Abstract
Background and aims
COVID-19 is a public world crisis, however, it is a self-limited infection. In COVID-19, the strength of immune and respiratory systems is a critical element. Thus, this review was conducted to demonstrate the short and long term effects of increasing the aerobic capacity on increasing the function and strength of immune and respiratory systems, particularly those essential for overcoming COVID-19 infections and associated disorders.
Methods
This review was carried out by searching in Web of Science, Scopus, EBSCO, Medline databases. The search was conducted over clinical trials and literature and systematic reviews on the effects of increasing the aerobic capacity on the function and strength of specific immune and respiratory elements essential for overcoming COVID-19 infections.
Results
This review found that increasing the aerobic capacity could produce short-term safe improvements in the function of immune and respiratory systems, particularly those specific for COVID-19 infections. This could be mainly produced through three mechanisms. Firstly, it could improve immunity by increasing the level and function of immune cells and immunoglobulins, regulating CRP levels, and decreasing anxiety and depression. Secondly, it could improve respiratory system functions by acting as an antibiotic, antioxidant, and antimycotic, restoring normal lung tissue elasticity and strength. Lastly, it could act as a protective barrier to decrease COVID-19 risk factors, which helps to decrease the incidence and progression of COVID-19.
Conclusion
This review summarizes that increasing the aerobic capacity is recommended because it has potential of improving immune and respiratory functions which would help counter COVID-19.
Keywords: Aerobic capacity, COVID-19, Immune system, Respiratory system
1. Introduction
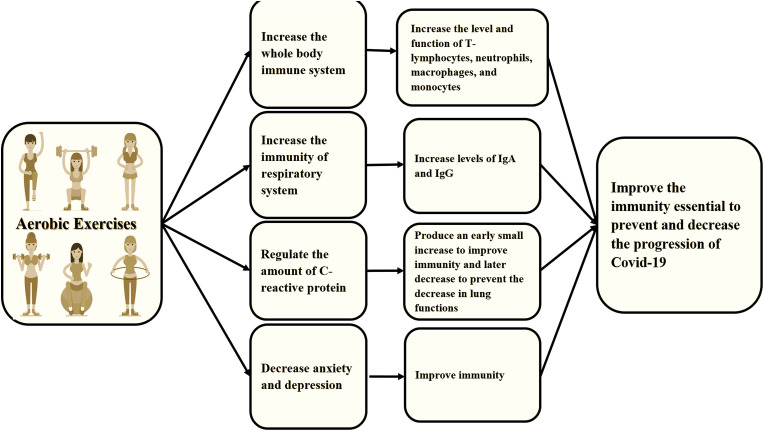
The new coronavirus or COVID-19, according to WHO, is a public world crisis. Nowadays, COVID-19 is quickly spreading from its origin in Wuhan City, China to all the world [1]. According to the WHO website, until April 6, 2020, around 1,136,862 cases have been confirmed to have COVID-19 and 63,025 of them died [2]. COVID-19 is a novel enveloped RNA beta-coronavirus and it is known as the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [3]. The common symptoms of COVID-2019 are fever and cough [4]. The fever exists in only 43.8% of the patients on admission, however, it increases by 88.7% through hospitalization. The cough is the second common symptom and occurs in about 67.8% [3]. Other symptoms include fatigue, myalgia, and dyspnea. (see Fig. 1, Fig. 2)
Fig. 1.
The effect of aerobic exercises on the immune system. Aerobic exercises improve the function of the immune system by increasing the level and function of T-lymphocytes, neutrophils, macrophages, and monocytes, which are essential elements in the body’s defense against infections, increasing levels of immunoglobulins “IgA, IgM, IgG”, particularly IgA and IgG because of its vital role in lung infections, regulating C-reactive protein levels, through producing a short-term small increase to fight lung viruses and a long–term decrease to prevent any drop in lung functions, and decreasing anxiety and depression to improve the immunity by rebalancing T-helper 1/T-helper 2 relationship.
Fig. 2.
The effect of aerobic exercises on the respiratory system. Aerobic exercises improve respiratory system functions by acting as antibiotic and antimycotic prophylaxis, to improve lung immunity, restoring normal lung tissue elasticity and increasing pulmonary muscles strength and endurance, acting as an antioxidant to limit free radical production and oxidative damage, and decreasing cough through an autonomic modulation.
COVID-19 is a self-limited infection, in which the strength of the host’s immune strength plays a significant role against it [5]. Increasing the aerobic capacity can produce immediate effects on the immune system activity. It has been shown that aerobic exercises significantly increase the function of the immune system in short periods and sometimes this increase occurs after only one session. The effect of increasing the aerobic capacity on improving the immune functions can be mainly produced through three mechanisms. Firstly, it can increase the level and function of T-lymphocytes, neutrophils, macrophages, and monocytes, which are essential elements in the body’s defense against infections [[6], [7], [8], [9], [10], [11]]. Secondly, it can increase the level of immunoglobulins (IgA, IgM, IgG), particularly IgA because of its vital role against lung infections [[12], [13], [14], [15]]. Lastly, it can regulate the level of C-reactive proteins (CRP), through inducing a short-term small increase [[16], [17], [18]] to fight lung infections and a long–term decrease to inhibit any decrease in lung functions [19,20], and lowering anxiety and depression to improve the immunity by re-balancing T-helper-1/T-helper-221−27.
Also, increasing the aerobic capacity can produce a preventive and curable role against respiratory infections and disorders. It can prevent or treat both pneumonia [[28], [29], [30]] and acute respiratory distress syndrome (ARDS) [11,31,32], which are the common disorders develop with COVID-19 and lead to a respiratory system failure. The effect of increasing the aerobic capacity on improving lung functions and preventing lung damage can be summarized into four mechanisms. The first mechanism includes the role of increasing the aerobic capacity as antibiotic and antimycotic prophylaxis to improve lung and body immunity [30]. The second mechanism includes the role of increasing the aerobic capacity on restoring normal lung tissue elasticity and increasing respiratory muscles strength and endurance, which help in increasing the ventilation, lung mechanics and decreasing lung damage [[33], [34], [35], [36]]. The third mechanism includes the role of increasing the aerobic capacity as an antioxidant to limit free radicals production and oxidative damage [37]. The fourth mechanism includes the role of increasing the aerobic capacity on decreasing the cough and clear respiratory airways through both improving the lung immunity [38] and producing an autonomic modulation [39,40]. The role of increasing the aerobic capacity on pulmonary functions and immunity is more important than breathing exercises and can accomplish more improvements in cough mechanism [36,41].
Furthermore, increasing the aerobic capacity can reduce COVID-19 risk factors. This helps in decreasing the incidence and progression of COVID-19. A recent study that has been published on March 13, 2020 [42] has demonstrated that risk factors linked with the occurrence of COVID-19 and its progression to death include aging, hypertension, diabetes, and heart problems. All these risk factors have been previously shown to be immediately or shortly improved by increasing the aerobic capacity [37,[43], [44], [45], [46], [47], [48], [49]].
Depending on the previous effects of increasing the aerobic capacity, particularly its immediate and short-term effects on the immune system, this review was developed to suggest a natural line of intervention for patients with COVID-19 through increasing their aerobic capacity to prevent or decrease the progression of accompanying disorders. Also, this review highlights the role of increasing the aerobic capacity as a recommended intervention during the lockdown or in mild cases (pre-fever stage). Increasing the aerobic capacity can be also used as an adjacent treatment to decrease hospitalization, which sometimes forces the medical staff to choose between treating some patients and leaving others to die. This review consists of three subtitles, the effects of increasing the aerobic capacity on improving the immune system functions, the effects of increasing the aerobic capacity on improving respiratory system functions and preventing its illnesses in patients with COVID-19, and the effects of increasing the aerobic capacity as a protective barrier against the occurrence of COVID-19.
2. The effect of increasing the aerobic capacity on improving the immune system functions
COVID-19 is a self-limited infection. Li et al. [50] have demonstrated that the immune response is crucial in controlling and treating coronavirus infections and any malfunction in the immune system may result in immunopathologies and impaired pulmonary functions. The development of a drug to treat patients with COVID-19 might take several months, thus the need for quick and safe interventions is a must to decrease its spreading and death rates. The subtopic is unique because it is the first subtopic that discusses in-depth the important role of increasing the aerobic capacity on improving COVID-19 specific immunity elements essential for decreasing its incidence and progression.
MERS-CoV, SARS-CoV, and COVID-19 are β-coronaviruses that can lead to serious lower respiratory tract infections and extra-pulmonary signs [50,51]. Among all immune cells, T-lymphocytes play major antiviral roles against pathogens [50]. CD4+ T-lymphocytes increase the secretion of virus-specific antibodies by stimulating T-dependent B-cells. Besides, T-lymphocytes can survive in the infected lungs and destroy the infecting viruses [52], this highlights the important role of T-lymphocytes in controlling the pathogenesis of coronaviruses infection. It has been shown that the response of cross-reactive T-lymphocytes is vital for the reduction in amounts of MERS-CoV [53]. The depletion of T-lymphocytes usually causes a decrease in the pulmonary recruitment of T-lymphocytes, neutralizing antibody, and cytokine production. This can cause a severe immune-mediated interstitial pneumonitis and late clearance of SARS-CoV from lungs [54]. Moreover, T-helper cells stimulate the release of pro-inflammatory cytokines through stimulating the NF-kB signaling pathway [55]. Pro-inflammatory cytokines stimulate the release and migration of monocytes and neutrophils to the location of infection to activate other downstream cytokine and chemokine cascades, including IL-21, IL-10, IL-6, IL-1, IL-8, MCP-1, and TNF-β [56,57]. MERS-CoVs can produce T-lymphocytes apoptosis by triggering the extrinsic and intrinsic and apoptosis pathways [58]. In the later stages of the lung infection, the diminution of T-lymphocytes could increase the period of infection and promote viral survival [59].
Increasing the aerobic capacity produces an immediate improvement in the action of T-cells. A recent systematic review conducted by Gonçalves et al. [6] has demonstrated that aerobic exercises can produce immediate and short-term improvements in the immune response of leukocytes, T-lymphocytes, lymphocyte subpopulations, interleukins, and immunoglobulins. Several authors have demonstrated that only a single session of aerobic exercises produces improvements in the utmost immune markers, such as T-lymphocytes, leukocytes, and immunoglobulins [[7], [8], [9], [10]]. Lippi et al. [7,8] have demonstrated that mid-distance running (21.1 km) increases the production of neutrophil, leukocyte, and monocyte amounts in unprofessional runners. Lira et al. [10] have shown that after just 60-min of 5-km running, an increase in cytokine IL-6 and IL-10 occurs. Li et al. [9] have demonstrated that a single session of prolonged aerobic exercises can increase the number of circulating leukocyte, neutrophil, and monocyte for up to 9 h. A more related study conducted by Gonçalves et al. [11], they investigated the protective short-term effect of aerobic exercises on artificially-induced acute lung injuries in mice. They found that performing low aerobic exercises for only 5-weeks produced a significant increase in the number of neutrophils in bronchoalveolar lavage (BAL) fluid and tissue, pulmonary elasticity and resistance, TNF-alpha in lung tissue, protein leakage, plasma levels of IL-10 and IL-6, and IL-1beta, IL-6 and KC levels.
The second defense mechanism to COVID-19 infection is the humoral immunity. The humoral B-cell subcategories have phenotypes properties of simple and non-isotype switched manner. They include memory cells and antibody-secreting cells, which increase with coronavirus infections [50,60]. The antigen activation of coronaviruses infection is elucidated by utilizing a specific 9-mer peptide “CYSSLILDY”, which rests at the region from 437 to 445 inside the area of the S-glycoprotein [50,60]. This structure has the maximum B-cell antigenicity scheme and can form an extreme number of interactions with MHCI alleles in a computerized model [61]. Several reports have shown that the humoral immunity is critical in regulating the persistent phase of coronaviruses infection [[62], [63], [64]].
The normal humoral immunity plays a critical role in the immune response of the host body to coronaviruses infections. The innate humoral immune response consists of multiple components, including the serum complements (C3–C9), the naturally occurring antibodies, or immunoglobulins (IgM, IgE, IgG, and IgA), pentraxins (CRP), and contact cascades (FXIIa) [65]. The complement system is vital in the antiviral response. The complement system is strongly regulated with inhibiting proteins secreted into the serum. Viruses usually have encoded proteins that help them to avoid their recognition by the complement system, particularly if this complement system has any impairment in its function [50,66]. Thus, the improvement of the function of the complement system might help in discovering the encoded proteins of these viruses.
To the best of our knowledge, there were rare human studies investigated the effects of aerobic exercises on the activities of serum complements and they focused more on athletes than normal individuals. Although the main aims of these studies were to study the effect of aerobic exercises on the complement activity to examine the role of overtraining syndrome in diminishing the immune response. In these studies, there were high contradictions in their results. Smith et al. [67] conducted a study to investigate the short-term effects of aerobic exercises on the complement activity in runners. They found that short-term aerobic exercises produced stimulation of C3 and C4 and consequent generation of C3a and C4a anaphylatoxins. They suggested that stimulation of the classical pathway of complement and selective downregulation of C3 production might be found in individuals commonly involved in mild aerobic exercises as runners. Karacabey et al. [68,69] investigated the effect of aerobic exercises on the humoral activity in professional athletes and sportswomen. They found that C3 and C4 levels suppressed immediately after aerobic exercises as compared to the pre-exercise results, while these values returned to be non-significant as compared to pre-exercise values after only 4 h. Wolach et al. [70] examined the effect of aerobic exercises on complement activity in young-female gymnasts. They found high contradictions in the results of complement components (C1Q, C1R, C1S, C2, C3, C4, C5, C6, C7, CA, and C9). These high contradictions might be raised because athletes usually participate in high strenuous and repeated activities, which can lead to adverse effects (overtraining syndrome) [71].
Immuunoglobins are very important in preventing COVID-19s infections. Immunoglobulins mainly include IgA, IgE, IgM, and IgG. The IgA and IgG are dominants immunoglobulins in the mucosal secretions and they play important roles in preventing respiratory tract infections (the more decrease in IgA and IgG concentrations, the more increase in upper tract infections) [[12], [13], [14]]. The role of increasing the aerobic capacity on increasing the amounts of immunoglobulins has been demonstrated in some studies. Karacabey et al. [68] have demonstrated that performing regular moderate aerobic exercises can increase hormone release and immunoglobulins IgA, IgG, and IgM. Mohamed and Taha [15] compared the long-term effects of aerobic and anaerobic exercises on the amounts of immunoglobulins in obese women. They found that unlike anaerobic exercises, aerobic exercises increased the amounts of immunoglobulins, particularly IgM and IgG.
Another subtype of humoral immunity components is pentraxins (CRP). Pentraxins play chief roles in inflammatory processes and body reactions to viral infections by stimulating the complement pathway, phagocytosis, apoptosis, nitric oxide secretion, and cytokines production [72]. Increasing CRP levels is a body defense mechanism against viral infections, however, persistent high CRP levels can significantly speed up lung damage because high CRP levels cause consequent drops in lung functions [73]. One of the main lab tests to discover COVID-19 is serum CRP. Patients with COVID-19 have very high levels of CRP [1,74]. Increasing the aerobic capacity plays an interesting role in regulating the CRP levels by producing a short-term small increase in CRP levels [[16], [17], [18]] to fight lung infections and a long-term decrease in CRP levels [19,20] to prevent the decrease in lung functions.
The immune activity can be significantly affected by the host mood. Patients with COVID-19 usually have higher degrees of anxiety and depression [75]. The negative effects of anxiety and depression on immunity have been demonstrated in several studies [21,76,77]. Stress plays a critical role in morbidity and mortality rates in immune-based diseases [21]. The actual relationship between stress and decreased immunity is not clear yet. Stress might decrease immunity by altering the balance between immune cells, such as altering the balance between T-helper-1/T-helper-2. Stress can cause this alteration through its effect on increasing the amounts of serum corticosteroids and catecholamines hormones, thus a decrease in the immunity response might occur [21].
Increasing the aerobic capacity can significantly improve the mood. This might be attributed to the effect of aerobic exercises on decreasing stress hormones, like corticosteroids and catecholamines hormones which can rebalance T-helper-1/T-helper-2 relationship [27]. It has been demonstrated that the association amid exercise duration and mood variation is non-linear and just performing 10- to 30-min aerobic exercises is sufficient to improve the mood [22,23]. Hogan et al. [24] have demonstrated that biking for 15 min has a positive effect on both youngers and elderlies with anxiety disorders. Broman-Fulks and Storey [25] have reported that walking with 50% of maximal heart rate or running with 60–90% of maximal heart rate for 20-min significantly decreases the sensitivity to anxiety. Crabbe et al. [26] have stated that aerobic exercises for just three days significantly decreases the emotional arousal to any unpleasant stimuli. Asmundson et al. [78] have shown that regular mild aerobic exercises significantly decrease anxiety disorders.
However, the performance of mild to moderate intensity aerobic exercises is beneficial in increasing the function of the immune system in patients with COVID-19, the performance of high intensity aerobic exercises should be avoided for those patients because of its adverse effects on suppressing the function of the immune system [79]. Also, aerobic exercises should be avoided in high fever because of its adverse effects on decreasing the immunity [80].
3. The effects of increasing the aerobic capacity on improving respiratory system functions and preventing its illnesses in patients with COVID-19
COVID-19 mainly affects the respiratory system causing pneumonia and ARDS [[81], [82], [83], [84], [85]]. Samples were taken from patients with COVID-19, in Wuhan-China, from their respiratory BAL fluid indicated that COVID-19 virus presented mainly in the BAL fluid [85,86], causing a novel coronavirus–infected pneumonia (NCIP) [[85], [86], [87], [88]]. In these samples, patients initially presented with common symptoms, like fever, cough, and pneumonia. In the later stages, there were both intra and extra respiratory changes [[81], [82], [83],85]. Early-stage chest CT scans showed significant intra-pulmonary small patch-like shadows presented in several lobes of both lungs. In the later stages, these small patch-like shadows converted to numerous ground-glass opacities along with infiltration shadows “large white lung” [77]. The median interval of progression of these symptoms from the initial onset of symptoms to dyspnea was five days, to hospital admission was seven days, and ARDS was eight days [81,84]. The subtopic is unique because it is the first subtopic that discusses in-depth the important role of increasing the aerobic capacity on improving COVID-19 specific respiratory system elements essential for decreasing its incidence, progression, and associated disorders and symptoms.
The respiratory pathological findings of COVID-19 in early stages are rare, however, Tian et al. [83], reported that infected patients might show proteinaceous exudate, edema, focal reactive hyperplasia of pneumocytes with sporadic inflammatory cellular infiltration, and multi-nucleated giant cells. Also, some differences were detected between these patients, as a reactive alveolar epithelial hyperplasia and fibroblastic proliferation (fibroblast plugs). The most common pulmonary disorders in patients with COVID-19 are pneumonia and ARDS, and they are considered life-threatening disorders and need immediate interventions [[1], [2], [3], [4], [5], [6], [7], [8]]. Also, the earliest and commonest signs in patients with COVID-19 are dry cough and dyspnea [[1], [2], [3], [4], [5], [6], [7], [8]]. Increasing the aerobic capacity can significantly help in preventing and treating pulmonary infections and pathological conditions [89]. The effects of aerobic exercises in pulmonary infections are more preferable than breathing exercises because of their associated immune and autonomic modulations [36,41].
Increasing the aerobic capacity can prevent pneumonia or decrease its progression from mild to severe. Baumann et al. [30] have demonstrated that short periods of aerobic exercises can prevent the occurrence of pneumonia and fever in patients with cancer. Williams [90] has stated that mild aerobic exercise, like running and walking, is associated with a lesser risk of pneumonia, respiratory diseases, and aspiration pneumonia mortality. Durigon et al. [37] have reported that increasing the aerobic capacity for 2-months can inhibit pseudomonas aeruginosa induced lung inflammation and bacterial colonization in elderly mice. Neuman et al. [28] have shown that increasing the aerobic capacity is very important in decreasing the occurrence of pneumonia in US women. Olivo et al. [29] have reported that mild aerobic exercises can produce a significant anti-inflammatory effect in patients with streptococcus pneumonia, which helps in attenuating pulmonary inflammation.
Also, increasing the aerobic capacity can prevent ARDS or decrease its progression from mild to severe. Rigonato-Oliveira et al. [31] have demonstrated that increasing the aerobic capacity inhibits acute lung inflammation through decreasing inflammatory cytokines and oxidative stress markers in mice and humans. Vieira et al. [91] have reported that increasing the aerobic capacity increases levels of IL-10, which is an essential element in immunity against acute lung inflammations and injuries. A very recent study conducted by Shi et al. [32] has shown that 5- weeks of mild aerobic exercises can prevent acute lung injury in mice by producing neutrophil extracellular traps (NETs) inhibition, which plays a vital role in acute lung injury. NETs are secreted by stimulated neutrophils and consist mainly of histones, DNA, myeloperoxidase, and neutrophil elastase (NE). The extracellular web-like structures can efficiently trap attacking pathogens and use highly local concentrations of anti-microbial peptides to destroy virulence components.
The effect of aerobic exercises over-breathing exercise in the treatment of respiratory dysfunctions that occur in patients with COVID-19 can be summarized in four mechanisms. The first mechanism includes the effect of aerobic exercises as antibiotic and antimycotic prophylaxis. It was previously mentioned in the section of “the effect of increasing the aerobic capacity on improving the immune system functions” that increasing the aerobic capacity increases the respiratory and body immunity through a) increasing the level and function of T-lymphocytes, neutrophils, macrophages, and monocytes which are essential elements in the body’s defense against infections, b) increasing the level of immunoglobulins “IgA, IgM, IgG”, particularly IgA because of its vital role in lung infections, C) regulating C-reactive protein levels, through producing a short-term small increase to fight lung viruses and a long–term decrease to prevent the drop in lung functions, and d) decreasing the anxiety and depression which helps to rebalance T-helper-1/T-helper-2 and improve the immunity.
The second mechanism includes the effect of aerobic exercises on restoring the elasticity of lung tissues and increasing strength and endurance of respiratory muscles. Increasing the aerobic capacity produces short-term effects on lung elasticity and recoil mechanism. Guimarães et al. [34], investigated the effect of 4-weeks of mild aerobic exercises on the occurrence of an artificially induced emphysema in mice. They found that aerobic exercises restored normal lung mechanics and flow acceleration rate through decreasing lung hyperinflation, and restoring normal lung tissue elasticity and strength. Park and Han [92] examined the effect of aerobic exercises on the maximum-expiratory lung capacity of older women. They found that 20-min of mild aerobic exercises for 12 weeks improved alveoli function and increased lung elasticity. Taskin et al. [93] studied the effect of aerobic exercises on the strength of respiratory muscles in patients with ankylosing spondylitis. They found that 40-min of mild aerobic exercises for 12-weeks significantly increased the strength of respiratory muscles, inspiratory muscle performance, and maximal exercise capacity and diminished dyspnea perception.
The third mechanism includes the effect of aerobic exercises as an antioxidant to limit free radical production and oxidative stress. Free radicals, like reactive oxygen species (ROS), are produced throughout the usual cellular function and are considered a part of normal physiological processes of all living animals. Free radicals have both beneficial and toxic effects. When levels of free radicals incredibly increase and cannot gradually be handled, they accumulate in the body creating a phenomenon called “oxidative stress” [94]. Commonly, oxidative stress is considered the initial point for the onset of several diseases, among them lung infections and diseases [94,95]. Mild aerobic exercises can help in processing these accumulated free radicals and prevent the initiation of lung infections and diseases as pneumonia and ARDS.
Besides, increasing the aerobic capacity can increase lung and body resistance to oxidative stress causing an elevation in the body resistance to consequent oxidative encounters by increasing the function of mitochondria and allowing better oxygenation to body and lung tissues [94,96]. Toledo et al. [33] investigated the effect of mild aerobic exercises performed for 24-weeks on the occurrence of pulmonary diseases in mice. They measured ROS as an indicator to the initiation of lung diseases. They found that mild aerobic exercises decreased ROS amounts in the BAL fluid in mic, which is important to prevent or decrease the progression of pneumonia and ARDS. Cunha et al. [97] studied the effect of aerobic exercises on the oxidative stress produced via an experimentally-induced lung injury in rats. They found that 20-min of mild aerobic exercises were able to inhibit the increase in reactive species, NF-кβ/p65 immuno-content, and nitrite levels. They suggested that mild aerobic exercises may have a significant role as a protector against the occurrence of acute lung injuries.
The fourth mechanism includes the effect of aerobic exercises in decreasing cough in patients with COVID-19. Increasing the aerobic capacity can decrease cough mainly through autonomic modulation more than mucociliary clearance [39,40]. Borghi-Silva et al. [40] examined the effect of 6-weeks of mild aerobic exercises on autonomic modulation in patients with chronic obstructive pulmonary disease (COPD). They found that aerobic exercises produced a decrease in respiratory rate and an increase in the tidal volume during exercise and these changes decreased in the heart rate [40,98]. Recently, Leite et al. [39] conducted a study to investigate the effect of 12-weeks of mild aerobic exercises on the autonomic nervous system and cough in patients with COPD. They found that mild aerobic exercises decreased cough and heart rate. The effect of increasing the aerobic capacity on decreasing the heart rate could be beneficial in decreasing dyspnea found in patients with COVID-1940. Thus, mild aerobic exercises could produce more significant effects on decreasing both cough and dyspnea, commonly seen in patients with COVID-19, than breathing exercises because they affect more the parasympathetic system causing a central decrease in these two symptoms.
4. The effects of increasing the aerobic capacity as a protective barrier against the occurrence of COVID-19
Increasing the aerobic capacity can significantly decrease COVID-19 risk factors in short periods, sometimes this effect occurs after one session only. This section focuses mainly on the immediate and short-term effects of increasing the aerobic capacity on COVID-19 risk factors because these risk factors can cause faster spreading and progression rates [81]. The common risk factors of COVID-19 include diabetes, hypertension, aging, and heart problems [42].
Diabetes Mellitus (DM) adversely affects many body systems [99], including immunity [100] and pulmonary systems [101]. Increasing the aerobic capacity can produce an immediate reduction in blood glucose in types I and II DM. Bacchi et al. [43] compared the acute effects of aerobic and resistance exercises on glucose concentrations in subjects with type II DM. They found that in contrast to resistance training, mild aerobic exercises decreased glucose concentration throughout the session and for the next whole night. Yardley et al. [44] compared the acute effects of aerobic and resistance exercises on glucose concentrations in subjects with type-1 DM. They found that 45-min of mild aerobic exercises decreased glucose concentrations more than resistance exercises, however, this decrease quickly increased after the exercise. Yokoyama et al. [45] investigated the short-term effects of aerobic exercises on arterial stiffness in subjects with type II DM. They found that 45-min of mild aerobic exercises performed for 3-weeks significantly decreased arterial stiffness in both femoral and common carotid arteries, and this reduction was associated with an enhancement in insulin resistance.
Increasing the aerobic capacity can produce an immediate significant reduction in higher BP [102]. Charlotte et al. [103] examined the immediate effects of aerobic exercises on ambulatory BP among female cleaners. They found that a single session of aerobic exercises significantly decreased ambulatory BP up to 25 h post-exercise. Ciolac et al. [104] investigated the acute effects of a single session of aerobic exercises on BP in subjects with long-term-treated hypertension. They found that a single session of aerobic exercises significantly decreased ambulatory BP. Nascimento et al. [105] studied the acute and chronic effects of aerobic exercises on BP in subjects with resistant hypertension. They found that 8-weeks of mild aerobic exercises significantly reduced BP. Guimaraes et al. [106] studied the effect of short-term heated water-based training on systemic BP in resistant-hypertensive patients. They found that 2-weeks of mild aerobic exercises significantly decreased both systolic and diastolic BP and cardiovascular load either after the exercise or during the following 24-h.
Aging significantly decreases the function of body systems, subjecting these systems to malfunctions and diseases. Elderlies with COVID-19 usually have higher death rates younger ones. Thus, decreasing the effect of aging on body systems might help in decreasing the high death rate. Cahapman et al. [46] investigated the short-term effects of mild aerobic exercises on cardiovascular fitness in elderlies. They found that 6-weeks of mild aerobic exercises significantly increased both VO2 max and perceived exertion rate. Francesco et al. [47] investigated the effect of mild aerobic exercises on heart rate recovery in elderlies. They found that 8-weeks of mild aerobic exercises significantly increased peak oxygen uptake, ventilatory aerobic threshold, and heart rate recovery and decreased the rate of increase in ventilation per unit of carbon dioxide production (VE/VCO2slope), they suggested that mild aerobic exercises can produce autonomic modulation through improving the vagal/sympathetic balance in older subjects. Durigon et al. [37] found that 5-weeks of aerobic exercises protected older mice from the occurrence of artificially-induced pneumonia.
Heart problems are usually associated with lung dysfunctions, this increases the deterioration rate of lung disorders and dysfunctions (heart-lung interaction) [[107], [108], [109]]. Increasing the aerobic capacity can produce short-term improvements in heart problems. The short-term improvements in heart volumes and rates were discussed in the previous paragraph [37,46,47]. Other animal studies have revealed that mild aerobic exercises produce significant improvements in heart functions. Tao et al. [48] examined the effect of mild aerobic exercises on preventing acute myocardial infarction in mice. They found that 3-weeks of mild aerobic exercises protected mice from acute myocardial infarction through improving myocardium energy metabolism and producing early adaptive alterations in mitochondrial biogenesis. Wisløff et al. [49] investigated the short-term effects of aerobic exercises on cardiomyocyte hypertrophy, cardiac muscle contractility, and Ca2+ sensitivity in mice after myocardial infarction. They found that 8-weeks of moderate aerobic exercises decreased cardiomyocyte hypertrophy and increased both cardiac contractility and Ca2+ sensitivity.
5. Conclusion
This review summarizes that increasing the aerobic capacity is recommended because it has potential of improving immune and respiratory functions which would help counter COVID-19. This can decrease the morbidity and mortality rates of COVID-19. Also, increasing the aerobic capacity of people in the lockdown period is strongly recommended to decrease risk factors of COVID-19 and improve the function of immunity and respiratory systems to allow better body functions against COVID-19. Thus, the performance of a routine of 10–30 min of mild to moderate aerobic exercises should be followed by all people in the lockdown or patients with mild pulmonary symptoms.
Role of authors
This manuscript included two authors Mohamed AA. and Alawna M.. Mohamed has developed the research idea and wrote all sections except the effect of increasing the aerobic capacity on the pulmonary system, which was written by Alawna.
References
- 1.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020:2019. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Corona- virus disease (COVID-19) outbreak.
- 3.Guan W.-J., Ni Z.-Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020:1–13. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He F., Deng Y., Li W. Coronavirus Disease 2019 (COVID-19): what we know? J Med Virol. 2020;2019 doi: 10.1002/jmv.25766. 0-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. 2020. Features, Evaluation and treatment coronavirus (COVID-19) [PubMed] [Google Scholar]
- 6.Gonçalves C.A.M., Dantas P.M.S., dos Santos I.K. Effect of acute and chronic aerobic exercise on immunological markers: a systematic review. Front Physiol. 2020;10(January):1–11. doi: 10.3389/fphys.2019.01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippi G., Banfi G., Montagnana M., Salvagno G.L., Schena F., Guidi G.C. Acute variation of leucocytes counts following a half-marathon run. Int J Lab Hematol. 2010;32(1 PART.2):117–121. doi: 10.1111/j.1751-553X.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- 8.Lippi G., Salvagno G.L., Danese E. Mean platelet volume (MPV) predicts middle distance running performance. PloS One. 2014;9(11):8–13. doi: 10.1371/journal.pone.0112892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li T.L., Cheng P.Y. Alterations of immunoendocrine responses during the recovery period after acute prolonged cycling. Eur J Appl Physiol. 2007;101(5):539–546. doi: 10.1007/s00421-007-0529-1. [DOI] [PubMed] [Google Scholar]
- 10.Lira F.S., dos Santos T., Caldeira R.S. Short-term high- and moderate-intensity training modifies inflammatory and metabolic factors in response to acute exercise. Front Physiol. 2017;8(OCT):1–8. doi: 10.3389/fphys.2017.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis Gonçalves C.T., Reis Gonçalves C.G., de Almeida F.M. Protective effects of aerobic exercise on acute lung injury induced by LPS in mice. Crit Care. 2012;16(5):R199. doi: 10.1186/cc11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodríguez A., Tjärnlund A., Ivanji J. Role of IgA in the defense against respiratory infections: IgA deficient mice exhibited increased susceptibility to intranasal infection with Mycobacterium bovis BCG. Vaccine. 2005;23(20):2565–2572. doi: 10.1016/j.vaccine.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 13.Hines M.T., Schott H.C., Bayly W.M., Leroux A.J. Exercise and immunity: a review with emphasis on the horse. J Vet Intern Med. 1996;10(10):280–289. doi: 10.1111/j.1939-1676.1996.tb02063.x. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham-Rundles C. vol. 157. Wiley-Blackwell; 2009. Lung disease, antibodies and other unresolved issues in immune globulin therapy for antibody deficiency; pp. 12–16. (Clinical and experimental immunology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamed G., Taha M. Comparison between the effects of aerobic and resistive training on immunoglobulins in obese women. Bull Fac Phys Ther. 2016;21(1):11. [Google Scholar]
- 16.Pedersen B.K., Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80(3):1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- 17.Marklund P., Mattsson C.M., Wåhlin-Larsson B. Extensive inflammatory cell infiltration in human skeletal muscle in response to an ultraendurance exercise bout in experienced athletes. J Appl Physiol. 2013;114(1):66–72. doi: 10.1152/japplphysiol.01538.2011. [DOI] [PubMed] [Google Scholar]
- 18.De Gonzalo-Calvo D., Dávalos A., Montero A. Circulating inflammatory miRNA signature in response to different doses of aerobic exercise. J Appl Physiol. 2015;119(2):124–134. doi: 10.1152/japplphysiol.00077.2015. [DOI] [PubMed] [Google Scholar]
- 19.Zheng G., Qiu P., Xia R. Effect of aerobic exercise on inflammatory markers in healthy middle-aged and older adults: a systematic review and meta-analysis of randomized controlled trials. Front Aging Neurosci. 2019;11(APR):98. doi: 10.3389/fnagi.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okita K., Nishijima H., Murakami T. Can exercise training with weight loss lower serum C-reactive protein levels? Arterioscler Thromb Vasc Biol. 2004;24(10):1868–1873. doi: 10.1161/01.ATV.0000140199.14930.32. [DOI] [PubMed] [Google Scholar]
- 21.Marshall G.D. The adverse effects of psychological stress on immunoregulatory balance: applications to human inflammatory diseases. Immunol Allergy Clin North Am. 2011;31(1):133–140. doi: 10.1016/j.iac.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed J., Buck S. The effect of regular aerobic exercise on positive-activated affect : a meta-analysis. Psychol Sport Exerc. 2009;10(6):581–594. [Google Scholar]
- 23.Chan J.S.Y., Liu G., Liang D., Deng K., Wu J., Yan J.H. Special issue–therapeutic benefits of physical activity for mood: a systematic review on the effects of exercise intensity, duration, and modality. J Psychol Interdiscip Appl. 2019;153(1):102–125. doi: 10.1080/00223980.2018.1470487. [DOI] [PubMed] [Google Scholar]
- 24.Hogan C.L., Mata J., Carstensen L.L. Exercise holds immediate benefits for affect and cognition in younger and older adults. 2013;28(2):587–594. doi: 10.1037/a0032634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broman-Fulks J.J., Storey K.M. Evaluation of a brief aerobic exercise intervention for high anxiety sensitivity. Hist Philos Logic. 2008;21(2):117–128. doi: 10.1080/10615800701762675. [DOI] [PubMed] [Google Scholar]
- 26.Crabbe J.B., Smith J.C., Dishman R.K. Emotional & electroencephalographic responses during affective picture viewing after exercise ☆. Physiol Behav. 2007;90:394–404. doi: 10.1016/j.physbeh.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Nabkasorn C., Miyai N., Sootmongkol A. Effects of physical exercise on depression, neuroendocrine stress hormones and physiological fitness in adolescent females with depressive symptoms. Eur J Publ Health. 2006;16(2):179–184. doi: 10.1093/eurpub/cki159. [DOI] [PubMed] [Google Scholar]
- 28.Neuman M.I., Willett W.C., Curhan G.C. Physical activity and the risk of community-acquired pneumonia in US women. Am J Med. 2010;123(3):1–10. doi: 10.1016/j.amjmed.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olivo C.R., Miyaji E.N., Oliveira M.L.S. Aerobic exercise attenuates pulmonary inflammation induced by Streptococcus pneumoniae. J Appl Physiol. 2014;117(9):998–1007. doi: 10.1152/japplphysiol.00290.2014. [DOI] [PubMed] [Google Scholar]
- 30.Baumann F.T., Zimmer P., Finkenberg K., Hallek M., Bloch W., Elter T. Influence of endurance exercise on the risk of pneumonia and fever in leukemia and lymphoma patients undergoing high dose chemotherapy. A pilot study. J Sports Sci Med. 2012;11(4):638–642. [PMC free article] [PubMed] [Google Scholar]
- 31.Rigonato-Oliveira N.C., Mackenzie B.A., Bachi A.L.L. Aerobic exercise inhibits acute lung injury: from mouse to human evidence exercise reduced lung injury markers in mouse and in cells. Exerc Immunol Rev. 2018;24:36–44. [PubMed] [Google Scholar]
- 32.Shi Y., Liu T., Nieman D.C. Aerobic exercise attenuates acute lung injury through NET inhibition. Front Immunol. 2020;11:409. doi: 10.3389/fimmu.2020.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toledo A.C., Magalhaes R.M., Hizume D.C. Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur Respir J. 2012;39(2):254–264. doi: 10.1183/09031936.00003411. [DOI] [PubMed] [Google Scholar]
- 34.Guimarães I., Padilha G., Lopes-Pacheco M. The impact of aerobic exercise on lung inflammation and remodeling in experimental emphysema. Eur Respir J. 2011;38(Suppl 55) [Google Scholar]
- 35.Dassios T., Katelari A., Doudounakis S., Dimitriou G. Aerobic exercise and respiratory muscle strength in patients with cystic fibrosis. Respir Med. 2013;107(5):684–690. doi: 10.1016/j.rmed.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Yamashina Y., Aoyama H., Hori H. Comparison of respiratory muscle strength in individuals performing continuous and noncontinuous walking exercises in water after the 6-week program. J Exerc Rehabil. 2019;15(4):566–570. doi: 10.12965/jer.1938260.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stravinskas Durigon T., Mackenzie B.A., Carneiro Oliveira-Junior M. Aerobic exercise protects from Pseudomonas aeruginosa -induced pneumonia in elderly mice. J Innate Immun. 2018;10(4):279–290. doi: 10.1159/000488953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vieira R.P., Toledo AC de, Ferreira S.C. Airway epithelium mediates the anti-inflammatory effects of exercise on asthma. Respir Physiol Neurobiol. 2011;175(3):383–389. doi: 10.1016/j.resp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Leite M.R., Ramos E.M.C., Kalva-Filho C.A. Effects of 12 weeks of aerobic training on autonomic modulation, mucociliary clearance, and aerobic parameters in patients with COPD. Int J COPD. 2015;10(1):2549–2557. doi: 10.2147/COPD.S81363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borghi-Silva A., Arena R., Castello V. Aerobic exercise training improves autonomic nervous control in patients with COPD. Respir Med. 2009;103(10):1503–1510. doi: 10.1016/j.rmed.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Evaristo K.B., Saccomani M.G., Martins M.A. Comparison between breathing and aerobic exercise on clinical control in patients with moderate-to-severe asthma: protocol of a randomized trial. BMC Pulm Med. 2014;14:160. doi: 10.1186/1471-2466-14-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern Med. 2020:1–10. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bacchi E., Negri C., Trombetta M. Differences in the acute effects of aerobic and resistance exercise in subjects with type 2 diabetes: results from the RAED2 randomized trial. PloS One. 2012;7(12) doi: 10.1371/journal.pone.0049937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yardley J.E., Kenny G.P., Perkins B.A. Resistance versus aerobic exercise. Diabetes Care. 2013;36(3):537–542. doi: 10.2337/dc12-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokoyama H., Emoto M., Fujiwara S. Short-term aerobic exercise improves arterial stiffness in type 2 diabetes. Diabetes Res Clin Pract. 2004;65(2):85–93. doi: 10.1016/j.diabres.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Chapman S.B., Aslan S., Spence J.S. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci. 2013;5(NOV) doi: 10.3389/fnagi.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giallauria F., Del Forno D., Pilerci F. Improvement OF heart rate recovery after exercise training IN older people. J Am Geriatr Soc. 2005;53(11):2037–2038. doi: 10.1111/j.1532-5415.2005.00479_4.x. [DOI] [PubMed] [Google Scholar]
- 48.Tao L., Bei Y., Lin S. Exercise training protects against acute myocardial infarction via improving myocardial energy metabolism and mitochondrial biogenesis. Cell Physiol Biochem. 2015;37(1):162–175. doi: 10.1159/000430342. [DOI] [PubMed] [Google Scholar]
- 49.Wisløff U., Loennechen J.P., Currie S., Smith G.L., Ellingsen Ø. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res. 2002;54(1):162–174. doi: 10.1016/s0008-6363(01)00565-x. [DOI] [PubMed] [Google Scholar]
- 50.Li G., Fan Y., Lai Y. Coronavirus infections and immune racesponses. J Med Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan J.F.W., Lau S.K.P., To K.K.W., Cheng V.C.C., Woo P.C.Y., Yue K.Y. Middle East Respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28(2):465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao J., Li K., Wohlford-Lenane C. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A. 2014;111(13):4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pascal K.E., Coleman C.M., Mujica A.O. Pre- and postexposure efficacy of fully human antibodies against Spike protein in a novel humanized mouse model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2015;112(28):8738–8743. doi: 10.1073/pnas.1510830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J., Lau Y.F., Lamirande E.W. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010;84(3):1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manni M.L., Robinson K.M., Alcorn J.F. A tale of two cytokines: IL-17 and IL-22 in asthma and infection. Expert Rev Respir Med. 2014;8(1):25–42. doi: 10.1586/17476348.2014.854167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bunte K., Beikler T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci. 2019;20(14) doi: 10.3390/ijms20143394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dutzan N., Abusleme L.T. Advances in experimental medicine and biology. 2019. Helper 17 cells as pathogenic drivers of periodontitis. [DOI] [PubMed] [Google Scholar]
- 58.Yang Y., Xiong Z., Zhang S. Bcl-xL inhibits T-cell apoptosis induced by expression of SARS coronavirus E protein in the absence of growth factors. Biochem J. 2005;392(1):135–143. doi: 10.1042/BJ20050698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mubarak A., Alturaiki W., Hemida M.G. Middle east respiratory syndrome coronavirus (mers-cov): infection, immunological response, and vaccine development. J Immunol Res. 2019;2019(Cdc) doi: 10.1155/2019/6491738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ababneh M., Alrwashdeh M., Khalifeh M. Recombinant adenoviral vaccine encoding the spike 1 subunit of the Middle East Respiratory Syndrome Coronavirus elicits strong humoral and cellular immune responses in mice. Vet World. 2019;12(10):1554–1562. doi: 10.14202/vetworld.2019.1554-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tuhin ali M., Morshed M.M., Musa M.A. Computer aided prediction and identification of potential epitopes in the receptor binding domain (RBD) of spike (S) glycoprotein of MERS-CoV. Bioinformation. 2014;10(8):533–538. doi: 10.6026/97320630010533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferreira D., Bernile G., Dockner E. Ultrapotent human neutralizing antibody repertoires against MERS-CoV from A recovered patient. J Infect Dis. 2018;8:1249–1260. doi: 10.1093/infdis/jiy311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niu P., Zhao G., Deng Y. A novel human mAb (MERS-GD27) provides prophylactic and postexposure efficacy in MERS-CoV susceptible mice. Sci China Life Sci. 2018;61(10):1280–1282. doi: 10.1007/s11427-018-9343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Z., Bao L., Chen C. Human neutralizing monoclonal antibody inhibition of Middle East respiratory syndrome coronavirus replication in the common marmoset. J Infect Dis. 2017;215(12):1807–1815. doi: 10.1093/infdis/jix209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shishido S.N., Varahan S., Yuan K., Li X., Fleming S.D. Humoral innate immune response and diease. Cinical iImunology. 2012;144(2):142–158. doi: 10.1016/j.clim.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baker S., Kessler E., Darville-Bowleg L., Merchant M. Different mechanisms of serum complement activation in the plasma of common (Chelydra serpentina) and alligator (Macrochelys temminckii) snapping turtles. PloS One. 2019;14(6):1–13. doi: 10.1371/journal.pone.0217626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith J.K., Chi D.S., Krish G., Reynolds S., Cambron G. Effect of exercise on complement activity. Ann Allergy. 1990;65(4):304–310. [PubMed] [Google Scholar]
- 68.Karacabey K., Peker, Saygın, Cıloglu F., Ozmerdivenli R., Bulut V. Effects of acute aerobic and anaerobic exercise on humoral immune factors in elite athletes. Biotechnol Biotechnol Equip. 2005;19(1):175–180. [Google Scholar]
- 69.Karacabey K., Saygin O., Ozmerdivenli R., Zorba E., Godekmerdan A., Bulut V. The effects of exercise on the immune system and stress hormones in sportswomen. Neuroendocrinol Lett. 2005;26(4):361–366. [PubMed] [Google Scholar]
- 70.Wolach B., Eliakim A., Gavrieli R. Aspects of leukocyte function and the complement system following aeeorbic exercise in young female gymnasts∗. Scand J Med Sci Sports. 2007;8(2):91–97. doi: 10.1111/j.1600-0838.1998.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 71.Mackinnon L.T. Overtraining effects on immunity and performance in athletes. Immunol Cell Biol. 2000;78(5):502–509. doi: 10.1111/j.1440-1711.2000.t01-7-.x. [DOI] [PubMed] [Google Scholar]
- 72.Sproston N.R., Ashworth J.J. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9(APR) doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rasmussen F., Mikkelsen D., Hancox R.J. High-sensitive C-reactive protein is associated with reduced lung function in young adults. Eur Respir J. 2009;33(2):382–388. doi: 10.1183/09031936.00040708. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J., Zhou L., Yang Y., Peng W., Wang W., Chen X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8(3):e11–e12. doi: 10.1016/S2213-2600(20)30071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang Y., Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 epidemic in China: a web-based cross-sectional survey. medRxiv. 2020 doi: 10.1101/2020.02.19.20025395. March 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gaspersz R., Lamers F., Wittenberg G. The role of anxious distress in immune dysregulation in patients with major depressive disorder. Transl Psychiatry. 2017;7(12) doi: 10.1038/s41398-017-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blume J., Douglas S.D., Evans D.L. Immune suppression and immune activation in depression. Brain Behav Immun. 2011;25(2):221–229. doi: 10.1016/j.bbi.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Asmundson G.J.G., Fetzner M.G., Deboer L.B., Powers M.B., Otto M.W., Smits J.A.J. Let’s get physical: a contemporary review of the anxiolytic effects of exercise for anxiety and its disorders. Depress Anxiety. 2013;30(4):362–373. doi: 10.1002/da.22043. [DOI] [PubMed] [Google Scholar]
- 79.Nieman D.C., Wentz L.M. The compelling link between physical activity and the body’s defense system. J Sport Heal Sci. 2019;8(3):201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dick N.A., Diehl J.J. Febrile illness in the athlete. Sports Health. 2014;6(3):225–231. doi: 10.1177/1941738113508373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.-Y. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu K., Fang Y.-Y., Deng Y. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020:1. doi: 10.1097/CM9.0000000000000744. February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. JAMA, J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hui D.S., I Azhar E., Madani T.A. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health — the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections-more than just the common cold. JAMA, J Am Med Assoc. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 89.Martin S.A., Pence B.D., Woods J.A. Exercise and respiratory tract viral infections. Exerc Sport Sci Rev. 2009;37(4):157–164. doi: 10.1097/JES.0b013e3181b7b57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Williams P.T. Dose-response relationship between exercise and respiratory disease mortality. Med Sci Sports Exerc. 2014;46(4):711–717. doi: 10.1249/MSS.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vieira R.P., Oliveira-Junior M.C., W Teixeira R. The crucial role of IL-10 in the anti-inflammatory effects of aerobic exercise in a model LPS-induced ARDS. Eur Respir J. 2013;42(Suppl 57) [Google Scholar]
- 92.Park J., Han D. Effects of high intensity aerobic exercise on treadmill on maximum-expiratory lung capacity of elderly women. J Phys Ther Sci. 2017;29(8):1454–1457. doi: 10.1589/jpts.29.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taskin H., Telli Atalay O., Pekesen Kurtca M. vol. 52. European Respiratory Society (ERS); 2018. The effects of aerobic training on respiratory muscle strength and exercise capacity in ankylosing spondylitis patients; p. PA1444. (European respiratory journal). [Google Scholar]
- 94.Simioni C., Zauli G., Martelli A.M. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018;9(24):17181–17198. doi: 10.18632/oncotarget.24729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trefler S., Rodríguez A., Martín-Loeches I. Oxidative stress in immunocompetent patients with severe community-acquired pneumonia. A pilot study. Med Intensiva. 2014;38(2):73–82. doi: 10.1016/j.medin.2013.01.004. English Ed. [DOI] [PubMed] [Google Scholar]
- 96.Gagnon D.D., Dorman S., Ritchie S. Multi-day prolonged low- to moderate-intensity endurance exercise mimics training improvements in metabolic and oxidative profiles without concurrent chromosomal changes in healthy adults. Front Physiol. 2019;10(September):1–12. doi: 10.3389/fphys.2019.01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Da Cunha M.J., Da Cunha A.A., Ferreira G.K. The effect of exercise on the oxidative stress induced by experimental lung injury. Life Sci. 2013;92(3):218–227. doi: 10.1016/j.lfs.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 98.Pöyhönen M., Syväoja S., Hartikainen J., Ruokonen E., Takala J. The effect of carbon dioxide, respiratory rate and tidal volume on human heart rate variability. Acta Anaesthesiol Scand. 2004;48(1):93–101. doi: 10.1111/j.1399-6576.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- 99.Deshpande A.D., Harris-Hayes M., Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88(11):1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Graves D.T., Kayal R.A. Diabetic complications and dysregulated innate immunity. Front Biosci. 2008;13(4):1227–1239. doi: 10.2741/2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ardigo D., Valtuena S., Zavaroni I., Baroni M.C., Delsignore R. Pulmonary complications in diabetes mellitus: the role of glycemic control. Curr Drug Targets - Inflamm Allergy. 2004;3(4):455–458. doi: 10.2174/1568010042634488. [DOI] [PubMed] [Google Scholar]
- 102.Hamer M. The anti-hypertensive effects of exercise: integrating acute and chronic mechanisms. Sport Med. 2006;36(2):109–116. doi: 10.2165/00007256-200636020-00002. [DOI] [PubMed] [Google Scholar]
- 103.Lund Rasmussen C., Nielsen L., Linander Henriksen M. Acute effect on ambulatory blood pressure from aerobic exercise: a randomised cross-over study among female cleaners. Eur J Appl Physiol. 2018;118(2):331–338. doi: 10.1007/s00421-017-3773-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ciolac E.G., Guimarães G.V., D’ávila V.M., Bortolotto L.A., Doria E.L., Bocchi E.A. Acute aerobic exercise reduces 24-H ambulatory blood pressure levels in long-term-treated hypertensive patients. Clinics. 2008;63(6):753–758. doi: 10.1590/S1807-59322008000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nascimento L.S., Santos A.C., Lucena J.M.S., Silva L.G.O., Almeida A.E.M., Brasileiro-Santos M.S. Acute and chronic effects of aerobic exercise on blood pressure in resistant hypertension: study protocol for a randomized controlled trial. Trials. 2017;18(1):1–8. doi: 10.1186/s13063-017-1985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guimarães G.V., Cruz L.G.B., Tavares A.C., Dorea E.L., Fernandes-Silva M.M., Bocchi E.A. Effects of short-term heated water-based exercise training on systemic blood pressure in patients with resistant hypertension. Blood Press Monit. 2013:1. doi: 10.1097/MBP.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 107.Agostoni P., Cattadori G., Bussotti M., Apostolo A. Cardiopulmonary interaction in heart failure. Pulm Pharmacol Therapeut. 2007;20(2):130–134. doi: 10.1016/j.pupt.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 108.Lee H.M., Liu M.A., Barrett-Connor E., Wong N.D. Association of lung function with coronary heart disease and cardiovascular disease outcomes in elderly: the Rancho Bernardo study. Respir Med. 2014;108(12):1779–1785. doi: 10.1016/j.rmed.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olson T.P., Beck K.C., Johnson B.D. Pulmonary function changes associated with cardiomegaly in chronic heart failure. J Card Fail. 2007;13(2):100–107. doi: 10.1016/j.cardfail.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]