Abstract
Objective:
Amputation is a devastating but preventable complication of diabetes and peripheral arterial disease (PAD). Multiple studies have focused on disparities in amputation rates based on race and socioeconomic status, but few focus on amputation trends in rural populations. The objective of this study was to identify the prevalence of major and minor amputation among patients admitted with diabetes and/or PAD in a rural, Appalachian state, and to identify geographic areas with higher than expected major and minor amputations using advanced spatial analysis while controlling for comorbidities and rurality.
Methods:
Patient hospital admissions of West Virginia residents with diagnoses of diabetes and/or PAD and with or without an amputation procedure were identified from the West Virginia Health Care Authority State Inpatient Database from 2011 to 2016 using relevant International Classification of Diseases, 9th edition and 10the edition codes. Bayesian spatial hierarchical modeling was conducted to identify areas of high risk, while controlling for important confounders for amputation.
Results:
Overall, there were 5557 amputations among 459,452 hospital admissions with diabetes and/or PAD from 2011 to 2016. The majority of the amputations were minor (61.7%; n = 3430), with a prevalence of 7.5 per 1000 and 40.4% (n = 2248) were major, with a prevalence of 4.9 per 1000. Geographic analysis found significant variation in risk for both major and minor amputation across the state, even after adjusting for the prevalence of risk factors. Analyses indicated an increased risk of amputation in the central and northeastern regions of West Virginia at the county level, although zip code-level patterns of amputation varied, with high-risk areas identified primarily in the northeastern and south central regions of the state.
Conclusions:
There is significant geographic variation in risk of amputation across West Virginia, even after adjusting for disease-related risk factors, suggesting priority areas for further investigation. The level of granularity obtained using advanced spatial analyses rather than traditional methods demonstrate the value of this approach, particularly when risk estimates are used to inform policy or public health intervention.
Keywords: Amputation, Rural health, Spatial analysis, Peripheral arterial disease, Diabetes
Amputation is a devastating but preventable complication of diabetes and peripheral arterial disease (PAD). The financial, physical and societal costs of amputation are high, with financial costs estimated at 8.7 billion dollars in 2013 alone.1 Amputation is also a marker for severe end-stage cardiovascular disease. Diabetic patients undergoing a PAD-related amputation have a 50% to 74% 5-year mortality primarily owing to associated cardiac and cerebrovascular complications,1 a prognosis worse than most forms of cancer.
Diabetes- and PAD-related amputations are largely preventable, a foot ulcer precedes 85% of diabetes-related amputations,2 and high-quality primary care with timely podiatric and vascular intervention can substantially decrease the risk of amputation.2-4 As a result, amputations have become an increasingly important measure to study disparities in the quality of diabetes and cardiovascular disease care in the United States.5,6 Previous studies have documented significant racial and economic disparities in amputation rates3-7; however, there are few data on rural disparities. This is of particular concern because rural populations tend to have multiple risk factors for amputation: they are older, economically depressed, with higher levels of chronic disease, riskier health behaviors, and greater barriers to accessing health care than their nonrural counterparts.8 These issues are further amplified in Appalachia, a highly rural region with higher overall cardiovascular disease deaths, diabetes prevalence rates and tobacco use compared to the rest of the United States.8-12
West Virginia is an ideal location to study rural and Appalachian health disparities, because 97% of its land mass is regarded as rural13 and it is the only state considered to be 100% Appalachian.9 West Virginia also has significant state-wide disparities in the prevalence of cardiovascular disease, diabetes, and other amputation risk factors.10 This makes location of patient residence (ie, spatial epidemiology) an important factor to consider in the identification of amputation disparities in the state. The objective of this study was to use advanced spatial epidemiology methods to identify areas with higher than expected major and minor amputation among patients with a diagnosis of diabetes and/or PAD in West Virginia, while controlling for relevant comorbid conditions and rurality.
METHODS
Data collection and management.
This study used 2011 to 2016 West Virginia Health Care Authority data, which is a part of the Healthcare Cost and Utilization Project of the Agency for Healthcare Research and Quality. The target population for the West Virginia Health Care Authority data includes inpatient discharges from community hospitals (excluding rehabilitation and longterm acute care hospitals) in West Virginia. The target population for this study was limited to adult (≥18 years of age) admissions for residents of West Virginia with diagnoses of diabetes and/or PAD. Cases were defined as admissions with amputations performed. Major amputation was defined as any below-the-knee or above-the-knee amputation, and minor amputation was defined as toe or partial foot amputation. Patients with trauma-related amputations, patients who did not have zip code/county data available, and patients who were residents of other states who underwent amputation in West Virginia were excluded from the analysis. All diagnoses and procedures were defined using International Classification of Diseases (ICD)-9 codes from January 1, 2011, to September 30, 2015, and ICD-10 codes from October 1, 2015, to December 31, 2016. A complete list of ICD-9 and ICD-10 codes used for inclusion and exclusion criteria is presented in Appendix (online only). The study was approved by the West Virginia University Institutional Review Board (protocol #1704554319) and a waiver of consent was granted.
Descriptive characteristics were summarized using χ2 tests and independent samples t-test for categorical and continuous variables respectively. Rurality was defined using Rural-Urban Commuting Area codes, a validated classification system of 33 codes used to classify the national census tracts according to rural and urban status. We used both the categorization A and C methods in our descriptive analysis.14 We generated descriptive statistics for the following outcomes: any amputation, major amputation, and minor amputation. All statistical analyses for this article were generated using SAS software, Version 9.4 (SAS Institute, Cary, NC).
For geographic analysis, data were aggregated to county and zip code levels of patient residence (not the facility where the amputation occurred). The relative risk of major and minor amputations was assessed separately as outcome variables in the model. Relative risk was estimated by dividing the rate of amputation at an individual location by the statewide rate. Relevant comorbid conditions included rate (per 1000 persons) of chronic obstructive pulmonary disease, congestive heart failure, coronary artery disease, renal failure, obesity, hypercholesterolemia, Medicaid status, diabetes, PAD, and diabetes with PAD. In addition, spatial patterns of these conditions were controlled in the model because of their association with major and minor amputation15 and known geographic variations in their prevalence.10 We calculated rates for the outcome and comorbid conditions using the total number of diabetes and/or PAD admissions in West Virginia as the denominator as opposed to the census estimates to better represent the population at risk.
Rurality was added as a covariate to the county- and zip code-level analyses owing to its potential role as a barrier to health care access. Rurality at the county level was defined as the proportion of rural census tracts within each county. Rurality was defined using Rural-Urban Commuting Area codes.16 County-level rurality was calculated at the census tract level as opposed to using the zip code level because of overlapping zip code boundaries at county lines.14 Zip code-level rurality was binary and a value of 1 was assigned to patients’ rural zip code of residence.
Data analysis.
Hierarchical Bayesian spatial models were fit using the integrated nested lattice approximation package in R.17,18 The relative risk of amputation given the relevant comorbid conditions and risk factors listed above was modeled using a Poisson gamma distribution, and as a function of (1) a random spatial effect accounting for spatial dependence, and (2) a nonspatial random effect accounting for residual variation that is not spatially dependent.19 The value of this method for quantitative health studies has been cited extensively elsewhere.20,21 Posterior predicted mean for relative risk of major and minor amputation as well as deviance information criteria (DIC) were exported from R, and visualized in ArcMap 10.5 using thematic maps. The use of DIC has been one of the most extensively cited measures used in both spatial and nonspatial Bayesian modeling.22-24 Generally, differences in DIC from 5 to 10 indicate potentially substantial change in model performance; lower DIC indicates a better model fit.23,25
RESULTS
Overall, there were 5557 amputations among 459,452 hospital admissions with diabetes and/or PAD registered in the database from 2011 to 2016. The majority of the amputations were minor (61.7%; n = 3430), with a prevalence of 7.5 per 1000 and the remaining were major 40.4% (n = 2248), with a prevalence of 4.9 per 1000. Amputation patients were on average younger (61.83 years vs 66.45 years), more likely to be male (64.98% vs 45.70%), have Medicaid insurance (20.87% vs 13.12%), renal failure (11.55% vs 5.60%), chronic kidney disease (CKD; 41.80% vs 29.47%), and have diabetes with PAD (66.53% vs 14.64%; Table). Descriptive results were similar for both major and minor amputations. However, patients undergoing major amputation were much more likely to have Medicaid insurance (80.2%).
Table.
Patient characteristics by no amputation and any amputation groups
| Characteristic | No amputation (n = 453,895) | Any amputation (n = 5557) | P value |
|---|---|---|---|
| Rurality (RUCA categorization A) | .1047 | ||
| Urban | 368,051 (81.21) | 4506 (81.20) | |
| Large rural city/town (micropolitan) | 31,252 (6.90) | 345 (6.22) | |
| Small rural town | 36,325 (8.01) | 463 (8.34) | |
| Isolated small rural town | 17,599 (3.88) | 235 (4.23) | |
| Rurality (RUCA categorization C) | .9955 | ||
| Urban | 368,051 (81.21) | 4506 (81.20) | |
| Rural | 85,176 (18.79) | 1043 (18.80) | |
| Age | 66.45 (14.73) | 61.83 (13.74) | <.0001 |
| Sex | <.0001 | ||
| Male | 207,448 (45.70) | 3611 (64.98) | |
| Female | 246,447 (54.30) | 1946 (35.02) | |
| Medicare | 318,774 (70.23) | 3440 (61.90) | <.0001 |
| Medicaid | 59,548 (13.12) | 1160 (20.87) | <.0001 |
| Obesity | 88,607 (19.52) | 1037 (18.66) | .1077 |
| Hypercholesterolemia | 234,401 (51.64) | 2493 (44.86) | <.0001 |
| Renal failure | 25,437 (5.60) | 642 (11.55) | <.0001 |
| CKD | 133,773 (29.47) | 2323 (41.80) | <.0001 |
| COPD | 143,904 (31.70) | 1119 (20.14) | <.0001 |
| CHF | 127,446 (28.08) | 1297 (23.34) | <.0001 |
| CAD | 225,089 (49.59) | 2454 (44.16) | <.0001 |
| Diabetes/PAD | <.0001 | ||
| Diabetes alone | 310,780 (68.47) | 818 (14.72) | |
| PAD alone | 76,650 (16.89) | 1042 (18.75) | |
| Diabetes with PAD | 66,465 (14.64) | 3697 (66.53) |
CAD, Coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; PAD, peripheral arterial disease; RUCA, rural urban commuting area code.
Values are presented as number (%).
The prevalence of any amputation (major or minor) was 2.6 per 1000 in patients with diabetes, 13.4 per 1000 in patients with PAD, and 52.7 per 1000 in patients with both diabetes and PAD. The absolute values of amputation prevalence between patients with diabetes, PAD, and diabetes with PAD differed between the any amputation, minor amputation, and major amputation groups; however, the overall pattern did not (ie, diabetes alone had the lowest prevalence of amputation in each category while PAD with diabetes had the highest).
The most common reason for admission were diseases of the circulatory system, which made up approximately 27% of admissions. Further classification of this reveals the most common diagnosis was diseases of the heart with 84,155 admissions (18.3%). This was followed by diseases of the respiratory system (13.9%), diseases of the digestive system (8.6%), and injury and poisoning (7.7%), with endocrine, nutritional, and metabolic diseases and immunity disorders (6.9%) rounding out the top five. The top Clinical Classification Software category for the amputation rate was for endocrine; nutritional and metabolic diseases and immunity disorders, which had a 7.4% rate of amputation. The Clinical Classification Software category with the second highest rate of amputation was diseases of the musculoskeletal system and connective tissue with an amputation rate of 3.2%.
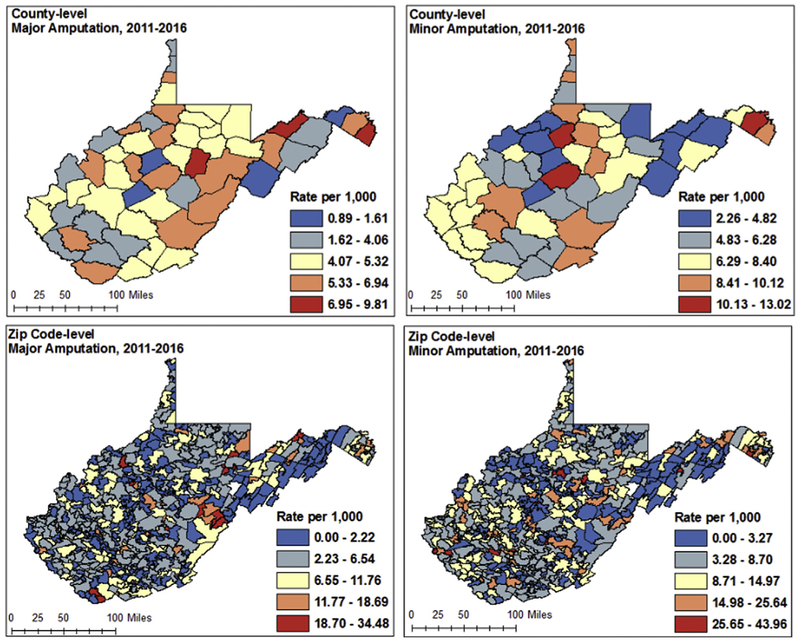
Geographic analysis revealed patterns of major and minor amputation differed at the county and zip code levels (Fig 1). At the county level, the rate of major amputation was highest (6.95-9.81 per 1000) among three counties in the north and northeast, and lowest (0.89-1.61 per 1000) in southern parts of the state. Patterns for minor amputations differed, with the highest risk counties (10.13-13.02 per 1000) located sporadically throughout the state, with potential clustering of high risk in the southern counties. Zip code-level choropleth maps displayed a higher degree of variation than county level mapping, but had similar results. Rates of major amputation remained highest (18.7-34.48 per 1000) in the eastern and northeastern parts of the state, whereas high-risk areas for minor amputation were found sporadically throughout the state.
Fig 1.
County and zip code-level choropleth maps displaying raw rate of major and minor amputation (each separately).
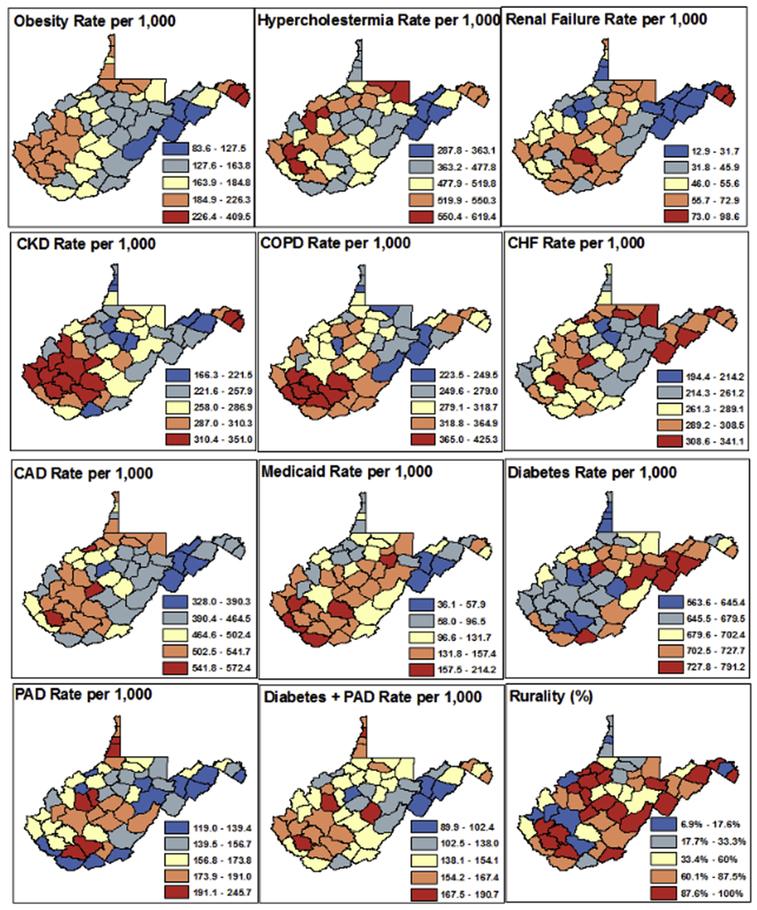
Model covariates were mapped by rate per 1000 for comorbidities and percent rural census tracts at the county level (Fig 2). Zip code-level maps are not included, because the highly granular images made it difficult to concisely assess. At the county level, the range of mapped classifications differed widely between the risk factors considered, and each map was given its own map legend. Overall, rate per 1000 of obesity, chronic obstructive pulmonary disease, and CKD were highest in the southern counties. Conditions such as congestive heart failure, coronary artery disease, and renal failure, diabetes, PAD, and diabetes with PAD had high-risk counties identified in multiple regions of the state. All conditions had lowest risk counties in the eastern and northeastern regions of West Virginia. Similarly, counties with a high proportion of rural census tracts were identified throughout the state.
Fig 2.
Choropleth maps of raw rate per 1000 of comorbid conditions and percent rural census tracts at the county level. CAD, Coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; PAD, peripheral arterial disease.
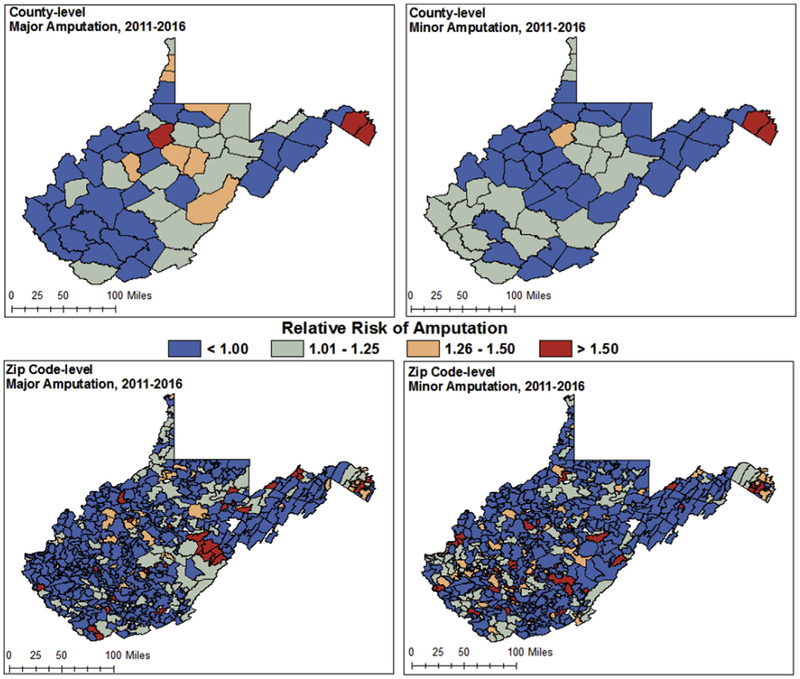
Separate amputation models at the county and zip code levels are displayed in Fig 3. For county-level models, the relative risk of amputation (major or minor) was not significantly associated with any covariates. Inclusion of the covariates and adjustment for average rate for West Virginia decreased the number of high-risk counties and increased the number of high-risk zip codes for amputation. For zip code-level models, rate of diabetes, PAD, and PAD with diabetes per 1000 were associated with major and minor amputation. Additionally, the CKD rate per 1000 was significantly associated with minor amputations at the zip code level. Posterior means and credible intervals for all independent variables considered in county and zip code-level models for major and minor amputation respectively are highlighted in Supplementary Tables I and II (online only). The impact of spatial smoothing was less pronounced in the zip code-level model owing to high variation at more granular mapped displays. High-risk zip codes were identified sporadically throughout the state in both models, with a higher frequency of zip codes in the highest risk category (relative risk, >1.5) around Pocahontas County for major amputation. DIC between counties and zip codes ranged between 1.8 and 4.0 for counties and between 0.40 and 13.7 for zip codes, indicating significant differences in model performance for zip code across the state.23,25 Significant differences in model performance detected in DIC were identified for zip codes in Pocahontas County for amputation (major and minor).
Fig 3.
County- and zip code-level model-fitted relative risk estimates for major and minor amputation (each separate model), adjusting for covariates.
DISCUSSION
In this study, the prevalence of overall amputation in West Virginia patients (2011-2016) with diabetes and/or PAD, was 12.4 per 1000, with a 7.5 per 1000 prevalence of minor amputation and a 4.9 per 1000 prevalence of major amputation. Significant geographic variations in risk for amputation across the state of West Virginia were noted, with some zip codes experiencing rates of major amputation as high as 34.5 per 1000. Advanced hierarchical spatial modeling (Bayesian modeling) techniques identified geographic variation at an unprecedented level of granularity to produce stable estimates of risk for amputation at both the county and zip code levels, despite the challenge of small number counts, which is a common issue in rural areas.
Bayesian modeling has been used previously in relevant health literature21,26; however, this study is the first to apply this method to investigate the risk of major and minor amputation in a population of patients with diabetes and/or PAD in a primarily rural state. Furthermore, it is the first study to apply hierarchical spatial modeling approaches at varying geographic scales to produce high-resolution estimates of amputation risk while controlling for associated comorbidities. Our results showed that the crude direct estimation of relative risk was comparable with model-fitted estimates in the county level model but not at the zip code level. The zip code-level model produced a much more granular illustration of risk, demonstrating the practical use of these advanced methods to identify high risk areas at small spatial scales where risk estimation would otherwise be exaggerated.21
Although our findings on amputation prevalence cannot be compared directly with other studies, they should be viewed in the context of the current national data on amputation. The Dartmouth Atlas identified the national prevalence of amputation (which included through-foot, below-the-knee, and above-the-knee amputations in the numerator) among Medicare patients with diabetes and/or PAD (from 2007 to 2011) to be 2.4 per 1000.27 Healthy People 2020, the federal framework that sets public health objectives for the country, documented the baseline rate of lower extremity amputation (any level of amputation) for persons diagnosed with diabetes from 2005 to 2007 to be 3.5 per 1000.5 Although not directly comparable, the prevalence of amputation identified in this work suggests that West Virginians may be undergoing amputation at higher rates than the rest of the country. This finding may be due to several factors; West Virginia is the third most rural state in the nation, with 91% of West Virginia counties qualifying as medically underserved with significant issues surrounding access to health care.28,29 West Virginia also has the second oldest, least educated and poorest population in the country, with high prevalence rates of chronic diseases that are known risk factors for amputation.10,11,27,30-32 These comorbid conditions, demographics, and socioeconomic factors serve to increase the overall risk of amputation in West Virginia, and variations in exposure to these risk factors across the state may serve to explain the variation in risk of amputation found in this study.
Significant geographic variation in the risk of amputation was identified in our spatial model despite adjusting for potential amputation risk factors, and illustrates the importance of considering geospatial risk factors in assessing disease risk. The notion that where you live is a key contributor to health outcomes is not a new concept, and is well-described in the literature concerning the social determinants of health.33,34 Our technique allowed for the inclusion of geospatial analysis in assessing the amputation risk of our population and confirmed the importance of its inclusion in analysis. This higher risk, even when adjusting for disease-related risk factors, may be due to disparities in access to services, health care providers, transportation, cost, and other barriers to care. This study illustrates the importance of identifying geographic variation in risk to better identify the etiology behind amputation risk and to help us address it.
This study identifies different patterns for major and minor amputation risk across the state, which suggests differences in the intensity and quality of foot care. Minor amputations are often a marker for aggressive foot care and our finding of different concentrations of risk for major and minor amputations are consistent with other studies.4 This concept has been described in the high-low amputation ratio model, which is the ratio of major amputation to minor amputation in a given region, and has been validated in the literature to be associated with improved limb salvage in areas where the ratio is less than 1,35 and has been used internationally to evaluate foot care program performance.36
This study adds to the existing body of literature that has examined the issue of geographic variation in amputation risk. Feinglass et al3 identified significant racial disparities in amputation rates in Northern Illinois based on the proportion of white, African American, and Latinx residents in zip codes aggregated by the North, South, and West-side neighborhoods of the region, but did not go on to perform spatial analysis for this data. Stevens et al37 created choropleth maps of amputations in aggregated zip codes in California and suggested a linear association between poverty and amputation. Choropleth maps (which we use in Fig 1 of this study), or heat maps are commonly used to provide a visualization of descriptive data, but should be approached with caution because they do not allow the determination of significant differences between two areas, despite highly suggestive color contrasts. The data from Stevens et al,37 much like other descriptive data, highlight an area where further spatial analysis with inferential statistical methods, such as those used in our study (illustrated in Fig 3) is warranted.
Other studies analyzing geographic disparities used the Medicare database and focused on Hospital Referral Regions, a geographic unit that represents regional health care markets for tertiary medical care and have a minimum population of 120,000,27 and found significant regional variations in amputation rates,38,39 even when controlling for variations in risk factors.40 These studies are useful for identifying that there are spatial issues at play, and also identify risk factors for amputations and consider issues surrounding practice patterns and access to care. However, these studies are limited to the Medicare population and only provide data on significantly larger geographic regions than zip code-level data. Our study addressed these limitations by incorporating advanced spatial epidemiologic modeling techniques developed for small area estimation to map the risk of amputation down to the zip code level. This approach is consistent with findings from Min et al,26 who demonstrated efficacy of these methods to identify spatial autocorrelation in risk of amputation. Our study built on Min et al’s work by applying a clinical perspective to the approach, using analytic epidemiologic methods to integrate spatial data with relevant covariates to identify high-risk areas for amputation while controlling for potential confounders.
Limitations to our study are those inherent to database research. The data are retrospective and cross-sectional in nature, and the patients do not have unique identifiers. Therefore, single patients may have had multiple admissions for amputations, which could skew the data because certain risk factors could result in more admissions for those individuals. Our results may also be impacted by inconsistencies in coding during patient hospitalizations. It should be noted, however, that the majority of studies on this subject are subject to these same limitations. Other limitations include under-reporting of commonly undiagnosed conditions incorporated in our spatial model (such as hypercholesterolemia), and the complexity of using zip code-level data. For example, although zip code-level data are often the highest level of granularity possible in health studies, patient post office (PO) box information is sometimes reported in place of residential address, which may be located in a different county or zip code. In our study, only 0.14% (n = 8) of cases were PO box associated and these PO box cases were allocated to the standard zip code containing the PO box zip code centroid. Finally, our study is limited by the potential for a geographic edge effect caused by patient leakage from border counties or zip codes to other states for amputation procedures. This issue is common for West Virginia patients owing to close proximity to major hospitals in Kentucky, Ohio, Virginia, Maryland, and Pennsylvania, and may lead to an underestimation of risk along our border counties.
Future directions for this research require a deeper analysis of the high-risk counties and zip codes identified in our study. This includes a thorough analysis of health care access and use, which requires evaluation of physical barriers (roads, transportation) to care, health care provider availability (presence of primary providers, specialists and clinics/hospitals), and cultural barriers to the access and use of care (which can be achieved using qualitative methods). In addition, patient-level database analysis using traditional biostatistical analyses such as multivariate logistic regression will help to identify independent risk factors for amputation in this population and should be performed to complement the geospatial findings. Gaining a better understanding of these issues will help to inform effective, evidence-based, community-level interventions and policy reform to decrease the risk of amputation in our state, and provide a model that can be used in other rural areas in the country.
CONCLUSIONS
Our study provides important information on the geographic patterns of amputation in West Virginia, and identifies highly specific areas for amputation risk, even after adjusting for covariates. These findings allow for the targeting of more detailed studies to optimize the allocation of resources for amputation prevention efforts and also directs further research for a greater understanding of the etiology of this issue. In particular, it gives direction for recruitment for qualitative analyses and allows for more rigorous quantitative analysis in these high-risk areas.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research: Retrospective analysis of the West Virginia Healthcare Authority State Inpatient Database (2011-2016)
Key Findings: The prevalence of amputation in West Virginia was 12.35 per 1000 (2011-2016). Advanced geographic analyses indicated increased risk of amputation in the central and northeastern regions of West Virginia at the county level. Zip code-level patterns of amputation varied, with high-risk areas identified primarily in the northeastern and south central regions of the state.
Take Home Message: West Virginians have a high prevalence of amputation, and there is significant geographic variation in amputation risk across the state. Advanced hierarchical spatial modeling methods are key for providing high-resolution spatial data on health outcomes like amputation, particularly in rural environments.
Acknowledgments
The authors would like to acknowledge Ashley Simmons, MBA, RHIA, CCS, Manager of Data Content and Integrity at West Virginia University Medicine for her assistance in the identification of ICD-9 and ICD-10 codes.
Supported by a grant from the Society for Vascular Surgery Foundation and in part by the following awards: National Institute of General Medical Sciences (2U54GM104942.), National Institute of Drug Abuse (R21DA040187 and 1UG3DA044825). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
APPENDIX (online only).
International Classification of Diseases (ICD) 9 and 10 codes
Diabetes definition
ICD-9 codes = 249.x, 250.x, 250.0x, 357.2, 362.0, 362.0x, 366.41, 648.0x
ICD-10 codes
E08.9, E09.9, E13.9, E08.65, E09.65, E08.10, E09.10, E13.10, E08.01, E09.01, E13.00, E08.11, E08.641, E09.11, E09.641, E13.11, E13.64, E08.21, E09.21, E08.311, E08.319, E08.36, E08.37X1, E08.37X2, E08.37X3, E08.37X9, E08.39, E09.39, E09.311, E09.319, E08.40, E08.41, E08.42, E08.43, E08.44, E08.49, E08.610, E09.40, E09.41, E09.42, E09.43, E08.51, E09.51, E13.59, E08.618, E08.620, E08.621, E08.622, E08.628, E08.630, E08.638, E08.65, E08.69, E09.618, E09.620, E09.621, E09.622, E09.628, E09.69, E09.630, E09.638, E08.649, E09.65, E09.69, E13.620, E13.630, E09.638, E09.649, E13.620, E13.621, E13.622, E13.628, E13.638, E13.649, E13.65, E13.69, E08.8, E09.8, E13.8, E09.9, E11.9, E10.9, E11.65, E10.65, E11.10, E11.69, E13.10, E10.10, E13.10, E11.00, E11.01, E10.69, E11.11, E11.641, E10.11, E10.641, E11.29, E10.29, E11.21, E10.21, E11.311, E11.319, E11.36, E11.39, E10.311, E10.319, E10.36, E10.37X1, E10.37X2, E10.37X3, E10.37X9, E10.39, E11.40, E10.40, E11.51, E10.51, E11.618, E11.620, E11.621, E11.622, E11.628, E11.630, E11.638, E11.649, E10.618, E10.620, E10.621, E10.622, E10.628, E10.630, E10.638, E10.649, E11.69, E10.69, E10.8, E11.8, E10.42, E11.42, E13.42, E11.319, E11.3591, E11.3592, E11.3593, E11.3599, E11.3291, E11.3292, E11.3293, E11.3299, E11.3391, E11.3392, E11.3393, E11.3399, E11.3491, E11.3492, E11.9493, E11.3499, E11.311, E08.36, E09.36, E10.36, E11.36, E13.36, O319, O24.32, O24.911, O24.912, O24.913, O24.92, O24.93
Peripheral arterial disease definition
ICD-9 codes = 429.2, 440.xx, 443.xx, 443.0, 443.1, 443.2, 443.22, 443.29, 443.8, 443.81, 443.82, 443.89, 443.9, 444.xx, 445.0, 445.02, 445.8, 445.89, 719.7, 730.0, 730.1, 730.2, 730.8, 730.9, 731.8, 736.7, 736.8, 736.9
ICD-10 codes
I25.10, I70.0, I70.1, I70.209, I70.219, I70.25, I70.269, I70.299, I70.339, I70.499, I70.599, I70.92, I70.8, I70.90, I73.00, I73.1, I77.72, I77.75, I77.76, I77.77, I77.79, I798, I73.81, I73.89, I73.9, I74.01, I74.09, I74.11, I74.2, I74.3, I74.5, I74.8, I74.9, I75.029, I75.89, R26.2, M86.10, M86.20, M86.119, M86.219, M86.129, M86.229, M86.139, M86.239, M86.149, M86.249, M86.259, M86.159, M86.169, M86.269, M86.179, M86.279, M86.18, M86.28, M86.19, M86.29, M86.60, M86.619, M86.629, M86.639, M86.642, M86.659, M86.669, M86.679, M86.68, M86.69, M86.9, M90.80, M90.819, M90.829, M90.839, M90.849, M90.859,M90.869, M90.879, M90.88, M90.89, M21.969, M21.549, M21.6X9, M21.539, M21.80, M21.759, M21.769, M21.90
Obesity
ICD-9 codes =278.00, 278.01, 278.03, v85.4x, v85.3x
ICD-10 codes
E66.9, E66.01, E66.2, Z68.41, Z68.42, Z68.43, Z68.44, Z68.45, Z68.31, Z68.32, Z68.33, Z68.34, Z68.35, Z68.36, Z68.37, Z68.38, Z68.39
Hypercholesterolemia
ICD-9 codes = 272.x
ICD-10 codes
E78.00, E78.01, E78.1, E78.2, E78.3, E78.4, E78.5, E78.6, E88.1, E75.21, E75.22, E75.249, E77.0, E77.1, E78.81, E78.89, E88.89, E78.9
Renal failure
ICD-9 codes = 403.01, 403.11, 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93, 585.5, 585.6, 586, 593.81, V45.11, V56x
ICD-10 codes
I12.0, I13.11, I13.2, N18.5, N18.6, N19, N28.0, Z99.2, Z49.31, Z49.01, Z49.02, Z49.32
Chronic kidney disease
ICD-9 codes = 249.4x, 250.4x, 403.x, 403.00, 403.10, 403.90, 404.x, 404.00, 404.01, 404.10, 404.11, 404.90, 404.91, 581.x, 581.8x, 582.x, 583.x, 585.1-585.4, 585.9
ICD-10 codes
E08.21, E09.21, E08.65, E11.22, E11.29, E10.29, E10.22, E11.21, E11.65, E10.21, E10.65, I12.9, I12.0, I13.0, I13.11, I13.2, I13.10, N04.4, N02.2, N04.3, N04.0, N08, N04.8, N04.9, N03.2, N03.3, N03.4, N03.8, N08, N03.9, N05.9, N05.2, N05.5, N17.1, N17.2, N05.8, I12.9, I13.10, I13.0, N18.1, N18.2, N18.3, N18.4, N18.9
COPD
ICD-9 codes = 491.xx, 492.xx, 494.xx, 496, 519.8
ICD-10 codes
J41.0, J41.1, J41.8, J42, J44.1, J44.0, J44.9, J43.0, J43.1, J43.2, J43.8, J43.9, J47, J47.1, J47.9, J98.8
Congestive heart failure
ICD-9 codes = 402.01, 402.11, 402.91, 404.01, 404.11, 404.91, 404.03, 404.13, 404.93, 425.4, 428.xx
ICD-10 codes
I13.0, I13.2, I42.5, I42.8, I50.814, I50.9, I50.1, I50.20, I50.21, I50.22, I50.30, I50.31, I50.32, I50.33, I50.40, I50.41, I50.42, I50.43, I50.810, I50.811, I50.812, I50.813, I50.82, I50.83, I50.84, I50.89
Coronary artery disease
ICD-9 codes = 410.xx, 411.xx, 412.xx, 414.x, 414.0x, 429.0, 429.1, 429.2, 429.3, 429.4, 429.5, 429.6, 429.7, 429.71, 429.79, 429.8, 429.81, 429.82, 429.89, 429.9, v45.81, V45.82
ICD-10 codes
I21.09, I21.19, I21.11, I21.29, I21.4, I21.3, I21.9, I21.A1, I21.A9, I24.1, I20.0, I24.0, I24.8, I25.2, I25.10, I25.810, I25.811, I25.812, I25.3, I25.41, I25.42, I25.82, I25.84, I25.5, I25.89, I25.9, I51.4, I51.5, I51.7, I97.0, I97.110, I97.130, I97.190, I51.1, I51.2, I51.0, I23.0, I51.89, I51.3, I51.9, Z95.1, Z95.5, Z98.61
Amputation
Major amputation.
ICD-9 codes: 84.10, 84.13, 84.14, 84.15, 84.16, 84.17
ICD-10 codes:
84.10= 0Y6C0Z1, 0Y6C0Z2, 0Y6C0Z3, 0Y6D0Z1, 0Y6D0Z2, 0Y6D0Z3, 0Y6H0Z1, 0Y6H0Z2, 0Y6H0Z3, 0Y6J0Z1, 0Y6J0Z2, 0Y6J0Z3
84.13= 0Y6M0Z0, 0Y6N0Z0
84.14= 0Y6H0Z3, 0Y6J0Z3
84.15= 0Y6H0Z1, 0Y6H0Z2, 0Y6H0Z3, 0Y6J0Z1, 0Y6J0Z2, 0Y6J0Z3
84.16= 0Y6F0ZZ, 0Y6G0ZZ
84.17= 0Y6C0Z1, 0Y6C0Z2, 0Y6C0Z3, 0Y6D0Z1, 0Y6D0Z2, 0Y6D0Z3
Minor amputation.
ICD-9 codes: 84.11 (toe amp), 84.12 (TMA)
ICD-10 codes:
84.11= 0Y6P0Z0, 0Y6P0Z1, 0Y6P0Z2, 0Y6P0Z3, 0Y6Q0Z0, 0Y6Q0Z1, 0Y6Q0Z2, 0Y6Q0Z3, 0Y6R0Z0, 0Y6R0Z1, 0Y6R0Z2, 0Y6R0Z3, 0Y6S0z0, 0Y6S0z1, 0Y6S0z2, 0Y6S0z3, 0Y6T0Z0, 0Y6T0Z1, 0Y6T0Z2, 0Y6T0Z3, 0Y6U0Z0, 0Y6U0Z1, 0Y6U0Z2, 0Y6U0Z3, 0Y6V0Z1, 0Y6V0Z2, 0Y6V0Z3, 0Y6W0Z1, 0Y6W0Z2, 0Y6W0Z3, 0Y6X0Z0, 0Y6X0Z1, 0Y6X0Z2, 0Y6X0Z3, 0Y6Y0Z0, 0Y6Y0Z1, 0Y6Y0Z2, 0Y6Y0Z3
84.12= 0Y6M0Z4, 0Y6M0Z5, 0Y6M0Z6, 0Y6M0Z7, 0Y6M0Z8, 0Y6M0Z9, 0Y6M0ZB, 0Y6M0ZC, 0Y6M0ZD, 0Y6M0ZF, 0Y6N0Z4, 0Y6N0Z5, 0Y6N0Z6, 0Y6N0Z7, 0Y6N0Z8, 0Y6N0Z9, 0Y6N0ZB, 0Y6N0ZC, 0Y6N0ZD, 0Y6N0ZF
Footnotes
Author conflict of interest: none.
Presented at the 2019 Vascular Annual Meeting of the Society for Vascular Surgery, Washington, D.C., June 13-15, 2019.
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Amputee Coalition: Limb Loss Task Force. Roadmap for stimulating limb loss research and improving care: recommendations from the 2015 Limb Loss Task Force Amputee Coalition Website 2015. Available at: www.amputee-coalition.org/wp-content/uploads/2016/01/roadmap-for-stimulation-limb-loss-research-and-improving-care-2015-lltf.pdf. Accessed October 13, 2017.
- 2.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 1990;13: 513–21. [DOI] [PubMed] [Google Scholar]
- 3.Feinglass J, Abadin S, Thompson J, Pearce WH. A census-based analysis of racial disparities in lower extremity amputation rates in Northern Illinois, 1987-2004. J Vasc Surg 2008;47:1001–7. [DOI] [PubMed] [Google Scholar]
- 4.Tseng CL, Helmer D, Rajan M, Tiwari A, Miller D, Crystal S, et al. Evaluation of regional variation in total, major, and minor amputation rates in a national health-care system. Int J Qual Health Care 2007;19:368–76. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2020. Washington, DC: Available at: www.healthypeople.gov/node/4121/data_details. Accessed November 27, 2018. [Google Scholar]
- 6.Chicago Department of Public Health. Healthy Chicago 2.0: partnering to improve health equity, 2016-2020. Chicago: Chicago Department of Public Health; 2016. [Google Scholar]
- 7.Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc 2002;94:666–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Harris JK, Beatty K, Leider JP, Knudson A, Anderson BL, Meit M. The double disparity facing rural local health departments. Annu Rev Public Health 2016;37:167–84. [DOI] [PubMed] [Google Scholar]
- 9.Marshall J, Thomas L, Lane N, Holmes G, Arcury T, Randolph R, et al. Health disparities in Appalachia. Report No. Washington D.C.: Appalachian Regional Commission; August 2017. [Google Scholar]
- 10.Centers for Disease Control and Prevention. Interactive atlas of heart disease and stroke 2014-2016. Available at: http://nccd.cdc.gov/DHDSPAtlas/Default.aspx?state=WV. Accessed November 6, 2018.
- 11.Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014 Atlanta, GA: US Department of Health and Human Services; 2014. Available at: www.cdc.gov/diabetes/pdfs/data/2014-report-estimates-of-diabetes-and-its-burden-in-the-united-states.pdf. Accessed August 7, 2018. [Google Scholar]
- 12.Centers for Disease Control and Prevention. State Tobacco Activities Tracking and Evaluation (STATE) system 2016. Available at: https://nccd.cdc.gov/STATESystem/rdPage.aspx?rdReport=OSH_State.CustomReports&rdAgCommand=OrderAdd&rdAgOrderColumn=Data_Value&rdAgOrderDirection=Ascending&rdAgCommandID=7b3c4b09-b6fe-4b73-98d4-7a743ac14870&rdScrollX=0&rdScrollY=900. Accessed October 13, 2017.
- 13.U.S. Census Bureau. Population and housing unit counts. West Virginia. Washington, DC: U.S. Government Printing Office; 2012. Contract No.: CPH-2-50. [Google Scholar]
- 14.Rural Health Research Center. RUCA Data: using RUCA Data UW School of Medicine: Rural Health Research Center website. Available at: http://depts.washington.edu/uwruca/ruca-approx.php. Accessed June 25, 2018.
- 15.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. TASC II Working Group: Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007;45:S5–67. [DOI] [PubMed] [Google Scholar]
- 16.USDA. Documentation - 2010 Rural Urban Commuting Area (RUCA) Codes United States Department of Agriculture - Economic Research Service 2016. Available at: www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/. Accessed July 23, 2018.
- 17.Rue HV, Martino S, Chopin N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J R Stat Soc B Stat Method 2009;71: 319–92. [Google Scholar]
- 18.Lindgren F, Rue H. Bayesian spatial modelling with R-INLA. J Stat Soft 2015;63:1–25. [Google Scholar]
- 19.Blangiardo M, Cameletti M, Baio G, Rue H. Spatial and spatio-temporal models with R-INLA. Spat Spatiotemporal Epidemiol 2013;7:39–55. [DOI] [PubMed] [Google Scholar]
- 20.Lawson A Bayesian disease mapping: hierarchical modeling in spatial epidemiology. New York: Chapman & Hall/CRC; 2013. [Google Scholar]
- 21.Rossen LM, Hedegaard H, Khan D, Warner M. County-level trends in suicide rates in the U.S., 2005-2015. Am J Prev Med 2018;55:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green PJ, Richardson S. Theory and methods - hidden Markov models and disease mapping. J Am Stat Assoc 2002;97:1055. [Google Scholar]
- 23.Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc B Stat Method 2002;64:583–639. [Google Scholar]
- 24.Spiegelhalter DJ, Best NG, Carlin BP, Linde A. The deviance information criterion: 12 years on. J R Stat Soc B Stat Method 2014;76:485–93. [Google Scholar]
- 25.Ribatet M Spatial extremes: modelling spatial extremes. R package version 2.0-72018. Vienna, Austria: The R Foundation; 2018. [Google Scholar]
- 26.Min X, Sun D, He Z, Schootman M. A Bayesian hierarchical model of nontraumatic lower-extremity amputation rates. Spat Spatiotemporal Epidemiol 2010;1:169–76. [DOI] [PubMed] [Google Scholar]
- 27.Dartmouth Institute for Health Policy and Clinical Practice. Dartmouth Atlas Project: research methods 2018. Available at: http://archive.dartmouthatlas.org/data/table.aspx?ind=307&tf=29&ch=35&loc=50,99,171,230&loct=3&fmt=352. Accessed December 19, 2018.
- 28.U.S. Census Bureau. State and County Quick Facts: West Virginia. Washington, D.C.: U.S. Census Bureau; 2012. [Google Scholar]
- 29.West Virginia Rural Health Association. Healthcare in West Virginia: a workforce analysis. Shady Spring, WV: West Virginia Rural Health Association; 2012. [Google Scholar]
- 30.Diebel J, Norda J, Kretchmer O. Educational attainment by state in the United States 2015. Available at: https://statisticalatlas.com/state/West-Virginia/Educational-Attainment#figure/state-in-united-states. Accessed November 6, 2017.
- 31.Diebel JN, Kretchmer O. Household income by state in the United States 2015. Available at: https://statisticalatlas.com/state/West-Virginia/Household-Income#figure/state-in-united-states. Accessed November 6, 2017.
- 32.Werner C. The older population: 2010: 2010 census briefs. Washington, DC: U.S. Census Bureau; 2011. [Google Scholar]
- 33.Center for Disease Control and Prevention. Social determinants of health: know what affects health Centers for Disease Control and Prevention 2018. Available at: www.cdc.gov/socialdeterminants/. n-citation>.
- 34.Bierman AS, Dunn JR. Swimming upstream. Access, health outcomes, and the social determinants of health. J Gen Intern Med 2006;21:99–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wrobel JS, Robbins J, Armstrong DG. The high-low amputation ratio: a deeper insight into diabetic foot care? J Foot Ankle Surg 2006;45:375–9. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt BM, Wrobel JS, Munson M, Rothenberg G, Holmes CM. Podiatry impact on high-low amputation ratio characteristics: a 16-year retrospective study. Diabetes Res Clin Pract 2017;126:272–7. [DOI] [PubMed] [Google Scholar]
- 37.Stevens CD, Schriger DL, Raffetto B, Davis AC, Zingmond D, Roby DH. Geographic clustering of diabetic lower-extremity amputations in low-income regions of California. Health Aff (Millwood) 2014;33:1383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margolis DJ, Hoffstad O, Nafash J, Leonard CE, Freeman CP, Hennessy S, et al. Location, location, location: geographic clustering of lower-extremity amputation among Medicare beneficiaries with diabetes. Diabetes Care 2011;34:2363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wrobel JS, Mayfield JA, Reiber GE. Geographic variation of lower-extremity major amputation in individuals with and without diabetes in the Medicare population. Diabetes Care 2001;24:860–4. [DOI] [PubMed] [Google Scholar]
- 40.Jones WS, Patel MR, Dai D, Subherwal S, Stafford J, Calhoun S, et al. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000-2008. J Am Coll Cardiol 2012;60:2230–6.Submitted Mar 28, 2019; accepted Jun 24, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.