Abstract
Objectives:
Factors that are responsible for age-related neurological deterioration of non-cognitive and cognitive processes may have a shared cause. We sought to examine the temporal, directional associations of handgrip strength and cognitive function in a national sample of aging Americans.
Design:
Longitudinal-Panel.
Setting:
Enhanced interviews that included physical, biological, and psychosocial measures were completed in person. Core interviews were often conducted over the telephone.
Participants:
The analytic sample included 14,775 Americans aged at least 50-years that participated in at least two waves of the 2006–2016 waves of the Health and Retirement Study.
Measures:
Handgrip strength was measured with a hand-held dynamometer. Participants were considered cognitively intact, mildly impaired, or severely impaired according to the Telephone Interview of Cognitive Status questionnaire. Separate lagged general estimating equations analyzed the directional associations of handgrip strength and cognitive function.
Results:
The overall time to follow-up was 2.1±0.4 years. Every 5-kilogram higher handgrip strength was associated with 0.97 (95% confidence interval (CI): 0.93, 0.99) lower odds for both future cognitive impairment and worse cognitive impairment. Those who were not-weak had 0.54 (CI: 0.43, 0.69) lower odds for future cognitive impairment and 0.57 (CI: 0.46, 0.72) lower odds for future worse cognitive impairment. Conversely, any (β=−1.09; CI: −1.54, −0.64), mild (β=−0.85; CI: −1.34, −0.36), and severe cognitive impairment (β=−2.34; CI: −3.25, −1.42) predicted decreased handgrip strength. Further, the presence of any, mild, and severe cognitive impairment was associated with 1.82 (CI: 1.48, 2.24), 1.65 (CI: 1.31, 2.08), and 2.53 (CI: 1.74, 3.67) greater odds for future weakness, respectively.
Conclusions/Implications:
Strength capacity and cognitive function may parallel each other, whereby losses of functioning in one factor may forecast losses of functioning in the other. Handgrip strength could be used for assessing cognitive status in aging Americans and strength capacity should be monitored in those with cognitive impairment.
Keywords: Alzheimer Disease, Dementia, Geriatrics, Muscle Strength, Muscle Weakness, Sarcopenia
Brief Summary:
A longitudinal study of nearly 15,000 older Americans revealed that muscle strength and cognitive function tend to parallel each other, whereby losses of functioning in one factor often predicts losses of functioning in the other.
INTRODUCTION
Factors that are responsible for age-related neurological deterioration of non-cognitive and cognitive processes may have a shared cause.1, 2 Muscle weakness, which is conveniently assessed with a hand-held dynamometer in aging adults,3 is associated with a variety of poor health outcomes including morbidity, functional limitations, and early mortality.4 Although age-related declines in muscle strength have been primarily attributed to physiological changes in the muscular system,5 emerging evidence suggests that weakness is more a product of diminished neural system functioning.6 For example, the amount of muscle force aging adults can produce during a grip force task is about half of what would be expected if the skeletal muscles were entirely activated by the nervous system, largely due to lower neuromuscular activation and motor unit recruitment.6, 7 Moreover, the cortical and subcortical regions of the brain that regulate hand dexterity are also linked to cognitive functions, which may explain why persons with a cognitive impairment have limited fine motor skills of the hands.8 Therefore, the same age-related neurodegeneration that contributes to decreased handgrip strength (HGS) may also be linked to cognitive impairment.
Several studies have investigated the association between HGS and cognitive function, but there has been debate regarding the direction of the association. For example, cross-sectional and longitudinal study designs have determined that increased HGS was associated with decreased risk for cognitive morbidity.9, 10 Conversely, others have found that poorer cognitive functioning was associated with decreased HGS.11, 12 Further, an analysis of adults aged at least 85-years revealed that better attention, memory, and processing speed were all associated with significantly less decline in HGS over a four-year period; whereas, weakness was only associated with decline in global cognitive performance.13 The inconsistent findings for the direction of the association between HGS and cognitive function have led to recommendations for studies to examine the bidirectional association between HGS and cognitive function.14 However, the findings from such studies are also unclear. The rate of decline in HGS has been shown to be associated with cognitive deficits,15 while other studies have not detected significant correlations between the rates of change in HGS and cognitive decline.16, 17
While low HGS and cognitive erosion are prevalent at older age,18, 19 incipient and accelerated declines in both HGS and cognitive function may occur at middle-age.20, 21 Thus, there is growing urgency to develop and maintain effective policies and programs both in the United States and globally that address the projected increase of cognitive impairment in the rapidly growing older adult population.22 Helping to disentangle the direction of the association between HGS and cognitive function will help healthcare providers better interpret the meaning of assessments for weakness and cognitive morbidity, while also providing guidance for interventions aiming to preserve muscle strength and cognitive function during aging. Accordingly, we sought to examine the longitudinal, directional associations of HGS and cognitive function in a national sample of aging Americans.
METHODS
Participants
This study utilized data from 28,980 adults aged at least 50 years who completed interviews without a proxy from the 2006–2016 Health and Retirement Study (HRS). Publicly-available HRS data files were joined with the RAND HRS dataset.23 The HRS is a continual longitudinal-panel study that monitors the health and financial status of aging Americans. Participants in the HRS were interviewed biennially and followed until mortality.24 Additional details for the HRS are published elsewhere.25
Starting in the 2006 wave, detailed face-to-face interviews that included physical and biological measures were performed on half of the sample; whereas, the other half sample only executed the core interview. The half-samples alternated completion of the detailed face-to-face interviews to minimize participant burden. Interview response rates for the HRS have been >80% at each wave.24 Written informed consent was provided by HRS participants prior to entering the study and the University’s Behavioral Sciences Committee Institutional Review Board approved study protocols.
Measures
Handgrip Strength
HGS was measured with a Smedley spring-type hand-held dynamometer (Scandidact, Denmark). Interviewers explained HGS protocols and fit the dynamometer to the hand size of each participant before they completed a practice trial. Beginning on the non-dominant hand, participants squeezed the dynamometer with maximal effort while their arm was at their side and elbow flexed at 90-degrees, and then released the muscle contractions. Each participant completed two HGS measurements, alternating between hands.
A 30-second break was allowed between measures if only a single hand could be used for testing. Those unable to stand or position their arm while grabbing the dynamometer could be seated and place their upper arm on a supporting object. Persons that had a surgical procedure, swelling, inflammation, extreme pain, or an injury in both hands did not engage in HGS assessments at baseline or follow-up. Participants that may not have been able to complete HGS assessments after baseline were only excluded from our analyses if they had not completed at least one follow-up measure. Those exceeding the highest possible value for HGS according to the HRS (>100.0 kilograms)23 and persons without HGS data were excluded (n=5,862).
Measurements of HGS were a part of the enhanced face-to-face interviews and were thereby measured at alternating waves. The wave in which HGS was first measured indicated when participants entered the study. More details about the HGS protocols are published elsewhere.26 The maximal HGS measurement from a single trial on either hand was included in the analyses. Gender-specific cut-points were used for determining weakness. Men and women with HGS <26-kilograms and <16-kilograms were considered as weak, respectively.27
Cognitive Function
Assessments of cognitive functioning were completed at each wave with the Telephone Interview of Cognitive Status, a validated screening tool from the Mini-Mental State Examination that was designed for population-based studies.28 A 27-point composite scale was used for those under 65-years of age that included immediate and delayed word recall from a list of 10 words (0–20 points), serial sevens subtraction test starting with the number 100 (0–5 points), and counting backward for 10 consecutive numbers at maximal speed starting from the number 20 (0–2 points). Those with scores of 7–11 were considered as having a mild cognitive impairment, participants with scores ≤6 had a severe cognitive impairment, and persons with scores ≤11 were classified as having any cognitive impairment.29
Given that age is prominent risk factor for cognitive declines, a 35-point composite scale with three additional assessment items is recommended and was used for those aged at least 65 years in our study.30 Additional assessments on the 35-point scale included object naming (0–2 points), date naming (0–4 points), and correctly identifying the current president and vice president of the United States (0–2 points). Those with scores between 8–10 points were considered as having a mild cognitive impairment, participants with scores ≤7 had a severe cognitive impairment, and persons with scores ≤10 were categorized as having any cognitive impairment.30 The age of a participant at each wave determined if they used the 27- or 35-point assessment.
Covariates
Participants self-reported their age, gender, race and ethnicity (Black, Hispanic, and White indicators), height, and body mass. Body mass index was calculated as body mass in kilograms divided by height in meter-squared and those with a body mass index ≥30 kilograms per meter-squared were considered obese. Obesity was included as a covariate because body composition impacts muscle strength,31 and is influential for our associations.32, 33 Respondents also told interviewers their highest level of education completed at baseline and were classified as not graduating from high school, passing a high school equivalency test, high school graduate, completed some college, and college graduate or above.
Participants reported their total household income (limited to respondent + spouse income) at each wave and the continuous amount reported was included in the analyses. Morbidity was collected by self-reported healthcare provider diagnosed hypertension, diabetes, cancer, lung disease, heart condition, stroke, emotional or psychiatric problems, and arthritis or rheumatism. The number of affirmative morbid diagnoses were summed at each wave and included in the analyses. At each wave, respondents indicated if they engaged in any physical activity at least once a week. Social engagement was examined by three variables: 1) volunteer activity at religious, educational, health-related or other organization for at least one hour in the past year, 2) weekly or greater contact with parents or in-laws, and 3) current employment status. Scores ranged from 0–3 with higher scores suggesting more social engagement.34 The continuous scores at each wave was included in the analyses.
The 8-item Center for the Epidemiologic Studies Depression (CES-D) scale was used for assessing depressive symptoms.35 Respondents told interviewers if they experienced any negative or positive emotions during the week before the interview date. Scores ranged from 0–8, with higher values suggesting more depressive symptoms. Continuous scores at each wave were included in the analyses.
Respondents indicated if they drink alcohol, were current smokers, and if they had ever smoked more than 100 cigarettes in their lifetime at each wave. A single item for self-rated health was used. Participants reported their health as either “excellent”, “very good”, “good”, “fair”, or “poor” at each wave. Those with missing covariates were excluded (n=234).
Statistical Analysis
All analyses were conducted with SAS 9.4 software (SAS Institute; Cary, NC). Independent t-tests and chi-squared tests were used to determine differences in the descriptive characteristics of weak and not-weak participants for continuous and categorical variables, respectively.
The outcomes from the cognitive functioning assessments were categorized as ordinal data for evaluating worse cognitive functioning. A generalized estimating equation (GEE) with independent correlation structures analyzed the marginal longitudinal associations of HGS (continuous predictor) and future cognitive impairment (dichotomous outcome), while another GEE examined the association between HGS and future worse cognitive functioning (ordinal outcome; no cognitive impairment, mild cognitive impairment, severe cognitive impairment). Similarly, separate GEEs determined the population-averaged association of not being weak (dichotomous predictor) and future 1) cognitive impairment, and 2) worse cognitive functioning. Each of these models were adjusted for cognitive function score at wave (Telephone Interview of Cognitive Status), age, gender, race and ethnicity, education, household income, alcohol consumption, physical activity participation, obesity, morbidity, depression score, social engagement, smoking status, self-rated health, and time.
The GEE models were also used to assess the marginal longitudinal association of cognitive impairment (dichotomous predictor) and future HGS (continuous outcome), and another GEE analyzed the association between worse cognitive impairment (categorical predictor) and future HGS. Further, distinct GEEs examined the association of 1) cognitive impairment, and 2) worst cognitive impairment with future weakness (dichotomous outcome). These models were also adjusted for HGS at wave, age, gender, race and ethnicity, education, household income, alcohol consumption, physical activity participation, obesity, morbidity, depression score, social engagement, smoking status, self-rated health, and time. The covariates in our GEE models were pre-specified by the investigators and analyses were shaped from another similar investigation.36
We chose to present our HGS and cognitive functioning variables differently (continuous, dichotomous, categorical) in each of our models to optimize the interpretability, robustness, and clinical meaningfulness of our findings.37 GEE models account for the temporal sequence of the data and the within-subject correlation. For all models, the outcome for the next wave participated was used, and the time between waves was adjusted for. Therefore, those who did not participate in at least two waves of the 2006–2016 waves of the HRS were excluded (n=8,109). More details about GEE models are published elsewhere.38
As an additional analysis, each of the GEE models for the directional association of HGS and cognitive function were stratified by gender and age (middle-aged: 50–64 years, older adults: ≥65 years). The results of these analyses were presented as an appendix because the gender and age stratified results were not part of our a priori purpose. An alpha level of 0.05 was used for all analyses.
RESULTS
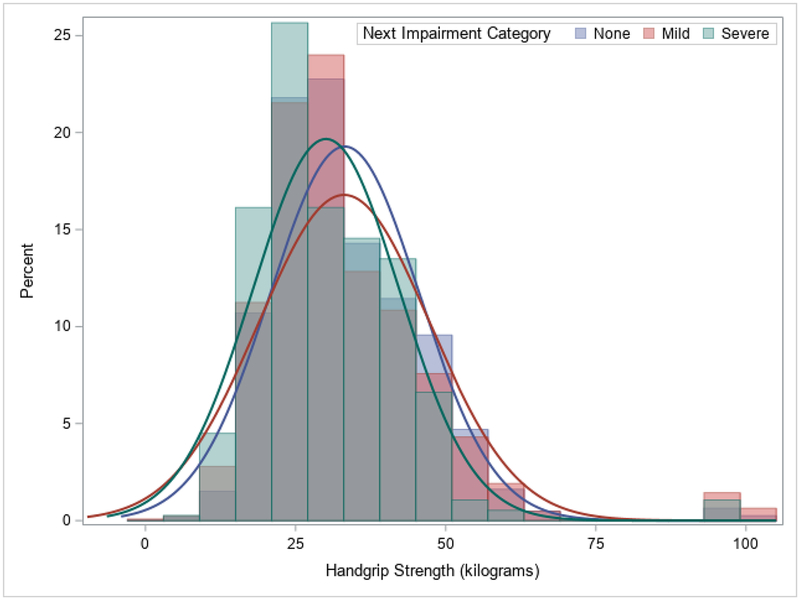
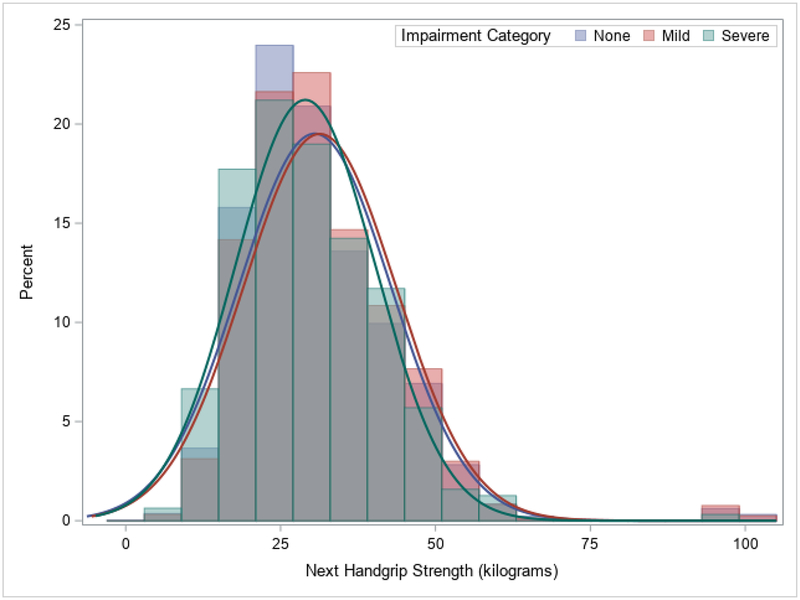
There were 14,775 participants included in the analyses and their descriptive characteristics are in Table 1. The overall time to follow-up was 2.1±0.4 years. Significant differences between the 14,189 (96.0%) not-weak and 586 (4.0%) weak participants existed for HGS, any cognitive impairment, and severe cognitive impairment (p<0.05). There were also significant differences between those who were not-weak and weak for nearly all of the other descriptive characteristics. Figure 1 shows a histogram of baseline HGS values by next ascertained cognitive function category, while Figure 2 portrays the next measured HGS values by baseline cognitive function category.
Table 1.
Descriptive Characteristics of the Participants.
| Overall (n=14,775) | Weak (n=586) | Not-Weak (n=14,189) | |
|---|---|---|---|
| Handgrip Strength (kilograms) | 33.6±12.3 | 16.0±5.3* | 34.3±11.9 |
| Any Cognitive Impairment (n (%)) | 1,575 (10.6%) | 86 (14.6%)* | 1,489 (10.4%) |
| Mild Cognitive Impairment (n (%)) | 1,309 (8.8%) | 63 (10.7%) | 1,246 (8.7%) |
| Severe Cognitive Impairment (n (%)) | 266 (1.8%) | 23 (3.9%)* | 243 (1.7%) |
| Age (years) | 64.1±9.5 | 71.3±10.9* | 63.9±9.3 |
| Female (n (%)) | 8,554 (557.9%) | 377 (64.3%)* | 8,177 (57.6%) |
| Black (n (%)) | 6,221 (42.1%) | 75 (12.8%)* | 2,544 (17.9%) |
| Hispanic (n (%)) | 1,737 (11.7%) | 103 (17.5%)* | 1,634 (11.5%) |
| White (n (%)) | 11,121 (75.2%) | 453 (77.3%) | 10,668 (75.1%) |
| Household Income ($) | 70,774.5±160.1 | 41,202.4±49.8* | 71,995.8±162.9 |
| Morbid Conditions | 1.7±1.3 | 2.4±1.4* | 1.7±1.3 |
| Depression Score | 1.4±1.9 | 2.1±2.2* | 1.3±1.9 |
| Social Activities | 1.5±0.9 | 1.0±0.8* | 1.5±0.9 |
| Education (n (%)) | |||
| No High School Graduation | 2,549 (17.2%) | 168 (28.7%)* | 2,381 (16.8%) |
| Passed a High School Equivalency Test | 756 (5.1%) | 26 (4.4%) | 730 (5.1%) |
| High School Graduate | 4,342 (29.4%) | 169 (28.8%) | 4,173 (29.4%) |
| Completed Some College | 3,672 (24.9%) | 124 (21.2%) | 3,548 (25.0%) |
| College Graduate or Above | 3,456 (23.4%) | 99 (16.9%)* | 3,357 (23.7%) |
| Drink Alcohol (n (%)) | 8,495 (57.5%) | 259 (44.2%)* | 8,236 (58.0%) |
| Current Smoker (n (%)) | 2,255 (15.2%) | 69 (11.7%)* | 2,186 (15.4%) |
| Previous Smoker (n (%)) | 6,007 (40.6%) | 225 (38.4%) | 5,782 (40.7%) |
| Obese (n (%)) | 5,178 (35.0%) | 162 (24.6%)* | 5,016 (35.3%) |
| Physical Activity Participation (n (%)) | 11,751 (79.5%) | 390 (66.5%)* | 11,361 (80.0%) |
| Self-Rated Health (n (%)) | |||
| Excellent | 1,777 (12.0%) | 31 (5.3%)* | 1,746 (12.3%) |
| Very Good | 4,724 (32.0%) | 114 (19.4%)* | 4,610 (32.5%) |
| Good | 4,702 (31.8%) | 171 (29.2%) | 4,531 (31.9%) |
| Fair | 2,788 (18.9%) | 183 (31.2%)* | 2,605 (18.4%) |
| Poor | 784 (5.3%) | 87 (14.9%)* | 6997 (4.9%) |
| Time to Follow-Up (years) | 2.1±0.4 | 2.1±0.5 | 2.1±0.4 |
p<0.05
Figure 1.
Histogram of Current Handgrip Strength by Next Ascertained Cognitive Impairment Category.
Note: Overall mean time to follow-up was 2.1±0.4 years.
Figure 2.
Histogram of Next Handgrip Strength Measure by Current Cognitive Impairment Category.
Note: Overall mean time to follow-up was 2.1±0.4 years.
Figure 3 presents the results for the lagged associations between the HGS predictor variables and change in cognitive function. Every 5-kilogram higher HGS was associated with 0.97 (95% confidence interval (CI): 0.93, 0.99) lower odds for future any cognitive impairment and future worse cognitive impairment. Those who were not-weak had 0.54 (CI: 0.43, 0.69) lower odds for future any cognitive impairment and 0.57 (CI: 0.46, 0.72) lower odds for future worse cognitive impairment.
Figure 3.
Association between Handgrip Strength and Change in Cognitive Function.
Note: Overall mean time to follow-up was 2.1±0.4 years. The models were lagged and adjusted for current cognitive functioning score, age, gender, race and ethnicity, education, household income, alcohol consumption, physical activity participation, obesity, morbidity, depression score, social engagement, smoking status, self-rated health, and time. Each arrow represents a model. 95% CI=95% confidence interval; OR=odds ratio.
The lagged associations between the cognitive function predictor variables and change in each HGS outcome are in Figure 4. Any (β=−1.09; CI: −1.54, −0.64), mild (β=−0.85; CI: −1.34, −0.36), and severe cognitive impairment (β=−2.34; CI: −3.25, −1.42) predicted decreased HGS. Further, any, mild, and severe cognitive impairment predicted 1.82 (CI: 1.48, 2.24), 1.65 (CI: 1.31, 2.08), and 2.53 (CI: 1.74, 3.67) greater odds for subsequent weakness, respectively.
Figure 4.
Association between Cognitive Function and Change in Handgrip Strength.
†Reference: no cognitive impairment. ‡Reference: no cognitive impairment.
Note: Overall mean time to follow-up was 2.1±0.4 years. The models were lagged and adjusted for current handgrip strength, age, gender, race and ethnicity, education, household income, alcohol consumption, physical activity participation, obesity, morbidity, depression score, social engagement, smoking status, self-rated health, and time. Each arrow represents a model. 95% CI=95% confidence interval; OR=odds ratio.
The results of the additional analyses for bidirectional association between HGS and cognitive function by gender and age are in Appendix 1. Appendix 2 presents the results for the bidirectional association of HGS and cognitive function by gender and age. Some of the gender and age stratified models showed null results.
DISCUSSION
The principal results of this investigation in our overall sample suggest that every 5-kilogram higher HGS was associated with 3% decreased odds for future cognitive impairment and worsening cognitive impairment. Older Americans who were not-weak had 46% decreased odds for future cognitive impairment and 43% decreased odds for subsequent worse cognitive impairment. Conversely, cognitive impairment predicted a 1.09-kilogram decrease in HGS; whereas, mild and severe cognitive impairment predicted a 0.85- and 2.34-kilogram decrease in HGS, respectively. Further, any, mild, and severe cognitive impairment was associated with 82%, 65%, and 153% increased odds for subsequent weakness, respectively. These findings are in alignment with the “common cause hypothesis” which suggests that common factors are responsible for age-related deterioration of non-cognitive and cognitive processes.1, 2 Examining possible systematic pathways may help to explain this bidirectional association.
Age-related weakness is considered a lifelong process with multiple etiological factors, and compromised nervous system function is an important contributor in this process.39 Our findings for the association of higher HGS (and not being considered weak) and cognitive dysfunction may be explained by those in our investigation who may have engaged in muscle strengthening activities before entering the study such as physical activity. Regular participation in physical activity is important for preserving strength during aging,40 and is beneficial for brain health.41 For example, lifespan physical activity is a pronounced gene modulator that stimulates structural and functional changes in the brain that benefit cognitive functioning, and protects against neurodegeneration.41 Such neural system deficits may help to explain why fine motor skills and coordinated movement are deficient in those who are weaker and have cognitive morbidity.42
Although the presence of a cognitive impairment impacts memory and processing,22 aging adults with cognitive impairment may be more vulnerable to becoming physically frail.43 Weakness best represents the beginning of the frailty phenotype.44 Neuronal degeneration at different levels of the nervous system drive cognitive deficits,45 and may also factor in diminished strength capacity. For example, those with a cognitive impairment may experience nervous system problems that will result in decreased muscle strength such as poor motor unit recruitment, low motor neuron firing, and reduced neuromuscular junction activity.39 Our results are in agreement with those who also found that lower cognitive functioning was associated with decreased strength.11, 12 Healthcare providers that have diagnosed their patients with a cognitive impairment should monitor muscle strength to prevent health outcomes related to weakness.
Gender may factor into dementia risk and cognitive function.46 Our additional analyses evaluating the associations of HGS and cognitive functioning may have also indicated gender differences. These findings align with calls to action for giving detection, treatment, and care of dementia a gender focus.47 Healthcare providers should consider the role of gender for the associations of HGS and cognitive functioning.
Some limitations should be acknowledged. Measures of HGS were part of the enhanced face-to-face interviews and collected from participants at alternating waves. Although proxy respondents may have had poorer levels of cognitive functioning, they were excluded because their interviews had limited measures. Self-report information from participants may have been subject to biases. Participants were required to have at least two observations to be included in the analyses and those who died shortly after being interviewed may have had rapid declines in muscle strength and cognitive functioning after being interviewed by the HRS. Data for biological factors that may have influenced the results (e.g., Apolipoprotein E4) were not publicly available. Causal inferences should not be made from our findings and direction of causation remains unclear because we analyzed observational data for this investigation. However, common processes between HGS and cognitive function may exist such as neurodegeneration, shared risk factors, and biological pathways (e.g., chronic inflammation).
Conclusions and Implications
We found a bidirectional association between HGS and cognitive function in a national sample of aging Americans. These findings indicate that strength capacity and cognitive function may parallel each other, such that losses of functioning in one factor may predict losses of functioning in the other. Our interpretation of these findings is that the muscular and neural systems that link HGS and cognitive function share a common cause. Healthcare providers should utilize measures of HGS in clinical and epidemiological settings to predict cognitive impairment risk in their aging adult patients, and for monitoring strength capacity in those with a cognitive impairment. Targeted interventions for muscle strength and cognitive functioning should also consider implementing measures of HGS as an outcome.
Supplementary Material
Funding Sources:
This research did not receive any funding from agencies in the public, commercial, or not-for-profit sectors. RM’s effort on this research was supported, in part by the College of Human Sciences and Education at North Dakota State University. BD’s effort was supported by grants from the National Institute on Aging (K01AG058789 and P30AG024832). BCC’s effort was partially supported by a grant from the National Institute on Aging (R01AG044424).
Conflicts of Interest
In the past 5-years BCC has received research funding from the NIH, Regeneron Pharmaceuticals, Astellas Pharma Global Development, Inc., RTI Health Solutions, and the Osteopathic Heritage Foundations. In the past 5-years BCC has received consulting fees from Regeneron Pharmaceuticals, Abbott Laboratories, and the Gerson Lehrman Group. Additionally, BCC is co-founder with equity, and serves as the Chief of Aging Research, of AEIOU Scientific, LLC. The other authors report no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Christensen H, Mackinnon AJ, Korten A, et al. The “common cause hypothesis” of cognitive aging: evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychol Aging. 2001;16:588–99. [DOI] [PubMed] [Google Scholar]
- 2.Salthouse TA, Hambrick DZ, McGuthry KE. Shared age-related influences on cognitive and noncognitive variables. Psychol Aging. 1998;13:486–500. [DOI] [PubMed] [Google Scholar]
- 3.Bohannon RW. Muscle strength: clinical and prognostic value of hand-grip dynamometry. Curr Opin Clin Nutr Metab Care. 2015;18:465–70. doi: 10.1097/MCO.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 4.McGrath RP, Kraemer WJ, Al Snih S, et al. Handgrip Strength and Health in Aging Adults. Sports Med. 2018;48:1993–2000. doi: 10.1007/s40279-018-0952-y. [DOI] [PubMed] [Google Scholar]
- 5.Zamboni M, Mazzali G, Fantin F, et al. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18:388–95. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Clark BC. Neuromuscular Changes with Aging and Sarcopenia. J Frailty Aging. 2019;8:7–9. doi: 10.14283/jfa.2018.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shinohara M, Latash M, Zatsiorsky V, et al. Effects of age and gender on finger coordination in MVC and submaximal force-matching tasks. J Appl Physiol. 2003;95:1361–69. [DOI] [PubMed] [Google Scholar]
- 8.Buchman AS, Bennett DA. Loss of motor function in preclinical Alzheimer’s disease. Expert Rev Neurother. 2011;11:665–76. doi: 10.1586/ern.11.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon D, Hwang S, Lee D, et al. Physical frailty and cognitive functioning in Korea rural community-dwelling older adults. J Clin Med. 2018;7:pii:E405. doi: 10.3390/jcm7110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchman AS, Wilson RS, Boyle PA, et al. Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology. 2007;29:66–73. [DOI] [PubMed] [Google Scholar]
- 11.van Dam R, Van Ancum JM, Verlaan S, et al. Lower Cognitive Function in Older Patients with Lower Muscle Strength and Muscle Mass. Dement Geriatr Cogn Disord. 2018;45:243–50. doi: 10.1159/000486711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raji MA, Kuo YF, Snih SA, et al. Cognitive status, muscle strength, and subsequent disability in older Mexican Americans. J Am Geriatr Soc. 2005;53:1462–68. [DOI] [PubMed] [Google Scholar]
- 13.Taekema DG, Ling CH, Kurrle SE, et al. Temporal relationship between handgrip strength and cognitive performance in oldest old people. Age Ageing. 2012;41:506–12. doi: 10.1093/ageing/afs013. [DOI] [PubMed] [Google Scholar]
- 14.Zammit AR, Robitaille A, Piccinin AM, et al. Associations between aging-related changes in grip strength and cognitive function in older adults: A systematic review. J Gerontol A Biol Sci Med Sci. 2018;74:519–27. doi: 10.1093/gerona/gly046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald SW, DeCarlo CA, Dixon RA. Linking biological and cognitive aging: toward improving characterizations of developmental time. J Gerontol B Psychol Sci Soc Sci. 2011;66:i59–70. doi: 10.1093/geronb/gbr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deary IJ, Johnson W, Gow AJ, et al. Losing one’s grip: a bivariate growth curve model of grip strength and nonverbal reasoning from age 79 to 87 years in the Lothian Birth Cohort 1921. J Gerontol B Psychol Sci Soc Sci. 2011;66:699–707. doi: 10.1093/geronb/gbr059. [DOI] [PubMed] [Google Scholar]
- 17.Ritchie SJ, Tucker-Drob EM, Starr JM, et al. Do cognitive and physical functions age in concert from age 70 to 76? Evidence from the Lothian Birth Cohort 1936. Span J Psychol. 2016;19:E90. [DOI] [PubMed] [Google Scholar]
- 18.Looker AC, Wang C-Y. Prevalence of reduced muscle strength in older US adults: United States, 2011–2012. https://www.medpagetoday.com/upload/2015/1/28/db179.pdf. Accessed June 20, 2019. [PubMed]
- 19.Geda YE. Mild cognitive impairment in older adults. Curr Pschiatry Rep. 2012;14:320–7. doi: 10.1007/s11920-012-0291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh-Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ. 2012;344:d7622. doi: 10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodds RM, Syddall HE, Cooper R, et al. Global variation in grip strength: a systematic review and meta-analysis of normative data. Age Ageing. 2016;45:209–16. doi: 10.1093/ageing/afv192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Cognitive impairment: A call for action, now. https://www.cdc.gov/aging/pdf/cognitive_impairment/cogimp_poilicy_final.pdf. Accessed June 20, 2019. [Google Scholar]
- 23.Health and Retirement Study. HRS Data Book. https://hrs.isr.umich.edu/about/data-book. Accessed June 20, 2019. [Google Scholar]
- 24.Sonnega A, Faul JD, Ofstedal MB, et al. Cohort profile: the health and retirement study (HRS). Int J Epidemiol. 2014;43:576–85. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Health and Retirement Study. HRS Data Book. https://hrs.isr.umich.edu/about/data-book. Accessed June 20, 2019. [Google Scholar]
- 26.Documentation of physical measures, anthropometrics and blood pressure in the Health and Retirement Study. https://hrs.isr.umich.edu/sites/default/files/biblio/dr-011.pdf. Accessed June 20, 2019.
- 27.Alley DE, Shardell MD, Peters KW, et al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559–66. doi: 10.1093/gerona/glu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plassman BL, Newman TT, Welsh KA, et al. Application in epidemiological and longitudinal studies. Cogn Behav Neurol. 1994;7:235–41. [Google Scholar]
- 29.Crimmins EM, Kim JK, Langa KM, et al. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66:i162–171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langa KM, Larson EB, Karlawish JH, et al. Trends in the prevalence and mortality of cognitive impairment in the United States: is there evidence of a compression of cognitive morbidity? Alzheimers Dement. 2008;4:134–44. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGrath R Comparing absolute handgrip strength and handgrip strength normalized to body weight in aging adults. Aging Clin Exp Res. 2019:1–3. doi: 10.1007/s40520-019-01126-5. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen JC, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci. 2014;8:375. doi: 10.3389/fnins.2014.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomlinson D, Erskine R, Morse C, et al. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology. 2016;17(3):467–83. doi: 10.1007/s10522-015-9626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Social Engagement and Cognitive Function of older Adults in Mexico and the United States: How Universal is the Health Concordance in Couples? [DOI] [PMC free article] [PubMed]
- 35.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–48. [DOI] [PubMed] [Google Scholar]
- 36.Kim GR, Sun J, Han M, et al. Evaluation of the directional relationship between handgrip strength and cognitive function: the Korean Longitudinal Study of Ageing. Age Ageing. 2019; 48:426–32. doi: 10.1093/ageing/afz013. [DOI] [PubMed] [Google Scholar]
- 37.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006;332(7549):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 39.Kwon YN, Yoon SS. Sarcopenia: neurological point of view. J Bone Metab. 2017;24:83–9. doi: 10.11005/jbm.2017.24.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langhammer B, Bergland A, Rydwik EJ. The Importance of Physical Activity Exercise among Older People. Biomed Res Int. 2018;2018:7856823. doi: 10.1155/2018/7856823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandolesi L, Polverino A, Montuori S, et al. Effects of physical exercise on cognitive functioning and wellbeing: biological and psychological benefits. Front Psychol. 2018;9:509. doi: 10.3389/fpsyg.2018.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carson RG. Get a grip: Individual variations in grip strength are a marker of brain health. Neurobiol Aging. 2018;71:189–222. doi: 10.1016/j.neurobiolaging.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 43.Brigola AG, Rossetti ES, Santos BR, et al. Relationship between cognition and frailty in elderly: A systematic review. Dement Neuropsychol. 2015;9:110–9. doi: 10.1590/1980-57642015DN92000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mufson EJ, Binder L, Counts SE, et al. Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol. 2012;123:13–30. doi: 10.1007/s00401-011-0884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laws KR, Irvine K, Gale TM. Sex differences in cognitive impairment in Alzheimer’s disease. World J Psychiatry. 2016;6(1):54–65. doi: 10.5498/wjp.v6.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nebel RA, Aggarwal NT, Barnes LL, et al. Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimers Dement. 2018;14(9):1171–83. doi: 10.1016/j.jalz.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.