Editor—Some patients with confirmed or suspected coronavirus disease 2019 (COVID-19) need emergency or urgent surgery, including Caesarean delivery. Vertical and perinatal mother-to-newborn transmission of COVID-19 has not yet been confirmed,1 , 2 although there are reports of COVID-19 infections in newborns.3, 4, 5, 6, 7 We report three mothers with COVID-19 and the outcomes of their newborns in Henan Province, China. The patients or their family members provided consent for this publication. All three mothers did not have significant past medical histories. Specimens from pharyngeal, laryngeal, throat, and tracheal tube tip were collected from the mothers and newborns with synthetic fibre swabs. The decision to perform Caesarean delivery was based on either a confirmed or suspected maternal diagnosis of COVID-19, and the obstetricians' desire to shorten the course of delivery. All three newborns were tested for COVID-19 in accordance with governmental policies requiring testing of newborns of mothers with confirmed or suspected COVID-19. In all three cases, the obstetricians, anaesthesiologists, neonatologists, and nurses wore full personal protective equipment (PPE), including an N95 mask, eye goggles, face shield, and a top-to-bottom tight-fitting gown, entering the operating theatres ∼5 min before the patients. Notably, only obstetricians touched both the mothers and newborns during the time of Caesarean delivery, handing the newborns off to the neonatologists after delivery. The resuscitation tables for newborns were ∼3 m away from the head of the mothers. The operating theatres in Cases 2 and 3 were equipped with negative pressure to minimise virus spread. The environmental surfaces of operating and inpatient rooms were routinely decontaminated with chlorine 2000 mg L−1 disinfectant for 30 min after the patients had exited the operating theatres or were discharged from inpatient rooms. Medical staff followed PPE doffing procedures according to standard guidelines, which included spraying the surfaces of PPE with ethanol 75% before removal and washing hands after doffing.
The first patient was a 28-yr-old nulliparous woman at 37 weeks gestation, who had lived in Wuhan and arrived in Xinyang, Henan Province the third week in January 2020. The next day she developed a fever, on Day 4 she developed a productive cough, and on Day 8 the mother tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by RT–PCR assay. Additional laboratory evaluation showed marked abnormalities (Supplementary Table S1). The mother's course was notable for fever, tachypnoea, and hypoxaemia. She had a Caesarean delivery under general anaesthesia with rapid sequence induction and easy intubation using video-assisted laryngoscopy on Day 9, and delivered a male with Apgar scores of 9 and 9 at 1 and 5 min, respectively. The mother did not have regional anaesthesia because of abnormal liver function and coagulopathy. The anaesthesia circuit had an electrostatic filter to avoid contaminating the machine and gas scavenging system. The operating theatre was not a negative-pressure airborne infection isolation room. The mother wore a face mask except during intubation and mechanical ventilation.
The newborn was taken from the operating theatre before extubation of the mother. For the remainder of his hospitalisation, he was in the parent's inpatient room, but was placed in a temperature-controlled isolator 3 m away from the mother's head. He was cared for by a nurse who was not in physical contact with the mother or other visitors after the delivery. Visitors wore masks in the mother's room, but were not allowed to be in contact with the newborn. The mother wore a face mask at all times after the surgery, and the medical staff wore PPE in the inpatient room as they did in the operating theatre. The newborn was discharged home 11 h after birth and tested positive for SARS-CoV-2 on Postnatal day (PND) 6. Three days later, his caregiver (grandmother) also tested positive for SARS-CoV-2. The mother recovered well and was discharged home on Day 26; 19 days after delivery, no caregivers had developed COVID-19. The hospital has since implemented a rooming policy to immediately isolate newborns from mothers with COVID-19.
The second patient was a 30-yr-old pregnant (G3P2) woman at 30.5 weeks gestation, who also lived in Wuhan and arrived in Henan Province the third week in January 2020. She had a cough, fever, dyspnoea, and abnormal clinical laboratory results (Supplementary Table S1), and tested positive for SARS-CoV-2 near the end of January 2020. On Day 4 of illness, the decision was made for urgent Caesarean delivery under spinal anaesthesia; the newborn boy had Apgar scores of 5 and 8 at 1 and 5 min, respectively. The mother wore a mask during the procedure and had mild coughing. The newborn was placed in an isolation room in the neonatal ICU shortly after delivery; he was intubated for 4 days because of prematurity and received surfactant treatment. The newborn tested negative for SARS-CoV-2 on PND 3. The mother's clinical condition deteriorated and she passed away on Day 35.
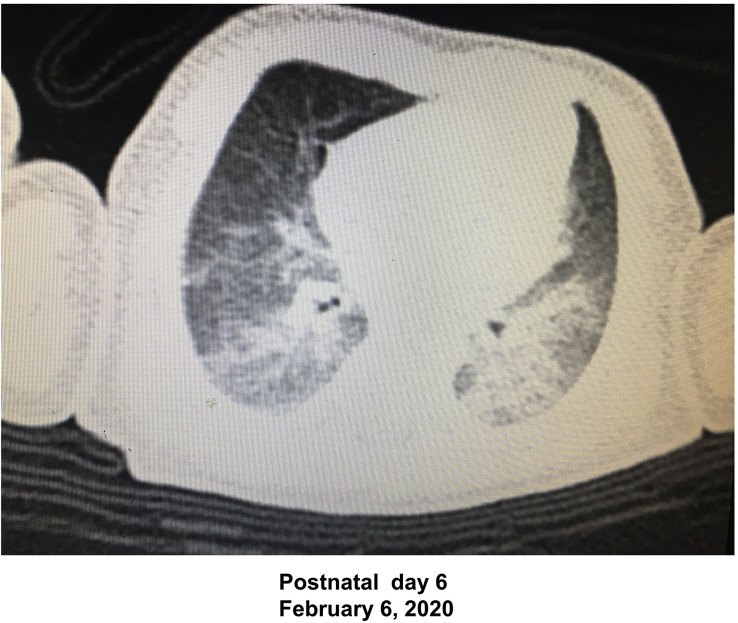
The third patient was a 29-yr-old pregnant woman at 36 weeks gestation who had a fever and cough, relatively normal clinical laboratory results (Supplementary Table S1), and tested positive for SARS-CoV-2 on Postpartum day 6. The woman had had an urgent Caesarean delivery under spinal anaesthesia on January 31, 2020, with the delivery of a newborn boy (Apgar scores of 3 and 5 at 1 and 5 min, respectively). The mother wore a mask during the procedure and had mild coughing. The newborn initially had asphyxia and was clinically managed in the operating theatre for ∼10 min without intubation. The newborn was then transferred to an isolation room in the neonatal ICU, and tested negative for SARS-CoV-2 on PND 8 and 20. Because he had a fever on PND 3, lung rales, and abnormal laboratory results (leucocytes 15.7×109 L−1, neutrophils 72.1%, lymphocytes 19.1%, C-reactive protein 25.1 mg L−1, erythrocyte sedimentation rate 31 mm, lactate dehydrogenase 528 U L−1, and creatine kinase muscle–brain fraction 38 U L−1], and because his mother had tested positive for SARS-CoV-2, a chest CT scan of the newborn was performed on PND 6 that showed findings suggestive of COVID-19 (Fig 1 ). Respiratory syncytial virus, adenovirus, influenza virus, Mycoplasma, Chlamydia, and other infections were excluded. COVID-19 was diagnosed based on the clinical and CT scan criteria.
Fig 1.
Chest CT scan of the newborn in Case 3. The CT scan shows the image consistent with COVID-19 in the newborn.
These three women infected with COVID-19 in the peripartum period had three different maternal and neonatal outcomes. The first case suggests mother-to-newborn transmission of SARS-CoV-2 during an urgent Caesarean delivery under general anaesthesia. The other two mothers both had urgent Caesarean deliveries under spinal anaesthesia; one baby never became infected and the other baby was diagnosed with COVID-19 despite negative SARS-CoV-2 testing. It is not clear whether transmission of COVID-19 to the newborn in Case 1 occurred in utero or in the operating theatre, recovery room, or community whilst being cared by his grandmother (wearing a mask), or whether the route of transmission was via airborne droplets, personal contact, or blood. The newborn in the third case who tested negative for SARS-CoV-2 had clinical symptoms and classical chest CT findings consistent with COVID-19, although other acute lung diseases could also cause the CT findings. Nevertheless, this report highlights the risk of mother-to-newborn transmission of SARS-CoV-2 and suggests that more systematic investigations are warranted to determine if vertical transmission is possible.
The lessons learnt from this case series are (i) the numbers of visitors should be minimised, and (ii) sufficient PPE is important to prevent caregivers from infection of COVID-19. Limitations in this case series include lack of testing of amniotic fluid and cord blood specimens in newborns, which may have detected the presence of SARS-CoV-2. We recommend consideration of routine testing from these sites when mothers have COVID-19 or are under investigation. Although the route of transmission in these newborns is not clear, it is important to maintain practices to minimise droplet and contact spread; we recommend isolating newborns from SARS-CoV-2-positive mothers immediately after birth and the use of PPE by visitors in the hospital and at home as long as community-based spread is considered a threat in a given geographical area.
Whilst there is a common belief that general anaesthesia, associated with more aerosol generation during intubation, may increase the risk of transmission of SARS-CoV-2,8 our case series included a case of potential transmission under regional anaesthesia. The limited case number in this series precludes conclusions about the association between risk of newborn SARS-CoV-2 transmission and type of anaesthesia, such that it is not yet known whether general or neuraxial anaesthesia9 for Caesarean delivery can lead to different outcomes.
Authors' contributions
Clinical data collection: MS, GX, YY, YT
Writing of article: MS, ZX, JZ
Critical revision: MP-S, VM
Approval of final version: all authors.
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
Joint Research Project of Medical Science and Technology of Henan Province (2018020414); National Natural Science Foundation of China (81901110) to MS; National Natural Science Foundation of China–Henan Joint Fund (U1704165) to JZ.
Acknowledgements
The authors would like to thank the patients and medical staff who provided the information for this report.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.04.066.
Contributor Information
Zhongcong Xie, Email: zxie@mgh.harvard.edu.
Jiaqiang Zhang, Email: jqzhang@henu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S., Guo L., Chen L. A case report of neonatal COVID-19 infection in China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa225. Adv Access Published March 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong L., Tian J., He S. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020 doi: 10.1001/jama.2020.4621. Adv Access Published March 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karimi-Zarchi M., Neamatzadeh H., Dastgheib S.A. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr Pathol. 2020 doi: 10.1080/15513815.2020.1747120. Adv Access published on April 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020 doi: 10.5858/arpa.2020-0901-SA. Adv Access Published March 12. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z.J., Yu X.J., Fu T. Novel coronavirus infection in newborn babies under 28 days in China. Eur Respir J. 2020 doi: 10.1183/13993003.00697-2020. Adv Access Published March 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odor P.M., Neun M., Bampoe S. Anaesthesia and COVID-19: infection control. Br J Anaesth. 2020 Apr 8 doi: 10.1016/j.bja.2020.03.025. pii: S0007-0912(20)30200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia H., Zhao S., Wu Z., Luo H., Zhou C., Chen X. Emergency Caesarean delivery in a patient with confirmed COVID-19 under spinal anaesthesia. Br J Anaesth. 2020;124:e216–e218. doi: 10.1016/j.bja.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.