Abstract
BACKGROUND
In the six Gulf Cooperation Council countries (GCCCs), Bahrain, Saudi Arabia, Kuwait, Oman, Qatar and the United Arab Emirates, breast cancer (BC) is the greatest cause of cancer incidence and mortality. Obesity and physical inactivity are established risk factors for BC globally and appear to be more of a problem in high income countries like the GCCCs.
AIM
To determine whether obesity and physical inactivity are associated with BC incidence in the GCCCs using the United Kingdom as a comparator.
METHODS
This systematic review was carried out according to PRISMA guidelines. A cancer registry and a statistical data search was done to identify the BC incidence over the past two decades and the prevalence of obesity and physical inactivity in the GCCCs. Additionally, a systematic search of the databases, MEDLINE, Web of Science, and PubMed between 1999 and 2019 was performed to determine whether obesity and physical inactivity are risk factors for BC in the GCCCs. All papers were critically appraised according to their research methods and were assessed for quality and risk of bias.
RESULTS
BC was the top malignancy in each GCC country. Women tended to be diagnosed with BC at a younger age than women in the United Kingdom. The greatest 10-year increase in BC incidence was seen in Saudi Arabia (54.2%), approximately seven times the rate of increase seen in the United Kingdom (7.6%). The prevalence of obesity and physical inactivity was greater in all the GCCCs in comparison to the United Kingdom. A total of 155 full studies were reviewed of which 17 were included. Of those, eight looked at the prevalence of obesity and physical inactivity in the Gulf States and nine looked at these as risk factors for BC. Only one study found an association between BC and obesity (odds ratio = 2.29). No studies looked solely at the link between physical inactivity and BC.
CONCLUSION
The prevalence of obesity and physical inactivity was high within the GCCCs, but the majority of the included studies found no positive correlation between obesity or physical inactivity and BC. A high proportion of women in this study were pre-menopausal which could contribute to the negative findings.
Keywords: Breast cancer, Obesity, Physical inactivity, Females, Gulf Cooperation Council Countries
Core tip: Breast cancer (BC) is the most prolific female cancer worldwide with an estimated 2.08 million new cases and over half a million deaths reported in 2018. The Gulf Cooperation Council countries are a largely under researched region yet have experienced an incline in BC incidence over the past 20 years. The prevalence of obesity and physical inactivity in the Gulf region is significantly high, both of which are known risk factors for BC. A positive correlation between BC and these risk factors in the Middle East is currently inconclusive, highlighting the importance of more research within the Gulf Cooperation Council countries.
INTRODUCTION
Breast cancer (BC) is the most common female cancer worldwide, with an estimated 2.08 million new cases and over half a million deaths reported in 2018[1]. Asia has the largest number of BC incidences and fatalities, closely followed by Europe[1]. Within Asia lie the six Middle Eastern countries, Bahrain, Kingdom of Saudi Arabia (KSA), Kuwait, Oman, Qatar, and the United Arab Emirates (UAE), which together form the Gulf Cooperation Council (GCC). Cancer research within the GCC countries (GCCCs) is minimal. A large review by Hamadeh et al[2] identified all the published literature on cancer in seven Arab countries, including Bahrain and Kuwait, between 2000-2013 and found that although research was increasing, the number of publications was considerably less than those from Europe, Japan and the United States[2]. Moreover, Hamadeh et al[2] discussed the need for future research within the Middle Eastern region, addressing obesity and physical inactivity as potential risk factors for cancer. This review intends to explore obesity and physical inactivity as possible risk factors for BC among females in the six Gulf States.
The Gulf Cooperation Council
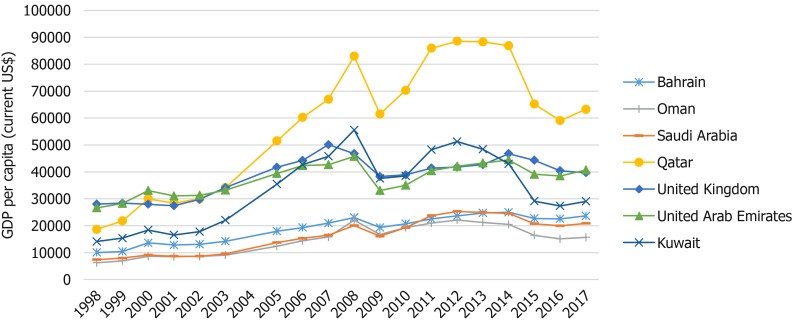
Since the discovery of oil in 1936, there has been a consistent and rapid economic growth across the GCCCs[3,4]. Figure 1 evidences the 20-year economic growth of all six GCCCs and the United Kingdom, all of which are very high human developmental index countries[5]. The gross domestic product (GDP) of all seven countries has grown between 1998 and 2017, however the estimated percentage increase in GDP per capita was greatest in Qatar (239% increase) and lowest in the United Kingdom (41% increase). Fast growing economies and movement towards a more westernized lifestyle has been closely associated with poor diet, lack of physical activity, increase in obesity and rise in non-communicable diseases, such as cancer[1,6,7].
Figure 1.
Gross domestic product per capita (current US$) over two decades. Data was taken from World Bank national accounts data, and Organization for Economic Co-operation and Development National Accounts data files. Access: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD.
Breast cancer risks
There are many risk factors for BC, notably female gender and old age, which are not modifiable. This review will be looking specifically at modifiable lifestyle choices as risk factors for BC. According to “Cancer Research United Kingdom” (2015) 23% of BC cases are preventable, of which 8% are associated to alcohol consumption and a further 8% to obesity. Obesity is a major problem in the United Kingdom. In 2016, 28.6% of adult females were reported obese [body mass index (BMI) ≥ 30], highlighting the extent of this public health problem[8,9]. Additionally, physical inactivity is a major modifiable risk factor for BC. Forty percent of female adults in the United Kingdom, in 2016, were insufficiently active[9]. Seeing as obesity and physical inactivity are so prevalent in the United Kingdom, comparable patterns could be present in the GCCCs. Similarly to the United Kingdom, the most frequent type of breast cancer among the GCCCs is invasive ductal carcinoma allowing patterns between BC and risk factors to be made[8,10].
High alcohol consumption is usually observed in high-income countries. However, the Gulf region is predominantly Muslim and alcohol consumption is prohibited[11]. The Eastern Mediterranean region (containing all 6 GCCCs) has the lowest alcohol consumption per capita in the world[12]. Consequently, this review will not be focusing on alcohol as one of the modifiable risk factors contributing to BC diagnosis in the GCCCs.
Breast cancer and obesity
Obesity is a global epidemic with an observational correlation with BC risk[13]. According to the World Health Organisations (2018) international classifications, obesity is defined as a BMI ≥ 30 kg/m2. Obesity is described as the excess accumulation of adipose tissue as a result of extra calorie consumption[14]. High-calorie intake results in greater energy storage and adipocyte swelling[13,14]. Although the exact molecular link between obesity and cancer is not fully known there are many studies revealing potential mechanisms that associate obesity with BC. The stromal cells of the breast tissue are predominantly composed of adipocytes and lipid storage cells[14]. One hypothesis described by Balaban et al[14] is that under the right stimulus these stromal cells will secrete metabolic substrates; fatty acids and glycerol. Cancer cells require these substrates as energy for proliferation, invasion and migration and they have the ability to reprogram themselves to aid their survival and adapt to oxidative stress[14]. In obese patients there are more triacylglycerides within the adipocytes, therefore, more fatty acids can be mobilized and used by cells as a metabolite[14]. Obesity also leads to chronic low-grade inflammation. A continuous systemic increase of inflammatory cytokines such as tumour necrosis factor-alpha (TNF-α) and C-reactive protein (CRP), are involved in cancer development[13,15]. This is further supported by a case-controlled study on 56 women with BC and 53 age and BMI matched controls, which found that glucose, triacylglycerides and inflammatory markers (TNF-α and CRP) were significantly raised in the BC group[16]. Obesity often co-exists with type 2 diabetes. Hyperinsulinemia, a characteristic of type 2 diabetes, can drive BC development and progression[17]. Breast tissue has a high expression of insulin receptors and their activation triggers cell proliferation and cell survival[17]. These are only several hypothesises of many, and more research is needed to determine the exact mechanism underlying the link between obesity and BC.
Breast cancer and physical inactivity
It has been established that physical inactivity is one of the main modifiable risks for BC and globally it contributes to 21%-25% of BC cases[18]. Lack of exercise is closely linked with elevated levels of circulating sex hormones[19]. In postmenopausal women, the main source of oestrogen comes from aromatization of adrenal androgens in the peripheral adipose tissues[19]. Oestrogen regulates transcription of genes controlling cell growth and differentiation[20]. Some women have a higher expression of oestrogen receptors on their breast tissue, increasing their risk of BC[20]. There is evidence that a lack of exercise in postmenopausal women can increase the levels of circulating sex hormones and lower the levels of sex-hormone binding globulin[21]. Low sex-hormone binding globulin enables free oestrogen to bind to its appropriate receptor in the breast tissue, act as a mitogen and promote cell survival[22]. Furthermore, exercise regulates the energy balance, prevents adiposity and has been shown to decrease circulating androgens, thus decreases the risk of BC[23]. As well as influencing androgen levels, exercise also increases insulin sensitivity and decreases adipokines and oxidative stress, all of which are risk factors for cancer[23].
Hypothesis, aim and objectives
Obesity and physical inactivity are major public health concerns in the United Kingdom. A large proportion of the British population are not meeting the recommended guidelines for physical activity or BMI. It is believed that the GCCCs are adapting a more western style of living, which may be influencing the health status in these Arab countries and effecting the BC incidence.
This study hypothesizes that the BC incidence in this Middle Eastern region has been increasing in a similar way to the United Kingdom. An economic and cultural shift towards a more westernized lifestyle could be encouraging a more sedentary lifestyle and a higher rate of obesity within the GCCCs. Thus, this study theorizes that obesity and physical inactivity are rising and that there is a correlated rise in BC incidence.
The aim was to determine whether obesity and physical inactivity are associated with BC incidence in the GCC countries, using the United Kingdom as a comparator. To achieve this, the study focused on three main objectives; (1) Establish an increase in BC incidence over the past two decades; (2) Determine whether there has been a rise in obesity and physical inactivity; and (3) Detect an association between obesity and physical inactivity individually as risk factors for BC in the GCCCs.
MATERIALS AND METHODS
This review has three objectives requiring a mixed method approach. The first objective was to understand the change in incidence of BC in the GCCCs, using the United Kingdom as a comparative. This involved a cancer registry search. The second and third objective was to determine the prevalence of obesity and physical inactivity and identify a correlation with these risk factors and BC in the GCCCs. This was done by a combination of searching the Global Health Observatory data repository for the prevalence of obesity and physical inactivity and a systematic search of the literature.
National cancer registry search
To access the BC incidence rate between 1998 and 2018 in both the United Kingdom and the 6 GCCCs, different sources were used. Age-standardized female BC incidence from Bahrain, Kuwait and the United Kingdom were available on the Global Cancer Observatory’s “cancer overtime” between 1998 and 2012[24]. The National Cancer Registry (online) database for Saudi Arabia provided data on BC incidence between 2001 and 2014, in the form of annual health reports[25]. The Qatar National Cancer Registry, the National Centre for Statistics and Information (Oman) and the Federal Competitiveness and Statistics Authority in Dubai (UAE) were contacted via email correspondence, due to missing data online. The 2018 BC incidence from all the countries were taken from the Global Cancer observatory’s “Cancer Today”[1].
Global health observatory data search for the prevalence of obesity and physical inactivity
Statistics on the prevalence of obesity and physical inactivity in the GCCCs and the United Kingdom were taken from the Global Health Observatory data repository published online[9].
Literature search
A systematic review of the literature between January 1999 and February 2019 was performed on the following databases: MEDLINE (OVID, 1996-present), Web of Science, and PubMed. The prevalence of obesity and physical inactivity among the GCCCs was determined by using the subsequent search terms in all the databases: (1) (“physical activity” or exercise) and (each GCCC individually); (2) (obes or weight or fat) and (each GCCC individually); To determine whether there was an association between the risk factors, obesity and physical inactivity with BC, the following search terms were used in all databases; (3) western and “breast cancer” and (“gulf cooperation council countries” or gulf or GCC); (4) “breast cancer” and (“gulf cooperation council countries” or gulf or GCC); (5) (“physical activity” or exercise) and “breast cancer” and (“gulf cooperation council countries” or gulf or GCC); (6) (obes or weight or fat) and “breast cancer” and (“gulf cooperation council countries” or gulf or GCC); (7) “breast cancer” and (each individual country); (8) (“physical activity” or exercise) and “breast cancer” and (each GCCC individually); and (9) (obes or weight or fat) and “breast cancer” and (each GCCC individually).
Inclusion/exclusion criteria: Two papers were identified under different titles, published in different journals but had the same study design and results [26,27] (Table 1). Consequently, Albawardi et al[26] (2016) was excluded from this review.
Table 1.
Inclusion/exclusion criteria: Two papers were identified under different titles, published in different journals but had the same study design and results
| Inclusion criteria | Exclusion criteria |
| Papers reporting on obesity of physical inactivity as a risk factor for BC within the GCCCs | Studies on countries outside the GCCCs |
| Studies looking at the prevalence of obesity and insufficient exercise within the GCCCs | Papers on metabolic syndrome, other cancers, BC awareness, screening and perceptions |
| Randomised controlled trials, case-controlled studies and observational studies | Systematic reviews, Meta-Analysis, Editorials, Letters and commentaries |
| Studies involving females aged ≥ 30 yr | Papers solely on children, adolescents (10-19 yr) and young adults (< 30 yr) |
GCCCs: Gulf Cooperation Council countries; BC: Breast cancer.
Data extraction: All data on the prevalence of obesity and physical inactivity in females was collected from all the papers.
Data extracted (where available): (1) Study design; (2) Age range and the mean age of participants; (3) Sample size; (4) Body mass index (BMI); (5) Waist circumference (cm); (6) Abdominal obesity; (7) Physical activity (Depending on the study design this was measured by quantitative or qualitative techniques); and (8) Menopausal status.
Appraisal: All papers were critically appraised using the appropriate quality assessment checklists developed by methodologists from the National Heart, Lung and Blood Institutes (NHLBI) and Research Triangle International Institute, available at https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools[28]. In accordance with the guidelines published by NHLBI, a quality rating of ”Good”, “Fair” or “Low” was subjectively given.
The GRADE (Grading of recommendations, assessment, development and evaluations) criteria was then used to assess the quality of evidence in five domains: Risk of bias, inconsistency, indirectness (relevance of the evidence to this review), imprecision (sample size) and publication bias. The GRADE handbook (https://gdt.gradepro.org/ app/handbook/handbook.html)[29] and chapter eight of the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0)[30] was used and adapted for this quality and bias assessment. Quality of evidence is categorized into four grades[29]: (1) High- “We are very confident that the true effect lies close to that of the estimate of the effect”; (2) Moderate- “The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different”; (3) Low- “The true effect may be substantially different from the estimate of the effect”; and (4) Very Low- “The true effect is likely to be substantially different from the estimate of effect”.
Randomised controlled trials are considered “high” quality studies and observational studies are considered “low” quality, though both can be modified depending on study strengths and limitations[29].
RESULTS
Cancer registry and breast cancer incidence results
In 2018, BC was the most common female cancer in all six GCCCs and the United Kingdom. It was also the chief malignancy across all ages and sexes[1]. Of the GCCCs, Kuwait had the highest age-standardized rate (ASR) for female BC in 2018, followed by the UAE, Bahrain, Qatar, Oman, and finally Saudi Arabia[1]. In comparison, the female BC incidence rate for the United Kingdom in 2018 was the highest by a factor of 2.6 times.
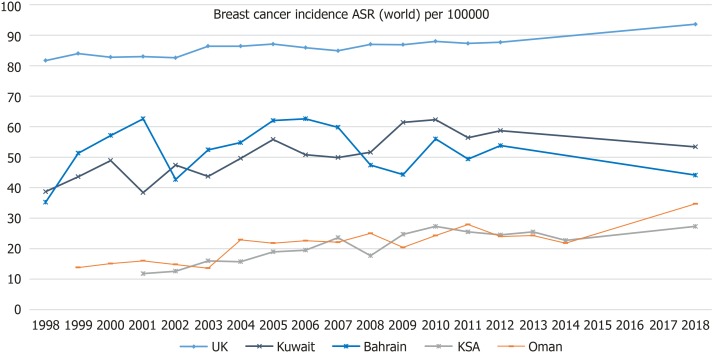
The trend of ASR BC incidence rates for four out of six GCCCs and the United Kingdom are illustrated in Figure 2. Data was available from 1998 for Bahrain, Kuwait and the United Kingdom. However, data was only available from 1999 and from 2001 in Oman and Saudi Arabia respectively. For all the countries shown in Figure 2, there is an upward trend in BC incidence over the past 20 years. The 20-year percentage increase in BC incidence in Kuwait, Bahrain and the United Kingdom are 38.0%, 25.3% and 14.6%, respectively. Over the past 10 years the BC incidence has increased in Saudi Arabia, Oman, United Kingdom and Kuwait by 54.2%, 38.8%, 7.6% and 3.49% respectively. BC incidence in Bahrain decreased by 7.0% over this period.
Figure 2.
Age-standardized rate (world) per 100000 breast cancer incidence; United Kingdom, Kuwait, Bahrain, Kingdom of Saudi Arabia and Oman. Sources: Global Cancer Observatory (United Kingdom, Kuwait and Bahrain), National centre for statistics and Information (Annual reports for cancer incidence in Oman) and Saudi Cancer Registry.
Data for Qatar and the UAE was only provided as “number of cases” and not ASR and therefore could not be added to the graph in Figure 2. The number of female BC cases in 2015 was 246 and 828 for Qatar and UAE respectively. According to the Global Cancer Observatory[1], the number of cases in 2018 was 190 and 1054 for Qatar and the UAE respectively.
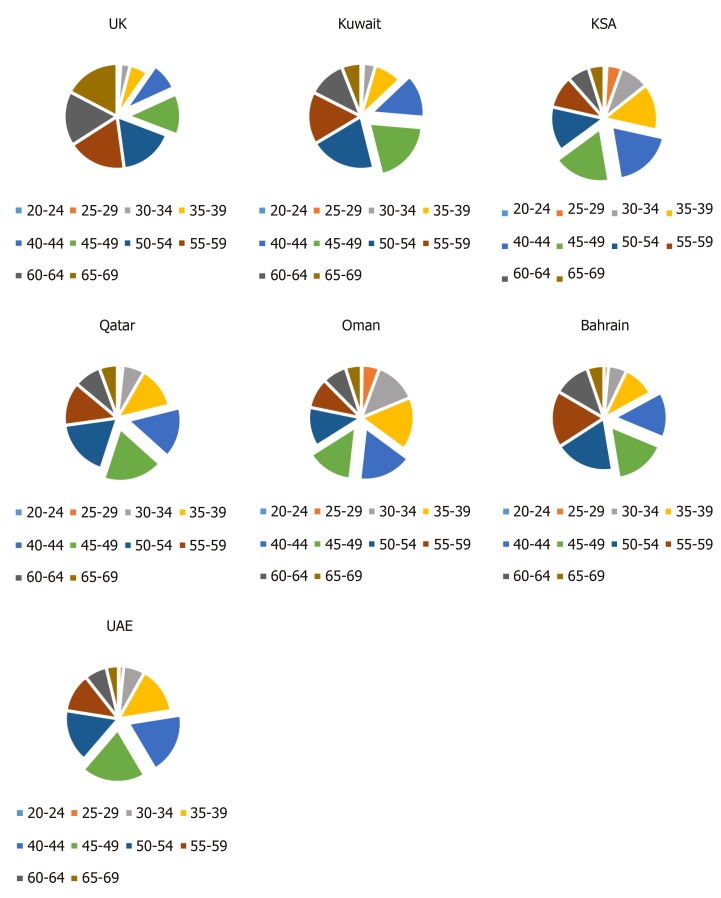
Incidence by age: Breast cancer was diagnosed at a younger age in females across the six GCCCs in comparison to females in the United Kingdom (Figure 3). The highest proportion of cases in Saudi Arabia and Oman was among the 40 to 44-year-olds. Followed by the 45 to 49-year-olds in the UAE and Qatar and the 50 to 54-year-olds in Kuwait and Bahrain. In the United Kingdom, BC was more prevalent among 55 to 59-year-old women, closely followed by the 65-69 age group.
Figure 3.
Number of cases of female breast cancer, by age group. Source: Global Cancer Observatory “cancer today” (2018). For comparison the 40-49 year age groups have been highlighted to show that females in the GCCCs are being diagnosed younger than females in the United Kingdom. UK: United Kingdom; KSA: Kingdom of Saudi Arabia, UAE: United Arab Emirates.
Global health observatory data on obesity and physical inactivity
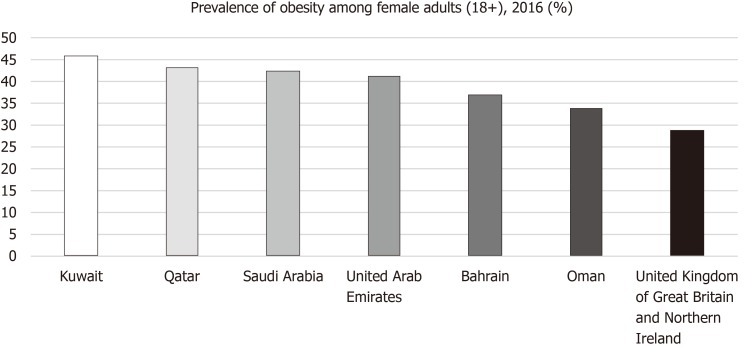
Figure 4 shows the prevalence of obesity (BMI ≥ 30) in female adults in 2016. All six GCCCs had a higher percentage of obese females than the United Kingdom. The highest prevalence of obesity was observed in Kuwait (45.6%) followed by Qatar (43.1%), Saudi Arabia (42.3%), UAE (41%), Bahrain (36.8%), Oman (33.7%) and lowest in the United Kingdom (28.6%).
Figure 4.
Age-standardized estimate of the percentage of female adults (+18) with obesity (Body mass index: 30) in 2016. Source: Global Health Observatory data repository available online at: https://www.who.int/gho/en/.
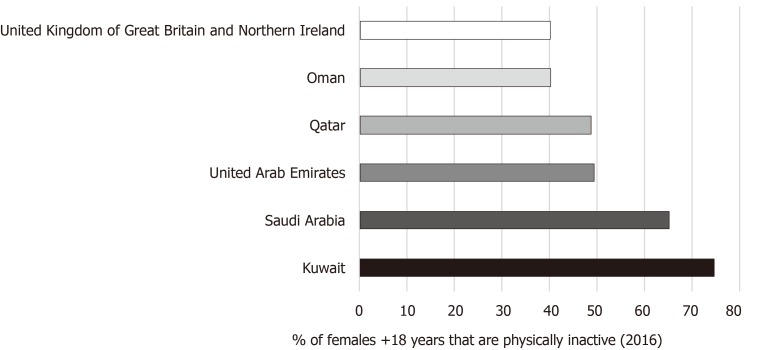
Figure 5 shows the prevalence of adult females that were physically inactive in 2016 in five of the GCCCs and the United Kingdom; data from Bahrain was missing. According to the WHO, insufficient exercise is not meeting the minimum recommendation of 150 min of moderate-intensity or 75 min of vigorous-intensity aerobic activity per week[31]. Again, Kuwait had the highest (74.6%) percentage of inactive females, followed by Saudi Arabia (65.1%), UAE (49.3%), Qatar (48.7%), Oman (40.2%) and the United Kingdom had the lowest (40%).
Figure 5.
Age-standardized estimate (%) of female adults (18+) physically inactive in 2016. Source Global Health Observatory data repository. Available online at: https://www.who.int/gho/en/.
Literature search
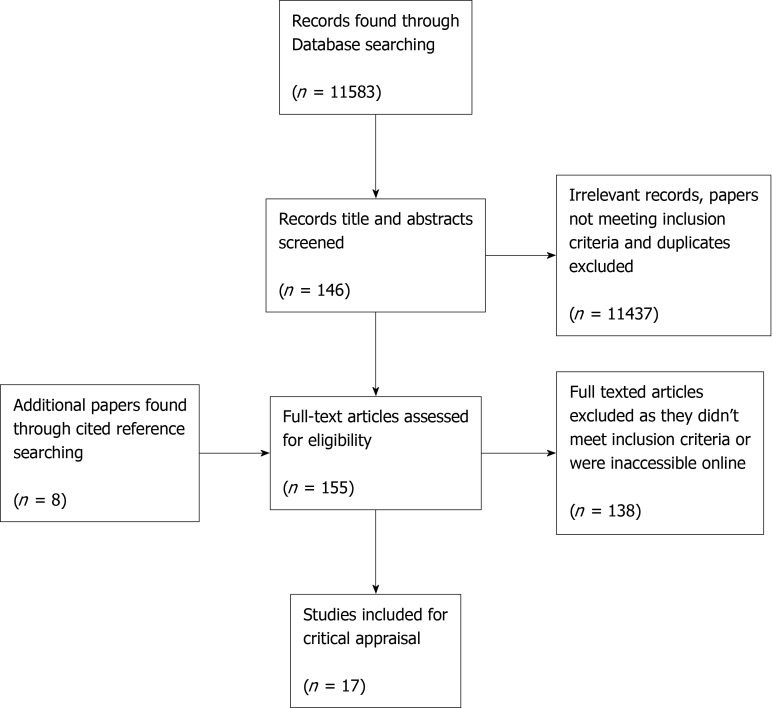
The flow diagram shown in Figure 6 illustrates the search strategy and results for this systematic review.
Figure 6.
Flow diagram of the search strategy for the evidence-based literature search. Diagram adapted from PRISMA flow diagram.
Prevalence of obesity and physical inactivity in the Gulf Cooperation Council countries: Tables 2 and 3 (below) presents the results of eight studies looking at the prevalence of physical inactivity and obesity in the GCCCs. In accordance with the inclusion criteria, all papers were female only studies and any study looking solely at women aged < 30 were excluded.
Table 2.
Results from papers looking at the prevalence of obesity and physical inactivity in the Gulf Cooperation Council countries
| Ref. | Sample size and characteristics | Participant age range (mean ± SD) | Exposure measures | Anthropometric measurements | Physical activity | Key findings | Other findings |
| [36] | 105 female volunteers recruited from Riyadh city, KSA | 18-45 yr (26.3 ± 7.1) | Pedometer used to measure daily steps; Weight and height measured accurately in the clinic | Mean BMI (± SD): 25 (± 4.2) | Mean steps (± SD) - 5114 (± 2213). Classified as “low-active” | There was no significant correlation between step count and any participant demographics | Step count had a strong correlation with self-efficacy |
| [32] | 277 healthy adult Omani women from 5/11 governates in Oman | 18-48 yr, IPAQ (n = 229) - 29.6 ± 7.3; D-SSTQ (n = 191) – 31 ± 7.1; Accelerometer (n = 80) – 29 ± 8.0 | 2 questionnaires and use of accelerometer to measure PA; IPAQ (n = 229); D-SSTQ (n = 191); Accelerometer (n = 80), weight and height measured accurately | IPAQ (n = 229) - Mean (± SD): 25.9 (± 6.3); 52.8% overweight/obese; D-SSTQ (n = 191) -Mean (± SD): 26.7 (± 5.9); 58.6% overweight/obese; Accelerometer (n = 80) - Mean (± SD): 25.1 (± 6.1) | IPAQ (n = 229) - 34% minimally active, 32% moderately active, 34% physically active; D-SSTQ (n = 191) - Mean self-reported sitting; 450 min on working day and 448 min on non-working day. Accelerometer (n = 80) - Mean time wearing was 813.7 ± 101.6 min/d. Time spent in sedentary behaviour was 62%, 35% in light PA and 3% in moderate-vigorous PA | From the IPAQ: a median ± IQR of 75 ± 249 min/wk spent in moderate PA, 0 ± 80 min/wk in vigorous PA and 120 ± 330 min/wk walking. Adults spent significantly (P ≤ 0.05) more time in moderate PA than the younger participants; There was no significance between PA levels and BMI. For the D-SSTQ: adults spent significantly (P < 0.001) more time watching television then the young adults. Generally, women 30-48 yr were more PA then younger adults | There was a significant decrease (P ≤ 0.0001) in the amount of PA in participants that had degree level education. Unemployed participated in more vigorous PA than employed (P ≤ 0.001). Postgraduate degree holders reported significantly more sitting time (P ≤ 0.001). There was no significant correlation between BMI and sitting time |
| [38] | 600 healthy Saudi females from Riyadh KSA | 16-45 yr (26.1 ± 7.7) | Weight and height measured by standard techniques | Mean BMI (± SD): 25.7 (± 5.6); 52.63% had a BMI > 24.9 (range was 14.7-50.3) | N/A | Majority of the participants were either overweight or obese | Married women had a significantly higher prevalence of overweight and obesity There is a statistically significant (P < 0.001) correlation between BMI and age. BMI increased with age and morbid obesity was greatest in the 36-45-year-old age group. There was no significant correlation in BMI between students and housewives |
| [33] | 237 female staff and students from Hail University, KSA | 18-30 yr (NB: 96% < 30) | The short version of the IPAQ for PA; Weight and height accurately measured | 42% overweight or obese | 57%- Inactive 41%- Moderate 2%- Physically active (health-enhancing PA level) | A high percentage of females were inactive | A significant correlation between increasing age and BMI and body fat (P < 0.0001); There was an inverse correlation between the intake of dietary fibre and BMI (P = 0.047) |
BMI > 24.9 is overweight and BMI-30 is obese. BMI: Body mass index; PA: Physical activity; WC: Waist circumference; SD: Standard deviation; IQR: Interquartile range; IPAQ: International physical activity questionnaire; D-SSTQ: Domain-specific sitting time questionnaire; KSA: Kingdom of Saudi Arabia.
Table 3.
Results from papers looking at the prevalence of obesity and physical inactivity in the Gulf Cooperation Council countries (continued Table 2)
| Ref. | Sample size and characteristics | Participant age range (mean ± SD) | Exposure measures | Anthropometric measurements | Physical activity | Key findings | General findings |
| [27] | 420 Saudi females, from 8 office-based worksites in Riyadh | 18-60 yr (31.7 ± 8.3) | PA questionnaire was completed then METs were calculated; Weight and height measured accurately and appropriately | Mean BMI (± SD): 27.1 (± 5.9) 58.3% overweight or obese | 52.1%- low-active 41.2%-moderately active 6.7%-Highly active | Sitting time significantly increased with increasing BMI (P = 0.008) | Majority of participants were aware that prolonged sitting was bad for health; The participants working in the private sector had a predicted 80-min increase in sitting time/day; Mean age at menopause was 47.5 ± 7.1 yr |
| [34] | 535 UAE female citizens living in the Urban area of Al Ain medical district. Surveyed September 2000 to August 2001 | 20-79 (34.3 ± 14.7), ~50% between 20-30 yr | Trained healthcare worker provided the questionnaire to assess PA; Weight and Height were accurately measured | 27% overweight; 35% obese | 84% report sufficiently active (above minimum recommendations for the elderly) | Prevalence of obesity declined with increasing age Women over the age of 40 were classified as obese by their % of body fat but not their BMI. Age was the only significant predictor of obesity is multivariate logistic regression analysis | Participants that had higher education were significantly more PA (P < 0.001); Younger females were significantly more active (P < 0.001); 84% of the sample are pre-menopausal |
| [37] | 438 non-pregnant married women. All Saudi and were born and resident in the Southwestern region of KSA | Divided into 2 age groups 18-39 yr (n = 305) and 40-60 yr (n = 133) | Weight and Height and WC measured accurately; Lipid Research Clinic questionnaire for strenuous exercise assessment | Mean BMI (± SD) of the 18-39 age group: 29.8 (± 6.5); Mean BMI (± SD) of 40-60 age group: 32.4 (± 5.9); Overall Mean BMI (± SD): 30.6 (± 6.5); 41.1% abdominally obese (WC > 88 cm); 52.2 % totally obese (BMI > 30) | Mean strenuous exercise score was 2.74 (score of 2 is “non-strenuous”, 4 is infrequently strenuous, 6 regularly strenuous) | Mean BMI and WC were significantly greater in the 40-60 age group (P < 0.0001); There was no significance found between abdominal obesity and strenuous exercise score, though the non-strenuous group contained the highest proportion of women with abdominal obesity | Women the 18-39 age group had a significantly higher level of education (P < 0.0001). The prevalence of abdominal obesity was greater in illiterate women (54.1%) |
| [35] | 549 female Qatari nationals. Recruited from the public, universities and companies | 18-64 yr (37.4 ± 11.7) | Weight and Height self-reported; Accelerometer to measure steps | Median BMI (IQR) - 28.8 (24.8-33.5) | 44%- Sedentary (< 5000 steps/d); 32.4%- low-active (5000-7499 steps/d); 23.5%- Physically active (≥ 7500 steps/d) | There was no significant difference between PA level and BMI; There was a significant difference (P < 0.0001) between activity level and age. Middle age females (45-64) were more PA | PA levels decreased during the summer months |
BMI: Body mass index; PA: Physical activity; WC: Waist circumference; SD: Standard deviation; IQR: Interquartile range; IPAQ: International physical activity questionnaire; D-SSTQ: Domain-specific sitting time questionnaire; KSA: Kingdom of Saudi Arabia.
All were cross-sectional in design. Four studies looked at both obesity and physical inactivity in the population[27,32-34], two papers primarily focused on physical activity (PA)[33,35,36] and two studies assessed the prevalence of obesity (Khalid[37], 2007 and Al-Malki et al[38], 2003). Al-Malki et al[38], (2003) was the only paper that did not report on PA.
The study population in all the results, except Al-Eisa and Al-Sobayel[36], were reported either overweight (BMI > 24.9) or obese (BMI ≥ 30). However, Al-Eisa and Al-Sobayel[36], contained the smallest sample size (n = 105) and had a low mean age (± SD) of 26.3 (± 7.1), which was younger than this reviews target population (> 30 year-olds). Al-Malki et al[38], (2003) and Al-Shammari et al[33], (2015) also had a predominantly younger sample population.
Across all the papers there was a common theme of low PA with a large amount of sedentary behaviour. Only Carter et al[34], (2004) reported a high percentage (84%) of PA. However, the questionnaire used to access PA was based on activity levels for “elderly” women when the age range of the cohort was 18-60 years (classified as “adults” by the WHO) and all the questions were in relation to walking[34]. Only three studies accurately measured PA levels using an accelerometer and of those studies, a high percentage of the females were either “low-active” or sedentary[32,35,36]. Sayegh et al[35], (2016) reported 44.1% of the female population as sedentary (achieving < 5000 steps/d) and only 23.5% were catergorized physically active (achieving ≥ 7500 steps/d).
Four of the studies[33,35-37] did not find a statistically significant correlation between BMI and PA level and the remaining four did not statistically access this relationship. Increasing age was significantly associated to obesity in three studies[33,37,38] but was negatively associated in one study[34]. However, Carter et al[34], (2004) found that % of body fatness was significantly associated with age and in the multivariate logistic analysis, age was the only significant risk factor for obesity.
Case-controlled studies: Table 4 (below) shows the results of four case-controlled studies looking at the relationship between BC and obesity[39-41]. By chance, all of the studies were looking at females living in Saudi Arabia. Al-Amri et al[40], (2015) was the only study to comment on the relationship between physical inacticity and BC but no significant correlation was noted, neither was there any data presented or mention of this in the methods. Elkum et al[39], (2014) was the only study to report obesity as a significant risk factor for BC (OR = 1.74, P < 0.0001), all the other case-controlled studies found no statistically significant correlation between BC and obesity. Additionally, Elkum et al[39], (2014) was the only study where the greatest proportion of females were postmenopausal. No case-controlled trials were found on the association between physical inactivity and BC in the GCCCs.
Table 4.
Paper results from case-control trials exploring the association of obesity and breast cancer
| Ref. | Sample size and characteristics |
Cases |
Controls |
Association between BC and obesity | Other findings | ||
| Age (mean ± SD) | Anthropometric Measurements | Age (mean ± SD) | Anthropometric measurements | ||||
| [40] | 348 Saudi women (58 newly diagnosed with BC and 290 controls) | 48.5 ± 7.1 | BMI > 30: 71.4% | 49.2 ± 6.9 | BMI > 30: 70.7% | There was no significant association between BMI and BC | BC was significantly correlated with age at marriage and age at menopause; There was no significant correlation between PA and BC; 62.1% of cases were pre-menopausal and 44.8% were post-menopausal |
| [41] | 500 women (250 newly diagnosed with BC, 250 no previous history of any cancer) from 2 hospitals in Riyadh, KSA | 45.7 ± 7.8 | Mean (± SD): 31.2 (± 7.0) | 43.9 ± 7.5 | Mean ± SD 30.7 ± 7.6 | No significant difference between the BMI of the cases and controls | There was a slight significance (P = 0.011) between the age of the 2 groups; Women with BC entered menopause significantly younger than the controls (P = 0.022); Mean (± SD) of menopause was 46.6 (± 6.4) for the controls and 48.7 (± 5.2) which was significant (P = 0.022) |
| [61] | 997 women from 1 research centre in Riyadh, KSA. 499 newly diagnosed and confirmed BC and 498 age-matched controls | 44.8 ± 11.5 | Mean (± SD); 29.5 (± 6.2) | 36.8 ± 12.8 | Mean ± SD 29.4 ± 6.2 | There was no significant difference between the BMI of the cases and controls | BC patients were significantly older than controls (P = 0.0001); A positive association between the highest quartile triglyceride level and BC risk (OR = 2.90); Mean ± SD menopausal age for cases was 48.2 ± 7.6 yr and 47.9 ± 8.1 yr for the controls |
| [39] | 1172 women aged 18+, 534 histologically confirmed primary BC cases and 638 unmatched controls that were BC free | 43.6 ± 8.3; 15% ≤ 35 yr, 85% > 35 yr | 29.4% overweight and 46.4% obese | Mean not provided; 31.5% ≤ 35 yr, 68.5% > 35 yr | 30.3% overweight and 31.0% obese | Overweight/ obese BMI significantly increased the BC risk compared to normal BMI (OR = 2.29). It is an independent risk factor for BC. Obesity/obese proportion was significantly high in BC group than controls (OR = 1.74 and P < 0.0001); Being overweight or obese in the pre- and postmenopausal ages were both significantly associated with increased BC risk compared to controls | Low education, unemployment and marriage were significantly associated with higher BMI (P < 0.0001); Low education was associated with an increased risk of BC (P < 0.0001); 49.7% of cases were premenopausal and 50.3% were postmenopausal. Post-menopausal women were found to have a positive association with BC risk |
BMI > 24.9 is overweight and BMI-30 is obese. BMI: Body mass index; PA: Physical activity; SD: Standard deviation; BC: Breast cancer; OR: Odds ratio; CRP: C-reactive protein; TNF-α: Tumour necrosis factor- alpha; KSA: Kingdom of Saudi Arabia.
Non-case-controlled studies on the association of obesity and physical inactivity with breast cancer: Tables 5 and 6 (below) presents the results of five papers that met the inclusion criteria but were not case-controlled studies. The participants in four of the studies had either a previous or present diagnosis of BC. Al Saeed et al[42], (2015), Alsaeed et al[43], (2017) and Rudat et al[44], (2012) reported over half their study population as obese (BMI ≥ 30), 53.6%, 56.4% and 51.5% respectively. Bener et al[45], (2017) was the only study that also assessed physical inactivity as a risk factor for BC. Of those studies, all of the participants had a BC diagnosis when the data was collected. Bener et al[45], (2017) assessed the BC risk in 1338 Arabian women using the Gail model. This BC risk assessment tool has been validated for many nationalities, though this study was the first to use this risk assessment in women from the Gulf region[45,46]. Results found that 72.8% of women were either overweight or obese; additionally, there was a statistically significant association between BMI and a high 5-year risk of BC (P < 0.001). However, when inserted into the linear regression model BMI was not a predictor for 5-year or lifetime risk of BC in Qatari women aged > 35 years (Bener et al[45], 2017). Bener et al[45], (2017) also found that 60.6% of women were postmenopausal and that age at menarche and age at menopause were significantly correlated with increased BC risk. PA was low, 60.5% of the Qatari women did no PA, though it was not measured as a risk factor for BC using the Gail model[45]. Alsaeed et al[43], (2015) found a significant association between being overweight (BMI: 25-29.9) and locoregional recurrence of BC (P = 0.002) but no significant correlation between obesity (BMI ≥ 30) and locoregional recurrence.
Table 5.
Paper results for non- case-controlled studies on obesity and physical activity in association with breast cancer
| Ref. | Type of study | Sample size and characteristics | Age range (mean ± SD) | Anthropometric measurements | PA | Key findings | Other findings |
| [81] | Single-institute retrospective study | 224 females (72.4% Saudi National) who underwent mastectomy, MRM or WLE with axillary dissection | 26-93 yr (48.8 ± 12.2); 61.7% of females < 50 yr | Mean BMI; 32; 38.3% overweight; 42.8% obese | N/A | Most of the participants in both age groups had a BMI > 30 | 92.6% of females had invasive BC; Ten-year survival rate did not differ significantly with females ≤ 45 or > 45. Only 12% of patients presented with early-stage disease |
| [42] | Data-analysis of patients treated with BCS and MRM between February 1988 and August 2008 | 112 Saudi women. Not included if had distant metastasis or neoadjuvant chemotherapy | 23-76 yr (47.0 ± 10.3) | Range: 15-52.8; Mean BMI (± SD): 31.8 (± 7.2); 28.6% overweight 53.6% obese | N/A | BMI < 18.5 was significantly associated (P = 0.002) to locoregional recurrences; BMI 26-30 (overweight) was significantly associated with locoregional recurrence (P = 0.002); In multivariate analysis age < 35 was an independent risk factor for locoregional recurrence. The risk of locoregional recurrence was not significant in obese females | Only 8.93% had locoregional recurrences, 83% of women were premenopausal and 17% were postmenopausal |
| [43] | Retrospective cross-sectional secondary data analysis study | 112 Saudi women diagnosed with BC that had either BCS with axillary lymph node dissection or MRM following neoadjuvant therapy | No range; 47 ± 10 | Mean BMI (± SD): 32 (± 7.16); 27.3% overweight 56.4% obese | N/A | BC receptor expression was not influenced by BMI | Obesity did not influence the TNM stage of the breast tumour; 82.7% of the sample were premenopausal and 17.3% were postmenopausal |
BMI > 24.9 is overweight and BMI-30 is obese. BMI: Body mass index; PA: Physical activity; SD: Standard deviation; BC: Breast cancer; KSA: Kingdom of Saudi Arabia; BCS: Breast-conserving surgery; MRM: Modified radical mastectomy; US: United States; WLE: Wide local excision.
Table 6.
Paper results for non- case-controlled studies on obesity and physical activity in association with breast cancer (continued Table 5)
| Ref. | Type of study | Sample size and characteristics | Age range (mean ± SD) | Anthropometric measurements | PA | Key findings | Other findings |
| [45] | Cross-section- Data collection from 10 randomly selected primary healthcare facilities | 1488 Qatar and Arab national women. 64.7% were Qatari and 35.3% were Arab expats | 35-65 yr (47 ± 10.8) | 42.8% overweight and 30.0% obese | PA walking per day: 27.5%-30 min, 12.0%- 60 min, 60.5%- none | 72.8% overweight/obese; Using the Gail model (n = 1338) BMI was significantly associated with a high 5-yr risk of BC (P < 0.001); In linear regression analysis, BMI was not associated with 5-yr or lifetime risk of BC. PA declined in the hot weather | Chronological age, age at menarche, menopausal age and occupation were all associated with a 5-yr risk of BC; 39.4% were premenopausal and 60.6% were postmenopausal |
| [44] | A retrospective epidemiological study. Results from KSA females compared with statistics from United States cancer registry (SEER) | 262 female patients in 1 hospital in the eastern provenience of KSA diagnosed with invasive BC | 24-94 yr, median age 48 | 31.9% overweight, 51.5% obese | N/A | The % of BC cases with a BMI > 30 was higher among the females in KSA than the females on the SEER database | BC diagnosis occurred at a significantly younger age when compared to females on the SEER database (United States); BC was significantly more aggressive than females on the SEER database, 58.7% were premenopausal and 41.3% were postmenopausal |
BMI: Body mass index; PA: Physical activity; SD: Standard deviation; BC: Breast cancer; KSA: Kingdom of Saudi Arabia; BCS: Breast-conserving surgery; MRM: Modified radical mastectomy; WLE: Wide local excision.
Other findings: Aside from the main objectives of this review, other interesting factors affecting obesity and PA were found. Women that were unemployed were significantly more active[32] and were at higher risk of obesity[39]. Women that worked in the private sector spent more time sitting than those that worked in the public domain[27]. Increasing age was associated with more obesity and body fat in three studies[33,37,38]. Numerous studies establish a relationship between low education, less PA and obesity[34,37,39]. Additionally, of the studies looking at women with BC a high proportion of cases were premenopausal[40-44].
Critical appraisal
Quality assessment tool results: Tables 7 and 8 (below) presents the critical appraisal of cross-sectionally designed studies. Al-Habsi et al[32], (2015) recorded the level of physical activity using three techniques; two separate questionnaires and an accelerometer. However, only 80 women agreed to wear an accelerometer which was a 31% response rate, thus the results are less likely to represent the whole population, increasing the risk of bias. Rudat et al[44], (2012) extracted data from 262 women in one hospital in KSA and compared it with 300000 females from across the United States, published on the SEER database, which is not comparable. Additionally, this study had no ethical statement, did not declare the researcher’s role, did not mention data analysis techniques and did not justify the sample sizes used. Al-Malki et al[38], (2003) was the only paper that did not follow the WHO classification for BMI. A BMI < 20 was classified “lean” and the “normal” BMI range was 20-24.9. Furthermore, no clear aim was stated and there were no ethical considerations. Two studies (Al Saeed et al[42], 2015 and Sayegh et al[35], 2016) did not justify their use of sample size and did not describe the data collection process in detail. Al-Eisa and Al-Sobayel[36] (2012) used telephone interviews to screen volunteers for inclusion, this lack of visual cues could be subject to bias[47].
Table 7.
Critical appraisal of observational cohort and cross-sectional studies using the National Institutes of health study quality checklists
| Al Saeed et al[42], 2015 | Al-Eisa and Al-Sobayel[36], 2012 | Al-Habsi et al[32], 2015 | Al-Malki et al[38], 2003 | Al-Shammari et al[33], 2015 | Alabdulkarim et al[81], 2018 | Albawardi et al[27], 2017 | Alsaeed et al[43], 2017 | Bener et al[45], 2017 | Carter et al[34], 2004 | Khalid[37], 2007 | Rudat et al[44], 2012 | Sayegh et al[35], 2016 | |
| Was the research question or objective clearly stated? | Y | Y | Y | N | N | N | Y | Y | Y | Y | Y | N | Y |
| Was the study population clearly specified and defined? | Y | N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Was the participation rate of eligible persons at least 50%? | CD | Y | Y (but N for accelerometer) | Y | Y | Y | Y | Y | Y | Y | Y | NA | NA |
| Were all subjects selected or recruited from the same or similar populations? Were inclusion/exclusion criteria prespecified? | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Was a sample size justification, power description or variance and effect estimates provided? | N | N | N | N | Y | N | Y | Y | Y | Y | Y | N | N |
| Was the exposure of interest measured prior to the outcome being measured? | Y | N | N | N | N | Y | N | Y | N | N | N | N | Y |
| Was the timeframe sufficient for an association to be seen? | Y | N | N | N | N | Y | N | N | N | N | N | N | Y |
| For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome? | Y | N | Y | NA | Y | NA | Y | NA | Y | Y | Y | Y | Y |
| Were the exposure measures (independent variables) clearly defined, valid and reliable and implemented consistently across all study participants? | Y | Y | Y | Y | Y | Y | Y | CD | Y | Y | Y | Y | Y |
| Was the exposure measured more than once over time? | N | Y | Y | N | N | Y | N | N | N | N | N | N | Y |
Available online at https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Y: Yes; N: No; CD: Cannot determine; NA: Not applicable; NR: Not reported; BMI: Body mass index; PA: physical activity.
Table 8.
Critical appraisal of observational cohort and cross-sectional studies using the National Institutes of health study quality checklists (continued Table 7)
| Al Saeed et al[42], 2015 | Al-Eisa and Al-Sobayel[36], 2012 | Al-Habsi et al[32], 2015 | Al-Malki et al[38], 2003 | Al-Shammari et al[33], 2015 | Alabdulkarim et al[81], 2018 | Albawardi et al[27], 2017 | Alsaeed et al[43], 2017 | Bener et al[45], 2017 | Carter et al[34], 2004 | Khalid[37], 2007 | Rudat et al[44], 2012 | Sayegh et al[35], 2016 | |
| Were the outcome measures (dependent variables) clearly defined, valid, reliable and implemented consistently across all study participants? | NR | Y | Y | NA | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Were the outcome assessors blinded to the exposure status of the participants | N | CD | N | N | N | CD | N | N | Y | Y | Y | N | N |
| Was loss to follow-up after baseline 20% or less? | NR | NA | NA | NA | NA | N | Y | NA | NA | NA | NA | NA | Y |
| Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure and outcome? | Y | N | N | Y | N | Y | Y | Y | Y | Y | Y | N | N |
| Quality rating | Poor | Poor | Poor | Poor | Poor | Fair | Good | Good | Fair | Fair | Good | Poor | Poor |
| Additional comments | Selection bias, no blinding | Confounding bias | Confounding and recall bias | Selection bias | Selection and confounding bias | Confounding and selection bias | Confounding and recall bias for BMI |
Y: Yes; N: No; CD: Cannot determine; NA: Not applicable; NR: Not reported; BMI: Body mass index; PA: Physical activity.
The critical appraisal of case-controlled studies is summarised in Table 9 (below). AlFaris et al[41], (2018) did not adjust for confounding factors in the data analysis, suggesting potential bias and decreasing the reliability of the results. Alothaimeen stated in the methods that controls were age-matched, but the BC patients were significantly older than the controls (P = 0.0001).
Table 9.
Critical appraisal of case-controlled studies using National Institutes of health study quality checklists
|
Critical assessment of case-controlled studies | ||||
| Al-Amri et al[40], 2015 | AlFaris et al[41], 2018 | Alothaimeen et al[61], 2004 | Elkum et al[39], 2014 | |
| Was the research question or objective clearly stated? | Y | Y | Y | Y |
| Was the study population clearly specified and defined? | Y | Y | Y | Y |
| Did the authors include a sample size justification? | Y | N | Y | N |
| Were controls selected or recruited from the same or similar population that gave rise to the cases? | Y | Y | Y | Y |
| Were the definitions, inclusion and exclusion criteria, algorithms or processes used to identify or select cases and controls valid, reliable and implemented consistently across all study participants? | Y | Y | N | Y |
| Were the cases clearly defined and differentiated from controls? | Y | Y | Y | Y |
| If less than 100% of eligible cases/controls were selected for the study, were the cases/controls randomly selected from those eligible? | NA | NA | NA | Y |
| Was there use of concurrent controls? | N | N | N | Y |
| Were the investigators able to confirm that the exposure/risk occurred prior to the development of the condition or event that defined a participant as a case? | Y | N | CD | N |
| Were the measures of exposure/risk clearly defined, valid, reliable and implemented consistently across all the study participants? | N | Y | y | Y |
| Were the assessors of exposure/risk blinded to the case to the case or control status of participants? | Y | N | N | Y |
| Were key potential confounding variables measured and adjusted statistically in the analyses? If matching was used, did the investigators account for matching during study analysis? | Y | N | Y | Y |
| Quality rating | Poor | Poor | Poor | Good |
| Additional comments | Controls not well defined and were not concurrent | High risk of bias and confounding not adjusted for | Cases were significantly older than the controls (P = 0.0001). High risk of bias | |
Available online at https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Y: Yes; N: No; CD: Cannot determine; NA: Not applicable; NR: Not reported.
GRADE results: Observational studies are considered “low” or “very low” quality evidence due to a higher risk of sampling and recall bias[48]. The quality of evidence using the GRADE criteria is summarised in Table 10. Since systematic reviews and meta-analyses were excluded from this paper, the inconsistency of studies and publication bias were not assessed using GRADE. Elkum et al[39], (2014) was the only study to score “low” quality, because it was not downgraded in any of the 3 domains; there was a low risk of bias, a sufficient sample size and the research questions addressed issues relevant for this study. The rest of the studies were of “very low” quality largely due to high recall bias and small sample sizes[29]. Recall bias was the most frequent form of bias and was associated with self-reported data on PA levels and anthropometric measurements.
Table 10.
Quality of evidence using the GRADE criteria for 3 domains; risk of bias, indirectness and imprecision
|
GRADE criteria | |||||
| Ref. | Study design | Risk of bias, No, serious (-1), very serious (-2) | Indirectness, No, serious (-1), very serious (-2) | Imprecision, No, serious (-1), very serious (-2) | Quality of evidence, RCT (starts at high quality), Non-RCT (starts at low) |
| Al Saeed et al[42], 2015 | Retrospective data analysis | No | -1 | -1 | Very Low |
| Al-Amri et al[40], 2015 | Case-control study | -1 | No | -1 | Very low |
| Al-Eisa and Al-Sobayel[36], 2012 | Cross-sectional | -2 | -1 | -2 | Very low |
| Al-Habsi et al[32], 2015 | Cross-sectional | -1 | -1 | -1 | Very low |
| Al-Malki et al[38], 2003 | Cross-sectional | No | -1 | No | Very low |
| Al-Shammari et al[33], 2015 | Cross-sectional | -1 | -2 | -1 | Very low |
| Alabdulkarim et al[81], 2018 | Single-institute retrospective | No | No | -1 | Very low |
| Albawardi et al[27], 2017 | Cross-sectional | -1 | -1 | No | Very low |
| AlFaris et al[41], 2018 | Case-control and cross-sectional design | -1 | -1 | No | Very low |
| Alothaimeen et al[61], 2004 | Case-control | -2 | No | -1 | Very low |
| Alsaeed et al[43], 2017 | Retrospective cross-sectional | No | -1 | -1 | Very low |
| Bener et al[45], 2017 | Cross-sectional | -1 | No | No | Very low |
| Carter et al[34], 2004 | Cross-sectional | -1 | -1 | No | Very low |
| Elkum et al[39], 2014 | Case-control | No | No | No | Low |
| Khalid[37], 2007 | Cross-sectional | -1 | No | No | Very low |
| Rudat et al[44], 2012 | Retrospective epidemiological | No | No | -1 | Very low |
| Sayegh et al[35], 2016 | Retrospective data analysis | -1 | -1 | No | Very low |
Taken from GRADE handbook, available at https://gdt.gradepro.org/app/handbook/handbook.html.
DISCUSSION
The aim of this study was to use a mixed method approach to (1) identify the BC incidence in the GCCCs; (2) determine the prevalence of obesity and physical inactivity in the GCCCs; and (3) demonstrate an association between these risk factors (obesity and physical inactivity) and BC, using the United Kingdom as a comparator.
It was hypothesised that there has been a significant increase in BC in all the GCCCs as a result of increasing obesity and physical inactivity caused by the rapid economic growth since the discovery of oil[3]. One of the Gulf Cooperation Councils’ main objectives is “to formulate similar regulations in various fields including the following…economic and financial affairs…social and health affairs”[49]. Thus, the health affairs among these populations is assumed to be similar, hence why they are being reviewed together. To the best of our knowledge, this is the first review of this kind.
Breast cancer incidence
This study found that in 2018, BC was responsible for 16.6% of all new cancer cases in the GCCCs and there has been an observed incline in BC incidence over the past 20 years, as was shown in Figure 2[1,24]. Although the United Kingdom had the greatest age-standardized incidence rate (93.6/10000), the largest 10-year increase in BC incidence was seen in Saudi Arabia (54.2%) but Saudi had the lowest incidence rate (27.3/100000). Since 1998, Saudi Arabia has experienced an estimated 182% increase in GDP per capita (Figure 1) which is almost 4.5 times greater than the growth seen in the United Kingdom during that time frame[4]. Furthermore, it was found that since 2008 the BC incidence in Saudi Arabia has increased at approximately seven times the rate of the United Kingdom[25] suggesting that the country’s economic status could be influencing the lifestyle of Arabic females causing an increased risk of BC.
A large empirical study looking at the relationship between economic development and cancer incidence in 122 countries, demonstrated a positive linear relationship between income per capita and BC incidence rate[50]. Rapid economic development leads to significant changes in diet and lifestyle and a rise in non-communicable diseases, such as cancer[7]. A parallel pattern could be occurring within the Gulf region and may contribute to the linear incline in BC incidence seen in this study. Similar to the United Kingdom, all the GCCCs offer free healthcare to all citizens in which BC screening is offered[51,52]. One could argue that the rise in BC incidence is related to better awareness and more screening. However, in Saudi Arabia screening uptake is very low, only 16.2% of 816 females aged ≥ 30 years had ever been screened in a study by Abdel-Aziz et al[53] and in a study by El Bcheraoui et al[51] (2015), 92% of 1001 females (aged 50-74 years) had never had a mammogram. Since the introduction of breast cancer screening programmes in the GCCCs, the uptake has been lower than expected and females are presenting with more advanced tumours[54]. This could be for a number of reasons including lack of health knowledge, cultural and religious reasons. Thus, the rise in BC incidence is more likely to be related to lifestyle changes rather than increased screening across a broader population.
It was hard to compare the BC incidence of the UAE and Qatar with the other GCCCs as the data provided by their cancer registries was limited and given as number of cases and not age-standardised rates. However, similar cultural, lifestyle and environmental factors exist between the GCCCs, thus BC incidence and risk factors are likely to be comparable.
Increasing age is a known risk factor for all cancers, including BC, with approximately nine in 10 cancers occurring in over 50-year-olds[55]. During the BC database search, it was found that in 2018, BC affected younger women in the GCCCs compared to the United Kingdom. The largest proportion of cases in Saudi Arabia and Oman occurred in 40 to 44-year-olds followed by 45 to 49-year- olds in Qatar and the UAE, as shown in Figure 3[1]. This puts the average age at diagnosis almost a decade younger than females in the United Kingdom, in which most cases occurred in the 55 to 59-year-olds[1]. Additionally, a large literature review analysing the age of diagnosis in a total of 7455 patients across 18 Arab nations found that the weighted average age of diagnosis was 49.8 and the age range was 43-52 years[56]. The average life expectancy for females in the GCCCs was approximately 78 years in 2016, in comparison to the United Kingdom which was 83 years[57]. The difference in age could explain why the BC data obtained from the Global Cancer Observatory[1] and the weighted average age of BC diagnosis found by Najjar and Easson[56] (2010) demonstrated a younger age of diagnosis in the GCCCs, than in the United Kingdom. A lower life expectancy is likely to be associated with a predominantly younger population which would influence the age of BC diagnosis. However, understanding why BC is affecting younger females in the GCCCs is beyond the scope of this study, which focuses on obesity and physical inactivity as risk factors for BC. Research to explain these findings is important to comprehend BC incidence patterns in the GCCCs.
Breast cancer and obesity
The percentage of adult females categorized “obese” in each Gulf country exceeds that of the United Kingdom (Figure 4). The prevalence of obesity was greatest in Kuwait, with 45.6% of females aged > 18 years having a BMI ≥ 30 (obese) and 75.1% either overweight or obese (Figure 4)[9]. The high numbers of overweight and obese females are further reflected in the participant characteristics in the majority of the studies found in the literature search. From the eight papers looking just at the prevalence of obesity and physical inactivity (Tables 2 and 3), over half the sample population were either overweight or obese, with the exception of Al-Eisa and Al-Sobayel[36] (2012) and Al-Shammari et al[33], (2015). The majority of BC cases in the GCCCs occur in > 40-year-olds but the mean age of participants in Al-Eisa and Al-Sobayel[36] (2012) study was 26.3 years and 96% of Al-Shammari et al[33], (2015) sample were aged < 30 years, making them less relevant for this study. Numerous other papers looking at obesity in the GCCCs on males and females reported similar rates of obesity[58-60]. Many factors could be contributing to the obesity crisis observed in the GCCCs such as diet and lifestyle and more research into the reasons why the rate of obesity is so high, would be beneficial as it has significant health risks for many non-communicable diseases.
Nine studies, across all three databases, looked at the relationship between BC and obesity in the GCCCs. Of them, four were case-controlled and the rest were cross-sectional in design (Tables 4-6). It is evident from all these studies that overweight and obesity is pointedly prevalent, as the mean BMI was either overweight or obese. Contradictory to many studies done outside the GCC, the majority of the papers in this review reported no significant correlation between obesity and BC[40,41,43,61]. Only two studies found obesity to be a significant risk factor for BC. Bener et al[45], (2017) used the Gail model, which has been validated for white, black/African, American, Hispanic and Asian women[62,63], to access BC risk in a sample of 1488 Qatari women. BMI was significantly associated with a 5-year risk of BC but in linear regression analysis, BMI was no longer a significant risk factor[45]. Elkum et al[39], (2014) in a case-controlled trial found that the odds of getting BC was 2.29 times greater in participants with a BMI ≥ 25 in comparison to a normal BMI and the proportion of females that were either overweight or obese was also significantly higher in the case group.
Copious United Kingdom-based studies have looked into the association between BC and obesity and it is well-established that being overweight or obese is a significant risk factor for breast cancer in postmenopausal women[64-67]. However, more recent research has found that obesity is inversely associated to breast cancer in premenopausal women[64,66,68]. Guo et al[64], (2016) conducted a large mendelian randomization analysis on European females to look at the association between genetically predicted BMI and BC risk. It was shown that being overweight from a child might actually be a protective factor for BC, due to less overall exposure to sex hormones than if you were to develop obesity in adulthood[64]. Obesity is a significant problem in children and adolescents in the Gulf Cooperation Council countries, such that the percentage of children and adolescents aged 5-19 years is greater in all the GCCCs in comparison to the United Kingdom where the estimated percentage of obesity is 10.2%[69]. The highest percentage observed in the GCCCs was in Kuwait (22.9%) and the lowest was Oman (14.9%)[69]. This may explain why this review could not identify a link between obesity and BC in the GCCCs.
Menopausal status was collected from all studies reviewed in this paper and of the studies that showed no significant association between obesity and BC, the highest proportion of women were premenopausal. Interestingly Elkum et al[39], (2014) was the only case-controlled trial to report a positive association between obesity and BC but it was also the only case-controlled study that had a predominately post-menopausal sample. As previously stated, women in the GCCCs are getting BC considerably younger than British females and many have not reached menopause at the time of diagnosis. Since, premenopausal obesity is thought to be a protective factor for BC, it may explain the lack of correlation observed in this study. The insignificant association between BC and obesity seen in the GCCC studies could also be due to the homogeneity of the study participants, as such a high percentage of the population are also overweight or obese.
Although this paper did not look at the age of menarche as a risk factor for BC in the GCCCs, the high prevalence of obesity in the adult population may also be present in younger females and children. A large study on female high-school students in Kuwait found that childhood obesity was significantly correlated with younger age of menarche[70] which itself is a major risk factor for BC[71]. If Arab girls are obese from a young age they may reach menarche earlier, which could explain the rapid incline in BC incidence seen over the last 20 years. Therefore, more research into childhood obesity, the age of menarche and BC risk in the GCCCs is required.
Another established protective factor for breast cancer is parity. The risk of BC decreases as the number of children each woman has increases[8]. According to the World Bank database the average number of births in 2017 among the GCCCs was similar to that in the United Kingdom[72]. However, in 1998 women in the GCCCs were having considerably more children than women in the United Kingdom. The average number of births per woman in the United Kingdom has remained relatively stable since 1998 in contrast to the 6 GCCCs which all demonstrated a significant decline within the same time period[72]. For example, in Saudi Arabia the births per women decreased from four to two between 1998 and 2017[72]. The observed pattern of decreased number if parity and the increasing BC incidence may explain why the GCCCs have experienced a large increase in BC incidence rate over the past 10-years in comparison to the United Kingdom.
Breast cancer and physical inactivity
This study showed that a large proportion of adult females were physically inactive, especially in Kuwait and Saudi Arabia where 74.6% and 65.1% are “inactive”, respectively (shown in Figure 5). Six studies presented in Tables 2 and 3 showed that the majority of females were “inactive” or “low-active” with one study not looking at PA (Al-Malki et al[38], 2003) and another study reporting 84% sufficiently active (Carter et al[34], 2004). However, the latter study based its findings on the minimum recommended PA levels for an elderly population, but the mean age of participants was 34.3 ± 14.7. Furthermore, half of the source’s results are subject to recall bias from self-reported activity levels. All the studies that looked at both obesity and physical inactivity in the population did not find a significant association between obesity and lack of exercise. There were limited studies looking at the association between BC and physical inactivity in the GCCCs. In the BC risk assessment carried out by Bener et al[45], (2017) on 1488 women from Qatar, 60.5% were “inactive” but this was not inserted into the Gail model for BC risk analysis. Al-Amri et al[40], (2015) was the only other study to report on the association between PA and BC, but no significance was found.
A large systematic review and meta-analysis looking at the association between leisure activity and BC in the United Kingdom found that the risk of BC was reduced by 3%, 6% and 14% in low-active (600-3999 METS), moderately active (4000-7999 METs) and highly active (≥ 8000 METS) individuals respectively when compared to inactive women[73]. There is a strong Arabic culture among the GCCCs where women are restricted by traditional and social norms[74]. Several studies looking at barriers to PA found that hot climate, lack of exercise facilities for females, limited public transport, social taboo and prohibition from husbands were strong factors for low activity in the GCCCs[18,75,76]. More research is required to determine whether there is a relationship between the low PA seen in the GCCCs and the high incidence of BC.
Limitations
Inaccessibility to databases, “Middle Eastern and Central Asian studies” and “Middle East and Africa database” limited the number of relevant studies included in this systematic review. Although the Middle Eastern and Central Asian studies database has a vast number of records, it predominately focuses on Arabic heritage and culture and less on science[77]. The Middle East and African database incorporate science and medical research, but it is a very small database with an estimated 320 papers[78]. Nonetheless, this should have a negligible effect on this study as the three databases searched provide access to several thousand accessible journals which include science and medical studies from across the Gulf region. The majority of the research on BC within the GCCCs was performed in Saudi Arabia, which somewhat limits the ability to apply these findings to the entire Gulf population. However, the alliance between the countries and their common objectives would suggest similar patterns of health between the Gulf states[49].
This study was inherently limited by the quality of evidence found during the systematic search of the literature. All of the studies found were observational (shown in Table 10), which provides a “low” quality of evidence. Moreover, the majority received a “very low” GRADE quality score. Although randomized controlled trials provide rigorous evidence, it would be difficult and unethical to use these trials to establish an association between BC and risk factors[79]. Furthermore, a larger systematic review looking at central, abdominal obesity and BC risk in the United Kingdom, found only observational studies[80,81].
Conclusions and future research
In conclusion, although the BC incidence rate in the Gulf Cooperation Council countries is lower than the United Kingdom it is still very prevalent, such that it is responsible for the largest number of cancer cases across all genders. Over the last two decades, the BC incidence rate in the GCCCs has been increasing considerably faster than in the United Kingdom. Furthermore, women in the GCCCs are being diagnosed at a much younger age than females in the United Kingdom. It was also found that obesity and physical inactivity were significantly high in women, especially in the adult age range when BC is most prevalent. However, this study could not conclusively demonstrate an association between obesity and BC. Moreover, there was insufficient evidence to support an association between physical inactivity and BC incidence seen in the GCCCs.
As the majority of women in the GCCCs appeared to get BC between the ages of 40-55, many of them had not yet reached menopause. This could impact on the results of this study and may contribute to this negative finding. If this study was to be repeated the literature search could be stratified according to menopausal status or age to determine whether obesity in premenopausal women increases or decreases the risk. Additionally, early menarche is a risk factor for BC, thus a study to establish whether Arab girls are reaching menarche earlier, due to factors like obesity, could help explain the findings of this review. This study raises a few questions for the Gulf Cooperation Council and its healthcare services; why has there been such a dramatic increase in both BC and obesity? Why are women in the GCCCs being diagnosed with BC younger than British females? Is this observation linked to genetics within the Arab population? Both obesity and physical inactivity are independent risk factors for numerous non-communicable diseases, such as cancer. Thus, more research and awareness into the risk factors for BC is needed in the GCCCs.
ARTICLE HIGHLIGHTS
Research background
Breast cancer (BC) is the most common female cancer worldwide and it is well established that lifestyle factors such as physical inactivity and obesity are attributed to most cancers. In the Gulf Cooperation Council countries (GCCCs), the BC incidence rate has been increasing over the past two decades and the prevalence of obesity and physical inactivity is very high.
Research motivation
BC in the GCCCs is under studied and more research is required to increase BC awareness and decrease the disease burden. Furthermore, if one can highlight the preventable lifestyle factors contributing to this incline in BC incidence public health actions can be made to address the issue and reduce the cases.
Research objectives
This study aimed to (1) establish an increase in BC incidence over the past two decades; (2) determine whether there has been a rise in obesity and physical inactivity; and (3) detect an association between obesity and physical inactivity individually as risk factors for BC in the GCCCs.
Research methods
A mixed methods approach was used, which included a systematic review of the literature and obtaining data from various cancer registries and databases.
Research results
This study found that BC was the top malignancy within the GCCCs and the incidence has been increasing at a significant rate over the past two decades. Obesity and physical inactivity were shown to be very prevalent there and exceeded the number seen in the United Kingdom. However, there was insufficient evidence to suggest that there is a correlation between these preventable risk factors and BC in the GCC. Furthermore, this study found that women in the GCCCs tended to be diagnosed at a younger age than women in the United Kingdom.
Research conclusions
This research was unable to determine a direct correlation between physical inactivity and obesity individually as risk factors for BC in the GCCCs. However, it did find that both obesity and physical inactivity was very prevalent in all the countries. This study unexpectantly found that women in the GCCCs are being diagnosed at a younger age than women in the United Kingdom, such that many were pre-menopausal at time of diagnosis. It is thought that premenopausal obesity may actually be a protective factor for BC. Therefore, more research to explain the trend in BC incidence is required.
Research perspectives
It is clear that BC in the GCCCs is under-researched. It is also evident that obesity and physical inactivity is a major public health concern, not just for cancer but for many other medical conditions. This study found that the majority of women in the GCCCs appeared to be diagnosed before they reached menopause. Therefore, if this study was to be repeated, the literature search could be stratified according to menopausal status or age to determine whether obesity in premenopausal women increases or decreases the risk.
Footnotes
Conflict-of-interest statement: All the authors declare that they have no competing interests.
PRISMA 2009 Checklist statement: The authors confirm that the manuscript was prepared according to the PRISMA 2009 checklist.
Manuscript source: Invited manuscript
Peer-review started: December 18, 2019
First decision: December 25, 2019
Article in press: March 26, 2020
Specialty type: Oncology
Country/Territory of origin: United Kingdom
Peer-review report´s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Razek AA, Papazafiropoulou A, Seeman MV S-Editor: Zhang L L-Editor: A E-Editor: Liu MY
Contributor Information
Lara Theresa Annette Tanner, School of Medicine, University of Nottingham, Derby DE22 3DT, United Kingdom.
Kwok Leung Cheung, School of Medicine, University of Nottingham, Derby DE22 3DT, United Kingdom. kl.cheung@nottingham.ac.uk.
References
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Cancer Today Lyon, France: International Agency for Research on Cancer; 2018. Available from: https://gco.iarc.fr/today.
- 2.Hamadeh RR, Borgan SM, Sibai AM. Cancer Research in the Arab World: A review of publications from seven countries between 2000-2013. Sultan Qaboos Univ Med J. 2017;17:e147–e154. doi: 10.18295/squmj.2016.17.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grassa R, Gazdar K. Financial development and economic growth in GCC countries. International Journal of Social Economics. 2014:41: 493–514. [Google Scholar]
- 4.World bank open data-High income 2018. In: The World Bank Group. [cited 2018 October 24] Available from: https://data.worldbank.org/income-level/high-income. [Google Scholar]
- 5.Human development reports 2017. In: United Nations Development Programme. [cited 2018 December 5] Available from: http://hdr.undp.org/en/composite/HDI. [Google Scholar]
- 6.Joseph S, Slyomovics S. Women and power in the Middle East. Philadelphia: University of Pennsylvania Press; 2001. Available from: https://static.www.upenn.edu/pennpress/book/13429.html. [Google Scholar]
- 7.Diet, nutrition and the prevention of chronic diseases 2002. In: World Health Organisation. [cited 2019 January 30] Available from: https://www.who.int/dietphysicalactivity/publications/trs916/intro/en/ [Google Scholar]
- 8.Breast cancer statistics 2015. In: Cancer Research UK. [cited 2018 October 24] Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer. [Google Scholar]
- 9.Global Health Observatory (GHO) data World Health Organisation 2016. In: Global Health Observatory. [cited 2019 March 25] Available from: https://www.who.int/gho/en/ [Google Scholar]
- 10.Albeshan SM, Mackey MG, Hossain SZ, Alfuraih AA, Brennan PC. Breast Cancer Epidemiology in Gulf Cooperation Council Countries: A Regional and International Comparison. Clin Breast Cancer. 2018;18:e381–e392. doi: 10.1016/j.clbc.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Rahim HF, Sibai A, Khader Y, Hwalla N, Fadhil I, Alsiyabi H, Mataria A, Mendis S, Mokdad AH, Husseini A. Non-communicable diseases in the Arab world. Lancet. 2014;383:356–367. doi: 10.1016/S0140-6736(13)62383-1. [DOI] [PubMed] [Google Scholar]
- 12.Global status report on alcohol and health 2011. In: World Health Organisation. [cited 2019 January 31] Available from: https://www.who.int/substance_abuse/publications/alcohol_2011/en/ [Google Scholar]
- 13.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 14.Balaban S, Shearer RF, Lee LS, van Geldermalsen M, Schreuder M, Shtein HC, Cairns R, Thomas KC, Fazakerley DJ, Grewal T, Holst J, Saunders DN, Hoy AJ. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017;5:1. doi: 10.1186/s40170-016-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alokail MS, Al-Daghri N, Abdulkareem A, Draz HM, Yakout SM, Alnaami AM, Sabico S, Alenad AM, Chrousos GP. Metabolic syndrome biomarkers and early breast cancer in Saudi women: evidence for the presence of a systemic stress response and/or a pre-existing metabolic syndrome-related neoplasia risk? BMC Cancer. 2013;13:54. doi: 10.1186/1471-2407-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang C, LeRoith D, Gallagher EJ. Diabetes, Obesity, and Breast Cancer. Endocrinology. 2018;159:3801–3812. doi: 10.1210/en.2018-00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly TT, Al-Thani ABM, Benjamin K, Al-Khater AH, Fung TS, Ahmedna M, Welch A. Arab female and male perceptions of factors facilitating and inhibiting their physical activity: Findings from a qualitative study in the Middle East. PLoS One. 2018;13:e0199336. doi: 10.1371/journal.pone.0199336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Roon M, May AM, McTiernan A, Scholten RJPM, Peeters PHM, Friedenreich CM, Monninkhof EM. Effect of exercise and/or reduced calorie dietary interventions on breast cancer-related endogenous sex hormones in healthy postmenopausal women. Breast Cancer Res. 2018;20:81. doi: 10.1186/s13058-018-1009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 21.Monninkhof EM, Velthuis MJ, Peeters PH, Twisk JW, Schuit AJ. Effect of exercise on postmenopausal sex hormone levels and role of body fat: a randomized controlled trial. J Clin Oncol. 2009;27:4492–4499. doi: 10.1200/JCO.2008.19.7459. [DOI] [PubMed] [Google Scholar]
- 22.Neilson HK, Conroy SM, Friedenreich CM. The Influence of Energetic Factors on Biomarkers of Postmenopausal Breast Cancer Risk. Curr Nutr Rep. 2014;3:22–34. doi: 10.1007/s13668-013-0069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res. 2011;188:125–139. doi: 10.1007/978-3-642-10858-7_11. [DOI] [PubMed] [Google Scholar]
- 24.Ferlay J, Colombet M, Bray F. Cancer Incidence in Five Continents, CI5 plus: IARC CancerBase No. 9 [Internet]. France: International Agency for Research on Cancer; 2018. [cited 2019 March 12] Available from: http://ci5.iarc.fr. [Google Scholar]
- 25.Saudi Health Council. Saudi Cancer Registry; In: Annual reports National Health Information Centre (Internet); 2019. [cited 2019 March 12] Available from: https://nhic.gov.sa/en.
- 26.Albawardi NM, Jradi H, Al-Hazzaa HM. Levels and correlates of physical activity, inactivity and body mass index among Saudi women working in office jobs in Riyadh city. BMC Womens Health. 2016;16:33. doi: 10.1186/s12905-016-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albawardi NM, Jradi H, Almalki AA, Al-Hazzaa HM. Level of Sedentary Behavior and Its Associated Factors among Saudi Women Working in Office-Based Jobs in Saudi Arabia. Int J Environ Res Public Health. 2017;14 doi: 10.3390/ijerph14060659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NHLBI. Study Quality Assessment Tools: National Health Institutes, U.S. Department of health & Human services 2019. [cited 2019 March 23] Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 29.GRADE. GRADE Handbook. GRADEpro2013. Available from: https://gdt.gradepro.org/app/handbook/handbook.htm.
- 30.Cochrane Handbook for Systematic Reviews of Interventions. In: The Cochrane Collaboration, Cochrane Library 2011. Available from: https://handbook-5-1.cochrane.org/front_page.htm. [Google Scholar]
- 31.Global recommendations on physical activity for health 2018. In: World Health Organisation. [cited 2019 October 31] Available from: http://www.who.int/dietphysicalactivity/factsheet_recommendations/en/ [Google Scholar]
- 32.Al-Habsi A, Kilani H. Lifestyles of Adult Omani Women: Cross-sectional study on physical activity and sedentary behaviour. Sultan Qaboos Univ Med J. 2015;15:e257–e265. [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Shammari E, Bano R, Al Rashidi SMN. Impact of Physical activity and Intake of fibre and fat in the anthropometric indices of university females in Hail city of Saudi Arabia. Curr Res Nutr Food Sci. 2015:3. [Google Scholar]
- 34.Carter AO, Saadi HF, Reed RL, Dunn EV. Assessment of obesity, lifestyle, and reproductive health needs of female citizens of Al Ain, United Arab Emirates. J Health Popul Nutr. 2004;22:75–83. [PubMed] [Google Scholar]
- 35.Sayegh S, Van Der Walt M, Al-Kuwari MG. One-year assessment of physical activity level in adult Qatari females: a pedometer-based longitudinal study. Int J Womens Health. 2016;8:287–293. doi: 10.2147/IJWH.S99943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Eisa ES, Al-Sobayel HI. Physical Activity and Health Beliefs among Saudi Women. J Nutr Metab. 2012;2012:642187. doi: 10.1155/2012/642187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalid ME. The prevalence of abdominal obesity and its associated risk factors in married, non-pregnant women born and living in high altitude, southwestern, Saudi Arabia. Saudi Med J. 2007;28:1875–80. [PubMed] [Google Scholar]
- 38.Al-Malki JS, Al-Jaser MH, Warsy AS. Overweight and obesity in Saudi females of childbearing age. Int J Obes Relat Metab Disord. 2003;27:134–139. doi: 10.1038/sj.ijo.0802181. [DOI] [PubMed] [Google Scholar]
- 39.Elkum N, Al-Tweigeri T, Ajarim D, Al-Zahrani A, Amer SM, Aboussekhra A. Obesity is a significant risk factor for breast cancer in Arab women. BMC Cancer. 2014;14:788. doi: 10.1186/1471-2407-14-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Amri FA, Saeedi MY, Al-Tahan FM, Ali AM, Alomary SA, Arafa M, Ibrahim AK, Kassim KA. Breast cancer correlates in a cohort of breast screening program participants in Riyadh, KSA. J Egypt Natl Canc Inst. 2015;27:77–82. doi: 10.1016/j.jnci.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 41.AlFaris NA, ALkehayez NM, AlMushawah FI, Al Naeem AN, Al-Amri ND, Almudawah ES. A descriptive study of vitamin D and other nutritional factors in breast cancer patients in Saudi Arabia. Saudi Med J. 2018;39:564–571. doi: 10.15537/smj.2018.6.21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al Saeed E, Al Ghabbban A, Tunio M. Impact of BMI on locoregional control among Saudi Patients with Breast cancer after breast conserving surgery and modified radical mastectomy. Gulf J Oncolog. 2015;1:7–14. [PubMed] [Google Scholar]
- 43.Alsaeed E, Albeeshi M, Alsari M, Alowaini F, Alsaawi A, Bahabri I. Diabetes mellitus, hypertension, hyperlipidemia and Obesity do not affect tumour expression of estrogen and progesterone receptors in Saudi breast cancer patients. Kuwait medical journal. 2017;49:17–21. [Google Scholar]
- 44.Rudat V, Brune-Erbe I, Noureldin A, Bushnag Z, Almuraikhi N, Altuwaijri S. Epidemiology of breast cancer patients at a tertiary care center in the Eastern Province of Saudi Arabia. Gulf J Oncolog. 2012:45–49. [PubMed] [Google Scholar]
- 45.Bener A, Çatan F, El Ayoubi HR, Acar A, Ibrahim WH. Assessing Breast Cancer Risk Estimates Based on the Gail Model and Its Predictors in Qatari Women. J Prim Care Community Health. 2017;8:180–187. doi: 10.1177/2150131917696941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Huang Y, Li L, Dai H, Song F, Chen K. Assessment of performance of the Gail model for predicting breast cancer risk: a systematic review and meta-analysis with trial sequential analysis. Breast Cancer Res. 2018;20:18. doi: 10.1186/s13058-018-0947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Novick G. Is there a bias against telephone interviews in qualitative research? Res Nurs Health. 2008;31:391–398. doi: 10.1002/nur.20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murad MH, Asi N, Alsawas M, Alahdab F. New evidence pyramid. Evid Based Med. 2016;21:125–127. doi: 10.1136/ebmed-2016-110401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The Cooperation Council for the Arab States of the Gulf. In: Secretariat General of the Gulf Cooperation Council. Objectives 2019. [cited 2019 March 25] Available from: http://www.gcc-sg.org/en-us/
- 50.Luzzati T, Parenti A, Rughi T. Economic Growth and Cancer Incidence. Ecological Economics. 2018;146:381–396. [Google Scholar]
- 51.El Bcheraoui C, Basulaiman M, Wilson S, Daoud F, Tuffaha M, AlMazroa MA, Memish ZA, Al Saeedi M, Mokdad AH. Breast cancer screening in Saudi Arabia: free but almost no takers. PLoS One. 2015;10:e0119051. doi: 10.1371/journal.pone.0119051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khoja T, Rawaf S, Qidwai W, Rawaf D, Nanji K, Hamad A. Health Care in Gulf Cooperation Council Countries: A Review of Challenges and Opportunities. Cureus. 2017;9:e1586. doi: 10.7759/cureus.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdel-Aziz SB, Amin TT, Al-Gadeeb MB, Alhassar AI, Al-Ramadan A, Al-Helal M, Bu-Mejdad M, Al-Hamad LA, Alkhalaf EH. Perceived Barriers to Breast Cancer Screening among Saudi Women at Primary Care Setting. Asian Pac J Cancer Prev. 2017;18:2409–2417. doi: 10.22034/APJCP.2017.18.9.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.So VHT, Channon AA, Ali MM, Merdad L, Al Sabahi S, Al Suwaidi H, Al Ajeel A, Osman N, Khoja TAM. Uptake of breast and cervical cancer screening in four Gulf Cooperation Council countries. Eur J Cancer Prev. 2019;28:451–456. doi: 10.1097/CEJ.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 55.Age and Cancer 2016. In: Cancer Research UK. [2019 cited March 25] Available from: https://www.cancerresearchuk.org/ [Google Scholar]
- 56.Najjar H, Easson A. Age at diagnosis of breast cancer in Arab nations. Int J Surg. 2010;8:448–452. doi: 10.1016/j.ijsu.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Data-Life expectancy at birth, female (years). In: The World Bank. [cited 2019 April 25] Available from: https://data.worldbank.org/indicator/SP.DYN.LE00.FE.IN?locations=BH-OM-KW-SA-AE-GB-QAview=chart. [Google Scholar]
- 58.Alsaif MA, Hakim IA, Harris RB, Alduwaihy M, Al-Rubeaan K, Al-Nuaim AR, Al-Attas OS. Prevalence and risk factors of obesity and overweight in adult Saudi population. Nutrition Research. 2002;22:1243–1252. [Google Scholar]
- 59.Borgan SM, Jassim GA, Marhoon ZA, Ibrahim MH. The lifestyle habits and wellbeing of physicians in Bahrain: a cross-sectional study. BMC Public Health. 2015;15:655. doi: 10.1186/s12889-015-1969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Kandari YY. Prevalence of obesity in Kuwait and its relation to sociocultural variables. Obes Rev. 2006;7:147–154. doi: 10.1111/j.1467-789X.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- 61.Alothaimeen A, Ezzat A, Mohamed G, Muammar T, Al-Madouj A. Dietary fat and breast cancer in Saudi Arabia: a case-control study. Eastern Mediterranean Health Journal. 2004;10:879–886 Available from: https://apps.who.int/iris/handle/10665/119492. [PubMed] [Google Scholar]
- 62.Breast cancer risk assessment tool. In: National Cancer Institute. [cited 2019 March 27] Available from: https://bcrisktool.cancer.gov/index.html. [Google Scholar]
- 63.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 64.Guo Y, Warren Andersen S, Shu XO, Michailidou K, Bolla MK, Wang Q, Garcia-Closas M, Milne RL, Schmidt MK, Chang-Claude J, Dunning A, Bojesen SE, Ahsan H, Aittomäki K, Andrulis IL, Anton-Culver H, Arndt V, Beckmann MW, Beeghly-Fadiel A, Benitez J, Bogdanova NV, Bonanni B, Børresen-Dale AL, Brand J, Brauch H, Brenner H, Brüning T, Burwinkel B, Casey G, Chenevix-Trench G, Couch FJ, Cox A, Cross SS, Czene K, Devilee P, Dörk T, Dumont M, Fasching PA, Figueroa J, Flesch-Janys D, Fletcher O, Flyger H, Fostira F, Gammon M, Giles GG, Guénel P, Haiman CA, Hamann U, Hooning MJ, Hopper JL, Jakubowska A, Jasmine F, Jenkins M, John EM, Johnson N, Jones ME, Kabisch M, Kibriya M, Knight JA, Koppert LB, Kosma VM, Kristensen V, Le Marchand L, Lee E, Li J, Lindblom A, Luben R, Lubinski J, Malone KE, Mannermaa A, Margolin S, Marme F, McLean C, Meijers-Heijboer H, Meindl A, Neuhausen SL, Nevanlinna H, Neven P, Olson JE, Perez JI, Perkins B, Peterlongo P, Phillips KA, Pylkäs K, Rudolph A, Santella R, Sawyer EJ, Schmutzler RK, Seynaeve C, Shah M, Shrubsole MJ, Southey MC, Swerdlow AJ, Toland AE, Tomlinson I, Torres D, Truong T, Ursin G, Van Der Luijt RB, Verhoef S, Whittemore AS, Winqvist R, Zhao H, Zhao S, Hall P, Simard J, Kraft P, Pharoah P, Hunter D, Easton DF, Zheng W. Genetically Predicted Body Mass Index and Breast Cancer Risk: Mendelian Randomization Analyses of Data from 145,000 Women of European Descent. PLoS Med. 2016;13:e1002105. doi: 10.1371/journal.pmed.1002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo W, Key TJ, Reeves GK. Adiposity and breast cancer risk in postmenopausal women: Results from the UK Biobank prospective cohort. Int J Cancer. 2018;143:1037–1046. doi: 10.1002/ijc.31394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rohan TE, Heo M, Choi L, Datta M, Freudenheim JL, Kamensky V, Ochs-Balcom HM, Qi L, Thomson CA, Vitolins MZ, Wassertheil-Smoller S, Kabat GC. Body fat and breast cancer risk in postmenopausal women: a longitudinal study. J Cancer Epidemiol. 2013;2013:754815. doi: 10.1155/2013/754815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 69.Prevalence of obesity among children and adolescents. In: World health organisation, Global Health observatory 2017. [cited 2019 December 27] Available from: https://www.who.int/gho/ncd/risk_factors/overweight_obesity/obesity_adolescents/en/ [Google Scholar]
- 70.Al-Awadhi N, Al-Kandari N, Al-Hasan T, Almurjan D, Ali S, Al-Taiar A. Age at menarche and its relationship to body mass index among adolescent girls in Kuwait. BMC Public Health. 2013;13:29. doi: 10.1186/1471-2458-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141–1151. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fertility Rate, total (births per woman). In: The world bank group 2019. Available from: https://data.worldbank.org/indicator/SP.DYN.TFRT.IN. [Google Scholar]
- 73.Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, Veerman JL, Delwiche K, Iannarone ML, Moyer ML, Cercy K, Vos T, Murray CJ, Forouzanfar MH. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857. doi: 10.1136/bmj.i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amin TT, Suleman W, Ali A, Gamal A, Al Wehedy A. Pattern, prevalence, and perceived personal barriers toward physical activity among adult Saudis in Al-Hassa, KSA. J Phys Act Health. 2011;8:775–784. doi: 10.1123/jpah.8.6.775. [DOI] [PubMed] [Google Scholar]
- 75.Amin A, Partin A, Epstein JI. Gleason score 7 prostate cancer on needle biopsy: relation of primary pattern 3 or 4 to pathological stage and progression after radical prostatectomy. J Urol. 2011;186:1286–1290. doi: 10.1016/j.juro.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 76.Pinelo Silva J, Akleh AZ. Investigating the relationships between the built environment, the climate, walkability and physical activity in the Arabian Peninsula: The case of Bahrain. Cogent Social Sciences. 2018 [Google Scholar]
- 77.Middle Eastern Central Asian Studies 2019. In: EBSCO. [cited 2019 April 10] Available from: https://www.ebsco.com/products/research-databases/middle-eastern-central-asian-studies. [Google Scholar]
- 78.Middle East and Africa Database 2019. In: ProQuest. [cited 2019 April 10] Available from: https://www.proquest.com/products-services/Middle-East-Africa-Database.html. [Google Scholar]
- 79.Hébert JR, Frongillo EA, Adams SA, Turner-McGrievy GM, Hurley TG, Miller DR, Ockene IS. Perspective: Randomized Controlled Trials Are Not a Panacea for Diet-Related Research. Adv Nutr. 2016;7:423–432. doi: 10.3945/an.115.011023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harvie M, Hooper L, Howell AH. Central obesity and breast cancer risk: a systematic review. Obes Rev. 2003;4:157–173. doi: 10.1046/j.1467-789x.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 81.Alabdulkarim B, Hassanain M, Bokhari A, AlSaif A, Alkarji H. Age distribution and outcomes in patients undergoing breast cancer resection in Saudi Arabia. A single-institute study. Saudi Med J. 2018;39:464–469. doi: 10.15537/smj.2018.5.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]