Abstract
Treatment of patients with chronic lymphocytic leukemia (CLL) with inhibitors of Bruton's tyrosine kinase (BTK), such as ibrutinib, is limited by primary or secondary resistance to this drug. Examinations of CLL patients with late relapses while on ibrutinib, which inhibits BTK's catalytic activity, revealed several mutations in BTK, most frequently resulting in the C481S substitution, and disclosed many mutations in PLCG2, encoding phospholipase C-γ2 (PLCγ2). The PLCγ2 variants typically do not exhibit constitutive activity in cell-free systems, leading to the suggestion that in intact cells they are hypersensitive to Rac family small GTPases or to the upstream kinases spleen-associated tyrosine kinase (SYK) and Lck/Yes-related novel tyrosine kinase (LYN). The sensitivity of the PLCγ2 variants to BTK itself has remained unknown. Here, using genetically-modified DT40 B lymphocytes, along with various biochemical assays, including analysis of PLCγ2-mediated inositol phosphate formation, inositol phospholipid assessments, fluorescence recovery after photobleaching (FRAP) static laser microscopy, and determination of intracellular calcium ([Ca2+]i), we show that various CLL-specific PLCγ2 variants such as PLCγ2S707Y are hyper-responsive to activated BTK, even in the absence of BTK's catalytic activity and independently of enhanced PLCγ2 phospholipid substrate supply. At high levels of B-cell receptor (BCR) activation, which may occur in individual CLL patients, catalytically-inactive BTK restored the ability of the BCR to mediate increases in [Ca2+]i. Because catalytically-inactive BTK is insensitive to active-site BTK inhibitors, the mechanism involving the noncatalytic BTK uncovered here may contribute to preexisting reduced sensitivity or even primary resistance of CLL to these drugs.
Keywords: calcium, drug resistance, phospholipase C, phosphoinositide, inositol phospholipid, inositol phosphate, Rac (Rac GTPase), tyrosine–protein kinase (tyrosine kinase), B-cell receptor (BCR), B-cell signaling, Bruton's tyrosine kinase (BTK), chronic lymphocytic leukemia (CLL), ibrutinib
Introduction
The two members of the γ subfamily of inositol phospholipid–specific phospholipase C (PLC),4 PLCγ1 and PLCγ2, have recently been found to be involved in a number of ostensibly different human disease states (1). These range from rare hereditary diseases caused by, or associated with, germline mutations in the PLCG2 gene, such as complex immunological diseases with an autoinflammatory hallmark (2, 3) and childhood-onset steroid-sensitive nephrotic syndrome (4), to acquired conditions associated with somatic PLCG1 or PLCG2 mutations, such as angiosarcoma and T-cell lymphomas (PLCG1) (5, 6), and endemic Burkitt lymphoma (PLCG2) (7). Point mutations in PLCG2 have also been reported to modify the risk of developing neurodegenerative diseases like Alzheimer's disease (8, 9) and to cause resistance of chronic lymphocytic leukemia (CLL) cells to catalytic-site inhibitors of Bruton's tyrosine kinase (BTK) (10).
BTK is one of the five members of the strongly-conserved Tec family of nonreceptor tyrosine kinases, also including the IL-2–inducible T-cell kinase ITK, TEC proper, RLK, and BMX (11). Although all five Tec variants are expressed in cells of the hematopoietic lineage, BTK and ITK are key regulators of B-cell and T-cell transmembrane signaling, respectively (12). Mutations of the human and mouse BTK and Btk genes functionally affecting BTK were found to be associated with human X-linked agammaglobulinemia (XLA) and murine X-linked immunodeficiency (11). BTK and the other Tec family members share an overall similar domain organization that closely resembles the organization of the Src family tyrosine kinases, with an SH3–SH2–protein-tyrosine kinase domain tandem at their C termini, but divergent N-terminal regions (11). In B cells, BTK couples antigen-activated B-cell receptors (BCR) to tyrosine phosphorylation of PLCγ2 at the four canonical tyrosine residues, Tyr-753, Tyr-759, Tyr-1197, and Tyr-1217 (13, 14). The latter modifications activate PLCγ2 to hydrolyze its substrate phosphatidylinositol 4,5-bisphosphate (PtdInsP2) to inositol 1,4,5-trisphosphate and diacylglycerol. All three of the latter act as intracellular second messengers (15, 16), which are involved in controlling key functions of both normal and neoplastic B lymphocytes. The activation of PLCγ2 is greatly facilitated by the adaptor protein BLNK/SLP-65 (17, 18) and requires membrane targeting of BTK via its N-terminal pleckstrin homology domain to phosphatidylinositol 3,4,5-trisphosphate generated from PtdInsP2 by phosphatidylinositol 3-kinases. For this reason, it can be blocked by the R28C and mimicked by the E41K mutation within its PH domain (19, 20). The K430R mutation of BTK, by replacing a crucial lysine coordinating the α- and β-phosphates of the substrate ATP and being present in this position in all protein kinases (21), renders the BTK protein catalytically inert (22).
Previous experimentation has shown that, during B-cell development, BTK partially acts as an adaptor molecule, independent of its catalytic activity (23), and that the tumor suppressor function of BTK identified in pre-B cells is independent of its catalytic activity (24). Specifically, kinase-inactive BTKK430R completely reconstituted λ usage in BTK-deficient mice and partially corrected the defective modulation of pre-B cell–surface markers, peripheral B-cell survival, and BCR-mediated NF-κB induction (23). Activation of BTK by pre-BCR stimulation was also shown to be independent of BLNK/SLP-65 (24). These effects have thus far been attributed to the observation that BTK associates with phosphatidylinositol-4-phosphate 5-kinases (PIP5Ks), the enzymes that synthesize the generic substrate of inositol phospholipid–specific phospholipase C, PtdInsP2 (25).
Irreversible BTK inhibitors such as ibrutinib have demonstrated remarkable activity in a number of B-cell malignancies with approval in CLL, mantle cell lymphoma, marginal zone lymphoma, and Waldenström's macroglobulinemia (26, 27). As a result, ibrutinib is being used with increasing frequency as frontline therapy, beginning to displace chemoimmunotherapy in this setting (28, 29). Currently, the drugs are also being evaluated for the treatment of other diseases, such as other malignancies and various autoimmune and/or inflammatory diseases (30–33). As is the case for other targeted tumor therapies, BTK inhibitor treatment of CLL is limited by either primary or secondary, i.e. acquired drug resistance (10). Thus, whole-exome sequencing of CLL cells causing late relapses revealed (i) C481S and a small number of other mutations in the same and in other positions in BTK and (ii) a higher variety of mutations in PLCG2. To date, some 25 point mutations of one or two adjacent residues are known in PLCG2, some of which cluster in specific regions of the encoded PLCγ2 protein, such as one of the two SH2 domains and the C2 domain (34–36). Collectively, the BTK and PLCG2 mutations are associated with about 85% of CLL cases with acquired resistance to BTK inhibitors (37).
Although the C481S mutation of BTK eliminates covalent binding of some BTK inhibitors, such as ibrutinib, to their target and hence markedly reduces their affinity to their binding site, the mechanism of resistance caused by the other BTK and the PLCG2 mutations is less clear. PLCγ2 is immediately downstream of BTK (26) leading to the suggestion that the variant proteins may be constitutively active. However, we have previously found that several PLCγ2 variant enzymes associated with ibrutinib resistance carrying mutations in various regions are not constitutively active when assayed, even as purified proteins, in a cell-free system employing artificial lipid vesicles as substrate (38, 39). The observation that PLCγ2 variants are, instead, hypersensitive in intact cells to the Rho GTPase RAC2, as well as the upstream protein-tyrosine kinases SYK and LYN (39, 40), has led us to suggest that the PLCG2 mutations found in BTK inhibitor–resistant cells may cause a rerouting of the transmembrane signals emanating from BCR to converge on and activate PLCγ2.
This work was undertaken to study the functional interaction of BTK with PLCγ2, WT, and ibrutinib resistance variants in a system reconstituted in intact cells from the two protein constituents. We chose enhanced inositol phosphate formation as a functional readout of a productive interaction between the two, rather than PLCγ2 phosphorylation, as it is the most relevant functional outcome of stimulated PLCγ2 in terms of B-cell signaling. Also PLCγ2 is phosphorylated at many other residues, for instance Tyr-733 or Tyr-1245, in addition to the four canonical sites utilized by activated BTK (41, 42), which may confound results. To our knowledge, this approach has not been reported previously. Here, we report that several PLCγ2 variants mediating BTK-inhibitor resistance in CLL patients are strikingly hypersensitive to activated and/or membrane-bound BTK and to BTK variants lacking protein-tyrosine kinase activity. Importantly, we demonstrate that the activity of the PLCγ2 variants in the context of catalytically-inactive BTK is not contingent on enhanced formation of the PLC substrate PtdInsP2 via stimulation of PtdInsP kinases, as postulated earlier. Hence, stimulation of PLCγ2 variants mediating BTK inhibitor resistance can be explained by their hypersensitivity to the noncatalytic actions of BTK. We suggest that this mechanism contributes to the acquired BTK inhibitor resistance observed in CLL patients with PLCG2 mutations and, potentially, to preexisting reduced sensitivity or even primary resistance of clonal populations of CLL cells with hyperactivated BCR.
Results
PLCγ2S707Y can be functionally reconstituted with and is hypersensitive to activation by BTK
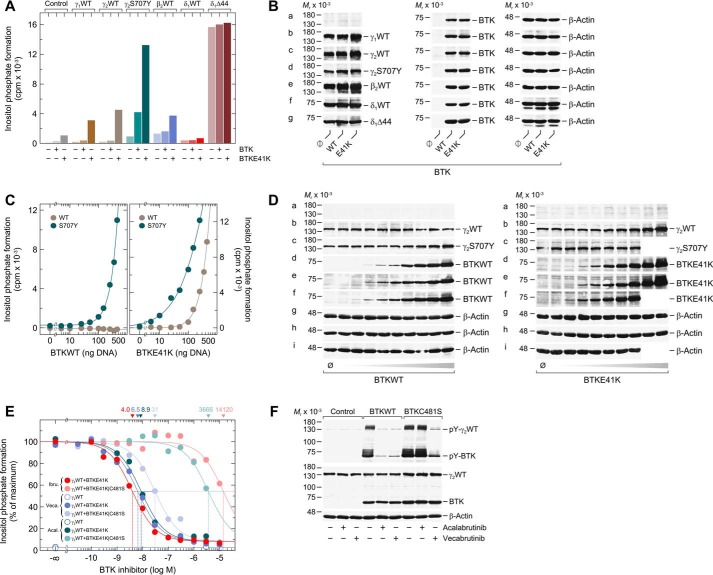
To investigate and compare the functional reconstitution of various PLC isozymes with WT BTK and its predominantly membrane-bound and, hence, active variant E41K, WT PLCγ1, PLCγ2, PLCβ2, WT, and Δ44 variant PLCδ1, and S707Y variant PLCγ2 were coexpressed with the two BTK variants in COS-7 cells radiolabeled with [3H]inositol for measurement of [3H]inositol phosphate formation (Fig. 1A). WT and constitutively-active Δ44 variants of the evolutionarily divergent PLC isoform PLCδ1, carrying a deletion of the autoinhibitory X-Y linker (43), were analyzed for comparison. PLCδ1 is not known to be regulated by heterotrimeric GTP-binding proteins or members of the Tec kinase family and is thus considered to be distinct from a mechanistic point of view. There was little effect of WT BTK on inositol phosphate formation in the absence of exogenous PLC enzymes and in the presence of any of the WT PLCs. In contrast, in the presence of PLCγ2S707Y, WT BTK caused a 4.6-fold increase in inositol phosphate formation. BTKE41K caused a minor increase in inositol phosphate formation in the absence of exogenous PLCs but triggered much more marked increases in the presence of WT PLCγ1, PLCγ2, PLCβ2, and, most strikingly, of PLCγ2S707Y. Neither WT nor Δ44 variant PLCδ1 activity was affected by WT or E41K variant BTK, suggesting that the stimulatory effects observed for the other PLC isozymes were not due to enhanced supply of their common substrate PtdInsP2. Fig. 1B shows that the differences in inositol phosphate formation observed in Fig. 1A were not explained by differences in protein expression. Note that there was no difference in the expression of WT PLCγ2 and PLCγ2S707Y when directly compared on the same immunoblots (cf. Fig. 3D and Fig. S3).
Figure 1.
Analysis of the functional reconstitution of PLCγ2 and BTK. A, COS-7 cells were transfected as indicated with 250 ng/well of either empty vector (Control) or 150 ng/well of vector encoding either WT PLCγ1 (γ1WT), WT PLCγ2 (γ2WT), WT PLCβ2 (β2WT), or WT PLCδ1 (δ1WT), 50 ng/well of vector encoding PLCγ2S707Y (γ2S707Y) or PLCδ1Δ44 (δ1Δ44), and 100 ng/well of vector encoding either WT BTK (BTKWT) or a constitutively-active version of BTK (BTKE41K). B, cells from one well each functionally analyzed in A were washed with 0.2 ml of Dulbecco's PBS and then lysed by addition of 100 μl of SDS-PAGE sample preparation buffer. Aliquots of the samples were subjected to SDS-PAGE, and immunoblotting was performed using an antibody reactive against the c-Myc epitope present on WT PLCγ1, WT PLCγ2, PLCγ2S707Y, WT PLCδ1, and PLCδ1Δ44 (a–d and f–g), antibody reactive against PLCβ2 (e), antibody reactive against BTK (a–g), or antibody reactive against β-actin (a–g). C, COS-7 cells were transfected as indicated with 50 ng/well of vector encoding WT PLCγ2 (γ2WT) or PLCγ2S707Y (γ2S707Y) and increasing amounts (0–450 ng/well) of vector encoding WT BTK (BTKWT) (left panel) or BTKE41K (right panel). A and C, 24 h after transfection, the cells were incubated for 18 h with myo-[2-3H]inositol, and inositol phosphate formation was then determined. D, cells from one well each functionally analyzed in C were washed with 0.2 ml of Dulbecco's PBS and then lysed by addition of 100 μl of SDS-PAGE sample preparation buffer. Aliquots of the samples were subjected to SDS-PAGE, and immunoblotting was performed using an antibody reactive against the c-Myc epitope present on WT PLCγ2 and PLCγ2S707Y (a–c), antibody reactive against BTK (d–f), or antibody reactive against β-actin (g–i). E, COS-7 cells were transfected as indicated with 150 ng/well of vector encoding WT PLCγ2 (γ2WT) and 100 ng/well of vector encoding BTKE41K or BTKE41K/C481S. Twenty four hours after transfection, the cells were incubated for 18 h with myo-[2-3H]inositol and either solvent (0.1% (v/v) DMSO) or increasing amounts of ibrutinib (0.1 nm to 10 μm), vecabrutinib (0.1 nm to 10 μm), or acalabrutinib (1 nm to 3 μm). Inositol phosphate formation was then determined. The results were normalized to percent of maximal inositol phosphate formation, which was 10,012, 9747, and 15,178 cpm for ibrutinib, acalabrutinib, and vecabrutinib, respectively. The IC50 values of ibrutinib, acalabrutinib, and vecabrutinib for the inhibition of BTKE41K and BTKE41K/C481S obtained by nonlinear curve fitting are shown above the graph in nm. F, COS-7 cells were transfected as indicated with 1.33 μg/well of vector encoding WT PLCγ2 (γ2WT) and 0.66 μg/well of empty vector (Control) or vector encoding BTKWT or BTKC481S. Twenty four hours after transfection, the cells were incubated for 18 h, as indicated with either solvent (0.1% (v/v) DMSO), acalabrutinib (1 μm), or vecabrutinib (1 μm). Cells were lysed, and immunoblotting was performed as indicated using an antibody reactive against the c-Myc epitope present on WT PLCγ2, antibody reactive against BTK, antibody reactive against β-actin, antibody reactive against phosphorylated tyrosine on PLCγ2 at position 759 (pY-PLCγ2), and antibody reactive against phosphorylated tyrosine on BTK at position 223 (pY-BTK). A, C, E, and F show representative results from three independent experiments each as mean values of three technical replicates.
Figure 3.
Activation of PLCγ2S707Y by catalytically-inert BTK is not mediated by augmented PLC substrate availability. A, COS-7 cells were transfected as indicated with 2 μg/well of empty vector or 0.5 μg/well of vector encoding WT BTK (WT) or BTK variants K430R, E41K, or E41K/K430R. Twenty four hours after transfection, the cells were incubated for 18 h with myo-[2-3H]inositol. Then, the amounts of radioactively labeled PtdIns (PI), PtdInsP (PIP), and PtdInsP2 (PIP2) in the intact COS-7 cells were determined by TLC analysis as described under “Experimental procedures.” Inset, cells from one well each were lysed and subjected to SDS-PAGE. Subsequent immunoblotting was performed using an antibody reactive against BTK or antibody reactive against β-actin. B, COS-7 cells were transfected as indicated with 500 ng of empty vector (Co.) or increasing amounts (15, 50, 150, or 500 ng) of vector encoding WT PLCγ1 (γ1WT), PLCγ1S729Y (γ1S729Y), WT PLCγ2 (γ2WT), or PLCγ2S707Y (γ2S707Y). C, COS-7 cells were transfected as indicated with 50 ng/well of vectors as described in B, and 100 ng/well of vector encoding WT BTK (WT) or BTK variants K430R, E41K, or E41K/K430R. Analysis of inositol phosphate formation was done as in Fig. 1. D, cells from one well each functionally analyzed in C were washed with 0.2 ml of Dulbecco's PBS and then lysed by addition of 100 μl of SDS-PAGE sample preparation buffer. Aliquots of the samples were subjected to SDS-PAGE, and immunoblotting was performed using an antibody reactive against the c-Myc epitope present on WT PLCγ1, WT PLCγ2, PLCγ1S729Y, and PLCγ2S707Y, antibody reactive against BTK, or antibody reactive against β-actin. A–C show representative results from three independent experiments each as mean values of three technical replicates.
Fig. 1C shows the dependence of WT versus S707Y variant PLCγ2 activity on increasing expression levels of WT versus E41K variant BTK after correction of inositol phosphate formation for background activity observed in the absence of exogenous PLCγ enzyme. In contrast to WT PLCγ2 activity, which was unaffected by WT BTK even at the highest expression level, PLCγ2S707Y variant activity was markedly stimulated by WT BTK in a concentration-dependent fashion. Furthermore, increased expression of BTKE41K caused striking increases in the activities of both WT and S707Y variant PLCγ2, although the variant was severalfold more sensitive to BTKE41K. The E41K mutation also rendered BTK more potent in activating PLCγ2S707Y (cf. Fig. 1C, left versus right panel). There were no differences in protein expression that would explain the functional differences shown in Fig. 1C (cf. Fig. 1D).
To determine whether the productive interaction between WT PLCγ2 and BTKE41K (Fig. 1C, right panel) was contingent on the protein-tyrosine kinase activity of BTKE41K, the effects of BTK protein-tyrosine kinase inhibitors, ibrutinib, acalabrutinib, and vecabrutinib, were investigated (Fig. 1E). In addition, the effect of the BTK C481S mutation was tested by incorporating this mutation into a BTKE41K/C481S compound variant. The C481S mutation is known to cause resistance to the two irreversible covalent BTK inhibitors, ibrutinib and acalabrutib, with limited, if any, impact on vecabrutinib activity, a novel noncovalent and reversible BTK inhibitor (44). Fig. 1E shows that both the E41K single and the E41K/C481S double variants caused a similar ∼38-fold increase in PLCγ2 activity. All three drugs showed potent inhibition of PLCγ2 activity in the E41K single variant cells. However, ibrutinib and acalabrutinib reduced inositol phosphate formation by only 50% in PLCγ2 and BTKE41K/C481S cells. Estimated IC50 values of these covalent inhibitors were ∼3530-fold (ibrutinib) and 410-fold (acalabrutinib) higher in cells expressing the BTK C481S acquired resistance mutation than in cells without this mutation. In contrast, this difference was only about 4.8-fold for vecabrutinib, which does not require BTK Cys-481 interaction for activity. We also tested the effects of acalabrutinib and vecabrutinib at a single concentration, 1 μm, on protein tyrosine phosphorylation of WT PLCγ2 and either WT BTK or its C481S variant. Fig. 1F shows that acalabrutinib was capable of substantially reducing both the autophosphorylation of WT BTK and the BTK-mediated phosphorylation of PLCγ2, but not of BTKC481S and PLCγ2, respectively, whereas vecabrutinib was strongly inhibitory in the presence of either WT or C481S variant BTK together with PLCγ2. Collectively, the results shown in Fig. 1, E and F, clearly support the view that activation of WT PLCγ2 by BTKE41K in the reconstituted systems requires the tyrosine kinase activity of BTK.
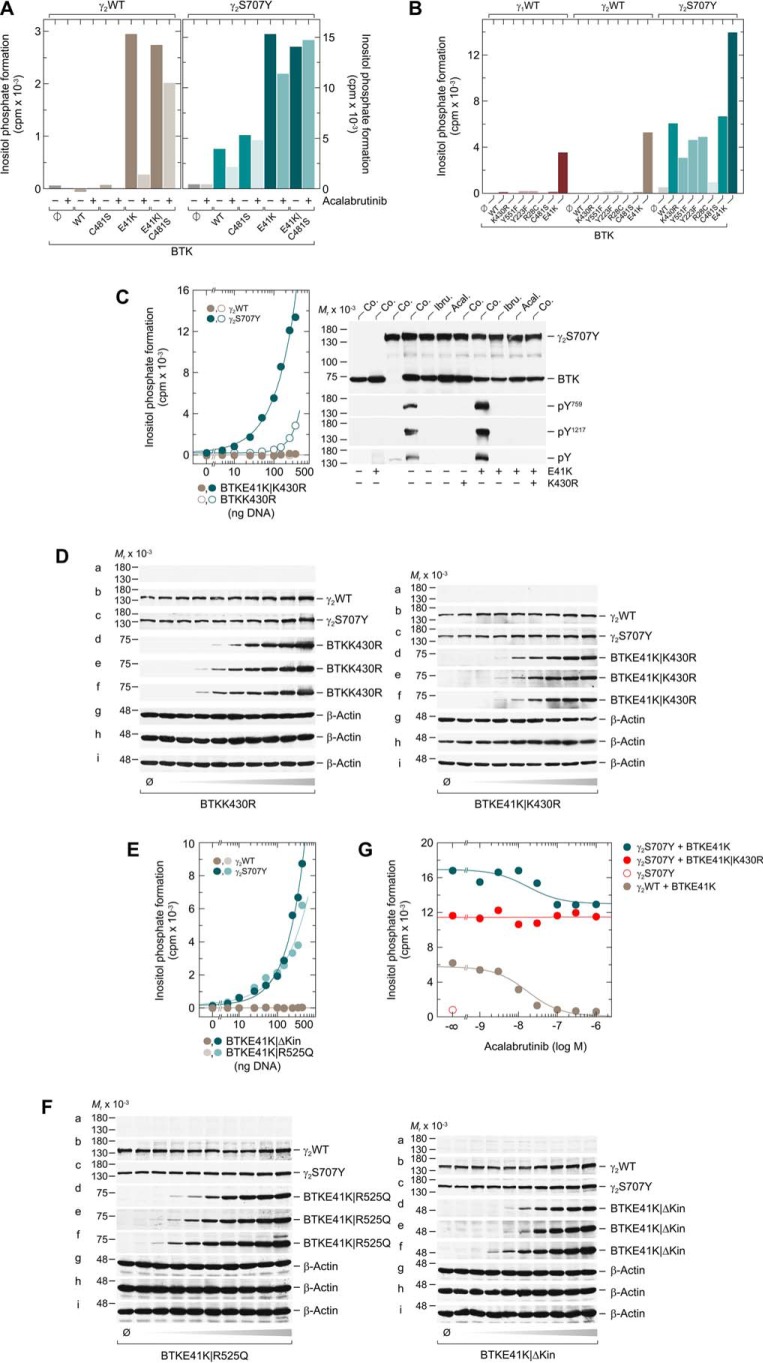
PLCγ2S707Y is stimulated by catalytically-inactive BTK independently of tyrosine phosphorylation and increased PtdInsP2 substrate supply
To interrogate the pharmacology of the BTK-mediated activation of WT and S707Y variant PLCγ2, both PLCγ2 variants were coexpressed with WT or variant BTK to examine the effects of 1 μm acalabrutinib on inositol phosphate formation (Fig. 2A). Acalabrutinib caused an almost complete loss of the PLCγ2 activity in BTKE41K cells but, as expected, had a much lower impact in BTKE41K/C481S cells. Surprisingly, acalabrutinib was much less effective in the context of PLCγ2S707Y-expressing BTKE41K cells, showing only ∼25% inhibition. Acalabrutinib had no activity at all in the PLCγ2S707Y-BTKE41K/C481S cells. Several point variants of BTK were coexpressed with PLCγ2S707Y to further investigate the functional differences between WT and S707Y variant PLCγ2 shown in Fig. 2A and to also extend this analysis to WT PLCγ1 (Fig. 2B). Point mutations of BTK, Y223F, or Y551F, which prevent tyrosine phosphorylation of BTK in cis or in trans by upstream protein kinases, and the covalent inhibitor binding site mutation C481S had only small, if any, effects on the ability of WT BTK to mediate PLCγ2S707Y activation. As expected, modulating the plasma membrane interaction through either inhibitory (R28C) or stimulatory (E41K) mutations caused considerable decreases and increases, respectively, in the reconstitutive activity of BTK. For both WT PLCγ1 and PLCγ2, enzyme activation was only observed for the E41K variant of BTK. Most interestingly, however, when BTK protein-tyrosine kinase activity was blocked by the K430R mutation, the BTK-mediated stimulation of PLCγ2S707Y was only reduced by ∼50%. This result, together with those shown in Fig. 2A, right panel, raised the distinct possibility that PLCγ2S707Y is sensitive to activation by BTK even in the absence of its tyrosine kinase activity.
Figure 2.
In contrast to the WT enzyme, the PLCγ2S707Y variant is activated by catalytically-inert BTK. A, COS-7 cells were transfected as indicated with 50 ng/well of vector encoding WT PLCγ2 (γ2WT) (left panel) or PLCγ2S707Y (γ2S707Y) (right panel) and 150 ng/well of empty vector (φ) or vector encoding WT BTK (WT) or the BTK variants C481S, E41K, or E41K/C481S. Twenty four hours after transfection, the cells were incubated for 18 h with myo-[2-3H]inositol and either solvent (0.1% (v/v) DMSO) (−) or 1 μm acalabrutinib (+) prior to determination of inositol phosphate formation. B, COS-7 cells were transfected as indicated with 50 ng/well of vector encoding WT PLCγ1 (γ1WT), WT PLCγ2 (γ2WT), or PLCγ2S707Y (γ2S707Y) and 150 ng/well of vector encoding WT BTK (WT) or the BTK variants K430R, Y551F, Y223F, R28C, C481S, or E41K. C, left panel, COS-7 cells were transfected as indicated with 50 ng/well of vector encoding WT PLCγ2 (γ2WT) or PLCγ2S707Y (γ2S707Y) and increasing amounts (0–450 ng/well) of vector encoding BTKE41K/K430R or BTKK430R. Right panel, COS-7 cells were transfected as indicated with 1.33 μg/well of vector encoding PLCγ2S707Y (γ2S707Y) and 0.66 μg/well of vector encoding WT BTK or BTK harboring the E41K or K430R mutations. Twenty four hours after transfection, the cells were incubated for 18 h with either solvent (Co.), 1 μm ibrutinib (Ibru.), or 1 μm acalabrutinib (Acal.). The cells were lysed, and immunoblotting was performed as indicated using an antibody reactive against the c-Myc epitope present on PLCγ2S707Y, antibody reactive against BTK, antibody reactive against phosphorylated tyrosine on PLCγ2 at position 759 (pY759), antibody reactive against phosphorylated tyrosine on PLCγ2 at position 1217 (pY1217), or antibody detecting tyrosine phosphorylated proteins in all species (pY). D, cells from one well each functionally analyzed in C were washed with 0.2 ml of Dulbecco's PBS and then lysed by addition of 100 μl of SDS-PAGE sample preparation buffer. Aliquots of the samples were subjected to SDS-PAGE, and immunoblotting was performed using an antibody reactive against the c-Myc epitope present on WT PLCγ2 and PLCγ2S707Y (a–c), antibody reactive against BTK (d–f), or antibody reactive against β-actin (g--i). E, COS-7 cells were transfected as indicated with 50 ng/well of vector encoding WT PLCγ2 (γ2WT) or PLCγ2S707Y (γ2S707Y) and increasing amounts (0–450 ng/well) of vector encoding BTKE41K/R525Q or BTKE41K/ΔKinase (BTKE41K/ΔKin). F, cells from one well each functionally analyzed in E were washed with 0.2 ml of Dulbecco's PBS and then lysed by addition of 100 μl of SDS-PAGE sample preparation buffer. Aliquots of the samples were subjected to SDS-PAGE and immunoblotting was performed using an antibody reactive against the c-Myc epitope present on WT PLCγ2 and PLCγ2S707Y (a–c), antibody reactive against BTK (d–f), or antibody reactive against β-actin (g–i). G, COS-7 cells were transfected as indicated with 150 ng/well of vector encoding WT PLCγ2 (γ2WT), 50 ng/well of vector encoding PLCγ2S707Y (γ2S707Y), and 100 ng/well of vector encoding BTKE41K or BTKE41K/K430R. Analysis of inositol phosphate formation and treatment with BTK inhibitor was done as in Fig. 1. A–C, E, and G show representative results from three independent experiments each as mean values of three technical replicates.
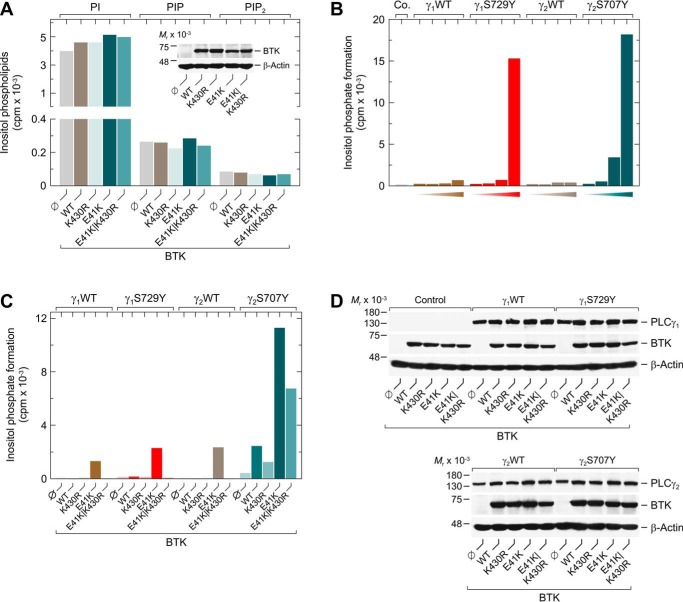
To further challenge this hypothesis, WT and S707Y variant PLCγ2 were coexpressed with increasing concentrations of BTKK430R and its membrane-targeted variant BTKE41K/K430R (Fig. 2C, left panel). Although the activity of WT PLCγ2 remained unaffected by the two BTK variants, inositol phosphate formation of the S707Y variant was markedly and concentration-dependently increased. Membrane targeting of even the catalytically incompetent BTK caused an almost 10-fold shift to the left of the concentration–response relationship. The functional effects observed in Fig. 2C were unrelated to changes in PLCγ2 expression (Fig. 2D). Analysis of protein tyrosine phosphorylation showed that the two BTK variants, K430R and E41K/K430R, similar to their catalytically-competent counterparts, WT and E41K, in the presence of ibrutinib or acalabrutinib were unable to mediate phosphorylation of the PLCγ2 residues Tyr-759 or Tyr-1217, i.e. known BTK phosphorylation sites, or other PLCγ2 tyrosine residues (Fig. 2C, right panel, subpanels labeled pY759 and pY1217, and pY, respectively). Importantly, stimulation of PLCγ2S707Y, but not WT PLCγ2, was not confined to the catalytically-inactive variant K430R and was also observed for another variant devoid of catalytic activity, R525Q (13). This stimulation was even seen for a BTK deletion variant (Δ amino acids 402–659) lacking the protein-tyrosine kinase domain (Fig. 2E). Fig. 2F shows that the effects of BTK variants on WT and variant PLCγ2 activity observed in Fig. 2E were not explained by differences in protein expression. We then determined how the differential sensitivity of the variant PLCγ2S707Y to the actions of both the catalytic and the noncatalytic functions of BTK translates into the concentration dependence of acalabrutinib-mediated BTK inhibition. It is clear from these results that the inhibitor did not affect the strikingly enhanced inositol phosphate formation in the presence of BTKE41K/K430R and PLCγ2S707Y (Fig. 2G). Furthermore, acalabrutinib reduced the activities of the BTKE41K/WT PLCγ2 and BTKE41K/PLCγ2S707Y pairs with very similar, if not identical, IC50 values (∼20 nm), but did so in a markedly different extent. Although enhanced activity was almost fully abrogated in the presence of WT PLCγ2, it was only partially reduced (∼23%) in the presence of PLCγ2S707Y, most interestingly to a level very similar to the one observed for catalytically-incompetent BTKE41K/K430R and PLCγ2S707Y. Notably, this level of activity, which was unaffected by acalabrutinib over the whole range of concentrations, was still ∼14-fold higher than that observed in the presence of PLCγ2S707Y alone. Therefore, the acalabrutinib resistance of the BTKE41K/PLCγ2S707Y pair is very likely based on the BTK variant exerting tyrosine phosphorylation–independent, stimulatory effects on PLCγ2S707Y.
To address the possibility that the stimulatory effect of membrane-targeted, catalytically-inert BTKE41K/K430R on inositol phosphate formation by PLCγ2S707Y is due to enhanced supply of the enzyme phospholipid substrate directly, the effects of WT as well as E41K, K430R, and E41K/K430R variant BTK on the abundance of the inositol phospholipids, PtdIns, PtdInsP, and PtdInsP2, in COS-7 cells were examined (Fig. 3A). No effect of any of the BTK variants on the abundance of these phospholipids was observed. To further examine potential effects of BTK on substrate availability rather than enzyme activity of PLCγ2, a close congener of PLCγ2S707Y, PLCγ1S729Y, which shares enhanced activity in intact cells with its PLCγ2 counterpart (Fig. 3B), was coexpressed with WT as well as variant BTK and functionally compared with PLCγ2S707Y. As shown in Fig. 3C, there was a marked difference between the two PLCγ2 variants vis à vis BTK, in that PLCγ1S729Y largely behaved like WT PLCγ1 and PLCγ2 and displayed a functional phenotype very different from that of PLCγ2S707Y, which was not due to differences in protein expression (Fig. 3D). These results are difficult, if not impossible, to explain by an effect of BTK on substrate availability to the two PLCγ variants in the reconstituted system. Interestingly, the PLCγ2-specific functional phenotype is not restricted to the S707Y variant. Instead, it is also observed for many of the PLCγ2 variants that have previously been associated with ibrutinib resistance of CLL cells: D334H (catalytic subdomain X), R665W, S707F, and S707P (all C-terminal SH2 domain), L845F (C-terminal half of split PH domain), D993H (catalytic subdomain Y), and M1141R (C2 domain) (34, 45, 46). All listed variants were found to be sensitive to stimulation by catalytically-inert BTK (activity ratios BTKE41K/K430R:BTKE41K ≥43.5%; Table 1). Notably, the highest activity ratios, maximal sensitivities for catalytically-inactive BTK, with no difference between BTKE41K and BTKE41K/K430R were observed for the PLCγ2 variants L845F and M1141R. Note that there are no differences in variant PLCγ2 protein expression in the presence of BTKE41K or BTKE41K/K430R. Therefore, the differences in activity ratios BTKE41K/K430R:BTKE41K analyzed for each row of data sets cannot be explained by differences in PLCγ2 and/or BTK protein expression (Fig. S4).
Table 1.
Activation of PLCγ2 by catalytically-inert BTK is not limited to PLCγ2S707Y but is also observed for other PLCγ2 variants mediating ibrutinib resistance in CLL cells
COS-7 cells were transfected as indicated with 150 ng/well vector encoding WT PLCγ2 (PLCγ2WT); 50 ng/well vector encoding the PLCγ2 variants D334H, R665W, S707Y, S707F, L845F, D993H, and M1141R; 15 ng of vector encoding PLCγ2S707P; and 100 ng/well of vector encoding BTKWT or BTK variants K430R, E41K, or E41K/K430R. The ratios of the PLC activities determined in the presence of BTKE41K/K430R and BTKE41K are specified in the right column. The data depicted in the table show representative results from three independent experiments each as mean values of three technical replicates.
| BTK | Inositol phosphate formation |
Ratio (E41K/K430R:E41K) | ||||
|---|---|---|---|---|---|---|
| Control | WT | K430R | E41K | E41K/K430R | ||
| cpm | % | |||||
| PLCγ2WT | 67 | 86 | 67 | 2358 | 121 | 5.2 |
| PLCγ2D334H | 40 | 1097 | 236 | 10089 | 4390 | 43.5 |
| PLCγ2R665W | 42 | 374 | 138 | 4420 | 1956 | 44.3 |
| PLCγ2S707Y | 231 | 1998 | 713 | 17442 | 8132 | 46.6 |
| PLCγ2S707F | 464 | 2561 | 1488 | 20150 | 11215 | 55.7 |
| PLCγ2S707P | 728 | 2991 | 2563 | 17734 | 11584 | 65.3 |
| PLCγ2L845F | 219 | 1660 | 455 | 8937 | 9668 | 108.2 |
| PLCγ2D993H | 702 | 3604 | 1888 | 10808 | 7892 | 73.0 |
| PLCγ2M1141R | 1966 | 5858 | 5395 | 14268 | 14187 | 99.4 |
Functional phenotype of PLCγ2S707Y vis à vis BTK is instigated by mutational preactivation of PLCγ2
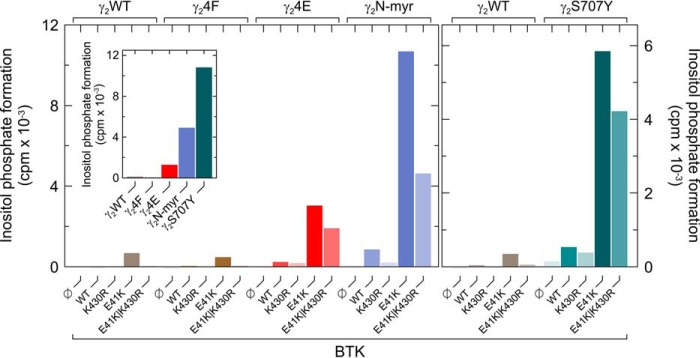
To determine whether the enhanced sensitivity of PLCγ2S707Y to catalytically-inert BTK is related to its augmented basal activity in intact cells, PLCγ2S707Y was compared with two other variants of PLCγ2 also showing this property, the phosphomimetic variant PLCγ24E, carrying glutamic acid residues in all four canonical positions of BTK-mediated tyrosine phosphorylation, Tyr-753, Tyr-759, Tyr-1197, and Tyr-1217, and the N-myr–PLCγ2 carrying an artificial N-terminal signal for myristoylation. The inset of Fig. 4 shows that the two variants caused specific enhancements of inositol phosphate formation in intact cells (PLCγ24F < PLCγ2 <<< PLCγ24E < N-myr-PLCγ2 < PLCγ2S707Y). Importantly, a very similar, if not identical, order was observed for the sensitivity of the five PLCγ2 variants to membrane-targeted BTKE41K, both catalytically-competent and -incompetent. Hence, preactivation of PLCγ2 appears to predispose the enzyme to further activation by catalytically-inert BTK.
Figure 4.
Preactivation of PLCγ2 sensitizes the enzyme to further activation by catalytically-inert BTK. COS-7 cells were transfected as indicated with 50 ng/well of vector encoding WT PLCγ2 (γ2WT), PLCγ24Y→F (γ24F), PLCγ24Y→E (γ24E), PLCγ2N-myr (γ2N-myr) (left panel), or PLCγ2S707Y (γ2S707Y) (right panel) and 100 ng/well of vector encoding WT BTK (WT) or BTK variants K430R, E41K, or E41K/K430R. Inset, COS-7 cells were transfected as indicated with 500 ng/well of vector to examine the influence of the PLCγ2 mutations on basal inositol phosphate formation in intact transfected cells. Analysis of inositol phosphate formation was done as in Fig. 1. The graph shows representative results from three independent experiments as mean values of three technical replicates.
Catalytically-inert BTK causes WT and S707Y variant to differentially interact with the plasma membrane
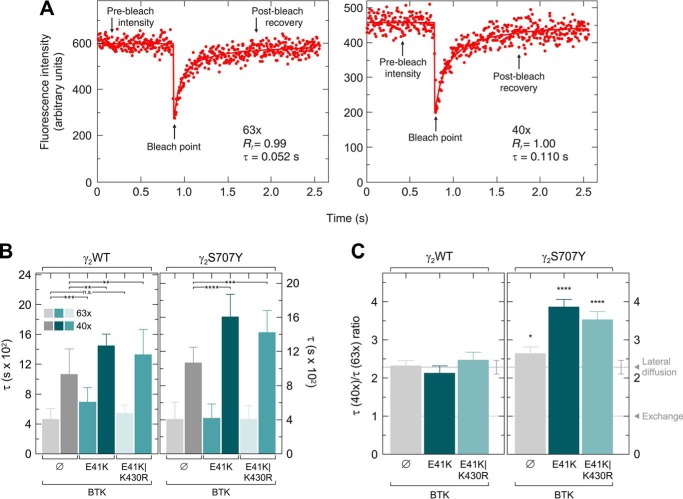
Next, fluorescence recovery after photobleaching (FRAP) static laser microscopy, employing a fixed-localization Gaussian beam and photon counting to follow fast recoveries (see under “Experimental procedures”) (47), was used to investigate the effects of BTKE41K and BTKE41K/K430R on the membrane interaction and diffusion of GFP-tagged WT and S707Y variant PLCγ2 in intact cells. A significant fraction of PLCγ2 is cytoplasmic and can therefore recover in FRAP by either lateral diffusion along the membrane surface or by exchange between membrane-associated and cytoplasmic populations. To evaluate the relative contribution of lateral diffusion and exchange (where the faster process contributes more), we previously developed the FRAP beam-size test (48, 49). This method is based on performing the FRAP experiment using two different laser beam sizes; if the fluorescence recovery is by exchange, the characteristic recovery time (τ) reflects the chemical relaxation time, which is independent of the beam size (48). Under such conditions, the ratio between the τ values measured with the two beam sizes generated in the current experiments by the two objectives equals 1 (τ(×40)/τ(×63) = 1). For lateral diffusion, τ is the characteristic diffusion time τD, which is proportional to the area bleached by the beam (τD = ω2/4D, where ω is the Gaussian radius of the beam, and D is the lateral diffusion coefficient). The results obtained with WT PLCγ2 (Fig. 5) are in line with recovery by lateral diffusion, because the τ(×40)/τ(×63) ratio (Fig. 5C, leftmost bar) is not significantly different from 2.28, which is the ratio between the beam sizes generated with these two objectives. However, the D value calculated from the FRAP studies, 3.1 μm2/s, is much faster than the D value of a lipid probe DiIC16(3) (1,1′-dihexadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) in the plasma membrane of the same cells (1 μm2/s) (50). This suggests that PLCγ2 membrane interactions are characterized by “gliding” along the inner membrane surface (i.e. it dissociates from the membrane but re-associates before it can diffuse away into the cytoplasm). The current results are similar to our earlier FRAP measurements of WT PLCγ2 with the smaller beam size (×63) (49), but we find a somewhat slower recovery with the beam generated by the ×40 objective. Slower recovery suggests stronger interactions with the membrane (because free GFP in the cytosol recovers at a rate faster by at least an order of magnitude). This difference may reflect a higher level of RAC activity in the current measurements, because overexpression of constitutively-active RAC2 increased to a similar level the τ(×40) of WT PLCγ2 (49). The effects of coexpressing BTKE41K or BTKE41K/K430R on the membrane interactions of WT PLCγ2–GFP are depicted in Fig. 5B, left panel. Both BTK variants induced increases in the τ(×40) values of WT PLCγ2–GFP and in the τ(×63) values; here, the effect of BTKE41K/K430R was weaker. Most importantly, neither of the BTK variants significantly affected the τ(×40)/τ(×63) ratio of WT PLCγ2–GFP (Fig. 5C, left), which remained only insignificantly different from the beam-size ratio throughout (2.28). This suggests that the mechanism of the FRAP remains by gliding, albeit with somewhat enhanced interactions with the membrane, as suggested by the larger τ values. We then compared the membrane interactions of PLCγ2S707Y–GFP with those of the WT enzyme (Fig. 5B, right panel). The FRAP results for this variant in the absence of BTK resembled those obtained for WT PLCγ2–GFP, suggesting recovery by gliding lateral diffusion. However, expression of BTKE41K or BTKE41K/K430R had a markedly different effect than in the case of WT PLCγ2. Here, only the τ(×40) values of PLCγ2S707Y–GFP became larger (slower recovery kinetics), resulting in a highly-significant deviation of the τ(×40)/τ(×63) ratio from the 2.28 beam-size ratio expected for recovery by lateral diffusion (Fig. 5C, right panel). The τ ratio dramatically increased, to 3.6–3.8, suggesting that the recovery becomes slower than expected for lateral diffusion with increasing distances. Such a phenomenon, termed subdiffusion or hop diffusion, may arise due to sensing of membrane heterogeneity by the diffusing molecule, which may reflect phase separation, potentially assisted by the cytoskeleton (51, 52). This indicates that the interactions of the variant PLCγ2S707Y with membrane heterogeneities, which give rise to the anomalous diffusion, are enhanced by BTK to a much higher degree than those of WT PLCγ2. Of note, BTKE41K and BTKE41K/K430R had a similar effect, indicating that the effect on PLCγ2S707Y–GFP arises due to interactions of BTK with the enzyme, but it does not require BTK tyrosine kinase activity.
Figure 5.
BTK mediates subdiffusion of PLCγ2S707Y but not of WT PLCγ2. COS-7 cells were transfected with a vector encoding PLCγ2–GFP (γ2WT) or PLCγ2S707Y–GFP (γ2S707Y) together with either empty vector or a vector encoding BTKE41K or BTKE41K/K430R. FRAP studies were conducted 24–26 h post-transfection at 22 °C. A, typical FRAP curves obtained using ×63 (smaller beam size) or ×40 (larger beam size) objectives. The time segments showing pre-bleach fluorescence intensity, the bleach point, and post-bleach recovery are indicated by arrows. Solid lines show the best fit of a nonlinear regression analysis (102), with the resulting τ and mobile fraction (Rf) values. B and C, FRAP beam-size analysis. Bars are means ± S.D. of multiple measurements. The τ data depicted in B are also used to calculate the τ ratios shown in C. The number of measurements for each condition was as follows: (i) PLCγ2, ×63 objective, n = 28; (ii) ×40 objective, n = 62; (iii) PLCγ2 with BTKE41K, ×63, n = 24; (iv) ×40, n = 22; (v) PLCγ2 with BTKE41K/K430R, ×63, n = 27; (vi) ×40, n = 29; (vii) PLCγ2S707Y–GFP, ×63, n = 30; (viii) ×40, n = 80; (ix) PLCγ2S707Y–GFP with BTKE41K, ×63, n = 63; (x) ×40, n = 58; (xi) PLCγ2S707Y–GFP with BTKE41K/K430R, ×63, n = 58; and (xii) ×40, n = 51. The studies employed ×40 and ×63 objectives, whose beam size measurements (n = 59) yielded a ω2(×40)/ω2(×63) ratio of 2.28 ± 0.15. Thus, a similar τ(×40)/τ(×63) ratio is expected for FRAP by lateral diffusion. A τ ratio of 1 is expected for recovery by exchange (48). The Rf values were high in all cases (0.98–1.00) and are given in Table S1. B displays the τ values, and C displays the τ(×40)/τ(×63) ratios. B, asterisks indicate significant differences between the sample without BTK and the samples with the two BTK variants; the comparison was done separately for the τ(×63) values and the τ(×40) values, comparing the same PLCγ2–GFP protein without BTK and with one of the BTK variants (n.s., not significant; **, p < 5 × 10−3; ***, p < 10−5; ****, p < 10−10; one-way ANOVA and Bonferroni post hoc test). C, bootstrap analysis (see under “Experimental procedures”; 1000 bootstrap resampling values for both τ and beam-size values) shows that the τ ratio of PLCγ2 (WT or the S707Y variant) in the absence of BTK fits FRAP by lateral diffusion. In the presence of BTK (either E41K or E41K/K430R), the τ ratio of PLCγ2S707Y, but not of WT PLCγ2, becomes significantly higher than the 2.28 beam size ratio (*, p < 0.05; ****, p < 10−20; bootstrap analysis and Student's two-tailed t test). The source data for the FRAP studies containing statistics (B and C) are provided in Table S1.
Activated RAC2 allows catalytically-inert BTK to activate WT PLCγ2
The previous demonstration that PLCγ2 variants mediating ibrutinib resistance in CLL, PLCγ2R665W, and PLCγ2L845F are hypersensitive to activated RAC endogenously present in COS-7 cells (38) prompted us to determine the contribution of RAC to the effects of catalytically-competent and -incompetent BTK on PLCγ2S707Y. Fig. 6A shows that blocking the functional interaction of PLCγ2S707Y with activated RAC in variant PLCγ2–F897Q caused conspicuous losses of inositol phosphate formation, which were particularly striking for the catalytically-inert BTK variants K430R (∼85%) and E41K/K430R (∼58%). Because the F897Q mutation, located in the C-terminal half of the split PH domain of PLCγ2, is known to neither affect the overall three-dimensional structure of the PH domain nor the catalytic activity of PLCγ2 in vitro (49, 53, 54), these results indicate that activated RAC may facilitate BTK-mediated PLCγ2 stimulation, in particular through the noncatalytic functions of BTK. Fig. 6B shows that the cellular abundance of exogenous, activated RAC2G12 exerts impressive effects on the activation of both WT and S707Y variant PLCγ2 by catalytically-inert BTKE41K/K430R. Thus, PLCγ2S707Y not only shows higher activity in the presence of RAC2G12V and catalytically-inactive BTKE41K/K430R, but also reveals a higher sensitivity to RAC2G12V in the presence of the latter. The basal activities of the constitutively-active PLC variants PLCγ2ΔSH (lacking the complete autoinhibitory SH2–SH2–SH3 tandem (55)) and PLCδ1Δ44 are shown in Fig. 6B, top panel, to demonstrate that the plateau observed for PLCγ2S707Y in the presence of BTKE41K/K430R at high RAC2G12V levels is not limited due to exhaustion of the enzyme substrate PtdInsP2. Increasing concentrations of RAC2G12V had no effect on the expression of PLCγ2 and BTKE41K/K430R (Fig. 6C). Strikingly, however, RAC2G12V appeared to unmask a conspicuous sensitivity of WT PLCγ2 to stimulation by the tyrosine phosphorylation–independent functions of BTK. This strikingly permissive effect of RAC is substantiated by the results shown in Fig. 6D, demonstrating insensitivity of PLCγ2 to BTKE41K/K430R in the absence of RAC2G12V, but with an ∼25-fold stimulation of inositol phosphate formation in its presence. The latter results indicate that coactivation of BTK and RAC by upstream regulators may render even WT PLCγ2 sensitive to the noncatalytic functions of BTK and, by extension, render WT BTK and WT PLCγ2 at least partially resistant to active-site inhibitors of BTK.
Figure 6.
Stimulation via RAC2 augments the responsiveness of the PLCγ2 enzyme to catalytically-inert BTK. A, COS-7 cells were transfected as indicated with 50 ng/well of vector encoding WT PLCγ2 (γ2WT), PLCγ2S707Y (γ2S707Y), and PLCγ2S707Y containing a Phe → Gln mutation at position 897, providing resistance toward RAC2 stimulation (F897Q) and 100 ng/well of vector encoding WT BTK (WT) or BTK variants K430R, E41K, or E41K/K430R. B, lower panel, COS-7 cells were transfected as indicated with 50 ng/well of vector encoding WT PLCγ2 (γ2WT) and BTKE41K/K430R or 25 ng/well of vector encoding PLCγ2S707Y (γ2S707Y) or increasing amounts (0–100 ng/well) of vector encoding RAC2G12V. Upper panel, COS-7 cells were transfected as indicated with 250 ng/well of vector encoding PLCδ1Δ44 (δ1Δ44) or PLCγ2ΔSH (γ2ΔSH). The ED50 values of vector encoding RAC2G12V for the stimulation of WT and variant PLCγ2 activity obtained by nonlinear curve fitting are shown above the graphs in ng/well. C, cells from one well each functionally analyzed in B were washed with 0.2 ml of Dulbecco's PBS and then lysed by addition of 100 μl of SDS-PAGE sample preparation buffer. Aliquots of the samples were subjected to SDS-PAGE, and immunoblotting was performed using an antibody reactive against the c-Myc epitope (a–b) present on WT PLCγ2 and PLCγ2S707Y, antibody reactive against BTK (c–d), antibody reactive against RAC2 (e–f), or antibody reactive against β-actin (g–h). D, COS-7 cells were transfected as indicated with 50 ng/well of vector encoding WT PLCγ2 (γ2WT) and RAC2G12V or increasing amounts (0–350 ng/well) of vector encoding BTKE41K/K430R. Analysis of inositol phosphate formation was done as in Fig. 1. A, B, and D show representative results from three independent experiments each as mean values of three technical replicates.
The ability of catalytically-inert BTK to mediate BCR-dependent Ca2+ mobilization in intact DT40 B-lymphoma cells is conditional on the degree of BCR stimulation
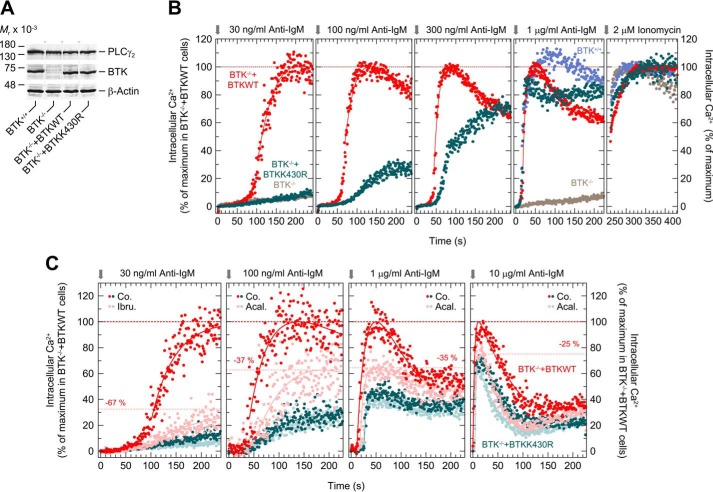
To investigate the functional significance of the catalytic functions of BTK, the WT enzyme and its catalytically-inactive variant BTKK430R were stably expressed in BTK-deficient DT40 B-lymphoma cells. Fig. 7A shows that BTK−/− cells lack immunoreactive BTK protein and that the two reconstituted cells lines, BTK−/−+BTKWT and BTK−/− + BTKK430R, express BTK at levels very similar, if not identical, to each other and to unmodified DT40 BTK+/+ cells. Fig. 7, B and C, shows the changes of intracellular free Ca2+ in response to increasing concentrations of anti-IgM, as determined by flow cytometry followed by statistical analysis of the results to allow for direct, quantitative comparison between the different experimental conditions. The most-right panel of Fig. 7B shows how the maximal Ca2+ concentration in response to 2 μm ionomycin was used to correct the traces shown in the other panels for differences in fluorescent dye loading. The corrected traces were then normalized to the maximal Ca2+ concentrations observed in BTK−/− + BTKWT cells (100%). Fig. 7B shows that there was little, if any, effect of anti-IgM in BTK−/− + BTKK430R cells compared with BTK−/− cells upon addition of anti-IgM at the lowest concentration, 30 ng/ml. Raising the concentration of anti-IgM over 100 and 300 ng/ml to 1000 ng/ml led to a gradual, distinct increase in the ability of the receptor ligand to increase free Ca2+. In addition, there was a progressive loss of the differences in the lag time of the Ca2+ responses from about ∼35 s at 100 ng/ml anti-IgM and ∼20 s at 300 ng/ml, such that there were only relatively minor differences in the kinetics of the Ca2+ responses at 1000 ng/ml anti-IgM between the two genetically-modified cell lines and unmodified BTK+/+ cells, both in terms of the lag time and the maximal Ca2+ concentrations. The results show that the ability of catalytically-inert BTK to mediate BCR-mediated increases in intracellular Ca2+ is conditional on the concentration of the BCR ligand, anti-IgM, ranging from no to very sizable responses between 30 and 1000 ng/ml of anti-IgM.
Figure 7.
Ability of catalytically-inert BTK to mediate intracellular Ca2+ mobilization depends on the level of BCR stimulation. A, DT40 cells (1.5 × 106) were lysed as described under “Experimental procedures,” and aliquots of the samples were subjected to SDS-PAGE, and immunoblotting was performed using an antibody reactive against both human and chicken BTK, antibody reactive against human and chicken PLCγ2, or antibody reactive against β-actin. One empty lane was present between each of the four lanes from the same immunoblot with the same length of exposure time. These lanes were removed by splicing. The positions of the splice sites are indicated by gray dotted lines and arrowheads. B, WT DT40 cells (BTK+/+), DT40 BTK−/− (BTK−/−), and DT40 BTK−/− cells expressing either human WT (BTK−/−+BTKWT) or human K430R BTK (BTK−/−+BTKK430R) were loaded with Indo-1-AM. After pre-warming to 37 °C, the cells were applied to the fluorescence analyzer and treated with anti-IgM followed by ionomycin as described under “Experimental procedures.” C, DT40 BTK−/− cells expressing either human WT (BTK−/−+BTKWT) or human K430R BTK (BTK−/−+BTKK430R) were either left unstained (BTK−/−+BTKWT) or stained with CFSE (BTK−/−+BTKK430R). After mixing the two populations at a 1:1 ratio, the cells were incubated for 1 h at 37 °C in the presence of either solvent (0.1% (v/v) of DMSO, Co.), or 1 μm ibrutinib (Ibr.), or acalabrutinib (Aca.) followed by washout. Afterward, cells were incubated with indo-1-AM, and the changes in [Ca2+]i were analyzed as described above. The individual points shown in B and C correspond to the times in full seconds versus the medians of all fluorescence ratios representing [Ca2+]i, following their correction for basal fluorescence intensity in the absence of anti-IgM and normalization according to the maximal values reached after addition of 2 μm ionomycin. In PLCγ2−/− DT40 cells expressing WT PLCγ2, type M4 anti-IgM causes BCR-mediated increases of [Ca2+]i at EC50 values between 30 and 50 ng/ml (49). Hence, there were no substantial changes in the [Ca2+]i responses in BTK−/− cells expressing WT BTK when the concentration of anti-IgM was increased to values higher than 30 ng/ml. Specifically, the values were 32.6, 42.9, 35.9, and 28.0% of the maximal Ca2+ concentrations upon addition of ionomycin following the addition of anti-IgM at concentrations of 30, 100, and 300 ng/ml and 1 μg/ml, respectively, in B), and 28.5, 18.0, 32.8, and 45.4% following the addition of anti-IgM at concentrations of 30 and 100 ng/ml and of 1 and 10 μg/ml, respectively, in C).
Because the actions of catalytically-inert BTKK430R are insensitive to catalytic site BTK inhibitors like ibrutinib or acalabrutinib (see above), the relative inhibitory effect of these drugs was tested as a function of the degree of BCR activation by anti-IgM. Fig. 7C, most left panel, shows that ibrutinib (1 μm) caused an ∼67% decrease in the maximum Ca2+ response in cells expressing WT BTK at the lowest anti-IgM concentration, 30 ng/ml, very close to the Ca2+ concentrations observed for cells expressing BTKK430R. In addition, there was a slight inhibitory effect of the drug in the latter cells, presumably due to the known off-target effects of ibrutinib (56). Therefore, acalabrutinib, with less off-target effects than ibrutinib (56), was used. It is evident from the comparison shown in Fig. 7C that, at increasing anti-IgM concentrations, not only was there a gradual increase in the ability of catalytically-inert BTK to reconstitute BCR-mediated Ca2+ increases, as before, but that, at the same time, there was a quantitative loss to BTK catalytic site inhibition, down to only ∼25% in the presence of the maximal anti-IgM concentration tested, 10 μg/ml. In addition, less inhibition at high-ligand concentrations also coincided with a continuous loss of the lag time of the Ca2+ response in BTK−/− + BTKWT cells in the absence versus presence of the BTK inhibitor. In other words, both the inhibitory efficacy and the kinetic effects on [Ca2+]i of BTK catalytic site inhibitors were found to be both conditional on and inversely related to the degree of BCR stimulation.
Discussion
Although the molecular basis of clinical resistance to BTK inhibitors, such as ibrutinib and acalabrutinib, caused by mutations at position Cys-481 of BTK are relatively clear, the mechanism of resistance caused by the other BTK and the PLCG2 mutations is less understood. Although PLCG2 mutations have been suggested to constitute, as a whole, gain-of-function mutations (40, 45, 57), we have previously found that several PLCγ2 variant enzymes carrying mutations in various regions are not constitutively active when assayed in cell-free systems (38, 39). The observation that these PLCγ2 variants are, instead, hypersensitive in intact cells to the Rho GTPase RAC2 led us to suggest that the PLCG2 mutations found in BTK inhibitor–resistant CLL cells may cause a rerouting of the transmembrane signals emanating from BCR and converging on as well as activating PLCγ2 (38, 39). In addition, Liu et al. (40) showed that PLCγ2R665W is sensitive to activation by the proximal tyrosine kinases SYK and LYN even in BTK-deficient chicken DT40 B cells. Even in the presence of BTK, PLCγ2R665W appeared to be resistant to ibrutinib in this system.
Here, we have established a reconstituted system allowing us to monitor the functional interaction of WT and variant forms of BTK and PLCγ2 in intact cells, placing special emphasis on a comparison between WT PLCγ2 and variants known to mediate clinical BTK inhibitor resistance in CLL patients. Although BTK has long been known to mediate protein tyrosine phosphorylation of PLCγ2 following coexpression in cells or cell-free preparations devoid of BLNK (58–60), information on the structural and functional requirements of this interaction to occur and result in enhanced PLCγ2 enzyme activity is limited. This is presumably due to the known requirement of the adaptor protein BLNK to observe BCR-mediated, BTK-dependent activation of PLCγ2 in B cells (61), together with the known difficulties to functionally reconstitute all three components outside the B cells context.
Our findings reveal that functional interaction of BTK and PLCγ2 in a non-B-cell environment is conditional on the introduction of mutations into the two coding regions and on the level of protein expression. Thus, although there was no productive interaction between the two WT components even at the highest expression level of BTK, mutational alteration of either PLCγ2 (S707Y) or BTK (E41K), or both, caused profound increases in PLCγ2 activity. The results obtained on WT PLCγ2 are consistent with the view that one of the roles of BLNK is to provide membrane targeting to PLCγ2 to make it more susceptible to BTK-mediated protein tyrosine phosphorylation. In fact, a membrane-targeted variant of PLCγ2 was sensitive to BCR stimulation even in the absence of BLNK (62, 63). According to this view, membrane targeting of BTK, such as introduced by the E41K mutation, would serve the same purpose. Extrapolating this concept to the altered functional properties of PLCγ2S707Y, which is highly sensitive to stimulation by even WT BTK, could indicate that this PLCγ2 variant may also be different from the WT enzyme in its membrane interaction.
The marked sensitivity of the functional BTKE41K–PLCγ2WT interaction to BTK inhibitors of all three generations shows that the protein kinase activity of BTKE41K is an important determinant of this interaction; phosphoblot analysis shows that PLCγ2 is a likely substrate of this activity. However, comparison of PLCγ2WT and PLCγ2S707Y revealed, unexpectedly, a fundamentally different dependence on protein tyrosine phosphorylation of the two PLCγ2 isoforms. Thus, the catalytically-inactive forms of BTKE41K and even BTKWT were clearly capable of causing marked activation of PLCγ2S707Y, but not of PLCγ2WT, in the absence of apparent protein tyrosine phosphorylation of the PLCγ2 variant. Importantly, this activity was also observed for another known catalytically-inactive variant of BTK, BTKE41K/R525Q (13), and even by a deletion variant of BTK lacking its catalytic domain.
In the absence of alternative regulatory pathways between BTK and PLCγ2, as is the case in the cell system employed here for transient functional reconstitution, activation of PLCγ2, both WT and the various point variants analyzed herein, are, on first approximation, expected to be similarly sensitive to inhibition by active-site inhibitors of BTK. However, as shown in Fig. 2, A and G, they are not. PLCγ2S707Y was inhibited by acalabrutinib only to the extent of its activity that depended on the kinase activity of BTKE41K, whereas the remaining activity, which results from BTK catalysis-independent functions, was completely acalabrutinib-resistant. This drug-resistant portion may even be higher for other PLCγ2 point variants with a higher BTK E41K/K430R:E41K ratio, such as the variants L845F or M1141R (Table 1). This mechanism provides an explanation, at least in part, for active-site BTK inhibitor resistance in tumor cells expressing PLCG2 point variants, without a requirement for constitutively-enhanced intrinsic activity, which is consistent with previous studies of the R665W and S707Y PLCγ2 variant proteins in vitro (38, 39).
Preactivation of PLCγ2, regardless whether by phosphomimetic mutations or by membrane targeting at its N terminus (64), rendered the variants hypersensitive to activation by both catalytically-active and -inactive BTK (cf. Fig. 4). This effect was particularly striking for the variant carrying the myristoylation tag of Rous sarcoma virus v-SRC at its N terminus, PLCγ2N-myr, which had a functional phenotype vis à vis BTK, WT, and variants, very similar to PLCγ2S707Y. Importantly, PLCγ1 phosphorylation and Ca2+ release were previously promoted in Xenopus laevis egg extracts by lipid rafts prepared from COS-7 cells expressing the Xenopus SRC protein, suggesting that COS-7 cells contain lipid raft microdomain structures and that SRC kinase is targeted to these structures (65, 66). Hence, the N-terminal SRC-derived myristoylation tag was likely to promote lipid raft targeting of PLCγ2N-myr to COS-7 cell lipid rafts. Membrane-targeting tags of lipid raft–associated proteins FcγRIII (62, 67, 68) or the Src family member LYN (63) have previously been attached to PLCγ2 and found to render BCR-mediated stimulation of PLCγ2 in DT40 cells independent of the adaptor protein BLNK. The similarity of the functional phenotypes vis à vis BTK, WT, as well as variants, between PLCγ2N-myr and PLCγ2S707Y (cf. Fig. 4) and the hop diffusion in the membrane detected by FRAP for PLCγ2S707Y in the presence of BTKE41K or BTKE41K/K430R (cf. Fig. 5), suggests that the latter protein functionally resembles a lipid raft–associated protein, possibly with acquired BLNK independence of activation by BTK. With regard to the FRAP results, an “anchored membrane-protein picket model” has been proposed by Fujiwara et al. (51), in which transmembrane proteins referred to as “pickets” are anchored to the membrane skeleton to act as posts along the membrane skeleton fence. The anchored protein pickets, observed in all examined cell types, including COS cells, were suggested to act as effective diffusion barriers due to steric hindrance as well as circumferential slowing (packing and frictional) effects even for phospholipids in the outer leaf of the membrane. According to this model, the long-range lateral diffusion of diffusible molecules in compartmentalized membranes is largely limited by their hop rate across the compartment boundaries. Importantly, compartmentalization of the cell membrane has also been suggested to occur for glycosylphosphatidylinositol-linked proteins and to be involved in modulating the size and lifetime of lipid rafts. Collectively, the results suggest that the point mutation S707Y promotes association with compartmentalized plasma membrane subdomains, possibly with lipid rafts. The importance of the latter for BCR-mediated signal transduction in B cells has previously been emphasized (69–72).
We have shown earlier that RAC, by physically interacting with the split PH domain of PLCγ2, amplifies BCR-induced Ca2+ signaling and markedly increases the sensitivity of B cells to BCR ligation, BCR-mediated Ca2+ release from intracellular stores, Ca2+ entry from the extracellular compartment, and nuclear translocation of the Ca2+-regulated nuclear factor of activated T cells (49). We also showed that various PLCγ2 variants are strikingly hypersensitive to activation by RAC2, such that even WT RAC2 suffices to activate the variant enzymes upon its introduction into intact cells (38, 39). These findings are highly consistent with the cooperative interaction between RAC2 and BTK as stimulators of PLCγ2 shown here, in particular with the striking permissive effect of activated RAC on the ability of catalytically-inert BTKE41K/K430R to activate even WT PLCγ2 (cf. Fig. 6, B and D). This result may indicate an “unleashing”, via VAV and RAC, of even WT PLCγ2 activity by the catalysis-independent functions of BTK. This effect could act in cis, i.e. within the BCR signal transduction pathway in a positive feed-forward regulatory loop, or in trans, via BCR coreceptors or integrins also capable of activating RAC (38), both enhancing BCR-mediated PLCγ2 activation. In either case, the sensitivity of BCR-mediated inositol phosphate formation to catalytic site–targeted inhibitors of BTK would be dependent on the relative penetrance of the pathways mediated by BTK independently of its protein kinase activity in the various physiological and pathophysiological environments of either normal or leukemic B cells. Of note, both VAV and RAC have been shown to localize to lipid rafts in B cells (70, 73).
An important extracellular variable encountered by B cells in different tissue environments is the abundance of the BCR antigen stimulus and, hence, the degree of BCR activation. The result shown herein indicate that this degree takes a profound influence on both the kinetics of the [Ca2+]i response and on the portion that may be contributed by the protein-tyrosine kinase–independent functions of BTK, which are resistant to catalytic site-targeted BTK inhibitors. Remarkably, in DT40 cells, this portion increased considerably when the concentration of anti-IgM was increased over the range of concentrations commonly used in the functional analysis of these cells.
At first glance, these findings may appear to contradict earlier seminal findings, such as the almost complete loss of the reconstitutive activity of catalytically-inert BTK variant R525Q in BTK−/− DT40 cells (K−/btk−), which was tested in response to type M4 anti-IgM at a nominal concentration of 2 μg/ml (13). Although it seems possible, as pointed out earlier (74), that the R525Q mutation used by Takata and Kurosaki (13) affects the catalytic capacity of the BTK kinase differently, the three kinase-inactive BTK variants analyzed herein, including the variant R525Q and even lacking the kinase domain, behaved very similarly in our experiments (cf. Fig. 2E). Notably, however, unlike Lys-430, Arg-525 is not conserved in the protein kinase superfamily and is already absent in this position in most of the human Src subfamily members, including LYN. Finally, in our hands, the anti-IgM concentration response of DT40 cells is sensitive to freezing and storage of the cells and, possibly, storage of the ligand at 4 °C, as well as somewhat variable with the particular batch of the ligand. For example, freshly-transfected cells that had not been frozen as such were used with freshly-prepared ligand in Fig. 7B. In Fig. 7C, the anti IgM-mediated [Ca2+]i increases were already reduced, e.g. at 1 μg/ml anti-IgM, after one freeze-thaw cycle. Therefore, it seems possible that the observations in Fig. 7C did in fact correspond to less-responsive cells and/or a less potent anti-IgM preparation. In vivo analysis of genetically-modified mice and experiments performed ex vivo on cultured cells from these animals have previously shown that the catalytically-inactive BTK variant K430R could either fully, only partially, only modestly, or not at all fulfill the functions of WT BTK, depending on the particular function tested (23, 24). These results have previously been explained by the finding, in primary and A20 cultured B cells, that BTK, regardless of its catalytic capabilities, associates with PIP5Ks, the enzymes that synthesize PtdInsP2 (25, 75). The findings were taken by the authors (25) to suggest that both forms of BTK act as shuttles to bring PIP5Ks to the plasma membrane and specifically to lipid rafts to enhance the availability of PtdInsP2 as a substrate for both PI3 kinases and PLCγ2 with subsequently enhanced membrane targeting of BTK (via its PH domain) and inositol phosphate formation, respectively. Although such a mechanism(s) appears persuasive, it is important to note that increased formation of PtdInsP2 by protein kinase–deficient BTK has not been shown in Saito et al. (25), neither in anti-IgM–stimulated DT40 cells nor in PIP5Kβ-overexpressing A20 cells. In addition, the observation herein that a number of PLCG2 point mutations associated with ibrutinib resistance of CLL cells cause markedly enhanced inositol phosphate formation in transfected cells (cf. Table 1), arguing strongly against a shortage of substrate PtdInsP2 at the site of catalysis. Furthermore, even activated BTKE41K did not alter the activity of WT or mutationally preactivated PLCδ1 in the experimental system utilized here (cf. Fig. 1A). There was also no change in the levels of the major inositol phospholipids in response to expression of various variant forms of BTK, including those capable of stimulating PLCγ2S707Y (cf. Fig. 3A). Finally, and perhaps most convincingly, there was a striking PLC isozyme specificity of the stimulatory effect of catalytically-inactive BTK, even within the PLCγ subfamily, where PLCγ1S729Y, the direct structural and functional counterpart of PLCγ2S707Y (39), did not show activation by BTKE41K/K430R (cf. Fig. 3C). Note that both PLCγ1 and PLCδ1 associate with lipid raft membrane microdomains (76, 77), and should, as such, be similarly if not equally sensitive, as compared with PLCγ2, to an enhanced availability of the PtdInsP2 substrate.
Tomlinson et al. (74) showed earlier that an inducible form of kinase-inactive BTK, in contrast to its kinase-active counterpart, was unable to induce an increase in [Ca2+]i in BTK-deficient DT40 cells on its own. However, in the context of BCR activation by 2 μg/ml anti-IgM, there was virtually no difference in the kinetics of [Ca2+]i between BTK−/− cells expressing kinase-active versus -inactive BTK. Hence, BCR-mediated activation of PLCγ2 via kinase-inactive BTK involves an additional mechanism. In the context of the results shown here (cf. Fig. 6, A–D), it is likely that RAC activated by the BCR, e.g. via VAV, permits the implementation of such a mechanism. We previously noted major losses of the proportion of singly analyzed DT40 cells carrying the RAC-resistant PLCγ2 variant F897Q in response to intermediate and high concentrations of anti-IgM, without an apparent change in the anti-IgM sensitivity of the cells. In addition, the integrated single-cell fluorescence intensities, as well as peak latencies, amplitudes, and frequencies were markedly (mostly up to >80%) reduced in these cells under these conditions (49). We therefore suggest that, following intermediate to high degree BCR activation by antigens, activated RAC physically interacts with WT PLCγ2 to sensitize the enzyme to the noncatalytic actions of BTK.
Considering the BCR-coupled multiprotein transmembrane-signaling chain as one functional unit coupling a ligand-binding receptor to a second messenger–generating enzyme such as PLCγ2, catalytic site–targeted BTK inhibitors can be categorized as variably effective, either irreversible (e.g. ibrutinib and acalabrutinib) or reversible (e.g. vecabrutinib) allosteric inhibitors: allosteric because they act at a site distinct from the (orthosteric) receptor–ligand-binding site. Based on the results presented herein, we suggest that inhibition by ibrutinib and acalabrutinib is only fully expressed at low degrees of BCR activation where only the catalysis-dependent effects of BTK are in effect. At high degrees of BCR activation, more and more of the catalysis-independent BTK functions come into effect, such that the BTK inhibitors acquire the properties of only partially-effective inhibitors. Whether or not the remaining relative inhibition suffices to block specific (patho)biologic functions of BCR depends on the “functional reserve” of the particular B-cell response distal to the increase in [Ca2+]i. In other transmembrane-signaling systems, such as those relying on enhanced intracellular formation of cAMP, this term refers to the fact that many receptor agonists are more powerful than they need to be, in that maximal responses of cellular functions are elicited at submaximal, in some cases only minimal degrees of receptor activation or second messenger generation (78, 79). For example, it is well-known that in adipocytes stimulated by lipolytic hormones the levels of cAMP are far in excess of those required for maximal lipolytic rates, even in the presence of inhibitors (80–82). This large “overshoot” of second messenger is also observed, e.g. in the heart and in adrenocortical cells, in many other biological systems with no direct correlation of second messenger concentration with the metabolic or physiological process under investigation (83, 84). Under these conditions, both orthosteric and allosteric inhibitors need to be capable of blocking a substantial portion, if not a great majority, or even all agonist effects to be efficacious (85, 86). Thus, as long as the BCR operates under a functional reserve arrangement in a given B cell, the changes in BTK inhibitor efficacy shown herein (cf. Fig. 7C) may take profound effects on and limit their therapeutic efficiency in cells expressing both WT BTK and WT PLCγ2, in particular at high degrees of BCR activation, as determined by high affinity and/or high abundance of the antigen.
In CLL cells, BCR activation may occur through engagement of the receptor by (auto)antigens (87, 88) or in a cell-autonomous way by BCR recognition of conserved epitopes within specific regions of the Ig heavy and light chains (89, 90). Either way, there are substantial differences in the degrees of this activation among samples of different patients, which in turn translates (at least in part) to the clinical heterogeneity of the disease (90, 91). Importantly, BTK inhibitor treatment of CLL has been reported to be limited by primary drug resistance in 13–30% (10). Partial efficacy of the drugs caused by the activation of PLCγ2 through catalysis-independent mechanisms of BTK provides a potential mechanism of this therapeutic problem. Novel drugs capable of interfering with the protein–protein interaction(s) of BTK with PLCγ2 may provide a way to address this treatment drawback in the future. Finally, clinical heterogeneity, referred to as “leaky” or “mild” phenotype in the less affected patients, is also known in XLA, even in patients from single pedigrees. It has previously been difficult or even impossible to establish a clear genotype–phenotype relationship for this disease caused by a large number of distinct mutations in BTK (RRID:SCR_013101). In some cases, these mutations either abrogate the catalytic functions of BTK (92) or cause a loss of its catalytic domain (93). We suggest that the noncatalytic functions of the variant BTK enzymes, possibly triggered in a sibling-specific fashion by high BCR activation, may contribute to this heterogeneity.
Experimental procedures
Materials
The rabbit polyclonal antibody 3872 reactive against human and chicken PLCγ2, the rabbit polyclonal antibody 3871 reactive against human PLCγ2 phosphorylated at tyrosine 1217, the rabbit polyclonal antibody 3874 reactive against human PLCγ2 phosphorylated at tyrosine 759, and the rabbit polyclonal antibody 5082 reactive against human BTK phosphorylated at tyrosine 223 were obtained from Cell Signaling Technology. The rabbit polyclonal antiserum reactive against human RAC2 (sc-96), the mouse mAb reactive against human BTK (sc-81735), and the mouse mAb (PY99) reactive against phosphotyrosine-containing proteins (sc-7020) were purchased from Santa Cruz Biotechnology. The rabbit polyclonal antibody reactive against human and chicken BTK (F52632) was obtained from NSJ Bioreagents. CFSE (eBioscienceTM, 65-0850-84) and Indo-1-AM (I1223) were obtained from Thermo Fisher Scientific. Mouse anti-chicken IgM (clone M-4) was obtained from Southern Biotech. The BTK inhibitors ibrutinib (PCI-32765) and acalabrutinib (ACP-196) were obtained from Selleckchem. The BTK inhibitor vecabrutinib (SNS-062) was kindly provided by Sunesis. The sources of other reagents have been described in Ref. 39, 94. BTK-deficient DT40 cells were kindly provided by J. Wienands, Institute of Cellular and Molecular Immunology, University of Göttingen.
Construction of vectors
The 4E variant (Y753E, Y759E, Y1197E, and Y1217E) of PLCγ2 was generated according to the 4F variant. The cDNA encoding PLCγ2N-myr was generated by N-terminal insertion of the first 15 amino acids from Rous sarcoma virus SRC (MGSSKSKPKDPSQRR) (95) and a small linker (PGIQ) providing a BamHI site into PLCγ2–pcDNA3.1(+). BTKΔkinase, lacking amino acids 402–659, was generated with help of in vitro mutagenesis by insertion of a stop codon (TAG) right in front of the kinase domain. The primer sequences and PCR protocols are available from the authors upon request. The cDNAs of n-FLAG epitope-tagged human BTK and its point variants R28C, E41K, Y223F, and K430R were provided by C. Brunner, Department of Otorhinolaryngology, Head and Neck Surgery, Ulm University Medical Center. The BTK variant Y551F was generated by O.-O. Wolz from the Department of Immunology, University of Tübingen. The generation of all other cDNAs has been detailed in Refs. 43, 49, 96.
Generation of stably-transfected DT40 cell clones, DT40 cell culture, and preparation of DT40 cell lysates
This was essentially done as described in Ref. 49. Proteomic analysis of whole-cell extract proteins, fractionated by SDS-PAGE, was used to verify the expression of WT and K430R variant BTK in the respective cell clones as described previously (97). MS/MS spectra were correlated with the UniProt chicken database complemented with the sequences of human WT and K430R variant BTK. Carbamidomethylated cysteine was considered as a fixed modification along with oxidation of methionine, and acetylated protein N termini as variable modifications. False discovery rates were set on both peptide and protein levels to 0.01.
Calcium flux measurements
Cells were suspended at a density of 107 cells/ml in FACS buffer (143 mm NaCl, 6 mm KCl, 1 mm MgSO4, 20 mm HEPES/NaOH, pH 7.4, 1 mm CaCl2, 5.6 mm glucose) and incubated for 45 min at 37 °C and 10% CO2 with 5 μm Indo-1-AM. The calcium measurements were performed on a BD FACS CelestaTM. In brief, 106 cells per sample in 0.5 ml of FACS buffer were pre-warmed at 37 °C for 10 min. Next, baseline was measured for 2 min before addition of the ligand anti-chicken IgM. After an additional 4 min, 2 μm ionomycin (I0634, Sigma) was added to the samples to obtain the maximal Ca2+ flux. Cells were kept at 37 °C during the measurements. The fluorescence ratio of Indo-1 violet (Ca2+-bound) to Indo-1 blue (Ca2+-free) was measured by using FACSDivaTM software (BD Biosciences). In some cases, two cell populations were analyzed simultaneously by staining half of the cells for 10 min at 37 °C and 10% CO2 with 20 nm CFSE (98), followed by washing of the cells (107 cells/ml) with 10 volumes of PBS. For BTK inhibitor treatment, unstained and CFSE-labeled DT40 cells were treated for 1 h at 37 °C with continuous shaking at a density of 107 cells/ml with 1 μm inhibitor or solvent in RPMI 1640 medium containing supplements. The inhibitor was washed out before adding the Ca2+ indicator. For data evaluation, the experimental results contained in FCS files generated by the FACSDivaTM software (BD Biosciences) were imported into the FlowPy Python tool (RRID:SCR_018195) and then transferred to Excel spread sheets. Following binning of the time attributes of the individual data points to integers (full seconds), the data of one experiment were assembled into a matrix M such that the first row listed the time attributes and the columns j in rows i + 1 tabulated the fluorescence ratios. The resulting matrices were typically made up of about 540 columns and up to about 1650 rows. In experiments analyzing two cell populations at the same time, DT40 cells expressing WT BTK (unstained) and DT40 cells expressing BTKK430R (stained) (Fig. 7C), the matrix was fractionated into two parts, M1 and M2, according to the fluorescence intensities determined for intracellular CFSE. The individual points shown in Fig. 7, B and C, correspond to the times in full seconds versus the medians of all fluorescence ratios representing [Ca2+]i, following their correction for basal fluorescence intensity in the absence of anti-IgM and normalization according to the maximal values reached after addition of 2 μm ionomycin. Between serial measurements, the latter values were normalized to 100%. Nonlinear least-squares curve fitting of selected portions of the data to equations of either one-phase exponential association, two-exponential association and decay, or log-normal distributions was done using GraphPad Prism®, versions 8.2.1 or 4.03, to determine the values corresponding to the maximal [Ca2+]i concentrations for normalization to allow for quantitative comparison between experiments.
Cell culture and transfection of COS-7 cells, extraction, and analysis of inositol phospholipids
Radiolabeling of inositol phospholipids and analysis of inositol phosphate formation
Twenty four hours after transfection, COS-7 cells were washed once with 0.3 ml/well of Dulbecco's PBS (PAA Laboratories) and then incubated for 18 h in 0.2 ml/well DMEM-containing supplements, as described previously (38), supplemented with 2.5 μCi/ml myo-[2-3H]inositol (NET1156005MC, PerkinElmer Life Sciences), and 10 mm LiCl. The cells were then washed once with 0.2 ml/well Dulbecco's PBS and lysed by addition of 0.2 ml/well 10 mm ice-cold formic acid. The analysis of inositol phosphate formation was performed as described previously (53) except that 15 ml of scintillation fluid Quicksafe A from Zinsser Analytic was used. To examine BTK inhibition via ibrutinib, acalabrutinib, or vecabrutinib, COS-7 cells were radiolabeled for 18 h in 0.2 ml/well DMEM-containing supplements as described previously (38), supplemented with 2.5 μCi/ml myo-[2-3H]inositol, 10 mm LiCl, 0.1% v/v DMSO (D2650, Sigma) or the respective BTK inhibitor.
Phosphoblot analysis
COS-7 cells were seeded into 6-well plates and transfected. After an additional medium change, the cells were incubated for a further 18 h at 37 °C and 10% CO2. The medium was removed, and the cells were washed with 0.5 ml of ice-cold TBS and then lysed by addition of 250 μl of phospholysis buffer (25 mm Tris-HCl, 100 mm NaCl, 1 mm EDTA, 0.5% (v/v) NP40, 0.2% (v/v) Triton X-100, 5% (v/v) glycerol, pH 7.5) supplemented with protease inhibitors (1 mm phenylmethylsulfonyl fluoride, 10 μm leupeptin, 2 μm pepstatin A, 2 μg/ml soybean trypsin inhibitor, 1 μg/ml aprotinin, and 3 mm benzamidine) and phosphatase inhibitors (2 mm sodium orthovanadate, 9.5 mm sodium fluoride, 10 mm β-glycerophosphate, and 10 mm tetrasodium pyrophosphate). After lysis, 250 μl of 2× SDS-PAGE sample preparation buffer were added, and aliquots of the samples were subjected to SDS-PAGE followed by immunoblotting.
FRAP experiments
FRAP studies (100, 101) were conducted as described earlier (49), using a fixed-localization Gaussian laser beam focused to a very small spot on the membrane for both bleaching and monitoring, continuously measuring the fluorescence intensity at the spot by photon counting (47). This method gives a much better time resolution (down to a few milliseconds) than FRAP of larger regions-of-interest in a confocal microscope, which employs nonsynchronous stepwise bleaching and monitoring, which take significantly longer (47). The improved time resolution is critical in the case of fast recovery contributed by exchange and/or gliding, as is the case in this study. The experiments were performed 24–26 h post-transfection on COS-7 cells transfected with PLCγ2–GFP derivatives. All experiments were conducted at 22 °C, in Hanks' balanced salt solution supplemented with 20 mm HEPES/NaOH, pH 7.2. The monitoring argon ion laser beam (488 nm, 1.2 microwatts; Innova 70C, Coherent) was focused through the microscope (AxioImager.D1, Carl Zeiss MicroImaging) to a Gaussian spot with a radius ω = 0.77 ± 0.03 μm (×63/1.4 NA oil-immersion objective) or 1.17 ± 0.05 μm (×40/1.2 NA water immersion objective). Experiments were conducted with each beam size (beam size analysis is described in Refs. 48, 49). The ratio between the illuminated areas (ω2(×40)/ω2(×63)) was 2.28 ± 0.15 (n = 59; S.D. calculated using bootstrap analysis as described below). After a brief measurement at the monitoring intensity, a 5-milliwatt pulse (4–6 or 10–20 ms for the ×63 and ×40xobjectives, respectively) bleached 50–70% of the fluorescence in the illuminated spot. Fluorescence recovery was followed by the monitoring beam. The apparent characteristic fluorescence recovery time (τ) and the mobile fraction (Rf) were derived from the FRAP curves by nonlinear regression analysis, fitting to a lateral diffusion process with a single τ value (102). The significance of differences between sets of τ values measured with the same beam size was evaluated by one-way ANOVA and Bonferroni post hoc test. To compare the τ(×40)/τ(×63) ratio of a given PLCγ2 variant with the ω2(×40)/ω2(×63) beam-size ratio (comparing a single pair of data sets), statistical significance was evaluated by two-tailed Student's t test using bootstrap analysis with 1000 bootstrap samples (103), which is preferable for comparison between ratios, as described by us previously (104).
Miscellaneous
SDS-PAGE and immunoblotting were performed according to standard protocols using antibodies reactive against the c-Myc epitope for WT and variant PLCγ1, PLCγ2, and PLCδ1 or antibodies reactive against BTK, PLCβ2, RAC2, and β-actin. Immunoreactive proteins were visualized using the Pierce ECL Western blotting detection system (catalog 32106, Thermo Fisher Scientific). Samples to be analyzed by Western blotting were taken, quasi as a fourth replicate, from the same plate as and immediately adjacent to the samples taken in triplicate for functional analysis. Using this protocol and paying meticulous attention to experimental detail, we were able to demonstrate for all results obtained in transiently-transfected COS-7 cells throughout this work that the differences observed in inositol phosphate formation were not explained by or related to differences in the respective protein expression (cf. Figs. 1, B and D, 2, D and F, 3D, and 6C and Figs. S1–S6). All experiments were performed at least three times. Similar results and identical trends were obtained each time. Data from representative experiments are shown as mean values of triplicate determinations. In the experiments shown in Figs. 1C, 2, A–C and E, 3C, 4, and 6A and Table 1, background inositol phosphate formation in response to PLC and/or BTK expression was determined in parallel on cells transfected with empty vector and subtracted from the corresponding values, with appropriate consideration of error propagation for differences and quotients to determine the standard deviations of means (S.D.) of triplicate determinations (105). Although the S.D. values of these technical replicates are not shown in Figs. 1, A, C, and E, 2, A–C, E, and G, 3, A–C, 4, and 6, A, B, and D, and Table 1, their relationships to the corresponding means were calculated in aggregate for all mean values shown in these representations (n = 500) and found to be distributed with a median of 9.93%, with 95% confidence limits of 8.97% and 10.93%. In the experiments displayed in Figs. 1, C and E, 2, C, E, and G, 6, B and D, and 7, B and C, the data were fitted by nonlinear least-squares curve fitting using all replicate values to three- or four-parameter dose-response equations using GraphPad Prism®, version 4.03. In certain cases, the global curve–fitting procedure contained in Prism was used to determine whether the best-fit values of selected parameters differed between data sets. The simpler model was selected unless the extra sum of squares F-test had a p value of less than 0.05.
Data availability
All data are contained within the manuscript. Raw data as well as computer subroutines used for data processing and equations used to calculate propagation of experimental errors are available on request from Peter Gierschik (peter.gierschik@uni-ulm.de).
Author contributions
M. W., P. G., and C. W. conceptualization; M. W. and Y. I. H. validation; M. W., L. M., O. G., J. H., S. E., Y. Z., R. R., S. W., and C. W. investigation; M. W., O. G., Y. I. H., and C. W. visualization; M. W. methodology; S. S., E. H., Y. I. H., P. G., and C. W. writing-review and editing; Y. I. H. and P. G. writing-original draft; P. G. and C. W. data curation; P. G. and C. W. supervision; C. W. formal analysis.
Supplementary Material
Acknowledgments
We thank Pietro Taverna and Judy Fox from Sunesis Pharmaceuticals for critically reading the manuscript and for the gift of vecabrutinib. We thank the expert technical assistance of Norbert Zanker, Anita Ruepp, and Susanne Gierschik. The International Graduate School in Molecular Medicine, Ulm (IGradU), Germany, was funded within the Excellence Initiative of the German Federal and State Governments Grant GSC 279.
This work was supported by Deutsche Forschungsgemeinschaft (DFG) Grants SFB 1074/TP A8 and GSC 279 and the Ministerium für Wissenschaft, Forschung, und Kunst Baden-Württemberg (PharmCompNet BW). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S6 and Table S1.
- PLC
- inositol phospholipid-specific phospholipase C
- RAC
- Ras-related C3 botulinum toxin substrate
- BTK
- Bruton's tyrosine kinase
- PIP5K
- phosphatidylinositol-4-phosphate 5-kinase
- BCR
- B-cell receptor
- [Ca2+]i
- intracellular calcium ion concentration
- PtdInsP3
- phosphatidylinositol 3,4,5-trisphosphate
- PtdInsP2
- phosphatidylinositol 4,5-bisphosphate
- CLL
- chronic lymphocytic leukemia
- WM
- Waldenström's macroglobulinemia
- FRAP
- fluorescence recovery after photobleaching
- NFAT
- Ca2+-regulated nuclear factor of activated T cells
- XLA
- human X-linked agammaglobulinemia
- ANOVA
- analysis of variance
- CFSE
- 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester
- SH
- Src homology
- PH
- pleckstrin homology
- v-SRC
- rous sarcoma virus tyrosine kinase.
References
- 1. Koss H., Bunney T. D., Behjati S., and Katan M. (2014) Dysfunction of phospholipase Cγ in immune disorders and cancer. Trends Biochem. Sci. 39, 603–611 [DOI] [PubMed] [Google Scholar]
- 2. Milner J. D. (2015) PLAID: a syndrome of complex patterns of disease and unique phenotypes. J. Clin. Immunol. 35, 527–530 10.1007/s10875-015-0177-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Lange K. M., Moutsianas L., Lee J. C., Lamb C. A., Luo Y., Kennedy N. A., Jostins L., Rice D. L., Gutierrez-Achury J., Ji S.-G., Heap G., Nimmo E. R., Edwards C., Henderson P., Mowat C., et al. (2017) Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 49, 256–261 10.1038/ng.3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lane B. M., Cason R., Esezobor C. I., and Gbadegesin R. A. (2019) Genetics of childhood steroid sensitive nephrotic syndrome: an update. Front. Pediatr. 7, 8 10.3389/fped.2019.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kogure Y., and Kataoka K. (2017) Genetic alterations in adult T-cell leukemia/lymphoma. Cancer Sci. 108, 1719–1725 10.1111/cas.13303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Megquier K., Turner-Maier J., Swofford R., Kim J.-H., Sarver A. L., Wang C., Sakthikumar S., Johnson J., Koltookian M., Lewellen M., Scott M. C., Schulte A. J., Borst L., Tonomura N., Alfoldi, et al. (2019) Comparative genomics reveals shared mutational landscape in canine hemangiosarcoma and human angiosarcoma. Mol. Cancer Res. 17, 2410–2421 10.1158/1541-7786.MCR-19-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaymaz Y., Oduor C. I., Yu H., Otieno J. A., Ong'echa J. M., Moormann A. M., and Bailey J. A. (2017) Comprehensive transcriptome and mutational profiling of endemic Burkitt lymphoma reveals EBV type-specific differences. Mol. Cancer Res. 15, 563–576 10.1158/1541-7786.MCR-16-0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sasner M., Williams H. M., Oblak A. L., O'Rourke R., Preuss C., Saykin A. J., Rizzo S. J. S., Ananda G., Philip V., Carter G. W., Lamb B. T., and Howell G. (2018) Novel models of late-onset Alzheimer's disease based on GWAS. Alzheimers Dement. 14, P1445 10.1016/j.jalz.2018.06.2433 [DOI] [Google Scholar]
- 9. van der Lee S. J., Conway O. J., Jansen I., Carrasquillo M. M., Kleineidam L., van den Akker E., Hernández I., van Eijk K. R., Stringa N., Chen J. A., Zettergren A., Andlauer T. F. M., Diez-Fairen M., Simon-Sanchez J., Lleó A., et al. (2019) A nonsynonymous mutation in PLCG2 reduces the risk of Alzheimer's disease, dementia with Lewy bodies and frontotemporal dementia, and increases the likelihood of longevity. Acta Neuropathol. 138, 237–250 10.1007/s00401-019-02026-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaur V., and Swami A. (2017) Ibrutinib in CLL: a focus on adverse events, resistance, and novel approaches beyond ibrutinib. Ann. Hematol. 96, 1175–1184 10.1007/s00277-017-2973-2 [DOI] [PubMed] [Google Scholar]
- 11. Berg L. J., Finkelstein L. D., Lucas J. A., and Schwartzberg P. L. (2005) Tec family kinases in T lymphocyte development and function. Annu. Rev. Immunol. 23, 549–600 10.1146/annurev.immunol.22.012703.104743 [DOI] [PubMed] [Google Scholar]
- 12. Andreotti A. H., Joseph R. E., Conley J. M., Iwasa J., and Berg L. J. (2018) Multidomain control over Tec kinase activation state tunes the T cell response. Annu. Rev. Immunol. 36, 549–578 10.1146/annurev-immunol-042617-053344 [DOI] [PubMed] [Google Scholar]
- 13. Takata M., and Kurosaki T. (1996) A role for Bruton's tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-γ2. J. Exp. Med. 184, 31–40 10.1084/jem.184.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watanabe D., Hashimoto S., Ishiai M., Matsushita M., Baba Y., Kishimoto T., Kurosaki T., and Tsukada S. (2001) Four tyrosine residues in phospholipase C-γ2, identified as Btk-dependent phosphorylation sites, are required for B cell antigen receptor-coupled calcium signaling. J. Biol. Chem. 276, 38595–38601 10.1074/jbc.M103675200 [DOI] [PubMed] [Google Scholar]
- 15. Harden T. K., and Sondek J. (2006) Regulation of phospholipase C isozymes by ras superfamily GTPases. Annu. Rev. Pharmacol. Toxicol. 46, 355–379 10.1146/annurev.pharmtox.46.120604.141223 [DOI] [PubMed] [Google Scholar]
- 16. Kadamur G., and Ross E. M. (2013) Mammalian phospholipase C. Annu. Rev. Physiol. 75, 127–154 10.1146/annurev-physiol-030212-183750 [DOI] [PubMed] [Google Scholar]
- 17. Fu C., Turck C. W., Kurosaki T., and Chan A. C. (1998) BLNK: a central linker protein in B cell activation. Immunity 9, 93–103 10.1016/S1074-7613(00)80591-9 [DOI] [PubMed] [Google Scholar]
- 18. Chiu C. W., Dalton M., Ishiai M., Kurosaki T., and Chan A. C. (2002) BLNK: molecular scaffolding through 'cis'-mediated organization of signaling proteins. EMBO J. 21, 6461–6472 10.1093/emboj/cdf658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li T., Rawlings D. J., Park H., Kato R. M., Witte O. N., and Satterthwaite A. B. (1997) Constitutive membrane association potentiates activation of Bruton tyrosine kinase. Oncogene 15, 1375–1383 10.1038/sj.onc.1201308 [DOI] [PubMed] [Google Scholar]
- 20. Várnai P., Rother K. I., and Balla T. (1999) Phosphatidylinositol 3-kinase–dependent membrane association of the Bruton's tyrosine kinase pleckstrin homology domain visualized in single living cells. J. Biol. Chem. 274, 10983–10989 10.1074/jbc.274.16.10983 [DOI] [PubMed] [Google Scholar]
- 21. Hubbard S. R., and Till J. H. (2000) Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 69, 373–398 10.1146/annurev.biochem.69.1.373 [DOI] [PubMed] [Google Scholar]
- 22. Márquez J. A., Smith C. I., Petoukhov M. V., Lo Surdo P., Mattsson P. T., Knekt M., Westlund A., Scheffzek K., Saraste M., and Svergun D. I. (2003) Conformation of full-length Bruton tyrosine kinase (Btk) from synchrotron X-ray solution scattering. EMBO J. 22, 4616–4624 10.1093/emboj/cdg448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Middendorp S., Dingjan G. M., Maas A., Dahlenborg K., and Hendriks R. W. (2003) Function of Bruton's tyrosine kinase during B cell development is partially independent of its catalytic activity. J. Immunol. 171, 5988–5996 10.4049/jimmunol.171.11.5988 [DOI] [PubMed] [Google Scholar]
- 24. Middendorp S., Zijlstra A. J., Kersseboom R., Dingjan G. M., Jumaa H., and Hendriks R. W. (2005) Tumor suppressor function of Bruton tyrosine kinase is independent of its catalytic activity. Blood 105, 259–265 10.1182/blood-2004-07-2708 [DOI] [PubMed] [Google Scholar]
- 25. Saito K., Tolias K. F., Saci A., Koon H. B., Humphries L. A., Scharenberg A., Rawlings D. J., Kinet J.-P., and Carpenter C. L. (2003) BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity 19, 669–678 10.1016/S1074-7613(03)00297-8 [DOI] [PubMed] [Google Scholar]
- 26. Hendriks R. W., Yuvaraj S., and Kil L. P. (2014) Targeting Bruton's tyrosine kinase in B cell malignancies. Nat. Rev. Cancer 14, 219–232 10.1038/nrc3702 [DOI] [PubMed] [Google Scholar]
- 27. Rogers K. A., Mousa L., Zhao Q., Bhat S. A., Byrd J. C., El Boghdadly Z., Guerrero T., Levine L. B., Lucas F., Shindiapina P., Sigmund A. M., Sullivan M., Wiczer T. E., Woyach J. A., and Awan F. T. (2019) Incidence of opportunistic infections during ibrutinib treatment for B-cell malignancies. Leukemia 33, 2527–2530 10.1038/s41375-019-0481-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mato A. R., Roeker L. E., Allan J. N., Pagel J. M., Brander D. M., Hill B. T., Cheson B. D., Furman R. R., Lamanna N., Tam C. S., Handunnetti S., Jacobs R., Lansigan F., Bhavsar E., and Barr P. M., et al. (2018) Outcomes of front-line ibrutinib treated CLL patients excluded from landmark clinical trial. Am. J. Hematol 93, 1394–1401 10.1002/ajh.25261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woyach J. A., Ruppert A. S., Heerema N. A., Zhao W., Booth A. M., Ding W., Bartlett N. L., Brander D. M., Barr P. M., Rogers K. A., Parikh S. A., Coutre S., Hurria A., Brown J. R., Lozanski G., et al. (2018) Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N. Engl. J. Med. 379, 2517–2528 10.1056/NEJMoa1812836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berglöf A., Hamasy A., Meinke S., Palma M., Krstic A., Månsson R., Kimby E., Österborg A., and Smith C. I. (2015) Targets for ibrutinib beyond B cell malignancies. Scand. J. Immunol. 82, 208–217 10.1111/sji.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ito M., Shichita T., Okada M., Komine R., Noguchi Y., Yoshimura A., and Morita R. (2015) Bruton's tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 6, 7360 10.1038/ncomms8360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Molina-Cerrillo J., Alonso-Gordoa T., Gajate P., and Grande E. (2017) Bruton's tyrosine kinase (BTK) as a promising target in solid tumors. Cancer Treat. Rev. 58, 41–50 10.1016/j.ctrv.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 33. Lv J., Wu J., He F., Qu Y., Zhang Q., and Yu C. (2018) Development of Bruton's tyrosine kinase inhibitors for rheumatoid arthritis. Curr. Med. Chem. 25, 5847–5859 10.2174/0929867325666180316121951 [DOI] [PubMed] [Google Scholar]
- 34. Maddocks K. J., Ruppert A. S., Lozanski G., Heerema N. A., Zhao W., Abruzzo L., Lozanski A., Davis M., Gordon A., Smith L. L., Mantel R., Jones J. A., Flynn J. M., Jaglowski S. M., Andritsos L. A., et al. (2015) Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 1, 80–87 10.1001/jamaoncol.2014.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Albitar A., Ma W., DeDios I., Estella J., Ahn I., Farooqui M., Wiestner A., and Albitar M. (2017) Using high-sensitivity sequencing for the detection of mutations in BTK and PLCγ2 genes in cellular and cell-free DNA and correlation with progression in patients treated with BTK inhibitors. Oncotarget 8, 17936–17944 10.18632/oncotarget.15316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones D., Woyach J. A., Zhao W., Caruthers S., Tu H., Coleman J., Byrd J. C., Johnson A. J., and Lozanski G. (2017) PLCG2 C2 domain mutations co-occur with BTK and PLCG2 resistance mutations in chronic lymphocytic leukemia undergoing ibrutinib treatment. Leukemia 31, 1645–1647 10.1038/leu.2017.110 [DOI] [PubMed] [Google Scholar]
- 37. Woyach J. A., Ruppert A. S., Guinn D., Lehman A., Blachly J. S., Lozanski A., Heerema N. A., Zhao W., Coleman J., Jones D., Abruzzo L., Gordon A., Mantel R., Smith L. L., McWhorter S., et al. (2017) BTKC481S-mediated resistance to ibrutinib in chronic lymphocytic leukemia. J. Clin. Oncol. 35, 1437–1443 10.1200/JCO.2016.70.2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walliser C., Hermkes E., Schade A., Wiese S., Deinzer J., Zapatka M., Désiré L., Mertens D., Stilgenbauer S., and Gierschik P. (2016) The phospholipase C-γ2 mutants R665W and L845F identified in ibrutinib-resistant chronic lymphocytic leukemia patients are hypersensitive to the Rho GTPase Rac2 protein. J. Biol. Chem. 291, 22136–22148 10.1074/jbc.M116.746842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Walliser C., Wist M., Hermkes E., Zhou Y., Schade A., Haas J., Deinzer J., Désiré L., Li S. S. C., Stilgenbauer S., Milner J. D., and Gierschik P. (2018) Functional characterization of phospholipase C-γ2 mutant protein causing both somatic ibrutinib resistance and a germline monogenic autoinflammatory disorder. Oncotarget 9, 34357–34378 10.18632/oncotarget.26173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu T.-M., Woyach J. A., Zhong Y., Lozanski A., Lozanski G., Dong S., Strattan E., Lehman A., Zhang X., Jones J. A., Flynn J., Andritsos L. A., Maddocks K., Jaglowski S. M., Blum K. A., et al. (2015) Hypermorphic mutation of phospholipase C-γ2 acquired in ibrutinib-resistant CLL confers BTK independency upon B-cell receptor activation. Blood 126, 61–68 10.1182/blood-2015-02-626846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brill L. M., Salomon A. R., Ficarro S. B., Mukherji M., Stettler-Gill M., and Peters E. C. (2004) Robust phosphoproteomic profiling of tyrosine phosphorylation sites from human T cells using immobilized metal affinity chromatography and tandem mass spectrometry. Anal. Chem. 76, 2763–2772 10.1021/ac035352d [DOI] [PubMed] [Google Scholar]
- 42. Rikova K., Guo A., Zeng Q., Possemato A., Yu J., Haack H., Nardone J., Lee K., Reeves C., Li Y., Hu Y., Tan Z., Stokes M., Sullivan L., Mitchell J., et al. (2007) Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 131, 1190–1203 10.1016/j.cell.2007.11.025 [DOI] [PubMed] [Google Scholar]
- 43. Hicks S. N., Jezyk M. R., Gershburg S., Seifert J. P., Harden T. K., and Sondek J. (2008) General and versatile autoinhibition of PLC isozymes. Mol. Cell 31, 383–394 10.1016/j.molcel.2008.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bond D. A., and Woyach J. A. (2019) Targeting BTK in CLL: beyond ibrutinib. Curr. Hematol. Malig. Rep. 14, 197–205 10.1007/s11899-019-00512-0 [DOI] [PubMed] [Google Scholar]
- 45. Woyach J. A., Furman R. R., Liu T.-M., Ozer H. G., Zapatka M., Ruppert A. S., Xue L., Li D. H., Steggerda S. M., Versele M., Dave S. S., Zhang J., Yilmaz A. S., Jaglowski S. M., Blum K. A., et al. (2014) Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N. Engl. J. Med. 370, 2286–2294 10.1056/NEJMoa1400029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Burger J. A., Landau D. A., Taylor-Weiner A., Bozic I., Zhang H., Sarosiek K., Wang L., Stewart C., Fan J., Hoellenriegel J., Sivina M., Dubuc A. M., Fraser C., Han Y., Li S., et al. (2016) Clonal evolution in patients with chronic lymphocytic leukemia developing resistance to BTK inhibition. Nat. Commun. 7, 11589 10.1038/ncomms11589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Berkovich R., Wolfenson H., Eisenberg S., Ehrlich M., Weiss M., Klafter J., Henis Y. I., and Urbakh M. (2011) Accurate quantification of diffusion and binding kinetics of non-integral membrane proteins by FRAP. Traffic 12, 1648–1657 10.1111/j.1600-0854.2011.01264.x [DOI] [PubMed] [Google Scholar]
- 48. Henis Y. I., Rotblat B., and Kloog Y. (2006) FRAP beam-size analysis to measure palmitoylation-dependent membrane association dynamics and microdomain partitioning of Ras proteins. Methods 40, 183–190 10.1016/j.ymeth.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 49. Walliser C., Tron K., Clauss K., Gutman O., Kobitski A. Y., Retlich M., Schade A., Röcker C., Henis Y. I., Nienhaus G. U., and Gierschik P. (2015) Rac-mediated stimulation of phospholipase Cγ2 amplifies B-cell receptor-induced calcium signaling. J. Biol. Chem. 290, 17056–17072 10.1074/jbc.M115.645739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Illenberger D., Walliser C., Strobel J., Gutman O., Niv H., Gaidzik V., Kloog Y., Gierschik P., and Henis Y. I. (2003) Rac2 regulation of phospholipase C-β2 activity and mode of membrane interactions in intact cells. J. Biol. Chem. 278, 8645–8652 10.1074/jbc.M211971200 [DOI] [PubMed] [Google Scholar]
- 51. Fujiwara T., Ritchie K., Murakoshi H., Jacobson K., and Kusumi A. (2002) Phospholipids undergo hop diffusion in compartmentalized cell membrane. J. Cell Biol. 157, 1071–1081 10.1083/jcb.200202050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ehrig J., Petrov E. P., and Schwille P. (2011) Near-critical fluctuations and cytoskeleton-assisted phase separation lead to subdiffusion in cell membranes. Biophys. J. 100, 80–89 10.1016/j.bpj.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Walliser C., Retlich M., Harris R., Everett K. L., Josephs M. B., Vatter P., Esposito D., Driscoll P. C., Katan M., Gierschik P., and Bunney T. D. (2008) Rac regulates its effector phospholipase Cγ2 through interaction with a split pleckstrin homology domain. J. Biol. Chem. 283, 30351–30362 10.1074/jbc.M803316200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bunney T. D., Opaleye O., Roe S. M., Vatter P., Baxendale R. W., Walliser C., Everett K. L., Josephs M. B., Christow C., Rodrigues-Lima F., Gierschik P., Pearl L. H., and Katan M. (2009) Structural insights into formation of an active signaling complex between Rac and phospholipase Cγ2. Mol. Cell 34, 223–233 10.1016/j.molcel.2009.02.023 [DOI] [PubMed] [Google Scholar]
- 55. Schade A., Walliser C., Wist M., Haas J., Vatter P., Kraus J. M., Filingeri D., Havenith G., Kestler H. A., Milner J. D., and Gierschik P. (2016) Cool-temperature–mediated activation of phospholipase C-γ2 in the human hereditary disease PLAID. Cell. Signal. 28, 1237–1251 10.1016/j.cellsig.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 56. Owen C., Berinstein N. L., Christofides A., and Sehn L. H. (2019) Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr. Oncol. 26, e233–e240 10.3747/co.26.4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou Q., Lee G.-S., Brady J., Datta S., Katan M., Sheikh A., Martins M. S., Bunney T. D., Santich B. H., Moir S., Kuhns D. B., Long Priel D. A., Ombrello A., Stone D., Ombrello M. J., et al. (2012) A hypermorphic missense mutation in PLCG2, encoding phospholipase Cγ2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am. J. Hum. Genet. 91, 713–720 10.1016/j.ajhg.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fluckiger A.-C., Li Z., Kato R. M., Wahl M. I., Ochs H. D., Longnecker R., Kinet J.-P., Witte O. N., Scharenberg A. M., and Rawlings D. J. (1998) Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 17, 1973–1985 10.1093/emboj/17.7.1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hashimoto S., Iwamatsu A., Ishiai M., Okawa K., Yamadori T., Matsushita M., Baba Y., Kishimoto T., Kurosaki T., and Tsukada S. (1999) Identification of the SH2 domain binding protein of Bruton's tyrosine kinase as BLNK—functional significance of Btk–SH2 domain in B-cell antigen receptor-coupled calcium signaling. Blood 94, 2357–2364 10.1182/blood.V94.7.2357.419k40_2357_2364 [DOI] [PubMed] [Google Scholar]
- 60. Rodriguez R., Matsuda M., Perisic O., Bravo J., Paul A., Jones N. P., Light Y., Swann K., Williams R. L., and Katan M. (2001) Tyrosine residues in phospholipase Cγ2 essential for the enzyme function in B-cell signaling. J. Biol. Chem. 276, 47982–47992 10.1074/jbc.M107577200 [DOI] [PubMed] [Google Scholar]
- 61. Kurosaki T., and Tsukada S. (2000) BLNK: connecting Syk and Btk to calcium signals. Immunity 12, 1–5 10.1016/S1074-7613(00)80153-3 [DOI] [PubMed] [Google Scholar]
- 62. Ishiai M., Kurosaki M., Pappu R., Okawa K., Ronko I., Fu C., Shibata M., Iwamatsu A., Chan A. C., and Kurosaki T. (1999) BLNK required for coupling Syk to PLCγ2 and Rac1–JNK in B cells. Immunity 10, 117–125 10.1016/S1074-7613(00)80012-6 [DOI] [PubMed] [Google Scholar]
- 63. Rodriguez R., Matsuda M., Storey A., and Katan M. (2003) Requirements for distinct steps of phospholipase Cγ2 regulation, membrane-raft-dependent targeting and subsequent enzyme activation in B-cell signalling. Biochem. J. 374, 269–280 10.1042/bj20021778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Udenwobele D. I., Su R.-C., Good S. V., Ball T. B., Varma Shrivastav S., and Shrivastav A. (2017) Myristoylation: an important protein modification in the immune response. Front. Immunol. 8, 751 10.3389/fimmu.2017.00751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Runft L. L., Watras J., and Jaffe L. A. (1999) Calcium release at fertilization of Xenopus eggs requires type I IP3 receptors, but not SH2 domain-mediated activation of PLCγ or Gq-mediated activation of PLCβ. Dev. Biol. 214, 399–411 10.1006/dbio.1999.9415 [DOI] [PubMed] [Google Scholar]
- 66. Sato K., Tokmakov A. A., He C.-L., Kurokawa M., Iwasaki T., Shirouzu M., Fissore R. A., Yokoyama S., and Fukami Y. (2003) Reconstitution of Src-dependent phospholipase Cγ phosphorylation and transient calcium release by using membrane rafts and cell-free extracts from Xenopus eggs. J. Biol. Chem. 278, 38413–38420 10.1074/jbc.M302617200 [DOI] [PubMed] [Google Scholar]
- 67. Cecchetti S., Spadaro F., Lugini L., Podo F., and Ramoni C. (2007) Functional role of phosphatidylcholine-specific phospholipase C in regulating CD16 membrane expression in natural killer cells. Eur. J. Immunol. 37, 2912–2922 10.1002/eji.200737266 [DOI] [PubMed] [Google Scholar]
- 68. Kondadasula S. V., Roda J. M., Parihar R., Yu J., Lehman A., Caligiuri M. A., Tridandapani S., Burry R. W., and Carson W. E. 3rd. (2008) Colocalization of the IL-12 receptor and FcγRIIIa to natural killer cell lipid rafts leads to activation of ERK and enhanced production of interferon-γ. Blood 111, 4173–4183 10.1182/blood-2007-01-068908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aman M. J., and Ravichandran K. S. (2000) A requirement for lipid rafts in B-cell receptor induced Ca2+ flux. Curr. Biol. 10, 393–396 10.1016/S0960-9822(00)00415-2 [DOI] [PubMed] [Google Scholar]
- 70. Guo B., Kato R. M., Garcia-Lloret M., Wahl M. I., and Rawlings D. J. (2000) Engagement of the human pre-B-cell receptor generates a lipid raft-dependent calcium signaling complex. Immunity 13, 243–253 10.1016/S1074-7613(00)00024-8 [DOI] [PubMed] [Google Scholar]
- 71. Pierce S. K. (2002) Lipid rafts and B-cell activation. Nat. Rev. Immunol. 2, 96–105 10.1038/nri726 [DOI] [PubMed] [Google Scholar]
- 72. Kroczek C., Lang C., Brachs S., Grohmann M., Dütting S., Schweizer A., Nitschke L., Feller S. M., Jäck H.-M., and Mielenz D. (2010) Swiprosin-1/EFhd2 controls B-cell receptor signaling through the assembly of the B-cell receptor, Syk, and phospholipase Cγ2 in membrane rafts. J. Immunol. 184, 3665–3676 10.4049/jimmunol.0903642 [DOI] [PubMed] [Google Scholar]
- 73. Phee H., Rodgers W., and Coggeshall K. M. (2001) Visualization of negative signaling in B cells by quantitative confocal microscopy. Mol. Cell. Biol. 21, 8615–8625 10.1128/MCB.21.24.8615-8625.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tomlinson M. G., Woods D. B., McMahon M., Wahl M. I., Witte O. N., Kurosaki T., Bolen J. B., and Johnston J. A. (2001) A conditional form of Bruton's tyrosine kinase is sufficient to activate multiple downstream signaling pathways via PLCγ2 in B cells. BMC Immunol. 2, 4 10.1186/1471-2172-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schwartzberg P. L. (2003) Amplifying Btk's signal. Immunity 19, 634–636 10.1016/S1074-7613(03)00304-2 [DOI] [PubMed] [Google Scholar]
- 76. Verí M.-C., DeBell K. E., Seminario M.-C., DiBaldassarre A., Reischl I., Rawat R., Graham L., Noviello C., Rellahan B. L., Miscia S., Wange R. L., and Bonvini E. (2001) Membrane raft-dependent regulation of phospholipase C-γ1 activation in T lymphocytes. Mol. Cell. Biol. 21, 6939–6950 10.1128/MCB.21.20.6939-6950.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Clarke C. J., Forman S., Pritchett J., Ohanian V., and Ohanian J. (2008) Phospholipase C-δ1 modulates sustained contraction of rat mesenteric small arteries in response to noradrenaline, but not endothelin-1. Am. J. Physiol. Heart Circ. Physiol. 295, H826–H834 10.1152/ajpheart.01396.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stephenson R. P. (1956) A modification of receptor theory. Br. J. Pharmacol. Chemother. 11, 379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Waud D. R. (1968) Pharmacological receptors. Pharmacol. Rev. 20, 49–88 [PubMed] [Google Scholar]
- 80. Butcher R. W., Baird C. E., and Sutherland E. W. (1968) Effects of lipolytic and antilipolytic substances on adenosine 3′,5′-monophosphate levels in isolated fat cells. J. Biol. Chem. 243, 1705–1712 [PubMed] [Google Scholar]
- 81. Yamamura H., and Rodbell M. (1976) Hydroxybenzylpindolol and hydroxybenzylpropranolol: partial β-adrenergic agonists of adenylate cyclase in the rat adipocyte. Mol. Pharmacol. 12, 693–700 [PubMed] [Google Scholar]
- 82. Drummond A. H., Baguley B. C., and Staehelin M. (1977) β-Adrenergic regulation of glycogen phosphorylase activity and adenosine cyclic 3′,5′-monophosphate accumulation in control and desensitized C-6 astrocytoma cells. Mol. Pharmacol. 13, 1159–1169 [PubMed] [Google Scholar]
- 83. Brooker G. (1975) Implications of cyclic nucleotide oscillations during the myocardial contraction cycle. Adv. Cyclic Nucleotide Res. 5, 435–452 [PubMed] [Google Scholar]
- 84. Ramachandran J. (1984) Corticotropin receptors, cyclic AMP and steroidogenesis. Endocr. Res. 10, 347–363 10.1080/07435808409036506 [DOI] [PubMed] [Google Scholar]
- 85. Prokocimer P. G., Maze M., Vickery R. G., and Hoffman B. B. (1988) Mechanism for desensitization of β-adrenergic receptor-stimulated lipolysis in adipocytes from rats harboring pheochromocytoma. Endocrinology 123, 528–533 10.1210/endo-123-1-528 [DOI] [PubMed] [Google Scholar]
- 86. Zhu B. T. (1993) The competitive and noncompetitive antagonism of receptor-mediated drug actions in the presence of spare receptors. J. Pharmacol. Toxicol. Methods 29, 85–91 10.1016/1056-8719(93)90055-J [DOI] [PubMed] [Google Scholar]
- 87. Ghia P., Chiorazzi N., and Stamatopoulos K. (2008) Microenvironmental influences in chronic lymphocytic leukaemia: the role of antigen stimulation. J. Intern. Med. 264, 549–562 10.1111/j.1365-2796.2008.02030.x [DOI] [PubMed] [Google Scholar]
- 88. Stamatopoulos K., Agathangelidis A., Rosenquist R., and Ghia P. (2017) Antigen receptor stereotypy in chronic lymphocytic leukemia. Leukemia 31, 282–291 10.1038/leu.2016.322 [DOI] [PubMed] [Google Scholar]
- 89. Dühren-von Minden M., Übelhart R., Schneider D., Wossning T., Bach M. P., Buchner M., Hofmann D., Surova E., Follo M., Köhler F., Wardemann H., Zirlik K., Veelken H., and Jumaa H. (2012) Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature 489, 309–312 10.1038/nature11309 [DOI] [PubMed] [Google Scholar]
- 90. Minici C., Gounari M., Übelhart R., Scarfò L., Dühren-von Minden M., Schneider D., Tasdogan A., Alkhatib A., Agathangelidis A., Ntoufa S., Chiorazzi N., Jumaa H., Stamatopoulos K., Ghia P., and Degano M. (2017) Distinct homotypic B-cell receptor interactions shape the outcome of chronic lymphocytic leukaemia. Nat. Commun. 8, 15746 10.1038/ncomms15746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ten Hacken E., Gounari M., Ghia P., and Burger J. A. (2019) The importance of B-cell receptor isotypes and stereotypes in chronic lymphocytic leukemia. Leukemia 33, 287–298 10.1038/s41375-018-0303-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Conley M. E., Fitch-Hilgenberg M. E., Cleveland J. L., Parolini O., and Rohrer J. (1994) Screening of genomic DNA to identify mutations in the gene for Bruton's tyrosine kinase. Hum. Mol. Genet. 3, 1751–1756 10.1093/hmg/3.10.1751 [DOI] [PubMed] [Google Scholar]
- 93. Kobayashi S., Iwata T., Saito M., Iwasaki R., Matsumoto H., Naritaka S., Kono Y., and Hayashi Y. (1996) Mutations of the Btk gene in 12 unrelated families with X-linked agammaglobulinemia in Japan. Hum. Genet. 97, 424–430 10.1007/BF02267060 [DOI] [PubMed] [Google Scholar]
- 94. Carozzi A. J., Kriz R. W., Webster C., and Parker P. J. (1992) Identification, purification and characterization of a novel phosphatidylinositol-specific phospholipase C, a third member of the β-subfamily. Eur. J. Biochem. 210, 521–529 10.1111/j.1432-1033.1992.tb17450.x [DOI] [PubMed] [Google Scholar]
- 95. Struppe J., Whiles J. A., and Vold R. R. (2000) Acidic phospholipid bicelles: a versatile model membrane system. Biophys. J. 78, 281–289 10.1016/S0006-3495(00)76591-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Piechulek T., Rehlen T., Walliser C., Vatter P., Moepps B., and Gierschik P. (2005) Isozyme-specific stimulation of phospholipase C-γ2 by Rac GTPases. J. Biol. Chem. 280, 38923–38931 10.1074/jbc.M509396200 [DOI] [PubMed] [Google Scholar]
- 97. Gross A., Kracher B., Kraus J. M., Kühlwein S. D., Pfister A. S., Wiese S., Luckert K., Pötz O., Joos T., Van Daele D., De Raedt L., Kühl M., and Kestler H. A. (2019) Representing dynamic biological networks with multi-scale probabilistic models. Commun. Biol. 2, 21 10.1038/s42003-018-0268-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fu G., and Gascoigne N. R. (2009) Multiplexed labeling of samples with cell tracking dyes facilitates rapid and accurate internally controlled calcium flux measurement by flow cytometry. J. Immunol. Methods 350, 194–199 10.1016/j.jim.2009.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Camps M., Hou C. F., Jakobs K. H., and Gierschik P. (1990) Guanosine 5′-[γ-thio]triphosphate-stimulated hydrolysis of phosphatidylinositol 4,5-bisphosphate in HL-60 granulocytes. Evidence that the guanine nucleotide acts by relieving phospholipase C from an inhibitory constraint. Biochem. J. 271, 743–748 10.1042/bj2710743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Axelrod D., Koppel D. E., Schlessinger J., Elson E., and Webb W. W. (1976) Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 16, 1055–1069 10.1016/S0006-3495(76)85755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., and Webb W. W. (1976) Dynamics of fluorescence marker concentration as a probe of mobility. Biophys. J. 16, 1315–1329 10.1016/S0006-3495(76)85776-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Petersen T. (1986) Fitting parametric survival models with time-dependent covariates. J. R. Stat. Soc. Ser. C Appl. Stat. 35, 281–288 10.2307/2348028 [DOI] [Google Scholar]
- 103. Efron B., and Tibshirani R. J. (1994) The Bootstrap Estimate of Standard Error. In An Introduction to the Bootstrap, pp. 45–59, Chapman and Hall/CRC Press, Boca Raton, FL [Google Scholar]
- 104. Gutman O., Walliser C., Piechulek T., Gierschik P., and Henis Y. I. (2010) Differential regulation of phospholipase C-β2 activity and membrane interaction by Gαq, Gβ1γ2, and Rac2. J. Biol. Chem. 285, 3905–3915 10.1074/jbc.M109.085100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Meyer S. L. (1975) Propagation of Error and Least Squares. In Data Analysis for Scientists and Engineers, pp. 39–48, Peer Management Consultants, Evanston, IL [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the manuscript. Raw data as well as computer subroutines used for data processing and equations used to calculate propagation of experimental errors are available on request from Peter Gierschik (peter.gierschik@uni-ulm.de).