Abstract
Coronavirus disease (COVID-19) was first diagnosed in Wuhan in December 2019. The World Health Organization defined the subsequent outbreak of COVID-19 worldwide as a public health emergency of international concern. Epidemiological data indicate that at least 20% of COVID-19 patients have severe disease. In addition to impairment of the respiratory system, acute kidney injury (AKI) is a major complication. Immune damage mediated by cytokine storms and concomitant AKI is a key factor for poor prognosis. Based on previous experience of blood purification for patients with severe acute respiratory syndrome and Middle East respiratory syndrome combined with clinical front-line practice, we developed a blood purification protocol for patients with severe COVID-19. This protocol is divided into four major steps. The first step is to assess whether patients with severe COVID-19 require blood purification. The second step is to prescribe a blood purification treatment for patients with COVID-19. The third step is to monitor and adjust parameters of blood purification. The fourth step is to evaluate the timing of discontinuation of blood purification. It is expected that blood purification will play a key role in effectively reducing the mortality of patients with severe COVID-19 through the standardized implementation of the present protocol.
Keywords: COVID-19, Blood purification treatment, SARS-CoV-2
In December 2019, a sudden public health incident (the coronavirus disease [COVID-19] epidemic) occurred in Wuhan, China.1 The disease caused by the pathogenic virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was named “COVID-19” by the World Health Organization (WHO) on February 11, 2020. Its original English name, “novel coronavirus pneumonia,” was revised by the National Health Commission of China on February 22, 2020 to “COVID-19,” which is consistent with the WHO naming, although its Chinese name remains unchanged. So far, more than 70,000 cases have been confirmed in China. According to reports in the literature, the proportions of patients with severe and critical COVID-19 are 13.8% and 4.7%, respectively, and the overall crude case fatality rate is approximately 2.3%.2 At present, the prevention and treatment of COVID-19 have entered critical stage, and the effective treatment of severe and critically ill patients is the key to reducing associate mortality. The “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Sixth Edition)”3 and “Diagnosis and Treatment Protocol for Severe and Critical Cases of Novel Coronavirus Pneumonia (Trial)”4 both mention that in addition to active oxygen therapy and respiratory support, circulatory monitoring and support, nutritional support treatment, occurrence of acute kidney injury (AKI), and multiple organ dysfunction syndrome (MODS) should be evaluated in a timely manner. For critically ill patients with high inflammatory response, plasma purification, absorption, perfusion, blood/plasma filtration, and other blood purification techniques should be performed if possible.
Excessive inflammatory response in severe COVID-19 and target organ involvement
The severity of COVID-19 depends on the patient's immune status and target organ involvement. Excessive immune response can trigger cytokine storms by multiple excessive inflammatory responses throughout the body and damage the multiple target organs involved.
Clinical studies have shown that the concentrations of serum inflammatory mediators, including interleukin (IL)-2, IL-7, IL-10, granulocyte colony-stimulating factor, interferon-inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1α, and tumor necrosis factor α (TNF-α), were significantly higher in the sera of patients with severe COVID-19 than in those of patients with mild disease.5 The first recent report on the pathological anatomy of dead COVID-19 patients showed that the lungs had diffuse alveolar damage with fibromucosal exudation and hyaline membrane formation, which was consistent with acute respiratory distress syndrome (ARDS). Flow cytometry revealed that the CD4+ and CD8+ cell counts in peripheral blood were greatly reduced; however, the double high positive ratios of human leukocyte antigen-DR (CD4 3.47%) and CD38 (CD8 39.4%) confirmed the simultaneous existence of lymphocyte hyperactivation.6 In addition, the number of highly proinflammatory CCR4+ CCR6+ Th17 cells increased, and CD8+ T cells demonstrated high cytotoxicity owing to their excessive activation, proving that cytokine storm-mediated immune injury is a key factor that leads to disease aggravation and a poor prognosis.
The lung is the main target organ of SARS-CoV-2. It has been reported that the incidence rate of ARDS among patients with COVID-19 ranges from 17% to 29%,7, 8, 9 thus requiring different degrees of respiratory support. Studies have shown that the S protein of SARS-CoV-2 may bind to the angiotensin-converting enzyme 2 (ACE2) receptor, thereby damaging target organs. ACE2 is highly expressed in the kidney, nearly 100 times higher than that in the respiratory tract, theoretically suggesting that the kidney is also a major target organ of COVID-19.10 Data from a clinical study of 59 patients with COVID-19 (including 28 severe cases) showed that 63% of patients had proteinuria (mild, 47%; moderate, 10%; severe, 6%); 27% of patients with COVID-19 had elevated urea nitrogen levels and 19% of patients with COVID-19 had elevated serum creatinine levels.11 Three recently published clinical studies showed that the incidence rate of AKI among patients with COVID-19 was 3%–7% and that 1.5%–9.0% of patients required continuous renal replacement therapy (CRRT). Among those patients, the incidence rates of AKI among patients with severe disease and critically ill patients admitted to the intensive care unit were significantly increased, ranging from 8.3% to 23.0%, and the proportion of patients who required CRRT was 5.6%–23.0%. The rate of CRRT among patients with AKI was as high as 66.7%–100%.7, 8, 9 This medical evidence indicates that AKI is not uncommon among patients with COVID-19, particularly among those with severe disease. Therefore, for the treatment of patients with COVID-19, there should be prompt screening for AKI risk factors; routine and dynamic monitoring of urine, urine sensitive kidney function, and serum creatinine; daily assessment of AKI complications; and optimization of volume and hemodynamics. The use of nephrotoxic drugs should be avoided whenever possible, and early blood purification interventions should be performed to reduce the occurrence and progression of AKI.
Basic principles of blood purification treatment for patients with severe COVID-19
Blood purification techniques include CRRT, blood/plasma perfusion, absorption, plasma replacement, and other modes of comprehensive blood purification. The basic principles of blood purification treatment for patients with severe COVID-19 mainly include the following: (1) removal of metabolic products such as creatinine and urea nitrogen; removal of various inflammatory mediators by convection, absorption, or plasma replacement; and reshaping of the immune homeostasis; (2) regulation of volume and correction of fluid overload to help maintain hemodynamic stability in critically ill patients; (3) correction of electrolyte and acid-base balance disorders to maintain internal environment stability; (4) control of high fever; and (5) combined treatment with extracorporeal membrane oxygenation (ECMO) for extracorporeal multiple organ support.
Based on the above principles, the internationally renowned critical care experts Claudio Ronco and Jean Louis Vincent jointly published an expert review titled “Coronavirus epidemic: preparing for extracorporeal organ support in intensive care” in the focus column of The Lancet Respiratory Medicine,12 emphasizing their position on blood purification treatment for severe COVID-19: CRRT is the most commonly used blood purification method in clinical practice, and for patients with severe COVID-19 with sepsis and ARDS, blood perfusion/plasma absorption treatment can also be selected to eliminate more inflammatory medium.
Blood purification treatment for patients with severe COVID-19
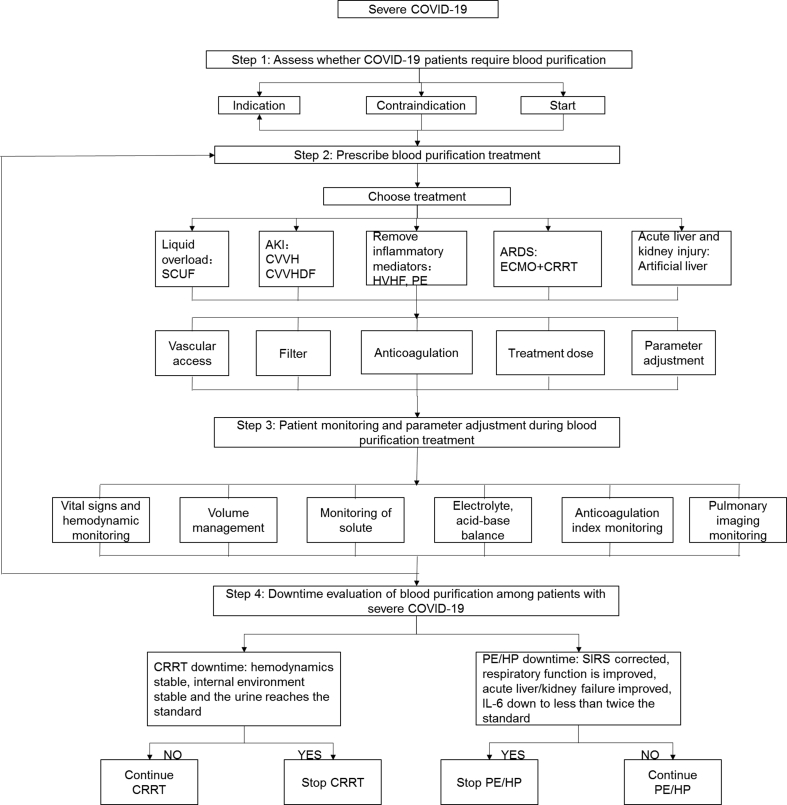
Based on the experiences of performing blood purification for patients with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome combined with the first-line clinical practice of blood purification in the treatment of patients with severe COVID-19, we formulated a process of blood purification treatment for patients with severe COVID-19. The process is divided into four major steps. The first step is to assess whether patients with severe COVID-19 require blood purification. The second step is to prescribe a blood purification treatment modality for patients with severe COVID-19. The third step is to monitor the management and adjust parameters during the blood purification treatment for patients with severe COVID-19. The fourth step involves the downtime evaluation of blood purification. Details of these processes are provided in Fig. 1.
Fig. 1.
Flow chart of blood purification treatment for patients with severe COVID-19. COVID-19: coronavirus disease 2019; SCUF: slow continuous ultrafiltration; AKI: acute kidney injury; CVVH: continuous venovenous hemofiltration; CVVHDF: continuous venovenous hemodiafiltration; HVHF: high volume blood filtration; PE: plasma exchange; HP: blood perfusion; CPFA: continuous plasma filtration absorption; ECMO: extracorporeal membrane oxygenation; CRRT: continuous renal replacement therapy; SIRS: systemic inflammatory response syndrome; IL-6: interleukin 6.
Step 1: Evaluate whether patients with severe COVID-19 require blood purification
This step mainly involves the assessment of the presence or absence of indications, contraindications, and timing of implementation.
-
1.
The indications for blood purification treatment for patients with severe COVID-19 are mainly renal and non-renal. (1) Renal indications: According to the consensus reached at the 17th Acute Disease Quality Initiative meeting,13 when metabolic and fluid management needs exceed renal capacity, acute renal replacement therapy needs to be considered. Blood purification treatment needs to be considered when patients with severe COVID-19 have ① AKI, particularly stage ≥2 that meets the criteria of Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline and ② severe fluid overload and electrolyte and acid-base balance disorder; ③ when maintenance hemodialysis patients have hemodynamic instability, the recommendation is to change to CRRT. (2) Non-renal indications: The following are non-renal indications: ① patients with severe ARDS, septic shock, severe acute liver failure, or MODS; ② patients with excessive inflammatory response; here, the concentration of serum inflammatory mediators (such as IL-6) is ≥5 times the normal value or there is a rate of daily increase of >1 time; and ③ patients with uncontrollable high fever (rectal temperature >39.5 °C).
-
2.
Contraindications to blood purification treatment for patients with severe COVID-19: There are no absolute contraindications to blood purification treatment for patients with severe COVID-19. Blood purification treatment should be cautiously performed when suitable vascular access cannot be established. It is recommended that temporary vascular access be established under ultrasound guidance or with the assistance of vascular surgery.
-
3.
The timing of blood purification treatment for patients with severe COVID-19: (1) When patients with severe COVID-19 have any of the following conditions, CRRT treatment should be initiated immediately: ① volume overload that is refractory to diuretics such as acute pulmonary edema; ② severe hyperkalemia (>6.5 mmol/L) or rapid increase in blood potassium levels with cardiotoxicity; and ③ severe metabolic acidosis (pH value <7.1). (2) When patients with severe COVID-19 develop AKI of KDIGO standard grade ≥2, particularly with sepsis, it is recommended that CRRT be initiated together with other blood purification treatment within 24 hours. (3) It is recommended that blood purification treatment with the main purpose of removing inflammatory mediators be initiated as soon as possible when the serum inflammatory mediator levels of patients with severe COVID-19 reach more than five times the upper limit of normal or increase >1 time within 24 hours.14
In clinical practice, physicians in charge should comprehensively evaluate the systemic inflammatory response, severity and progress of the disease, severity of comorbid AKI, and progress of AKI among patients with COVID-19, and they should consider local medical resources and qualifications of blood purification operators to make reasonable choices.
Step 2: Prescribe blood purification treatment for patients with severe COVID-19
The prescription of blood purification treatment for patients with severe COVID-19 must be formulated according to the needs of patients and physiological goals. The specific contents include the choice of mode of blood purification treatment, establishment of vascular access, choice of blood purification filter, anticoagulation scheme, treatment dose, and initial parameter settings.
-
1.
Mode of blood purification treatment for patients with severe COVID-19: It is recommended that the choice of mode of treatment be goal-oriented. Different modes of treatment should be selected for different treatment goals.15 (1) In patients with severe COVID-19 combined with AKI or severe electrolyte and acid-base balance disorders, CRRT is recommended as first-line treatment. Continuous venovenous hemofiltration (CVVH) or continuous venovenous hemodiafiltration (CVVHDF) can be used. (2) In patients with severe COVID-19 combined with simple volume overload and acute pulmonary edema, slow continuous ultrafiltration (SCUF) is recommended. (3) When the main purpose is to remove inflammatory mediators in patients with severe COVID-19, the following treatment modalities are recommended: high volume hemofiltration (HVHF), high cutoff molecular weight hemofiltration, blood/plasma perfusion, absorption, or continuous plasma filtration absorption. (4) When severe COVID-19 coexists with severe ARDS, ECMO combined with CRRT is recommended. (5) When severe COVID-19 is complicated by acute liver failure, artificial liver therapies such as plasma exchange are recommended. (6) CVVH or CVVHDF may be performed for maintenance hemodialysis among patients with severe COVID-19, and the recommended treatment time is 6–8 hours every other day.
-
2.
Establishment of vascular access: (1) With reference to the KDIGO Clinical Practice Guideline for Acute Kidney Injury,16 it is recommended that temporary central venous access be the first choice. The order of choices of sites for vascular access is first, the right internal jugular vein, and second, the femoral vein. Vascular access via the subclavian vein is not recommended. To ensure hemodynamic monitoring channels among patients with COVID-19 and minimize the risk of infection among medical staff, femoral venous catheterization may be preferred. In addition, the difficulty of tube placement is obviously increased as the medical staff requires at least level 2 protection when placing the catheter. Therefore, it is recommended that vascular access be established under ultrasound guidance. (2) If patients with severe COVID-19 require simultaneous treatment with ECMO, it is recommended that CRRT be integrated into the ECMO system as the preferred option. Specific methods of access are as follows: the arterial and vein lines used in CRRT are connected before the ECMO centrifugal pump, the venous line in front and the arterial line behind; the arterial and venous lines used in CRRT are connected between the ECMO centrifugal pump and the oxygenator, the arterial line in front and the venous line behind; and the arterial line used in CRRT is connected to the Luer lock of the arterial line of the oxygenator and the venous line is connected to the Luer lock of the venous end of the oxygenator.17 Different methods of connecting the ECMO and CRRT components have their own advantages and disadvantages. It is recommended that the appropriate connection method be selected according to the familiarity of each medical unit with the connection method and the feasibility of the technology.
-
3.
Selection of a blood purification filter in blood purification treatment for patients with severe COVID-19: It is recommended that different blood purification filters be selected according to the blood purification method. (1) When administering CRRT, refer to the “Blood purification standard operating procedures (Chinese Edition, 2010)”.18 It is recommended that synthetic membrane filters with sufficient ultrafiltration coefficient (usually ≥50 ml·h−1 mmHg−1) (1 mmHg = 0.133 kPa) and good blood compatibility be used. When the purpose is to remove inflammatory mediators, it is recommended that filters with absorption properties (such as the AN69ST membrane or oXiris membrane) be selected. High molecular weight cutoff filters (membrane pore size up to 8–10 nm, which is approximately 2–3 times the pore size of ordinary high-throughput membranes) can also be used. However, such filters can increase the removal of albumin and other large molecules that should be monitored and supplemented in time. (2) When performing plasma replacement and blood/plasma absorption, the corresponding plasma separator, blood perfusion device, or absorber can be selected according to different methods.
-
4.Anticoagulation scheme of blood purification treatment for patients with severe COVID-19: First, fully evaluate the coagulation function and bleeding risk of patients with COVID-19 and then select the appropriate anticoagulation scheme according to the patient's coagulation status and risk of bleeding.15,19
-
(1)Patients with severe COVID-19 without a risk of bleeding: Patients with severe COVID-19 have a high risk of hypercoagulability. It has been reported that the activated partial thromboplastin time (APTT) and prothrombin time are reduced by 16% and 30%, respectively, 36% of patients present with elevated D-dimer levels,9 and patients with severe COVID-19 often have accompanying severe hypoxemia and tissue hypoperfusion. Therefore, for patients with no active bleeding and normal coagulation or hypercoagulation, heparin or low molecular weight heparin is recommended as anticoagulant. ① Use of heparin: Patients with pre-dilution should receive intravenous injection, with the first dose being 15–20 mg, followed by continuous intravenous infusion of 5–10 mg/h. Patients with post-dilution should receive intravenous injection, with the first dose being 20–30 mg, followed by continuous intravenous infusion of 8–15 mg/h. The infusion should be stopped 30–60 min before the end of treatment. When administering blood perfusion/absorption therapy, the heparin dose can be appropriately increased to maintain APTT at 1.5–2.0 times of the normal value. The activated clotting time can also be monitored and maintained at 200–250 s. ② Use of low molecular weight heparin: The first dose to be intravenously administered is 60–80 U/kg, and an additional dose of 30–40 U/kg can be intravenously administered every 4–6 h. The activity level of anti-factor Xa should be monitored and maintained at 0.25–0.35 U/ml.
-
(2)Patients with severe COVID-19 with active bleeding or a high bleeding risk: ① Patients with an international normalized ratio (INR) ≥1.5 do not require anticoagulation. ② Patients with an INR <1.5 before treatment: For patients with no contraindications to citric acid therapy (hypoxemia and/or insufficient tissue perfusion has been corrected and there is no serious liver dysfunction), it is recommended that the local citric acid anticoagulation scheme be followed. A 4% trisodium citrate solution should be continuously administered at a dose of 1.2–1.5 times of the blood flow rate (ml/min) before the filter is applied. The citrate concentration should reach 3–5 mmol/L before the filter is applied, the free calcium concentration after the filter is applied should be maintained at 0.25–0.35 mmol/L, and the venous free calcium concentration should be maintained at 1.00–1.35 mmol/L. The dose of citric acid should be adjusted according to the ionized calcium concentration after the filter, and the dose of calcium chloride or calcium gluconate solution should be adjusted according to the serum concentration of ionized calcium. For patients with contraindications to citric acid but no contraindications to argatroban, argatroban can be used as an anticoagulant: continuous infusion at 1–2 μg·kg−1·min−1 before the filter or an exact first dose (250 μg/kg) should be administered to control APTT or INR of the blood sample obtained from the peripheral vein or the arterial end of the CRRT line at less than 1.5 times of the base value, and the APTT or INR of the blood sample obtained at the venous end of the CRRT line should be maintained at 1.5–2.5 times of the base value. For patients with contraindications to the use of argatroban, heparin can be used for local anticoagulation: pre-fill the filter with 2 L of normal saline +2500 U of plain heparin. The initial dose of plain heparin is 30 U/kg followed by 1000–1500 U/h through the arterial input. Protamine should be administered via the venous end at the initial ratio of 1 mg protamine: 100 U heparin, to adjust the ratio of heparin to protamine according to the APTT in the body and the circulation circuit and to keep the APTT in the body within the normal range and the APTT in the circulation circuit at 2.0 times of the normal value.
-
(3)Anticoagulation combined with ECMO: ECMO mainly uses systemic heparinization as the main anticoagulation method; thus, it is not necessary to administer anticoagulants during CRRT.
-
(1)
-
5.Treatment dose and parameter setting.
-
(1)CRRT: The KDIGO AKI guidelines16 recommend that when patients with COVID-19 are treated with CRRT, the therapeutic dose is 20–25 ml·kg−1·h−1 when using post-dilution and 25–30 ml·kg−1·h−1 when using pre-dilution. When using HVHF to remove inflammatory mediators, the recommended dose is >35 ml·kg−1·h−1. When SCUF is used, the ultrafiltration rate is generally set to 2–5 ml/min, which can be adjusted in time according to the patient's volume status and hemodynamic stability. In principle, the total amount of SCUF ultrafiltrate should not exceed 4 L.
-
(2)Plasma replacement: In the article titled “Expert Consensus on the Application of Artificial Liver Blood Purification System for the Treatment of Novel Coronavirus Pneumonia of Severe and Critical Conditions,” Li Lanjuan14 reported that the amount of plasma replacement (L) = body weight (kg) × (1/13) × (100–hematocrit) and that at least 2000 ml of plasma should be administered when plasma is scarce.
-
(3)Plasma absorption: The single adsorption treatment dose is usually 1.5–2.0 times the plasma volume. The patient's plasma volume can be calculated and estimated using the following formulas based on the patient's gender, hematocrit, and body weight. ① Plasma volume = (1–hematocrit) × [b + (c × body weight)], where the unit of plasma volume is ml, the unit of body weight is kg, and b and c are constants. The value of b is 1530 for men and 864 for women, and the value of c is 41 for males and 47.2 for females. ② Plasma volume (L) = 0.065 × body weight × (1–hematocrit), where the unit of body weight is kg. The duration of treatment is 2–4 hours. At the beginning of treatment, the blood flow rate in general increases gradually from 50 to 80 ml/min to 100–150 ml/min. The separated plasma flows through the absorption column at a flow rate of 25–50 ml/min and is returned to the body.
-
(1)
Step 3: Patient monitoring and parameter adjustment of blood purification treatment for severe COVID-19
During blood purification treatment for patients with severe COVID-19, patient monitoring should be strengthened, and the treatment parameters should be adjusted according to the monitoring content by titration.
-
1.
Vital signs and hemodynamic monitoring: Closely monitor vital signs based on the actual situation of the individual medical unit and choose a method of hemodynamic monitoring that is as simple and manageable as possible, such as the central venous pressure (CVP), central venous oxygen saturation, and arteriovenous partial pressure of carbon dioxide. When conditions permit, noninvasive ultrasound and dynamic assessment of patients with severe disease are recommended.
-
2.
Volume management during CRRT: Patients with severe COVID-19 usually have ARDS. Under the premise of not affecting tissue perfusion, it is recommended that a negative balance be maintained as much as possible. It is recommended that goal-oriented titration with three-level volume management be used; i.e., when appropriate under hemodynamic monitoring (such as CVP, ultrasound for severe disease), calculate fluid input and output per hour. In particular, pay attention to the amount of insignificant water lost from the respiratory tracts of patients with COVID-19 in respiratory distress.
-
3.
Monitoring of solute clearance: Serum creatinine levels should be monitored daily to ensure that the serum creatinine level on each day of CRRT is lower than that of the previous day. When the purpose is to remove inflammatory mediators, daily monitoring of the blood count, lymphocyte count, and C-reactive protein level and dynamic monitoring of IL-6, IL-10, TNF-α, lymphocyte subsets, and other indicators should be performed. In clinical practice, we should also pay attention to the effect of CRRT on the clearance of drugs used in the treatment of COVID-19. Currently, the most commonly used anti-SARS-CoV-2 drugs (lopinavir/ritonavir and)arbidol are cleared mainly through non-renal routes, and the protein binding rate is above 90%; therefore, there is no need to adjust the dose of these antiviral drugs during CRRT.
-
4.
Electrolyte and acid-base balance monitoring: The blood levels of potassium, sodium, and bicarbonate should be measured every 4–6 hours during CRRT, and the blood levels of magnesium and phosphorus should be measured at least every 24 hours. The replacement fluid/dialysate formula should be adjusted in time according to the test results.
-
5.
Anticoagulation index monitoring: Monitor different indicators according to different anticoagulation methods (see the anticoagulation scheme for details). When there is no anticoagulation, mainly check the coagulation of the filter and help judgment based on the transmembrane pressure and filter front pressure.
-
6.
Pulmonary imaging monitoring: Patients with severe COVID-19 show rapid changes on lung imaging. It is recommended that chest radiographs or lung computed tomography images be monitored at least every 3 days to determine the efficacy.
Step 4: Downtime evaluation of blood purification for patients with severe COVID-19
There is no consensus regarding indications for downtime during blood purification treatment for patients with severe COVID-19. Downtime is recommended in the following situations.
-
1.
Downtime of CRRT: The patient's vital signs are stable, hemodynamics are normal, ventilator conditions are significantly reduced, water electrolyte and acid-base balance disorders are corrected, and the daily urine output without diuretics is ≥500–1000 ml and with diuretics is ≥1500–2000 ml.
-
2.
Downtime of plasma exchange and plasma perfusion/absorption: The performance of systemic inflammatory response syndrome is improved, respiratory function is improved, acute liver failure is obviously corrected, and the levels of serum inflammatory mediators such as IL-6 drop to less than twice the normal value.14
Finally, it should be emphasized that medical personnel must protect and disinfect blood purification equipment and venues when performing blood purification treatment for patients with COVID-19.20
Funding
Zhejiang Provincial Science and Technology Department 2019 Provincial Key R&D Program Project (2019C03024).
Conflicts of interest
None.
Edited by Yan-Gang Ren and Yi Cui
Footnotes
The Chinese version of this article was first published on Natl Med J China, 2020, 100. https://doi.org/10.3760/cma.j.cn112137-20200224-00420.
Peer review under responsibility of Chinese Medical Association.
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z.Y., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020 Feb 24 doi: 10.1001/jama.2020.2648. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.National Health Commission of the People's Republic of China, National Administration of Tranditional Chinese Medicine . 2020. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia.http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml (Trial Version 6) [Google Scholar]
- 4.National Health Commission of the People's Republic of China . 2020. Diagnosis and Treatment Protocol for Severe and Critical Cases of Novel Coronavirus Pneumonia (Trial)http://www.nhc.gov.cn/xcs/zhengcwj/202001/9fbefc9a5fe747e98ea5baeedfb68158.shtml [Google Scholar]
- 5.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y.C., Luo R., Wang K. 2020. Kidney Impairment Is Associated with In-Hospital Death of COVID-19 Patients.https://www.medrxiv.org/content/10.1101/2020.02.18.20023242v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D.W., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7 doi: 10.1001/jama.2020.1585. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan C.B., Li K., Ding Y.H., Wei L.L., Wang J.Q. 2020. ACE2expressioninkidneyandtestismaycausekidneyandtestisdamageafter2019-nCoVinfection.https://www.medrxiv.org/content/10.1101/2020.02.12.20022418v1 [Google Scholar]
- 11.Li Z., Wu M., Guo J., Yao J.W. 2020. Caution on Kidney Dysfunctions of 2019-nCoV Patients.https://www.medrxiv.org/content/10.1101/2020.02.08.20021212v1 [Google Scholar]
- 12.Ronco C., Navalesi P., Vincent J.L. Coronavirus epidemic: preparing for extracorporeal organ support in intensive care. Lancet Respir Med. 2020;8:240–241. doi: 10.1016/S2213-2600(20)30060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostermann M., Joannidis M., Pani A. Patient selection and timing of continuous renal replacement therapy. Blood Purif. 2016;42:224–237. doi: 10.1159/000448506. [DOI] [PubMed] [Google Scholar]
- 14.National Clinical Research Center for Infectious Diseases, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases Expert consensus on the application of artificial liver blood purification system in the treatment of severe and critical COVID-19 (in Chinese) Chin J Clin Infect Dis. 2020;13:1–3. doi: 10.3760/cma.j.issn.1674-2397.2020.01.001. [DOI] [Google Scholar]
- 15.National Renal Disease Professional Medical Quality Management and Control Center, China Medical Promotion Association Blood Purification Treatment and Engineering Technology Branch. PLA Blood Purification Therapy Specialized Committee . 2020. Expert Opinion on the Application of CRRT in the Treatment of Novel Coronavirus Pneumonia.http://www.cnrds.net/Static/file/%E6%96%B0%E5%9E%8B%E5%86%A0%E7%8A%B6%E7%97%85%E6%AF%92%E8%82%BA%E7%82%8E%E6%95%91%E6%B2%BB%E4%B8%ADCRRT%E5%BA%94%E7%94%A8%E7%9A%84%E4%B8%93%E5%AE%B6%E6%84%8F%E8%A7%81%2020200206.pdf [Google Scholar]
- 16.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 17.Ostermann M., Connor M., Jr., Kashani K. Continuous renal replacement therapy during extracorporeal membrane oxygenation: why, when and how. Curr Opin Crit Care. 2018;24:493–503. doi: 10.1097/MCC.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 18.Chen X.M. People's Military Medical Press; Beijing: 2010. Standard Operating Procedures for Blood Purification. [Google Scholar]
- 19.Yang X.H., Zhang L.N., Hu B. Standardized treatment procedure for continuous renal replacement therapy (in Chinese) Chin J Crit Care Med. 2019;5:27–31. doi: 10.3877/cma.j.issn.2096-1537.2019.01.006. [DOI] [Google Scholar]
- 20.Expert team of Chinese Medical Association Nephrology Branch Recommendations for prevention and control of novel coronavirus infection in blood purification center (room) from the Chinese Medical Association Nephrology Branch (in Chinese) Chin J Neahrol. 2020;36:82–84. doi: 10.3760/cma.j.issn.1001-7097.2020.02.002. [DOI] [Google Scholar]