset of meiosis II, the signal at the inner pole of the meiosis I spindle splits into two foci (00:00, insets 3 and 4), which then localize to the two poles of the meiosis II spindle (07:20, insets 5 and 6). We noted a difference in fluorescence intensity between these two mEGFP::pmPlk1 foci, with a brighter signal for the outer focus, closer to the plasma membrane (07:20; compare inset 5 with inset 6). In addition, we found that the inner focus of mEGFP::pmPlk1 is no longer detected shortly after extrusion of the second polar body (27:32, inset 7). Similar dynamics was observed for the human hsPlk1:mEGFP fusion protein (Supplemental Figure S1A). Such distributions mirror those reported for pancentriolar components in P. miniata oocytes (Borrego-Pinto et al., 2016a), indicating that Plk1 localizes initially to all centrioles or their immediate vicinity, albeit to different degrees depending on the centriole in question. Moreover, we observed that both mEGFP::pmPlk1 and hsPlk1::mEGFP also label what appears to be kinetochores (Figure 1C, –25:15 and 07:20; Supplemental Figure S1A, –19:34 and 16:00; arrows), in line with the distributions reported in other systems for this kinase family (reviewed in Archambault and Glover, 2009). We found analogous localizations at spindle poles and kinetochores when examining hsPlk1::mEGFP in A. forbesi oocytes (Supplemental Figure S1B).
Overall, we conclude that Plk1 is present initially at all four oocyte-derived centrioles in starfish oocytes, with more protein detected at mother centrioles, and is then lost from daughter centrioles before MTOC activity ceases.
Plk1 activity does not protect centrioles from elimination in P. miniata oocytes
We set out to test whether Plk1 protects centrioles from elimination in P. miniata oocytes. Plk1 is required for bipolar spindle formation in a wide range of systems, including in the starfish Asterina pectinifera, where function-blocking antibodies result in monopolar spindle assembly during meiosis I (Okano-Uchida et al., 2003). However, the fate of centrioles was not addressed in that study. Here, analyzing oocytes expressing hsEB3::mCherry3 and the pancentriolar marker pmCentrin2::mEGFP, we established that treatment with 10 µM of the Plk1 inhibitor BI-2536 (Lénárt et al., 2007) at the time of 1-MA addition prevents bipolar spindle assembly during meiosis I (compare Figure 2A with Figure 2B). Instead of a bipolar spindle (Figure 2A), a diffuse crown of microtubules forms transiently around centrioles (Figure 2B). Thereafter, centrioles in drug-treated specimens typically move deeper into the oocyte proper and lose microtubule nucleation activity (Figure 2B 01:36:04; compare with Figure 2A, 01:40:55), although sometimes hsEB3::mCh3 can be present around one centriole at later times (Figure 2B, 02:02:52, inset 9).
FIGURE 2:
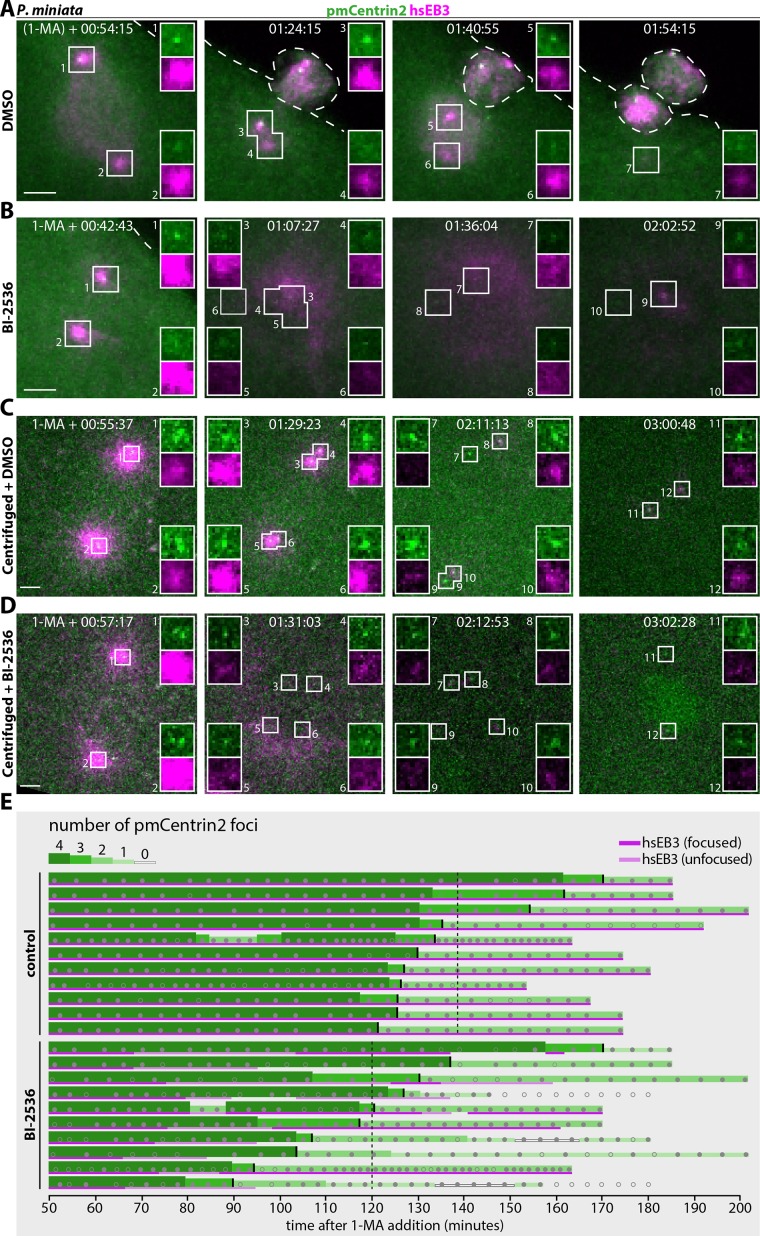
Plk1 inhibition does not provoke centriole elimination in P. miniata. (A–D) Still images from dual color time-lapse confocal microscopy of P. miniata oocytes expressing hsEB3::mCherry3 to mark microtubules and mEGFP::pmCentrin2 to mark centrioles, treated with either 0.1% DMSO as a control or 10 µM BI-2536 in 0.1% DMSO, both added simultaneously with 1-MA. A, B: noncentrifuged oocytes; note that gray levels were adjusted differently in A and B, which stem from independent experimental series. C, D: centrifuged oocytes. (E) Oocyte-derived centriole number (green) and MTOC activity (magenta) over time as monitored by mEGFP::pmCentrin2 foci and hsEB3::mCherry3 (magenta), respectively, in DMSO (C) or BI-2536-treated (D) centrifuged oocytes. Each line corresponds to one oocyte. Centriole number is indicated with different shades of green and different line thicknesses. Dark or light magenta lines indicate whether microtubules are focused around centrioles or diffuse, respectively. In BI-2536 oocytes, hsEB3::mCh3 is usually diffuse before disappearing completely (n = 5/10) or sometimes refocusing in a very limited area around centrioles (n = 5/10). Small gray discs: actual data points; gray circles: ambiguous data points, either because foci are out-of-focus or because foci disappear and reappear within three frames. Small vertical black marks indicate for each oocyte when the number of detected foci drops below 3, and vertical dashed lines the resulting average time of dropping below 3 for each condition (138 min for DMSO control, 120 min for BI-2536-treated). Time is indicated in hours:minutes:seconds after 1-MA addition, as meiosis II onset is difficult to discern in BI-2536 treated oocytes. In A, the parentheses around 1-MA denote the fact that timing in this particular oocyte is merely estimated from the actual timing of NEBD and the observation that NEBD typically occurs ∼30 min after 1-MA addition.
If Plk1 activity were to protect centrioles from elimination, then BI-2536 treatment should provoke disappearance of the two mother centrioles and potentially precocious elimination of daughter centrioles. To best determine whether this is the case, we analyzed mildly centrifuged oocytes expressing hsEB3::mCherry3 and pmCentrin2::mEGFP. Centrifugation leads to nuclear detachment from the animal pole, where centrioles remain cortically anchored (Figure 2C; Supplemental Figure S1, C–F). Even though centrosomes nucleate microtubules in such centrifuged oocytes (Figure 2C, 01:29:23), the meiotic spindles do not capture the distant chromosomes, and consequently, polar bodies are not extruded. As a result, all four oocyte-derived centrioles remain typically close to the plasma membrane, thus facilitating their monitoring throughout meiosis (Matsuura and Chiba, 2004; Borrego-Pinto et al., 2016a). In centrifuged control oocytes, two of the four pmCentrin2::mEGFP foci persist and nucleate microtubules, whereas the other two lose MTOC activity and are no longer detectable by the end of meiosis II (Figure 2C, 02:11:13 and 03:00:48; Figure 2E; Supplemental Figure S1G). Importantly, in centrifuged BI-2536–treated oocytes, all four centrioles have essentially no MTOC activity as early as meiosis I, but at least one and often two of the four retained pmCentrin2::mEGFP foci persist until the end of meiosis II (Figure 2D, 01:31:03 and 03:02:28; Figure 2E; Supplemental Figure S1H). Moreover, we observed that loss of the pmCentrin2::mEGFP foci corresponding to daughter centrioles occurred on average ∼18 min earlier in BI-2536 treated oocytes than in the control condition (Figure 2E). This may not reflect a bona fide temporal shift, but instead reflect the loss of focused hsEB3::mCh3 signal and the typically dimmer pmCentrin2::mEGFP signal upon BI-2536 treatment, rendering daughter centriole tracking more challenging (Supplemental Figure S1H).
We cannot exclude the possibility that a potential Plk1-dependent mechanism modulating centriole elimination requires only minute kinase activity. Moreover, whereas the Drosophila genome encodes a single Polo kinase, there is a second Polo-like kinase in P. miniata that is ∼40% identical to Plk1 (sequences IDs PMI_003306 and PMI_004640 from EchinoBase aligned with ClustalW), which could potentially act redundantly with Plk1. However, 10 µM BI-2536 is expected to readily inhibit both Plk1 and Plk2, considering that the corresponding IC50s for their human counterparts are 0.83 and 3.5 nM, respectively (Steegmaier et al., 2007).
Overall, within the time frame of this experiment, these findings lead us to conclude that inhibiting Plk1 activity in P. miniata oocytes is sufficient to provoke loss of MTOC activity but not to trigger the elimination of mother centrioles nor a drastic precocious disappearance of daughter centrioles.
Mother centrioles are extruded into polar bodies of A. forbesi oocytes
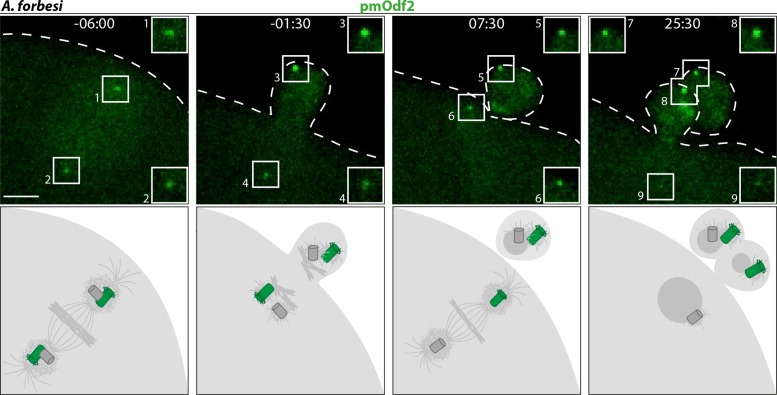
Early observations raised the possibility that centriole fate may differ in the radiate star A. forbesi from that in the bat star P. miniata (Sluder et al., 1989, 1993; see the Introduction). We set out to investigate this potential difference using molecular markers. As a first step, we tested whether the two mother centrioles are systematically directed to the first and second polar bodies in A. forbesi, as they are in P. miniata (Borrego-Pinto et al., 2016a). We injected oocytes with mRNA encoding a fusion protein between mEGFP and the mother centriole-specific component Odf2. We found that, as in P. miniata, the strong focused signals of pmOdf2::mEGFP were systematically present on both poles of the meiosis I spindle and on the pole closest to the plasma membrane of the meiosis II spindle (Figure 3). Therefore, mother centrioles are systematically extruded in polar bodies in A. forbesi, as they are in P. miniata.
FIGURE 3:
Mother centrioles are extruded into polar bodies in A. forbesi oocytes. Top: still images from time-lapse confocal microscopy of A. forbesi oocyte expressing the mother centriole marker pmOdf2::mEGFP. A bright focus is observed in each polar body. In some oocytes, as illustrated here, a weak focus can also be detected at the innermost centriole at the end of meiosis II. Bottom: corresponding schematic representation.
Retained oocyte-derived centrioles lose MTOC activity upon meiosis II exit in A. forbesi
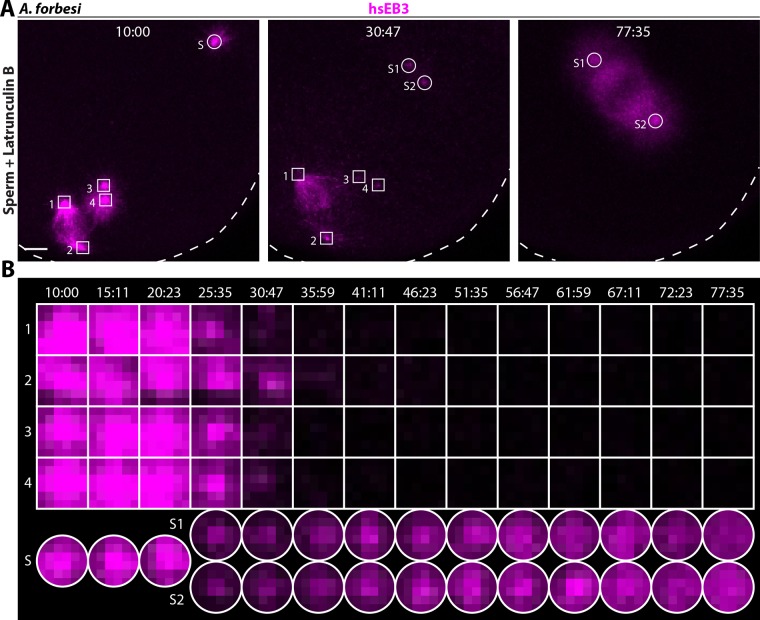
We next set out to investigate whether oocyte-derived centrioles experimentally retained in A. forbesi zygotes exhibit MTOC activity. To this end, we injected oocytes with mRNA encoding hsEB3::mCherry3 to label growing microtubules. Following 1-MA-induced maturation and NEBD, we added sperm to activate development and then Latrunculin B to block extrusion of the two polar bodies. MTOC activity of the centrioles thus retained in the zygote was assessed from meiosis I until the first mitotic division by time-lapse confocal microscopy. We found that the meiosis I spindle forms normally, with a pair of centrioles at each pole, and that centrioles disengage normally thereafter (unpublished data); this is followed by the formation of two small bipolar spindles with one centriole at each pole (Figure 4A, 10:00). Importantly, we found that all oocyte-derived centrioles lose associated MTOC activity upon exit from meiosis II (Figure 4A, 30:47 and Figure 4B), leading to the formation of a bipolar spindle driven exclusively by sperm-derived centrioles during the first mitotic division in the zygote (Figure 4A, 77:35). This is in stark contrast to P. miniata, where a tetrapolar spindle assembles following retention of oocyte-derived centrioles in the fertilized zygote (Borrego-Pinto et al., 2016a). We conclude that mother centrioles retained in the A. forbesi zygote do not function as MTOCs, in contrast to the situation in P. miniata.
FIGURE 4:
Oocyte-derived centrioles lose MTOC activity when retained in A. forbesi zygotes. (A) Still images from time-lapse confocal microscopy of A. forbesi zygote treated with Latrunculin B to retain all centrioles, monitoring MTOC activity using hsEB3::Cherry3 from meiosis II onset until mitosis. Here, as well as in Figure 5, oocyte-derived centrioles are bounded by squares and sperm-derived centrioles by circles. Note that hsEB3::mCherry3 is lost from the surroundings of all four oocyte-derived centrioles, whereas the two sperm-derived centrioles (S and then S1, S2) exhibit MTOC activity. Scale bar: 10 µm. (B) Single confocal z-planes corresponding to the zygote shown in A illustrating differential MTOC activity of oocyte-derived versus sperm-derived centrioles from meiosis II to mitosis. Each image is 3.73 × 3.73 µm.
Centriolar markers persist at retained oocyte-derived centrioles in A. forbesi zygotes
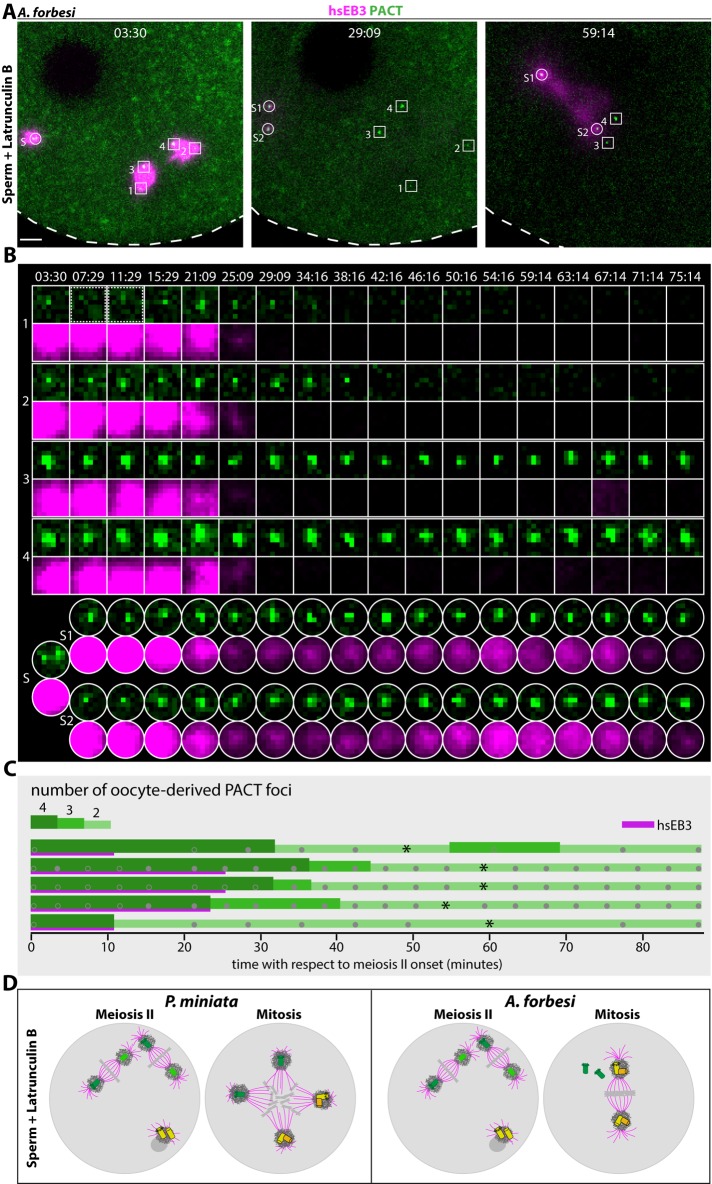
At least three scenarios could explain the lack of MTOC activity of centrioles retained in A. forbesi zygotes. First, all four centrioles could be eliminated. Second, all four centrioles could be retained, but without exhibiting MTOC activity. Third, daughter centrioles could be removed but mother centrioles could persist without nucleating microtubules. To distinguish between these possibilities, we set out to monitor centriolar markers from meiosis II until the first mitosis in A. forbesi zygotes treated with Latrunculin B (Figure 5 and S2; Movie S1). To follow centrioles, we injected mRNAs coding for a fusion protein between mEGFP and PACT, a small protein fragment derived from human Pericentrin and AKAP450 that marks centrioles across a range of organisms (Gillingham and Munro, 2000). As shown in Figure 5, we found that mEGFP::PACT labels initially all four centrioles localizing at the poles of the two bipolar spindles present during meiosis II upon Latrunculin B treatment (Figure 5A, 3:30; Figure 5B, centrioles 1–4; Figure 5C). After meiosis II exit, the MTOC activity of all four centrioles is lost, as monitored with hsEB3::mCherry3, whereas all four mEGFP::PACT foci persist initially (Figure 5A, 29:09; Figure 5B, centrioles 1–4; Figure 5C). Thereafter, two of the four mEGFP::PACT foci, presumably corresponding to the daughter centrioles, are no longer detectable (Figure 5A, 59:14; Figure 5B, centrioles 1 and 2; Figure 5C). Importantly, we found in addition that the two other foci, which we surmise correspond to the two mother centrioles, remain present at least until the first mitosis and are still observed as far as the second mitosis in some oocytes that were analyzed until then (unpublished data). Upon onset of the first mitotic division, these two oocyte-derived units approach the sperm-derived bipolar spindle, probably through the action of minus-end directed motors, without regaining MTOC activity (Figure 5A, 59:14; Figure 5B, centrioles 3 and 4; Figure 5C).
FIGURE 5:
Two retained foci of mEGFP::PACT persist until mitosis in A. forbesi. (A) Still images from dual-color time-lapse confocal microscopy of A. forbesi zygote treated with Latrunculin B to retain all centrioles, monitoring microtubules labeled with hsEB3::mCherry3 and centrioles marked with mEGFP::PACT from meiosis II onset until mitosis. Scale bar: 10 µm; see also Supplemental Movie S1. (B) Single confocal z-planes showing MTOC activity and the presence of mEGFP::PACT foci in oocyte-derived and sperm-derived centrioles from meiosis II until mitosis. Images boxed by a dashed line for centriole 1 indicate time frames when this centriole was slightly out of focus. Note that the little signal for hsEB3::mCherry3 of centriole 3 at time point 67:14 stems from sperm-derived MTOC activity. Each image is 3.98 × 3.98 µm. (C) Oocyte-derived centriole number over time as monitored by mEGFP::PACT foci (green) and MTOC activity as monitored by hsEB3::mCherry3 (magenta). Each line corresponds to one oocyte, with asterisks indicating mitosis onset. Centriole number is indicated with different shades of green and line thicknesses. Gray filled discs: data points; gray circles: ambiguous data points due to mEGFP::PACT not being yet detected at all centrioles, to foci being out-of-focus, or to the presence of multiple, probably spurious, foci. (D) Schematic representation of centriole fate in P. miniata based on Borrego-Pinto et al. (2016a) and A. forbesi zygotes treated with Latrunculin B. The same color code is used as in Figure 1. See text for details.
Movie S1.
Two retained foci of PACT::mEGFP persist in A. forbesi. Movie from dual color time-lapse confocal microscopy from meiosis II onset until mitosis of A. forbesi zygote treated with Latrunculin B and expressing hsEB3::mCherry3 (magenta) to label microtubules as well as PACT::mEGFP (green) to mark centrioles (related to Figure 5A and 5B). Sperm asters are on the left and maternal bipolar MII spindles are on the right. Time is indicated in hours:minutes:seconds. Scale bar: 10 μm.
We sought to extend the above observation to another centriolar marker. Therefore, we investigated oocytes injected with mRNA coding for pmPoc1::mEGFP, a centriolar component and microtubule binding protein conserved from green algae to human beings, including starfish (Keller et al., 2005; Borrego-Pinto et al., 2016a). As reported in Supplemental Figure S2, we found that pmPoc1::mEGFP labels all four centrioles and microtubules in A. forbesi zygotes treated with Latrunculin B. Although the weaker pmPoc1::mEGFP centriolar signal as compared with mEGFP::PACT sometimes prevented us from determining with certainty the number of oocyte-derived centrioles that persisted until mitosis, there was usually at least one left (Supplemental Figure S2C, Supplemental Table S1). Analysis of the minute foci harboring mEGFP::PACT and pmPoc1::mEGFP using serial section electron microscopy would be needed to ascertain whether they retained the full native architecture of centrioles. Regardless, the findings to date establish that foci of oocyte-derived centriolar components can remain present until mitosis in A. forbesi.
Concluding remarks
We set out to investigate whether the persistence of MTOC activity from the PCM surrounding oocyte-derived centrioles retained in P. miniata zygotes (Borrego-Pinto et al., 2016a) may reflect a mechanism analogous to that operating during Drosophila oogenesis (Pimenta-Marques et al., 2016). However, we found that centrioles can persist in P. miniata zygotes with impaired Plk1 activity, as well as in A. forbesi without nucleating microtubules, uncovering diversity in the mechanisms governing centriole removal across metazoan organisms. We note that whereas centrioles are eliminated during oogenesis in Drosophila, removal occurs after the meiotic divisions in starfish. Such differential timing may explain why Plks play a role in flies and seemingly not in starfish for ensuring proper centriole number at fertilization.
What mechanisms ensure that oocyte- and sperm-derived centrioles are endowed with different fates in the newly fertilized zygote? In P. miniata, MAP kinase activity suppresses the formation of sperm asters during meiosis and thus prevents them from interfering with active oocyte-derived centrioles driving meiotic spindle formation (Stephano and Gould, 2000). A related phenomenon operates in the clam Spisula soldissima, where γ-tubulin and MTOC activity of sperm centrioles are lost during meiosis I and regained only during meiosis II (Wu and Palazzo, 1999). Moreover, during physiological polyspermy in the newt Cynops pyrrhogaster, multiple sperm cells initially enter the oocyte, but only two centrioles develop a large aster that then drive mitotic spindle formation, with the remaining centrioles degenerating (Iwao et al., 2002). It will be interesting to unravel how specific centrioles can be earmarked for retention or removal in the same cell in these diverse settings. In starfish, it will also be interesting to explore whether variations in PCM and centriolar components, as well as in activities present in the cytoplasm, could explain the differential ability of persisting mother centrioles to nucleate microtubules during mitosis in the two species (Figure 5D), and thus further unravel the diversity of mechanisms governing centriole removal.
MATERIALS AND METHODS
mRNA generation
mRNAs were synthesized and purified as described by Borrego-Pinto et al. (2016b). Briefly, the ORF of the protein of interest in frame with either an N- or C-terminal fluorescent tag was subcloned into pGEMHE for in vitro transcription reactions (Borrego-Pinto et al., 2016b). Capped mRNAs were synthesized from linearized templates using the AmpliCap-Max T7 High Yield Message Maker kit (CellScript), and a poly-A tail was added using the A-Plus Poly(A) Polymerase Tailing Kit (CellScript). Purified mRNAs (typically 2–6 μg/μl) were diluted in 11 μl RNAse-free water (Borrego-Pinto et al., 2016b).
Starfish and gamete collection
Patiria miniata (previously known as Asterina miniata) was purchased from Monterey Abalone Company (Monterey, CA), and Asterias forbesi was collected from the waters of Cape Cod by the Marine Resource Center (MRC) of the Marine Biological Laboratory (MBL; Woods Hole, MA). Animals were maintained in seawater tanks at 16–20°C at the MRC or at EMBL’s Marine Facility (Heidelberg, Germany). Ovaries were dissected from female animals and washed for 20 min in calcium-free seawater supplemented with phenylalanine (437 mM NaCl, 9 mM KCl, 22.9 mM MgCl2, 25.5 mM MgSO4, 2.1 mM NaHCO3, 50 mM phenylalanine; pH 8), after which oocytes were collected upon treatment of ovary pieces with ∼100 µM acetylcholine as described by as described by Terasaki (1994) and Borrego-Pinto et al., (2016b). Healthy-looking oocytes were selected and kept in filtered seawater at ∼14°C to be used within 2 days of extraction. Sperm was obtained by extracting testis fragments from male animals; such fragments were kept dry for several days at 4°C and tested before use.
Microinjection, maturation, drug treatments and fertilization
Oocytes were mounted in Kiehart–Ellis chambers and injected using mercury back-filled needles (Terasaki, 1994; Borrego-Pinto et al., 2016b). The amount of injected mRNA was calibrated using an ocular micrometer and the optimum was determined empirically for each mRNA. Injected oocytes were incubated overnight at ∼14°C in a humidified Petri dish to allow protein expression. Meiosis resumption was then induced by 10 μM 1-methyladenine (1-MA, Acros Organics). To prevent polar body extrusion, oocytes were treated with 250 nM Latrunculin B (EMD Biosciences) in seawater 5–10 min after sperm addition, since fertilization depends on actin polymerization. BI-2536 (Medchem express, Sweden, HY-50698) was diluted 1:1000 in seawater from a 10 mM stock in 100% DMSO, and added to oocytes simultaneously with 1-MA. Control oocytes were exposed to 1:1000 DMSO in seawater. We noted that treatment with 10 µM BI-2536 did not result in a noticeable difference in the timing of NEBD from that for DMSO control in P. miniata oocytes observed under a dissecting microscope. For in vitro fertilization, sperm was diluted 1:1000–1:8000 in seawater depending on the motility on the day of the experiment and added 30–45 min after 1-MA addition.
Centrifugation and drug treatments
Oocyte centrifugation was performed in a clinical centrifuge at 2400 rpm for 1 h at 4°C (Multifuge 3 L-R, Heraeus) after the oocyte chamber was placed in a plastic holder in a 50-ml Falcon tube filled with seawater (see Figure S1B) (Matsuura and Chiba, 2004; Borrego-Pinto et al., 2016a). After centrifugation, the oocyte chamber was placed into a 35-mm μ-dish (Vitaris) for 1-MA addition and BI-2536 treatment.
Microscopy and image processing
After overnight incubation and experiment-specific handling following maturation, injected oocytes were transferred to a confocal microscope for multiposition dual-color time-lapse imaging. Depending on the experiment, data were acquired with a 40 × 1.25NA water immersion lens on a Nikon A1+ confocal, a 40 × HCX PL APO 1.10 NA water immersion lens on a Leica SP5II, or a 40 × C-Apochromat NA 1.2 water immersion lens on a Zeiss LSM780 confocal microscope. Z-stacks of the relevant portion of the oocyte were captured typically every micrometer at intervals of ∼3–5 min. Brightness/contrast adjustments were performed using Fiji in a uniform manner for all time points in a given oocyte, and panels were assembled using Adobe Illustrator.
Supplementary Material
Acknowledgments
We are grateful to Mark Terasaki (University of Connecticut Health Center, Farmington, CT) and Kip Sluder (University of Massachusetts Medical School, Worcester, MA) for their generous support, to John Allen (Nikon) for imaging support at the Marine Biological Laboratory (MBL, Woods Hole, MA), and the Marine Resource Center of the MBL for supplying A. forbesi, as well as the MPI-BPC Live-Cell Imaging Facility. We also thank Kalman Somogyi (EMBL), Andrea Callegari (EMBL), and Jasmin Jakobi (MPI-BPC, Göttingen) for preparing plasmid constructs and mRNAs. Jasmin Jakobi is also acknowledged for help in injecting mRNAs. We are grateful to Alexandra Bezler, Mark Terasaki, and Kip Sluder for comments on the manuscript. This work was funded in part by an EMBO postdoctoral fellowship to M.P. (ALTF 1426-2016), by EMBL and by the EMBL PhD Programme to J.P. and P.L., as well as by MBL Whitman Center Research Awards to P.G. in 2015, 2017, and 2018.
Abbreviations used:
- hs
Homo sapiens
- 1-MA
1-methyladenine
- MTOC
microtubule-organizing center
- NEBD
nuclear envelope breakdown
- PCM
pericentriolar material
- Plk1
Polo-like kinase 1
- pm
Patiria miniata
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E19-06-0346) on February 19, 2020.
REFERENCES
- Archambault V, Glover DM. (2009). Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol , 265–275. [DOI] [PubMed] [Google Scholar]
- Bornens M. (2012). The centrosome in cells and organisms. Science , 422–426. [DOI] [PubMed] [Google Scholar]
- Borrego-Pinto J, Somogyi K, Karreman MA, König J, Müller-Reichert T, Bettencourt-Dias M, Gönczy P, Schwab Y, Lénárt P. (2016a). Distinct mechanisms eliminate mother and daughter centrioles in meiosis of starfish oocytes. J Cell Biol , 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrego-Pinto J, Somogyi K, Lénárt P. (2016b). Live imaging of centriole dynamics by fluorescently tagged proteins in starfish oocyte meiosis. Methods Mol Biol , 145–166. [DOI] [PubMed] [Google Scholar]
- Crowder ME, Strzelecka M, Wilbur JD, Good MC, von Dassow G, Heald R. (2015). A comparative analysis of spindle morphometrics across metazoans. Curr Biol , 1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delattre M, Gönczy P. (2004). The arithmetic of centrosome biogenesis. J Cell Sci , 1619–1630. [DOI] [PubMed] [Google Scholar]
- Fırat-Karalar EN, Stearns T. (2014). The centriole duplication cycle. Philos Trans R Soc Lond B Biol Sci , rstb.2013.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DL. (1994). Gamma-tubulin is asymmetrically distributed in the cortex of Xenopus oocytes. Dev Biol , 131–140. [DOI] [PubMed] [Google Scholar]
- Gillingham AK, Munro S. (2000). The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep , 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönczy P, Hatzopoulos GN. (2019). Centriole assembly at a glance. J Cell Sci , jcs228833. [DOI] [PubMed] [Google Scholar]
- Iwao Y, Murakawa T, Yamaguchi J, Yamashita M. (2002). Localization of γ-tubulin and cyclin B during early cleavage in physiologically polyspermic newt eggs. Dev Growth Differ , 489–499. [DOI] [PubMed] [Google Scholar]
- Kanatani H, Shirai H, Nakanishi K, Kurokawa T. (1969). Isolation and identification of meiosis inducing substance in starfish Asterias amurensis. Nature , 273–274. [DOI] [PubMed] [Google Scholar]
- Keller LC, Romijn EP, Zamora I, Yates JR, Marshall WF. (2005). Proteomic analysis of isolated chlamydomonas centrioles reveals orthologs of ciliary-disease genes. Curr Biol , 1090–1098. [DOI] [PubMed] [Google Scholar]
- Lénárt P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters J-M. (2007). The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol , 304–315. [DOI] [PubMed] [Google Scholar]
- Loncarek J, Bettencourt-Dias M. (2018). Building the right centriole for each cell type. J Cell Biol , 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald AP, Strassheim JM. (1970). Intercellular migration of centrioles in the germarium of Drosophila melanogaster. An electron microscopic study. J Cell Biol , 306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manandhar G, Schatten H, Sutovsky P. (2005). Centrosome reduction during gametogenesis and its significance. Biol Reprod , 2–13. [DOI] [PubMed] [Google Scholar]
- Matsuura R, Chiba K. (2004). Unequal cell division regulated by the contents of germinal vesicles. Dev Biol , 76–86. [DOI] [PubMed] [Google Scholar]
- Mikeladze-Dvali T, von Tobel L, Strnad P, Knott G, Leonhardt H, Schermelleh L, Gönczy P. (2012). Analysis of centriole elimination during C. elegans oogenesis. Development , 1670–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima S, Kato KH. (2001). Centriole behavior during meiosis in oocytes of the sea urchin Hemicentrotus pulcherrimus. Dev Growth Differ , 437–445. [DOI] [PubMed] [Google Scholar]
- Okano-Uchida T, Okumura E, Iwashita M, Yoshida H, Tachibana K, Kishimoto T. (2003). Distinct regulators for Plk1 activation in starfish meiotic and early embryonic cycles. EMBO J , 5633–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta-Marques A, Bento I, Lopes CAM, Duarte P, Jana SC, Bettencourt-Dias M. (2016). A mechanism for the elimination of the female gamete centrosome in Drosophila melanogaster. Science , aaf4866. [DOI] [PubMed] [Google Scholar]
- Sathananthan AH, Ratnasooriya WD, de Silva PK, Menezes J. (2001). Characterization of human gamete centrosomes for assisted reproduction. Ital J Anat Embryol , 61–73. [PubMed] [Google Scholar]
- Shirato Y, Tamura M, Yoneda M, Nemoto S-I. (2006). Centrosome destined to decay in starfish oocytes. Development , 343–350. [DOI] [PubMed] [Google Scholar]
- Sluder G, Miller FJ, Lewis K. (1993). Centrosome inheritance in starfish zygotes II: selective suppression of the maternal centrosome during meiosis. Dev Biol , 58–67. [DOI] [PubMed] [Google Scholar]
- Sluder G, Miller FJ, Lewis K, Davison ED, Rieder CL. (1989). Centrosome inheritance in starfish zygotes: selective loss of the maternal centrosome after fertilization. Dev Biol , 567–579. [DOI] [PubMed] [Google Scholar]
- Steegmaier M, Hoffmann M, Baum A, Lénárt P, Petronczki M, Krssák M, Gürtler U, Garin-Chesa P, Lieb S, Quant J, et al. (2007). BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol , 316–322. [DOI] [PubMed] [Google Scholar]
- Stephano JL, Gould MC. (2000). MAP kinase, a universal suppressor of sperm centrosomes during meiosis? Dev Biol , 420–428. [DOI] [PubMed] [Google Scholar]
- Szollosi D, Calarco P, Donahue RP. (1972). Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci , 521–541. [DOI] [PubMed] [Google Scholar]
- Terasaki M. (1994). Redistribution of cytoplasmic components during germinal vesicle breakdown in starfish oocytes. J Cell Sci (Pt 7), 1797–1805. [DOI] [PubMed] [Google Scholar]
- Wu X, Palazzo RE. (1999). Differential regulation of maternal vs. paternal centrosomes. Proc Natl Acad Sci , 1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.