Abstract
Infectious bronchitis virus (IBV), an avian coronavirus, significantly affects the performance of both the egg-laying and meat-type birds causing the foremost of economic loss in poultry industry. This paper aims to develop a rapid, low-cost, and sensitive biosensor for IBV detection by using molybdenum disulfide (MoS2). MoS2 is a 2-D nanosheet which has strong high fluorescence-quenching ability when applied to a dye-labeled antibody (Ab). In this paper, we developed an Ab-functionalized MoS2-based fluorescent immunosensor, which utilized the fluorescence resonance energy transfer (FRET) between the MoS2 and fluorescence dye during the Ab–antigen interaction. The assay was performed on a low-cost cotton thread-based microfluidic platform due to the good wicking property and flexibility. Upon the optimization of assay conditions, the immunosensor demonstrated remarkable sensitivity of 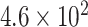 EID50 per mL and specificity with a dynamic linear response range of 102–106 EID50 per mL for IBV standard solutions. The developed immunoassay successfully detected the IBV spiked chicken serum with satisfactory results. The foregoing presents its potential application for on-farm detection.
EID50 per mL and specificity with a dynamic linear response range of 102–106 EID50 per mL for IBV standard solutions. The developed immunoassay successfully detected the IBV spiked chicken serum with satisfactory results. The foregoing presents its potential application for on-farm detection.
Keywords: Infectious bronchitis virus, molybdenum disulfide, FRET, immunosensor, cotton thread
I. Introduction
Coronaviruses are enveloped, positive-strand RNA viruses of birds and mammals including humans. As a representative of the Gamma-coronavirus genus, the avian coronavirus (AvCoV) infectious bronchitis virus (IBV) may cause highly contagious respiratory disease in chickens and other galliforme birds [1]. IBV spread fast between individuals may cause a morbidity rate of 100% in bird populations which have not been vaccinated [2]. Chickens infected with IBV exhibit the symptoms of mild respiratory such as coughing, gasping, rales, and nasal discharge, and appearing depressed, or severe kidney and oviduct disease [3], [4], which may result in a decrease in egg production or quality. Development of affordable and highly sensitive detection methods for rapid monitoring and screening of IBV is extremely important, especially in less-developed countries. The enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) test have now been used as gold standard for nucleic acid biomarker diagnostics. However, conventional ELISA can only work on a higher concentration of target molecules hence makes the approach not quite suitable for highly sensitive detection, while PCR usually requires expensive reagents and equipment as well as skilled personnel for the complicated process [5]. All these limitations restrict their applications in on-site detection.
Nanomaterials have become powerful element in constructing new biosensors due to their unique optical, electronic and catalytic properties [6]–[8]. Recently, transition metal dichalcogenides (TMDs) have gained world-wide attention as a group of 2D layered nanomaterials analogous to graphene owing to their excellent optoelectronics, nanoelectronics, and energy-harvesting properties. TMDs have been widely used in many research including DNA detection, transistors, photodetectors and photovoltaic devices [9], [10]. Molybdenum disulfide (MoS2) is an emerging material and one of such TMDs that can be synthesized in large scale and directly dispersed in aqueous solution and no treatment of surfactants or oxidation is required [11], [12]. Moreover, a higher fluorescence-quenching ability than graphene and the hydrophobicity property of surface make MoS2 become promising material in biosensing platforms and finding various applications [13]–[18]. The hydrophobicity of the MoS2 surface is a key enabling feature, because it enables strong affinity of protein-surface adsorption [11]. Kong et al.
[11] made an aptamer-functionalized MoS2 biosensor by using the high fluorescence-quenching ability between MoS2 and dye-labeled single-stranded DNA probe for prostate specific antigen detection. High sensitivity and high selectivity with a detection limit for the PSA of 0.2 ng/mL were achieved. Tuteja et al.
[18] reported a MoS2-based electrochemical immunosensor for detecting 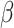 -hydroxybutyrate, which is biomarker of subclinical ketosis. The immunosensor is based on the immmunodetection of the anti-
-hydroxybutyrate, which is biomarker of subclinical ketosis. The immunosensor is based on the immmunodetection of the anti-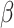 HBA antibodies immobilized on the MoS2-modified electrodes and
HBA antibodies immobilized on the MoS2-modified electrodes and 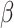 HBA antigen. Geldert et al.
[19] developed fluorescence resonance energy transfer (FRET)-based MoS2 aptasensor for the detection of the malarial biomarker Plasmodium lactate dehydrogenase (pLDH). Zhang et al.
[20] demonstrated a sandwich electrochemiluminescence immunosensor based on MoS2 for alpha fetal protein detection. MoS2 nanosheets surface was modified using polyethylenimine (PEI) polymer and gold nanopaticles. The immunosensor was able to analyze AFP in real human serum samples with limit of detection of 1.0
HBA antigen. Geldert et al.
[19] developed fluorescence resonance energy transfer (FRET)-based MoS2 aptasensor for the detection of the malarial biomarker Plasmodium lactate dehydrogenase (pLDH). Zhang et al.
[20] demonstrated a sandwich electrochemiluminescence immunosensor based on MoS2 for alpha fetal protein detection. MoS2 nanosheets surface was modified using polyethylenimine (PEI) polymer and gold nanopaticles. The immunosensor was able to analyze AFP in real human serum samples with limit of detection of 1.0 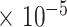 ng/mL. These research demonstrate the huge protetial of MoS2 in biosensor applications.
ng/mL. These research demonstrate the huge protetial of MoS2 in biosensor applications.
The thread-based microfluidics is promising alternative to conventional microfluidic systems due to it many special characters. The thread-based biomedical devices are low cost and broadly available, flexible, easily to handle, lightweight, easy to manipulate and facilitate to transported or stored in any forms. In addition, the wicking properties of thread enable the low volumes of sample solution through it efficiently. All above mentioned makes thread-based microfluidics an attractive matrix for the fabrication of low-cost and low-volume microfluidic diagnostic devices for handheld on-site diagnosis applications [21]–[23].
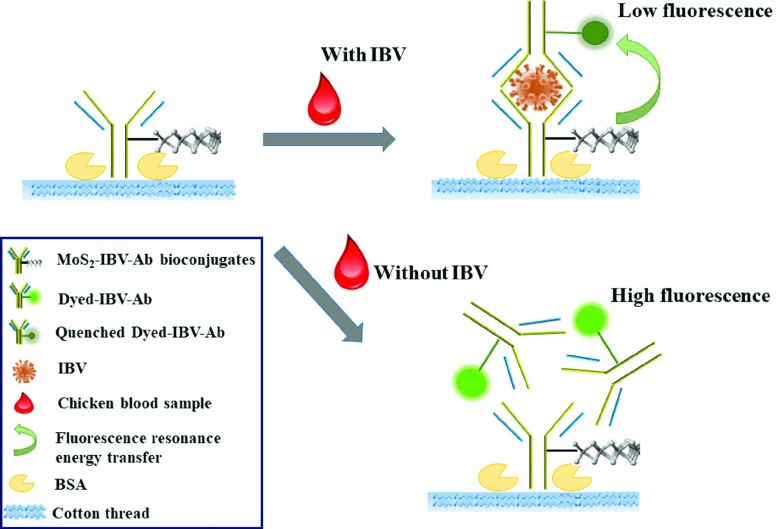
Here, we aimed at developing a biosensor for rapid detection of IBV. We developed a single-step immunosensor on cotton thread based on antibody-functionalized MoS2 for the detection of IBV. The principle is based on a homogeneous FRET immunoassay, the sensing mechanism is shown in Fig. 1. The distinct quenching property of MoS2 on the fluorophore during the antigen-antibody reaction was adopted. The antibody (Ab) probes are modified with fluorescent dye labelling (dyed-IBV-Ab) and MoS2(MoS2-IBV-Ab bioconjugates), respectively. In the presence of IBV, both the dyed-IBV-Ab and the MoS2-IBV-Ab bioconjugates will specifically bind with the target IBV due to the antigen-antibody reaction. After binding, the fluorescence of the dyed-IBV-Ab probe is largely quenched owing to the transfer of electrons or energy between the closely connected dye molecules and the MoS2. In the absence of IBV, the fluorescence of the dyed-IBV-Ab probe will not change. Therefore, the concentration of the IBV in the chicken blood sample can be quantitatively determined by analysis the fluorescence intensity in an assay.
Fig. 1.
Schematic of the single-step homogeneous immunoassay on cotton thread using MoS2-based FRET for IBV detection.
II. Immunosensor Principle and Experiment
A. Materials
MoS2 nano-sheet dispersion, ovine serum albumin (lyophilized powder, ≥96%), hydrogen peroxide (H2O2), poly(L-lysine) (PLL), chicken serum and all other mentioned chemicals and solvents were purchased from Sigma-Aldrich (Oakville, ON, Canada). Anti-infectious bronchitis virus (Massachusetts) (IBV) was purchased MyBioSource, Inc. (San Diego, CA, USA). Infectious bronchitis virus, low pathogenic avian influenza virus A H4N6 and H9N2 were kindly provided by our collaborator (Ontario Veterinary College, Canada) and the detailed culture procedure can be found elsewhere [24]. Briefly, isolate of IBV was propagated in embryonated specific pathogen free (SPF) chicken eggs followed by the titration determined by the method of Reed and Muench. The viral titer of the stock solution was 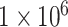 EID50 per mL (egg infectious dose 50%). Avian influenza virus A H4N6 (Avian influenza virus A/Duck/Czech/56 (H4N6)) was propagated in 11-day-oldembryonated chicken eggs by inoculation into the allantoic cavity [25]. Inactivated Avian influenza virus A H9N2 (A/Turkey/Ontario/1/66) was propagated in 10-day-old embryonated SPF chicken eggs followed by inactivating with formalin (final concentration 0.02%) for 72 h at 37 °C [26]. Alexa Fluor 488 Antibody Labeling Kit was purchased from Life Technologies Inc. (Burlington, ON, Canada). Chicken whole blood (Cat. no. IR1-080N) was obtained from Innovation Research, Michigan, USA. Milli-Q water (18.2
EID50 per mL (egg infectious dose 50%). Avian influenza virus A H4N6 (Avian influenza virus A/Duck/Czech/56 (H4N6)) was propagated in 11-day-oldembryonated chicken eggs by inoculation into the allantoic cavity [25]. Inactivated Avian influenza virus A H9N2 (A/Turkey/Ontario/1/66) was propagated in 10-day-old embryonated SPF chicken eggs followed by inactivating with formalin (final concentration 0.02%) for 72 h at 37 °C [26]. Alexa Fluor 488 Antibody Labeling Kit was purchased from Life Technologies Inc. (Burlington, ON, Canada). Chicken whole blood (Cat. no. IR1-080N) was obtained from Innovation Research, Michigan, USA. Milli-Q water (18.2 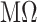 ) was used in all experiments.
) was used in all experiments.
B. Preparation of Dyed-IBV-Ab
The labelling of IBV Ab with fluorescent dye is conducted by using the Alexa Fluor 488 Antibody Labeling Kit. Briefly, 1.0 mg/mL IBV Ab was mixed with 1 M sodium bicarbonate solution. Then 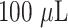 of the mixture was added into the vial of Alexa FLuo dye followed by an incubation of 1 h at room temperature. The labeled antibody was then obtained via purification using a purification column with resin bed and centrifuged at
of the mixture was added into the vial of Alexa FLuo dye followed by an incubation of 1 h at room temperature. The labeled antibody was then obtained via purification using a purification column with resin bed and centrifuged at 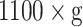 for 5 min. The labeled antibody was stored at 4 °C for further use.
for 5 min. The labeled antibody was stored at 4 °C for further use.
C. Preparation of MoS2-IBV-Ab Bioconjugates
The MoS2 acted as a nanoplatform to adsorb IBV Ab and was further employed as a biological probe. The MoS2 (0.5 mg/mL) dispersion in aqueous was first concentrated by centrifugation at 8000 rpm for 30 min to remove the surfactant. The MoS2 was resuspended in Milli-Q water and sonicated for 1 h to provide a homogeneous solution. Aqueous poly(L-lysine) solution was added to the resulting MoS2 at a concentration of 1 mg/mL and stirred thoroughly for 1 h followed by overnight incubation at 4 °C. The poly(L-lysine) backbone is electrostatically adsorbed to MoS2 surface. The PLL-MoS2 mixture was purified and concentrated by centrifugation three times at 8000 rpm for 30 min to remove the solvent and resuspended in PBS. IBV Ab was then added at a desired concentration into the obtained mixture followed by overnight incubation at 4 °C to produce the MoS2-IBV-Ab bioconjugates. With the chemical modification by poly(L-lysine), the antibodies can be bind to MoS2 via electrostatic and covalent interactions [27]. The resulting mixture was purified at 5000 rmp at 4 °C and resuspended in PBS with desired concentrations for further use.
D. Preparation of Thread-Based Microfluidics
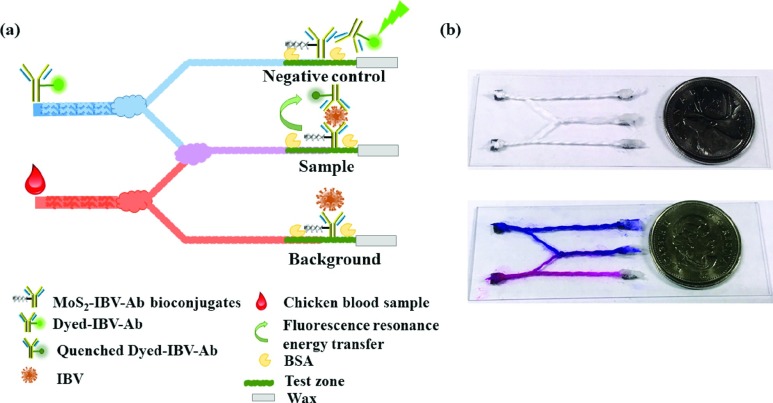
Cotton threads (100%) were boiled by 2 M NaCl for 30 min, and then soaked in 0.01% H2O2 and 0.01 M HCl for 5 min respectively, followed by washing with a large amount of ultrapure water to remove the residual acid. Finally, cotton threads were dried at 37 °C for at least 2 h and stored for further use. Cotton threads were rendered hydrophilic with an air plasma (Harrick Plasma, Ithaca, NY, USA). The design of the thread network is shown in Fig. 2. Two individual thread for sample and probe reagent dispensing were split to two streams. One of the two stream was then recombined with knot for mixing. Noted that the length of the split stream should be identical to achieve good mixing ratio between two streams flowing into it through the interwoven knot [21]. The other two streams are used for negative control (dyed-IBV-Ab solution, no IBV presented) and background testing, respectively. A test zones were defined on each branch thread. A test zone was coated with 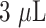 (applied as three aliquots of
(applied as three aliquots of 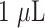 , with 10 min drying at 37 °C after each step) of MoS2-IBV-Ab bioconjugates. The cotton thread was blocked with 1% BSA in PBS for 30 min, rinsed with PBS (0.1% Tween 20) and Milli-Q water, and quickly dried under a stream of N2. Paraffin wax was applied on the end of the each thread channels to create isolated zones to prevent the fluid flowing. The cotton thread was stretched on the glass slide with the paper support using double adhesive tape. The cotton threads with MoS2-IBV-Ab bioconjugates were stored at 4 °C and used within one week.
, with 10 min drying at 37 °C after each step) of MoS2-IBV-Ab bioconjugates. The cotton thread was blocked with 1% BSA in PBS for 30 min, rinsed with PBS (0.1% Tween 20) and Milli-Q water, and quickly dried under a stream of N2. Paraffin wax was applied on the end of the each thread channels to create isolated zones to prevent the fluid flowing. The cotton thread was stretched on the glass slide with the paper support using double adhesive tape. The cotton threads with MoS2-IBV-Ab bioconjugates were stored at 4 °C and used within one week.
Fig. 2.
(a) Schematic of the thread network for immunosensor (not to scale). (b) A picture of the cotton thread microfluidics.
E. Characterization
The morphology of MoS2 was characterized by the FEI-Tecani G2 transmission electron microscope (TEM) operating at 200 kV. The hydrodynamic diameters, size distribution and Zeta (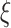 ) potentials of the MoS2, PLL-MoS2 and MoS2-IBV-Ab bioconjugates were measured in water by a dynamic light scattering (DLS) system (Malvern Zetasizer Nano ZS, UK). The UV-Vis absorption spectra were analyzed on DR6000TM UV-Vis spectrophotometer (HACH, Loveland, Colorado, CA, USA) with a resolution of 1 nm. The fluorescence spectra were recorded by the Cytation 5 Multi-mode Reader (BioTek, Winooski, VT, USA). The fluorescent imaging (Ex/Em=488 nm/519 nm) was taken on a fluorescent microscopy (Nikon Eclipse Ti, Nikon Canada Inc., Mississauga, ON, Canada) under the same settings, namely exposure time, magnification, etc. The fluorescence intensity was then analyzed by analyzed by ImageJ.
) potentials of the MoS2, PLL-MoS2 and MoS2-IBV-Ab bioconjugates were measured in water by a dynamic light scattering (DLS) system (Malvern Zetasizer Nano ZS, UK). The UV-Vis absorption spectra were analyzed on DR6000TM UV-Vis spectrophotometer (HACH, Loveland, Colorado, CA, USA) with a resolution of 1 nm. The fluorescence spectra were recorded by the Cytation 5 Multi-mode Reader (BioTek, Winooski, VT, USA). The fluorescent imaging (Ex/Em=488 nm/519 nm) was taken on a fluorescent microscopy (Nikon Eclipse Ti, Nikon Canada Inc., Mississauga, ON, Canada) under the same settings, namely exposure time, magnification, etc. The fluorescence intensity was then analyzed by analyzed by ImageJ.
F. Operational Procedure
In a typical assay, a small volume (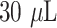 ) of the fluorescence probe (dyed-IBV-Ab) and sample solution were added into the inlets of functionalized threads, respectively. An incubation time of 0~12 min at room temperature was then given to ensure the antigen-antibody reaction. After incubation, the fluorescence measurements were taken and recorded to analyze the change in fluorescence intensity using a fluorescence microscope equipped with a charge-coupled device camera. The intensity of the control zone and the background zone were used for calculation. Fluorescence images were converted into a numerical response using ImageJ software, a region of interest on the thread was drawn on the thread to perform the measurement.
) of the fluorescence probe (dyed-IBV-Ab) and sample solution were added into the inlets of functionalized threads, respectively. An incubation time of 0~12 min at room temperature was then given to ensure the antigen-antibody reaction. After incubation, the fluorescence measurements were taken and recorded to analyze the change in fluorescence intensity using a fluorescence microscope equipped with a charge-coupled device camera. The intensity of the control zone and the background zone were used for calculation. Fluorescence images were converted into a numerical response using ImageJ software, a region of interest on the thread was drawn on the thread to perform the measurement.
G. Validation and Optimization of Detection Conditions
The validation and optimization of the sensing mechanism was performed by fluorescent spectra analysis on a microplate reader. The effect of the concentrations of dyed-IBV-Ab (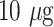 /mL,
/mL, 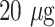 /mL and
/mL and 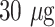 /mL), MoS2-IBV-Ab bioconjugates and incubation time on the fluorescence quenching were studied. Ten microliter of MoS2-IBV-Ab, sample and dyed-IBV-Ab were sequentially added in the microwell followed by fluorescence intensity analysis. All experiment was conducted in triplicate.
/mL), MoS2-IBV-Ab bioconjugates and incubation time on the fluorescence quenching were studied. Ten microliter of MoS2-IBV-Ab, sample and dyed-IBV-Ab were sequentially added in the microwell followed by fluorescence intensity analysis. All experiment was conducted in triplicate.
H. Immunosensing of IBV in Chicken Whole Blood and Specificity
The performance of the presented immunosensor for detecting real blood sample was validated. IBV of various concentration (1, 5, 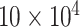 EID50 per mL) was spiked in the chicken whole blood and loaded into the sensor. The average fluorescence intensity is then calculated to determine the sample concentration. To evaluate the specificity of the presented immunosensor, avian influenza A H4N6 and H9N2 were detected. The fluorescence signals with these non-specific analytes were recorded.
EID50 per mL) was spiked in the chicken whole blood and loaded into the sensor. The average fluorescence intensity is then calculated to determine the sample concentration. To evaluate the specificity of the presented immunosensor, avian influenza A H4N6 and H9N2 were detected. The fluorescence signals with these non-specific analytes were recorded.
III. Results and Discussion
A. Characterization
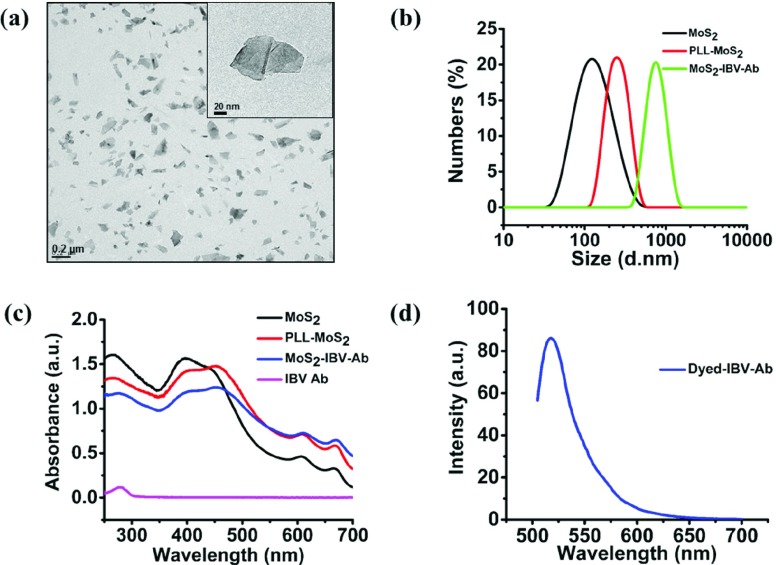
The structure and morphology of the MoS2 dispersion was investigated with the use of TEM (Fig. 3 (a)). TEM of the MoS2 nanosheets emphasized the uniformly sized nano MoS2 with a sheet-like morphology. The size distribution and zeta (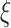 ) potential of the MoS2, PLL-MoS2 dispersion and MoS2-IBV-Ab bioconjugates were characterized to evaluate the binding by dynamic light scattering (DLS) system (Malvern Zetasizer Nano ZS, UK). As shown in Fig. 3 (b), the size distribution of MoS2, PLL-MoS2 dispersion and MoS2-IBV-Ab bioconjugates are 113.5±0.32 nm, 162.1±1.07 nm and 983±84.5 nm, respectively. The Zeta potential (
) potential of the MoS2, PLL-MoS2 dispersion and MoS2-IBV-Ab bioconjugates were characterized to evaluate the binding by dynamic light scattering (DLS) system (Malvern Zetasizer Nano ZS, UK). As shown in Fig. 3 (b), the size distribution of MoS2, PLL-MoS2 dispersion and MoS2-IBV-Ab bioconjugates are 113.5±0.32 nm, 162.1±1.07 nm and 983±84.5 nm, respectively. The Zeta potential (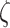 ) values of MoS2, PLL-MoS2 dispersion and MoS2-IBV-Ab bioconjugates, as shown in Fig. S1, are −37.9±0.7 mV, +37.3±1.18 mV and −3.6±0.9 mV, respectively, which indicate the bind of the antibody and moderate dispersion of the antibody conjugated nanoparticles in aqueous medium [28]–[30]. The optical properties of MoS2 dispersion in DI water, PLL-MoS2, MoS2-IBV-Ab and pure IBV Ab was observed by UV-vis absorption measurement. As shown in Fig. 3(c), four characteristic peaks at ~398 nm, ~450 nm, ~612 nm and ~670 nm appeared which are due to band gap energies of 3.18 eV, 2.76 eV, 2.03 eV and 1.85 eV, respectively. The spectra is consistent with [31] and [32]. Fig. 3 (d) presents the fluorescence spectra of the dyed-IBV-Ab.
) values of MoS2, PLL-MoS2 dispersion and MoS2-IBV-Ab bioconjugates, as shown in Fig. S1, are −37.9±0.7 mV, +37.3±1.18 mV and −3.6±0.9 mV, respectively, which indicate the bind of the antibody and moderate dispersion of the antibody conjugated nanoparticles in aqueous medium [28]–[30]. The optical properties of MoS2 dispersion in DI water, PLL-MoS2, MoS2-IBV-Ab and pure IBV Ab was observed by UV-vis absorption measurement. As shown in Fig. 3(c), four characteristic peaks at ~398 nm, ~450 nm, ~612 nm and ~670 nm appeared which are due to band gap energies of 3.18 eV, 2.76 eV, 2.03 eV and 1.85 eV, respectively. The spectra is consistent with [31] and [32]. Fig. 3 (d) presents the fluorescence spectra of the dyed-IBV-Ab.
Fig. 3.
(a) TEM image of MoS2. (b) Particle size distribution of pure MoS2, PLL-MoS2, MoS2-IBV-Ab by DLS. The mean hydration diameter of the MoS2, PLL-MoS2, MoS2-IBV-Ab are 21.9 nm and 47.9 nm, respectively. (c) UV-Vis absorption spectrum for MoS2 dispersion in DI water, PLL-MoS2, MoS2-IBV-Ab and pure IBV Ab. (d) Fluorescence spectra of the dyed-IBV-Ab.
B. Evaluation of Sensing Mechanism and Optimization
The validation and optimization of the biosensor was performed by a microplate reader. Standard IBV solution was made at a series of concentration of 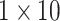 (IBV1),
(IBV1), 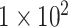 (IBV2),
(IBV2), 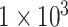 (IBV3),
(IBV3), 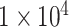 (IBV4),
(IBV4), 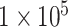 (IBV5) and
(IBV5) and 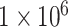 (IBV6) EID50 per mL.
(IBV6) EID50 per mL.
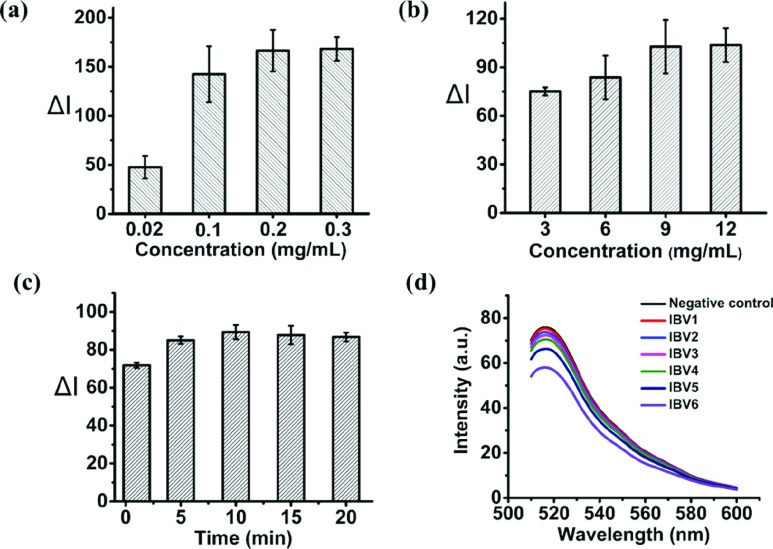
When preparing the thread, the MoS2-IBV-Ab conjugates was dropped on the thread, it filled up the micro-voids between thread fibers due to capillary action and got trapped between them. Additionally, upon drying, the conjugates also wrapped around the fibers. The effect of concentration of MoS2-IBV-Ab and dyed-IBV-Ab were investigated as well as the reaction time duration. A series of concentrations of MoS2-IBV Ab, 0.02 mg/mL, 0.1 mg/mL, 0.2 mg/mL and 0.3 mg/mL were carried out while keeping the concentration of dyed-IBV-Ab at 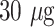 /mL and the reaction time with 10 min. Fig. 4 (a) shows the relative fluorescence intensity difference (
/mL and the reaction time with 10 min. Fig. 4 (a) shows the relative fluorescence intensity difference (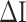 ) compared to negative control on the IBV standard of
) compared to negative control on the IBV standard of 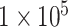 (IBV5) EID50 per mL. Result was taken at the moment of 10 min. A significant increase when the concentration of MoS2-IBV-Ab goes higher, however, no distinguishable difference is observed for the concentration higher than 0.2 mg/mL. Therefore, 0.2 mg/mL MoS2-IBV-Ab was selected as the optimized concentration. Similarly, the optimized concentration of the dyed-IBV-Ab was investigated by carried out the detection with a series of concentration,
(IBV5) EID50 per mL. Result was taken at the moment of 10 min. A significant increase when the concentration of MoS2-IBV-Ab goes higher, however, no distinguishable difference is observed for the concentration higher than 0.2 mg/mL. Therefore, 0.2 mg/mL MoS2-IBV-Ab was selected as the optimized concentration. Similarly, the optimized concentration of the dyed-IBV-Ab was investigated by carried out the detection with a series of concentration, 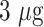 /mL,
/mL, 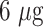 /mL,
/mL, 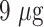 /mL and
/mL and 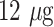 /mL. The result with a reaction time of 10 min is shown in Fig. 4 (b), the quenching effect increases as the increase of the concentration of the dyed-IBV-Ab while becoming “constant” when it goes above to
/mL. The result with a reaction time of 10 min is shown in Fig. 4 (b), the quenching effect increases as the increase of the concentration of the dyed-IBV-Ab while becoming “constant” when it goes above to 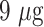 /mL. The reaction time duration was also studied, an experiment was carried out by detecting the IBV standard of
/mL. The reaction time duration was also studied, an experiment was carried out by detecting the IBV standard of 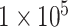 (IBV5) EID50 per mL using the optimized concentrations of MoS2-IBV-Ab and dyed-IBV-Ab, 0.2 mg/mL
(IBV5) EID50 per mL using the optimized concentrations of MoS2-IBV-Ab and dyed-IBV-Ab, 0.2 mg/mL 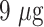 /mL, respectively. Time duration of 1 min, 5 min, 10 min and 20 min were investigated, the response of the sensing method is shown in Fig. 4 (c). The result shows that 10 min is more than sufficient to complete the quenching. Three triplicates were carried out for each data point and then the average of three independent measurements was calculated, the error bars indicate the standard deviation of the mean (n = 3). Under the optimized settings, fluorescence analysis for a series of IBV standard solution (from 0 to 106 EID50 per mL, in PBS buffer of pH 7.4) was conducted, the result of which is shown in Fig. 4 (d). The result highlights a decrease in fluorescence intensity with the increased IBV concentration.
/mL, respectively. Time duration of 1 min, 5 min, 10 min and 20 min were investigated, the response of the sensing method is shown in Fig. 4 (c). The result shows that 10 min is more than sufficient to complete the quenching. Three triplicates were carried out for each data point and then the average of three independent measurements was calculated, the error bars indicate the standard deviation of the mean (n = 3). Under the optimized settings, fluorescence analysis for a series of IBV standard solution (from 0 to 106 EID50 per mL, in PBS buffer of pH 7.4) was conducted, the result of which is shown in Fig. 4 (d). The result highlights a decrease in fluorescence intensity with the increased IBV concentration.
Fig. 4.
Sensing mechanism optimization and validation: (a) effect of concentrations of MoS2-IBV-Ab on the biosensor; (b) effect of concentrations of dyed-IBV-Ab (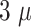 g/mL,
g/mL, 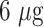 /mL,
/mL,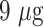 /mL and
/mL and 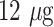 /mL) on the biosensor; (c) effect of reaction time duration (1 min, 5 min, 10 min, 15 min and 20 min) on the biosensor; and (d) illustrative fluorescent spectra of the biosensor tested with multiple concentration of IBV standards ranging from 10~106 EID50 per mL.
/mL) on the biosensor; (c) effect of reaction time duration (1 min, 5 min, 10 min, 15 min and 20 min) on the biosensor; and (d) illustrative fluorescent spectra of the biosensor tested with multiple concentration of IBV standards ranging from 10~106 EID50 per mL.
C. Detection of IBV on Cotton Thread
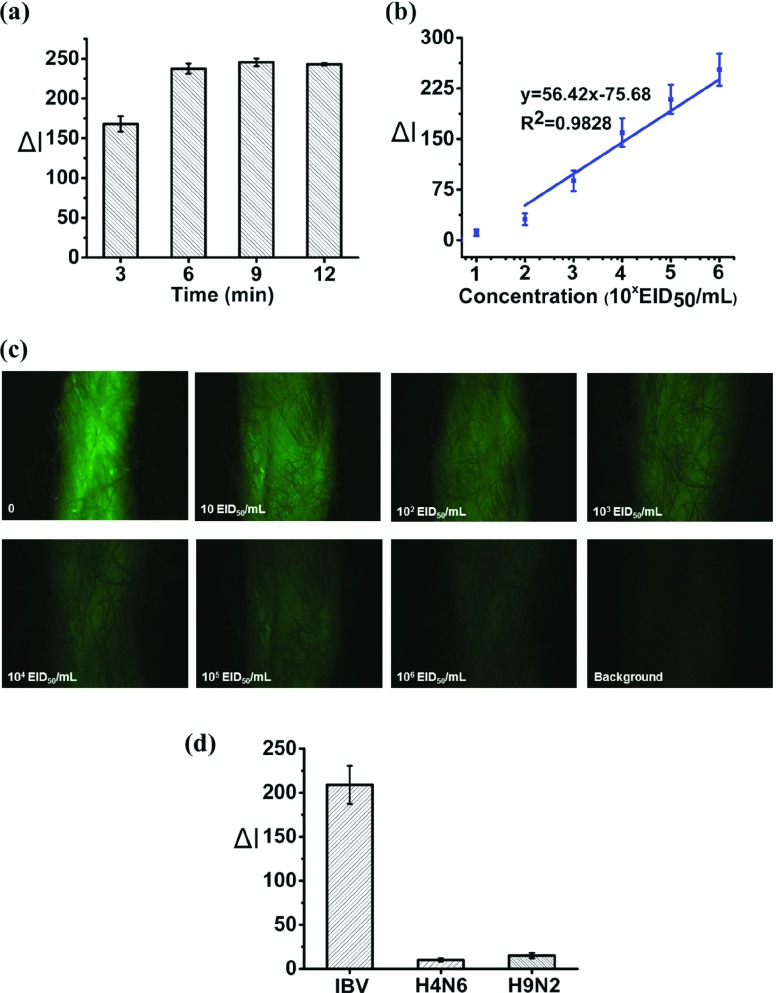
With the optimization settings as demonstrated in previous section, quantitative analysis of IBV was carried out on the cotton thread. The transportation of fluidic was investigated firstly by using food color and it was observed that the fluid could reach the test zones within 1 min due to the capillary wicking. In addition, a time duration of 6 min was found to be sufficient for a complete reaction, as shown in Fig. 5 (a). The reaction time is slightly shorter that conducted in microplate. This may be attributed to the faster kinetics due to the better mixing effect on the thread. A wide range of IBV standard solution was detected. The IBV standard solution ranging from 0~106 EID50 per mL was analyzed by the presented thread immunosensor to obtain the standard curve and calculate the limit of detection. A linear fit of the obtained relative fluorescence intensity difference with respect to varying concentration of IBV standards is plotted in Fig. 5 (b). The values denote average relative fluorescence intensity difference (n = 3) compared to negative control with standard deviation as error bars. It is shown that a distinct change presents starting from 102 EID50 per mL with a correlation coefficient (R2) of 0.9828. The limit of detection calculated based on 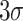 [33] of the blank is
[33] of the blank is 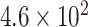 EID50 per mL. The different fluorescence responses of the test zones on the cotton thread upon the detection of various concentrations of IBV standard solution are shown in Fig. 5 (c). It is clearly seen that the fluorescence of the dyed-IBV-Ab was significantly quenched for all concentrations of IBV standard solution. With the concentration of IBV increases, the fluorescence is quenched more because more “sandwich” complex of dyed-IBV-Ab / IBV / MoS2-IBV-Ab are formed, resulting in largely quenched owing to the transfer of electrons or energy between the closely connected dye molecules and the MoS2.
EID50 per mL. The different fluorescence responses of the test zones on the cotton thread upon the detection of various concentrations of IBV standard solution are shown in Fig. 5 (c). It is clearly seen that the fluorescence of the dyed-IBV-Ab was significantly quenched for all concentrations of IBV standard solution. With the concentration of IBV increases, the fluorescence is quenched more because more “sandwich” complex of dyed-IBV-Ab / IBV / MoS2-IBV-Ab are formed, resulting in largely quenched owing to the transfer of electrons or energy between the closely connected dye molecules and the MoS2.
Fig. 5.
Detection of IBV on cotton thread. (a) Time effect on cotton thread under optimized settings. (b) Standard fluorescence intensity calibration plot against varying concentrations of IBV in standard PBS buffer (pH 7.4). (c) Representative fluorescence images of the test zones on the cotton thread upon the detection of various concentrations of IBV standard solution. (d) Specificity of the developed cotton biosensor with different influenza viruses. Error bars indicate the standard deviation of the mean (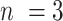 ).
).
D. Accuracy of the Biosensor
The specificity of the developed cotton thread biosensor was evaluated against non-specific virus, avian influenza A H4N6 (100 HAU/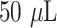 ) and H9N2 (100 HAU/
) and H9N2 (100 HAU/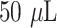 ). As shown in Fig. 5 (d), no distinguishable singles were obtained in the presence of the introduced interferents, which indicates that the highly specific towards to IBV.
). As shown in Fig. 5 (d), no distinguishable singles were obtained in the presence of the introduced interferents, which indicates that the highly specific towards to IBV.
ELISA was carried out side by side to validate the accuracy of the biosensor. Briefly, a ninety-six-well Maxisorp microtiter plate (Life Technologies Inc., Burlington, ON, Canada) was coated with the IBV Ab at 10 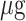 /mL diluted in filtered PBS (pH 7.4), and incubated overnight at 4 °C. The wells were washed with 0.05% Tween-20 (
/mL diluted in filtered PBS (pH 7.4), and incubated overnight at 4 °C. The wells were washed with 0.05% Tween-20 (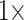 PBS, pH 7.4) three times followed by being blocked with
PBS, pH 7.4) three times followed by being blocked with 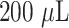 of 3% BSA in PBS at room temperature for 2 h. After washing for three times,
of 3% BSA in PBS at room temperature for 2 h. After washing for three times, 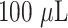 of standards and spiked chicken blood were added, incubated for 1 h at room temperature and washed for three times with 0.05% Tween-20 (
of standards and spiked chicken blood were added, incubated for 1 h at room temperature and washed for three times with 0.05% Tween-20 (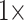 PBS, pH 7.4). Into each well
PBS, pH 7.4). Into each well 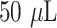 of detection antibody dyed-IBV-Ab was added followed by incubation for 1 h at room temperature and washed with 0.05% Tween-20 (
of detection antibody dyed-IBV-Ab was added followed by incubation for 1 h at room temperature and washed with 0.05% Tween-20 (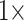 PBS, pH 7.4) three times.
PBS, pH 7.4) three times. 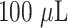 of filtered PBS (pH 7.4) was then added into each well and followed by the fluorescence intensity analysis (Ex=488 nm, Em=519 nm) on a microplate reader. All samples were tested in triplicate. Table I summarizes the results of the detection of IBV spiked chicken blood by presented immunosensor and ELISA. A good recoveries and consistency of the spiked IBV are presented. The standard derivations (SD) were 1.0~10% for both of methods. The total detection time from adding a sample was around 10 min. The results clearly demonstrated that the presented immunosensor is capable of the single-step detection of IBV in chicken blood sample and its high accuracy.
of filtered PBS (pH 7.4) was then added into each well and followed by the fluorescence intensity analysis (Ex=488 nm, Em=519 nm) on a microplate reader. All samples were tested in triplicate. Table I summarizes the results of the detection of IBV spiked chicken blood by presented immunosensor and ELISA. A good recoveries and consistency of the spiked IBV are presented. The standard derivations (SD) were 1.0~10% for both of methods. The total detection time from adding a sample was around 10 min. The results clearly demonstrated that the presented immunosensor is capable of the single-step detection of IBV in chicken blood sample and its high accuracy.
TABLE I. Comparison of Avian Detection Using Presented Immunosensor and ELISA Method.
Spiked concentration (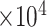 EID50 per mL) EID50 per mL) |
Immunosensor (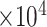 EID50 per mL) EID50 per mL) |
SD (%) | ELISA (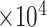 EID50 per mL) EID50 per mL) |
SD (%) |
|---|---|---|---|---|
| 1.0 | 0.98 | 3.1 | 0.96 | 2.2 |
| 5.0 | 4.63 | 9.3 | 4.83 | 6.8 |
| 10.0 | 9.80 | 6.2 | 9.76 | 5.1 |
| 50.0 | 46.5 | 1.0 | 46.1 | 1.6 |
IV. Conclusions
In this study, we reported a proof-of-concept MoS2-based immunosensor on a thread-based microfluidic network for rapid IBV detection. The distinct quenching property of MoS2 on the fluorophore during the antigen-antibody reaction was adopted. The thread-based network interconnected by knots to achieve the fluid mixing and separation. IBV standards and spiked chicken blood sample were successfully detection with high specificity and a detection of limit of 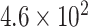 EID50 per mL. The present immunosensor demonstrated a good linearity and validated with ELISA method. In comparison with conventional immunological tests, the presented method have many advantages, such as ease of local manufacture, small consumption of reagents and samples, high sensitive and short time of analysis. All these characteristics allow for the use of this technology for rapid, prompt on-site IBV detection.
EID50 per mL. The present immunosensor demonstrated a good linearity and validated with ELISA method. In comparison with conventional immunological tests, the presented method have many advantages, such as ease of local manufacture, small consumption of reagents and samples, high sensitive and short time of analysis. All these characteristics allow for the use of this technology for rapid, prompt on-site IBV detection.
Biographies
Xuan Weng received the Ph.D. degree in mechatronics engineering from the University of Waterloo, Canada, in 2014. She is currently a Research Engineer with the BioNano Laboratory, University of Guelph. Her research interests are in microfluidics and development of biosensors for food safety applications.
Suresh Neethirajan is currently an Associate Professor with the Biological Engineering Program, University of Guelph. He is the Director of the BioNano Laboratory, University of Guelph, and has extensively published in the development of biosensing solutions for agriculture, food safety, and animal health sector.
Funding Statement
This work was supported in part by the Natural Sciences and Engineering Research Council of Canada under Grant 400705.
Contributor Information
Xuan Weng, Email: xuanw@uoguelph.ca.
Suresh Neethirajan, Email: sneethir@uoguelph.ca.
References
- [1].He Y.et al. , “Responses of the toll-like receptor and melanoma differentiation-associated protein 5 signaling pathways to avian infectious bronchitis virus infection in chicks,” Virol. Sin., vol. 31, no. 1, pp. 57–68, Feb. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Awad F., Chhabra R., Baylis M., and Ganapathy K., “An overview of infectious bronchitis virus in chickens,” World’s Poultry Sci. J., vol. 70, no. 2, pp. 375–384, Jun. 2014. [Google Scholar]
- [3].Cavanagh D., “Coronavirus avian infectious bronchitis virus,” Vet. Res., vol. 38, no. 2, pp. 281–297, Mar. 2007. [DOI] [PubMed] [Google Scholar]
- [4].Promkuntod N., Van Eijndhoven R. E. W., de Vrieze G., Gröne A., and Verheije M. H., “Mapping of the receptor-binding domain and amino acids critical for attachment in the spike protein of avian coronavirus infectious bronchitis virus,” Virology, vol. 448, pp. 26–32, Jan. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cheng Y., Tang H., and Jiang J., “Enzyme mediated assembly of gold nanoparticles for ultrasensitive colorimetric detection of hepatitis C virus antibody,” Anal. Methods, vol. 9, pp. 3777–3781, May 2017. [Google Scholar]
- [6].Kumar S., Ahlawat W., Kumar R., and Dilbaghi N., “Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare,” Biosensors Bioelectron., vol. 70, pp. 498–503, Aug. 2015. [DOI] [PubMed] [Google Scholar]
- [7].Holzinger M., Le Goff A., and Cosnier S., “Nanomaterials for biosensing applications: A review,” Front. Chem., vol. 2, p. 63, Aug. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ge X., Asiri A. M., Du D., Wen W., Wang S., and Lin Y., “Nanomaterial-enhanced paper-based biosensors,” TrAC Trends Anal. Chem., vol. 58, pp. 31–39, Jun. 2014. [Google Scholar]
- [9].Lv R.et al. , “Transition metal dichalcogenides and beyond: Synthesis, properties, and applications of single- and few-layer nanosheets,” Accounts Chem. Res., vol. 48, no. 1, pp. 56–64, Dec. 2014. [DOI] [PubMed] [Google Scholar]
- [10].Li H., Wu J., Yin Z., and Zhang H., “Preparation and applications of mechanically exfoliated single-layer and multilayer MoS2 and WSe2 nanosheets,” Accounts Chem. Res., vol. 47, no. 4, pp. 1067–1075, Apr. 2014. [DOI] [PubMed] [Google Scholar]
- [11].Kong R. M., Ding L., Wang Z., You J., and Qu F., “A novel aptamer-functionalized MoS2 nanosheet fluorescent biosensor for sensitive detection of prostate specific antigen,” Anal. Bioanal. Chem., vol. 407, no. 2, pp. 369–377, Jan. 2015. [DOI] [PubMed] [Google Scholar]
- [12].Kalantar-Zadeh K. and Ou J. Z., “Biosensors based on two-dimensional MoS2,” ACS Sensors, vol. 1, no. 1, pp. 5–16, Nov. 2015. [Google Scholar]
- [13].Zang Y., Lei J., Hao Q., and Ju H., “CdS/MoS2 heterojunction-based photoelectrochemical DNA biosensor via enhanced chemiluminescence excitation,” Biosensors Bioelectron., vol. 77, pp. 557–564, Mar. 2016. [DOI] [PubMed] [Google Scholar]
- [14].Hassanzadeh J. and Khataee A., “Ultrasensitive chemiluminescent biosensor for the detection of cholesterol based on synergetic peroxidase-like activity of MoS2 and graphene quantum dots,” Talanta, vol. 178, pp. 992–1000, Feb. 2018. [DOI] [PubMed] [Google Scholar]
- [15].Loan P. T. K., Zhang W., Lin C.-T., Wei K.-H., Li L.-J., and Chen C.-H., “Graphene/MoS2 heterostructures for ultrasensitive detection of DNA hybridisation,” Adv. Mater., vol. 26, no. 28, pp. 4838–4844, Jul. 2014. [DOI] [PubMed] [Google Scholar]
- [16].Chen H.et al. “Quasi-two-dimensional metal oxide semiconductors based ultrasensitive potentiometric biosensors,” ACS Nano, vol. 11, no. 5, pp. 4710–4718, Apr. 2017. [DOI] [PubMed] [Google Scholar]
- [17].Lin K. C., Jagannath B., Muthukumar S., and Prasad S., “Sub-picomolar label-free detection of thrombin using electrochemical impedance spectroscopy of aptamer-functionalized MoS2,” Analyst, vol. 142, no. 15, pp. 2770–2780, 2017. [DOI] [PubMed] [Google Scholar]
- [18].Tuteja S. K., Duffield T., and Neethirajan S., “Liquid exfoliation of 2D MoS2 nanosheets and their utilization as a label-free electrochemical immunoassay for subclinical ketosis,” Nanoscale, vol. 9, no. 30, pp. 10886–10896, 2017. [DOI] [PubMed] [Google Scholar]
- [19].Geldert A., Kenry K., Zhang X., Zhang H., and Lim C. T., “Enhancing the sensing specificity of a MoS2 nanosheet-based FRET aptasensor using a surface blocking strategy,” Analyst, vol. 142, pp. 2570–2577, May 2017. [DOI] [PubMed] [Google Scholar]
- [20].Zhang X.et al. , “A sandwich electrochemiluminescence immunosensor for highly sensitive detection of alpha fetal protein based on MoS2-PEI-Au nanocomposites and Au@BSA core/shell nanoparticles,” Sens. Actuators B, Chem., vol. 253, pp. 470–477, Dec. 2017. [Google Scholar]
- [21].Safavieh R., Mirzaei M., Qasaimeh M. A., and Juncker D., “Yarn based microfluidics: From basic elements to complex circuits,” in Proc. MicroTAS, 13th Int. Conf. Miniaturized Syst. Chem. Life Sci., 2009. [Google Scholar]
- [22].Mao X., Du T. E., Wang Y., and Meng L., “Disposable dry-reagent cotton thread-based point-of-care diagnosis devices for protein and nucleic acid test,” Biosensors Bioelectron., vol. 65, pp. 390–396, Mar. 2015. [DOI] [PubMed] [Google Scholar]
- [23].Du T. E., Wang Y., Zhang Y., Zhang T., and Mao X., “A novel adenosine-based molecular beacon probe for room temperature nucleic acid rapid detection in cotton thread device,” Anal. Chim. Acta, vol. 861, pp. 69–73, Feb. 2015. [DOI] [PubMed] [Google Scholar]
- [24].Grgić H., Hunter D. B., Hunton P., and Nagy E., “Pathogenicity of infectious bronchitis virus isolates from Ontario chickens,” Can. J. Vet. Res., vol. 72, no. 5, p. 403, Oct. 2008. [PMC free article] [PubMed] [Google Scholar]
- [25].St. Paul M.et al. , “Effects of ligands for toll-like receptors 3, 4, and 21 as adjuvants on the immunogenicity of an avian influenza vaccine in chickens,” Viral Immunol., vol. 27, no. 4, pp. 167–173, May 2014. [DOI] [PubMed] [Google Scholar]
- [26].Singh S. M.et al. , “Characterization of immune responses to an inactivated avian influenza virus vaccine adjuvanted with nanoparticles containing CpG ODN,” Viral Immunol., vol. 29, no. 5, pp. 269–275, Jun. 2016. [DOI] [PubMed] [Google Scholar]
- [27].Angenendt P., Glökler J., Murphy D., Lehrach H., and Cahill D. J., “Toward optimized antibody microarrays: A comparison of current microarray support materials,” Anal. Biochem., vol. 309, no. 2, pp. 253–260, Oct. 2002. [DOI] [PubMed] [Google Scholar]
- [28].Yao Y.et al. , “High-concentration aqueous dispersions of MoS2,” Adv. Funct. Mater., vol. 23, no. 28, pp. 3577–3583, Jul. 2013. [Google Scholar]
- [29].Marsich L., Bonifacio A., Mandal S., Krol S., Beleites C., and Sergo V., “Poly-L-lysine-coated silver nanoparticles as positively charged substrates for surface-enhanced Raman scattering,” Langmuir, vol. 28, no. 37, pp. 13166–13171, Sep. 2012. [DOI] [PubMed] [Google Scholar]
- [30].Yadav S., Liu J., Shire S. J., and Kalonia D. S., “Specific interactions in high concentration antibody solutions resulting in high viscosity,” J. Pharmaceutical Sci., vol. 99, no. 3, pp. 1152–1168, Mar. 2010. [DOI] [PubMed] [Google Scholar]
- [31].Posudievsky O. Y.et al. , “Improved dispersant-free liquid exfoliation down to the graphene-like state of solvent-free mechanochemically delaminated bulk MoS2,” J. Mater. Chem. C, vol. 1, no. 39, pp. 6411–6415, 2013. [Google Scholar]
- [32].Forsberg V.et al. , “Exfoliated MoS2 in water without additives,” PLoS ONE, vol. 11, no. 4, pp. e0154522, Apr. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Thomsen V., Schatzlein D., and Mercuro D., “Limits of detection in spectroscopy,” Spectroscopy, vol. 18, no. 12, pp. 112–114, Dec. 2003. [Google Scholar]