Graphical abstract
Keywords: Schinus terebinthifolius Raddi, Photoprotection, Sunscreen formulation, Zika virus, Placenta protection, Antiviral activity
Highlights
-
•
Extracts from Schinus terebenthifolius successfully inhibited Zika virus entry in trophoblast cells.
-
•
They present a potential early antiviral effect.
-
•
Present data represent a potential hope in a scarce therapeutical field.
-
•
The extract and formulations are excellent for photoprotection.
-
•
Resveratrol was evidenced for the first time in whole fruit and peel ethanolic extracts.
Abstract
Schinus terebinthifolius Raddi is a well-known medicinal plant native of South America. This species has demonstrated important biological activities such as antihypertensive and vasodilator, antimicrobial, anti-inflammatory and antioxidant. However, no studies have been, so far, reported with the fruits of S. terebinthifolius as a protector of the placenta against Zika virus infection and as sunscreen agents. The present study aimed to investigate new uses for the ethanolic fruit extracts of S. terebinthifolius, from fruits’peel (STPE) and from the whole fruits (STWFE). Zika virus (ZIKV) has been linked to several fetal malformations, such as microcephaly and other central nervous system abnormalities. Thus, the potential of these natural extracts against ZIKV infection was evaluated, using an in vitro method. The photoprotective potential, determined by spectrometry, along with phenolic content, antioxidant capacity, and chemical composition of both extracts were also evaluated. The chemical composition of the extracts was evaluated by HPLC-UV / vis. The cytotoxicity of peel and whole fruit extracts in vero E6 cell lines, in placental cell lines and placental explant cultures were evaluated by the MTT assay. The infectivity of placental cells and explants was evaluated by qRT-PCR and the effects of extracts on ZIKV infection were investigated using HTR-8/SVneo cells, pre-treated with 100 μg mL−1 of STWFE for 1 h, and infected with MR766 (AD) or PE243 (EH) ZIKV strains. STFE and STWFE were well-tolerated by both placental-derived trophoblast cell line HTR-8/SVneo as well as by term placental chorionic villi explants, which indicate absence of cytotoxicity in all analysed concentrations. Two strains of ZIKV were tested to access if pre-treatment of trophoblast cells with the STWFE would protect them against infection. Flow cytometry analysis revealed that STWFE extract greatly reduced ZIKV infection. The extracts were also photoprotective with SPF values equivalent to the standard, benzophenone-3. The formulations prepared in different concentrations of the extracts (5–10 %) had shown maximum SPF values of 32.21. STWFE represents a potential natural mixture to be used in pregnancy in order to restrain placental infection by ZIKV and might potentially protect fetus against ZIKV-related malformations. The extracts exhibited photoprotective activity and some of the phenolic compounds, mainly resveratrol, catechin and epicatechin, are active ingredients in all assayed activities. The development of biotechnological/medical products, giving extra value to products from family farming, is expected, with strong prospects for success.
1. Introduction
Herbs and spices are of great commercial importance. In particular, Schinus terebinthifolius Raddi (Anacardiaceae), a South America-native plant, widely found on the Brazilian coast and popularly known as “pimenta-rosa” or “aroeira-vermelha”. This plant provides a fruit, used as a refined spice in world cuisine, due to its soft taste and good appearance (Pagani et al., 2014; Silva et al., 2017a, b). Re-examination of safe medicinal natural products toward new targets is strategic.
S. terebinthifolius Raddi is inserted in the Brazilian Relation of Essential Medicines (RENISUS) (Brazilian Ministry of Health, 2017), in which there are 71 medicinal plants that present the potential to generate products of interest to the Brazilian Unified Health System (SUS) (DiCiaula et al., 2014; Torres et al., 2016). This species is present in the Phytotherapeutic Form of the Brazilian Pharmacopoeia (2011) (Anvisa, 2011). Important biological activities, such as antihypertensive and vasodilatory (Glória et al., 2017), antimicrobial (Degaspari et al., 2005; Silva et al., 2017a, b); anti-allergic (Cavalher-Machado et al., 2008), antioxidant, antiproliferative and anti-inflammatory (Silva et al., 2017a, b); antioxidant and protective against doxorubicin-induced cardiotoxicity (Rocha et al., 2018) and, more recently, against multidrug-resistant strains of hospital origin (Gomes et al., 2019) were already reported.
Zika virus (ZIKV) and dengue virus (DENV), viral infections transmitted by vectors, are threats to health and reasons for international concern, for instance, microcephaly among newborns and incidences of Guillain-Barré syndrome among adults. As such, we decided to evaluate the ability of this medicinal plant to inhibit ZIKV infection, especially in placenta.
The placenta is a unique immunological site, responsible for maternal tolerance to the fetus and for maternal and fetal defense against possible pathogens. Maternal decidual cells and several leukocytes in the basal decidua of the placenta are involved in local and systemic immunomodulation, but trophoblast cells also express different receptors from the innate immune response, which are known to bind to a plethora of pathogens, one of them the Zika virus (ZIKV) (Tabata et al., 2016; Aagard et al., 2017).
ZIKV belongs to the Flaviviridae family and was first isolated in Nigeria, in 1954. Since then, several outbreaks were reported mainly between 2007 and 2015, when it lastly hitted the Americas, causing a worrying number of newborns with brain malformations (Rodriguez-Morales et al., 2018). During pregnancy, the ZIKV can be vertically transmitted, and infects the fetus, which may develop the congenital Zika syndrome, characterized by stillbirth/miscarriage, fetal growth restriction, microcephaly, ocular abnormalities, ventriculomegaly and other brain malformations (França et al., 2016).
Diverse studies using mice models were published in recent years (Miner et al., 2016; Adibi et al., 2016; Aliota et al., 2016; Cao et al., 2017). The animal models have successfully demonstrated that ZIKV infects the placenta and fetal brain, during early pregnancy, with human placentas’ comparable histopathological findings, represented by poor pregnancy outcomes, as fetal growth restriction, abortions and fetal demise (Miner et al., 2016; Adibi et al., 2016; Aliota et al., 2016). Nevertheless, the placenta is morphologically and physiologically different between rodents and humans, and results should be carefully analysed (Chaouat and Clarke, 2015).
Furthermore, experimental infection models using in vitro human placental explants, and bi- and tri-dimensional culture models, have also demonstrated ZIKV infection and replication in the placenta. Mainly, Hofbauer cells, mesenchymal cells, fibroblasts and cytotrophoblast cells (both villous and extravillous) are prone to be infected and act as virus reservoirs, as wells as decidual cells and decidual macrophages in the maternal compartment (Tabata et al., 2016; Aagard et al., 2017). As such, trophoblast cells that compose the placental barrier can be infected and, besides primary cultures, several trophoblast cell lines were also successfully proven to be infected by ZIKV, as Swan 71, JEG-3, BeWo and HTR-8/SVneo cells. As such, they provide good models to test ZIKV molecular interactions and potential interveners (Aldo et al., 2016; Arumugasaamy et al., 2018; Luo et al., 2018; Cao et al., 2017).
In this context, inhibiting ZIKV infection of trophoblast cells and its crossing of the placental barrier would be important to prevent ZIKV deleterious effects to the developing fetus, and several natural products have been investigated, due to their antiviral effects on viral entrance and cellular replication (Batista et al., 2019).
Additionally, phytochemical studies have reported the presence of phenolic compounds as the main constituents of extracts of Schinus terebinthifolius Raddi (Santana et al., 2012; Pagani et al., 2014; Feuereisen et al., 2014, 2017; Gomes et al., 2019). They exert important biological activities as antioxidant and photoprotective compounds, due to their capacity to fight against reactive oxygen and nitrogen species and through absorbing ultraviolet (UV) radiation (Saewan and Jimtaisong, 2013; Agati et al., 2013; Souza et al., 2015). Ultraviolet A (320−400 nm) and B (290−320 nm) radiations are associated with a number of irreversible damages to cutaneous tissue, such as photoaging and skin cancer, while UVB radiation is related with immune dysfunction, erythema and sunburn, triggering oxidative stress and DNA damage (Afaq, 2011; Gregoris et al., 2011). One of the preventive measures against these radiations is the use of sunscreens. There is a renewed interest in the incorporation of natural extracts with antioxidant properties, in a sunscreen. The reasons for that are the perspective of reducing oxidative damage induced by UV rays, increasing physical and chemical stabilities of the sunscreens, and complementing the photoprotective action (Scalia and Mezzena, 2010; Prá et al., 2017), as recently demonstrated (Afonso et al., 2014; Działo et al., 2016; De Oliveira-Júnior et al., 2017). Protective formulations prepared with the crude extract of the leaves of S. terebinthifolius showed absorption on the UVB photoprotection area (Bulla et al., 2015).
Hitherto, up to our knowledge, there are no studies with the peel and fruits of S. terebinthifolius, as placenta protector against Zika virus infection and as sunscreen agents. Thus, the objectives of this work are to reveal new and relevant properties of the ethanolic extracts of the peel and whole fruit of S. terebinthifolius and to contribute insights into the development of ZIKV therapeutics and photoaging, adding potential value for this product from family farming.
2. Materials and methods
2.1. Materials
The analytical grade compounds were purchased from Sigma-Aldrich (St. Louis, USA): DPPH (α,α-diphenyl-picrylhydrazyl radical), TPTZ (2,4,6-tripyridyl-s-triazine), Folin-Ciocalteau reagent (FC), quercetin, catechin, resveratrol, gallic, coumaric and acetic acids, DMEM/F12 culture medium, L-15 medium, Hank's balanced salt solution (HBSS), phosphate buffered saline (PBS) and trypsin-EDTA. Fetal bovine serum (FBS) and l-glutamine were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Benzophenone-3 was purchased from Pharma Nostra (São Paulo, Brazil). Chloroquine was provided by Farmanguinhos (Fundação Oswaldo Cruz, Rio de Janeiro, Brazil). The Lanette base used as a vehicle for the preparation of the formulations was purchased from local merchandising pharmacy. Acetonitrile and ethanol were acquired from Merck (Germany). All the reagents were of analytical grade and the stock solutions and buffers were prepared with Milli-Q purified water.
2.2. HTR-8/SVneo cell line culture
The HTR-8/SVneo (ATCC® CRL-3271™) was kindly donated by Prof. Estela Bevilacqua from University of Sao Paulo (USP, São Paulo, Brazil). It is a placenta cell line derived from first trimester extravillous trophoblast cells, routinely cultured in DMEM/F12 medium supplemented with 10 % fetal bovine serum (FBS) and 2 mM l-glutamine and kept in humid incubator at 37 °C and 5 % CO2. Cells were subcultured every 5 days with 80 % confluence for HTR-8/SVneo cells and the medium is replenished every 2 days.
2.3. Ethical issues
The use of placentas was approved by the ethical committee from Federal University of Alagoas, throughout the national Plataforma Brasil unified system (CAEE 57828616.3.0000.5013), and with signed informed consent from all patients according to Brazilian Health Ministry guidelines.
2.4. Placenta collection and chorionic villi explants culture
Placentas derived from term pregnancies (37–40 weeks of pregnancy) were obtained at the Obstetrics Service from Prof. Alberto Antunes University Hospital of the Federal University of Alagoas, Brazil (HUPAA/UFAL) by elective cesarean section. Three term placentas from healthy pregnant women were collected from women without detectable infections, hypertensive disorders, chronic diseases or other conditions. After placenta collection, fresh samples from the chorionic villi were extensively washed in 0.1 mol L−1 PBS and in HBSS, followed by the separation of terminal chorionic villi for explants culture in 24-well plates with DMEM/F12 supplemented medium, and kept in humid incubator at 37 °C and 5 % CO2, where they can be cultured until 12 days, without loosing significant viability, as described previously (Miller et al., 2005) and routinely used worldwide.
2.5. Sample preparation and extraction
The fruit peels and whole fruits from S. terebinthifolius Raddi are craft trade products, furnished by familiar farmers of Aroeira Association, supervised by Eco Engenho Institute, in Maceió, Alagoas State, Northeast of Brazil, kept in aluminium bags, under vacuum. The plants were identified by Mrs. Rosângela P. L. Lemos and samples were deposited in the herbarium at the Instituto do Meio Ambiente, Alagoas State, Brazil. The crude extracts of the whole fruits (STWFE) and the fruit’s peel of S. terebinthifolius (STPE) were prepared using ethanol, in a Soxhlet apparatus, heated for a period of 6 h. The solvent was removed in a rotary evaporator, to obtain STPE and STWFE. The extracts were stored in amber glass, under refrigeration (5 °C).
2.6. Crude extracts analysis
2.6.1. Determination of total phenolic content
The total phenolic contents (TPC) of the ethanolic extracts were determined using FC reagent, as described by Cicco et al. (2009) with the following modifications: aliquots (120 μL) of ethanolic solutions of dried extract (10 μg mL−1), were placed in test tubes, followed by the addition of 180 μL of water. Then, 300 μL of FC reagent were added to each tube. After 2 min, 2.4 mL of a 5 % (w/v) sodium carbonate solution were added. The mixture was shaken and heated, at 40 °C, in a water bath, for 20 min. The tubes were, then, rapidly cooled and the developed color analysed at 760 nm, in a MultiSpec–1501 UV–vis spectrophotometer (Shimadzu, Japan). The same procedure was performed, using 120 μL of ethanol as a blank. The concentration of phenolic compounds was estimated using a calibration curve traced with gallic acid (GA) in ethanol (1.5–13 × 10−4 mol L−1) as a polyphenol reference, in triplicate. The results are expressed as mg of GA equivalents g−1 of dry extract (mg GAE g−1 dry extract).
2.6.2. Radical (DPPH)• scavenging activity (RAS – DPPH•)
The antioxidant capacities of the extracts were measured in terms of their radical scavenging ability (RSA), using the DPPH• method (Saânchez-Moreno et al., 1999). The solution obtained from 0.30 mL of the extract dissolved in ethanol was mixed with 2.7 mL of DPPH• radical solution (40 μg mL−1 in methanol) to give a final concentration of sample of approximately 10 μg mL−1. The mixture was homogenized and stored in the dark, prior to analysis. The percentage of DPPH• radical – scavenging activity (%RSA - DPPH•) of sample was calculated as follows %RSA = (1- AC/AD) x 100, where AC is the absorbance of the solution when the sample was added at a particular level; AD is the absorbance of the original DPPH• solution. The IC50 (half maximal inhibitory concentration) was calculated graphically, using a calibration curve in the linear range, by plotting the extract concentration versus the corresponding scavenging effect (I% inhibition percentage), at 30 min. The value of I% was calculated using the equation: I% = [(A0 – A1) A0] × 100, where A0 is the absorbance of the control and A1, the absorbance in the presence of the extract.
2.6.3. FRAP assay
The assay was performed according to the method described by Benzie and Strain (1996), with some modifications. Briefly, the FRAP reagent is prepared by mixing 2.5 mL of FeCl3 (20 mmol L−1), 2.5 mL of a solution of TPTZ (10 mmol L−1) in 40 mmol L−1 HCl and 25 mL of 0.30 mol L−1 acetate buffer (pH 3.6). Sample aliquots (90 μL) were mixed with 270 μL of distilled water and 2.7 mL of FRAP reagent and incubated at 37 °C for 30 min, resulting in a final concentration of 10 μg mL−1 of extract. The absorbance of the reaction mixture was measured at 595 nm and a calibration curve was obtained, using Trolox (0.15–30 μmol L−1). The results are expressed as Trolox Equivalent Antioxidant Capacities (TEACFRAP), in μmol of Trolox g−1 of dry extract.
2.6.4. Sun protection factor (SPF)
The photoprotective capacity of the ethanolic extracts was determined according to the method described by Mansur et al. (1986). Aliquots of the stock solution of the crude extract (0.1 g mL−1) were used in the preparation of the following concentrations: 0.2, 2, 5, 10 and 15 mg mL−1. These solutions were read in triplicate, in a spectrophotometer (MultiSpec–1501 UV–vis - Shimadzu, Japan), at the wavelength range of 290−320 nm with 5 nm increments. Ethanol was used as a blank. The SPF values of the ethanol extracts of S. terebinthifolius (STWFE and STPE) were calculated using Eq. (1).
| (1) |
where CF is the correction factor (equal to 10); EE (λ) is the erythemal effect spectrum; I (λ) is the solar intensity spectrum and Abs (λ) is the absorbance of the solution at a wavelength (λ). The values of EE x I are constant and were determined, following Sayre et al. (1979).
2.6.5. HPLC analysis
The identification of phenolic compounds was carried out, using a liquid chromatograph Shimadzu (VP series, Kyoto, Japan), a system controller (CBM-20A), a pump (LC-20AT vp) and a column oven (CTO-20A/C). The column used was a Shimadzu VP-ODS C18 column (250 L x4.6 mm), with an UV/vis detector (SPD-M20A) and a computer software (LC-solution). The reversed-phase HPLC analyses were adapted from Glória et al. (2017). The mobile phase was composed by 0.5 % acetic acid in purified water (solvent A), and acetonitrile (solvent B). The solvent gradient was programmed as: 0’: 100 % A; 5’: 85 % A; 10’: 80 % A; 15’: 70 % A; 20’: 60 % A; 25’: 50 % A; 30’: 70 % A; 40’: 85 % A; 41’: stop. A flow rate of 1.0 mL min−1 and a column temperature of 36 °C. STPE and STWFE extracts were dissolved in the mobile phase (5 mg mL−1) and filtered through a 0.45 μm nylon membrane, prior to HPLC injection. The injection volume was 20 μL, using a detection wavelength of 280 nm. The phenolic compounds present in the extracts were identified by comparing the retention times (Rt) of the standards, as well as by co-injection of the samples with standards. The standards were dissolved in the mobile phase to reach a concentration of 0.5 mg mL−1. The phenolic standards were analysed using the same conditions of the extract.
2.7. Preparation of photoprotective formulations
STPE and STWFE extracts were solubilized in ethanol and incorporated into the Lanette base, with two concentrations 5 and 10 %. Likewise, formulations were prepared containing the chemical filter benzophenone, at 5%, for comparative purposes. Thus, five formulations were prepared and nominated, STPE = Schinus terebinthifolius Peel Extract; STWFE = Schinus terebinthifolius whole Fruit Extract; CSTPE5 (STPE incorporated to lanette cream in 5%); CSTPE10 (STPE incorportaed to lanette cream in 10 %); CSTWFE5 (STWFE incorporated to lanette cream in 5%), CSTWFE10 (STWFE incorporated to lanette cream in 10 %) and CB5 (benzophenone incorporated to lanette cream in 5 %).
2.7.1. In vitro sun protection factor (SPF) evaluation
Aliquots of the stock solution of the formulations (0.1 g mL−1) were used in the preparation of the following concentrations: 0.2, 2, 5, 10 and 15 mg mL−1. The methodology used for the SPF measurements of formulations was the same as described for the crude extract (Section 2.6.4). The Lanette base was used as a blank. The analyses were performed in triplicate.
2.8. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay
The MTT assay was used to verify possible cytotoxicity of the peel and the whole fruit extracts, on both placenta cell lines and placenta explants cultures. As such, placenta explants were weighted and plated in equal proportion to each well, and Vero E6 cells or HTR-8/SVneo cells were plated at 2.5 × 105 cells and treated with the peel and the whole fruit extracts diluted in DMEM/F12 medium in different concentrations (0.01, 0.1, 1, 10, and 100 μg mL−1), and further cultured for 24 h. Since chloroquine diphosphate has been described as a potent anti-ZIKV drug (Delvecchio et al., 2016; Li et al., 2017; Shiryaev et al., 2017), we tested if it would affect cell lines or explants viability in different concentrations based on Delvecchio et al. (2016) (6.25, 12.5, 25, 50 and 100 μg mL−1), in order to use it, as a positive anti-ZIKV control, added 1 h after ZIKV incubation. The control group had no addition of extracts. The medium was replaced with a fresh culture medium containing 5 mg/mL of MTT and the supernatant was discarded after a 4 h incubation period at 37 °C, followed by the addition of 150 μL of DMSO. The absorbance of the dissolved MTT formazan product was spectrophotometrically measured at 540 nm. The viability percentage was determined in relation to the controls [(absorbance of treated cells/absorbance of untreated cells) × 100].
2.9. Zika virus (ZIKV) propagation and titration
Two stock strains of ZIKV were kindly donated by Dr. Juliano Bordignon, from Instituto Carlos Chagas of Fundação Oswaldo Cruz (ICC/FIOCRUZ-PR, Rio de Janeiro, Brazil): the MR766 strain (isolated in Uganda in 1947, third passage, stock titulation of 6.25 × 107 FFU/mL, GenBank accession KX421193), and the PE243 strain (isolated in Pernambuco, Brazil in 2016, third passage, stock titulation of 1 × 108 FFU/mL, GenBank accession MF352141.1). To perform viral propagation, both strains were cultured, in separate, in Aedes albopictus derived C6/36 cells with l-15 medium supplemented with 10 % SBF, 10 % tryptose and 1% PSA until 80 % confluence. Cell-free supernatants were harvested 5 days post infection (dpi), aliquoted and stored at −80 °C. Titration was performed after viral infection using green monkey kidney-derived Vero E6 cells cultured with supplemented DMEM for 1 h under gentle agitation. The media was changed and cells were incubated with 1.5 % carboxymethylcellulose (CMC) in DMEM with 2 % FBS at 1:1 (v/v). Plaque formation was observed at 5 dpi, after 10 % formalin fixation and 2 % violet crystal staining. Viral titration of MR766 strain was stocked at 5 × 105 PFU/mL and PE243 at 3 × 105 PFU/mL.
2.10. ZIKV infection of HTR-8/SVneo cells and treatment
Cells were plated at 3 × 105 cells in 24 well plates for 24 h and received 100 μg mL−1 of STPE or STWFE extracts for 1 h. Afterwards, cells were infected with one multiplicity of infection (MOI) of MR766 or PE243 ZIKV strains for 1 h. Supernatant was removed and cells were thoroughly washed with PBS. New culture medium was added and cells were cultured for 24 h. Positive controls were performed with the addition of 100 μg mL−1 of chloroquine diphosphate to the cultures, after ZIKV incubation and culture medium change. As such, cultures after the medium change were nominated as 0 h and all time points afterwards have only the effects derived from the 1 h ZIKV incubation.
2.11. Cytopathic effect in HTR-8/SVneo cells
After ZIKV incubation and treatment, pictures were taken in an inverted optical microscope coupled with camera (Olympus, Japan) at 0 h, 2 h, 24 h and 48 h (200 × magnification), and images were analysed for morphological cytopathic effects.
2.12. Immunofluorescence and phalloidin staining
All culture groups were fixed with 4 % paraformaldehyde in PBS and permeabilized with 0.1 % Triton X-100 in PBS (v/v). Phalloidin-fluorescein (FITC) conjugated staining (1:100; Abcam, Cambridge, UK). Then, it was performed unspecific antigens blockade with 0.05 % fish skin gelatin (Merck/Sigma-Aldrich, Germany) in PBS for 1 h. It was followed by monoclonal primary mouse antibody anti-ZIKV NS1 (1:100, E107, MA5−24585, Invitrogen, UK) incubation with Zenon Alexa 594 conjugated secondary anti-mouse antibody (1:250; Thermo Fisher Scientific) for 5 min to form an immunocomplex, which was later incubated with Zenon Blocking Reagent (1:250; Thermo Fisher Scientific, Wathlam, MA, USA) for further 5 min at room temperature. The immunocomplex mixture was added to cells for 1 h at 37 °C. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; 1:1000 in PBS; Merck/Sigma-Aldrich), and mounting was performed with PBS/glycerol (1:9, v/v) under glass slides. The results were visualized with a fluorescence microscope Nikon DS-Ri1 (Nikon, Japan) and images were acquired using the DP2-BSW software (Nikon).
2.13. Flow cytometry analysis of ZIKV infection
To access the percentage of cells infected by ZIKV, cells were detached with 2.5 % trypsin-EDTA and fixation and permeabilization were performed using commercial flow cytometry kits (e-Bioscience, San Diego, CA, EUA). A monoclonal primary mouse antibody anti-ZIKV NS1 (1:100, E107, MA5-24585, Invitrogen) was incubated with Zenon Alexa 488 conjugated secondary anti-mouse antibody (1:250; Thermo Fisher Scientific) for 5 min to form an immunocomplex with further addition of Zenon Blocking Reagent for further 5 min. The immunocomplex was later incubated with cells for 1 h at 37 °C. The percentage of ZIKV infected cells was analysed with FACS CantoTM® II flow cytometer (BD Biosciences, San Jose, CA, USA), using the software FlowJo (TreeStar, Ashland, OR, USA).
2.14. Quantitative reverse transcription polymerase Chain reaction (qRT-PCR)
The RNA viral load from cells’ supernatants was extracted using PureLink™ Viral RNA/DNA Mini Kit (Invitrogen), following the manufacturer’s instructions. The cDNA was synthesized and amplified using M-MLV Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). Next, ZIKV RNA copies were quantified using StepOnePlus™ Real-Time PCR System (Applied Biosystems, Life Technologies, Foster City, CA, USA). For each single-well amplification reaction, a threshold cycle (Ct) was evaluated in the exponential phase.The primers used were described by Lanciotti et al. (2007) (ZIKV 1086 and ZIKV 1162c).
2.15. Statistical analyses
The assays were performed in triplicate for each sample and the results were expressed as mean and standard deviations. The statistical evaluation was determined by analysis of variance ANOVA with Geisser-Greenhouse correction, followed by Dunnett post test, at the level of significance of 95 %, using the software Graphpad Prism 8.3.0. All results are depicted as Mean ± SEM.
3. Results and discussion
3.1. HPLC analysis
HPLC technique has been widely used for the detection of phenolic compounds in plant extracts, due to their versatility and accuracy. In general, the phenolic compounds are analysed in an HPLC instrument, using reverse phase C18 columns, a diode arrangement detector (PDA), and acidified polar organic solvents. The composition profile of the phenolic compounds in S. terebinthifolius was analysed using HPLC. In both extracts it was possible to verify the presence of phenols as gallic and p-coumaric acids, catechin, epicatechin and resveratrol. The compounds were identified in the extract from the comparison of the retention time (Rt) (Table 1 ) and by co-injection of the correspondent phenolic standards (figure not shown).
Table 1.
Phenolic standards and retention times (Rt/min) present in the extracts of STPE and STWFE.
| Peak | Phenolic compound | Rt/min |
|---|---|---|
| 1 | Gallic acid | 9.05 |
| 2 | Catechin | 12.91 |
| 3 | Epicatechin | 14.34 |
| 4 | p-Coumaric acid | 16.99 |
| 5 | Resveratrol | 22.19 |
Resveratrol was found for the first time in S. terebinthifolius. All these identified phenols had already their antioxidant and biological properties reported in the literature (see later). In addition, these compounds also present photoprotective characteristics, being able to act as active ingredient in sunscreen formulations (Polonini et al., 2013; Stevanato et al., 2014; Bulla et al., 2015; Alencar-Filho et al., 2016).
STWFE extract presents the phenolic compounds gallic acid, catechin, epicatechin, p-coumaric acid and resveratrol, all of them already detected in STPE. However, the concentrations of these compounds were lower under the same analytical conditions.
Some authors have reported the presence of phenolic compounds in S. terebinthifolius by use of HPLC. Glória et al. (2017) reported the presence of gallic acid and naringenin, in the ethyl acetate fraction of fruits, while Bernardes et al. (2014) identified apigenin in the fruit peel’s extract and Tlili et al. (2018), the presence of gallic acid, catechin, vanillic and coumaric acids, epicatechin, rutin, luteolin and kaempferol, in the extracts of fruits of S. terebinthifolius and S. molle.
Phytochemical studies performed with the leaf and fruit extracts of S. terebinthifolius revealed the presence of phenolic compounds, such as methyl gallate, gallic acid, quercetin, luteolin, catechin, epicatechin and naringin in their composition (Degaspari et al., 2005; Santana et al., 2012; Pagani et al., 2014; Feuereisen et al., 2014, 2017; Glória et al., 2017; Tlili et al., 2018; Gomes et al., 2019).
3.2. Analyses of aqueous extracts in HTR-8/SVneo trophoblast cell line, in Vero E6 cells and placenta viability
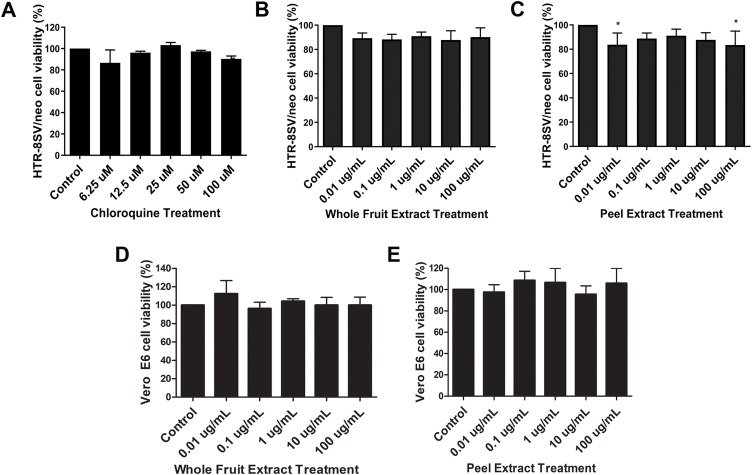
Firstly, the dry STWFE and STPE extracts were dissolved in aqueous solution and analysed in HTR-8/SVneo trophoblast cell line. Regarding the whole fruit aqueous extract, none of the concentrations altered cell viability (Fig. 1 B), the same occurring to chloroquine diphosphate-treated cells (Fig. 1A). The aqueous peel extract slightly reduced cell viability, only at concentrations of 0.01 μg mL−1 and 100 μg mL−1 (0.01 μg mL−1, cell viability = 83.72 % ± 5.58 %, p < 0.05; 100 μg mL−1 cell viability = 83.4 ± 6.64, p < 0.05, when compared to Control group that was cultured only with DMEM/F12), whereas at 0.1 μg mL−1, 1 μg mL−1 and 10 μg mL−1, the extract unchanged cell viability (Fig. 1C). Afterwards, we tested STWFE and STPE extracts in Vero E6 cell line, and no changes in cell viability were observed whatsoever (Fig. 1D and E)
Fig. 1.
Cellular viability under different concentrations of peel and whole fruit extracts. HTR-8/SVneo cells were treated with DMEM/F12 solution (Control), and with 0.01 μg mL−1, 0.1 μg mL−1, 1 μg mL−1, 10 μg mL−1 and 100 μg mL−1 chloroquine diphosphate (positive control) (A), whole fruit extract (STWFE) (B) and peel extract (STPE) (C). Vero E6 cells were treated with STFWE (D) and STFE (E) at the same concentrations. All different concentrations of whole fruit (STWE) aqueous extract have not altered trophoblast cell viability, the same occurring for both STFWE and STFE treatments in Vero E6 cells. The bar graphs represent the mean values ± S.E.M.; n = 3 in triplicate. *, p < 0.05.
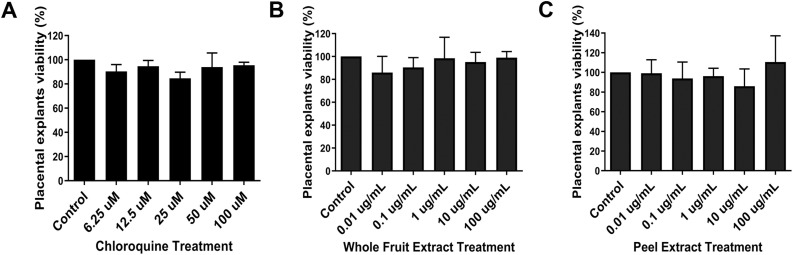
Then, we analysed the aqueous extracts action on placental tissue explants culture. Both peel and whole fruit extracts, as well as chloroquine diphosphate in all different concentrations analysed were unable to change cell viability (Fig. 2 A–C), indicating that they are not cytotoxic to placental chorionic villi.
Fig. 2.
Placental tissue explants viability under different concentrations of peel and whole fruit extracts. Placental explants were treated with DMEM/F12 solution (Control), and with 0.01 μg mL−1, 0.1 μg mL−1, 1 μg mL−1, 10 μg mL−1 and 100 μg mL-1 of whole fruit extract (STWFE) (B) and peel extract (STPE) (C). None of the different concentrations of the studied extracts or chloroquine diphosphate reduced placental tissue explants viability. The bar graphs represent the mean values ± S.E.M.; n = 3, in triplicate.
3.3. Analyses of ZIKV cytopathic effects on HTR-8/SVneo cells
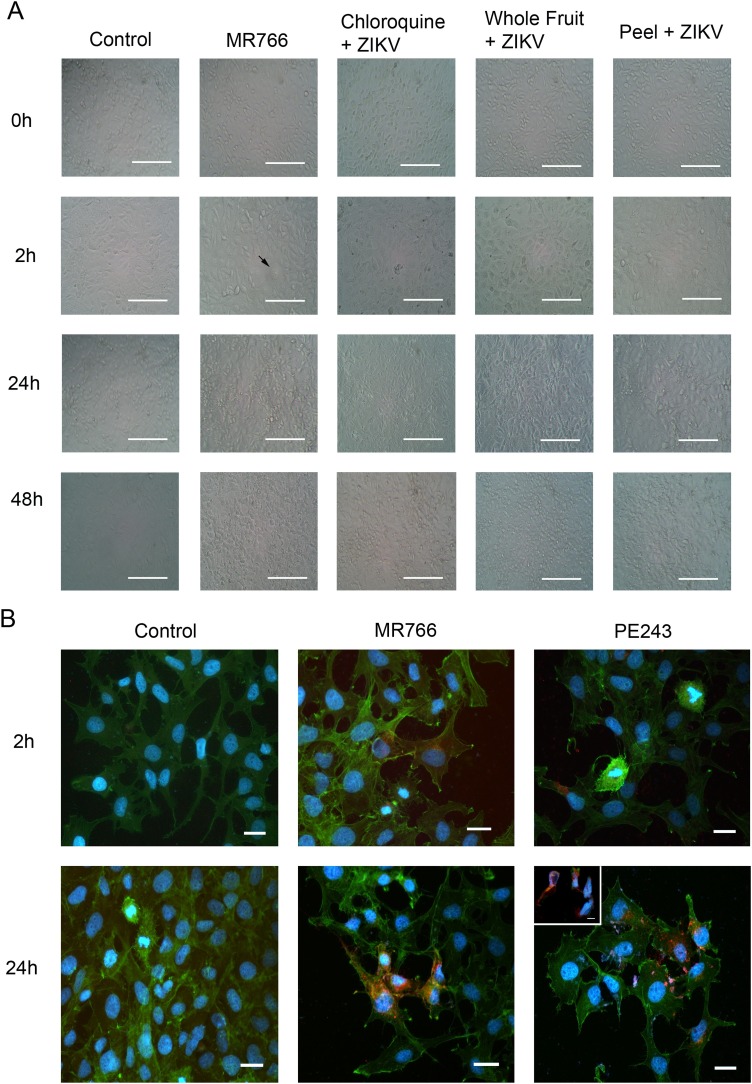
Cytopathic effects are morphological/structural changes observed in host cells caused by viral invasion. These effects can range from cytoplasmic inclusion bodies to total cell destruction. Herein, we observed cell morphology from 0 h to 48 h after ZIKV MR766 infection and with association to the treatments with chloroquine diphospate, and STWFE and STPE extracts (Fig. 3 A). After 2 h we observed the formation of scarce syncytium in the MR766 group, while none was observed in all the treament groups. Also in the MR766 group, from 24 to 48 h, we observed an incidence in cell destruction and possible apoptotic bodies, more present at 48 h. Nevertheless, we also observed the same effects in all other groups, although in lesser amounts (Fig. 3A).
Fig. 3.
Cytophatic effects on HTR-8/SVneo cells. Live cell images were acquired at 0 h, 2 h, 24 h and 48 h after ZIKV MR766 strain incubation. Tratments were made with DMEM/F12 solution (Control), and with 100 μg mL−1 of chloroquine diphosphate 1 h after viral incubation or 10 μg mL-1 of whole fruit extract (STWFE) or peel extract (STPE) 1 h prior viral incubation. Arrow indentifies syncytium formation (A). Phalloidin staining (green) of polymerized F-actin, immnulocalization of ZIKV NS1 protein (red) and nuclei staining (blue) of HTR-8/SVneo cells control or infected with ZIKV MT766 or PE243 strains, after 2 h and 24 h incubation, showing reduced cellular confluence and shrinking cytoplasm of infected cells after 24 h infection with MR766 strain, and cell destruction with extracellular DNA and NS1 proteins after 24 h infection with PE243 strain. Inset demonstrates severe cellular degeneration (B). Scale bars in A represent 400 μm for 100 × magnifications. Scale bars in B represent 100 μm for 400 × magnifications and in the inset represents 50 μm for 1000 × magnification.
To evaluate cellular alterations with more detail and to analyse how these trophoblast cells were affected morphologically, we stained cells for phalloidin to observe F-actin polymerization detailing cytoplasm limits, filipodia and stress fibers formation, together with the immunolocalization of ZIKV NS1 protein. Control cells presented large cytoplasms with cellular extensions, with more intensity after 24 h culture, possibly due to increased F-actin polymerization, with the formation of stress fibers, also with a number of cells in different stages of mitosis (Fig. 3B). Nevertheless, cells infected after 2 h with MR766 strain already presented NS1 protein positivity inside some cells, with no observed cytopathic effects, although cells appear to have brighter phalloidin staining in comparison to control, with more stress fibers and F-actin aggregates in protusion tips, indicating focal adhesion points (Fig. 3B). After 24 h, around 60 % of cells had massive positivity for ZIKV NS1 protein, more pronounced in the endoplasmic reticulum area, although visible in other compartments and the tips of filopodia. Also interesting to observe, the cellular confluence was reduced in comparison to control and infected cells had smaller area of cytoplasm with more condensed and smaller nuclei, the first cytopathic effects we observed with MR766 strain in these cells. Regarding the PE243 strain, after 2 h, we have also observed few cells positive for ZIKV NS1 protein, and some adhered to the cell membranes, with the presence of some atypic mitosis figures, which had viral particles in the cytoplasm. After 24 h, around 40 % of cells were positive for ZIKV NS1 protein, and we no longer observed mitosis. However, we did observed several clusters of extracellular DNA mixed with NS1 proteins, and cytoplasm small pieces scattered, indicating total cell rupture. Apoptotic bodies were also observed and, when cells were infected, but still preserved their structures, NS1 protein was more located perinuclearly, close to the endoplasmic reticulum (Fig. 3B). Also important to highlight the increased amount of cells without cytoplasm or with scarce cytoplasm residues, greatly stained for NS1 ZIKV protein (Fig. 3B inset), indicating that in only 24 h, the PE243 strain at MOI 1 induced more pronounced cytopathic effects in comparison to the MR766 strain.
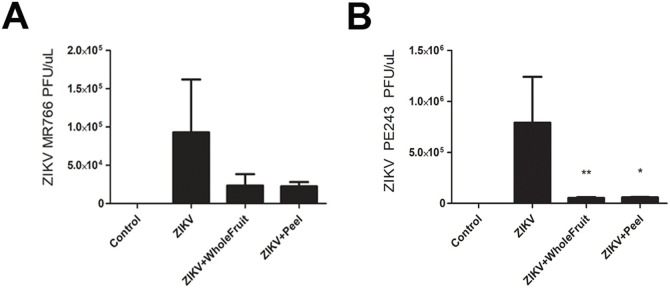
3.4. Viral load qRT-PCR analyses
HTR-8/SVneo cells were plated at 1 × 106 cells per well and treated as described before, with only 1 h of ZIKV incubation, culture medium removal and PBS washes with the addition of new medium. After 24 h, the MR766 strain had an average viral load of 0.929 × 105 PFU/μL (± 0.399 × 105 PFU/μL), and both treatments were not statistically significant in reducing the viral load, although a clear tendency could be observed, as treatment with STWFE reduced to 0.234 × 105 PFU/μL (± 0.086 × 105 PFU/μL), and with STPE to 0.223 × 105 PFU/μL (± 0.033 × 105 PFU/μL) (Fig. 4 A). Nevertheless, the PE243 strain had an average of 7.901 × 105 PFU/μL (± 4.505 × 105 PFU/μL), and a remarkable reduction of viral load, as treatment with STWFE reduced to 0.519 × 105 PFU/μL (± 0.052 × 105 PFU/μL, p = 0.0074), and with STPE to 0.579 × 105 PFU/μL (± 0.025 × 105 PFU/μL; p < 0.05) (Fig. 4B). As such, both treatments seem to consistently reduce the viral load at least for the PE243 strain.
Fig. 4.
Quantitative RT-PCR analyses of ZIKV viral load after HTR-8/SVneo cells infection and treatments efficiency. fter ZIKV MR766 strain incubation. Tratments were made with DMEM/F12 solution (Control), and with 10 μg mL−1 of whole fruit extract (STWFE) or peel extract (STPE) 1 h prior viral incubation for MR766 strain (A) or PE243 strain (B) of 1 MOI for 1 h. After culture medium renewal, cells were left for 24 h and their supernatants were analysed to observe ZIKV viral load. As such, we had higher viral load in the PE243 strain group, with great reduction of in the groups treated with STFWE or STPE. The bar graphs represent the mean values ± S.E.M.; n = 3.
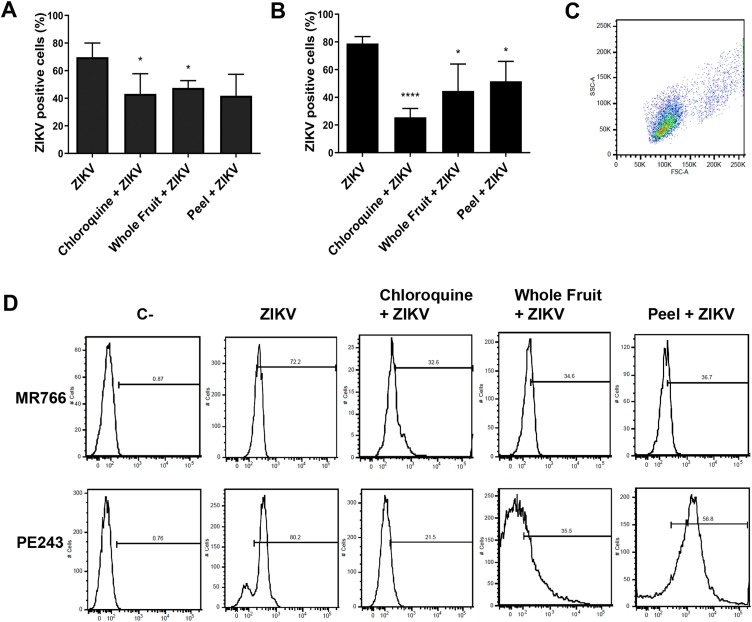
3.5. Flow cytometry analysis on ZIKV infection of HTR-8/SVneo cells
Since both extracts are well tolerated by trophoblast cells and chorionic villi explants, we tested whether treatments of 100 μg mL−1 chloroquine diphosphate, or 100 μg mL−1 of STWF aqueous extract or peel (STP) aqueous extract 1 h prior to ZIKV infection with 1 MOI of MR766 or PE243 strains would prevent and reduce ZIKV infection of trophoblast cells. As such, the MR766 strain infected an average of 69.81 % (± 4.19 %) of cells in the control group (Fig. 5 A–D). Regarding treatments, the chloroquine diphosphate group had 43.2 % (± 5.96 %), the whole fruit group had 47.53 % (± 2.14 %) and the peel group had 41.9 % (± 6.31 %) infected cells, meaning that all different treatments had similar effects, substantially reducing ZIKV infected cells (n = 6, respectively p = 0.0226, p = 0.0194 and p = 0.0447 in comparison to MR766 ZIKV group) (Fig. 5A–D).
Fig. 5.
Flow cytometry analysis on whole fruit extract treatment on ZIKV placenta infection. HTR-8/SVneo cells were pre-treated with 100 μg mL−1 of chloroquine diphosphate or 10 μg mL-1 of whole fruit extract or peel extract for 1 h, and further infected with MR766 (A and D) or PE243 (B and D) ZIKV strains. Cellular gate for size and granularity of HTR-8/SVneo cells is depicted in C. In D, first line depicts MR766 strain infection and second line PE243 infection, both with negative controls (C-), ZIKV group, chloroquine diphosphate + ZIKV, whole fruit + ZIKV and peel + ZIKV. The treatments were able to prevent infection of both strains of ZIKV in trophoblast cells (A, B and D). Experiments were performed in n = 6.
The PE243 strain infected an average of 78.89 % (± 2.03 %) in the control group (Fig. 5B–D). The chloroquine diphosphate group had 25.58 % (± 2.62 %), the whole fruit (STWF) group had 44.65 % (± 7.9 %) and the peel (STP) group had 51.65 % (± 5.84 %) infected cells. As such, all treatments also reduced ZIKV infection of trophoblast cells, although chloroquine diphosphate was more efficient (n = 6, respectively p < 0.0001, p = 0.023 and p = 0.0178 in comparison to PE243 ZIKV group) (Fig. 5B and D).
Inhibiting ZIKV infection of trophoblast cells and its crossing of the placental barrier would be remarkable to prevent ZIKV deleterious effects to the developing fetus. Some natural products have already been described to inhibit ZIKV infection or its replication in different cell types. In addition to the promising extracts from S. terebinthifolius Raddi, we have used the antimalarial chloroquine diphosphate as a positive control of antiviral activity, as it has been described by different sources (Delvecchio et al., 2016; Li et al., 2017; Shiryaev et al., 2017; Kao et al., 2018). Other active plants have been already described, such as Doratoxylon apetalum, an indigenous medicinal plant from Mascarene Island, which inhibited Zika and Dengue virus infection in leukocytes (Haddad et al., 2019). One polyphenol present in large amounts in the green tea, (−)-epigallocatechin gallate (EGCG), has been also described as a potent antiviral molecule, able to inhibit ZIKV entry in Vero E6 cells (Carneiro et al., 2016). Delphinidin (D) and epigallocatechin gallate (EGCG)] were shown to inhibit more effectively the virus production, on flaviviruses West Nile Virus infection (Vázquez-Calvo et al., 2017). Curcumin and suramin can also inhibit ZIKV infection on different cells (Wah et al., 2017; Mounce et al., 2017) and other compounds such as GSK126, nanchangmycin, obatoclax, pentagalloylglucose, saliphenylhalamide (SaliPhe) and 25-hydroxycholesterol have been also described to inhibit ZIKV endocytosis or entry in different models (Silva et al., 2018). Nevertheless, it is important to highlight that our study is pioneer in showing antiviral candidates against ZIKV infection with action in trophoblast cells, without cellular toxicity.
3.6. Total phenolic content and antioxidant capacity
Phenolic compounds are known as naturally occurring antioxidants and are widely distributed in plants. Several assays have been applied to evaluate antioxidant activity of the fruit, peel, seed and pulp extracts of different plants and agroindustrial residues (Oliveira et al., 2009). In the present work, the total phenolic content (TPC) was determined using the FC reagent and antioxidant capacity by DPPH• and FRAP methods.
Table 2 shows the results of TPC, DPPH• and FRAP obtained with STPE and STWFE extracts, with TPC values of 452 and 73.6 mg g−1 dry extract, respectively.
Table 2.
Total phenolic content (TPC), DPPH• (RSA % and IC50) and FRAP of ethanolic extracts of peel and whole fruit of Schinus terebinthifolius Raddi.
| Extracts | TPC (mg GAE* g−1 dry extract) | DPPH• |
FRAP (TEAC***FRAP μmol TE g−1) | |
|---|---|---|---|---|
| RSA %** | IC50 (μg mL−1) | |||
| STPE | 452.5 ± 10.2 | 78.4 ± 0.9 | 6.1 ± 0.4 | 3484.7 ± 255.5 |
| STWFE | 73.6 ± 10.4 | 24.1 ± 0.5 | – | 488.6 ± 81.1 |
STPE = Schinus terebinthifolius Peel Extract; STWFE = Schinus terebinthifolius whole Fruit Extract.
Gallic acid equivalents.
Percentage of DPPH• radical-scavenging ability in 30 min.
Trolox equivalent antioxidant capacity.
Some works report that fruit peels and seeds present higher amounts of total phenols and antioxidant activity compared to the edible portions (Omena et al., 2012). Concerning Punica granatum, Li et al. (2006) found 249.4 mg g−1 dry extract for peel and 24.7 mg g−1 dry extract for pulp.
Several factors such as climatic conditions, cultivation regions and extraction methods may influence the results of TPC (Oliveira et al., 2009). Costa et al. (2015) reported the effects of the extraction processes (Soxhlet extraction and maceration) on TPC for fruit extracts of S. terebinthifolius, with a variation of 5−110 mg g−1 dry extract. According to the studies of Tlili et al. (2018), extracts of the fruits of S. terebenthifolius from two localities in Tunisia were analysed by the FC reagent method and presented 35.23 and 32.39 mg g−1 dry extract, being these values much lower than the values obtained in the present work.
For the DPPH• method, STWFE and STPE extracts exhibited RSA of 24.1 and 78.4 %, respectively (Table 2).
Degaspari et al., 2004, analysed the aqueous and ethanolic extracts of fruits of S. terebenthifolius, and found RSA values of 25 % and 53 %, respectively. These values are below the value obtained for STPE and superior to the values of STWFE. However, our results are inferior to the results obtained by Pagani et al. (2014) with 83.33 % for the methanolic extract of fruit of S. terebinthifolius. In relation to the concentration required to reduce the DPPH• by 50 %, the STPE extract showed an IC50 value of 6.1 μg mL−1, while the STWFE extract did not reach 50 % inhibition at the studied concentrations. The obtained value is in agreement with the literature. Bernardes et al. (2014), found IC50 value < 10 μg mL−1 for the methanolic extract of the fruit’s peel of S. terebinthifolius. For the FRAP method, values of 488.6 and 3484.7 μmol TE g−1 were obtained for STWFE and STPE extracts, respectively (Table 2). The higher ferric reducing power exhibited by the peel extract can be attributed to its higher phenolic content. Along this line, Ali et al. (2014) evaluated the methanolic extracts of different parts of Punica granatum L. (peel, pulp, seeds and whole fruits). The peel extract exhibited the highest ferric reducing capacity. According to the authors, the higher antioxidant capacity exhibited by extract may be due to the presence of the phenolic compounds chlorogenic acid, rutin, coumaric acid and pyrogallol.
In summary, STPE extract has shown the best results in all antioxidant assays.
3.7. Analyses of crude extract and formulations towards photoprotection
3.7.1. In vitro sun protection factor (SPF) of the crude extracts and formulations
The extracts of S. terebinthifolius and respective formulations presented photoprotective potential, evaluated through the method developed by Mansur et al. (1986). SPF values (Table 3 ) determined for the extracts and formulations as a function of the concentration and percentages of extract incorporated into the base cream are listed. They were compared to the results obtained with the standard benzophenone-3. It was observed that SPF values of peel and whole fruit extracts ranged from 20.15–26.82 and 5.08–16.41, respectively. For the STPE formulations, the values of SPF varied from 1.25–32.40 and for STWFE formulations ranged from 0.52 to 41.58. The formulations with benzophenone-3 ranged from 9.76 to 35.90.
Table 3.
Sun protection factor of crude extracts and formulations.
| Concentration (mg mL−1) | |||||
|---|---|---|---|---|---|
| Formulation | 0.2 | 2 | 5 | 10 | 15 |
| STPE | 24.84 ± 0.76a | 26.82 ± 1.15b | 23.80 ± 0.94b | 21.72 ± 0.71b | 20.15 ± 0.53b |
| CSTPE5 | 1.25 ± 0.56d | 15.38 ± 0.53c | 28.36 ± 2.50 a, b | 32.21 ± 2.32a | 31.89 ± 2.40a |
| CSTPE10 | 2.70 ± 0.35d | 25.37 ± 1.47b | 32.40 ± 2.44a | 31.67 ± 1.93a | 29.67 ± 2.45a |
| STWFE | 5.08 ± 0.49c | 16.41 ± 1.33c | 14.86 ± 0.70c | 13.14 ± 0.48c | 11.50 ± 0.37c |
| CSTWFE5 | 0.52 ± 0.02e | 2.35 ± 0.07d | 5.60 ± 0.08d | 11.36 ± 0.32c | 17.40 ± 2.65b |
| CSTWFE10 | 0.24 ± 0.08e | 5.86 ± 0.08d | 14.89 ± 0.13c | 31.25 ± 0.21a | 31.58 ± 0.30a |
| CB5 | 9.76 ± 1.24b | 33.09 ± 2.69a | 35.90 ± 0.90a | 33.73 ± 0.21a | 28.31 ± 3.61a |
STPE = Schinus terebinthifolius Peel Extract; STWFE = Schinus terebinthifolius whole Fruit Extract; CSTPE5 (STPE incorporated to lanette cream in 5%); CSTPE10 (STPE incorportaed to lanette cream in 10 %); CSTWFE5 (STWFE incorporated to lanette cream in 5%), CSTWFE10 (STWFE incorporated to lanette cream in 10 %); CB5 (benzophenone incorporated to lanette cream in 5%). Results expressed as mean ± standard deviation. The level of significance was p < 0.05. Tukey: equal letters represent statistically similar values for the same concentration.
According to the Resolution of the Collegiate Board of Directors of June, the 30th, 2012, determined by the Brazilian Regulatory Agency (ANVISA), only FPS ≥ 6 is considered suitable for use in photoprotective formulations (Anvisa, 2012).
In the present study, SPF values varied according to the concentration, where, the decrease of concentration results in the increase of the SPF. However, when saturation levels are reached, the SPF values kept constant (Ribeiro, 2006). The extracts and formulations had shown satisfactory SPF values, being higher than the value established by ANVISA. In addition, they can be compared with the SPF values of benzophenone-3. By statistical analysis, it was possible to observe that CSTPE5 and CSTPE10 showed values very close to the benzophenone-3 at 5 mg mL−1 dilution, similarly to CSTWFE10 at 10 mg mL−1 dilution. In this study, it was possible to observe STPE and its formulations presented values of SPF higher than the values found for STWFE, with saturation levels of UVB radiation absorption at around 2 mg mL−1. The potential photoprotection exhibited by the extracts can be attributed to the presence of phenolic compounds in the samples, as shown in topic 3.6.
Recent studies have demonstrated the photoprotective potential of natural active principles such as plant extracts, which, due to their chemical composition, most often present bioactive constituents such as phenolic compounds, in particular flavonoids, which exert antioxidant action, neutralizing the actions of free radicals and inhibiting oxidation processes. In addition, these compounds possess the ability to absorb UV radiation (Saewan and Jimtaisong, 2013; De Oliveira-Júnior et al., 2017). Extracts of leaves of S. terebinthifolius have been studied in relation to their photoprotective potential. Bulla et al. (2015), analysed the in vitro photoprotective potential and the in vivo percutaneous penetration of the hydroethanolic extracts and leaf formulations of S. terebinthifolius. The methanolic solutions of extracts at 10 and 25 % (w/v) presented UV absorption with UVB photoprotective potential, with SPF values of 2.40 and 6.89, respectively. Spectroscopic measurements confirmed absorption in the UV region and the topical application of the formulations did not cause histological changes in the skin of the mice.
Other plant species also showed photoprotective action. Nunes et al. (2018) demonstrated that the ethanolic extracts of the leaves and barks of Amburana cearenses and Curatella americana presented values of SPF of 12.60–14.74, at concentration of 0.2 mg mL−1, below the SPF value found for the present peel extract, at the same concentration (24.84 ± 0.76, Table 3), and the ethanolic extract of Camellia sinensis (Kaur and Saraf, 2011), which presented a value of SPF 18.10 ± 0.05 at the same concentration. Silva et al. (2016) investigated the crude peptone extract of Spondias purpurea at 10 mg mL-1 and obtained SPF values of the formulations prepared, with the extracts in 10, 20 and 30 % of 4.13, 7.82 and 13.99, respectively, inferior to the present data (Table 3).
Considering Table 1, Table 2, Table 3 and the results against ZIKV infection, we searched biological activities of the chemical constituents of both extracts. The results are presented in Table 4 , with special emphasis on antiviral and anti-aging activities.
Table 4.
Reported biological activities of the natural compounds present in the ethanolic extract of Schinus terebenthifolius.
| Compound | Activity | References |
|---|---|---|
| Gallic acid | Antitumoral | (Varela-Rodríguez et al., 2020) |
| Antidiabetic and cardioprotective | (Uddin et al., 2020) | |
| Antimicrobial, anticancer and against gastrointestinal, cardiovascular, neuropsychological and metabolic diseases | (Kahkeshani et al., 2018) | |
| Antioxidant, anticancer, anti-inflammatory, antimicrobial, antimelanogenic, and anti-allergic and antiviral (showed inhibition of replication of human immunodeficiency virus type 1 (HIV-1) and herpes simplex virus type 1 and 2 (HSV)) | (Badhani et al., 2015) | |
| Antimicrobial and antiviral (evaluated against HSV-1 viruses as a representative of DNA viruses and PI-3 as a representative of RNA viruses, with significant activity) | (Özcelik et al., 2010) | |
| Antiviral (fraction of Rubus coreanus seed extract and its gallic acid derivative were evaluated against strains of influenza A and B. Both showed potential and broad antiviral activity. | (Lee et al., 2016) | |
| Catechin | Antimicrobial and antiviral (showed inhibition against Herpes simplex virus type 1 (HSV-1) | (Musarra-Pizzo et al., 2019) |
| Anti-oxidant, antitumor, antibacterial, antifungal, antidiabetic, anti-inflammatory, antiproliferative and antitumor | (Cosarcã et al., 2019) | |
| Antioxidant, UV protective, anti-allergenic, anti-inflammatory, anti-microbial, anti-viral and anti-cancer | (Bae et al., 2020) | |
| Antiviral (showed inhibition against pandemic influenza A (H1N1) | (You et al., 2018) | |
| Epicatechin | Antiviral (showed inhibitory effect against Mayaro (MAYV) virus and other alphavirus viruses | (Ferreira et al., 2018) |
| Antinociceptive and anti-inflammatory | (Dias et al., 2018) | |
| Antibacterial | (Ezhil and Lakshmi, 2017) | |
| Antidiabetic | (Ibrahim et al., 2018) | |
| Anti-infective | (Song, 2017) | |
| Anti-oxidant, antimicrobial, anti-inflammatory, antitumor, antidiabetic, anticancer and cardioprotective | (Prakash et al., 2019) | |
| Antioxidant and anticollagenase (showed a high inhibitory power against the collagenase enzyme, responsible for the degradation of matrix collagen and the photo-aging process. | (Geeta et al., 2019) | |
| UV protection | (Prasanth et al., 2020) | |
| Coumaric acid | Anti-oxidant and antihyperlipidemic | (Shen et al., 2019) |
| Anti-inflammatory | (Sabitha et al., 2019) | |
| Cytotoxic and leishmanicidal | (Arruda et al., 2020) | |
| Antioxidant, antimelanogenic, antimicrobial | (Boo, 2019) | |
| Antioxidant, antimicrobial, antimutagenesis, anticancer and antiviral (inhibition against the hepatitis C virus) | (Pei et al., 2015) | |
| Antioxidant and anti-aging (showed inhibitory activity against the enzymes elastase, collagenase and hyaluronidase) | (Widowati et al., 2020) | |
| Resveratrol | Antioxidant, antifungal and anti-staphylococcal | (Dwibedi and Saxena, 2019) |
| Anti-inflammatory, antioxidant, anti hyperlipidemic, immunomodulator, anticarcinogenic, cardioprotective, vasorelaxant, and neuroprotective | (Shaito et al., 2020) | |
| Antiviral (showed MERS-CoV infection and prolonged cellular survival after virus infection, in addition, decreased the expression of the nucleocapsid (N) protein essential for the duplication of the virus. | (Lin et al., 2017) | |
| Antiviral (exerted antiviral effects against Zika virus (ZIKV) replication in a dose-dependent manner) | (Mohd et al., 2019) | |
| UV protective (inhibited UVB-induced apoptosis by promoting HSP27 expression) | (Zhou et al., 2018) |
The investigation of the mechanism of antiviral action is very complex (Jassim and Naii, 2003; De Clercq, 2002; Rodriguez et al., 2019), is under way and is not possible to define, at the time being. Antiviral drug analysis has as main targets viral or cellular proteins or the search of broader activity spectrum, with less chance of resistance development.
Among the chemical constituents analysed, resveratrol was shown to exhibit direct virucidal activity against ZIKV, with anti-ZIKV replication properties (Mohd et al., 2019). In relation to the replication of herpes simplex virus (HSV), it was shown that it reduces mRNA of glycoprotein C and HSV late gene. In the studies by Faith et al. (2006), the authors observed how resveratrol negatively alters a host factor, NF-B, in HSV infected cells resulting in inhibition of virus replication, the impairment of activation of essential immediate-early, early and late genes and the inhibition of DNA synthesis.
4. Conclusions
Up to our knowledge, no studies have been so far conducted showing a natural product with absent or low cytotoxicity to the placenta and with such a good potential of inhibition of ZIKV entrance in trophoblast cells. As such, our study is pioneer as we show not only STPE and STWFE extracts are well-tolerated by trophoblast cells and placental tissue explants, but also both extracts successfully inhibited ZIKV entry in trophoblast cells, presenting a potential early antiviral effect. Since the extracts presented such similar response to inhibiting ZIKV infection, we must now search for molecules present in both extracts to identify which one(s) would be responsible for such promising effect. As such, present data represent a potential hope in a scarce therapeutical field of such sensible and neglected populations of pregnant women.
Additionally, it is also the first to report the photoprotective potential of the peel and whole fruit of this species. The formulations incorporated with low concentrations of the extracts presented values of SPF equivalent to the standard benzophenone-3, used in the production of commercial sunscreens. The present activities may be related to the presence of the phenolic compounds gallic acid, catechin, epicatechin, p-coumaric acid and resveratrol, the last one, being described for the first time in this species. As such, STPE and STWFE can be considered active ingredients in the preparation of photoprotective formulations. Further studies are required, including biological tests in vivo, definition of the mechanism of antiviral activities, among others, to ensure a cosmetic/medicinal application with quality, safety and efficacy.
Possible applications of this material for the development of biotechnological/medical products are expected, with consequent aggregated value to a product from family farming.
Credit author statement
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge the financial support of the Brazilian research funding agencies CNPq, CAPES/RENORBIO/PROAP, CAPES/PROCAD, INCT-Bioanalysis and FAPEAL (PPSUS/Decit/MS/CNPq/SESAU-AL/FAPEAL n.06/2016:60030 000819/2016). The authors wish to thank Aldy dos Santos and Juliane Pereira Silva Barreto for technical assistance, and Dr José Roberto Fonseca for providing the Schinus terebinthifolius Raddi samples. We thank Dr. Alessandra Borges for the donation of Vero E3 cells and C6/36 cells, and Dr. Ênio Bassi and the IMMUNOREG group for the help with viral growing and titration. We are also thankful to Farmanguinhos for providing chloroquine diphosphate.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.indcrop.2020.112503.
Contributor Information
Alexandre U. Borbely, Email: alexandre.borbely@icbs.ufal.br.
Marília O.F. Goulart, Email: mofg@qui.ufal.br.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aagard K.M., Lahon A., Suter M.A., Arya R.P., Seferovic M.D., Vogt M.B., Hu M., Stossi F., Mancini M.A., Harris R.A., Kahr M., Eppes C., Rac M., Belfort M.A., Park C.S., Lacorazza D., Rico-Hesse R. Primary human placental trophoblasts are permissive for Zika virus replication. Sci. Rep. 2017;7:41389. doi: 10.1038/srep41389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi J.J., Zhao Y., Cartus A.R., Gupta P., Davidson L.A. Placental mechanics in the Zika-microcephaly relationship. Cell Host Microbe. 2016;20:9–11. doi: 10.1016/j.chom.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Afaq F. Natural agents: cellular and molecular mechanisms of photoprotection. Arch. Biochem. Biophys. 2011;508:144–151. doi: 10.1016/j.abb.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso S., Horita K., Silva J.P.S., Almeida I.F., Amaral M.H., Lobão P.A., Costa P.C., Miranda M.S., Silva J.C.G.E., Lobo J.M. Photodegradation of avobenzone: stabilization effect of antioxidants. J. Photochem. Photobiol. B Biol. 2014;140:36–40. doi: 10.1016/j.jphotobiol.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Agati G., Brinetti C., Ferdinando M.D., Ferrini F., Pollastri S., Tattini M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013;72:35–45. doi: 10.1016/j.plaphy.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Aldo P., You Y., Szigeti K., Horvath T.L., Lindenbach B., Mor G. HSV-2 enhances ZIKV infection of the placenta and induces apoptosis in first-trimester trophoblast cells. Am. J. Reprod. Immunol. 2016;76:348–357. doi: 10.1111/aji.12578. [DOI] [PubMed] [Google Scholar]
- Alencar-Filho J.M.T., Sampaio P.A., Pereira E.C.V., Junior R.G.O., Silva F.S., Almeida J.R.G.S., Rolim L.A., Nunes X.P., Araújo E.C.C. Flavonoids as photoprotective agents: a systematic review. J. Med. Plant Res. 2016;10:848–864. doi: 10.5897/JMPR2016.6273. [DOI] [Google Scholar]
- Ali S.I., El-Baz F.K., El-Emary A.E., Khan E.A., Mohamed A.A. HPLC-analysis of polyphenolic compounds and free radical scavenging activity of pomegranate fruit (Punica granatum L.) Intern. J. Pharm. Clin. Res. 2014;6:348–355. [Google Scholar]
- Aliota M.T., Caine E.A., Walker E.C., Larkin K.E., Camacho E., Osorio J.E. Characterization of lethal Zika virus infection in AG129 mice. PLoS Negl. Trop. Dis. 2016:1–11. doi: 10.1371/journal.pntd.0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anvisa . 1.ed. 2011. Formulário de Fitoterápicos Farmacopeia Brasileira. URL http://portal.anvisa.gov.br/documents/33832/259456/Formulario_de_Fitoterapicos_da_Farmacopeia_Brasileira.pdf (Accessed 25 June 2019) [Google Scholar]
- Anvisa . Agência Nacional de Vigilância Sanitária (ANVISA); Diário Oficial da União, DF: 2012. RDC nº 30, de 01 de junho de 2012. Brasil, Ministério da Saúde.http://portal.anvisa.gov.br/legislacao/?inheritRedirect=true#/visualizar/28857 [Google Scholar]
- Arruda C., Ribeiro V.P., Mejía J.A.A., Almeida O.M., Goulart M.O., Candido A.C.B.B., Santos R.A., Magalhães L.G., Martins C.H.G., Bastos J.K. Green propolis: cytotoxic and leishmanicidal activities of artepillin C, p-coumaric acid, and their degradation products. Rev. Bras. Farmacogn. 2020 doi: 10.1007/s43450-020-00043-3. [DOI] [Google Scholar]
- Arumugasaamy N., Ettehadieh L.E., Huo C.Y., Paquin-Proulx D., Kitchen S.M., Santoro M., Placone J.K., Silveira P.P., Aguiar R.S., Nixon D.F., Fisher J.P., Kim P.C.W. Biomimetic placenta-fetus model demonstrating maternal-fetal transmission and fetal neural toxicity of Zika virus. Ann. Biomed. Eng. 2018 doi: 10.1007/s10439-018-2090-y. [DOI] [PubMed] [Google Scholar]
- Badhani B., Sharma N., Kakkar R. Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015;5:27540–27557. doi: 10.1039/c5ra01911g. [DOI] [Google Scholar]
- Bae J., Kim N., Shin Y., Kim S., Kim Y. Activity of catechins and their applications. Biomed. Derm. 2020 doi: 10.1186/s41702-020-0057-8. [DOI] [Google Scholar]
- Batista M.N., Braga A.C.S., Campos G.R.F., Souza M.M., Matos R.P.A., Lopes T.Z., Candido A.M., Lima M.L.D., Machado F.C., Andrade S.T.Q., Bittar C., Nogueira M.L., Carneiro B.M., Mariutti R.B., Arni R.K., Calmon M.F., Rahal P. Natural products isolated from oriental medicinal herbs inactive Zika virus. Viruses. 2019;11:49. doi: 10.3390/v11010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie I.E.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bernardes N.R., Heggdorne-Araújo M., Borges I.F.J.C., Almeida F.M., Amaral E.P., Lasunskaia E.B., Muzitano M.F., Oliveira D.B. Nitric oxide production, inhibitory, antioxidant and antimycobacterial activities of the fruits extract and flavonoid content of Schinus terebinthifolius. Braz. J. Pharmacogn. 2014;24:644–650. doi: 10.1016/j.bjp.2014.10.012. [DOI] [Google Scholar]
- Boo Y.C. p-Coumaric acid as an active ingredient in cosmetics: a review focusing on its antimelanogenic effects. Antioxidants. 2019;8:275. doi: 10.3390/antiox8080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazilian Ministry of Health . 2017. Brasília. Relação Nacional de Medicamentos Essenciais: RENAME. 210. [Google Scholar]
- Bulla M.K., Hernandes L., Baesso M.L., Nogueira A.C., Bento A.C., Bortoluzzi B.B., Serra L.Z., Cortezi D.A.G. Evaluation of photoprotective potential and percutaneous penetration by photoacoustic spectroscopy of the Schinus terebinthifolius Raddi extract. J. Photochem. Photobiol. 2015;91:558–566. doi: 10.1111/php.12419. [DOI] [PubMed] [Google Scholar]
- Cao B., Parnell L.A., Diamond M.S., Mysorekar I.U. Inhibition of autophagy limits vertical transmission of Zika virus in pregnant mice. J. Exp. Med. 2017;214(8):2303–2313. doi: 10.1084/jem.20170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro B.M., Batista M.N., Braga A.C.S., Nogueira M.L., Rahal P. The green tea molecule EGCG inhibits Zika vírus entry. Virology. 2016;496:215–218. doi: 10.1016/j.virol.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Cavalher-Machado S.C., Rosas E.C., Brito F.A., Heringe A.P., Oliveira R.R., Kaplan M.A.C., Figueiredo M.R., Henriques M.G.M. The anti-allergic activity of the acetate fraction of Schinus terebinthifolius leaves in IgE induced mice paw edema and pleurisy. Int. Immunopharmacol. 2008;8:1552–1560. doi: 10.1016/j.intimp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Chaouat G., Clark D.A. Are animal models useful or confusing in understanding the human feto-maternal relationship? A debate. J. Reprod. Immunol. 2015;108:56–64. doi: 10.1016/j.jri.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Cicco N., Lanorte M.T., Paraggio M., Viggiano M., Lattanzio V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009;91:107–110. doi: 10.1016/j.microc.2008.08.011. [DOI] [Google Scholar]
- Cosarcã S., Tanase C., Muntean D.L. Therapeutic aspects of catechin and its derivatives – an update. Acta Biol. Marisiensis. 2019;2(1):21–29. doi: 10.2478/abmj-2019-0003. [DOI] [Google Scholar]
- Costa C.O.S., Ribeiro P.P., Loureiro M.B., Simões R.C., Castro R.D., Fernandez L.G. Phytochemical screening, antioxidant and antibacterial activities of extracts prepared from different tissues of Schinus terebinthifolius Raddi that occurs in the coast of Bahia, Brazil. Pharmacogn. Mag. 2015;11:607–614. doi: 10.4103/0973-1296.160459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Strategies in the design of antiviral drugs. Nat. Rev. Drug Discovery. 2002;1:13–25. doi: 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]
- De Oliveira-Júnior R.G., Souza G.R., Adrielly C., Ferraz A., De Oliveira A.P., Araújo C.D.S., De Lima-saraiva S.R.G., Alan S., Bomfim G., Gonçalves T.M., Rolim L.A., Rolim-neto P.J., Celise F., César S., Roberto J., Almeida S. Development and evaluation of photoprotective O / W emulsions containing hydroalcoholic extract of Neoglaziovia variegata (bromeliaceae) Sci. World J. 2017:1–8. doi: 10.1155/2017/5019458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degaspari C.H., Waszczynskyj N., Santos R.J. Antioxidant activity of extracts from fruit of aroeira (Schinus terebenthifolius Raddi) Visão Acadêmica. 2004;5:83–89. [Google Scholar]
- Degaspari C.H., Waszczynskyj N., Prado M.R.M. Atividade antimicrobiana de Schinus terebinthifolius Raddi. Ciênc Agrotec. 2005;3:617–622. doi: 10.1590/S1413-70542005000300016. [DOI] [Google Scholar]
- Delvecchio R., Higa L.M., Pezzuto P., Valadão A.L., Garcez P.P., Monteiro F.L., Loiola E.C., Rehen S., Campanati L., Aguiar R.S., Tanuri A. Chloroquine, an endocytosis blocking agent, inhibits Zika virus infection in different cell models. Viruses. 2016;8:322. doi: 10.3390/v8120322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias T.L.M.F., Melo G.M.A., Silva Y.K.C., Queiroz A.C., Goulart H.F., Alexandre-Moreira M.S., Santana A.E.G., Uchôa V.T. Antinociceptive and anti-inflammatory activities of the ethanolic extract, of fractions and of epicatechin isolated from the stem bark of Ximenia americana L. (Oleacaceae) RVQ. 2018;10(1):86–101. doi: 10.21577/1984-6835.20180009. [DOI] [Google Scholar]
- DiCiaula M.C., Lopes C.G., Ieda S.S., Mello J.C. Optimization of solvent mixtures for extraction from bark of Schinus terebinthifolius by a statistical mixture-design technique and development of a UV-VIS spectrophotometric method for analysis of total polyphenols in the extract. Quim. Nova. 2014;37:158–163. doi: 10.1590/S0100-40422014000100026. [DOI] [Google Scholar]
- Dwibedi V., Saxena S. In vitro anti-oxidant, anti-fungal and anti-staphylococcal activity of resveratrol-producing endophytic fungi. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019;90(1):207–219. doi: 10.1007/s40011-019-01098-6. [DOI] [Google Scholar]
- Działo M., Mierziak J., Korzun U., Preisner M., Szopa J., Kulma A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016;17:1–41. doi: 10.3390/ijms17020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhil I., Lakshmi T. Antibacterial efficacy of epicatechin and rutin from Acacia catechu leaf extract against Enterococcus faecalis and Streptococcus mutans - an in vitro study. J. Adv. Pharm. Educ. Res. 2017;7(1):22–24. [Google Scholar]
- Faith S.A., Sweet T.J., Bailey E., Booth T., Docherty J.J. Resveratrol suppresses nuclear factor-B in herpes simplex virus infected cells. Antiviral Res. 2006;72:242–251. doi: 10.1016/j.antiviral.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Ferreira P.G., Ferraz A.C., Figueiredo J.E., Lima C.F., Rodrigues V.G., Taranto A.G., Ferreira J.M.S., Brandão G.C., Vieira-Filho S.A., Duarte L.P., Magalhães C.L.B., Magalhães J.C. Detection of the antiviral activity of epicatechin isolated from Salacia crassifolia (Celastraceae) against Mayaro virus based on protein C homology modelling and virtual screening. Arch. Virol. 2018;163:1567–1576. doi: 10.1007/s00705-018-3774-1. [DOI] [PubMed] [Google Scholar]
- Feuereisen M.M., Hoppe J., Zimmermann B.F., Weber F., Schulze-Kaysers N., Schieber A. Characterization of phenolic compounds in Brazilian pepper (Schinus terebinthifolius Raddi) exocarp. J. Agric. Food Chem. 2014;62:6219–6226. doi: 10.1021/jf500977d. [DOI] [PubMed] [Google Scholar]
- Feuereisen M.M., Zimmermann B.F., Schulze-Kaysers N., Schieber A. Differentiation of Brazilian peppertree (Schinus terebinthifolius Raddi) and Peruvian peppertree (Schinus molle L.) fruits by UHPLC-UV-MS analysis of their anthocyanin and biflavonoid profiles. J. Agric. Food Chem. 2017;65:5330–5338. doi: 10.1021/acs.jafc.7b00480. [DOI] [PubMed] [Google Scholar]
- França G.V., Schuler-Faccini L., Oliveira W.K., Henriques C.M., Carmo E.H., Pedi V.D., Nunes M.L., Castro M.C., Serruya S., Silveira M.F., Barros F.C., Victora C.G. Congenital zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388:891–897. doi: 10.1016/S0140-6736(16)30902-3. [DOI] [PubMed] [Google Scholar]
- Geeta W.S.W., Widowati W., Ginting C.N., Lister N., Armansyah A., Girsang E. Comparison of antioxidant and anti-collagenase activity of genistein and epicatechin. Pharm. Sci. Res. 2019;6(2):111–117. doi: 10.7454/prs.v6i2.4510. [DOI] [Google Scholar]
- Glória L.L., Arantes M.B.S., Junior A.R.C., Antunes F., Braz-Filho R., Vieira I.J.C., Cruz L.L., Chaves D.S.A., Freitas S.P., Oliveira D.B. Phenolic compounds present in Schinus terebinthifolius raddi influence the lowering of blood pressure in rats. Molecules. 2017;22:10. doi: 10.3390/molecules22101792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes R.B.A., Souza E.S.S., Barraqui N.S.G., Tosta C.L., Nunes A.P.F., Shuenck R.P., Ruas F.G., Ventura J.A., Filgueiras P.R., Kuster R.M. Residues from the Brazilian pepper tree (Schinus terebinthifolia Raddi) processing industry: chemical profile and antimicrobial activity of extracts against hospital bacteria. Ind. Crops Prod. 2019 doi: 10.1016/j.indcrop.2019.05.079. [DOI] [Google Scholar]
- Gregoris E., Fabris S., Bertelle M., Grassato L., Stevanato R. Propolis as potential cosmeceutical sunscreen agent for its combined photoprotective and antioxidant properties. Int. J. Pharm. 2011;405:97–101. doi: 10.1016/j.ijpharm.2010.11.052. [DOI] [PubMed] [Google Scholar]
- Haddad L.G., Koishi A.C., Gaudry A., Santos C.N.D., Viranaicken W., Desprès P., El Kalamouni C. Doratoxylon apetalum, an indigenous medicinal plant from Mascarene Islands, is a potent inhibitor of Zika and dengue virus infection in human cells. Int. J. Mol. Sci. 2019;20(2382):1–13. doi: 10.3390/ijms20102382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim G.M., Ahmedi O.M., Abbast N.H., El Fated M.M. Evaluation of the anti-diabetic effects of epicatechin and/or gallic acid in STZ/NA- induced diabetic Wister rats. Res. J. Appl. Biotech. 2018;4:87–104. doi: 10.21608/rjab.2018.57527. [DOI] [Google Scholar]
- Jassim S.A.A., Naii M.A. A review. Novel antiviral agents: a medicinal plant perspective. J. Appl. Microbiol. 2003;95:412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- Kahkeshani N., Farzaei F., Fotouhi M., Alavi S.S., Bahramsoltani R., Naseri R., Momtaz S., Abbasabadi Z., Rahimi R., Farzaei M.H., Bishayee A. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J. Basic. Med. Sci. 2018;22:225–237. doi: 10.22038/ijbms.2019.32806.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J.C., Fu W.C.H., Tsai T.T., Ho M.R., Jhan M.K., Shen T.J., Tseng P.C., Wang Y.T., Lin C.F. The antiparasitic drug niclosamide inhibits dengue virus infection by interfering with endosomal acidification independent of mTOR. Plos Negl. Trop. Dis. 2018;12(8) doi: 10.1371/journal.pntd.0006715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C.D., Saraf S. Photochemoprotective activity of alcoholic extract of Camellia sinensis. Int. J. Pharm. 2011;7:400–404. doi: 10.3923/ijp.2011.400.404. [DOI] [Google Scholar]
- Lanciotti R.S., Kosoy O.L., Laven J.J., Velez J.O., Lambert A.J., Johnson A.J., Stanfield S.M., Duffy M.R. Genetic and serologic properties of Zika virus associated with and epidemic, Yap State, Micronesia. Emerg. Infect Dis. 2007;14(8):1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Oh M., Seok J.H., Kim S., Lee D.B., Bae G., Bae H., Bae S.Y., Hong Y., Kwon S., Lee D.H., Song C.S., Mun J.Y., Chung M.S., Kim K.H. Antiviral effects of black raspberry (Rubus coreanus) seed and its gallic acid against influenza virus infection. Viruses. 2016;8:157. doi: 10.3390/v8060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Guo C., Yang J., Wei J., Xu J., Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96:254–260. doi: 10.1016/j.foodchem.2005.02.033. [DOI] [Google Scholar]
- Li C., Zhu X., Ji X., Quanquin N., Deng Y.-O., Tian M., Alivari R., Zuo X., Yuan L., Afridi S.K., Li X.F., Jung J.U., Nielsen-Saines K., Qin F.X.F., Qin C.F., Xu Z., Cheng G. Chloroquine, a FDA-approved drug, preventes Zika virus infection and its associated congenital microcephaly in mice. EBioMedicine. 2017;24:189–194. doi: 10.1016/j.ebiom.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.C., Ho C.T., Chuo W.H., Li S., Wangs T.T., Lin C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017;17:144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Winklelmann E.R., Fernandez-Salas I., Li L., Mayer S.V., Danis-Lozano R., Sanchez-Casas R.M., Vasilakis N., Tesh R., Barret A.D., Weaver S.C., Wang T. Zika, dengue and yellow fever viruses induce differential anti-viral immune responses in human monocytic and first trimester trophoblast cells. Antiviral Res. 2018;151:55–62. doi: 10.1016/j.antiviral.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur J.S., Breder M.N.R., Mansur M.C.A., Azulay R.D. Determinacão do fator de proteção solar por espectrofotometria. An. Bras. Dermatol. 1986;61:121–124. (in Portuguese) [Google Scholar]
- Miller R.K., Genbacev O., Turner M.A., Aplin J.D., Caniggia I., Huppertz B. Human placental explants in culture: approaches and assessments. Placenta. 2005;6:439–448. doi: 10.1016/j.placenta.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Miner J.J., Cao B., Govero J., Smith A.M., Fernandez E., Cabrera O.H., Garber C., Noll M., Klein R.S., Noguchi K.K., Mysorekar I.U., Diamond M.S. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd A., Zainal N., Tan K., Abubakar S. Resveratrol effects Zika virus replication in vitro. Sci. Rep. 2019;9:14336. doi: 10.1038/s41598-019-50674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounce B.C., Cesar T., Carrau L., Vallet T., Vignuzzi M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antiviral Res. 2017;142:148. doi: 10.1016/j.antiviral.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Musarra-Pizzo M., Ginestra G., Smeriglio A., Pennisi R., Sciortino M.T., Mandalari G. The antimicrobial and antiviral activity of polyphenols from almond (Prunus dulcis L.) Skin. Nutrients. 2019;11:2355. doi: 10.3390/nu11102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes A.R., Rodrigues A.L.M., Queiróz D.B., Vieira I.G.P., Neto J.F.C., Junior J.T.C., Tintino S.R., Morais S.M., Coutinho H.D.M. Photoprotective potential of medicinal plants from Cerrado biome (Brazil) in relation to phenolic content and antioxidant activity. J. Photochen. Photobiol. B Biol. 2018;189:119–123. doi: 10.1016/j.jphotobiol.2018.10.013. [DOI] [PubMed] [Google Scholar]
- Oliveira A.C., Valentim I.B., Silva C.A., Bechara E.J.H., De Barros M.P., Mano C.M., Goulart M.O.F. Total phenolic content and free radical scavenging activities of methanolic extract powders of tropical fruit residues. Food Chem. 2009;115:469–475. doi: 10.1016/j.foodchem.2008.12.045. [DOI] [Google Scholar]
- Omena C.M.B., Valentim I.B., Guedes G.S., Rabelo L.A., Mano C.M., Bechara E.J.H., Sawaya A.C.H.F., Trevisan M.T.S., Da Costa J.G., Ferreira R.C.S., Santana A.E.G., Goulart M.O.F. Antioxidant, antiacetylcholinesterase and cytotoxic activities of ethanol extracts of peel, pulp and seed of exotic Brazilian fruits. Food Res. Intern. 2012;49:334–344. doi: 10.1016/j.foodres.2012.07.010. [DOI] [Google Scholar]
- Özcelik B., Kartal M., Orhan I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2010;49(4):396–402. doi: 10.3109/13880209.2010.519390. [DOI] [PubMed] [Google Scholar]
- Pagani A.A.C., Souza A.L.G., Souza D.S., Batista R.A., Xavier A.C.R., Pagani G.D. Quantification of bioactive compounds of pink pepper (Schinus terebinthifolius Raddi) Intern. J. Eng. Innov. Tech. 2014;4:37–41. [Google Scholar]
- Pei K., Ou J., Huang J., Ou S. p-Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2015;96:2952–2962. doi: 10.1002/jsfa.7578. [DOI] [PubMed] [Google Scholar]
- Polonini H.C., Larissa L.L., Gonçalves K.M., Carmo A.M.R., Silva A.D., Raposo N.R.B. Photoprotective activity of resveratrol analogues. Bioorg. Med. Chem. 2013;21:964–968. doi: 10.1016/j.bmc.2012.11.052. [DOI] [PubMed] [Google Scholar]
- Prá V.D., Lunellli F.C., Vendruscolo R.G., Martins R., Wagner R., Lazzartti A.P., Freire D.M.G., Alexandri M., Koutinas A., Mazutti M.A., Rosa M.B. Ultrasound-assisted extraction of bioactive compounds from palm pressed fiber with high antioxidant and photoprotective activities. Ultras. Sonochem. 2017;36:362–366. doi: 10.1016/j.ultsonch.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Prakash M., Basavaraj B.V., Murthy C.K.N. Biological functions of epicatechin: plant cell to human cell health. J. Funct. Foods. 2019;52:14–24. doi: 10.1016/j.jff.2018.10.021. [DOI] [Google Scholar]
- Prasanth B., Soman A., Johnson J., Narayanan P.S., Jhon P.A. Plants and phytoconstituents having sunscreen activity. World J. Curr. Med. Pharm. Res. 2020;2:14–20. doi: 10.37022/WJCMPR.2020.02019. [DOI] [Google Scholar]
- Ribeiro C. Second ed. Pharmabooks; São Paulo (in Portuguese): 2006. Cosmetologia aplicada a dermoestética. [Google Scholar]
- Rocha P.S., Campos J.F.C., Nunes-Souza V., Vieira M.C., Boleti A.P.A., Rabelo L.A., Santos E.L., Souza K.P. Antioxidant and protective effects of Schinus terebinthifolius Raddi against doxorubicin-induced toxicity. Appl. Biochem. Biotechnol. 2018:869–884. doi: 10.1007/s12010-017-2589-y. [DOI] [PubMed] [Google Scholar]
- Rodriguez A.K., Ana Luisa Muñoz A.L., Nidya Alexandra Segura N.A., Héctor Rafael Rangel H.R., Bello F. Molecular characteristics and replication mechanism of dengue, zika and chikungunya arboviruses, and their treatments with natural extracts from plants: an updated review. EXCLI J. 2019;18:988–1006. doi: 10.17179/excli2019-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Morales A.J., Cardona-Ospina J.A., Ramirez-Jaramillo V., Gaviria J.A., Gonzalez-Moreno G.M., Castrillón-Spitia J.D., López-Villegas A., Morales-Jimenez E., Ramirez-Zapata V., Rueda-Merchan G.E., Trujillo A.M., Tabares-Villa F.A., Henao-SanMartin V., Murillo-Garcia D.R., Herrera-Soto J.A., Buitrago-Cañas M.L., Collins M.H., Sepúlveda-Arias J.C., Londoño J.J., Bedoya-Rendón H.D., Cárdenas-Pérez J.J., Olaya S.X., Lagos-Grisales G.J. Diagnosis and outcomes of pregnant women with Zika virus infection in two municipalities of Risaralda, Colombia: second report of the ZIKERNCOL study. Travel. Med. Infect. Dis. 2018;25:20–25. doi: 10.1016/j.tmaid.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Saânchez-Moreno C., Larrauri J., Saura-Calixto F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res. Intern. 1999;32:407–412. [Google Scholar]
- Sabitha R., Nishi K., Gunasekaran V.P., Annamalai G., Agilan B., Ganeshan M. p-Coumaric acid ameliorates ethanol-induced kidney injury by inhibiting inflammatory cytokine production and NF-κB signaling in rats. Asian Pac. J. Trop. Biomed. 2019;9(5):188–195. doi: 10.4103/2221-1691.258998. [DOI] [Google Scholar]
- Saewan N., Jimtaisong A. Photoprotection of natural flavonoids. J. Appl. Pharm. Sci. 2013;3:129–141. doi: 10.7324/JAPS.2013.3923. [DOI] [Google Scholar]
- Santana J.S., Sartorelli P., Guadagnim R.C., Matsuo A.A., Figueiredo C.R., Soares M.G., Da Silva A.M., Lago J.H. Essential oils from Schinus terebinthifolius leaves chemical composition and in vitro cytotoxicity evaluation. Pharm. Biol. 2012;50:1248–1253. doi: 10.3109/13880209.2012.666880. [DOI] [PubMed] [Google Scholar]
- Sayre R.M., Agin P.P., Levee G.J., Marlowe E. Comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979;29:559–565. doi: 10.1111/j.1751-1097.1979.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Scalia S., Mezzena M. Photostabilization effect of quercetin on the UV filter combination, butyl methoxydibenzoylmethane-octyl methoxycinnamate. Photochem. Photobiol. 2010;86:273–278. doi: 10.1111/J.1751-1097.2009.00655.x. [DOI] [PubMed] [Google Scholar]
- Shaito A., Posadino A.M., Younes N., Hasan H., Halabi S., Alhababi D., Al-Mohannadi A., Abdel-Rahman W.M., Eid A.H., Nasrallah G.K., Pintus G. Potential adverse effects of resveratrol: a literature review. Int. J. Mol. Sci. 2020;21:2084. doi: 10.3390/ijms21062084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Songa X., Li L., Sunc J., Jaiswali Y., Huange J., Liug C. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019;111:579–587. doi: 10.1016/j.biopha.2018.12.074. [DOI] [PubMed] [Google Scholar]
- Shiryaev S.A., Mesci P., Pinto A., Fernandes I., Sheets N., Shresta S., Farhy C., Huang C.T., Strongin A.Y., Muotri A.R., Terskikh A.V. Repurposing of the anti-malaria drug chloroquine for Zika virus treatment and prophylaxis. Sci. Rep. 2017;15771 doi: 10.1038/s41598-017-15467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R.V., Costa S.C.C., Branco C.R.C., Branco A. In vitro photoprotective activity of the Spondias purpurea L. peel crude extract and its incorporation in a pharmaceutical formulation. Ind. Crops Prod. 2016;83:509–514. doi: 10.1016/j.indcrop.2015.12.077. [DOI] [Google Scholar]
- Silva B.G., Fileti A.M.F., Foglio M.A., Rosa P.T.V., Taranto O.P. Effects of different drying conditions on key quality parameters of pink peppercorns (Schinus terebinthifolius Raddi) J. Food Quality. 2017 doi: 10.1155/2017/3152797. Article ID 3152797, 12 pages. [DOI] [Google Scholar]
- Silva M.M., Iriguchi E.K.K., Kassuya C.A.L., Vieira M.C., Foglio M.A., Carvalho J.E., Ruiz A.L.T.G., Souza K.P., Formagio A.S.N. Schinus terebinthifolius: phenolic constituents and in vitro antioxidant, antiproliferative and in vitro anti-inflammatory activities. Rev. Bras. Farmacogn. 2017;27:445–452. doi: 10.1016/j.bjp.2016.12.007. [DOI] [Google Scholar]
- Silva S., Martins D.O.S., Jardim C.G. A review of the ongoing research on Zika virus treatment. Viruses. 2018;10:1–18. doi: 10.3390/v10050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J. Anti-infective potential of catechins and their derivatives against viral hepatitis. Clin. Exp. Vaccine Res. 2017;7:37–42. doi: 10.7774/cevr.2018.7.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza S.K.M., Santos T.A., Alves A.C.F., Oliveira A.P., Almeida A.V.H., Alves J.F.S., Cruz E.C.A., Silva J.R.S., Santos N.D.S., Pereira X.N. Identification of flavonol glycosides and in vitro photoprotective and antioxidant activities of Triplaris gardneriana Wedd. J. Med. Plants Med. 2015;9:207–215. doi: 10.5897/JMPR2014.5555. [DOI] [Google Scholar]
- Stevanato R., Bertelle M., Fabris S. Photoprotective characteristics of natural antioxidant polyphenols. Regul. Toxicol. Pharmacol. 2014;69:71–77. doi: 10.1016/j.yrtph.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Tabata T., Petitt M., Puerto-Guardo H., Michlmayr D., Wang C., Fang-Hoover J., Harris E., Pereira L. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe. 2016;20:155–166. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlili N., Yahia Y., Feriani A., Labidi A., Ghazouani L., Nasri N., Saadoui E., Khaldi A. Schinus terebinthifolius vs Schinus molle: a comparative study of the effect of species and location on the phytochemical content of fruits. Ind. Crops Prod. 2018;122:559–565. doi: 10.1016/j.indcrop.2018.05.080. [DOI] [Google Scholar]
- Torres K.A.M., Lima S.M.R.R., Ueda S.M.Y. Activity of the aqueous extract of Schinus terebinthifolius Raddi on strains of the Candida genus. Rev. Bras. Ginecol. Obst. 2016:593–599. doi: 10.1055/s-0036-1597694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin S.J., Afroz M., Zihad N.S.M., Rahman S.Md., Akter S., Khan I.N., Al-Rabbi S., Rouj R., Islam M.T., Shilpi J.A., Nahar L., Tiralongo E., Sarker S.D. a systematic review on anti-diabetic and cardioprotective potential of gallic acid: a widespread dietary phytoconstituent. Food. Rev. Int. 2020 doi: 10.1080/87559129.2020.1734609. [DOI] [Google Scholar]
- Varela-Rodríguez L., Sánchez-Ramírez B., Hernández-Ramírez V.I., Varela-Rodríguez H., Castellanos-Mijangos R.D., González-Horta C., Chávez-Munguía B., Talamás-Rohana P. Effect of Gallic acid and Myricetin on ovarian cancer models: a possible alternative antitumoral treatment. BMC Comp. Med. Ther. 2020;20:110. doi: 10.1186/s12906-020-02900-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Calvo A., Oya N.J., Martín-Acebes M.A., Garcia-Moruno E., Saiz J.C. Antiviral properties of the natural polyphenols delphinidin and epigallocatechin gallate against the flaviviruses west nile virus, zika virus, and dengue virus. Front. Microbiol. 2017;8:1314. doi: 10.3389/fmicb.2017.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wah T.C., Sam I.C., Lim C.W., Vannajan S.L., Chan Y.F. Polysulfonate suramin inhibits Zika virus infection. Antiviral Res. 2017;143:186–194. doi: 10.1016/j.antiviral.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Widowati W., Fauziah N., Herdiman H., Afni M., Affah E., Kusuma H.S.W., Nufus H., Arumwardana S., Rihibiha D.D. antioxidant and anti-aging assays of Oryza Sativa extracts, vanillin and coumaric acid. J. Nat. Remed. 2020;16(3) doi: 10.18311/jnr/2016/7220. [DOI] [Google Scholar]
- You H.L., Huang C.C., Chen C.J., Chang C.C., Liao P.L., Huang S.T. Anti-pandemic influenza a (H1N1) virus potential of catechin and gallic acid. J. Chin. Med. Assoc. 2018;81:458–468. doi: 10.1016/j.jcma.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Huang X., Pan Y., Di Cao, Liu C., Liu Y., Chen A. Resveratrol protects HaCaT cells from ultraviolet B-induced photoaging via upregulation of HSP27 and modulation of mitochondrial caspase-dependent apoptotic pathway. Biochem. Biophys. Res. Commun. 2018:1–7. doi: 10.1016/j.bbrc.2018.03.207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.