Abstract
Objectives:
Quantifying the association between muscle weakness and mortality with carefully matched cohorts will help to better establish the impact of weakness on premature death. We utilized a matched cohort analysis in a national sample of older Americans to determine if those who were weak had a higher risk for mortality compared to control groups with incrementally higher strength capacities.
Design:
Longitudinal-Panel.
Setting:
Detailed interviews that included physical measures were conducted in person, while core interviews were often performed over the telephone.
Participants:
Data from 19,729 Americans aged at least 50-years from 2006-2014 waves of the Health and Retirement Study were analyzed.
Measures:
A hand-grip dynamometer was used to assess handgrip strength (HGS) in each participant. Men with HGS <26-kilograms were considered weak, ≥26-kilograms were considered not-weak, and ≥32-kilogrmas were considered strong. Women with HGS <16-kilograms were classified as weak, ≥16-kilograms were classified as not-weak, and ≥20-kilograms were classified as strong. The National Death Index and postmortem interviews determined date of death. The greedy matching algorithm was used to match cohorts.
Results:
Of the 1,077 weak and not-weak matched pairs, 401 (37.2%) weak and 296 (27.4%) not-weak older Americans died over an average 4.4±2.5 year follow-up. There were 392 (37.0%) weak and 243 (22.9%) strong persons that died over a mean 4.5±2.5 year follow-up from the 1,057 weak and strong matched pairs. Those in the weak cohort had a 1.40 (95% confidence interval (CI): 1.19, 1.64) and 1.54 (CI: 1.30, 1.83) higher hazard for mortality relative to persons in the not-weak and strong control cohorts, respectively.
Conclusions/Implications:
Our findings may indicate a causal association between muscle weakness and mortality in older Americans. Healthcare providers should include measures of HGS as part of routine health assessments and discuss the health risks of muscle weakness with their patients.
Keywords: Aging, Epidemiology, Geriatrics, Hand Strength, Muscle Strength, Sarcopenia
Brief Summary:
A longitudinal study of a national sample of older Americans determined that muscle weakness may have a casual association with early mortality, thereby helping to provide more so confirmatory evidence for this association.
INTRODUCTION
Frailty is generally a geriatric syndrome reflecting a state of susceptibility to acute and chronic stressors from age-related declines across several physiological systems.1, 2 As such, frailty carries an increased risk for many poor health outcomes, including early mortality.1, 3, 4 Weakness, as measured by handgrip strength (HGS), is an important component of frailty screenings. Weakness is the most common initial indicator of frailty onset and introduces vulnerability to stressors; thus, recognizing weakness helps to identify populations for targeted frailty prevention interventions.1, 5 Given that reduced neural and muscle system functioning during aging contributes to weakness,6-8 measures of HGS have been considered a “vital sign” and powerful biomarker of aging.9, 10
Previous epidemiological investigations have utilized longitudinal, observational data and determined that weakness was associated with premature death in aging adults.11-13 While observational studies are generally the most viable option for cause-effect research questions, the inability to make causal inferences from some longitudinal study designs poses challenges.14 For example, in longitudinal-panel studies, participants are often not randomly allocated to exposure and non-exposure groups, which may result in systematic differences between the groups. Therefore, differences in observed outcomes that exist between such groups may be due to confounding variables rather than the exposure.15 Depending on how variables are measured and included in analyses, the association between an exposure and outcome may then become biased due to differences between exposure groups or underlying confounders that may predicate assignment to the exposure group.15
Propensity score analyses are gaining popularity for assessing cause-effect hypotheses in observational studies, and allows for a multitude of confounders to be matched between exposure and “control” groups, thereby emulating some characteristics of a controlled trial.14 Matched cohort analyses such as propensity score matching often provides exceptional balance in covariates and analyses that are easy to analyze, present and interpret.16 Accordingly, a call to use matched cohort analyses has been made to help improve our understanding of the causal inferences of weakness with observational data.6 Although previous research has found that weakness was associated with early mortality during aging, the association between weakness and mortality should not be viewed as purely causal because the presence of other risk factors and poor health outcomes may mediate the association.6 However, utilizing matched cohort analyses would effectively allow for a comparison of survival between weak and not-weak, or weak and strong older adults after matching for important covariates known to be predictive of premature death. Utilizing matched cohort analyses may help to provide a deeper understanding as to whether weakness causes early mortality during aging. For this investigation, a matched cohort analysis was utilized in a national sample of older Americans to determine if persons who were weak had a higher risk for early mortality compared to a matched not-weak or strong control group.
METHODS
Participants
Data from 23,207 adults aged at least 50-years who completed interviews without a proxy from the 2006-2014 waves of the Health and Retirement Study (HRS) were analyzed. Publicly-available HRS data files were joined with the RAND HRS dataset.17 The HRS is an ongoing longitudinal-panel study that monitors the health and financial status of aging Americans.18 Participants in the HRS are interviewed biennially and followed until death. Additional information for the HRS is published elsewhere.19
Beginning in the 2006 wave, enhanced face-to-face interviews that included physical and biological measures were collected on half of the sample, while the other half sample only completed the core interview. Interview response rates for each wave of the HRS have been >80%.18 Participants provided written informed consent before entering the HRS and the University’s Behavioral Sciences Committee Institutional Review Board approved study protocols.
Measures
Outcome Variable
Date of death was verified through linkage to the National Death Index. Postmortem interviews with a surviving family member or other informant were also completed for death verification. Mortality validation for the HRS has shown that the National Death Index and postmortem interviews capture 99% of participant deaths.20
Exposure Variable
A Smedley spring-type hand-grip dynamometer (Scandidact; Odder, Denmark) was held in the hand of each tested individual for assessing HGS. Beginning on the non-dominant hand, participants squeezed the hand-grip dynamometer with their hand at maximal effort, and released the muscle contractions. Two HGS measures were performed on each hand, alternating between hands. More information for HGS protocols are published elsewhere.21
Participants who underwent a surgical procedure, had swelling, inflammation, intense pain, or an injury in both hands did not complete HGS assessments. Those with missing HGS data for all waves (n=2,987) and implausible HGS values (HGS=350 kg; n=1) were excluded. The highest recorded HGS measurement from a single trial on either hand was included in the analyses and the wave at which HGS was first measured was used as the baseline. Sex-specific thresholds were utilized for determining HGS cohorts.22 Men with HGS <26-kilograms and women with HGS <16-kilograms were classified as weak. Likewise, men with HGS ≥26-kilograms and women with HGS ≥16-kilograms were considered not-weak; whereas, men with HGS >32-kilograms and women with HGS >20-kilograms were considered strong.
Covariates
Age, sex, race, height, and body mass were self-reported. Body mass index (BMI) was calculated as body mass in kilograms divided by height in meters-squared. Respondents told interviewers if they had ever smoked more than 100-cigarettes in their lifetime (smoking history) and if they were current cigarette smokers. A single-item self-rated health measure determined if participants perceived their health as either “excellent”, “very good”, “good”, “fair”, or “poor”. Respondents also revealed if a physician diagnosed them with a chronic lung disease, stroke, heart condition, emotional or psychiatric problems, cancer, diabetes, and arthritis.
Participants reported their ability to complete six activities of daily living (ADL): dressing, eating, transferring in or out of bed, toileting, bathing, and walking across a small room. Anyone reporting difficulty or an inability in completing an ADL were considered as having an ADL disability. Each participant also told interviewers if they were a patient in a hospitalization or nursing home for at least an overnight stay in the past two-years.
Cognitive functioning was assessed with a variety of tests from the modified Telephone Interview of Cognitive Status (TICS), a validated cognitive screening tool from the Mini-Mental State Examination that was designed for population-based studies.23 A 27-point composite scale was used for those under age 65-years. Participants with scores ≤11 were considered as having a cognitive impairment.21 A 35-point composite scale was used for assessing cognitive functioning in participants aged at least 65-years. Respondents with scores ≤10 were considered as having a cognitive impairment.24 Those with one or more missing covariates were excluded (n=490).
Statistical Analysis
All analyses were conducted with SAS 9.4 software (SAS Institute; Cary, NC). The greedy matching algorithm was used to match 1) those in the weak cohort 1:1 to older Americans in the not-weak control cohort, and 2) those in the weak cohort 1:1 to older Americans in the strong control cohort.25 An exact match was performed for sex, race, smoking history, current smoking status, cognitive functioning, stroke diagnosis, diabetes diagnosis, arthritis diagnosis, cancer diagnosis, the diagnosis of any heart condition, and nursing home stay. Additionally, the match that minimized the logit of the propensity score for age, BMI, chronic lung disease diagnosis, ADL disability, emotional or psychiatric diagnosis, hospitalization, and self-rated health were found using the PSMATCH procedure in SAS. All matched covariates were pre-specified by investigators for the association between weakness and early mortality. A standardized mean difference between the cohorts of −0.25 to 0.25 was used for each matched covariate to determine if covariates were balanced between exposure and non-exposure control groups.14.
After the weak participants were matched on relevant-covariates to the not-weak or strong control group, separate Kaplan-Meier estimators were executed to generate survival curves using either the matched weak and not-weak cohorts or weak and strong cohorts as the strata. Distinct Cox proportional hazard regression models were used to determine the association between weakness and time to mortality using either the not-weak or strong control cohort as the reference group. To further reduce bias, misspecification, and imbalances in matched covariates, a doubly robust estimation was performed for the Cox models by adjusting for all of the matched covariates.15, 26 Data were left-truncated because participants entered the HRS at different ages and had to be at least 50-years to be included. The age when HGS was first measured was the entry variable for both models. Participants were right censored if they did not die during the study period or were lost to follow-up.
In order to address potential differences in participant characteristics from limitations due to imbalanced sample sizes for those who were weak and not-weak or weak and strong, older Americans in the weak cohort were matched 1:2 to 1) those in the not-weak control group, and 2) persons in the strong control group (when possible to find an additional suitable match) as a sensitivity analysis. The same exact and closest match methods, and standardized difference protocol from the 1:1 matching procedure was used for the 1:2 matching procedure. After the 1:2 matching procedures were completed, doubly robust estimation was again applied to the Cox models for examining the association between weakness and early mortality using either the not-weak or strong control matched cohort as the reference group. If the hazard ratios from the Cox models for the 1:2 matching procedure did not differ by >10% from the hazard ratios of the Cox models for the 1:1 matching procedure, participant characteristic biases from imbalanced sample sizes between weak and not-weak, or weak and strong cohorts were not influential for the results.27 An alpha level of 0.05 was used for all analyses.
RESULTS
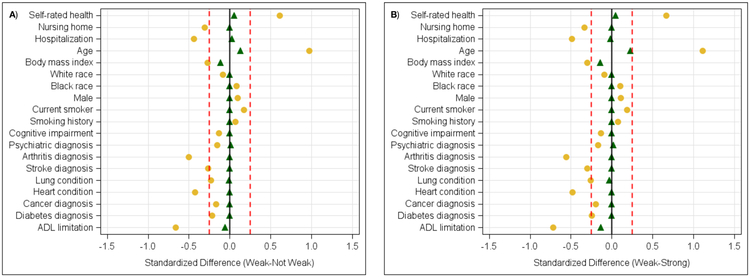
Of the 19,729 participants, 1,138 were weak (5.8%), 18,591 were not-weak (94.2%), and 16,120 (81.7%) were strong. After performing the exact and closest 1:1 match, there were 1,077 matched pairs (94.6% matched) for the weak and not-weak cohorts, and 1,057 matched pairs (92.9% matched) for the weak and strong cohorts. The descriptive characteristics for each of the matched pairs are in Table 1. To make comparisons on descriptive characteristics between the 1:1 matched cohorts, the means and 95% confidence intervals (CI) are provided in Appendix 1. Figure 1A shows the standardized differences for each covariate between the weak and not-weak cohorts while Figure 1B shows the standardized differences for each covariate between the weak and strong cohorts. The covariates were considered balanced between cohorts because no matched covariate exceeded the standardized bias thresholds.
Table 1.
Baseline Characteristics of the Participants for 1:1 Matching.
| Variable | Weak (n=1,077) |
Not-Weak (n=1,077) |
Weak (n=1,057) |
Strong (n=1,057) |
|---|---|---|---|---|
| Handgrip Strength (kilograms) | 15.9±5.3 | 29.5±11.5 | 16.0±5.3 | 31.8±10.9 |
| Age (years) | 74.5±12.1 | 73.0±10.6 | 74.5±12.1 | 72.0±10.2 |
| Body Mass Index (kg/m2) | 27.0±6.6 | 27.7±5.9 | 27.0±6.6 | 27.9±5.9 |
| ADL Disability (n (%)) | 431 (40.0) | 404 (37.5) | 420 (39.7) | 360 (34.0) |
| White Race (n (%)) | 846 (78.5) | 846 (78.5) | 830 (78.5) | 830 (78.5) |
| Black Race (n (%)) | 163 (15.1) | 163 (15.1) | 160 (15.1) | 160 (15.1) |
| Other Race (n (%)) | 68 (6.4) | 68 (6.4) | 67 (6.4) | 67 (6.4) |
| Male (n (%)) | 398 (36.9) | 398 (36.9) | 393 (37.1) | 393 (37.1) |
| Previous Smoker (n (%)) | 581 (53.9) | 581 (53.9) | 570 (53.9) | 570 (53.9) |
| Current Smoker (n (%)) | 119 (11.0) | 119 (11.0) | 117 (11.0) | 117 (11.0) |
| Cognitive Impairment (n (%)) | 143 (13.2) | 143 (13.2) | 138 (13.0) | 138 (13.0) |
| Stroke Diagnosis (n (%)) | 111 (10.3) | 111 (10.3) | 104 (9.8) | 104 (9.8) |
| Chronic Lung Disease Diagnosis (n (%)) | 185 (17.1) | 181 (16.8) | 180 (17.0) | 169 (15.9) |
| Heart Condition Diagnosis (n (%)) | 415 (38.5) | 415 (38.5) | 402 (38.0) | 402 (38.0) |
| Emotional or Psychiatric Diagnosis (n (%)) | 241 (22.3) | 247 (22.9) | 231 (21.8) | 239 (22.6) |
| Cancer Diagnosis (n (%)) | 189 (17.5) | 189 (17.5) | 184 (17.4) | 184 (17.4) |
| Diabetes Diagnosis (n (%)) | 299 (27.7) | 299 (27.7) | 288 (27.2) | 288 (27.2) |
| Arthritis Diagnosis (n (%)) | 823 (76.4) | 823 (76.4) | 804 (76.0) | 804 (76.0) |
| Hospitalization (n (%)) | 445 (41.3) | 457 (42.4) | 430 (40.6) | 422 (39.9) |
| Nursing Home Stay (n (%)) | 56 (5.2) | 56 (5.2) | 44 (4.1) | 44 (4.1) |
| Self-Rated Health (n (%)) | ||||
| Excellent | 44 (4.1) | 38 (3.5) | 43 (4.1) | 32 (3.0) |
| Very Good | 192 (17.8) | 182 (16.9) | 191 (18.1) | 186 (17.6) |
| Good | 309 (28.7) | 367 (34.0) | 304 (28.7) | 351 (33.2) |
| Fair | 332 (30.8) | 332 (30.9) | 324 (30.6) | 342 (32.4) |
| Poor | 200 (18.6) | 158 (14.7) | 195 (18.5) | 146 (13.8) |
| Deaths (n (%)) | 401 (37.2) | 296 (27.4) | 392 (37.0) | 243 (22.9) |
| Age at Death (years) | 84.2±9.3 | 82.4±9.6 | 84.2±9.3 | 81.7±9.0 |
Note: ADL=Activities of Daily Living; kg/m2=kilograms per meters-squared.
Figure 1.
Standardized Bias for the 1:1 Matched Covariates.
Note: A=Weak vs. Not-Weak, B=Weak vs. Strong; Yellow=Pre-Matched, Green=Post-Matched.
Survival curves for the weak and not-weak cohorts are in Figure 2A and survival curves for the weak and strong cohorts are in Figure 2B. The survival probability for the weak cohort was significantly lower compared to both their not-weak or strong matched peers (Wilcoxon p<0.001; log-rank p<0.001). Table 2 reveals the results of the Cox models for the association between weakness and time to mortality for the 1:1 matched cohorts. After an average 4.4±2.5 year follow-up, 401 (37.2%; 90.6 per 1,000 person-years) and 296 (27.4%; 57.8 per 1,000 person-years) older Americans in the weak and not-weak cohorts died, respectively. Relative to the not-weak control, the weak cohort had a 1.40 (CI: 1.19-1.64) higher hazard for mortality. Moreover, there were 392 weak older Americans that died (37.0%; 89.6 per 1,000 person-years) and 243 strong older Americans that died (22.9%; 46.7 per 1,000 person-years) over an average 4.5±2.5 year follow-up. Compared to the strong control, the weak cohort had a 1.54 (CI: 1.30-1.83) higher hazard for mortality. Full results for each of the Cox models are presented in Appendices 2 and 3.
Figure 2.
Kaplan-Meier Curves for the 1:1 Matched Cohorts.
Note: A=Weak vs. Not-Weak, B=Weak vs. Strong; Red=Weak, Blue=Not-Weak (Figure 2A) or Strong (Figure 2B).
Table 2.
Weakness and Risk for All-Cause Mortality Using 1:1 Matching.
| Number of Deaths |
Mean and 95% CI Follow-Up (Years) |
Mortality Rate per 1,000 Person-Years |
Hazard Ratio (95% CI) |
|
|---|---|---|---|---|
| Weak vs. Not-Weak | ||||
| Not-Weak Cohort | 296 | 4.7 (4.6, 4.9) | 57.8 | Reference |
| Weak Cohort | 401 | 4.1 (3.9, 4.2) | 90.6 | 1.40 (1.19, 1.64) |
| Weak vs. Strong | ||||
| Strong Cohort | 243 | 4.9 (4.7, 5.0) | 46.7 | Reference |
| Weak Cohort | 392 | 4.1 (3.9, 4.2) | 89.6 | 1.54 (1.30, 1.83) |
Note: Each Cox model was adjusted for race, sex, age, body mass index, current smoking status, smoking history, cognitive impairment, stroke diagnosis, chronic lung disease diagnosis, heart condition diagnosis, cancer diagnosis, diabetes diagnosis, activities of daily living disability, arthritis, emotional or psychiatric diagnosis, hospitalization, nursing home stay, and self-rated health. CI=Confidence Interval.
After executing the 1:2 match, there were 1,077 persons in the weak cohort and 2,100 older Americans in the not-weak control (94.6% of the weak cohort had two not-weak matched controls); whereas, there were 1,057 persons in the weak cohort and 2,040 older Americans in the strong control (92.9% of the weak cohort had two strong matched controls). The results for the association between weakness and time to mortality for the 1:2 matched cohorts using the not-weak cohort as the reference are in Appendix 4. Compared to the not-weak control, older Americans in the weak cohort had a 1.38 (CI: 1.20-1.59) higher hazard for mortality. Appendix 5 shows the results for the association between weakness and time to mortality for the 1:2 matched cohorts using the strong control as the reference. Relative to the strong control, those in the weak cohort had a 1.57 (CI: 1.35-1.83) higher hazard for mortality. The hazard ratios for the association between weakness and time to mortality did not differ by >10% for the 1:1 and 1:2 matched cohorts, suggesting that participant characteristic biases from imbalanced sample sizes between cohorts were not influential for the results.
DISCUSSION
This investigation was designed to determine the association between weakness and time to mortality in older Americans using a robust matched cohort analysis. Those in the weak cohort had a 40% greater risk for early mortality relative to older Americans who were in the not-weak control, despite these cohorts being balanced on modifiable and non-modifiable factors associated with mortality. The risk for mortality increased to 54% when comparing weak older Americans to the strong control group. Although our findings do not confirm that weakness directly causes death, our results indicate that a causal association between weakness and early mortality may indeed exist.
The association between weakness and mortality has been shown throughout the life course, including adolescence,28 adulthood,12 middle-age,13 older adulthood,29 and among the oldest old populations.30 Some have postulated that those who are weak tend to have poorer metabolic profiles.31 This may help to explain why weakness is associated with greater risk for acute health events that may lead to sudden unexpected death. For example, weakness is associated with myocardial infarction and stroke.12, 32 Weak older Americans who were not diagnosed with such acute health outcomes at baseline for our investigation may not have had the strength to survive complications from these critical health events if they experienced them during the study period. Similarly, those who have already sustained an acute health outcome such as myocardial infarction or stroke are at an increased risk for the reoccurrence of the same acute health event.33 Older Americans who were weak and had an acute health outcome diagnosis at baseline in our study may not have been able to survive the possible reoccurrence of such health events.
Others have suggested that the association between weakness and mortality is partially attributed to diminished neural system functioning.7, 8 Age-related deterioration to the neural system may have further explained our findings because sudden deaths could have occurred in the weak cohort from acute outcomes related to poor physical function or falls.34, 35 Regardless of the mechanisms that explain the association between weakness and mortality, healthcare providers and their patients should acknowledge how weakness may potentiate the risk for an acute health event that abruptly ends life.
While matched cohort analyses allowed us to match weak and not-weak, or weak and strong cohorts on chronic factors that may accelerate time to mortality, older Americans in the weak cohort still had a greater mortality risk. Those who were in the weak cohort may have had more difficulty preventing exposure to stressors from daily life or chronic disease.1 For example, persons who were weak and had chronic obstructive pulmonary disease may not be as capable of overcoming complications due to pneumonia.36 Similarly, older Americans who were weak and had a diabetes diagnosis may be especially vulnerable to chronic stressors that increase mortality risk from diabetes complications.37 Therefore, although weakness itself may not cause mortality in those who experience daily life or chronic disease stressors, weakness makes resilience to such stressors more challenging, which in turn, may exacerbate mortality risk.
Our findings further support the notion that measures of HGS are a “vital sign” and powerful biomarker of aging.9, 10 Our results also lend support to muscle strength being an important component in the concept of intrinsic capacity which merits lifespan monitoring.38-40 It should also be acknowledged that the findings for the association between self-rated health and mortality in our models were strong. Self-rated health is a powerful, subjective predictor of poor health status, and HGS is associated with self-reported health.41 Thus, HGS could be viewed as an objective measure of self-rated health. While the biologic mechanisms that may explain why weakness is linked to mortality remain unclear,42 more research is needed to understand why weakness increases mortality risk. Examining how weakness is measured in more detail, including HGS, may help to uncover such pathways. Healthcare providers should include measures of HGS as part of routine health assessments, communicate the hazards of weakness to their patients, and discuss options for improving strength. Other domains of intrinsic capacity should be considered for determining risk and strategy of care for addressing weakness and functional abilities.38
Some limitations should be noted. Although self-report data are common in large, population-based studies such as the HRS, biases from self-report may exist. Specificity for certain health factors and diagnoses may have helped to improve matching covariates between the weak and not-weak, or weak and strong cohorts. Likewise, information for specific medications that may have contributed to weakness was not available. Measures of physical activity were not included because the physical activity questionnaire used in the HRS is not well validated and cardiorespiratory fitness indicators were not measured. While a matched cohort analysis allowed us to emulate some characteristics of a randomized controlled trial, our findings do not provide definitive evidence that weakness directly causes early mortality. Certain factors related to each research question should be considered before employing matched cohort analyses or covariate-adjustment in regression models to other investigations.16
Conclusions and Implications
Our findings indicate that a causal association between weakness and mortality may exist; however, the physiological basis for how exactly weakness is linked to mortality remains somewhat unclear. Nevertheless, healthcare providers should integrate measures of HGS into routine health assessments, discuss the health risks of weakness with their patients, and communicate strategies for preserving strength to their older patients in effort to extend longevity.
Acknowledgments
Funding Sources: RM’s effort in this research was partially supported by funding from the College of Human Sciences and Education at North Dakota State University. BCC’s effort in this research was supported, in part, by a grant from the National Institute on Aging (R01AG044424).
Appendix
Appendix 1.
Mean and 95% Confidence Intervals for the Baseline Characteristics of the Participants for 1:1 Matching.
| Variable | Weak | Not-Weak | Weak | Strong |
|---|---|---|---|---|
| HGS (kilograms) | 15.9 (15.6, 16.3) | 29.5 (28.8, 30.2) | 16.0 (15.6, 16.3) | 31.8 (31.1, 32.4) |
| Age (years) | 74.5 (73.7, 75.2) | 73.0 (72.3, 73.6) | 74.5 (73.7, 75.2) | 72.0 (71.4, 72.6) |
| Body Mass Index (kg/m2) | 27.0 (26.6, 27.4) | 27.7 (27.3, 28.0) | 27.0 (26.6, 27.4) | 27.9 (27.5, 28.2) |
| ADL Disability (%) | 40.0 (37.0, 42.9) | 37.5 (34.6, 40.4) | 39.7 (36.7, 42.6) | 34.0 (31.2, 36.9) |
| White Race (%) | 78.5 (76.1, 81.0) | 78.5 (76.1, 81.0) | 78.5 (76.0, 81.0) | 78.5 (76.0, 81.0) |
| Black Race (%) | 15.1 (12.9, 17.2) | 15.1 (12.9, 17.2) | 15.1 (12.9, 17.3) | 15.1 (12.9, 17.3) |
| Other Race (%) | 6.4 (4.8, 7.7) | 6.4 (4.8, 7.7) | 6.4 (4.8, 7.7) | 6.4 (4.8, 7.7) |
| Male (%) | 36.9 (34.0, 39.8) | 36.9 (34.0, 39.8) | 37.1 (34.2, 40.0) | 37.1 (34.2, 40.0) |
| Previous Smoker (%) | 53.9 (50.9, 56.9) | 53.9 (50.9, 56.9) | 53.9 (50.9, 56.9) | 53.9 (50.9, 56.9) |
| Current Smoker (%) | 11.0 (9.1, 12.9) | 11.0 (9.1, 12.9) | 11.0 (9.1, 12.9) | 11.0 (9.1, 12.9) |
| Cognitive Impairment (%) | 13.2 (11.2, 15.3) | 13.2 (11.2, 15.3) | 13.0 (11.0, 15.0) | 13.0 (11.0, 15.0) |
| Stroke Diagnosis (%) | 10.3 (8.4, 12.1) | 10.3 (8.4, 12.1) | 9.8 (8.0, 11.6) | 9.8 (8.0, 11.6) |
| CLD Diagnosis (%) | 17.1 (14.9, 19.4) | 16.8 (14.5, 19.0) | 17.0 (14.7, 19.3) | 15.9 (13.7, 18.2) |
| HC Diagnosis (%) | 38.5 (35.6, 41.4) | 38.5 (35.6, 41.4) | 38.0 (35.1, 40.9) | 38.0 (35.1, 40.9) |
| EP Diagnosis (%) | 22.3 (19.8, 24.8) | 22.9 (20.4, 25.4) | 21.8 (19.3, 24.3) | 22.6 (20.0, 25.1) |
| Cancer Diagnosis (%) | 17.5 (15.2, 19.8) | 17.5 (15.2, 19.8) | 17.4 (15.1, 19.6) | 17.4 (15.1, 19.6) |
| Diabetes Diagnosis (%) | 27.7 (25.0, 30.4) | 27.7 (25.0, 30.4) | 27.2 (24.5, 29.9) | 27.2 (24.5, 29.9) |
| Arthritis Diagnosis (%) | 76.4 (73.8, 78.9) | 76.4 (73.8, 78.9) | 76.0 (73.4, 78.6) | 76.0 (73.4, 78.6) |
| Hospitalization (%) | 41.3 (38.3, 44.2) | 42.4 (39.4, 45.3) | 40.6 (37.7, 43.6) | 39.9 (36.9, 42.8) |
| Nursing Home Stay (%) | 5.2 (3.8, 6.5) | 5.2 (3.8, 6.5) | 4.1 (2.9, 5.3) | 4.1 (2.9, 5.3) |
| Self-Rated Health (%) | ||||
| Excellent | 4.1 (2.9, 5.2) | 3.5 (2.4, 4.6) | 4.1 (2.8, 5.2) | 3.0 (1.9, 4.0) |
| Very Good | 17.8 (15.5, 20.1) | 16.9 (14.6, 19.1) | 18.1 (15.7, 20.3) | 17.6 (15.3, 19.8) |
| Good | 28.7 (25.9, 31.3) | 34.0 (31.2, 36.9) | 28.7 (26.0, 31.4) | 33.2 (30.3, 36.0) |
| Fair | 30.8 (28.0, 33.5) | 30.9 (28.0, 33.5) | 30.6 (27.8, 33.4) | 32.4 (29.5, 35.1) |
| Poor | 18.6 (16.2, 20.8) | 14.7 (12.5, 16.7) | 18.5 (16.1, 20.7) | 13.8 (11.7, 15.8) |
| Follow-Up (years) | 4.7 (4.6, 4.9) | 4.1 (3.9, 4.2) | 4.9 (4.7, 5.0) | 4.1 (3.9, 4.2) |
| Deaths (%) | 37.2 (34.3, 40.1) | 27.4 (24.8, 30.1) | 37.0 (34.1, 40.0) | 22.9 (20.4, 25.5) |
| Age at Death (years) | 84.2 (83.3, 85.1) | 82.4 (81.3, 83.5) | 84.2 (83.3, 85.1) | 81.7 (80.6, 82.8) |
Note: ADL=Activities of Daily Living; CLD=Chronic Lung Disease; EP=Emotional or Psychiatric; HC=Heart Condition; HGS=Handgrip Strength; kg/m2=kilograms per meters-squared.
Appendix 2.
Association Between Weakness and Time to Mortality for 1:1 Matching Using the Not-Weak Cohort as the Reference.
| Hazard Ratio | 95% Confidence Interval | |
|---|---|---|
| Weakness (Ref: Not-Weak) | 1.40 | 1.19, 1.64 |
| White Race (Ref: Not-White) | 1.77 | 1.03, 3.05 |
| Black Race (Ref: Not-Black) | 1.76 | 0.98, 3.17 |
| Male (Ref: Not-Male) | 1.65 | 1.38, 1.96 |
| Age | 0.93 | 0.89, 0.96 |
| Body Mass Index | 0.98 | 0.96, 0.99 |
| Current Smoker (Ref: Non-Smoker) | 1.56 | 1.13, 2.16 |
| Previous Smoker (Ref: Non-Smoker) | 1.32 | 1.11, 1.58 |
| Cognitive Impairment (Ref: No Impairment) | 1.72 | 1.25, 2.35 |
| Stroke (Ref: No Stroke) | 1.07 | 0.84, 1.36 |
| Chronic Lung Disease (Ref: No Lung Disease) | 1.18 | 0.96, 1.45 |
| Heart Condition (Ref: No Heart Condition) | 1.05 | 0.88, 1.25 |
| Cancer (Ref: No Cancer) | 1.01 | 0.83, 1.24 |
| Diabetes (Ref: No Diabetes) | 1.28 | 1.05, 1.56 |
| ADL Disability (Ref: No ADL Disability) | 1.50 | 1.26, 1.79 |
| Arthritis (Ref: No Arthritis) | 0.74 | 0.60, 0.91 |
| Emotional or Psychiatric Diagnosis (Ref: No Diagnosis) | 1.20 | 0.97, 1.49 |
| Hospitalization (Ref: No Hospitalization) | 1.54 | 1.29, 1.82 |
| Nursing Home Stay (Ref: No Nursing Home) | 1.08 | 0.78, 1.49 |
| Self-Rated Health (Ref: Excellent) | ||
| Very Good | 0.98 | 0.59, 1.64 |
| Good | 1.27 | 0.77, 2.08 |
| Fair | 1.62 | 0.98, 2.66 |
| Poor | 2.24 | 1.32, 3.77 |
Note: ADL=Activities of Daily Living, Ref=Reference.
Appendix 3.
Association Between Weakness and Time to Mortality for 1:1 Matching Using the Strong Cohort as the Reference.
| Hazard Ratio | 95% Confidence Interval | |
|---|---|---|
| Weakness (Ref: Strong) | 1.54 | 1.30, 1.83 |
| White Race (Ref: Not-White) | 1.35 | 0.80, 2.26 |
| Black Race (Ref: Not-Black) | 1.30 | 0.74, 2.30 |
| Male (Ref: Not-Male) | 1.67 | 1.40, 1.99 |
| Age | 0.93 | 0.90, 0.97 |
| Body Mass Index | 0.98 | 0.97, 1.00 |
| Current Smoker (Ref: Non-Smoker) | 1.55 | 1.12, 2.14 |
| Previous Smoker (Ref: Non-Smoker) | 1.38 | 1.15, 1.65 |
| Cognitive Impairment (Ref: No Impairment) | 1.85 | 1.33, 2.57 |
| Stroke (Ref: No Stroke) | 1.13 | 0.89, 1.44 |
| Chronic Lung Disease (Ref: No Lung Disease) | 1.11 | 0.90, 1.37 |
| Heart Condition (Ref: No Heart Condition) | 1.03 | 0.86, 1.23 |
| Cancer (Ref: No Cancer) | 1.11 | 0.90, 1.35 |
| Diabetes (Ref: No Diabetes) | 1.25 | 1.02, 1.53 |
| ADL Disability (Ref: No ADL Disability) | 1.49 | 1.24, 1.78 |
| Arthritis (Ref: No Arthritis) | 0.70 | 0.58, 0.86 |
| Emotional or Psychiatric Diagnosis (Ref: No Diagnosis) | 1.26 | 1.01, 1.58 |
| Hospitalization (Ref: No Hospitalization) | 1.49 | 1.26, 1.78 |
| Nursing Home Stay (Ref: No Nursing Home) | 1.12 | 0.78, 1.61 |
| Self-Rated Health (Ref: Excellent) | ||
| Very Good | 0.92 | 0.54, 1.55 |
| Good | 1.30 | 0.79, 2.15 |
| Fair | 1.49 | 0.90, 2.47 |
| Poor | 2.03 | 1.20, 3.45 |
Note: ADL=Activities of Daily Living, Ref=Reference.
Appendix 4.
Association Between Weakness and Time to Mortality for 1:2 Matching Using the Not-Weak Cohort as the Reference.
| Hazard Ratio | 95% Confidence Interval | |
|---|---|---|
| Weakness (Ref: Not-Weak) | 1.38 | 1.20, 1.59 |
| White Race (Ref: Not-White) | 1.82 | 1.12, 2.95 |
| Black Race (Ref: Not-Black) | 1.85 | 1.09, 3.12 |
| Male (Ref: Not-Male) | 1.62 | 1.39, 1.89 |
| Age | 0.91 | 0.89, 0.94 |
| Body Mass Index | 0.97 | 0.96, 0.98 |
| Current Smoker (Ref: Non-Smoker) | 1.65 | 1.24, 2.18 |
| Previous Smoker (Ref: Non-Smoker) | 1.26 | 1.08, 1.48 |
| Cognitive Impairment (Ref: No Impairment) | 1.82 | 1.37, 2.42 |
| Stroke (Ref: No Stroke) | 1.09 | 0.87, 1.35 |
| Chronic Lung Disease (Ref: No Lung Disease) | 1.28 | 1.07, 1.53 |
| Heart Condition (Ref: No Heart Condition) | 1.05 | 0.90, 1.23 |
| Cancer (Ref: No Cancer) | 1.16 | 0.98, 1.39 |
| Diabetes (Ref: No Diabetes) | 1.45 | 1.22, 1.72 |
| ADL Disability (Ref: No ADL Disability) | 1.57 | 1.35, 1.83 |
| Arthritis (Ref: No Arthritis) | 0.71 | 0.60, 0.85 |
| Emotional or Psychiatric Diagnosis (Ref: No Diagnosis) | 1.10 | 0.91, 1.32 |
| Hospitalization (Ref: No Hospitalization) | 1.36 | 1.17, 1.57 |
| Nursing Home Stay (Ref: No Nursing Home) | 1.12 | 0.82, 1.51 |
| Self-Rated Health (Ref: Excellent) | ||
| Very Good | 1.16 | 0.73, 1.85 |
| Good | 1.45 | 0.93, 2.26 |
| Fair | 1.91 | 1.22, 2.99 |
| Poor | 2.63 | 1.64, 4.21 |
Note: ADL=Activities of Daily Living, Ref=Reference.
Appendix 5.
Association Between Weakness and Time to Mortality for 1:2 Matching Using the Strong Cohort as the Reference.
| Hazard Ratio | 95% Confidence Interval | |
|---|---|---|
| Weakness (Ref: Strong) | 1.57 | 1.35, 1.83 |
| White Race (Ref: Not-White) | 1.44 | 0.90, 2.32 |
| Black Race (Ref: Not-Black) | 1.49 | 0.89, 2.49 |
| Male (Ref: Not-Male) | 1.61 | 1.37, 1.89 |
| Age | 0.92 | 0.89, 0.95 |
| Body Mass Index | 0.98 | 0.97, 0.99 |
| Current Smoker (Ref: Non-Smoker) | 1.62 | 1.22, 2.16 |
| Previous Smoker (Ref: Non-Smoker) | 1.37 | 1.16, 1.63 |
| Cognitive Impairment (Ref: No Impairment) | 1.82 | 1.33, 2.48 |
| Stroke (Ref: No Stroke) | 1.08 | 0.85, 1.35 |
| Chronic Lung Disease (Ref: No Lung Disease) | 1.29 | 1.07, 1.54 |
| Heart Condition (Ref: No Heart Condition) | 1.10 | 0.93, 1.30 |
| Cancer (Ref: No Cancer) | 1.16 | 0.96, 1.39 |
| Diabetes (Ref: No Diabetes) | 1.39 | 1.16, 1.67 |
| ADL Disability (Ref: No ADL Disability) | 1.42 | 1.20, 1.68 |
| Arthritis (Ref: No Arthritis) | 0.68 | 0.56, 0.81 |
| Emotional or Psychiatric Diagnosis (Ref: No Diagnosis) | 1.04 | 0.85, 1.27 |
| Hospitalization (Ref: No Hospitalization) | 1.39 | 1.19, 1.63 |
| Nursing Home Stay (Ref: No Nursing Home) | 1.07 | 0.75, 1.55 |
| Self-Rated Health (Ref: Excellent) | ||
| Very Good | 0.95 | 0.61, 1.49 |
| Good | 1.27 | 0.82, 1.95 |
| Fair | 1.62 | 1.05, 2.51 |
| Poor | 2.40 | 1.52, 3.81 |
Note: ADL=Activities of Daily Living, Ref=Reference.
REFERENCES
- 1.Xue Q-L. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fedarko NS. The biology of aging and frailty. Clin Geriatr Med. 2011;27(1):27–37. doi: 10.1016/j.cger.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilardi F, Scarcella P, Proietti MG, et al. Frailty as a predictor of mortality and hospital services use in older adults: a cluster analysis in a cohort study. Eur J Public Health. 2018;28(5):842–6. doi: 10.1093/eurpub/cky006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 2017;47(2):193–200. doi: 10.1093/ageing/afx162. [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. [DOI] [PubMed] [Google Scholar]
- 6.McGrath RP, Kraemer WJ, Al Snih S, et al. Handgrip Strength and Health in Aging Adults. Sports Med. 2018;48(9):1993–2000. doi: 10.1007/s40279-018-0952-y. [DOI] [PubMed] [Google Scholar]
- 7.Clark BC. Neuromuscular Changes with Aging and Sarcopenia. J Frailty Aging. 2018;8(1):7–9. doi: 10.14283/jfa.2018.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carson RG. Get a grip: Individual variations in grip strength are a marker of brain health. Neurobiol Aging. 2018;71:189–222. doi: 10.1016/j.neurobiolaging.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Bohannon RW. Muscle strength: clinical and prognostic value of hand-grip dynamometry. Curr Opin Clin Nutr Metab Care. 2015;18(5):465–70. doi: 10.1097/MCO.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 10.Sayer AA, Kirkwood T. Grip strength and mortality: a biomarker of ageing? Lancet. 2015;386(9990):226–7. doi: 10.1016/S0140-6736(14)62349-7. [DOI] [PubMed] [Google Scholar]
- 11.Duchowny K Do nationally representative cutpoints for clinical muscle weakness predict mortality? Results from 9 years of follow-up in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2019;18:1070–75. doi: 10.1093/gerona/gly169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–73. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki H, Kasagi F, Yamada M, et al. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med. 2007;120(4):337–42. [DOI] [PubMed] [Google Scholar]
- 14.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods. 2010;15(3):234–49. doi: 10.1037/a0019623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin DB. Matched sampling for causal effects. Cambridge, United Kingdom: Cambridge University Press; 2006. [Google Scholar]
- 16.Elze MC, Gregson J, Baber U, et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol. 2017;69(3):345–57. doi: 10.1016/j.jacc.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 17.Health and Retirement Study. HRS data products. https://hrs.isr.umich.edu/data-products. Accessed June 21, 2019.
- 18.Sonnega A, Faul JD, Ofstedal MB, et al. Cohort profile: the health and retirement study (HRS). Int J Epidemiol. 2014;43(2):576–85. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Health and Retirement Study. HRS data book. https://hrs.isr.umich.edu/about/data-book?_ga=2.177450149.1489958521.1509473800-353572931.1501594459. Accessed June 21, 2019.
- 20.Weir DR. Validating mortality ascertainment in the health and retirement study. https://hrs.isr.umich.edu/publications/biblio/9022. Accessed September 17, 2019. [Google Scholar]
- 21.Crimmins E, Guyer H, Langa K, et al. Documentation of physical measures, anthropometrics and blood pressure in the Health and Retirement Study. HRS Documentation Report DR-011. 2008;14:47–59. [Google Scholar]
- 22.Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69(5):559–66. doi: 10.1093/gerona/glu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plassman BL, Newman TT, Welsh KA, et al. Properties of the Telephone Interview for Cognitive Status: Application in epidemiological and longitudinal studies. Cogn Behav Neurol. 1994;7(3):235–41. [Google Scholar]
- 24.Langa KM, Larson EB, Karlawish JH, et al. Trends in the prevalence and mortality of cognitive impairment in the United States: is there evidence of a compression of cognitive morbidity? Alzheimers Dement. 2008;4(2):134–44. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NCSS Statistical Software. Data Matching-Optimal and Greedy. http://ncss.wpengine.netdna-cdn.com/wp-content/themes/ncss/pdf/Procedures/NCSS/Data_Matching-Optimal_and_Greedy.pdf. Accessed June 21, 2019.
- 26.Funk MJ, Westreich D, Wiesen C, et al. Doubly robust estimation of causal effects. Am J Epidemiol. 2011;173(7):761–767. doi: 10.1093/aje/kwq439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan BL, Koval J, Corbett B. Assessing the impact of potentially influential observations in weighted logistic regression. In: The Research Data Centres Information and Technical Bulletin: Statistics Canada. http://www.isr.umich.edu/src/smp/asda/weighted_influence_logistic_2015.pdf. Accessed June 21, 2019.
- 28.Ortega FB, Silventoinen K, Tynelius P, et al. Muscular strength in male adolescents and premature death: cohort study of one million participants. BMJ. 2012;345:e7279. doi: 10.1136/bmj.e7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Buyser SL, Petrovic M, Taes YE, et al. Physical function measurements predict mortality in ambulatory older men. Eur J Clin Invest. 2013;43(4):379–86. doi: 10.1111/eci.12056. [DOI] [PubMed] [Google Scholar]
- 30.Crimmins E, Ailshire J. Physical and biological indicators of health and functioning in US oldest old. Annu Rev Gerontol Geriatr. 2013;33(1):193–215. [Google Scholar]
- 31.Lawman HG, Troiano RP, Perna FM, et al. Associations of relative handgrip strength and cardiovascular disease biomarkers in US adults, 2011–2012. Am J Prev Med. 2016;50(6):677–83. doi: 10.1016/j.amepre.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor J Hand grip strength predicts myocardial infarction and stroke. Eur Heart J. 2015;36(29):1845. doi: 10.1093/eurheartj/ehv249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Prevention of Recurrences of Myocardial Infarction and Stroke Study. https://www.who.int/cardiovascular_diseases/priorities/secondary_prevention/country/en/index1.html. Accessed June 21, 2019.
- 34.Arvandi M, Strasser B, Volaklis K, et al. Mediator effect of balance problems on association between grip strength and falls in older adults: results from the KORA-Age Study. Gerontol Geriatr Med. 2018;4:2333721418760122. doi: 10.1177/2333721418760122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Restrepo MI, Sibila O, Anzueto A. Pneumonia in patients with chronic obstructive pulmonary disease. Tuberc Respir Dis (Seoul). 2018;81(3):187–97. doi: 10.4046/trd.2018.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cusick M, Meleth AD, Agrón E, et al. Associations of mortality and diabetes complications in patients with type 1 and type 2 diabetes: early treatment diabetic retinopathy study report no. 27. Diabetes Care. 2005;28(3):617–25. [DOI] [PubMed] [Google Scholar]
- 38.Cesari M, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, et al. Evidence for the domains supporting the construct of intrinsic capacity. J Gerontol A Biol Sci Med Sci. 2018;73(12):1653–60. [DOI] [PubMed] [Google Scholar]
- 39.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–46. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. Operationalising the concept of intrinsic capacity in clinical settings. https://www.who.int/ageing/health-systems/clinical-consortium/CCHA2017-backgroundpaper-1.pdf. Accessed September 12, 2019.
- 41.Musalek C, Kirchengast S. Grip strength as an indicator of health-related quality of life in old age—a pilot study. Int J Environ Res Public Health. 2017;14(12): ppi:1447. doi: 10.3390/ijerph14121447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rantanen T, Volpato S, Ferrucci L, et al. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51(5):636–641. [DOI] [PubMed] [Google Scholar]