Abstract
Over the last several years it has become clear that higher order assemblies on membranes, exemplified by signalosomes, are a paradigm for the regulation of many membrane signaling processes. We have recently combined two-color direct stochastic optical reconstruction microscopy (dSTORM) with the (Clus-DoC) algorithm that combines cluster detection and colocalization analysis to observe the organization of 5-lipoxygenase (5-LO) and 5-lipoxygenase–activating protein (FLAP) into higher order assemblies on the nuclear envelope of mast cells; these assemblies were linked to leukotriene (LT) C4 production. In this study we investigated whether higher order assemblies of 5-LO and FLAP included cytosolic phospholipase A2 (cPLA2) and were linked to LTB4 production in murine neutrophils. Using two- and three-color dSTORM supported by fluorescence lifetime imaging microscopy we identified higher order assemblies containing 40 molecules (median) (IQR: 23, 87) of 5-LO, and 53 molecules (62, 156) of FLAP monomer. 98 (18, 154) molecules of cPLA2 were clustered with 5-LO, and 77 (33, 114) molecules of cPLA2 were associated with FLAP. These assemblies were tightly linked to LTB4 formation. The activation-dependent close associations of cPLA2, FLAP, and 5-LO in higher order assemblies on the nuclear envelope support a model in which arachidonic acid is generated by cPLA2 in apposition to FLAP, facilitating its transfer to 5-LO to initiate LT synthesis.
Keywords: cell biology, cell signaling, cytokine, leukotriene, neutrophil, nuclear envelope, single-molecule localization microscopy, superresolution microscopy, supramolecular complex, direct stochastic optical reconstruction microscopy (dSTORM)
Introduction
Leukotriene B4 (LTB4),4 the product of the arachidonate 5-lipoxygenase (5-LO) (UniProtKB P09917) pathway in neutrophils (1, 2), plays a pivotal role in initiating and amplifying the inflammatory response. LTB4 is chemotactic for neutrophils and functions to amplify the recruitment of these cells by complement component 5a (C5a), f-Met-Leu-Phe, and selected chemokines (3–7). LTB4 also regulates neutrophil swarming (8) and the recruitment of monocytes and lymphocytes (9–12). The synthesis of LTB4 by neutrophils is governed by two key regulatory constraints that apply to all pro-inflammatory signaling in these cells. First, activation must occur concurrently with recruitment to the sites of infection or injury; otherwise systemic activation of neutrophils would generate a sepsis-like microenvironment (13–16). This constraint means that on a molecular level, pro-inflammatory pathways must not be sensitive to low or sporadic levels of initiating signals but require a strong and persistent initiating signal. These conditions allow a very high degree of assurance that host defense mechanisms are appropriately engaged. The second constraint is that the overall synthesis of LTB4 must be governed by a proportional rather than a binary “on-off” response to surface signals; the latter would lead to excessive tissue injury which would be difficult to repair. A corollary of both constraints is that neutrophils must have a molecular mechanism that allows the integration of multiple simultaneous cell surface signals to synthesize an appropriate amount of LTB4.
The spatial segregation of the initial enzyme of LT synthesis, 5-LO, the nuclear envelope scaffold protein arachidonate 5-lipoxygenase-activating protein (FLAP) (UniProtKB P20292) (17–21), and the substrate AA is one mechanism that prevents LT generation in circulating neutrophils. 5-LO is localized in the cytosol and the nucleoplasm. Upon cell activation and calcium mobilization, 5-LO translocates to the nuclear envelope (17, 21, 22). FLAP is an integral nuclear envelope protein and functions as a trimer (18). Cytosolic phospholipase A2 (cPLA2) (UniProtKB P47712) has been linked to the formation of LTs from endogenous AA (23, 24). In circulating neutrophils, cPLA2 is localized in the cytosol where it is segregated from AA esterified in membranes. Following cell activation and an increase in cytosolic calcium, cPLA2 moves to cell membranes and releases AA, which subsequently serves as a substrate for eicosanoid production (25, 26).
Our group identified the formation of the LT synthetic complex (27) and defined its assembly in mast cells, bone marrow neutrophils, and synovial neutrophils in a model of arthritis (27). We subsequently characterized the changes in the relationship between 5-LO and FLAP that occur on the membrane using fluorescence lifetime imaging microscopy (FLIM) (4). These studies showed that a change in the association of AA with FLAP causes changes in the spatial arrangement of FLAP domains, allowing a closer association of 5-LO and FLAP. These basic principles have been confirmed by others (28).
Over the last several years it has become clear that higher order supramolecular assemblies, such as signalosomes, are a paradigm for the regulation of many signaling processes (29–31). These include complexes of MyD88, IRAK2, and IRAK4 (32). Fas is incorporated into large helical supramolecular complexes that contain multiple hundreds to thousands of molecules (33). One advantage of these assemblies is the ability to block responses to weak signals, preventing adventitious activation. Another advantage is to modulate graded responses to signaling by regulating assembly size. This concept has immense appeal in the context of 5-LO and other lipoxygenases and bioactive lipids in general. However, technical approaches to identify higher order assemblies of LT biosynthetic proteins and to assign functionality to organization per se have not been established. Proving a link between organization and mechanism is an extremely complex problem. For tumor necrosis factor receptors, the role of higher order assemblies has been identified using cryo-EM structures combined with modeling (31). Although these studies are elegant, they do not provide direct evidence from cell-based data and membrane measurements.
Overexpression systems of 5-LO and FLAP, and the use of artificial stimuli such as calcium ionophore A23187 (28), can both be misleading because they are not physiologically relevant and can distort membrane organization. Although crystal structures of 5-LO (34) and FLAP (18) have been solved, there is no evidence that regions of potential contact between FLAP and 5-LO deduced from these studies would govern the assembly of higher order assemblies, nor do they exclude the possibility that other proteins are involved. Although FLIM detects interactions at ∼10 nm, the resolution is limited by diffraction and therefore only bulk paired interactions occurring within a ∼250 nm distance can be reliably detected. Thus, these techniques cannot identify higher order assemblies.
This manuscript represents the initial critical step in a multistep complex process of assigning biological function to higher order assemblies of 5-LO and FLAP on the nuclear envelope. Here we employed two- and three-color dSTORM with Clus-DoC analysis supported by fluorescence lifetime imaging microscopy to achieve this goal.
An additional question is whether higher order assemblies can participate in the integration of signals from more than one surface receptor. For example, a combination of GM-CSF and C5a leads to augmented LTB4 generation from neutrophils when compared with C5a alone (4). This combination of ligands is highly relevant to inflammatory diseases in which endothelial cells generate GM-CSF (35–37). C5a initiates the formation of LTB4-dependent signaling networks (3) and clinical pathology relevant to vasculitis (38, 39). Finally, how do cells efficiently couple cPLA2-generated AA to FLAP, and then ultimately to 5-LO?
We have previously combined two-color dSTORM analysis with the Clus-DoC cluster algorithm to identify higher order assemblies of 5-LO and FLAP on the nuclear envelope that are linked to LTC4 formation in the RBL-2H3 mast cell line (40). These assemblies averaged 20 molecules of 5-LO and the same number of FLAP molecules. In this study we not only identify the formation of higher order assemblies of 5-LO and FLAP in neutrophils that are linked to LTB4 synthesis but have also identified cPLA2 as a third member of these assemblies and show that its interactions with 5-LO and FLAP are also linked to the formation of LTB4. These observations indicate a process by which AA generation occurs in apposition to 5-LO and FLAP. Finally, we provide evidence that the organization of these structures is linked to the high levels of LTB4 formed by combinations of GM-CSF and C5a.
Results
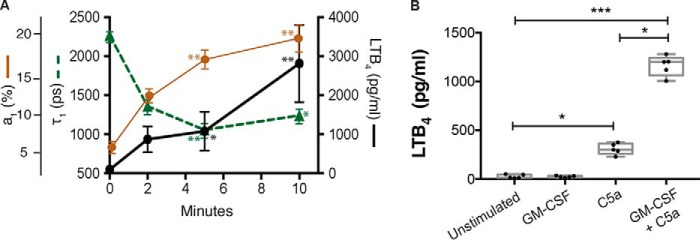
As we were interested in the organization of 5-LO and FLAP during LTB4 production, we initially conducted a time-course measuring stimulated LTB4 production to determine the appropriate time frames for imaging analysis. Neutrophils were primed with 2 ng/ml GM-CSF for 45 min and then stimulated with C5a for 0, 2, 5, and 10 min. The concentration of LTB4 released in the media increased over time from 0 to 5 min (black circles, solid line) and again at 10 min (Fig. 1A). The relationship between 5-LO and FLAP was then measured by FLIM. The progressive decrease in the interacting lifetime (τ1) from 2220 ± 66 ps (mean ± S.E.) at 0 min to 1421 ± 93 ps at 2 min, and 1068 ± 88 ps at 5 min indicated an increasingly close association between 5-LO and FLAP (green triangles, dashed line). The percentage of interacting molecules a1 (%) increased over time from 7% ± 0.6 at 0 min (control cells) to 13% ± 0.8 at 2 min, 17% ± 1 at 5 min, and 19% ± 1.5 at 10 min (brown circles, solid line). From these data, we chose 5 min as an optimal stimulation time for dSTORM analysis. At 5 min, we measured LTB4 production with different combinations of priming and stimulus. We found that LTB4 production greatly increased from 15 pg/ml (median) (IQR: 11, 47) at time zero to 301 pg/ml (258, 363) after stimulation with C5a and 1201 pg/ml (1063, 1281) when cells were primed with GM-CSF prior to the addition of C5a compared with C5a alone (Fig. 1B). GM-CSF alone did not result in significant LTB4 synthesis (22 pg/ml (16, 33)) (Fig. 1B).
Figure 1.
5-LO:FLAP interactions are correlated with LTB4 generation. Neutrophils were treated with 1 ng/ml GM-CSF for 45 min prior to the addition of 100 nm C5a for 0 (unstimulated), 2, 5, and 10 min. A, 5-LO and FLAP association is correlated with LTB4 generation. At each time point media were collected and analyzed by enzyme immunoassay for LTB4 (right axis, solid line with circles). The cells were then washed and fixed and the 5-LO:FLAP relationship determined by FLIM: Interaction lifetime (τ1 (ps)) (left axis, dashed green line with triangles) and fraction interacting (a1 (%)) (far left axis, solid brown line with circles). Data are expressed as mean ± S.E. B, LTB4 generation at 5 min. The boxes show the median and interquartile range (25%, 75%). Experiments were performed using five separate mice. In each experiment, 5–20 cells were used in each experimental condition. One-way ANOVA with Bonferroni post hoc test was performed to determine significance where *, p < 0.05; **, p < 0.0.01; and ***, p < 0.001.
Neutrophil stimulation was conducted in chambered cover glass slides, so that we could directly associate LTB4 levels with the molecular organization of 5-LO, cPLA2, and FLAP in the same cells. We imaged the cells with two-color dSTORM, employing the workflow demonstrated in our recent publication where dSTORM localization data were converted to .txt files then analyzed by Clus-DoC in MATLAB (40).
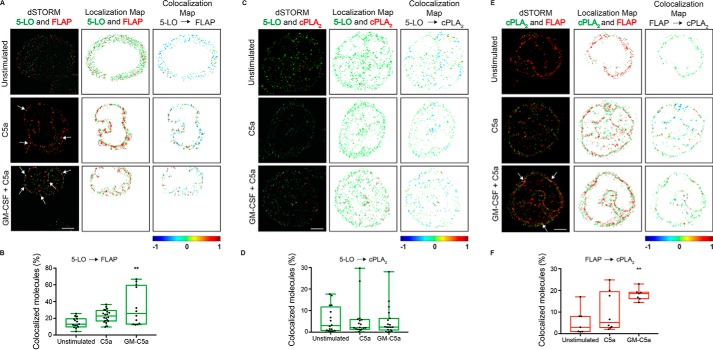
Two-color dSTORM images of localizations for 5-LO (green) and FLAP (red) are shown in the panels in the left column of Fig. 2A. Cells stimulated with C5a, or cells primed with GM-CSF and stimulated with C5a show a clear redistribution of 5-LO to the nuclear envelope. We verified this localization in separate imaging of 5-LO and FLAP with markers for the nuclear envelope (Nup98) and endoplasmic reticulum (calnexin) (Fig. S1). To probe the relationship between 5-LO and FLAP we analyzed dSTORM data by Clus-DoC (41). Localizations associated with the nuclear envelope are plotted in the middle column and emphasize the nonuniform distribution of 5-LO and FLAP on the nuclear envelope. Clus-DoC measures the degree of colocalization (DoC) of 5-LO and FLAP on the nuclear envelope. DoC scores range from −1 (anticorrelated, likely differently localized) to 0 (uncorrelated) to +1 (correlated, likely colocalized). Localizations with a score of at least 0.4 were considered colocalized. DoC scores are directional, and we selected the direction showing the biggest difference in colocalization under the reasoning that due to distribution or number, one molecule may be more sensitive to changes in localization than the other. The right column shows the localization map of the cell pseudocolored by DoC score. The uneven spatial distribution of colocalized molecules is evident in activated cells when compared with unstimulated cells. Nuclear membrane localizations from 6 to 20 cells were analyzed and the percentage of 5-LO molecules that were colocalized with FLAP doubled from 13 (11, 19) to 26 (13, 59) under maximal conditions where LTB4 was made (Fig. 2B). The frequency distribution of DoC scores for 5-LO and FLAP show a clear increase in the frequency of molecules with a very high degree of colocalization after neutrophils were treated with a combination of GM-CSF and C5a (Fig. 3A).
Figure 2.
Localization and interactions of 5-LO, FLAP, and cPLA2 on the nuclear envelope. Neutrophils were cultured in the presence or absence of 1 ng/ml GM-CSF for 45 min prior to stimulation with 100 nm C5a for 0 (unstimulated) or 5 min. The cells were washed, fixed, and stained with indicated antibodies. Localizations were identified by two-color dSTORM and analyzed using Clus-DoC. A, analysis of 5-LO and FLAP. Two-color dSTORM images of 5-LO (Alexa Fluor 561, green) and FLAP (Alexa Fluor 647, red) (left panels), Clus-DoC localization maps for 5-LO (green) and FLAP (red) (middle panels), and Clus-DoC colocalization maps for 5-LO relative to FLAP (right panels). 5-LO localizations are color coded according to their DoC scores (score bar at bottom of right panels). B, percent colocalization of 5-LO molecules with FLAP. Data from ROIs of all cells were analyzed (graph in green). C, two-color dSTORM images of 5-LO (green) and cPLA2 (red) at 0 and 5 min after GM-CSF and C5a stimulation (left panels), localization maps for 5-LO (green) and cPLA2 (red) (middle panels), and colocalization maps for 5-LO relative to cPLA2 (right panels). 5-LO molecules are color coded according to their DoC scores (score bar at bottom of right panels). D, percent colocalization of 5-LO molecules with cPLA2 from all ROIs (graph in green). E, two-color dSTORM images of cPLA2 (green) and FLAP (red) at 0 and 5 min after GM-CSF and C5a stimulation (left panels), localization maps for cPLA2 (green) and FLAP (red) (middle panels), and colocalization maps for FLAP relative to cPLA2 (right panels). FLAP molecules are color coded according to their DoC scores (score bar at bottom of right panels). F, percent colocalization of FLAP molecules with cPLA2 from all ROIs (graph in red). White arrows point to areas of interaction. Scale bar represents 1.5 μm. The boxes show the median and interquartile range. B–F, one-way ANOVA with Bonferroni post hoc test was performed to determine significance where **, p < 0.01. Experiments were performed using five separate mice. In each experiment, 5–20 cells were used in each experimental condition. Representative cells for each condition are shown.
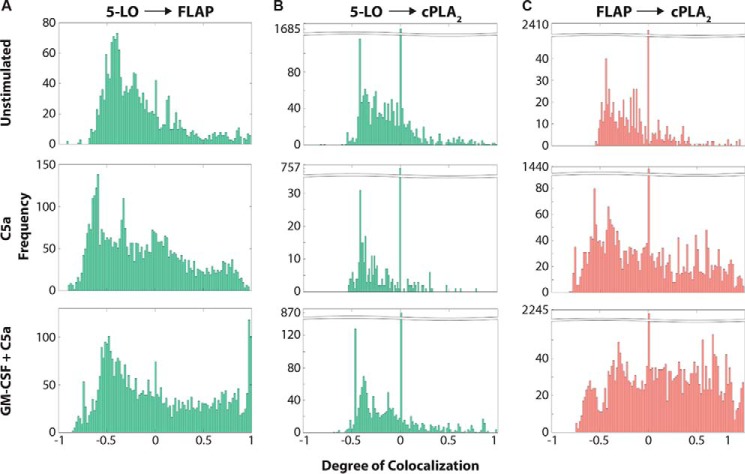
Figure 3.
Analysis of the colocalization of 5-LO, FLAP, and cPLA2 on the nuclear envelope. Neutrophils were cultured with or without 1 ng/ml GM-CSF for 45 min then activated with 100 nm C5a for 0 (unstimulated) or 5 min. Localization data were collected by two-color dSTORM and analyzed with Clus-DoC. The data shown are from the same cells shown in Fig. 2. A, histograms of DoC scores of all molecules for 5-LO relative to FLAP (5-LO:FLAP). B, histograms of DoC scores of all molecules for 5-LO relative to cPLA2 (5-LO:cPLA2). C, histograms of DoC scores of all molecules for FLAP relative to cPLA2 (FLAP:cPLA2).
We employed the same approach to analyzing the relationship between the other protein pairs. Although both 5-LO and cPLA2 showed movement to the nuclear envelope after cell activation (Fig. 2C, left and middle columns), unlike with 5-LO and FLAP, there was no apparent change in amount of 5-LO and cPLA2 colocalized on the nuclear envelope (right column and Fig. 2D). This observation was supported by the frequency distributions (Fig. 3B). In contrast, both relocation of cPLA2 to the nuclear envelope and a clear increase in the colocalization of FLAP and cPLA2 was observed on the nuclear envelope after neutrophil stimulation from 3 (0.7, 11) to 18 (16, 19) (Fig. 2E, right column, and Fig. 2F). A clear increase in the frequencies at high DoC scores is also apparent in the frequency distribution for FLAP relative to cPLA2 (Fig. 3C). To provide more information on the three-way interactions, we applied three-color dSTORM acquisition. As shown in Fig. 4, the stimulus-dependent organization observed with two-color dSTORM was retained in three-color dSTORM analysis.
Figure 4.
Localization and interaction of 5-LO, FLAP, and cPLA2 on the nuclear envelope determined by three-color dSTORM. Neutrophils were treated with 1 ng/ml GM-CSF for 45 min then activated with 100 nm C5a for 5 min. Localization data were collected by three-color dSTORM and analyzed pairwise by Clus-DoC. A, localization maps for 5-LO (green), cPLA2 (red), and FLAP (yellow). B, percent colocalization of 5-LO molecules with FLAP from all ROIs. C, percent colocalization of 5-LO molecules with cPLA2 from all ROIs. D, percent colocalization of cPLA2 molecules with FLAP from all ROIs. Percent colocalized was defined as the fraction of localizations with a DoC score of at least 0.4. The boxes show the median and interquartile range. Scale bar represents 2.5 μm. One-way ANOVA with Bonferroni post hoc test was used to determine significance indicated by *, p < 0.05; ***, p < 0.001. Experiments were performed using five separate mice. In each experiment, 5–20 cells were used in each experimental condition. Representative cells for each condition are shown.
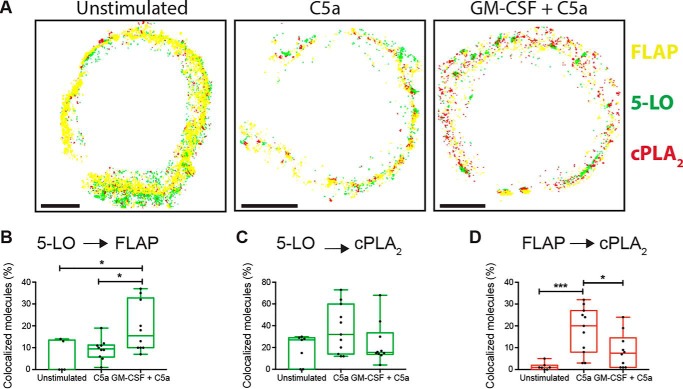
Colocalized molecules can simply be close, or alternatively, they may be organized in more complex relationships that can be defined and then detected by cluster analysis. Coclusters can be further divided into subsets based on parameters such as the degree of interaction. In mast cells, Clus-DoC allowed us to link the properties of clusters of 5-LO and FLAP to the generation of LTC4 (40). We applied the same algorithm to determine cluster properties in neutrophils. Initially we set a threshold of >5 localizations for 5-LO and cPLA2, or >10 localizations for FLAP to define true clusters. A higher threshold was chosen for FLAP because it functions as a homotrimer (18), increasing the chance that identified clusters contain at least three functional FLAP proteins. A higher level of stringency was used to identify clusters with a high degree of colocalization (HIC, high interaction clusters) denoting at least five localizations with a DoC score of ≥0.4). The properties of 5-LO:FLAP, 5-LO:cPLA2, and FLAP:cPLA2 HIC were compared at 0 and 5 min post cell activation.
We determined the relationships between the three possible pairs of proteins on the nuclear envelope and found that several HIC properties correlated with LTB4 synthesis, similarly to the generation of LTC4 in mast cells. The number of 5-LO:FLAP HIC on the nuclear envelope increased from 4 (2, 13) to 52 (19, 56) in cells stimulated with C5a and to 25 (20, 128) when first primed with GM-CSF compared with control (Fig. 5A). The median area of 5-LO:FLAP HIC increased from 0.72 (0.52, 0.97) to 0.9 (0.60, 1.51) nm2 in cells primed with GM-CSF and stimulated with C5a (Fig. 5B). The number of 5-LO localizations in 5-LO:FLAP HIC increased from 26 (12, 35) to 40 (23, 87) in cells primed with GM-CSF and then treated with C5a (Fig. 5C). The number of FLAP localizations in 5-LO:FLAP HIC increased 2-fold under both conditions, 35 (16, 42) to 53 (40, 104) (Fig. 5D). The percentage of 5-LO localizations interacting with FLAP inside 5-LO:FLAP HIC increased from 13 (IQR 0, 45) to 60 (22, 85) after priming with GM-CSF followed by stimulation with C5a (Fig. 5F). The percentage of FLAP localizations interacting with 5-LO inside 5-LO:FLAP HIC did not appreciably change under these conditions (Fig. 5G). Thus, these data show that C5a alone causes 5-LO:FLAP clustering, whereas priming with GM-CSF is necessary for maximal 5-LO recruitment to 5-LO:FLAP HIC.
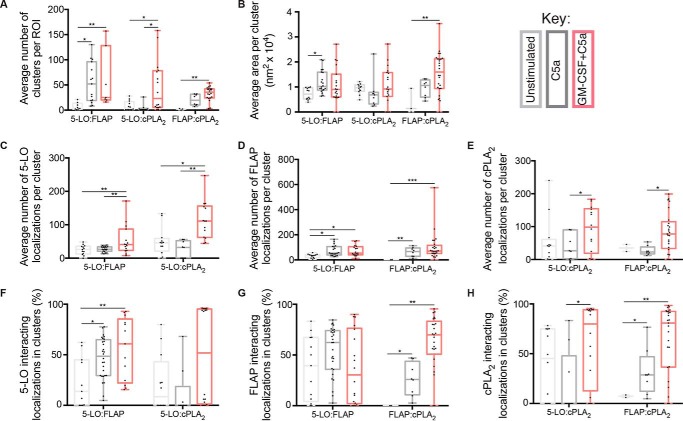
Figure 5.
Properties of HIC containing 5-LO, FLAP, and cPLA2. Neutrophils were treated with 1 ng/ml GM-CSF for 45 min then activated with 100 nm C5a for 5 min. Localization data were collected by two-color dSTORM and analyzed by Clus-DoC. True clusters were defined as having ≥5 5-LO localizations, ≥5 cPLA2 localizations or ≥10 FLAP localizations, and a subset of HICs were selected if ≥ 5 localizations in the cluster had a degree of colocalization (DoC) score ≥0.4. All values are for HICs. A, average number of clusters. B, average cluster area (nm2 × 104). C, average number of 5-LO localizations per cluster. D, average number of FLAP localizations per cluster. E, average number of cPLA2 localizations per cluster. F, percent of 5-LO interacting localizations in clusters with FLAP or cPLA2. G, percent of FLAP interacting localizations in clusters with 5-LO or cPLA2. H, percent of cPLA2 interacting localizations in clusters with 5-LO or FLAP. The boxes show the median and interquartile range. One-way ANOVA with multiple comparisons test was performed to determine significance. *, p < 0.05; **, p < 0.01; and ***, p < 0.001. Experiments were performed using five separate mice. In each experiment, 5–20 cells were used in each experimental condition.
When analyzing the clustering of cPLA2 with either 5-LO and FLAP, the patterns for each measure were generally similar to those observed for 5-LO:FLAP clustering, with some important differences. The number of FLAP:cPLA2 HIC increased from 2 (0, 3) to 19 (10, 31) after stimulation with C5a and then to 35 (24, 42) after priming with GM-CSF. However, the number of 5-LO:cPLA2 HIC increased only after priming with GM-CSF and C5a, 4 (1, 16) to 23 (5, 78), but not with treatment by C5a alone (Fig. 5A). No appreciable change in the area of 5-LO:cPLA2 HIC was observed after stimulation with C5a or a combination of C5a and GM-CSF (Fig. 5B). However, for FLAP:cPLA2 HICs an increase from 0.1 (0, 0.9) to 1.6 (0.9, 2.1) was found with GM-CSF priming followed by C5a but not with C5a alone (Fig. 5B). The number of 5-LO localizations in 5-LO:cPLA2 HIC did not increase from unstimulated to C5a alone (46 (21, 59) to 32 (0, 52), respectively) (Fig. 5C), but increased to 111 (62, 156) after priming with GM-CSF prior to C5a. The number of FLAP localizations in FLAP:cPLA2 HIC increased from 1 (0, 2) to 66 (28, 94) with C5a and increased up to 70 (51, 115) with GM-CSF priming (Fig. 5D). The number of cPLA2 localizations in 5-LO:cPLA2 HIC increased from 40 (2, 60) to 98 (18, 154) after cells were primed with GM-CSF and stimulated with C5a, but not with C5a alone (Fig. 5E). The percent of 5-LO interacting with cPLA2 inside 5-LO:cPLA2 HIC increased from 9% (0, 43) to 52% (1, 95) with GM-CSF and C5a, but no change was seen with C5a alone (Fig. 5F). The fraction of FLAP interacting with cPLA2 inside cPLA2:FLAP HIC increased from 0% (0, 0) to 25% (10, 43) in C5a alone and up to 69% (50, 83) with priming and C5a (Fig. 5G). The fraction of cPLA2 interacting with 5-LO in 5-LO:cPLA2 HIC increased with priming and C5a from 45 (0, 74) to 70 (12, 94) but not with C5a alone 0 (0, 47). The fraction of cPLA2 interacting with FLAP in FLAP-cPLA2 HIC increased from 7 (6, 8) to 28 (12, 46) with C5a alone and peaking at 80 (36, 98) with GM-CSF and C5a (Fig. 5H). Collectively, these data demonstrate that whereas C5a alone leads to moderate clustering of cPLA2 with FLAP, priming with GM-CSF boosts recruitment and association of cPLA2 with both FLAP and 5-LO, achieving maximal interactions between proteins. These data are summarized in Table S1.
Discussion
We have previously shown that higher order assemblies of 5-LO and FLAP are tightly linked to the formation of LTC4 in mast cells (40). In this study we extend this principle to the formation of LTB4 in neutrophils and identify the incorporation of cPLA2 into higher order assemblies with 5-LO and FLAP. Both the pseudocolor images and the analysis of colocalization by Clus-DoC indicate that the combination of GM-CSF and C5a stimulates the colocalization of 5-LO and FLAP and the colocalization of cPLA2 and FLAP, but not of 5-LO and cPLA2 (Figs. 2 and 3). In the case of 5-LO and FLAP, exposure to GM-CSF led to a clear increase in molecules of 5-LO that had the highest degree of being colocalized with FLAP (Fig. 3A, lower panel). In the case of cPLA2 and FLAP, this increased shift was even more pronounced (Fig. 3B, lower panel). It is worth noting that an even distribution of two molecules in the same area might occur if both are recruited to the same area but are not directly associated, resulting in a DoC score near 0. These data support a model in which 5-LO, FLAP, and cPLA2 all become localized within the same small regions of the nuclear envelope. Our results are distinct from previous studies suggesting that cPLA2 is localized on the Golgi (42). The previous studies, however, were performed using overexpressed fusion proteins.
Further support for our concept was provided by three-color dSTORM, analyzed pairwise with Clus-DoC (Fig. 4). In these experiments 5-LO and FLAP were identical. However, the colocalization of cPLA2 and FLAP was seen with C5a, rather than after treatment with both GM-CSF and C5a. The reason for the small differences that were observed between these approaches is not clear, but the results indicate that colocalization between cPLA2 and FLAP is a central component of the process. Although three-color dSTORM allows the measurements between three different components to be made within the same cells (compared with combinations of two-color dSTORM), available analytical methods are limited to pairwise interactions. Development of three-way interaction analysis will be necessary to further probe multi-protein clustering.
Fig. 5 demonstrates that the relationships of 5-LO, FLAP, and cPLA2 within LT synthetic complexes provide a subtle way of organizing signaling that is linked to LT generation. In response to both GM-CSF and C5a, 5-LO and FLAP and cPLA2 and FLAP form HIC and the percentage of 5-LO interacting in clusters increases. The number of 5-LO and cPLA2 molecules in HIC increases with priming and stimulation. The data suggest a critical role for the interaction of cPLA2 and FLAP. Further, FLAP-interacting cPLA2 and 5-LO are both enriched in FLAP HICs (Fig. 5, F and H). Taken together, these data indicate FLAP mediates the observed 5-LO:cPLA2 clustering, supporting the model of FLAP as the organizing component of leukotriene synthesis centers that locally concentrate the proteins involved in releasing and processing AA into bioactive compounds. One intriguing inference is that disassembly could be a mechanistic component of terminating pro-inflammatory signaling.
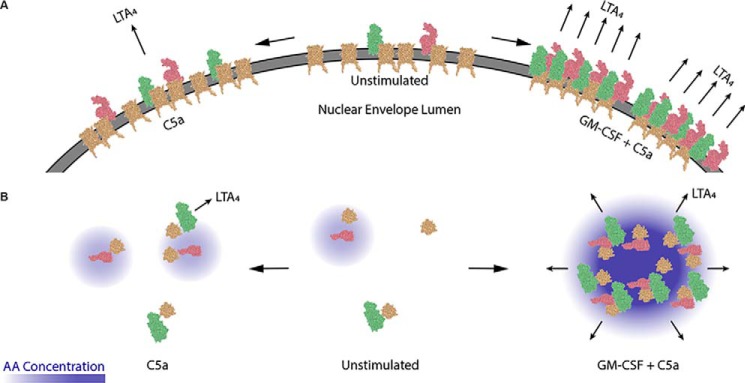
As described in the introduction, the regulated formation of higher order assemblies of receptors and interacting proteins on the plasma membrane is now recognized as an important regulatory mechanism in immune signaling. Besides signalosomes (31, 32), other examples in which similar reorganization plays an important role is the control of integrin avidity (43) and the segregation of active and inactive integrin molecules into defined clusters. Until recently, suitable technological approaches were not available to test this principle in the biosynthesis of eicosanoids and other bioactive lipids. Based on our data, we have developed a model of how 5-LO, FLAP, and cPLA2 form into clusters along the nuclear envelope following cell activation (Fig. 6): In unstimulated conditions (middle), FLAP (gold) is loosely organized on the nuclear envelope and 5-LO (red) and cPLA2 (green) largely remain cytosolic. When cells are activated with C5a (left), FLAP forms small clusters with 5-LO or cPLA2, producing a small amount of AA and therefore low amounts of LTA4 (the immediate precursor of LTB4). When GM-CSF primes the cells prior to C5a exposure (right), large clusters of FLAP, 5-LO, and cPLA2 form and produce high levels of locally focused AA, leading to larger quantities of LTA4. Fig. 6B presents a vertical view of this process looking down on the membrane. How a priming factor like GM-CSF leads to such a striking reorganization of these proteins remains to be determined (44).
Figure 6.
Proposed model of the 5-LO:FLAP:cPLA2 supramolecular complex. A, view of 5-LO (green), cPLA2 (red), and FLAP (gold) on the nuclear envelope in neutrophils. Minimal interactions among 5-LO:cPLA2:FLAP and no LTA4 production in unstimulated conditions (middle). C5a activation increases 5-LO:FLAP and cPLA2:FLAP clustering, resulting in minimal LTA4 production (left). C5a activation with GM-CSF priming results in maximal 5-LO:FLAP, cPLA2:FLAP, and 5-LO:cPLA2 clustering, suggestive that 5-LO, cPLA2, and FLAP interact and are localized in clusters with maximal LTA4 production (right). B, vertical view of the formation and function HIC with increased locally concentrated AA in response to GM-CSF. Antibody graphic was created using NGL Viewer (44) from RCSB PDB: 5-LO (3O8Y), cPLA2 (1CJY), and FLAP (2Q7M).
Our work firmly establishes the principle that higher order assemblies are a critical regulatory principle in eicosanoid biosynthesis. In addition, it lays the groundwork for a broader understanding of how the overall balance of proteins and enzyme substrate available in space and time determine the synthesis of eicosanoids and other bioactive lipids. We recognize that even more precise imaging and the ability to specifically disrupt assemblies will ultimately be required to definitively assign a biological role to organization.
Experimental procedures
Mice
C57BL/6 mice, 8 to 12 weeks old, were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained under specific-pathogen–free conditions at the animal facilities at the Massachusetts General Hospital Charlestown facility. This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animals were handled by approved institutional animal care and use committee (IACUC) protocols. The protocol was approved by the Committee on the Ethics of Animal Experiments of Massachusetts General Hospital (Animal Welfare Assurance Number: A3596-01).
Reagents
Poly-l-lysine solution was purchased from Sigma-Aldrich. Recombinant mouse GM-CSF was obtained from Millipore (Billerica, MA) and C5a was from R&D Systems (Minneapolis, MN). VECTASHIELD with DAPI was purchased from Vector Laboratories, Inc. (Burlingame, CA). Anti-FLAP (NB 300–891, Antibody Registry: AB_577652, targeting C terminus) goat polyclonal antibody was purchased from Novus Biologicals (Littleton, CO). Anti–5-LO rabbit polyclonal antibody (H-120, sc-20785, Antibody Registry: AB_2226938, targeting N terminus) was purchased from Santa Cruz Biotechnology (Dallas, TX). Anti–5-LO mouse polyclonal antibody was from BD Biosciences (San Jose, CA). Anti-Nup98 rat antibody and anti-calnexin rabbit antibody were from Abcam (Cambridge, MA). Affipure donkey anti-rabbit, donkey anti-goat, and donkey anti-mouse IgG were from Jackson ImmunoResearch Laboratories (West Grove, PA); Alexa Fluor 488–conjugated donkey anti-mouse, Alexa Fluor 594–conjugated donkey anti-rabbit secondary antibodies, and Alexa Fluor 647 and 488 and Cy3B dyes were purchased from Life Technologies (Grand Island, NY).
Purification, preparation, and activation of mouse neutrophils
Bone marrow neutrophils were purified from the tibia and femur of 8- to 12-week-old C57BL6 WT mice by density gradient centrifugation with Histopaque 1077 and 1119 (Sigma-Aldrich) as the separation media (45). 1.0 × 106 neutrophils were suspended in 300 μl of DMEM (phenol red-free) containing 1.5 mm calcium and magnesium and transferred to 8-well Millicell EZ slides (EMD Millipore; Billerica, MA) or 8-chamber Lab-Tek II slides (Thermo Scientific) pre-coated with poly-l-lysine (per manufacturer's instructions, 1:10 in deionized water). The cells adhered to the slides for 10 min at room temperature (RT). For priming, neutrophils were incubated with 1 ng/ml GM-CSF for 45 min at 37 °C in an atmosphere of 5% CO2; neutrophils for control and C5a-only conditions were incubated in media alone for 45 min at 37 °C. Neutrophils were then stimulated with 100 nm C5a for 5 min at 37 °C and the supernatants were removed from all wells at the same time and kept on ice for subsequent LTB4 analysis.
Time-correlated single-photon counting (TCSPC) FLIM analysis
Neutrophils were seeded onto poly-l-lysine–treated 8-well Millicell EZ slides adhered for 10 min. Cells were treated or left untreated (control) and washed twice in PBS. Cells were fixed in 4% paraformaldehyde, washed twice in PBS, and permeabilized for 5 min at room temperature with 0.1% Triton X-100 in PBS. The permeabilized cells were washed twice in PBS and blocked with 5% FBS in PBS for 60 min at RT. Anti–5-LO antibody (1:20) and anti-FLAP (1:100) were diluted in blocking solution overnight at 4 °C. The next day, cells were washed twice in PBS and incubated with Alexa Fluor 488– and Alexa Fluor 594–conjugated secondary antibodies (1:1000 in blocking buffer) for 60 min at room temperature in the dark. Labeled cells were washed twice in PBS and mounted with VECTASHIELD containing DAPI using a 24 × 60–1.5 glass coverslip. Slides were sealed with nail polish and stored at 4 °C until use.
Protein interactions were measured by TCSPC-FLIM with the instrument modifications noted below (27, 46). The baseline lifetimes of Alexa Fluor 488 (donor fluorophore, 5-LO) were calculated by single exponential decay fitting of fluorescence emission in the absence of Alexa Fluor 594 (acceptor fluorophore, FLAP). For samples stained for both donor and acceptor, lifetimes were fit to a bi-exponential decay with lifetime of one component fixed to the donor-only lifetime. The lifetime for the interacting component, τ1, as well as fractional contributions for the percent of interacting fluorophores, a1 (%), and noninteracting component were determined. At least three separate experiments were performed, and at least 15 different pixels along the nuclear envelope were used to determine the mean value.
All experiments were performed at room temperature. A Nikon Ti-E inverted microscope using a Plan APO VC 60× oil DC N2 objective with a N.A. of 1.4 was used in all microscopy experiments. The microscope base was equipped with Becker and Hickl DCS-120 TCSPC system, including Becker and Hickl BDL-488-SMC Picosecond Diode Laser, long-pass (HQ500LP) and band-pass (HQ435/50) emission filters, a hybrid detector (HPM-100-40 GaAsP Hybrid Detector integrated with a Hamamatsu R10467-40 photomultiplier tube). SPCImage 7.4 (Becker and Hickl GmbH) software was used for FLIM analysis.
Direct stochastic optical reconstruction microscopy (dSTORM)
Two-color dSTORM experiments were performed using a Nikon system (details below). Purified cells were seeded onto poly-l-lysine–treated Lab-Tek II slides (Thermo Scientific) and were adhered for 10 min. Cells were treated or left untreated (control) and then washed twice in PBS. Cells were fixed and prepared for superresolution microscopy (47). Primary antibodies, anti–5-LO (Santa Cruz Technology, 1:20), anti-FLAP (1:100), anti–5-LO (BD Biosciences, 1:100), and anti-cPLA2 (1:100) were diluted in blocking solution overnight at 4 °C. The anti–5-LO from Santa Cruz used in FLIM experiments was discontinued, therefore the two-color dSTORM experiments were performed using anti–5-LO from BD Biosciences. The day following primary antibody incubation, cells were washed twice in PBS and incubated with in-house conjugated secondary antibodies (3 μg/ml) for 60 min at RT in the dark. For two-color dSTORM experiments, donkey anti–rabbit Cy3B, donkey anti–goat Alexa Fluor 647, and donkey anti–mouse Alexa Fluor 488 were used.
Preparation of antibodies for dSTORM
Donkey anti-goat, donkey anti-mouse, and donkey anti-rabbit affinity purified secondary antibodies (H+L chains) were purchased from The Jackson Laboratory. They were conjugated to Cy3B (GE Healthcare), Alexa Fluor 488, or Alexa Fluor 674 dye (Life Technologies), both with carboxylic acid succinimidyl ester moieties. For the conjugation reactions, 240 μg of the secondary antibody was reacted with 6 μg of dye in 56 mm carbonic buffer for 2 h at RT. After the reaction, the antibodies were separated from unconjugated dye using gravity filtration through Sephadex G-25 DNA grade columns (GE Healthcare). The antibody and dye concentrations were measured using NanoDrop spectroscopy. Concentrations and the dye/antibody ratio were calculated using correction factors for the dye absorbance at 280 nm, the molar extinction coefficient, and the equation to calculate the dye/antibody ratio based on the manufacturer's specifications. The final ratio for the donkey anti-rabbit:Cy3B was 1:1.7 with antibody concentration of 285 μg/μl. The final ratio for the donkey anti-goat:AF647 was 1:2.7 with antibody concentration of 236 μg/μl. The final ratio for the donkey anti-mouse:AF488 was 1:0.98 with antibody concentration of 385 μg/μl. The final ratio for the donkey anti-rabbit:Cy3B was 1:2.1 with antibody concentration of 176 μg/μl.
The imaging buffer contained 100 mm 2-mercaptoethanolamine and 1% (v/v) GLOX (glucose oxidase and catalase solution) was used to promote photoswitching and reduce photobleaching (47). An inverted Nikon Ti2 Eclipse/STORM 5.0 system with Perfect Focus focal plane lock was used for image acquisition. This system contains a NSTORM quadband filter and 405, 488, 561, and 647 nm lasers and was equipped with an HP APO TIRF AC 100×/1.49 NA oil objective and ORCA-Flash4.0v2 S-CMOS camera (Hamamatsu Photonics). 15,000 frames for each dye were collected at 30-ms exposure time with continuous activation. Localizations were identified with NIS Elements 5.0 (Nikon Instruments) and exported as tab-delimited text files.
Clus-DoC analysis of single molecule localizations
We employed Clus-DoC (41) to quantify colocalization of individual proteins and molecules (localizations) and cluster properties in regions of interest (ROI), the output of which we determined using custom scripts. ROIs were drawn around nuclear envelope when FLAP was being detected and whole cells otherwise. As each molecule may be associated with multiple localizations because of labeling and multiple blinking, grouping algorithms are often used to reduce redundancy. However, grouping can lead to undercounting and STORM detection efficiency is at most 60% (48). As such, we proceeded without grouping so that relative changes in clustering could be robustly detected, with the understanding that cluster localization counts may not reflect the true molecule count. We only considered clusters having at least 5 localizations for 5-LO, 5 for cPLA2, and 10 for FLAP (more because it functions as a trimer).
LTB4 assay
To analyze LTB4 generation, an enzyme immunoassay kit for LTB4 (Cayman Chemical Co.) was used per manufacturer's instructions. Culture media were removed at desired times and stored at −80 °C until use.
Statistics
All statistics were performed using Prism GraphPad 7 (GraphPad Software, La Jolla, CA). The median and IQR (25–75%) was determined. The data are expressed as median (25%, 75%). Illustrations were built in Adobe Illustrator CC (22.0.1). Enzyme immunoassay, FLIM, and cluster property results were further analyzed using one-way ANOVA followed by Bonferroni multiple comparison or a Tukey post test where p < 0.05 was considered significant. A Student's t test with Welch's correction was performed to determine to significance where two conditions were compared.
Data availability
Raw STORM data files are stored on a local server. STORM localization list text files and custom scripts are available on GitHub (doi:10.5281/zenodo.3701524). All remaining data are contained within the article.
Author contributions
A. B. S. and R. J. S. conceptualization; A. B. S., N. C. B., H. S., and M. D. G. data curation; A. B. S. and N. C. B. formal analysis; A. B. S. supervision; A. B. S. and R. J. S. funding acquisition; A. B. S., N. C. B., and P. A. N. validation; A. B. S. and N. C. B. investigation; A. B. S., N. C. B., and G. E. E. visualization; A. B. S., N. C. B., M. D. G., and G. E. E. methodology; A. B. S. and N. C. B. writing-original draft; A. B. S., N. C. B., H. S., G. E. E., and R. J. S. writing-review and editing; N. C. B., H. S., and J. T. L. software; H. S., J. T. L., and P. A. N. resources; R. J. S. project administration.
Supplementary Material
This work was supported by NIDDK, National Institutes of Health Grants K01DK089145 (to A. B. S.), R01DK062472 (to A. B. S. and R. J. S.), P30AR070253 (P. A. N., R. J. S., and A. B. S.), and T32DK007540 (to N. C. B. and R. J. S.); by NIAMS, National Institutes of Health Grant R01AR065538 (to A. B. S., P. A. N., and R. J. S.); and by NCRR, National Institutes of Health Grant S10RR027931 (to R. J. S.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Fig. S1 and Table S1.
- LT
- leukotriene
- 5-LO
- arachidonate 5-lipoxygenase
- AA
- arachidonic acid
- ANOVA
- analysis of variance
- C5a
- complement component 5a
- cPLA2
- cytosolic phospholipase A2
- DoC
- degree of colocalization
- dSTORM
- direct stochastic optical reconstruction microscopy
- FLAP
- arachidonate 5-lipoxygenase-interacting protein
- FLIM
- fluorescence lifetime imaging microscopy
- GM-CSF
- granulocyte-macrophage colony-stimulating factor
- HIC
- high interaction cluster
- IQR
- interquartile range
- ROI
- regions of interest
- RT
- room temperature
- TCSPC
- time-correlated single-photon counting.
References
- 1. Borgeat P., and Samuelsson B. (1979) Arachidonic acid metabolism in polymorphonuclear leukocytes: Unstable intermediate in formation of dihydroxy acids. Proc. Natl. Acad. Sci. U.S.A. 76, 3213–3217 10.1073/pnas.76.7.3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weller P. F., Lee C. W., Foster D. W., Corey E. J., Austen K. F., and Lewis R. A. (1983) Generation and metabolism of 5-lipoxygenase pathway leukotrienes by human eosinophils: Predominant production of leukotriene C4. Proc. Natl. Acad. Sci. U.S.A. 80, 7626–7630 10.1073/pnas.80.24.7626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Afonso P. V., Janka-Junttila M., Lee Y. J., McCann C. P., Oliver C. M., Aamer K. A., Losert W., Cicerone M. T., and Parent C. A. (2012) LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell 22, 1079–1091 10.1016/j.devcel.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bair A. M., Turman M. V., Vaine C. A., Panettieri R. A. Jr., and Soberman R. J. (2012) The nuclear membrane leukotriene synthetic complex is a signal integrator and transducer. Mol. Biol. Cell 23, 4456–4464 10.1091/mbc.e12-06-0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clark S. R., Guy C. J., Scurr M. J., Taylor P. R., Kift-Morgan A. P., Hammond V. J., Thomas C. P., Coles B., Roberts G. W., Eberl M., Jones S. A., Topley N., Kotecha S., and O'Donnell V. B. (2011) Esterified eicosanoids are acutely generated by 5-lipoxygenase in primary human neutrophils and in human and murine infection. Blood 117, 2033–2043 10.1182/blood-2010-04-278887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DiPersio J. F., Billing P., Williams R., and Gasson J. C. (1988) Human granulocyte-macrophage colony-stimulating factor and other cytokines prime human neutrophils for enhanced arachidonic acid release and leukotriene B4 synthesis. J. Immunol. 140, 4315–4322 [PubMed] [Google Scholar]
- 7. Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., and Smith M. J. (1980) Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 286, 264–265 10.1038/286264a0 [DOI] [PubMed] [Google Scholar]
- 8. Lämmermann T., Afonso P. V., Angermann B. R., Wang J. M., Kastenmüller W., Parent C. A., and Germain R. N. (2013) Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375 10.1038/nature12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Czarnetzki B. (1983) Increased monocyte chemotaxis towards leukotriene B4 and platelet activating factor in patients with inflammatory dermatoses. Clin. Exp. Immunol. 54, 486–492 [PMC free article] [PubMed] [Google Scholar]
- 10. Ott V. L., Cambier J. C., Kappler J., Marrack P., and Swanson B. J. (2003) Mast cell-dependent migration of effector CD8+ T cells through production of leukotriene B4. Nat. Immunol. 4, 974–981 10.1038/ni971 [DOI] [PubMed] [Google Scholar]
- 11. Tager A. M., Bromley S. K., Medoff B. D., Islam S. A., Bercury S. D., Friedrich E. B., Carafone A. D., Gerszten R. E., and Luster A. D. (2003) Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat. Immunol. 4, 982–990 10.1038/ni970 [DOI] [PubMed] [Google Scholar]
- 12. Goodarzi K., Goodarzi M., Tager A. M., Luster A. D., and von Andrian U. H. (2003) Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat. Immunol. 4, 965–973 10.1038/ni972 [DOI] [PubMed] [Google Scholar]
- 13. Carmona-Rivera C., and Kaplan M. J. (2013) Low-density granulocytes: A distinct class of neutrophils in systemic autoimmunity. Semin. Immunopathol. 35, 455–463 10.1007/s00281-013-0375-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng Q., Sarris M., Bennin D. A., Green J. M., Herbomel P., and Huttenlocher A. (2013) Localized bacterial infection induces systemic activation of neutrophils through Cxcr2 signaling in zebrafish. J. Leukoc. Biol. 93, 761–769 10.1189/jlb.1012534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mócsai A. (2013) Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 210, 1283–1299 10.1084/jem.20122220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X., Qiu L., Li Z., Wang X. Y., and Yi H. (2018) Understanding the multifaceted role of neutrophils in cancer and autoimmune diseases. Front. Immunol. 9, 2456 10.3389/fimmu.2018.02456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dixon R. A., Diehl R. E., Opas E., Rands E., Vickers P. J., Evans J. F., Gillard J. W., and Miller D. K. (1990) Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature 343, 282–284 10.1038/343282a0 [DOI] [PubMed] [Google Scholar]
- 18. Ferguson A. D., McKeever B. M., Xu S., Wisniewski D., Miller D. K., Yamin T. T., Spencer R. H., Chu L., Ujjainwalla F., Cunningham B. R., Evans J. F., and Becker J. W. (2007) Crystal structure of inhibitor-bound human 5-lipoxygenase-activating protein. Science 317, 510–512 10.1126/science.1144346 [DOI] [PubMed] [Google Scholar]
- 19. Reid G. K., Kargman S., Vickers P. J., Mancini J. A., Léveillé C., Ethier D., Miller D. K., Gillard J. W., Dixon R. A., and Evans J. F. (1990) Correlation between expression of 5-lipoxygenase-activating protein, 5-lipoxygenase, and cellular leukotriene synthesis. J. Biol. Chem. 265, 19818–19823 [PubMed] [Google Scholar]
- 20. Miller D. K., Gillard J. W., Vickers P. J., Sadowski S., Leveille C., Mancini J. A., Charleson P., Dixon R. A., Ford-Hutchinson A. W., Fortin R., Gauthier J. Y., Rodkey J., Rosen R., Rouzer C., Sigal I. S., Strader C. D., and Evans J. F. (1990) Identification and isolation of a membrane protein necessary for leukotriene production. Nature 343, 278–281 10.1038/343278a0 [DOI] [PubMed] [Google Scholar]
- 21. Woods J. W., Evans J. F., Ethier D., Scott S., Vickers P. J., Hearn L., Heibein J. A., Charleson S., and Singer I. I. (1993) 5-lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. J. Exp. Med. 178, 1935–1946 10.1084/jem.178.6.1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brock T. G., McNish R. W., Bailie M. B., and Peters-Golden M. (1997) Rapid import of cytosolic 5-lipoxygenase into the nucleus of neutrophils after in vivo recruitment and in vitro adherence. J. Biol. Chem. 272, 8276–8280 10.1074/jbc.272.13.8276 [DOI] [PubMed] [Google Scholar]
- 23. Panini S. R., Yang L., Rusinol A. E., Sinensky M. S., Bonventre J. V., and Leslie C. C. (2001) Arachidonate metabolism and the signaling pathway of induction of apoptosis by oxidized LDL/oxysterol. J. Lipid Res. 42, 1678–1686 [PubMed] [Google Scholar]
- 24. Gijón M. A., and Leslie C. C. (1999) Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J. Leukoc. Biol. 65, 330–336 10.1002/jlb.65.3.330 [DOI] [PubMed] [Google Scholar]
- 25. Ghosh M., Tucker D. E., Burchett S. A., and Leslie C. C. (2006) Properties of the group IV phospholipase A2 family. Prog. Lipid Res. 45, 487–510 10.1016/j.plipres.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 26. Leslie C. C. (2015) Cytosolic phospholipase A2: Physiological function and role in disease. J. Lipid Res. 56, 1386–1402 10.1194/jlr.R057588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mandal A. K., Jones P. B., Bair A. M., Christmas P., Miller D., Yamin T. T., Wisniewski D., Menke J., Evans J. F., Hyman B. T., Bacskai B., Chen M., Lee D. M., Nikolic B., and Soberman R. J. (2008) The nuclear membrane organization of leukotriene synthesis. Proc. Natl. Acad. Sci. U.S.A. 105, 20434–20439 10.1073/pnas.0808211106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gerstmeier J., Newcomer M. E., Dennhardt S., Romp E., Fischer J., Werz O., and Garscha U. (2016) 5-Lipoxygenase-activating protein rescues activity of 5-lipoxygenase mutations that delay nuclear membrane association and disrupt product formation. FASEB J. 30, 1892–1900 10.1096/fj.201500210R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hao Y. H., Doyle J. M., Ramanathan S., Gomez T. S., Jia D., Xu M., Chen Z. J., Billadeau D. D., Rosen M. K., and Potts P. R. (2013) Regulation of WASH-dependent actin polymerization and protein trafficking by ubiquitination. Cell 152, 1051–1064 10.1016/j.cell.2013.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hou F., Sun L., Zheng H., Skaug B., Jiang Q. X., and Chen Z. J. (2011) MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146, 448–461 10.1016/j.cell.2011.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu H., and Fuxreiter M. (2016) The structure and dynamics of higher-order assemblies: amyloids, signalosomes, and granules. Cell 165, 1055–1066 10.1016/j.cell.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin S. C., Lo Y. C., and Wu H. (2010) Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 465, 885–890 10.1038/nature09121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang L., Yang J. K., Kabaleeswaran V., Rice A. J., Cruz A. C., Park A. Y., Yin Q., Damko E., Jang S. B., Raunser S., Robinson C. V., Siegel R. M., Walz T., and Wu H. (2010) The Fas-FADD death domain complex structure reveals the basis of DISC assembly and disease mutations. Nat. Struct. Mol. Biol. 17, 1324–1329 10.1038/nsmb.1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gilbert N. C., Bartlett S. G., Waight M. T., Neau D. B., Boeglin W. E., Brash A. R., and Newcomer M. E. (2011) The structure of human 5-lipoxygenase. Science 331, 217–219 10.1126/science.1197203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stock A. T., Hansen J. A., Sleeman M. A., McKenzie B. S., and Wicks I. P. (2016) GM-CSF primes cardiac inflammation in a mouse model of Kawasaki disease. J. Exp. Med. 213, 1983–1998 10.1084/jem.20151853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Campbell I. K., van Nieuwenhuijze A., Segura E., O'Donnell K., Coghill E., Hommel M., Gerondakis S., Villadangos J. A., and Wicks I. P. (2011) Differentiation of inflammatory dendritic cells is mediated by NF-κB1-dependent GM-CSF production in CD4 T cells. J. Immunol. 186, 5468–5477 10.4049/jimmunol.1002923 [DOI] [PubMed] [Google Scholar]
- 37. Codarri L., Gyülvészi G., Tosevski V., Hesske L., Fontana A., Magnenat L., Suter T., and Becher B. (2011) RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12, 560–567 10.1038/ni.2027 [DOI] [PubMed] [Google Scholar]
- 38. Jayne D. R. W., Bruchfeld A. N., Harper L., Schaier M., Venning M. C., Hamilton P., Burst V., Grundmann F., Jadoul M., Szombati I., Tesa V., Segelmark M., Potarca A., Schall T. J., Bekker P., and Clear Study Group. (2017) Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J. Am. Soc. Nephrol. 28, 2756–2767 10.1681/ASN.2016111179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schreiber A., Xiao H., Jennette J. C., Schneider W., Luft F. C., and Kettritz R. (2009) C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J. Am. Soc. Nephrol. 20, 289–298 10.1681/ASN.2008050497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schmider A. B., Vaught M., Bauer N. C., Elliott H. L., Godin M. D., Ellis G. E., Nigrovic P. A., and Soberman R. J. (2019) The organization of leukotriene biosynthesis on the nuclear envelope revealed by single molecule localization microscopy and computational analyses. PLoS One 14, e0211943 10.1371/journal.pone.0211943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pageon S. V., Nicovich P. R., Mollazade M., Tabarin T., and Gaus K. (2016) Clus-DoC: A combined cluster detection and colocalization analysis for single-molecule localization microscopy data. Mol. Biol. Cell 27, 3627–3636 10.1091/mbc.e16-07-0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghosh M., Loper R., Ghomashchi F., Tucker D. E., Bonventre J. V., Gelb M. H., and Leslie C. C. (2007) Function, activity, and membrane targeting of cytosolic phospholipase A2ζ in mouse lung fibroblasts. J. Biol. Chem. 282, 11676–11686 10.1074/jbc.M608458200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spiess M., Hernandez-Varas P., Oddone A., Olofsson H., Blom H., Waithe D., Lock J. G., Lakadamyali M., and Strömblad S. (2018) Active and inactive β1 integrins segregate into distinct nanoclusters in focal adhesions. J. Cell Biol. 217, 1929–1940 10.1083/jcb.201707075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rose A. S., Bradley A. R., Valasatava Y., Duarte J. M., Prlić A., and Rose P. W. (2018) NGL viewer: Web-based molecular graphics for large complexes. Bioinformatics 34, 3755–3758 10.1093/bioinformatics/bty419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feng Y., Zou L., Si R., Nagasaka Y., and Chao W. (2010) Bone marrow MyD88 signaling modulates neutrophil function and ischemic myocardial injury. Am. J. Physiol. Cell Physiol. 299, C760–C769 10.1152/ajpcell.00155.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mandal A. K., Skoch J., Bacskai B. J., Hyman B. T., Christmas P., Miller D., Yamin T. T., Xu S., Wisniewski D., Evans J. F., and Soberman R. J. (2004) The membrane organization of leukotriene synthesis. Proc. Natl. Acad. Sci. U.S.A. 101, 6587–6592 10.1073/pnas.0308523101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dempsey G. T. (2013) A user's guide to localization-based super-resolution fluorescence imaging. Methods Cell Biol. 114, 561–592 10.1016/B978-0-12-407761-4.00024-5 [DOI] [PubMed] [Google Scholar]
- 48. Feher K., Halstead J. M., Goyette J., and Gaus K. (2019) Can single molecule localization microscopy detect nanoclusters in T cells? Curr. Opin. Chem. Biol. 51, 130–137 10.1016/j.cbpa.2019.05.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw STORM data files are stored on a local server. STORM localization list text files and custom scripts are available on GitHub (doi:10.5281/zenodo.3701524). All remaining data are contained within the article.