Abstract
Recent advances in stem cell biology, synthetic biology, bioengineering, and biotechnology have included significant work leading to the development of stem cell-derived organoids. The growing popularity of organoid research and their use is widely due to the fact that these three-dimensional cellular structures better model human physiology compared to traditional in vitro and in vivo methods by recapitulating many biologically relevant parameters. Organoids show great promise for a wide range of applications, such as for use in disease modeling, drug discovery, and regenerative medicine. However, many challenges associated with reproducibility and scale up still remain. Identification of the conditions which generate a robust environment that predictably promotes cellular self-assembly and organization leading to organoid formation is critical and requires a multidisciplinary approach. To accomplish this we need to identify a cellular source, engineer a matrix to stimulate cell–cell and cell–matrix interactions, and provide the biochemical and biophysical cues which mimic that of the in vivo environment. Discussion of the components needed for organoid development and formation is reviewed herein, as well as specific organoid examples and the promise of this research for the future.
Keywords: Biomaterials, Brain, Cardiac, Intestine, Stem cells, Self-assembly
INTRODUCTION
Organoids are three-dimensional (3D) cellular structures derived from pluripotent stem cell (PSC) or multipotent stem cells. These constructs have gained recent attention in the biomedical research field over the last decade due to their ability to be used as tools to investigate many biological processes, as model systems continue to be the driving force of current biomedical research. Organoids have shown great promise for modeling human physiology over traditional animal and two-dimensional (2D) cellular models due to the ability of stem cells to self-organize into 3D tissue structures. These tissue models now serve as a new biological class of their own, replicating both tissues and organs.32 Organoids recapitulate many biological parameters, such as the spatial organization and physiological functions of tissue-specific cells, in addition to cell–cell and cell–matrix interactions of both undifferentiated and differentiated cell types.58 Organoids continue to bridge the gaps between existing model systems by providing a controlled environment susceptible to manipulation, while also being more representative of the in vivo physiology.58
Organoids have been used to investigate the processes involved in disease development and progression, for drug discovery applications and high throughput screening methods, in addition to serving as tissue alternatives for regenerative medicine applications. Although many stem cell-derived organoids have been developed, most organoid systems represent individual or partial tissue components. For this reason, engineers and scientists strive to investigate the conditions that direct cell behavior and organization (the vital processes needed for organoid formation) while elucidating the underlying mechanism responsible for such actions. Using multidisciplinary approaches, organoids have been constructed and deconstructed to provide enhanced control of organoid formation for specific applications. The components needed for organoid formation (Fig. 1) and examples of such are discussed, specifically the cellular source, extracellular matrix (ECM) components, and niche factors.
FIGURE 1.
Process of organoid formation. Organoid formation can be initiated from pluripotent stem cells or multipotent adult stem cells. When provided with the appropriate biophysical and biochemical cues to that of the specific in vivo environment, cell aggregates form and then are embedded in a hydrogel matrix. When cultured in the presence of niche factors, the cells begin to self-assemble and differentiate generating organoids with several cell types. The derived organoids can be used to recapitulate organogenesis and serve as systems for disease modeling and high throughput drug testing.
ENGINEERING ORGANOID SYSTEMS
Cell Source
Organoid formation is governed by the self-assembly and organization of stem cells which depends on their origin and stage of development.47 Thus, one important consideration in the design of organoid systems is the source of stem cells. Organoids can be derived from adult stem cells (ASCs), which reside in adult tissue, or from PSCs, which include induced PSCs (iPSCs), or embryonic stem cells (ESCs). ASCs are obtained through a tissue biopsy, which is then treated with enzymes, such as collagenase, elastase, or dispase, to isolate single cells.19 While ASCs have been used in many organoid systems including intestinal,17,22 liver,7,25 and lung,46 PSCs must be used when tissue biopsy is not feasible, such as for brain and cardiac organoids.
Organoids derived from either ASCs and PSCs differ, in that ASCs are cultured in a physiological milieu of the native tissue, while PSCs are cultured in an environment that mimics the conditions under which in vivo development takes place.5 The use of PSCs enables a broader potency due to the coordination of cells to form multiple germ layers. Despite the success of the developed lung,20 gastric,36 and intestinal54 organoids, PSC-derived organoids have yet to be predictably reproducible.5,44 In contrast to PSCs, the potency of ASCs is limited due to the dependence on their tissue origin. While this may seem to be a limitation, ASC-derived organoids have to ability to recapitulate the conditions and regenerative processes exhibited by the specific adult tissue. However, the restricted potency does lack the necessary interactions to promote complex organ like architectures. Although there are advantages and limitations of using ASCs vs. PSCs for organoid development, an in between approach has also been investigated.5 This approach aims to partially differentiate PSCs toward a specific progenitor cell lineage before aggregation with differentiated cells. These directed stem cells have the ability of being proliferative with a high differentiation potential while still having the capability to prevent the development of non-desired structure due to their partial commitment.5
Extracellular Matrix
Another important aspect of organoid development is providing an environment for distribution of key biochemical and biophysical signals, while also promoting cellular organization. While organoids have the potential to be useful tools as models of disease and human development, they must be cultured in a reproducible manner, especially when used for clinical translation.
Organoids are developed from stem cell aggregates which can be formed using either a scaffold-based or scaffold-free approach. A scaffold-based approach relies on the use of either a porous structure or a hydrogel, which provides a foundation for the cells to grow and differentiate. The scaffold serves as a substitute for the ECM that cells naturally interact with in vivo and aims to mimic the biophysical and biochemical microenvironment of the native tissue. Because cell–ECM interactions play an important role in the migration, proliferation, and differentiation of stem cells, several scaffold parameters must be considered in the design. Material properties, such as material type, stiffness, and pore size affect the ability of cells to interact with the matrix, and thus must be carefully selected when designing 3D organoid cultures.35
Organoids developed to date have primarily relied on the use of animal-derived ECMs, such as Matrigel and collagen. Matrigel is composed of numerous ECM proteins, primarily laminin, collagen IV, and enactin, extracted from Engelbreth–Holm–Swarm tumors in mice.26 Because these materials have components that are found in native ECM, they have an advantage in that they can readily interact with cells via integrin binding. For instance, Matrigel components such as fibronectin and laminin contain RGD (Arg-Gly-Asp) peptides, which provide cells with binding sites on the matrix via integrin interactions. It has been shown that these sites are necessary for intestinal stem cell (ISC) proliferation and organoid formation.22 However, natural materials are associated with a high degree of batch-to-batch variability and immunogenicity, limiting their use in clinical applications. Synthetic materials pose as promising alternatives due to their homogeneity, reproducibility, and ability to modulate many biochemical and biophysical components. In addition, their influence on biological processes can be tested systematically to determine their influence on stem cell fate.11 For example, polyethylene glycol (PEG) is a hydrophilic polymer that has been successful in the development of several organoid types.14,22,41,52 Although such matrices are resistant to protein adhesion which limits cell interactions,8 they can be easily functionalized with biochemical cues to promote cell attachment and proliferation. Furthermore, these synthetic matrices allow for chemical modulation to incorporate specific material bioactivity and degradability.
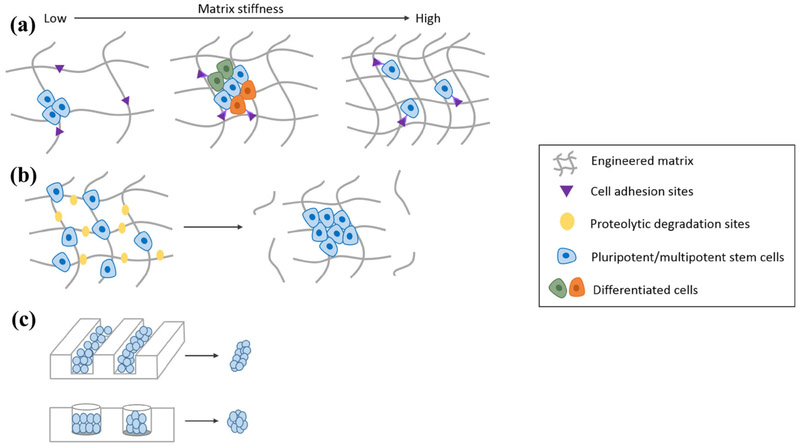
Mechanical properties of the scaffold play a large role in stem cell migration, proliferation and differentiation,51,56,57 and thus can affect the ability of an organoid to develop and survive (Table 1). It is well established that mechanical stresses and strains regulate cell behavior including stem cell differentiation.21 Therefore, to ensure the cells are receiving mechanical cues that mimic those present in vivo, the optimal stiffness of the matrix will be comparable to that of the native tissue, and thus will vary depending on the organ of interest. In fact, several studies have shown that a stiffness too high or too low can hinder cell proliferation, and differentiation, and organoid formation (Fig. 2a). For example, Shkumatov et al. showed that matrix stiffness can affect cardiac organoid formation by limiting cell differentiation.53 They found that the degree of cardiomyogenic differentiation was dependent on the shear modulus of the matrix, with the maximum differentiation occurring on gels of 6 kPa, which is in the range of native cardiac muscle (6–10 kPa). Cells cultured on gels with low modulus showed limited differentiation, suggesting that differentiation into cardiomyocytes requires a minimum mechanical signal.53 In general, softer matrices limit cell migration on the matrix, causing cells to cluster, limiting proliferation.23 However, matrices that are too stiff limit cell–cell interactions by activating cellular integrin expression, leading to the dissociation of cell aggregates needed to form organoids.18,53 A delicate balance between cell–ECM interaction and cell–cell interactions is thus needed to ensure proper cell growth and morphogenesis. In fact, optimal organoid formation may require a dynamic mechanical environment to allow stem cells to first proliferate on a stiff matrix and then aggregate on a softer matrix. Gjorevski et al. found that ISC proliferation is optimal at a stiffness around 1.3 kPa; however, organoid formation only occurred in matrices with a stiffness of 190 Pa.22 This suggests that a dynamic mechanical environment is needed for optimal organoid formation, which can be achieved by designing matrices capable of degradation (Fig. 2b). A stiff matrix encourages cell proliferation and as it degrades, the stiffness decreases, making it more conducive to organoid formation. DiMarco et al. found that in an elastin-like matrix, primary intestinal organoids showed increased matrix metalloproteinase (enzymes which degrade ECM proteins) activity in stiffer matrices, suggesting that the cells were attempting to degrade the matrix in response to the stiff environment.17 Thus, stiffer matrices may be suitable for organoid formation if they are designed with degradation properties, which can be systematically modulated through addition of proteolytic degradation sites.
TABLE 1.
Summary of the influence of matrix mechanical properties on organoid formation.
| Organ/ tissue |
Cell source | 3D biomaterial matrix | Matrix mechanical properties |
Effects of material properties on organoid for mation |
Reference |
|---|---|---|---|---|---|
| Intestine | Murine ASCs | Functionalized PEG | 1.3 kPa (shear modulus) | Optimal ISC expansion achieved within PEG matrices in the presence of 1 mM RGD | 22 |
| Murine ASCs | Functionalized and hydrolytically degradable PEG | 0.19 kPa (shear modulus) | ISC differentiation and organoid formation was achieved in soft PEG matrices in the presence of RGD and laminin-111 | 22 | |
| Murine ASCs | Recombinant engineered matrices (containing an elastin-like structural backbone) | 0.18 kPa (shear modulus) | Highest incidence of organoid formation was achieved within engineered matrices in the presence of 3.2 mM RGD | 17 | |
| Human iPSCs | Functionalized four-armed PEG with maleimide terminals | ~ 0.10 kPa (storage modulus) | Highest organoid viability was obtained in 4% PEG-4MAL matrices in the presence of 2 mM RGD | 14 | |
| Brain | Human iPSCs | Hyaluronic acid and chitosan | 10 kPa (Young’s modulus) | Evidence of organoids with neuronal morphologies were observed on the matrix surface | 34 |
| Cardiac | Murine ESCs | Collagen-conjugated polyacrylamide | 6 kPa (elastic modulus) | Percentage of contractile EBs, levels of cardiomyogenic differentiation, and frequency of cardiomyoblasts division were highest when cultured on this matrix | 53 |
| Immune | Primary B cells (isolated from murine spleen) and 40LB stromal cells | Gelatin with SiNP | 1.9 kPa (storage modulus) | Enhanced spreading and network formation of 40LB cells was observed in 2% gelatin matrices with 1.5% SiNP | 40 |
ASCs adult stem cells, EBs embryoid bodies, ESCs embryonic stem cells, iPSCs induces pluripotent stem cells, ISC intestinal stem cell, PEG poly(ethylene glycol), RGD Arg-Gly-Asp, SiNP silicate nanoparticles, 40LB BALB/c 3T3 fibroblasts transfected with both CD40L and BAFF.
FIGURE 2.
Aggregation of stem cells in/on 3D engineered matrices. (a) Synthetic materials must be modified with cell adhesion sites, such as the RGD peptide, to promote cell–ECM interactions via integrin binding. In addition, mechanical stiffness of the scaffold affects cell migration, proliferation, and differentiation due to different degrees of cell–cell and cell–ECM interactions. Soft matrices limit cell–ECM interactions whereas stiff matrices limit cell–cell interactions. A matrix of intermediate stiffness, optimizes cell–cell and cell–ECM interactions, promoting cell proliferation, organization, and differentiation. (b) Proteolytic degradation sites are another important component for organoid culture as they allow for dynamic mechanical properties. A stiffer matrix can be used to promote cell proliferation and can then degrade to yield a softer matrix, which promotes cell organization and organoid formation. (c) Alternatively, stem cells can form aggregates in microwells where the cells take the shape of the well. This allows for formation of homogenous aggregates where the size and shape of the aggregates can be controlled. The aggregates are then seeded in 3D culture for organoid formation.
Stem cells can also form aggregates [i.e., spheroids or embryoid bodies (EBs)] in a scaffold-free approach, where the size and shape of the organoid is defined by the environment in which the cells first begin to aggregate (Fig. 2c). One method is to seed cells in microwells, typically made up of weakly adhesive materials such as polydimethylsiloxane, PEG, or agarose in order to prevent cell attachment to the surface and promote cell aggregation.5 The shape and size of the spheroid can be controlled by the cell seeding density and microwell structures, as the cells will take the shape of the well. For example, Mori et al. found that HepG2 organoids grew larger in diameter when cultured in circular microwells compared to rectangular microwells due to more efficient diffusion of oxygen and nutrients.38 In addition, Choi et al. found that, compared to cylindrical microwells, concave wells formed more homogenous EB aggregates (aggregates of PSCs) due to the curved surface of the aggregates.13 In fact, one advantage of using microwells is that the spheroids formed are more homogenous, in size and shape as well as expression of differentiation markers, compared to those formed in suspension.30
Controlling the size of the cell aggregates is important in the formation of organoids, as it has been shown that the size of EBs influences stem cell fate. For example, Hwang et al. found that larger EBs formed cardiomyocytes while smaller aggregates formed endothelial cells.28 Although microwells can serve as useful tools to control cell fate, size, and the shape of cell aggregates, they lack the biochemical and biomechanical signals that are needed to fully recapitulate native tissue. Thus, cell aggregates formed in microwells are typically transferred into 3D culture environments to continue to grow and differentiate until organoids are fully formed.39 Alternatively, microwells can be formed directly on a hydrogel by stamping a micropatterned mold prior to curing the gel. This technique can be used to screen the effects of different microenvironment components, such as hydrogel stiffness and biochemical composition, on organoid growth and viability.10
Modulation of the Stem Cell Niche
The assembly of cellular organoids is a highly sophisticated process which requires detailed control of cellular differentiation, the cellular microenvironment, and the tissue architecture. The ability of stem cells to self-assemble to form constructs which recapitulate key characteristics of their in vivo tissue origin warrants the underlying mechanisms behind these functions to be investigated and further elucidated. The stem cell niche (the microenvironment that surrounds the stem cell) directly influences cell behavior and cell fate. Some even believe the stem cell niche, as opposed to the cell alone, is the functional unit which controls cell fate.39,50 The components which make up the stem cell niche and methods to control the cellular microenvironment are thus highly sought-after for investigation and promotion of organogenesis.
The individual components which make up the stem cell niche vary depending on the specific tissue, however, all include several factors such as ECM components, differentiated cells, biochemical cues, and biophysical cues. While many of these niche components have been identified for ASC populations, the exact mechanisms by which these individual components regulate the local microenvironment, owing to their interdependence, remains unknown. Therefore, methods to determine and quantify niche factors are necessary in an effort to engineer the stem cell niche. For example, Devaud et al. demonstrated that a tandem mass spectrometry (LC–MS/MS) method could be used to selectively identify proteins deposited in a protein-free 3D PEG hydrogel following various growth factor treatments.15 Moreover, Roch et al. utilized single-cell multigene expression analysis and time-lapse microscopy to define gene expression profiles and cell cycle hallmarks of murine hematopoietic stem cells to bioengineer artificial stem cell niches.45 Building upon the current technologies used to identify various niche factors will have a major impact in the development of stem cell microenvironments to promote cellular organization and organoid formation.
Since the emergence of organoids, the ability to investigate human stem cell niche interactions using these models has been at the forefront; their use offers a controlled, high throughput environment to assess the contributions of multiple niche factors. The use of droplet based microfluidics exemplifies a high throughput screening method which allows for multiple combinations of hydrogel precursors and biochemical signals (forming artificial microniches) to be fabricated and selected.1 Microfluidic technologies can also be used to generate spatiotemporally controlled chemical gradients by delivering signaling molecules to match the presentation of those in vivo.4,27 The ability to control specific material parameters enables modulation of the stem cell–ECM and therefore, niche. Further development of these matrices could lead to the synthesis of tunable dynamic hydrogels to influence stem cell organization. Elucidation of these interactions would enable further understanding of tissue homeostasis, disease, and regeneration.
EXAMPLES OF ORGANOID DEVELOPMENT
The ability to modulate cell signaling has the potential to increase morphogenesis of stem cell-derived organoids and even organogenesis in culture. To accomplish this, biomaterial-based synthetic, recombinant, and animal-derived matrices have been utilized to provide control of the stem cell microenvironment and support for cellular organization (Table 2). Since the development of organoid models, their use has been able to demonstrate key organ functions, in addition to providing critical information which was not previously possible using conventional 2D cellular models. Biomaterial matrices have been used and models such as, intestine,6,9,14,22 brain,34,52 and select immune organs.40-42 A summary of the biomaterials used to aid in such applications is provided herein in regards to their use for modeling human development and disease.
TABLE 2.
Natural and synthetic hydrogels used to promote organoid formation.
| Type of material | Organ/ tissue |
Rationale | Reference |
|---|---|---|---|
| Natural | |||
| Alginate | Intestine | Serve as a natural alternative to conventional tumor-derived matrices for translational applications | 9 |
| Fibrin | Intestine | Serve as a natural alternative to conventional tumor-derived matrices for translational applications | 6 |
| Collagen | Cardiac | Serve as a natural substrate for translational applications | 53 |
| Hyaluronic acid and chitosan | Brain | Serve as a chemically defined matrix to control tissue organization | 34 |
| Synthetic | |||
| Functionalized and hydrolytically degradable PEG | Intestine | Provide control for presentation of biochemical and biophysical cues | 22 |
| Four-arm maleimide-functionalized PEG | Intestine | Provide control for presentation of biochemical and biophysical cues, serve as a robust and reproducible matrix | 14 |
| Four-arm maleimide-functionalized PEG | Immune | Provide control for presentation of biochemical and biophysical cues, enables patient specific signaling | 41 |
| Eight-arm PEG | Brain | Provides control for presentation of biochemical and biophysical cues | 52 |
Intestinal Organoids
The small and large intestines span approximately 8 m in length, and are the longest organs within the digestive tract. The small intestine in divided into three distinct regions, the duodenum, jejunum, and ileum, and the large intestine is divided into two, the cecum and colon. While their roles are primarily associated with digestion, nutrient absorption and waste removal, the intestines also regulate metabolism and feeding behavior in conjunction with other organ systems through endocrine hormones.55 In addition, the epithelium of the intestinal tract serves as a protective barrier by restricting microbes to the gut lumen. The intestinal epithelium is also highly regenerative, driven by its resident population of ISCs. This finding came about through the discovery of Lgr5+ crypt-base columnar ISCs which reside at the base of intestinal crypts.3 Since the discovery of these cells, intestinal organoids were among the first to be derived, and make up one of the most abundant topics in the organoid literature.14,17,22,48 Through this, research efforts have focused on constructing a functional epithelium to further investigate intestinal development and dysfunction, in addition to drug absorption and toxicity.
Since the identification of murine Lgr5+ ISCs, intestinal organoids were created based on ISC self-assembly with the appropriate stem cell niche components provided in the culture conditions, namely, Wnt agonist R-spondin 1, epidermal growth factor (EGF), and the BMP inhibitor Noggin.49 It must also be noted that laminin rich Matrigel was used to mimic the intestinal crypts and provide structural integrity to the nutrient rich environment. In this environment, single crypts formed into multiple crypts through fission events, all while generating villus-like epithelial domains where differentiated cell types are present.49
Moreover, PSCs have been used to form intestinal organoids by mimicking steps of intestinal development.37 McCracken et al. demonstrated that PSCs first need to be directed towards the endoderm germ layer before spheroid culture in Matrigel with prointestinal growth factors.37 This was the first method established to direct differentiation of PSCs through stages that emulate embryonic intestinal development. This gave rise to development of a system for understanding and modeling human intestinal development, homeostasis and disease.
In addition to the previously mentioned Matrigel used to support the intestinal organoid ECM, synthetic hydrogels have also been used.14,22 Gjorevski et al. used functionalized PEG hydrogels to define key ECM parameters that control ISC expansion and organoid formation.22 Specifically, the authors found that fibronectin-based adhesion was sufficient for ISC survival and proliferation. However, the expansion of ISCs was impacted by the mechanical properties of the matrix, where a high matrix stiffness resulted in increased ISC expansion. In contrast, a soft matrix with laminin-based adhesions is required for ISC differentiation and organoid formation. These findings greatly impact intestinal organoid research by determining the biomaterial-based ECM properties needed to investigate several aspects of intestinal organoid formation from ISCs, in addition to providing the necessary information for developing well-defined alternatives to animal-derived matrices for the culture of mouse and human stem cell-derived organoids.22 Furthermore, functionalized PEG macromers have been shown to support the growth and expansion of human intestinal organoids derived from PSC spheroids.14 Cruz-Acuña et al. synthesized a four-armed, maleimide-terminated PEG macromer, with similar mechanical properties to that of Matrigel, functionalized with a RGD adhesive peptide and crosslinked with a protease degradable peptide to promote cell migration and growth.14 This synthetic hydrogel network provided an environment which supported human intestinal organoid growth and expansion similar to that when cultured in Matrigel. However, the benefit of the previously described synthetic matrices is that they offer translation of organoid technologies for regenerative medicine applications, as tumor-derived matrices (e.g., Matrigel) would not be considered for such applications.
Cerebral Organoids
The brain is one of the most complex organs in the body, making it difficult to study and model, outside of animal models. Recent advances, however, have made it possible to develop models of the human brain in a 3D culture setting. Human brain development begins with the folding and fusion of the neural plate, which is comprised of neural ectoderm, to form the neural tube. Morphogenic gradients result in ventral–dorsal and rostral–caudal axes that give rise to the four major brain regions, the forebrain, midbrain, hindbrain, and spinal cord.33 Many of the current methods for creating brain organoids exploit the ability of iPSCs to self-organize under controlled culture conditions. The iPSCs aggregate to form EBs, which are then treated with dual SMAD inhibition or WNT inhibitors to form neuroectoderm.16 From here, specific brain regions can be formed as the cell fate can be controlled through the addition of specific signaling molecules. For example, Qian et al. treated neuroectodermal EBs with a GSK-3β inhibitor, a WNT2A protein, and a SMAD inhibitor to direct the fate to a forebrain identity.43
Alternatively, if the neural progenitors are not directed to a specific fate and instead undergo self-patterning and organization, there is a potential to develop whole brain organoids, consisting of multiple brain regions.44 Lancaster et al. formed cerebral organoids without the use of signaling molecules by culturing EBs in Matrigel droplets, which were then placed in a spinning bioreactor.33 They found that the organoids expressed gene markers for multiple, distinct regions of the brain, including hindbrain, midbrain, and forebrain. While this approach has the clear advantage of generating in vitro structures containing multiple brain regions, it suffers from a lack of reproducibility and formation of non-ectodermal tissue.44 Thus, the advantage of using signaling molecules to direct cell fate rather than relying on intrinsic signaling is that there is more control over the types of cells produced. This controls the formation of non-neural tissue, such as the endoderm and mesoderm and can reduce the variability between organoid samples, resulting in more homogenous and reproducible structures.
To enhance scalability and even spatial control of biochemical cues, the microenvironment can be adapted to better form cerebral organoids. Lindborg et al. developed a chemically defined hydrogel material and culture medium to obtain cerebral organoids derived from human PSCs.34 Sodium hyaluronan and protonated chitosan were used to form a hydrogel network in which iPSCs were embedded. In the absence of additional neural induction components, cerebral organoids formed on the hydrogel surface within 10–14 days. The organoids also showed neural rosette and tube-like structures, as well as evidence of early corticogenesis.34 Furthermore, these cerebral organoids demonstrated protein and gene expression representative of forebrain, midbrain, and hindbrain development, in addition to responding to glutamate and depolarization in many cells. This study showcased the need for chemically defined matrices to control the stem cell microenvironment leading to the formation of cerebral organoids with results that are consistent with neural behavior.
Cardiac Organoids
Cardiac organoids have primarily been developed to study early human cardiogenesis, as little is known about this process. Current approaches for engineering cardiac organoids primarily rely on formation of tissues from direct differentiation of EBs or from predifferentiated cardiomyocytes.24 The native myocardial tissue is composed of cardiomyocytes, fibroblasts, and endothelial cells, thus, most methods to obtain cardiac organoids focus on the incorporation of all three cell types. Iyer et al. found that when cardiomyocytes were cultured on preformed networks of endothelial cells and fibroblasts, the resulting organoids resembled native myofibers and had comparable cardiac function to that of organoids formed solely from cardiomyocytes.29 The pre-culture allowed the non-myocytes to secrete ECM components and growth factors as they reorganized and elongated, resulting in a structure that more closely resembled the heart, compared to when the three cell types were cultured simultaneously.29
Microfabrication is commonly used in the formation of cardiac organoids as it can be used to mimic the dimensions of cardiac myofibers, such as through the use of microchannels12,24,29 and patterning.2,31 Chiu et al. formed cardiac organoids by seeding cardiomyocytes, fibroblasts, and endothelial cells in microchannels created on PEG discs.12 The microchannels were coated with Matrigel to allow for cell attachment and assembly. In addition, Khademhosseini et al. created contractile cardiac organoids using microfluidic patterning of hyaluronic acid on a glass substrate.31 The micropatterns served as templates for organoid formation, allowing cardiomyocytes to elongate and align along the patterns. Primary cardiomyocytes adhered to the interface between fibronectin coated glass and the hyaluronic acid pattern and elongated to a length on the order of 100 μm.31 The resulting organoids spontaneously detached from the substrate and exhibited contractile activity. This micropatterning technique has the potential to be directly applied to 3D scaffolds to give cells topographical cues to organize in a 3D environment, rather than on a 2D substrate, which can allow for formation of larger organoids that more closely recapitulate in vivo tissues.
Many of the early approaches to create cardiac organoids used tissue-specific cells isolated from nonhuman mammals and therefore, have limitations on their potential to accurately model the human heart. Hoang et al. then developed a technique to create cardiac organoids directly from iPSCs.24 iPSCs were seeded onto micropatterned PEG substrates and differentiated into cardiomyocytes through activation of the WNT/β-catenin pathway. While this technique is novel in its ability to direct self-organization and cardiac differentiation of human iPSCs by providing biophysical cues, it is limited in that the resulting organoids are only representative of early stage development.
PERSPECTIVE
Organoids have the ability to simulate important aspects of human organs due to their 3D architecture, cellular compositions, and functionality. Thus, these constructs hold great promise for a wide range of biomedical research and translational applications. Although the ability to recapitulate in vivo organ functionality using organoids has been previously explored through basic science research, one of the most exciting areas of current and future investigations is the ability to study human development and disease processes. Advances in stem cell biology in combination with bioengineering tools have granted much success towards the current status and future potential of organoid research, however, many challenges still remain. The current major obstacles involve modulating cellular assembly and organization to predict and reproduce the formation of intended organoids, determining the conditions to generate robust organoids at physiologically relevant shapes and sizes, and creating environments which extend the longevity and functionality of organoids.
A multidisciplinary approach to cellular self-assembly and organoid formation focuses on identifying a cell source, engineering an ECM to promote cell–cell and cell–ECM interactions, directing cellular symmetry, and providing the biochemical and biophysical cues which mimic that of the in vivo environment for cellular organization.5 Identifying the components which lead to optimal organoid development could address limitations regarding design and scalability. In addition, use of microtechnologies, imaging techniques, organs-on-a-chip, and advances in synthetic biology and tissue engineering will allow researchers to develop enhanced model systems to address fundamental questions on what drives cellular organization to tissue and organ formation. Elucidating the underlying mechanisms involved in these processes will require the need for simplified systems where many variables can be manipulated and isolated (i.e., high throughput techniques) to address fundamental questions. By combining the use of novel technologies, bioengineering tools, and advances in stem cell and synthetic biology multidisciplinary approaches can be used to overcome the existing limitations thus transforming the future of organoid research and its applications.
ACKNOWLEDGMENTS
We acknowledge support from the National Institutes of Health under Award Number R01-EB022025. In addition, N.A.P. acknowledges support from the Cockrell Family Chair Foundation, the Office of the Dean of the Cockrell School of Engineering at the University of Texas at Austin (UT) for the Institute for Biomaterials, Drug Delivery, and Regenerative Medicine, and the UT-Portugal Collaborative Research Program. During this work, M.E.W. was supported by a National Science Foundation Graduate Research Fellowship (DGE-1610403).
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Allazetta S, and Lutolf MP. Stem cell niche engineering through droplet microfluidics. Curr. Opin. Biotechnol 35:86–93, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Annabi N, Tamayol A, Shin SR, Ghaemmaghami AM, Peppas NA, and Khademhosseini A. Surgical materials: current challenges and nano-enabled solutions. Nano Today 9:574–589, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, and Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449:1003–1007, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia SN, and Ingber DE. Microfluidic organs-on-chips. Nat. Biotechnol 32:760–772, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Brassard JA, and Lutolf MP. Engineering stem cell self-organization to build better organoids. Cell Stem Cell 24:860–876, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Broguiere N, Isenmann L, Hirt C, Ringel T, Placzek S, Cavalli E, Ringnalda F, Villiger L, Züllig R, Lehmann R, Rogler G, Heim MH, Schüler J, Zenobi-Wong M, and Schwank G. Growth of epithelial organoids in a defined hydrogel. Adv. Mater 30:1801621, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Broutier L, Andersson-Rolf A, Hindley CJ, Boj SF, Clevers H, Koo BK, and Huch M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc 11:1724–1743, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Burdick JA, and Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. A 15:205–219, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capeling MM, Czerwinski M, Huang S, Tsai Y-H, Wu A, Nagy MS, Juliar B, Sundaram N, Song Y, Han WM, Takayama S, Alsberg E, Garcia AJ, Helmrath M, Putnam AJ, and Spence JR. Nonadhesive alginate hydrogels support growth of pluripotent stem cell-derived intestinal organoids. Stem Cell Rep. 12:381–394, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casey J, Yue X, Nguyen TD, Acun A, Zellmer VR, Zhang S, and Zorlutuna P. 3D hydrogel-based microwell arrays as a tumor microenvironment model to study breast cancer growth. Biomed. Mater 12:025009, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Cha C, Liechty WB, Khademhosseini A, and Peppas NA. Designing biomaterials to direct stem cell fate. ACS Nano 6:9353–9358, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu LLY, Iyer RK, King J-P, and Radisic M. Biphasic electrical field stimulation aids in tissue engineering of multicell-type cardiac organoids. Tissue Eng. A 17:1465–1477, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi YY, Chung BG, Lee DH, Khademhosseini A, Kim J-H, and Lee S-H. Controlled-size embryoid body formation in concave microwell arrays. Biomaterials 31:4296–4303, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Acuña R, Quirós M, Farkas AE, Dedhia PH, Huang S, Siuda D, García-Hernández V, Miller AJ, Spence JR, Nusrat A, and García AJ. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol 19:1326–1335, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devaud YR, Avilla-Royo E, Trachsel C, Grossmann J, Martin I, Lutolf MP, and Ehrbar M. Label-free quantification proteomics for the identification of mesenchymal stromal cell matrisome inside 3D poly(ethylene glycol) hydrogels. Adv. Healthc. Mater 7:1800534, 2018. [DOI] [PubMed] [Google Scholar]
- 16.Di Lullo E, and Kriegstein AR. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci 18:573–584, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiMarco RL, Dewi RE, Bernal G, Kuo C, and Heilshorn SC. Protein-engineered scaffolds for in vitro 3D culture of primary adult intestinal organoids. Biomater. Sci 3:1376–1385, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Discher DE, Mooney DJ, and Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science 324:1673–1677, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta D, Heo I, and Clevers H. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med 23:393–410, 2017. [DOI] [PubMed] [Google Scholar]
- 20.Dye BR, Hill DR, Ferguson MA, Tsai Y-H, Nagy MS, Dyal R, Wells JM, Mayhew CN, Nattiv R, Klein OD, White ES, Deutsch GH, and Spence JR. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 2015. 10.7554/eLife.05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gjorevski N, Ranga A, and Lutolf MP. Bioengineering approaches to guide stem cell-based organogenesis. Development 141:1794–1804, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, Clevers H, and Lutolf MP. Designer matrices for intestinal stem cell and organoid culture. Nature 539:560–564, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Guo WH, Frey MT, Burnham NA, and Wang YL. Substrate rigidity regulates the formation and maintenance of tissues. Biophys. J 90:2213–2220, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoang P, Wang J, Conklin BR, Healy KE, and Ma Z. Generation of spatial-patterned early-developing cardiac organoids using human pluripotent stem cells. Nat. Protoc 13:723–737, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huch M, Dorrell C, Boj SF, Van Es JH, Li VSW, Van De Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, and Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494:247–250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes CS, Postovit LM, and Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10:1886–1890, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Huh D, Hamilton GA, and Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 21:745–754, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang Y-S, Chung BG, Ortmann D, Hattori N, Moeller HC, and Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc. Natl Acad. Sci. USA 106:16978–16983, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyer RK, Chiu LLY, and Radisic M. Microfabricated poly(ethylene glycol) templates enable rapid screening of triculture conditions for cardiac tissue engineering. J. Biomed. Mater. Res. A 89:616–631, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Karp JM, Yeh J, Eng G, Fukuda J, Blumling J, Suh K-Y, Cheng J, Mahdavi A, Borenstein J, Langer R, and Khademhosseini A. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip 7:786–794, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Khademhosseini A, Eng G, Yeh J, Kucharczyk PA, Langer R, Vunjak-Novakovic G, and Radisic M. Microfluidic patterning for fabrication of contractile cardiac organoids. Biomed. Microdevices 9:149–157, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Lancaster MA, and Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345:1247125, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, and Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature 501:373–379, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindborg BA, Brekke JH, Vegoe AL, Ulrich CB, Haider KT, Subramaniam S, Venhuizen SL, Eide CR, Orchard PJ, Chen W, Wang Q, Pelaez F, Scott CM, Kokkoli E, Keirstead SA, Dutton JR, Tolar J, and O’Brien TD. Rapid induction of cerebral organoids from human induced pluripotent stem cells using a chemically defined hydrogel and defined cell culture medium. Stem Cells Transl. Med 5:970–979, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthys OB, Hookway TA, and McDevitt TC. Design principles for engineering of tissues from human pluripotent stem cells. Curr. Stem Cell Rep 2:43–51, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCracken KW, Catá EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai Y-H, Mayhew CN, Spence JR, Zavros Y, and Wells JM. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516:400–404, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCracken KW, Howell JC, Wells JM, and Spence JR. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc 6:1920–1928, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mori R, Sakai Y, and Nakazawa K. Micropatterned organoid culture of rat hepatocytes and HepG2 cells. J. Biosci. Bioeng 106:237–242, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Murrow LM, Weber RJ, and Gartner ZJ. Dissecting the stem cell niche with organoid models: an engineering-based approach. Development 144:998–1007, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purwada A, Jaiswal MK, Ahn H, Nojima T, Kitamura D, Gaharwar AK, Cerchietti L, and Singh A. Ex vivo engineered immune organoids for controlled germinal center reactions. Biomaterials 63:24–34, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purwada A, Shah SB, Beguelin W, Melnick AM, and Singh A. Modular immune organoids with integrin ligand specificity differentially regulate ex vivo B cell activation. ACS Biomater. Sci. Eng 3:214–225, 2017. [DOI] [PubMed] [Google Scholar]
- 42.Purwada A, and Singh A. Immuno-engineered organoids for regulating the kinetics of B-cell development and antibody production. Nat. Protoc 12:168–182, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian X, et al. Brain-region-specific organoids using minibioreactors for modeling ZIKV exposure. Cell 165:1238–1254, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quadrato G, Brown J, and Arlotta P. The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat. Med 22:1220–1228, 2016. [DOI] [PubMed] [Google Scholar]
- 45.Roch A, Giger S, Girotra M, Campos V, Vannini N, Naveiras O, Gobaa S, and Lutolf MP. Single-cell analyses identify bioengineered niches for enhanced maintenance of hematopoietic stem cells. Nat. Commun 8:221, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, and Hogan BLM. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl Acad. Sci. USA 106:12771–12775, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossi G, Manfrin A, and Lutolf MP. Progress and potential in organoid research. Nat. Rev. Genet 19:671–687, 2018. [DOI] [PubMed] [Google Scholar]
- 48.Sato T, Stange DE, Ferrante M, Vries RGJ, van Es JH, van den Brink S, van Houdt WJ, Pronk A, van Gorp J, Siersema PD, and Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141:1762–1772, 2011. [DOI] [PubMed] [Google Scholar]
- 49.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, and Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Scadden DT Nice neighborhood: emerging concepts of the stem cell niche. Cell 157:41–50, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schultz KM, Kyburz KA, and Anseth KS. Measuring dynamic cell–material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc. Natl Acad. Sci. USA 112:E3757–E3764, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz MP, Hou Z, Propson NE, Zhang J, Engstrom CJ, Santos Costa V, Jiang P, Nguyen BK, Bolin JM, Daly W, Wang Y, Stewart R, Page CD, Murphy WL, and Thomson JA. Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc. Natl Acad. Sci. USA 112:12516–12521, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shkumatov A, Baek K, and Kong H. Matrix rigidity-modulated cardiovascular organoid formation from embryoid bodies. PLoS ONE 9:e94764, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, and Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470:105–109, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wells JM, Spence JR, and Le Douarin NM. How to make an intestine. Development 141:752–760, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang C, DelRio FW, Ma H, Killaars AR, Basta LP, Kyburz KA, and Anseth KS. Spatially patterned matrix elasticity directs stem cell fate. Proc. Natl Acad. Sci. USA 113:E4439–E4445, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang C, Tibbitt MW, Basta L, and Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat. Mater 13:645–652, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin X, Mead BE, Safaee H, Langer R, Karp JM, and Levy O. Engineering stem cell organoids. Cell Stem Cell 18:25–38, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]