Abstract
In recent years, electronic cigarettes (e-cigs) have been falsely advertised as safe alternatives to conventional smoking. We report a case involving a 16-year-old female who presented with fever, nonproductive cough, and shortness of breath after vaping e-cig/tetrahydrocannabinol dab pen. Her symptoms rapidly deteriorated and met diagnostic criteria for pediatric acute respiratory distress syndrome. Chest radiograph revealed extensive patchy airspace disease and computed tomography scan showed bilateral ground glass opacities. Bronchoalveolar lavage fluid revealed increased neutrophils, lymphocytosis, but absent eosinophilia. After the results of a comprehensive workup for infectious etiology returned negative, she was diagnosed with hypersensitivity pneumonitis and started on systemic corticosteroids.
Keywords: pediatric acute respiratory distress syndrome, hypersensitivity pneumonitis, electronic cigarette
Introduction
Electronic cigarettes (e-cigs) are battery-powered devices that deliver aerosolized nicotine, flavorings, and other substances to the vaping user. E-cigs were developed in 2003 and introduced to the US and European markets in 2006 as safe alternatives to traditional cigarettes. 1 2 3 By 2014, e-cigs became the most commonly used tobacco-containing products among US middle and high school students. 1 Between 2017 and 2018 alone, the Centers for Disease Control and Prevention reported an astounding 38.3% increase among youth e-cig consumers (or 1.5 million more users). 3 Recently, e-cigs have been modified into vaporizer dab pens (vape pens) that deliver liquid tetrahydrocannabinol (THC). Emerging vaping-associated pulmonary illnesses (VAPIs) in adult patients include bronchiectasis, acute eosinophilic pneumonia (AEP), lipoid pneumonia (LP), organizing pneumonia (OP), and diffuse alveolar hemorrhage. 2 This case report describes a previously healthy 16-year-old girl who developed pediatric acute respiratory distress syndrome (PARDS) and acute hypersensitivity pneumonitis (HP) due to inhalation injury related to e-cig vaping.
Case Report
A previously healthy 16-year-old female presented to the emergency department (ED) with chief complaints of fever, nonproductive cough, and shortness of breath for 5 days. On the day prior, she was evaluated at an urgent care and diagnosed with bilateral lower lobes pneumonia ( Fig. 1A ). She received an injection of ceftriaxone and was prescribed oral azithromycin. She developed progressive dyspnea and difficulty breathing, which prompted a return to the ED. The patient has a medical history of mild intermittent asthma. There is no history of atopic dermatitis. There are no sick contacts or dust inhalation. Her immunizations are up to date.
Fig. 1.
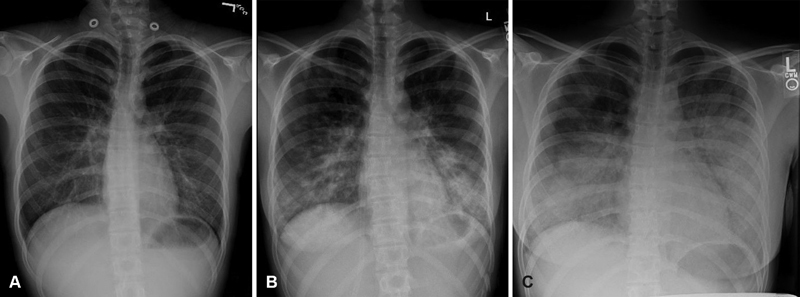
( A ) Initial chest radiograph revealed bilateral lower lobes pneumonia. ( B ) Radiograph on day 1 of admission showed worsening patchy airspace opacities. ( C ) Radiograph on day 2 of admission revealed more extensive patchy airspace disease in the bilateral lung bases.
The patient lives at home with her parents and brother. The household has one cat, two dogs, and one sparrow. The patient traveled to a US territory island on a family cruise about 2 months ago, but denied exposure to pathogens. Six months prior to presentation, she started vaping e-cigs several times daily. She also experimented with a vape pen containing liquid concentrates of THC. Upon further questioning, the patient endorsed purchasing a THC cartridge for her vape pen from an anonymous street vendor just days prior.
Initial vitals were remarkable for oxygen saturation of 90%, temperature of 101.8 F, heart rate of 134 beats/min, respirations of 28 breaths/min, and blood pressure of 122/74 mm Hg. Her cardiac examination did not reveal any rubs nor murmurs. Her lung examination was notable for use of accessory muscles, diffuse fine crackles, and diminished breath sounds at the bases. There was no hepatosplenomegaly. There were no track marks on her arms. No evidence of lower extremity edema or calf tenderness.
A complete blood count (CBC) differential revealed white blood cell of 19.7 (×10 3 /mL), 85% neutrophilic predominance, 2% lymphocytes, 1% monocytes, 23% bands, and absent eosinophilia. Her hemoglobin level was 13.6 g/dL with a platelet count of 343,000/mL. Her C-reactive protein (CRP) was 26.79 mg/dL. Electrolytes and transaminases were normal. Rapid antigen detection assay and polymerase chain reaction (PCR) from a nasopharyngeal swab were both negative for influenza A H1N1. Chest radiograph showed worsening patchy airspace opacities ( Fig. 1B ). She received albuterol/ipratropium treatments, systemic corticosteroids, and broad-spectrum antibiotics. She was admitted to the general pediatric floor for management of presumed asthma exacerbation and superimposed community-acquired bacterial pneumonia.
Her respiratory symptoms deteriorated within 12 hours upon being admitted to the pediatric unit. She required a maximum of 15 L/min supplemental oxygen via nonrebreather mask for hypoxemia and respiratory distress. Early venous blood gas showed pH of 7.38, pCO 2 of 37, base deficit of −2.7, and PaO 2 of 75.8 mm Hg. Repeat radiograph showed new and more extensive patchy airspace disease in the bilateral lung bases ( Fig. 1C ). Computed tomography (CT) scan of the chest showed smooth interlobular septal thickening, bilateral interstitial infiltrates, and ground glass opacities ( Fig. 2 ).
Fig. 2.
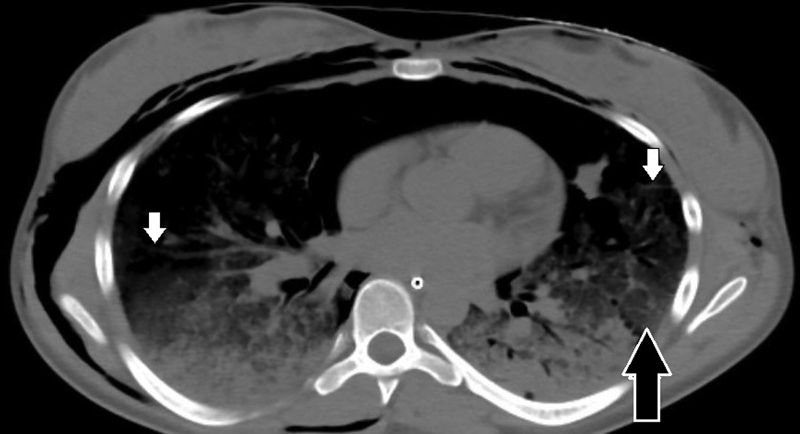
Computed tomography scan of chest showed smooth interlobular septal thickening ( white arrows ), bilateral interstitial infiltrates, and ground glass opacities ( black arrow ).
The patient was transferred to the pediatric intensive care unit where she was placed on a trial of noninvasive bilevel positive airway pressure (BiPAP) with positive end-expiratory pressure (PEEP) of 5. After the trial, she did not tolerate BiPAP weaning and continued to have hypoxemia. She was intubated after her radiographs revealed increasing subcutaneous emphysema throughout the soft tissues of chest/neck, alongside findings of new and worsening pulmonary infiltrates. A chest tube was inserted due to the risks of PARDS and pulmonary air leak syndrome.
After 24 hours of mechanical ventilation (with PEEP of 8 and FiO 2 as high as 87%), her mean airway pressure (MAP) was still below 60 mm Hg, while PaO 2 was 96 mm Hg, reflective of significant rightward shift of the oxygen–hemoglobin dissociation curve. She continued to have severe hypoxemia as evidenced by an oxygenation index (OI) of 21.8. She was escalated to oscillatory therapy using a high-volume strategy that consisted of incremental increases in MAP and lung volumes to maintain PaO 2 ≥ 90 mm Hg. Hemodynamic parameters including cardiac index (CI), systemic vascular resistance index (SVRI), pulmonary vascular resistance index (PVRI), and OI were closely monitored. The patient's CI was 3.7 L/m/m 2 , SVRI was 1,500 dyne·second/cm 5 , and PVRI was 300 dyne·second/cm 5 . She was started on nitric oxide to help decrease pulmonary artery pressures, PVRI, and increase oxygen saturation.
Gross examination from a bedside bronchoscopy revealed edematous and erythematous mucosa of the trachea and mainstem bronchi with thick copious inspissated secretions. Bronchoalveolar lavage (BAL) fluid revealed glandular epithelial and reactive mononuclear cells. BAL cytology was notable for mildly increased neutrophils, lymphocytosis, but absent eosinophilia. The results of BAL testing for Mycoplasma PCR and Legionella direct fluorescent antibody were negative. Aerobic and fungal BAL cultures were negative. Sputum culture was negative for bacterial, viral, and fungal organisms. Other infectious etiologies including tuberculosis, Strongyloides , Coccidioides , Pneumocystis , and Histoplasma were all negative.
Given her rapid onset of PARDS, negative infectious workup, clinical manifestations, and the absence of BAL eosinophilia, a diagnosis of acute HP was made. She was started on high-dose intravenous methylprednisolone and was extubated to room air 7 days afterward. She was transitioned to oral prednisone taper dose and was discharged in good condition.
Discussion
In general, acute respiratory distress syndrome (ARDS) refers to diffuse inflammatory alveolar injury that results in impaired gaseous exchange, decreased pulmonary compliance, and increased pulmonary artery pressure. 4 Etiologies for ARDS include: pneumonia, sepsis, gastric content aspiration, smoke inhalation injury, environmental chemical exposure, drugs, alcohol intoxication, trauma, nonfatal drowning, chemotherapeutic/immunosuppressant agents, or lung stem cell transplant. 4 According to a contemporary Berlin criteria from 2012, ARDS should be suspected in patients with progressive worsening symptoms of dyspnea, an increasing requirement for supplemental oxygen, respiratory failure not explained by fluid overload or congestive heart failure (CHF), and bilateral alveolar infiltrates on radiographs within 6 to 72 hours of presentation. 5 6
A modified set of criteria geared toward PARDS was utilized for this patient, as detailed by the Pediatric Acute Lung Injury Consensus Conference (PALICC) in 2015. Compared with the Berlin definition, which required the presence of “bilateral alveolar infiltrates,” the PALICC criteria for PARDS instead required the presence of “new” infiltrates with an “acute” element attached (bilateral or not). 7 In addition, the PALICC criteria incorporated OI as a marker of degree of hypoxemia in pediatric patients (to account for any potential challenges/difficulties of gaining arterial access). 7 Our patient met all of the PALICC criteria: (1) her time of onset was within 1 week from known clinical insult (i.e., e-cig vaping), (2) her respiratory failure was not due to fluid overload or CHF, (3) she developed “new” infiltrates consistent with “acute” pulmonary parenchymal disease, and (4) her OI of 21.8 was consistent with severe hypoxemia. 7
The incidence of PARDS is low, only 2 to 12 cases per 100,000 person-years. 8 9 Due to the paucity of evidence-based guidelines for PARDS, the stepwise management for this patient was extrapolated from previous adult recommendations and anecdotal reports. The use of PEEP has been an integral part of the standard of treatment in both adults and children with ARDS for decades. 10 In theory, a higher PEEP setting should aid in lung recruitment by keeping alveoli open through the ventilation cycle, while lower PEEP setting will propagate further lung injury as suggested by refractory hypoxemia. 8 In practice, the adverse effects of prolonged excessive PEEP may outweigh the benefits, particularly in pediatric patients. We were cognizant of the fact that end-inspiratory alveolar over-distention associated with prolonged high PEEP (and mechanical ventilation) may worsen intrapulmonary shunt, pulmonary vascular resistance, and increase alveolar dead space. 11 12 Hence, the patient only received a short duration of higher PEEP before she was escalated to oscillatory therapy. One meta-analysis study actually found no statistical reduction in mortality of (adult) patients with ARDS using higher PEEP for a prolonged period of time. 13 To our knowledge, there are currently no ongoing clinical trials on PARDS, so the benefits of providing higher (and prolonged) PEEP for pediatric patients remain to be validated.
HP is defined as an inflammatory disease of the lung parenchyma (i.e., alveoli, terminal bronchioli, and alveolar interstitium) caused by an immunologic response to inhaled antigens such as moldy hay or grains, agricultural dusts, fungus, toxins, or proteins found in bird droppings. 14 Much of the epidemiologic roots of HP has formerly been linked to farmers, dairy cattle workers, bird/poultry handlers (avian-related HP), and lumber milling workers. 14 In most instances, a thorough inquiry regarding occupational, residential, and environmental exposures can help uncover the cause of HP. For our patient, the possibility of having avian-related HP was thoughtfully considered because there was a pet sparrow in the household. After our patient was discharged home, she was appropriately referred to an outpatient allergy/immunology specialist for further workup. Routine serum antibodies against a wide variety of antigens including the sparrow's droppings and feathers were collected with pending results. The main caveat in obtaining serum antibodies is that they are detectable in only 35% of patients who develop chronic and insidious illness, 15 which means that the presence (or absence) of specific antibodies against avian antigens may be insufficient to conclusively rule in (or rule out) a formal diagnosis of avian-related HP. The next most reliable diagnostic approach for identifying avian-related HP is to perform a provoked inhalation challenge . 16 Pigeon dropping extract and pigeon serum are used as antigens for inhalation provocation testing to measure the antigen-specific responses. Unfortunately, there is a lack of an experienced testing location in our geographic region for this type of procedure.
The clinical manifestations of HP are classified as acute , subacute , or chronic depending on the frequency, intensity of exposure, and duration of illness. 17 The acute form corresponds to intermittent, intense allergen exposure. Symptoms can present similarly to a self-limiting viral respiratory tract infection. The subacute form corresponds to continued, but less intense allergen exposure. Common symptoms include dyspnea on exertion, asthenia, and weight loss. The chronic form corresponds to prolonged, insidious inhalation of low concentration of antigen, without prior history of acute disease. It can lead to irreversible lung injury such as pulmonary fibrosis. 5 8 18 19 Some confusion still surrounds the classification of HP in medical literature. The distinction between acute and subacute HPs is often difficult to distinguish as both likely represent different manifestations of a single disease that may be related more to the pattern of antigen exposure than to the offending antigen itself. 20
Overall, there is no pathognomonic diagnostic test for HP. A plausible diagnosis of HP was made for this patient based on a high index of suspicion, evidence of exposure, clinical features, diagnostic imaging findings, laboratory results, BAL fluid analysis, and pulmonary function testing (PFT) results. Schuyler and Cormier provided general major criteria for diagnosing HP, which our patient exhibited: (1) evidence of exposure to chemicals/antigens (i.e., e-cigs), (2) radiograph and CT findings consistent with HP, and (3) lymphocytosis in BAL fluid. 21 The minor criteria included fine crackles auscultated at the lung bases and presence of arterial hypoxemia. 8 CT scan (in the acute and subacute forms) consisted of nodular, ground glass, or airspace opacities. 22 Laboratory results are nonspecific for HP—leukocytosis, increased erythrocyte sedimentation rate/CRP, and positive serum antibodies (immunoglobulin G) to the specific antigens. Of note, the e-cig and THC vape pen in this case report were sent to the Oklahoma Health Department for chemical identification and serologic testing. Once again, positive serologic results would be helpful, but not diagnostic of HP, while the absence of specific antibodies (especially in the acute and subacute forms) does not completely rule out HP either. 23 BAL fluid cytology can be beneficial in diagnosing HP—BAL cell count differential may reveal lymphocytosis, neutrophil predominance, and occasionally eosinophilia. Our patient had BAL counts notable for mildly increased neutrophils, lymphocytosis, but absent eosinophilia. Finally, our patient's PFT cumulative averages were consistent with a restrictive form of interstitial lung disease (i.e., decreased forced vital capacity and decreased total lung capacity).
BAL eosinophilia can also be identified in AEP, which presents with analogous symptoms to that of HP. AEP is a sporadic disease with only 200 cases reported worldwide. 24 The exact etiology of AEP is unknown in most patients; however, a recent change/alteration in tobacco smoking habits, e-cig vaping, or drug intake may trigger this condition. 25 26 Initial symptoms include fever, nonproductive cough, dyspnea, and malaise. 26 A CBC with differential may reveal a neutrophilic leukocytosis early in the course, followed by an eosinophilic predominance with disease progression. 27 Like HP, CT findings of AEP can show patchy ground glass opacities along the bronchovascular bundles. 26 A diagnosis of AEP can be made on the basis of clinical features, CT findings, and BAL fluid sample with > 25% eosinophilia (which was not present in our patient). The mechanism by which vaping can induce AEP is thought to be triggered by a strong inflammatory stimulus that recruits macrophages and neutrophils to the lung tissues. This further induces the release of proinflammatory cytokines (e.g., interleukin [IL]-5, IL-6, and IL-7) and tumor necrosis factor, which may be the inciting event producing eosinophil-rich exudates within the alveoli. 28 29 Suffice it to say that e-cigs in general may induce HP, AEP, and other rare lung diseases in susceptible individuals, as vaping seems to exacerbate the same alveolar inflammatory process previously seen in traditional cigarette smoking. 30
The treatment for HP (and AEP) is centered on avoidance of the inciting agent(s) during the acute phase, which can promptly reverse the course of illness. In more severely ill patients during the subacute and chronic phases, corticosteroids have been shown to accelerate lung recovery. 26 31 The advantage of corticosteroid is a more rapid therapeutic response, while the disadvantage is that it introduces the impossibility to monitor the completeness of allergen avoidance measures. 26 Lack of strict antigenic avoidance and/or nonadherence to corticosteroid therapy may lead to pulmonary fibrosis, 18 the need for lung transplant at a young age, 32 or end-stage lung disease with death. 33 Our patient had good clinical outcomes after corticosteroid administration in the short term. The ultimate impact of vaping on her overall long-term pulmonary function is yet to be determined.
Aside from HP and AEP, the authors considered two other disease processes in the differential diagnosis that have overlapping pathological features and imaging findings attributable to VAPI, including LP and OP. LP is an inflammatory response to the presence of lipids within the alveolar space and typically results from inhalation of oil-based products or hydrocarbons. Recently, LP has been well documented in numerous cases of VAPI (e.g., vitamin E acetate additive found in cannabis vape pens). 34 35 36 Chest radiographs of LP include the typical ground glass opacities and lower lobe consolidations. 37 Macroscopic fat attenuation on CT chest imaging (<−30 Hounsfield units) and BAL specimen with lipid-laden macrophages are the two main hallmark features of LP. 34 35 36 OP, previously called bronchiolitis obliterans , is a rare type of chronic pediatric lung disease characterized by patchy areas of inflammation and infiltrates causing obstruction of the airways via intraluminal polyps of fibrous tissues. 38 The OP histopathologic lesions typically comprise of fibroblast proliferation of granulation tissues within small airways (proliferative bronchiolitis) and alveolar ducts, associated with chronic inflammation in the surrounding alveoli. 39 Even though our patient shared similar imaging patterns with LP, we were able to successfully rule out both diagnoses of LP and OP after careful investigation.
The authors strongly support the following multilevel approach to help curtail the e-cig epidemic: (1) cohesive federal legislative actions, (2) focused state oversight regulations, and (3) local emphasis on counter promotion strategies. 30 In 2015, the federal government regulated manufacturing of e-cigs with the Child Nicotine Poisoning Prevention Act. The Deeming Rule of 2016 extended the Federal Drug Administration's (FDA) regulatory authority over all tobacco products. 40 FDA regulations of e-cig products include: (1) banning sales to consumers < 18 years of age, (2) requiring photo identification verification from consumers < 27 years of age, (3) banning free samples and vending machine sales, and (4) including a warning statement on e-cig packaging explaining that nicotine is addictive. 40 Following the Deeming Rule, manufacturers would be required to submit a premarket review application , allowing the FDA to assess the public health impact of e-cigs. 40 In 2017, implementation of the Deeming Rule was temporarily delayed, which allowed e-cigs to remain on the market without any formal premarket review until the year 2022. 40 Following the 2019 outbreak of VAPI cases linked to e-cigs that sickened thousands of people in the United States, the federal government (as of September 2019) has been working on plans to ban e-cig liquid flavors. There are new policies being proposed to prevent youth access to, and appeal of, flavored tobacco products, e-cigs, and cigars. 41 42 At the time this case report was being written, the FDA has mandated manufacturers of all flavored e-cigs to submit their premarket review applications by August 8, 2021, which is a positive step in the right direction.
While the federal government can regulate the manufacturing of tobacco products, individual states themselves have the ability to regulate the number of tobacco products sold and used. In Oklahoma, where this case report is based, there are no official regulations in place for e-cig packaging, no state excise or special tax (nonsales tax), and no retail license/permit is required to sell. The term “e-cig” is not even mentioned anywhere in the definition of tobacco products in Oklahoma's statutes. 43 The problem is further compounded by the vast marketplace for e-cig products—one that is filled with many counterfeiters, illicit street suppliers, and numerous create-it-at-home vendors. This makes it incredibly challenging for regulators and scientists to pinpoint specific harmful ingredients being distributed. But with the exponential rise in sales especially among US youth consumers, 44 there needs to be more state oversight regulations of all e-cig products.
By early 2018, the US surgeon general declared an “E-cigarette Epidemic” among youth […] and advocated for actions to be taken by parents, teachers, and health care providers. 45 When interviewing pediatric patients about conventional tobacco usage, the clinician should now incorporate queries about vaping into the conversation. 46 Clinicians are encouraged to familiarize themselves with the different e-cig products (e.g., “vape pens,” “pod mods,” “tanks”) and various names for the liquid content(s) they may contain (e.g., “e-juice,” “e-liquid,” “THC cartridges,” “pods”). 47 It is important to remain approachable and use nonjudgmental language to allow a safe environment for discussion. Furthermore, a reiteration to the pediatric patients that their developing brains are more vulnerable to the addictive effects of nicotine […] and that tobacco consumption of any form is not safe. 48
The widespread popularity of e-cigs is attributable to its strategic marketing and frequent presence on television advertisements, internet campaigns, and social media platforms. 2 Therein lies a unique opportunity for clinicians to provide face-to-face counter promotion strategies during regularly scheduled well visits or acute encounters. An adolescent patient who has not experimented with vaping should still recognize the potential dangers and be educated to continue strict avoidance. For those who admit to vaping, there are resources available to aid with cessation. The FDA's “The Real Cost Youth E-cigarette Prevention Campaign” focuses on adolescent (ages 12–17 years) who have either used e-cigs or are interested in trying them by providing educational materials on the dangers of e-cigs. 49 The American Lung Association (ALA) offers a variety of informative resources: (1) “The Vape Talk” is a conversational guide for parents to address this issue with their adolescent kids; (2) “Not on Tobacco” is a 10-week program designed for teens (ages 14–19 years) to help self-identify reason(s) for smoking and provide community resources that will support their efforts to quit. The ALA's Intervention for Nicotine Dependence: Education, Prevention, Tobacco, and Health is a program in which students participate in a series of educational sessions; this program is an alternative for students who face suspension for violation of their school's policies on possession of tobacco, vaping, or nicotine products. 49
Conclusion
Clinicians should be aware of the early symptoms of PARDS and consider HP as a diagnosis in previously healthy adolescents presenting with respiratory failure and concurrent history of e-cig vaping. Although we continue to learn and discover new cases of VAPI every day, we now possess enough knowledge to take immediate actions. At the local level, clinicians should start integrating discussion on the potential harms of vaping with their pediatric patients and provide effective counter promotion strategies when applicable. United bipartisan efforts at the state and federal levels must focus on oversight regulations and enact timely legislative actions because the dramatic rise in e-cig consumption among young adults will soon threaten to unravel decades of public health progress toward tobacco cessation.
Acknowledgment
We thank the patient and her family for their contribution to this case report.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Centers for Disease Control and Prevention.Tobacco use in youth is rising: E-cigarettes are the main reasonAvailable at:https://www.cdc.gov/vitalsigns/youth-tobacco-use. Accessed September 18, 2019
- 2.Wild L G, Lopez M. Hypersensitivity pneumonitis: a comprehensive review. J Investig Allergol Clin Immunol. 2001;11(01):3–15. [PubMed] [Google Scholar]
- 3.Rom O, Pecorelli A, Valacchi G, Reznick A Z. Are E-cigarettes a safe and good alternative to cigarette smoking? Ann N Y Acad Sci. 2015;1340:65–74. doi: 10.1111/nyas.12609. [DOI] [PubMed] [Google Scholar]
- 4.Cheifetz I M. Year in review 2015: pediatric ARDS. Respir Care. 2016;61(07):980–985. doi: 10.4187/respcare.05017. [DOI] [PubMed] [Google Scholar]
- 5.Ranieri V M, Rubenfeld G D, Thompson B T et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 6.Bernard G R, Artigas A, Brigham K L et al. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. Intensive Care Med. 1994;20(03):225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 7.Pediatric Acute Lung Injury Consensus Conference Group.Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference Pediatr Crit Care Med 20151605428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hough R F. Recent advances in pediatric acute respiratory distress syndrome. Curr Pediatr Rep. 2017;5(04):228–236. [Google Scholar]
- 9.Schouten L R, Veltkamp F, Bos A P et al. Incidence and mortality of acute respiratory distress syndrome in children. Crit Care Med. 2016;44(04):819–829. doi: 10.1097/CCM.0000000000001388. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Loh S W, Lee J H. Paediatric acute respiratory distress syndrome: progress over the past decade. J Emerg Crit Care Med. 2018;2:24. [Google Scholar]
- 11.Rouby J J, Brochard L. Tidal recruitment and overinflation in acute respiratory distress syndrome: yin and yang. Am J Respir Crit Care Med. 2007;175(02):104–106. doi: 10.1164/rccm.200610-1564ED. [DOI] [PubMed] [Google Scholar]
- 12.Hubmayr R D. Perspective on lung injury and recruitment: a skeptical look at the opening and collapse story. Am J Respir Crit Care Med. 2002;165(12):1647–1653. doi: 10.1164/rccm.2001080-01CP. [DOI] [PubMed] [Google Scholar]
- 13.Cavalcanti B, Suzumura E A, Laranjeira L N et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs. low PEEP on mortality in patients with acute respiratory syndrome: a randomized clinical trial. JAMA. 2017;318(14):1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez M, Salvaggio J E. Epidemiology of hypersensitivity pneumonitis/allergic alveolitis. Monogr Allergy. 1987;21:70–86. [PubMed] [Google Scholar]
- 15.Richerson H B, Bernstein I L, Fink J Net al. Guidelines for the clinical evaluation of hypersensitivity pneumonitis. Report of the Subcommittee on Hypersensitivity Pneumonitis J Allergy Clin Immunol 198984(5 Pt 2):839–844. [DOI] [PubMed] [Google Scholar]
- 16.Ishizuka M, Miyazaki Y, Tateishi T, Tsutsui T, Tsuchiya K, Inase N. Validation of inhalation provocation test in chronic bird-related hypersensitivity pneumonitis and new prediction score. Ann Am Thorac Soc. 2015;12(02):167–173. doi: 10.1513/AnnalsATS.201408-350OC. [DOI] [PubMed] [Google Scholar]
- 17.Selman M. Shelton, CT: People's Medical Publishing House; 2011. Hypersensitivity pneumonitis; p. 597. [Google Scholar]
- 18.Girard M, Lacasse Y, Cormier Y. Hypersensitivity pneumonitis. Allergy. 2009;64(03):322–334. doi: 10.1111/j.1398-9995.2009.01949.x. [DOI] [PubMed] [Google Scholar]
- 19.Hirschmann J V, Pipavath S N, Godwin J D. Hypersensitivity pneumonitis: a historical, clinical, and radiologic review. Radiographics. 2009;29(07):1921–1938. doi: 10.1148/rg.297095707. [DOI] [PubMed] [Google Scholar]
- 20.Ohtani Y, Saiki S, Sumi Y et al. Clinical features of recurrent and insidious chronic bird fancier's lung. Ann Allergy Asthma Immunol. 2003;90(06):604–610. doi: 10.1016/S1081-1206(10)61863-7. [DOI] [PubMed] [Google Scholar]
- 21.Schuyler M, Cormier Y. The diagnosis of hypersensitivity pneumonitis. Chest. 1997;111(03):534–536. doi: 10.1378/chest.111.3.534. [DOI] [PubMed] [Google Scholar]
- 22.Guillerman R P. Imaging of childhood interstitial lung disease. Pediatr Allergy Immunol Pulmonol. 2010;23(01):43–68. doi: 10.1089/ped.2010.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourke S J, Dalphin J C, Boyd G, McSharry C, Baldwin C I, Calvert J E. Hypersensitivity pneumonitis: current concepts. Eur Respir J Suppl. 2001;32:81s–92s. [PubMed] [Google Scholar]
- 24.Cottin V, Cordier J F. Eosinophilic lung diseases. Immunol Allergy Clin North Am. 2012;32(04):557–586. doi: 10.1016/j.iac.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Uchiyama H, Suda T, Nakamura Y et al. Alterations in smoking habits are associated with acute eosinophilic pneumonia. Chest. 2008;133(05):1174–1180. doi: 10.1378/chest.07-2669. [DOI] [PubMed] [Google Scholar]
- 26.Rhee C K, Min K H, Yim N Y et al. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J. 2013;41(02):402–409. doi: 10.1183/09031936.00221811. [DOI] [PubMed] [Google Scholar]
- 27.Allen J N, Pacht E R, Gadek J E, Davis W B. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N Engl J Med. 1989;321(09):569–574. doi: 10.1056/NEJM198908313210903. [DOI] [PubMed] [Google Scholar]
- 28.Allen J N, Liao Z, Wewers M D, Altenberger E A, Moore S A, Allen E D. Detection of IL-5 and IL-1 receptor antagonist in bronchoalveolar lavage fluid in acute eosinophilic pneumonia. J Allergy Clin Immunol. 1996;97(06):1366–1374. doi: 10.1016/s0091-6749(96)70206-3. [DOI] [PubMed] [Google Scholar]
- 29.Kuschner W G, D'Alessandro A, Wong H, Blanc P D. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur Respir J. 1996;9(10):1989–1994. doi: 10.1183/09031936.96.09101989. [DOI] [PubMed] [Google Scholar]
- 30.Jenssen B P, Walley S C; SECTION ON TOBACCO CONTROL.E-cigarettes and similar devices Pediatrics 201914302e20183652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kokkarinen J I, Tukiainen H O, Terho E O. Effect of corticosteroid treatment on the recovery of pulmonary function in farmer's lung. Am Rev Respir Dis. 1992;145(01):3–5. doi: 10.1164/ajrccm/145.1.3. [DOI] [PubMed] [Google Scholar]
- 32.Selman M, Pardo A, King T E., Jr Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186(04):314–324. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 33.Wiatr E, Radzikowska E, Pawłowski J.Pulmonary fibrosis in young patients with hypersensitivity pneumonitis [in Polish] Pneumonol Alergol Pol 200472(3-4):111–116. [PubMed] [Google Scholar]
- 34.Modi S, Sangani R, Alhajhusain A.Acute lipoid pneumonia secondary to E-cigarette use: an unlikely replacement for cigarettes Chest 2015148(4_Supplement):382A25569856 [Google Scholar]
- 35.McCauley L, Markin C, Hosmer D. An unexpected consequence of electronic cigarette use. Chest. 2012;141(04):1110–1113. doi: 10.1378/chest.11-1334. [DOI] [PubMed] [Google Scholar]
- 36.Viswam D, Trotter S, Burge P S, Walters G I. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep. 2018;2018:bcr-2018–224350. doi: 10.1136/bcr-2018-224350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betancourt S L, Martinez-Jimenez S, Rossi S E, Truong M T, Carrillo J, Erasmus J J. Lipoid pneumonia: spectrum of clinical and radiologic manifestations. AJR Am J Roentgenol. 2010;194(01):103–109. doi: 10.2214/AJR.09.3040. [DOI] [PubMed] [Google Scholar]
- 38.Battistini E, Dini G, Savioli C et al. Bronchiolitis obliterans organizing pneumonia in three children with acute leukaemias treated with cytosine arabinoside and anthracyclines. Eur Respir J. 1997;10(05):1187–1190. doi: 10.1183/09031936.97.10051187. [DOI] [PubMed] [Google Scholar]
- 39.Kligerman S J, Franks T J, Galvin J R. From the radiologic pathology archives: organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. Radiographics. 2013;33(07):1951–1975. doi: 10.1148/rg.337130057. [DOI] [PubMed] [Google Scholar]
- 40.US Food and Drug Administration.The facts on the FDA's new tobacco ruleAvailable at:https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm506676.htm. Accessed September 8, 2019
- 41.US Food and Drug Administration.Statement from FDA commissioner Scott Gottlieb, MD on pivotal public health step to dramatically reduce smoking rates by lowering nicotine in combustible cigarettes to minimally or non-addictive levelsAvailable at:https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-pivotal-public-health-step-dramatically-reduce-smoking. Accessed September 8, 2019
- 42.Office of the Commissioner C for TP.Press Announcements – Statement from the FDA Commissioner Scott Gottlieb, MD., on proposed new steps to protect youth by preventing access to flavored tobacco products and banning menthol in cigarettes 2018. Available at:https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-proposed-new-steps-protect-youth-preventing-access. Accessed September 8, 2019
- 43.Centers for Disease Control and Prevention.State Tobacco Activities Tracking and Evaluation (STATE) System 2018. Available at:https://www.cdc.gov/statesystem/index.htm. Accessed September 8, 2019
- 44.King B A, Gammon D G, Marynak K L, Rogers T. Electronic cigarette sales in the United States, 2013-2017. JAMA. 2018;320(13):1379–1380. doi: 10.1001/jama.2018.10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. Adams Jones. “Surgeon General's Advisory on E-Cigarette Use Among Youth.” Know the Risks: E-Cigarettes & Young People,2018. Available at:https://e-cigarettes.surgeongeneral.gov. Accessed September 18, 2019
- 46.Wyckoff A.Addiction expert offers insight on screening for E-cigarette useAAP. Available at:https://www.aappublications.org/news/2018/11/05/nce18plenary1110518. Accessed September 8, 2019
- 47.American Academy of Pediatrics.AAP Policy Statement: E-cigarettes need stronger regulations to prevent youth access and useAvailable at:https://www.healthychildren.org/English/news/Pages/E-Cigarettes-Need-Stronger-Regulations.aspx. Published January 28, 2019. Accessed September 8, 2019
- 48.US Food and Drug Administration.The Real Cost Campaign 2019. Available at:https://www.fda.gov/tobacco-products/public-health-education-campaigns/real-cost-campaign#1. Accessed September 8, 2019
- 49.American Lung Association.Stop Smoking. “Helping Teens Quit” 2019. Available at:https://www.lung.org/stop-smoking/helping-teens-quit/. Accessed September 8, 2019


