Abstract
Introduction
Completion of the SARC-F questionnaire constitutes the obligatory first step in the diagnostic process of sarcopenia, according to the revised European consensus on the definition and diagnosis of sarcopenia published by the European Working Group on Sarcopenia in Older People2 (EWGSOP2). SARC-F has been recognized as the most up-to-date and coherent screening tool for sarcopenia. The aim of the study was to translate and to validate the Polish version of the SARC-F questionnaire.
Materials and Methods
The validation process was performed in two stages: 1) translation and intercultural adaptation and 2) clinical validation. The inclusion criteria were as follows: age ≥65 years, unimpaired mobility, and no cognitive impairment. The EWGSOP2 criteria were used to diagnose sarcopenia. Hand grip strength measurement, physical fitness test, and body weight composition analysis were conducted. Sensitivity, specificity, accuracy-likelihood ratios, and SARC-F predictive values were calculated using the EWGSOP2 criteria.
Results
Sixty-seven people participated in the study of whom 21% were diagnosed with sarcopenia (SARC-F score: ≥4). The reliability of the questionnaire based on the Cronbach’s alpha coefficient was 0.784. Sensitivity, specificity, and negative predictive values were 92.9%, 98.1%, and 98.1%, respectively.
Conclusion
The process of validating the SARC-F questionnaire against Polish conditions demonstrated its applicability as a simple and reliable tool for diagnosing sarcopenia in daily clinical practice with older adults.
Keywords: sarcopenia, older adults, muscle mass, screening
Introduction
The term “sarcopenia” was first introduced in 1995 by Rosenberg and Roubenoff to describe age-related loss in skeletal muscle.1 Since then, a considerable amount of research on sarcopenia has been conducted globally. Over time, a test of muscle strength and function has been added to the diagnostic process of sarcopenia, which originally consisted in measuring muscle mass. Since 2018, sarcopenia has been considered a disease (ICD 10-MC, code M62.84),2 which is associated with functional decline, falls and fractures, the fragility syndrome and an increased risk of mortality.3 Resistance training, combined with high-protein diet4 and dietary supplements, plays a vital role in the prevention and treatment of sarcopenia.5 The literature offers an accumulating body of evidence indicating that therapeutic interventions can improve the physical condition of older people and prevent or reverse sarcopenia-related adverse changes.6
The number of patients with sarcopenia is expected to increase significantly over the next 30 years, posing a serious challenge for public health.7 Early diagnosis should become a priority when assessing the health status of older people, as early detection of sarcopenia allows to undertake timely actions to prevent negative health effects in the future.8
However, the diagnosis of sarcopenia remains challenging. For a long time, sarcopenia was defined as “loss of muscle mass”. It was not until 2010 that the European Working Group on Sarcopenia in Older People (EWGSOP) recommended diagnostic criteria for sarcopenia to include both, low muscle mass and impaired muscle function, defined by low muscle strength or reduced physical fitness. In line with the European approach, the International Working Group of Sarcopenia (IWGS) and the Asian Working Group for Sarcopenia (AWGS) have also included similar criteria into their definitions of sarcopenia.
The diagnosis of sarcopenia requires the assessment of muscle mass and strength as well as their function, which was confirmed by numerous international studies.9 Muscle mass assessment can be performed using a variety of methods, e.g. Bioelectrical Impedance Analysis (BIA), dual-energy X-ray absorptiometry (DXA), computed tomography (CT), or magnetic resonance imaging (MRI), but it always requires the use of advanced technologies. Muscle function is assessed by measuring muscle strength and physical fitness. The most commonly used methods include the following tests: hand grip strength (HGS), leg muscle strength, repeated chair stand, Timed Up and Go (TUG), 6-min walk distance or 400-m walk, stair climb power, and Short Physical Performance Battery (SPPB).10 Time and adequate space are needed to assess for example the strength of the lower limb muscles (leg muscle strength test) or to perform the 6-min walk distance test. The use of sarcopenia tests is limited in everyday clinical practice due to the necessity of having either access to specialized equipment and/or adequate amount of time to conduct the test.11 Hence, routine screening for sarcopenia among older subjects is often unfeasible.
New, more easily applicable and cheaper screening methods are necessary and the SARC-F questionnaire has been suggested as a practical screening tool for sarcopenia in everyday practice.6 SARC-F evaluates the simple functions and the risk for sarcopenia, indicating whether further and more detailed analysis, using the above-mentioned tests, is needed.12 Originally developed in English, SARC-F is a simple, self-report tool for rapid detection of sarcopenia. It consists of 5 components (strength, assistance with walking, rise from a chair, climb stairs and falls) which can easily be evaluated by healthcare professionals.7 Predictability of physical limitations using SARC-F is comparable with the criteria used by EWGSOP, IWGS and AWGS. EWGSOP2 recommends SARC-F as the most up-to-date and coherent tool and a mandatory first step in the diagnostic process of sarcopenia. The questionnaire has already been approved in the United States, China and Hong Kong.7
The aim of the study was to translate and validate the Polish version of the SARC-F questionnaire.
Materials and Methods
SARC-F Questionnaire
The SARC-F questionnaire consists of 5 items which evaluate 5 parameters: strength, assistance with walking, rise from a chair, climbing stairs, and falls. The subject can receive from 0 to 2 points for each item. The total result ranges from 0 to 10 points (worst and best score, respectively). The score of 0–3 points indicates no risk of sarcopenia, while the score of ≥4 points indicates sarcopenia.13 The SARC-F questionnaire is presented in Table 1.
Table 1.
SARC-F Score
| Component | Question | Scoring |
|---|---|---|
| Strength | How much difficulty do you have in lifting and carrying 4.5 kg? | None = 0; Some = 1; A lot or unable = 2 |
| Assistance with walking | How much difficulty do you have walking across a room? | None = 0; Some = 1; A lot, use aids, or unable = 2 |
| Rise from a chair | How much difficulty do you have transferring from a chair or a bed? | None = 0; Some = 1; A lot or unable without help = 2 |
| Climb stairs | How much difficulty do you have climbing a flight of 10 stairs? | None = 0; Some = 1; A lot or unable = 2 |
| Falls | How many times did you fall in the past year? | None = 0; 1–3 falls = 1; ≥4 falls = 2 |
Study Design
A cross-sectional descriptive study using the two-step World Health Organization (WHO) methodology for translating and intercultural adaptation of health questionnaires was conducted. The EWGSOP recommendations for the validation of SARC-F in European languages were applied.10
Procedure
The validation was carried out in two stages: 1) translation and intercultural adaptation and 2) clinical validation.9 The first phase consisted of 8 different steps based on the guidelines recommended by WHO.14 The translation was carried out by a multidisciplinary team consisting of three specialists: a physiotherapist, a physician working with elderly patients, and an independent bilingual translator. After translating SARC-F into Polish, a questionnaire validation study was conducted. The protocol was based on the original validation of the English version of the SARC-F questionnaire.6 A cross-sectional study was conducted to assess the specificity and sensitivity of SARC-F to the definitions of sarcopenia using the EWGSOP2 criteria.10
Study Population
A total of 67 subjects (54 women and 13 men), aged 65 years or more, who were Polish community-dwelling elderly persons from the Wielkopolska region were included in the study. Written informed consent was obtained from all participants. The inclusion criteria were as follows: age of ≥65 years, independent movement, and no cognitive impairment determined using the Mini-Cog test.15
Ethics
The consent of the Bioethics Committee of the University of Medical Sciences in Poznań was obtained for conducting tests (Resolution No. 995/18). The study protocol followed the Declaration of Helsinki Ethical Principles for Medical Research.
Physical Measurements
The following anamnestic and demographic data were collected: sex, age (years), height (cm), weight (kg), shoulder circumference (cm), shoulder span (cm), waist circumference (cm), assistance with walking (yes/no), education (no qualifications, elementary, secondary, post-secondary education, doctoral degree).
The SARC-F scale was also verified in relation to other measurements associated with sarcopenia, i.e. Geriatric Depression Scale (GDS)16 for assessing depression, Katz Scale for assessing Activities of Daily Living (ADL),17 Lawton Instrumental Activities of Daily Living Scale for assessing instrumental activities of daily living (IADL),18 Mini Nutritional Assessment (MNA)19 for measuring the nutritional status, and history of falls in the past year (yes/no, if yes - provide the number).
Clinical Diagnosis of Sarcopenia
The clinical diagnosis of sarcopenia was made on the same day by the same member of the medical staff. The following tests were conducted: measurement of the total muscle mass and appendicular lean mass using the BIA method,20 and hand grip strength measured using a hydraulic hand dynamometer (Jamar, Duluth, MN).21 Three readings were taken, and the highest measurement for each hand was taken into consideration. The SPPB test was used to assess physical fitness.22
Definitions of Sarcopenia
According to EWGSOP2, sarcopenia is defined as low muscle strength and low muscle mass, and additionally its progression is measured with physical performance score. The cut-off points were determined based on the lowest quintile values in the current study population using the following parameters: Appendicular Lean Mass index (ALM/ht2) for men ≤ 7.0 kg/m2 and for women ≤ 5.5 kg/m2; hand grip strength ≤27 kg for men and ≤16 kg for women; and gait speed ≤ 0.8 m/s for men and women or SPPB ≤ 8 point score.10
Statistical Analysis
Mean measurement and standard deviation were used for the descriptive analysis of the group. The normality of variable distribution was determined using the Shapiro–Wilk test. The t-Student test was used to compare normally distributed variables, while the Mann–Whitney test was used for non-normally normally distributed and for ordinal variables. Sensitivity (Se), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+) in relation to the sarcopenia scores according to EWGSOP2 are expressed as percentages. Receiver-operating characteristic (ROC) analysis was performed to calculate the area under the curve (ROC- AUC). The internal coherence of the SARC-F questionnaire was assessed using the Cronbach’s Alpha test, and the correlations of each SARC-F component with the overall score were checked using the Spearman test. Relationships between SARC-F and other variables (hand grip strength, basic and instrumental daily life activities, nutrition level, body dimensions and composition, as well as physical performance) were also investigated. The p-value <0.05 was considered as statistically significant. Statistical analysis was performed using Statistica 13.
Results
Patient Characteristics
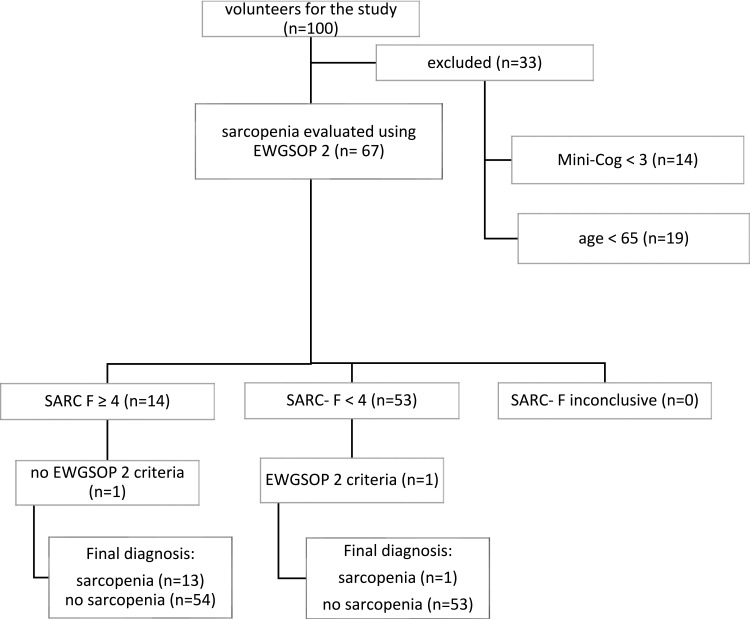
A total of 100 individuals volunteered to participate and 67 subjects were deemed eligible for the study. The stages of the eligibility process are presented in Figure 1. Patient characteristics are presented in Table 2.
Figure 1.
Classification of the study participants.
Abbreviation: EWGSOP 2, European Working Group on Sarcopenia in Older People 2.
Table 2.
Patient Characteristics
| Parameter | SARC- F <4 n=53 (79%) W=44; M=9 | SARC- F ≥4 n=14 (21%) W=10; M=4 | p-value | ||
|---|---|---|---|---|---|
| Mean | ± SD | Mean | ± SD | ||
| Age (years) | 68.08 | 3.67 | 75.00 | 5.11 | <0.001 |
| SARC-F strength | 0.25 | 0.59 | 1.21 | 0.58 | <0.001 |
| SARC-F assistance with walking | 0.00 | 0.00 | 0.79 | 0.58 | <0.001 |
| SARC-F rising from a chair | 0.00 | 0.00 | 1.14 | 0.53 | <0.001 |
| SARC-F climbing stairs | 0.00 | 0.00 | 1.00 | 0.68 | <0.001 |
| SARC-F falls | 0.26 | 0.49 | 0.86 | 0.95 | 0.035 |
| SARC-F total score | 0.60 | 0.86 | 5.00 | 1.52 | <0.001 |
| HGS D (kg) | 22.72 | 6.55 | 14.50 | 5.26 | <0.001 |
| HGS ND (kg) | 22.30 | 7.12 | 13.07 | 6.07 | <0.001 |
| SPPB chair stand test (points) | 3.57 | 0.84 | 1.29 | 1.14 | <0.001 |
| SPPB balance test (points) | 3.75 | 0.59 | 1.71 | 1.38 | <0.001 |
| SPPB gait speed test (points) | 3.85 | 0.50 | 2.00 | 1.11 | <0.001 |
| SPPB total score (points) | 11.19 | 1.58 | 5.00 | 3.42 | <0.001 |
| Height (cm) | 161.03 | 7.76 | 164.62 | 7.62 | 0.127 |
| Weight (kg) | 78.69 | 13.64 | 68.01 | 11.16 | 0.009 |
| Calf circumference (cm) | 36.34 | 3.48 | 32.16 | 2.12 | <0.001 |
| Arm circumference (cm) | 31.16 | 3.97 | 28.90 | 4.37 | 0.068 |
| Waist circumference (cm) | 96.90 | 12.94 | 73.95 | 6.17 | <0.001 |
| BMI (kg/m2) | 30.30 | 4.54 | 25.03 | 3.34 | <0.001 |
| ADL/8 (points) | 6.00 | 0.00 | 5.29 | 0.47 | <0.001 |
| IADL/27 (points) | 27.00 | 0.00 | 22.79 | 3.66 | <0.001 |
| MNA (points) | 13.92 | 0.38 | 12.21 | 0.97 | <0.001 |
| Fat mass (%) | 39.02 | 7.41 | 39.76 | 6.68 | 0.736 |
| Total muscle mass (kg) | 26.13 | 5.32 | 21.29 | 4.76 | 0.003 |
| ALM/ht2 (kg/m2) | 7.27 | 1.01 | 5.54 | 1.07 | <0.001 |
Note: Statistically significant differences are marked in bold.
Abbreviations: W, women; M, men; SD, standard deviations; HGS D, grip strength of the dominant hand; HGS ND, grip strength of the non-dominant hand; SPPB, Short Physical Performance Battery; BMI, Body Mass Index; ADL, Activities of Daily Living; IAD, Instrumental Activities of Daily Living; MNA, Mini Nutritional Assessment; ALM, ratio of Appendicular Lean Mass over height squared.
Reliability
Correlations of individual questions with the total SARC-F score are presented in Table 3. Internal coherence assessed using Cronbach’s Alpha ratio test is 0.784.
Table 3.
Internal Coherence of the Polish Version of the SARC-F Questionnaire
| SARC- F Item | Correlation | p-value |
|---|---|---|
| Strength | 0.833 | <0.001 |
| Assistance with walking | 0.647 | <0.001 |
| Rising from a chair | 0.735 | <0.001 |
| Climbing stairs | 0.678 | <0.001 |
| Falls | 0.625 | <0.001 |
Notes: Cronbach’s alpha= 0.784. The item-total score correlations were analyzed using the Spearman test.
Validity
The 5-item SARC-F questionnaire was tested and the score of ≥4 indicated sarcopenia. Se, Sp, PPV, NPV, LR + were calculated and subsequently compared with the EWGSOP2 criteria. Table 4 shows the distribution of patients with and without sarcopenia, using the SARC-F score (≥4) and the EWGSOP 2 criteria.
Table 4.
Contingency Table Showing Sample Distribution, According to the Polish Version of the SARC-F Questionnaire and the EWGSOP2 Criteria (N= 67)
| EWGSOP 2 criteria | p-value | ||||
|---|---|---|---|---|---|
| Sarcopenia | No Sarcopenia | Total | |||
| SARC-F | ≥ 4 | 13 | 1 | 14 | p<0.001 |
| < 4 | 1 | 52 | 53 | ||
| Total | 14 | 53 | 67 | ||
Abbreviation: EWGSOP 2, European Working Group on Sarcopenia in Older People 2.
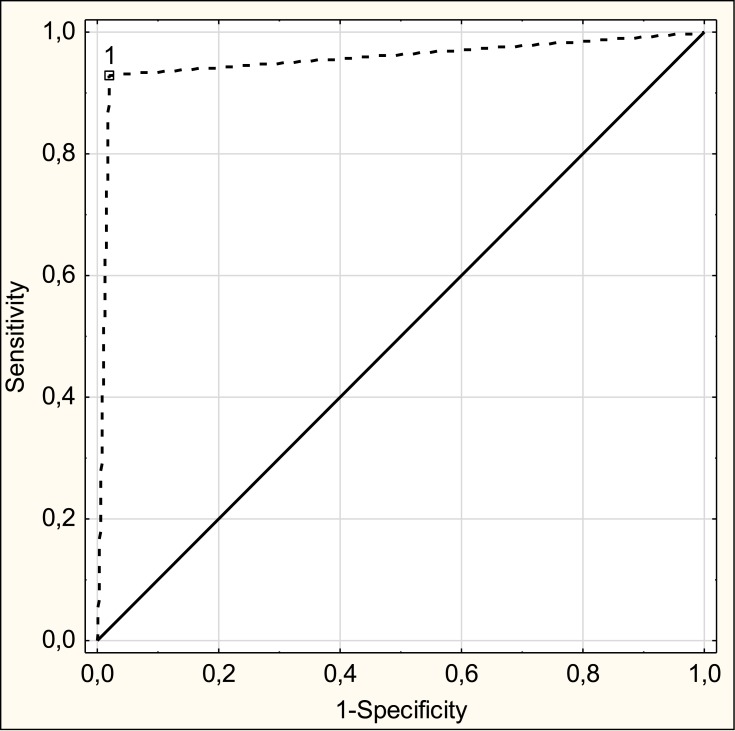
Table 5 presents the values of Se, Sp, PPV, NPV, LR+ for the SARC-F questionnaire using the EWGSOP2 criteria as the reference standard. Sensitivity and specificity of the SARC-F questionnaire were 92.9% and 98.1%, respectively. The ROC curve of SARC-F against the EWGSOP2 criteria is shown in Figure 2. ROC- AUC is 0.955 (95% CI: 0.0873–1, p<0.001), which demonstrates high diagnostic potential of the SARC-F questionnaire.
Table 5.
Criterion Validity Between the Polish Version of the SARC-F Questionnaire and the EWGSOP 2 Criteria
| Se (%) | Sp (%) | PPV (%) | NPV (%) | Accuracy (%) | LR+ | |
|---|---|---|---|---|---|---|
| EWGSOP 2 | 92.9 | 98.1 | 92.9 | 98.1 | 97 | 49.2 |
Abbreviations: EWGSOP 2, European Working Group on Sarcopenia in Older People 2; Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; LR +, positive likelihood ratio.
Figure 2.
The ROC curve of SARC-F against the EWGSOP 2 criteria.
Abbreviation: EWGSOP 2, European Working Group on Sarcopenia in Older People 2.
Correlations between individual SARC-F items and clinical and functional measurements (age, hand grip strength, basic and instrumental daily life activities, nutrition level, body dimensions and composition, physical performance) are presented in Table 6. Statistically significant correlation was found between the total SARC-F score and all of the investigated parameters with the exception of height and fat mass. Statistically significant correlation was found between SARC-F scores in all of the investigated domains and the following parameters: age, SPPB gait speed, total muscle mass.
Table 6.
Validation Between the SARC-F (Each Domain and Total Score) and Other Related Measurements
| Parameters | Strength | Assistance with Walking | Rising from a Chair | Climbing Stairs | Falls | Total Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation | p-value | Correlation | p-value | Correlation | p-value | Correlation | p-value | Correlation | p-value | Correlation | p-value | |
| Age (years) | 0.342 | 0.005 | 0.472 | <0.001 | 0.516 | <0.001 | 0.460 | <0.001 | 0.289 | 0.018 | 0.491 | <0.001 |
| HGS D (kg) | −0.028 | 0.825 | 0.103 | 0.405 | 0.127 | 0.306 | 0.102 | 0.412 | −0.157 | 0.204 | −0.731 | <0.001 |
| HGS ND (kg) | −0.270 | 0.027 | −0.183 | 0.138 | −0.303 | 0.013 | −0.238 | 0.052 | −0.286 | 0.019 | −0.717 | <0.001 |
| SPPB chair stand test (points) | −0.497 | <0.001 | −0.502 | <0.001 | −0.510 | <0.001 | −0.506 | <0.001 | −0.224 | 0.069 | −0.708 | <0.001 |
| SPPB balance test (points) | −0.225 | 0.067 | −0.105 | 0.399 | −0.183 | 0.139 | −0.155 | 0.210 | −0.244 | 0.046 | −0.634 | <0.001 |
| SPPB gait speed test (points) | −0.540 | <0.001 | −0.563 | <0.001 | −0.654 | <0.001 | −0.583 | <0.001 | −0.320 | 0.008 | −0.746 | <0.001 |
| SPPB total score (points) | −0.310 | 0.011 | −0.260 | 0.033 | −0.408 | 0.001 | −0.312 | 0.010 | −0.214 | 0.081 | −0.708 | <0.001 |
| Height (cm) | −0.577 | <0.001 | −0.999 | <0.001 | −0.864 | <0.001 | −0.947 | <0.001 | −0.056 | 0.653 | −0.071 | 0.568 |
| Weight (kg) | −0.571 | <0.001 | −0.993 | <0.001 | −0.857 | <0.001 | −0.940 | <0.001 | −0.053 | 0.669 | −0.354 | 0.003 |
| Calf circumference (cm) | −0.483 | <0.001 | −0.831 | <0.001 | −0.859 | <0.001 | −0.781 | <0.001 | −0.203 | 0.100 | −0.541 | <0.001 |
| Arm circumference (cm) | −0.091 | 0.464 | −0.031 | 0.805 | 0.064 | 0.608 | 0.029 | 0.817 | 0.272 | 0.026 | −0.270 | 0.027 |
| Waist circumference (cm) | −0.350 | 0.004 | −0.342 | 0.005 | −0.379 | 0.002 | −0.383 | 0.001 | −0.295 | 0.015 | −0.633 | <0.001 |
| BMI (kg/m2) | −0.492 | <0.001 | −0.543 | <0.001 | −0.599 | <0.001 | −0.566 | <0.001 | −0.239 | 0.051 | −0.355 | 0.003 |
| ADL (points) | −0.577 | <0.001 | −0.999 | <0.001 | −0.864 | <0.001 | −0.947 | <0.001 | −0.056 | 0.653 | −0.647 | <0.001 |
| IADL (points) | −0.571 | <0.001 | −0.993 | <0.001 | −0.857 | <0.001 | −0.940 | <0.001 | −0.053 | 0.669 | −0.641 | <0.001 |
| MNA (points) | −0.483 | <0.001 | −0.831 | <0.001 | −0.859 | <0.001 | −0.781 | <0.001 | −0.203 | 0.100 | −0.642 | <0.001 |
| Fat mass (%) | −0.091 | 0.464 | −0.031 | 0.805 | 0.064 | 0.608 | 0.029 | 0.817 | 0.272 | 0.026 | 0.084 | 0.501 |
| Total muscle mass (kg) | −0.350 | 0.004 | −0.342 | 0.005 | −0.379 | 0.002 | −0.383 | 0.001 | −0.295 | 0.015 | −0.456 | <0.001 |
| ALM/ht2 (kg/m2) | −0.492 | <0.001 | −0.543 | <0.001 | −0.599 | <0.001 | −0.566 | <0.001 | −0.239 | 0.051 | −0.583 | <0.001 |
Abbreviations: HGS D, grip strength of the dominant hand; HGS ND, grip strength of the non-dominant hand; SPPB, Short Physical Performance Battery; BMI, Body Mass Index; ADL, Activities of Daily Living; IAD, Instrumental Activities of Daily Living; MNA, Mini Nutritional Assessment; ALM, ratio of Appendicular Lean Mass over height squared.
Discussion
In the present study, we found that the 5-item SARC-F questionnaire for the diagnosis of sarcopenia does not require any measurements and its specificity and the positive predictive value of adverse physical changes are comparable to the EWGSOP2 criteria.
Perra-Rodriguez11 reported that the SARC-F scale was successfully adapted to Spanish and validated in Mexican community-dwelling elderly persons. These authors showed that the Spanish version of the questionnaire was associated with high reliability (Cronbach’s Alpha = 0.641). All items of the scale correlated to the total score, criterion validity when compared to the consensus panel criteria (high specificity and negative predictive values). The scale also correlated to other measures related to sarcopenia such as age, quality of life, self-rated health status, cognition, dependence in daily living activities, nutritional status, depression, gait speed, grip strength, peak torque and power for knee extension, SPPB, balance, and SMI.
In the present study, the level of reliability was higher. Both, high sensitivity and specificity have been demonstrated, indicating that SARC-F successfully identifies people with sarcopenia. Also, a correlation was found between individual SARC-F questions and other measures related to sarcopenia.
Sánchez-Rodríguez et al,23 considered SARC-F to be a feasible tool, suitable for bedside assessment in community-dwelling older patients, and expected it to be widely distributed for the assessment of sarcopenia in Spain. The level of reliability in their research was 0.779, and the sensitivity and specificity were 78.3% and 50.8%, respectively.
In the present study, high sensitivity and specificity of SARC-F were observed (92.9% and 98.1%, respectively), which proves that the tool detects cases with no sarcopenia and identifies individuals with sarcopenia. Similar specificity values were reported by Woo et al (98.7% for men, and 94.4% for women),24 Kera et al (97.3%)25 and Kim et al (91.2%).26 However, the sensitivity values in the present study were very high as compared to the abovementioned authors.
SARC-F was originally developed in English but EWGSOP2 recommends for it to be translated and validated into other languages as well. SARC-F has already been translated into Brazilian Portuguese,27 Chinese,28 Japanese,25 Korean,26 Mexican Spanish,11 and numerous other language validations in Europe are currently in progress.
In the preset study, mean age of the patients with sarcopenia was 70±5 years. The age of patients with sarcopenia diagnosed using SARC-F was statistically significant higher as compared to disease-free subjects. In Spanish validation studies, patient age was higher by 11 years on average (± 6 years), and sarcopenia was diagnosed using SARC-F in 51 out of 90 subjects.11 In the present study, sarcopenia was diagnosed only in 14 individuals, which might have resulted from younger age of our study population.
Limitations
The study is not without limitations, chief among them a relatively small sample size, although it is compliant with the methodological report from the European Union Geriatric Medicine Society Sarcopenia Special Interest Group.9 Also, the number of people classified as suffering from sarcopenia constitutes only a small proportion of the entire study population and may be biased towards those without sarcopenia. Since participation in the study was voluntary and participants had to be independent to be able to reach the research center, the group of people without sarcopenia was larger.
Another limitation of the study was the considerable disproportion in the number of female and male participants, even taking into account the feminization of aging, which is described as a tendency for women to outlive men.29 According to the Statistics Poland (formerly known as Central Statistical Office), the feminization index in Poland increases with age and is the consequence of higher mortality among the males.30 Further validation of SARC-F should include elderly people with health problems in hospitals or nursing homes.
Conclusions
Sarcopenia is a well-known negative effect of the aging process which reduces functional performance and increases the risk for disability and mortality. In light of the global aging of the population, screening for sarcopenia should become a public health priority. Early detection of sarcopenia can prevent or reduce its negative effects by introducing therapeutic measures. The SARC-F questionnaire is a recommended, translated and validated tool for assessing sarcopenia in everyday practice in Polish conditions. It is an inexpensive, easily applicable and non-invasive method which does not require the use of specialized equipment.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rosenberg IH, Roubenoff R. Stalking sarcopenia. Ann Intern Med. 1995;123(9):727–728. doi: 10.7326/0003-4819-123-9-199511010-00014 [DOI] [PubMed] [Google Scholar]
- 2.Vellas B, Fielding RA, Bens C, et al. Implications of ICD-10 for sarcopenia clinical practice and clinical trials: report by the international conference on frailty and sarcopenia research task force. J Frailty Aging. 2018;7(1):2–9, 2. doi: 10.14283/jfa.2017.30 [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanimoto Y, Watanabe M, Sun W, et al. Sarcopenia and falls in community-dwelling elderly subjects in Japan: defining sarcopenia according to criteria of the European working group on sarcopenia in older people. Arch Gerontol Geriatr. 2014;59(2):295–299. doi: 10.1016/j.archger.2014.04.016 [DOI] [PubMed] [Google Scholar]
- 5.Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J Cachexia Sarcopenia Muscle. 2016;7(3):290–298. doi: 10.1002/jcsm.12073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14(8):531–532. doi: 10.1016/j.jamda.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 7.Malmstrom TK, Miller DK, Simonsick EM, et al. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7(1):28–36. doi: 10.1002/jcsm.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc. 2015;16(3):247–252. doi: 10.1016/j.jamda.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 9.Bahat G, Yilmaz O, Oren MM, et al. Cross-cultural adaptation and validation of the SARC-F to assess sarcopenia: methodological report from European Union geriatric medicine society sarcopenia special interest group. Eur Geriatr Med. 2018;9(1):23–28. doi: 10.1007/s41999-017-0003-5 [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parra-Rodríguez L, Szlejf C, García-González AI, et al. Cross-cultural adaptation and validation of the Spanish-language version of the SARC-F to assess sarcopenia in Mexican community-dwelling older adults. J Am Med Dir Assoc. 2016;17(12):1142–1146. doi: 10.1016/j.jamda.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 12.Beaudart C, McCloskey E, Bruyère O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16:170. doi: 10.1186/s12877-016-0349-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishii S, Tanaka T, Shibasaki K, et al. Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int. 2014;14(1):93–101. doi: 10.1111/ggi.12197 [DOI] [PubMed] [Google Scholar]
- 14.WHO. Process of translation and adaptation of instruments. Available from: https://www.who.int/substance_abuse/research_tools/translation/en/. Accessed November04.
- 15.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive “vitals signs” measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021. doi: [DOI] [PubMed] [Google Scholar]
- 16.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- 17.Katz S, Down TD, Cash HR, et al. Progress in development of the Index of ADL. Gerontologist. 1970;10(1 Part 1):20–30. doi: 10.1093/geront/10.1_Part_1.20 [DOI] [PubMed] [Google Scholar]
- 18.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3 Part 1):179–186. doi: 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 19.Vellas B, Guigoz Y, Garry PJ, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15(2):116–122. doi: 10.1016/S0899-9007(98)00171-3 [DOI] [PubMed] [Google Scholar]
- 20.Yamada Y, Nishizawa M, Uchiyama T, et al. Developing and validating an age-independent equation using multi-frequency bioelectrical impedance analysis for estimation of appendicular skeletal muscle mass and establishing a cutoff for sarcopenia. Int J Environ Res Public Health. 2017;14(7):809. doi: 10.3390/ijerph14070809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figueiredo IM, Sampaio RF, Mancini MC, et al. Teste de força de preensão utilizando o dinamômetro Jamar. Acta Fisiátrica. 2007;14(2):104–110. [Google Scholar]
- 22.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sánchez-Rodríguez D, Marco E, Dávalos-Yerovi V, et al. Translation and validation of the Spanish version of the SARC-F questionnaire to assess sarcopenia in older people. J Nutr Health Aging. 2019;23(6):518–524. doi: 10.1007/s12603-019-1204-z [DOI] [PubMed] [Google Scholar]
- 24.Woo J, Leung J, Morley JE. Validating the SARC-F: a suitable community screening tool for sarcopenia? J Am Med Dir Assoc. 2014;15(9):630–634. doi: 10.1016/j.jamda.2014.04.021 [DOI] [PubMed] [Google Scholar]
- 25.Kera T, Kawai H, Hirano H, et al. SARC-F: a validation study with community-dwelling older Japanese adults. Geriatr Gerontol Int. 2019;19(11):1172–1178. doi: 10.1111/ggi.13768 [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Kim M, Won CW. Validation of the Korean version of the SARC-F questionnaire to assess sarcopenia: Korean frailty and aging cohort study. J Am Med Dir Assoc. 2018;19(1):40–45. doi: 10.1016/j.jamda.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 27.Barbosa-Silva TG, Menezes AMB, Bielemann RM, et al. Enhancing SARC-F: improving sarcopenia screening in the clinical practice. J Am Med Dir Assoc. 2016;17(12):1136–1141. doi: 10.1016/j.jamda.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 28.Cao L, Chen S, Zou C, et al. A pilot study of the SARC-F scale on screening sarcopenia and physical disability in the Chinese older people. J Nutr Health Aging. 2014;18(3):277–283. doi: 10.1007/s12603-013-0410-3 [DOI] [PubMed] [Google Scholar]
- 29.Davidson PM, Digiacomo M, McGrath SJ. The feminization of aging: how will this impact on health outcomes and services? Health Care Women Int. 2011;32(12):1031–1045. doi: 10.1080/07399332.2011.610539 [DOI] [PubMed] [Google Scholar]
- 30.Central Statistical Office [homepage on the Internet]. Information on the situation of the older based on surveys of the central statistical office; 2018. Available from: https://stat.gov.pl/obszary-tematyczne/osoby-starsze/osoby-starsze/informacja-o-sytuacji-osob-starszych-na-podstawie-badan-glownego-urzedu-statystycznego,1,2.html. Accessed March3, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- WHO. Process of translation and adaptation of instruments. Available from: https://www.who.int/substance_abuse/research_tools/translation/en/. Accessed November04.
- Central Statistical Office [homepage on the Internet]. Information on the situation of the older based on surveys of the central statistical office; 2018. Available from: https://stat.gov.pl/obszary-tematyczne/osoby-starsze/osoby-starsze/informacja-o-sytuacji-osob-starszych-na-podstawie-badan-glownego-urzedu-statystycznego,1,2.html. Accessed March3, 2020.